- 1Institute of General Electrical Engineering, University of Rostock, Rostock, Germany
- 2Department of Electronics Engineering, The Islamia University of Bahawalpur, Bahawalpur, Pakistan
- 3Faculty of Mechanical Engineering and Marine Technology, University of Rostock, Rostock, Germany
- 4Department Life, Light and Matter, University of Rostock, Rostock, Germany
- 5Department of Ageing of Individuals and Society, Interdisciplinary Faculty, University of Rostock, Rostock, Germany
Electrosensitive hydrogels are smart biomaterials that swell, shrink, deform, and bend when an external electric field is applied. These hydrogels have enormous potential for the controlled therapeutic delivery of biochemical substances to the affected area, thus promoting tissue regeneration. Computational modeling and simulation approaches have provided researchers with cost-effective predictive models that can be used to optimize in vitro and in vivo experimental protocols. In this article, we present a review of the modeling theories that can be used for the modeling and numerical simulation of electrosensitive hydrogels immersed in a solution with an applied electric field for cartilage tissue engineering. Each theory presents tradeoffs for the numerical modeling of cartilage repair implants. The selection of an appropriate theory depends on the required accuracy, time-dependent application, and deformation behavior. Although most simulations are limited to one-dimensional cases, multidimensional simulations are crucial. By reviewing the modeling theories of electrosensitive hydrogels, this article aims to inspire researchers to model the electrical stimulation of electrosensitive hydrogels for various applications, including cartilage tissue engineering.
1 Introduction
Articular cartilage is a specialized connective tissue devoid of blood vessels, nerves, and lymphatics (Ngo and Knothe Tate, 2020). Chondrocytes are the primary cells in the extracellular network of articular cartilage and are responsible for repair and maintenance. An essential feature of articular cartilage is an extremely low cell count (Bhosale and Richardson, 2008). The primary function of articular cartilage is to facilitate smooth articulation in the diarthrodial joints of the body (Xu et al., 2022). Cartilage tissue is constantly degraded owing to injury, immobility, or aging, eventually leading to osteoarthritis (Van Gelder et al., 2023). Unlike other connective tissues, articular cartilage has very little cell growth; therefore, it does not heal easily when damaged (Bhosale and Richardson, 2008).
The electrochemical properties of articular cartilage originate from the flow of electrolytes relative to the fixed negative charges attached to the proteoglycans (Jackson and Gu, 2009). The resulting electromechanical events in the tissue can be distinguished by diffusion potential, streaming potential, and the Donnan osmotic swelling pressure (Buschmann and Grodzinsky, 1995; Lai et al., 2000). Thus, the charged nature of the tissue is responsible for its electrokinetic properties. Upon applying a mechanical load, electric potentials are generated owing to the movement of electrolytes across the proteoglycans. Alternatively, the application of an external electric field generates stress in the cartilage tissue (Frank and Grodzinsky, 1987a; b; Liu et al., 2025). Consequently, it was hypothesized that external electric fields similar to those generated in native articular cartilage can positively affect the synthesis of necessary extracellular matrix components (Brighton et al., 2006; Culma et al., 2025).
Polyelectrolyte hydrogels can be used as substitute materials to restore and regenerate articular cartilage (Romischke et al., 2022). Hydrogels are three-dimensional (3D) polymers that can absorb large quantities of water or biological fluids without being soluble in appropriate physiological environments (Lei et al., 2022; Kaith et al., 2021). They are extensively used in drug delivery (Qureshi et al., 2019), tissue engineering (El-Husseiny et al., 2022), sensors/biosensors (Hu et al., 2019), actuators (Hu et al., 2019), and cell-based therapies (Mohamed et al., 2019). They are also being utilized for novel biomedical applications, such as alleviating postoperative pancreatic fistulas, which are distinguished by the leakage of digestive enzymes (He et al., 2024), treating diabetic wounds using cross-linked multifunctional hydrogels (Shi et al., 2023), and promoting Achilles tendon repair while preventing adhesion using injectable lubricative hydrogels (Cheng et al., 2025). Moreover, hydrogels are widely used in the development of multifunctional personal protective equipment (Zhang et al., 2024) and the preparation of efficient and sustainable flame-retardant materials (Zuo et al., 2024). Hydrogels in general, and electrosensitive hydrogels in particular, are widely used to repair and regenerate the articular hyaline cartilage (Ni et al., 2023; Hashemi-Afzal et al., 2025). Stimuli-responsive hydrogels respond to external environmental stimuli and can be used in various applications (Roy et al., 2022). Several stimulation types exist for hydrogels, which can be classified into physical (stress, temperature, light, ultrasound, and electric and magnetic fields), chemical (ionic strength, pH), and biological (enzyme, glucose) stimuli-responsive hydrogels (Qureshi et al., 2019; El-Husseiny et al., 2022).
The prominent representatives of electrosensitive hydrogels are conjugated polymer-based compounds, such as polyaniline (PANi), polypyrrole (PPy), polythiophene, poly (3,4-ethylenedioxythiophene) (PEDOT), carbon-based materials, e.g., derivatives of graphene and carbon nanotubes (CNTs), hydrogels having gold and silver nanoparticles, and bio-ionic liquids (Carayon et al., 2020; Kanaan and Piedade, 2022; Walker et al., 2019). Electroresponsive hydrogels are gaining popularity because they have the distinct advantage of the precise and software-programmable control of electrical parameters (Erol et al., 2019). These hydrogels engineered with electrosensitive characteristics can revive the intrinsic electrochemical communication among cells, thereby augmenting tissue biofunction, which is hindered by injury (Lavrador et al., 2021; Gaspar et al., 2020). Such hydrogels are widely used in various biomedical applications (Ali et al., 2019; Rogers et al., 2020; Zou et al., 2022), including articular cartilage regeneration (Ni et al., 2023; Miguel et al., 2022; Zhou et al., 2023). Polymer-based electroactive hydrogels, such as PANi, PPy, and PEDOT, have been used for chondrocyte proliferation and differentiation (Hosseini et al., 2019; Uzieliene et al., 2023; Liu et al., 2023). Similarly, electrosensitive hydrogels containing gold and silver nanoparticles have been used for cartilage tissue engineering (Bai et al., 2023; He et al., 2015). Graphene, its derivatives (graphene oxide and reduced graphene oxide), and CNTs are widely used for the repair and regeneration of articular cartilage and osteochondral defects (Liu et al., 2021; Amiryaghoubi et al., 2023).
Intrinsic electric fields are involved in various tissue functions including regeneration, disease expression, and progression (Zhao et al., 2020). Thus, extrinsic electric fields, similar to intrinsic electric fields, can be applied to cell-seeded scaffolds in vitro to develop therapeutic interventions (Ryan et al., 2021). These hydrogel scaffolds can then be implanted at the defect site, as illustrated in Figure 1. In this review, we focus on the in vitro electrical stimulation part of the tissue engineering approach and review the different theories that can be utilized for its modeling and simulation, as depicted in Figure 1. Such in vitro direct electrical stimulation setups have been used in various cartilage studies (MacGinitie et al., 1994; Chao et al., 2000; Hiemer et al., 2018). Similarly, capacitively coupled in vitro cartilage stimulation setups have been widely investigated (Brighton et al., 2008; Krueger et al., 2021; Esfandiari et al., 2014; Vaca-González et al., 2020). For a detailed description of electrical stimulation studies involving the cartilage tissue, interested readers can refer to our recent review (Zimmermann et al., 2024) or elsewhere (Vaca-González et al., 2019).
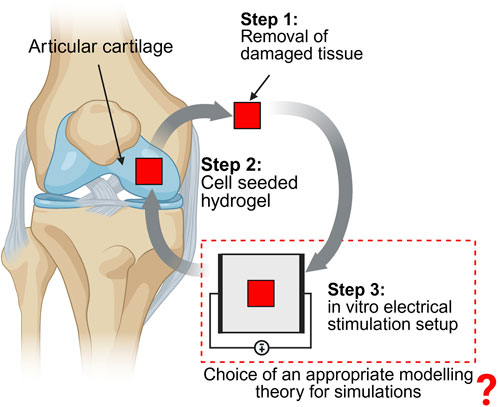
Figure 1. Different steps involved in articular cartilage tissue engineering using electrical stimulation (Created in Biorender).
The swelling mechanism of hydrogels owing to stimulation can be explained by Flory’s osmotic pressure theory (Shiga and Kurauchi, 1990; Yang et al., 2000). As the hydrogel was immersed in the solution, the ionic species diffused between the hydrogel and the solution. Fixed charges are also present within the hydrogel. Thus, varying ionic concentrations were created between the hydrogel and the surrounding buffered solution. These variations in concentration result in osmotic pressure, which determines the swelling behavior of the hydrogel. The swelling and shrinking of the hydrogel caused a redistribution of the ionic species within the hydrogel until an equilibrium was established.
Several theories have been proposed to mathematically estimate the behavior of hydrogels placed in a solution under external electrical stimulation. These theories can be classified as the multiphasic or multi-effect-coupling electric-stimulus (MECe) theory (Li et al., 2004c; Chen et al., 2005), transport theory (Bassetti et al., 2005; Suthar et al., 2007; Wallmersperger and Ballhause, 2008), and porous media theory (PMT) (Wallmersperger et al., 2013b; Ehlers, 2002). The multiphasic theory arises from the multiphase mixture model of hydrated soft tissues (Gu et al., 1998). Mixture models are further classified into biphasic (Mow et al., 1980), triphasic (Lai et al., 1991), quadphasic (Huyghe and Janssen, 1997), and multiphasic (Gu et al., 1998) theories. The multiphasic model provides a detailed description of the solvent phase, ionic concentration, and deformation of the polymer network.
Three types of transport models exist in the literature, proposed by Li et al. (2004a), Wallmersperger (2003); Wallmersperger et al. (2004a), and Bassetti et al. (2005), and named Li’s, Wallmersperger’s, and Bassetti’s transport models, respectively. Different fluid or osmotic pressure equations lead to different transport models. In the following sections, we briefly describe the various theories used to describe the dynamics of electrosensitive hydrogels and compare them. All the mathematical models for the simulation of the electrosensitive hydrogels presented in this study are summarized in Figure 2.
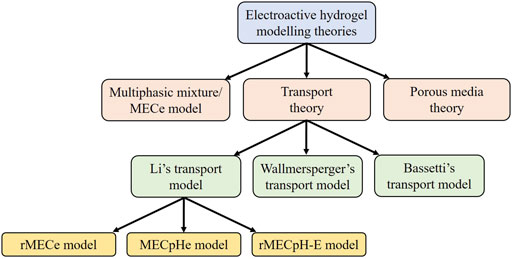
Figure 2. Modeling theories of the electrosensitive hydrogels. MECe is the multi-effect-coupling electric-stimulus model, rMECe is the refined multi-effect-coupling electric-stimulus model, MECpHe is the multi-effect-coupling pH-electric-stimuli model, and rMECpH-E represents the refined multi-effect-coupling pH-electric-stimuli model.
We previously presented a numerical model for the simulation of electrosensitive hydrogels in the context of cartilage tissue engineering (Farooqi et al., 2019b; 2020). Such models have also been employed for the electrical stimulation of scleral tissue (Mehr and Hatami-Marbini, 2022; 2023; Hatami-Marbini and Mehr, 2022). Reviews of modeling theories for the electrical stimulation of hydrogels have been reported (Saunders et al., 2008; Wallmersperger and Leichsenring, 2016). However, to date, no review has provided a comprehensive and systematic overview of modeling approaches for the electrical stimulation of hydrogels, which reflects the current state of research. While previous reviews have laid important groundwork, they have not captured the significant theoretical and methodological advances made in recent years. This review fills this gap by critically analyzing the latest scientific contributions and integrating them with earlier findings. Thus, it offers the first consolidated and up-to-date reference in this rapidly evolving field, helping researchers navigate existing models and identify directions for future research.
In the following section, we review the modeling theories of electrosensitive hydrogels immersed in a solution and subjected to an external electric field. First, the constitutive equations of the involved chemo-electro-mechanical fields involved in each theory are introduced. Subsequently, the significant parameters and properties are described. The important features and limitations of these theories are summarized for readers interested in designing and optimizing experimental protocols for cartilage tissue engineering. Moreover, the experimental verification of the reported modeling theories and the type of hydrogel used for this purpose are discussed. Finally, conclusions are drawn, and possible directions for future research regarding the modeling theories of electrosensitive hydrogels for cartilage repair are highlighted.
2 Multiphasic mixture/multi-effect-coupling electric-stimulus (MECe) model
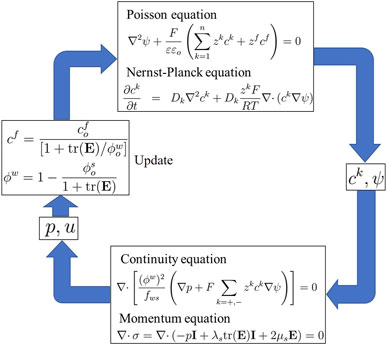
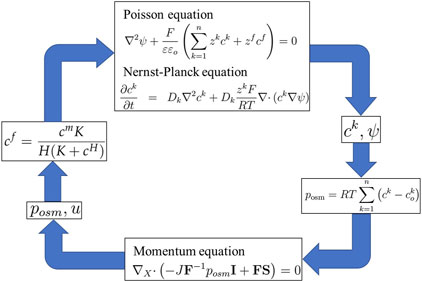
The MECe model includes the Poisson, Nernst–Planck, and continuum equations (including the continuity and momentum equations) for the deformation of hydrogels (Chen, 2004). The nonlinear MECe model is also known as a multiphasic mixture model. The Poisson equation corresponds to the electric potential and is coupled with the Nernst–Planck equations to calculate the ionic concentrations (Li et al., 2004c). The change in ionic concentration was then used to evaluate the hydrogel deformation using continuity and momentum equations (Saunders, 2013). The mathematical relationships of the MECe model, including the Poisson, Nernst–Planck, continuity, and momentum equations are discussed below.
2.1 Poisson equation
To determine the electric potential
where
2.2 Nernst–Planck equation
The sum of the diffusion flux, electric transference, and convection-induced transfer of an ion
Here,
The continuity equation of the ionic flux for the entire domain is (Chen and Ma, 2006),
In the absence of convection and chemical reactions, Equation 3 can be written as (Nernst, 1888; 1889; Planck, 1890),
where
2.3 Continuity equation
According to the law of mass conservation, the solid, liquid, and ionic phases have velocities (Li et al., 2007a)
where
From the triphasic theory (Lai et al., 1991), the fixed charge density is dependent upon the tissue deformation of tissue and the volume fraction of fluid
which is represented with the tissue deformation as
and the solidity of the tissue
where
The magnitudes of
and we have
The saturation condition (9) for the reference configuration can be written as,
Ignoring the inertial and body forces, the governing equations of the multiphasic theory can be written as
where
Thus, the continuity equation of the multiphasic theory for the fluid pressure
where
Only a few important equations relevant to the continuity equation were presented. The detailed derivation of the continuity equation can be found in our previous study (Farooqi et al., 2019a).
2.4 Momentum equation
The momentum equation for estimating the displacement when the deformations are small is written as (Chandrasekharaiah and Debnath, 1994; Tanaka et al., 1973)
where
The redistribution of ions occurs at a much lower speed than that of hydrogel deformation. Thus, a quasi-static condition can be considered, and Equation 12 is reduced to
In addition, the electrostatic stress was neglected because of the small potential difference applied. Using Equation 8, the above equation becomes
where
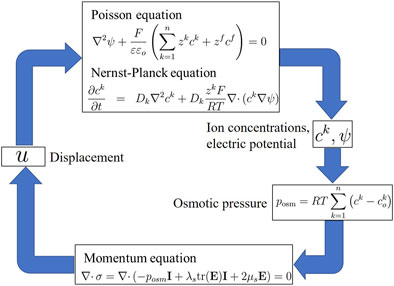
3 Wallmersperger’s transport model
The transport model for the electrical stimulation of hydrogels proposed by Wallmersperger et al. (2004a), (2004c) consists of the Poisson–Nernst–Planck, osmotic pressure, and momentum equations. The Poisson, Nernst–Planck, and momentum equations have been expressed in detail in Equations 1, 4 and 13, respectively. The remaining osmotic pressure equation is as follows:
3.1 Osmotic pressure equation
In the Wallmersperger transport model, the fluid pressure in Equation 10 is modified by the osmotic pressure
where
Thus, Equations 1, 4 and 13, and 14 collectively constitute the transport model proposed by Wallmersperger, as shown in the simulation flow diagram in Figure 4.
4 Li’s transport model
Several variations of the transport model proposed by Yew (2006), Luo (2008) exist for the electrical excitation of hydrogels in a solution. These variations of the transport model are classified into the refined multi-effect-coupling electric-stimulus model (rMECe) (Lam et al., 2006), the multi-effect-coupling pH-electric-stimuli model (MECpHe) (Luo et al., 2007b), and the refined multi-effect-coupling pH-electric-stimuli model (rMECpH-E) (Li et al., 2004b; 2005) to include nonlinear deformation under the simultaneous variation of pH and electric field (Yew et al., 2007). All these transport models use the Poisson equation to evaluate the electric potential, the Nernst–Planck equation for ionic concentration profiles, and the equation of motion to estimate the deformation of the hydrogels. These models differ in how the fluid/osmotic pressure is coupled from the Poisson–Nernst–Planck equations to the equation of motion. A mathematical description of each Li transport model is provided below.
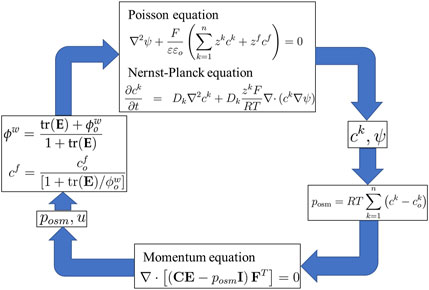
4.1 Refined multi-effect-coupling electric-stimulus (rMECe) model
The multi-effect-coupling pH-stimulus model (MECpH) (Li et al., 2004b; 2005) describes hydrogel swelling behavior, which is sensitive to pH variation. The rMECe is a refined version of the MECpH model that can be extended to electroresponsive hydrogels (Luo et al., 2007a; Li et al., 2007d). The rMECe model has a reformulated fixed-charge density to accommodate an external electric field. Additionally, the refined model could incorporate large deformations of the hydrogels at a high applied voltage.
From Equations 6 and 7, the volume fraction of the fluid phase is
Combining the triphasic theory (Lai et al., 1991) and Equation 15 yields
where
To include the effects of large deformations in the rMECe model, the nonlinear governing equations were described using the total Lagrangian formulation as (Li et al., 2007c)
where
where
Substituting Equation 20 into Equation 17, the hydrogel governing equation for large deformation becomes (Luo et al., 2008a)
Owing to the low applied voltage, the hydrogels exhibited small deformations. The linear theory is sufficient and can be evaluated using Equation 13. Thus, Equations 1, 2, 15, 16 and 21 collectively constitute Li’s transport model or the rMECe model, as shown in the simulation flow diagram in Figure 5.
4.2 Multi-effect-coupling pH-electric-stimuli (MECpHe) model
The MECpHe model for simulating hydrogel swelling and deformation with the simultaneous effects of the applied electric field and solution pH is presented herein (Li et al., 2007b; Luo et al., 2008b). The hydrogel is considered to be a triphasic mixture of an ionic species, a solid matrix, and an interstitial fluid.
When an electric field is applied, mobile ions move to the opposite electrode, creating a gradient of ionic concentrations. The hydrogel structure has a fixed negative charge. An ionic concentration difference is created at the interface between the hydrogel and the buffer solution, resulting in osmotic pressure. The diffusion of cations in the hydrogel dominates that of anions. At the interface, the increase in osmotic pressure was greater near the anode than near the cathode, and the hydrogel swelled more at the anode than at the cathode.
The Langmuir absorption theory (Grimshaw et al., 1990) was used to represent the fixed charge density as
where
Using the relations between the volume fractions and hydration, we obtain
The fluid- and solid-phase volume fractions derived from Equation 23 are
The relation of fluid and solid volume fractions, ignoring the ionic volume fraction
where
where
Using Equations 24 and 25,
Rearranging Equation 26, the local hydration of the hydrogel is
Substituting Equations 26 and 27 into Equation 22, the fixed charge density can be rewritten as
The current MECpHe model is based on the finite elastic deformation theory instead of the small deformation theory to establish a mechanics equation. If pH-electric-sensitive hydrogels experience large deformations with the application of a high electric field, the linear elastic theory cannot provide reasonably accurate results (Luo et al., 2009). This is because the deviation between the initial and deformed states can no longer be ignored, as is the case for the linear elastic behavior. Consequently, for the case of nonlinear large deformation, mechanical equations based on the total Lagrangian description are used, as described in Equations 17–21 (Li et al., 2009; Luo et al., 2010). Thus, Equations 1, 2, 14, 21 and 28 collectively constitute Li’s transport/MECpHe model, as depicted in the simulation flow diagram in Figure 6.
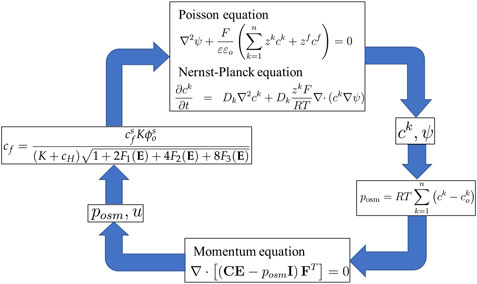
4.3 Refined multi-effect-coupling pH-electric-stimuli (rMECpH-E) model
rMECpH-E is an improved version of the MECpH model that includes a finite/nonlinear deformation formulation in the equilibrium mechanical equation (Yew et al., 2007; Li et al., 2007e). An equation was developed based on the Langmuir absorption isotherm that describes the relationship between fixed charges and diffusing hydrogen ions (Grimshaw, 1989). The fixed charge concentration is given by
The initial and final configuration differences cannot be ignored if the elastic body undergoes large deformation. Green–Lagrangian strain and second Piola–Kirchhoff stress tensors were incorporated for this type of analysis. The Lagrangian formulation provides the momentum equation for a large deformation as follows:
where
where
The deformation gradient
The second Piola–Kirchhoff stress tensor
where the symbol “:” represents the second-order inner product of two tensors (Zohdi, 2017). The Green–Lagrange strain
Similarly, for an elastic isotropic substance, the material tensor in Equation 32 can be expressed as
Substituting Equation 31 into Equation 30, the mechanical deformation equation for the rMECpH-E model becomes
If the inertial and body forces are ignored, Equation 33 can be simplified as
The relevant boundary conditions are prescribed in Equations 18 and 19 (Ng et al., 2007). Hence, Equations 1, 2, 15, 29 and 34 collectively constitute Li’s transport model or the rMECpH-E model (Ng et al., 2010), as is evident from the simulation flow diagram in Figure 7.
5 Bassetti’s transport model
This model relies on the work of De et al. (2002), De and Aluru (2004), which was initially utilized for modeling pH-stimulated hydrogels, and later extended by Bassetti et al. (2005) for electrically stimulated hydrogels. Similar to other transport models, it also starts with the Poisson equation, described in Equation 1, for the external electric field and its interaction with mobile and fixed ions. The hydrogel has an effective dielectric constant
where
The porosity of a hydrogel is related to its hydration state, as shown in Equation 24. The Nernst–Planck Equation 2 is written specifically for hydrogen ions, assuming that neither chemical reactions nor convection occur (Saunders et al., 2008; Nussbaum, 1986)
One way that this model differs from other transport models is how hydrogen ions (
where
where
The flux of hydrogen ions attached to the solution inside the hydrogel is related to the flux of the hydrogen ions (Chu et al., 1995; Mackie and Meares, 1955)
where
By combining Equations 35 and 36 with Equations 37–39, we obtain the following continuity equations for hydrogen ions:
The mechanical equations controlling the hydrogel polymer network displacement can be described using the equation of motion in Equation 11, which is reduced to Equation 13 with some simplifications. Considering the swelling process in two dimensions, the components of the stress tensor are.
where
where
Bassetti’s model also incorporates the Young’s modulus variation with hydration and an external electric field. The change in the Young’s modulus is given by the following equation (Okay and Durmaz, 2002)
where
The following equation provides the change in shear modulus
where
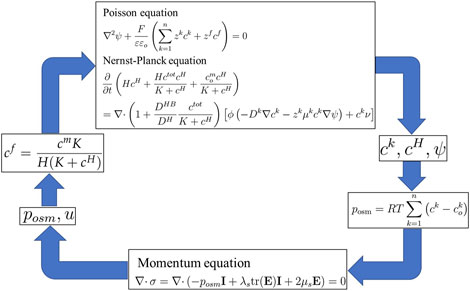
6 Porous media theory (PMT)
The PMT (de Boer, 1996) is a macroscopic theory that is an extension of the theory of mixtures (TM) (Mow et al., 1980; Bowen, 1980) using volume fractions (Ehlers, 2009). This theory does not require local porous microstructures or the actual geometric distribution of all the constituents. This theory is a valuable tool for modeling mixtures with immiscible components, indicating that they can be individually identified macroscopically (Acartürk, 2009). Most of the work on stimuli-responsive and, in particular, electroresponsive hydrogels placed in a solution has been performed by Wallmersperger et al. (Attaran, 2017; Sobczyk, 2018).
Continuum-based chemoelectromechanical relations were obtained using Maxwell’s equations, the balance laws of hydrogels, and constitutive relations (Attaran, 2017). The mathematical description includes chemical, electrical, and mechanical field equations combined with the relevant initial, boundary, and jump conditions. This is known as the initial boundary value problem. The complete domain is decomposed into different components, and the domain
6.1 Chemical field
The equations for the mobile ions, bound charges, and reference configuration of the chemical field are as follows:
6.2 Electric field
The characteristic equations for the mobile ions, bound charges, and reference configuration of the electric field are as follows:
6.3 Mechanical field
Finally, the mechanical field equations are given as.
Thus, Equations 44–49 collectively constitute the PMT (Attaran et al., 2015; 2018).
7 Discussion and comparison
Three different scenarios can be used for the numerical simulation of the chemoelectromechanical equations (Wallmersperger, 2009). The first involves solving each equation separately and then updating the values of the unknowns. This weak-coupling scheme does not converge, and numerous iterations may be required for a single time step. The second scenario involves solving the chemoelectrical fields simultaneously, and then solving the mechanical equation while considering the differential osmotic pressure resulting from the differences in ionic concentrations. It is called sequential, one-way, or semi-coupling, where the mechanical field is usually considered only for the hydrogel domain. In the third case, all three field equations are solved simultaneously; this is referred to as a strong or full coupling scheme. The solution and hydrogel domains were simultaneously modeled chemoelectromechanically; however, the two domains had different material properties.
Doi et al. (1992), and Shiga and Kurauchi (1990), Shiga (1997) were among the pioneers who studied the behavior of electrosensitive hydrogels immersed in solutions under an external electric field. They incorporate Flory and Rehner’s statistical theory (Flory and Rehner, 1943a; b; Flory, 1953) and Donnan’s equilibrium theory (Donnan, 1924). Grimshaw et al. (1990), (1989) provided a macroscopic continuum explanation for the dynamic response of polyelectrolyte hydrogels under an electric field. However, these models are unsuitable for accurately describing the behavior of hydrogels in a solution under an applied electric field. A major contribution to the modeling of electrically stimulated hydrogels depends on the biphasic (Mow et al., 1980), triphasic (Lai et al., 1991), quadphasic (Huyghe and Janssen, 1997), and generalized multiphasic theory (Gu et al., 1998). These theories are relevant, as they were initially proposed to characterize the electrochemical properties of cartilage tissue. Hon et al. (2000), Zhou et al. (2002) used the multiphasic theory to model hydrogels immersed in a solution with an applied electric field. This method has limitations because the computational domain is limited to hydrogel samples.
Subsequently, multiphasic, transport, and porous media theories were reported, which are comprehensive for describing the behavior of electrosensitive hydrogels. All the modeling theories reported in this paper for electrosensitive hydrogels placed in a solution under an external electric field are summarized in Table 1. Initially, these theories were simulated using in-house software, which is not openly available. Subsequently, the simulations were also performed using commercial finite element solvers, i.e., ABAQUS and COMSOL MultiphysicsⓇ. We employed the open-source finite element software, FEniCS (Logg et al., 2012) to solve the Wallmerperger transport model for cartilage tissue engineering (Farooqi et al., 2020). Recently, Mehr and Hatami-Marbini (2022) have used FEniCS to solve a similar chemoelectromechanical model to investigate the electroactive response of scleral tissue (Mehr and Hatami-Marbini, 2022).
Much work on modeling theories for electrosensitive hydrogels has been conducted by Li Hua’s research group at the National University of Singapore. The group reported a multiphase mixture chemoelectromechanical model called the MECe model and three variants of the transport model, namely, the rMECe, MECpHe, and rMECpH-E models. All these models were solved using the meshless HermiteCloud method (Li et al., 2003) with custom-built software; however, they did not report the details of this software. The MECe model can consider the time-dependent behavior of hydrogels; however, transport models lack this property. However, the evaluation of time-dependent articular cartilage tissue behavior under various conditions provides important insights (Hosseini et al., 2010; Murakami et al., 2021).
Another major contribution to the modeling of hydrogels under electrical stimulation was made by the Wallmersperger group at the Technical University of Dresden, Germany. Initially, they reported the studies where only the electrochemical effect was studied (Wallmersperger et al., 2001; 2002) using the Poisson–Nernst–Planck equations. Subsequently, they coupled it with mechanical field equations to estimate hydrogel deformation (Wallmersperger et al., 2009). This group conducted numerous studies using the transport model and PMT (Wallmersperger et al., 2013a), which were solved using the finite element method. Finite element simulations were performed using custom-built programs and the commercial software ABAQUS. The nonlinear equations of these models were solved numerically using a sequential scheme as well as a fully coupled approach, where all the unknowns were evaluated simultaneously. However, these models cannot simulate large hydrogel deformations at high applied voltages. This is because the linear elastic theory has been used for mechanical deformation. However, the analysis of the large deformation behavior of articular cartilage arising from its unique composition, structure, and nonlinear characteristics is preferred (Woo et al., 1979; Zhang et al., 2015).
Another contribution to the modeling theories of electrosensitive hydrogels is the transport model proposed by Bassetti et al. (2005). This model is based on the studies by De et al. (2002), De and Aluru (2004) initially proposed for modeling pH-sensitive hydrogels. This model incorporates the electrostatic stress of the hydrogel. Unlike transport models, the PMT considers each constituent separately, following the principles of continuum mechanics (Wallmersperger and Leichsenring, 2016). Implementing the PMT can be computationally expensive owing to the number of constituents, but it can simulate transient behavior.
The presented theories were evaluated to determine whether they could simulate large deformations of hydrogels or if they were valid only for small deformations. The possibility of steady-state or transient simulation was also investigated. We also evaluated whether one- or two-dimensional geometries were considered in the simulation domain. It is evident from Table 1 that no theory has been used to simulate three-dimensional geometries. Finally, the hydrogel fixation was evaluated to determine whether the fixation was center- or edge-fixed to avoid rigid-body motion in the mechanical simulation.
A limitation of Wallmersperger’s transport model is that a small deformation is assumed, even at high applied potentials (Luo, 2008), as is evident from the data in Table 1. However, it has been experimentally confirmed that hydrogels undergo large deformations when a high electric potential is applied (Zhou et al., 2002). In addition, the effect of fixed charges was ignored (Li, 2009b). The osmotic pressure calculation in this model depends only on the ionic concentrations. However, electric potential should also be considered when calculating the osmotic pressure with an applied electric field.
In transport models, hydrogel swelling and deformation depend on ionic diffusion, whereas fluid flow inside the hydrogel is ignored. In addition, osmotic pressure instead of fluid pressure was used to calculate the stress of the hydrogel mixture. This osmotic pressure can be determined from the ionic concentration differences between the solution bath and the interior of the hydrogel. Osmotic and fluid pressures are essential parameters for cartilage characterization (Farooq and Siddique, 2021; Warren and Bajpayee, 2022).
The model proposed by Wallmersperger and Ballhause (2008) for electrosensitive hydrogels is approximate and difficult to extend to two- and three-dimensional geometries. The system, which is composed of a hydrogel scaffold placed in a solution with an applied electric field, cannot attain thermodynamic equilibrium even in a steady state. Moreover, the chemical potential of the water in the system was nonuniform. Thus, the osmotic pressure considered in the model proposed by Wallmersperger and Ballhause (2008) is not the same as the fluid pressure. Therefore, it cannot be used as a mechanical parameter for thermodynamic equilibrium (Feng et al., 2011).
An experimental verification of these theories is also provided. While most theories have been compared quantitatively, others have been compared qualitatively. The theoretical transport model proposed by Grimshaw et al. (1990) was experimentally verified by comparing it with the measurements of the chemically and electrically induced swelling and shrinking of crosslinked polymethacrylic acid (PMAA) membranes. Shiga and Kurauchi (1990) qualitatively compared the theoretical deformation behavior under an electric field with the experimental findings of a poly (sodium acrylate) (PAANa) hydrogel placed in a NaOH solution between two electrodes (without touching the electrodes). The numerical results of the transport model by Doi et al. (1992) were qualitatively compared with experimental results focusing on acryl acid–acrylamide copolymer hydrogels (Shiga and Kurauchi, 1990). Zhou et al. (2002) theoretically and experimentally investigated the deformation response of chitosan and poly (ethylene glycol) (PEG) hydrogel strips immersed in an acidic solution under an external electric field. The numerical simulation results of the multiphasic mixture/MECe theory (Li et al., 2004c; a; Chen et al., 2005) were quantitatively compared with the experimental results of the variation of the average PEG hydrogel curvature versus the applied voltage (Zhou et al., 2002) and experimentally measured endpoint displacement of the PAANa hydrogel strip at different time steps (Shiga and Kurauchi, 1990). Wallmersperger et al. (2004a), (2004b) qualitatively compared the transport theory results with the experimental results on poly (acrylamide/acrylic acid) (PAAm/PAA) hydrogel under an electric field (Gülch et al., 2000). The numerical results of the rMECe theory involving polyelectrolyte hydrogels (Lam et al., 2006; Luo et al., 2007a; Li et al., 2007d) were quantitatively validated using the experimental results of the PEG hydrogel strip (Zhou et al., 2002). Similarly, the numerical results of the MECpHe theory (Li et al., 2007b; Luo et al., 2007b) were compared with the bending behavior of an interpenetrating polymer network composed of PMAA and a poly (vinyl alcohol) (PVA) hydrogel upon application of an electric field, as reported by Kim et al. (2004) and were observed to be consistent with each other. Li’s transport/rMECpH-E theory (Yew et al., 2007; Li et al., 2007e; Ng et al., 2007) was experimentally verified by comparing it with the results reported in the literature (Zhou et al., 2002; De et al., 2002; Kim et al., 2003; 2004). Bassetti’s transport theory (Bassetti et al., 2005) was quantitatively benchmarked against the experimental results. There is a significant increase in the swelling of electroresponsive hydroxyethyl methacrylate (HEMA) gels with increasing applied voltages across the electrodes (Chatterjee et al., 2003b; Chatterjee, 2003). Finally, the PMT for polyelectrolyte hydrogels (Wallmersperger et al., 2013a) was qualitatively compared with the experimental results reported previously for PAAm/PAA (Gülch et al., 2000) and PAANa (Shiga and Kurauchi, 1990) hydrogels. The nature of the experimental validation of these theories is summarized in Table 1.
8 Conclusions and outlook
This review presents complete mathematical formulations of various theories available in the literature to describe the mechanism of electrical stimulation of polyelectrolyte hydrogels under an electric field. The application of these theories as models for cartilage tissue engineering using electrical stimulation has been explained in detail. First, the kinematics, balance laws of continuum chemoelectromechanics, and constitutive equations of the fields involved in each theory are presented. Several other important features, such as the modeling approach, coupling scheme, solution method, and simulation software, are outlined. Moreover, the large or small deformation capability, transient or stationary behavior, geometric dimensions, and fixation type are summarized. All these theories are then compared, and their essential features are described.
The coupled multifield models provide an excellent description of the electrical stimulation of polyelectrolyte gels and can effectively be used for the numerical simulation of cartilage repair implants under electrical stimulation. Wallmersperger’s transport model (Wallmersperger, 2003) is the simplest of all the chemoelectromechanical models because it has the least number of unknowns. However, it has certain disadvantages that limit its use in accurately simulating electrosensitive hydrogels. The multiphasic theory/MECe model (Li et al., 2004c) is the most comprehensive theory that can be used for simulation; however, the number of unknowns involved is the highest compared with all others. It also has the ability to simulate time-dependent behaviors. However, it has the disadvantage of not being applicable to large deformations of hydrogels with high input voltages. The transport models proposed by Lam et al. (2006), Li et al. (2007b), Yew et al. (2007) are the most appropriate choices for implementation. However, the disadvantage of these models is that they cannot simulate time-dependent behavior.
Generally, multidimensional simulations are essential for a comprehensive understanding of electrosensitive hydrogels under electrical stimulation. A significant contribution was made regarding the modeling theories of electrosensitive hydrogels to replicate the experimental procedures. However, the available modeling theories mainly consider hydrogels as general polyelectrolyte materials. Further work is necessary so that a modeling approach can be selected depending on the characteristics of the electrosensitive hydrogels, for example, natural versus synthetic, macromolecular versus supramolecular, conjugated polymers versus hydrogels with nanofillers, or the choice of models based on the physical or chemical crosslinking of the hydrogel. To achieve this, modeling studies on the microscale characterization of electrosensitive hydrogels are necessary (Bassetti et al., 2005).
Author contributions
AF: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft. HS: Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing – review and editing. UvR: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The study was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – SFB 1270/1,2 – 299150580 in the scope of the CRC 1270 “Electrically Active Implants” ELAINE.
Acknowledgments
We thank Julius Zimmermann, Synthetic Physiology Lab, Dipartimento di Ingegneria Civile e Architettura, Università di Pavia, Italy, for the fruitful discussions and valuable comments on the manuscript.
Conflict of interest
The authors declare that the study was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Correction note
This article has been corrected with minor changes. These changes do not impact the scientific content of the article.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
1The readers not familiar with basic tensor theory can have a look at some relevant book, e.g., (Zohdi, 2017).
References
Acartürk, A. (2009). Simulation of charged hydrated porous materials. Ph.D. thesis. Stuttgart: University of Stuttgart.
Ali, I., Xudong, L., Xiaoqing, C., Zhiwei, J., Pervaiz, M., Weimin, Y., et al. (2019). A review of electro-stimulated gels and their applications: present state and future perspectives. Mater. Sci. Eng. C 103, 109852. doi:10.1016/j.msec.2019.109852
Amiryaghoubi, N., Fathi, M., Barar, J., Noroozi-Pesyan, N., Omidian, H., and Omidi, Y. (2023). Application of graphene in articular cartilage tissue engineering and chondrogenic differentiation. J. Drug Deliv. Sci. Technol. 83, 104437. doi:10.1016/j.jddst.2023.104437
Attaran, A. (2017). Modeling and numerical simulation of electroactive and magnetoactive polymer gels. Ph.D. thesis. Dresden: Technical University Dresden.
Attaran, A., Brummund, J., and Wallmersperger, T. (2015). Modeling and simulation of the bending behavior of electrically-stimulated cantilevered hydrogels. Smart Mater. Struct. 24, 035021. doi:10.1088/0964-1726/24/3/035021
Attaran, A., Keller, K., and Wallmersperger, T. (2018). Modeling and simulation of hydrogels for the application as finger grippers. J. Intelligent Material Syst. Struct. 29, 371–387. doi:10.1177/1045389X17708040
Bai, X., Sun, H., Jia, L., Xu, J., Zhang, P., Zhang, D., et al. (2023). Chondrocyte targeting gold nanoparticles protect growth plate against inflammatory damage by maintaining cartilage balance. Mater. Today Bio 23, 100795. doi:10.1016/j.mtbio.2023.100795
Bassetti, M. J., Chatterjee, A. N., Aluru, N. R., and Beebe, D. J. (2005). Development and modeling of electrically triggered hydrogels for microfluidic applications. J. Microelectromechanical Syst. 14, 1198–1207. doi:10.1109/jmems.2005.845407
Bhosale, A. M., and Richardson, J. B. (2008). Articular cartilage: structure, injuries and review of management. Br. Med. Bull. 87, 77–95. doi:10.1093/bmb/ldn025
Biot, M. A. (1956). Theory of deformation of a porous viscoelastic anisotropic solid. J. Appl. Phys. 27, 459–467. doi:10.1063/1.1722402
Bowen, R. M. (1980). Incompressible porous media models by use of the theory of mixtures. Int. J. Eng. Sci. 18, 1129–1148. doi:10.1016/0020-7225(80)90114-7
Brighton, C. T., Wang, W., and Clark, C. C. (2006). Up-regulation of matrix in bovine articular cartilage explants by electric fields. Biochem. Biophys. Res. Commun. 342, 556–561. doi:10.1016/j.bbrc.2006.01.171
Brighton, T., Wang, W., and Clark, C. C. (2008). The effect of electrical fields on gene and protein expression in human osteoarthritic cartilage explants. J. Bone Jt. Surg. 90, 833–848. doi:10.2106/jbjs.f.01437
Buschmann, M. D., and Grodzinsky, A. J. (1995). A molecular model of proteoglycan-associated electrostatic forces in cartilage mechanics. J. Biomechanical Eng. 117, 179–192. doi:10.1115/1.2796000
Carayon, I., Gaubert, A., Mousli, Y., and Philippe, B. (2020). Electro-responsive hydrogels: macromolecular and supramolecular approaches in the biomedical field. Biomaterials Sci. 8, 5589–5600. doi:10.1039/d0bm01268h
Chandrasekharaiah, D. S., and Debnath, L. (1994). Continuum mechanics. San Diego: Academic Press. doi:10.1016/C2009-0-21209-8
Chao, P.-H. G., Roy, R., Mauck, R. L., Liu, W., Valhmu, W. B., and Hung, C. T. (2000). Chondrocyte translocation response to direct current electric fields. J. Biomechanical Eng. 122, 261–267. doi:10.1115/1.429661
Chatterjee, A. N. (2003). Modeling and simulation of polymeric hydrogels and hydrogel based devices for MEMS/Bio-MEMS applications. Ph.D. thesis. Urbana, Illinois: University of Illinois at Urbana-Champaign.
Chatterjee, A. N., De, S. K., and Aluru, N. (2003a). “Electrically triggered hydrogels: mathematical models and simulations,” in 2003 nanotechnology conference and trade show-nanotech 2003, Cambridge, Massachusetts: TechConnect. 130–133.
Chatterjee, A. N., Yu, Q., Moore, J. S., and Aluru, N. R. (2003b). Mathematical modeling and simulation of dissolvable hydrogels. J. Aerosp. Eng. 16, 55–64. doi:10.1061/(ASCE)0893-1321(2003)16:2(55)
Chen, J. (2004). Multiphasic model development and meshless simulations of electric-sensitive hydrogels. Ph.D. thesis. Singapore: National University of Singapore.
Chen, J., and Ma, G. (2006). Modelling deformation behaviour of polyelectrolyte gels under chemo-electro-mechanical coupling effects. Int. J. Numer. Methods Eng. 68, 1052–1071. doi:10.1002/nme.1752
Chen, J., Li, H., and Lam, K. Y. (2005). Transient simulation for kinetic responsive behaviors of electric-sensitive hydrogels subject to applied electric field. Mater. Sci. Eng. C 25, 710–712. doi:10.1016/j.msec.2005.06.020
Cheng, S., Yang, J., Song, J., Cao, X., Zhou, B., Yang, L., et al. (2025). A motion-responsive injectable lubricative hydrogel for efficient achilles tendon adhesion prevention. Mater. Today Bio 30, 101458. doi:10.1016/j.mtbio.2025.101458
Chu, Y., Varanasi, P. P., McGlade, M. J., and Varanasi, S. (1995). pH-Induced swelling kinetics of polyelectrolyte hydrogels. J. Appl. Polym. Sci. 58, 2161–2176. doi:10.1002/app.1995.070581203
Culma, J. J. S., Morales, J. M. G., Uribe, Y. A. H., Garzón-Alvarado, D. A., Leal-Marin, S., Glasmacher, B., et al. (2025). Effects of electric fields on the modulation of chondrocytes dynamics in gelatin scaffolds: a novel approach to optimize cartilage tissue engineering. J. Biomaterials Sci. Polym. Ed., 1–20. doi:10.1080/09205063.2025.2466971
De, S. K., and Aluru, N. R. (2004). A chemo-electro-mechanical mathematical model for simulation of pH sensitive hydrogels. Mech. Mater. 36, 395–410. doi:10.1016/S0167-6636(03)00067-X
de Boer, R. (1996). Highlights in the historical development of the porous media theory: toward a consistent macroscopic theory. Appl. Mech. Rev. 49, 201–262. doi:10.1115/1.3101926
De, S. K., Aluru, N. R., Johnson, B., Crone, W. C., Beebe, D. J., and Moore, J. S. (2002). Equilibrium swelling and kinetics of pH-responsive hydrogels: models, experiments, and simulations. J. Microelectromechanical Syst. 11, 544–555. doi:10.1109/JMEMS.2002.803281
Doi, M., Matsumoto, M., and Hirose, Y. (1992). Deformation of ionic polymer gels by electric fields. Macromolecules 25, 5504–5511. doi:10.1021/ma00046a058
Donnan, F. G. (1924). The theory of membrane equilibria. Chem. Rev. 1, 73–90. doi:10.1021/cr60001a003
Ehlers, W. (2002). “Foundations of multiphasic and porous materials,” in Porous media: theory, experiments and numerical applications (Berlin, Heidelberg: Springer), 3–86.
Ehlers, W. (2009). Challenges of porous media models in geo- and biomechanical engineering including electro-chemically active polymers and gels. Int. J. Adv. Eng. Sci. Appl. Math. 1, 1–24. doi:10.1007/s12572-009-0001-z
El-Husseiny, H. M., Mady, E. A., Hamabe, L., Abugomaa, A., Shimada, K., Yoshida, T., et al. (2022). Smart/stimuli-responsive hydrogels: cutting-edge platforms for tissue engineering and other biomedical applications. Mater. Today Bio 13, 100186. doi:10.1016/j.mtbio.2021.100186
Erol, O., Pantula, A., Liu, W., and Gracias, D. H. (2019). Transformer hydrogels: a review. Adv. Mater. Technol. 4, 1900043–27. doi:10.1002/admt.201900043
Esfandiari, E., Roshankhah, S., Mardani, M., Hashemibeni, B., Naghsh, E., Kazemi, M., et al. (2014). The effect of high frequency electric field on enhancement of chondrogenesis in human adipose-derived stem cells. Iran. J. Basic Med. Sci. 17, 571–576. doi:10.22038/ijbms.2014.3188
Farooq, U., and Siddique, J. (2021). Compressive stress relaxation behavior of articular cartilage and its effects on fluid pressure and solid displacement due to non-newtonian flow. Comput. Methods Biomechanics Biomed. Eng. 24, 161–172. doi:10.1080/10255842.2020.1817408
Farooqi, A. R. (2020). Computational modeling of electroactive hydrogels for cartilage – tissue repair using electrical stimulation. Ph.D. thesis. Rostock: University of Rostock.
Farooqi, A. R., Bader, R., and van Rienen, U. (2019a). Numerical study on electromechanics in cartilage tissue with respect to its electrical properties. Tissue Eng. Part B Rev. 25, 152–166. doi:10.1089/ten.teb.2018.0214
Farooqi, A. R., Zimmermann, J., Bader, R., and van Rienen, U. (2019b). Numerical simulation of electroactive hydrogels for cartilage – tissue engineering. Materials 12, 2913. doi:10.3390/ma12182913
Farooqi, A. R., Zimmermann, J., Bader, R., and van Rienen, U. (2020). Computational study on electromechanics of electroactive hydrogels for cartilage-tissue repair. Comput. Methods Programs Biomed. 197, 105739. doi:10.1016/j.cmpb.2020.105739
Feng, L., Jia, Y., Li, X., and An, L. (2011). Comparison of the multiphasic model and the transport model for the swelling and deformation of polyelectrolyte hydrogels. J. Mech. Behav. Biomed. Mater. 4, 1328–1335. doi:10.1016/j.jmbbm.2011.05.001
Flory, P. J., and Rehner, J. (1943a). Statistical mechanics of cross-linked polymer networks i. rubberlike elasticity. J. Chem. Phys. 11, 512–520. doi:10.1063/1.1723791
Flory, P. J., and Rehner, J. (1943b). Statistical mechanics of cross-linked polymer networks ii. swelling. J. Chem. Phys. 11, 521–526. doi:10.1063/1.1723792
Frank, E. H., and Grodzinsky, A. J. (1987a). Cartilage electromechanics-i. electrokinetic transduction and the effects of electrolyte ph and ionic strength. J. Biomechanics 20, 615–627. doi:10.1016/0021-9290(87)90282-X
Frank, E. H., and Grodzinsky, A. J. (1987b). Cartilage electromechanics-ii. a continuum model of cartilage electrokinetics and correlation with experiments. J. Biomechanics 20, 629–639. doi:10.1016/0021-9290(87)90283-1
Gaspar, V. M., Lavrador, P., Borges, J., Oliveira, M. B., and Mano, J. F. (2020). Advanced bottom-up engineering of living architectures. Adv. Mater. 32, 1903975. doi:10.1002/adma.201903975
Grimshaw, P. E. (1989). Electrical control of solute transport across polyelectrolyte membranes. Ph.D. thesis. Cambridge, MA: Massachusetts Institute of Technology.
Grimshaw, P. E., Grodzinsky, A. J., Yarmush, M. L., and Yarmush, D. M. (1989). Dynamic membranes for protein transport: chemical and electrical control. Chem. Eng. Sci. 44, 827–840. doi:10.1016/0009-2509(89)85256-X
Grimshaw, P. E., Nussbaum, J. H., Grodzinsky, A. J., and Yarmush, M. L. (1990). Kinetics of electrically and chemically induced swelling in polyelectrolyte gels. J. Chem. Phys. 93, 4462–4472. doi:10.1063/1.458729
Gu, W. Y., Lai, W. M., and Mow, V. C. (1998). A mixture theory for charged-hydrated soft tissues containing multi-electrolytes: passive transport and swelling behaviors. J. Biomechanical Eng. 120, 169–180. doi:10.1115/1.2798299
Gülch, R. W., Holdenried, J., Weible, A., Wallmersperger, T., and Kröplin, B. (2000). “Polyelectrolyte gels in electric fields: a theoretical and experimental approach,” Proceedings of SPIE, Bellingham, Washington, March 6-9 2000. 3987, 193–202. doi:10.1117/12.387778
Hashemi-Afzal, F., Fallahi, H., Bagheri, F., Collins, M. N., Eslaminejad, M. B., and Seitz, H. (2025). Advancements in hydrogel design for articular cartilage regeneration: a comprehensive review. Bioact. Mater. 43, 1–31. doi:10.1016/j.bioactmat.2024.09.005
Hatami-Marbini, H., and Mehr, J. A. (2022). Modeling and experimental investigation of electromechanical properties of scleral tissue; a CEM model using an anisotropic hyperelastic constitutive relation. Biomechanics Model. Mechanobiol. 21, 1325–1337. doi:10.1007/s10237-022-01590-5
He, W., Kienzle, A., Liu, X., Müller, W. E., and Feng, Q. (2015). In vitro 30 nm silver nanoparticles promote chondrogenesis of human mesenchymal stem cells. RSC Adv. 5, 49809–49818. doi:10.1039/c5ra06386h
He, W., Wang, Y., Li, X., Ji, Y., Yuan, J., Yang, W., et al. (2024). Sealing the pandora’s vase of pancreatic fistula through entrapping the digestive enzymes within a dextrorotary (d)-peptide hydrogel. Nat. Commun. 15, 7235. doi:10.1038/s41467-024-51734-7
Hiemer, B., Krogull, M., Bender, T., Ziebart, J., Krueger, S., Bader, R., et al. (2018). Effect of electric stimulation on human chondrocytes and mesenchymal stem cells under normoxia and hypoxia. Mol. Med. Rep. 18, 2133–2141. doi:10.3892/mmr.2018.9174
Hon, Y. C., Lu, M. W., Xue, W. M., and Zhou, X. (1999). A new formulation and computation of the triphasic model for mechano-electrochemical mixtures. Comput. Mech. 24, 155–165. doi:10.1007/s004660050448
Hon, Y. C., Lu, M. W., Mak, A. F. T., and Zhou, X. (2000). “Mechano-electrochemical response analysis of a hydrogel strip under electric field,” in Advances in computational engineering and sciences (Palmdale, California: Tch Science Press), 1681–1686.
Horkay, F., Tasaki, I., and Basser, P. J. (2000). Osmotic swelling of polyacrylate hydrogels in physiological salt solutions. Biomacromolecules 1, 84–90. doi:10.1021/bm9905031
Hosseini, A., Van de Velde, S. K., Kozanek, M., Gill, T. J., Grodzinsky, A. J., Rubash, H. E., et al. (2010). In-vivo time-dependent articular cartilage contact behavior of the tibiofemoral joint. Osteoarthr. Cartil. 18, 909–916. doi:10.1016/j.joca.2010.04.011
Hosseini, F. S., Saburi, E., Enderami, S. E., Ardeshirylajimi, A., Bagherabad, M. B., Marzouni, H. Z., et al. (2019). Improved chondrogenic response of mesenchymal stem cells to a polyethersulfone/polyaniline blended nanofibrous scaffold. J. Cell. Biochem. 120, 11358–11365. doi:10.1002/jcb.28412
Hu, L., Zhang, Q., Li, X., and Serpe, M. J. (2019). Stimuli-responsive polymers for sensing and actuation. Mater. Horizons 6, 1774–1793. doi:10.1039/c9mh00490d
Huyghe, J. M., and Janssen, J. D. (1997). Quadriphasic mechanics of swelling incompressible porous media. Int. J. Eng. Sci. 35, 793–802. doi:10.1016/S0020-7225(96)00119-X
Jackson, A. R., and Gu, W. Y. (2009). Transport properties of cartilaginous tissues. Curr. Rheumatol. Rep. 5, 40–50. doi:10.2174/157339709787315320
Kaith, B. S., Singh, A., Sharma, A. K., and Sud, D. (2021). Hydrogels: synthesis, classification, properties and potential applications—A brief review. J. Polym. Environ. 29, 3827–3841. doi:10.1007/s10924-021-02184-5
Kanaan, A. F., and Piedade, A. P. (2022). Electro-responsive polymer-based platforms for electrostimulation of cells. Mater. Adv. 3, 2337–2353. doi:10.1039/d1ma01012c
Kim, S. J., Lee, K. J., Kim, S. I., Lee, Y. M., Chung, T. D., and Lee, S. H. (2003). Electrochemical behavior of an interpenetrating polymer network hydrogel composed of poly(propylene glycol) and poly(acrylic acid). J. Appl. Polym. Sci. 89, 2301–2305. doi:10.1002/app.12327
Kim, S. J., Yoon, S. G., Lee, S. M., Lee, S. H., and Kim, S. I. (2004). Electrical sensitivity behavior of a hydrogel composed of polymethacrylic acid/poly(vinyl alcohol). J. Appl. Polym. Sci. 91, 3613–3617. doi:10.1002/app.13597
Krueger, S., Riess, A., Jonitz-Heincke, A., Weizel, A., Seyfarth, A., Seitz, H., et al. (2021). Establishment of a new device for electrical stimulation of non-degenerative cartilage cells in vitro. Int. J. Mol. Sci. 22, 394–397. doi:10.3390/ijms22010394
Lai, W. M., Hou, J. S., and Mow, V. C. (1991). A triphasic theory for the swelling and deformation behaviors of articular cartilage. J. Biomechanical Eng. 113, 245–258. doi:10.1115/1.2894880
Lai, W. M., Mow, V. C., Sun, D. D., and Ateshian, G. A. (2000). On the electric potentials inside a charged soft hydrated biological tissue: streaming potential versus diffusion potential. J. Biomechanical Eng. 122, 336–346. doi:10.1115/1.1286316
Lam, K. Y., Li, H., Ng, T. Y., and Luo, R. (2006). Modeling and simulation of the deformation of multi-state hydrogels subjected to electrical stimuli. Eng. Analysis Bound. Elem. 30, 1011–1017. doi:10.1016/j.enganabound.2006.03.011
Lavrador, P., Esteves, M. R., Gaspar, V. M., and Mano, J. F. (2021). Stimuli-responsive nanocomposite hydrogels for biomedical applications. Adv. Funct. Mater. 31, 2005941. doi:10.1002/adfm.202005941
Lei, Y., Zhang, Q., Kuang, G., Wang, X., Fan, Q., and Ye, F. (2022). Functional biomaterials for osteoarthritis treatment: from research to application. Smart Med. 1, e20220014–e20220014. doi:10.1002/smmd.20220014
Li, H. (2009a). Kinetics of smart hydrogels responding to electric field: a transient deformation analysis. Int. J. Solids Struct. 46, 1326–1333. doi:10.1016/j.ijsolstr.2008.11.001
Li, H., Ng, T. Y., Cheng, J. Q., and Lam, K. Y. (2003). Hermite-Cloud: a novel true meshless method. Comput. Mech. 33, 30–41. doi:10.1007/s00466-003-0497-1
Li, H., Chen, J., and Lam, K. Y. (2004a). Multiphysical modeling and meshless simulation of electric-sensitive hydrogels. J. Polym. Sci. Part B Polym. Phys. 42, 1514–1531. doi:10.1002/polb.20025
Li, H., Yew, Y. K., Lam, K. Y., and Ng, T. Y. (2004b). Numerical simulation of pH-stimuli responsive hydrogel in buffer solutions. Colloids Surfaces A Physicochem. Eng. Aspects 249, 149–154. doi:10.1016/j.colsurfa.2004.08.068
Li, H., Yuan, Z., Lam, K. Y., Lee, H. P., Chen, J., Hanes, J., et al. (2004c). Model development and numerical simulation of electric-stimulus-responsive hydrogels subject to an externally applied electric field. Biosens. Bioelectron. 19, 1097–1107. doi:10.1016/j.bios.2003.10.004
Li, H., Ng, T. Y., Yew, Y. K., and Lam, K. Y. (2005). Modeling and simulation of the swelling behavior of ph-stimulus-responsive hydrogels. Biomacromolecules 6, 109–120. doi:10.1021/bm0496458
Li, H., Chen, J., and Lam, K. Y. (2006). A transient simulation to predict the kinetic behavior of hydrogels responsive to electric stimulus. Biomacromolecules 7, 1951–1959. doi:10.1021/bm060064n
Li, H., Chen, J., and Lam, K. Y. (2007a). Transient simulation of kinetics of electric-sensitive hydrogels. Biosens. Bioelectron. 22, 1633–1641. doi:10.1016/j.bios.2006.07.016
Li, H., Luo, R., Birgersson, E., and Lam, K. Y. (2007b). Modeling of multiphase smart hydrogels responding to pH and electric voltage coupled stimuli. J. Appl. Phys. 101, 114905. doi:10.1063/1.2736862
Li, H., Luo, R., and Lam, K. Y. (2007c). Modeling and simulation of deformation of hydrogels responding to electric stimulus. J. Biomechanics 40, 1091–1098. doi:10.1016/j.jbiomech.2006.04.012
Li, H., Luo, R., and Lam, K. Y. (2007d). Modeling of ionic transport in electric-stimulus-responsive hydrogels. J. Membr. Sci. 289, 284–296. doi:10.1016/j.memsci.2006.12.011
Li, H., Ng, T. Y., Yew, Y. K., and Lam, K. Y. (2007e). Meshless modeling of pH-sensitive hydrogels subjected to coupled pH and electric field stimuli: young modulus effects and case studies. Macromol. Chem. Phys. 208, 1137–1146. doi:10.1002/macp.200600620
Li, H., Luo, R., and Lam, K. Y. (2009). Multiphysics modeling of electrochemomechanically smart microgels responsive to coupled pH/electric stimuli. Macromol. Biosci. 9, 287–297. doi:10.1002/mabi.200800139
Liu, H., Chen, J., Qiao, S., and Zhang, W. (2021). Carbon-based nanomaterials for bone and cartilage regeneration: a review. ACS Biomaterials Sci. Eng. 7, 4718–4735. doi:10.1021/acsbiomaterials.1c00759
Liu, C. T., Yu, J., Lin, M. H., Chang, K. H., Lin, C. Y., Cheng, N. C., et al. (2023). Biophysical electrical and mechanical stimulations for promoting chondrogenesis of stem cells on pedot:pss conductive polymer scaffolds. Biomacromolecules 24, 3858–3871. doi:10.1021/acs.biomac.3c00506
Liu, C., Zhao, L., Dong, H., Hua, Z., Wang, Y., Wang, Y., et al. (2025). Experimental investigation on the reverse mechano-electrical effect of porcine articular cartilage. Front. Bioeng. Biotechnol. 13, 1485593. doi:10.3389/fbioe.2025.1485593
Logg, A., Mardal, K.-A., and Wells, G. (2012). Automated solution of differential equations by the finite element method: the FEniCS book, 84. Berlin, Heidelberg: Springer Science and Business Media.
Luo, R. (2008). Modeling and simulations of equilibrium of environmentally sensitive hydrogels. Ph.D. thesis. Palmdale, California: National University of Singapore.
Luo, R., Li, H., and Lam, K. Y. (2007a). Coupled chemo-electro-mechanical simulation for smart hydrogels that are responsive to an external electric field. Smart Mater. Struct. 16, 1185–1191. doi:10.1088/0964-1726/16/4/029
Luo, R., Li, H., and Lam, K. Y. (2007b). Modeling and simulation of chemo-electro-mechanical behavior of ph-electric-sensitive hydrogel. Anal. Bioanal. Chem. 389, 863–873. doi:10.1007/s00216-007-1483-9
Luo, R., Li, H., Birgersson, E., and Khin, Y. L. (2008a). Modeling of electric-stimulus-responsive hydrogels immersed in different bathing solutions. J. Biomed. Mater. Res. - Part A 85, 248–257. doi:10.1002/jbm.a.31586
Luo, R., Li, H., and Lam, K. Y. (2008b). Modeling and analysis of ph-electric-stimuli-responsive hydrogels. J. Biomaterials Sci. Polym. Ed. 19, 1597–1610. doi:10.1163/156856208786440532
Luo, R., Li, H., and Ng, T. Y. (2009). Analysis of ionic transport interaction between soft smart hydrogel and solution in BioMEMS channel. Adv. Mater. Res. 74, 125–128. doi:10.4028/www.scientific.net/AMR.74.125
Luo, R., Li, H., Ng, T. Y., and Lam, K. Y. (2010). Computational analysis of influence of ionic strength on smart hydrogel subject to coupled pH-electric environmental stimuli. Mech. Adv. Mater. Struct. 17, 573–583. doi:10.1080/15376490903556568
MacGinitie, L. A., Gluzband, Y. A., and Grodzinsky, A. J. (1994). Electric field stimulation can increase protein synthesis in articular cartilage explants. J. Orthop. Res. 12, 151–160. doi:10.1002/jor.1100120202
Mackie, J. S., and Meares, P. (1955). The diffusion of electrolytes in a cation-exchange resin membrane. I. Theoretical. Proc. R. Soc. A Math. Phys. Eng. Sci. 232, 498–509. doi:10.1098/rspa.1955.0234
Mehr, J. A., and Hatami-Marbini, H. (2022). Experimental and numerical analysis of electroactive characteristics of scleral tissue. Acta Biomater. 143, 127–137. doi:10.1016/j.actbio.2022.01.017
Mehr, J. A., and Hatami-Marbini, H. (2023). Finite deformation of scleral tissue under electrical stimulation: an arbitrary lagrangian-eulerian finite element method. Bioengineering 10, 920. doi:10.3390/bioengineering10080920
Miguel, F., Barbosa, F., Ferreira, F. C., and Silva, J. C. (2022). Electrically conductive hydrogels for articular cartilage tissue engineering. Gels 8, 710. doi:10.3390/gels8110710
Mohamed, M. A., Fallahi, A., El-Sokkary, A. M. A., Salehi, S., Akl, M. A., Jafari, A., et al. (2019). Stimuli-responsive hydrogels for manipulation of cell microenvironment: from chemistry to biofabrication technology. Prog. Polym. Sci. 98, 101147. doi:10.1016/j.progpolymsci.2019.101147
Mow, V. C., Kuei, S. C., Lai, W. M., and Armstrong, C. G. (1980). Biphasic creep and stress relaxation of articular cartilage in compression: theory and experiments. J. Biomechanical Eng. 102, 73–84. doi:10.1115/1.3138202
Murakami, T., Sakai, N., Yarimitsu, S., Nakashima, K., Yamaguchi, T., Sawae, Y., et al. (2021). Evaluation of influence of changes in permeability with aging on friction and biphasic behaviors of artificial hydrogel cartilage. Biotribology 26, 100178. doi:10.1016/j.biotri.2021.100178
Nemat-Nasser, S. (2002). Micromechanics of actuation of ionic polymer-metal composites. J. Appl. Phys. 92, 2899–2915. doi:10.1063/1.1495888
Nemat-Nasser, S., and Li, J. Y. (2000). Electromechanical response of ionic polymer-metal composites. J. Appl. Phys. 87, 3321–3331. doi:10.1063/1.372343
Nernst, W. (1888). Zur Kinetik der in Lösung befindlichen Körper. Z. für Phys. Chem. 2U, 613–637. doi:10.1515/zpch-1888-0274
Nernst, W. (1889). Die elektromotorische Wirksamkeit der Jonen. Z. für Phys. Chem. 4U, 129–181. doi:10.1515/zpch-1889-0412
Ng, T. Y., Li, H., Yew, Y. K., and Lam, K. Y. (2007). Effects of initial-fixed charge density on pH-sensitive hydrogels subjected to coupled pH and electric field stimuli: a meshless analysis. J. Biomechanical Eng. 129, 148–155. doi:10.1115/1.2472370
Ng, T. Y., Li, H., and Yew, Y. K. (2010). Computational analysis of smart soft hydrogels subjected to pH-electrical coupled stimuli: effects of initial geometry. Int. J. Solids Struct. 47, 614–623. doi:10.1016/j.ijsolstr.2009.10.024
Ngo, L., and Knothe Tate, M. L. (2020). Osteoarthritis: new strategies for transport and drug delivery across length scales. ACS Biomaterials Sci. Eng. 6, 6009–6020. doi:10.1021/acsbiomaterials.0c01081
Ni, X., Xing, X., Deng, Y., and Li, Z. (2023). Applications of stimuli-responsive hydrogels in bone and cartilage regeneration. Pharmaceutics 15, 982. doi:10.3390/pharmaceutics15030982
Nussbaum, J. H. (1986). Electric field control of mechanical and electrochemical properties of polyelectrolyte gel membranes. Ph.D. thesis. Cambridge, MA: Massachusetts Institute of Technology.
Okay, O., and Durmaz, S. (2002). Charge density dependence of elastic modulus of strong polyelectrolyte hydrogels. Polymer 43, 1215–1221. doi:10.1016/S0032-3861(01)00723-6
Planck, M. (1890). Ueber die Erregung von Electricität und Wärme in Electrolyten. Ann. Phys. 275, 161–186. doi:10.1002/andp.18902750202
Qureshi, D., Nayak, S. K., Maji, S., Anis, A., Kim, D., and Pal, K. (2019). Environment sensitive hydrogels for drug delivery applications. Eur. Polym. J. 120, 109220. doi:10.1016/j.eurpolymj.2019.109220
Rogers, Z. J., Zeevi, M. P., Koppes, R., and Bencherif, S. A. (2020). Electroconductive hydrogels for tissue engineering: current status and future perspectives. Bioelectricity 2, 279–292. doi:10.1089/BIOE.2020.0025
Romischke, J., Scherkus, A., Saemann, M., Krueger, S., Bader, R., Kragl, U., et al. (2022). Swelling and mechanical characterization of polyelectrolyte hydrogels as potential synthetic cartilage substitute materials. Gels 8, 296. doi:10.3390/gels8050296
Roy, A., Manna, K., and Pal, S. (2022). Recent advances in various stimuli-responsive hydrogels: from synthetic designs to emerging healthcare applications. Mater. Chem. Front. 6, 2338–2385. doi:10.1039/d2qm00469k
Ryan, C. N. M., Doulgkeroglou, M. N., and Zeugolis, D. I. (2021). Electric field stimulation for tissue engineering applications. BMC Biomed. Eng. 3, 1–9. doi:10.1186/s42490-020-00046-0
Saunders, J. R. (2013). AC frequency-based cyclical electrical stimulation of hydrogel microactuators. Ph.D. thesis. Edmonton, Alberta: University of Alberta.
Saunders, J. R., Abu-Salih, S., Khaleque, T., Hanula, S., and Moussa, W. A. (2008). Modeling theories of intelligent hydrogel polymers. J. Comput. Theor. Nanosci. 5, 1942–1960. doi:10.1166/jctn.2008.1001
Shi, T., Lu, H., Zhu, J., Zhou, X., He, C., Li, F., et al. (2023). Naturally derived dual dynamic crosslinked multifunctional hydrogel for diabetic wound healing. Compos. Part B Eng. 257, 110687. doi:10.1016/j.compositesb.2023.110687
Shiga, T. (1997). “Deformation and viscoelastic behavior of polymer gels in electric fields,” in Neutron spin echo spectroscopy viscoelasticity rheology (Berlin, Heidelberg: Springer), 131–163.
Shiga, T., and Kurauchi, T. (1990). Deformation of polyelectrolyte gels under the influence of electric field. J. Appl. Polym. Sci. 39, 2305–2320. doi:10.1002/app.1990.070391110
Sobczyk, M. (2018). Modellierung und Simulation von Hydrogelen und hydrogelbasierten Schichtsystemen. Ph.D. thesis. Dresden: Technical University Dresden.
Suthar, K. J., Ghantasala, M. K., and Mancini, D. C. (2007). Simulation of hydrogel micro-actuation. Proc. SPIE 6798, Microelectron. Des. Technol. Packag. III, 67980P–9. doi:10.1117/12.769925
Tanaka, T., Hocker, L. O., and Benedek, G. B. (1973). Spectrum of light scattered from a viscoelastic gel. J. Chem. Phys. 59, 5151–5159. doi:10.1063/1.1680734
Uzieliene, I., Popov, A., Lisyte, V., Kugaudaite, G., Bialaglovyte, P., Vaiciuleviciute, R., et al. (2023). Stimulation of chondrocyte and bone marrow mesenchymal stem cell chondrogenic response by polypyrrole and polypyrrole/gold nanoparticles. Polymers 15, 2571. doi:10.3390/polym15112571
Vaca-González, J. J., Escobar, J. F., Guevara, J. M., Hata, Y. A., Gallego Ferrer, G., and Garzón-Alvarado, D. A. (2019). Capacitively coupled electrical stimulation of rat chondroepiphysis explants: a histomorphometric analysis. Bioelectrochemistry 126, 1–11. doi:10.1016/j.bioelechem.2018.11.004
Vaca-González, J. J., Clara-Trujillo, S., Guillot-Ferriols, M., Ródenas-Rochina, J., Sanchis, M. J., Ribelles, J. L. G., et al. (2020). Effect of electrical stimulation on chondrogenic differentiation of mesenchymal stem cells cultured in hyaluronic acid – Gelatin injectable hydrogels. Bioelectrochemistry 134, 107536. doi:10.1016/j.bioelechem.2020.107536
Van Gelder, P., Audenaert, E., Calders, P., and Leybaert, L. (2023). A new look at osteoarthritis: threshold potentials and an analogy to hypocalcemia. Front. Aging 4, 977426–6. doi:10.3389/fragi.2023.977426
Walker, B. W., Lara, R. P., Mogadam, E., Yu, C. H., Kimball, W., and Annabi, N. (2019). Rational design of microfabricated electroconductive hydrogels for biomedical applications. Prog. Polym. Sci. 92, 135–157. doi:10.1016/j.progpolymsci.2019.02.007
Wallmersperger, T. (2003). Modellierung und Simulation stimulierbarer polyelektrolytischer Gele. Ph.D. thesis. Düsseldorf: VDI Verlag.
Wallmersperger, T. (2009). “Modelling and simulation of the chemo-electro-mechanical behaviour,” in Hydrogel sensors and actuators. Editors G. Gerlach, and K.-F. Arndt 1 edn. (Berlin, Heidelberg: Springer-Verlag Berlin Heidelberg), 137–163. chap. 4.
Wallmersperger, T., and Ballhause, D. (2008). Coupled chemo-electro-mechanical finite element simulation of hydrogels: II. Electrical stimulation. Smart Mater. Struct. 17, 045012–10. doi:10.1117/12.774639
Wallmersperger, T., and Leichsenring, P. (2016). “Polymer gels as EAPs: models,” in Electromechanically active polymers: a concise reference. Editor F. Carpi (Cham: Springer), 277–278. chap. 3. doi:10.1002/pi.2790
Wallmersperger, T., Kröplin, B., Holdenried, J., and Gülch, R. W. (2001). “A coupled multi-field-formulation for ionic polymer gels in electric fields,” Proceedings of SPIE, Bellingham, Washington, March 4-8 2001. 264–275. doi:10.1117/12.432655
Wallmersperger, T., Kröplin, B., and Gülch, R. W. (2002). “Numerical simulation of a coupled chemo-electric-formulation for ionic polymer gels in electric fields,” Proceedings of SPIE, Bellingham, Washington, March 17-21 2002. 4695, 303–314. doi:10.1117/12.475177
Wallmersperger, T., Kröplin, B., and Gülch, R. W. (2004a). Coupled chemo-electro-mechanical formulation for ionic polymer gels - numerical and experimental investigations. Mech. Mater. 36, 411–420. doi:10.1016/s0167-6636(03)00068-1
Wallmersperger, T., Kröplin, B., and Gülch, R. W. (2004b). “Modeling and analysis of chemistry and electromechanics,” in Electroactive polymer (EAP) actuators as artificial muscles: reality, potential, and challenges. Editor Y. Bar-Cohen 2 edn. (Bellingham, Washington: SPIE Press), 335–362. chap. 11.
Wallmersperger, T., Kröplin, B., and Gülch, R. W. (2004c). “Polyelectrolyte gels - basics, modelling and simulation,” in Chemo-mechanical couplings in porous media geomechanics and biomechanics. Editors B. Loret, and J. M. Huyghe 1 edn. (Vienna: Springer), 333–382.
Wallmersperger, T., Ballhause, D., Kröplin, B., Günther, M., Shi, Z., and Gerlach, G. (2008). Coupled chemo-electro-mechanical simulation of polyelectrolyte gels as actuators and sensors. Electroact. Polym. Actuators Devices (EAPAD) 2008 (SPIE) 6927, 293–302.
Wallmersperger, T., Ballhause, D., Kröplin, B., Günther, M., and Gerlach, G. (2009). Coupled multi-field formulation in space and time for the simulation of intelligent hydrogels. J. Intelligent Material Syst. Struct. 20, 1483–1492. doi:10.1177/1045389X09105236
Wallmersperger, T., Attaran, A., Keller, K., Brummund, J., Günther, M., and Gerlach, G. (2013a). “Modeling and simulation of hydrogels for the application as bending actuators. In Intelligent Hydrogels,” in Progress in colloid and polymer science. Editor W. R. Gabriele Sadowski 1 edn. (Cham: Springer), 189–204.
Wallmersperger, T., Keller, K., and Attaran, A. (2013b). Polyelectrolyte gels as bending actuators: modeling and numerical simulation. Proceedings of SPIE, Bellingham, Washington, March 10-14 2013. 8687, 86872X. doi:10.1117/12.2009662
Warren, M. R., and Bajpayee, A. G. (2022). Modeling electrostatic charge shielding induced by cationic drug carriers in articular cartilage using donnan osmotic theory. Bioelectricity 4, 248–258. doi:10.1089/bioe.2021.0026
Woo, S.-Y., Lubock, P., Gomez, M., Jemmott, G., Kuei, S., and Akeson, W. (1979). Large deformation nonhomogeneous and directional properties of articular cartilage in uniaxial tension. J. biomechanics 12, 437–446. doi:10.1016/0021-9290(79)90028-9
Xu, W., Zhu, J., Hu, J., and Xiao, L. (2022). Engineering the biomechanical microenvironment of chondrocytes towards articular cartilage tissue engineering. Life Sci. 309, 121043. doi:10.1016/j.heliyon.2024.e38112
Yang, Y., Zhao, Y., and Engberts, J. B. (2000). Stimuli response of polysoap hydrogels in aqueous solution and DC electric fields. Colloids Surfaces A Physicochem. Eng. Aspects 169, 85–94. doi:10.1016/S0927-7757(00)00420-9
Yew, Y. K. (2006). Model development for numerical simulaiton of the behaviors of pH-stimulus responsive hydrogels. Ph.D. thesis. Singapore: National University of Singapore.
Yew, Y. K., Ng, T. Y., Li, H., and Lam, K. Y. (2007). Analysis of pH and electrically controlled swelling of hydrogel-based micro-sensors/actuators. Biomed. Microdevices 9, 487–499. doi:10.1007/s10544-007-9056-4
Yuan, Z., and Li, H. (2013). Modeling development and numerical simulation of transient nonlinear behaviors of electric sensitive hydrogel membrane under an external electric field. J. Biochips Tissue Chips 03. doi:10.4172/2153-0777.1000103
Yuan, Z., Yin, L., and Jiang, H. (2007). Numerical simulation of transient nonlinear behaviors of electric-sensitive hydrogel membrane under an external electric field. Microfluid. BioMEMS, Med. Microsystems V (SPIE) 6465, 282–288. doi:10.1117/12.697085
Zhang, L., Miramini, S., Smith, D. W., Gardiner, B. S., and Grodzinsky, A. J. (2015). Time evolution of deformation in a human cartilage under cyclic loading. Ann. Biomed. Eng. 43, 1166–1177. doi:10.1007/s10439-014-1164-8
Zhang, X., Wang, S., Chen, X., Cui, Z., Li, X., Zhou, Y., et al. (2024). Bioinspired flexible kevlar/hydrogel composites with antipuncture and strain-sensing properties for personal protective equipment. ACS Appl. Mater. and Interfaces 16, 45473–45486. doi:10.1021/acsami.4c08659
Zhao, S., Mehta, A. S., and Zhao, M. (2020). Biomedical applications of electrical stimulation. Cell. Mol. Life Sci. 77, 2681–2699. doi:10.1007/s00018-019-03446-1
Zhou, X., Hon, Y. C., Sun, S., and Mak, A. F. T. (2002). Numerical simulation of the steady-state deformation of a smart hydrogel under an external electric field. Smart Mater. Struct. 11, 459–467. doi:10.1088/0964-1726/11/3/316
Zhou, Z., Zheng, J., Meng, X., and Wang, F. (2023). Effects of electrical stimulation on articular cartilage regeneration with a focus on piezoelectric biomaterials for articular cartilage tissue repair and engineering. Int. J. Mol. Sci. 24, 1836. doi:10.3390/ijms24031836
Zimmermann, J., Farooqi, A. R., and van Rienen, U. (2024). Electrical stimulation for cartilage tissue engineering - a critical review from an engineer’s perspective. Heliyon 10, e38112. doi:10.1016/j.heliyon.2024.e38112
Zohdi, T. I. (2017). Modeling and simulation of functionalized materials for additive manufacturing and 3d printing: continuous and discrete media: continuum and discrete element methods, 60. Cham: Springer.
Zou, F., Xu, J., Yuan, L., Zhang, Q., and Jiang, L. (2022). Recent progress on smart hydrogels for biomedicine and bioelectronics. Biosurface Biotribology 8, 212–224. doi:10.1049/bsb2.12046
Keywords: cartilage tissue engineering, electrical stimulation, electrosensitive hydrogels, scaffolds, transport theory, multiphasic theory, porous media theory, computational modeling
Citation: Farooqi AR, Seitz H and van Rienen U (2025) Review on modeling theories of electrosensitive hydrogels for cartilage tissue engineering. Front. Bioeng. Biotechnol. 13:1631725. doi: 10.3389/fbioe.2025.1631725
Received: 20 May 2025; Accepted: 28 July 2025;
Published: 12 August 2025; Corrected: 18 August 2025.
Edited by:
Paolo Gargiulo, Reykjavík University, IcelandReviewed by:
Mahmoud Ebrahimi, University of Maragheh, IranJoão C. Silva, Polytechnic University of Turin, Italy
Copyright © 2025 Farooqi, Seitz and van Rienen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abdul Razzaq Farooqi , YWJkdWwuZmFyb29xaUB1bmktcm9zdG9jay5kZQ==
 Abdul Razzaq Farooqi
Abdul Razzaq Farooqi Hermann Seitz
Hermann Seitz Ursula van Rienen
Ursula van Rienen