- Genetics of Prokaryotes, Faculty of Biology and Center for Biotechnology (CeBiTec), Bielefeld University, Bielefeld, Germany
Terpenes are biomolecules of significant industrial relevance, with applications in pharmaceuticals, cosmetics, and the food industry. Their biotechnological production is emerging, with Corynebacterium glutamicum, a Gram-positive bacterium traditionally employed for large-scale amino acid production, serving as a promising host. While metabolic engineering strategies have been extensively applied to enhance terpene titers in C. glutamicum, the role of medium composition, particularly trace elements, remains underexplored. In this study, the impact of trace element composition on trans-nerolidol production by engineered C. glutamicum was investigated. A Design of Experiments (DoE) approach identified MgSO4 as a critical factor, and the refined trace element composition led to a 34% increase in trans-nerolidol production. Further metabolic engineering efforts resulted in a final titer of 28.1 mg L-1. Subsequent fed-batch fermentation achieved a trans-nerolidol titer of 0.41 g L-1, representing the highest reported sesquiterpene titer being produced by C. glutamicum to date. Additionally, the refined trace element composition was successfully applied to patchoulol- and (+)-valencene-producing strains, leading to production increases of 15% and 72%, respectively. These findings demonstrate that trace element refinement and metabolic engineering act as complementary strategies for enhancing terpene production in a microbial production host.
1 Introduction
With more than 80,000 characterized molecules, terpenes are the largest and structurally most diverse group of natural products (Christianson, 2017). Fulfilling a myriad of functions in nature, terpenes are, for example, involved in photosynthesis (Zulfiqar et al., 2021), regulation of biotic and abiotic stress (Wang et al., 2023), defense and attraction (Gershenzon and Dudareva, 2007), as well as membrane fluidity (Zhou et al., 2015). Terpenes are also of significant industrial interest as they find application in pharmaceuticals, cosmetics, food, and biofuels (Caputi and Aprea, 2011; Wang et al., 2015; Fan et al., 2023). Most of these compounds have to be extracted from plants, which poses significant challenges due to low concentrations in plant tissues, seasonal and geographical fluctuations, and the extensive agricultural areas required. To avoid this, biotechnological production offers a sustainable and efficient alternative to produce terpenes (Moser and Pichler, 2019; Zhang and Hong, 2020). Among the different microbes available, the Gram-positive industrial workhorse Corynebacterium glutamicum, extensively used for the large-scale production of amino acids (Wendisch, 2020), is a promising cell factory for natural compounds as its deriving products are classified as GRAS (Cankar et al., 2023). In recent years, C. glutamicum has been engineered for the production of various terpenes including hemi-, mono-, sesqui-, di- and triterpenes (Kang et al., 2014; Henke et al., 2018a; Henke et al., 2018b; Sasaki et al., 2019; Lim et al., 2020; Luckie et al., 2024; Li et al., 2025; Lee et al., 2025). To increase the product titer, mostly metabolic engineering strategies were pursued. Competing pathways like carotenogenesis (Henke et al., 2018b) or in the central carbon metabolism (Li et al., 2025) were deleted. Lim et al. (2020) identified and overexpressed key enzymes of the methylerythritol 4-phosphate (MEP) pathway to enhance precursor supply. In contrast to that, Luckie et al. (2024) and Sasaki et al. (2019) introduced the heterologous mevalonate (MVA) pathway to circumvent endogenous regulation of the MEP pathway that might limit carbon flux. In addition, shake flasks cultivation conditions have been optimized (Li et al., 2025).
Metabolic engineering efforts have to be combined with process intensification: cultivation conditions and the cultivation medium may impact the overall performance of the strain, as the medium can influence cell growth and productivity (Galbraith et al., 2018). Since there is no universal approach for media optimization, different methods have been used. The one-factor-at-a-time (OFAT) experiments might be the most commonly applied technique for media optimization (Singh et al., 2017). Here, only one factor is varied while the other variables are kept constant. This approach was used to investigate the effect of several media additives for a poly (3-hydroxybutyrate) producer (Nikel et al., 2008). Although the OFAT technique is simple and convenient, the large number of experiments are laborious, time consuming, and costly. Furthermore, interactions between the variables cannot be detected and the optimum might be missed completely (Singh et al., 2017). By using statistical approaches like the design of experiments (DoE), the limitations of the OFAT can be overcome. This systematic approach allows to vary multiple parameters simultaneously, thereby, identifying significant variables, their interactions, and optimal conditions with minimal experimental effort (Fisher, 1926). The two-level fractional factorial Plackett-Burman design (PBD) aims to identify major effects while neglecting interactions. Due to the low number of experiments, this experimental design is often applied in early-stage development to identify significant parameters (Singh et al., 2017). PDB has been used for media optimization for enzyme productions like chitinase and β-amylase (Rama et al., 1999; Vaidya et al., 2003). To uncover interactions among the different variables and to determine optimum conditions, response surface methodology (RSM) becomes necessary. Therefore, experimental designs such as the Box-Behnken or central composite design (CCD) are required. The CCD consists of a (fractional) factorial core, star points that extend the design beyond the factorial levels as well as center points (Gündogdu et al., 2016; Singh et al., 2017). In terms of media optimization, CCD and RSM have been successfully used to improve the production of oxytetracycline and actinorhodin (Elibol, 2004; Singh et al., 2012).
The standard cultivation medium for C. glutamicum is the CGXII minimal medium (Keilhauer et al., 1993). Initially established for amino acid production, the components have been adapted to different production scenarios (Jeon et al., 2013; Hoffmann and Altenbuchner, 2014; Buchholz et al., 2014; Ko et al., 2018). To date, there is no medium dedicated for the production of terpenes by C. glutamicum. Variations in glucose concentration and C:N ratio have been investigated for isopentenol production, but exhibited effects comparable to standard CGXII conditions (Sasaki et al., 2019). Consequently, other media components might be worth to be investigated. Given that trace elements, despite their low concentrations, have been shown to enhance the production of l-lysine and carotenoids in C. glutamicum (Weuster-Botz et al., 1997; Meyer et al., 2025), as well as surfactin in Bacillus subtilis (Wei et al., 2007), xylitol in Debaryomyces hansenii (Bustos Vázquez et al., 2017), proteins in Pichia pastoris (Isidro et al., 2016; Tavasoli et al., 2017), and lipids and citric acid in Yarrowia lipolytica (Kumar et al., 2021), their potential to improve terpene biosynthesis should be further explored.
Therefore, the focus of this study was a DoE-based approach to optimize the trace element composition of CGXII medium for the production of terpenes with C. glutamicum. To study the effects of the trace elements on terpene production, trans-nerolidol was chosen as a model terpene. This sesquiterpene is used in decorative cosmetics like perfumes and shampoos as well as non-cosmetic products such as household cleansers and has been approved by the U.S. Food and Drug Administration as food flavoring agent (Chan et al., 2016). Furthermore, nerolidol exhibits antioxidant (Zhao et al., 2020), antitumor (Ambrož et al., 2015), and antidiabetic (Jiang and Zhang, 2022) activities, making it interesting for the pharmaceutical industry. Metabolic engineering was combined with media optimization to improve the overall production titer. In addition, the transferability of the refined trace elements was tested with patchoulol and (+)-valencene.
2 Materials and methods
2.1 Bacterial strains and growth conditions
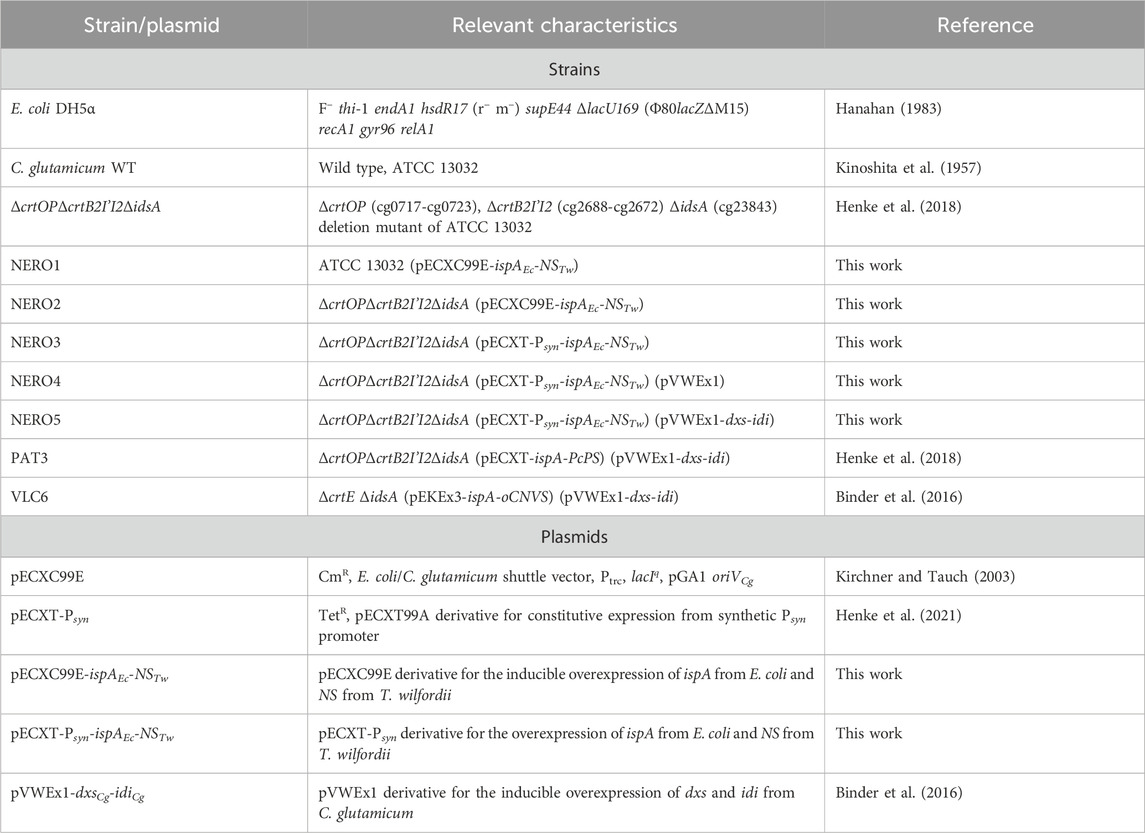
Strains and plasmids used in this study are listed in Table 1. Escherichia coli was used as cloning host and was cultivated in lysogeny broth (LB (Bertani, 1951)) at 37°C and 180 rpm. C. glutamicum was used for production experiments. Precultures were cultivated in 100 mL baffled shake flasks containing LB supplemented with 10 g L-1 glucose at 30°C and 120 rpm. The pre-cultivated cells were used to inoculate the main culture to an initial optical density (wavelength: 600 nm, OD600) of 1 determined by using the V-1200 spectrophotometer (VWR, Radnor, PA, United States). All trans-nerolidol producing experiments, including response surface methodology (see Section 2.3), were performed in 48-well FlowerPlates (Beckman Coulter, Brea, CA, United States) with a filling volume of 1 mL per well at 1,100 rpm and 30°C in the BioLector XT microcultivation system (Beckman Coulter, Brea, CA, United States) for 24 h. For the transfer of the refined trace elements to patchoulol and (+)-valencene, 100 mL baffled shake flasks with a filling volume of 10 mL and additional 10% (v v−1) dodecane as organic overlay were used instead of the BioLector. Cultivation lasted 48 h at 30°C and 120 rpm. 40 g L-1 glucose was added to the CGXII medium as carbon and energy source (Keilhauer et al., 1993) for all main cultures. If appropriate, the medium was supplemented with kanamycin (25 μg mL-1), tetracycline (5 μg mL-1), chloramphenicol (7.5 μg mL-1 for C. glutamicum or 30 μg mL-1 for E. coli). 1 mM isopropyl-β-D-1-thiogalactopyranoside (IPTG) was added at the start of the main cultivation to induce gene expression.
2.2 Molecular biological techniques
Primers for DNA amplification and sequencing (Supplementary Table S1) were purchased from Sigma-Aldrich (St. Louis, MO, United States). Plasmid DNA isolation (QIAwave Plasmid Miniprep, Qiagen, Venlo, Netherlands) and PCR clean-up (NucleoSpin® Gel and PCR Clean-up, Macherey-Nagel, Düren, Germany) were performed according to the manufacturer’s instructions. Nerolidol synthase (NS) gene from Tripterygium wilfordii (GenBank: KU588405) (Su et al., 2017) was codon optimized (Codon Optimization Tool, 2025) and synthesized by Twist Bioscience (San Francisco, CA, United States). Gene fragments were amplified using the Allin™ HiFi DNA polymerase (highQu GmbH, Kraichtal, Germany) and cloned into BamHI-digested (Thermo Fisher Scientific, Waltham, MA, United States) plasmids using Gibson Assembly (Gibson et al., 2009). CaCl2 chemocompetent E. coli DH5α were transformed with the Gibson Assembly reaction mix by heat shock at 42°C (Sambrook and Russell, 2001). Cloned DNA fragments were verified by DNA sequencing. Trans-Nerolidol producing C. glutamicum strains were created by transforming electrocompetent cells via electroporation and subsequent heat shock at 46°C with the respective plasmids (Eggeling and Bott, 2005).
2.3 Design of experiment and response surface methodology
Cultivation was performed as described in Section 2.1. Due to the total amount of 1 mL per well, the trace elements were prepared separately as 100x stock solutions to facilitate pipetting. Design and analysis of the experiment was performed by using R 4.4.1 (R Studio Team 2024) and the rsm package version 2.10.5 (Lenth, 2009). Within the experiment, the effect of the trace elements MgSO4, CaCl2, FeSO4, MnSO4, and ZnSO4 on the trans-nerolidol titer was investigated. The central composite design consisted of a 25 full factorial design as cube portion, ten face-centered star points and six center points. The concentration of each trace element at the center point was regarded as 100%. 50% and 150% of the center point concentration were chosen for the cube portion of the model. For the star points, only one out of the five trace elements was set to either 0% and 200%, respectively, while the others remained at 100%. The overall workflow was: first CCD with standard CGXII trace elements as center points, followed by a steepest ascent experiment. The best trace elements composition of the steepest ascent experiment was used as the center point for the second CCD. All combinations and volumes can be found in the Supplementary Material. The other media components remained constant as described in Section 2.1.
2.4 Terpene extraction and quantification
For the extraction of trans-nerolidol, 1 mL of hexane was added to 330 µL of cultivation broth and incubated at 50°C and 1,000 rpm for 30 min (ThermoMixer C, Eppendorf, Hamburg, Germany). The extraction mixture was centrifuged, the organic phase was removed, dried with sodium sulphate and transferred to GC-MS analysis. For patchoulol and (+)-valencene, the dodecane overlay was separated from the aqueous phase by centrifugation and was directly used for analysis. All terpenes were quantified using a TRACE GC Ultra gas chromatograph and a ISQ single quadrupole mass spectrometer equipped with an AS 3000 autosampler and a TraceGOLD™ TG-5 MS column (30 m × 0.25 mm x 0.25 µm) (Thermo Fisher Scientific, Waltham, MA, United States). 1 μL was injected in splitless mode. Helium was used as carrier gas at a constant flow of 1 mL min-1. Temperatures were set as the following: injector (250°C), interface (250°C), and ion source (220°C). The oven profile was set as the following: 80°C for 1 min, increased to 120°C at a rate of 10°C min-1, followed by 3°C min-1–160°C, and a further increase at 10°C min-1–270°C, which was held for 2 min. Mass spectra were recorded after a solvent cutoff at 14 min using a scanning range of 50–750 m/z at 20 scans s-1. Chromatograms were evaluated using Xcalibur 2.1.0 (Thermo Fisher Scientific, Waltham, MA, United States). Extracted-ion chromatograms at m/z = 93 were used for quantification of trans-nerolidol, m/z = 138 and m/z = 220 for patchoulol, and m/z = 161 for (+)-valencene. Analytical standards for trans-nerolidol, patchoulol, and (+)-valencene were purchased from Extrasynthese (Lyon, France), Biosynth (Staad, Switzerland), and Sigma-Aldrich (St. Louis, MO, United States), respectively.
2.5 Bioreactor fed-batch fermentation
Fed-batch fermentation using strain NERO5 was performed in a bioreactor with a total volume of 3.7 L (KLF, Bioengineering AG, Wald, Switzerland). The aspect ratio of the reactor was 2.6:1.0. Three six-bladed Rushton turbines were placed along the stirrer axis at 6, 12, and 18 cm from the bottom of the reactor with a stirrer to reactor diameter ratio of 0.39. 1 L of the high cell density medium based on Knoll et al. (2007) was used with some alterations: all trace elements were replaced by the refined trace elements in a 20x excess compared to the regular cultivation in CGXII (see Supplementary Table S2). Antibiotics and IPTG were added accordingly and the medium was inoculated to an OD600 of 1. 500 mL of a 600 g L-1 glucose solution was used as feed medium. A headspace overpressure of 0.5 bar was applied. The temperature was kept at 30°C during fermentation. The pH of 7 was automatically maintained by the addition of 10% (v v−1) H3PO4 and 25% (v v−1) NH3. An initial airflow of 0.25 NL min-1 was provided from the bottom through a ring sparger, which was manually increased during the fermentation to 0.75 NL min-1 when oxygen supply became limiting. The stirrer speed increased automatically in a stepwise manner from 400 rpm to 1,500 rpm, every time the relative dissolved oxygen saturation (rDOS) fell below 30%. The feed pump was primed when the rDOS fell below 60% for the first time. Subsequently, the feed pump was activated every time the rDOS exceeded 50% and stopped as soon as the rDOS fell below 50% thereby preventing overfeeding. To control foam formation, 0.6 mL L-1 of antifoam 204 was already added to the batch medium. If required, an antifoam probe controlled the supply of antifoam 204 during the process. Samples were automatically taken over the course of the fermentation and stored at 4°C until further use.
3 Results
3.1 Establishment of trans-nerolidol production in C. glutamicum
The genome of C. glutamicum codes for two geranylgeranyl pyrophosphate (GGPP) synthases (crtE and idsA), but neither of them synthesizes significant amounts of the sesquiterpene precursor farnesyl pyrophosphate (FPP) from the end products of the MEP pathway isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP) (Frohwitter et al., 2014). Trans-Nerolidol production in C. glutamicum wild type was enabled by overexpressing FPP synthase ispA from E. coli together with a codon-optimized nerolidol synthase from T. wilfordii from the plasmid pECXC99E (NERO1, see Figures 1, 2). To prevent further conversion of FPP to GGPP and subsequently to carotenoids, a metabolically engineered wild type derivative lacking both carotenoid operons as well as the GGPP synthase idsA (Henke et al., 2018b) was transformed with the plasmid pECXC99E-ispAEc-NSTw (NERO2). This strain did not synthesize carotenoids, thus increasing the trans-nerolidol titer 5.9-fold from 1.9 ± 0.1 to 11.2 ± 0.8 mg L-1. The engineered C. glutamicum strain NERO2 was subsequently used to study the effects of the trace element composition.
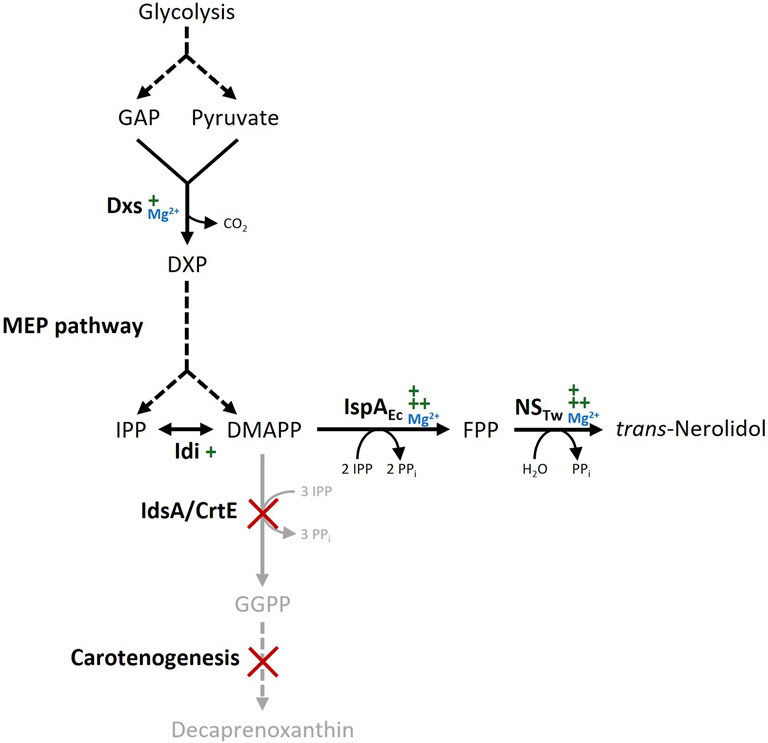
Figure 1. Biosynthetic pathway for trans-nerolidol production in Corynebacterium glutamicum. Dashed arrows represent multiple enzymatic steps, gene deletions are marked with red crosses, Mg2+ dependency of key enzymes is indicated in blue. Gene overexpression is indicated by green plus symbols; the number of symbols refers to expression strength. Abbreviations: GAP, glyceraldehyde 3-phosphate; DXP, 1-deoxy-D-xylulose 5-phosphate; IPP, isopentenyl pyrophosphate; DMAPP, dimethylallyl pyrophosphate; FPP, farnesyl pyrophosphate; GGPP, geranylgeranyl pyrophosphate; DXS, DXP synthase; MEP, 2-C-methyl-D-erythritol 4-phosphate; Idi, IPP isomerase; IspAEc, FPP synthase from Escherichia coli; NSTw, Nerolidol synthase from Tripterygium wilfordii; IdsA, GGPP synthase; CrtE, GGPP synthase.
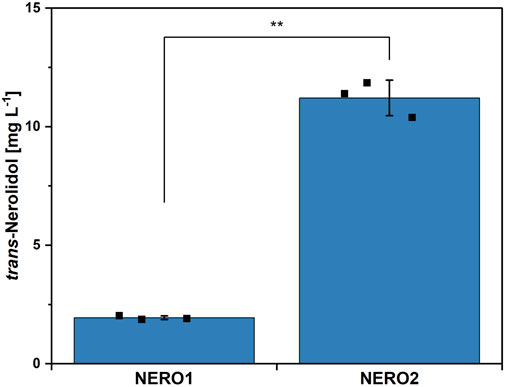
Figure 2. trans-Nerolidol production by C. glutamicum NERO1 and NERO2. Means, standard deviations as error bars, and single points of triplicate cultivations are given. Statistical significance was calculated with a Students’ t-test p < 0.05 (*), p < 0.01 (**), p < 0.005 (***).
3.2 Design of experiments for refinement of trace element composition
Although some media formulations for C. glutamicum contain additional trace elements like Na2MoO4 and H3BO3 (Weuster-Botz et al., 1997), the classical CGXII medium contains the seven trace elements MgSO4, CaCl2, FeSO4, MnSO4, ZnSO4, CuSO4, and NiCl2 (Keilhauer et al., 1993). To reduce the number of experiments, it was decided to neglect the two trace elements with the lowest concentration, being CuSO4 and NiCl2. This is in accordance with the fact that out of the seven trace elements in CGXII, copper and nickel ions are the metal ions used least as cofactors in enzymes (Waldron et al., 2009). Therefore, it was hypothesized that variation of the copper and nickel ion concentrations might influence terpene biosynthesis the least. A CCD approach, with the standard CGXII trace elements concentrations (see Supplementary Table S3) as center points, was performed in the BioLector microcultivation system using strain NERO2 (see Section 2.3).
The analysis of the trans-nerolidol titer showed no significant two-factor interactions between the trace elements (data not shown). This is why the data was subsequently evaluated considering only first order and quadratic effects. Significant first-order effects of MgSO4 as well as quadratic effects of MgSO4 and FeSO4 were observed (see Supplementary Table S4). Although the model possessed no significant lack of fit, the quadratic effects might be just artificial as the star points containing no MgSO4 or FeSO4 did not grow and therefore did not produce any trans-nerolidol (see boxplots in Supplementary Figure S1 and Supplementary Table S3). This is why only first order effects were considered, showing strong positive effects of MgSO4 (Table 2).
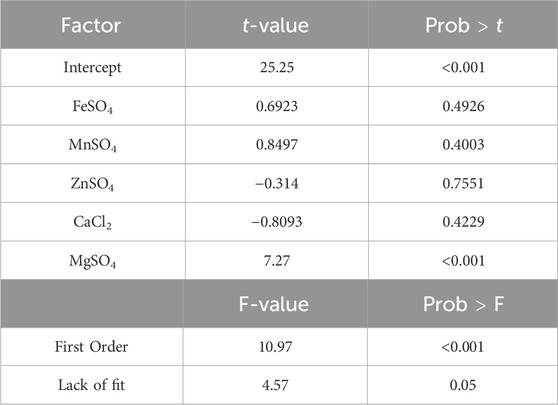
Table 2. Effects of the trace elements on trans-nerolidol titer considering first order effects. The effect of each factor, the F-values and their probabilities are given.
By applying only a first order model, no stationary point with a predicted optimum could be obtained. Furthermore, it was expected to gain more insight into the interactions between the different trace elements and their effects on trans-nerolidol production. A reasonable next step therefore is to follow the direction along where the response increases the fastest to end up at trace element concentrations that result in higher titers than the starting condition. This position would be the new center point used for a second round of CCD (Roberts et al., 2020). The so-called path of steepest ascent was calculated by the results of the first order model out of the CCD, yielding a set of trace element compositions and predicted titers (see Supplementary Table S5). The results of the steepest ascent analysis are shown in Figure 3. Although fluctuating, a trend of increasing production along the path of steepest ascent was observed. At a distance of 4.5, the highest titer (17.1 ± 0.7 mg L-1) was observed, being significantly higher than the control. This trace element composition was subsequently used as a new center point for another CCD, allowing to investigate concentration ranges that might be closer to an optimum (see Supplementary Table S6). After having performed the second CCD experiment, neither two-factor interactions, quadratic effects or first order models could be fitted significantly (data not shown).
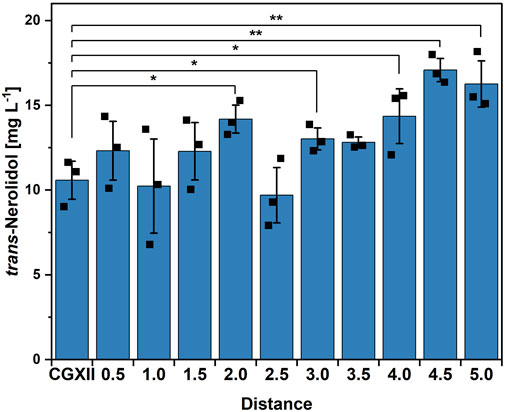
Figure 3. Steepest ascent experiment based on the first CCD. Means, standard deviations as error bars, and single points of triplicate cultivations are given. Statistical significance was calculated with a Students’ t-test p < 0.05 (*), p < 0.01 (**), p < 0.005 (***).
3.3 Verification of trace element composition
The second round of CCD did not result in a significant result. However, some of the tested trace element combinations within this new range of concentration showed a clearly increased titer compared to the control. To validate these observations, the best composition from the second CCD (see Supplementary Table S6, run 45) was compared to the standard trace elements and to the best combination of the steepest ascent experiment, which was used as the center point of the second CCD. As MgSO4 showed a strong positive effect in the first CCD (Table 2), the influence of a 4x increased MgSO4 concentration was investigated as well. The tested trace element compositions are listed in Supplementary Table S2 and the results are shown in Figure 4. All three trace element compositions resulted in significantly increased titers. The highest titer of 14.3 ± 0.4 mg L-1, corresponding to an improvement of 34%, was achieved using the trace element composition found within the second CCD. No statistical significance was found when comparing the high MgSO4 composition with the one from the second CCD. However, as the overall amount of trace elements changed the least when compared to the other compositions (see Supplementary Table S2), the combination obtained within the second CCD was now chosen to be the refined trace element combination.
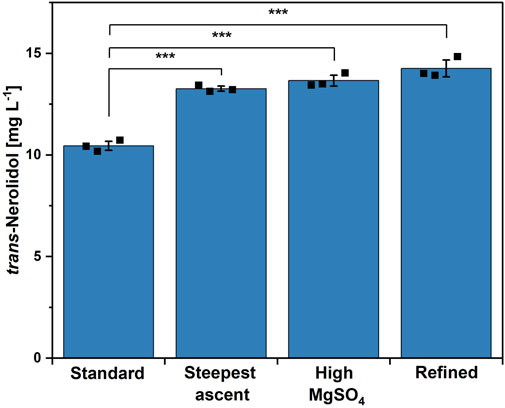
Figure 4. Comparison of different trace element compositions using strain NERO2. From left to right: standard CGXII trace elements, best composition of steepest ascent experiment, standard CGXII trace elements with 4x MgSO4 concentration, best composition of second CCD experiment (=refined). Means, standard deviations as error bars, and single points of triplicate cultivations are given. Statistical significance was calculated with a Students’ t-test p < 0.05 (*), p < 0.01 (**), p < 0.005 (***).
3.4 Improved gene overexpression of terminal biosynthesis enzymes and of MEP pathway genes
Trace element refinement improved trans-nerolidol production of the base strain NERO2. To answer the question, if the refined trace elements would also support higher trans-nerolidol production, strain NERO2 was further metabolically engineered (Figure 1) before standard and refined media were compared.
To improve the terminal biosynthesis, the synthetic operon of FPP synthase and trans-nerolidol synthase genes was cloned into the pECXT-Psyn vector containing the strong constitutive Psyn promoter (Henke et al., 2021) yielding strain NERO3. Using the standard trace elements, the stronger expression increased the titer significantly to 14.5 ± 0.3 mg L-1 (compare strains NERO2 and NERO3 in Figure 5). The addition of the empty vector pVWEx1 to NERO3 (NERO4; empty vector control) slightly decreased the titer. To improve the entry reaction into the MEP pathway as well as the interconversion of IPP and DMAPP, dxs and idi were overexpressed as a synthetic operon (NERO5) resulting in a titer of 25.1 ± 0.7 mg L-1, which was 2.4 times higher compared to strain NERO2 (Figure 5). Next, it was tested, if the refined traces would have a positive impact on trans-nerolidol production by strain NERO5. Indeed, the refined trace elements further significantly improved trans-nerolidol production to the titer to 28.1 ± 1.0 mg L-1 (+12%).
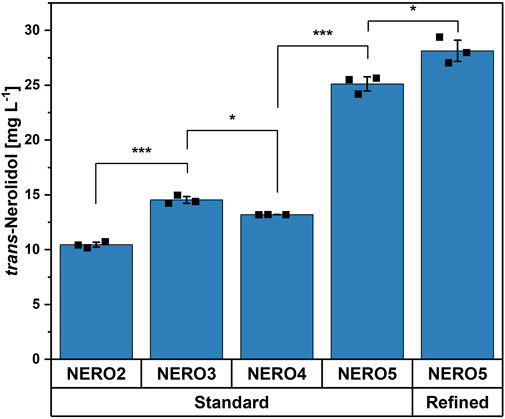
Figure 5. Comparison of different trans-nerolidol producing strains with standard and refined trace elements. Means, standard deviations as error bars, and single points of triplicate cultivations are given. Statistical significance was calculated with a Students’ t-test p < 0.05 (*), p < 0.01 (**), p < 0.005 (***).
3.5 Bioreactor fed-batch fermentation
Since the combination of trace element refinement and metabolic engineering was successful in a microcultivation system, it was evaluated whether the trans-nerolidol production using C. glutamicum strain NERO5 can be stably transferred to a 1.5 L fed-batch process, corresponding to a 1,500-fold volume increase. The high cell density medium based on Knoll et al. (2007) was used for fermentation. However, the trace element composition of this medium corresponds neither to the standard CGXII nor to the refined trace elements. Hence, the refined trace element composition was used instead, while all other media components remained unchanged. To account for the higher cell density, the concentration of refined trace elements was increased proportionally 20-fold relative to standard CGXII cultivations in BioLector or shake flasks (see Supplementary Table S2). The glucose feed was consumed after 74 h resulting in a biomass titer of 57.5 gCDW L-1. The trans-nerolidol titer reached 0.41 g L-1, corresponding to a volumetric productivity of 5.6 mg L-1 h-1 (Figure 6).
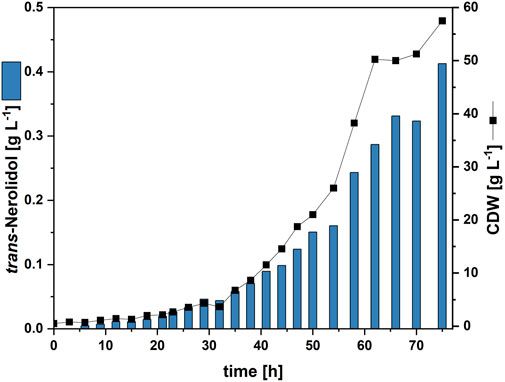
Figure 6. Fed-batch fermentation of NERO5. Cell dry weight concentration (CDW) and trans-nerolidol titer are given.
3.6 Transfer of refined trace elements to the production of other sesquiterpenes
To test if the refined medium can be transferred as a general strategy for the production of other sesquiterpenes, it was used to cultivate previously established strains overproducing patchoulol (Henke et al., 2018b) and (+)-valencene (Binder et al., 2016). The refined trace elements increased the (+)-valencene titer from 6.0 ± 0.3 mg L-1 using standard CGXII trace elements to 10.3 ± 1.7 mg L-1, corresponding to a 72% improvement. Also, the patchoulol titer was increased, i.e., from 10.4 ± 0.4 mg L-1 to 11.9 ± 0.2 mg L-1, corresponding to a 15% improvement (Figure 7). Thus, the medium with refined trace elements developed for trans-nerolidol production also significantly improved production of two other sesquiterpenes.
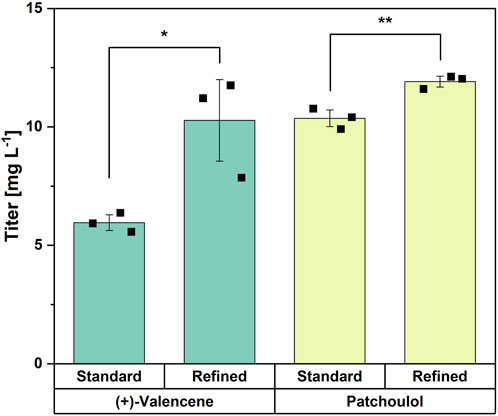
Figure 7. Comparison of standard CGXII with refined trace elements for the production of (+)-valencene and patchoulol. Means, standard deviations as error bars, and single points of triplicate cultivations are given. Statistical significance was calculated with a Students’ t-test p < 0.05 (*), p < 0.01 (**), p < 0.005 (***).
4 Discussion
In this study, trans-nerolidol production in C. glutamicum was enabled, thereby expanding the repertoire of C15 terpenes beyond the previously reported compounds patchoulol (Henke et al., 2018b) (+)-valencene (Binder et al., 2016), and α-farnesene (Lim et al., 2020). To improve its production a combined approach of metabolic engineering and media refinement was chosen which increased the product titer about 15 fold from 1.9 ± 0.1 to 28.1 ± 1.0 mg L-1. Significant improvements by applying the refined trace elements were also demonstrated for the production of patchoulol and (+)-valencene.
Instead of employing an OFAT approach to investigate the effects of the trace element composition on trans-nerolidol production, a DoE methodology was implemented, reducing the number of experiments required while simultaneously enabling the investigation of interactions. In an initial round of experiments, MgSO4 was identified as a significant positive factor influencing trans-nerolidol production. A subsequent steepest ascent experiment was followed by a second round of CCD, which did not yield statistically significant results. This outcome might be associated with broad concentration ranges in the experimental design. While magnesium, iron, and zinc are essential for the growth of C. glutamicum, the remaining trace elements exhibit less pronounced effects (Nakayama et al., 1964; Yang et al., 2021; Meyer et al., 2025). To improve future studies, experimental conditions should be chosen to avoid complete growth inhibition. Nevertheless, the positive effect of MgSO4 was confirmed by using a high MgSO4 medium.
In general, terpene synthases require divalent cations, typically Mg2+, as cofactors (Rudolf and Chang, 2020). Some terpene synthases are also known to use alternative metal cofactors such as manganese or iron (Steele et al., 1998). The metal cofactor mediates the abstraction of the diphosphate, thereby creating a reactive carbocation, and additionally neutralizes the diphosphate over the course of the reaction (Vattekkatte et al., 2018). In the absence of an divalent metal ion, terpene synthases are not active (Degenhardt and Gershenzon, 2000). However, it is crucial for the activity of the terpene synthase, that the correct metal cofactor is bound, which has been shown for magnesium and manganese particularly (Steele et al., 1998; Whitehead et al., 2023). Strong Mg2+ dependency was shown for the trans-nerolidol synthase used in this study (Su et al., 2017). Since unbound metals compete for a binding site, a phenomenon known as mismetalation can occur, leading to reduced enzyme activity (Foster et al., 2022). It was shown for the trans-nerolidol synthases from maize and kiwifruit that Mg2+ supported the highest activity, whereas Mn2+ or Zn2+ diminished activity (Degenhardt and Gershenzon, 2000; Schnee et al., 2002; Green et al., 2012). Hence, it can be hypothesized that a refined trace element composition, with increased Mg2+, but reduced Mn2+, Fe2+, and Zn2+, mitigated unfavorable mismetalation, thereby enhancing enzymatic activity and increasing terpene titers. This favorable ratio was successfully achieved either by supplying a high surplus of Mg2+ (high MgSO4 medium) or by moderately increasing Mg2+ while reducing Mn2+, Fe2+, and Zn2+ concentrations (refined medium). An OFAT approach might have resulted in a similar result as obtained for the high MgSO4 medium. However, the specific combination of an elevated magnesium ion concentration along the reduction of other components was only identifiable through a DoE-based approach, highlighting the advantage of this methodology over conventional optimization strategies.
Although the terpene synthases expressed in the patchoulol- and (+)-valencene-producing strains have been shown to utilize Mg2+ as a cofactor (Munck and Croteau, 1990; Sharon-Asa et al., 2003), the refined trace element composition resulted in varying degrees of improvement, potentially related to differences in cofactor affinity and preferences among the enzymes. Besides terpene synthases, other enzymes involved in terpene biosynthesis may also benefit from increased Mg2+ availability, supporting the broader applicability of the refined trace element formulation for the production of diverse terpene compounds. Dxs, which catalyzes the rate-limiting step of the MEP pathway and, thus, supply of the precursor substrates IPP and DMAPP, requires Mg2+ (Xiang et al., 2007). Enhanced Mg2+ availability may therefore contribute to an overall increase in MEP pathway flux. In addition, FPP synthase ispA from E. coli, which was heterologously overexpressed in this study as well as in the patchoulol- and (+)-valencene-producing strains, was also shown to utilize Mg2+ as a cofactor (Hosfield et al., 2004). Recently, the importance of zinc ions for α-bisabolene production in Rhodosporidium toruloides was shown, which was associated with its role as a cofactor for the isopentenyl diphosphate isomerase and in lipid synthesis (Adamczyk et al., 2025). However, magnesium was not investigated in this study (Adamczyk et al., 2025).
In terms of metabolic engineering, three steps were applied in this study to enhance trans-nerolidol biosynthesis. First, a strain lacking the carotenogenesis was selected to prevent conversion of FPP to GGPP and onwards to carotenoids. In the second step, two expression levels of the terminal biosynthetic enzymes were evaluated, since terpene synthase activity is often the rate-limiting step under high-flux conditions (Whitehead et al., 2023). In addition to the enhanced enzymatic activity facilitated by the optimized trace element composition, the stronger expression showed a positive effect on trans-nerolidol titer. Instead of solely increasing the expression strength, the application of a translational fusion of FFP synthase and NS was successfully shown to enhance nerolidol production in yeast (Cheah et al., 2023). Additionally, the choice of the synthase is important as trans-nerolidol synthases exhibit considerable variability in activity (Tan et al., 2023), which can be further improved by enzyme engineering (Liu et al., 2022). Furthermore, the overexpression of dxs and idi, encoding the key enzymes of the MEP pathway, almost doubled the product titer. Previous studies have demonstrated further MEP pathway optimization, such as the overexpression of ispD and ispF or dxr along with dxs and idi (Lim et al., 2020). However, no universal approach has yet been identified, as the most effective target genes vary depending on the specific terpene product making additional fine-tuning necessary (Lim et al., 2020). Although not as prominent as the 34% increase for the basic strain NERO2, the refined trace elements still achieved a significant 12% improvement when applied for the engineered strain NERO5, underlining the importance of combined strain and media optimization.
Strain NERO5, in combination with the refined trace element composition, was subsequently implemented in a fed-batch fermentation process, yielding 0.41 g L-1 trans-nerolidol. As so far 60 mg L-1 of patchoulol were produced in a fed-batch fermentation by C. glutamicum (Henke et al., 2018b), the process shown here reached the highest titer of a sesquiterpene produced by C. glutamicum reported to date. In contrast, 16 g L-1, 4.2 g L-1, and 11.1 g L-1 of trans-nerolidol have been produced by E. coli, Saccharomyces cerevisiae, and Y. lipolytica, respectively. (Liu et al., 2022; Cheah et al., 2023; Tan et al., 2023). Given that the refined trace element composition appears to influence multiple steps within the terpene biosynthetic pathway and its applicability to the production of other sesquiterpenes, existing terpene-producing strains may benefit from medium adaptation.
Instead of using a chemically defined glucose-based medium as used in this study, alternative substrates could be employed. C. glutamicum was shown to grow on a variety of complex substrates derived from agro-industrial side streams, including residues from wheat processing and aquaculture as well as hydrolysates from rice straw, oat spelts, orange peel and hazelnut husk (Buschke et al., 2011; Burgardt et al., 2021; Sasikumar et al., 2021; Pakalın et al., 2023; Schmitt et al., 2023; Junker et al., 2024). Notably, some of these complex substrates were capable of replacing the trace elements and micronutrients typically provided by CGXII medium. However, the composition of trace elements in these substrates may not be optimal for terpene biosynthesis. While the addition of beneficial trace elements (i.e., Mg2+) is possible, the removal of undesired trace elements such as Mn2+ or chelating compounds from the substrate might be challenging. Additionally, the presence of endogenous terpenes, such as limonene in orange peels (Wikandari et al., 2015) may interfere with downstream processing by complicating the purification of a target terpene product such as trans-nerolidol. Although these hydrolysates represent a sustainable and cost-effective alternative to conventional carbon sources, substrate selection remains critical.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
JS: Methodology, Investigation, Conceptualization, Writing – review and editing, Writing – original draft. SL: Writing – original draft, Investigation. FS: Writing – original draft, Investigation. NH: Conceptualization, Writing – review and editing, Funding acquisition. VW: Funding acquisition, Writing – review and editing, Conceptualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Part of this research was funded by the German Federal Ministry of Education and Research (BMBF) project KaroTec (grant number: 03VP09460). We acknowledge the financial support of the German Research Foundation (DFG) and the Open Access Publication Fund of Bielefeld University for the article processing charge. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fbioe.2025.1621955/full#supplementary-material
Abbreviations
CCD, central composite design; CDW, cell dry weight; DMAPP, dimethylallyl pyrophosphate; DoE, design of experiment; FPP, farnesyl pyrophosphate; GGPP, geranylgeranyl pyrophosphate; IPP, isopentenyl pyrophosphate; IPTG, isopropyl-β-D-1-thiogalactopyranoside; MEP, methylerythritol 4-phosphate; MVA, mevalonate; OFAT, one-factor-at-a-time; PDB, Plackett-Burman design; rDOS, relative dissolved oxygen saturation; RSM, response surface methodology.
References
Adamczyk, P. A., Hwang, H. J., Chang, T.-H., Gao, Y., Baidoo, E. E. K., Kim, J., et al. (2025). The oleaginous yeast Rhodosporidium toruloides engineered for biomass hydrolysate-derived (E)-α-bisabolene production. Metab. Eng. 90, 92–105. doi:10.1016/j.ymben.2025.02.014
Ambrož, M., Boušová, I., Skarka, A., Hanušová, V., Králová, V., Matoušková, P., et al. (2015). The influence of sesquiterpenes from Myrica rubra on the antiproliferative and pro-oxidative effects of doxorubicin and its accumulation in cancer cells. Molecules 20, 15343–15358. doi:10.3390/molecules200815343
Bertani, G. (1951). Studies on lysogenesis I. J. Bacteriol. 62, 293–300. doi:10.1128/jb.62.3.293-300.1951
Binder, D., Frohwitter, J., Mahr, R., Bier, C., Grünberger, A., Loeschcke, A., et al. (2016). Light-controlled cell factories: employing photocaged isopropyl-β-D-thiogalactopyranoside for light-mediated optimization of lac promoter-based gene expression and (+)-valencene biosynthesis in Corynebacterium glutamicum. Appl. Environ. Microbiol. 82, 6141–6149. doi:10.1128/aem.01457-16
Buchholz, J., Graf, M., Blombach, B., and Takors, R. (2014). Improving the carbon balance of fermentations by total carbon analyses. Biochem. Eng. J. 90, 162–169. doi:10.1016/J.BEJ.2014.06.007
Burgardt, A., Prell, C., and Wendisch, V. F. (2021). Utilization of a wheat sidestream for 5-aminovalerate production in Corynebacterium glutamicum. Front. Bioeng. Biotechnol. 9, 732271. doi:10.3389/fbioe.2021.732271
Buschke, N., Schröder, H., and Wittmann, C. (2011). Metabolic engineering of Corynebacterium glutamicum for production of 1,5-diaminopentane from hemicellulose. Biotechnol. J. 6, 306–317. doi:10.1002/biot.201000304
Bustos Vázquez, G., Pérez-Rodríguez, N., Salgado, J. M., Oliveira, R. P. D. S., and Domínguez, J. M. (2017). Optimization of salts supplementation on xylitol production by Debaryomyces hansenii using a dynthetic medium or corncob hemicellulosic hydrolyzates and further scaled up. Ind. Eng. Chem. Res. 56, 6579–6589. doi:10.1021/acs.iecr.7b01120
Cankar, K., Henke, N. A., and Wendisch, V. F. (2023). Functional food additives/ingredients production by engineered Corynebacterium glutamicum. Syst. Microbiol. Biomanuf 3, 110–121. doi:10.1007/S43393-022-00141-4
Caputi, L., and Aprea, E. (2011). Use of terpenoids as natural flavouring compounds in food industry. Recent Pat. Food Nutr. Agric. 3, 9–16. doi:10.2174/2212798411103010009
Chan, W. K., Tan, L. T. H., Chan, K. G., Lee, L. H., and Goh, B. H. (2016). Nerolidol: a sesquiterpene alcohol with multi-faceted pharmacological and biological activities. Molecules 21, 529. doi:10.3390/molecules21050529
Cheah, L. C., Liu, L., Stark, T., Plan, M. R., Peng, B., Lu, Z., et al. (2023). Metabolic flux enhancement from the translational fusion of terpene synthases is linked to terpene synthase accumulation. Metab. Eng. 77, 143–151. doi:10.1016/j.ymben.2023.03.012
Christianson, D. W. (2017). Structural and chemical biology of terpenoid cyclases. Chem. Rev. 117, 11570–11648. doi:10.1021/acs.chemrev.7B00287
Codon Optimization Tool (2025). IDT. Available online at: https://eu.idtdna.com/pages/tools/codon-optimization-tool (Accessed February 21, 2025).
Degenhardt, J., and Gershenzon, J. (2000). Demonstration and characterization of (E)-nerolidol synthase from maize: a herbivore-inducible terpene synthase participating in (3E)-4,8-dimethyl-1,3,7-nonatriene biosynthesis. Planta 210, 815–822. doi:10.1007/S004250050684
Eggeling, L., and Bott, M. (2005). Handbook of Corynebacterium glutamicum. 1st Edn. Boca Raton: CRC Press. doi:10.1201/9781420039696
Elibol, M. (2004). Optimization of medium composition for actinorhodin production by Streptomyces coelicolor A3(2) with response surface methodology. Process Biochem. 39, 1057–1062. doi:10.1016/S0032-9592(03)00232-2
Fan, M., Yuan, S., Li, L., Zheng, J., Zhao, D., Wang, C., et al. (2023). Application of terpenoid compounds in food and pharmaceutical products. Fermentation 9, 119. doi:10.3390/fermentation9020119
Fisher, R. A. (1926). The arrangement of field experiments. J. Ministry Agric. 33, 503–515. Available online at: https://repository.rothamsted.ac.uk/item/8v61q/the-arrangement-of-field-experiments (Accessed February 26, 2025).
Foster, A. W., Young, T. R., Chivers, P. T., and Robinson, N. J. (2022). Protein metalation in biology. Curr. Opin. Chem. Biol. 66, 102095. doi:10.1016/j.cbpa.2021.102095
Frohwitter, J., Heider, S. A. E., Peters-Wendisch, P., Beekwilder, J., and Wendisch, V. F. (2014). Production of the sesquiterpene (+)-valencene by metabolically engineered Corynebacterium glutamicum. J. Biotechnol. 191, 205–213. doi:10.1016/j.jbiotec.2014.05.032
Galbraith, S. C., Bhatia, H., Liu, H., and Yoon, S. (2018). Media formulation optimization: current and future opportunities. Curr. Opin. Chem. Eng. 22, 42–47. doi:10.1016/j.coche.2018.08.004
Gershenzon, J., and Dudareva, N. (2007). The function of terpene natural products in the natural world. Nat. Chem. Biol. 3 (7), 408–414. doi:10.1038/nchembio.2007.5
Gibson, D. G., Young, L., Chuang, R. Y., Venter, J. C., Hutchison, C. A., and Smith, H. O. (2009). Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 6, 343–345. doi:10.1038/nmeth.1318
Green, S. A., Chen, X., Nieuwenhuizen, N. J., Matich, A. J., Wang, M. Y., Bunn, B. J., et al. (2012). Identification, functional characterization, and regulation of the enzyme responsible for floral (E)-nerolidol biosynthesis in kiwifruit (Actinidia chinensis). J. Exp. Bot. 63, 1951–1967. doi:10.1093/jxb/err393
Gündogdu, T. K., Deniz, I., Çalişkan, G., Şahin, E. S., and Azbar, N. (2016). Experimental design methods for bioengineering applications. Crit. Rev. Biotechnol. 36, 368–388. doi:10.3109/07388551.2014.973014
Hanahan, D. (1983). Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166, 557–580. doi:10.1016/S0022-2836(83)80284-8
Henke, N. A., Frohwitter, J., Peters-Wendisch, P., and Wendisch, V. F. (2018a). “Carotenoid production by recombinant Corynebacterium glutamicum: strain construction, cultivation, extraction, and quantification of carotenoids and terpenes,” in Microbial carotenoids: methods and protocols. Editors C. Barreiro,, and J.-L. Barredo (New York: Humana Press Inc.), 127–141. doi:10.1007/978-1-4939-8742-9
Henke, N. A., Krahn, I., and Wendisch, V. F. (2021). Improved plasmid-based inducible and constitutive gene expression in Corynebacterium glutamicum. Microorganisms 9, 204–215. doi:10.3390/microorganisms9010204
Henke, N. A., Wichmann, J., Baier, T., Frohwitter, J., Lauersen, K. J., Risse, J. M., et al. (2018b). Patchoulol production with metabolically engineered Corynebacterium glutamicum. Genes (Basel) 9, 219. doi:10.3390/genes9040219
Hoffmann, J., and Altenbuchner, J. (2014). Hyaluronic acid production with Corynebacterium glutamicum: effect of media composition on yield and molecular weight. J. Appl. Microbiol. 117, 663–678. doi:10.1111/jam.12553
Hosfield, D. J., Zhang, Y., Dougan, D. R., Broun, A., Tari, L. W., Swanson, R. V., et al. (2004). Structural basis for bisphosphonate-mediated inhibition of isoprenoid biosynthesis. J. Biol. Chem. 279, 8526–8529. doi:10.1074/jbc.C300511200
Isidro, I. A., Portela, R. M., Clemente, J. J., Cunha, A. E., and Oliveira, R. (2016). Hybrid metabolic flux analysis and recombinant protein prediction in Pichia pastoris X-33 cultures expressing a single-chain antibody fragment. Bioproc Biosyst. Eng. 39, 1351–1363. doi:10.1007/S00449-016-1611-Z
Jeon, J. M., Thangamani, R., Song, E., Lee, H. W., and Yang, Y. H. (2013). Media optimization of corynebacterium glutamicum for succinate production under oxygen-deprived condition. J. Microbiol. Biotechnol. 23, 211–217. doi:10.4014/jmb.1206.06057
Jiang, N., and Zhang, Y. (2022). Antidiabetic effects of nerolidol through promoting insulin receptor signaling in high-fat diet and low dose streptozotocin-induced type 2 diabetic rats. Hum. Exp. Toxicol. 41, 9603271221126487–212. doi:10.1177/09603271221126487
Junker, N., Sariyar Akbulut, B., and Wendisch, V. F. (2024). Utilization of orange peel waste for sustainable amino acid production by Corynebacterium glutamicum. Front. Bioeng. Biotechnol. 12, 1419444. doi:10.3389/fbioe.2024.1419444
Kang, M. K., Eom, J. H., Kim, Y., Um, Y., and Woo, H. M. (2014). Biosynthesis of pinene from glucose using metabolically-engineered Corynebacterium glutamicum. Biotechnol. Lett. 36, 2069–2077. doi:10.1007/S10529-014-1578-2
Keilhauer, C., Eggeling, L., and Sahm, H. (1993). Isoleucine synthesis in Corynebacterium glutamicum: molecular analysis of the ilvB-ilvN-ilvC operon. J. Bacteriol. 175, 5595–5603. doi:10.1128/jb.175.17.5595-5603.1993
Kinoshita, S., Udaka, S., and Shimono, M. (1957). Studies on the amino acid fermentation part I. Production of L-glutamic acid by various microorganisms. J. Gen. Appl. Microbiol. 3, 193–205. doi:10.2323/jgam.3.193
Kirchner, O., and Tauch, A. (2003). Tools for genetic engineering in the amino acid-producing bacterium Corynebacterium glutamicum. J. Biotechnol. 104, 287–299. doi:10.1016/S0168-1656(03)00148-2
Knoll, A., Bartsch, S., Husemann, B., Engel, P., Schroer, K., Ribeiro, B., et al. (2007). High cell density cultivation of recombinant yeasts and bacteria under non-pressurized and pressurized conditions in stirred tank bioreactors. J. Biotechnol. 132, 167–179. doi:10.1016/j.jbiotec.2007.06.010
Ko, Y. J., Joo, Y. C., Hyeon, J. E., Lee, E., Lee, M. E., Seok, J., et al. (2018). Biosynthesis of organic photosensitizer Zn-porphyrin by diphtheria toxin repressor (DtxR)-mediated global upregulation of engineered heme biosynthesis pathway in Corynebacterium glutamicum. Sci. Rep. 8, 14460. doi:10.1038/S41598-018-32854-9
Kumar, L. R., Yellapu, S. K., Tyagi, R. D., and Drogui, P. (2021). Optimization of trace elements in purified glycerol for microbial lipid and citric acid production by Yarrowia lipolytica SKY7. Syst. Microbiol. Biomanufacturing 1, 76–89. doi:10.1007/S43393-020-00006-8
Lee, H. J., Kim, C., Heo, Y. B., Kim, S. E., and Woo, H. M. (2025). Bacterial biosynthesis of abietane-type diterpene ferruginol from glucose. Microb. Cell Fact. 24, 67. doi:10.1186/S12934-025-02691-3
Lenth, R. (2009). Response-surface methods in R, using rsm. J. Stat. Softw. 32, 1–17. doi:10.18637/jss.v032.i07
Li, J., Wang, X., Xokat, X., Wan, Y., Gao, X., Wang, Y., et al. (2025). Metabolic engineering of Corynebacterium glutamicum for producing different types of triterpenoids. ACS Synth. Biol. 14, 819–832. doi:10.1021/acssynbio.4C00737
Lim, H., Park, J., and Woo, H. M. (2020). Overexpression of the key enzymes in the methylerythritol 4-phosphate pathway in Corynebacterium glutamicum for improving farnesyl diphosphate-derived terpene production. J. Agric. Food Chem. 68, 10780–10786. doi:10.1021/acs.jafc.0C04307
Liu, F., Liu, S. C., Qi, Y. K., Liu, Z., Chen, J., Wei, L. J., et al. (2022). Enhancing trans-nerolidol productivity in Yarrowia lipolytica by improving precursor supply and optimizing nerolidol synthase activity. J. Agric. Food Chem. 70, 15157–15165. doi:10.1021/acs.jafc.2C05847
Luckie, B. A., Kashyap, M., Pearson, A. N., Chen, Y., Liu, Y., Valencia, L. E., et al. (2024). Development of Corynebacterium glutamicum as a monoterpene production platform. Metab. Eng. 81, 110–122. doi:10.1016/j.ymben.2023.11.009
Meyer, F., Schmitt, I., Wendisch, V. F., and Henke, N. A. (2025). Response surface-based media optimization for astaxanthin production in Corynebacterium glutamicum. Front. Bioeng. Biotechnol. 13, 1516522. doi:10.3389/fbioe.2025.1516522
Moser, S., and Pichler, H. (2019). Identifying and engineering the ideal microbial terpenoid production host. Appl. Microbiol. Biotechnol. 103, 5501–5516. doi:10.1007/S00253-019-09892-Y
Munck, S. L., and Croteau, R. (1990). Purification and characterization of the sesquiterpene cyclase patchoulol synthase from Pogostemon cablin. Arch. Biochem. Biophys. 282, 58–64. doi:10.1016/0003-9861(90)90086-E
Nakayama, K., Sato, Z., and Kinoshita, S. (1964). Growth of a glutamic acid producing bacterium and related bacteria I. Effect of iron salts, ferrichrome, amino acids and some other compounds. J. Gen. Appl. Microbiol. 10, 143–155. doi:10.2323/jgam.10.143
Nikel, P. I., Pettinari, M. J., Galvagno, M. A., and Méndez, B. S. (2008). Poly(3-hydroxybutyrate) synthesis from glycerol by a recombinant Escherichia coli arcA mutant in fed-batch microaerobic cultures. Appl. Microbiol. Biotechnol. 77, 1337–1343. doi:10.1007/S00253-007-1255-7
Pakalın, B., Kurpejović, E., Bastem, G. M., Sayar, N. A., Wendisch, V. F., and Akbulut, B. S. (2023). Valorization of hazelnut husk as a carbon source for l-DOPA production with Corynebacterium glutamicum. Biochem. Eng. J. 190, 108768. doi:10.1016/j.bej.2022.108768
Rama, P., Reddy, M., Reddy, G., and Seenayya, G. (1999). Production of thermostable β-amylase and pullulanase by Clostridium thermosulfurogenes SV2 in solid-state fermentation: screening of nutrients using Plackett-Burman design. Bioprocess Eng. 21, 175–179. doi:10.1007/PL00009069
Roberts, T. M., Kaltenbach, H. M., and Rudolf, F. (2020). Development and optimisation of a defined high cell density yeast medium. Yeast 37, 336–347. doi:10.1002/yea.3464
R Studio Team (2024). A language and environment for statistical computing. Available online at: http://www.r-project.org.
Rudolf, J. D., and Chang, C. Y. (2020). Terpene synthases in disguise: enzymology, structure, and opportunities of non-canonical terpene synthases. Nat. Prod. Rep. 37, 425–463. doi:10.1039/c9np00051h
Sambrook, J., and Russell, D. W. (2001). Molecular cloning: a laboratory manual. 3rd Edn. New York: Cold Spring Harbor Laboratory Press.
Sasaki, Y., Eng, T., Herbert, R. A., Trinh, J., Chen, Y., Rodriguez, A., et al. (2019). Engineering Corynebacterium glutamicum to produce the biogasoline isopentenol from plant biomass hydrolysates. Biotechnol. Biofuels 12, 41–15. doi:10.1186/S13068-019-1381-3
Sasikumar, K., Hannibal, S., Wendisch, V. F., and Nampoothiri, K. M. (2021). Production of biopolyamide precursors 5-amino valeric acid and putrescine from rice straw hydrolysate by engineered Corynebacterium glutamicum. Front. Bioeng. Biotechnol. 9, 635509. doi:10.3389/fbioe.2021.635509
Schmitt, I., Meyer, F., Krahn, I., Henke, N. A., Peters-Wendisch, P., and Wendisch, V. F. (2023). From aquaculture to aquaculture: production of the fish feed additive astaxanthin by Corynebacterium glutamicum using aquaculture sidestream. Molecules 28, 1996. doi:10.3390/molecules28041996
Schnee, C., Köllner, T. G., Gershenzon, J., and Degenhardt, J. (2002). The maize gene terpene synthase 1 encodes a sesquiterpene synthase catalyzing the formation of (E)-β-farnesene, (E)-nerolidol, and (E,E)-farnesol after herbivore damage. Plant Physiol. 130, 2049–2060. doi:10.1104/pp.008326
Sharon-Asa, L., Shalit, M., Frydman, A., Bar, E., Holland, D., Or, E., et al. (2003). Citrus fruit flavor and aroma biosynthesis: isolation, functional characterization, and developmental regulation of Cstps1, a key gene in the production of the sesquiterpene aroma compound valencene. Plant J. 36, 664–674. doi:10.1046/J.1365-313X.2003.01910.X
Singh, N., Rai, V., and Tripathi, C. K. M. (2012). Production and optimization of oxytetracycline by a new isolate Streptomyces rimosus using response surface methodology. Med. Chem. Re 21, 3140–3145. doi:10.1007/S00044-011-9845-4
Singh, V., Haque, S., Niwas, R., Srivastava, A., Pasupuleti, M., and Tripathi, C. K. M. (2017). Strategies for fermentation medium optimization: an in-depth review. Front. Microbiol. 7, 2087. doi:10.3389/fmicb.2016.02087
Steele, C. L., Crock, J., Bohlmann, J., and Croteau, R. (1998). Sesquiterpene synthases from grand fir (Abies grandis): Comparison of constitutive and wound-induced activities, and cDNA isolation characterization, and bacterial expression of δ-selinene synthase and γ-humulene synthase. J. Biol. Chem. 273, 2078–2089. doi:10.1074/jbc.273.4.2078
Su, P., Hu, T., Liu, Y., Tong, Y., Guan, H., Zhang, Y., et al. (2017). Functional characterization of NES and GES responsible for the biosynthesis of (E)-nerolidol and (E,E)-geranyllinalool in Tripterygium wilfordii. Sci. Rep. 7, 40851–40859. doi:10.1038/srep40851
Tan, N., Ong, L., Shukal, S., Chen, X., and Zhang, C. (2023). High-yield biosynthesis of trans-nerolidol from sugar and glycerol. J. Agric. Food Chem. 71, 8479–8487. doi:10.1021/ACS.JAFC.3C01161
Tavasoli, T., Arjmand, S., Siadat, S. O. R., Shojaosadati, S. A., and Lotfi, A. S. (2017). Enhancement of alpha 1-antitrypsin production in Pichia pastoris by designing and optimizing medium using elemental analysis. Iran. J. Biotechnol. 15, 224–231. doi:10.15171/ijb.1808
Vaidya, R., Vyas, P., and Chhatpar, H. S. (2003). Statistical optimization of medium components for the production of chitinase by Alcaligenes xylosoxydans. Enzyme Microb. Technol. 33, 92–96. doi:10.1016/S0141-0229(03)00100-5
Vattekkatte, A., Garms, S., Brandt, W., and Boland, W. (2018). Enhanced structural diversity in terpenoid biosynthesis: enzymes, substrates and cofactors. Org. Biomol. Chem. 16, 348–362. doi:10.1039/c7ob02040f
Waldron, K. J., Rutherford, J. C., Ford, D., and Robinson, N. J. (2009). Metalloproteins and metal sensing. Nature 460, 823–830. doi:10.1038/nature08300
Wang, Q., Zhao, X., Jiang, Y., Jin, B., and Wang, L. (2023). Functions of representative terpenoids and their biosynthesis mechanisms in medicinal plants. Biomolecules 13, 1725. doi:10.3390/biom13121725
Wang, X., Ort, D. R., and Yuan, J. S. (2015). Photosynthetic terpene hydrocarbon production for fuels and chemicals. Plant Biotechnol. J. 13, 137–146. doi:10.1111/pbi.12343
Wei, Y. H., Lai, C. C., and Chang, J. S. (2007). Using Taguchi experimental design methods to optimize trace element composition for enhanced surfactin production by Bacillus subtilis ATCC 21332. Process Biochem. 42, 40–45. doi:10.1016/j.procbio.2006.07.025
Wendisch, V. F. (2020). Metabolic engineering advances and prospects for amino acid production. Metab. Eng. 58, 17–34. doi:10.1016/j.ymben.2019.03.008
Weuster-Botz, D., Kelle, R., Frantzen, M., and Wandrey, C. (1997). Substrate controlled fed-batch production of l-lysine with Corynebacterium glutamicum. Biotechnol. Prog. 13, 387–393. doi:10.1021/bp970034J
Whitehead, J. N., Leferink, N. G. H., Johannissen, L. O., Hay, S., and Scrutton, N. S. (2023). Decoding catalysis by terpene synthases. ACS Catal. 13, 12774–12802. doi:10.1021/acscatal.3C03047
Wikandari, R., Nguyen, H., Millati, R., Niklasson, C., and Taherzadeh, M. J. (2015). Improvement of biogas production from orange peel waste by leaching of limonene. Biomed. Res. Int. 2015, 1–6. doi:10.1155/2015/494182
Xiang, S., Usunow, G., Lange, G., Busch, M., and Tong, L. (2007). Crystal structure of 1-deoxy-d-xylulose 5-phosphate synthase, a crucial enzyme for isoprenoids biosynthesis. J. Biol. Chem. 282, 2676–2682. doi:10.1074/jbc.m610235200
Yang, P., Chen, Y., and Gong, A. (2021). Development of a defined medium for Corynebacterium glutamicum using urea as nitrogen source. 3 Biotech. 11, 405. doi:10.1007/S13205-021-02959-6
Zhang, C., and Hong, K. (2020). Production of terpenoids by synthetic biology approaches. Front. Bioeng. Biotechnol. 8, 347. doi:10.3389/fbioe.2020.00347
Zhao, M., Zhang, N., Gao, T., Jin, J., Jing, T., Wang, J., et al. (2020). Sesquiterpene glucosylation mediated by glucosyltransferase UGT91Q2 is involved in the modulation of cold stress tolerance in tea plants. New Phytol. 226, 362–372. doi:10.1111/NPH.16364
Zhou, Y., Stuart-Williams, H., Grice, K., Kayler, Z. E., Zavadlav, S., Vogts, A., et al. (2015). Allocate carbon for a reason: priorities are reflected in the 13C/12C ratios of plant lipids synthesized via three independent biosynthetic pathways. Phytochemistry 111, 14–20. doi:10.1016/j.phytochem.2014.12.005
Keywords: Corynebacterium glutamicum, trans-nerolidol, terpenes, design of experiment, media optimization, metabolic engineering
Citation: Seeger J, Lohoff S, Schmitfranz F, Henke NA and Wendisch VF (2025) Enabling and improving trans-nerolidol production by Corynebacterium glutamicum: combining metabolic engineering and trace elements medium refinement. Front. Bioeng. Biotechnol. 13:1621955. doi: 10.3389/fbioe.2025.1621955
Received: 02 May 2025; Accepted: 06 June 2025;
Published: 23 June 2025.
Edited by:
Heng Song, Wuhan University, ChinaReviewed by:
Jiangang Yang, Chinese Academy of Sciences (CAS), ChinaChengsen Cui, Chinese Academy of Sciences (CAS), China
Copyright © 2025 Seeger, Lohoff, Schmitfranz, Henke and Wendisch. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Volker F. Wendisch, dm9sa2VyLndlbmRpc2NoQHVuaS1iaWVsZWZlbGQuZGU=
†Present addresses: Nadja A. Henke, Institute for Process Engineering in Life Sciences, Karlsruhe Institute of Technology (KIT), Karlsruhe, Germany
 Jan Seeger
Jan Seeger Nadja A. Henke
Nadja A. Henke Volker F. Wendisch
Volker F. Wendisch