- 1Center of Cell Technologies, Institute of Cytology Russian Academy of Science, Saint-Petersburg, Russia
- 2Centre of Traumatology and Orthopedics, Department of Wound Infection, Vreden National Medical Research, Saint-Petersburg, Russia
- 3North-Western State Medical University named after I.I. Mechnikov, Saint Petersburg, Russia
- 4International Laboratory of Bioinformatics, Faculty of Computer Science, HSE University, Moscow, Russia
Hyaline cartilage (HC) is a specialized connective tissue that covers the surfaces of major joints and is characterized by its limited regenerative capacity. Modern therapeutic approaches to HC restoration often do not provide complete regeneration of damaged tissue. Developed tissue engineering methods show promise as effective approaches for restoring various types of HC damage. Due to the rapid evolution of various technologies in research practice, the range of methods available for analysis of TE constructs has expanded, including for the study of tissue engineering of hyaline cartilage (TEHC). Because of the complexity of the HC’s structure, a whole range of methods is needed to assess characteristics of the scaffold, such as structure and strength. It is also important to study the behavior of cells inside the TE construct at all stages of cultivation, including post transplantation into the damaged area. The opacity of the scaffold and the complexity of its architecture often cause issues with the cell visualization and assessment of their viability. Therefore, there is a need to optimize each specific method for each specific scaffold. Despite the active study of TEHC, the results remain unsatisfactory. In this study, we have systematized data on the effectiveness and feasibility of methods to analyze structure, mechanical characteristics, cell interaction with the scaffold, and their ability to form new tissue before and after transplantation.
Introduction
The goal of this study is to analyze experimental techniques dedicated to research tissue engineering of hyaline cartilage (TEHC). The concept of tissue engineering, proposed by Langer (Langer and Vacanti, 1993) in 1993, involves the development of–Cell-engineered construct CECs based on biodegradable scaffolds, cell cultures, and chemical patterns for modulating cell proliferation or hyaline cartilage recovery. The article describes methods for evaluating different stages of research in tissue engineering of hyaline cartilage, comparing their advantages, disadvantages, areas of application, and the final results achieved.
Materials and methods
Literature search and selection criteria
A literature review was conducted to identify existing methods for assessing the effectiveness of tissue engineering of hyaline cartilage in PubMed (MEDLINE), eLIBRARY, ScienceDirect, and Google Scholar databases, retrieving literature available up to mid-2024. The article reviews original works devoted to various methods for assessing the effectiveness of hyaline cartilage tissue engineering. The search was conducted in two stages. In the first stage, we analyzed the articles and searched for fundamental methods for assessing the effectiveness of hyaline cartilage tissue engineering. In the second stage, we deepened the search and detailed the information on each of these methods. Studies were included if they simultaneously met the following criteria: (1) included experimental hyaline cartilage tissue engineering 2) included a performance evaluation method 3) were in open access. Studies were excluded if 1) the full text was not available 2) there was no connection with hyaline cartilage 3) there was no connection with tissue engineering 4) there were no methods to evaluate the experimental performance 5) the work was clinical and did not contain experimental data with animals. Subsequently, experimental studies were examined, and relevant review articles in the field were used to identify additional literature for further analysis. Experimental studies from the past 5 years were prioritized. In parallel, the searches focused on specific methods and their applications in research tissue engineering of hyaline cartilage were carried out (Figure 1).
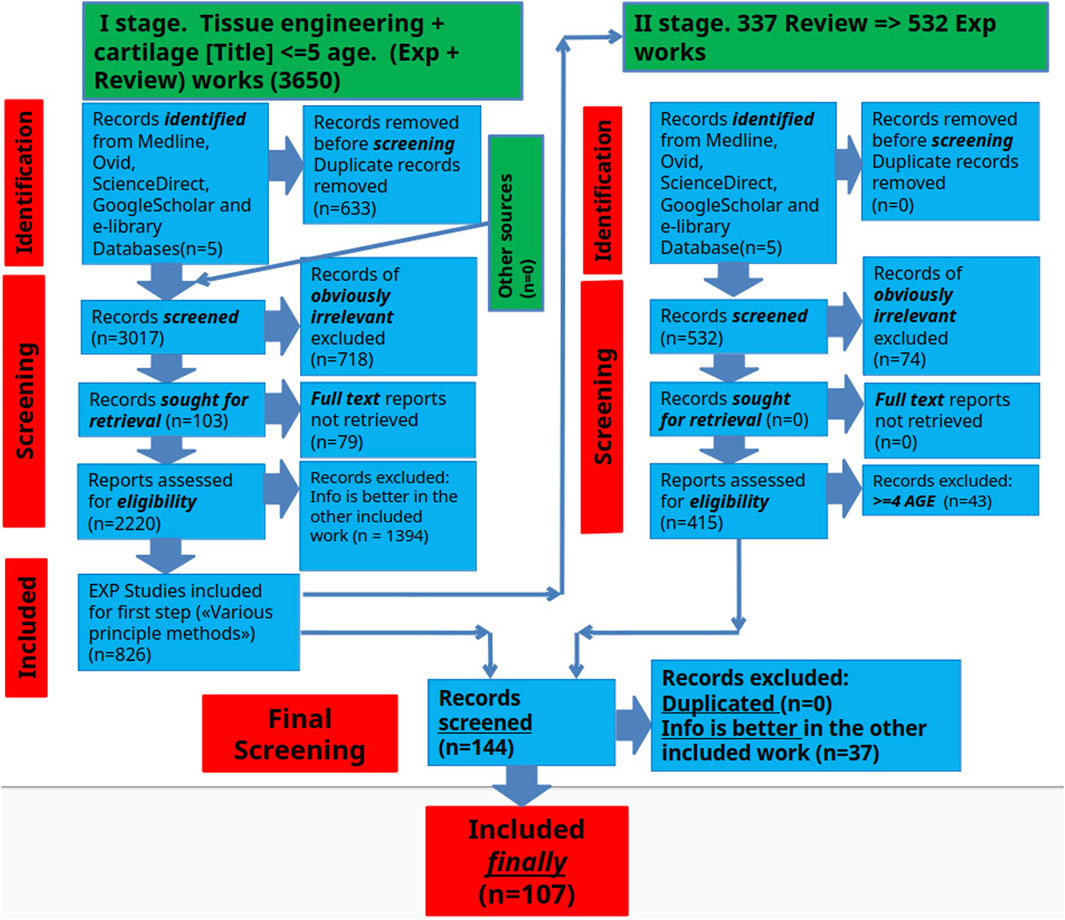
Figure 1. Schematic representation of the selection process for scientific articles included in this review.
Study selection process
The search results underwent a rigorous screening process to identify and eliminate duplicates according to the predefined inclusion criteria. This assessment aimed to select the articles that would ultimately be included in the final information extraction. The screening involved 6 independent reviewers working in pairs who carefully assessed the titles, abstracts and full texts of the manuscripts during two separate screening phases.
Data collection
The included articles were added to a spreadsheet. The selected studies were categorized according to the principle of the method. The authors did not aim to analyze all publications currently available on this topic. However, we believe that the adopted search and analysis strategy ultimately achieved the primary objectives of the study: to consolidate data on research methodologies and to evaluate their advantages and disadvantages. It is important to note that the authors define a “research method” as a “block and set of specific techniques for evaluating the effectiveness of tissue engineering of hyaline cartilage in a particular area of study.”
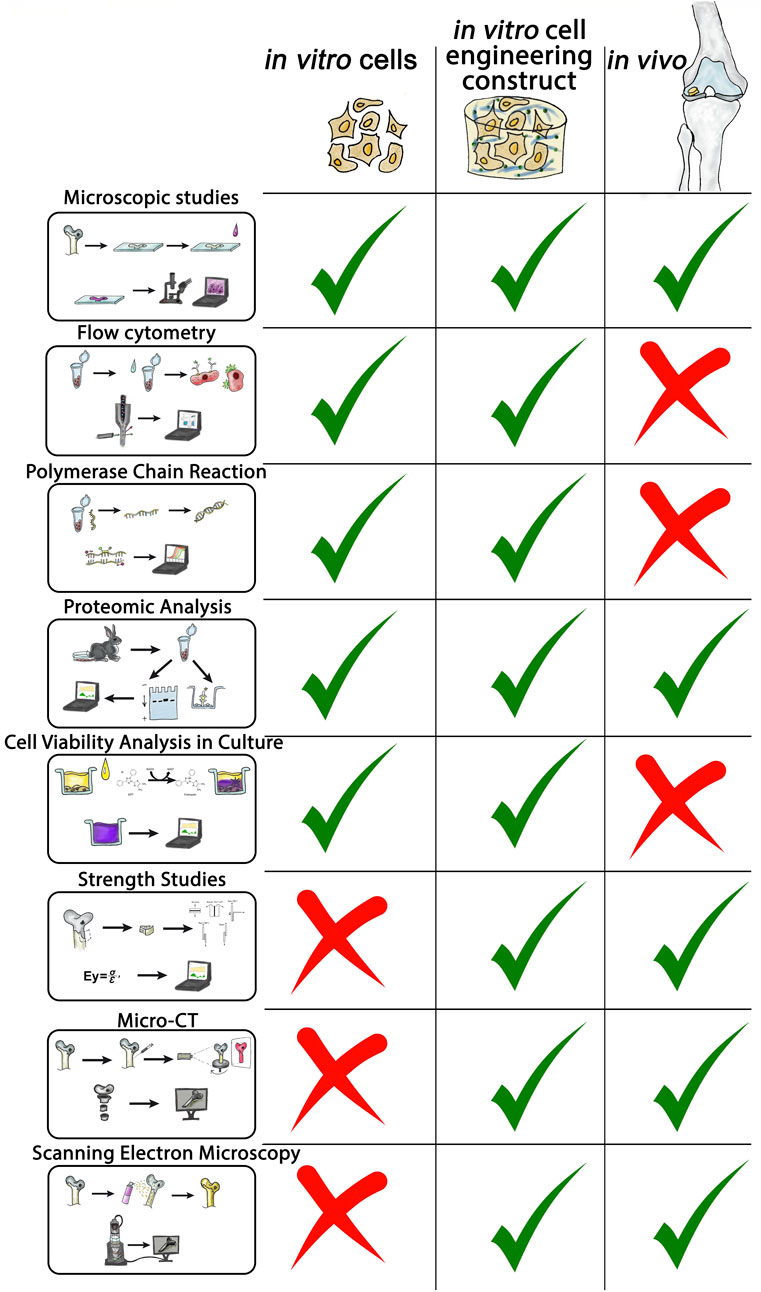
Microscopic studies
Among the methods used to assess the effectiveness of tissue engineering of hyaline cartilage, histological and/or microscopic studies hold a central position. This basic method requires specialized skills; however, it is economically affordable. Despite their two-century history (Karamanou et al., 2010) microscopy remains one of the most widely used and unbiased methods for evaluating experimental outcomes (Figure 2A). Microscopy is widely applied in ex vivo experiments to assess cell status within cultures and CECs during cultivation stages and to monitor cell proliferation and aggregation during modifications or enhancing extracellular. Matrix (ECM) synthesis (Figure 2B) (Nikolai et al., 2020). Cross-sections of cell-engineering constructs are stained with various dyes also to analyze cell proliferation and protein synthesis within designated zones (Figure 2C) (Zhou et al., 2020; Korpayev et al., 2020). Standard in vivo experiments histological analysis involves sample fixation and preparation of tissue sections containing the region of interest. (Figure 2C) (Zhou et al., 2020; Korpayev et al., 2020).
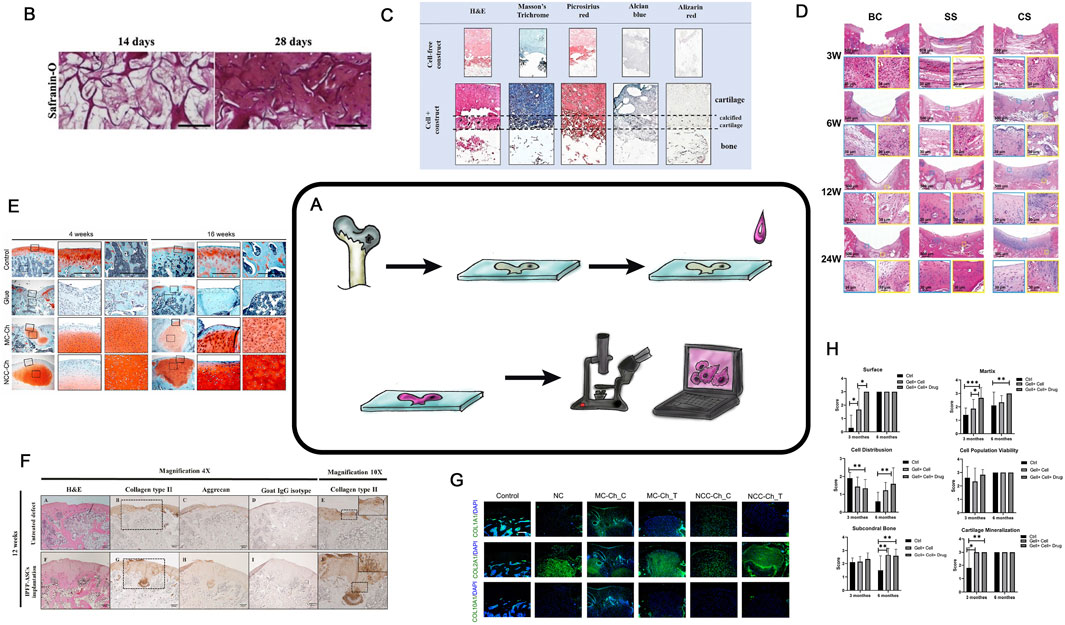
Figure 2. Histological analysis in TEHC. (A) is a schematic diagram of a histological examination. (B) Safranin-O staining of BMSCs in the FC-CS scaffolds for in vitro 14 or 28 days. Taken from the article (Nikolai et al., 2020; Zhou et al., 2020). (C) Histological examination of themulti-layered osteochondral scaffolds after 21 days of co-culture. The paraffin mounted scaffolds were sectioned and stained with hematoxylin and eosin (H,E), Masson’s trichrome and Picrosirius red for total collagen, Alcian blue for GAGs and Alizarin red for mineralization. Cell-free scaffolds were also stained as control. Taken from the article (Zhou et al., 2020; Korpayev et al., 2020). (D) Microscopic appearance of H&E-stained blank control, simple scaffold, and composite scaffold groups after 3, 6, 12 and 24 weeks. BC, Blank Control; SS, Simple Scaffold; CS, Composite Scaffold; H&E, hematoxylin and eosin. Taken from the article (Han et al., 2021a). (E) Rat joints analyzed by Safranin O/Fast Green staining. Solid boxed (superficial) and dashed boxed (subchondral bone) areas in the left column are shown at a higher magnification in the central and right column, respectively. Taken from the article (Lee et al., 2021). (F) Representative histological results obtained from the untreated defect and implanted IPFP-ASCs group at 4 weeks post-operation. H&E staining (A,F), immunohistochemistry staining for type II collagen (B,G), aggrecan (C,H), and goat IgG isotype as a negative control (D,I). Magnification 4×. Scale bars at 200 μm as indicated. Higher magnifications of both groups were enlarged from the black dotted square in the images B and G, respectively (E,J). Magnification 10×. Scale bars at 100 μm as indicated. Taken from the article (Sriwatananukulkit et al., 2022). (G) Immunofluorescence staining of regenerated cartilage for detection of COL1A1, COL2A1, and COL10A1. Nuclear DNA was labeled with DAPI Taken from the article (Lee et al., 2021). (H) Results of in vivo cartilage defect repair: International Cartilage Repair Society (ICRS) scoring of the gross appearance of the regenerated cartilage; n = 12, Taken from the article (Naghizadeh et al., 2021).
In some cases, special microscopic films are used for the preparation of sections from hard samples such as in this works (Morodomi et al., 2019; Bozhokin et al., 2021a).
Special attention should be paid to consistent 3D positioning of the sample before microtomy (Han et al., 2021a; Al-Sabah et al., 2019) (Figure 2D). The selection of fixation and decalcification protocols as well effects the state and parameters of the regenerated area, making the preanalytical stage of research critically important (Király et al., 1996). To determine the precise localization of various ECM proteins within the structural components of TEHC, histochemical analysis with stains specific to the protein properties can be also employed (Lee et al., 2021; Sriwatananukulkit et al., 2022) (Figures 2E,F). Currently, good practice declares that all images are processed to yield numerical parameters, such as ECM quantity, cellular morphology or calcification (Figure 2H) (Naghizadeh et al., 2021). Histochemical analysis can be performed on culture plastic or glass, while evaluating chondrogenic differentiation in vitro (Albert and Creech, 1941 ; Bozhokin. et al., 2021a). For detailed information on chondrogenesis, confocal microscopy is used (Figure 2G) (Lee et al., 2021). And so it is possible to assess the chondrocyte viability in their natural 3D arrangement or capture the images at different depths within native tissue or cell-engineering construct (Al-Sabah et al., 2019; Galarraga et al., 2021).
Microscopic tissue analysis results can be quantitatively evaluated using various scoring systems, based on staining intensity or ratio of stained structures or cells to total area or nuclear count (O’Driscoll et al., 1986). Images, that are evaluated using histological scoring systems (International Cartilage Repair Society grading system (ICRS), O'Driscoll), can conclude the overall experiment’s effectiveness (Chen et al., 2020; Murata et al., 2022; Sun et al., 2021; Guo et al., 2021). Unfortunately, all “semi-quantitative” histological evaluation systems are observer-dependent. In 1994, it was proposed an automated cartilage assessment based on color differences in safranin-O-stained specimens. Modern software for histological image analysis can automatically calculate numerous histological parameters (specific cell types, amounts of ECM proteins, the area of defect filling by the regenerate, and many others) with minimal time investment (Farshid Moussavi-Harami et al., 2009; Yang et al., 2019; Rutgers et al., 2010). Specialized software or scoring systems help to transform histological research results from subjective qualitative assessments to statistically significant numerical parameters. The application of AI in the analysis of histological preparations is developing dynamically. For example, in the article (Nagarajan et al., 2024) authors use an algorithm that involves evaluating histological images (e.g., safranin O staining and chondrocyte distribution) using automatic classification methods based on artificial intelligence (such as deep learning). The progression of such work over the last few years is significant; while in 2022 such software could automatically recognize individual elements on histological images (such as the lateral and medial condyles (Mori et al., 2022)), in 2023 the software learned to automatically assess the degree of OA from histological preparations (Khader and Alquran, 2023), by 2024 AI-based software was already reliably and objectively assessing the degree of hyaline cartilage repair. In the authors’ view, such tools will continue to improve and we could see an explosive growth in such work in the near future.
At present, microscopic studies in tissue engineering of hyaline cartilage are the most important reference methods by which the effectiveness of the whole experiment can be unequivocally assessed, without which no modern scientific article is published. In the near future, in our opinion, online tools using AI to evaluate experimental images using a unified algorithm are likely to appear which will greatly simplify the comparison of studies performed by different teams.
Flow cytometry
Flow cytometry (FC) is a modern technology that enables rapid, multiparametric analysis of individual cells in an automated manner (Figure 3A) (Moldavan, 1934; Gucker et al., 1947; Walles, 1956). The method is based on detecting fluorescence and light scattering (i.e., physically and biologically determining antigens on different cell types and inside the cell bodies) (Figure 3B). Quantitative characteristics of cell populations used in the TEHC projects include cell shape, proliferative and clonogenic potentials, immunological profile, morphology, phenotype (Piagnerelli et al., 2007), size (Jerald et al., 2002), viability (Ouyang et al., 2019; Rasouli et al., 2003), nucleic acid content (Lebaron et al., 2002), and intracellular processes (Darzynkiewicz et al., 2001; Piotr and Darzynkiewicz, 2004). Additionally, this method can be used to assess cell aggregation, native fluorescence, expression of surface markers, and last but not least, the cells can be sorted (Figure 3C) (Rasouli et al., 2003; Zhao et al., 2018; Jolene and Bradford, 2011).
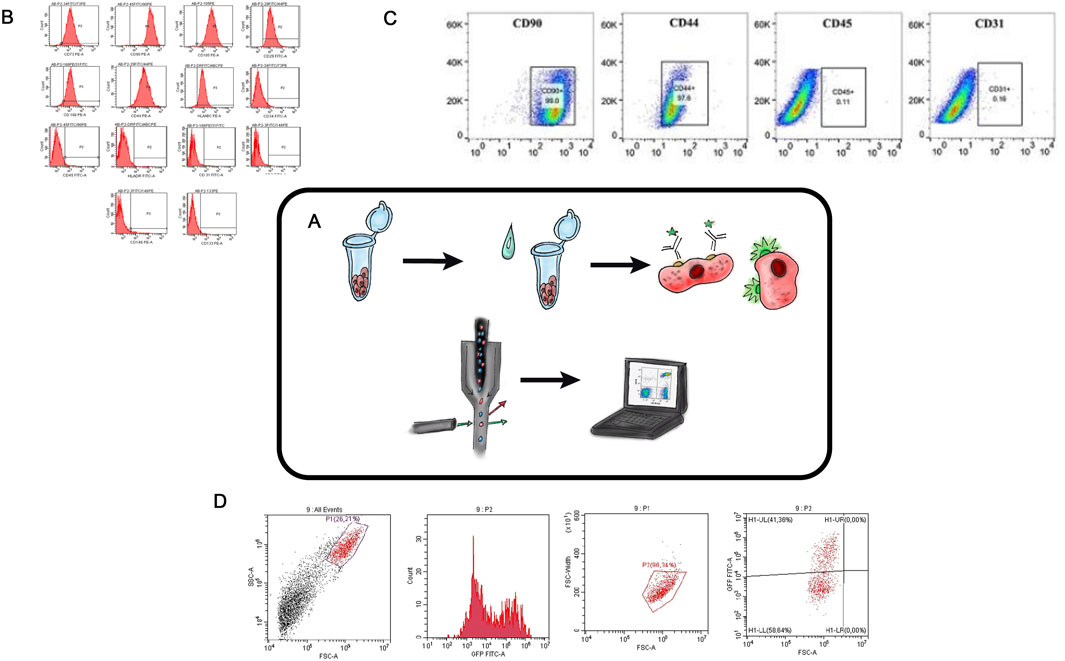
Figure 3. Flow Cytometry in TEHC (A) – Schematic diagram of flow cytometry. (B) – Collective flow cytometry histograms for mesenchymal stem cells markers (CD105, CD44, CD166, CD29, CD90, and CD73 and HLA-ABC) and antibodies specific to haematopoietic cells (CD45, CD34, CD14 and HLA-DR) were displayed for the hASCs retrieved from the confluent cultures at the 7th day of passage 2. Areas in red color indicate stained cells. hASC, human adipose-derived stem cell. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article. Taken from the article (Piagnerelli et al., 2007). (C) – FCM analysis of the expression of stem cell identification-related antibodies in rASCs. Taken from the article (Liu et al., 2022a). (D) – Cytofluorometric analysis of human dermal fibroblasts with lentiviral transduction Tgfb3 gene (authors unpublished data).
FC was used to determine the immunophenotypic profile of adipose stem cell (ASC) by analyzing the presence of mesenchymal stem cell surface markers connected to the cell-engineering constructs development (Tulin, 2020) (Figure 3B). In another study (Liu et al., 2022) the identification of chondrogenic, osteogenic, and adipogenic differentiation potentials of stem cells derived from rat adipose tissue (Figure 3C) was performed. Flow cytometry kits are now available on the market, and so the determination of immunophenotypic profiles, proliferative capacities, and DNA content assessment for different types of cells can be done simultaneously and with minimal activity (Jolene and Bradford, 2011). Flow cytometry is an efficient and precise method for evaluating the effectivity of genetic cell modifications, especially when fluorescent genes are inserted in the expression plasmid (Figure 3D) (Bozhokin et al., 2021c). A crucial practical application of FC lies in its ability to sort cells into distinct subpopulations. For example, Chen-Shuang Li investigated the chondrogenic differentiation potential of human perivascular stem cells under the influence of a combination of growth factors that were previously isolated from the human stromal vascular fraction using fluorescence-activated cell sorting (FACS) (Li et al., 2016).
An important consideration for cell-engineering constructs materials is their biocompatibility with the native tissue microenvironment and with proliferating cells. In a study which aimed to create a composite hydrogel for an HC defect repair, a composite hydrogel based on strontium alginate was compared with a strontium alginate/chondroitin sulfate composite hydrogel (Ma et al., 2019). An MTT assay was used to determine the cytotoxicity of the material and its ability to support chondrocyte proliferation, while FC was used to assess apoptosis levels in chondrocytes and the viability of cell populations depending on the hydrogel material used.
FC has extensive potential applications in studies aimed at developing new tissue engineering of hyaline cartilage approaches. The advantages of flow cytometry in this field include the ability to simultaneously analyze a large number of cells with minimal time investment, as well as the automated evaluation of cell viability and proliferative activity. FC can be used to assess the effectivity of TE constructs modifications based on fluorescent signal levels or the expression of specific surface markers. Additionally, FC enables cell sorting into distinct subpopulations, which may have varying potentials for tissue regeneration and chondrogenic differentiation. Thus, flow cytometry is an easy-to-use, economically available numerical technique for evaluating various cell subpopulations and cell modification methods.
Reverse transcription - Polymerase chain reaction
The classical polymerase chain reaction. (PCR) method was introduced by Kary Mullis in 1983 (Mullis, 1990). Reverse transcription PCR (RT-PCR) has broad applications in various fields, such as disease diagnosis, virus genotyping (Paulina Rajko-Nenow and Batten, 2022), detection and quantification of microorganisms in food products (Kingsley et al., 2010), studies of gene expression changes during cellular processes, pathological conditions, wound healing, and many more. It is also actively and routinely used in tissue engineering of hyaline cartilage (Figure 4A) (Ma et al., 2019; Nour-Eldeen et al., 2020).
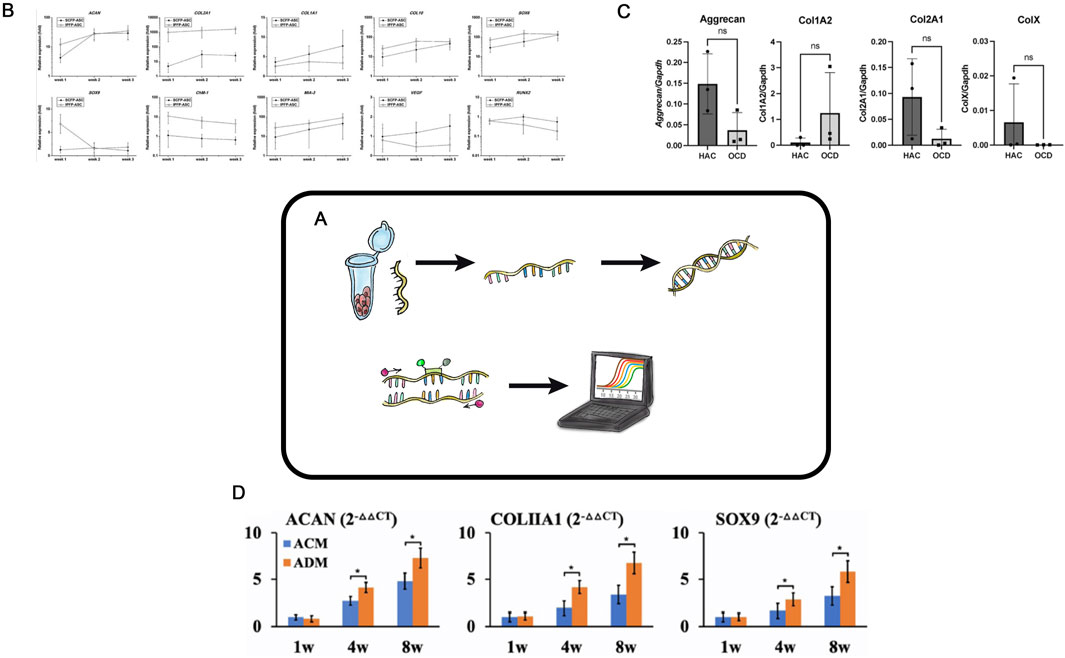
Figure 4. Reverse transcription PCR in TEHC (A) – Schematic representation of the real-time polymerase chain reaction (PCR) in hyaline cartilage tissue engineering. (B) – mRNA expression profile of the ASC-derived chondrocytes in 3D matrix. The IPFP-ASC-derived chondrocytes had higher ACAN mRNA expression than did the SCFP-ASC-derived chondrocytes at week 1 and extremely high COL2A1 expression. By contrast, the SCFP-ASC-differentiated chondrocytes exhibited significantly higher COL1A1 expression at weeks 2 and 3. Although the IPFP-ASC-derived chondrocytes had high COL10 level at weeks 1 and 2, they also had significantly higher SOX6 (weeks 1 and 2), SOX9 (week 1), ChM-1 (weeks 1, 2, and 3), and MIA-3 (weeks 1, 2, and 3) levels and lower VEGF (weeks 2 and 3) and RUNX2 (weeks 2 and 3) levels than the SCFP-ASC-derived chondrocytes in 3D matrix. ASCs, adipose tissue–derived stem cells; IPFP, infrapatellar fat pads; SCFP, subcutaneous fat pads. Taken from the article (Wang et al., 2021a). (C)– Real-time PCR analyses of OCD chondrocytes and healthy articular chondrocytes. Three OCD and HAC cartage donors were analyzed for expression of Aggrecan, Collagen type I (Col1A2); Collagen type II (Col2A1); and Collagen type X (ColX). Ns no statistically significant difference. The CT values were normalized to GAPDH housekeeping gene. All assays were performed in triplicates. Taken from the article (Vapniarsky et al., 2022). (D)– QPCR analysis of in vitro ECs in ACM and ADM groups. Expression of ACAN, COLIA1 , and SOX9 genes in ACM and ADM groups after 1, 4, and 8 weeks of in vitro culture. *P < 0.05. Taken from the article (Wang et al., 2021b).
In tissue engineering of hyaline cartilage, RT-PCR is used to evaluate changes in the relative expression of key chondrogenesis genes, including Col2A1, Col1A1, ACAN, Sox9, TGF-β3, and Comp (Figures 4B–D). The advantages of RT-PCR are its simplicity, cost-effectiveness, and the quantitative nature of results, making RT-PCR particularly suitable for the initial screenings. In a study by Ye Sun aimed at developing a CEC containing growth differentiation factor 5 and bone marrow-derived stem cells (Sun et al., 2019), RT-PCR was used to compare marker gene expression levels across experimental groups. In another work, allogenic chondrocytes were transplanted using a hybrid scaffold made of chitosan hydrogel and demineralized bone matrix to repair rabbit cartilage defects (Chen et al., 2016). RT-PCR analysis revealed increased mRNA levels of insulin-like growth factor 1, bone morphogenetic protein 7, and hepatocyte growth factor 1 month after transplantation, and so indicating activation of these genes.
RT-PCR is widely used in tissue engineering of hyaline cartilage research to select the optimal matrix for constructs, identify the best cell donors, or choose cell cultures with specific chondrogenic differentiation parameters. For example, chondrocytes isolated from various tissue regions can exhibit these various chondrogenic differentiation potentials when cultured in 3D scaffolds. In a study (Wang et al., 2021a), the expression profile of mRNA in ASCs cultured in a gelatin-based 3D scaffold was analyzed using RT-PCR, which helped to identify the most suitable cell source for TEHC (Figure 4B). In another study, authors used RT-PCR to evaluate the expression of key chondrogenesis genes in chondrocytes isolated from cartilage fragments of donors with osteochondritis dissecans compared to healthy donors (Figure 4C) (Vapniarsky et al., 2022). RT-PCR is widely used to select the most suitable scaffold for creating tissue engineering of hyaline cartilage constructs: Wang (Wang et al., 2021b) employed this method to compare the expression of some genes and demonstrated that an acellular cartilage matrix was superior to an acellular dermal matrix (Figure 4D).
Many researchers in tissue engineering of hyaline cartilage intentionally manipulate the proliferation and differentiation of cells used in CECs, modifying them via various methods to enhance their effectiveness. RT-PCR is essential in such studies where cell modification is used and where researchers evaluate the resulting chondrogenic differentiation of cells. RT-PCR is rapid and economically accessible method; it provides quantitative data to evaluate cell proliferation and chondrogenic modification, cytotoxicity and biocompatibility of matrices and scaffolds, underscoring its necessity for the primary analysis of novel tissue engineering of hyaline cartilage techniques.
RNA-seq analysis
A further development of RT-PCR method is RNAseq analysis. This method is employed to ascertain the palette and expression profiles of a variety of genes in a cell culture or subpopulation of cells. However, it is expensive and requires mandatory subsequent bioinformatic analysis. This approach is preliminary BEFORE directly experimenting with tissue engineering of hyaline cartilage. It takes quite a lot of effort to direct this tool to solve practical experimental problems.
A paucity of research has been found on the use of RNAseq analysis in tissue engineering of hyaline cartilage, which can be attributed to the complexity and economic cost of the experiment. The primary objective is to determine the most effective method of cartilage regeneration. Cell modification, encompassing the analysis of gene expression changes, constitutes a secondary yet equally significant undertaking. In article 2025, an injectable hydrogel for cartilage regeneration was investigated, and an increase in the expression of genes responsible for hyaline cartilage metabolism was shown by RNA-seq method (Zhou et al., 2025). Utilizing this methodology in study of the application of ascorbic acid to costal chondrocytes made it possible to precisely determine the alteration in gene expression profile and observe potential osteogenic differentiation and cartilage hypertrophy (Zheng et al., 2024). The method also allows to clarify which cell types are affected in OA, which gene networks regulate OA progression, and which there are cell subtypes are present in hyaline cartilage at different stages of OA. (Gu et al., 2023). Currently, this technique allows for more accurate and efficient selection of a specific cell line for use in tissue engineering of hyaline cartilage (Jiang and Tuan, 2015). Another potential use of this technique is the preliminary analysis of cell culture by scRNA-seq of banked and already described cell culture. (Gu et al., 2023). However, the impact of the methodology and its potential for implementation in tissue engineering of hyaline cartilage is still indirect. The methodology is complex, economically unprofitable and requires solving a large number of technical and computational problems. However, it is worth noting that it is possible to use AI learning technologies to help with the decoding of the data obtained and thus, perhaps, one of the difficulties of using this method will be solved (Gu et al., 2023).
Proteomic analysis
The primary functional role in HC is performed by ECM proteins. Assessing the protein composition of the regenerate or developed CECs is a key analytical task for evaluating the efficacy of tissue engineering of hyaline cartilage. Methods for studying protein composition can be divided into semi-quantitative (“presence or absence” of specific proteins) and quantitative (determining the amount of protein per mass or volume unit) (Figure 5A).
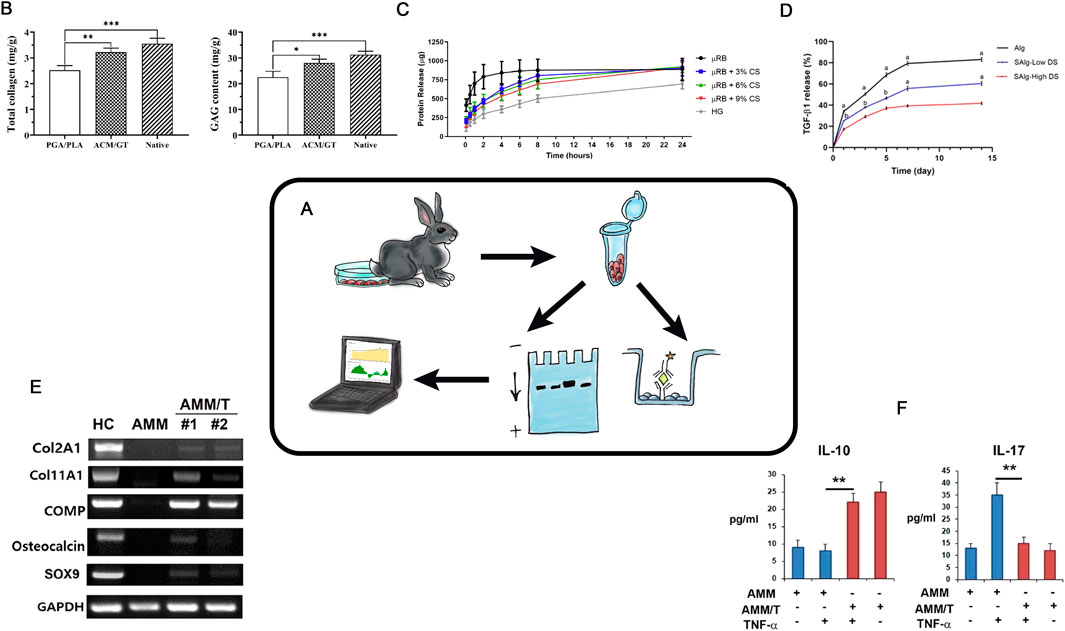
Figure 5. Protein Analysis in TEHC (A) – Schematic representation of the protein analysis in hyaline cartilage tissue engineering. (B)– Total collagen, GAG content. Taken from the article (Jia et al., 2021). (C)– Protein elution profile of BSA from all scaffolds over 24 h (n = 5/group). Taken from the article (Rogan et al., 2020). (D)– Percent cumulative transforming growth factor- β1 (TGF-β1) release from hydrogels over 14. Taken from the article (Payam et al., 2021). (E)– Western blot. Protein synthesis level. Taken from the article (Chae et al., 2021). (F)- ELISA results showed that coculture with AMM/T resulted in significantly higher IL-10 and lower IL-17A levels in cell culture supernatant compared with AMMs. Taken from the article (Chae et al., 2021).
Currently, polyacrylamide gels (PAGEs) are widely used to separate protein (Burnette, 1981). After the protein separation, staining and semi-quantitative assessment can be performed with immunoblotting (i.e., Western blotting) (Alwine et al., 1977; Hawkes et al., 1982) (Figure 5E). The determination of the protein here relies on the specific interaction between antigen and antibody. Currently, commercially available kits allow for the semi-quantitative assessment of specific protein release even at the in vitro stage (Figures 5B–D,F). Depending on the modifications and the use of different cell-engineering constructs, the content of specific ECM proteins can be evaluated both in vitro and in vivo with precision down to nanograms.
In HC defects repairing studies, the selection and evaluation of cell-engineering construct parameters are impossible without the analysis of the protein composition. Researchers use semi-quantitative methods as the PAGE and immunoblotting, but more commonly (and preferably), highly accurate quantitative methods based on ELISA (Enzyme-linked immunosorbent assay) should be applied. ELISA can precisely determinate the absolute ECM protein amounts in the given CECs, providing critical insights into the effectiveness of the entire methodology at both intermediate and final stages of research. For tissue engineering of hyaline cartilage, it is the optimal protein composition that is important for the formation of a regenerate resistant to mechanical stress. In studies where cell culture modification is used, it is a prerequisite to confirm this by analysing the changes in protein composition. These analyses are simple, economically accessible methods for the evaluation of the quantitative protein composition in both in vitro and in vivo experiments.
Biosensors
A new and dynamically developing area is the use of biosensors for the determination of protein composition. To date, existing methods for detection of specific proteins are mainly based on ELISA assay. However, ELISA assays have the following disadvantages, such as lack of accuracy in detecting small amounts of proteins, false positive results, and significant analysis duration. Such methods cannot be used for early diagnosis of diseases and/or for detection of small amounts of proteins. A biosensor is a device that provides an electrical pulse reading from a test preparation depending on the concentration of a particular protein. For the current period biosensors for detection and sensing are fundamentally divided into are electrochemical, optical, Quartz crystal microbalance (QCM), molecular and wearable biosensors (Wang et al., 2010). The advantages of such biosensors are as follows: ease of use, accuracy of measurements, possibility of mass production, low cost (when they are put into mass production). This new direction is dynamically developing; however, it has not yet received mass use due to the complexity of development and design. An example of such a biosensor for the detection of an early marker of osteoarthritis was given in a clinical article, where a measurement accuracy of 0.2 * 10−18 per ml of solution was reported (Lv et al., 2024), (Ahn et al., 2011). For some diseases associated with OA where additional detection accuracy is required (such as juvenile idiopathic arthritis, for example), such biosensors may be the only solution (Rodovalho et al., 2018). Thus, these findings are being actively applied already in clinical practice and allow for precision assessment of parameters such as protein composition, etc. We could not find works that combine classical tissue engineering experiments on animals and the use of biosensors; however, this is apparently a matter for the near future.
Cell viability analysis
An essential stage in tissue engineering of hyaline cartilage involves a development of cell-engineering construct composed of a cell culture and a biodegradable scaffold. However, achieving a proliferating cell culture on the surface or within the scaffold remains a challenging technological task. Therefore, evaluating the viability of cells cultured in 3D conditions is a critical and necessary intermediate step in modern tissue engineering of hyaline cartilage (Figure 6A).
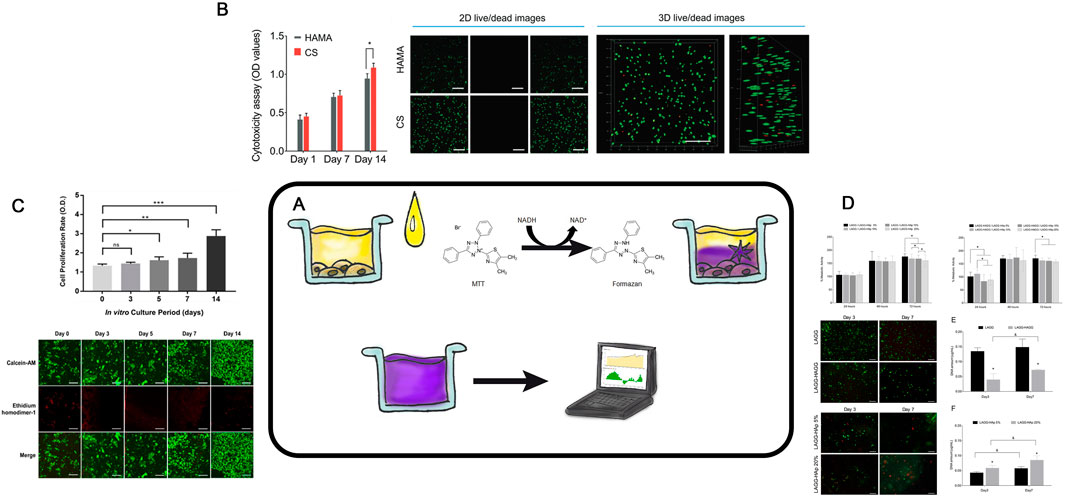
Figure 6. Cell Viability Analysis (A)– Schematic representation of the MTT assay procedure. (B)– CCK8 assay of hBMSCs encapsulated in the indicated hydrogels and 2D live/dead staining images of hBMSCs after encapsulation in the HAMA and CS hydrogels for 7 days. Taken from the article (Liu et al., 2020). (C)– In vitro Cell proliferation and viability assay for human chondrocyte in Silk-GMA hydrogel. CCK 8 assay for cell proliferation rate increasedaccording to culture period, gradually. Data are shown as the mean ± SD (*p < 0.05, **p < 0.005 and ***p > 0.0005, respectively). Confocal microscopic images for Live & Dead assay with Calcein-AM (live cells, green fluorescence) and ethidium homodimer-1 (dead cells, red fluorescence) staining showed that human chondrocytes were proliferated well in 30% of Silk-GMA hydrogel up to 2 weeks cultivation (Scale bar = 500 μm). Taken from the article (Hong et al., 2020). (D)- In-vitro screening of cytotoxicity of BHC. Metabolic activity of L929 exposed to LAGG/LAGG-HAp and LAGG-HAGG/LAGG-HAp extracts respectively, for a period of 72 h. (*) Indicates a significant difference between groups for the same time point (p < 0.05). Live/dead staining by Calcein AM/PI, of chondrocytes within LAGG and LAGG-HAGG and osteoblasts within LAGG-HAp 5% and 20%. OC-derived cells were cultured for 7 days. Scale bar represents 200 m. Proliferation of the embedded chondrocytes and osteoblasts within respective formulations up to 7 days. (*) Indicates a significant difference between groups for the same time point and (&) indicates a significant difference between time points for the same formulation (p < 0.05). Taken from the article (Pereira et al., 2018).
The simplest and the most accessible method to recognize the potential cytotoxic effects of a scaffold on the living cells involves an analysis of exudates after the scaffold co-incubation. During the incubation, scaffold components may leach into the media, negatively affecting cell viability (Lee et al., 2020a). The most commonly, the MTT assay is used. It measures the ability of NADPH- dependent cellular oxidoreductases to reduce the tetrazolium dye 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide into insoluble formazan (Figure 6B) with can later be detected spectrophotometrically. For example, Gögele evaluated the potential cytotoxicity of glass-containing polylactide-glycolide copolymer scaffolds using the MTT assay (Gögele et al., 2022). The most accurate method is to analyze the viability of cells directly in contact with the scaffold, often using again the modified MTT assay. Haghighi (Paniz and Shamloo, 2021) used it to evaluate the viability of chondrocytes cultured in silk fibroin-based scaffolds. This method has limitations, such as the potential sorption of formazan by the scaffold, which may decrease the optical density of the analyzed solution.
The MTT assay also enables comparative analysis of how many viable cell within the scaffold (Vinod et al., 2019). Cell viability in the gels was determined via gel staining, which is only possible with optically transparent gels. An alternative to the MTT assay is the MTS assay (Payam et al., 2021), which works on a similar principle. Sun et al. assessed chondrocytes in scaffolds based on methacrylated polyethylene glycol using MTS, analyzing optical density at 492 nm after a 4-h incubation (Sun et al., 2015).
Calcein staining is another accurate and specific method to determine the cell viability for both cultured on the scaffold surface and within it (limited to optically transparent gels) (Liu et al., 2020; Vinod et al., 2019) (Figures 6C,D). This reagent can penetrate the cell membrane of living cells, where intracellular esterases cleave its acetoxymethyl group, causing calcein to fluoresce in the green spectrum. Simultaneously, propidium iodide can be added to identify dead cells. For instance, using fluorescence microscopy, Gögele and authors assessed the viability of chondrocytes on the surface of a polylactide-glycolide copolymer-based scaffold after calcein and propidium iodide staining (Gögele et al., 2022). The rate of cell viability, expressed as the ratio of alive cells to the total number of cells (both live and dead), was analyzed in Acar’s work with polymer (Karabıyık Acar et al., 2021), as well as in a series of studies involving gels (Hong et al., 2020; Pereira et al., 2018; Oyadomari et al., 2021). The transparency of chitosan- and hyaluronic acid-based gels allows for evaluating of chondrocytes not only on the scaffold surface but also throughout the entire gel depth. Sun and colleagues in their study assessed cell viability using calcein staining (Sun et al., 2015). Another solution suitable for FC and microscopy is the commercial ViaQuant™ Far-Red Dead Cell Staining Kit, designed to distinguish live and dead cells. This kit relies on a reaction of a fluorescent dye with cellular amines and emits light in far-red range, being applicable for in vivo studies (He et al., 2021). For the effective implantation of cell-engineering constructs and regeneration of the modelled defect, it is important to achieve minimal cytotoxicity of the scaffold for the cell culture used and to use methods to assess cell viability at the in vitro stage. This type of method is a simple, cost-effective way to obtain numerical data on cytotoxicity and cell viability in combination with the scaffold. Currently, evaluating the precise viability of cell cultures within opaque 3D objects remains a complex and unresolved challenge. Traditional methods are not fully reliable in such cases and no universal method has been developed for this purpose.
Strength studies
Due to its high content of proteoglycans and collagen fibrils, hyaline cartilage is characterized by high strength, resilience, elasticity, and density. Thus, it is enabling to withstand significant loads during the body’s physiological activity (Liu and Karan, 2021). A key task for the full recovery is to create an implant with physical and mechanical properties similar to those of hyaline cartilage. Therefore, during the development of cell-engineering constructs, it is critically important to evaluate their mechanical properties, specifically the ability of the experimental construct to endure mechanical loads (Galarraga et al., 2021) both in vitro and in vivo (Figure 7A).
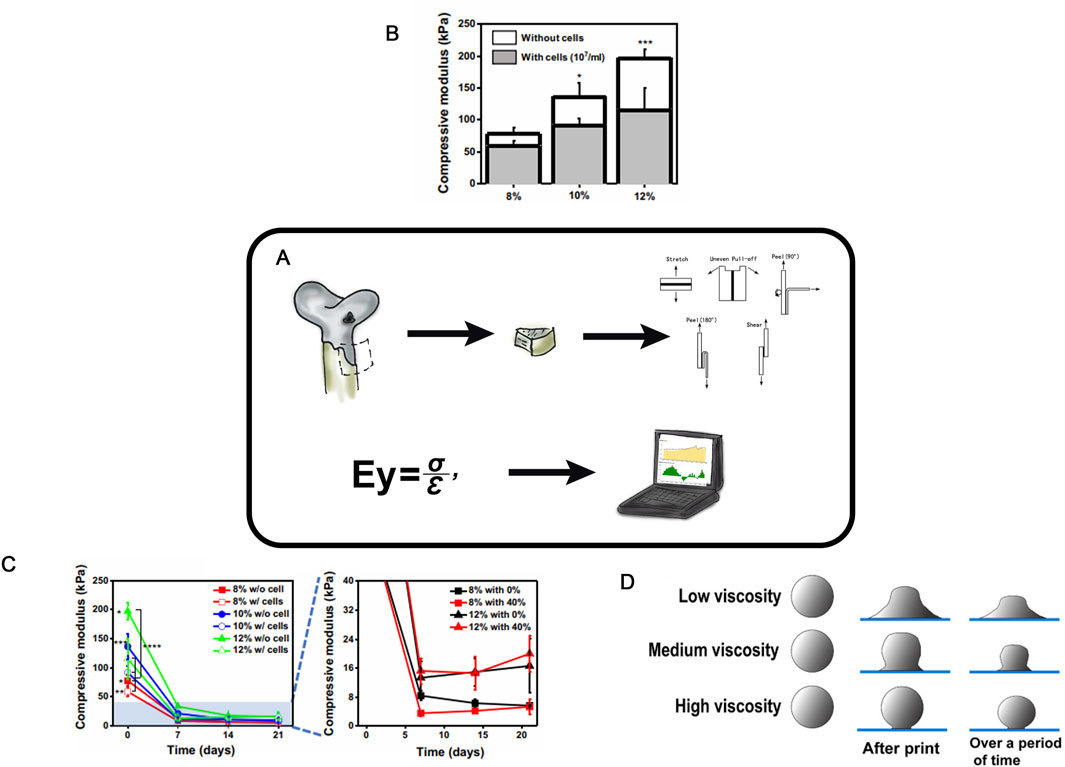
Figure 7. Strength Studies in TEHC (A)– Schematic of strength testing of cellular-engineered constructs (B)- Compressive modulus of PEG/OMA hydrogels (8, 10, and 12% PEG/OMA) with or without cells on day 0 (n = 5) (One-way ANOVA with Tukey’s significant difference post hoc test; *P < 0.05 and ***P < 0.005 compared with 8% without cells). Taken from the article (Lee et al., 2020b). (C)- Time profile of hydrogel degradation without compression for 21 days (n = 5). (One-way ANOVA with Tukey’s significant difference post hoc test; *P < 0.05 compared with 10% without cell group, **P < 0.05 compared with 12% with cell group, ***P < 0.005 compared with 8% with cells, and ****P < 0.005 compared with 12% without cell group at day 0.) Taken from the article (Lee et al., 2020b). (D)- Schematic diagram of MEW electrospinning with different viscosities. Taken from the article (Han et al., 2021a).
There are certain examples of dynamic changes in the physical properties of cell-engineering constructs depending on their composition (cellular, gel-based, etc.) (Figures 7B,C). The creation of mechanically resilient cell-engineering constructs, for instance, from gels, can be achieved by increasing the polymer concentration in the scaffold or enhancing crosslinking via higher levels of a crosslinking agent (Loebel et al., 2020; Stephanie and Anseth, 2002), as well as by the formation of composite hydrogels with polymers of diverse chemical structures and mechanical properties (Hashemibeni et al., 2020; Ciardulli et al., 2020; Ali et al., 2020). These solutions increase the mechanical stability of the scaffold by enhancing its density and, typically, reducing the pore size. However, such dense constructs can impair cell migration into the scaffold, thereby reducing cell viability (Liu et al., 2022b; Han et al., 2021b).
Hoenig et al. assessed the effect of subchondral bone permeability on the properties of CEC (Hoenig et al., 2013). Native cartilage-bone cylinders retrieved from pigs were cultured for 2 weeks in a bioreactor under a mechanical load, with and without restricted bone permeability. The Young’s modulus and stiffness of each cartilage sample were determined using an unconfined compression test consisting of five sequential deformation loads and six loading cycles.
Middendorf investigated the complex mechanical behavior, function, and temporal changes in cultured in vitro tissue engineering of hyaline cartilage via compression, friction, and shear tests (Middendorf et al., 2017). The compression and friction tests revealed improved properties of the construct with prolonged culture time. The elasticity correlated with glycosaminoglycan (GAG) content, while the improved friction coefficients were associated with increased lubrication of the construct surface (Gregorio et al., 2019). The elasticity or compressive modulus of HC scaffolds were evaluated via uniaxial compression tests (Lee et al., 2020b). Typically, cylindrical samples are prepared under physiological conditions in a swollen state to mimic in vivo situation. The compressive modulus is calculated as the slope of the stress-strain curve during deformation, and can be improved by modifying the cell-engineering construct structure or components (Figure 7B). A decrease in scaffold stiffness—and thus a reduction in the compressive modulus—can be caused by intra-scaffold cell culturing (Figure 7C).
A precise control of polymer viscosity, elasticity, and phase transitions is particularly important during the scaffold formation (Gregorio et al., 2019). Phase transitions can be evaluated by the rheological properties with a rheometer (Figure 7D) (Han et al., 2021a). Polymer rheology can indicate mechanical resilience as well as confirms an increased crosslinking in hydrogels.
By using different scaffolds in combination with different cell cultures (possibly pre-modified), researchers obtain different physical and mechanical properties of cell-engineering construct. In order to analyze the influence of each of these factors on the final parameters of the resulting objects, it is necessary to apply just this set of techniques.
The primary goal is the quantitative analysis of the mechanical parameters of developed cell-engineering construct to create optimized constructs for HC repair and to study the effects of various cell-engineering construct parameters (or cell modifications) on these properties. Specialized equipment is required for the mechanical tests, but the experiments themselves are simple and economically accessible.
Micro-computed tomography (MCT)
MCT, first developed in the early 1980s (Flannery et al., 1987; Feldkamp et al., 1989) is an X-ray imaging method that enables the acquisition of 3D images of objects (Figure 8A). It is now actively used in tissue engineering of hyaline cartilage research, both in experimental in vivo research (Ki et al., 2018; Batiste et al., 2004), and in assessment of scaffold and construct characteristics (Tsai et al., 2015; Bertoldi et al., 2011; Liao et al., 2015; Swieszkowski et al., 2007) and morphological changes in articular cartilage under physical stress (Rapagna et al., 2022) and during aging (Moncayo-Donoso et al., 2019). MCT provides a detailed imaging of tissue engineering of hyaline cartilage (Figure 8B) in animal models (Figure 8C) with high resolution, making it particularly suitable for the small size experimental animals. Another important feature of the technique is an opportunity to create 3D models of the study area (Figure 8E).
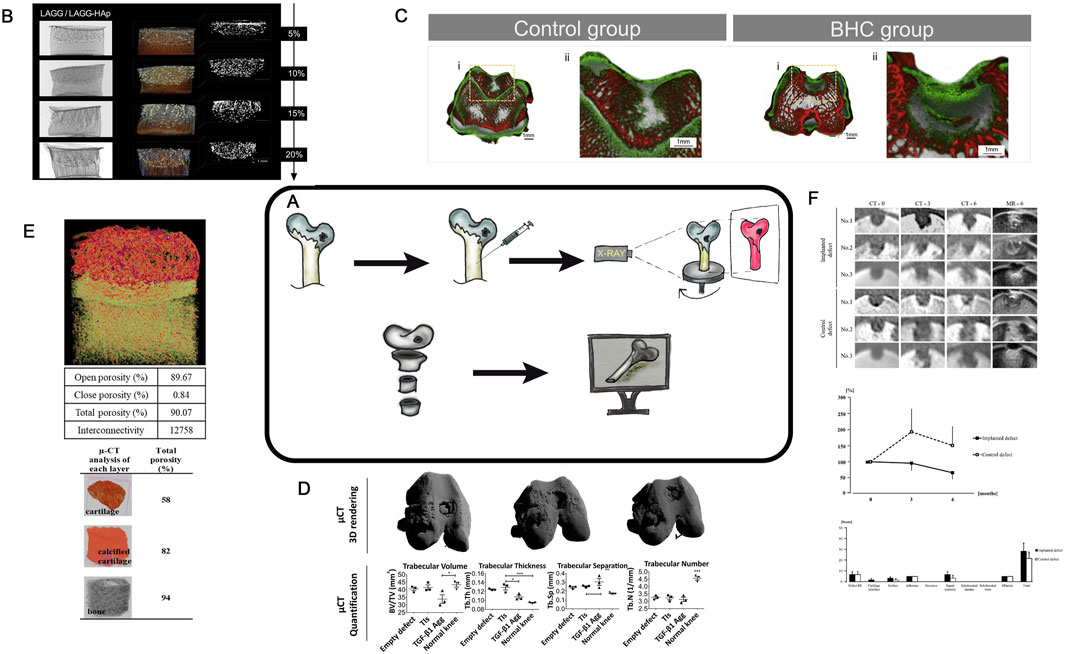
Figure 8. (A)– Schematic diagram of MCT approach. (B)- MCT analysis of different experimental CEC. Scale bar represents 1 mm. Taken from the article [71=60]. (C)- MCT micrographs of the explanted rabbit’s knees at 4 weeks after surgery for Control group and Experimental group. 3D explant images showing hard tissue (bone-like tissue , red colour) and soft tissue (cartilagelike tissue & hydrogel, green colour). Taken from the article (Pereira et al., 2018). (D)- MCT 3D rendering showed subchondral bone regeneration at the osteochondral defect site. Quantification and characterization of new subchondral bone formation at the defect site was performed by analyzing a region of interest 1.5 mm diameter × 1 mm depth. n = 3 animals per condition. One-way ANOVA followed by Tukey’s multiple comparison test was used to analyze the results. *P < 0.05, **P < 0.01, ***P < 0.001. Taken from the article (Mendes et al., 2018). (E)- MCT representation and porosity evaluation of multi-layered scaffold and each distinct layer. The red dots in the multi-layered view represent the nHA particles. Taken from the article (Korpayev et al., 2020). (F)- MCT assessment of osteochondral defects. MCT images show one cross-section of the multi-planar reconstruction images at one (CT-0), three (CT-3), and six (CT-6) months after the surgery in No. 1, No. 2, and No. 3, and MR images (MR-6) show the images corresponding to the MCT images at 6 months after surgery. Line graph shows the averages of RV (radiolucent volume) percentages at the third and sixth months against those at month zero in both defects. Bar graph shows the averages of the items in the Modified 2D-MOCART scores based on the images of MR-6 Taken from the article (Murata et al., 2022).
MCT enables both the visualization and quantitative assessment of bone and cartilage 3D tissue formation during the implantation of tissue engineering of hyaline cartilage in animal models (Murata et al., 2022; Duke et al., 2009; Jaecques et al., 2004; Hutmacher, 2005). This technique helps to examine samples in vitro, in vivo, and ex vivo (Figures 8B–E). Saey Tuan Ho et al. compared MCT with other methods for characterizing scaffolds in TE and highlighted several advantages of the technique (Ho and Hutmacher, 2006). These include the ability to assess scaffold porosity, its interconnections, surface, and permeability (Figures 8D–F). MCT was used to evaluate and visualize different regions of a chitosan- and collagen-based construct after implantation (Figure 8E) (Korpayev et al., 2020). In another study (Swieszkowski et al., 2007), MCT was employed to evaluate similar parameters for biphasic scaffolds composed of polycaprolactone and fibrin, as well as polycaprolactone and tricalcium phosphate. The authors seeded cells into these constructs, created defects, implanted the constructs and evaluated the recovery with MCT. Mendes et al. used MCT to compare various constructs, enabling both visual and quantitative assessment of the cell density in regenerative regions (Figure 8E) (Mendes et al., 2018).
For a more detailed visualization of soft tissues such as cartilage, MCT can be combined with Equilibrium Partitioning of Ionic Contrast agents (EPIC- MCT) (Frank et al., 2005). Recently, contrast agents such as iothalamate (Cysto-Conray® II) (Entezari et al., 2014) and ioxaglate (Hexabrix®) (Kerckhofs et al., 2014) have been developed, enabling MCT imaging of unmineralized cartilage due to the charged nature of the cartilage ECM. Xiao-Fei Li used EPIC-MCT to confirm age-related changes in sulfated glycosaminoglycan (sGAG) to describe cartilage degeneration (Li et al., 2015). Palmer et al. demonstrated that EPIC- MCT is a quantitative, non-biased, noninvasive, and highly accurate method to assess cartilage composition and 3D morphology in cartilage degeneration studies (Palmer et al., 2006).
MCT is a high-precision, noninvasive approach for quantitative evaluation of regenerative changes and in vivo morphology in studies of cartilage degeneration and repair after experimental interventions (both in vivo and in vitro). This method is simple and cost-effective, but requires specialized equipment, especially for the cell-engineering construct analysis. MCT helps to quantify the defect progression and replacement in HC of animal models with a high resolution (up to 6 µm). The simultaneous visualization of soft tissues can be achieved via contrast enhancement. MCT holds a great potential for further application, as it enables noninvasive computation of numerous numerical parameters related to the internal structure of constructs. By date, there are certain steps in development of automated multiparametric analysis protocols for constructs using MCT (Mendes et al., 2018), including the use of AI to analyze such regenerative changes has become widespread.
Scanning electron microscopy (SEM)
SEM visualizes of the surface of a sample up to 10 nm range (Figure 9A). The potential for applying SEM to HC research was first reported in 1971 (Clarke, 1971). The sample surface is coated with a conductive layer and then placed in an electromagnetic field. By analyzing the deflection of the electron beam generated by an electron gun, it becomes possible to visualize the surface.
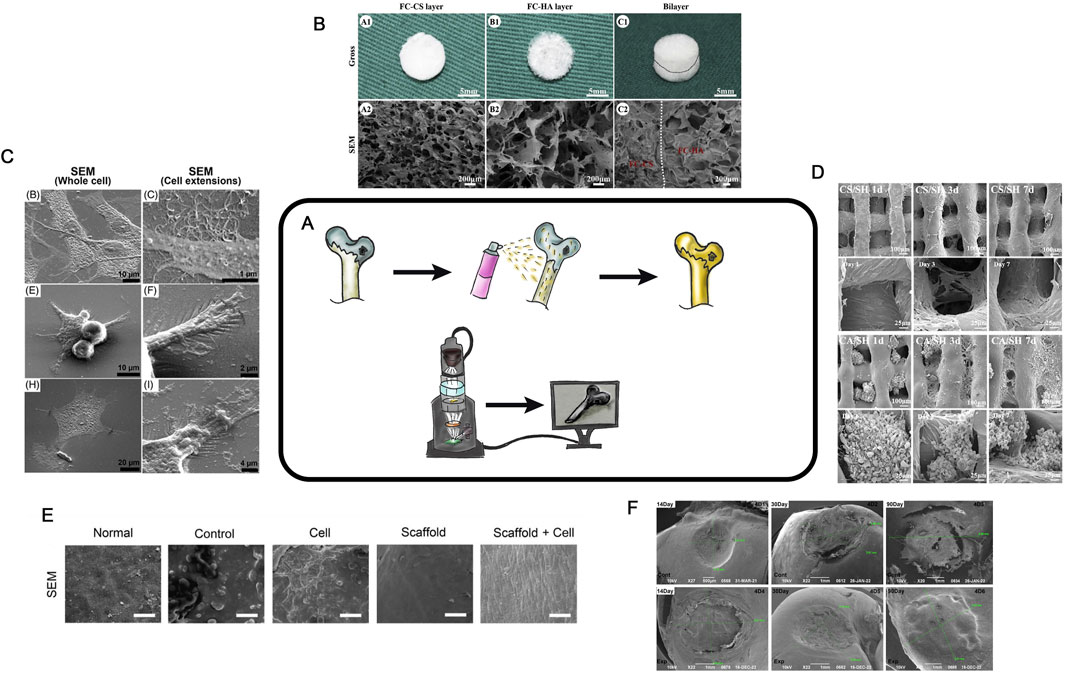
Figure 9. SEM in TEHC (A)– Schematic diagram of the SEM method. (B)– Macroscopic and microscopic views of fish collagen chondroitin sulfate and fish collagen hydroxyapatite scaffolds. The gross (A1-C1), SEM (A2-C2). Taken from the article Zhou et al. (2020). (C)– SEM micrographs taken after 3 days of culture (middle and right) of chondrocytes grown in different groups. (Katrín et al., 2022) (D)– SEM of different groups at 1, 3 and 7 days. Taken from the article Li et al. (2021) (E)– SEM images of Surface area, normal group is smooth, MF and cell group was smoother than those of the other three groups, and in scaffold cell groups the cells were attached to scaffold in cartilage tissue is visualized (scale bar = 30 μm). Taken from the article Dadgar et al. (2021) (F)- SEM images of Surface area, measurement of resulting hyaline cartilage defects. (authors unpublished data).
SEM is now widely used for examining scaffold porosity (Figure 9B) (Zhou et al., 2020). It can also be employed to analyze individual cells on the surface (Figure 9C) (Katrín et al., 2022). After cell seeding and the formation of CEC, SEM enables the analysis of cell proliferation, migration, and distribution both on the surface and within the scaffold over various cultivation periods (Figures 9D,E). During in vivo studies, SEM can be used to evaluate the regeneration area after CEC implantation. It allows assessing of the surface structure, the defected or regenerated area, the contact zone of the scaffold and surrounding tissues at the defect margin, and provides data for scoring systems (e.g., International Cartilage Repair Society - ICRS) (Figure 9F) (Zheng et al., 2019). This technique enables a detailed visualization of the structures. The advantages of SEM include the simplicity of sample preparation (typically limited to drying and dehydration), the ability to obtain numerical data (e.g., pore sizes, sample or defect dimensions, cell distribution on the surface, and surface layer structure), and its high-resolution imaging. This technique is cost-effective, but requires a direct scanning electron microscope. SEM is not strictly necessary, but allows good visualization of the area of interest at all stages.
FDA-approved methods
The significant progress made in experimental tissue engineering of hyaline cartilage implies the introduction of similar techniques, with some time lag, into clinical practice. The set of techniques for analyzing clinical efficacy differs somewhat between experimental and clinical practice. For logical and understandable reasons, non-invasive or minimally invasive techniques take precedence in clinical practice. In clinical practice mainly used are: MRI (CT) diagnosis, X-rays, questionnaires and arthroscopy in rare cases. Protein assay or RT-PCR methods are sometimes used and biosensors have begun to be introduced to provide a wide range of data. The authors are aware of only a few studies involving the use of histological (invasive) methods of analysis. SEM and histology are not used at all. Flow cytometry is also not investigated directly from the area of interest (at the site of cell-engineering construct transplantation). We believe that invasive methods of analysis should ideally be used at the stage of cell-engineering construct preparation to confirm its safety and efficacy, whereas after implantation only minimally invasive methods should be used to minimize additional trauma to the patient.
Conclusion
Experimental tissue engineering of hyaline cartilage is a technologically complex field that requires researchers to apply a wide range of methods, both during the experimental phase and in the evaluation of results. In this work, we highlighted the main methods used to evaluate the experimental effectiveness of tissue engineering of hyaline cartilage (Figure 10).
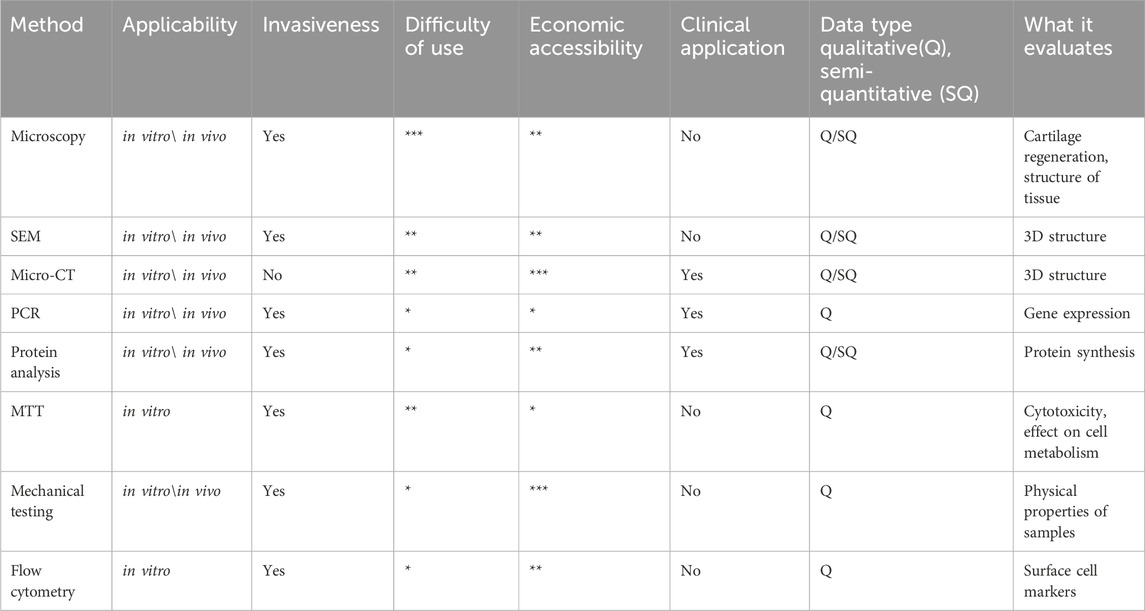
The listed and analyzed methodologies allow creating of a summary table outlining their applications, advantages, and limitations (Table 1). The relationship between the various methods is shown additionally in the attached filе (Supplementary Figure S1).
All the employed methods have become quantitative, suitable for comparative analysis across studies. Among these methods, histological analysis remains particularly important. In our opinion, histology is an essential reference method to be compared to all other techniques. Modern tissue engineering of hyaline cartilage studies employ a wide variety of techniques and require access to advanced equipment or close collaboration of multiple laboratories to work in one direction. Thus, a defining feature of contemporary experimental tissue engineering of hyaline cartilage studies is the comprehensive application of all (or nearly all) of the aforementioned methods for effective analysis.
Author contributions
MB: Project administration, Conceptualization, Methodology, Investigation, Supervision, Writing – review and editing, Writing – original draft, Visualization, Formal Analysis, Data curation. YK: Writing – original draft, Conceptualization, Data curation. SB: Data curation, Formal Analysis, Conceptualization, Writing – review and editing, Writing – original draft, Supervision. EM: Methodology, Visualization, Writing – original draft. DM: Data curation, Formal Analysis, Methodology, Writing – original draft. BR: Writing – original draft, Data curation, Methodology, Validation. YN: Formal Analysis, Data curation, Methodology, Conceptualization, Writing – original draft. MK: Writing – review and editing, Supervision, Funding acquisition, Methodology, Data curation, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the Ministry of Science and Higher Education of the Russian Federation (state contract №075-15-2025-482).
Acknowledgments
The authors are grateful to Boris Margulis, Nataly Bildyug, Oksana Malikova and Maria Rubel for their advice while writing the article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fbioe.2025.1595116/full#supplementary-material
Abbreviations
HC, Hyaline cartilage; TEHC, Tissue engineering of hyaline cartilage; CEC, Cell-engineered construct; ECM, Extracellular matrix; ICRS, International Cartilage Repair Society grading system; FC, Flow cytometry; ASC, adipose stem cell; FACS, fluorescence-activated cell sorting; RT-PCR, Reverse transcription polymerase chain reaction; PCR, Polymerase chain reaction; BMSCs, Bone marrow-derived stem cells; GDF5, Growth differentiation factor-5; hMSCs, Human mesenchymal multipotent stromal cells; IVFC, In vivo flow cytometry; ELISA, Enzyme-linked immunosorbent assay; GAG, Glycosaminoglycans; MCT, Micro-computed tomography; EPIC, equilibrium partitioning of ionic contrast agents; SEM, Scanning electron microscopy; ICRS, International Cartilage Repair Society.
References
Ahn, K. Y., Kwon, K., Huh, J., Kim, G. T., Lee, E. B., Park, D., et al. (2011). A sensitive diagnostic assay of rheumatoid arthritis using three-dimensional ZnO nanorod structure. Biosens. Bioelectron. 28, 378–385. doi:10.1016/j.bios.2011.07.052
Albert, H. C., and Creech, H. J. (1941). Immunological properties of an antibody containing a fluorescent group. Exp. Biol. Med. 47 (2). doi:10.3181/00379727-47-1308
Ali, H., Saeed, K., Hashemibeni, B., and Setayeshmehr, M. (2020). Physicomechanical and biological properties of polycaprolactone/fibrin hybrid scaffold fabricated by 3D-printing and salt-leaching methods. Mater Technol. 9 (1268). doi:10.1080/10667857.2020.1824148
Al-Sabah, A., Burnell, S. E. A., Simoes, I. N., Jessop, Z., Badiei, N., Blain, E., et al. (2019). Structural and mechanical characterization of crosslinked and sterilised nanocellulose-based hydrogels for cartilage tissue engineering. Carbohydr. Polym. 15, 242–251. doi:10.1016/j.carbpol.2019.02.057
Alwine, J. C., Kemp G, D. J., and Stark, G. R. (1977). Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc. Natl. Acad. Sci. U. S. A. 74 (12), 5350–5354. doi:10.1073/pnas.74.12.5350
Batiste, D. L., Kirkley, A., Laverty, S., Thain, L. M., Spouge, A. R., and Holdsworth, D. W. (2004). Ex vivo characterization of articular cartilage and bone lesions in a rabbit ACL transection model of osteoarthritis using MRI and micro-CT. Cart. Osteoarthr. 12 (12), 986–996. doi:10.1016/j.joca.2004.08.010
Bertoldi, S., Silvia, F. M., and Tanzi, M. C. (2011). Assessment of scaffold porosity: the new route of micro-CT. J. Appl. Biomater. Biomech. 9 (3), 165–175. doi:10.5301/JABB.2011.8863
Bozhokin, M. S., Bozhkova, S., Naschekina, Y., Sopova, Y., Rubel, A., and Khotin, M. (2021). Transfection of mesenchymal stem cells (msc) for modifying cell culture for recovery hyaline cartilage defects. Mod. Problems Sci. Educ. 4 (90), 100. doi:10.17513/spno.31052
Bozhokin, M. S., Bozhkova, S. A., Rubel, A. A., Sopova, J. V., Nashchekina, Y. A., Bildyug, N. B., et al. (2021). Specificities of scanning electron microscopy and histological methods in assessing cell-engineered construct effectiveness for the recovery of hyaline cartilage. Methods Protoc. 4, 77(4). doi:10.3390/mps4040077
Bozhokin, M. S., Vcherashnii, D. B., Yastrebov, S. G., Beilinson, L. L., Zherebtsova, J. V., and Khotin, M. G. (2021). Low-intensity photobiomodulation at 632.8 nm increases tgfβ3, col2a1, and sox9 gene expression in rat bone marrow mesenchymal stem cells in vitro. Lasers Med. Sci. 37, 435–441. doi:10.1007/s10103-021-03279-0
Burnette, W. N. (1981). “western blotting”: electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal. Biochem. 112 (2), 195–203. doi:10.1016/0003-2697(81)90281-5
Chae, D.-S., Han, Ju H., Young-Jin Park, S. K., and Kim, S. (2021). TGF-β1 overexpressing human MSCs generated using gene editing show robust therapeutic potential for treating collagen-induced arthritis. J. Tissue Eng. Regen. Med. 5, 513–523. doi:10.1002/term.3191
Chen, H., Li, Z., Li, X., Huang, H., Meng, Q., Zhang, X., et al. (2016). Transplantation of allogenic chondrocytes with chitosan hydrogel-demineralized bone matrix hybrid scaffold to repair rabbit cartilage injury. Biomaterials 108, 157–167. doi:10.1016/j.biomaterials.2016.09.002
Chen, W., Xu, Y., Li, Y., Jia, L., Mo, X., Jiang, G., et al. (2020). 3D printing electrospinning fiber-reinforced decellularized extracellular matrix for cartilage regeneration. Chem. Eng. J. 382, 122986. doi:10.1016/j.cej.2019.122986
Ciardulli, M. C., Marino, L., Lovecchio, J., Giordano, E., Forsyth, N. R., Selleri, C., et al. (2020). Tendon and cytokine marker expression by human bone marrow mesenchymal stem cells in a hyaluronate/poly-lactic-co-glycolic acid (PLGA)/fibrin three-dimensional (3D) scaffold. Cells 20 (9), 1268. doi:10.3390/cells9051268
Clarke, I. (1971). Articular cartilage: a review and scanning electron microscope study. 1. The interterritorial fibrillar architecture. J. Bone Jt. Surg. - Ser. B, 732–750. doi:10.1302/0301-620X.53B4.732
Dadgar, N., Ali, G., Irani, S., Rabbani, S., Tafti, S. H. A., Soufizomorrod, M., et al. (2021). Cartilage tissue engineering using injectable functionalized demineralized bone matrix scaffold with glucosamine in PVA carrier, cultured in microbioreactor prior to study in rabbit model. Mater Sci. Eng. C Mater Biol. Appl. 120 (111677), 111677. doi:10.1016/j.msec.2020.111677
Darzynkiewicz, Z., Bedner P, E., and Smolewski, P. (2001). Flow cytometry in analysis of cell cycle and apoptosis. Semin. Hematol. 38 (2), 179–193. doi:10.1016/s0037-1963(01)90051-4
Duke, P. J., Doan, L., Luong, H., Kelley, C., Leboeuf, W., Diep, Q., et al. (2009). Correlation between Micro-Ct sections and histological sections of mouse skull defects implanted with engineered cartilage. Gravit. Sp. Biol. Bull. 22 (2), 45–50.
Entezari, V., Bansal, P. N., Stewart, R. C., Lakin, B. A., Grinstaff, M. W., and Snyder, B. D. (2014). Effect of mechanical convection on the partitioning of an anionic iodinated contrast agent in intact patellar cartilage. J. Orthop. Res. 32 (10), 1333–1340. doi:10.1002/jor.22662
Farshid Moussavi-Harami, S., Pedersen, D. R., Martin, J. A., Hillis, S. L., and Brown, T. D. (2009). Automated objective scoring of histologically apparent cartilage degeneration using a custom image analysis program. J. Orthop. Res. 27 (4), 522–528. doi:10.1002/jor.20779
Feldkamp, L. A., Goldstein, S. A., Parfitt, A. M., Jesion, G., and Kleerekoper, M. (1989). The direct examination of three-dimensional bone architecture in vitro by computed tomography. J. Bone Min. Res. 4 (1), 3–11. doi:10.1002/jbmr.5650040103
Flannery, B. P., Deckman, H. W., and Roberge K, W. G. (1987). Three-dimensional X-ray microtomography. Sci. (80-) 18 (237), 1439–1444. doi:10.1126/science.237.4821.1439
Frank, W. R., Mohr, A., Lynch, J. A., Meta, M. D., Guermazi, A., and Genant, H. K. (2005). Micro-CT arthrography: a pilot study for the ex vivo visualization of the rat knee joint. AJR Am. J. Roentgenol. 184 (4), 1215–1219. doi:10.2214/ajr.184.4.01841215
Galarraga, J. H., Locke, R. C., Witherel, C. E., Stoeckl, B. D., Castilho, M., Mauck, R. L., et al. (2021). Fabrication of MSC-Laden composites of hyaluronic acid hydrogels reinforced with MEW scaffolds for cartilage repair. Biofabrication 14 (1), 014106. doi:10.1088/1758-5090/ac3acb
Gögele, C., Müller, S., Belov, S., Pradel, A., Wiltzsch, S., Lenhart, A., et al. (2022). Biodegradable Poly(D-L-lactide-co-glycolide) (PLGA)-infiltrated bioactive glass (CAR12N) scaffolds maintain mesenchymal stem cell chondrogenesis for cartilage tissue engineering. Cells 11 (9), 1577. doi:10.3390/cells11091577
Gregorio, M., Matteo, B., Marco, B. G., and Filardo, G. (2019). Cartilage mechanical tests: evolution of current standards for cartilage repair and tissue engineering. A literature review. Clin. Biomech. Bristol, Avon. 68, 58–72. doi:10.1016/j.clinbiomech.2019.05.019
Gu, Y., Hu, Y., Zhang, H., Wang, S., Xu, K., and Su, J. (2023). Single-cell RNA sequencing in osteoarthritis. Rev. Cell Prolif. 56 (12), e13517. doi:10.1111/cpr.13517
Gucker, F. T., Pickard, H. B., and O'Konski, C. T. (1947). A photoelectric instrument for comparing the concentrations of very dilute aerosols, and measuring low light intensities. J. Am. Chem. Soc. 69 (2), 429–438. doi:10.1021/ja01194a070
Guo, J. L., Kim, Y. S., Koons, G. L., Lam, J., Navara, A. M., Barrios, S., et al. (2021). Bilayered, peptide-biofunctionalized hydrogels for in vivo osteochondral tissue repair. Acta Biomater. 1 (128), 120–129. doi:10.1016/j.actbio.2021.04.038
Han, Y, Jia, B, Lian, M., Sun, B., Wu, Q., Sun, B., et al. (2021a). High-precision, gelatin-based, hybrid, bilayer scaffolds using melt electro-writing to repair cartilage injury. Bioact. Mater 6 (7), 2173–2186. doi:10.1016/j.bioactmat.2020.12.018
Han, Y, Lian, M., Wu, Q., Qiao, Z., Sun, B., and Dai, K. (2021b). Effect of pore size on cell behavior using melt electrowritten scaffolds. Front. Bioeng. Biotechnol. 9 (629270), 629270. doi:10.3389/fbioe.2021.629270
Hashemibeni, B., Mardani, M., Bahrami, M., and Valiani, A. (2020). Comparison of fibrin and PLGA/Fibrin scaffolds for chondrogenesis of human adipose derived stem cells by icariin. J. Kerman Univ. Med. Sci. 27 (1). doi:10.22062/jkmu.2020.89592
Hawkes, R., E Niday, J., and Gordon, J. (1982). A dot-immunobinding assay for monoclonal and other antibodies. Anal. Biochem. 119 (1), 142–147. doi:10.1016/0003-2697(82)90677-7
He, T., Li, B., Colombani, T., Joshi-Navare, K., Mehta, S., Kisiday, J., et al. (2021). Hyaluronic acid-based shape-memory cryogel scaffolds for focal cartilage defect repair. Tissue Eng. Part A 27 (11-12), 748–760. doi:10.1089/ten.TEA.2020.0264
Ho, S. T., and Hutmacher, D. W. (2006). A comparison of micro CT with other techniques used in the characterization of scaffolds. Biomaterials 27, 1362–1376. doi:10.1016/j.biomaterials.2005.08.035
Hoenig, E., Leicht, U., Winkler, T., Mielke, G., Beck, K., Peters, F., et al. (2013). Mechanical properties of native and tissue-engineered cartilage depend on carrier permeability: a bioreactor study. Tissue Eng. Part A 19 (13-14), 1534–1542. doi:10.1089/ten.TEA.2012.0538
Hong, H., Seo, Ye B., Kim, Do Y., Lee, J. S., Lee, Y. J., Lee, H., et al. (2020). Digital light processing 3D printed silk fibroin hydrogel for cartilage tissue engineering. Biomaterials 232 (119679), 119679. doi:10.1016/j.biomaterials.2019.119679
Hutmacher, D. W. (2005). Application of micro CT and computation modeling in bone tissue engineering. Comput. Des. 37 (11), 1151–1161. doi:10.1016/j.cad.2005.02.006
Jaecques, S. V. N., Van Oosterwyck, H., Muraru, L., Van Cleynenbreugel, T., De Smet, E., Wevers, M., et al. (2004). Individualised, micro CT-based finite element modelling as a tool for biomechanical analysis related to tissue engineering of bone. Biomaterials 25 (9), 1683–1696. doi:10.1016/s0142-9612(03)00516-7
Jerald, Z. G., Williams, D. C., Katharine, L. C. J., and Jones, C. (2002). Fine-needle aspiration in Non-Hodgkin lymphoma: evaluation of cell size by cytomorphology and flow cytometry. Am. J. Clin. Pathol. 117 (6), 880–888. doi:10.1309/16UL-W4PX-HRL7-7V88
Jia, L., Zhang, P., Zheng, C, Zhang, W., Liu, Y., Jiang, H., et al. (2021). Immune-inflammatory responses of an acellular cartilage matrix biomimetic scaffold in a xenotransplantation goat model for cartilage tissue engineering. Front. Bioeng. Biotechnol. 9 (667161), 667161. doi:10.3389/fbioe.2021.667161
Jiang, Y., and Tuan, R. S. (2015). Origin and function of cartilage stem/progenitor cells in osteoarthritis. Nat. Rev. Rheumatol. 11, 206–212. doi:10.1038/nrrheum.2014.200
Jolene, A., and Bradford, S. (2011). Panel development for multicolor flow-cytometry testing of proliferation and immunophenotype in hMSCs. Methods Mol. Biol. 698, 367–385. doi:10.1007/978-1-60761-999-4_27
Karabıyık Acar, Ö., Bedir, S., Kayitmazer, A. B., and Kose, G. T. (2021). Chondro-inductive hyaluronic acid/chitosan coacervate-based scaffolds for cartilage tissue engineering. Int. J. Biol. Macromol. 188, 300–312. doi:10.1016/j.ijbiomac.2021.07.176
Karamanou, M., Poulakou-Rebelakou, E., Tzetis, M., and Androutsos, G. (2010). Anton van leeuwenhoek (1632-1723): father of micromorphology and discoverer of spermatozoa. Rev. Argent. Microbiol. 42 (4), 311–314. doi:10.1590/S0325-75412010000400013
Katrín, L. E., Scott, L., Rebecca, L. I., and Jurewicz, I. (2022). Biomimetic approach to articular cartilage tissue engineering using carbon nanotube-coated and textured polydimethylsiloxane scaffolds. Ann. N. Y. Acad. Sci. 1513 (1), 48–64. doi:10.1111/nyas.14769
Kerckhofs, G., Sainz, J., Marécha, M., Wevers, M., Van de Putte, T., Geris, L., et al. (2014). Contrast-enhanced nanofocus X-Ray computed tomography allows virtual three-dimensional histopathology and morphometric analysis of osteoarthritis in small animal models. Cartilage 5 (1), 55–65. doi:10.1177/1947603513501175
Khader, A., and Alquran, H. (2023). Automated prediction of osteoarthritis level in human osteochondral tissue using histopathological images. Bioeng. (Basel) 10 (7), 764. doi:10.3390/bioengineering10070764
Kim, Ji E., Song, D.-H., Kim, S. H., Jung, Y., and Kim, S. J. (2018). Development and characterization of various osteoarthritis models for tissue engineering. PLoS One 13 (3), e0194288. doi:10.1371/journal.pone.0194288
Kingsley, K. A., Goji, N., Macmillan, T., Said, K. B., Druhan, S., Tanaka, E., et al. (2010). Development of multitarget real-time PCR for the rapid, specific, and sensitive detection of Yersinia pestis in milk and ground beef. J. Food Prot. 73, 18–25. doi:10.4315/0362-028x-73.1.18
Király, K., Lammi, M., Arokoski, J., Lapveteläinen, T., Tammi, M., Helminen, H., et al. (1996). Safranin O reduces loss of glycosaminoglycans from bovine articular cartilage during histological specimen preparation. Histochem J. 28 (2), 99–107. doi:10.1007/BF02331414
Korpayev, S., Kaygusuz, G., Şen, M., Orhan, K., Oto, Ç., and Karakeçili, A. (2020). Chitosan/collagen based biomimetic osteochondral tissue constructs: a growth factor-free approach. Int. J. Biol. Macromol. 1 (156), 681–690. doi:10.1016/j.ijbiomac.2020.04.109
Langer, R., and Vacanti, J. (1993). Tissue engineering. Science 260 (80), 920–926. doi:10.1126/science.8493529
Lebaron, P., Servais, P., Baudoux, A.-C., Bourrain, M., Courties, C., and Parthuisot, N. (2002). Variations of bacterial-specific activity with cell size and nucleic acid content assessed by flow cytometry. Aquat. Microb. Ecol. 28 (2), 131–140. doi:10.3354/ame028131
Lee, C., O’Connell, C. D., Onofrillo, C., Choong, P. F. M., Di Bella, C., and Duchi, S. (2020a). Human articular cartilage repair: sources and detection of cytotoxicity and genotoxicity in photo-crosslinkable hydrogel bioscaffolds. Stem Cells Transl. Med. 9 (3), 302–315. doi:10.1002/sctm.19-0192
Lee, J., Jeon, O., Kong, M., Abdeen, A. A., Shin, J. Y., Lee, H. N., et al. (2020b). Combinatorial screening of biochemical and physical signals for phenotypic regulation of stem cell-based cartilage tissue engineering. Sci. Adv. 6 (21), eaaz5913. doi:10.1126/sciadv.aaz5913
Lee, M.-S., Stebbins, M. J., Jiao, H., Huang, H. C., Leiferman, E. M., Walczak, B. E., et al. (2021). Comparative evaluation of isogenic mesodermal and ectomesodermal chondrocytes from human iPSCs for cartilage regeneration. Sci. Adv. 7 (21), eabf0907. doi:10.1126/sciadv.abf0907
Li, C.-S., Zhang, X., Péault, B., Jiang, J., Ting, K., Soo, C., et al. (2016). Accelerated chondrogenic differentiation of human perivascular stem cells with NELL-1. Tissue Eng. Part A 22 (3-4), 272–285. doi:10.1089/ten.TEA.2015.0250
Li, Q., Xu, S., and Qi, F. (2021). 3D printed silk-gelatin hydrogel scaffold with different porous structure and cell seeding strategy for cartilage regeneration. Bioact. Mater 19 (6), 3396–3340. doi:10.1016/j.bioactmat.2021.03.013
Li, X.-F., Cai, X.-R., Fan, F., Niu, H. J., Li, S. Y., Li, D. Y., et al. (2015). Observation of sGAG content of human hip joint cartilage in different old age groups based on EPIC micro-CT. Connect. Tissue Res. 56 (2), 99–105. doi:10.3109/03008207.2015.1009052
Liao, J. F., Qu, Y., Chu, B. Y., Zhang, X., and Qian, Z. (2015). Biodegradable CSMA/PECA/Graphene porous hybrid scaffold for cartilage tissue engineering. Sci. Rep. 11 (5), 9879. doi:10.1038/srep09879
Liu, F., Wang, X, Li, Y., Ren, M., He, P., Wang, L., et al. (2022a). Dendrimer-modified gelatin methacrylate hydrogels carrying adipose-derived stromal/stem cells promote cartilage regeneration. Stem Cell Res. Ther. 13 (1), 26. doi:10.1186/s13287-022-02705-6
Liu, X., Wei, Y., Xuan, C., Liu, L., Lai, C., Chai, M., et al. (2020). A biomimetic biphasic osteochondral scaffold with layer-specific release of stem cell differentiation inducers for the reconstruction of osteochondral defects. Adv. Heal Mater 9 (23), e2000076. doi:10.1002/adhm.202000076
Liu, X., Zhang, Z., Shi, Y., Meng, X., Qiu, Z., Qu, X., et al. (2022b). Effect of electrohydrodynamic printing scaffold with different spacing on chondrocyte dedifferentiation. Ann. Transl. Med. 10 (13), 743. doi:10.21037/atm-22-2796
Liu, Y., and Karan, M. S. J. (2021). Strategies for articular cartilage repair and regeneration. Front. Bioeng. Biotechnol. 17 (9). doi:10.3389/fbioe.2021.770655
Loebel, C., Kwon, Mi Y., Wang, C., Han, L., Mauck, R. L., and Burdick, J. A. (2020). Metabolic labeling to probe the spatiotemporal accumulation of matrix at the chondrocyte-hydrogel interface. Adv. Funct. Mater 30 (44), 1909802. doi:10.1002/adfm.201909802
Lv, T., Liu, J., Li, F., Ma, S., Wei, X., Li, X., et al. (2024). Label-free and ultrasensitive detection of cartilage acidic protein 1 in osteoarthritis using a single-walled carbon nanotube field-effect transistor biosensor. ACS Appl. Mater Interfaces 16 (28), 36804–36810. doi:10.1021/acsami.4c05638
Ma, F., Ge, Y., Liu, N., Pang, X., Shen, X., and Tang, B. (2019). In situ fabrication of a composite hydrogel with tunable mechanical properties for cartilage tissue engineering. J. Mater Chem. B 7 (15), 2463–2473. doi:10.1039/c8tb01331d
Mendes, L. F., Katagiri, H., Tam, W. L., Chai, Y. C., Geris, L., Roberts, S. J., et al. (2018). Advancing osteochondral tissue engineering: bone morphogenetic protein, transforming growth factor, and fibroblast growth factor signaling drive ordered differentiation of periosteal cells resulting in stable cartilage and bone formation in vivo. Stem Cell Res. Ther. 21 (9), 42. doi:10.1186/s13287-018-0787-3
Middendorf, J. M., Griffin, D. J., Shortkroff, S., Dugopolski, C., Kennedy, S., Siemiatkoski, J., et al. (2017). Mechanical properties and structure-function relationships of human chondrocyte-seeded cartilage constructs after in vitro culture. J. Orthop. Res. 35 (10), 2298–2306. doi:10.1002/jor.23535
Moldavan, A. (1934). Photo-electric technique for the counting of microscopical cells. Sci. (80) 80 (2069), 188–189. doi:10.1126/science.80.2069.188
Moncayo-Donoso, M., Guevara, J. M., Márquez-Flórez, K., R. Fontanilla, M., Barrera, L. A., and Garzón-Alvarado, D. A. (2019). Morphological changes of physeal cartilage and secondary ossification centres in the developing femur of the house mouse (mus musculus): a micro-CT based study. Anat. Histol. Embryol. 48 (2), 117–124. doi:10.1111/ahe.12417
Mori, Y., Oichi, T., Enomoto-Iwamoto, M., and Saito, T. (2022). Automatic detection of medial and lateral compartments from histological sections of mouse knee joints using the single-shot multibox detector algorithm. Cartilage 13 (1), 19476035221074009. doi:10.1177/19476035221074009
Morodomi, Y., Kanaji, S., Won, E., Kawamoto, T., and Kanaji, T. (2019). Modified application of Kawamoto’s film method for super-resolution imaging of megakaryocytes in undecalcified bone marrow. Res. P. R. Thromb. Haemost. 4 (1), 86–91. doi:10.1002/rth2.12276
Mullis, K. B. (1990). The unusual origin of the polymerase chain reaction. Sci. Am. 262 (4), 56–65. doi:10.1038/scientificamerican0490-56
Murata, D., Ishikawa, S., Sunaga, T., Saito, Y., Sogawa, T., Nakayama, K., et al. (2022). Osteochondral regeneration of the femoral medial condyle by using a scaffold-free 3D construct of synovial membrane-derived mesenchymal stem cells in horses. BMC Vet. Res. 18 (1), 53. doi:10.1186/s12917-021-03126-y
Nagarajan, L., Khatri, A., Sudan, A., Vaishya, R., and Ghosh, S. (2024). Deep learning augmented osteoarthritis grading standardization. Tissue Eng. Part A 30 (19-20), 591–604. doi:10.1089/ten.TEA.2023.0206
Naghizadeh, Z., Karkhaneh, A., Nokhbatolfoghahaei, H., Farzad-Mohajeri, S., Rezai-Rad, M., Dehghan, M. M., et al. (2021). Cartilage regeneration with dual-drug-releasing injectable hydrogel/microparticle system: in vitro and in vivo study. J. Cell Physiol. 236 (3), 2194–2204. doi:10.1002/jcp.30006
Nikolai, P. O., Karalkin, P. A., Bulanova, E. A., Koudan, E. V., Parfenov, V. A., Rodionov, S. A., et al. (2020). Extracellular matrix determines biomechanical properties of chondrospheres during their maturation in vitro. Cartilage 11 (4), 521–531. doi:10.1177/1947603518798890
Nour-Eldeen, G., Abdel-Rasheed, M., El-Rafei, A. M., Azmy, O., and El-Bassyouni, G. T. (2020). Adipose tissue-derived mesenchymal stem cells and chitosan/poly (vinyl alcohol) nanofibrous scaffolds for cartilage tissue engineering. Cell Regen. 9 (1), 7. doi:10.1186/s13619-020-00045-5
O’Driscoll, S. W., Keeley, F. R., and Salter, R. B. (1986). The chondrogenic potential of free autogenous periosteal grafts for biological resurfacing of major full-thickness defects in joint surfaces under the influence of continuous passive motion. An experimental investigation in the rabbit. J. Bone Jt. Surg. Am. 68, 1017–1035. doi:10.2106/00004623-198668070-00008
Ouyang, X., Yongfang, X. G. W., and Wang, G. (2019). Mechanical stimulation promotes the proliferation and the cartilage phenotype of mesenchymal stem cells and chondrocytes co-cultured in vitro. Biomed. Pharmacother. 117, 109146. doi:10.1016/j.biopha.2019.109146
Oyadomari, S., Brown, W. E., Kwon, H., Otarola, G., Link, J. M., Athanasiou, K. A., et al. (2021). In vitro effects of bupivacaine on the viability and mechanics of native and engineered cartilage grafts. Am. J. Sport Med. 49 (5), 1305–1312. doi:10.1177/0363546521995184
Palmer, A. W., Guldberg, R. E., and Levenston, M. E. (2006). Analysis of cartilage matrix fixed charge density and three-dimensional morphology via contrast-enhanced microcomputed tomography. Proc. Natl. Acad. Sci. U. S. A. 103(51):19255–19260. doi:10.1073/pnas.0606406103
Paniz, H. A., and Shamloo, A. (2021). Fabrication of a novel 3D scaffold for cartilage tissue repair: in-vitro and in-vivo study. Mater Sci. Eng. C Mater Biol. Appl. 128 (112285), 112285. doi:10.1016/j.msec.2021.112285
Paulina Rajko-Nenow, C. B., and Batten, C. (2022). Genotyping of African swine fever virus. Methods Mol. Biol. 2503, 119–132. doi:10.1007/978-1-0716-2333-6_8
Payam, B., Hamed, D., Farhad, M., Azam Sayahpour, F., Baharvand, H., and Baghaban Eslaminejad, M. (2021). A tough polysaccharide-based cell-laden double-network hydrogel promotes articular cartilage tissue regeneration in rabbits. Chem. Eng. J. 418, 129277. doi:10.1016/j.cej.2021.129277
Pereira, D. R., Canadas, R. F., Silva-Correia, J., da Silva Morais, A., Oliveira, M., Dias, I., et al. (2018). Injectable gellan-gum/hydroxyapatite-based bilayered hydrogel composites for osteochondral tissue regeneration. Appl. Mater 12, 309–321. doi:10.1016/j.apmt.2018.06.005
Piagnerelli, M., Zouaoui Boudjeltia, K., Brohee, D., Vereerstraeten, A., Piro, P., Vincent, J. L., et al. (2007). Assessment of erythrocyte shape by flow cytometry techniques. J. Clin. Pathol. 60 (5), 549–554. doi:10.1136/jcp.2006.037523
Piotr, P. Z., and Darzynkiewicz, Z. (2004). Analysis of cell cycle by flow cytometry. Methods Mol. Biol. 281 (301), 301–311. doi:10.1385/1-59259-811-0:301
Rapagna, S., Roberts, B. C., Solomon, L. B., Reynolds, K. J., Thewlis, D., and Perilli, E. (2022). Relationships between tibial articular cartilage, in vivo external joint moments and static alignment in end-stage knee osteoarthritis: a micro-CT study. J. Orthop. Res. 40 (5), 1125–1134. doi:10.1002/jor.25140
Rasouli, A., Sun, C.-H., Reshmi, B. B., and Wong, B. J. (2003). Quantitative assessment of chondrocyte viability after laser mediated reshaping: a novel application of flow cytometry. Lasers Surg. Med. 32 (1), 3–9. doi:10.1002/lsm.10142
Rodovalho, V. R., Araujo, G. R., Vaz, E. R., Ueira-Vieira, C., Goulart, L., Madurro, J., et al. (2018). Peptide-based electrochemical biosensor for juvenile idiopathic arthritis detection. Biosens. Bioelectron. 100, 577–582. doi:10.1016/j.bios.2017.10.012
Rogan, H., Ilagan, F., Tong, X., Chu, C. R., and Yang, F. (2020). Microribbon-hydrogel composite scaffold accelerates cartilage regeneration in vivo with enhanced mechanical properties using mixed stem cells and chondrocytes. Biomaterials 228 (119579), 119579. doi:10.1016/j.biomaterials.2019.119579
Rutgers, M., van Pelt, M. J., Dhert, W. J., Creemers, L., and Saris, D. (2010). Evaluation of histological scoring systems for tissue-engineered, repaired and osteoarthritic cartilage. Osteoarthr. Cartil. 18, 12–23. doi:10.1016/j.joca.2009.08.009
Sriwatananukulkit, O., Tawonsawatruk, T., Rattanapinyopituk, K., Luangwattanawilai, T., Srikaew, N., and Hemstapat, R. (2022). Scaffold-free cartilage construct from infrapatellar fat pad stem cells for cartilage restoration. Tissue Eng. Part A 28 (5-6), 199–211. doi:10.1089/ten.TEA.2020.0167
Stephanie, J. B., and Anseth, K. S. (2002). Hydrogel properties influence ECM production by chondrocytes photoencapsulated in poly(ethylene glycol) hydrogels. J. Biomed. Mater Res. 59 (1), 63–72. doi:10.1002/jbm.1217
Sun, A. X., Lin, H., Beck, A. M., Kilroy, E. J., and Tuan, R. S. (2015). Projection stereolithographic fabrication of human adipose stem Cell- incorporated biodegradable scaffolds for cartilage tissue engineering. Front. Bioeng. Biotechnol. 3 (115), 115. doi:10.3389/fbioe.2015.00115
Sun, J., Xing, F., Zou, M., Gong, M., Li, L., and Xiang, Z. (2021). Comparison of chondrogenesis-related biological behaviors between human urine-derived stem cells and human bone marrow mesenchymal stem cells from the same individual. Stem Cell Res. Ther. 12 (1), 366. doi:10.1186/s13287-021-02370-1
Sun, Y, You, Y., Jiang, W., Zhai, Z., and Dai, K. (2019). 3D-bioprinting a genetically inspired cartilage scaffold with GDF5-conjugated BMSC-laden hydrogel and polymer for cartilage repair. Theranostics 9 (23), 6949–6961. doi:10.7150/thno.38061
Swieszkowski, W., Tuan, B. H. S., Kurzydlowski, K. J., and Hutmacher, D. W. (2007). Repair and regeneration of osteochondral defects in the articular joints. Biomol. Eng. 24 (5), 489–495. doi:10.1016/j.bioeng.2007.07.014
Tkachev, S., Chepelova, N., Galechyan, G., Ershov, B., Golub, D., Popova, E., et al. (2024). Three-Dimensional cell culture Micro-CT visualization within collagen scaffolds in an aqueous environment. Cells 13 (15), 1234. doi:10.3390/cells13151234
Tsai, M.-C., Hung, K.-C., Shih-Chieh, H. S., and Hsu, S. h. (2015). Evaluation of biodegradable elastic scaffolds made of anionic polyurethane for cartilage tissue engineering. Colloids Surf. B Biointerfaces 125, 34–44. doi:10.1016/j.colsurfb.2014.11.003
Tulin, I. A. (2020). Decellularized biological scaffold and stem cells from autologous human adipose tissue for cartilage tissue engineering. Methods 15 (171), 97–107. doi:10.1016/j.ymeth.2019.04.020
Vapniarsky, N., Moncada, L., Garrity, C., Wong, A., Filliquist, B., Chou, P. Y., et al. (2022). Tissue engineering of canine cartilage from surgically debrided osteochondritis dissecans fragments. Ann. Biomed. Eng. 50 (1), 56–77. doi:10.1007/s10439-021-02897-7
Vinod, E., Vinod Francis, D., Jacob, T., Amirtham, S. M., Sathishkumar, S., Kanthakumar, P., et al. (2019). Autologous platelet rich fibrin as a scaffold for chondrocyte culture and transplantation: an in vitro bovine study. J. Clin. Orthop. Trauma 10 (1), 26–31. doi:10.1016/j.jcot.2019.04.023
Walles, H. (1956). High speed automatic blood cell counter and cell size analyzer. Proc. Natl. Electron Conf. 12, 1034–1040.
Wang, C.-C., Chen, I.-H, Yang, Y.-T., and Yang, K. C. (2021a). Infrapatellar fat pads-derived stem cell is a favorable cell source for articular cartilage tissue engineering: an in vitro and Ex Vivo study based on 3D organized self-assembled biomimetic scaffold. Cartilage 13, 508–520. doi:10.1177/1947603520988153
Wang, S. H., Shen, C. Y., Weng, T. C., Lin, P. H., Yang, J. J., Chen, I. F., et al. (2010). Detection of cartilage oligomeric matrix protein using a quartz crystal microbalance. Sensors 10, 11633–11643. doi:10.3390/s101211633
Wang, Y., Xu, Y., Zhou, G., Liu, Y., and Cao, Y. (2021b). Biological evaluation of acellular cartilaginous and dermal matrixes as tissue engineering scaffolds for cartilage regeneration. Front. Cell Dev. Biol. 8 (624337), 624337. doi:10.3389/fcell.2020.624337
Yang, L., Coleman, M. C., Hines, M. R., Kluz, P. N., Brouillette, M. J., and Goetz, J. E. (2019). Deep learning for chondrocyte identification in automated histological analysis of articular cartilage. Iowa Orthop. J. 39 (2), 1–8.
Zhao, D., Li, Y., Xizong, Z. Z., and Yang, Z. (2018). Peripheral blood mesenchymal stem cells combined with modified demineralized bone matrix promote pig cartilage defect repair. Cells Tissues Organs 206 (1-2), 26–34. doi:10.1159/000493210
Zheng, K., Ma, Y., Cheng, C., Xue, M., Zhang, C., and Du, D. (2024). Enhanced articular cartilage regeneration using costal chondrocyte-derived scaffold-free tissue engineered constructs with ascorbic acid treatment. J. Orthop. Transl. 45, 140–154. doi:10.1016/j.jot.2024.02.005
Zheng, P., Hu, X., Lou, Y., and Tang, K. (2019). A rabbit model of osteochondral regeneration using three-dimensional printed polycaprolactone-hydroxyapatite scaffolds coated with umbilical cord blood mesenchymal stem cells and chondrocytes. Med. Sci. Monit. 25, 7361–7369. doi:10.12659/msm.915441
Zhou, H., Chen, R, Wang, J., Lu, J., Yu, T., Wu, X., et al. (2020). Biphasic fish collagen scaffold for osteochondral regeneration. Mater Des. 195 (108947), 108947. doi:10.1016/j.matdes.2020.108947
Keywords: tissue engineering, methods, review, hyaline cartilage, recovery
Citation: Bozhokin MS, Korneva YS, Bozhkova SA, Mikhaylova ER, Marchenko DM, Rakhimov BR, Nashchekina YA and Khotin MG (2025) Modern experimental methods for assessing the effectiveness of tissue-engineered products for hyaline cartilage regeneration. Front. Bioeng. Biotechnol. 13:1595116. doi: 10.3389/fbioe.2025.1595116
Received: 17 March 2025; Accepted: 25 June 2025;
Published: 21 July 2025.
Edited by:
Denghui Xie, Southern Medical University, ChinaReviewed by:
Francesco De Francesco, Azienda Ospedaliero Universitaria Ospedali Riuniti, ItalyFilippo Cipriani, New York Stem Cell Foundation, United States
Copyright © 2025 Bozhokin, Korneva, Bozhkova, Mikhaylova, Marchenko, Rakhimov, Nashchekina and Khotin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: M. S. Bozhokin, d3JpdGViYWNrQG1haWwucnU=
†ORCID: M. S. Bozhokin, orcid.org/0000-0002-9931-3394; J. S. Korneva, orcid.org/0000-0002-8080-904X; S. A. Bozhkova, orcid.org/0000-0002-2083-2424; E. R. Mikhaylova, orcid.org/0000-0001-8576-3717; D. M. Marchenko, orcid.org/0000-0002-7940-9444; B. R. Rakhimov, orcid.org/0009-0003-5807-5750; Y. A. Nashekina, orcid.org/0000-0002-4371-7445; M. G. Khotin, orcid.org/0000-0002-8293-6368
 M. S. Bozhokin
M. S. Bozhokin Yu. S. Korneva2,3†
Yu. S. Korneva2,3† E. R. Mikhaylova
E. R. Mikhaylova B. R. Rakhimov
B. R. Rakhimov M. G. Khotin
M. G. Khotin