- 1The First Affiliated Hospital of Harbin Medical University, School of Stomatology, Harbin Medical University, Harbin, China
- 2School of Materials Science and Engineering, Harbin Institute of Technology, Harbin, China
Introduction: Guided bone regeneration (GBR) is widely used for maxillofacial defects, but fabricating membranes that enhance osteoinduction and antimicrobial resistance remains challenging. This study addresses critical bone defect therapy by developing collagen (Col) and zeolitic imidazolate framework-8 (ZIF-8) reinforced poly(lactide-co-glycolide) (PLGA) nanofibrous membranes.
Methods: Material characterization analyzed the composite nanofibrous membranes’ morphology, structure, wettability, tensile strength, in vitro degradation, and ion release. Biocompatibility and osteogenesis were evaluated using live/dead staining, cytoskeleton staining, CCK-8 assay, alkaline phosphatase (ALP) activity quantification, ALP staining, alizarin red S (ARS) staining and ARS quantification. Antibacterial efficacy was assessed via agar plate counting and bacterial growth kinetics. In vivo bone regeneration was examined in a rat cranial critical defect model treated with the membrane; bone formation was evaluated by Micro-CT and hematoxylin-eosin staining after 4 weeks.
Results: Optimization of the PLGA/Col weight ratio (100:3) yielded composite membranes demonstrating superior tensile strength. The PLGA/Col/ZIF-8 nanofibrous composite incorporating 1 wt% ZIF-8 nanoparticles exhibited optimal biocompatibility with sustained Zn2+ release kinetics. In vitro experiments demonstrated that sustained release of Zn2+ has the dual effects of stimulating osteogenic differentiation and effectively preventing early bacterial infection. In vivo a rat calvarial defect model further confirmed the positive bone regeneration effect of the PLGA/Col/ZIF-8 composite nanofibrous membrane.
Discussion: PLGA/Col/ZIF-8 composite nanofibrous membranes have great potential for application in guiding bone tissue regeneration.
1 Introduction
The incidence of alveolar and maxillofacial bone defects caused by trauma, tumor and inflammation is high, and such pathological bone defects often lead to biomechanical stability and functional reconstruction of conventional restorations and implant-supported prosthetic solutions (Visscher et al., 2016; Yang et al., 2022). In this context, revolutionary advances in bone tissue engineering technologies are providing breakthrough solutions for the regenerative repair of bone defects. Guided bone regeneration (GBR) biomaterials can significantly improve the effectiveness of structural and functional reconstruction of critical-sized bone defects (Urban and Monje, 2019). The core principle of GBR is to create a physical barrier between the bone defect area and the soft tissue by using a biological barrier membrane that selectively inhibits the infiltration of fibrous connective tissue and epithelial cells based on a spatial competition mechanism, while creating a preferential microenvironment for the proliferation of osteogenic progenitor cells. The barrier membrane maintains the stability of the clot and the morphology of the defect area through mechanical isolation and relieves pressure on the soft tissues, thereby supporting the process of directed migration, differentiation and mineralization of osteoblasts (Laney, 2017; Sheikh et al., 2017).
Currently, GBR membranes are mainly divided into non-resorbable and resorbable materials. Although polytetrafluoroethylene (PTFE) is clinically effective as a non-resorbable membrane, its non-resorbability requires secondary surgical removal, which significantly increases the risk of postoperative infection and limits its clinical use (Carbonell et al., 2014; Garcia et al., 2018; Trobos et al., 2018). In contrast, resorbable collagen (Col) membranes, with their excellent biocompatibility, biodegradability and natural bioactivity, have become an important alternative in bone augmentation therapy, promoting bone repair through tissue regeneration mechanisms while avoiding secondary surgery (Dimitriou et al., 2012; Liu and Kerns, 2014). However, the poor mechanical properties and rapid degradation behavior of collagen membranes have hindered their development, as they are unable to maintain their spatial configuration (Garcia et al., 2017; Rothamel et al., 2005). Micro- and nanoscale fiber structures produced by electrospinning technology have been widely used in the development of GBR membranes due to their highly biomimetic properties. These structures can mimic the topological characteristics of the natural extracellular matrix (ECM) and effectively promote cell adhesion, proliferation and metabolic exchange, while the micro-barrier formed by the nanofiber network can selectively block the invasion of fibrous connective tissue and ensure the stability of the osteogenic microenvironment in the bone defect region. However, these electrostatically spun GBR membranes have not yet been clinically translated (Martins et al., 2020; Wang et al., 2019; Wang et al., 2013). Therefore, the development of GBR membranes with good mechanical properties, degradation rate, osteoinductive activity and antimicrobial functionality required to withstand the multidimensional effects of mechanical stress, microbial colonization and biomolecular metabolism in the dynamic oral cavity bioenvironment is a core research direction.
Col, a core component of connective tissue, is widely used in biomaterial design due to its low immunogenicity, biodegradability and structural support function (Ren et al., 2022). Poly (lactide-co-glycolide) (PLGA), an FDA-approved biodegradable polyester, has proven to be the preferred substrate for bone tissue engineering scaffolds due to its combination of excellent mechanical strength and histocompatibility (Brown et al., 2018). Based on this, this study aims to improve the interfacial compatibility and mechanical stability of electrostatically spun fiber membranes through intermolecular interactions by the composite of Col and PLGA (Ahi et al., 2020; Kwak et al., 2016; Yang et al., 2018). Prior research shows that PLGA/Col composite systems have been validated in the field of nerve axon guidance scaffolds, brain injury repair carriers and bone defect filling materials (Park et al., 2018). However, the composite system still suffers from significant functional deficiencies in terms of osteoinductive differentiation efficiency and antimicrobial activity in guided bone regeneration membrane applications.
The dual role of bioactive ions in tissue repair and bacterial resistance modulation is gradually becoming a research focus. Zinc, an essential trace element, plays a key biological function by regulating osteoblast differentiation, matrix mineralization and maintenance of bone homeostasis (Yamaguchi and Weitzmann, 2011). In addition, its antimicrobial activity has been widely demonstrated (Nagata and Lonnerdal, 2011). However, excessive zinc ions have the potential to cause toxicity and to inhibit the expression of bone-related genes. Therefore, a controlled slow-release system is required to achieve zinc ion delivery (Giliopoulos et al., 2020; Yang and Yang, 2020). Metal-organic frameworks (MOFs) are a class of porous materials self-assembled by coordination between organic ligands and metal ions, among which zeolite imidazolate framework-8 (ZIF-8) is composed of zinc ions coordinated with dimethylimidazole, which combines excellent biocompatibility and osteoinductive properties (Pan et al., 2011). The material exhibits stability in neutral environments, yet rapidly dissociates under acidic conditions, thereby facilitating the prompt release of Zn2+ in bacterial infection microenvironments (Sun et al., 2012). It has been demonstrated that nano ZIF-8 activates the MAPK signaling pathway in bone marrow mesenchymal stem cells through the process of endocytosis, thereby significantly promoting osteogenic differentiation (Gao et al., 2021; Wang et al., 2016). Therefore, ZIF-8 nanoparticles can be introduced into GBR membranes as antimicrobial-osteogenic functional modification materials to achieve the membrane material to play a precise regulatory role of antimicrobial and osteogenic in the process of bone repair.
In this study, a novel PLGA/Col/ZIF-8 composite nanofibrous membrane was fabricated by electrospinning technology for bone defect repair. Initially, a mechanically adapted substrate was constructed by means of gradient doping Col in a PLGA matrix, followed by the subsequent integration of ZIF-8 into PLGA/Col to achieve a microenvironment-triggered release mechanism of zinc ions, thereby resulting in both antimicrobial and osteogenic effects. Furthermore, a rat cranial defect model was utilized to confirm its capacity to repair bone tissue defects in vivo, thereby assessing the potential of this composite fibrous membrane in bone regeneration strategies.
2 Materials and methods
2.1 Materials and reagents
Poly (lactic-co-glycolic acid) (PLGA; LA/GA = 75:25, Mw = 66–107 kDa) was procured from Sigma Aldrich. Type I collagen (Col I) was obtained from Macklin, while 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) and 2-methylimidazole (2-MIM) were sourced from Aladdin. Zinc nitrate hexahydrate was procured from Innochem. All of the aforementioned chemical reagents were of analytical grade and did not undergo any further purification.
2.2 Fabrication of PLGA/col/ZIF-8 electrospun membrane
The synthesis and characterization of the ZIF-8 nanoparticles were conducted in accordance with the following procedure (Supplementary Figures S1, S2). The electrospun solution was prepared by dissolving PLGA in HFIP (20% w/v) under magnetic stirring at room temperature. To prepare the PLGA/Col electrospun solution, 1 g of PLGA was dissolved in 5 mL of HFIP. Subsequently, the different weight ratios of Col/PLGA (1:100, 3:100, 5:100 and 10:100; abbreviated as PC1, PC3, PC5 and PC10, respectively) were added under stirring until complete dissolution. The electrospun solution of PLGA/Col/ZIF-8 was prepared in the following manner: ZIF-8 at varying weight ratios (0.5 wt%, 1 wt%, 1.5 wt% and 2 wt%; designated as PC3Z0.5, PC3Z1, PC3Z1.5 and PC3Z2, respectively) was subjected to sonication and dispersion in 5 mL HFIP. Subsequently, 1 g of PLGA and 0.03 g of Col I were added to the solution, which was then magnetically stirred until complete dissolution of the PLGA and Col I had occurred. The solution was then sonicated for a further 0.5 h to ensure uniform dispersion of the nano-ZIF-8 within the spinning solution. The electrospun solutions were introduced into the system via a 23 G flat-tipped needle at a constant injection rate of 0.5 mL/h, using a plastic syringe. The distance between the needle and the flat collector was 15 cm, the applied positive voltage was 10–15 kV, and the humidity was approximately 21%–31%.
2.3 Characterization
The microscopic morphology of the membranes was examined using a scanning electron microscope (SEM, Gemini 560, ZEISS, Germany). The chemical compositions of the membranes were established through the use of a Fourier transform infrared spectrometer (FTIR, Nicolet iS5, Thermo, United States). Moreover, Nano Measurer was employed to quantify the diameter and distribution of the membranes from the acquired SEM image. The presence of ZIF-8 crystals in the membranes was confirmed through X-ray diffraction (XRD, X’PERT, Panalytical, Holland). Thermogravimetric analysis (TG, STA449F3, NETZSCH, Germany) was employed to ascertain the thermal stability of the membranes. All samples were subjected to a heating process, increasing the temperature from 30°C to 500°C at a rate of 10°C/min, in a nitrogen-enriched environment. The wettability of the membranes (n = 3) was evaluated using a contact angle meter (SDC 350, SINDIN, China). In order to evaluate the impact of Col and ZIF-8 NPs incorporation on the mechanical properties of the membranes (n = 3), an electronic universal testing machine (Model 6103, MTS, China) with a 50 N load cell was employed to conduct a series of tests on the membranes under dry and wet condition (soaking in deionized water for 24 h), which had overall dimensions of 50 mm × 10 mm × 0.2 mm. The crosshead speed was set at 15 mm/min. For degradation test, membrane samples (n = 3) were precisely sectioned into 10 mm × 10 mm squares. Initial dry mass (W0) was determined using an analytical balance. Specimens were subsequently immersed in 10 mL of phosphate-buffered saline (PBS, pH 7.4) and incubated at 37°C. The PBS was replenished weekly to maintain ionic stability. The samples were removed at each predetermined time point, at days 1, 3, 7, 14, 21, 28 and 56, and then placed in a vacuum drying oven until completely dry and weighed (Wt). Weight loss (%) = (W0 - Wt)/W0 × 100. The release of zinc ions from the composite membranes (n = 3) was quantified using inductively coupled plasma emission spectroscopy (ICP-OES, iCAP 7,400, Thermo, United States). The membranes were immersed in PBS with a specified pH value (pH 7.4) for 1, 3, 5, and 7 days at 37°C without agitation, the extracts were collected at various time points and the amount of released Zn2+ ions was measured.
2.4 Biocompatibility in vitro
2.4.1 Cell culture
The mouse bone marrow mesenchymal stem cells (BMSCs) were procured from Immocell Biotechnology. The BMSCs were cultured in Dulbecco’s Modified Eagle Medium: F-12 (DMEM/F12, Pricella, China) supplemented with 10% Fetal Bovine Serum (FBS, Gibco, United States) and 1% mixture of penicillin-streptomycin (Gibco, United States), and passaged every 2 days. The osteogenic differentiation of BMSCs was induced using an osteogenic induction medium (OriCell, China). All cells were cultured in accordance with standard conditions (37°C, 5% CO2, 95% relative humidity). The fiber membranes employed in all cell experiments were subjected to a pre-processing step involving immersion in a 75% ethanol solution for 1 hour. Thereafter, the prepared membranes underwent a sterilization procedure involving exposure to ultraviolet light.
2.4.2 Cell proliferation
BMSCs were seeded on the fiber membranes (n = 4) in 96-well plates at a cell density of 2.5 × 103 cells per well. Following a period of cultivation lasting for 1, 3, and 5 days, the extent of cellular proliferation was gauged through the utilization of the cell counting kit-8(CCK-8, MedChemExpress, China). At the designated time point, the cells were rinsed with PBS and cultured in a medium containing 10% CCK-8 solution at 37°C for 2 h. Subsequently, the optical density (OD) value at 450 nm was determined using a microplate reader.
2.4.3 Cell live/dead staining
The cells were seeded on the fiber membranes (n = 4) in 24-well plates at a density of 2 × 104 cells per well. Following a 24 h incubation period, the working solution was prepared at a ratio of 1:1:1,000, comprising Calcein AM, PI and detection buffer. Subsequently, the solution was added to each well and incubated at 37°C for 30 min in the dark. The presence of live (green) and dead (red) cells were observed using fluorescence microscopy.
2.4.4 Cell morphology
The cells were seeded on the membranes (n = 4) in 24-well plates at a density of 2 × 104 cells per well for a 24 h culture period. Subsequently, the cells were fixed with 4% paraformaldehyde and rinsed with PBS. They were then permeabilized with 0.5% Triton X-100 and rinsed with PBS. Subsequently, F-actin (red) and nuclei (blue) were stained with TRITC-phalloidin and 4, 6-diamidino-2-phenylindole dihydrochloride (DAPI) in the dark, respectively, and observed using fluorescence microscopy.
2.5 Osteogenic ability in vitro
2.5.1 ALP activity
BMSCs were seeded at a density of 2 × 104 cells per well on the membranes (n = 4) in 24-well plates. The osteogenic induction medium was replenished every 2 days. Following a 7-day and 14-day cultivation period, a quantitative evaluation of ALP activity was conducted in accordance with the instructions provided in the ALP detection kit (Beyotime, China). For qualitative analysis of ALP, BMSCs were fixed with 4% paraformaldehyde for 15 min at room temperature and subsequently stained with the BCIP/NBT ALP kit for 30 min in the absence of light.
2.5.2 Alizarin red S staining
BMSCs were seeded at a density of 2 × 104 cells per well on the membranes (n = 4) in 24-well plates. Following a 21-day period of cultivation in an osteogenic induction medium, the extracellular matrix mineralization capacity was evaluated through the use of ARS staining. The BMSCs were fixed with 4% paraformaldehyde for a period of 30 min, after which they were stained with alizarin red S (OriCell, China) for a further 10 min at 37°C. The stained samples were thoroughly washed with PBS to remove any residual dye, and images were acquired under a stereomicroscope. 10% cetylpyridinium chloride was added to each well, and the stained mineralized nodules were solubilized by shaking the bed for 30 min. The absorbance at 630 nm was then detected by a microplate reader.
2.6 Antibacterial assay
2.6.1 Bacterial culture
Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus) were retrieved from the −80°C refrigerator, with individual colonies selected and dispersed in medium. The suspension was then shaken and cultured at 37°C with 130 rpm agitation, resulting in a bacterial concentration of 1 × 106 CFU/mL, which was subsequently utilized in subsequent experiments.
2.6.2 Agar plate count
At 37°C, samples (n = 3) were cocultured with E. coli and S. aureus for 24 h. Thereafter, 60 μL of broth was aspirated and diluted to specific concentrations as needed. Subsequently, 100 μL of the diluted broth was spread evenly on solid agar plates. After 12 h of continuous incubation at 37°C, the number of colonies on the plates was counted and photographed.
2.6.3 Bacterial growth curve
The three groups of nanofiber membranes (n = 3) were co-cultured with E. coli or S. aureus and placed in a bacterial shaking incubator at 37°C. At specific time points (0, 2, 4, 6, 8, 10, and 12 h), an appropriate volume of bacterial suspension was aspirated, transferred to a 96-well plate, and the OD at 600 nm was measured using a microplate reader. Bacterial growth curves were then plotted based on the OD values.
2.7 Animal experiments
2.7.1 Animal grouping and surgery
All animal procedures were approved by the Ethical Committee on Animal Experiments of Harbin Medical University. Twenty male Sprague-Dawley rats were randomly divided into four groups (n = 5): blank control group, P, PC, and PCZ. Under general anesthesia, the dorsal area was shaved and disinfected, followed by the creation of a 2 cm skin incision to form a subcutaneous pocket. After implantation of the corresponding materials, the wound was closed using non-absorbable monofilament sutures. Peripheral blood samples were collected 3 days post-surgery for biochemical analysis.
Twenty Sprague Dawley rats were anaesthetized, the surgical site on the top of the head was prepared and disinfected, and a longitudinal incision was made in the middle of the skull, with the periosteum elevated to expose the cranial surface. A circular bone defect measuring 5 mm in diameter was created on the side of the middle cranial suture using a ring bone extraction drill. The bone defect was full-length and did not damage the dura mater. During the procedure, saline rinsing of the ring drill was ensured for cooling protection. Twenty Sprague-Dawley rats were randomized into four groups (Blank; P; PC; PCZ). Calvarial defects in three treatment groups (n = 5) received membrane implantation, while the blank control group remained non-interventional. Following the implantation of the membrane material, the subcutaneous tissue was closed and the skin layer incision was sutured. The rats were euthanized 4 weeks after the surgical procedure. The removal and fixation of the cranial tissues, as well as the heart, liver, spleen, lung and kidney organs, was conducted using 4% paraformaldehyde for subsequent analysis.
2.7.2 Blood biochemistry assay
Blood samples were collected for the purpose of quantifying various hematological parameters, including white blood cells (WBC), red blood cells (RBC), hemoglobin (HGB), platelets (PLT), aspartate aminotransferase (AST), alanine aminotransferase (ALT), blood urea nitrogen (UREA) and creatinine (CREA).
2.7.3 Micro-CT analysis
Micro-CT was utilised to scan the fixed cranial tissue blocks, thus facilitating analysis of bone regeneration at the defect site in each group. The cranial scanning parameters comprised a voltage of 70 kV, a current of 114 μA, a voxel of 10 μm and a 0.5 mm aluminium filter. The 3D images were reconstructed by software, after which the bone volume fraction (bone volume/total volume, BV/TV) was measured and analysed.
2.7.4 Histology staining
Following micro-CT scanning, the samples were subjected to decalcification using a 10% EDTA solution. Thereafter, they underwent a process of gradient dehydration, followed by paraffin embedding, sectioning (4 μm thickness), staining with hematoxylin and eosin (H&E), and imaging under a light microscope. For the purpose of visceral histological assessment, heart, liver, spleen, lung and kidney tissues were collected from each group and fixed in 4% paraformaldehyde. The tissues were then dehydrated in ethanol and embedded in paraffin. The tissues were sectioned into 4 μm and stained for H&E.
2.8 Statistical analysis
All experiments were repeated at least three times, and the data were calculated as the mean ± standard deviation. Statistical analysis of the date was performed using one-way ANOVA (GraphPad Prism 8, United States). The level of statistical significance of differences was set P < 0.05.
3 Results and discussion
3.1 Optimization of col and ZIF-8 content
In order to prepare PCZ composite fibrous membranes with both excellent mechanical properties and good cytocompatibility, different contents of Col and ZIF-8 were added to the PLGA fibrous membranes (the optimization process is illustrated in Supplementary Figures S3, S4, and the PCZ composite fibrous membranes with the optimal performance were selected for subsequent analysis.
The morphology of the membrane is illustrated in Figures 1A,B,D, where the fiber exhibit random orientation and form an interconnected porous structure. The P membranes exhibited an average fiber diameter of 1,301 ± 312 nm, demonstrating homogeneous morphological characteristics with smooth surfaces devoid of bead-like or spindle-shaped structural defects. Increasing Col content from 1% to 15% induced a progressive fiber diameter reduction from 1,057 ± 272 nm to 451 ± 80 nm in PC membranes. This inverse correlation originated from collagen’s viscosity-lowering effect on the electrospinning solution, thereby decreasing the mean fiber dimensions (Lee et al., 2009). Supplementary Figure S3 demonstrates a biphasic tensile strength response to elevated Col concentrations in the membranes. The PC3 formulation, corresponding to the tensile strength maximum, was subjected to subsequent ZIF-8 parametric analysis to optimize mechanical performance. A moderate increase in Col content can enhance the tensile properties of the material by optimizing the fiber network structure through intermolecular cross-linking; beyond the critical concentration, excess collagen triggers phase separation or crystallization defects, leading to stress concentration and reduced mechanical strength.
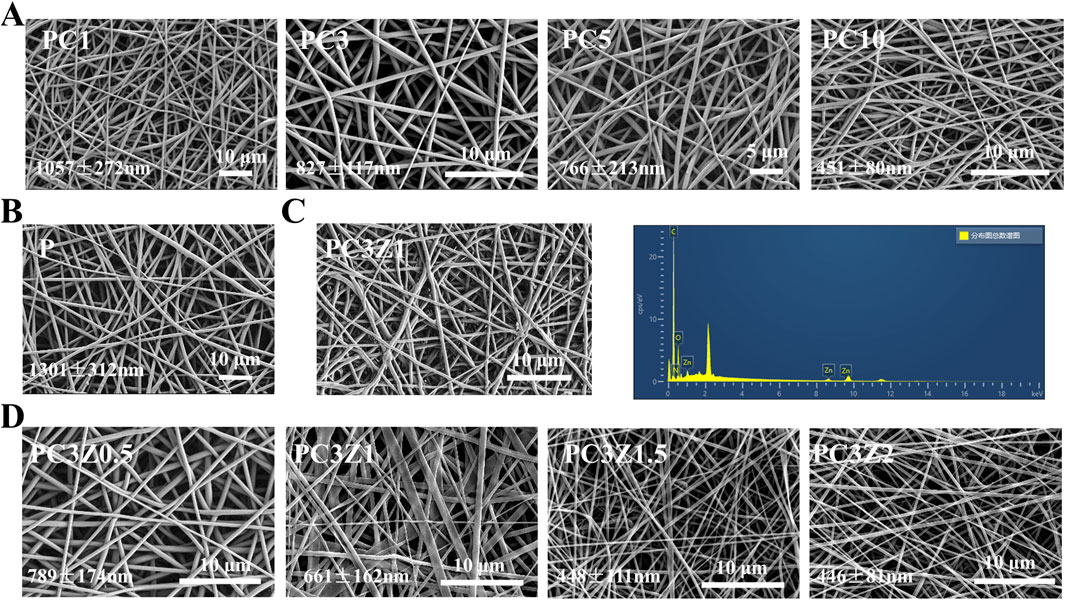
Figure 1. SEM images and corresponding diameter of membranes with different Col contents (A,B) and different ZIF-8 contents (D). (C) Corresponding EDS spectrogram of PC3Z1 fibers.
To determine the optimal ZIF-8 NPs loading, PC3 fibers were modified with four ZIF-8 NPs weight fractions (0.5-2 wt%). Supplementary Figure S4 demonstrated maximal proliferative activity at 1 wt% ZIF-8 on days 3/5, establishing this concentration for PCZ membrane fabrication. As shown in Figure 1D, the doping of ZIF-8 NPs led to a further decrease in the average diameter of fibers in the PCZ membrane. This originated from ZIF-8-induced viscosity reduction in the spinning solution. The initial 10 kV voltage insufficiently countered surface tension, causing droplet ejection, necessitating voltage elevation to 15 kV for stabilized fiber formation. Furthermore, EDS analysis demonstrated the presence of C, N, O and Zn, thereby further indicating the presence of ZIF-8 NPs in the nanofibers (Figure 1C). Therefore, Pure PLGA (P), PC3 (PC), and PC3Z1 (PCZ) membranes were prioritized for detailed investigation in this study.
3.2 Characterizations of the composite fibrous membranes
As shown in Figure 2A, the XRD analysis of the fibrous membrane revealed that pure PLGA and Col belong to the amorphous structure, presenting only one broad peak. The incorporation of Col resulted in the diffusion and broadening of the peaks corresponding to PC and PCZ. This phenomenon may be attributed to the interaction between PLGA and Col molecules, which impedes the arrangement of PLGA chains. The XRD pattern of PCZ exhibited distinctive peaks at 7.07°, 10.03°, 12.30°, 14.32°, 16.01° and 17.64°. These peaks correspond to the ZIF-8 crystal face at (011), (002), (112), (022), (013) and (222), which indicates the presence of ZIF-8 dispersion within the nanofibers. The FTIR spectra (Figure 2B) revealed that the PLGA, PLGA/Col, and PLGA/Col/ZIF-8 exhibited stretching bands of C=O and C-O at 1752 cm−1 and 1,083 cm−1, respectively, which corresponded to the characteristic peaks of PLGA. The absorption peaks of am-ide I and amide II in Col were observed at 1,657 cm−1 and 1,529 cm−1, respectively. The absorption peaks of PLGA/Col and PLGA/Col/ZIF-8 exhibited slight shifts towards lower wavelengths, appearing at 1,644 cm−1 and 1,526 cm−1, respectively. This suggests that the amino group in Col most likely formed hydrogen bonds with the PLGA molecular chain. Additionally, PLGA/Col/ZIF-8 displayed a Zn-N stretching vibration at 427 cm−1.
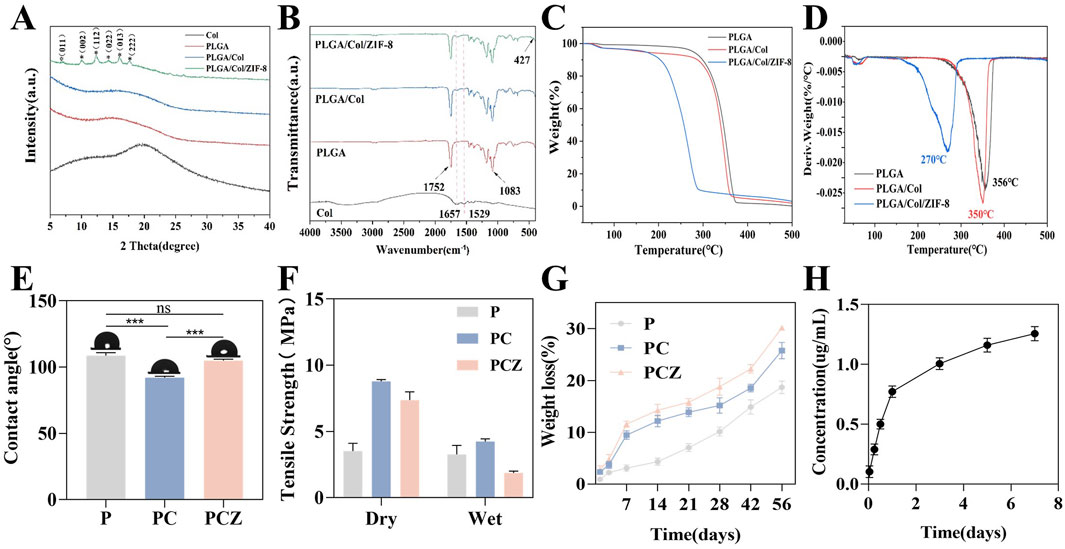
Figure 2. Characterization of fibrous membrane. (A) XRD patterns of the nanofibers. (B) FTIR spectra of the nanofibers. (C) TG analysis of the nanofibers. (D) DTG curve of the nanofibers. (E) Water contact angles of the nanofibers. (F) Tensile strength of the nanofibers in the dry and wet conditions. (G) Degradation behavior of the nanofibers after soaking in PBS solution. (H) Cumulative Zn2+ release from the PCZ nanofibers. Data are presented as mean ± SD (n = 3, *P < 0.05, **P < 0.01, ***P < 0.001).
The thermal analysis of the fibrous membrane is illustrated in Figures 2C,D. The TG curve with a first-order derivative demonstrates that the fastest thermal decomposition temperature of PLGA is 356°C, while the fastest thermal decomposition temperatures of Col and ZIF-8 are 350°C and 270°C, respectively. It can be concluded that the introduction of Col and ZIF-8 resulted in a reduction of the fastest thermal decomposition temperature of the membranes, while still meeting the thermal stability requirements of GBR membranes. The initial stage of mass loss can be attributed to the evaporation of physically adsorbed water, while the subsequent stage may be attributed to the collapse of the molecular chain structure and thermal decomposition. As displayed in Figure 2E, the PLGA membrane exhibited hydrophobic behavior, with a water contact angle of 100.43°. The incorporation of Col markedly enhanced the hydrophilicity of the membrane, reducing the surface contact angle of the material to 90.97°. This was predominantly attributed to the hydrophilic nature of the amino and carboxyl functional groups of Col. The contact angle of PCZ was 104.27°.
The mechanical strength of a material is also an important performance indicator. Accordingly, the objective of this experiment was to evaluate the mechanical properties of various membranes through a tensile strength test. As demonstrated in Figure 2F, the tensile strength of the pure P membrane in the dry state was found to be 3.52 ± 0.58 MPa. In contrast, the tensile strength of the PC composite membrane and the PCZ composite membrane was significantly higher, reaching approximately 2.5 and 2 times that of the pure P membrane, respectively. It is important to acknowledge the considerable impact of ambient humidity on the mechanical properties of materials. While the pure P membrane exhibited only 7% strength attenuation under wet conditions, the tensile strength of the PC and PCZ composite membrane decreased sharply by 52% and 75%, respectively. Specifically, the tensile strength of the PCZ membrane was measured at 7.37 ± 0.63 MPa in the dry condition, which decreased to 1.87 ± 0.14 MPa after 24 h of water immersion. A comparison with commercially available products demonstrated that the tensile strength of the Bio-Gide resorbable barrier membrane in the dry/wet state was 4.6 ± 0.94 MPa versus 1.68 ± 0.54 MPa, respectively (Raz et al., 2019). This comparison demonstrates that the mechanical properties of PCZ membranes in the wet state are still superior to those of clinically available products, which is crucial for guided bone regeneration applications.
Studies have indicated that the diameter of electrospun fibers critically influences their mechanical properties, with reduced diameters typically accompanied by enhanced strength and reduced ductility (Stachewicz et al., 2012; Tan et al., 2005). The introduction of col significantly decreased fiber diameter (Figure 1), thereby enhancing the mechanical strength of the composites. This improvement stems from two aspects. Firstly, active groups such as amino/carboxyl groups in col molecules form intermolecular hydrogen-bonding networks with PLGA, which stabilize molecular conformations and enhance macroscopic mechanical performance. As previously mentioned, XRD and FTIR results partially support this perspective. Secondly, the incorporation of ZIF-8 nanoparticles may cause disordering of polymer chain arrangements and particle agglomeration phenomena, resulting in slightly lower tensile strength in the PCZ group compared to the PC group. Nevertheless, the overall tensile performance of PCZ membranes remains significantly superior to that of pure PLGA membranes and fully meets the mechanical strength requirements for GBR applications.
In order to evaluate the in vitro degradability of the prepared membranes, the mass loss during their immersion was detected. As demonstrated in Figure 2G, the mass loss rate of PCZ was marginally higher than that of PC. However, the degradation kinetic curves of these two samples exhibited analogous trends. The experimental data demonstrated that the degradation rate of the pure PLGA material was significantly lower than that of the other fibrous membranes at all the detected time nodes, thereby confirming that the addition of Col changes the material properties and significantly accelerates the degradation process of the composite fibers. This phenomenon can be attributed to the formation of continuous structural defects within the composite material due to the rapid degradation of Col. This, in turn, facilitates the penetration of hydrolysis media by increasing the contact surface between the fibrous membrane and the soaking solution. Furthermore, the progressive dissolution and release of ZIF-8 NPs from the PCZ fiber membrane occurred with the extension of the degradation time, which further led to a slightly faster degradation rate of the PCZ than that of the PC membrane. Subsequently, the release behavior of Zn2+ from PCZ nanofibrous membranes was evaluated. As shown in Figure 2H, under simulated physiological conditions (pH 7.4, 37°C), the Zn2+ release profile exhibits a typical two-stage pattern: an initial burst release occurs within the first 24 h (cumulative release: 0.77 ± 0.05 μg/mL), followed by a sustained-release phase, reaching a cumulative concentration of 1.26 ± 0.06 μg/mL by day 7. Notably, this release amount is significantly lower than the internationally recognized safe threshold range for zinc ions (0.65–16.25 ppm) (Nagata and Lönnerdal, 2011). Studies have shown that zinc-based sustained-release materials generally exhibit an initial rapid release (1–7 days) followed by exponential decay, rather than a linear accumulation trend (Xue et al., 2021). With regard to the toxicity threshold, it has been confirmed in vitro that ZIF-8 concentrations less than 50 μg/mL possess anti-apoptotic properties and improve cell survival (Gao et al., 2021). Importantly, the peak release level of this system at 7 days was only 2.5% of that safety threshold, further corroborating its safety advantage.
3.3 In vitro biocompatibility
Cell-material interactions initiate with attachment, potentially progressing to adhesion, followed by proliferation and spreading phases (Anselme, 2000). BMSCs cultured on the surface of fibrous membranes exhibited proliferation behaviors quantified via CCK-8 assay. Enhanced proliferative capacity, a cornerstone of cellular physiology, directly supports differentiation potential in biomaterial interfaces. Figure 3A indicated that the proliferation of cells on the fibrous membranes exhibited an overall increasing trend over time. There was no statistically significant difference in cell proliferation between the groups on days 1 and 3, indicating that the fibrous membranes exhibited good biocompatibility. The proliferation of BMSCs was significantly enhanced by PCZ nanofiber membranes compared to the P and PC groups on day 5. The enhanced cell proliferation capacity may be attributed to the release of zinc ions at optimal concentrations, which facilitated the proliferation of BMSCs (Yusa et al., 2011). Furthermore, cell adhesion and nutrient exchange on membranes is found to be highly correlated with fiber diameter, morphology and pore size (Zhou et al., 2015). Previous studies have shown that the number of cells adhering to nanofibers is greater than that adhering to microfibers.
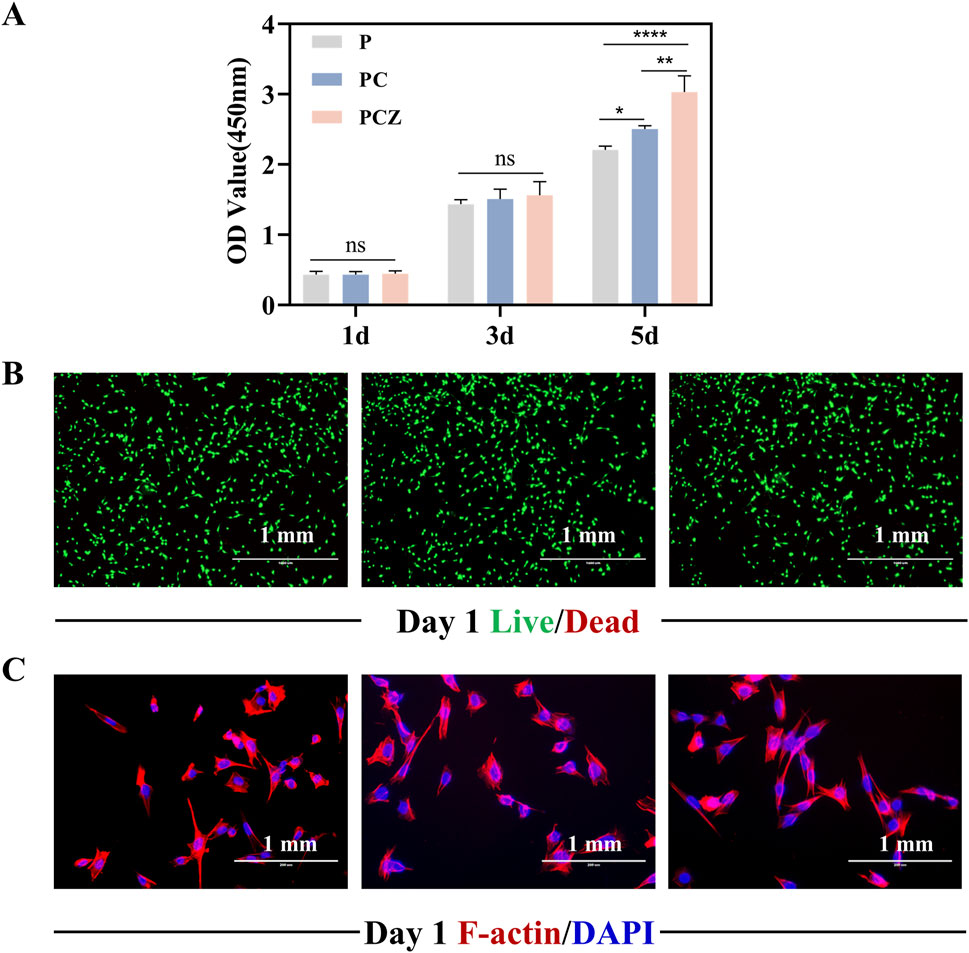
Figure 3. Biocompatibility of BMSCs cultured on the nanofibers in vitro. (A) Proliferation of BMSCs on the surface of the nanofibers measured by CCK-8 assay on day 1, 3, and 5. (B) Fluorescent staining of live/dead of BMSCs on the nanofibers at day 1. (C) Fluorescent staining of the cytoskeleton and nuclei of BMSCs on the nanofibers at day 1. Data are presented as mean ± SD (n = 4, *P < 0.05, **P < 0.01, ***P < 0.001).
Live/dead staining revealed cell viability on fibrous membranes (Figure 3B). Following 24 h culture, all groups exhibited predominantly viable cells (green) with minimal red fluorescence (dead cells), confirming the material was not significantly toxic to the cells. Given the critical role of cell adhesion in proliferation and differentiation, this study assessed the impact of fibrous membranes on the adhesion behavior and morphological spreading of BMSCs by means of a cytoskeleton-specific staining technique. Dual-channel fluorescent labeling (phalloidin for F-actin, red; DAPI for nuclei, blue) was employed (Figure 3C). After 24 h incubation, a certain number of BMSCs were observed to be adhered to the surface of the P, PC and PCZ membranes. It was evident that the incorporation of ZIF-8 NPs had a significant impact on the spreading area of the cells within the material. The cells exhibited a characteristic polygonal morphology, accompanied by filamentous pseudopod extension features, suggesting that the cells had established stable extracellular matrix interactions. It was further demonstrated that PCZ fibrous membranes would construct a growth-promoting microenvironment via strengthened cell-matrix interactions.
3.4 In vitro osteogenic differentiation
As a characteristic biomarker of early stages of osteogenic differentiation, ALP expression levels were used to systematically assess the effect of nanofibrous membranes on the efficacy of early osteogenic induction in BMSCs (Ren et al., 2021). ALP staining (Figures 4A,B) and quantitative analysis of ALP activity (Figures 4D,E) demonstrated that following a 7-day and 14-day culture period, cells in all groups showed the phenomenon of violet-blue precipitate deposition, with the PCZ group showing the densest staining areas and the deepest staining intensity due to synergistic effect of ZIF-8 NPs. The PC group demonstrated significantly higher staining intensity and activity at 14 days (P < 0.01) in comparison to the P group. However, no statistical difference was observed between the two groups at 7 days (P > 0.05). Quantitative analysis further demonstrated that ALP activity in the PCZ group increased in a time-dependent manner, and its 14-day activity value was elevated approximately 1.6-fold in comparison with that of the P group (P < 0.001). The results indicated that ZIF-8 NPs significantly strengthened the early osteogenic differentiation ability of PCZ composite fibrous membranes by regulating osteogenesis-related signaling pathways.
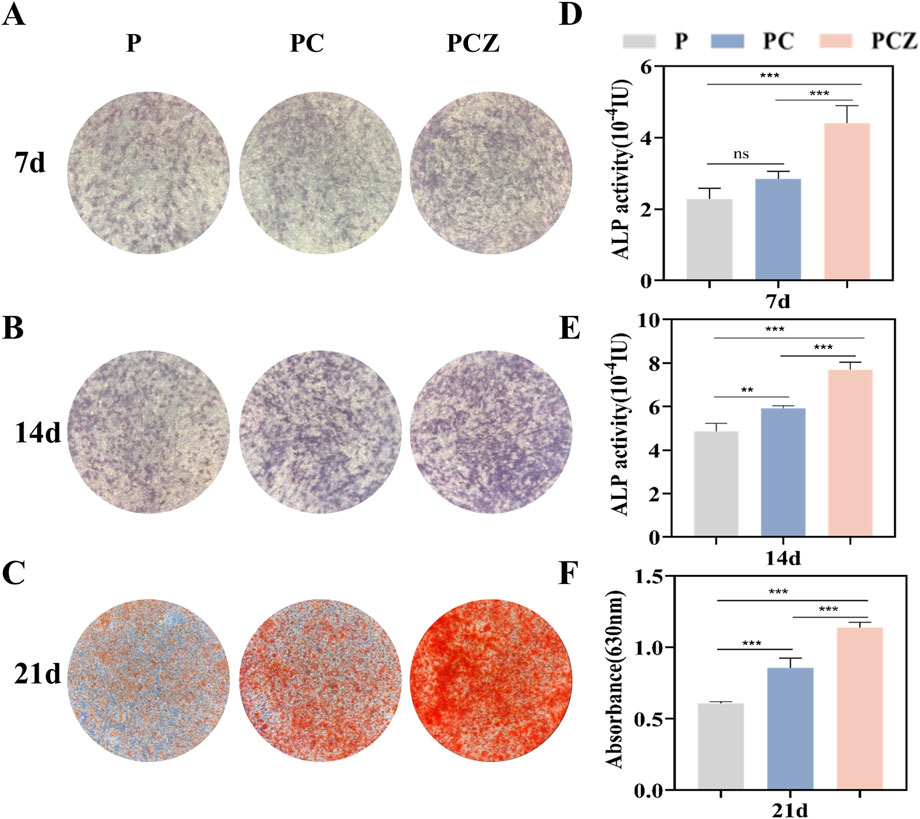
Figure 4. Osteogenic differentiation of BMSCs cultured on the nanofibers in vitro. (A,B) ALP staining of BMSCs cultured on the nanofibers at day 7 and 14 after osteogenic differentiation stimulation, and (D,E) the determination of ALP activity. (C) Qualitative and (F) quantitative characterization of Alizarin Red S staining of BMSCs cultured on different membranes at day 21. Data are presented as mean ± SD (n = 4, *P < 0.05, **P < 0.01, ***P < 0.001).
The capacity of the extracellular matrix to mineralize was systematically assessed by means of ARS calcium nodule staining. This is a key morphological marker of advanced osteogenic differentiation (Sculean et al., 2008). Following a 21-day incubation period, the presence of orange mineralized nodule deposition was observed in all groups (Figure 4C). The PCZ group demonstrated the highest mineralization density and strongest staining intensity, followed by the PC group. In contrast, the P group exhibited the lightest staining and the lowest number of calcium nodules. The results of the semi-quantitative analysis conducted by ARS (Figure 4F) demonstrated a consistent trend with staining, with significantly higher OD values observed in the PCZ group compared to the PC group (P < 0.01) and the P group (P < 0.001). The results obtained above demonstrate that the prepared PCZ composite fiber membrane has the capacity to promote extracellular matrix mineralization and calcium deposition.
3.5 In vitro antibacterial ability
In the aftermath of GBR, the regenerative outcome of hard and soft tissues is subject to the influence of bacterial colonization and infection (Wang et al., 2020). The introduction of antimicrobial components has been demonstrated to be efficacious in the prevention of infection, as well as in the reduction of inflammation (Brown et al., 2018). In the context of bacterial research, E. coli (Gram-negative bacteria) and S. aureus (Gram-positive bacteria) have been selected as models to assess the antimicrobial capacity of PCZ nanofiber membranes.
As demonstrated in Figure 5A, the antibacterial properties of distinct fiber membranes were examined through agar plate counting. In comparison to P and PC, PCZ nanofiber membrane exhibited substantial bacterial inhibition, evidenced by a substantial decrease in the number of bacterial colonies following 12 h of inoculation with E. coli and S. aureus (Figure 5B). Moreover, OD values of bacterial strains co-cultured with the fiber membranes were investigated. As depicted in Figures 5C,D, after 12 h of co-incubation, the OD value of the P showed no significant difference compared to that of the PC, indicating that Col contributes minimally to the antibacterial activity of the PC membrane. In contrast, the OD values for E. coli and S. aureus in the PCZ were substantially lower than those in the P and PC. This demonstrates a significantly enhanced antibacterial effect, further confirming that the Zn2+ released from the PCZ fiber membrane demonstrates certain inhibitory activity against E. coli and S. aureus.
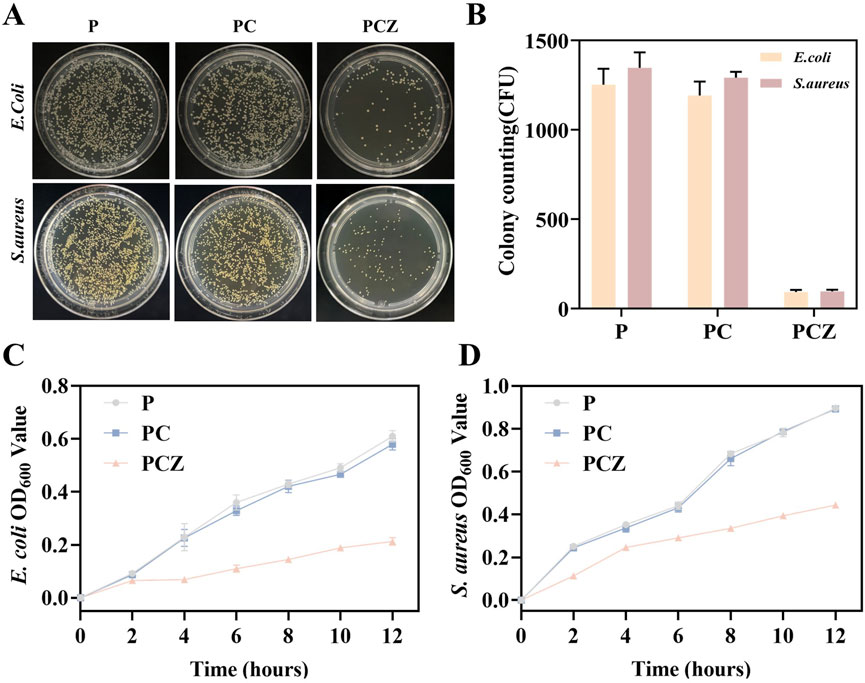
Figure 5. Antibacterial ability of the nanofibers in vitro. (A) Agar plate images for E. coli and S. aureus treated with the different membranes. (B) Colony counts for E. coli and S. aureus. OD values of bacterial solution for (C) E. coli and (D) S. aureus cocultured with different membranes. Data are presented as mean ± SD (n = 3, *P < 0.05, **P < 0.01, ***P < 0.001).
Membrane materials have been shown to carry a high risk of infection during the initial week following implantation. To prevent further bacterial colonization, it is essential to ensure a sustained release of Zn2+ (Xue et al., 2014). ZIF-8 nanoparticles, derived from PCZ nanofiber membranes, have been demonstrated to be effective in preventing bacterial colonization, with a sustained release capacity of at least 7 days (Figure 2F). This property is crucial in the early stages of bacterial colonization, where it can play a pivotal role in preventing the establishment of bacterial communities (Wu, B. et al., 2019).
3.6 In vivo bone regeneration
The study evaluated the efficacy of PCZ nanofiber membranes on bone tissue regeneration in a rat cranial defect model (diameter: 5 mm). Three types of membrane materials, P, PC and PCZ membranes, were tested in parallel and the results were compared with a “no membrane control” group (Figure 6A). The rats were euthanized 4 weeks after surgery, and the cranial defects were collected and assessed for bone regeneration by Micro-CT and histological analysis.
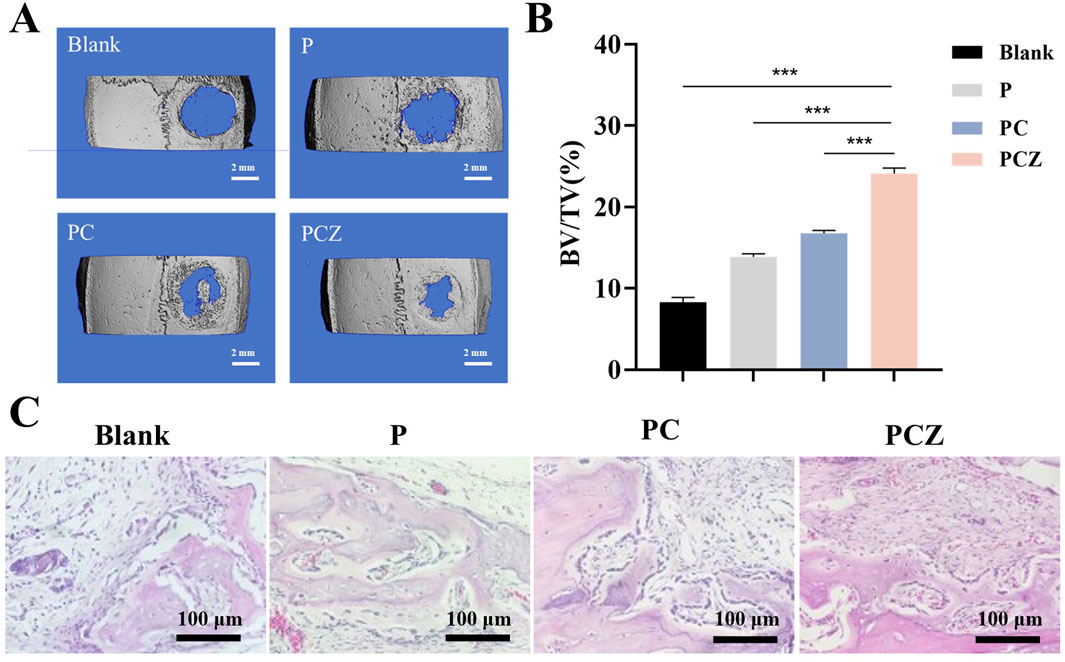
Figure 6. New bone formation in a rat cranial defect model after implantation of the nanofibers. (A) Micro-CT reconstructions of the defects after 4 weeks post-surgery in different groups (Blank, P, PC, and PCZ). (B) Bone volume to tissue volume (BV/TV) in the calvarial defect determined by micro-CT. (C) H&E staining images of sections of different groups after 4 weeks post-surgery. Data are presented as mean ± SD (n = 5, *P < 0.05, **P < 0.01, ***P < 0.001).
A reconstructed Micro-CT scan image is presented in Figure 6A, showing newly formed bone within the defect at 4 weeks postoperatively. The 3D reconstructed image demonstrated a significant increase in bone regeneration in the defect treated with PCZ fibrous membrane. Specifically, a significant increase in bone volume to tissue volume (BV/TV) was observed in the PCZ fiber membrane treated site compared to the untreated site and the other fiber membrane treated sites (P < 0.01) (Figure 6B).
Moreover, the results of H&E staining further validated the results of the Micro-CT analysis. As demonstrated in Figure 6C, in the blank control group, connective tissue occupied the majority of the bone defect area, with only a minimal amount of new bone formation observable at the edges. Observations revealed the presence of new bone formation on the inner face of the fibrous membrane material in all three groups (P, PC and PCZ). The study demonstrated that the osteoconductive ability of the membrane was indicated by the growth of new bone from the edge of the defect. A significant number of osteoblasts could be seen surrounding the bone defect area in the PCZ group, indicating a promising bone regeneration ability. Furthermore, new bone was discovered beneath the membrane, predominantly originating from its base. This phenomenon can be attributed to the presence of stem cells beneath the dura matter, a characteristic commonly observed in models of cranial defects. For histological assessment of visceral organs, examination of critical organs (heart, liver, spleen, lungs, and kidneys) via H&E staining revealed no significant toxicity or injury (Supplementary Figure S5A). Additionally, in the blood biochemical tests, no abnormalities were detected in WBC, RBC, HGB, PLT, AST, ALT, UREA, or CREA at day 3 (Supplementary Figure S5B). The implanted membranes demonstrated neither hematological nor organ toxicity, providing critical evidence supporting their biological applications. This study has limitations in detecting inflammatory indicators; future research must further examine the pathological manifestations of inflammatory responses.
While this study confirms the macroscopic efficacy of composite materials in promoting bone regeneration through multi-scale characterization, the following limitations must be acknowledged. First, the molecular regulatory mechanisms by which the material mediates bone repair remain incompletely elucidated. Second, although electrospinning technology successfully constructed a biomimetic nanofiber scaffold, scalable production remains constrained by the low throughput of single-nozzle spinning and its sensitivity to processing parameters. Furthermore, this study focused on the early stage of bone repair, primarily monitoring the 7-day Zn2+ release kinetics, but the risk of Zn2+ accumulation during the later degradation phase and its coupling effect with new bone remodeling rates require further validation through long-term experiments. To address these issues, subsequent investigations will build upon previous signal transduction pathway research to examine whether canonical osteogenic signaling pathways participate in PCZ-induced BMSCs osteogenic differentiation. Simultaneously, the in vivo observation period will be prolonged to 12 weeks to further evaluate the spatiotemporal matching between material degradation and bone functional reconstruction, thereby facilitating the material’s advancement toward clinical translation.
4 Conclusion
In this study, a novel Col and ZIF-8 reinforced PLGA fiber-guided bone regeneration membrane was successfully prepared by electrospinning. The incorporation of Col into the PLGA-based membranes resulted in a substantial enhancement of their mechanical strength, while the addition of ZIF-8 nanoparticles facilitated the sustained release of zinc ions from the fibrous membranes. The incorporation of ZIF-8 and Col enhanced the cytocompatibility of PLGA membranes to BMSCs; notably, doped ZIF-8 considerably promoted osteogenic differentiation of BMSCs. PCZ fibrous membranes have been demonstrated to provide a basic microenvironment for osteoblasts and have been shown to promote bone defect repair in a rat cranial defect model. Therefore, it is considered that electrostatically spun PCZ fiber membranes are potential candidates for guided bone tissue regeneration applications.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by the Ethical Committee on Animal Experiments of Harbin Medical University (Approval No. 2024007). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
TW: Conceptualization, Writing – original draft, Methodology, Formal Analysis. QX: Formal Analysis, Supervision, Writing – review and editing, Validation. HL: Methodology, Writing – review and editing, Software, Resources. YS: Writing – review and editing, Validation, Supervision, Resources. WX: Funding acquisition, Project administration, Conceptualization, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the Construction of Oral Experimental Teaching Base (Grant No. 31993190299).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fbioe.2025.1611948/full#supplementary-material
References
Ahi, Z. B., Assunçao-Silva, R. C., Salgado, A. J., and Tuzlakoglu, K. (2020). A combinatorial approach for spinal cord injury repair using multifunctional collagen-based matrices: development, characterization and impact on cell adhesion and axonal growth. Biomed. Mat. 15 (5), 055024. doi:10.1088/1748-605X/ab924d
Anselme, K. (2000). Osteoblast adhesion on biomaterials. Biomaterials 21 (7), 667–681. doi:10.1016/S0142-9612(99)00242-2
Brown, J. H., Das, P., DiVito, M. D., Ivancic, D., Tan, L. P., and Wertheim, J. A. (2018). Nanofibrous plga electrospun scaffolds modified with type I collagen influence hepatocyte function and support viability in vitro. Acta. Biomater. 73, 217–227. doi:10.1016/j.actbio.2018.02.009
Carbonell, J. M., Martín, I. S., Santos, A., Pujol, A., Sanz-Moliner, J. D., and Nart, J. (2014). High-density polytetrafluoroethylene membranes in guided bone and tissue regeneration procedures: a literature review. Int. J. Oral. Maxillofac. Surg. 43 (1), 75–84. doi:10.1016/j.ijom.2013.05.017
Dimitriou, R., Mataliotakis, G. I., Calori, G. M., and Giannoudis, P. V. (2012). The role of barrier membranes for guided bone regeneration and restoration of large bone defects: current experimental and clinical evidence. BMC Med. 10, 81. doi:10.1186/1741-7015-10-81
Gao, X. M., Xue, Y. Y., Zhu, Z., Chen, J. Y., Liu, Y. H., Cheng, X. T., et al. (2021). Nanoscale zeolitic imidazolate Framework-8 activator of canonical mapk signaling for bone repair. ACS Appl. Mat. Interfaces. 13 (1), 97–111. doi:10.1021/acsami.0c15945
Garcia, J., Dodge, A., Luepke, P., Wang, H. L., Kapila, Y., and Lin, G. H. (2018). Effect of membrane exposure on guided bone regeneration: a systematic review and meta-analysis. Clin. Oral. Implants. Res. 29 (3), 328–338. doi:10.1111/clr.13121
Garcia, J. J., Berghezan, S., Caramês, J. M. M., Dard, M. M., and Marques, D. N. S. (2017). Effect of cross-linked Vs non-cross-linked collagen membranes on bone: a systematic review. J. Periodontal. Res. 52 (6), 955–964. doi:10.1111/jre.12470
Giliopoulos, D., Zamboulis, A., Giannakoudakis, D., Bikiaris, D., and Triantafyllidis, K. (2020). Polymer/metal organic framework (MOF) nanocomposites for biomedical applications. Molecules 25 (1), 185. doi:10.3390/molecules25010185
Kwak, S., Haider, A., Gupta, K. C., Kim, S., and Kang, I. K. (2016). Micro/nano multilayered scaffolds of plga and collagen by alternately electrospinning for bone tissue engineering. Nanoscale Res. Lett. 11, 323–16. doi:10.1186/s11671-016-1532-4
Laney, W. R. (2017). Glossary of oral and maxillofacial implants. Int. J. Oral Maxillofac. Implants. 32 (4), G200–G200. doi:10.11607/jomi.2017.4.gomi
Lee, J. J., Yu, H. S., Hong, S. J., Jeong, I., Jang, J. H., and Kim, H. W. (2009). Nanofibrous membrane of collagen-polycaprolactone for cell growth and tissue regeneration. J. Mat. Sci. Mat. Med. 20 (9), 1927–1935. doi:10.1007/s10856-009-3743-z
Liu, J., and Kerns, D. G. (2014). Mechanisms of guided bone regeneration: a review. Open Dent. J. 8, 56–65. doi:10.2174/1874210601408010056
Martins, A., Reis, R. L., and Neves, N. M. (2020). Biofunctional nanostructured systems for regenerative medicine. Nanomedicne 15 (16), 1545–1549. doi:10.2217/nnm-2020-0147
Nagata, M., and Lönnerdal, B. (2011). Role of zinc in cellular zinc trafficking and mineralization in a murine osteoblast-like cell line. J. Nutr. Biochem. 22 (2), 172–178. doi:10.1016/j.jnutbio.2010.01.003
Pan, Y. C., Liu, Y. Y., Zeng, G. F., Zhao, L., and Lai, Z. P. (2011). Rapid synthesis of zeolitic imidazolate Framework-8 (Zif-8) nanocrystals in an aqueous system. Chem. Commun. 47 (7), 2071–2073. doi:10.1039/c0cc05002d
Park, H. K., Joo, W., Gu, B. K., Ha, M. Y., You, S. J., and Chun, H. J. (2018). Collagen/Poly(D,L-Lactic-Co-Glycolic acid) composite fibrous scaffold prepared by independent nozzle control multi-electrospinning apparatus for dura repair. J. Ind. Eng. Chem. 66, 430–437. doi:10.1016/j.jiec.2018.06.010
Raz, P., Brosh, T., Ronen, G., and Tal, H. (2019). Tensile properties of three selected collagen membranes. Biomed. Res. Int. 2019, 1–8. doi:10.1155/2019/5163603
Ren, C. X., Hao, X. Q., Wang, L., Hu, Y., Meng, L., Zheng, S. Z., et al. (2021). Metformin carbon dots for promoting periodontal bone regeneration Via activation of erk/ampk pathway. Adv. Healthc. Mat. 10 (12), e2100196. doi:10.1002/adhm.202100196
Ren, Y. R., Fan, L., Alkildani, S., Liu, L., Emmert, S., Najman, S., et al. (2022). Barrier membranes for guided bone regeneration (GBR): a focus on recent advances in collagen membranes. Int. J. Mol. Sci. 23 (23), 14987. doi:10.3390/ijms232314987
Rothamel, D., Schwarz, F., Sager, M., Herten, M., Sculean, A., and Becker, J. (2005). Biodegradation of differently cross-linked collagen membranes: an experimental study in the rat. Clin. Oral. Implants. Res. 16 (3), 369–378. doi:10.1111/j.1600-0501.2005.01108.x
Sculean, A., Nikolidakis, D., and Schwarz, F. (2008). Regeneration of periodontal tissues: combinations of barrier membranes and grafting materials - biological foundation and preclinical evidence: a systematic review. J. Clin. Periodontol. 35, 106–116. doi:10.1111/j.1600-051X.2008.01263.x
Sheikh, Z., Qureshi, J., Alshahrani, A. M., Nassar, H., Ikeda, Y., Glogauer, M., et al. (2017). Collagen based barrier membranes for periodontal guided bone regeneration applications. Odontology 105 (1), 1–12. doi:10.1007/s10266-016-0267-0
Stachewicz, U., Bailey, R. J., Wang, W., and Barber, A. H. (2012). Size dependent mechanical properties of electrospun polymer fibers from a composite structure. Polymer 53 (22), 5132–5137. doi:10.1016/j.polymer.2012.08.064
Sun, C. Y., Qin, C., Wang, X. L., Yang, G. S., Shao, K. Z., Lan, Y. Q., et al. (2012). Zeolitic imidazolate Framework-8 as efficient Ph-Sensitive drug delivery vehicle. Dalton Trans. 41 (23), 6906–6909. doi:10.1039/c2dt30357d
Tan, E. P. S., Ng, S. Y., and Lim, C. T. (2005). Tensile testing of a single ultrafine polymeric fiber. Biomaterials 26 (13), 1453–1456. doi:10.1016/j.biomaterials.2004.05.021
Trobos, M., Juhlin, A., Shah, F. A., Hoffman, M., Sahlin, H., and Dahlin, C. (2018). in vitro evaluation of barrier function against oral bacteria of dense and expanded polytetrafluoroethylene (ptfe) membranes for guided bone regeneration. Clin. Implant. Dent. Relat. Res. 20 (5), 738–748. doi:10.1111/cid.12629
Urban, I. A., and Monje, A. (2019). Guided bone regeneration in alveolar bone reconstruction. Oral Maxillofac. Surg. Clin. North Am. 31 (2), 331–338. doi:10.1016/j.coms.2019.01.003
Visscher, D. O., Farré-Guasch, E., Helder, M. N., Gibbs, S., Forouzanfar, T., van Zuijlen, P. P., et al. (2016). Advances in bioprinting technologies for craniofacial reconstruction. Trends Biotechnol. 34 (9), 700–710. doi:10.1016/j.tibtech.2016.04.001
Wang, C. Y., Wang, J., Zeng, L. D., Qiao, Z. W., Liu, X. C., Liu, H., et al. (2019). Fabrication of electrospun polymer nanofibers with diverse morphologies. Molecules 24 (5), 834. doi:10.3390/molecules24050834
Wang, J., Wang, Y. M., Zhang, Y. T., Uliana, A., Zhu, J. Y., Liu, J. D., et al. (2016). Zeolitic imidazolate framework/graphene oxide hybrid nanosheets functionalized thin film nanocomposite membrane for enhanced antimicrobial performance. ACS Appl. Mat. Interfaces. 8 (38), 25508–25519. doi:10.1021/acsami.6b06992
Wang, J. L., Zhan, L. L., Zhang, X. H., Wu, R. F., Liao, L., and Wei, J. C. (2020). Silver nanoparticles coated Poly(L-Lactide) electrospun membrane for implant associated infections prevention. Front. Pharmacol. 11, 431. doi:10.3389/fphar.2020.00431
Wang, X. F., Ding, B., and Li, B. Y. (2013). Biomimetic electrospun nanofibrous structures for tissue engineering. Mat. Today. 16 (6), 229–241. doi:10.1016/j.mattod.2013.06.005
Wu, B., Fu, J., Zhou, Y., Shi, Y., Wang, J., Feng, X., et al. (2019). Metal-organic framework-based chemo-photothermal combinational system for precise, rapid, and efficient antibacterial therapeutics. Pharmaceutics 11, 463. doi:10.3390/pharmaceutics12020148
Xue, J., He, M., Liu, H., Niu, Y., Crawford, A., Coates, P. D., et al. (2014). Drug loaded homogeneous electrospun PCL/Gelatin hybrid nanofiber structures for anti-infective tissue regeneration membranes. Biomaterials 35 (34), 9395–9405. doi:10.1016/j.biomaterials.2014.07.060
Xue, Y., Zhu, Z., Zhang, X., Chen, J., Yang, X., Gao, X., et al. (2021). Accelerated bone regeneration by MOF modified multifunctional membranes through enhancement of osteogenic and angiogenic performance. Adv. Healthc. Mat. 10 (6), e2001369. doi:10.1002/adhm.202001369
Yamaguchi, M., and Weitzmann, M. N. (2011). Zinc stimulates osteoblastogenesis and suppresses osteoclastogenesis by antagonizing Nf-Κb activation. Mol. Cell. Biochem. 355 (1-2), 179–186. doi:10.1007/s11010-011-0852-z
Yang, J., and Yang, Y. W. (2020). Metal-organic frameworks for biomedical applications. Small 16 (10), e1906846. doi:10.1002/smll.201906846
Yang, X., Li, Y. Y., He, W., Huang, Q. L., Zhang, R. R., and Feng, Q. L. (2018). Hydroxyapatite/collagen coating on plga electrospun fibers for osteogenic differentiation of bone marrow mesenchymal stem cells. J. Biomed. Mat. Res. A 106 (11), 2863–2870. doi:10.1002/jbm.a.36475
Yang, Z., Wu, C., Shi, H. X., Luo, X. Y., Sun, H., Wang, Q., et al. (2022). Advances in barrier membranes for guided bone regeneration techniques. Front. Bioeng. Biotechnol. 10, 921576. doi:10.3389/fbioe.2022.921576
Yusa, K., Yamamoto, O., Fukuda, M., Koyota, S., Koizumi, Y., and Sugiyama, T. (2011). In vitro prominent bone regeneration by release zinc ion from Zn-Modified implant. Biochem. Biophys. Res. Commun. 412 (2), 273–278. doi:10.1016/j.bbrc.2011.07.082
Zhou, T., Wang, N. P., Xue, Y., Ding, T. T., Liu, X., Mo, X. M., et al. (2015). Retraction: development of biomimetic tilapia collagen nanofibers for skin regeneration through inducing keratinocytes differentiation and collagen synthesis of dermal fibroblasts. ACS Appl. Mat. Interfaces. 7 (35), 19863. doi:10.1021/acsami.5b07490
Keywords: electrospinning, nanofibrous membrane, ZIF-8, tensile strength, guided bone regeneration
Citation: Wang T, Xie Q, Liang H, Sun Y and Xie W (2025) Multifunctional PLGA/collagen/zeolitic imidazolate framework-8 composite nanofibrous membranes for guided bone regeneration. Front. Bioeng. Biotechnol. 13:1611948. doi: 10.3389/fbioe.2025.1611948
Received: 15 April 2025; Accepted: 13 June 2025;
Published: 27 June 2025.
Edited by:
Dan Lin, Shanghai University of Medicine and Health Sciences, ChinaReviewed by:
Yuanzhang Jiang, Sichuan University, ChinaMarco Friuli, National Research Council (CNR), Italy
Copyright © 2025 Wang, Xie, Liang, Sun and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weili Xie, eGlld2xAaHJibXUuZWR1LmNu
 Tianqi Wang1
Tianqi Wang1 Weili Xie
Weili Xie