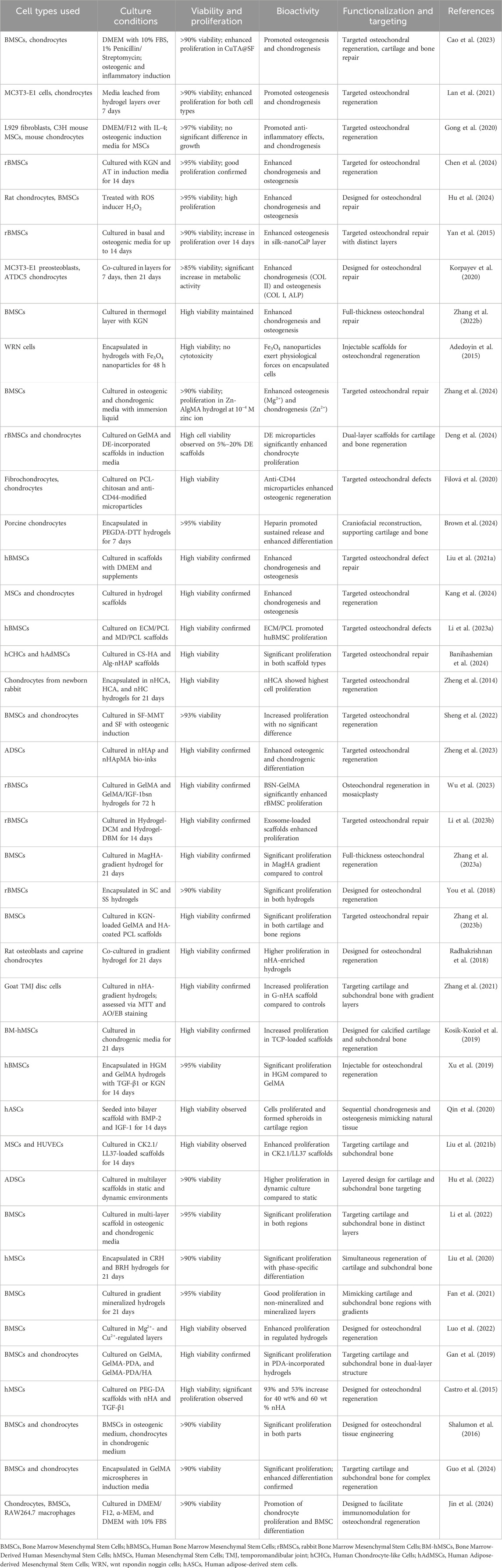
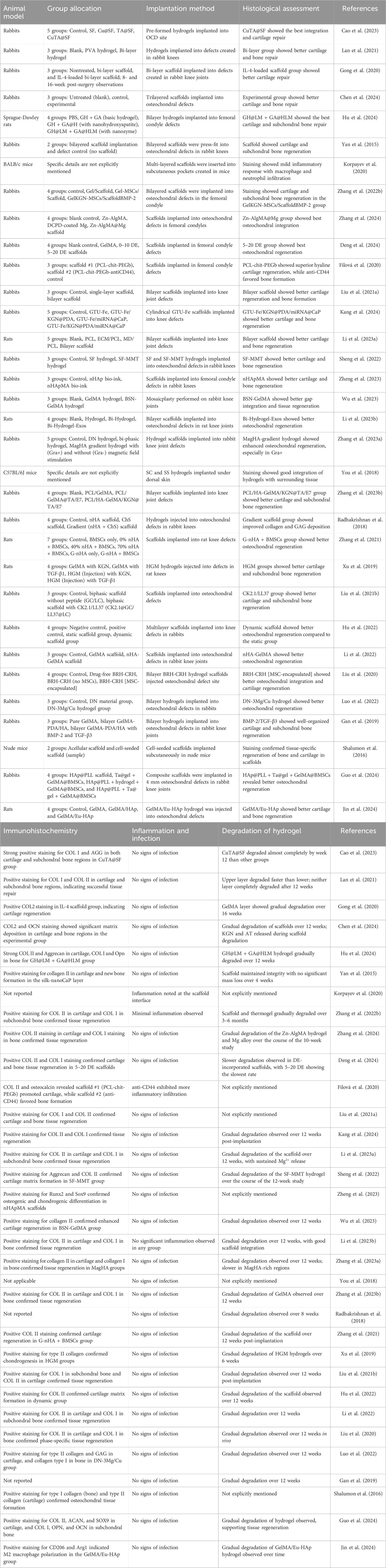
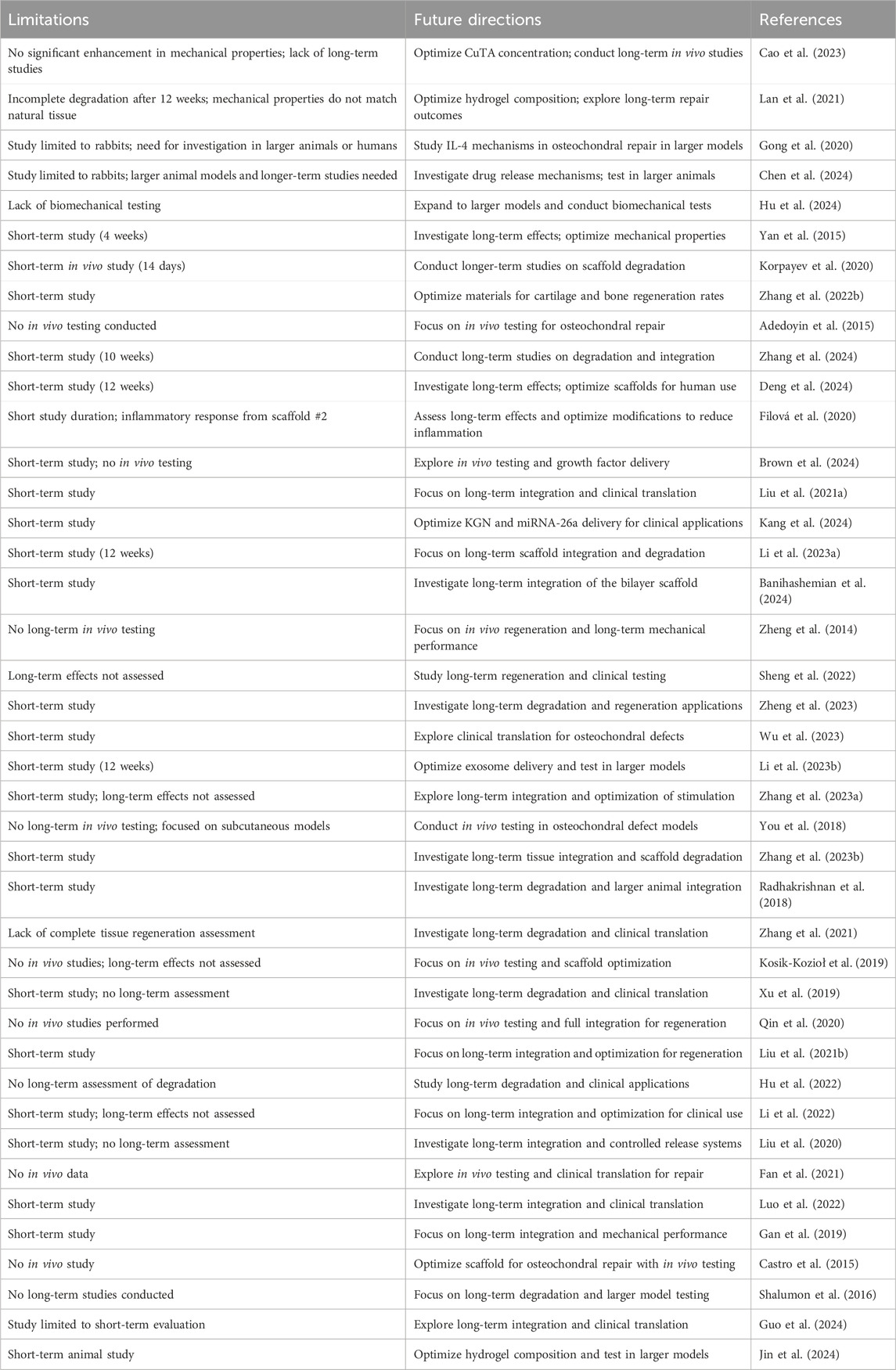
- 1Comprehensive Orthopedic Surgery Department, The Second Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China
- 2School of Public Health, Xi’an Jiaotong University Health Science Center, Xi’an, Shaanxi, China
- 3Department of Joint Surgery, Honghui Hospital, Xi’an Jiaotong University, Xi’an, Shaanxi, China
Background: Osteochondral defects, involving both cartilage and subchondral bone, remain clinically challenging due to the poor intrinsic healing capacity of cartilage and the limited durability of traditional treatments. This systematic review aims to evaluate current advancements in nano-hydrogel formulations for osteochondral repair, focusing on their composition, preparation methods, mechanical properties, biocompatibility, and regenerative outcomes.
Methods: Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, a comprehensive literature search was conducted across PubMed, Web of Science, and Scopus. Eligible studies were screened based on predefined inclusion and exclusion criteria. The methodological quality and risk of bias of included studies were assessed using CAMARADES checklist, which considered factors such as randomization, blinding, animal welfare compliance, outcome reporting, and study reproducibility. Data synthesis was performed through structured tabulation and subgroup stratification by scaffold structure (single-phase, bilayered, trilayered, gradient), formulation type (injectable vs. preformed), and polymer origin (natural, synthetic, hybrid).
Results: A total of 41 studies were included, encompassing both in vitro and in vivo models, with participant numbers ranging from small animal models (e.g., rabbits, rats) to larger preclinical systems. Studies varied in scaffold design, bioactive integration, and fabrication techniques. Most nano-hydrogels demonstrated high biocompatibility, tunable degradation, and enhanced tissue integration. However, heterogeneity in design parameters, lack of standardized outcome measures, and variable reporting quality limited direct comparisons.
Conclusion: Nano-hydrogels show strong potential as biomimetic scaffolds for osteochondral repair, offering customizable mechanical and biological properties. Nevertheless, the evidence base is limited by study heterogeneity, moderate risk of bias, and lack of standardized protocols, which complicates direct comparison and clinical extrapolation. Future work should focus on long-term validation, functional outcome measures, and development of smart, adaptive materials to support clinical translation.
1 Introduction
Osteochondral defects, characterized by damage to both cartilage and the underlying bone, present a significant clinical challenge due to the limited regenerative capacity of cartilage tissue and the complex architecture of the osteochondral unit (Mano and Reis, 2007; Dinoro et al., 2019; Davis et al., 2021; Liu et al., 2021a). These defects are commonly caused by trauma, osteoarthritis, and other degenerative conditions, leading to pain, reduced mobility, and a decreased quality of life (Verhagen et al., 2003; Martin et al., 2007; Liu et al., 2020). Traditional treatments, such as microfracture surgery, autologous chondrocyte implantation, and osteochondral allografts, often fail to provide long-term solutions, particularly for larger lesions, due to complications such as donor site morbidity, limited graft availability, and incomplete integration with host tissues (Hjelle et al., 2002; Cavendish et al., 2019; Chahla et al., 2019). Consequently, there is a critical need for innovative therapeutic strategies that can effectively promote the regeneration of both cartilage and subchondral bone in a coordinated manner (De Leon-Oliva et al., 2023; Li et al., 2023b).
Recent advances in tissue engineering and regenerative medicine have highlighted the potential of biomaterials to overcome the limitations of conventional therapies (Lynch et al., 2021; Zhang et al., 2021; Cao and Ding, 2022; Luo et al., 2022). Among the various biomaterials explored, nano-hydrogel systems have garnered significant attention due to their unique physicochemical properties and versatility (Chander et al., 2021; Ahmad et al., 2022; Sethi et al., 2023; Rana and De la Hoz Siegler, 2024). Nano-hydrogels are three-dimensional, water-swollen polymeric networks that can be engineered to mimic the native extracellular matrix (ECM) of osteochondral tissues (Liu and Hsu, 2018; Zengin et al., 2021; Hwang and Lee, 2024). Their nano-scale features, high surface area, and tunable mechanical properties make them ideal candidates for supporting cell adhesion, proliferation, and differentiation (Quazi and Park, 2022; Hwang and Lee, 2024). Additionally, nano-hydrogels can be easily functionalized to deliver therapeutic agents, such as growth factors, cytokines, and nanoparticles, in a controlled and sustained manner, further enhancing their regenerative potential (Lee, 2018; Soni et al., 2022).
The design and development of nano-hydrogels for osteochondral repair involve several key considerations, including mechanical strength, biodegradability, biocompatibility, and the ability to support dual regeneration of cartilage and bone (Yue et al., 2020; Xiang et al., 2022; Yao et al., 2023). Successful regeneration requires a scaffold that not only mimics the structural and functional properties of the native tissue but also degrades at a rate that matches the pace of tissue formation, thereby providing support throughout the healing process (Yue et al., 2020; Hwang and Lee, 2024). Furthermore, the incorporation of bioactive molecules that can modulate the local cellular environment is essential for promoting chondrogenic and osteogenic differentiation, ensuring effective integration of the scaffold with host tissues (Yue et al., 2020; Xiang et al., 2022).
While numerous studies have reported the development of nano-hydrogel systems for osteochondral repair, there remains a lack of comprehensive understanding regarding the optimal design parameters and functionalization strategies (Wang et al., 2022b). Additionally, the variability in experimental models and evaluation criteria across studies has made it challenging to compare outcomes and draw definitive conclusions about the efficacy of different approaches (Hwang and Lee, 2024). To address these gaps, this systematic review aims to provide a detailed overview of the current status of nano-hydrogel preparations for osteochondral repair, with a focus on their composition, preparation methods, mechanical properties, biocompatibility, and in vitro and in vivo efficacy.
This review analyzes and synthesizes findings from recent literature, highlighting key advancements and identifying existing challenges in the field. It offers insights into the design principles that guided the development of next-generation nano-hydrogel systems, ultimately contributing to the advancement of more effective and reliable therapeutic solutions for osteochondral defects.
2 Materials and methods
This systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Moher et al., 2009; Moher et al., 2015). A protocol was specified and registered on the database International Prospective Register of Systematic Reviews (PROSPERO) (registration number CRD42024586563) and is available from: https://www.crd.york.ac.uk/prospero/#myprospero.
2.1 Search strategy
A comprehensive search was conducted across three English-language databases: PubMed, Scopus, and Web of Science. The search focused on identifying studies related to nano-hydrogel systems for osteochondral repair. Search terms included combinations of MeSH and free-text keywords: (“nanohydrogel” OR “nanogel” OR “nano-hydrogel scaffold” OR “nanoscale hydrogel” OR “nano-sized hydrogel” OR “nanocomposite hydrogel”) AND (“osteochondral repair” OR “cartilage regeneration” OR “cartilage repair” OR “osteochondral defect”). Filters were applied to include only English-language publications. A detailed list of search terms and strategies for each database is provided in Supplementary Table S1.
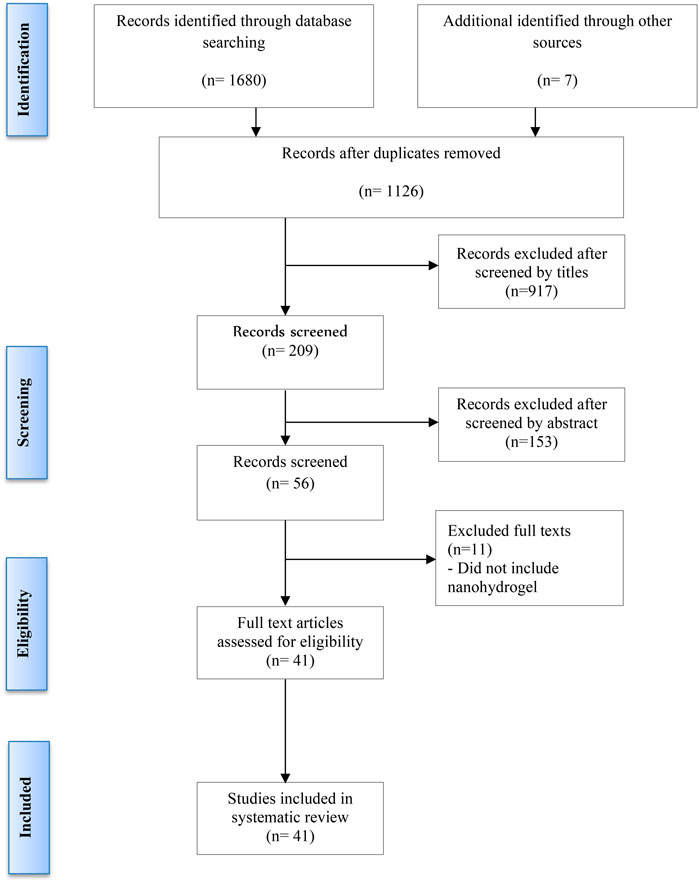
Additionally, reference lists of retrieved articles were manually reviewed to identify any further relevant studies. Two authors (AFA and LQ) independently screened titles and abstracts to assess eligibility based on the inclusion criteria. Full-text articles were further reviewed to exclude any duplicates or studies that did not meet the criteria (Figure 1). Discrepancies were resolved through discussion with a third reviewer (JH). The last update search was conducted on 29 September 2024.
2.2 Focused question
This systematic review was performed to address the following focused question: “What is the current status of nano-hydrogel preparations in promoting osteochondral repair, specifically regarding their composition, preparation methods, mechanical properties, biocompatibility, and therapeutic efficacy?”
2.3 Selection criteria
To ensure the inclusion of high-quality and relevant studies, specific eligibility criteria were established prior to the screening process. Studies were included if they were original research articles published in peer-reviewed journals, written in English, and focused on the preparation and application of nano-hydrogel systems specifically for osteochondral or cartilage repair. Eligible studies were required to provide sufficient detail on the hydrogel’s composition, crosslinking or functionalization strategies, and report at least one form of biological or functional evaluation, whether in vitro, ex vivo, or in vivo.
Studies were excluded if they were review articles, conference abstracts, dissertations, clinical case reports, editorials, or other forms of grey literature. Additionally, publications that did not focus on osteochondral repair, or those that lacked essential data on hydrogel characterization or biological performance, were omitted. There were no restrictions on publication year; however, only articles published in English were considered. These criteria were designed to ensure methodological rigor and relevance to the focused research question.
2.4 Screening methods and data extraction
Titles and abstracts were screened by two independent reviewers (AFA and LQ), followed by full-text assessments for studies that met the inclusion criteria. Disagreements on study eligibility were resolved through consultation with a third reviewer (JH). The extracting data were following PICO (P: sources, I: interventions, C: control study, O: outcomes) standards.
The data extraction process focused on gathering information about general study characteristics, including nano-hydrogel composition, types of nanoparticles, preparation methods, crosslinking strategies, and controlled release mechanisms. It also covered mechanical and bioactivity properties, such as mechanical strength, degradation rates, biocompatibility, swelling ratios, and functionalization aspects. For in vitro studies, details on cell types, culture conditions, cell viability, and proliferation were collected. In vivo studies were evaluated based on animal models, group allocation, implantation techniques, histological assessments, and outcomes related to subchondral bone and cartilage regeneration, including immunohistochemical findings, inflammation, infection, and hydrogel degradation. Lastly, the extraction included identification of research limitations and recommendations for future studies, ensuring a comprehensive overview of each study’s approach and findings.
2.5 Quality assessment and analysis of the data
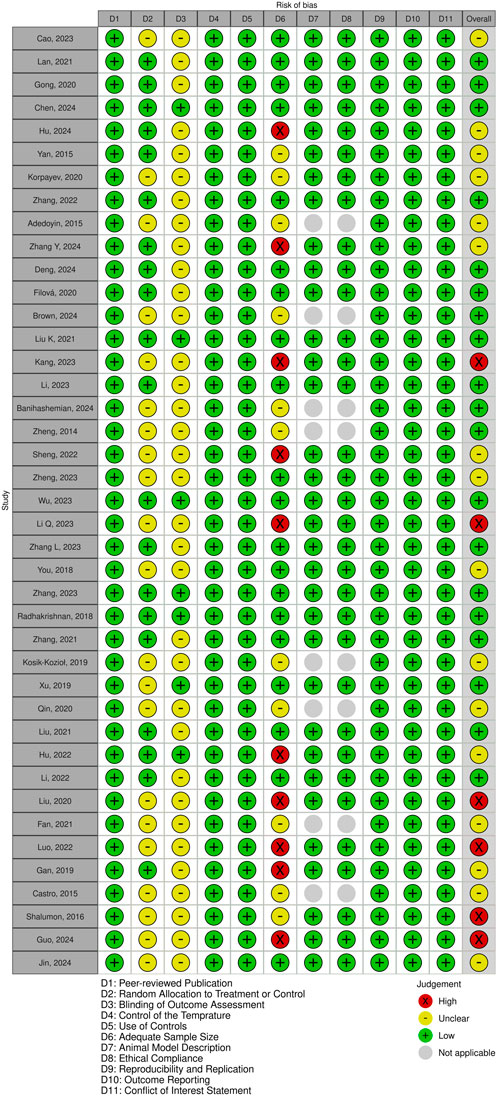
The methodological quality of the included studies was evaluated using a customized CAMARADES checklist, which I adapted to better assess the relevance of each study (Macleod et al., 2004). The adapted checklist incorporated 11 key criteria to assess study relevance: (1) publication in a peer-reviewed journal, (2) random allocation to treatment or control groups, (3) blinded outcome assessment, (4) Control of the temperature in the animal facilities, (5) use of appropriate controls, (6) adequate sample size, (7) clear description of the animal model, (8) adherence to animal welfare guidelines, (9) reproducibility and replication of findings, (10) thorough outcome reporting, and (11) disclosure of any potential conflicts of interest. Given the nature of the data, analysis was conducted descriptively, as the variability across studies precluded meta-analysis.
3 Results and discussion
3.1 Search outcomes
Following the removal of duplicates, a total of 1,126 unique publications were identified through database screening. Title and abstract screening narrowed these to 56 articles for full-text evaluation. After applying the inclusion criteria, 11 studies were excluded. Consequently, 41 studies were included in this systematic review (Figure 1). Of these, 34 studies employed both in vitro and in vivo methodologies, while seven were limited to in vitro experiments (Adedoyin et al., 2015; Castro et al., 2015; Kosik-Kozioł et al., 2019; Qin et al., 2020; Fan et al., 2021; Banihashemian et al., 2024; Brown et al., 2024). The assessment of bias showed a spectrum from low to high risk, and detailed findings on methodological quality are illustrated in Figures 2,3, .
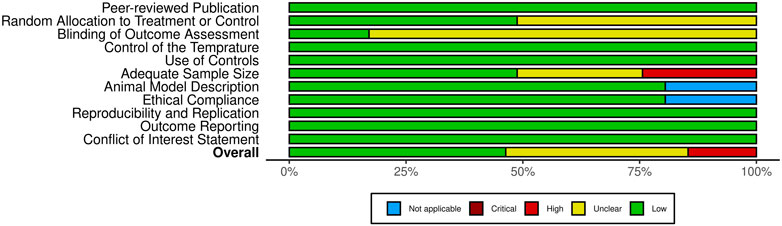
Figure 3. Overview of risk of bias assessment for included studies using a modified CAMARADES checklist.
3.2 Nano-hydrogel composition and preparation methods
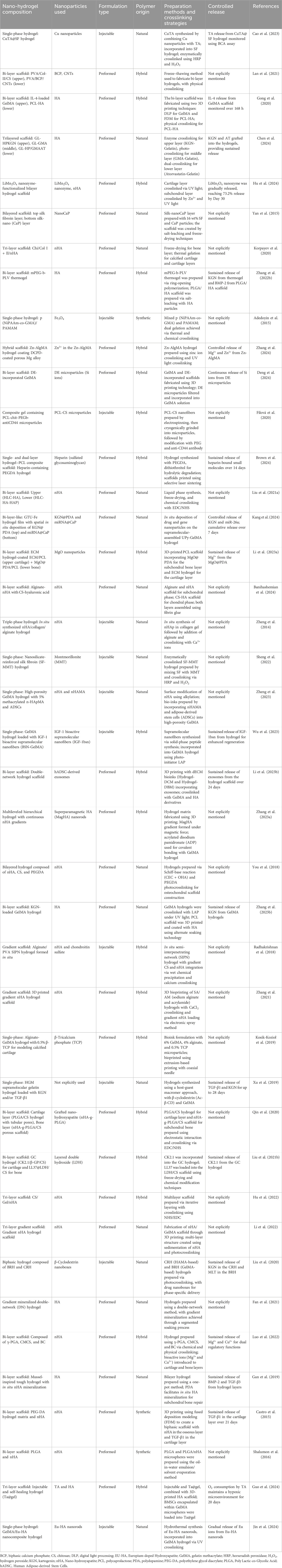
The studies summarized in Table 1 highlight the structural and compositional diversity of nano-hydrogel systems used for osteochondral repair. These range from simple, single-phase injectable formulations to more complex preformed multilayered scaffolds—each engineered to address distinct mechanical and biological requirements. Scaffold configurations were stratified into single-phase, bilayered, trilayered, and gradient systems. Many bilayered and trilayered constructs were designed to emulate the zonal architecture of osteochondral tissue, allowing site-specific modulation of chondrogenesis and osteogenesis.
Integration of nanoparticles such as hydroxyapatite (HA), chitosan montmorillonite, silica, and polydopamine (PDA) has been shown to enhance the mechanical integrity, osteoconductivity, and cellular interactions of hydrogels (Shalumon et al., 2016; Gong et al., 2020; Korpayev et al., 2020; Sheng et al., 2022; Hu et al., 2024; Jin et al., 2024). For instance, a study by Cao et al. (2023) utilized Cu-based nanoparticles embedded in a silk fibroin (SF) matrix via enzymatic crosslinking to create a single-phase injectable hydrogel with antioxidative and immunomodulatory properties Similarly, preformed bilayer hydrogels composed of polyvinyl alcohol (PVA), biphasic calcium phosphate (BCP), and carbon nanotubes (CNTs) were fabricated through a freeze-thawing process to generate a gradient interface, mimicking native cartilage–bone transition zones (Lan et al., 2021). These examples illustrate how both formulation type and nanoparticle selection directly influence the functional performance of nano-hydrogels.
The choice of crosslinking strategy is another determinant of scaffold performance, affecting mechanical stability, degradation behavior, and cellular response. Studies included a wide array of crosslinking approaches, enzymatic, photo-initiated, thermal, chemical, ionic, and dual-crosslinking methods, each tailored to the specific polymer systems and application needs (Adedoyin et al., 2015; Xu et al., 2019; Zhang et al., 2022b; Cao et al., 2023; Wu et al., 2023; Chen et al., 2024). For instance, photo-crosslinking has been employed to allow spatially controlled gelation, ideal for constructing gradient or multi-layered hydrogels (Zhang et al., 2024). However as highlighted in multiple reports, optimization is needed to reduce cytotoxicity from residual initiators, which may impact cell viability and tissue integration (Berry et al., 2019; Hu et al., 2019; Tomal and Ortyl, 2020). In terms of polymer origin, systems were broadly classified as natural, synthetic, or hybrid. Natural polymers like chitosan, gelatin (GelMA), alginate, and hyaluronic acid offer favorable biocompatibility and degradation profiles. Synthetic polymers such as PEGDA, PVA, and PLGA provide enhanced mechanical tunability and process control. Hybrid systems, which combine the strengths of both natural and synthetic components, emerged as especially promising in balancing bioactivity with structural integrity, several trilayered and bilayered scaffolds utilized such combinations to achieve distinct zone-specific functions.
Moreover, the application of advanced fabrication methods such as 3D printing, electrospinning, microsphere sintering, and solvent casting enabled precise spatial organization of materials. These techniques facilitated the development of functionally graded scaffolds, often incorporating nano-hydroxyapatite (nHA) or exosome-loaded layers, to mimic the mechanical and biochemical gradients of native osteochondral tissue (Zhang et al., 2022b; Brown et al., 2024). Several preformed multilayered systems were constructed with dual or triple layers, each designed with distinct pore architectures, ion release kinetics, and biofunctional molecules to modulate regeneration in a zone-specific manner.
Collectively, the reviewed studies demonstrate how scaffold architecture (e.g., single-phase, bilayered, trilayered), formulation type (injectable vs. preformed), polymer composition (natural, synthetic, hybrid), nanoparticle inclusion, crosslinking strategy, and fabrication technique can be tailored in concert to engineer next-generation nano-hydrogels for osteochondral repair. This multi-dimensional classification, as summarized in Table 1, provides a comparative framework to inform rational scaffold design and translational scaffold development.
3.3 Mechanical properties and degradation behaviour
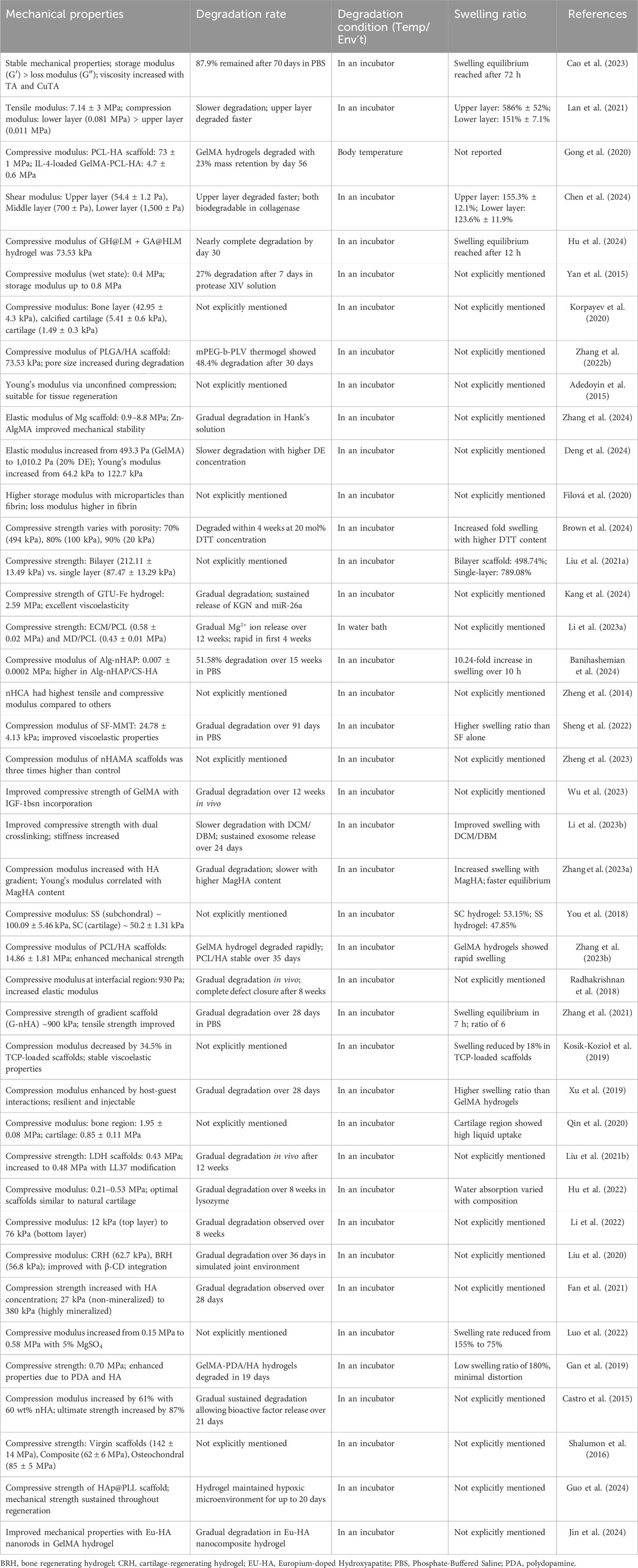
Mechanical properties are essential for nano-hydrogel systems, particularly for osteochondral repair, where the scaffold must withstand the mechanical stresses of both cartilage and subchondral bone environments. As observed in Table 2, studies report varied mechanical strengths, with compressive moduli ranging from 0.4 MPa (Mpa) to over 73 MPa depending on the hydrogel composition (Gong et al., 2020; Zhang et al., 2022b; Brown et al., 2024; Hu et al., 2024; Kang et al., 2024). For instance, polycaprolactone-hydroxyapatite (PCL-HA) scaffolds have demonstrated compressive moduli as high as 73 ± 1 MPa, while IL-4-loaded GelMA-PCL-HA composites exhibit lower values around 4.7 ± 0.6 MPa (Gong et al., 2020). These scaffold values are within the range of trabecular (cancellous) bone, which exhibits compressive moduli typically between 10 and 200 MPa, depending on site and density. In contrast, the modulus of natural cortical bone is substantially higher, with a longitudinal elastic modulus ranging from 17.2 to 23.2 GPa and a transverse modulus ranging from 10.8 to 13.9 GPa, as demonstrated through multiscale modeling validated by nanoindentation and ultrasound measurements (Hamed et al., 2010). These comparisons highlight the potential of HA-containing scaffolds to approximate native bone behavior in osteochondral repair applications, particularly when enhanced with structural reinforcements like hydroxyapatite.
Biomimetic designs incorporating GelMA and HA have shown promise in enhancing mechanical stability and bioactivity for bone regeneration applications. GelMA hydrogels, while beneficial for tissue engineering, lack sufficient mechanical strength and osteogenic factors (Wang et al., 2022a). Incorporating HA into GelMA hydrogels improves their mechanical properties, biocompatibility, and osteogenic potential (Suvarnapathaki et al., 2020). Mineralized HA nanofibers further enhance the mechanical and bone regenerative performances of GelMA composites (Wang et al., 2022a). GelMA-based biomaterials can be tailored to overcome challenges in bone tissue engineering, such as insufficient mechanical properties and uncontrolled degradation (Dong et al., 2019). Advanced designs combining GelMA with other materials, like methacrylated HA nanoparticles and l-arginine-based unsaturated poly (ester amide), can create periosteum-mimicking scaffolds with improved mechanical strength, tissue adhesion, and osteogenic-angiogenic coupling effects (Yang et al., 2021). Double-crosslinking and freeze-drying methods have also been widely applied, producing physically and chemically reinforced structures that retain mechanical properties under physiological conditions (Yan et al., 2015; Filová et al., 2020; Zheng et al., 2023).
Balancing degradation rates with tissue regeneration remains another core challenge. An ideal scaffold degrades gradually, transferring mechanical load to newly forming tissue to aid integration (Hu et al., 2022; Li et al., 2022; Banihashemian et al., 2024; Chen et al., 2024). Studies have shown that adjusting crosslinking density and introducing bioactive molecules can customize degradation profiles for specific applications (Radhakrishnan et al., 2018; Zhang et al., 2023a; Zhang et al., 2023b; Deng et al., 2024). For example, Chen et al. developed a trilayered hydrogel with varied degradation rates across layers to replicate the native tissue gradient from cartilage to bone, facilitating sustained cell infiltration and extracellular matrix formation (Chen et al., 2024). Recent research has focused on developing multilayered hydrogel scaffolds to mimic the zonal organization of native cartilage tissue. These scaffolds feature gradients in mechanical properties, extracellular matrix composition, and bioactive factors across layers to guide cell differentiation and tissue formation (Brady et al., 2017; Qiao et al., 2021). Furthermore, a study demonstrated that layer-specific biomaterial compositions could direct a single stem cell population into zone-specific chondrocytes, resulting in native-like cartilage with varying mechanical and biochemical properties (Nguyen et al., 2011). In addition, a study further showed that stiffness gradient hydrogels could induce zone-specific responses in both chondrocytes and mesenchymal stem cells, mimicking cartilage zonal organization (Zhu et al., 2018). These approaches offer promising strategies for engineering complex osteochondral tissues with spatially-varying properties that more closely resemble native tissue structure and function.
Future advancements will likely focus on refining crosslinking techniques, such as enzyme-catalyzed, thermal, and photo-crosslinking, to develop materials that meet both mechanical and degradation needs for effective tissue engineering.
3.4 Biocompatibility and functional characteristics
Nano-hydrogel systems have consistently demonstrated excellent biocompatibility and functional characteristics, making them highly suitable for applications in tissue engineering, particularly in osteochondral regeneration. Studies have reported cell viability rates exceeding 90% and enhanced cell proliferation, supporting the potential of these materials to promote tissue growth and regeneration (Table 3). For example, a study showed that LiMn2O4 nanozyme-functionalized hydrogels effectively supported the proliferation of rat chondrocytes and bone marrow-derived mesenchymal stem cells (BMSCs), promoting cell adhesion and growth (Hu et al., 2024). In addition, in vitro studies have highlighted that nano-hydrogels, such as GH@LM + GA@HLM and Zn-AlgMA, significantly enhance the proliferation of both chondrocytes and BMSCs, while maintaining high levels of cell viability (Hu et al., 2024; Zhang et al., 2024). Similarly, functionalized scaffolds, including those with CK2.1/LL37 and SF-MMT, further promote the regenerative processes of BMSCs and chondrocytes, reinforcing the critical role of scaffold composition in optimizing cellular responses (Liu et al., 2021b; Sheng et al., 2022).
Nano-hydrogels mimicking the extracellular matrix (ECM) have emerged as promising scaffolds for tissue engineering and regenerative medicine. These biomimetic materials create a three-dimensional (3D) environment that closely resembles the native ECM’s nanoscale architecture (Geckil et al., 2010; Gough et al., 2012; Brown et al., 2024). By incorporating nanostructured components, such as nanofibers or nanosilicates, these hydrogels can actively modulate cellular responses, including attachment, proliferation, and differentiation (Wei and Ma, 2008). For instance, nanoengineered collagen-based hydrogels reinforced with disk-shaped nanosilicates have been shown to enhance osteogenic differentiation of human mesenchymal stem cells without the need for exogenous growth factors (Paul et al., 2016). These ECM-mimicking hydrogels not only provide structural support but also create a regulatory milieu that guides tissue formation and organization (Geckil et al., 2010). Furthermore, their biocompatibility and ability to induce regenerative processes make them promising candidates for various biomedical applications, including bone tissue engineering and in vitro disease modeling (Wei and Ma, 2008; Paul et al., 2016).
Furthermore, functionalization techniques are crucial for enhancing the bioactivity of hydrogels in osteochondral tissue engineering. By incorporating growth factors, bioactive molecules, and nanoparticles, these hydrogels can promote both osteogenesis and chondrogenesis. For example, research has shown that embedding polydopamine-encapsulated kartogenin (KGN) and calcium phosphate-encapsulated miRNA-26a within hydrogels effectively promotes regeneration in both cartilage and bone layers (Kang et al., 2024). Additionally, KGN has been grafted onto ultrasmall superparamagnetic iron-oxide nanoparticles, which are then integrated into hydrogels for cartilage repair while enhancing MRI contrast (Yang et al., 2019). Another study developed microscaffold-hydrogel composites containing KGN and peptides to accelerate osteochondral repair through endochondral ossification (Zhang et al., 2022a). Moreover, a versatile hydrogel system using click chemistry has been created to provide tissue-specific cues for either chondrogenesis or osteogenesis (You et al., 2018; Guo et al., 2020; Liu et al., 2021a; Li et al., 2023a). These approaches highlight the potential of functionalized hydrogels in addressing the complex requirements of osteochondral tissue regeneration.
Recent studies demonstrate the effectiveness of functionalized biomaterials in advancing osteochondral repair, primarily by supporting both osteogenic and chondrogenic differentiation. Composite hydrogels with anti-CD44-labeled microparticles have shown to significantly improve osteogenic regeneration in animal models of osteochondral defects (Filová et al., 2020). Likewise, bilayer scaffolds that guide stem cell differentiation spatially have been effective in directing cells into osteogenic and chondrogenic lineages, enhancing repair outcome (Kang et al., 2024; Lowen et al., 2024). Furthermore, microscaffold-hydrogel composites, incorporating bioactive modifications like RGD peptides, have demonstrated accelerated osteochondral repair through endochondral ossification, achieved by controlled delivery of bioactive molecules within the scaffold layers (Zhang et al., 2022a; Brown et al., 2024; Deng et al., 2024). Other studies reinforce these findings, with functionalized hydrogels designed for dual osteogenic and chondrogenic applications showing sustained, layer-specific release of growth factors and bioactive ions, thus promoting cell proliferation and tissue integration (Cao et al., 2023; Wu et al., 2023).
These findings underscore the potential of multi-functionalized nano-hydrogels in tissue engineering, with customizable layers enabling the spatially controlled release of bioactive agents that foster site-specific tissue regeneration. Such approaches pave the way for advanced therapies for osteochondral defects and other complex tissue engineering applications (Wu et al., 2023; Brown et al., 2024).
These findings suggest that nano-hydrogels are capable of providing a supportive 3D microenvironment that mimics the native ECM. However, achieving consistent differentiation and integration remains challenging, particularly when translating in vitro success to in vivo conditions. Variability in cell behavior across studies suggests that more standardized protocols are needed to optimize cell-scaffold interactions, ensuring predictable outcomes in clinical settings.
3.5 In vivo efficacy and regeneration outcomes
The in vivo studies summarized in Table 4 illustrate the promising efficacy of nano-hydrogels in promoting osteochondral repair, using diverse animal models such as rabbits, rats, and mice to assess the regenerative potential of these systems. Significant cartilage regeneration and subchondral bone repair were observed in a rabbit model using a bi-layered GelMA-PCL-HA scaffold, where histological analyses confirmed the formation of a smooth cartilage surface and well-integrated bone layer (Gong et al., 2020). Similarly, a bilayer hydrogel containing GH@LM + GA@HLM demonstrated notable regeneration, with micro-CT and histological assessments indicating smooth hyaline cartilage formation and robust subchondral bone repair (Hu et al., 2024) (Table 4). These advanced hydrogel systems have demonstrated improvements in defect filling, cartilage thickness, and bone regeneration compared to control groups (Gan et al., 2019; Guo et al., 2021). However, a critical review of in vivo cartilage repair studies highlights the need for standardized experimental designs and careful interpretation of results (Vilela et al., 2015).
Histological assessments across various studies frequently highlighted improved tissue integration. A GTU-Fe/KGN@PDA/miRNA@CaP scaffold led to enhanced chondrogenic and osteogenic marker expression, indicating successful differentiation and maturation of regenerated tissue, with elevated glycosaminoglycans (GAG) and collagen deposition contributing to effective cartilage and bone regeneration (Table 4) (Kang et al., 2024). Further corroborating these findings, a Zn-AlgMA@Mg scaffold achieved significant osteochondral integration, facilitating seamless cartilage repair and trabecular bone formation within femoral condyle defects in rabbits (Zhang et al., 2024) (Table 4). Despite these advancements, scaffold-cartilage integration remains a significant challenge in tissue engineering. Recent strategies to address this issue include manipulating cellular, material, and biomolecular composition of engineered tissue (Jelodari et al., 2022). These findings highlight the potential for improved cartilage repair and integration using advanced scaffolds and tissue engineering techniques.
Many studies achieved substantial subchondral bone regeneration, suggesting that functionalization strategies including the incorporation of miRNAs, bioactive molecules, and structurally adaptive hydrogels play a crucial role in promoting dual regeneration for osteochondral repair. For example, bi-layer hydrogels and trilayered scaffolds demonstrated enhanced bone volume and trabecular thickness, ultimately supporting comprehensive osteochondral regeneration (Lan et al., 2021; Chen et al., 2024). Moreover, these studies predominantly used femoral condyle defect models, effectively showing that nano-hydrogels, when tailored to recreate the native extracellular environment, support robust tissue regeneration over extended periods. Functionalization strategies, such as incorporating tissue-specific peptides or drugs, have shown enhanced chondrogenesis and osteogenesis both in vitro and in vivo (Guo et al., 2021; Chen et al., 2024). These advanced scaffolds have demonstrated improved bone volume, trabecular thickness, and overall defect filling in femoral condyle defect models, supporting comprehensive osteochondral regeneration (Cao et al., 2024; Chen et al., 2024; Guo et al., 2024).
The variability in regenerative outcomes observed across studies, characterized by differing degrees of bone density and cartilage smoothness, highlights the necessity for a standardized approach to evaluating scaffold performance. Future research should focus on adopting consistent animal models, such as femoral defect models, and harmonized assessment criteria, such as specific histological markers and imaging techniques, to enable comparative evaluations across various hydrogel systems. Such standardization could accelerate the translation of nano-hydrogel-based technologies into clinical settings, supporting more predictable outcomes and broader applicability.
3.6 Key limitations in osteochondral repair studies and prospective innovations
Recent advances in osteochondral tissue engineering have focused on developing scaffolds that support cell growth and tissue regeneration. Scaffold degradation plays a crucial role in the repair process, with different degradation modalities and speeds influencing outcomes (Tortorici et al., 2022). Despite considerable advances in osteochondral repair, several critical limitations remain across studies, as outlined in Table 5. One major challenge involves inconsistent degradation rates in scaffold materials. Achieving a uniform degradation timeline has proven difficult, with some hydrogel systems degrading faster than intended, reducing structural support for newly forming tissue, while others degrade too slowly, limiting cell infiltration and impeding tissue remodeling. For instance, study conducted by Adedoyin et al. noted this inconsistency in their dual-gelation scaffold, where uneven degradation impacted overall regenerative outcomes (Adedoyin et al., 2015). To address this, further research should investigate advanced crosslinking techniques to fine-tune degradation kinetics, ensuring scaffold resorption aligns more closely with native tissue growth.
Another prevalent issue is the variability in scaffold mechanical strength, particularly when scaling up for larger defects. Achieving a mechanical resilience that closely mimics native tissue properties remains challenging. Li et al. reported that preserving compressive strength in bilayer scaffolds was difficult over long-term in vivo applications, highlighting a critical need for more durable biomaterials (Li et al., 2023a). Novel scaffold compositions and innovative crosslinked structures could offer the increased load-bearing capacities necessary to provide robust support in osteochondral applications, particularly those involving weight-bearing joints.
Additionally, there is limited long-term in vivo data on the efficacy and safety of these scaffolds. While short-term successes are frequently observed, the potential for chronic inflammation or complications related to scaffold degradation requires longer follow-up. Studies highlight the necessity for prolonged trials to thoroughly assess scaffold stability, biocompatibility, and integration with native tissue structures, all critical for achieving successful clinical translation (Brown et al., 2024; Hu et al., 2024).
To overcome these challenges, future research could focus on innovative materials and scaffold designs. The use of in situ forming hydrogels, which adapt to irregular defect sites during implantation, may enhance scaffold integration (Zheng et al., 2014; Park and Park, 2018; Kang et al., 2024). Smart, stimuli-responsive hydrogels capable of controlled therapeutic release could also support sustained regeneration and more effective clinical outcomes. Additionally, combining nano-hydrogels with synergistic regenerative approaches such as gene therapy, bioelectronics, or cell-based treatments may lead to multifunctional scaffolds that facilitate not only osteogenesis and chondrogenesis but also angiogenesis (Kumar et al., 2022; Chen et al., 2023). Together, these integrated approaches have the potential to advance osteochondral repair, bringing the field closer to scalable, reliable therapeutic solutions.
4 Conclusion
This systematic review underscores the diverse and evolving strategies employed in nano-hydrogel-based scaffolds for osteochondral repair. By systematically stratifying the included studies according to formulation type (injectable vs. preformed), structural design (single-phase, bilayered, trilayered, or gradient), and polymer origin (natural, synthetic, hybrid), we identified key trends linking scaffold architecture to biological performance. Notably, bilayered and trilayered systems that emulate the native osteochondral zonation more effectively support site-specific chondrogenesis and osteogenesis. Similarly, hybrid scaffolds integrating natural and synthetic polymers often demonstrate superior synergy between mechanical strength and bioactivity.
Despite promising preclinical outcomes, translational challenges persist. The field is hindered by variability in fabrication methods, inconsistencies in mechanical robustness and degradation profiles, and a lack of long-term in vivo validation. Moreover, the absence of standardized animal models and outcome measures limits direct comparison across studies, thereby impeding regulatory progression and clinical adoption.
To address these limitations, we propose a scaffold design framework emphasizing biomimetic zoning, controlled delivery of bioactive cues, stimuli-responsive behavior, and compliance with good manufacturing practice (GMP) standards. Comparative evaluations using unified scoring systems, load-bearing models, and long-term functional assessments will be critical to bridge the gap between laboratory innovation and clinical implementation.
In conclusion, while nano-hydrogels offer clear advantages in mimicking the extracellular matrix and modulating the local microenvironment, their future lies in rational design guided by translational benchmarks. With sustained interdisciplinary collaboration and regulatory foresight, these systems have the potential to evolve into clinically viable, patient-specific therapies for osteochondral regeneration.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
AFA: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Resources, Writing – original draft, Writing – review and editing. LQ: Conceptualization, Formal Analysis, Investigation, Resources, Writing – review and editing. HD: Formal Analysis, Investigation, Methodology, Resources, Writing – review and editing. JL: Formal Analysis, Investigation, Methodology, Writing – review and editing. JW: Formal Analysis, Supervision, Writing – review and editing. WW: Supervision, Writing – review and editing. JH: Supervision, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fbioe.2025.1611522/full#supplementary-material
References
Adedoyin, A., Kumar, R., Sridhar, S., and Ekenseair, A. (2015). Injectable bionanocomposite hybrid scaffolds with responsive control for enhanced osteochondral tissue regeneration. IEEE, Troy, NY, USA, 1–2.
Ahmad, Z., Salman, S., Khan, S. A., Amin, A., Rahman, Z. U., Al-Ghamdi, Y. O., et al. (2022). Versatility of hydrogels: from synthetic strategies, classification, and properties to biomedical applications. Gels 8 (3), 167. doi:10.3390/gels8030167
Banihashemian, A., Zamanlui Benisi, S., Hosseinzadeh, S., Shojaei, S., and Abbaszadeh, H. (2024). Structural and biological investigation of alginate-nano-hydroxyapatite with chitosan-hyaluronic acid for potential osteochondral regeneration. Int. J. Polym. Mater. Polym. Biomaterials 73 (10), 851–865. doi:10.1080/00914037.2023.2215378
Berry, D. R., Díaz, B. K., Durand-Silva, A., and Smaldone, R. A. (2019). Radical free crosslinking of direct-write 3D printed hydrogels through a base catalyzed thiol-Michael reaction. Polym. Chem. 10 (44), 5979–5984. doi:10.1039/c9py00953a
Brady, M. A., Talvard, L., Vella, A., and Ethier, C. R. (2017). Bio-inspired design of a magnetically active trilayered scaffold for cartilage tissue engineering. J. tissue Eng. Regen. Med. 11 (4), 1298–1302. doi:10.1002/term.2106
Brown, N. E., Ellerbe, L. R., Hollister, S. J., and Temenoff, J. S. (2024). Development and characterization of heparin-containing Hydrogel/3D-Printed scaffold composites for craniofacial reconstruction. Ann. Biomed. Eng. 52, 2287–2307. doi:10.1007/s10439-024-03530-z
Cao, D., and Ding, J. (2022). Recent advances in regenerative biomaterials. Regen. Biomater. 9, rbac098. doi:10.1093/rb/rbac098
Cao, Y., Zhang, H., Qiu, M., Zheng, Y., Shi, X., and Yang, J. (2024). Biomimetic injectable and bilayered hydrogel scaffold based on collagen and chondroitin sulfate for the repair of osteochondral defects. Int. J. Biol. Macromol. 257, 128593. doi:10.1016/j.ijbiomac.2023.128593
Cao, Z., Wang, H., Chen, J., Zhang, Y., Mo, Q., Zhang, P., et al. (2023). Silk-based hydrogel incorporated with metal-organic framework nanozymes for enhanced osteochondral regeneration. Bioact. Mater. 20, 221–242. doi:10.1016/j.bioactmat.2022.05.025
Castro, N. J., Patel, R., and Zhang, L. G. (2015). Design of a novel 3D printed bioactive nanocomposite scaffold for improved osteochondral regeneration. Cell. Mol. Bioeng. 8, 416–432. doi:10.1007/s12195-015-0389-4
Cavendish, P. A., Everhart, J. S., Peters, N. J., Sommerfeldt, M. F., and Flanigan, D. C. (2019). Osteochondral allograft transplantation for knee cartilage and osteochondral defects: a review of indications, technique, rehabilitation, and outcomes. JBJS Rev. 7 (6), e7. doi:10.2106/jbjs.rvw.18.00123
Chahla, J., Sweet, M. C., Okoroha, K. R., Nwachukwu, B. U., Hinckel, B., Farr, J., et al. (2019). Osteochondral allograft transplantation in the patellofemoral joint: a systematic review. Am. J. sports Med. 47 (12), 3009–3018. doi:10.1177/0363546518814236
Chander, S., Kulkarni, G. T., Dhiman, N., and Kharkwal, H. (2021). Protein-based nanohydrogels for bioactive delivery. Front. Chem. 9, 573748. doi:10.3389/fchem.2021.573748
Chen, H., Huang, J., Li, X., Zhao, W., Hua, Y., Song, Z., et al. (2024). Trilayered biomimetic hydrogel scaffolds with dual-differential microenvironment for articular osteochondral defect repair. Mater. Today Bio 26, 101051. doi:10.1016/j.mtbio.2024.101051
Chen, W., Ming, Y., Wang, M., Huang, M., Liu, H., Huang, Y., et al. (2023). Nanocomposite hydrogels in regenerative medicine: applications and challenges. Macromol. Rapid Commun. 44 (15), 2300128. doi:10.1002/marc.202300128
Davis, S., Roldo, M., Blunn, G., Tozzi, G., and Roncada, T. (2021). Influence of the mechanical environment on the regeneration of osteochondral defects. Front. Bioeng. Biotechnol. 9, 603408. doi:10.3389/fbioe.2021.603408
De Leon-Oliva, D., Boaru, D. L., Perez-Exposito, R. E., Fraile-Martinez, O., García-Montero, C., Diaz, R., et al. (2023). Advanced hydrogel-based strategies for enhanced bone and cartilage regeneration: a comprehensive review. Gels 9 (11), 885. doi:10.3390/gels9110885
Deng, C., Qin, C., Li, Z., Lu, L., Tong, Y., Yuan, J., et al. (2024). Diatomite-incorporated hierarchical scaffolds for osteochondral regeneration. Bioact. Mater. 38, 305–320. doi:10.1016/j.bioactmat.2024.05.004
Dinoro, J., Maher, M., Talebian, S., Jafarkhani, M., Mehrali, M., Orive, G., et al. (2019). Sulfated polysaccharide-based scaffolds for orthopaedic tissue engineering. Biomaterials 214, 119214. doi:10.1016/j.biomaterials.2019.05.025
Dong, Z., Yuan, Q., Huang, K., Xu, W., Liu, G., and Gu, Z. (2019). Gelatin methacryloyl (GelMA)-based biomaterials for bone regeneration. RSC Adv. 9 (31), 17737–17744. doi:10.1039/c9ra02695a
Fan, Z., Chen, Z., Zhang, H., Nie, Y., and Xu, S. (2021). Gradient mineralized and porous double-network hydrogel effectively induce the differentiation of BMSCs into osteochondral tissue in vitro for potential application in cartilage repair. Macromol. Biosci. 21 (3), 2000323. doi:10.1002/mabi.202000323
Filová, E., Tonar, Z., Lukášová, V., Buzgo, M., Litvinec, A., Rampichová, M., et al. (2020). Hydrogel containing anti-CD44-labeled microparticles, guide bone tissue formation in osteochondral defects in rabbits. Nanomaterials 10 (8), 1504. doi:10.3390/nano10081504
Gan, D., Wang, Z., Xie, C., Wang, X., Xing, W., Ge, X., et al. (2019). Mussel-inspired tough hydrogel with in situ nanohydroxyapatite mineralization for osteochondral defect repair. Adv. Healthc. Mater. 8 (22), 1901103. doi:10.1002/adhm.201901103
Geckil, H., Xu, F., Zhang, X., Moon, S., and Demirci, U. (2010). Engineering hydrogels as extracellular matrix mimics. Nanomedicine 5 (3), 469–484. doi:10.2217/nnm.10.12
Gong, L., Li, J., Zhang, J., Pan, Z., Liu, Y., Zhou, F., et al. (2020). An interleukin-4-loaded bi-layer 3D printed scaffold promotes osteochondral regeneration. Acta Biomater. 117, 246–260. doi:10.1016/j.actbio.2020.09.039
Gough, J. E., Saiani, A., and Miller, A. F. (2012). Peptide hydrogels: mimicking the extracellular matrix. Bioinspired, Biomim. Nanobiomaterials 1 (1), 4–12. doi:10.1680/bbn.11.00007
Guo, C., Su, Z., Zhao, L., Chen, R., Wang, Y., Wu, Y., et al. (2024). Customized triphasic cartilage composite scaffold simulating hypoxic microenvironment for osteochondral regeneration. Compos. Part B Eng. 271, 111161. doi:10.1016/j.compositesb.2023.111161
Guo, J. L., Kim, Y. S., Koons, G. L., Lam, J., Navara, A. M., Barrios, S., et al. (2021). Bilayered, peptide-biofunctionalized hydrogels for in vivo osteochondral tissue repair. Acta Biomater. 128, 120–129. doi:10.1016/j.actbio.2021.04.038
Guo, J. L., Li, A., Kim, Y. S., Xie, V. Y., Smith, B. T., Watson, E., et al. (2020). Click functionalized, tissue-specific hydrogels for osteochondral tissue engineering. J. Biomed. Mater. Res. Part A 108 (3), 684–693. doi:10.1002/jbm.a.36848
Hamed, E., Lee, Y., and Jasiuk, I. (2010). Multiscale modeling of elastic properties of cortical bone. Acta Mech. 213 (1), 131–154. doi:10.1007/s00707-010-0326-5
Hjelle, K., Solheim, E., Strand, T., Muri, R., and Brittberg, M. (2002). Articular cartilage defects in 1,000 knee arthroscopies. Arthrosc. J. Arthrosc. and Relat. Surg. 18 (7), 730–734. doi:10.1053/jars.2002.32839
Hu, C., Huang, R., Xia, J., Hu, X., Xie, D., Jin, Y., et al. (2024). A nanozyme-functionalized bilayer hydrogel scaffold for modulating the inflammatory microenvironment to promote osteochondral regeneration. J. nanobiotechnology 22 (1), 445. doi:10.1186/s12951-024-02723-x
Hu, W., Wang, Z., Xiao, Y., Zhang, S., and Wang, J. (2019). Advances in crosslinking strategies of biomedical hydrogels. Biomaterials Sci. 7 (3), 843–855. doi:10.1039/c8bm01246f
Hu, X., Zheng, S., Zhang, R., Wang, Y., Jiao, Z., Li, W., et al. (2022). Dynamic process enhancement on chitosan/gelatin/nano-hydroxyapatite-bone derived multilayer scaffold for osteochondral tissue repair. Biomater. Adv. 133, 112662. doi:10.1016/j.msec.2022.112662
Hwang, H. S., and Lee, C.-S. (2024). Nanoclay-composite hydrogels for bone tissue engineering. Gels 10 (8), 513. doi:10.3390/gels10080513
Jelodari, S., Ebrahimi Sadrabadi, A., Zarei, F., Jahangir, S., Azami, M., Sheykhhasan, M., et al. (2022). New insights into cartilage tissue engineering: improvement of tissue-scaffold integration to enhance cartilage regeneration. BioMed Res. Int. 2022 (1), 7638245. doi:10.1155/2022/7638245
Jin, Y., Shu, M., Liu, Z., Li, H., Liu, C., Zhu, C., et al. (2024). Bio-functional immunomodulatory europium-doped hydroxyapatite nanorods for osteochondral repair via CDH5-RAS-RAF-MEK-ERK-CSF1 axis. Chem. Eng. J. 484, 149311. doi:10.1016/j.cej.2024.149311
Kang, J., Li, Y., Qin, Y., Huang, Z., Wu, Y., Sun, L., et al. (2024). In situ deposition of drug and gene nanoparticles on a patterned supramolecular hydrogel to construct a directionally osteochondral plug. Nano-Micro Lett. 16 (1), 18. doi:10.1007/s40820-023-01228-w
Korpayev, S., Kaygusuz, G., Şen, M., Orhan, K., Oto, Ç., and Karakeçili, A. (2020). Chitosan/Collagen based biomimetic osteochondral tissue constructs: a growth factor-free approach. Int. J. Biol. Macromol. 156, 681–690. doi:10.1016/j.ijbiomac.2020.04.109
Kosik-Kozioł, A., Costantini, M., Mróz, A., Idaszek, J., Heljak, M., Jaroszewicz, J., et al. (2019). 3D bioprinted hydrogel model incorporating β-tricalcium phosphate for calcified cartilage tissue engineering. Biofabrication 11 (3), 035016. doi:10.1088/1758-5090/ab15cb
Kumar, A., Sood, A., Singhmar, R., Mishra, Y. K., Thakur, V. K., and Han, S. S. (2022). Manufacturing functional hydrogels for inducing angiogenic–osteogenic coupled progressions in hard tissue repairs: prospects and challenges. Biomaterials Sci. 10 (19), 5472–5497. doi:10.1039/d2bm00894g
Lan, W., Xu, M., Qin, M., Cheng, Y., Zhao, Y., Huang, D., et al. (2021). Physicochemical properties and biocompatibility of the bi-layer polyvinyl alcohol-based hydrogel for osteochondral tissue engineering. Mater. and Des. 204, 109652. doi:10.1016/j.matdes.2021.109652
Lee, J. H. (2018). Injectable hydrogels delivering therapeutic agents for disease treatment and tissue engineering. Biomaterials Res. 22 (1), 27. doi:10.1186/s40824-018-0138-6
Li, C., Zhang, W., Nie, Y., Jiang, D., Jia, J., Zhang, W., et al. (2023a). Integrated and bifunctional bilayer 3D printing scaffold for osteochondral defect repair. Adv. Funct. Mater. 33 (20), 2214158. doi:10.1002/adfm.202214158
Li, M., Song, P., Wang, W., Xu, Y., Li, J., Wu, L., et al. (2022). Preparation and characterization of biomimetic gradient multi-layer cell-laden scaffolds for osteochondral integrated repair. J. Mater. Chem. B 10 (22), 4172–4188. doi:10.1039/d2tb00576j
Li, Q., Yu, H., Zhao, F., Cao, C., Wu, T., Fan, Y., et al. (2023b). 3D printing of microenvironment-specific bioinspired and exosome-reinforced hydrogel scaffolds for efficient cartilage and subchondral bone regeneration. Adv. Sci. 10 (26), 2303650. doi:10.1002/advs.202303650
Liu, K., Liu, Y., Duan, Z., Ma, X., and Fan, D. (2021a). A biomimetic bi-layered tissue engineering scaffolds for osteochondral defects repair. Sci. China Technol. Sci. 64 (4), 793–805. doi:10.1007/s11431-020-1597-4
Liu, P., Li, M., Yu, H., Fang, H., Yin, J., Zhu, D., et al. (2021b). Biphasic CK2. 1-coated β-glycerophosphate chitosan/LL37-modified layered double hydroxide chitosan composite scaffolds enhance coordinated hyaline cartilage and subchondral bone regeneration. Chem. Eng. J. 418, 129531. doi:10.1016/j.cej.2021.129531
Liu, X., Chen, Y., Mao, A. S., Xuan, C., Wang, Z., Gao, H., et al. (2020). Molecular recognition-directed site-specific release of stem cell differentiation inducers for enhanced joint repair. Biomaterials 232, 119644. doi:10.1016/j.biomaterials.2019.119644
Liu, Y., and Hsu, S.-H. (2018). Synthesis and biomedical applications of self-healing hydrogels. Front. Chem. 6, 449. doi:10.3389/fchem.2018.00449
Lowen, J. M., Wheeler, E. E., Shimamoto, N. K., Ramos-Rodriguez, D. H., Griffin, K. H., Bond, G. C., et al. (2024). Functionalized annealed microgels for spatial control of osteogenic and chondrogenic differentiation. Adv. Funct. Mater. 34, 2311017. doi:10.1002/adfm.202311017
Luo, M., Chen, M., Bai, J., Chen, T., He, S., Peng, W., et al. (2022). A bionic composite hydrogel with dual regulatory functions for the osteochondral repair. Colloids Surfaces B Biointerfaces 219, 112821. doi:10.1016/j.colsurfb.2022.112821
Lynch, C. R., Kondiah, P. P., and Choonara, Y. E. (2021). Advanced strategies for tissue engineering in regenerative medicine: a biofabrication and biopolymer perspective. Molecules 26 (9), 2518. doi:10.3390/molecules26092518
Macleod, M. R., O’collins, T., Howells, D. W., and Donnan, G. A. (2004). Pooling of animal experimental data reveals influence of study design and publication bias. Stroke 35 (5), 1203–1208. doi:10.1161/01.str.0000125719.25853.20
Mano, J., and Reis, R. (2007). Osteochondral defects: present situation and tissue engineering approaches. J. tissue Eng. Regen. Med. 1 (4), 261–273. doi:10.1002/term.37
Martin, I., Miot, S., Barbero, A., Jakob, M., and Wendt, D. (2007). Osteochondral tissue engineering. J. biomechanics 40 (4), 750–765. doi:10.1016/j.jbiomech.2006.03.008
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., and Prisma, G. T. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann. Intern. Med. 151 (4), 264–269. doi:10.7326/0003-4819-151-4-200908180-00135
Moher, D., Shamseer, L., Clarke, M., Ghersi, D., Liberati, A., Petticrew, M., et al. (2015). Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 4, 1–9. doi:10.1186/2046-4053-4-1
Nguyen, L. H., Kudva, A. K., Saxena, N. S., and Roy, K. (2011). Engineering articular cartilage with spatially-varying matrix composition and mechanical properties from a single stem cell population using a multi-layered hydrogel. Biomaterials 32 (29), 6946–6952. doi:10.1016/j.biomaterials.2011.06.014
Park, K. M., and Park, K. D. (2018). In situ cross-linkable hydrogels as a dynamic matrix for tissue regenerative medicine. Tissue Eng. Regen. Med. 15, 547–557. doi:10.1007/s13770-018-0155-5
Paul, A., Manoharan, V., Krafft, D., Assmann, A., Uquillas, J. A., Shin, S. R., et al. (2016). Nanoengineered biomimetic hydrogels for guiding human stem cell osteogenesis in three dimensional microenvironments. J. Mater. Chem. B 4 (20), 3544–3554. doi:10.1039/c5tb02745d
Qiao, Z., Lian, M., Han, Y., Sun, B., Zhang, X., Jiang, W., et al. (2021). Bioinspired stratified electrowritten fiber-reinforced hydrogel constructs with layer-specific induction capacity for functional osteochondral regeneration. Biomaterials 266, 120385. doi:10.1016/j.biomaterials.2020.120385
Qin, Y., Li, G., Wang, C., Zhang, D., Zhang, L., Fang, H., et al. (2020). Biomimetic bilayer scaffold as an incubator to induce sequential chondrogenesis and osteogenesis of adipose derived stem cells for construction of osteochondral tissue. ACS Biomaterials Sci. and Eng. 6 (5), 3070–3080. doi:10.1021/acsbiomaterials.0c00200
Quazi, M. Z., and Park, N. (2022). Nanohydrogels: advanced polymeric nanomaterials in the era of nanotechnology for robust functionalization and cumulative applications. Int. J. Mol. Sci. 23 (4), 1943. doi:10.3390/ijms23041943
Radhakrishnan, J., Manigandan, A., Chinnaswamy, P., Subramanian, A., and Sethuraman, S. (2018). Gradient nano-engineered in situ forming composite hydrogel for osteochondral regeneration. Biomaterials 162, 82–98. doi:10.1016/j.biomaterials.2018.01.056
Rana, M. M., and De La Hoz Siegler, H. (2024). Evolution of hybrid hydrogels: next-generation biomaterials for drug delivery and tissue engineering. Gels 10 (4), 216. doi:10.3390/gels10040216
Sethi, S., Medha, T. S., Singh, A., Kaith, B. S., and Khullar, S. (2023). Handbook of green and sustainable nanotechnology: fundamentals, developments and applications. Springer Nature, 1–31.
Shalumon, K., Sheu, C., Fong, Y. T., Liao, H.-T., and Chen, J.-P. (2016). Microsphere-based hierarchically juxtapositioned biphasic scaffolds prepared from poly (lactic-co-glycolic acid) and nanohydroxyapatite for osteochondral tissue engineering. Polymers 8 (12), 429. doi:10.3390/polym8120429
Sheng, R., Chen, J., Wang, H., Luo, Y., Liu, J., Chen, Z., et al. (2022). Nanosilicate-reinforced silk fibroin hydrogel for endogenous regeneration of both cartilage and subchondral bone. Adv. Healthc. Mater. 11 (17), 2200602. doi:10.1002/adhm.202200602
Soni, S. S., D'elia, A. M., Alsasa, A., Cho, S., Tylek, T., O'brien, E. M., et al. (2022). Sustained release of drug-loaded nanoparticles from injectable hydrogels enables long-term control of macrophage phenotype. Biomaterials Sci. 10 (24), 6951–6967. doi:10.1039/d2bm01113a
Suvarnapathaki, S., Wu, X., Lantigua, D., Nguyen, M. A., and Camci-Unal, G. (2020). Hydroxyapatite-incorporated composite gels improve mechanical properties and bioactivity of bone scaffolds. Macromol. Biosci. 20 (10), 2000176. doi:10.1002/mabi.202000176
Tomal, W., and Ortyl, J. (2020). Water-soluble photoinitiators in biomedical applications. Polymers 12 (5), 1073. doi:10.3390/polym12051073
Tortorici, M., Petersen, A., Duda, G. N., and Checa, S. (2022). The degradation of synthetic polymeric scaffolds with strut-like architecture influences the mechanics-dependent repair process of an osteochondral defect in silico. Front. Bioeng. Biotechnol. 10, 846665. doi:10.3389/fbioe.2022.846665
Verhagen, R. A., Struijs, P. A., Bossuyt, P. M., and Van Dijk, C. N. (2003). Systematic review of treatment strategies for osteochondral defects of the talar dome. Foot ankle Clin. 8 (2), 233–242. doi:10.1016/s1083-7515(02)00064-5
Vilela, C., Correia, C., Oliveira, J. M., Sousa, R. A., Espregueira-Mendes, J., and Reis, R. L. (2015). Cartilage repair using hydrogels: a critical review of in vivo experimental designs. ACS Biomaterials Sci. and Eng. 1 (9), 726–739. doi:10.1021/acsbiomaterials.5b00245
Wang, H., Hu, B., Li, H., Feng, G., Pan, S., Chen, Z., et al. (2022a). Biomimetic mineralized hydroxyapatite nanofiber-incorporated methacrylated gelatin hydrogel with improved mechanical and osteoinductive performances for bone regeneration. Int. J. Nanomedicine Vol. 17, 1511–1529. doi:10.2147/ijn.s354127
Wang, S., Qiu, Y., Qu, L., Wang, Q., and Zhou, Q. (2022b). Hydrogels for treatment of different degrees of osteoarthritis. Front. Bioeng. Biotechnol. 10, 858656. doi:10.3389/fbioe.2022.858656
Wei, G., and Ma, P. X. (2008). Nanostructured biomaterials for regeneration. Adv. Funct. Mater. 18 (22), 3568–3582. doi:10.1002/adfm.200800662
Wu, H., Shang, Y., Sun, W., Ouyang, X., Zhou, W., Lu, J., et al. (2023). Seamless and early gap healing of osteochondral defects by autologous mosaicplasty combined with bioactive supramolecular nanofiber-enabled gelatin methacryloyl (BSN-GelMA) hydrogel. Bioact. Mater. 19, 88–102. doi:10.1016/j.bioactmat.2022.03.038
Xiang, C., Zhang, X., Zhang, J., Chen, W., Li, X., Wei, X., et al. (2022). A porous hydrogel with high mechanical strength and biocompatibility for bone tissue engineering. J. Funct. Biomaterials 13 (3), 140. doi:10.3390/jfb13030140
Xu, J., Feng, Q., Lin, S., Yuan, W., Li, R., Li, J., et al. (2019). Injectable stem cell-laden supramolecular hydrogels enhance in situ osteochondral regeneration via the sustained co-delivery of hydrophilic and hydrophobic chondrogenic molecules. Biomaterials 210, 51–61. doi:10.1016/j.biomaterials.2019.04.031
Yan, L.-P., Silva-Correia, J., Oliveira, M. B., Vilela, C., Pereira, H., Sousa, R. A., et al. (2015). Bilayered Silk/silk-nanoCaP scaffolds for osteochondral tissue engineering: in vitro and in vivo assessment of biological performance. Acta Biomater. 12, 227–241. doi:10.1016/j.actbio.2014.10.021
Yang, W., Zhu, P., Huang, H., Zheng, Y., Liu, J., Feng, L., et al. (2019). Functionalization of novel theranostic hydrogels with kartogenin-grafted USPIO nanoparticles to enhance cartilage regeneration. ACS Appl. Mater. and interfaces 11 (38), 34744–34754. doi:10.1021/acsami.9b12288
Yang, Y., Xu, T., Zhang, Q., Piao, Y., Bei, H. P., and Zhao, X. (2021). Biomimetic, stiff, and adhesive periosteum with osteogenic–angiogenic coupling effect for bone regeneration. Small 17 (14), 2006598. doi:10.1002/smll.202006598
Yao, H., Wang, C., Zhang, Y., Wan, Y., and Min, Q. (2023). Manufacture of bilayered composite hydrogels with strong, elastic, and tough properties for osteochondral repair applications. Biomimetics 8 (2), 203. doi:10.3390/biomimetics8020203
You, B., Li, Q., Dong, H., Huang, T., Cao, X., and Liao, H. (2018). Bilayered HA/CS/PEGDA hydrogel with good biocompatibility and self-healing property for potential application in osteochondral defect repair. J. Mater. Sci. and Technol. 34 (6), 1016–1025. doi:10.1016/j.jmst.2017.11.016
Yue, S., He, H., Li, B., and Hou, T. (2020). Hydrogel as a biomaterial for bone tissue engineering: a review. Nanomaterials 10 (8), 1511. doi:10.3390/nano10081511
Zengin, A., Castro, J., Habibovic, P., and Van Rijt, S. (2021). Injectable, self-healing mesoporous silica nanocomposite hydrogels with improved mechanical properties. Nanoscale 13 (2), 1144–1154. doi:10.1039/d0nr07406c
Zhang, H., Huang, H., Hao, G., Zhang, Y., Ding, H., Fan, Z., et al. (2021). 3D printing hydrogel scaffolds with nanohydroxyapatite gradient to effectively repair osteochondral defects in rats. Adv. Funct. Mater. 31 (1), 2006697. doi:10.1002/adfm.202006697
Zhang, H., Li, Q., Xu, X., Zhang, S., Chen, Y., Yuan, T., et al. (2022a). Functionalized microscaffold–hydrogel composites accelerating osteochondral repair through endochondral ossification. ACS Appl. Mater. and interfaces 14 (47), 52599–52617. doi:10.1021/acsami.2c12694
Zhang, L., Dai, W., Gao, C., Wei, W., Huang, R., Zhang, X., et al. (2023a). Multileveled hierarchical hydrogel with continuous biophysical and biochemical gradients for enhanced repair of full-thickness osteochondral defect. Adv. Mater. 35 (19), 2209565. doi:10.1002/adma.202209565
Zhang, P., Chen, J., Sun, Y., Cao, Z., Zhang, Y., Mo, Q., et al. (2023b). A 3D multifunctional bi-layer scaffold to regulate stem cell behaviors and promote osteochondral regeneration. J. Mater. Chem. B 11 (6), 1240–1261. doi:10.1039/d2tb02203f
Zhang, Y., Dong, Q., Zhao, X., Sun, Y., Lin, X., Zhang, X., et al. (2024). Honeycomb-like biomimetic scaffold by functionalized antibacterial hydrogel and biodegradable porous Mg alloy for osteochondral regeneration. Front. Bioeng. Biotechnol. 12, 1417742. doi:10.3389/fbioe.2024.1417742
Zhang, Y., Han, Y., Peng, Y., Lei, J., and Chang, F. (2022b). Bionic biphasic composite scaffolds with osteochondrogenic factors for regeneration of full-thickness osteochondral defects. Biomaterials Sci. 10 (7), 1713–1723. doi:10.1039/d2bm00103a
Zheng, L., Jiang, X., Chen, X., Fan, H., and Zhang, X. (2014). Evaluation of novel in situ synthesized nano-hydroxyapatite/collagen/alginate hydrogels for osteochondral tissue engineering. Biomed. Mater. 9 (6), 065004. doi:10.1088/1748-6041/9/6/065004
Zheng, S., Li, D., Liu, Q., Tang, C., Hu, W., Ma, S., et al. (2023). Surface-modified nano-hydroxyapatite uniformly dispersed on high-porous GelMA scaffold surfaces for enhanced osteochondral regeneration. Int. J. Nanomedicine Vol. 18, 5907–5923. doi:10.2147/ijn.s428965
Keywords: nano-hydrogel, osteochondral repair, tissue engineering, biomaterials, osteochondral
Citation: Amhare AF, Qiao L, Deng H, Lin J, Wang J, Wang W and Han J (2025) The current status of nano-hydrogel preparations for osteochondral repair: Systematic Review. Front. Bioeng. Biotechnol. 13:1611522. doi: 10.3389/fbioe.2025.1611522
Received: 14 April 2025; Accepted: 23 June 2025;
Published: 01 July 2025.
Edited by:
Dongxu Ke, Wake Forest University, United StatesReviewed by:
Yogendra Pratap Singh, VIT University, IndiaMiguel Fuentes Chandia, Case Western Reserve University, United States
Copyright © 2025 Amhare, Qiao, Deng, Lin, Wang, Wang and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Wang, ZHIud2FuZ3dlaUB4anR1LmVkdS5jbg==; Jing Han, YmJiaXNob3BAMTI2LmNvbQ==
 Abebe Feyissa Amhare
Abebe Feyissa Amhare Lichun Qiao
Lichun Qiao Huan Deng
Huan Deng Jinyan Lin1
Jinyan Lin1 Wei Wang
Wei Wang Jing Han
Jing Han