- 1Department of Orthopaedics, The Second Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 2National Engineering Research Center for Tissue Restoration and Reconstruction, South China University of Technology, Guangzhou, China
Bone infection is a disease with high treatment cost, long period and high disability rate in orthopedics. The development of antibacterial implant materials in vivo can alleviate the pain of patients and social burden. How to make the antibacterial substances in the implant materials release regularly as needed is a key technical breakthrough that antibacterial bone repair implants have been trying to optimize. It has been proved that metal silver ions sputtering can form higher specific surface area on the surface of implanted materials, and can produce electron biological effect to realize in situ sterilization. Antibacterial peptide crosslinked with hyaluronic acid can be hydrolyzed by hyaluronidase during bacterial infection, thus killing free bacteria, producing immune regulation. At present, it is planned to use titanium dioxide nanotubes to construct a double-layer nanotube structure, fill osteogenic drugs into the nanotubes by vacuum-assisted physical adsorption, and sputter deposit metal silver ions on the surface of the nanotubes. The outer layer of the material is prepared by covalent grafting of antibacterial peptides and hyaluronic acid to prepare a layer-by-layer assembly technology shell to prepare a bone implant scaffold material with dual sterilization response systems of electronic biological antibacterial response and enzymatic hydrolysis antibacterial response.
Introduction
After the aging of the population, the problem of bone injury caused by external violence and internal inflammation, tumor, osteoporosis and other diseases is becoming more and more serious. Regeneration and repair after bone injury is a complex physiological process including reconstruction and replacement of damaged tissue.
From the occurrence of bone injury to the final healing, the whole process can be roughly divided into three stages: inflammation stage, repair stage and remodeling stage. Although the repair can be divided into three stages according to the main events during the repair of bone injury, these three stages are not strictly distinguished and do not exist completely independently. As a continuous process, these events will occur simultaneously at the junction in different stages of bone injury repair (Wang et al., 2020; Claes et al., 2012). For example, when the inflammatory period and the repair period are in transition, although the activity of immune cells has been relatively reduced, the gradually accelerated proliferation of osteoblasts in the repair is still accompanied by the cellular behavior of immune cells to help repair various damaged tissues at the injury site. To sum up, the implantation of bone repair materials and their corresponding functions in different periods will affect the bone repair process in different periods and produce different effects.
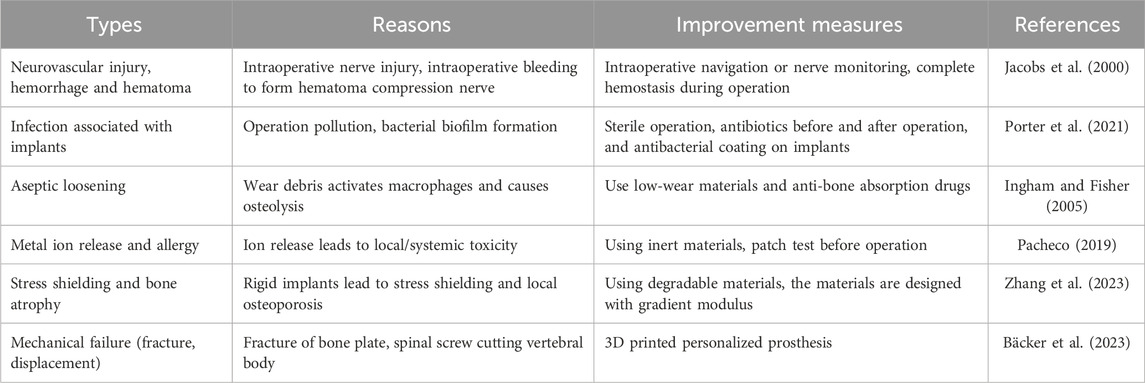
With the aging population and the increasing incidence of metabolic diseases related to bone repair, such as diabetes, the application and demand of various bioactive materials in bone defect repair-related surgery are increasing, and the types of materials that can be used to promote bone repair and regeneration are also increasing (Guo et al., 2023; Saul and Khosla, 2022). Appropriate bioactive materials can increase the adhesion, differentiation and proliferation of cells by combining with macromolecules, cells and other elements, and finally promote bone repair and regeneration (Tang et al., 2021; Sovova et al., 2021). Bone repair materials lacking biological activity are at risk of bacterial infection after implantation, and also face the problem of poor bone integration (Table 1) (Jacobs et al., 2000; Porter et al., 2021; Ingham and Fisher, 2005; Pacheco, 2019; Zhang et al., 2023; Bäcker et al., 2023). In the failure cases of implant materials, 25% cases were caused by bacterial infection, and 16% cases were caused by loose implant materials (Bozic et al., 2010). For implant surgery, bacterial infection is a very serious clinical problem. When bacterial infection occurs at the implant site, it will cause a series of serious complications for patients, and may even lead to sepsis. Poor osseointegration will loosen the implant material, which is also the key factor to cause the failure of the implant material (Raphel et al., 2016). In addition, it is found that when bacteria are infected, the environment of inflammation and bone absorption will lead to the wear of implant materials and produce particles, which will lead to poor bone integration. However, the surface of implant materials with poor osseointegration is more likely to adhere to bacteria, which leads to more bacteria proliferation on the surface and more serious bacterial infection (Costerton et al., 1999). These two factors cooperate with each other to further improve the failure rate of implant materials. Therefore, designing functional implant materials that can prevent bacterial infection and promote bone integration is the key to successful surgery.
Porous structure can make tissues and cells grow, adhere and maintain cell activity in the material space, and even form osteon structure, which is beneficial to enhance the stable combination of materials and body tissues and avoid the problems of loosening and falling off after transplantation (Sovova et al., 2021; Dec et al., 2022). Scaffold material is an important center of tissue engineering bone. The ideal scaffold needs to simulate the three-dimensional structure of extracellular matrix (ECM), and the best bone repair material should have the following advantages: Firstly, the scaffold needs to own good biocompatibility to support the adhesion and proliferation of osteoblasts. Secondly, it must have good mechanical properties. At the same time, it is necessary to have pore connectivity suitable for transporting nutrients and oxygen. Moreover, it should also meet the requirements of signal molecules that are conducive to the directional differentiation of cells into ideal types of morphology, so as to promote cell adhesion, proliferation, metabolism and differentiation. It should also have the function of promoting vascularization to meet the nutritional supply and waste removal of tissue growth. Degradable scaffold materials also need to meet the biodegradability or bioresorbability, so as to provide growth space for new bone tissue (Li et al., 2017; Alghamdi and Jansen, 2020).
Inorganic materials include metallic materials and nonmetallic materials. Compared with natural materials, metal materials are widely used in bone repair because of their excellent hardness and stiffness mechanical properties, especially when the bone tissue to be repaired still needs to bear strong pressure. Available metal materials include titanium (Ti), tantalum, cobalt, magnesium alloy, etc (Niinomi, 2008; Elias et al., 2008; Albrektsson and Johansson, 2001). When pure metal is transplanted into the body bone environment, it will face some problems, such as insufficient flexibility, corrosion of metal body, failure to form a stable link with bone tissue cells, etc. In order to solve these pain points, methods such as coating alloy materials with metal surface is easy to establish tissue connectors have been developed. Bioactive materials can promote the rate and efficiency of inflammatory reaction, bone formation, angiogenesis and other processes in bone injury repair by influencing immune cells, bone formation-related cells, osteoblast progenitor cells, vascular endothelial cells and so on (Wei et al., 2023; Zhao et al., 2023). At present, a large number of studies on Ti materials show that the rough surface of Ti can improve the integration of exogenous implant materials and bulk bone tissue, and promote the osteogenic differentiation of mesenchymal stem cells (MSCs) compared with smooth surfaces (Vermeulen et al., 2022; Solier et al., 2023; Buser et al., 2004). The regulation of cell differentiation ability by surface roughness treatment of this metal material may be realized by directly changing cell shape and adhesion state and further affecting integrin signaling pathway (Dalby et al., 2007). Anodizing is the most commonly used way to modify Ti-based surface at present. Anodizing can form titanium dioxide (TiO2) nanotubes on Ti and its surface. By adjusting the time and voltage of anodizing, a multi-layer nanotube structure can be constructed on Ti-based surface.
Although artificial bone repair materials have the advantages of flexible design and considerable output, there are still some problems such as lack of activity and insufficient bone conductivity. The research and development of ideal artificial bone replacement materials is still one of the main challenges facing clinical and basic research in orthopedics (Bharadwaz and Jayasuriya, 2020; Bashir et al., 2023). At present, it is the preferred strategy in the field of bone tissue engineering to combine various materials and combine their advantages to prepare scaffolds with biological activity, suitable porosity and excellent mechanical properties. Growth factors play an important regulatory role in the process of bone formation, reconstruction and regeneration, mainly focusing on enhancing the biological functions of bone grafts, such as the recruitment of endogenous MSCs, endothelial cell migration and osteogenic differentiation (Martino et al., 2015). Different growth factors are needed in different stages of bone regeneration, so different kinds of growth factors can be loaded in different materials and play a role in time, thus playing a role in specific stages, simulating the bone regeneration process more accurately and obtaining better functional performance in vivo. The controlled release of drugs and bioactive substances plays an important role in bone defect repair, and it is particularly critical to realize controlled and sustainable delivery through reasonable material structure design, which can be combined with bioactive factors to promote bone repair.
At present, the clinically approved orthopedic antibacterial implant system mainly covers joint replacement, trauma fixation and spinal fusion, and its antibacterial mechanism involves antibacterial coating, sustained release of antibiotics and material modification. Antibiotic joint cement can slowly release antibiotics and reduce the risk of postoperative infection (Xu et al., 2020). Antibacterial coating orthopedic implants can play a role through material surface coating and inhibit biofilm formation through contact sterilization (Akay and Yaghmur, 2024). Absorbable antibacterial internal fixation system can continuously release antibacterial components through degradation process (Lin Z. et al., 2019). The composite antibacterial bone repair material can combine drug loading and antibacterial, and has both bone conduction and antibacterial functions (Li et al., 2022). Among them, the currently approved antibacterial implants are mainly antibiotic bone cement and antibacterial coated metal implants. The extensive use of antibiotics in orthopedic perioperative period can easily lead to bacterial resistance and increase the economic burden of patients (Parvizi et al., 2010). Therefore, it is the pursuit and vision of many scientists at home and abroad to develop antibacterial bone repair materials through non-antibiotic routes. At present, non-antibiotic loaded bone repair materials mainly use composite inorganic antibacterial agents, composite organic antibacterial agents and bionic nano-structured surface antibacterial agents to achieve antibacterial function. The growth rate of bone tissue is slow, so it is challenging to treat bone defects and other related diseases through self-repair. The risk of bacterial infection in bone injury is as high as 15%–55% (Morgenstern et al., 2018), while the infection rate of bone joint implantation can reach 4.9% (Kazimierczak et al., 2022). More serious bone infection even requires amputation. The most effective way to solve this problem is that bone repair materials have antibacterial properties themselves.
After biomaterials are implanted into the body, biological cascade reactions such as bacterial adhesion-biofilm formation and cell adhesion-proliferation-differentiation-tissue formation all occur at the material interface (Raphel et al., 2016; Kazimierczak et al., 2022; Kaneko et al., 2021; Busscher et al., 2012). The interface design of material is very important for the research and development of biomaterials and implant equipment. The adhesion of bacteria and cells on the surface of implants is associated and competitive. Gristina put forward the theory of “race for the surface”, pointing out that bacteria and cells compete for living space on the surface of implants (Gristina, 1987). If the host cells can reach and occupy the implant surface, it can not only achieve stronger tissue integration at first, but also establish a defense barrier to resist microbial adhesion and reproduction. After bacteria adhere and stabilize, biofilm will form within 12–18 h, leading to implant infection. In the process of biofilm formation, bacterial colonies will form and secrete polysaccharide layers to protect them from host immune response and attack of therapeutic drugs. The dosage of antibiotics required to kill bacteria in biofilm is 1,000 times higher than that required to kill suspended/initial adhesion bacteria (Hetrick and Schoenfisch, 2006). Inhibition of initial bacterial adhesion is the core of antibacterial implant design. It is very important to optimize the implant interface design under the guidance of the core, endow the implant with excellent antibacterial performance and promote the integration of the implant with surrounding bone.
The ideal graft material should have osteoinductivity, osteoconductivity and osseointegration, good biocompatibility and biodegradability, and meet the requirements of mechanical properties as well as biological properties (Holzapfel et al., 2017). A single material is compounded into a composite material with better mechanical properties by bioengineering technology, and the prepared biological scaffold is supplemented with cytokines to improve its biological properties, which can greatly meet the microenvironment requirements of bone regeneration (Cunniffe et al., 2017).
Hypothesis
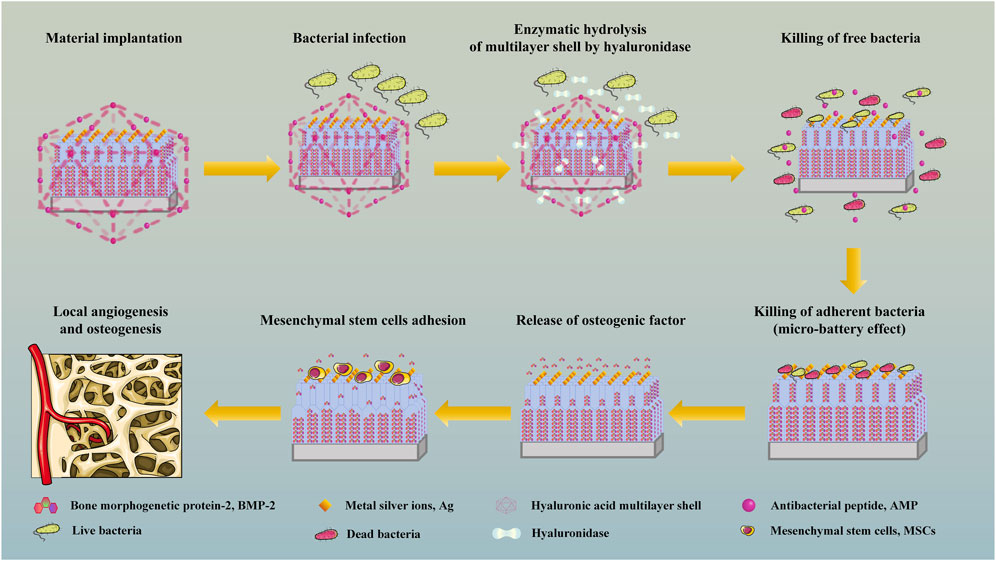
TiO2 nanotubes are prepared on the surface of Ti by anodic oxidation and construct a double-layer nanotube structure, and osteogenic drugs are filled into the nanotubes by vacuum-assisted physical adsorption, so as to realize long-term sustained release of osteogenic drugs and promote continuous osteogenesis. Metal silver ions (Ag) are deposited on the surface of the material by sputtering, which makes the implanted material have higher specific surface area, produces electron biological effect, realizes in situ sterilization and promotes angiogenesis. The outer layer of the material is prepared from covalently grafted antibacterial peptide (AMP) and hyaluronic acid (HA) by layer-by-layer assembly technology (LBL). When local bacterial infection occurs, the multilayer shell will be hydrolyzed by enzymes secreted by bacteria, thus releasing AMP to kill free bacteria, produce immune regulation and repair tissue damage (Figure 1). The antibacterial material has a dual sterilization response system of electronic biological antibacterial response and enzymatic hydrolysis antibacterial response, which can eradicate planktonic bacteria and adherent bacteria at the injured part after the material is implanted, assist the double-layer tubular structure to continuously and fully release osteogenic drugs, and assist the sputtering deposition ion structure with high specific surface area on the surface of the material to accelerate the formation of in situ bone tissue in multiple directions (Figure 2).
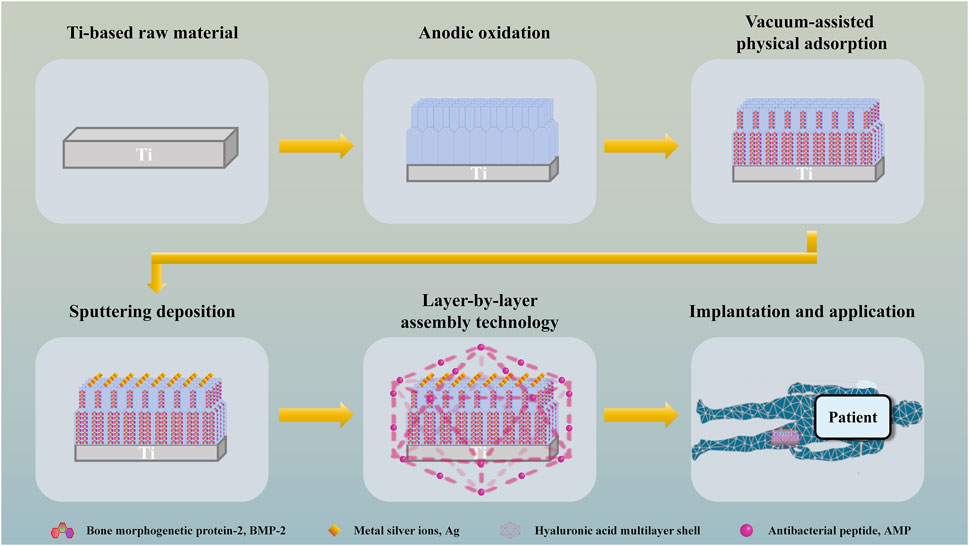
Figure 1. Schematic depiction of the preparation and application of bone implant scaffold with dual sterilization response systems of electronic biological antibacterial response and enzymatic hydrolysis antibacterial response.
Application and modification of Ti
Metal materials have good biocompatibility, strong corrosion resistance and excellent mechanical properties, so they are mainly used in parts that need mechanical support, such as bone defects of long bones. Metal scaffolds can effectively avoid extremely low intraosseous stress change and bone absorption between implants and cortical bones, thus promoting the growth of cells in the interface of implants and enhancing the bonding strength between bone tissues and implants (Dec et al., 2022; Bakhshandeh B. et al., 2017). The main problem of metal implant is that the corrosion of physiological environment will change the physical and chemical properties of the material, make the implant loose and damaged, and the increase of metal ion level will have potential toxic and side effects on the body. Currently, the most researched and widely used non-degradable materials are mainly Ti and tantalum, and the degradable metal scaffold material is mainly magnesium.
After Ti is implanted into the body, the surface will become the niche of the surrounding cells, and the niche is the place where the biological response events of the host and the surrounding cells and tissues are concentrated. However, due to the biological inertia on the surface of Ti, the effective integration of Ti with surrounding bone tissue is greatly limited (Fukuda et al., 2011; He et al., 2018). Therefore, how to biologically activate the inert Ti surface, enhance the long-term stability of Ti-based implants and promote the in situ osseointegration has become a huge challenge in the current research field. After Ti is implanted into the body, it faces two major challenges, aseptic loosening and bacterial infection. After Ti is implanted into the body, the process of effective bone repair and reconstruction between bone and graft surface is the positive result of the synergistic interaction and precise regulation of many cells and factors, which has many similarities with bone growth and remodeling and fracture repair (Fukuda et al., 2011; Panetta et al., 2010; Einhorn, 2006).
Brammer et al. (Brammer et al., 2009) prepared TiO2 nanotubes on the surface of Ti by anodic oxidation, and studied the pore sizes of nanotubes. The results showed that nanotubes with small diameters (less than 30 nm in diameter) were beneficial to cell adhesion, but not to cell differentiation. Large diameter nanotubes (between 70 and 100 nm in diameter) were not conducive to cell adhesion, but were beneficial to cell elongation, induced cytoskeleton stress to increase, and enhanced osteogenic differentiation. Zhang et al. (Zhang et al., 2017) prepared a double-layer nanotube drug storage pool system using Ti, with the upper nanotube diameter of 70 nm and the lower nanotube diameter of 140 nm, and loaded AMP by vacuum-assisted physical adsorption. The research results showed that the double-layer nanotube system could release drugs for up to 60 days, and could exert antibacterial activity for a long time without obvious cytotoxicity. To sum up, nanotube system and double-layer nanotube system have obvious advantages in sustained release and sustained action of drugs (Figure 3).
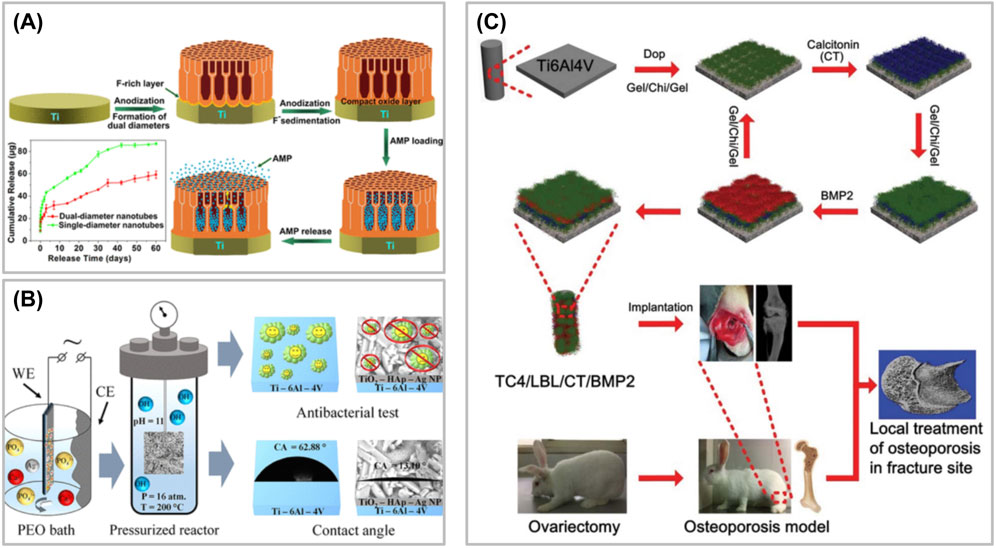
Figure 3. (A) Preparation of a double-layer nanotube drug storage pool system. (B) Preparation of Ti alloy system with Ag nanoparticles and calcium hydroxyphosphate by plasma electrolytic oxidation. (C) Schematic illustration of biofunctional multilayer coating on a Ti6Al4V (TC4) implant through layer-by-layer electrostatic assembly technique and investigation its regulation on local bone remodeling in the femur of an osteoporotic rabbit.
As a bone implant, solid Ti itself is dense and stable, and its elastic modulus is significantly higher than that of human bone, which will lead to stress shielding effect (Niinomi, 2008; Geetha et al., 2009). Under physiological conditions, the mechanical load can not be well transmitted from the implant to the surrounding bone tissue, which leads to bone absorption and movement at the material interface, and finally leads to the failure of material implantation (Tang et al., 2016). Porous Ti scaffold has a broad application prospect because of its suitable mechanical strength and a large number of coherent pore structures. Its large number of pores are beneficial to the circulation of body fluids, the transport of oxygen and nutrients and the formation of vascular tissue, and provide growth space for bone tissue. After the bone tissue grows into the scaffold, it forms a stable “mechanical lock” with the implant, which improves the interface bonding strength, reduces the stress shielding effect, and improves the long-term stability and life of the implant.
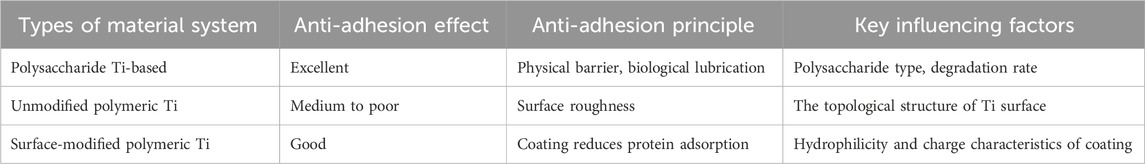
In the polysaccharide Ti-based system, the anti-adhesion polysaccharide Ti-based system often has high biocompatibility, and its degradation products are non-toxic and have good affinity with tissues. Anti-adhesion polysaccharide can form a physical barrier, reduce postoperative adhesion and produce significant anti-adhesion effect. At the same time, by adjusting the proportion of polysaccharide, the degradation rate of material coating can be changed to match the time of tissue healing (García-Robledo et al., 2024; Kasi et al., 2023; Pérez-Anes et al., 2015; Soylu et al., 2021; G et al., 2020; Ding et al., 2023; Tardelli et al., 2024; Xiong et al., 2025; Wennerberg and Albrektsson; Wang R. et al., 2022; Amirtharaj Mosas et al., 2022) (Table 2). Most polysaccharides have natural bacteriostatic effect, but their bacteriostatic effect is weak, so they are often combined with antibacterial components to achieve synergistic bactericidal effect to enhance antibacterial spectrum (Liu et al., 2025). At the same time, the sustained-release design of the reinforced material can prolong the antibacterial time of the material, thus reducing the number of times of administration. Some polysaccharides, such as carboxymethyl cellulose, have the ability to absorb exudate, maintain moist environment and promote healing (Ramezanzade et al., 2024; Osman et al., 2025; Wang et al., 2023). However, the mechanical strength of pure polysaccharide material is too low, so it needs to be compounded with Ti-based system to enhance its mechanical properties. At the same time, the antibacterial spectrum of materials dependent on polysaccharide type is limited, which may have poor effect on drugresistant bacteria, so at present, materials using polysaccharide alone are rare. For poly Ti without antibacterial drugs, it may have stable chemical properties and high-strength supporting ability, but its inert surface and no antibacterial property often lead to the lack of biological activity of the material. Polymeric Ti-based system loaded with antibiotics usually has strong antibacterial ability and local targeting effect, thus avoiding systemic drug side effects (Choi et al., 2024; Lv et al., 2024). However, the sudden release risk of drugs in this kind of materials may lead to insufficient drug concentration in the later period due to the sudden release of antibacterial drugs in the early stage, and the sudden release of drugs may lead to local toxic and side effects. Therefore, the optimization focus of this kind of materials is not only to improve the mechanical properties of Ti-based materials, but also to consider the drug release kinetics (Oliver et al., 2019).
Ti-based orthopedic implant system is at the critical turning point of technological innovation, and the paradigm shift from “passive replacement” to “active repair” and from “single function” to “system intelligence” will be ushered in the next decade. With the cross-integration of materials science, bioengineering, nanotechnology and artificial intelligence, a new generation of Ti-based implant system will break through the traditional limitations and achieve a qualitative leap in bone integration efficiency, infection prevention and control and long-term service performance. Despite the broad prospects, the innovative development of Ti-based orthopedic implant system still faces multiple transformation barriers. The complexity of regulatory approval is the primary obstacle. FDA classifies smart drug release implants as “combined products”, which need to meet the double standards of drugs and medical devices at the same time. Also, the newly promulgated MDR regulations in the European Union put forward stricter biocompatibility requirements for nano-material implants, forcing enterprises to invest more resources in safety evaluation. Therefore, perfecting and innovating Ti-based orthopedic implant system as much as possible is a task that the entire orthopedic industry and even the industrial chain need to continue to develop and adhere to.
Sustained release of osteogenic factors
The process of bone metabolism and repair after injury is highly complex, involving the interaction of various growth factors. Growth factors that can increase the growth activity of bone cells, regulate the growth and differentiation of bone cells and promote bone remodeling are called osteogenic factors. Osteogenic factors play an important role in the process of bone repair. Osteogenic factors can bind with target cell receptors and regulate various biological processes in cells (Tian et al., 2016). The process of bone repair after bone injury is a continuous process of bone repair-related cells such as osteoclasts and osteoblasts under the regulation of osteogenic factors. At the initial stage of bone injury, various osteogenic factors will be released, activating osteoblasts and osteoclasts, stimulating the proliferation and differentiation of osteoblasts, synthesizing bone protein and growth factors, and inducing bone formation (Q et al., 2017; Shirani et al., 2017). At present, there are many osteogenic factors studied, including bone morphogenetic protein (BMP), transforming growth factor-β, fibroblast growth factors, plateletderived growth factor and so on (Safari et al., 2021; Lowery and Rosen, 2018).
BMP is derived from bone and bone-derived cells and belongs to the transforming growth factor β superfamily. BMP is one of the most widely studied growth factors at present, and more than 20 BMP subtypes have been found, among which BMP-2, BMP-4 and BMP-7 have osteogenic ability, BMP-8 and BMP-9 are related to the formation of cartilage, and BMP-12, BMP-13 and BMP-14 are involved in the formation and repair of ligaments and tendons (Pallotta et al., 2018). BMP can participate in the expression of multiple signal pathways in the body and regulate the formation of cartilage, bone and blood vessels. BMP can activate Smad signaling pathway and mediate bone differentiation. BMP owns two kinds of transmembrane kinase receptors. When BMP binds to the transmembrane receptor on the cell membrane, it can activate Smad protein in cells, enter the nucleus, and activate downstream target factors such as osteocalcin, thus exerting its biological effects (Yoo et al., 2017; Zhao et al., 2016; Zhang et al., 2022; Lin et al., 2022). BMP has good bone induction ability, which can directionally induce MSCs to differentiate into osteoblasts, induce the proliferation and differentiation of various cells with osteogenic potential in bone tissue, synthesize collagen, promote the formation of cartilage and bone matrix, and form calcified bone tissue (Srouji et al., 2011), and owns the ability of cross-species induction of bone (Kloos et al., 2002). It has a good application effect on the treatment of fracture, bone defect and nonunion, and its clinical application in orthopedics has attracted extensive attention of researchers.
By vacuum-assisted physical adsorption, drugs promoting osteogenesis are filled into nanotubes, and appropriate solvents can be selected according to the physical and chemical properties of drugs, and the storage amount of drugs in nanotubes can be controlled. Controlling the degradation mode and rate of drug-coated materials can realize the degradation of materials under different response modes, thus realizing the release of osteogenic drugs and promoting local osteogenesis.
The exertion of antibacterial effect
The incidence and economic burden of bone infection are shocking. The infection rate of joint replacement accounts for 0.3%–2.4% of total hip replacement and 1%–3% of total knee replacement (Qayoom et al., 2020). The most commonly used treatment for osteomyelitis is to fill the bone nest with polymethylmethacrylate cement impregnated with gentamicin (Gen) to eradicate bone infection. The main motivation of using bone active carrier to deliver antibiotics locally is to achieve controlled and sustained delivery of drugs at the infected site, so as to overcome the need for long-term systemic administration and induce the formation of bone structure at the surgically removed bone. In the osteomyelitis environment, the dead space produced by extensive debridement will be filled with hematoma and fibrous tissue, which will easily lead to fractures and further complications (Osmon et al., 2013). If the surrounding soft tissue capsule is damaged, the degree of vascularization will also be damaged, and the self-healing ability of bone tissue will be reduced, so it is very important to fill the residual dead space. Implants act as biocompatible and bioactive fillers in the dead space, which is helpful for the regeneration of bone tissue lost during surgical debridement. The implanted carrier can also be used as a drug carrier, which can maintain a high drug concentration locally, thus better managing infection, bone formation and defect healing. Using high concentration strategy to achieve satisfactory drug delivery and effective antibiotics to prevent infection may have side effects (Yu et al., 2022). Local antibacterial therapy is beneficial to treat local infection, improve bioavailability and deliver related substances to bone infection site in a targeted manner. Inflammation and impaired bone healing are common clinical symptoms of infection, and cytokines and growth factors secreted by attacked osteoblasts can also promote inflammation and thus affect bone regeneration.
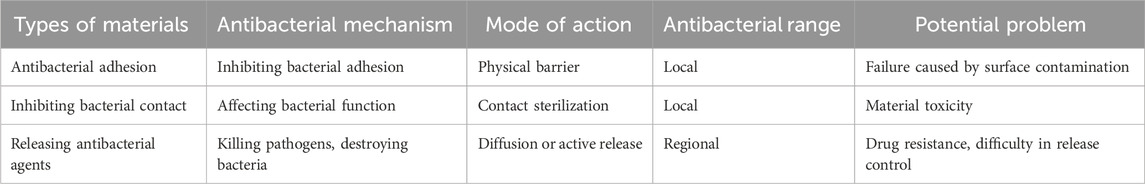
According to the classification of antibacterial mechanism of implanted materials, the antibacterial function of materials can be divided into three categories: antibacterial adhesion, inhibiting bacterial contact and releasing antibacterial agents (Table 3) (Ran et al., 2018). The mechanism of antibacterial adhesion implant materials is to inhibit the adhesion of bacteria on the implant surface during the process of bacterial adhesion and biofilm formation. This antibacterial concept can be realized by improving the physical topology, biocompatibility and chemical groups of materials and drugs carried by materials (Kummer et al., 2013; Yu et al., 2015; Godoy-Gallardo et al., 2015). The mechanism of inhibiting bacterial contact implant materials is mainly to affect bacteria through the charge and chemical composition on the surface of the materials, thus affecting the physiological functions of bacteria such as enzyme activity and respiration, so as to achieve contact bacteriostasis (Chen et al., 2016; Lin et al., 2015; Yue et al., 2014). Special attention should be paid to the negative charge on the surface of bacteria, so it is a breakthrough for materials to exert antibacterial effect by using strong positive charge groups or quaternized polymers to interact with bacteria. Killing the pathogens around the implant by releasing antibacterial agents is the most commonly used strategy for releasing antibacterial implant materials, and the most commonly used way in clinic is to use antibiotics to inhibit or kill pathogenic microorganisms (Campoccia et al., 2010). AMP, a broad-spectrum antibacterial polypeptide, can physically penetrate and permanently destroy the bacterial membrane of bacteria, making them lose the integrity of structure and function, leading to the overflow of their contents, thus killing bacteria. AMP has good biocompatibility with eukaryotic cells, and has certain immune regulation and injury repair functions (Levy and Marshall, 2004; Spellberg et al., 2004). The antibacterial stability of AMP is affected by many factors, including structural characteristics, environmental conditions and microbial species (Zeng et al., 2016). For example, in the microenvironment of bacterial infection, the local environment is acidic, and the activity of AMP will increase in acidic environment, but it will decrease in neutral or alkaline environment, which just makes AMP play an effective bactericidal role under the condition of bacterial infection (Chen and Jiang, 2023). Effective combination of AMP and delivery system can not only protect AMP from degradation, but also realize sustained release of AMP and maintain local high concentration activity. Kazemzadeh-Nabbat et al. (Kazemzadeh-Narbat et al., 2010) electrodeposited micron-sized porous CaP coating on Ti surface, and then applied AMP to the surface, and found that the material had strong antibacterial effect. Most studies have confirmed that AMP plays an obvious role in local antibacterial of biomaterials.
Metal ions play an important role in bone tissue engineering. Metal ions not only play a significant role in promoting bone tissue growth, but also play an antibacterial role locally. Many studies have confirmed that metal ion modified composite scaffolds can effectively resist the infection of local bone defects and effectively promote bone regeneration (Lu et al., 2021; Wang F. et al., 2022; Kumagai et al., 2019; Yedekçi et al., 2021). Metal ion antibacterial agents have strong lethality to microorganisms such as bacteria. Nano-Ag was prepared in situ by plasma immersion ion implantation technology and embedded on the surface of Ti. The immobilized Ag in this system can overcome the toxicity caused by free Ag entering cells (Cao et al., 2011; Cao et al., 2013). Ions on the surface of Ti material can produce “micro-battery effect”, that is, electronic biological response occurs (Figure 2). The surface of bacteria is negatively charged, but there is no electron conduction chain on the surface of cells, so bacteria can play a charge attraction and aggregation reaction at the ions on the surface of materials without affecting the adhesion between materials and cells.
Ag nanoparticles have excellent broad-spectrum bactericidal efficacy, which can kill Gram-positive bacteria, Gram-negative bacteria, fungi and some viruses (Geng et al., 2017). Ag nanoparticles have great potential in the treatment of nonunion and bone defect caused by chronic infection. Because of its special sterilization mechanism, the related drug resistance events are rare (Rai et al., 2009). The combination of Ag nanoparticles and composite nanoparticles assembled from other materials can effectively reduce toxicity and maintain strong anti-infection performance. Ag nanoparticles are a broad-spectrum antibacterial agent, and their antibacterial stability is affected by chemical state, environmental conditions and carrier materials. Ag nanoparticles destroy microorganisms by destroying cell membranes, interfering with bacterial metabolism and producing reactive oxygen species, making it difficult to produce drug resistance (Guo et al., 2024). Free Ag nanoparticles have strong antibacterial activity, but they are easy to combine with anions to form insoluble precipitate, thus losing activity. The side effects caused by ion dissociation can be greatly avoided by fixing free Ag nanoparticles on TiO2 nanotubes caliber by sputtering spraying technology, and the cytotoxicity caused by excessive free ions can be greatly reduced. Sobolev et al. (2019) prepared a Ti alloy system with Ag nanoparticles and calcium hydroxyphosphate by plasma electrolytic oxidation, which could be transformed into silver sulfide nanoparticles in vivo, thus exerting anti-infection characteristics. Bakhshandeh S. et al. (2017) electrodeposited chitosan-gelatin composite layers doped with Ag and vancomycin on the surface of 3D printed porous Ti. In this system, vancomycin could be released continuously for at least 21 days, and played a synergistic antibacterial role, thus achieving the goal of completely eradicating planktonic bacteria and adherent bacteria, and there was no cytotoxicity. It has been pointed out that after cells are exposed to Ag nanoparticles, the expression of protein molecules related to bone cell pathway in stem cells, such as BMP-4, BMP-6 and FOS-like antigen, increases significantly (Qing et al., 2018), so Ag nanoparticles are often used as an important component to modify other materials because of their excellent antibacterial ability (Figure 3). Although ion doping endows bone repair materials with antibacterial ability, the sudden release effect of metal ions in vivo can not be overcome at present, and a large number of metal ions released in a short time have dose-toxic effects. Electroplating surface deposition technology can stabilize ions on the surface of materials, which is an excellent solution to solve the problem of sudden release of ions. In silver ion sputtering deposition, particle size is an important parameter to control the characteristics of materials. By adjusting the parameters of silver ion sputtering, controlling the target material and gas, and monitoring the surface in real time, the ion size can be accurately adjusted, so as to directionally design the material function. It can effectively reduce the problems of particle agglomeration and uneven edge effect (Kelly et al., 2003). HA, as the material of LBL, has normal degradation behavior after HA crosslinking for 4–8 weeks, which is just conducive to the release of BMP in TiO2 nanotubes to play a role in promoting bone, while Ag ions on the surface continue to play an antibacterial role (Lin H. et al., 2019). In the case of bacterial infection, bacterial secretions quickly act on LBL coating to achieve the bactericidal effect of AMP, improve the local infection environment, and facilitate tissue repair and osteogenesis.
Application of LBL
There are many ways for local drug delivery. No matter which way is used to achieve effective local drug delivery, the ultimate goal of sustained antibacterial implant system is to achieve local sustained and stable drug release. The existing implant drug delivery technologies can use thin films (Rakib et al., 2022), hydrogels (Meng et al., 2023), microparticles (Kim et al., 2024), nanoparticles (Tengjisi et al., 2022), electrospun fibers (Krysiak et al., 2023) and TiO2 nanotubes (Xu et al., 2016), which have different characteristics and can be used in different scenarios (Table 4).
LBL is a kind of technology based on polyelectrolyte multilayer films formed by alternating adsorption and deposition of oppositely charged polyelectrolytes through electrostatic action. With this technology, many proteins, drugs and so on can be encapsulated in materials to regulate cell behavior (Figure 3) (Guo et al., 2017; Shen et al., 2016a; Huang et al., 2016). The surface polyelectrolyte multilayer films prepared by LBL are similar to natural ECM, and have the characteristics of strong biocompatibility and are beneficial to cell adhesion, proliferation and differentiation. At the same time, the degradation of multilayer films can release the drugs wrapped in them, thus further stimulating and regulating the biological behavior of cells. LBL has been widely used in the preparation of bone repair materials because of its simple preparation technology and simple operation process. At present, chitosan is the most common medium for LBL. Chitosan widely exists in ECM, which is one of the main components connecting collagen fibers. Chitosan can provide microenvironment for cell proliferation and ECM production, and has the potential to promote osteogenesis (Zang et al., 2017). Chitosan not only has good biocompatibility, safety and low antigenicity, but also has antibacterial and antifungal properties, which can inhibit the reproduction of bacteria, fungi and viruses. Therefore, chitosan is often used as an antibacterial material in combination with other materials to treat infectious bone defects. Because simple chitosan has weak mechanical properties, poor water solubility and low biodegradation rate, chitosan is often used as scaffold coating or modified by chemical modification to prepare injectable hydrogel, giving full play to its advantages. Shen et al. (2016b) used silkworm-free peptide to load TiO2 nanotubes and covered them with LBL multilayer structure with silkworm-free peptide/chitosan coupling HA, which could achieve short-term and long-term antibacterial effect of hyaluronidase triggered by potential bacteria. Studies have shown that the HA-Gen derivatives with hyaluronidase response were formed by covalently grafting Gen onto HA molecules, and then the multilayer membrane structure of chitosan/HA-Gen derivatives was constructed by LBL to encapsulate the nanotube storage pool of deferoxamine drugs. During local bacterial infection, a large number of hyaluronidase secreted by bacteria could hydrolyze HA-Gen derivative multilayer layers, which on the one hand released Gen fragments for sterilization, and on the other hand released deferoxamine in nanotubes to promote bone formation and angiogenesis, thus accelerating bone repair.
After the antibacterial osteogenesis system constructed in this study is implanted, LBL coating will not be hydrolyzed by enzyme in a non-infectious environment, achieving the effect of gradual decomposition, and slowly released AMP will be gradually metabolized. At the same time, the release of BMP in nanotubes will promote local bone repair, thus achieving the effects of preventing infection and promoting bone regeneration. In the case of local bacterial infection, LBL coating is hydrolyzed by enzyme, which quickly hydrolyzes and releases a large amount of AMP for antibacterial effect. At the same time, Ag nanoparticles sputtered on the surface of nanotubes achieve continuous antibacterial effect, and BMP in nanotubes will also be released to accelerate local bone integration, thus realizing the organic combination of antibacterial and promoting bone. The loading amount of the drugs used should be strictly controlled, so as to achieve effective antibacterial and bone-promoting effects, and at the same time, it will not produce additional toxic and side effects on cells.
The challenge of the antibacterial drug delivery system
The purpose of this study is to develop a material: Ti-based bone repair material with dual-response antibacterial system and sustained drug. The purpose is to prevent infection and promote local bone repair under the condition of no local bacterial infection after the implant is implanted in the body, and to respond to bacteria and accelerate local bone repair under the condition of local bacterial infection. The existing technology and materials can completely realize the expected construction of the above materials, but the main challenge is to find the best composition ratio of bone factor and antibacterial components loaded in the implanted materials, and to strive to achieve the maximum drug loading of the two drugs without mutual influence, and at the same time, the drug content has no toxic effect on the body, and to play the most lasting and effective osteogenesis and antibacterial role. Under the exploration of basic experiments, this study should not only overcome the above problems, but also explore the relationship between antibacterial effect and osteogenic effect after implantation of this material as much as possible.
Conclusion
The application of materials provides a scaffold for bone regeneration-related cell growth for bone defect repair, and also helps to maintain the integrity of bone structure with its own mechanical properties. In this hypothesis, the Ti scaffold with double-layer nanotube structure with slow-release osteogenic factors not only provides a niche for osteoblasts to adhere, but also stores osteogenic factors to the greatest extent and continues to play a slow-release role. The existence of the scaffold fills the gap conducive to hematoma formation and provides favorable conditions for further osteogenesis. As for antibacterial, the enzymatic antibacterial system in the scaffold can release AMP in the case of bacterial infection to eradicate free bacteria. The ion antibacterial system located on the surface of Ti scaffold can not only destroy adhesion bacteria, but also provide a rougher and broader site for cell adhesion, which provides favorable conditions for cell proliferation, differentiation and osteogenesis in the next step. At the same time, BMP-2 slowly released from the double-layer tubular structure has a strong osteogenic and angiogenic effect, which promotes the osteogenic efficiency of the system. This kind of Ti-based bone implant with sustained drug release and high specific surface area wrapped by LBL of dual-responsive antibacterial system has a strong development prospect. The preparation of the scaffold combines the cross-integration of material science, bioengineering and nanotechnology, breaks through the traditional limitations, and improves the optimization of Ti-based materials in bone integration efficiency, infection prevention and control and long-term service performance.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
WZ: Writing – original draft, Software. ZG: Writing – review and editing, Resources. ZH: Methodology, Writing – review and editing. HZ: Writing – review and editing, Methodology. HL: Writing – review and editing, Methodology. XP: Methodology, Writing – review and editing. XY: Methodology, Writing – review and editing. RZ: Conceptualization, Writing – review and editing. WH: Writing – review and editing, Conceptualization. LC: Writing – review and editing, Supervision, Funding acquisition. ZD: Supervision, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The authors acknowledge financial support from The International Postdoctoral Exchange Fellowship Program of Chongqing (2021JLPY004), Natural Science Foundation Postdoctoral Science Foundation Project of Chongqing (cstc2021jcyj-bsh0019), Chongqing Medical University Future Medical Youth Innovation Team Project (No. W0108), Kuanren Talent Project (NO. kryc-gg-2204), the Chongqing Graduate Research Innovation Project (No. CYB23200), National Key R&D Program of China (2023YFB3809901) and the Science and Technology Program of Guangzhou (202206040001 and SL2023A04J00808).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Akay, S., and Yaghmur, A. (2024). Recent advances in antibacterial coatings to combat orthopedic implant-associated infections. Molecules 29 (5), 1172. doi:10.3390/molecules29051172
Albrektsson, T., and Johansson, C. (2001). Osteoinduction, osteoconduction and osseointegration. Eur. Spine J. 10 (2), S96–S101. doi:10.1007/s005860100282
Alghamdi, H. S., and Jansen, J. A. (2020). The development and future of dental implants. Dent. Mater. J. 39 (2), 167–172. doi:10.4012/dmj.2019-140
Amirtharaj Mosas, K. K., Chandrasekar, A. R., Dasan, A., Pakseresht, A., and Galusek, D. (2022). Recent advancements in materials and coatings for biomedical implants. Gels 8 (5), 323. doi:10.3390/gels8050323
Bäcker, H. C., Wu, C. H., Kienzle, A., Perka, C., and Gwinner, C. (2023). Mechanical failure of total hip arthroplasties and associated risk factors. Archives Orthop. trauma Surg. 143 (2), 1061–1069. doi:10.1007/s00402-022-04353-0
Bakhshandeh, B., Zarrintaj, P., Oftadeh, M. O., Keramati, F., Fouladiha, H., Sohrabi-Jahromi, S., et al. (2017a). Tissue engineering; strategies, tissues, and biomaterials. Biotechnol. and Genet. Eng. Rev. 33 (2), 144–172. doi:10.1080/02648725.2018.1430464
Bakhshandeh, S., Gorgin Karaji, Z., Lietaert, K., Fluit, A. C., Boel, C. H. E., Vogely, H. C., et al. (2017b). Simultaneous delivery of multiple antibacterial agents from additively manufactured porous biomaterials to fully eradicate planktonic and adherent Staphylococcus aureus. ACS Appl. Mater. and interfaces 9 (31), 25691–25699. doi:10.1021/acsami.7b04950
Bashir, M. H., Korany, N. S., Farag, D. B. E., Abbass, M. M. S., Ezzat, B. A., Hegazy, R. H., et al. (2023). Polymeric nanocomposite hydrogel scaffolds in craniofacial bone regeneration: a comprehensive review. Biomolecules 13 (2), 205. doi:10.3390/biom13020205
Bharadwaz, A., and Jayasuriya, A. C. (2020). Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration. Mater. Sci. and Eng. C, Mater. Biol. Appl. 110, 110698. doi:10.1016/j.msec.2020.110698
Bozic, K. J., Kurtz, S. M., Lau, E., Ong, K., Chiu, V., Vail, T. P., et al. (2010). The epidemiology of revision total knee arthroplasty in the United States. Clin. Orthop. Relat. Res. 468 (1), 45–51. doi:10.1007/s11999-009-0945-0
Brammer, K. S., Oh, S., Cobb, C. J., Bjursten, L. M., van der Heyde, H., and Jin, S. (2009). Improved bone-forming functionality on diameter-controlled TiO(2) nanotube surface. Acta biomater. 5 (8), 3215–3223. doi:10.1016/j.actbio.2009.05.008
Buser, D., Broggini, N., Wieland, M., Schenk, R. K., Denzer, A. J., Cochran, D. L., et al. (2004). Enhanced bone apposition to a chemically modified SLA titanium surface. J. Dent. Res. 83 (7), 529–533. doi:10.1177/154405910408300704
Busscher, H. J., van der Mei, H. C., Subbiahdoss, G., Jutte, P. C., van den Dungen, J. J., Zaat, S. A., et al. (2012). Biomaterial-associated infection: locating the finish line in the race for the surface. Sci. Transl. Med. 4 (153), 153rv10. doi:10.1126/scitranslmed.3004528
Campoccia, D., Montanaro, L., Speziale, P., and Arciola, C. R. (2010). Antibiotic-loaded biomaterials and the risks for the spread of antibiotic resistance following their prophylactic and therapeutic clinical use. Biomaterials 31 (25), 6363–6377. doi:10.1016/j.biomaterials.2010.05.005
Cao, H., Liu, X., Meng, F., and Chu, P. K. (2011). Biological actions of silver nanoparticles embedded in titanium controlled by micro-galvanic effects. Biomaterials 32 (3), 693–705. doi:10.1016/j.biomaterials.2010.09.066
Cao, H., Qiao, Y., Liu, X., Lu, T., Cui, T., Meng, F., et al. (2013). Electron storage mediated dark antibacterial action of bound silver nanoparticles: smaller is not always better. Acta biomater. 9 (2), 5100–5110. doi:10.1016/j.actbio.2012.10.017
Chen, N., and Jiang, C. (2023). Antimicrobial peptides: structure, mechanism, and modification. Eur. J. Med. Chem. 255, 115377. doi:10.1016/j.ejmech.2023.115377
Chen, R., Willcox, M. D., Ho, K. K., Smyth, D., and Kumar, N. (2016). Antimicrobial peptide melimine coating for titanium and its in vivo antibacterial activity in rodent subcutaneous infection models. Biomaterials 85, 142–151. doi:10.1016/j.biomaterials.2016.01.063
Choi, H. S., Yun, J., Jeong, Y., Jo, Y. K., and Cha, H. J. (2024). Self-controllable proteinic antibacterial coating with bacteria-triggered antibiotic release for prevention of periprosthetic infection. Biomaterials 305, 122457. doi:10.1016/j.biomaterials.2023.122457
Claes, L., Recknagel, S., and Ignatius, A. (2012). Fracture healing under healthy and inflammatory conditions. Nat. Rev. Rheumatol. 8 (3), 133–143. doi:10.1038/nrrheum.2012.1
Costerton, J. W., Stewart, P. S., and Greenberg, E. P. (1999). Bacterial biofilms: a common cause of persistent infections. Sci. (New York, NY) 284 (5418), 1318–1322. doi:10.1126/science.284.5418.1318
Cunniffe, G. M., Díaz-Payno, P. J., Ramey, J. S., Mahon, O. R., Dunne, A., Thompson, E. M., et al. (2017). Growth plate extracellular matrix-derived scaffolds for large bone defect healing. Eur. cells and Mater. 33, 130–142. doi:10.22203/eCM.v033a10
Dalby, M. J., Gadegaard, N., Tare, R., Andar, A., Riehle, M. O., Herzyk, P., et al. (2007). The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat. Mater. 6 (12), 997–1003. doi:10.1038/nmat2013
Dec, P., Modrzejewski, A., and Pawlik, A. (2022). Existing and novel biomaterials for bone tissue engineering. Int. J. Mol. Sci. 24 (1), 529. doi:10.3390/ijms24010529
Ding, Y., Ma, R., Liu, G., Li, X., Xu, K., Liu, P., et al. (2023). Fabrication of a new hyaluronic Acid/Gelatin nanocomposite Hydrogel coating on titanium-based implants for treating biofilm infection and excessive inflammatory response. ACS Appl. Mater. and interfaces 15 (10), 13783–13801. doi:10.1021/acsami.2c23320
Elias, C. N., Lima, J. H. C., Valiev, R., and Meyers, M. A. (2008). Biomedical applications of titanium and its alloys. JOM 60 (3), 46–49. doi:10.1007/s11837-008-0031-1
Fukuda, A., Takemoto, M., Saito, T., Fujibayashi, S., Neo, M., Yamaguchi, S., et al. (2011). Bone bonding bioactivity of Ti metal and ti-zr-nb-ta alloys with Ca ions incorporated on their surfaces by simple chemical and heat treatments. Acta biomater. 7 (3), 1379–1386. doi:10.1016/j.actbio.2010.09.026
G, P. H., Kariduraganavar, M. Y., and Mitchell, G. R. (2020). Crosslinked nanocomposite sodium alginate-based membranes with titanium dioxide for the dehydration of isopropanol by pervaporation. Molecules 25 (6), 1298. doi:10.3390/molecules25061298
García-Robledo, H., García-Fernández, L., Parra, J., Martín-López, R., Vázquez-Lasa, B., and de la Torre, B. (2024). Ti/Ta-based composite polysaccharide scaffolds for guided bone regeneration in total hip arthroplasty. Int. J. Biol. Macromol. 271 (Pt 1), 132573. doi:10.1016/j.ijbiomac.2024.132573
Geetha, M., Singh, A. K., Asokamani, R., and Gogia, A. K. (2009). Ti based biomaterials, the ultimate choice for orthopaedic implants – a review. Prog. Mater. Sci. 54 (3), 397–425. doi:10.1016/j.pmatsci.2008.06.004
Geng, H., Poologasundarampillai, G., Todd, N., Devlin-Mullin, A., Moore, K. L., Golrokhi, Z., et al. (2017). Biotransformation of silver released from nanoparticle coated titanium implants revealed in regenerating bone. ACS Appl. Mater. and interfaces 9 (25), 21169–21180. doi:10.1021/acsami.7b05150
Godoy-Gallardo, M., Mas-Moruno, C., Yu, K., Manero, J. M., Gil, F. J., Kizhakkedathu, J. N., et al. (2015). Antibacterial properties of hLf1-11 peptide onto titanium surfaces: a comparison study between silanization and surface initiated polymerization. Biomacromolecules 16 (2), 483–496. doi:10.1021/bm501528x
Gristina, A. G. (1987). Biomaterial-centered infection: microbial adhesion versus tissue integration. Sci. (New York, NY) 237 (4822), 1588–1595. doi:10.1126/science.3629258
Guo, S., Zhu, X., and Loh, X. J. (2017). Controlling cell adhesion using layer-by-layer approaches for biomedical applications. Mater. Sci. and Eng. C, Mater. Biol. Appl. 70 (Pt 2), 1163–1175. doi:10.1016/j.msec.2016.03.074
Guo, Y., Wei, J., Liu, C., Li, X., and Yan, W. (2023). Metformin regulates bone marrow stromal cells to accelerate bone healing in diabetic mice. eLife 12, e88310. doi:10.7554/elife.88310
Guo, S., Liu, X., Chen, H., Wang, J., Qiao, Y., Zhang, T., et al. (2024). Antibacterial effect of the metal nanocomposite on Escherichia coli. J. Hazard Mater. 476, 135149. doi:10.1016/j.jhazmat.2024.135149
He, Y., Mu, C., Shen, X., Yuan, Z., Liu, J., Chen, W., et al. (2018). Peptide LL-37 coating on micro-structured titanium implants to facilitate bone formation in vivo via mesenchymal stem cell recruitment. Acta biomater. 80, 412–424. doi:10.1016/j.actbio.2018.09.036
Hetrick, E. M., and Schoenfisch, M. H. (2006). Reducing implant-related infections: active release strategies. Chem. Soc. Rev. 35 (9), 780–789. doi:10.1039/b515219b
Holzapfel, B. M., Rudert, M., and Hutmacher, D. W. (2017). Scaffold-based bone tissue engineering. Der Orthopade 46 (8), 701–710. doi:10.1007/s00132-017-3444-0
Huang, L., Luo, Z., Hu, Y., Shen, X., Li, M., Li, L., et al. (2016). Enhancement of local bone remodeling in osteoporotic rabbits by biomimic multilayered structures on Ti6Al4V implants. J. Biomed. Mater. Res. Part A 104 (6), 1437–1451. doi:10.1002/jbm.a.35667
Ingham, E., and Fisher, J. (2005). The role of macrophages in osteolysis of total joint replacement. Biomaterials 26 (11), 1271–1286. doi:10.1016/j.biomaterials.2004.04.035
Jacobs, R., Quirynen, M., and Bornstein, M. M. (2000). Neurovascular disturbances after implant surgery. Periodontology 66 (1), 188–202. doi:10.1111/prd.12050
Kaneko, M., Shigeno, K., Wakatsuki, M., Nakada, A., Inada, Y., and Nakamura, T. (2021). Bone regeneration utilizing a newly developed octacalcium phosphate/weakly denatured collagen scaffold. Int. J. Artif. organs 44 (2), 139–145. doi:10.1177/0391398820924045
Kasi, P. B., Azar, M. G., Dodda, J. M., Bělský, P., Kovářík, T., Šlouf, M., et al. (2023). Chitosan and cellulose-based composite hydrogels with embedded titanium dioxide nanoparticles as candidates for biomedical applications. Int. J. Biol. Macromol. 243, 125334. doi:10.1016/j.ijbiomac.2023.125334
Kazemzadeh-Narbat, M., Kindrachuk, J., Duan, K., Jenssen, H., Hancock, R. E., and Wang, R. (2010). Antimicrobial peptides on calcium phosphate-coated titanium for the prevention of implant-associated infections. Biomaterials 31 (36), 9519–9526. doi:10.1016/j.biomaterials.2010.08.035
Kazimierczak, P., Golus, J., Kolmas, J., Wojcik, M., Kolodynska, D., and Przekora, A. (2022). Noncytotoxic zinc-doped nanohydroxyapatite-based bone scaffolds with strong bactericidal, bacteriostatic, and antibiofilm activity. Biomater. Adv. 139, 213011. doi:10.1016/j.bioadv.2022.213011
Kelly, K. L., Coronado, E., Zhao, L. L., and Schatz, G. C. (2003). The optical properties of metal nanoparticles: the influence of size, shape, and dielectric environment. J. Phys. Chem. B 107 (3), 668–677. doi:10.1021/jp026731y
Kim, K. J., Hwang, M. J., Choe, S. W., Jeong, K. C., and Yoon, S. D. (2024). Drug release profile of phenytoin-loaded starch-based biomaterials incorporating hierarchical microparticles with photothermal effects. Int. J. Biol. Macromol. 282 (Pt 2), 136803. doi:10.1016/j.ijbiomac.2024.136803
Kloos, D. U., Choi, C., and Wingender, E. (2002). The TGF-beta--Smad network: introducing bioinformatic tools. Trends Genet. TIG 18 (2), 96–103. doi:10.1016/s0168-9525(02)02556-8
Krysiak, Z. J., and Stachewicz, U. (2023). Electrospun fibers as carriers for topical drug delivery and release in skin bandages and patches for atopic dermatitis treatment. Wiley Interdiscip. Rev. Nanomed Nanobiotechnol 15 (1), e1829. doi:10.1002/wnan.1829
Kumagai, H., Makihara, T., Funayama, T., Sato, K., Noguchi, H., Abe, T., et al. (2019). Angiogenesis and new bone formation in novel unidirectional porous beta-tricalcium phosphate: a histological study. J. Artif. organs official J. Jpn. Soc. Artif. Organs 22 (4), 294–299. doi:10.1007/s10047-019-01120-8
Kummer, K. M., Taylor, E. N., Durmas, N. G., Tarquinio, K. M., Ercan, B., and Webster, T. J. (2013). Effects of different sterilization techniques and varying anodized TiO2 nanotube dimensions on bacteria growth. J. Biomed. Mater. Res. Part B, Appl. biomaterials 101 (5), 677–688. doi:10.1002/jbm.b.32870
Levy, S. B., and Marshall, B. (2004). Antibacterial resistance worldwide: causes, challenges and responses. Nat. Med. 10 (12 Suppl. l), S122–S129. doi:10.1038/nm1145
Li, C., Jiang, C., Deng, Y., Li, T., Li, N., Peng, M., et al. (2017). RhBMP-2 loaded 3D-printed mesoporous silica/calcium phosphate cement porous scaffolds with enhanced vascularization and osteogenesis properties. Sci. Rep. 7, 41331. doi:10.1038/srep41331
Li, W., Li, S., Zhang, J., Zhong, H., Liang, J., Huang, S., et al. (2022). Fabrication and evaluation of bone morphogenetic protein-2 microspheres coated black phosphorus nanosheets@polylactic-glycolic acid copolymers scaffold: a multifunctional antibacterial photothermal scaffold for bone regeneration. Int. J. Biol. Macromol. 210, 350–364. doi:10.1016/j.ijbiomac.2022.05.028
Lin, W., Junjian, C., Chengzhi, C., Lin, S., Sa, L., Li, R., et al. (2015). Multi-biofunctionalization of a titanium surface with a mixture of peptides to achieve excellent antimicrobial activity and biocompatibility. J. Mater. Chem. B 3 (1), 30–33. doi:10.1039/c4tb01318b
Lin, Z., Wu, S., Liu, X., Qian, S., Chu, P. K., Zheng, Y., et al. (2019a). A surface-engineered multifunctional TiO(2) based nano-layer simultaneously elevates the corrosion resistance, osteoconductivity and antimicrobial property of a magnesium alloy. Acta biomater. 99, 495–513. doi:10.1016/j.actbio.2019.09.008
Lin, H., Beck, A. M., Shimomura, K., Sohn, J., Fritch, M. R., Deng, Y., et al. (2019b). Optimization of photocrosslinked gelatin/hyaluronic acid hybrid scaffold for the repair of cartilage defect. J. tissue Eng. Regen. Med. 13 (8), 1418–1429. doi:10.1002/term.2883
Lin, S., Yin, S., Shi, J., Yang, G., Wen, X., Zhang, W., et al. (2022). Orchestration of energy metabolism and osteogenesis by Mg(2+) facilitates low-dose BMP-2-driven regeneration. Bioact. Mater. 18, 116–127. doi:10.1016/j.bioactmat.2022.03.024
Liu, X., Liu, L., and Zeng, Z. (2025). Effectiveness of a local drug delivery System based on antimicrobial peptides in early treatment of Peri-implantitis. Int. Dent. J. 75 (2), 1400–1408. doi:10.1016/j.identj.2024.11.001
Lowery, J. W., and Rosen, V. (2018). The BMP pathway and its inhibitors in the skeleton. Physiol. Rev. 98 (4), 2431–2452. doi:10.1152/physrev.00028.2017
Lu, T., Zhang, J., Yuan, X., Tang, C., Wang, X., Zhang, Y., et al. (2021). Enhanced osteogenesis and angiogenesis of calcium phosphate cement incorporated with zinc silicate by synergy effect of zinc and silicon ions. Mater. Sci. and Eng. C, Mater. Biol. Appl. 131, 112490. doi:10.1016/j.msec.2021.112490
Lv, Z., Ji, Y., Wen, G., Liang, X., Zhang, K., and Zhang, W. (2024). Structure-optimized and microenvironment-inspired nanocomposite biomaterials in bone tissue engineering. Burns Trauma 12, tkae036. doi:10.1093/burnst/tkae036
Martino, M. M., Briquez, P. S., Maruyama, K., and Hubbell, J. A. (2015). Extracellular matrix-inspired growth factor delivery systems for bone regeneration. Adv. drug Deliv. Rev. 94, 41–52. doi:10.1016/j.addr.2015.04.007
Meng, S., Hu, H., Qiao, Y., Wang, F., Zhang, B. N., Sun, D., et al. (2023). A Versatile hydrogel with antibacterial and sequential drug-releasing capability for the programmable healing of infectious keratitis. ACS nano 17 (23), 24055–24069. doi:10.1021/acsnano.3c09034
Morgenstern, M., Kühl, R., Eckardt, H., Acklin, Y., Stanic, B., Garcia, M., et al. (2018). Diagnostic challenges and future perspectives in fracture-related infection. Injury 49 (Suppl. 1), S83–s90. doi:10.1016/s0020-1383(18)30310-3
Niinomi, M. (2008). Mechanical biocompatibilities of titanium alloys for biomedical applications. J. Mech. Behav. Biomed. Mater. 1 (1), 30–42. doi:10.1016/j.jmbbm.2007.07.001
Oliver, J. N., Su, Y., Lu, X., Kuo, P. H., Du, J., and Zhu, D. (2019). Bioactive glass coatings on metallic implants for biomedical applications. Bioact. Mater. 4, 261–270. doi:10.1016/j.bioactmat.2019.09.002
Osman, H., Tang, X., Wei, Q., Liu, B., Gao, J., and Wang, Y. (2025). In situ electrochemical fabrication of photoreactive Ag-Cu bimetallic nanocomposite coating and its antibacterial-osteogenic synergy. ACS Appl. Bio Mater 8 (7), 6326–6338. doi:10.1021/acsabm.5c00802
Osmon, D. R., Berbari, E. F., Berendt, A. R., Lew, D., Zimmerli, W., Steckelberg, J. M., et al. (2013). Diagnosis and management of prosthetic Joint Infection: clinical Practice Guidelines by the infectious diseases Society of Americaa. Clin. Infect. Dis. official Publ. Infect. Dis. Soc. Am. 56 (1), e1–e25. doi:10.1093/cid/cis803
Pacheco, K. A. (2019). Allergy to surgical implants. Clin. Rev. allergy and Immunol. 56 (1), 72–85. doi:10.1007/s12016-018-8707-y
Pallotta, I., Sun, B., Lallos, G., Terrenoire, C., and Freytes, D. O. (2018). Contributions of bone morphogenetic proteins in cardiac repair cells in three-dimensional in vitro models and angiogenesis. J. tissue Eng. Regen. Med. 12 (2), 349–359. doi:10.1002/term.2460
Panetta, N. J., Gupta, D. M., and Longaker, M. T. (2010). Bone regeneration and repair. Curr. stem cell Res. and Ther. 5 (2), 122–128. doi:10.2174/157488810791268618
Parvizi, J., Pawasarat, I. M., Azzam, K. A., Joshi, A., Hansen, E. N., and Bozic, K. J. (2010). Periprosthetic joint infection: the economic impact of methicillin-resistant infections. J. arthroplasty 25 (6 Suppl. l), e42–e47. doi:10.1016/j.arth.2010.01.050
Pérez-Anes, A., Gargouri, M., Laure, W., Van Den Berghe, H., Courcot, E., Sobocinski, J., et al. (2015). Bioinspired Titanium drug eluting platforms based on a Poly-β-cyclodextrin-Chitosan layer-by-layer self-assembly targeting infections. ACS Appl. Mater. and interfaces 7 (23), 12882–12893. doi:10.1021/acsami.5b02402
Porter, G. C., Schwass, D. R., Tompkins, G. R., Bobbala, S. K. R., Medlicott, N. J., and Meledandri, C. J. (2021). AgNP/Alginate Nanocomposite hydrogel for antimicrobial and antibiofilm applications. Carbohydr. Polym. 251, 117017. doi:10.1016/j.carbpol.2020.117017
Quinlan, E., López-Noriega, A., Thompson, E. M., Hibbitts, A., Cryan, S. A., and O'Brien, F. J. (2017). Controlled release of vascular endothelial growth factor from spray-dried alginate microparticles in collagen-hydroxyapatite scaffolds for promoting vascularization and bone repair. J. tissue Eng. Regen. Med. 11 (4), 1097–1109. doi:10.1002/term.2013
Qayoom, I., Teotia, A. K., Panjla, A., Verma, S., and Kumar, A. (2020). Local and sustained delivery of Rifampicin from a bioactive ceramic carrier treats bone infection in Rat Tibia. ACS Infect. Dis. 6 (11), 2938–2949. doi:10.1021/acsinfecdis.0c00369
Qing, T., Mahmood, M., Zheng, Y., Biris, A. S., Shi, L., and Casciano, D. A. (2018). A genomic characterization of the influence of silver nanoparticles on bone differentiation in MC3T3-E1 cells. J. Appl. Toxicol. JAT 38 (2), 172–179. doi:10.1002/jat.3528
Rai, M., Yadav, A., and Gade, A. (2009). Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 27 (1), 76–83. doi:10.1016/j.biotechadv.2008.09.002
Rakib, H. K. M., Shankar Hazra, R., Nair, G., Mohammad, J., Jiang, L., Reindl, K., et al. (2022). Cellulose nanofibers as Scaffold-forming materials for thin film drug delivery systems. Int. J. Pharm. 627, 122189. doi:10.1016/j.ijpharm.2022.122189
Ramezanzade, V., Dinari, M., and Mehvari, F. (2024). Investigation study of methyl violet photodegradation over alginate-carboxymethyl cellulose/titanium(IV) oxide/covalent organic frameworks bio-nanocomposite beads under ultraviolet irradiation. Int. J. Biol. Macromol. 277 (Pt 3), 134287. doi:10.1016/j.ijbiomac.2024.134287
Ran, Q., Yang, W., Hu, Y., Shen, X., Yu, Y., Xiang, Y., et al. (2018). Osteogenesis of 3D printed porous Ti6Al4V implants with different pore sizes. J. Mech. Behav. Biomed. Mater 84, 1–11. doi:10.1016/j.jmbbm.2018.04.010
Raphel, J., Holodniy, M., Goodman, S. B., and Heilshorn, S. C. (2016). Multifunctional coatings to simultaneously promote osseointegration and prevent infection of orthopaedic implants. Biomaterials 84, 301–314. doi:10.1016/j.biomaterials.2016.01.016
Safari, B., Davaran, S., and Aghanejad, A. (2021). Osteogenic potential of the growth factors and bioactive molecules in bone regeneration. Int. J. Biol. Macromol. 175, 544–557. doi:10.1016/j.ijbiomac.2021.02.052
Saul, D., and Khosla, S. (2022). Fracture healing in the setting of endocrine diseases, aging, and cellular senescence. Endocr. Rev. 43 (6), 984–1002. doi:10.1210/endrev/bnac008
Shen, X., Zhang, Y., Hu, Y., Luo, Z., Ma, P., Li, L., et al. (2016a). Regulation of local bone remodeling mediated by hybrid multilayer coating embedded with hyaluronan-alendronate/BMP-2 nanoparticles on Ti6Al7Nb implants. J. Mater. Chem. B 4 (44), 7101–7111. doi:10.1039/c6tb01779g
Shen, X., Zhang, F., Li, K., Qin, C., Ma, P., Dai, L., et al. (2016b). Cecropin B loaded TiO2 nanotubes coated with hyaluronidase sensitive multilayers for reducing bacterial adhesion. Mater. and Des. 92 (Feb), 1007–1017. doi:10.1016/j.matdes.2015.12.126
Shirani, G., Abbasi, A. J., Mohebbi, S. Z., and Moharrami, M. (2017). Comparison between autogenous iliac bone and freeze-dried bone allograft for repair of alveolar clefts in the presence of plasma rich in growth factors: a randomized clinical trial. J. cranio-maxillo-facial Surg. 45 (10), 1698–1703. doi:10.1016/j.jcms.2017.08.001
Sobolev, A., Valkov, A., Kossenko, A., Wolicki, I., Zinigrad, M., and Borodianskiy, K. (2019). Bioactive coating on Ti alloy with high osseointegration and antibacterial Ag nanoparticles. ACS Appl. Mater. and interfaces 11 (43), 39534–39544. doi:10.1021/acsami.9b13849
Solier, S., Müller, S., Cañeque, T., Versini, A., Mansart, A., Sindikubwabo, F., et al. (2023). A druggable copper-signalling pathway that drives inflammation. Nature 617 (7960), 386–394. doi:10.1038/s41586-023-06017-4
Sovova, S., Abalymov, A., Pekar, M., Skirtach, A. G., and Parakhonskiy, B. (2021). Calcium carbonate particles: synthesis, temperature and time influence on the size, shape, phase, and their impact on cell hydroxyapatite formation. J. Mater. Chem. B 9 (39), 8308–8320. doi:10.1039/d1tb01072g
Soylu, H. M., Chevallier, P., Copes, F., Ponti, F., Candiani, G., Yurt, F., et al. (2021). A novel strategy to coat dopamine-functionalized titanium surfaces with agarose-based hydrogels for the controlled release of gentamicin. Front. Cell Infect. Microbiol. 11, 678081. doi:10.3389/fcimb.2021.678081
Spellberg, B., Powers, J. H., Brass, E. P., Miller, L. G., and Edwards, J. E. (2004). Trends in antimicrobial drug development: implications for the future. Clin. Infect. Dis. official Publ. Infect. Dis. Soc. Am. 38 (9), 1279–1286. doi:10.1086/420937
Srouji, S., Ben-David, D., Lotan, R., Livne, E., Avrahami, R., and Zussman, E. (2011). Slow-release human recombinant bone morphogenetic protein-2 embedded within electrospun scaffolds for regeneration of bone defect: in vitro and in vivo evaluation. Tissue Eng. Part A 17 (3-4), 269–277. doi:10.1089/ten.tea.2010.0250
Tang, D., Tare, R. S., Yang, L. Y., Williams, D. F., Ou, K. L., and Oreffo, R. O. (2016). Biofabrication of bone tissue: approaches, challenges and translation for bone regeneration. Biomaterials 83, 363–382. doi:10.1016/j.biomaterials.2016.01.024
Tang, G., Liu, Z., Liu, Y., Yu, J., Wang, X., Tan, Z., et al. (2021). Recent trends in the development of bone regenerative biomaterials. Front. cell Dev. Biol. 9, 665813. doi:10.3389/fcell.2021.665813
Tardelli, J. D. C., Schiavon, M. A., and Dos Reis, A. C. (2024). Chitosan coatings on titanium-based implants - from development to characterization and behavior: a systematic review. Carbohydr. Polym. 344, 122496. doi:10.1016/j.carbpol.2024.122496
Tengjisi, L. Y., Zou, D., Yang, G., and Zhao, C. X. (2022). Bioinspired core-shell silica nanoparticles monitoring extra- and intra-cellular drug release. J. Colloid Interface Sci. 624, 242–250. doi:10.1016/j.jcis.2022.05.099
Tian, H., Du, J., Wen, J., Liu, Y., Montgomery, S. R., Scott, T. P., et al. (2016). Growth-Factor nanocapsules that enable tunable controlled release for bone regeneration. ACS nano 10 (8), 7362–7369. doi:10.1021/acsnano.5b07950
Vermeulen, S., Tahmasebi Birgani, Z., and Habibovic, P. (2022). Biomaterial-induced pathway modulation for bone regeneration. Biomaterials 283, 121431. doi:10.1016/j.biomaterials.2022.121431
Wang, L., You, X., Lotinun, S., Zhang, L., Wu, N., and Zou, W. (2020). Mechanical sensing protein PIEZO1 regulates bone homeostasis via osteoblast-osteoclast crosstalk. Nat. Commun. 11 (1), 282. doi:10.1038/s41467-019-14146-6
Wang, R., Ni, S., Ma, L., and Li, M. (2022a). Porous construction and surface modification of titanium-based materials for osteogenesis: a review. Front. Bioeng. Biotechnol. 10, 973297. doi:10.3389/fbioe.2022.973297
Wang, F., Sun, P., Xie, E., Ji, Y., Niu, Y., Li, F., et al. (2022b). Phytic acid/magnesium ion complex coating on PEEK fiber woven fabric as an artificial ligament with anti-fibrogenesis and osteogenesis for ligament-bone healing. Biomater. Adv. 140, 213079. doi:10.1016/j.bioadv.2022.213079
Wang, S., Zhao, X., Hsu, Y., He, Y., Wang, F., Yang, F., et al. (2023). Surface modification of titanium implants with Mg-containing coatings to promote osseointegration. Acta biomater. 169, 19–44. doi:10.1016/j.actbio.2023.07.048
Wei, X., Zhou, W., Tang, Z., Wu, H., Liu, Y., Dong, H., et al. (2023). Magnesium surface-activated 3D printed porous PEEK scaffolds for in vivo osseointegration by promoting angiogenesis and osteogenesis. Bioact. Mater. 20, 16–28. doi:10.1016/j.bioactmat.2022.05.011
Wennerberg, A., and Albrektsson, T. (2009). Effects of titanium surface topography on bone integration: a systematic review. Clin. Oral Implants Res. 20 (Suppl. 4), 172–184. doi:10.1111/j.1600-0501.2009.01775.x
Xiong, J., Lu, Q., Liu, Z., Hu, J., Cao, H., Wang, M., et al. (2025). Bioinspired hierarchical PEEK and amorphous silicon nitride composite coatings on titanium alloy biomedical implants. Small 21 (26), e2410313. doi:10.1002/smll.202410313
Xu, J., Zhou, X., Gao, Z., Song, Y. Y., and Schmuki, P. (2016). Visible-Light-Triggered drug release from TiO2 Nanotube arrays: a controllable antibacterial platform. Angew. Chem. Int. Ed. Engl. 55 (2), 593–597. doi:10.1002/anie.201508710
Xu, Y. M., Peng, H. M., Feng, B., and Weng, X. S. (2020). Progress of antibiotic-loaded bone cement in joint arthroplasty. Chin. Med. J. Engl. 133 (20), 2486–2494. doi:10.1097/cm9.0000000000001093
Yedekçi, B., Tezcaner, A., Alshemary, A. Z., Yılmaz, B., Demir, T., and Evis, Z. (2021). Synthesis and sintering of B, Sr, Mg multi-doped hydroxyapatites: structural, mechanical and biological characterization. J. Mech. Behav. Biomed. Mater 115, 104230. doi:10.1016/j.jmbbm.2020.104230
Yoo, H. S., Kim, G. J., Song, D. H., Chung, K. H., Lee, K. J., Kim, D. H., et al. (2017). Calcium supplement derived from gallus gallus domesticus promotes BMP-2/RUNX2/SMAD5 and suppresses TRAP/RANK expression through MAPK signaling activation. Nutrients 9 (5), 504. doi:10.3390/nu9050504
Yu, K., Lo, J. C., Mei, Y., Haney, E. F., Siren, E., Kalathottukaren, M. T., et al. (2015). Toward infection-resistant surfaces: achieving high antimicrobial peptide potency by modulating the functionality of polymer brush and peptide. ACS Appl. Mater. and interfaces 7 (51), 28591–28605. doi:10.1021/acsami.5b10074
Yu, K. E., Kwon, H. K., Dussik, C. M., Cahill, S. V., Back, J., Alder, K. D., et al. (2022). Enhancement of impaired MRSA-Infected fracture healing by combinatorial antibiotics and modulation of sustained inflammation. J. bone mineral Res. official J. Am. Soc. Bone Mineral Res. 37 (7), 1352–1365. doi:10.1002/jbmr.4570
Yue, C., Kuijer, R., Kaper, H. J., van der Mei, H. C., and Busscher, H. J. (2014). Simultaneous interaction of bacteria and tissue cells with photocatalytically activated, anodized titanium surfaces. Biomaterials 35 (9), 2580–2587. doi:10.1016/j.biomaterials.2013.12.036
Zang, S., Zhu, L., Luo, K., Mu, R., Chen, F., Wei, X., et al. (2017). Chitosan composite scaffold combined with bone marrow-derived mesenchymal stem cells for bone regeneration: in vitro and in vivo evaluation. Oncotarget 8 (67), 110890–110903. doi:10.18632/oncotarget.22917
Zeng, D., Debabov, D., Hartsell, T. L., Cano, R. J., Adams, S., Schuyler, J. A., et al. (2016). Approved glycopeptide antibacterial drugs: mechanism of action and resistance. Cold Spring Harb. Perspect. Med. 6 (12), a026989. doi:10.1101/cshperspect.a026989
Zhang, Y., Zhang, L., Li, B., and Han, Y. (2017). Enhancement in sustained release of antimicrobial peptide from dual-diameter-structured TiO(2) nanotubes for long-lasting antibacterial activity and cytocompatibility. ACS Appl. Mater. and interfaces 9 (11), 9449–9461. doi:10.1021/acsami.7b00322
Zhang, W., Wang, N., Yang, M., Sun, T., Zhang, J., Zhao, Y., et al. (2022). Periosteum and development of the tissue-engineered periosteum for guided bone regeneration. J. Orthop. Transl. 33, 41–54. doi:10.1016/j.jot.2022.01.002
Zhang, C., Zeng, C., Wang, Z., Zeng, T., and Wang, Y. (2023). Optimization of stress distribution of bone-implant interface (BII). Biomater. Adv. 147, 213342. doi:10.1016/j.bioadv.2023.213342
Zhao, Y. G., Meng, F. X., Li, B. W., Sheng, Y. M., Liu, M. M., Wang, B., et al. (2016). Gelatinases promote calcification of vascular smooth muscle cells by up-regulating bone morphogenetic protein-2. Biochem. biophysical Res. Commun. 470 (2), 287–293. doi:10.1016/j.bbrc.2016.01.067
Keywords: bone infection, antibacterial, electronic biological response, enzymatic hydrolysis response, bone repair, titanium-based materials
Citation: Zhang W, Guo Z, He Z, Zhou H, Liu H, Peng X, Yang X, Zhong R, Huang W, Chu L and Deng Z (2025) An ideal biomaterial: Ti-based bone repair material with dual-response antibacterial system and sustained drug release. Front. Bioeng. Biotechnol. 13:1619084. doi: 10.3389/fbioe.2025.1619084
Received: 27 April 2025; Accepted: 26 August 2025;
Published: 02 September 2025.
Edited by:
Raj Hazra, North Dakota State University, United StatesReviewed by:
Fei Xu, McMaster University, CanadaOlawale Alimi, University of Nebraska Medical Center, United States
Copyright © 2025 Zhang, Guo, He, Zhou, Liu, Peng, Yang, Zhong, Huang, Chu and Deng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhongliang Deng, ZGVuZ3psQGNxbXUuZWR1LmNu; Lei Chu, Y2h1bGVpQGhvc3BpdGFsLmNxbXUuZWR1LmNu
†These authors share first authorship
 Wentao Zhang
Wentao Zhang Zhaoyang Guo
Zhaoyang Guo Zhongyuan He
Zhongyuan He Hang Zhou1
Hang Zhou1