- 1Joint and Sports Medicine Center, Ningbo No. 6 Hospital, Ningbo, Zhejiang, China
- 2Ningbo Clinical Research Center for Orthopedics, Sports Medicine & Rehabilitation, Ningbo, Zhejiang, China
- 3Department of Orthopaedic Surgery, Beijing Jishuitan Hospital, Capital Medical University, Fourth Clinical College of Peking University, National Center for Orthopaedics, Beijing, China
- 4Beijing Research Institute of Traumatology and Orthopaedics, Beijing Jishuitan Hospital, Capital Medical University, Fourth Clinical College of Peking University, National Center for Orthopaedics, Beijing, China
- 5Department of Spinal Surgery, Shihezi General Hospital of the Eighth Division, Shihezi, China
- 6Department of Clinical Laboratory, Beijing Jishuitan Hospital, Capital Medical University, Fourth Clinical College of Peking University, National Center for Orthopaedics, Beijing, China
Object: Our previous systematic review of either computed tomography (CT)-based or magnetic resonance imaging (MRI)-based patient-specific instrumentation (PSI) systems in total knee arthroplasty (TKA) included literature up to June 2016. However, the quickly evolving field warranted an update. Therefore, the aim of this systematic review and meta-analysis was to provide updated, evidence-based insights comparing the outcomes of CT-based versus MRI-based PSI systems in TKA.
Methods: We conducted comprehensive searches of PubMed, Embase, and the Cochrane Library databases from inception to February 2025. Prospective comparative trials that compared CT-based versus MRI-based PSI systems for TKA were included. Our predefined primary outcome was the incidence of outliers in overall coronal limb alignment. Secondary outcomes encompassed the accuracy of component alignment, operation time, and clinical outcomes.
Results: Nine publications reporting seven eligible trials were identified. Six trials involving a total of 407 knees were included for qualitative analysis, with five trials suitable for quantitative meta-analysis. The integrated results revealed no significant differences between CT- and MRI-based PSI systems concerning the outlier incidence of coronal overall limb alignment, the outlier incidence of coronal/sagittal alignment of the femoral/tibial component, the angular errors of coronal overall limb alignment, the angular errors of the femoral/tibial component in the coronal plane, or incidence of change of implant size of the femoral/tibial component. However, CT-based PSI systems were associated with significantly greater angular errors in coronal limb alignment (mean difference [MD]: 0.69°; 95% CI, 0.03°–1.36°; P = 0.04) and a prolonged operative time (MD: 5.02 min; 95% CI, 1.26min–8.79 min; P = 0.009) when compared to MRI-based systems. Clinical outcomes, while not amenable to meta-analysis due to clinical heterogeneity, showed no significant differences between groups during short-to mid-term follow-up.
Conclusion: This finding is inconsistent with our previous study. Contrary to our previous findings, current evidence indicates no significant difference in alignment outcomes between CT-based and MRI-based PSI systems for TKA. Additionally, short-to mid-term clinical outcomes were comparable between the two imaging modalities.
Systematic Review Registration: identifier CRD42022339910.
Background
The past decade has witnessed remarkable advancements in digital orthopedic technologies, highlighted by the rise of robot-assisted total joint replacement as a breakthrough (Halm-Pozniak et al., 2023; Youssef et al., 2024; Bini et al., 2020). However, the prohibitive initial investment required for robotic equipment, combined with additional costs associated with disposable instruments and consumables, has predominantly restricted robotic-assisted procedures to high-volume medical centers. Middle- and low-volume hospitals, constrained by financial barriers and limited economic returns, consequently encounter significant challenges in adopting this advanced technology (Barbash and Glied, 2010; Ng et al., 2023; Hua and Salcedo, 2022). In this context, patient-specific instrumentation (PSI) has emerged as a compelling alternative for total knee arthroplasty (TKA), which offers customized surgical cutting guides generated from preoperative imaging data (Mattei et al., 2016). This individualized approach provides distinct advantages, including personalized implant alignment, improved surgical accuracy, and enhanced cost-effectiveness (Gong et al., 2019; Vide et al., 2017; Jaffry et al., 2014; Hickey et al., 2023). Robotic systems typically involve high initial capital investments (approximately USD 1.2 million), whereas PSI generally requires negligible capital investment but incurs per-case expenses comparable to robotic systems (e.g., USD 1520 vs. USD 1390) (Hickey et al., 2023).
Currently, PSI blocks can be fabricated based on either magnetic resonance imaging (MRI) or computed tomography (CT), both serving as foundational imaging modalities for the preoperatively design of PSI jigs (Lionberger et al., 2014). MRI typically offers superior visualization of cartilage and soft tissues but has inherent limitations in clearly defining bony anatomy. Conversely, CT excels in accurately depicting bone structures but lacks sufficient detail in visualizing cartilage and soft tissues, potentially influencing the precision of preoperative modeling and surgical planning. Despite the widespread use of these imaging techniques, uncertainty persists regarding which modality is more effective in accurately restoring the mechanical axis and ensuring precise component alignment in TKA. Although multiple prospective trials have been conducted on this issue (Fritschy and Messerli, 2011; Asada et al., 2014; Ensini et al., 2014; Pfitzner et al., 2014; Silva et al., 2016; Schotanus et al., 2016; Kang et al., 2020; Thijs et al., 2020; Theeuwen et al., 2024), their findings have been inconsistent, emphasizing the necessity for further investigation.
To address this uncertainty, we have previously conducted a systematic review and meta-analysis to compare MRI-based PSI systems with CT-based PSI systems (Wu et al., 2017). Our initial findings indicated that MRI-based PSI systems achieved superior accuracy compared to CT-based systems regarding coronal limb axis alignment in TKA (Wu et al., 2017). Given the rapid evolution of PSI technology and the continuous influx of new evidence, we have since updated our systematic review and meta-analysis to provide clinicians and researchers with the latest available data. Furthermore, this updated review aims to offer a quantitative evaluation of the effects of PSI on short-to mid-term clinical outcomes, an aspect that has not been comprehensively addressed in previous literature.
Methods
This updated systematic review and meta-analysis was conducted in accordance with the guidelines outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins and Green, 2011) and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Moher et al., 2009).
Protocol registration
The review was prospectively registered in the PROSPERO database (CRD42023393302).
Literature search
Two independent reviewers designed and executed a comprehensive literature search across PubMed, Embase, and the Cochrane Library databases, covering all publications from inception to February 2025, without language restrictions. The search strategy integrated exploded Medical Subject Headings (MeSH) terms and relevant keywords related to TKA, PSI, CT, and MRI, along with their variants. The detailed search strategy is provided in Supplementary Material. Additionally, the ClinicalTrials.gov registry (https://clinicaltrials.gov/) was also queried to identify ongoing or unpublished clinical trials. Reference lists of eligible studies and relevant reviews were also examined to capture additional pertinent studies.
Selection criteria
Records were managed using EndNote® version 21 (Clarivate Analytics). After removing duplicates, titles and abstracts were screened independently by two reviewers, and the full texts of potentially eligible studies were subsequently assessed by both reviewers. Disagreements were resolved through discussion until a consensus was reached. Studies were included if they fulfilled the following criteria:
Population: Patients diagnosed with end-stage knee osteoarthritis scheduled for primary TKA.
Intervention: CT-based PSI for TKA.
Comparison: MRI-based PSI for TKA.
Outcomes: At least one of the predefined outcome measures.
Study Design: Prospective randomized controlled trials (RCTs) or prospective non-randomized controlled trials (non-RCTs).
Data extraction
Data abstraction was independently performed by the same reviewers who conducted the initial study selection. The collected information included: first author, publication year, location of study, publication journal, study design, clinical setting, number of participants, characteristics of study participants, manufacturers and imaging protocols for both CT- and MRI-based PSI, types of implants used, methods for evaluating alignment accuracy, surgeon experience, dimensional accuracy, and outcome measurements. If any aspect of the study design and conduct was unclear, the corresponding authors of the study were contacted for clarification.
Outcome measurements
The predefined primary outcome was the incidence of outliers in coronal overall limb alignment, defined as deviations exceeding 3° from the preoperative plan (applicable to both overall alignment and individual components) (Jeffery et al., 1991; Ritter et al., 1994; Fang et al., 2009; Gromov et al., 2014). This threshold was chosen because previous studies consistently associate alignment deviations greater than 3° with increased risks of adverse clinical outcomes, including implant failure, poorer functional outcomes, and higher revision rates (Jeffery et al., 1991; Ritter et al., 1994; Fang et al., 2009; Gromov et al., 2014). Secondary outcomes included the incidence of outliers in the coronal/sagittal alignment of the femoral and tibial components, angular errors in coronal overall limb alignment, angular errors of the femoral and tibial components in the coronal plane, operation time, perioperative changes in the implant sizes of the femoral and tibial components, and clinical outcomes at follow-up.
Quality assessment
The risk of bias for each included study was independently evaluated by two reviewers using the Cochrane risk-of-bias tool (Higgins et al., 2011). The assessment covered key domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other potential sources of bias. Studies were classified as having a low risk of bias if all domains (excluding the blinding of participants and personnel) were rated as low risk; otherwise, they were deemed to have an unclear or high risk of bias (Higgins and Green, 2011). Any discrepancies were resolved by discussion.
Statistical analysis
We combined the studies included in this update with those included in our previous review to determine the feasibility of performing a meta-analysis. For dichotomous outcomes, risk ratios (RRs) with 95% confidence intervals (CIs) were calculated. For continuous outcomes, mean differences (MDs) with 95% CIs were computed. Statistical heterogeneity was assessed using the I2 statistic; a P-value ≥0.05 indicated non-significant heterogeneity, while I2 values > 50% were considered indicative of substantial heterogeneity (Higgins and Thompson, 2002; Higgins et al., 2003). In account of clinical heterogeneity (e.g., variability in surgeon experience with PSI), a random-effects model was applied for data pooling. All statistical tests were two-sided, with a P-value <0.05 considered statistically significant. Potential publication bias was examined by visually inspecting funnel plots and statistically using Egger’s test (Macaskill et al., 2001; Egger et al., 1997). All analyses were performed using Stata version 14.0 (StataCorp LP) and Review Manager version 5.4 (Nordic Cochrane Center).
Results
Study selection
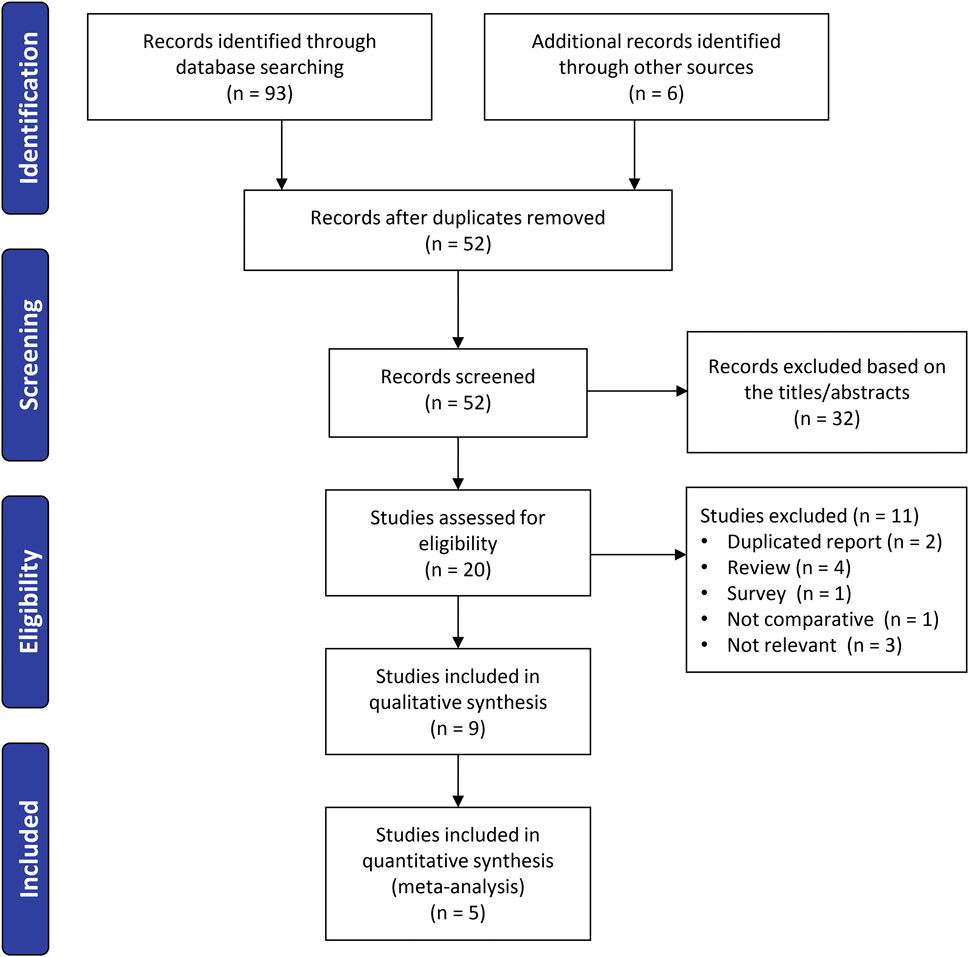
The initial literature search identified 93 records. After deduplication, 52 unique abstracts were screened. Of these, 20 full-text articles were reviewed in detail, and after applying the inclusion criteria, nine studies (Fritschy and Messerli, 2011; Asada et al., 2014; Ensini et al., 2014; Pfitzner et al., 2014; Silva et al., 2016; Schotanus et al., 2016; Kang et al., 2020; Thijs et al., 2020; Theeuwen et al., 2024) published between 2011 and 2024 were included in the systematic review. Among these, five studies (Asada et al., 2014; Ensini et al., 2014; Pfitzner et al., 2014; Schotanus et al., 2016; Kang et al., 2020) provided sufficient data for inclusion in the final meta-analysis (Figure 1).
Description of the studies
Table 1 summarizes the main characteristics of the included trials. The nine studies represented seven distinct clinical trials conducted across seven different countries (Switzerland, Japan, Italy, Germany, Portugal, Netherlands, and South Korea). Four trials were RCTs (Ensini et al., 2014; Pfitzner et al., 2014; Schotanus et al., 2016; Theeuwen et al., 2024) while three trials were prospective non-RCTs (Fritschy and Messerli, 2011; Asada et al., 2014; Silva et al., 2016). Three trials employed both CT- and MRI-based PSI systems from the same manufacturer (Silva et al., 2016; Schotanus et al., 2016; Theeuwen et al., 2024), whereas three trials used PSI systems from different manufacturers (Asada et al., 2014; Ensini et al., 2014; Pfitzner et al., 2014), and one study did not specify the manufacturer (Fritschy and Messerli, 2011). Variations existed among manufacturers in terms of radiographic imaging protocols and prosthesis types. Furthermore, postoperative radiographic measurements used to assess lower-limb mechanical alignment accuracy and component positioning were heterogeneous, and the surgeons’ experience with conventional TKA and PSI-assisted TKA varied considerably.
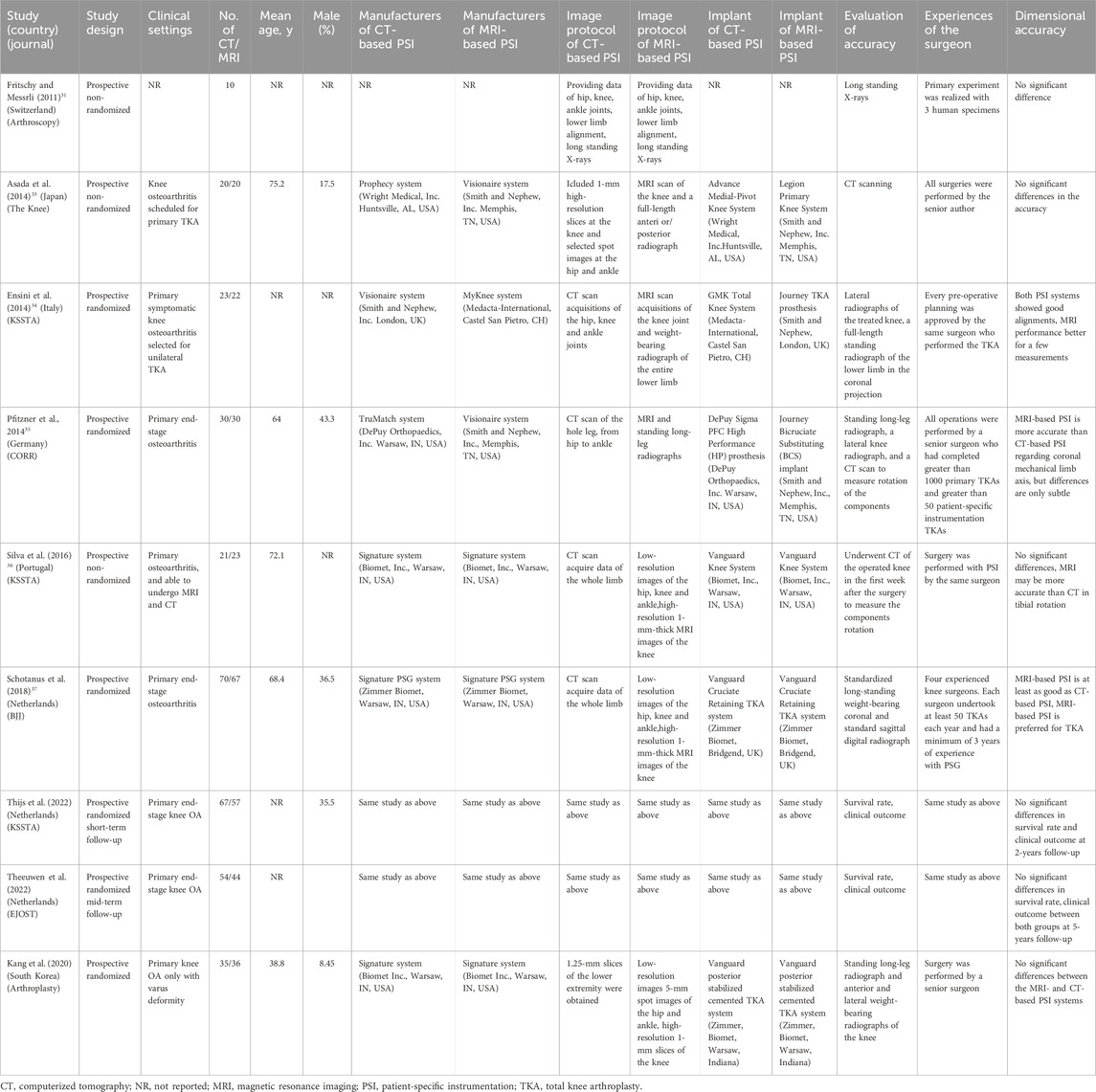
Table 1. Characteristics of included prospective studies comparing CT-based versus MRI-based patient-specific instrumentation for total knee arthroplasty.
Quality assessment
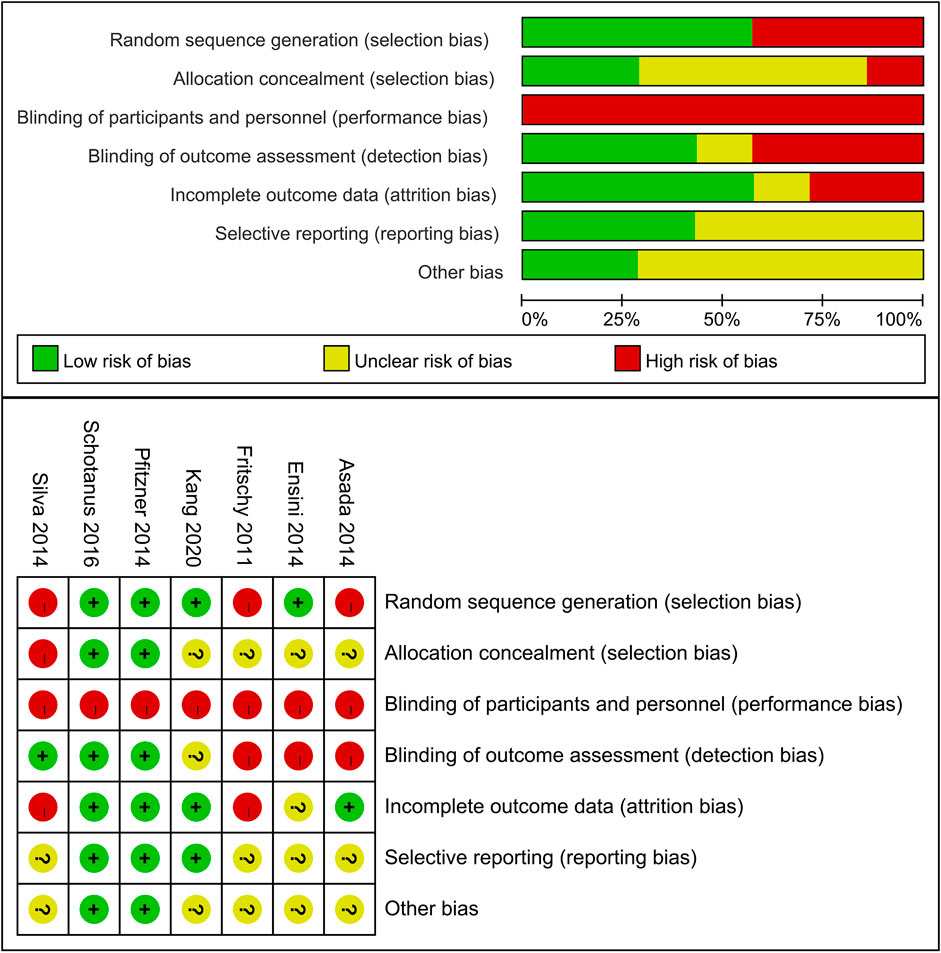
Figure 2 summarizes the risk of bias assessment for the included studies. For the non-RCTs (Fritschy and Messerli, 2011; Asada et al., 2014; Silva et al., 2016), random sequence generation and allocation concealment were rated as unclear or high risk. Due to inherent differences between CT- and MRI-based PSI systems, especially when sourced from different manufacturers, blinding of participants was challenging. However, blinding of outcome assessment was feasible and successfully implemented in three trials (Ensini et al., 2014; Pfitzner et al., 2014; Silva et al., 2016). Overall, two trials (Pfitzner et al., 2014; Schotanus et al., 2016) were categorized as low risk of bias, whereas four (Fritschy and Messerli, 2011; Asada et al., 2014; Ensini et al., 2014; Silva et al., 2016) were considered to have a high risk of bias. Nevertheless, radiological outcomes, being relatively objective, were considered less susceptible to bias related to insufficient blinding.
Primary outcome
Data on the primary outcome were available from five trials (Fritschy and Messerli, 2011; Asada et al., 2014; Ensini et al., 2014; Silva et al., 2016), encompassing a total of 353 knees. Meta-analysis indicated no significant difference in the outlier incidence of coronal overall limb alignment between MRI-based PSI and CT-based PSI was associated with a (RR: 1.54; 95% CI: 1.00–2.37; P = 0.055) with no heterogeneity detected (I2 = 0%) (Figure 3).
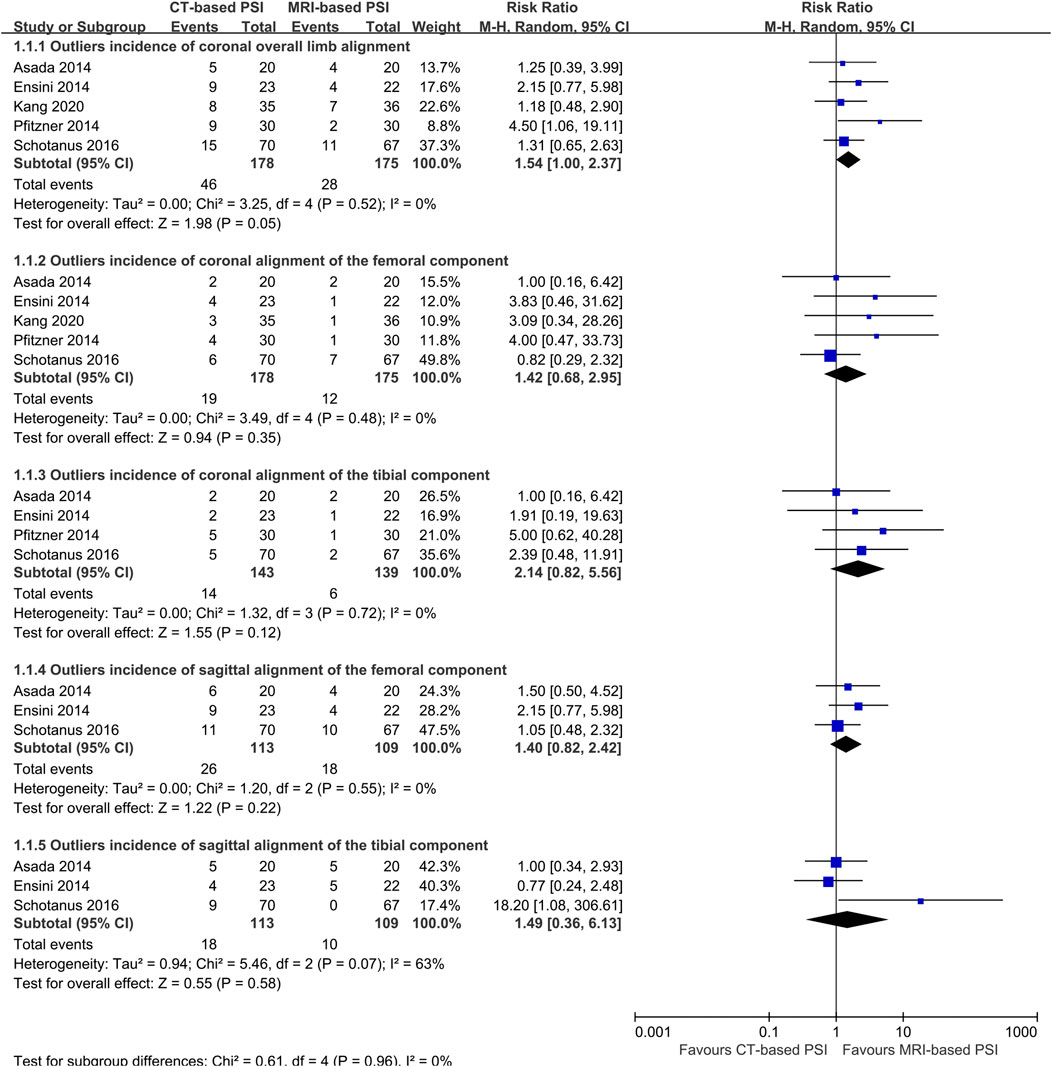
Figure 3. Forest plots illustrating the pooled effects comparing CT-versus MRI-based Patient-Specific Instrumentation for: outliers incidence of the coronal overall limb alignment; - outliers incidence of the coronal alignment of the femoral component; - outliers incidence of the coronal alignment of the tibial component; - outliers incidence of the sagittal alignment of the femoral component; - outliers incidence of the sagittal alignment of the tibial component; CI, confidence interval; M-H, Mantel-Haenszel.
Secondary outcomes
Five trials reported the accuracy of the component alignment (Fritschy and Messerli, 2011; Asada et al., 2014; Ensini et al., 2014; Silva et al., 2016). There were no statistically significant differences between CT- and MRI-based PSI regarding the outlier incidence for:
Coronal alignment of the femoral component (RR: 1.42; 95% CI: 0.68–2.80; P = 0.52); coronal alignment of the tibial component (RR: 2.14; 95% CI: 0.82–5.56; P = 0.12); sagittal alignment of the femoral component (RR: 1.40; 95% CI: 0.82–2.42; P = 0.22); sagittal alignment of the tibial component (RR: 1.49; 95% CI: 0.36–6.13; P = 0.58); (Figure 3).
In contrast, CT-based PSI was associated with significantly greater angular errors in coronal overall limb alignment (MD: 0.69°; 95% CI: 0.0.03°–1.36°; P = 0.04). No significant differences were found regarding angular errors of the femoral component (MD: 0.06°; 95% CI: 0.22°–0.35°; P = 0.65) or the tibial component (MD: 0.02°; 95% CI: 0.25°–0.21°; P = 0.86) in the coronal plane (Figure 4). Furthermore, CT-based PSI was associated with a longer operation time (MD: 5.02 min; 95% CI: 1.26–8.79; P = 0.009) (Figure 5). No statistically significant differences were detected in the incidence of changes in implant size for either femoral (RR: 1.35; 95% CI: 0.63–2.90; P = 0.44) or tibial components (RR: 1.33; 95% CI: 0.78–2.27; P = 0.30) (Figure 6).
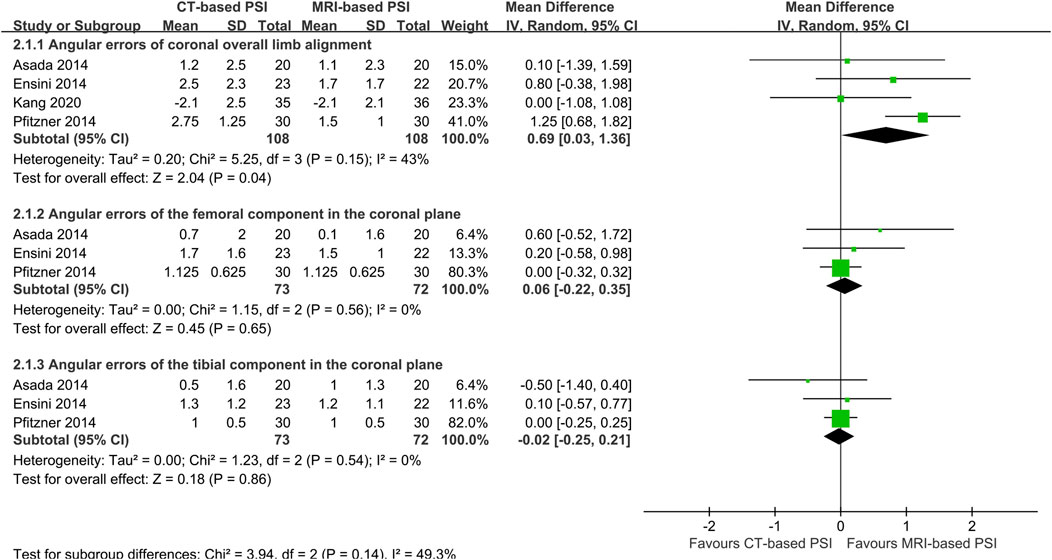
Figure 4. Forest plots demonstrating pooled effects comparing CT-versus MRI-based Patient-Specific Instrumentation for: - angular errors of coronal overall limb alignment; - angular errors of the femoral component in the coronal plane; - angular errors of the tibial component in the coronal plane; CI, confidence interval; M-H, Mantel-Haenszel.
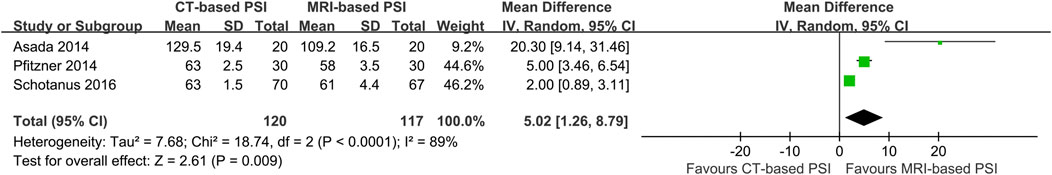
Figure 5. Forest plot illustrating the pooled effect of CT-versus MRI-based PSI on operation time. CI, confidence interval; I-V, Inverse Variance.
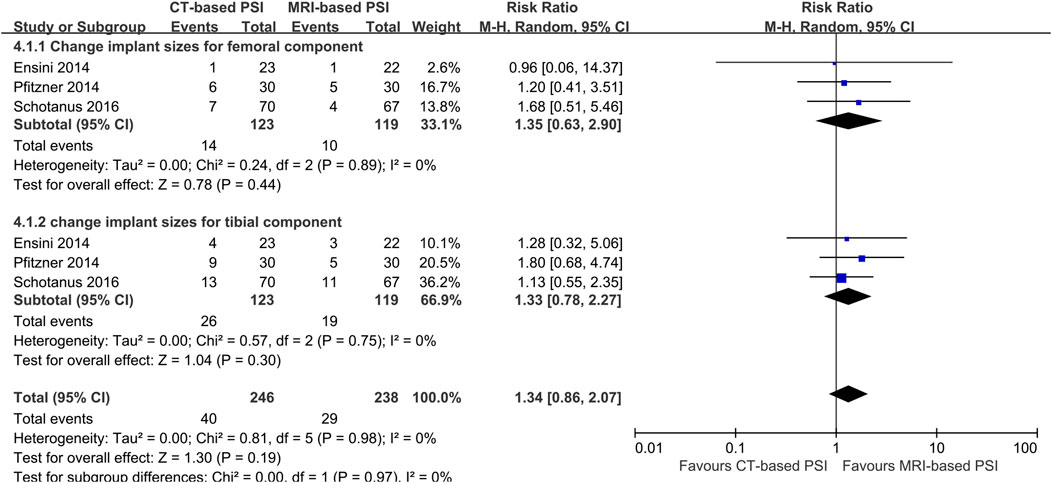
Figure 6. Forest plots illustrating the pooled effects comparing CT-versus MRI-based Patient-Specific Instrumentation for: - change implant size of the femoral component; - change implant size of the tibial component. CI, confidence interval; M-H, Mantel-Haenszel.
Functional outcomes
Four studies provided clinical follow-up data. Pfitzner et al. reported no differences in the postoperative Knee Society pain and function scores or WOMAC scores between CT- and MRI-based PSI at 3 months postoperatively (Pfitzner et al., 2014). Similarly, Kang et al. identified no difference in Knee Society knee and functional scores, 36-item Short Form Survey scores, perioperative complications, or periprosthetic fracture rates between groups at 2 years follow-up (Kang et al., 2020). Thijs et al. observed no significant differences for the patient-reported outcome measurements (PROMs) or revision rate at 2-year follow-up (Thijs et al., 2020). Theeuwen et al. reporting mid-term follow-up on the same cohort, found a statistically significant difference only in the EuroQol-Visual Analog Scale, favoring MRI-based PSI (P < 0.040), while the Forgotten Joint Score and survival rates were comparable between both groups at the 5-year follow-up (Theeuwen et al., 2024).
Publication bias
Visual inspection of the funnel plot for the primary outcome (Figure 7) revealed no clear evidence of publication bias, which was further supported by a non-significant Egger’s test (P = 0.18).
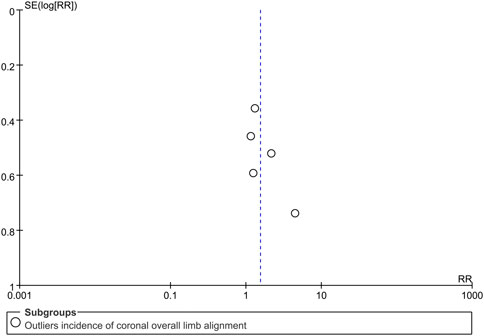
Figure 7. Funnel plot assessing potential publication bias for the outliers incidence of the coronal overall limb alignment. RR, risk ratio.
Discussion
Main findings
In this updated systematic review and meta-analysis, we have summarized all published evidence available between 2016 and 2025 comparing CT- and MRI-based PSI for TKA. Encouragingly, recent studies provided additional clinical follow-up data not previously available (Wu et al., 2017). Contrary to our previous review, the updated analysis suggested no significant differences between CT- and MRI-based PSI regarding either radiological or functional outcomes. Although MRI-based PSI demonstrated marginal benefits, such as a slightly lower incidence of coronal limb alignment outliers, shorter operative times, and improved EuroQol-Visual Analog Scale scores, these differences, while statistically significant, are likely clinically negligible. Specifically, a mean alignment difference of 0.69° or an operative time difference of approximately 5 min falls below commonly accepted thresholds for clinical relevance (>3° for alignment and >15 min for operative time), and thus are unlikely to meaningfully impact patient outcomes. Thus, both CT- and MRI-based PSI systems appear to provide comparable outcomes in TKA.
Comparison with previous studies
Several previous systematic reviews and meta-analyses have compared CT- and MRI-based PSI systems, yielding inconsistent results. In 2015, Stirling et al. performed a literature review and included animal studies, cadaveric studies, and clinical studies, and infer CT-based PSI might be preferable due to shorter scanning times, wider availability, and lower cost (Stirling et al., 2015). Conversely, in 2017, An et al. performed a meta-analysis of retrospective and prospective studies, and concluded that MRI-based PSI was superior regarding a lower proportion of outliers in the overall coronal limb alignment (An et al., 2017). Subsequently, our previous meta-analysis further supported the superiority of MRI-based PSI over CT-based PSI for TKA (Wu et al., 2017). In 2018, Schotanus et al. conducted an indirect comparison of CT- and MRI-based PSI for TKA, indicating that MRI-based PSI alignment was at least equivalent or potentially superior to CT-based PSI (Schotanus et al., 2018). However, in 2020, Li et al. found that CT-based PSI was preferable due to the lower rate of femoral rotational alignment outliers (Li et al., 2020).
These conflicting conclusions highlight the ongoing debate regarding the optimal imaging modality for PSI systems in TKA. Notably, our current study comprehensively integrates recent evidence and demonstrates that there were no significant differences in short-to mid-term clinical outcomes between CT-based and MRI-based PSI. Although MRI-based PSI showed statistically minor advantages, such as slightly reduced angular alignment errors and shorter operative times, the clinical significance of these findings remains limited, as these differences do not translate into meaningful variations in functional scores or revision rates. Given these uncertainties, additional long-term clinical studies are critical to clarify whether subtle differences observed between imaging modalities ultimately impact long-term implant survival and patient outcomes, providing clearer guidance for clinical practice (Klasan et al., 2020).
Implications for clinical practice
The findings of our study bear significant implications for clinical practice. Although the results we obtained are promising, they warrant further consideration and validation to ensure their applicability and robustness in a clinical context. Based on our results, clinicians, patients, and manufacturers can confidently select either CT- or MRI-based PSI systems for TKA without significant concern for differences in radiological or functional outcomes. The comparable performance of both modalities might result from balancing advantages and limitations inherent in each imaging approach.
The comparable outcomes observed between CT- and MRI-based PSI systems may be attributed to several potential mechanisms and influencing factors. In theory, MRI provides superior visualization of cartilage and soft tissues, which can enhance the accuracy of preoperative modeling and potentially result in improved alignment outcomes. Conversely, CT provides a clearer and more detailed depiction of bone anatomy, although it inadequately visualizes cartilage. This limitation in CT imaging could lead to mismatches between the planned cutting guides and the actual resected bone surfaces, potentially affecting surgical precision. Despite these theoretical distinctions, recent comparative studies have produced inconsistent findings, possibly due to heterogeneity in study designs, varying surgeon expertise, and differences in imaging protocols and PSI manufacturers. These complexities and potential confounders highlight why previous studies, including our earlier meta-analysis, reported advantages favoring MRI-based PSI systems (Kwon et al., 2015; Koh et al., 2025), while current evidence no longer demonstrates significant clinical differences between these modalities.
However, these potential advantages of MRI-based PSI may be mitigated by factors such as the learning curve associated with PSI system design and use, as the learning curve exists for both engineers and surgeons in designing the PSI (Thi et al., 2013; Wen et al., 2022). Wen et al. proposed that engineer-based and surgeon-directed PSI designs could enhance accuracy, particularly in kinematically aligned TKA (Wen et al., 2022). Moreover, the surgeon’s experience and surgical technique can also significantly influence the clinical outcomes (Gaukel et al., 2022).
In addition, variability among manufacturers in PSI system design, including differences in imaging protocols, prosthetic designs, proprietary algorithms, and cutting-guide technologies, could introduce substantial heterogeneity, potentially masking true differences between CT- and MRI-based PSI systems. For instance, the current design philosophy of the PSI systems primarily relies on bone landmarks and rarely incorporates functional parameters adequately. However, strategies for achieving appropriate soft tissue balance, such as soft tissue releases, are typically underrepresented in PSI designs (Moerenhout et al., 2021; Deng et al., 2021). Due to these confounding factors, differences between CT- and MRI-based PSI have been diluted, resulting in minimal detectable differences in radiological and functional outcomes as demonstrated by our meta-analysis.
Future perspectives
Innovations in TKA constantly aim to enhance surgical outcomes, improve patient functionality, and optimize cost-effectiveness. Despite the rapid global adoption of robot-assisted arthroplasty, PSI systems remain valuable. Notably, robot-assisted arthroplasty presents several drawbacks, including pin-related complications, registration errors, longer operative times, higher associated costs, and steep learning curves (Thomas et al., 2022; Fontalis et al., 2024; Pagan et al., 2024) In contrast, PSI involves the use of preoperative customized cutting guides, eliminating the necessity for intraoperative landmark registration, avoiding steps related to the intramedullary alignment of the femoral component, thus potentially reducing operation time. However, in recent years, robotic technologies have seen continuous advancements, such as the integration of sensor technologies for improved soft tissue balancing and augmented reality for intraoperative navigation (Gordon et al., 2021; Ding et al., 2024). Meanwhile, PSI has experienced relatively limited technological advancements in recent years. At present, PSI could benefit from integration with emerging technologies, such as the VERASENSE sensor, to enhance clinical outcomes in TKA (Deng et al., 2021). Further research and development efforts are necessary to explore such combinations to optimize surgical outcomes.
Strengths and limitations
A significant strength of our meta-analysis is the inclusion of recent clinical follow-up data, providing valuable insights for clinical decision-making. Besides, we included both prospective RCTs and prospective non-RCTs clarified, which may introduce additional risk of bias but allowed a broader overview of real-world clinical practice, thus enhancing the applicability and generalizability of our findings.
However, several limitations should be acknowledged. First, the included studies utilized CT- and MRI-based PSI systems from various manufacturers, each possibly following different design philosophies, proprietary algorithms, cutting guide designs, and implant systems, thus introducing potential confounding factors and diluting observable differences between CT- and MRI-based PSI systems. Secondly, our meta-analysis is further limited by the considerable heterogeneity across included studies regarding surgeons’ experience levels and the methodologies employed for postoperative radiographic measurement, which may also affect the robustness and generalizability of our findings. Thirdly, four of the seven included trials were classified as having a high risk of bias, and inadequate blinding could further bias radiological and functional outcome assessments. Therefore, these factors necessitate a cautious interpretation of our meta-analysis results. Fourthly, the absence of long-term outcome data, particularly concerning implant survival and revision rates, necessitates a cautious interpretation of the current findings. Lastly, due to the relatively limited number of included studies and their small sample sizes, the possibility of small-study bias cannot be entirely excluded. This limitation may impact the validity and generalizability of our meta-analysis findings, warranting cautious interpretation.
Conclusion
Our updated systematic review and meta-analysis indicate no significant differences between CT-based and MRI-based PSI systems regarding alignment accuracy or short-to mid-term clinical outcomes in TKA. This suggests that both imaging modalities are suitable for clinical utilization in TKA, although further long-term studies are required to fully validate these findings.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
LS: Resources, Funding acquisition, Conceptualization, Writing – review and editing, Project administration, Investigation, Validation, Methodology, Visualization, Supervision, Writing – original draft, Data curation, Formal Analysis, Software. X-DW: Writing – review and editing, Funding acquisition, Writing – original draft, Visualization, Conceptualization, Supervision, Validation, Data curation, Investigation. YL: Investigation, Writing – original draft, Conceptualization, Software, Methodology, Formal Analysis, Writing – review and editing, Data curation. XW: Writing – original draft, Supervision, Funding acquisition, Formal Analysis, Writing – review and editing, Methodology, Data curation, Software, Investigation, Conceptualization. CL: Project administration, Data curation, Validation, Conceptualization, Writing – review and editing, Supervision, Methodology, Writing – original draft, Resources, Investigation, Visualization. KT: Software, Writing – original draft, Formal Analysis, Resources, Visualization, Supervision, Writing – review and editing, Methodology, Investigation, Data curation. SW: Writing – review and editing, Conceptualization, Writing – original draft, Investigation, Methodology, Software, Project administration, Visualization, Resources, Formal Analysis, Data curation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Zhejiang Medical Health Science and Technology plan (2022RC068 to LS), Zhejiang Medical Health Science and Technology plan (2022RC248 to LS), Capital’s Funds for Health Improvement and Research (2024-2-1122 to X-D. W.), Beijing Natural Science Foundation (L244059 to X-D. W.), Beijing Jishuitan Hospital Natural Fund Incubation Program (ZR-202304 to X-D. W.), Beijing Jishuitan Hospital Youth Fund (QN-202410 to X-D. W.), Young Elite Scientist Sponsorship Program By BAST (BYESS2024341 to X-D. W.), School Nature Science Foundation of Capital Medical University (PYZ23129 to X-D. W.), Scientific and Technological Research Plan in Key areas of Shihezi (2023SF04 to XW), and Ningbo Clinical Research Center for Orthopedics, Sports Medicine & Rehabilitation (2024L004 to SL).
Acknowledgments
All authors have reviewed and approved the final revised version of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fbioe.2025.1624600/full#supplementary-material
Abbreviations
CI, confidence interval; CT, computed tomography; MD, mean difference; MeSH, Medical Subject Headings; MRI, magnetic resonance imaging; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; PROM, patient-reported outcome measurements; PSI, patient-specific instrumentation; RCT, randomized controlled trial; RR, relative risk; TKA, total knee arthroplasty.
References
An, V. V., Sivakumar, B. S., Phan, K., Levy, Y. D., and Bruce, W. J. (2017). Accuracy of MRI-Based vs. CT-based patient-specific instrumentation in total knee arthroplasty: a meta-analysis. J. Orthop. Sci. 22 (1), 116–120. doi:10.1016/j.jos.2016.10.007
Asada, S., Mori, S., Matsushita, T., Nakagawa, K., Tsukamoto, I., and Akagi, M. (2014). Comparison of MRI- and CT-based patient-specific guides for total knee arthroplasty. Knee 21 (6), 1238–1243. doi:10.1016/j.knee.2014.08.015
Barbash, G. I., and Glied, S. A. (2010). New technology and health care costs--the case of robot-assisted surgery. N. Engl. J. Med. 363 (8), 701–704. doi:10.1056/nejmp1006602
Bini, S. A., Schilling, P. L., Patel, S. P., Kalore, N. V., Ast, M. P., Maratt, J. D., et al. (2020). Digital orthopaedics: a glimpse into the future in the midst of a pandemic. J. Arthroplasty 35 (7S), S68–S73. doi:10.1016/j.arth.2020.04.048
Deng, T., Liu, T., Lei, Q., Cai, L., and Chen, S. (2021). Patient-specific instrumentation combined with a new tool for gap balancing is useful in total knee replacement: a 3-year follow-up of a retrospective study. J. Orthop. Surg. Res. 16 (1), 309. doi:10.1186/s13018-021-02467-6
Ding, H., Sun, W., and Zheng, G. (2024). Robot-assisted augmented reality (AR)-guided surgical navigation for periacetabular osteotomy. Sensors (Basel) 24 (14), 4754. doi:10.3390/s24144754
Egger, M., Davey Smith, G., Schneider, M., and Minder, C. (1997). Bias in meta-analysis detected by a simple, graphical test. BMJ 7109, 629–634. doi:10.1136/bmj.315.7109.629
Ensini, A., Timoncini, A., Cenni, F., Belvedere, C., Fusai, F., Leardini, A., et al. (2014). Intra- and post-operative accuracy assessments of two different patient-specific instrumentation systems for total knee replacement. Knee Surg. Sports Traumatol. Arthrosc. 22 (3), 621–629. doi:10.1007/s00167-013-2667-9
Fang, D. M., Ritter, M. A., and Davis, K. E. (2009). Coronal alignment in total knee arthroplasty: just how important is it? J. Arthroplasty 24 (6 Suppl. l), 39–43. doi:10.1016/j.arth.2009.04.034
Fontalis, A., Hansjee, S., Giebaly, D. E., Mancino, F., Plastow, R., and Haddad, F. S. (2024). Troubleshooting robotics during total hip and knee arthroplasty. Orthop. Clin. North Am. 55 (1), 33–48. doi:10.1016/j.ocl.2023.06.004
Fritschy, D., and Messerli, G. (2011). Paper # 3: patient-specific cutting block in TKR: Comparison between CT and MRI 3D planning. Arthroscopy 27, e70–e71. doi:10.1016/j.arthro.2011.08.004
Gaukel, S., Vuille-Dit-Bille, R. N., Schläppi, M., and Koch, P. P. (2022). CT-based patient-specific instrumentation for total knee arthroplasty in over 700 cases: single-use instruments are as accurate as standard instruments. Knee Surg. Sports Traumatol. Arthrosc. 30 (2), 447–455. doi:10.1007/s00167-020-06150-x
Gong, S., Xu, W., Wang, R., Wang, Z., Wang, B., Han, L., et al. (2019). Patient-specific instrumentation improved axial alignment of the femoral component, operative time and perioperative blood loss after total knee arthroplasty. Knee Surg. Sports Traumatol. Arthrosc. 27 (4), 1083–1095. doi:10.1007/s00167-018-5256-0
Gordon, A. C., Conditt, M. A., and Verstraete, M. A. (2021). Achieving a balanced knee in robotic TKA. Sensors (Basel) 21 (2), 535. doi:10.3390/s21020535
Gromov, K., Korchi, M., Thomsen, M. G., Husted, H., and Troelsen, A. (2014). What is the optimal alignment of the tibial and femoral components in knee arthroplasty? An overview of the literature. Acta Orthop. 85, 480–487. doi:10.3109/17453674.2014.940573
Halm-Pozniak, A., Lohmann, C. H., Zagra, L., Braun, B., Gordon, M., and Grimm, B. (2023). Best practice in digital orthopaedics. EFORT Open Rev. 8 (5), 283–290. doi:10.1530/eor-23-0081
Hickey, M. D., Masri, B. A., and Hodgson, A. J. (2023). Can technology assistance be cost effective in TKA? A simulation-based analysis of a risk-prioritized, practice-specific framework. Clin. Orthop. Relat. Res. 481 (1), 157–173. doi:10.1097/corr.0000000000002375
Higgins, J. P., Altman, D. G., Gøtzsche, P. C., Jüni, P., Moher, D., Oxman, A. D., et al. (2011). The cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343, d5928. doi:10.1136/bmj.d5928
Higgins, J. P., and Thompson, S. G. (2002). Quantifying heterogeneity in a meta-analysis. Stat. Med. 21 (11), 1539–1558. doi:10.1002/sim.1186
Higgins, J. P., Thompson, S. G., Deeks, J. J., and Altman, D. G. (2003). Measuring inconsistency in meta-analyses. BMJ 327 (7414), 557–560. doi:10.1136/bmj.327.7414.557
Higgins, J. P. T., and Green, S. (2011). Cochrane handbook for systematic reviews of interventions version 5.1.0. United Kingdom: The Cochrane Collaboration. Available online at: http://www.cochrane.org/handbook. (Accessed on July, 2025)
Hua, Y., and Salcedo, J. (2022). Cost-effectiveness analysis of robotic-arm assisted total knee arthroplasty. PLoS One 17 (11), e0277980. doi:10.1371/journal.pone.0277980
Jaffry, Z., Masjedi, M., Clarke, S., Harris, S., Karia, M., Andrews, B., et al. (2014). Unicompartmental knee arthroplasties: robot vs. patient specific instrumentation. Knee 21 (2), 428–434. doi:10.1016/j.knee.2013.11.017
Jeffery, R. S., Morris, R. W., and Denham, R. A. (1991). Coronal alignment after total knee replacement. J. Bone Jt. Surg. Br. 73 (5), 709–714. doi:10.1302/0301-620x.73b5.1894655
Kang, D. G., Kim, K. I., and Bae, J. K. (2020). MRI-Based or CT-based patient-specific instrumentation in total knee arthroplasty: how do the two systems compare? Arthroplasty 2 (1), 1. doi:10.1186/s42836-019-0020-6
Klasan, A., de Steiger, R., Holland, S., Hatton, A., Vertullo, C. J., and Young, S. W. (2020). Similar risk of revision after kinematically aligned, patient-specific instrumented total knee arthroplasty, and all other total knee arthroplasty: combined results from the Australian and New Zealand joint replacement registries. J. Arthroplasty 35 (10), 2872–2877. doi:10.1016/j.arth.2020.05.065
Koh, Y. G., Nam, J. H., Kim, J. K., Suh, D. S., Chung, J. H., Park, K. K., et al. (2025). Enhancing surgical efficiency and radiological outcomes through advances in patient-specific instrument design. J. Clin. Med. 14 (2), 307. doi:10.3390/jcm14020307
Kwon, O. R., Kang, K. T., Son, J., Choi, Y. J., Suh, D. S., and Koh, Y. G. (2015). The effect of femoral cutting guide design improvements for patient-specific instruments. Biomed. Res. Int. 2015, 1–8. doi:10.1155/2015/978686
Li, Z., Yang, Z., Liao, W., Wang, W., Zou, Y., Pan, Y., et al. (2020). Fewer femoral rotational outliers produced with CT-than with MRI-Based patient-specific instrumentation in total knee arthroplasty. Knee Surg. Sports Traumatol. Arthrosc. 28 (9), 2930–2941. doi:10.1007/s00167-019-05678-x
Lionberger, D. R., Crocker, C. L., and Chen, V. (2014). Patient specific instrumentation. J. Arthroplasty 29 (9), 1699–1704. doi:10.1016/j.arth.2014.03.019
Macaskill, P., Walter, S. D., and Irwig, L. (2001). A comparison of methods to detect publication bias in meta-analysis. Stat. Med. 4, 641–654. doi:10.1002/sim.698
Mattei, L., Pellegrino, P., Calò, M., Bistolfi, A., and Castoldi, F. (2016). Patient specific instrumentation in total knee arthroplasty: a state of the art. Ann. Transl. Med. 4 (7), 126. doi:10.21037/atm.2016.03.33
Moerenhout, K., Allami, B., Gkagkalis, G., Guyen, O., and Jolles, B. M. (2021). Advantages of patient-specific cutting guides with disposable instrumentation in total knee arthroplasty: a case control study. J. Orthop. Surg. Res. 16 (1), 188. doi:10.1186/s13018-021-02310-y
Moher, D., Liberti, A., Tetzlaff, J., and Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339, b2535. doi:10.1136/bmj.b2535
Ng, A. P., Sanaiha, Y., Bakhtiyar, S. S., Ebrahimian, S., Branche, C., and Benharash, P. (2023). National analysis of cost disparities in robotic-assisted versus laparoscopic abdominal operations. Surgery 173 (6), 1340–1345. doi:10.1016/j.surg.2023.02.016
Pagan, C. A., Karasavvidis, T., Siljander, B., Debbi, E. M., DeCook, C. A., and Vigdorchik, J. (2024). Operative time learning curve for an image-free robotic arm assisted total knee arthroplasty: a cumulative sum analysis. Arthroplast Today 31, 101588. doi:10.1016/j.artd.2024.101588
Pfitzner, T., Abdel, M. P., von Roth, P., Perka, C., and Hommel, H. (2014). Small improvements in mechanical axis alignment achieved with MRI versus CT-based patient-specific instruments in TKA: a randomized clinical trial. Clin. Orthop. Relat. Res. 472 (10), 2913–2922. doi:10.1007/s11999-014-3784-6
Ritter, M. A., Faris, P. M., Keating, E. M., and Meding, J. B. (1994). Postoperative alignment of total knee replacement. Its effect on survival. Clin. Orthop. Relat. Res. 299, 153–156. doi:10.1097/00003086-199402000-00021
Schotanus, M. G., Sollie, R., van Haaren, E. H., Hendrickx, R. P., Jansen, E. J., and Kort, N. P. (2016). A radiological analysis of the difference between MRI- and CT-based patient-specific matched guides for total knee arthroplasty from the same manufacturer: a randomised controlled trial. Bone Jt. J. 98-B (6), 786–792. doi:10.1302/0301-620x.98b6.36633
Schotanus, M. G. M., Thijs, E., Heijmans, M., Vos, R., and Kort, N. P. (2018). Favourable alignment outcomes with MRI-Based patient-specific instruments in total knee arthroplasty. Knee Surg. Sports Traumatol. Arthrosc. 26 (9), 2659–2668. doi:10.1007/s00167-017-4637-0
Silva, A., Pinto, E., and Sampaio, R. (2016). Rotational alignment in patient-specific instrumentation in TKA: MRI or CT? Knee Surg. Sports Traumatol. Arthrosc. 24 (11), 3648–3652. doi:10.1007/s00167-014-3394-6
Stirling, P., Valsalan Mannambeth, R., Soler, A., Batta, V., Malhotra, R. K., and Kalairajah, Y. (2015). Computerised tomography vs magnetic resonance imaging for modeling of patient-specific instrumentation in total knee arthroplasty. World J. Orthop. 6 (2), 290–297. doi:10.5312/wjo.v6.i2.290
Theeuwen, D. M. J., Haveman, I., Boonen, B., van Haaren, E. H., Hendrickx, R. P. M., and Schotanus, M. G. M. (2024). No differences in mid-term survival and clinical outcome between CT- and MRI-Based patient-specific instrumentation for total knee arthroplasty, a randomized controlled trial. Eur. J. Orthop. Surg. Traumatol. 34 (7), 3529–3534. doi:10.1007/s00590-023-03680-1
Thienpont, E., Bellemans, J., Delport, H., Van Overschelde, P., Stuyts, B., Brabants, K., et al. (2013). Patient-specific instruments: industry's innovation with a surgeon's interest. Knee Surg. Sports Traumatol. Arthrosc. 21 (10), 2227–2233. doi:10.1007/s00167-013-2626-5
Thijs, E., Theeuwen, D., Boonen, B., van Haaren, E., Hendrickx, R., Vos, R., et al. (2020). Comparable clinical outcome and implant longevity after CT- or MRI-Based patient-specific instruments for total knee arthroplasty: a 2-year follow-up of a RCT. Knee Surg. Sports Traumatol. Arthrosc. 28 (6), 1821–1826. doi:10.1007/s00167-019-05616-x
Thomas, T. L., Goh, G. S., Nguyen, M. K., and Lonner, J. H. (2022). Pin-related complications in computer navigated and robotic-assisted knee arthroplasty: a systematic review. J. Arthroplasty 37 (11), 2291–2307.e2. doi:10.1016/j.arth.2022.05.012
Vide, J., Freitas, T. P., Ramos, A., Cruz, H., and Sousa, J. P. (2017). Patient-specific instrumentation in total knee arthroplasty: simpler, faster and more accurate than standard instrumentation-a randomized controlled trial. Knee Surg. Sports Traumatol. Arthrosc. 25 (8), 2616–2621. doi:10.1007/s00167-015-3869-0
Wen, L., Wang, Z., Ma, D., and Qu, T. (2022). Surgeon dominated design can improve the accuracy of patient-specific instruments in kinematically aligned TKA. J. Pers. Med. 12 (8), 1192. doi:10.3390/jpm12081192
Wu, X. D., Xiang, B. Y., Schotanus, M. G. M., Liu, Z. H., Chen, Y., and Huang, W. (2017). CT-versus MRI-Based patient-specific instrumentation for total knee arthroplasty: a systematic review and meta-analysis. Surgeon 15 (6), 336–348. doi:10.1016/j.surge.2017.06.002
Keywords: patient-specific instrumentation, total knee arthroplasty, computed tomography, magnetic resonance imaging, alignment
Citation: Shao L, Wu X-D, Liu Y, Wang X, Li C, Tao K and Wang S (2025) No difference between CT- and MRI-based patient-specific instrumentation for total knee arthroplasty: an updated systematic review and meta-analysis. Front. Bioeng. Biotechnol. 13:1624600. doi: 10.3389/fbioe.2025.1624600
Received: 07 May 2025; Accepted: 02 July 2025;
Published: 23 July 2025.
Edited by:
Yaying Sun, Shanghai General Hospital, ChinaReviewed by:
Cheng Huang, China-Japan Friendship Hospital, ChinaChaoxin Wang, First Affiliated Hospital of Fujian Medical University, China
Copyright © 2025 Shao, Wu, Liu, Wang, Li, Tao and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiang-Dong Wu, d3hkNTYxQGdtYWlsLmNvbQ==
†These authors share first authorship
 Long Shao
Long Shao Xiang-Dong Wu
Xiang-Dong Wu Yuhao Liu3†
Yuhao Liu3† Chunyan Li
Chunyan Li Shicheng Wang
Shicheng Wang