- 1Department of Radiology, Anam Hospital, Korea University College of Medicine, Seoul, Republic of Korea
- 2Department of Mathematics, Korea University, Seoul, Republic of Korea
- 3Department of Ophthalmology, Dongguk University Ilsan Hospital, Goyang, Republic of Korea
- 4Department of Ophthalmology, Ansan Hospital, Korea University College of Medicine, Ansan, Republic of Korea
- 5Department of Radiology, Ansan Hospital, Korea University College of Medicine, Ansan, Republic of Korea
- 6Department of Computer Science and Engineering, Soonchunhyang University, Asan, Republic of Korea
Orbit fractures under 20 years are a medical emergency requiring urgent surgery with the gold standard modality being high-resolution CT. If radiography could be used to identify patients without fractures, the number of unnecessary CT scans could be reduced. The purpose of this study was to develop and validate a deep learning-based multi-input model with a novel cross-sequence learning method, which outperforms the conventional single-input models, to detect orbital fractures on radiographs of young patients. Development datasets for this retrospective study were acquired from two hospitals (n = 904 and n = 910). The datasets included patients with facial trauma who underwent orbital rim view and CT. The development dataset was split into training, tuning, and internal test sets in 7:1:2 ratios. A radiology resident, pediatric radiologist, and ophthalmic surgeon participated in a two-session observer study examining an internal test set, with or without model assistance. The area under the receiver operating characteristic curve (AUROC), sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and 95% confidence intervals (CIs) were obtained. Our proposed model detected orbital fractures with an AUROC of 0.802. The sensitivity, specificity, PPV, and NPV of the model achieved 65.8, 86.5, 70.9, and 83.5%, respectively. With model assistance, all values for orbital fracture detection improved for the ophthalmic surgeon, with a statistically significant difference in specificity (P < 0.001). For the radiology resident, specificity exhibited significant improvement with model assistance (P < 0.001). Our proposed model was able to identify orbital fractures on radiographs, reducing unnecessary CT scans and radiation exposure.
1 Introduction
Orbital fractures typically occur due to blunt force trauma, with the relatively thin structures of the orbital floor and medial wall making them more prone to fracture (Gerber et al., 2013). In young pediatric patients, the presence of relatively greater bone elasticity may be associated with “trapdoor” fractures; in these cases, in which the extraocular muscles become entrapped, urgent surgery to prevent permanent muscle damage is required (Joseph and Glavas, 2011). The gold standard modality to detect orbital fractures is thin-sliced high-resolution computed tomography (CT), which provides detailed images of the facial bones (Caranci et al., 2012). Although frequently used to detect orbital fractures, orbital radiographs present a relatively high false–negative rate, ranging (9–28) % (Iinuma et al., 1993).
Deep learning through artificial intelligence (AI) is rapidly advancing, and the medical field is no exception. Deep learning-based fracture detections in various locations, including the shoulder (Uysal et al., 2021), scaphoid (Ozkaya et al., 2022), ribs (Zhou et al., 2020), spine (Murata et al., 2020), and hip joint (Cheng et al., 2019), can achieve high accuracy, with sensitivity and specificity both reaching 91%. Advances in imagery analysis have demonstrated that computer models can assist, and even outperform humans in detecting features of radiographs (Soffer et al., 2019). These models use deep convolutional neural networks (DCNNs), which enable computers to learn features and data patterns that are not readily visible to the human eye. DCNN applications are increasingly used for disease detection and segmentation in medical image analysis. Building on these advances, the development of transformer architectures has further expanded the capabilities of medical image analysis. In particular, Vision Transformers (ViTs) have emerged as powerful alternatives to traditional DCNNs by processing image patches and capturing long-range dependencies through self-attention mechanisms (Dosovitskiy et al., 2020).
Recently, multi-input learning for medical images has gained increased interest. Multi-input models are designed to simultaneously analyze different data formats, such as different imaging modalities or resolutions. However, due to cost and/or time constraints, the simultaneous acquiring of different types of data is not always feasible. To address these limitations, some studies have focused on improving the performance of individual image data using cropping techniques. A two-stage network approach has been proposed (Park et al., 2019; Cho et al., 2021), in which the model first learns from small random patches of the original input images, and then performs transfer learning with whole images. However, this method requires two separate steps, and considerable training time.
This study assumed that DCNNsour multi-input ViT architecture combined with a novel cross-sequence learning technique could assist physicians in identifying orbital fractures and improve patient outcomes. Therefore, we developed and validated a straightforward deep learning-based multi-input model with cross-sequence learning to detect orbital fractures in the plain radiographs of patients under 20 years.
2 Materials and methods
2.1 Dataset
This retrospective study was approved by the Institutional Review Boards of Korea University Anam Hospital (IRB no. 2022AN0214) and Korea University Ansan Hospital (IRB no. 2022AS0130), and the requirement for informed consent was waived.
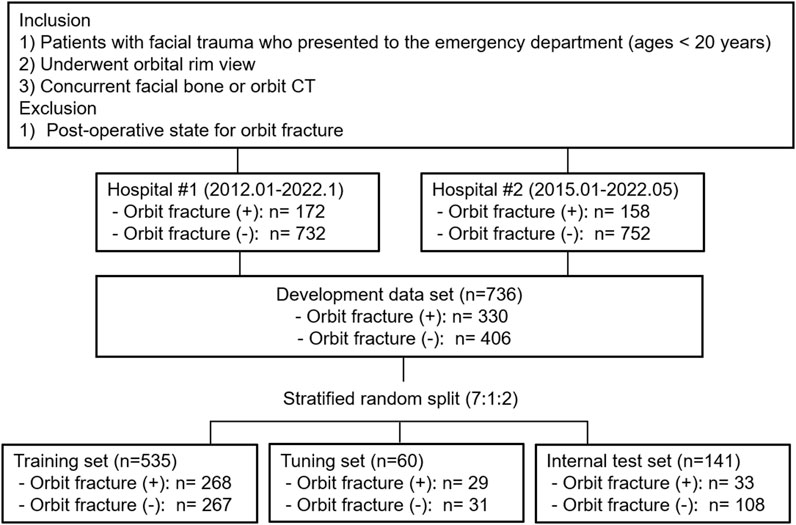
Orbit radiographs in DICOM format were collected from hospital #1 (January 2012 − January 2022), and from hospital #2 (January 2015 − May 2022). The inclusion criteria for the institution’s computerized medical databases were: 1) patients younger than 20 years who had facial trauma and presented to the emergency department, and 2) patients who underwent an orbital rim view and concurrent facial bone or orbit CT. Orbital rim view is an AP view of the orbit where the orbital rim aligned horizontal to the detector and the central ray enters the head at a 10–15°. CT images were obtained with various CT scanners at two institutions (hospital #1: Somatom Definition AS and Somatom Definition Flash, Siemens Healthcare, Forchheim, Germany, or Brilliance 64, Philips Healthcare, Amsterdam, Netherlands and hospital #2: Aquilion ONE, Toshiba, Minato, Japan or Revolution, GE Healthcare, Chicago, IL, USA). The most frequently used scanning parameters were as follows: tube voltage, 120 kVp; effective tube current, 250 mAs; section thickness, 2 mm; pitch, 0.8; rotation time, 1.0 s; and collimation, 128 × 0.6 mm.Patients with postoperative state for orbital fracture were excluded. The reference standard for orbital fracture diagnosis is facial bone or orbit CT. Two radiologists (S.O. and G.C., with 9 and 7 years experience, respectively, of pediatric imaging interpretation) were blinded to the clinical information, and reviewed the CT scans independently. They evaluated the presence of orbital fractures, and recorded their locations. The locations were classified as superior, medial, lateral, floor, or multiple. The reviewers resolved any disagreements by consensus. The development dataset was randomly split into training, tuning, and internal test sets in approximate ratios of 7:1:2 at the patient level, in a stratified manner based on the labels. Additionally, we employed stratified 10-fold cross validation. To improve training quality and balance, patients without orbital fractures were randomly selected for the training and tuning sets. Figure 1 presents the details of the data set.
2.2 Classification model
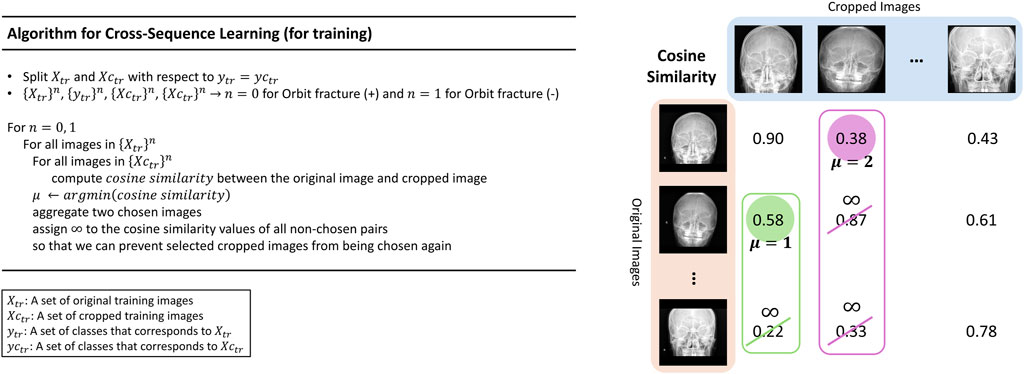
This study proposes a new learning technique termed cross-sequence learning for multi-input image classification models. The approach involves two parallel ViTs without specially designed feature fusion encoders, except for one concatenation layer. A cropped image based on the region of interest (ROI) was generated for each image. Each cropped image was generated by manually isolating only the head region based on the ROI and the orbital area, excluding the neck and any background elements. Since the cropping process only excludes regions irrelevant to diagnosis, the label of each cropped image remains consistent with its corresponding original image. Input image diversity was increased by matching different input types based on their lowest cosine similarity. To compare the performance of multi-input models with cross-sequence learning, single- and multi-input models without cross-sequence learning were designed, respectively.
Multi-input image classification models with cross-sequence learning use two images for each input: the original, and the cropped image based on the ROI (Figure 2). In a multi-input model with two ViTs in parallel, the first ViT processes the original image, while the second ViT processes the cropped image as the input. The features extracted from both ViTs are concatenated, and passed through the fully connected layers for binary classification. Cross-sequence learning consists of two steps (Figures 3, 4): Step 1 determines the pair of images by selecting the index of the cropped image having the lowest cosine similarity with each original image. In this step, we ensure that the matched images share the same given label to prevent mixing data from different classes during training, which may cause label confusion. It is also noteworthy that we calculated the cosine similarity based on the raw pixel values of each image. In step 2) prohibits the selected cropped images from being chosen again to increase input diversity. The matched original and cropped images were then combined and used as inputs for the multi-input classification model. Both the ViTs processed the original and the ROI-cropped images. The extracted features were concatenated sequentially for the final classification. Cross-sequence learning was not necessary for the validation and testing processes. The original and ROI-cropped images with the same indices were used as inputs for the multi-input model. More algorithmic details can be found in the Appendix.
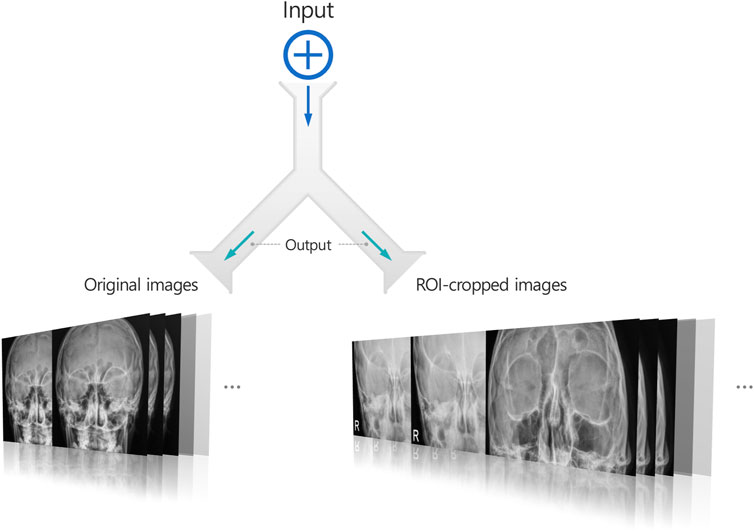
Figure 2. A multi-input image classification model generating two images for each input image: The original image and a cropped image based on the ROI.
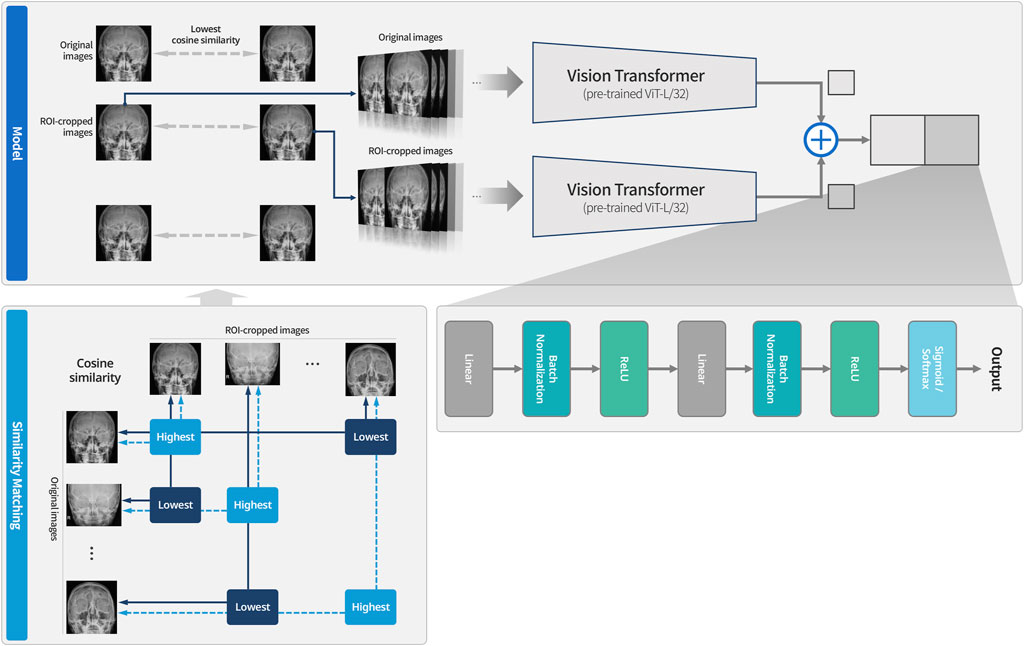
Figure 3. System overview of multi-input models with cross-sequence learning for orbital fracture detection. The dotted line indicates cases that could not be matched due to having the highest values, while the solid line represents cases that were matched because they had the lowest values. After the similarity matching process, the paired images are used as input to the model. Training is then conducted using the Vision Transformer for original images and the Vision Transformer for ROI-cropped images, respectively.
2.3 Clinical validation
Clinical validation was performed using internal validation test. Three readers participated in a two-session review of the orbital rim view: a pediatric radiologist with 9 years of experience (reader 1), an ophthalmic surgeon with 9 years of experience (reader 2), and a radiology resident with 2 years of experience (reader 3). Anonymized original DICOM files (excluding age and sex) were provided. Readers were informed that the study included young patients with facial trauma. Radiographs only were obtained in the first session. The second session was held 1 month after the first session. Readers were provided with model assistance, and the review order of the patients was altered. High-probability areas were highlighted as the most likely fracture sites in the original image. In both sessions, the readers recorded the final reading of each patient’s orbital fracture (with or without AI results) on a five-point scale (1 = definitely normal; 2 = probably normal; 3 = indeterminate; 4 = probable fracture; 5 = definite fracture).
2.4 Statistical analysis
Fracture detection accuracy was evaluated using the area under the receiver operating characteristic curve (AUROC), sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV). Optimal cutoff values for sensitivity and specificity were assessed using the Youden index (Youden, 1950), which is the point on the ROC that maximizes both sensitivity and specificity.
For human readers, AUROC values were obtained using five-point diagnostic confidence levels, and then dichotomized into normal (scores (1–3)) and fractured (scores 4 and 5) for binary diagnosis. Sensitivity, specificity, PPV, and NPV were obtained from the confusion matrices. The DeLong method (DeLong et al., 1988) was used to compare individual AUROC values, and McNemar’s test was used to compare the sensitivity and specificity values. A P value of less than 0.05 was considered statistically significant. MedCalc version 22.007 (MedCalc Software BVBA) was used for all statistical analyses.
3 Results
3.1 Patients
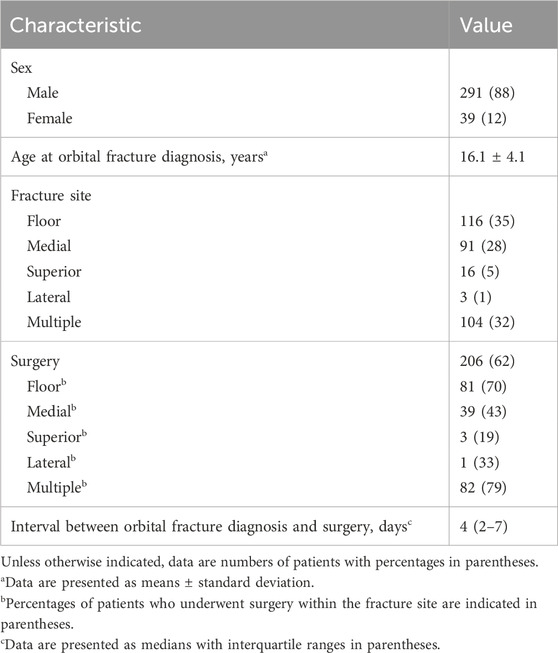
This study included 172 patients with orbital fracture, and 732 without, from Hospital #1; and 158 with, and 752 without, from Hospital #2. Of the 1,814 included radiographs (330 [18%] with orbital fracture, and 1,484 [82%] without), the most common site of orbital fractures was the orbital floor (35%, 116/330), followed by multiple fractures (32%, 104/330), and medial wall fractures (28%, 91/330). A total of 206 patients (62%) underwent surgery for orbital fracture repair, with a median interval of 4 days (interquartile range, 2–7 days) between diagnosis and surgery. The most common surgical site was multiple orbital walls (40%, 82/206), with 70% (81/116) of the patients undergoing surgery for orbital floor fractures (Table 1).
3.2 Standalone performance of the deep learning model
The single-input model created with ViT achieved an AUROC of 0.670 and a specificity of 0.871 (Table 2; Figure 5), and a low sensitivity (0.387), with PPV of 0.600 and an NPV of 0.740. The multi-input model without cross-sequence learning showed improved sensitivity (0.580) and AUROC (0.800), with slightly improved PPV and NPV (0.666 and 0.803, respectively).
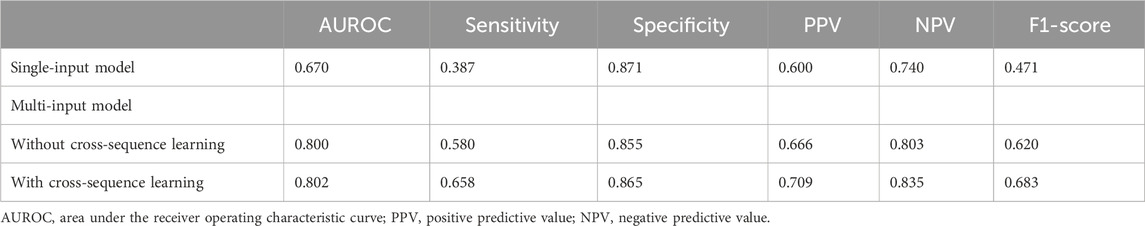
Table 2. Model performance to detect orbital fractures according to input and cross-sequence learning.
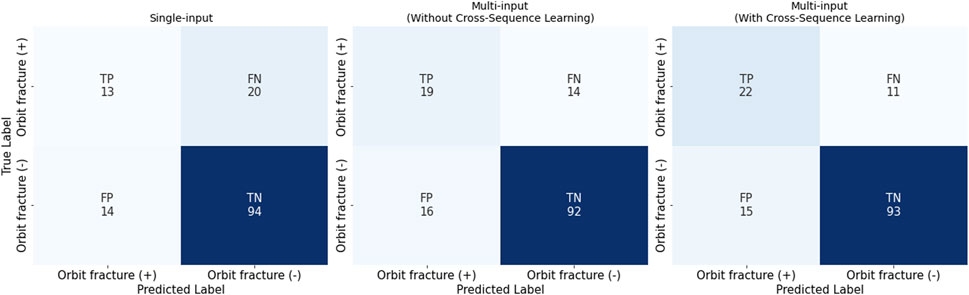
Figure 5. Confusion Matrices for the Three Settings: single-input, multi-input without/with cross-sequence learning. It is noteworthy that we have conducted stratified 10-fold cross validation.
Multi-input image classification models using cross-sequence learning matched the original images with the cropped images of other patients to increase learning diversity. By setting the number of epochs to 400, all values exhibited slight improvement (AUROC, 0.802; sensitivity, 0.658; specificity, 0.865; PPV, 0.709; NPV, 0.835). Figure 6 presents the representative true–positive and true–negative cases from the internal test set.
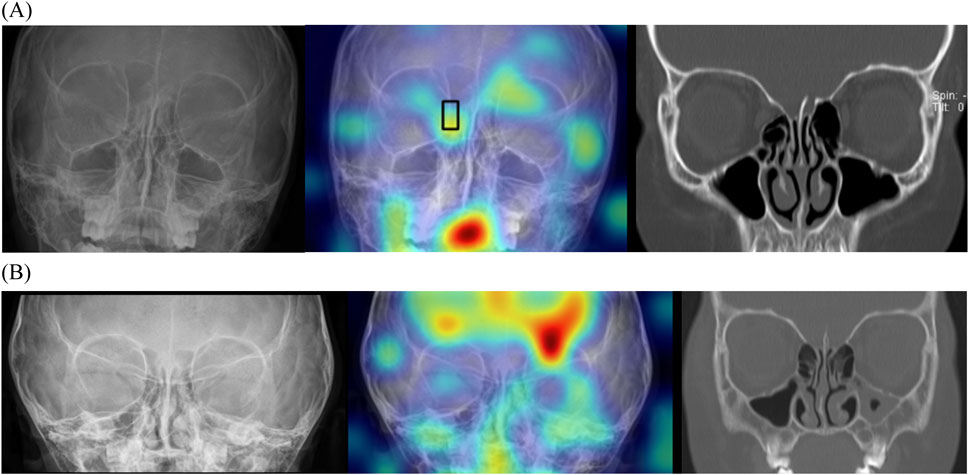
Figure 6. Localizing fracture sites using gradient-weighted class activation mapping. (A) Representative true-positive case of a 10-year-old boy with orbital fracture. Right medial wall fracture of the orbit was correctly localized by the model (black box). (B) Representative true-negative case of a 3-year-old girl without an orbital fracture. This model does not identify any fractures; therefore, no bounding box is offered.
3.3 Observer performance with and without deep learning model assistance
Table 3 and Figure 7 show the diagnostic performance of human readers in the internal test set with, and without, model assistance. In the first session, reader AUROCs ranged (0.611–0.676). The sensitivity and specificity of the observers ranged (38.7–64.5) %, and (63.6–80.9) %, respectively. The range of PPV of the readers was relatively low at (28.6–38.2) %, compared to that of NPV at (80.8–86.4) %.
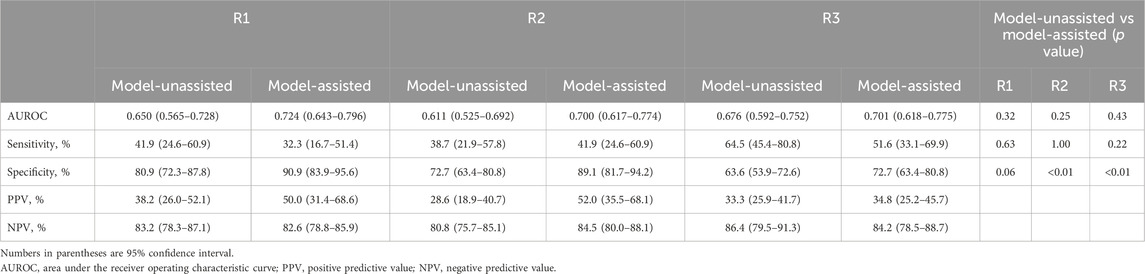
Table 3. Diagnostic performance of human readers in the external test set with and without the model’s assistance.
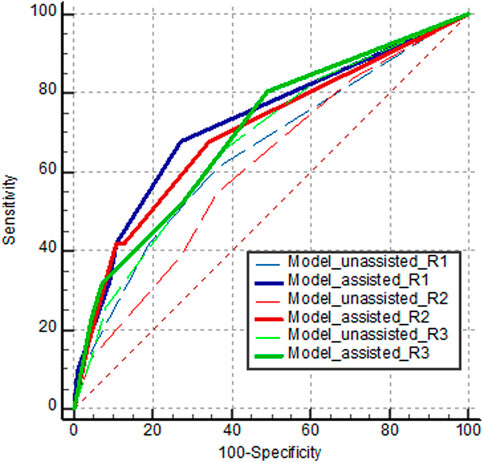
Figure 7. AUROC curves of human readers diagnosing orbital fracture on the internal test set. Dashed and solid lines indicate the first session without model assistance and the second session with model assistance, respectively.
In the second session with model assistance, some performance parameters improved for some readers. All readers exhibited higher specificities (improvements of (9.1–16.4) %), with statistical significance for two readers (P < 0.001 for reader 2, and P < 0.001 for reader 3). Compared to the first session, all readers showed AUROC improvements (reader 1: 0.075; 95% confidence interval [CI], (−0.073–0.222); P = 0.323; reader 2: 0.089; 95% CI, (−0.061–0.240); P = 0.245; reader 3: 0.025; 95% CI, (−0.038–0.088); P = 0.432), without statistical significance. Readers 1 and 2 yielded significant improvements in PPV at (11.8 and 23.4) %, respectively, whereas reader 3 exhibited minimal improvement in PPV (1.5%).
4 Discussion
This study developed and validated deep learning-based models to differentiate normal and fractured orbits on plain radiographs. With an AUROC of 0.802, the results of our multi-input model with cross-sequence learning suggest that deep learning methods, such as those analyzing orbital fractures, can detect bone fractures that are difficult for human readers to evaluate. This study also demonstrated the feasibility and clinical validity of a deep learning algorithm to diagnose orbital fractures on plain radiographs. Several recent studies have applied convolutional neural network training models to detect different types of fractures in radiographs (Yang et al., 2020; Gan et al., 2019). Their performance in long bone or limb joint fractures achieved excellent accuracy of approximately 90% (Lindsey et al., 2018; Chung et al., 2018; Pelka et al., 2018; Kuo et al., 2022). Few studies have investigated the pediatric population (Choi et al., 2020; Hayashi et al., 2022; Zech et al., 2023), and only one study involved fractures other than those in the long bones (Choi et al., 2022). To the best of our knowledge, this is the first study to develop a deep neural network model to detect orbital fractures on plain radiographs in a population under 20 years. Li et al. reported an AUROC of 0.958 to detect orbital fractures using orbital CT scans in an adult population (Li et al., 2020).
Orbital fractures have various presentations and clinical severities. The anatomical complexity of the orbital and intraorbital structures also creates confusion (Caranci et al., 2012). Radiography has a sensitivity of (64–78) % for fractures. Currently, radiographic examination of the orbits is rarely performed (Iinuma et al., 1994). CT is considered the imaging modality of choice to evaluate orbital trauma (Kubal, 2008). Three-dimensional reformation is a useful tool for guide treatment (Rhea et al., 1999), though it requires prolonged hospitalization and radiation exposure. Pediatric orbital fractures differ from those in adults, with diplopia, muscle entrapment, and trapdoor configuration fractures being more common in children (Lane et al., 2007). Urgent surgery is indicated to prevent soft tissue scarring and its long-term sequelae (Bansagi and Meyer, 2000). Our study demonstrated a short interval between orbital fracture diagnosis and surgery in clinical practice. Additionally, orbital fractures were present in only 18% of the patients who underwent CT at our institutions, suggesting unnecessary radiation exposure. If radiography could be used to identify patients without fractures, the number of unnecessary CT scans and radiation could be reduced.
Deep learning-based models can learn features and data patterns that are invisible to the human eye. Training deep learning-based models for medical image analysis requires access to substantial, high-quality, and well-annotated datasets. A multi-input approach was proposed to improve the quality of the dataset. This approach can be applied to various tasks that include image classification, segmentation, and restoration (Duarte et al., 2018; Chen et al., 2022; Yang et al., 2022). For example, a multi-modal fusion method (Chen et al., 2022) combines the analysis of images, graphs, and genomic data, whereas a multi-resolution fusion model (Yang et al., 2022) examines the same object from various perspectives. However, due to cost and/or time constraints, most multi-input models that simultaneously obtain different types of data are infeasible. Furthermore, most multi-input models typically involve specialized preceding architectures for feature fusion. Previous studies (Duarte et al., 2018; Chen et al., 2022) introduced Siamese networks and Kronecker products for multi-input image analysis. However, when compared to single models, these models tend to be more complex. Other studies have proposed a two-stage network approach (Park et al., 2019; Cho et al., 2021), in which the model first learns from small random patches of the original input images, and then performs transfer learning with whole images. Nevertheless, this method requires two separate steps and considerable training time, as sufficient patches are required to achieve a meaningful performance.
This study developed a multi-input ViT architecture with a similarity-matching mechanism to identify the original and ROI-cropped images with the lowest cosine similarity for multi-input image classification. Our experiments validate the effectiveness of the proposed framework using two different datasets. We also considered that similarity matching could reduce unintended errors, and provide important information regarding ROIs. This matching can quantitatively and qualitatively enhance the performance of multi-input models. Future work will incorporate graph neural networks to create a trainable similarity function rather than a cosine similarity function, and use multiple parallel architectures to generate cropped images of various sizes from the original images.
This study has limitations. Our models were trained on large datasets from two academic institutions. Hence, to improve the accuracy and generalization of models, further assessment of large datasets from other centers is required. Despite balancing the training and tuning sets, our model exhibited a relatively low sensitivity, possibly due to the small number of orbital fractures included in the study. However, the improved sensitivity of multi-input models with cross-sequence learning indicates that our proposed models can detect non-visible fractures. Although the sensitivity of our model is relatively low, it remains comparable to that of expert clinicians. We anticipate that, in emergency settings, our model could still provide more reliable results than those obtained by individuals who are less experienced in interpreting orbital radiographs. Finally, as not all patients with facial trauma undergo both orbital radiography and CT, indication bias is another potential limitation, considering that the patients included in the training set had a high likelihood of orbit fractures and underwent CT.
In this study, our multi-input ViTs with cross-sequence learning method were developed to identify orbital fractures using radiography. Sensitivity and specificity at encouraging levels were achieved, suggesting that the models can detect bone fractures that are difficult for human readers to evaluate. This study also determined that the multi-input models with cross-sequence learning could improve the detection of fractures that are not readily visible to physicians. This enhanced diagnostic capacity can help solve medical problems with high monetary or quality-of-life costs, and improve fracture care.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Korea University Anam Hospital (IRB no. 2022AN0214) and Korea University Ansan Hospital (IRB no. 2022AS0130). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because This study was a retrospective analysis that posed no risk to the participants; therefore, informed consent was waived. Written informed consent was not obtained from the minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article because the likelihood of identifying patients from their X-ray images is extremely low, the requirement for informed consent was waived.
Author contributions
JK: Data curation, Formal Analysis, Validation, Writing – original draft. SL: Investigation, Methodology, Visualization, Writing – original draft. SA: Validation, Writing – review and editing. GC: Resources, Writing – review and editing. B-KJ: Supervision, Writing – review and editing. BP: Funding acquisition, Supervision, Writing – review and editing. YC: Funding acquisition, Investigation, Methodology, Visualization, Writing – review and editing. SO: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Methodology, Resources, Validation, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by a grant from Korea University, Seoul, Republic of Korea (grant number: K2314141, K2426651), a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (grant number: HR22C1302) and the Ministry of Education of the Republic of Korea and the National Research Foundation of Korea (grant number: NRF-RS-2023-00239603, RS-2023-00218176).
Acknowledgments
Kyung Sook Yang (Department of Biostatistics, Korea University College of Medicine) kindly provided statistical advice for this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bansagi, Z. C., and Meyer, D. R. (2000). Internal orbital fractures in the pediatric age group: characterization and management. Ophthalmology 107, 829–836. doi:10.1016/s0161-6420(00)00015-4
Caranci, F., Cicala, D., Cappabianca, S., Briganti, F., Brunese, L., and Fonio, P. (2012). Orbital fractures: role of imaging. Semin. Ultrasound CT MR 33, 385–391. doi:10.1053/j.sult.2012.06.007
Chen, R. J., Lu, M. Y., Wang, J., Williamson, D. F. K., Rodig, S. J., Lindeman, N. I., et al. (2022). Pathomic fusion: an integrated framework for fusing histopathology and genomic features for cancer diagnosis and prognosis. IEEE Trans. Med. Imaging 41, 757–770. doi:10.1109/tmi.2020.3021387
Cheng, C. T., Ho, T. Y., Lee, T. Y., Chang, C. C., Chou, C. C., Chen, C. C., et al. (2019). Application of a deep learning algorithm for detection and visualization of hip fractures on plain pelvic radiographs. Eur. Radiol. 29, 5469–5477. doi:10.1007/s00330-019-06167-y
Cho, Y., Park, B., Lee, S. M., Lee, K. H., Seo, J. B., and Kim, N. (2021). Optimal number of strong labels for curriculum learning with convolutional neural network to classify pulmonary abnormalities in chest radiographs. Comput. Biol. Med. 136, 104750. doi:10.1016/j.compbiomed.2021.104750
Choi, J. W., Cho, Y. J., Ha, J. Y., Lee, Y. Y., Koh, S. Y., Seo, J. Y., et al. (2022). Deep learning-assisted diagnosis of pediatric skull fractures on plain radiographs. Korean J. Radiol. 23, 343–354. doi:10.3348/kjr.2021.0449
Choi, J. W., Cho, Y. J., Lee, S., Lee, J., Lee, S., Choi, Y. H., et al. (2020). Using a dual-input convolutional neural network for automated detection of pediatric supracondylar fracture on conventional radiography. Invest Radiol. 55, 101–110. doi:10.1097/rli.0000000000000615
Chung, S. W., Han, S. S., Lee, J. W., Oh, K. S., Kim, N. R., Yoon, J. P., et al. (2018). Automated detection and classification of the proximal humerus fracture by using deep learning algorithm. Acta Orthop. 89, 468–473. doi:10.1080/17453674.2018.1453714
DeLong, E. R., DeLong, D. M., and Clarke-Pearson, D. L. (1988). Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44, 837–845. doi:10.2307/2531595
Dosovitskiy, A., Beyer, L., Kolesnikov, A., Weissenborn, D., Zhai, X., Unterthiner, T., et al. (2020). An image is worth 16x16 words: transformers for image recognition at scale. arXiv Prepr. Arxiv2010.11929. doi:10.48550/arXiv.2010.11929
Duarte, D., Nex, F., Kerle, N., and Vosselman, G. (2018). Multi-resolution feature fusion for image classification of building damages with convolutional neural networks. Remote Sens. 10, 1636. doi:10.3390/rs10101636
Gan, K., Xu, D., Lin, Y., Shen, Y., Zhang, T., Hu, K., et al. (2019). Artificial intelligence detection of distal radius fractures: a comparison between the convolutional neural network and professional assessments. Acta Orthop. 90, 394–400. doi:10.1080/17453674.2019.1600125
Gerber, B., Kiwanuka, P., and Dhariwal, D. (2013). Orbital fractures in children: a review of outcomes. Br. J. Oral Maxillofac. Surg. 51, 789–793. doi:10.1016/j.bjoms.2013.05.009
Hayashi, D., Kompel, A. J., Ventre, J., Ducarouge, A., Nguyen, T., Regnard, N. E., et al. (2022). Automated detection of acute appendicular skeletal fractures in pediatric patients using deep learning. Skelet. Radiol. 51, 2129–2139. doi:10.1007/s00256-022-04070-0
Iinuma, T., Hirota, Y., and Ishio, K. (1994). Orbital wall fractures. Conventional views and CT. Rhinology 32, 81–83.
Iinuma, T., Ishio, K., Yoshinami, H., Kuriyama, J., and Hirota, Y. (1993). Orbital wall fractures: a comparison of computed tomography and conventional views. Nihon Jibiinkoka Gakkai Kaiho 96, 175–181,361. doi:10.3950/jibiinkoka.96.175
Joseph, J. M., and Glavas, I. P. (2011). Orbital fractures: a review. Clin. Ophthalmol. 12, 95–100. doi:10.2147/opth.s14972
Kubal, W. S. (2008). Imaging of orbital trauma. Radiographics 28, 1729–1739. doi:10.1148/rg.286085523
Kuo, R. Y. L., Harrison, C., Curran, T. A., Jones, B., Freethy, A., Cussons, D., et al. (2022). Artificial intelligence in fracture detection: a systematic review and meta-analysis. Radiology 304, 50–62. doi:10.1148/radiol.211785
Lane, K., Penne, R. B., and Bilyk, J. R. (2007). Evaluation and management of pediatric orbital fractures in a primary care setting. Orbit 26, 183–191. doi:10.1080/01676830701519374
Li, L., Song, X., Guo, Y., Liu, Y., Sun, R., Zou, H., et al. (2020). Deep convolutional neural networks for automatic detection of orbital blowout fractures. J. Craniofac Surg. 31, 400–403. doi:10.1097/scs.0000000000006069
Lindsey, R., Daluiski, A., Chopra, S., Lachapelle, A., Mozer, M., Sicular, S., et al. (2018). Deep neural network improves fracture detection by Clinicians. Proc. Natl. Acad. Sci. U. S. A. 6, 11591–11596. doi:10.1073/pnas.1806905115
Murata, K., Endo, K., Aihara, T., Suzuki, H., Sawaji, Y., Matsuoka, Y., et al. (2020). Artificial intelligence for the detection of vertebral fractures on plain spinal radiography. Sci. Rep. 18, 20031. doi:10.1038/s41598-020-76866-w
Ozkaya, E., Topal, F. E., Bulut, T., Gursoy, M., Ozuysal, M., and Karakaya, Z. (2022). Evaluation of an artificial intelligence system for diagnosing scaphoid fracture on direct radiography. Eur. J. Trauma Emerg. Surg. 48, 585–592. doi:10.1007/s00068-020-01468-0
Park, B., Cho, Y., Lee, G., Lee, S. M., Cho, Y. H., Lee, E. S., et al. (2019). A curriculum learning strategy to enhance the accuracy of classification of various lesions in Chest-PA X-ray screening for pulmonary abnormalities. Sci. Rep. 25, 15352. doi:10.1038/s41598-019-51832-3
Pelka, O., Nensa, F., and Friedrich, C. M. (2018). Annotation of enhanced radiographs for medical image retrieval with deep convolutional neural networks. PLoS One 12, e0206229. doi:10.1371/journal.pone.0206229
Rhea, J. T., Rao, P. M., and Novelline, R. A. (1999). Helical CT and three-dimensional CT of facial and orbital injury. Radiol. Clin. North Am. 37, 489–513. doi:10.1016/s0033-8389(05)70108-1
Soffer, S., Ben-Cohen, A., Shimon, O., Amitai, M. M., Greenspan, H., and Klang, E. (2019). Convolutional neural networks for radiologic images: a radiologist's guide. Radiology 290, 590–606. doi:10.1148/radiol.2018180547
Uysal, F., Hardalac, F., Peker, O., Tolunay, T., and Tokgoz, N. (2021). Classification of shoulder X-ray images with deep learning ensemble models. Appl. Sci. 11, 2723. doi:10.3390/app11062723
Yang, S., Yin, B., Cao, W., Feng, C., Fan, G., and He, S. (2020). Diagnostic accuracy of deep learning in orthopaedic fractures: a systematic review and meta-analysis. Clin. Radiol. 75, 713.e17–713.e28. doi:10.1016/j.crad.2020.05.021
Yang, S. X., Wu, X., Ge, S., Zhou, S. K., and Xiao, L. (2022). Knowledge matters: chest radiology report generation with general and specific knowledge. Med. Image Anal. 80, 102510. doi:10.1016/j.media.2022.102510
Youden, W. J. (1950). Index for rating diagnostic tests. Cancer 3, 32–35. doi:10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3
Zech, J. R., Carotenuto, G., Igbinoba, Z., Tran, C. V., Insley, E., Baccarella, A., et al. (2023). Detecting pediatric wrist fractures using deep-learning-based object detection. Pediatr. Radiol. 53, 1125–1134. doi:10.1007/s00247-023-05588-8
Zhou, Q. Q., Wang, J., Tang, W., Hu, Z. C., Xia, Z. Y., Li, X. S., et al. (2020). Automatic detection and classification of rib fractures on thoracic CT using convolutional neural network: accuracy and feasibility. Korean J. Radiol. 21, 869–879. doi:10.3348/kjr.2019.0651
Appendix
Configuration of Vision Transformer (ViT): We use the pre-trained ViT-L/32 as our backbone network, which is a large variant of Vision Transformer with a patch size of
Feature Representations: We extract the full sequence of patch token embeddings from the final transformer layer of ViT-L/32, excluding the CLS token. Especially, we extract features from the last normalization layer of ViT, before the final attention block. An input resolution of
Concatenating two outputs of ViTs: We concatenate the outputs along the patch dimension, resulting in a combined shape of
Final Classifier Architecture, Final Classifier Architecture: The concatenated feature vector is fed into a fully connected (FC) classifier with the following architecture.
(1) FC Layer (
(2) FC Layer (
(3) FC Layer (
Training details: The goal of our experiments is to reduce the binary cross entropy loss, using a batch size of 64 and an initial learning rate of 0.001. We use the Adam optimizer and apply learning rate decay via a LambdaLR scheduler, which multiplies the learning rate by 0.95 after every epoch. The training is conducted for 100 epochs. All experiments are performed on a GPU server running Ubuntu 20.04 with CUDA 11.2 and three 24 GB Titan RTX graphics cards. All models are implemented using PyTorch 1.8.0.
Data Preprocessing: For all images, including cropped ones, we apply standard scaling and then resize images to
Algorithmic Details of Cosine Similarity-based Matching: The entire pipeline is illustrated in Figure 4, along with the algorithm and corresponding graphics.
Keywords: orbital fractures, artificial intelligence, deep learning, radiography, pediatrics
Citation: Kim J, Lee S, Ahn SM, Choi G, Je B-K, Park BJ, Cho Y and Oh S (2025) Deep learning model using cross-sequence learning to identify orbital fractures in radiographs of patients under 20 Years. Front. Bioeng. Biotechnol. 13:1613417. doi: 10.3389/fbioe.2025.1613417
Received: 17 April 2025; Accepted: 17 July 2025;
Published: 02 September 2025.
Edited by:
Farzad Pakdel, Tehran University of Medical Sciences, IranReviewed by:
Mehrnoush Momeni Roochi, TUMS, IranHuan-Chih Wang, National Taiwan University Hospital, Taiwan
Copyright © 2025 Kim, Lee, Ahn, Choi, Je, Park, Cho and Oh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Saelin Oh, bmlub2xpbjJAZ21haWwuY29t; Yongwon Cho, ZHJhZ29uMXdvbkBzY2guYWMua3I=
†These authors have contributed equally to this work and share first authorship
 Joohui Kim1†
Joohui Kim1† Yongwon Cho
Yongwon Cho Saelin Oh
Saelin Oh