- Department of Mechanical and Aerospace Engineering, University of Texas at Arlington, Arlington, TX, United States
Introduction: Head and neck injuries, including traumatic brain injuries (TBI), are a leading cause of disability and death worldwide. It affects millions of people worldwide, from automobiles to sports to military personnel. This study investigates the influence of impact locations, severities, and neck strength on head and neck injury parameters using a musculoskeletal head-neck model in OpenSim software.
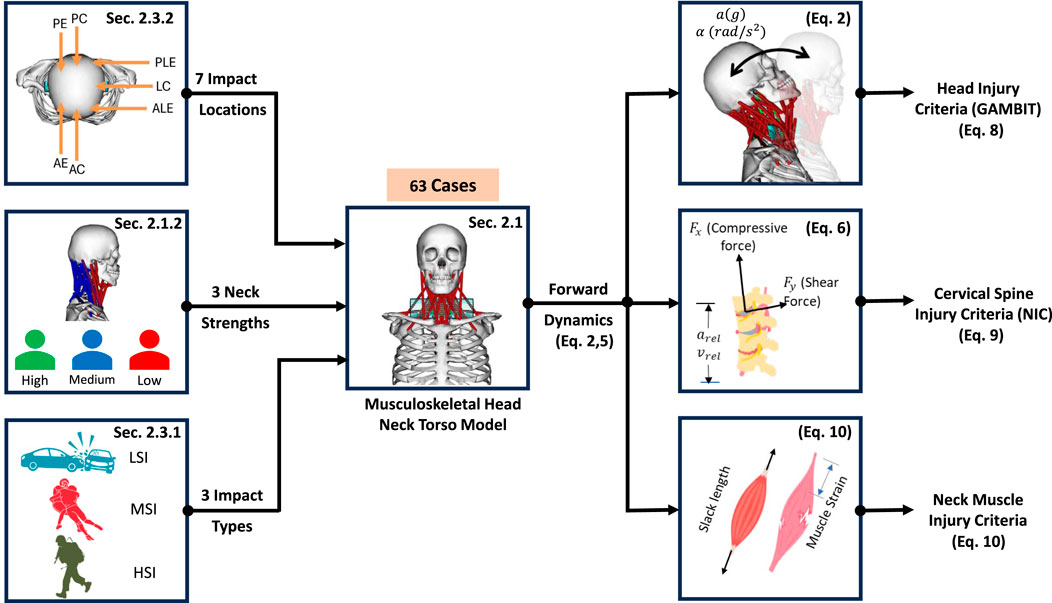
Methods: We hypothesize that eccentric impacts, particularly those on the anterolateral side, increase GAMBIT and Neck Injury Criteria (NIC) due to elevated rotational accelerations, and that higher neck strength mitigates GAMBIT and NIC under these impacts. To test our hypotheses, we investigated a total of 63 cases in which seven impact locations (two from the anterior side, two from the posterior side, and three from lateral sides), three neck strengths (low, mid, high strength capacity), and three impact severities (low, moderate, and high) were explored. Seven output parameters were analyzed: linear and rotational accelerations, the Generalized Acceleration Model for Brain Injury Threshold (GAMBIT), neck force, neck moment, and Neck Injury Criteria (NIC) and neck muscle strain.
Results: Results reveal that anterolateral eccentric impacts pose the greatest risk, with rotational acceleration reaching 4,176 that is 4.75 times higher than anterior central impacts (879 rad/s2). GAMBIT values for moderate and high severity impacts are 1.44 and 1.54 times greater than low severity impacts, respectively. Head and neck injury parameters vary minimally (10) with neck strength.
Discussion: In summary, the severities and location of impacts had a significant role in GAMBIT and NIC, and the anterolateral eccentric impact had a higher probability of head and neck injury than the other six impact locations. These findings underscore the critical role of impact location and severity in injury risk and suggest helmet padding in lateral and anterolateral zones with energy-absorbing materials to reduce rotational acceleration.
1 Introduction
An estimated 1.6 to 3.8 million sport and recreation-related traumatic brain injuries (TBIs) occur in the USA annually (Borich et al., 2013). Over 6 million passenger car accidents take place every year, and more than 42,514 people lost their lives in 2022 in the USA (Insurance Institute for Highway Safety and Highway Loss Data Institute, 2024). According to the CDC, in 2019, 60,611 TBI-related deaths occurred in the USA (Centers for Disease Control and Prevention, 2019). Along with the TBI, neck injuries are also common in high-energy events like blasts, automobile accidents, and sports impacts. It is reported that half a million people faced cervical spine injuries in high-energy accidents in the USA from 2005 to 2013, and the trend of incidents has increased from 4.1% to 5.4% (Johnson et al., 2020). A major portion of these head and neck injury events has been widely underreported. Even in fully equipped settings, like sports, 66% of TBI go unreported (McCrea et al., 2004). According to the Defense Health Agency, 505,896 service members faced mild to severe head and neck injuries from 2000 to 2024 (Defense Health Agency, 2024). In a study, it was found that 57% of service members who faced head and neck injuries did not seek medical care (Escolas et al., 2020). So, the percentage of the civil population that ignores head and neck injuries is really a concern. Moreover, the complications from TBI and neck injuries have long-term effects and can alter a person’s thinking, sensation, language, or emotions, which may not be immediately apparent. That’s why TBI and neck injuries are frequently referred to as a “silent epidemic” (Takhounts et al., 2011).
TBI is a complex pathophysiological process induced by mechanical loading of the brain. TBI can occur because of a direct impact or blow to the head or an indirect inertial response due to a non-cranial impact on the human body. Most of the life-threatening head and neck injuries from contact sports, automobiles, and blasts involve the torso, spine, and head. The rotational response of the head has been correlated to the risk of TBIs such as subdural hematomas and Diffuse Axonal Injury (DAI) (Browne et al., 2011). The effect of neck strength on concussions is still under investigation. Neck resistance and strength have an effect on the human head response during and after an impact (Farmer et al., 2022). Additionally, certain brain structures exhibit directional response dependencies, such as the falx and tentorium cerebri, particularly in the corpus callosum and brainstem, which undergo significant strain during axial rotations (Ho et al., 2017). Most studies indicate that stronger and stiffer necks reduce the risk of concussion (Fanta et al., 2013; Eckner et al., 2014; Hasegawa et al., 2014; Mang et al., 2015). However, some studies disagree with this (Mihalik et al., 2011). To gain a comprehensive understanding of the effects of neck strength and impact dynamics on head and neck injuries, three major parameters need to be carefully investigated. First, a computational or experimental head-neck model that can mimic head-neck response with high fidelity. Second, the input data for the analysis should be free from bias. Third, impacts from all around the head should be considered, as they can cause head and neck injuries.
A very detailed model of the human head and neck is a vital tool to assess any injury. The cervical spine is a pivotal structure of head dynamics and controls the head’s response after external loading. It establishes the mechanical linkage between the torso and head, and stabilizes the head using cervical disc and neck muscle tissue resistance. The most popular experimental and computational tools for reconstructing head and neck injury events are anthropomorphic test devices (ATD), multibody models, finite element models (FEM), and musculoskeletal models. Since all ATD use pin joints to connect the vertebrae, it allows limited rotation and bending in a single plane (Farmer et al., 2022). ATDs have 2-10 times stiffer necks and are mainly used for frontal, rear, and near-side automotive impact (Farmer et al., 2022). It lacks the natural compliance and multi-planar motion of human intervertebral discs and ligaments. ATDs can not mimic the intervertebral rotation with high biofidelity. Therefore, the evaluation of the rotational acceleration-based head injury criterion and Neck Injury Criterion (NIC) is not fully reliable with ATD experiments. Moreover, ATDs are expensive for repetitive blast and severe impact experiments. For these reasons, there is a growing interest in using fast and high bio-fidelity computational models. At the initial stages, lumped parameter models (Bumberger et al., 2025; Merrill et al., 1984; Deng and Goldsmith, 1987) and multibody models (Garcia and Ravani, 2003; Huo and Wu, 2017) of head and neck were developed. Most of those models are based on simplified rigid linkages. With the increase in computational efficiency, finite element models (FEM) became popular (Kleiven, 2007; Pramudita et al., 2009; Zhang et al., 2011; Gabler et al., 2019; Wu et al., 2019; Dymek et al., 2021; Liu et al., 2022; Putra et al., 2022; Singh et al., 2022; Tang et al., 2023; Wang et al., 2023). Finite element models were widely used to study the head and neck motion for whiplash (Zhang et al., 2011; Putra et al., 2022). However, FEM models are computationally very heavy and require predefined boundary conditions. Also, it is very difficult for the FEM model to exactly mimic the nature of muscle tissues and their interactions with the surrounding anatomical structures, such as muscle wrapping, dynamics, muscle excitations and contractions, and interactions with tendons and skeletons. The reliability of muscle force-generating capacity is a contributing factor in determining the head-neck model response. Not only does the shape and alignment of the cervical spine stabilize the head-neck joints, but the ligaments and voluntarily controlled muscles also play a contributing factor (Fanta et al., 2013). To understand the response of the head and neck under external loadings, it is essential to integrate all these physiological and biomechanical properties into the model to estimate a model’s muscle force-generating capacity. However, FEM model lacks those properties, hindering its ability to accurately simulate anatomical interactions.
Computational musculoskeletal models offer enhanced biofidelity compared to FEM and multibody models, as they incorporate dynamic inputs, thereby reducing reliance on static assumptions. These steps are critical for accurately capturing the biomechanical response to impact (Halloran et al., 2010). Musculoskeletal models incorporate time-dependent dynamic inputs such as muscle activations, contractions, wrapping, tendon compliances, and joint movement. Furthermore, computational musculoskeletal models are easily scalable to subject-specific anthropometric details and can provide segmental acceleration, velocity, and force information for a specific subject. These features reduce the kinematic errors at the early stage that can propagate throughout a biomechanical analysis. Musculoskeletal models can help us study the cause-and-effect relationship between various muscle and joint physiological properties and head and neck injuries, which is a vital part of this study. Additionally, computational musculoskeletal models are less time-consuming and computationally less expensive than FEM, cadaveric, or dummy tests. Considering all these factors and hypotheses of this study, we employed a musculoskeletal model to investigate the effects of neck strength and impact dynamics on head and neck injury criteria. In our model, we considered three neck strengths: low force capacity (sedentary or untrained), mid force capacity (recreationally active), and high force capacity (Athletic).
To perform a detailed injury analysis, sources of kinematics and kinetics data should be free from bias and cover a broad spectrum of events. The acceleration loading curve on the head has a significant effect on TBI (Post et al., 2014). Musculoskeletal models have been widely used for low-speed human movements like weight lifting, walking, running, etc. Recently, there have been some studies where the musculoskeletal model has been used for moderate severity impacts, such as NFL (Kleiven, 2007; Jin et al., 2017; Mortensen et al., 2018; Kuo et al., 2019; Mortensen et al., 2020) and rugby impacts (Silvestros et al., 2024). Head and neck injuries, because of low and high severity impacts, were not studied much using computational musculoskeletal models. In all those studies, sports impacts were the only source of real-life input data to study the effect of impact dynamics and neck strength on head and neck injury. However, sports data has visual perception and risk compensation phenomenon error (Schmidt et al., 2014). That means players with stronger and larger cervical musculature may be confident that they are more protected from head and neck injuries and may engage in more violent collisions. On the other hand, weaker subjects tend to avoid violent collisions. Furthermore, it was also reported that without visual perception, muscle activation takes 0.027s after an impact, while with visual perception, muscle activation takes 0.127s before the impact (Fanta et al., 2013). It is also reported in the same study that head injury criteria show about 30% decrease with visual perception. For these reasons, the studies and decisions about the effect of impact dynamics and neck strength on head injuries, based only on sports data, may mislead the injury prediction. To fill up these gaps, in our study, we consider three types of impact data: automobile impact test as low severity impact (LSI), NFL as moderate severty impact (MSI), and blast as high severity impact (HSI). Thus, we make our data independent of visual perception error. Details about the severity classification of impact are mentioned in Section 2.3.1.
Another research gap in the literature is that the impacts from all around the head are not widely studied for both head and neck injuries in terms of neck strength. Impact locations influence the head and neck injury metric (Mychasiuk et al., 2016; Yu et al., 2022). Impacts on the frontal or occipital region increase the risk of subdural hematoma, whereas parenchymal contusions are more likely due to the side impacts on the head (Post et al., 2015). In a study, the effects of anterior central, posterior central, lateral central, and posterolateral impacts were analyzed for sports-related concussive and sub-concussive impacts (Mortensen et al., 2020). They found that neck strength has statistical significance in terms of head injury criteria. In another study, they considered cranial anterior, cranial posterior, lateral mid-posterior, mid-anterior, and lateral interior impacts from rugby players (Silvestros et al., 2024). However, they studied only neck injuries; head injuries were not included. Anterior eccentric and posterior eccentric impacts were not studied for both head and neck injuries. However, our hypothesis is that eccentric impact may create higher accelerations and momentum around the head than concentric impacts. To fix this research gap, we considered seven impact locations for all the above-mentioned impact severities and neck strengths: anterior concentric, anterior eccentric, posterior concentric, posterior eccentric, lateral concentric, posterolateral eccentric, and anterolateral eccentric.
To investigate the effect of neck strength and impact dynamics on head and neck injury, a comprehensive study is necessary to examine different neck strengths and consider varying impact severities from all directions to reach a conclusive decision. We hypothesize that eccentric impacts will result in higher GAMBIT and NIC values compared to concentric impacts due to the increased rotational accelerations associated with eccentric loading. In this study, we designed 63 cases guided by these hypotheses (3 impact severities × 3 neck strengths × 7 impact locations). In Section 2, we will provide details about the head-neck dynamics framework, injury criteria, impact dynamics, and simulation workflow. In Section 3, we will present the results, and in Section 4, we will discuss the influence of impact locations, neck strengths, and impact severities on head and neck injury criteria.
2 Methods
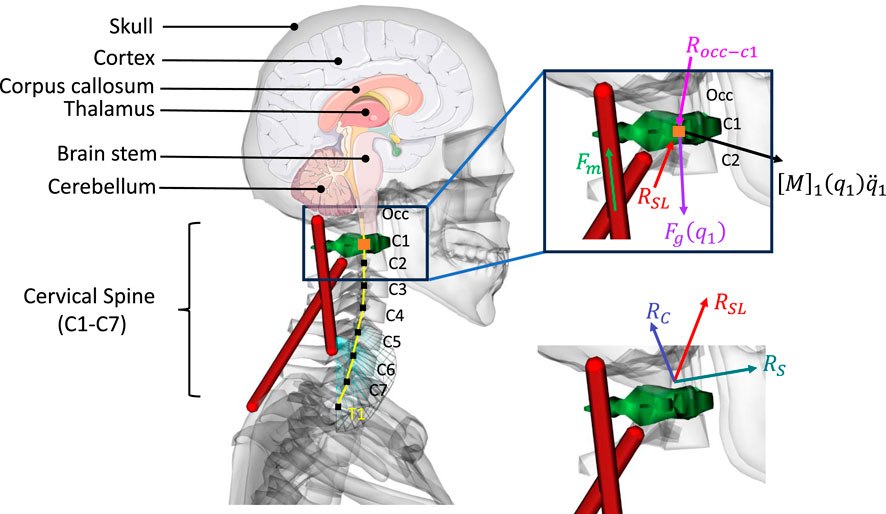
The musculoskeletal head-neck model is a muscle-driven model. Neck muscles generate forces based on complex head-neck muscle activation and musculo-tendon dynamics. Under an accelerative environment, all neck muscles collectively generate a muscle-driven head-neck response (head acceleration, muscle strain, and joint reaction load of cervical spine), which is crucial in head and neck injury prediction with high bio-fidelity. The head-neck dynamics framework is provided below in Figure 1.
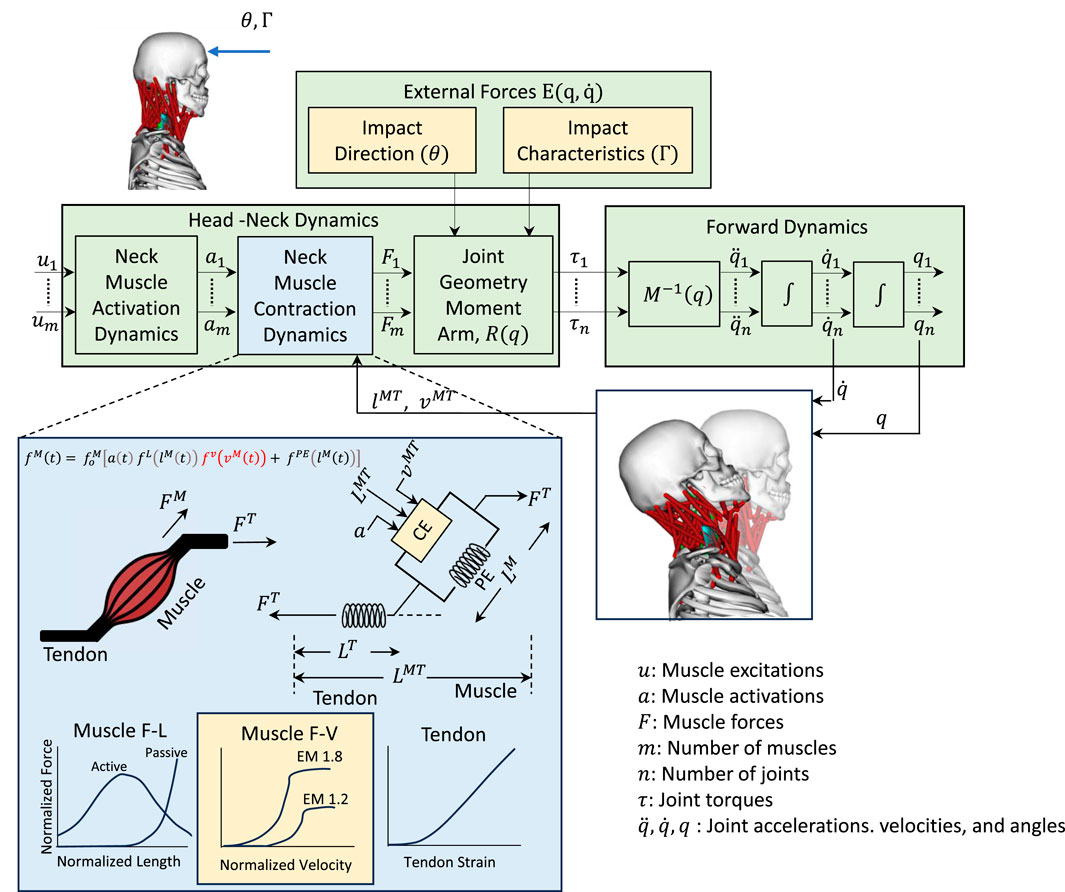
Figure 1. Head-neck dynamics framework uses a muscle-driven simulation to determine the head accelerations. Impact direction (
2.1 Head-neck dynamics framework
2.1.1 Head-neck dynamics
The dynamics of the human musculoskeletal system can be formulated with the Euler-Lagrange equations (Zuo et al., 2024) as per Equation 1:
Where,
The acceleration of the body in response to muscle forces and other loads can be computed using the equations of motion for the body as per Equation 2.
The process to get the muscle force vectors is shown in Equation 4.
2.1.2 Muscle-force generating parameters
Muscle activation dynamics takes muscle excitation as the input and muscle activation as the output. Muscle excitation
Neck muscle forces can be computed from the activation
Where
The total generated neck muscle force is a function of muscle fiber length and muscle velocity. The force-length curve,
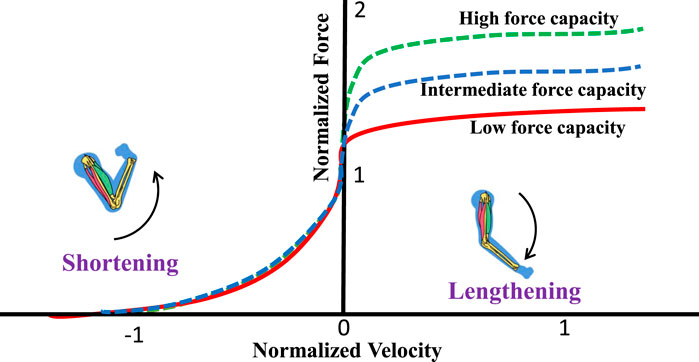
Figure 2. Force-velocity relationship. A higher eccentric multiplier results in higher forces during eccentric (lengthening) contractions. Positive velocity indicates lengthening, and negative velocity indicates shortening.
Based on the eccentric multiplier, we consider three subjects (neck strengths): (i) Low force capacity (Sedentary or untrained): EM = 1.2, (ii) Mid force capacity (Recreationally active): EM = 1.4, and (iii) High force capacity (Athletic): EM 1.8.
2.1.3 Forward dynamics
Forward dynamics takes the musculoskeletal model, initial conditions, muscle excitations, and external forces as the input and provides kinematic data over time, muscle states, and joint torques as the output. It computes muscle force using the Hill-type muscle model (Hill, 1938) based on current states and excitations, solves Equation 2 to get the accelerations, and integrates accelerations over time intervals
2.1.4 Joint reaction load
Joint reaction load analysis provides the forces and moments between two consecutive bodies based on the output of forward dynamics. This analysis determines the forces and moments acting on each cervical joint to maintain equilibrium, accounting for external forces, inertial forces, and internal forces, such as force generated from muscles and tendons. It is calculated by solving Newton-Euler equations where all translational and rotational dynamics are presented between those two consecutive bodies. The Newton-Euler Equations for estimating the atlantoaxial (C1-C2) joint reaction load is shown in Equation 6
where
2.2 Integration of head, neck, and muscle injury criteria with the framework
2.2.1 Head injury criteria
Acceleration is measured in multiples of the acceleration of gravity (g), and time is measured in seconds. Head Injury Criteria (HIC) is proposed by the US National Highway Traffic Safety Administration (NHTSA), as shown in Equation 7
Where,
However, rotational acceleration is responsible for shear stress that damages brain tissue. HIC does not consider rotational acceleration of the head in its injury evaluation. The Generalized Acceleration model Brain Injury Threshold (GAMBIT) model was developed by Newman (Newman, 1986) by combining translational and rotational components of head acceleration as shown in Equation 8.
Where
Margulies and Thibault (1992) reported that the threshold value of shear strain has been reported to be about 5%–10% in case of DAI. It was observed that 50 percent concussion probability is observed within the corpus callosum at 21 percent strain (Kleiven, 2007). Deck and willinager proposed a 31% for maxmimum principal strain threshold and a 25% shear strain to indicate the onset of axonal damage. Rika carlsen adopted tissue level threshold 18% as the axonal strain injury tolerance. Bain et al. considered 18% strain as for the onset of electrophysiological impairment and 21% as morphological damage of axon. Kimapra et al. found out that at 63 g and 4,267 rad/s^2 accelerations, the brain tissue strain is about 29% for 25% mTBI. However, the macro level paramters and tissue level paraterms interactions requuiires further researche to devleop the bridge.
2.2.2 Neck injury criteria
The existing understanding of neck injury criteria is based on load combinations of axial force and flexion-extension moments that are measured with ATDs. Studies have shown that a compressive force of 1750 N–4800 N can cause cervical disc injury (Myers and Winkelstein, 1995; Whyte et al., 2020). One of the most utilized neck injury criteria is
2.2.3 Muscle-tendon strain
Muscle-tendon strain is expressed as a percentage increase of its current length compared to the length when it is at rest and developing no force. The resting length is called the slack length. The strain of muscle-tendon at any given instant can be expressed in Equation 10.
Here,
2.3 Impact parameters
2.3.1 Impact severities (
We adopted the classification of impact severity from Beeman et al. (2012). Based on impact durations and force, we classified external forces into three categories: (i) Low-severity impact (LSI),
2.3.2 Impact locations (
The selection of impact locations was driven by both clinical injury prevalence and biomechanical considerations. These locations encompass a comprehensive range of impact directions observed in high-energy events such as automotive crashes, sports collisions, and blasts (Pellman et al., 2003; Post et al., 2015; National Highway Traffic Safety Administration, 2020). Post et al. (2015) reported that subdural hematoma occurred from impacts to the frontal and occipital regions, whereas impacts to the sides of the head produced parenchymal contusions than the impacts to the front and occipital regions of the head. Pellman et al. (2003) indicated that 78% of NFL impacts happen from the frontal left and right sides.
We categorized the location of head contact into seven impact locations (
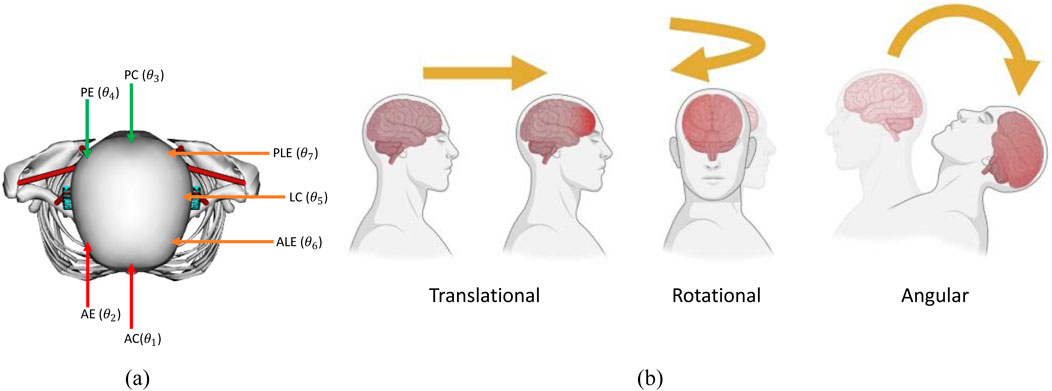
Figure 4. (a) Impact locations (AC = Anterior Concentric, AE = Anterior Eccentric, PC = Posterior Concentric, PE = Posterior Eccentric, LC = Lateral Concentric, PLE = Posterolateral Eccentric, ALE = Anterolateral Eccentric), (b) types of head acceleration.
There are three different types of acceleration that affect the skull and the head: translational, rotational, and angular acceleration, as shown in Figure 4b. For the translational type, also known as linear, the center of gravity of the head will move in a straight line without rotation, and this usually results in focal injury, while in rotational acceleration, there is no movement of the center, but the head will rotate around it, resulting in diffuse shear strain. Lastly, the angular type is a combination of both rotational and linear properties, and the center of gravity moves in an angular manner. The angular type is the most common type and is responsible for the bulk of the TBI cases.
2.4 Simulation workflow
2.4.1 Data collection
We collected three datasets to analyze the relationship of neck strength, impact locations, and impact severities on head and neck injury parameters. For the low-severity impacts (LSI), we collected an automotive crash test from the NHTSA database. The test number was 11,149 (National Highway Traffic Safety Administration, 2020). A frontal rigid barrier impact test was conducted on a 2020 Toyota Highlander SUV. The impact velocity of the vehicle was 56.35 km/h (35 mph). A 50th percentile male Hybrid III ATD was placed in the driver sitting position. The impact duration was about 75 ms, and the impact force was 1210N. For the moderate-severity impacts (MSI), we used extrapolated American Football data (Mortensen et al., 2018). The impact duration was 13 ms, and the maximum impact force was 1510N. For the high-severity impacts (HSI), we used simulated blast data (Kulkarni et al., 2014). The impact duration was 5 ms and the impact force was 3455N. The exact force and time duration data were also used to validate the model.
2.4.2 Musculoskeletal model simulation
A modified head-neck musculoskeletal model was employed in OpenSim software (Delp et al., 2007; Seth et al., 2018). The model was verified using 10 subjects’ data for moderate severity impacts. The head neck model is based on Mortensen (Mortensen et al., 2018) and Vasavada’s model (Vasavada et al., 1998). The model has a total of 72 Millard muscle actuators (Millard et al., 2013; Kuo et al., 2019). Neck muscles such as multifidus and Semispinalis Cervicis are short and highly pennated. The Millard muscles better capture muscle-specific behaviors, such as tendon compliance and fiber pennation angles. Moreover, the Millard model has detailed non-linear tendon dynamics. Therefore, it reflects neck muscle activation dynamics better than traditional hill-type muscle. The musculoskeletal model incorporates soft-tissue damping and rate-dependent ligaments. It also includes passive cervical spine ligaments and viscoelastic properties of muscles. These passive components stabilize the cervical spine during dynamic loading, ensuring biofidelic GAMBIT and NIC predictions for lateral and flexion-extension impacts. These properties are crucial for accurately simulating energy dissipation during rapid stretching in impacts. The details about the model can be found in (Kuo et al., 2018; Mortensen et al., 2018; Kuo et al., 2019; Mortensen et al., 2020).
Muscle activation data for neck stiffness were collected from the literature (Kuo et al., 2018; Kuo et al., 2019). Three neck strengths, three impact severities, and seven impact locations, a total of 63 cases, were performed. A MATLAB script was used to run these 63 cases in OpenSim forward dynamics simulations. We used OpenSim’s adaptive time step size for the forward dynamics. The maximum step size was set to 1, and the minimum step size was set to 1 × 10−7. The integrator tolerance was set to 1 × 10−5. During the forward dynamics, the integrator dynamically chooses a step size between these bounds to satisfy the integrator error tolerance. For slower and steadier motion, it will increase the step size to save computation time. For highly dynamic simulations, the step size shrinks to capture fast-changing dynamics. The workflow diagram is shown in Figure 5.
2.4.3 Statistical analyses
Statistical analyses were performed using IBM SPSS Statistics version 29.0 (IBM Corp., Armonk, NY, USA). Initially, normality of the data was assessed with the Shapiro-Wilk test. Parametric one-way analysis of variance (ANOVA) was employed for normally distributed data, while the nonparametric Kruskal–Wallis test was utilized for datasets deviating from normality. For pairwise comparisons, the Mann-Whitney U test was conducted.
A post hoc power analysis for GAMBIT, conducted in SPSS using an effect size of
3 Results
3.1 Head injury risk analysis based on linear and rotational accelerations
The linear accelerations are presented in Figure 6a. The rotational accelerations are presented in Figure 6b. The linear and rotational accelerations of the head are calculated at its center of mass.
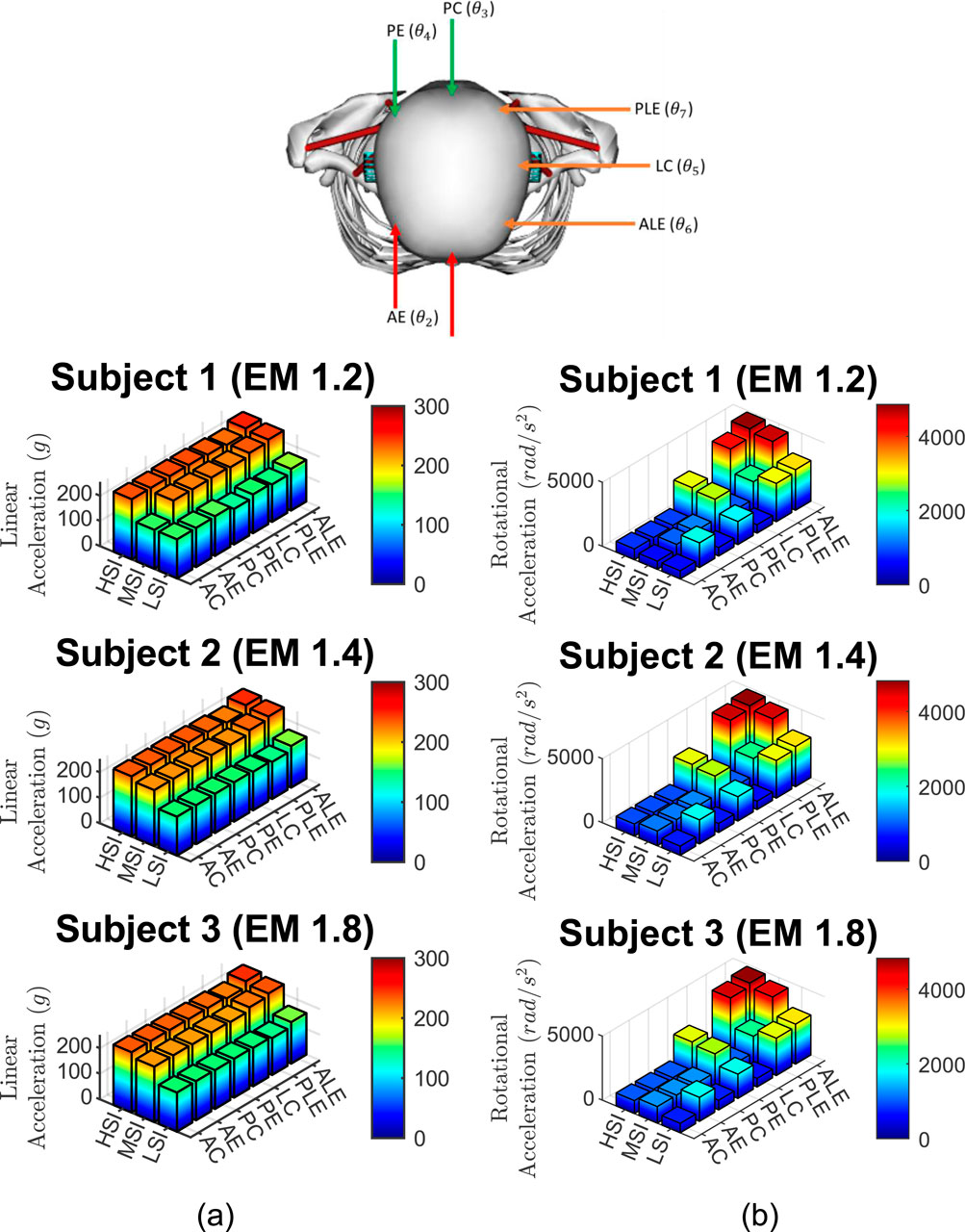
Figure 6. Accelerations from 63 cases, (a) Linear accelerations and (b) rotational accelerations for three subjects, three impact severities, and seven impact locations.
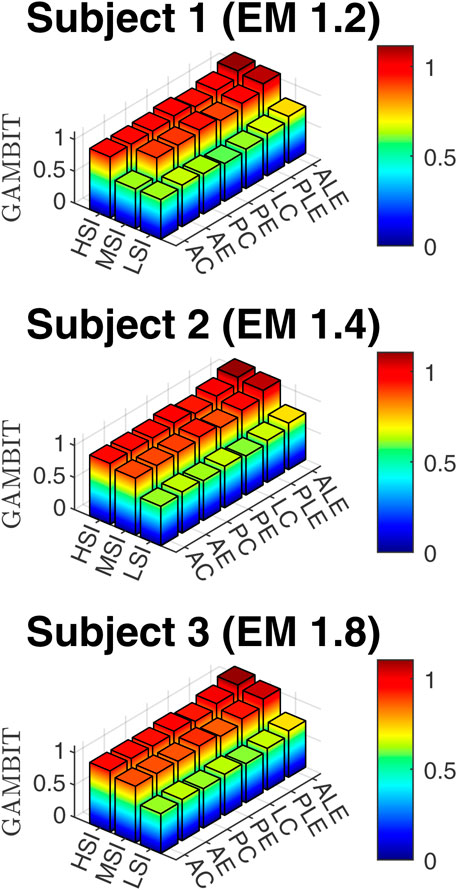
3.2 Head injury risk analysis based on GAMBIT
The GAMBIT results, based on Equation 7 are presented in Figure 7.
3.3 Neck injury risk assessment based on cervical spine force and moment
The calculated neck force and moment data are presented in Figure 8.
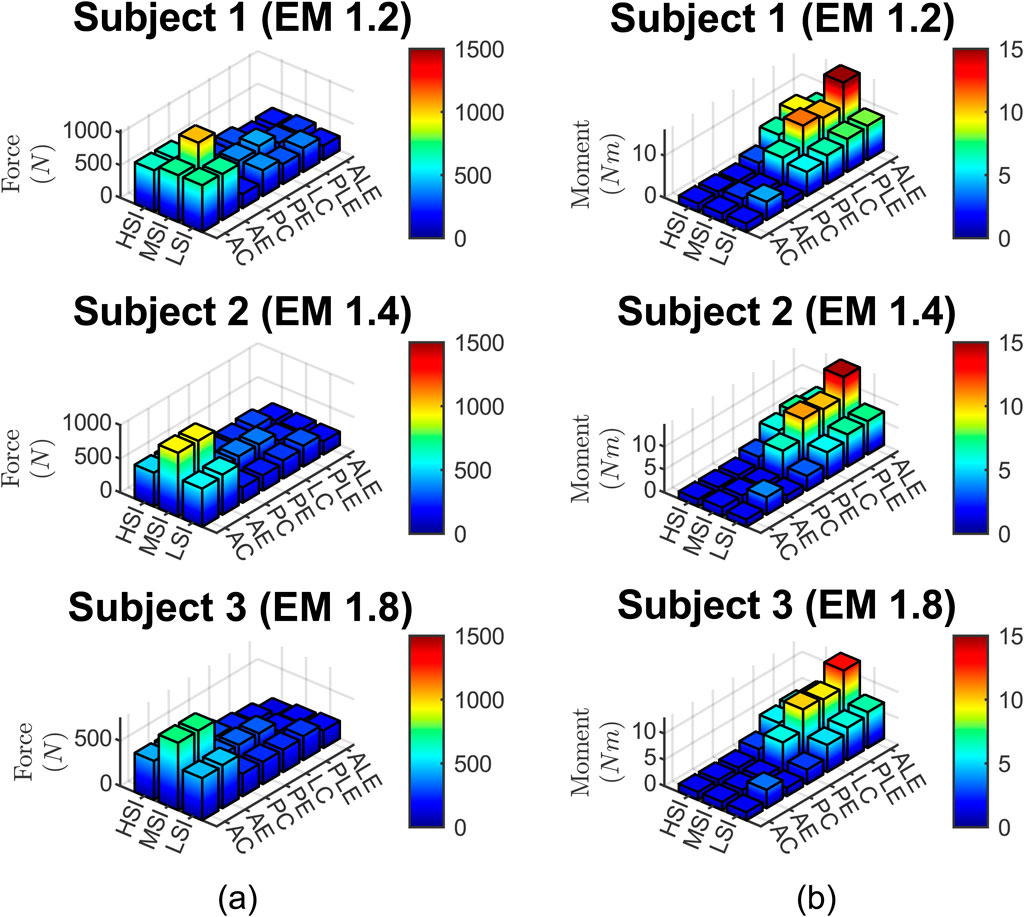
Figure 8. (a) neck force, (b) neck moment for three subjects, three impact severities, and seven impact locations.
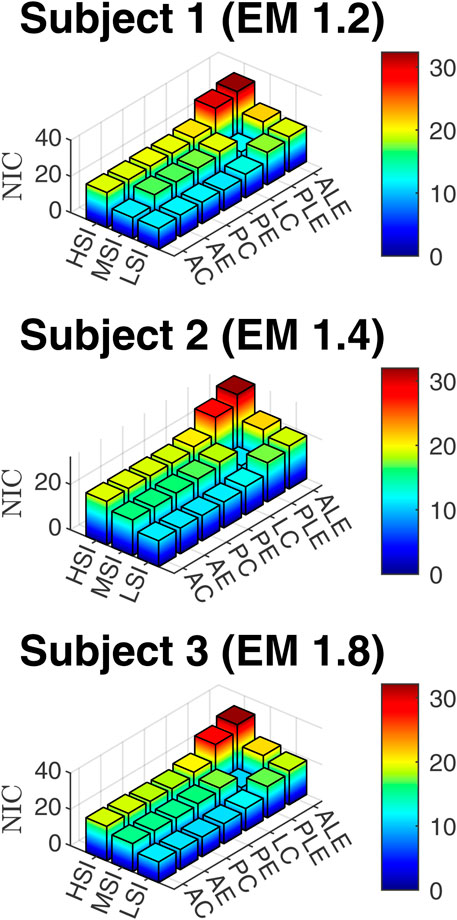
3.4 Neck injury assessment based on NIC
The NIC data, based on Equation 8 are presented in Figure 9.
3.5 Muscle strain injury
The strains for six different muscles, that take part in resistive action of the neck are presented in Figure 10.
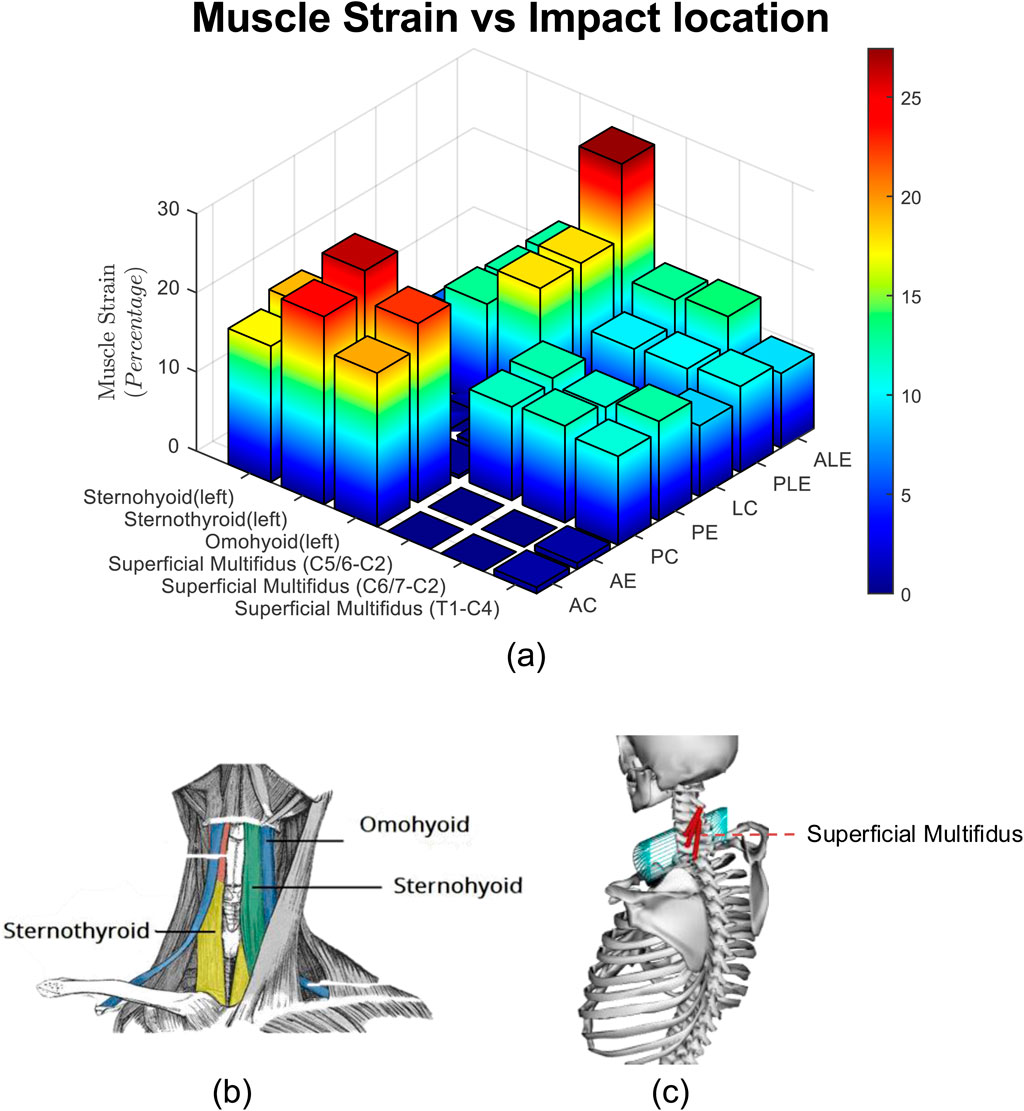
Figure 10. (a) Muscle Strain for three subjects, three impact severities, and seven impact locations. (b) Hyoid muscles, (c) superficial multifidus muscles.
4 Discussion
In this study, we developed a computational musculoskeletal head-neck model using OpenSim software to investigate the effects of impact location, neck strength, and impact severity on head and neck injury parameters. We designed a total of 63 simulation cases, varying across seven impact locations, three neck strength levels, and three impact severities.
4.1 Effect of impact location
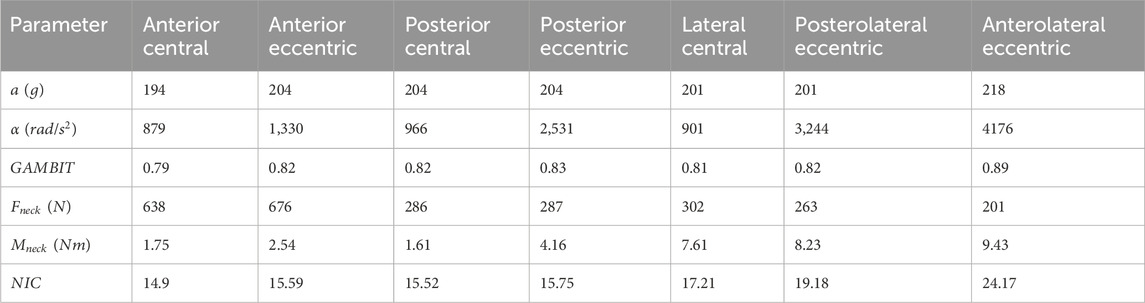
The box plots in Figure 11 illustrate the linear and rotational accelerations, GAMBIT, neck force, neck moment, and NIC across various impact locations, with data taken from three subjects. The boxes represent the interquartile range (IQR), the red horizontal lines indicate the medians, and the red circles show the mean values, while the whiskers display the minimum and maximum values within the data range. Table 1 shows the mean value of each data for different locations. It can be seen from 9 (a) that linear accelerations do not vary much based on impact directions and locations and stay within 194 g–218 g. Eccentric impacts create higher linear acceleration than central impacts however, the increment is less than 10%. The lateral impacts generate about 10% more linear accelerations than anterior and posterior impacts. The hyoid muscles are highly stretched (>10%) during frontal and ALE impacts, whereas the multifidus muscles were highly stretched during posterior and lateral impacts.
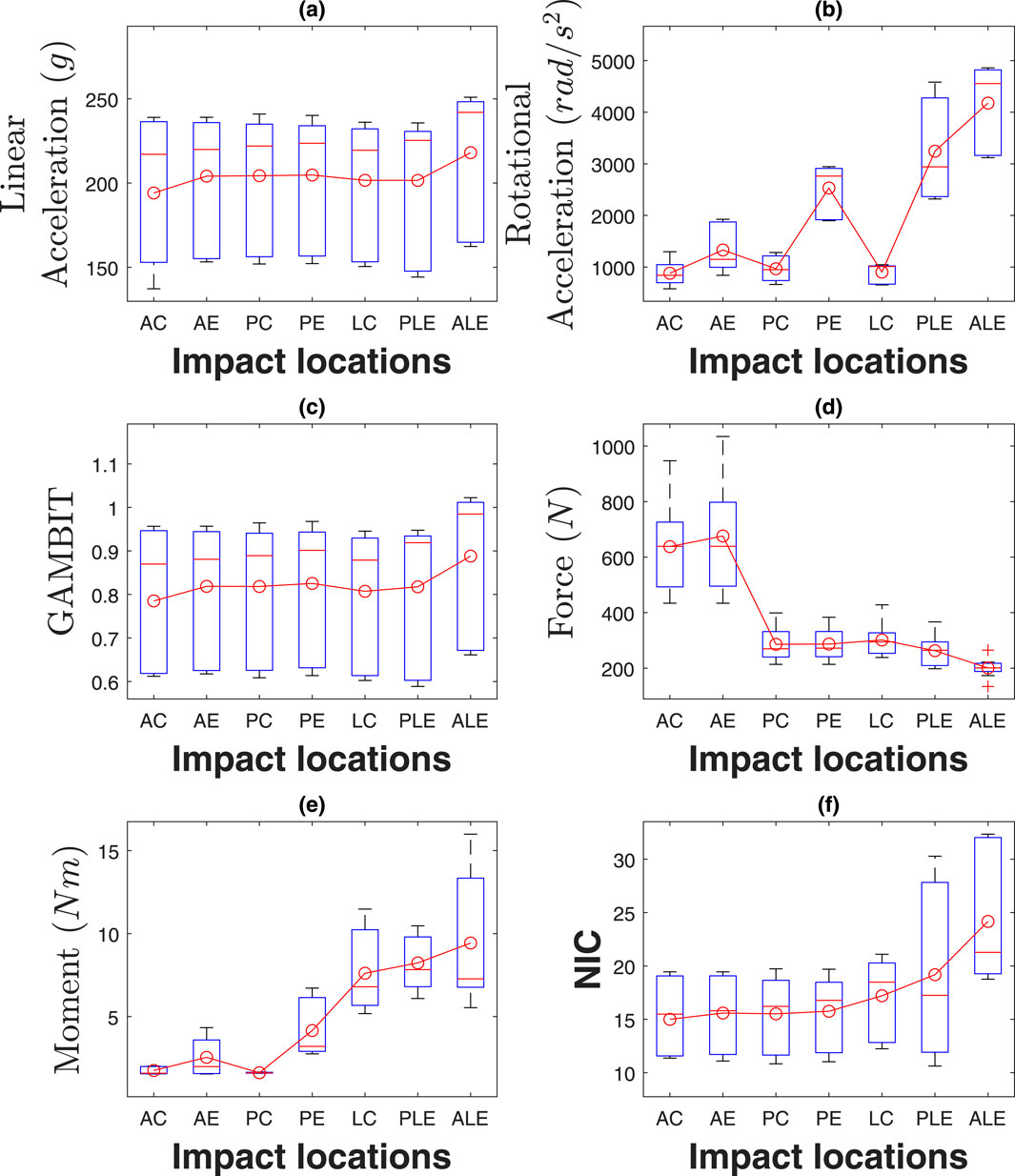
Figure 11. Effect of impact locations on (a) linear acceleration, (b) rotational acceleration, (c) GAMBIT, (d) neck force, (e) neck moment, and (f) NIC.
To quantify the effect of impact location on injury parameters, we conducted Kruskal–Wallis tests due to normality violations (Shapiro-Wilk p < 0.05 for most variables). Significant main effects were found for NIC (
The rotational accelerations in Figure 11b vary significantly based on impact directions and locations. The eccentric impacts generated about 1.51, 2.61, and 4.11 times more rotational acceleration than central impacts, for the anterior, posterior, and lateral sides, respectively. Posterior and lateral impacts create 1.58 and 2.51 times more rotational acceleration than anterior impacts, respectively. The maximum average rotation acceleration is for anterolateral eccentric impact (4,176
The GAMBIT values in Figure 11c do not vary very much with the impact directions and locations similar to linear accelerations. It was within 0.79–0.83 for all impact locations except for anterolateral eccentric, which was 0.89. In summary, impact locations have a significant effect on rotational acceleration, and anterolateral impact is the riskiest impact location for both head and neck.
The average neck forces for the anterior impacts were about 2.5 times higher than for posterior and lateral impacts. The maximum average neck force was for the anterior eccentric impacts (676N). Neck moments in Figure 11e vary significantly based on impact locations and directions. The highest average neck moment was for anterolateral eccentric (9.43Nm), which was 5.85 times more than the posterior central neck moment (1.61Nm). Also, eccentric impacts generate about 157% more neck moments than central impacts. NIC values in Figure 11f were within 15–24
4.2 Effect of neck strength
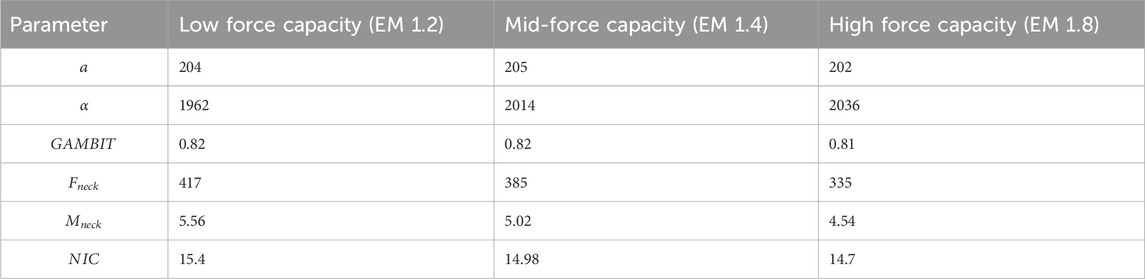
The effects of neck strength are presented in Figure 12 and Table 2. Average linear accelerations in Figure 12a do not vary much with neck muscle strength and were within 202
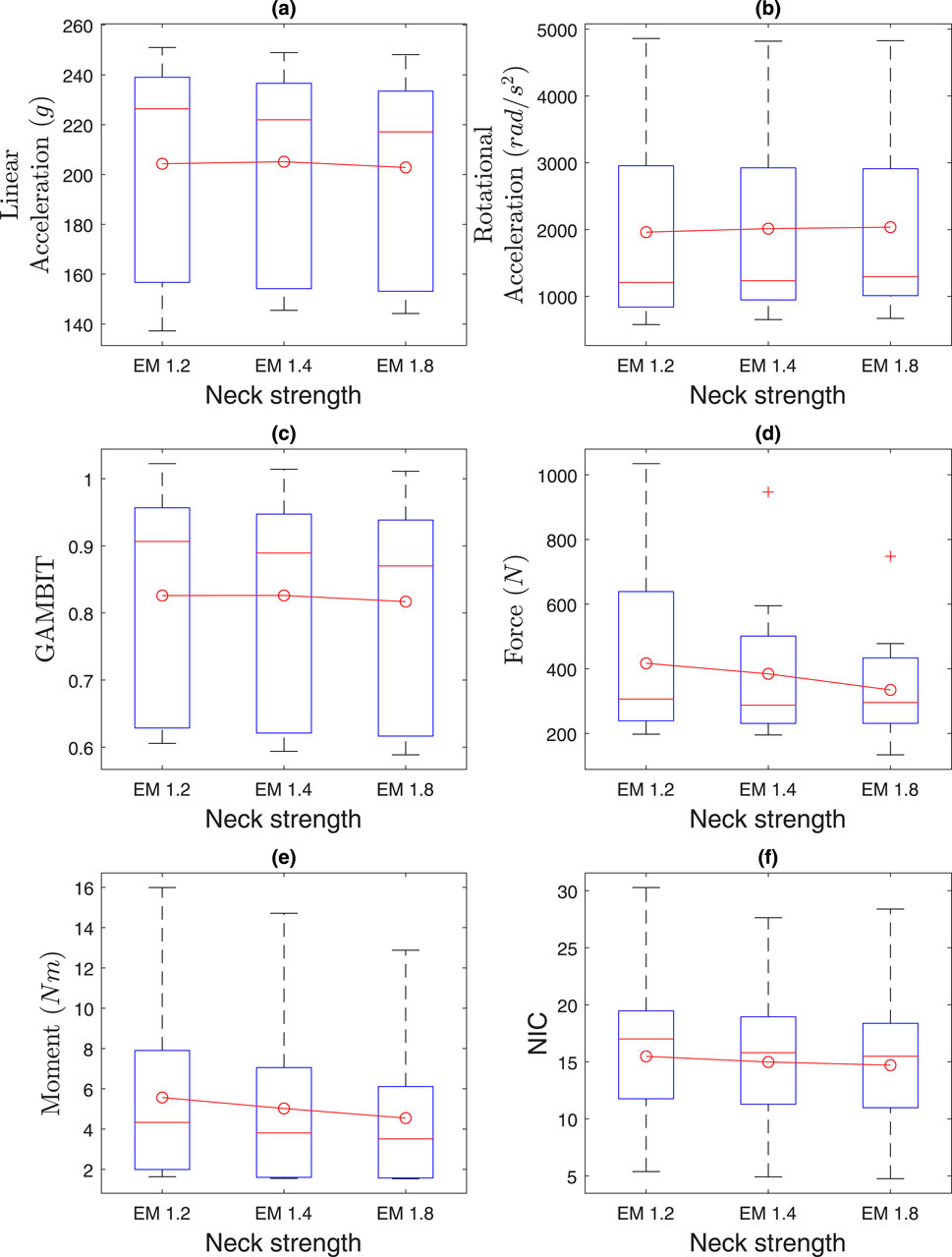
Figure 12. Effect of neck strength on (a) linear acceleration, (b) rotational acceleration, (c) GAMBIT, (d) neck force, (e) neck moment, and (f) NIC.
Kruskal–Wallis tests showed no significant effects of neck strength (EM 1.2, 1.4, 1.8) on any injury parameters (
The average neck force in Figure 12d ranges from 334N to 417N. The average neck moment in Figure 12e ranges from 4.5Nm to 5.6Nm. The average NIC value ranges from 14.7 to 15.5. In summary, the average head and neck injury parameters do not vary by more than 10% based on neck strength, which agrees with the literature (Mortensen, Vasavada et al., and statistical analysis.
4.3 Effect of impact severities
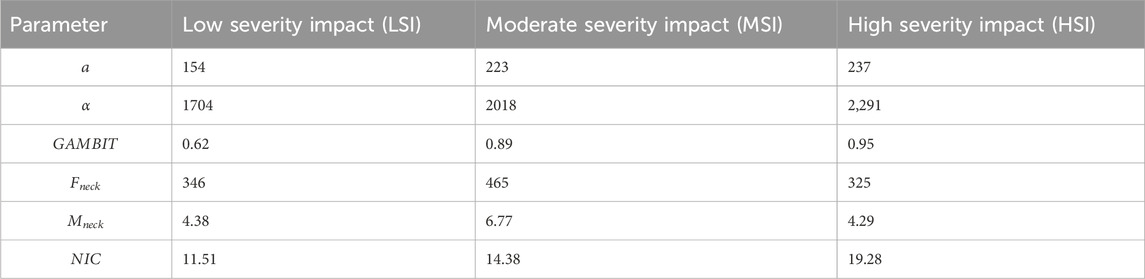
The effects of impact severities are presented in Figure 13 and Table 3. Kruskal–Wallis tests revealed significant effects of impact severities (LSI, MSI, HSI) on NIC (
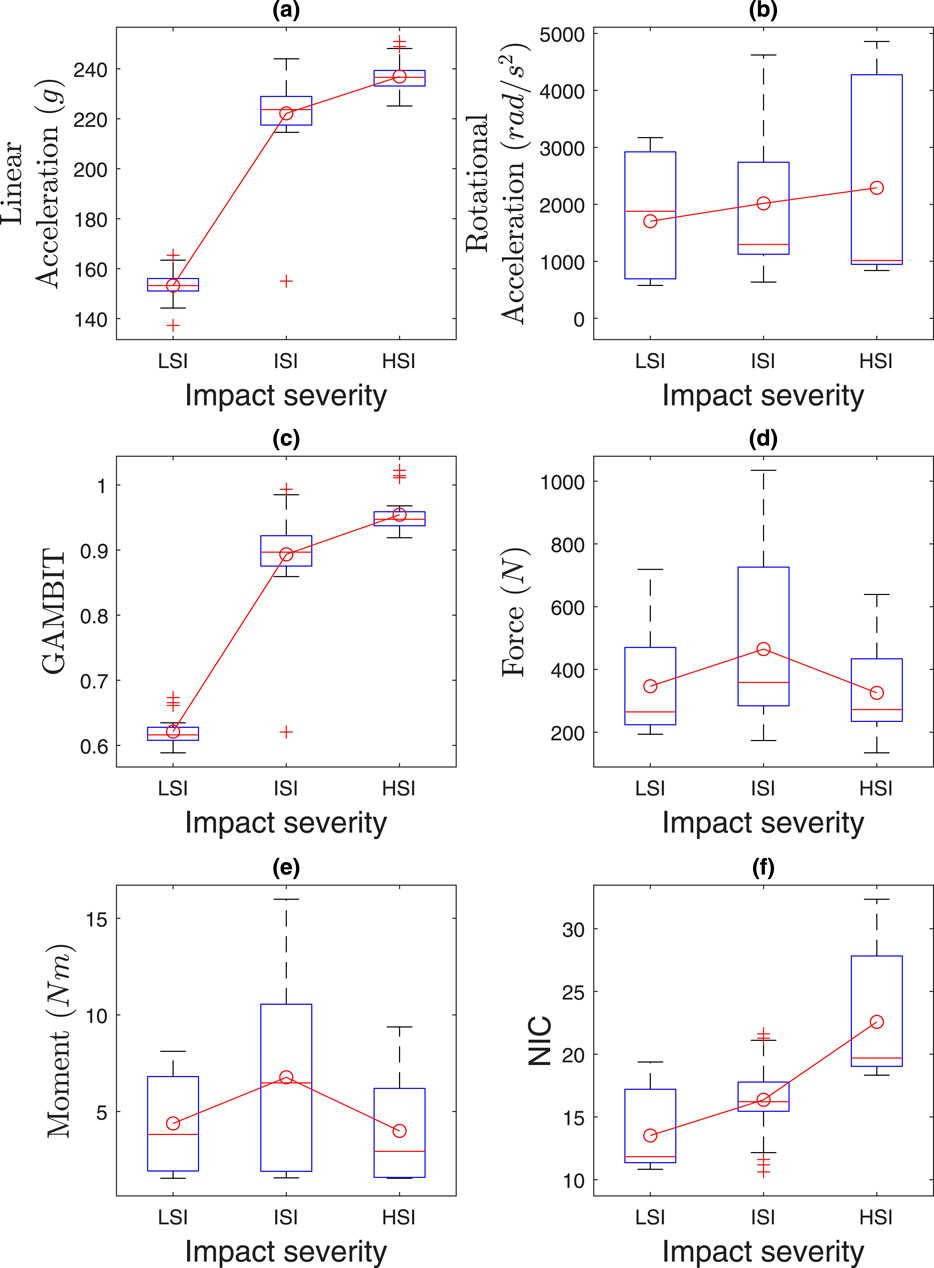
Figure 13. Effect of impact severities on (a) linear acceleration, (b) rotational acceleration, (c) GAMBIT, (d) neck force, (e) neck moment, and (f) NIC.
Our model incorporates both passive and active muscle responses, as detailed in Section 2.1.2 (Equation 3), using muscle activation data from literature (Kuo et al., 2018; Kuo et al., 2019). However, in HSI, the rapid onset limits active muscle activation, making passive responses dominant. For LSI and MSI impacts, active muscle responses contribute significantly, reflecting anticipatory stiffening in automotive or sports scenarios. NIC and GAMBIT may be conservatively high due to limited active response time for HSI. However, we ignored pre-activation in all 63 cases, as it is reported that pre-activation can reduce head acceleration by ∼30% in sports contexts (Fanta et al., 2013). This was omitted to standardize responses across all impact severities. It ensures consistency across the 63 cases and avoids bias from sports-specific pre-activation data.
The average linear accelerations in Figure 13a for MSI and HSI are 1.45 and 1.54 times higher than LSI, and range from 153
The neck forces and moments did not vary much based on the impact severities, as in Figures 13d,e. The NIC in Figure 13f for MSI and HSI were 1.24 and 1.68 times more than LSI. The maximum NIC value was 30.28 for HSI. In summary, the risk probability of head and neck injuries is higher for HSI and MSI than LSI.
4.4 Validation
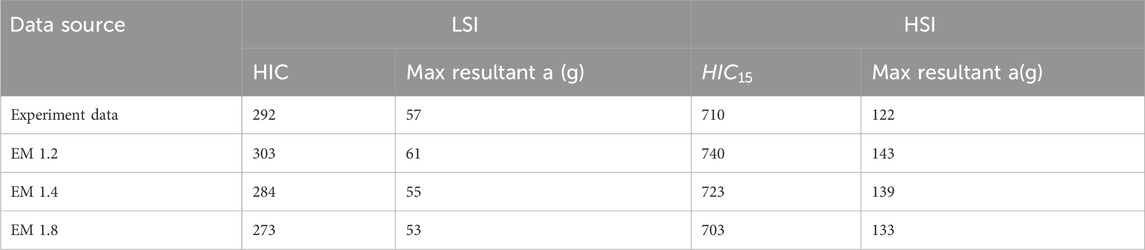
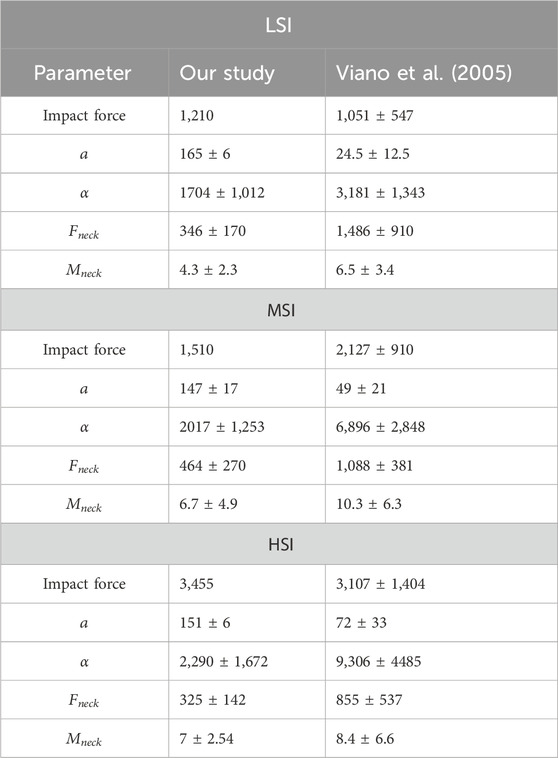
The musculoskeletal model was validated in previous studies for MSI using NFL (Mortensen et al., 2018; Kuo et al., 2019; Mortensen et al., 2020) and rugby (Silvestros et al., 2024) data. However, it was not validated for LSI and HSI. To validate the model for LSI and HSI, we ran the simulation using the literature data for LSI and HSI for all neck strength. The literature only has the HIC value and max resultant linear acceleration, which we compared with our result for validation. However, the neck strengths of the experimental subjects were not mentioned in the literature. So, we ran the simulation for all neck strength. We can see from Table 4, for LSI, the experimental data indicates that the subject’s (ATD’s) neck strength was between EM 1.2 and EM 1.4. For HSI, the experimental data indicates that the subject’s (ATD’s) neck strength was between 1.4 and 1.8. The simulation results were consistent with the literature data.
We also compared our average results with Hybrid III ATD results (Viano, 2005) as shown in Table 5. Although the experiment design and impact forces differ, the means of most results are within one standard deviation for rotational accelerations, neck force, and moments.
From our results, the rotational accelerations of the anterolateral eccentric and posterolateral eccentric are 4.7 and 3.7 times higher than the anterior central impacts, respectively. It is mentioned in the literature that spin rotational acceleration (anterolateral eccentric or posterolateral eccentric) of the head is 3.5 times higher than whiplash acceleration (anterior impact) (Razzaghi, 2019). Our acceleration data is consistent with the literature. The distance ratio from the front of the head to the center of mass of the head and from back of the skull to the center of mass of the skull is 0.52 and 0.48. That means the anterolateral impact will create more acceleration and moment than the posterolateral impact. Viano et al. (2007) studied twenty-five helmet collisions involving NFL players and found that the maximum rotation occurred around the superior-inferior axis. The maximum acceleration was 9,678
4.5 Limitation
There are some limitations to this study. First, we ignored torso velocity and accelerations on the impact of head and neck injuries. We considered the torso as a fixed body. Secondly, we did not consider the whole blast impact on the head and neck injuries. We considered an idealized blast scenario, which did not account for multidimensional waveforms. This does not perfectly reproduce the experimental environment and head impacts. As a result, it may underestimate the rotational kinematics. Future models should incorporate these complexities to improve the realism. Thirdly, we consider height, weight, and anthropometric data for all tests to be the same, in order to consider the effects of neck strength, impact locations, and impact severities on head and neck injuries. However, with different anthropometric and muscle activation data, head-neck responses after an incident may vary. Also, we used the MSI muscle activation data for all cases. The muscles’ activations data were collected from (Mortensen et al., 2018; Mortensen et al., 2020).
Fourth, while
Figure 14. (a) Lobes and functional areas of the brain, (b) cross-section of the brain. Images modified and used with permission from Servier Medical Art, Creative Commons Attribution 4.0 Unreported License.
Also, the biomechanical model utilized in this study is based on a 50th percentile male, which may limit its generalizability to female populations. Sex-based differences in neck musculature and TBI susceptibility suggest that females may exhibit distinct injury responses due to variations in anatomy and physiology (Putra et al., 2022). This study does not quantify the differences in inter-subject variability, such as age, sex, and muscle composition. Moreover, in this study, we did not quantify the spatial distribution of impact eccentricity, such as moment arm distances. This limitation may restrict a detailed dose-response analysis that future research could explore, if relevant relationships are identified. Careful consideration should be given to the limitations outlined in this study during interpretation of the presented data and decision making. This study provides valuable insights. However, the limitations of those study should be kept in mind for any conclusion in a broader context. Further works are necessary to enhance the robustness and generalizability of the outcomes.
5 Conclusion
In this study, we investigated the varying relationship of neck strength, impact locations, and impact severities with head and neck injury criteria. We employed a musculoskeletal model and used forward dynamics to run 63 cases. The results were validated using different literature data. It was found that impact locations have a significant effect on head and neck injury parameters, and anterolateral eccentric impact is the riskiest impact location for both head and neck. We also found that average head and neck injury parameters do not vary more than 10% based on neck strength. Finally, the risk probability of head and neck injuries is higher for HSI and MSI than for LSI. These findings indicate that people who face an anterolateral eccentric impact are at higher risk than the person who faces an impact from other directions and should seek medical attention. The biomechanical findings of this study suggest a need for enhanced lateral impact protection in helmet design, potentially through energy-absorbing materials. These recommendations are preliminary and require clinical validation to ensure efficacy. To our knowledge, this is the first musculoskeletal model based study, where head and neck injury parameters were assessed for multiple impact locations and for all three types of severity impacts. Although the musculoskeletal-model-based framework has limitations, it provides vital information about head and neck injuries for future researchers. The high biofidelity data from this study can be utilized as the input for tissue-level and molecular-level head and neck injury studies. That will enhance our understanding about the cause-and-effects of upper extremity organ-level injuries.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
RZ: Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft. MH: Data curation, Formal Analysis, Writing – original draft. AR: Formal Analysis, Visualization, Writing – original draft. SC: Formal Analysis, Visualization, Writing – original draft. AJ: Writing – review and editing. AK: Writing – review and editing. AA: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. AA acknowledges the financial support from Office of Naval Research (ONR)’s Force Health Protection (FHP) program (through the Award # ONR: N00014-21-1-2051 and ONR:N000142412324 Dr. Timothy Bentley, Program Manager).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bain, A. C., and Meaney, D. F. (2000). Tissue-level thresholds for axonal damage in an experimental model of central nervous system white matter injury. J. Biomech. Eng. 122 (6), 615–622. doi:10.1115/1.1324667
Beeman, S. M., Kemper, A. R., Madigan, M. L., Franck, C. T., and Loftus, S. C. (2012). Occupant kinematics in low-speed frontal sled tests: human volunteers, hybrid III ATD, and PMHS. Accid. Analysis and Prev. 47, 128–139. doi:10.1016/j.aap.2012.01.016
Bohman, K., Haland, Y., H Land, Y., and Kullgren, A. (2000). A study of AIS1 neck injury parameters in 168 frontal collisions using a restrained hybrid III dummy. SAE Tech. Pap. 44, 103–116. doi:10.4271/2000-01-sc08
Borich, M. R., Cheung, K. L., Jones, P., Khramova, V., Gavrailoff, L., Boyd, L. A., et al. (2013). Concussion: current concepts in diagnosis and management. J. Neurologic Phys. Ther. 37 (3), 133–139. doi:10.1097/npt.0b013e31829f7460
Boström, O., and Kullgren, A. (2007). Characteristics of anti-whiplash seat designs with good real-life performance. Proc. IRCOBI Conf.
Browne, K. D., Chen, X.-H., Meaney, D. F., and Smith, D. H. (2011). Mild traumatic brain injury and diffuse axonal injury in swine. J. neurotrauma 28 (9), 1747–1755. doi:10.1089/neu.2011.1913
Bumberger, R., Acar, M., and Bouazza-Marouf, K. (2025). Parametric computational head-and-neck model and anthropometric effects on whiplash loading.
Burdi, A. R., Huelke, D. F., Snyder, R. G., and Lowrey, G. H. (1969). Infants and children in the adult world of automobile safety design: pediatric and anatomical considerations for design of child restraints. J. Biomechanics 2 (3), 267–280. doi:10.1016/0021-9290(69)90083-9
Chinn, B., Canaple, B., Derler, S., Doyle, D., Otte, D., Schuller, E., et al. (2001). COST 327 motorcycle safety helmets, 3. Belgium: European Commission, Directorate General for Energy and Transport, 1135–1148.
Deck, C., and Willinger, R. (2008). Improved head injury criteria based on head FE model. Int. J. Crashworthiness 13 (6), 667–678. doi:10.1080/13588260802411523
Defense Health Agency (DHA) (2024). DOD numbers for traumatic brain injury worldwide. Def. Health Agency (DHA).
Delp, S. L., Anderson, F. C., Arnold, A. S., Loan, P., Habib, A., John, C. T., et al. (2007). OpenSim: open-source software to create and analyze dynamic simulations of movement. IEEE Trans. Biomed. Eng. 54 (11), 1940–1950. doi:10.1109/tbme.2007.901024
Deng, Y.-C., and Goldsmith, W. (1987). Response of a human head/neck/upper-torso replica to dynamic loading—II. analytical/Numerical model. J. biomechanics 20 (5), 487–497. doi:10.1016/0021-9290(87)90249-1
Dymek, M., Ptak, M., Ratajczak, M., Fernandes, F. A., Kwiatkowski, A., and Wilhelm, J. (2021). Analysis of HIC and hydrostatic pressure in the human head during NOCSAE tests of American football helmets. Brain Sci. 11 (3), 287. doi:10.3390/brainsci11030287
Eckner, J. T., Oh, Y. K., Joshi, M. S., Richardson, J. K., and Ashton-Miller, J. A. (2014). Effect of neck muscle strength and anticipatory cervical muscle activation on the kinematic response of the head to impulsive loads. Am. J. sports Med. 42 (3), 566–576. doi:10.1177/0363546513517869
Escolas, S. M., Luton, M., Ferdosi, H., Chavez, B. D., and Engel, S. D. (2020). Traumatic brain injuries: unreported and untreated in an army population. Mil. Med. 185 (Suppl. ment_1), 154–160. doi:10.1093/milmed/usz259
Fanta, O., Hadraba, D., Lopot, F., Kubový, P., Bouček, J., and Jelen, K. (2013). Pre-activation and muscle activity during frontal impact in relation to whiplash associated disorders. Neuroendocrinol. Lett. 34 (7), 708–716.
Farmer, J., Mitchell, S., Sherratt, P., and Miyazaki, Y. (2022). A human surrogate neck for traumatic brain injury research. Front. Bioeng. Biotechnol. 10, 854405. doi:10.3389/fbioe.2022.854405
Feist, F., Gugler, J., Arregui-Dalmases, C., Del Pozo de Dios, E., López-Valdés, F., Deck, D., et al. (2009). “Pedestrian collisions with flat-fronted vehicles: injury patterns and importance of rotational accelerations as a predictor for traumatic brain injury (TBI),” in 21st international technical conference on the enhanced safety of vehicles (ESV).
Fernandes, F. A., and Sousa, R. J. A. d. (2015). Head injury predictors in sports trauma–a state-of-the-art review. Proc. Institution Mech. Eng. Part H J. Eng. Med. 229 (8), 592–608. doi:10.1177/0954411915592906
Funk, J. R., Duma, S., Manoogian, S., and Rowson, S. (2007). “Biomechanical risk estimates for mild traumatic brain injury,” in Annual proceedings/association for the advancement of automotive medicine (Melbourne, VIC: Association for the Advancement of Automotive Medicine).
Gabler, L. F., Crandall, J. R., and Panzer, M. B. (2019). Development of a second-order system for rapid estimation of maximum brain strain. Ann. Biomed. Eng. 47, 1971–1981. doi:10.1007/s10439-018-02179-9
Garcia, T., and Ravani, B. (2003). A biomechanical evaluation of whiplash using a multi-body dynamic model. J. Biomech. Eng. 125 (2), 254–265. doi:10.1115/1.1556856
Halloran, J. P., Ackermann, M., Erdemir, A., and Van den Bogert, A. J. (2010). Concurrent musculoskeletal dynamics and finite element analysis predicts altered gait patterns to reduce foot tissue loading. J. biomechanics 43 (14), 2810–2815. doi:10.1016/j.jbiomech.2010.05.036
Hasegawa, K., Takeda, T., Nakajima, K., Ozawa, T., Ishigami, K., Narimatsu, K., et al. (2014). Does clenching reduce indirect head acceleration during rugby contact? Dent. Traumatol. 30 (4), 259–264. doi:10.1111/edt.12082
Hernandez, F., Shull, P. B., and Camarillo, D. B. (2015). Evaluation of a laboratory model of human head impact biomechanics. J. biomechanics 48 (12), 3469–3477. doi:10.1016/j.jbiomech.2015.05.034
Hill, A. V. (1938). The heat of shortening and the dynamic constants of muscle. Proc. R. Soc. Lond. Ser. B-Biological Sci. 126 (843), 136–195.
Ho, J., Zhou, Z., Li, X., and Kleiven, S. (2017). The peculiar properties of the falx and tentorium in brain injury biomechanics. J. biomechanics 60, 243–247. doi:10.1016/j.jbiomech.2017.06.023
Huo, F., and Wu, H. (2017). Predicting the head-neck posture and muscle force of the driver based on the combination of biomechanics with multibody dynamics. SAE Tech. Pap. doi:10.4271/2017-01-0407
Insurance Institute for Highway Safety and Highway Loss Data Institute (IIHS-HLDI) (2024). Fatality facts 2022 yearly snapshot.
Jin, X., Feng, Z., Mika, V., Li, H., Viano, D. C., and Yang, K. H. (2017). The role of neck muscle activities on the risk of mild traumatic brain injury in American football. J. biomechanical Eng. 139 (10), 101002. doi:10.1115/1.4037399
Johnson, D., Koya, B., and Gayzik, F. S. (2020). Comparison of neck injury criteria values across human body models of varying complexity. Front. Bioeng. Biotechnol. 8, 985. doi:10.3389/fbioe.2020.00985
Kimpara, H., and Iwamoto, M. (2012). Mild traumatic brain injury predictors based on angular accelerations during impacts. Ann. Biomed. Eng. 40 (1), 114–126. doi:10.1007/s10439-011-0414-2
Kleiven, S. (2007). Predictors for traumatic brain injuries evaluated through accident reconstructions. SAE Tech. Pap. 51, 81–114. doi:10.4271/2007-22-0003
Kulkarni, K. B., Ramalingam, J., and Thyagarajan, R. (2014). Assessment of the accuracy of certain reduced order models used in the prediction of occupant injury during under-body blast events. SAE Int. J. Transp. Saf. 2 (2), 307–319. doi:10.4271/2014-01-0752
Kuo, C., Fanton, M., Wu, L., and Camarillo, D. (2018). Spinal constraint modulates head instantaneous center of rotation and dictates head angular motion. J. biomechanics 76, 220–228. doi:10.1016/j.jbiomech.2018.05.024
Kuo, C., Sheffels, J., Fanton, M., Yu, I. B., Hamalainen, R., and Camarillo, D. (2019). Passive cervical spine ligaments provide stability during head impacts. J. R. Soc. Interface 16 (154), 20190086. doi:10.1098/rsif.2019.0086
Liu, J., Jin, J. J., Eckner, J. T., Ji, S., and Hu, J. (2022). Influence of morphological variation on brain impact responses among youth and young adults. J. biomechanics 135, 111036. doi:10.1016/j.jbiomech.2022.111036
Mackay, M. (2007). The increasing importance of the biomechanics of impact trauma. Sadhana 32 (4), 397–408. doi:10.1007/s12046-007-0031-9
Mang, D. W., Siegmund, G. P., Brown, H. J., Goonetilleke, S. C., and Blouin, J.-S. (2015). Loud preimpact tones reduce the cervical multifidus muscle response during rear-end collisions: a potential method for reducing whiplash injuries. spine J. 15 (1), 153–161. doi:10.1016/j.spinee.2014.08.002
Margulies, S. S., and Thibault, L. E. (1992). A proposed tolerance criterion for diffuse axonal injury in man. J. biomechanics 25 (8), 917–923. doi:10.1016/0021-9290(92)90231-o
McCrea, M., Hammeke, T., Olsen, G., Leo, P., and Guskiewicz, K. (2004). Unreported concussion in high school football players: implications for prevention. Clin. J. sport Med. 14 (1), 13–17. doi:10.1097/00042752-200401000-00003
Merrill, T., Goldsmith, W., and Deng, Y. (1984). Three-dimensional response of a lumped parameter head-neck model due to impact and impulsive loading. J. biomechanics 17 (2), 81–95. doi:10.1016/0021-9290(84)90126-x
Mihalik, J. P., Guskiewicz, K. M., Marshall, S. W., Greenwald, R. M., Blackburn, J. T., and Cantu, R. C. (2011). Does cervical muscle strength in youth ice hockey players affect head impact biomechanics? Clin. J. sport Med. 21 (5), 416–421. doi:10.1097/jsm.0b013e31822c8a5c
Millard, M., Uchida, T., Seth, A., and Delp, S. L. (2013). Flexing computational muscle: modeling and simulation of musculotendon dynamics. J. Biomechanical Eng. 135 (2), 021005. doi:10.1115/1.4023390
Mortensen, J. D., Vasavada, A. N., and Merryweather, A. S. (2018). The inclusion of hyoid muscles improve moment generating capacity and dynamic simulations in musculoskeletal models of the head and neck. PloS one 13 (6), e0199912. doi:10.1371/journal.pone.0199912
Mortensen, J. D., Vasavada, A. N., and Merryweather, A. S. (2020). Sensitivity analysis of muscle properties and impact parameters on head injury risk in American football. J. biomechanics 100, 109411. doi:10.1016/j.jbiomech.2019.109411
Mychasiuk, R., Hehar, H., Candy, S., Ma, I., and Esser, M. J. (2016). The direction of the acceleration and rotational forces associated with mild traumatic brain injury in rodents effect behavioural and molecular outcomes. J. Neurosci. methods 257, 168–178. doi:10.1016/j.jneumeth.2015.10.002
Myers, B. S., and Winkelstein, B. A. (1995). Epidemiology, classification, mechanism, and tolerance of human cervical spine injuries. Crit. Reviews™ Biomed. Eng. 23 (5-6), 307–409. doi:10.1615/critrevbiomedeng.v23.i5-6.10
National Highway Traffic Safety Administration (2020). “New car assessment program (NCAP), frontal barrier impact test, Toyota Motor manufacturing, Indiana, INC, 2020 Toyota highlander,” in Five door suv, nhtsa no: m20205100, u. s. department of transportation, national highway traffic safety administration, office of crashworthiness standards.
Newman, J. (1986). “A generalized acceleration model for brain injury threshold (GAMBIT),” in Proceedings of international IRCOBI conference, 1986.
Pellman, E. J., Viano, D. C., Tucker, A. M., and Casson, I. R. (2003). Concussion in professional football: location and direction of helmet impacts—Part 2. Neurosurgery 53 (6), 1328–1341. doi:10.1227/01.neu.0000093499.20604.21
Post, A., Hoshizaki, T. B., Gilchrist, M. D., Brien, S., Cusimano, M. D., and Marshall, S. (2014). The influence of acceleration loading curve characteristics on traumatic brain injury. J. biomechanics 47 (5), 1074–1081. doi:10.1016/j.jbiomech.2013.12.026
Post, A., Hoshizaki, T. B., Gilchrist, M. D., Brien, S., Cusimano, M., and Marshall, S. (2015). Traumatic brain injuries: the influence of the direction of impact. Neurosurgery 76 (1), 81–91. doi:10.1227/neu.0000000000000554
Pramudita, J. A., Ujihashi, S., Ono, K., Ejima, S., Sato, F., Yamazaki, K., et al. (2009). Development of a head-neck finite element model and analysis of intervertebral strain response during rear impact. Trans. Jpn. Soc. Mech. Eng. Ser. A 75 (759), 1549–1555. doi:10.1299/kikaia.75.1549
Prasad, P., and Daniel, R. P. (1984). A biomechanical analysis of head, neck, and torso injuries to child surrogates due to sudden torso acceleration. SAE Trans., 784–799.
Putra, I. P. A., Iraeus, J., Sato, F., Svensson, M. Y., and Thomson, R. (2022). Finite element human body models with active reflexive muscles suitable for sex based whiplash injury prediction. Front. Bioeng. Biotechnol. 10, 968939. doi:10.3389/fbioe.2022.968939
Razzaghi, M. (2019). Mechanical analysis of head impact. Open Access J. Neurology and Neurosurg. 10 (3), 47–51. doi:10.19080/oajnn.2019.10.555787
Rowson, S., Duma, S. M., Beckwith, J. G., Chu, J. J., Greenwald, R. M., Crisco, J. J., et al. (2012). Rotational head kinematics in football impacts: an injury risk function for concussion. Ann. Biomed. Eng. 40 (1), 1–13. doi:10.1007/s10439-011-0392-4
Schmidt, J. D., Guskiewicz, K. M., Blackburn, J. T., Mihalik, J. P., Siegmund, G. P., and Marshall, S. W. (2014). The influence of cervical muscle characteristics on head impact biomechanics in football. Am. J. sports Med. 42 (9), 2056–2066. doi:10.1177/0363546514536685
Schmitt, K.-U., Niederer, P. F., Cronin, D. S., Morrison, B., Muser, M. H., and Walz, F. (2019). (Book) trauma biomechanics: an introduction to injury biomechanics. Springer.
Seth, A., Hicks, J. L., Uchida, T. K., Habib, A., Dembia, C. L., Dunne, J. J., et al. (2018). OpenSim: simulating musculoskeletal dynamics and neuromuscular control to study human and animal movement. PLoS Comput. Biol. 14 (7), e1006223. doi:10.1371/journal.pcbi.1006223
Silvestros, P., Quarrington, R. D., Preatoni, E., Gill, H. S., Jones, C. F., and Cazzola, D. (2024). An extended neck position is likely to produce cervical spine injuries through buckling in accidental head-first impacts during rugby tackling. Ann. Biomed. Eng. 52, 3125–3139. doi:10.1007/s10439-024-03576-z
Singh, A., Harmukh, A., and Ganpule, S. (2022). Investigation of role of falx and tentorium on brain simulant strain under impact loading. J. Biomechanics 144, 111347. doi:10.1016/j.jbiomech.2022.111347
Takhounts, E. G., Hasija, V., Ridella, S. A., Rowson, S., and Duma, S. M. (2011). “Kinematic rotational brain injury criterion (BRIC),” in Proceedings of the 22nd enhanced safety of vehicles conference. Paper.
Tang, J., Zhou, Q., Shen, W., Chen, W., and Tan, P. (2023). Can we reposition finite element human body model like dummies? Front. Bioeng. Biotechnol. 11, 1176818. doi:10.3389/fbioe.2023.1176818
Uchida, T. K., and Delp, S. L. (2021). “Biomechanics of movement: the science of sports,” in Robotics, and rehabilitation (MIT Press).
Vasavada, A. N., Li, S., and Delp, S. L. (1998). Influence of muscle morphometry and moment arms on the moment-generating capacity of human neck muscles. Spine 23 (4), 412–422. doi:10.1097/00007632-199802150-00002
Viano, D. C. (2005). “Head impact biomechanics in sport: implications to head and neck injury tolerances,” in IUTAM symposium on impact biomechanics: from fundamental insights to applications (Springer).
Viano, D. C., and Pellman, E. J. (2005). Concussion in professional football: biomechanics of the striking player—part 8. Neurosurgery 56 (2), 266–280. doi:10.1227/01.neu.0000150035.54230.3c
Viano, D. C., Casson, I. R., Pellman, E. J., Bir, C. A., Zhang, L., Sherman, D. C., et al. (2005). Concussion in professional football: comparison with boxing head impacts—part 10. Neurosurgery 57 (6), 1154–1172. doi:10.1227/01.neu.0000187541.87937.d9
Viano, D. C., Casson, I. R., and Pellman, E. J. (2007). Concussion in professional football: biomechanics of the struck player—part 14. Neurosurgery 61 (2), 313–328. doi:10.1227/01.neu.0000279969.02685.d0
Wang, Y., Jiang, H., Teo, E. C., and Gu, Y. (2023). Finite element analysis of head–neck kinematics in rear-end impact conditions with headrest. Bioengineering 10 (9), 1059. doi:10.3390/bioengineering10091059
Whyte, T., Melnyk, A. D., Van Toen, C., Yamamoto, S., Street, J., Oxland, T. R., et al. (2020). A neck compression injury criterion incorporating lateral eccentricity. Sci. Rep. 10 (1), 7114. doi:10.1038/s41598-020-63974-w
Wright, R. M. (2012). A computational model for traumatic brain injury based on an axonal injury criterion. Baltimore, Maryland. The Johns Hopkins University.
Wright, R. M., Post, A., Hoshizaki, B., and Ramesh, K. T. (2013). A multiscale computational approach to estimating axonal damage under inertial loading of the head. J. neurotrauma 30 (2), 102–118. doi:10.1089/neu.2012.2418
Wu, T., Alshareef, A., Giudice, J. S., and Panzer, M. B. (2019). Explicit modeling of white matter axonal fiber tracts in a finite element brain model. Ann. Biomed. Eng. 47, 1908–1922. doi:10.1007/s10439-019-02239-8
Yu, X., Halldin, P., and Ghajari, M. (2022). Oblique impact responses of hybrid III and a new headform with more biofidelic coefficient of friction and moments of inertia. Front. Bioeng. Biotechnol. 10, 860435. doi:10.3389/fbioe.2022.860435
Zaman, R., Rifat, M. N. I., Maliha, F., Hossain, M. N. B., Akhtaruzzaman, R., and Adnan, A. (2024). Multiscale structure of brain and challenges in traumatic brain injury risk prediction. Multiscale Sci. Eng. 6, 124–146. doi:10.1007/s42493-024-00117-7
Zhan, X., Li, Y., Liu, Y., Domel, A. G., Alizadeh, H. V., Raymond, S. J., et al. (2021). The relationship between brain injury criteria and brain strain across different types of head impacts can be different. J. R. Soc. Interface 18 (179), 20210260. doi:10.1098/rsif.2021.0260
Zhang, J.-G., Wang, F., Zhou, R., and Xue, Q. (2011). A three-dimensional finite element model of the cervical spine: an investigation of whiplash injury. Med. and Biol. Eng. and Comput. 49, 193–201. doi:10.1007/s11517-010-0708-9
Keywords: traumatic brain injury, neck injury, injury criteria, head impact, injury biomechanics, musculoskeletal modeling, neck strength, impact biomechanics
Citation: Zaman R, Hossain MI, Raiyan AZ, Chowdhury S, Jackson A, Koster AT and Adnan A (2025) A biomechanical study of neck strength and impact dynamics on head and neck injury parameters. Front. Bioeng. Biotechnol. 13:1597267. doi: 10.3389/fbioe.2025.1597267
Received: 20 March 2025; Accepted: 14 August 2025;
Published: 03 September 2025.
Edited by:
Daniele Cafolla, Swansea University, United KingdomCopyright © 2025 Zaman, Hossain, Raiyan, Chowdhury, Jackson, Koster and Adnan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ashfaq Adnan, YWFkbmFuQHV0YS5lZHU=, YXNoZmFxYWRuYW5AZ21haWwuY29t
 Rahid Zaman
Rahid Zaman Mohammad Ibrahim Hossain
Mohammad Ibrahim Hossain Ashfaq Adnan
Ashfaq Adnan