- 1 School of Stomatology, Jiangxi Medical College, Nanchang University, Nanchang, Jiangxi, China
- 2 Jiangxi Provincial Key Laboratory of Oral Diseases, Nanchang, Jiangxi, China
- 3 Jiangxi Provincial Clinical Research Center for Oral Diseases, Nanchang, Jiangxi, China
The rising prevalence of antibiotic resistance necessitates innovative alternatives for managing polymicrobial oral infections. Photothermal therapy (PTT) emerges as a revolutionary approach that transcends conventional antimicrobial limitations by leveraging near-infrared (NIR)-activated photothermal agents to generate localized hyperthermia, enabling precise biofilm eradication while circumventing systemic drug resistance. The modality capitalizes on the anatomical accessibility of oral tissues and the optical transparency of dental structures, allowing spatiotemporal control over pathogenic niches from superficial caries biofilms to deep periodontal pockets. Recent advances in nanoplatform engineering have unlocked multifunctional PTT systems capable of synergizing thermal ablation with immunomodulation, biofilm matrix penetration, and even tissue regeneration, addressing the dual challenges of microbial persistence and host inflammatory damage. However, clinical translation remains hindered by unresolved technical barriers, including optimal thermal dosage calibration, lesion-specific material design, and long-term biosafety assessment. This review systematically dissects cutting-edge photothermal strategies across the oral infectious spectrum (dental caries, endodontic infections, periodontitis, and peri-implantitis) while critically evaluating their mechanistic innovations in overcoming antibiotic limitations. We further propose a roadmap for next-generation smart PTT systems integrating stimulus-responsive materials and microbiome-aware therapeutic paradigms to achieve personalized oral infection management.
1 Introduction
As one of the most critical anatomical regions in the body, the oral cavity constitutes the initial segment of the digestive tract and maintains direct exposure to the external environment (Madani et al., 2014; Kunath et al., 2024). This unique anatomical and physiological positioning renders it highly susceptible to colonization by a diverse array of microorganisms. Structures such as teeth, gingival sulci, and mucosal surfaces provide a nutrient-rich ecological niche for these microbial communities to colonize, flourish, and thrive (Deo and Deshmukh, 2019; Brookes et al., 2023). The oral microbiome, recognized as the second most complex microbial ecosystem in the human body, predominantly colonizes the surface of oral mucosa and dentition (Kilian et al., 2016; Xiao et al., 2020; Baker et al., 2024). The maintenance of oral microbial homeostasis is critical for preserving both oral and systemic health. Multiple exogenous and endogenous factors—including dietary patterns, tobacco use, suboptimal oral hygiene practices, systemic comorbidities, and pharmacological interventions—can perturb the equilibrium of the oral microbiota, predisposing to pathogenic shifts (Sedghi et al., 2021; Gupta et al., 2024). Such dysbiosis states enable the proliferation of opportunistic pathogens, precipitating polymicrobial infections exemplified by periodontitis (Lamont et al., 2018; Jiang et al., 2021; Sedghi et al., 2021; Belibasakis et al., 2024). As a global health priority, oral infections rank among the most prevalent human infections, imposing significant socioeconomic burdens on healthcare infrastructure and international economies (Peres et al., 2019; Bernabe et al., 2020; Collaborators, 2025; Zheng et al., 2025). Clinically, these diseases often present with symptoms such as toothache and gingival inflammation, which can significantly impair mastication, communication, and aesthetic function, ultimately diminishing people’s quality of life (Spanemberg et al., 2019; Popescu et al., 2024). Moreover, emerging evidence underscores a compelling association between oral infections and an elevated risk of systemic disorders, including diabetes mellitus, atherosclerosis, and Alzheimer’s disease (Scannapieco and Cantos, 2016; Altamura et al., 2024; Popescu et al., 2024; Villoria et al., 2024). Consequently, the prevention, management, and therapeutic intervention of oral infections have garnered considerable scientific and clinical attention, underscoring the imperative for interdisciplinary research and innovative strategies to mitigate their global impact.
Oral infections comprise a diverse group of highly prevalent conditions, including dental caries, endodontics, periodontitis, and peri-implantitis, etc (Gondivkar et al., 2019; Peres et al., 2019). The management and treatment of these diseases are characterized by three distinct features: First, the affected organs (such as the pulp chamber, root canal, and periodontal tissues) are small in volume yet anatomically complex, making it difficult to completely eradicate infections, which often results in suboptimal treatment outcomes or even failure. Second, most of the oral infections are oral biofilm infection-associated diseases (Muras et al., 2022; Pan et al., 2025). Extracellular polymeric substances (EPS) in microbial biofilms offer adhesion and protection, rendering innate immune cells and conventional antimicrobials ineffective at breaking down oral biofilms and eradicating the microbes they contain (Bowen et al., 2018; Chen et al., 2023a). Third, the oral and maxillofacial region, being crucial for speech, mastication, respiration, and aesthetics, possesses complex physiological and psychological functions, necessitating minimally invasive treatment approaches that preserve function. Oral infections typically necessitate the removal of pathogenic bacteria and their biofilms (Ertem et al., 2017; Pitts and Mayne, 2021; Alawaji et al., 2022). Current clinical approaches are based on mechanical removal supplemented by antibiotics, such as scaling and root planning (SRP) therapy combined with minocycline for periodontitis (Mombelli, 2018; Sanz et al., 2020; Laforgia et al., 2024). However, the effectiveness of the traditional mechanical bacteria and biofilms removal method is primarily compromised by the intricate and small anatomical structures of the organs (Li et al., 2021; Lin et al., 2024). Meanwhile, inappropriate antibiotic use has led to bacterial resistance, including multidrug-resistant bacteria (Hernando-Amado et al., 2019; Rams et al., 2020). Oral biofilms significantly contribute to drug resistance, as their matrix effectively bars the penetration and activation of antibiotics. Moreover, as bacteria expand and metabolic residues accumulate, the resulting acidic shift within the biofilm environment not only inactivates antibiotics but also compromises their overall effect. Therefore, alternative non-antibiotic-dependent antimicrobial strategies are required to address these issues.
Widely utilized across various fields, including antimicrobial applications, photothermal therapy (PTT) represents a promising strategy for the treatment of oral infections (He et al., 2023; Wang et al., 2024; Liang et al., 2025; Zhang and Chen, 2025). PTT operates by exposing photothermal agents (PTAs) to light at a specific wavelength (e.g., visible or near-infrared, NIR), which facilitates the interaction of photons with the PTAs’ surface (Overchuk et al., 2023). This interaction induces molecular vibrations and rotations, converting the absorbed energy into heat and elevating the local temperature (Fang et al., 2025; Zhang et al., 2025). Elevated temperatures disrupt bacterial cell membranes, compromising their structural integrity and increasing permeability, which leads to the leakage of essential intracellular components (Cao et al., 2024; Mondal et al., 2024). Concurrently, crucial bacterial proteins involved in replication, metabolic processes, and survival are denatured, ultimately leading to bacterial demise (Yin et al., 2019). Unlike antibiotics, PTT’s physical antibacterial mechanism offers broad-spectrum capabilities, a low likelihood of inducing drug resistance, and the ability to circumvent pre-existing drug-resistant bacterial strains (Cao et al., 2024). PTT has also demonstrated significant advantages in combating biofilms. PTAs, especially in nanoparticle form, can readily traverse the EPS to access the embedded microbial cells (Pinto et al., 2020). Moreover, hyperthermia exhibits significant potential to disrupt the intrinsic physiological microenvironment of biofilms by inactivating their inherently bioactive substrates, such as nucleic acids and proteins, which contribute to the degradation of oral biofilms (Liu et al., 2021; Chen et al., 2023b; Mammari and Duval, 2023). It has also been reported that PTT can disrupt pathogen co-aggregation via the Cbe-Ltp1-Ptk1-fimA signaling pathway, thereby preventing biofilm development (Lin et al., 2024). While high temperatures (>50 °C) inhibit bacterial growth, mild PTT (mPTT; <45 °C) can modulate host immune responses and promote tissue regeneration (Sheng et al., 2021; Zhang et al., 2021; Huang et al., 2022; Li et al., 2022; Xue et al., 2022; Xue et al., 2023). Extensive studies have demonstrated that periodic mild PTT, by maintaining local temperatures at approximately 40 °C–43 °C for short durations (e.g., 3–5 min) repeated several times, can substantially mitigate inflammation and accelerate both angiogenesis and osteogenesis (Zhang et al., 2019; Li et al., 2022; Wu et al., 2022; Zeng et al., 2023; You et al., 2024). Thermal stimulation could regulate macrophage polarization by activating the PI3K-AKT1 signaling pathway, which promotes the phenotypic transition of pro-inflammatory M1 macrophages towards an anti-inflammatory and pro-reparative M2 state. Angiogenesis might be fostered through the vascular endothelial growth factor (VEGF), heat shock protein 90 (HSP90)/endothelial nitric oxide synthase (eNOS) pathways, while osteogenesis might be promoted by enhancing bone morphogenetic protein-2 (BMP-2) expression and activating the Wnt signaling pathway (Zhang et al., 2019; Sheng et al., 2021). Therefore, PTT offers a powerful and versatile approach, integrating potent antimicrobial activity with desirable anti-inflammatory and pro-regenerative functions, making it a valuable strategy when combined with other antimicrobial and regenerative strategies (Chen et al., 2023b). As a non-antibiotic-dependent antimicrobial strategy, PTT offers antibacterial performance superior to that of antibiotics. This advantage stems from its non-invasive, spatiotemporal, and site-selective characteristics, strong tissue penetration, low side effects, broad-spectrum antibacterial properties, inherent resistance to the development of drug resistance, and versatile nature as a therapeutic platform (Wu J. et al., 2019) (Figure 1).
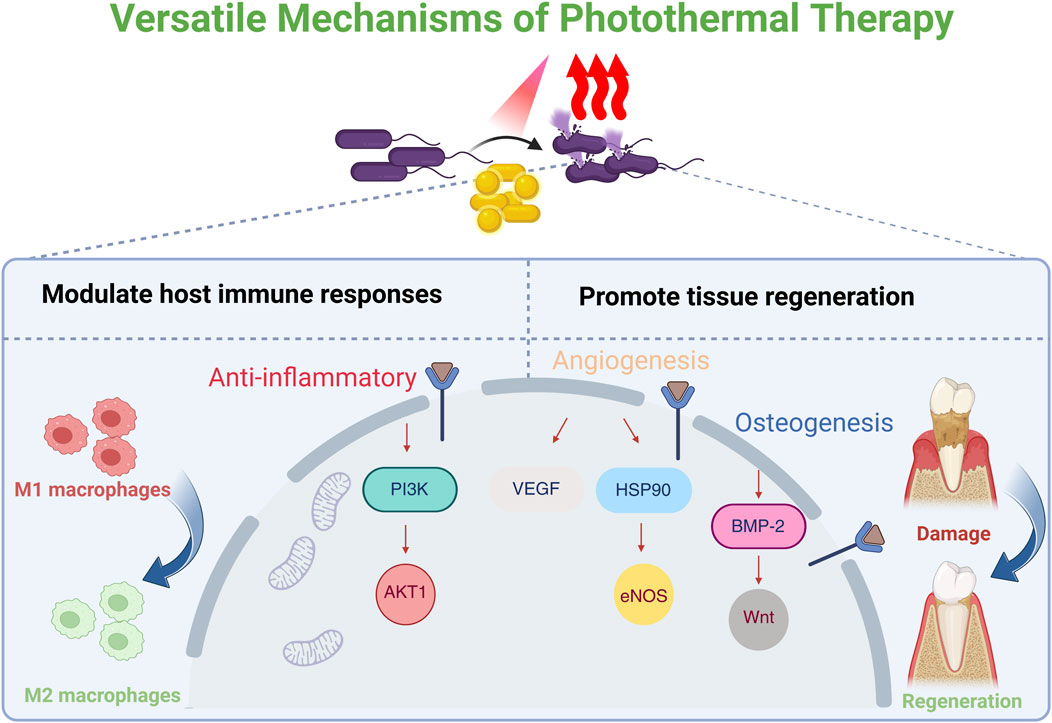
Figure 1. Schematic illustration of the versatile mechanisms of photothermal therapy (PTT). PTT could modulate the host’s biological response. This includes promoting the polarization of pro-inflammatory M1 macrophages towards an anti-inflammatory M2 phenotype via the PI3K-AKT1 signaling pathway. The resulting pro-regenerative microenvironment enhances angiogenesis through the VEGF/HSP90/eNOS pathway and promotes osteogenesis by activating the BMP-2 and Wnt signaling pathways. Created in BioRender. Liang, J. (2025) https://BioRender.com/6vgs15f.
The efficacy of PTT for oral infections hinges on two factors: the PTAs and the light sources. While the superficial anatomical location of oral tissue mitigates concerns regarding penetration depth, PTT’s overall effectiveness is primarily limited by the photothermal conversion efficiency (PCE) of PTAs and the risk of collateral thermal damage to healthy tissues from overheating (Liu et al., 2019; Yu S. et al., 2024). Over the past few decades, advancements in nanomaterials have significantly improved the PCE of PTAs (Zhao et al., 2024). Research has gradually shifted from focusing solely on the photothermal properties of individual materials to strategically designing and synthesizing multifunctional platforms. These platforms integrate features like targeted drug delivery, synergistic antibacterial action, and combinational immune modulation, thereby enhancing therapeutic efficacy while minimizing adverse effects. This field has rapidly progressed, yielding encouraging results. While existing reviews primarily focus on specific materials [e.g., gold-based nanomaterials (Zhang S. et al., 2024; Qi et al., 2025)] or single diseases [e.g., dental caries (Xu et al., 2025) and periodontitis (Li J. et al., 2024)], a comprehensive, interdisciplinary overview of the broader spectrum of major oral infections is still missing. Here, we summarize the advances of PTT in oral infections with a focus on dental caries, endodontics, periodontics, and peri-implantitis, highlight the design concepts and mechanisms, address the challenges PTT faces, and suggest future directions (Figure 2). We aim to provide a foundational framework for advancing PTT research for the treatment of oral infections and to catalyze the development of precise, efficient, and clinically viable therapeutic strategies (Table 1).
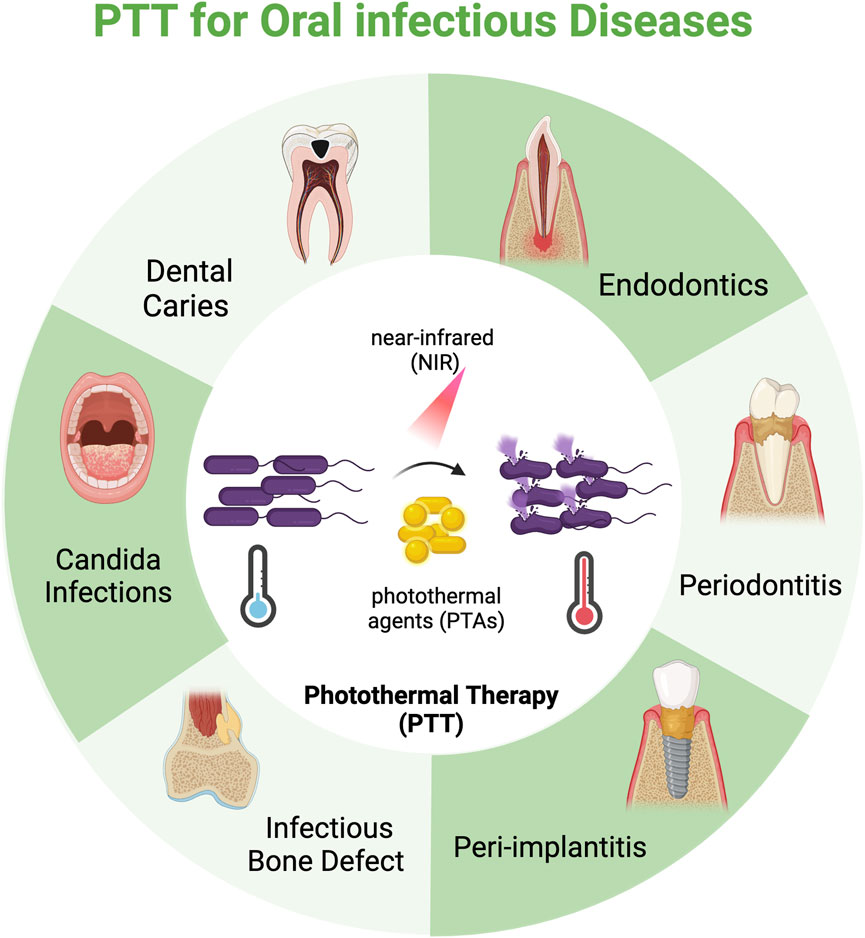
Figure 2. Schematic illustration of photothermal therapy (PTT) for oral infectious diseases. By leveraging photothermal agents (PTAs), PTT converts near-infrared (NIR) laser into localized hyperthermia to induce pathogen mortality. The application of PTT to oral infectious diseases has predominantly seen progress in the areas of dental caries, endodontics, periodontics, and peri-implantitis, but also holds significant promise for treating other infections like infectious bone defects and candida infections. Created in BioRender. Liang, J. (2025) https://BioRender.com/6vgs15f.
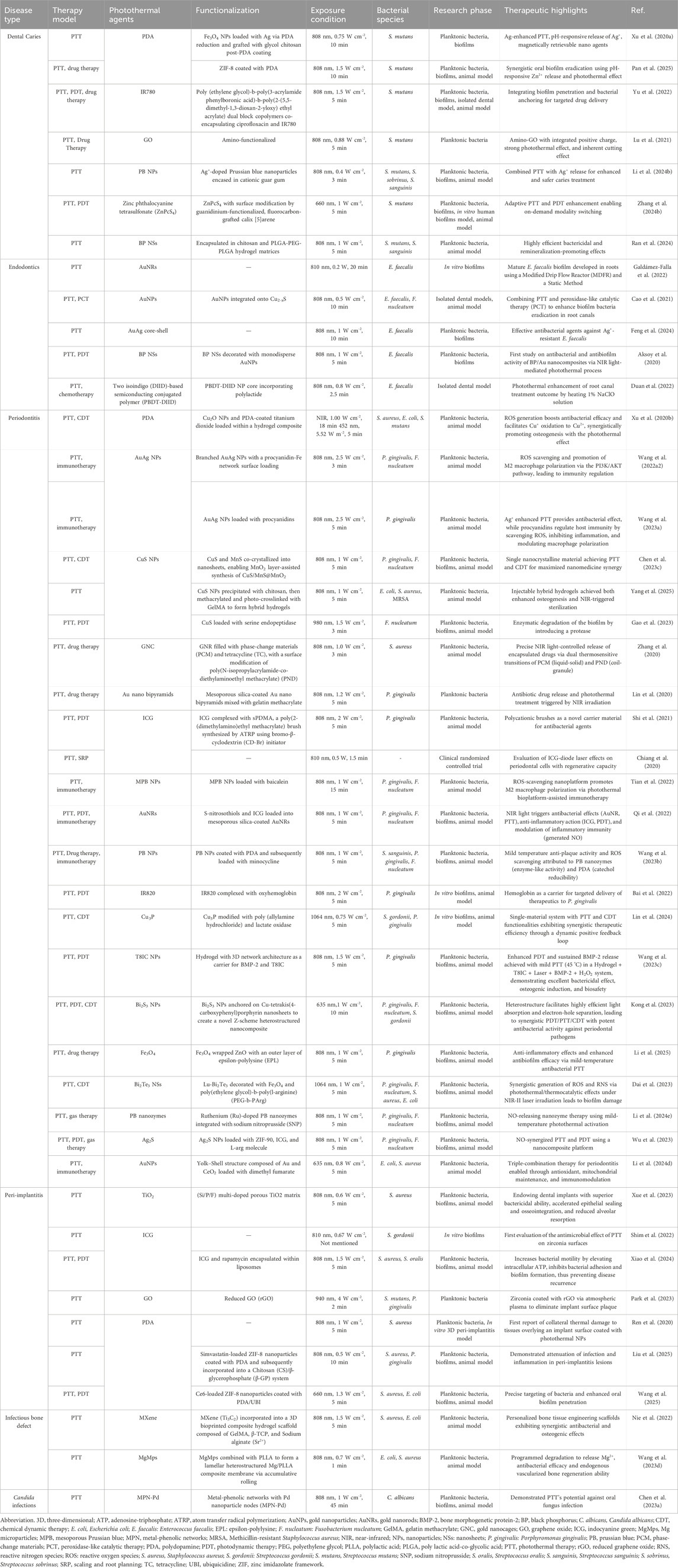
Table 1. Summaries of photothermal therapy (PTT) for oral infectious diseases. This section comprehensively summarizes the details of recent research reported in the literature, emphasizing the functionalization of photothermal agents (PTAs), their therapeutic highlights, and the underlying design concepts and mechanisms.
2 PTT for dental caries
Dental caries is a chronic condition precipitated by the accumulation of dental plaque, metabolic acid production, and subsequent localized demineralization of hard tissues, which can lead to severe tooth defects (Pitts et al., 2017; Shen et al., 2024; Zhao et al., 2024). Dental plaque, composed of cariogenic microbial biofilms, serves as a critical etiological driver in dental caries development, with Streptococcus mutans (S. mutans) being the primary cariogenic pathogen (Li et al., 2024d; Mazurel et al., 2025). The primary approach to preventing and treating caries involves using antimicrobial drugs combined with mechanical removal of decayed tissue (Akindele et al., 2025; Song et al., 2025). However, biofilms impede the penetration of antimicrobial drugs and enable bacteria to adapt their metabolic states to the biofilm microenvironment, thereby fostering antibiotic resistance (Jakubovics et al., 2021; Hajishengallis et al., 2023). Moreover, mechanical removal often damages healthy tooth structures and is prone to caries recurrence (AlSahafi et al., 2022). There have been innovative approaches for caries prevention and treatment, including therapeutics to prevent the demineralization caused by dental biofilm (Li et al., 2024d) (novel chemoprophylactic agents, antimicrobial peptides, probiotics and replacement therapy, etc.) as well as therapeutics to promote the remineralization process (fluoride and casein phosphopeptides, etc.) (Chen and Wang, 2010). However, the implementation of these methods is limited by factors such as mucosal irritation, systemic toxicity, the development of drug-resistant microbes, and the inability to maintain adequate drug concentrations in the oral cavity.
The unique properties of PTT, such as its ability to deliver localized and controlled thermal energy, make it a promising alternative to conventional methods for managing dental caries. By leveraging the photothermal effect, PTT can selectively target cariogenic biofilms without causing significant damage to surrounding healthy tissues (Zhu et al., 2014; Tan et al., 2018; Yang et al., 2018). Since cariogenic bacteria generate an acidic microenvironment through biofilm formation and acid production on tooth surfaces, pH-responsive targeting strategies have been developed for PTAs (Xu H. et al., 2022; Yu et al., 2022). A photothermal antibacterial “warm paste” was fabricated by loading Ag onto the surface of Fe3O4 nanoparticles by polydopamine (PDA) reduction, followed by a second PDA coating and subsequent grafting with glycol chitosan. Under normal physiological conditions, the PDA layer inhibits the excessive release of Ag+ and reduces its damage to normal tissues. However, within a cariogenic acidic environment, the protonation of amine groups on glycol chitosan leads to a positive charge on the nanoparticles, which enhances their strong adhesion to negatively charged cariogenic bacteria at the intended site. When irradiated by NIR, the increased temperature promotes Ag + release, leading to a high local concentration in the cavitated dental tissue. This thereby achieves effective targeted antimicrobial action through an Ag-assisted PTT strategy (Figure 3A) (Xu H. et al., 2022). Other pH-responsive agents tailored for acidic oral niches include zinc imidazolate framework-8 (ZIF-8) and Poly (ethylene glycol) (PEG) etc (Yu et al., 2022; Pan et al., 2025). The positive charge of some modified PTAs like amino-functionalized graphene oxide (GO) ensures their strong interaction with the negatively charged bacterial cells, which can also be helpful for the target of cariogenic bacteria (Lu et al., 2021). This precision is particularly advantageous in the complex and delicate environment of the oral cavity, where preserving tooth structure and minimizing collateral damage is critical. Furthermore, PTT’s ability to generate heat at specific depths reduces the production of exopolysaccharides—the main component endowing biofilm architecture and stability (Yao W. et al., 2025). This mechanism aids in the disintegration of biofilms and ensures effective penetration, addressing a major limitation of traditional antimicrobial therapies. Emerging studies have also highlighted the potential of PTT to synergize with other therapeutic modalities (Xu X. et al., 2022; Li et al., 2024c). One key feature of PTT, its efficient thermal generation at desired locations, can enhance combination therapies in various ways. For instance, PTT can be combined with photodynamic therapy (PDT) or antimicrobial agents, where the photothermal effect promotes the controlled release of these agents (Xu H. et al., 2022; Pan et al., 2025) or enables on-demand modality switching between PTT and PDT (Zhang Y. et al., 2024), thereby boosting their efficacy in eradicating biofilms. An adaptive supramolecular nanoformulation (ZnPcS4@GC5AF5, GFZ) switchable from PTT to PDT under the trigger of adenosine triphosphate (ATP) was reported. The activation of the photothermal properties of GFZ through visible irradiation led to bacterial cell membrane rupture and intracellular ATP release. Subsequently, ATP reduced the photothermal activity (low state) and restored the photodynamic activity (ON state). A large number of reactive oxygen species (ROS)were generated while avoiding high local temperatures, which not only resulted in eradicating pathogenic bacteria biofilms but also minimized heat damage to normal pulp tissues (Figure 3B) (Zhang Y. et al., 2024).
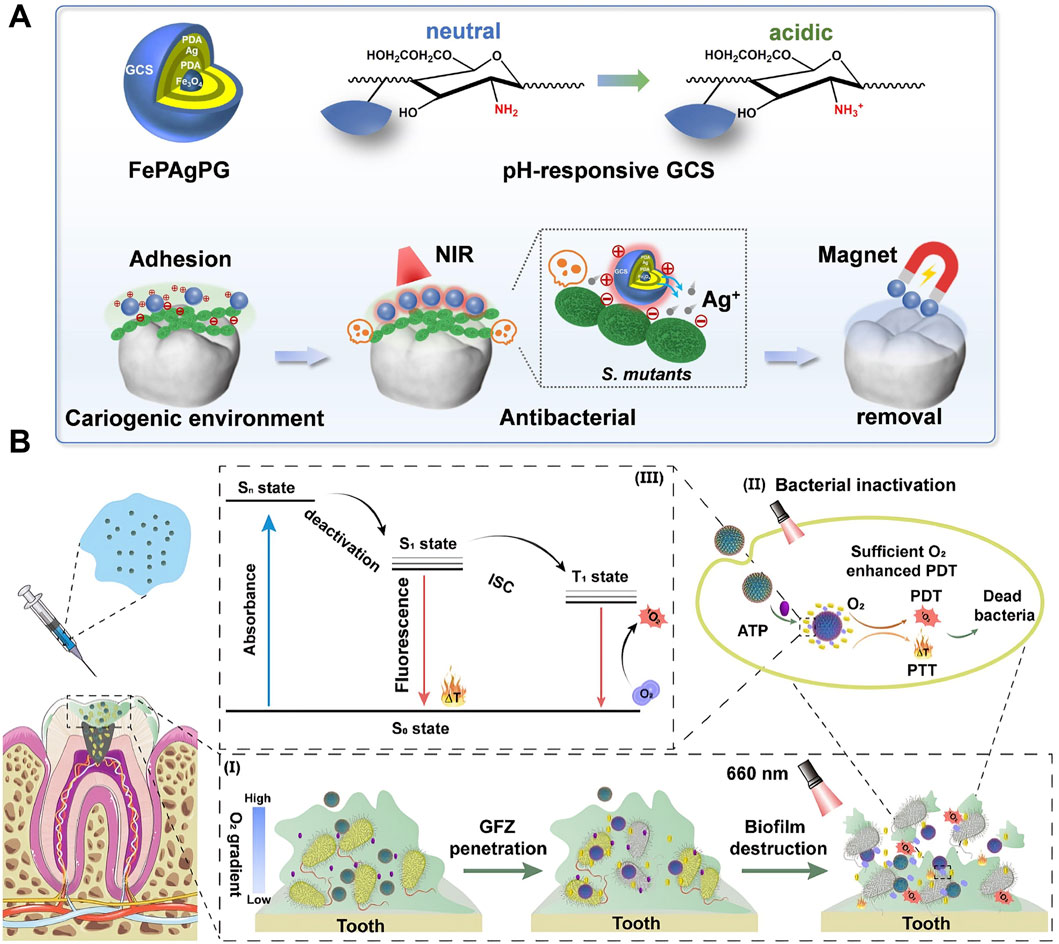
Figure 3. Photothermal therapy (PTT) for dental caries. (A) Schematic illustration depicting a removable photothermal antibacterial “warm paste” designed to target cariogenic bacteria. Reproduced with permission (Xu H. et al., 2022). Copyright 2021, Elsevier Group. (B) Schematic illustration of effective biofilm removal using a supramolecular nanoformulation featuring adaptive photothermal/photodynamic conversion. Reproduced with permission (Zhang Y. et al., 2024). Copyright 2024, American Chemical Society.
Despite these promising advances, several challenges remain in translating PTT into clinical practice for caries treatment. Key issues include optimizing the parameters of light irradiation (e.g., wavelength, intensity, and duration) to achieve effective biofilm eradication without causing thermal damage to oral tissues. Additionally, PTAs’ long-term safety and biocompatibility must be rigorously evaluated to confirm their suitability in the oral cavity. The prevention and management of dental caries represent a protracted process, so further research is also needed to investigate the potential of PTT in preventing caries’ recurrence and addressing the complex microbial ecology of dental biofilms. Developing composite materials capable of inhibiting the proliferation of cariogenic bacteria and facilitating the remineralization of early-stage demineralized dental tissues constitutes a promising research trajectory for the future (Zhu et al., 2022). In conclusion, PTT represents a groundbreaking approach to combating dental caries, offering a combination of precision, efficacy, and minimal invasiveness that addresses the limitations of current therapies. As research in this field continues to advance, PTT holds the potential to revolutionize the prevention and treatment of dental caries, ultimately improving oral health outcomes and alleviating the global burden of this pervasive disease.
3 PTT for endodontics
The dental pulp comprises sterile connective tissue and is protected by the surrounding enamel, dentin, and cementum (Pohl et al., 2024). Exposure resulting from factors including trauma, dental caries, or tooth wear can precipitate endodontics, characterized by symptoms such as pain, sinus tracts, and swelling (Karamifar et al., 2020). The elimination of bacteria and their biofilms assumes a pivotal role in the treatment of endodontics (Neelakantan et al., 2017). In clinical practices, root canal therapy (RCT) represents a commonly employed approach for removing microorganisms that instigate or exacerbate this ailment (Burns et al., 2022; Huang et al., 2024). Although biomechanical root canal preparation and chemical sterilization of irrigants could effectively eradicate the microbes, achieving thorough debridement and eradicating tenacious infections persist as formidable challenges in root canal treatment (Moradi Eslami et al., 2019). Additionally, High concentrations of chemical irrigants may cause serious damage by irritating periodontal soft and periapical tissues (Xu H. et al., 2022). Enterococcus faecalis (E. faecalis) is a key bacterial species frequently isolated from root canals afflicted with refractory endodontic infections, contributing to 20%–70% of RCT failures (Manoil et al., 2023). This is attributed to its capacity to form biofilms which can adapt to external alterations as an integrated entity (Pourhajibagher et al., 2018; Cao et al., 2021). Consequently, various studies aim to explore novel materials, encompassing irrigants and intracanal dressings, to eliminate E. faecalis in the biofilm phase (Aksoy et al., 2020; Cao et al., 2021; Duan et al., 2022; Galdámez-Falla et al., 2022; Feng et al., 2024). During PTT, hyperthermia aids in biofilm disintegration and induces bacterial demise (Mei et al., 2023; Zhou et al., 2024). Crucially, it remains confined within the root canal, as tooth hard tissues impede the complete transfer of heat to the periodontal tissues, thereby reducing potential damage to these tissues (Duan et al., 2022). Studies have demonstrated that PTT exhibits remarkable efficacy against E. faecalis and its biofilms without compromising dentin strength, supporting its potential as a prospective antibacterial therapy during RCT (Castillo-Martínez et al., 2015; Khantamat et al., 2015; Bermúdez-Jiménez et al., 2020; Galdámez-Falla et al., 2022).
The thermal generation of PTT not only directly inhibits E. faecalis and its biofilms, but can also be combined with root canal conventional irrigants, such as sodium hypochlorite (NaClO) and hydrogen peroxide (H2O2), to augment the overall efficacy of root canal disinfection. Cao et al. (2021) constructed Au@Cu2-xS NPs by integrating Cu2-xS with peroxidase-like activity and Au NPs with photothermal effect to augment the capacity to eliminate biofilms. It not only exhibits strong photothermal activity but also catalyzes H2O2 to generate hydroxyl radicals (·OH), which are more effective for biofilm degradation. Mechanistic studies demonstrated that the treatment effectively degrades proteins and polysaccharides—the primary components of biofilm EPS. The synergistic strategy combining PTT and peroxidase-like catalytic treatment with H2O2 holds significant potential for eradicating bacteria and biofilms within root canals (Figure 4A). Heating 1% NaClO—another irrigant extensively used clinically—within the root canal during PTT also significantly enhances its antibacterial efficacy (Abou-Rass and Oglesby, 1981; Tosić et al., 2016). A temperature increase of <10 °C on the external root surface achieved 99.7% antimicrobial efficacy against E. faecalis using heated 1% NaClO solution (Figure 4B) (Duan et al., 2022). Additionally, scanning electron microscopy (SEM) reveals that the teeth treated in the experimental group exhibit regular exposure of dentinal tubules. Conversely, the dentin in the control groups exhibited a rough surface, characterized by a profusion of bacteria and smear layers, with the majority of dentinal tubules remaining occluded (Figure 4C) (Duan et al., 2022). These studies manifested the potential of safely and efficaciously improve the RCT outcome by heating the irrigants.
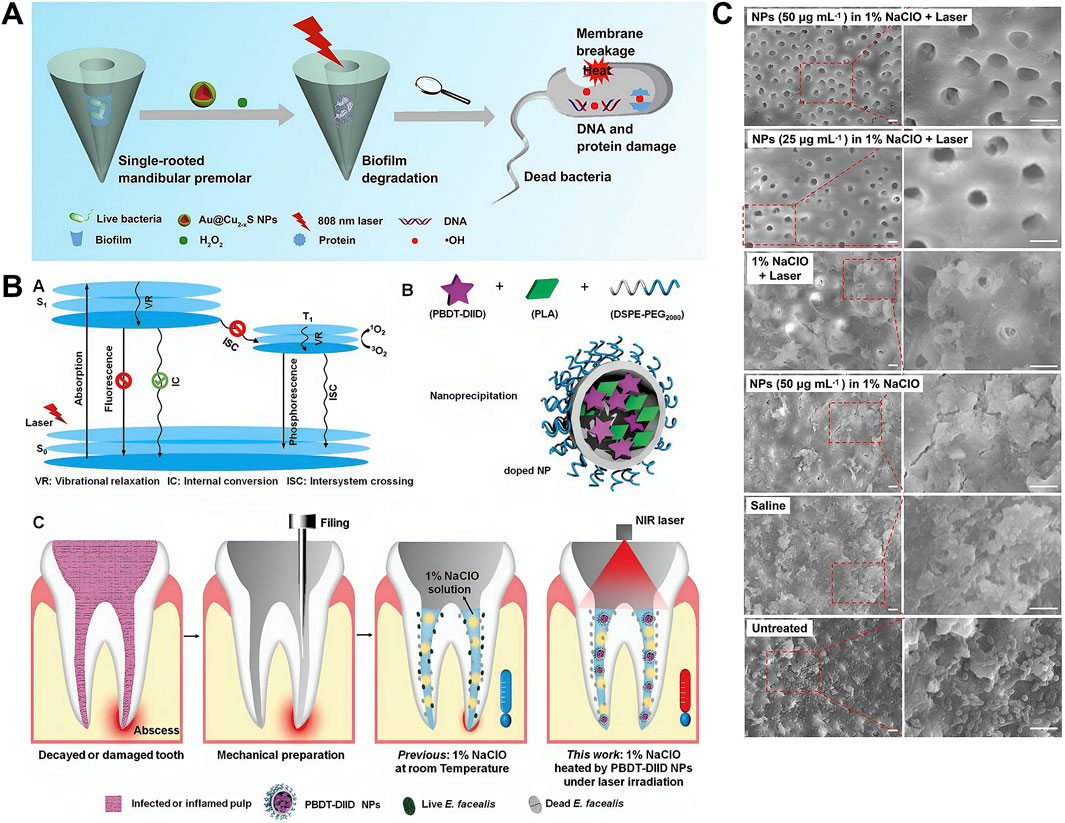
Figure 4. Photothermal therapy (PTT) for endodontics. (A) Schematic illustration of Au@Cu2-xS nanoparticles with NIR photothermal and peroxidase (POD) catalytic activities for antibiofilm-oriented root canal therapy. Reproduced with permission (Cao et al., 2021). Copyright 2021, Elsevier Group. (B) Schematic diagram illustrating the heating of a 1% NaClO solution under 808 nm laser irradiation in the presence of PBDT-DIID nanoparticles to enhance intracanal sterilization. (C) Representative SEM images of the middle of the sample tooth in different groups. Reproduced with permission (Duan et al., 2022). Copyright 2022, John Wiley and Sons Group.
Current research on PTT for endodontic diseases focuses primarily on its antibacterial role as an adjunct to root canal therapy, predominantly targeting E. faecalis. As effective treatments for refractory and recurrent root canal infections remain lacking, PTT offers a promising therapeutic approach. Nevertheless, the tooth root canal is complex and contains many small branching canals. The root canal biofilm is a very complex, organized entity (Neelakantan et al., 2017). Single-rooted mandibular premolar models and monospecies biofilms used in previous studies may oversimplify the root canals and the ecological phenomenon of biofilms. They may not truly reflect the results achievable in the clinical scenario. After the photothermal material is injected into the root canal and exerts its function, the challenge of effectively removing it without impeding subsequent root canal filling represents major hurdle confronting research in this field.
4 PTT for periodontitis
Among all oral infectious diseases, the PTT for periodontitis has garnered the most extensive attention. Periodontitis represents a chronic inflammatory disorder instigated by bacteria (Kwon et al., 2021). The establishment of pathogenic bacteria within subgingival dental plaque provokes the host immune response, resulting in the generation of a significant amount of ROS and subsequent oxidative stress (Sczepanik et al., 2020; Kwon et al., 2021; Iniesta et al., 2023). Consequently, this process leads to the degradation of tooth-supporting tissues, eventually resulting in the development of periodontal pockets, alveolar bone resorption, and subsequent tooth loosening (Kuboniwa et al., 2017; Tóthová and Celec, 2017; Heitz-Mayfield, 2024). Currently, in clinical practice, mechanical debridement and antibiotics are commonly employed (Mombelli, 2018; Cobb and Sottosanti, 2021). Nevertheless, in most cases, mechanical debridement proves arduous to comprehensively eliminate periodontitis infections within deep-seated periodontal pockets, furcation, and irregular root surface regions (Umeda et al., 2004). Additionally, the protracted administration of antibiotics engenders numerous issues, such as the development of drug-resistant bacteria, bacillary dysentery, and gastrointestinal disorders (Rams et al., 2020). Beyond bacterial factors, biofilm-induced immune dysregulation constitutes another major contributor to impaired bacterial clearance and disease persistence in periodontitis. Consequently, periodontitis treatment represents a key research focus in dentistry, with current strategies targeting not only antibacterial action but also anti-inflammatory effects and periodontal regeneration (Kong et al., 2023; Wang F. et al., 2023; Li T. et al., 2024; Li Z. et al., 2024; Yang et al., 2025). PTT offers distinct advantages for periodontitis treatment, as it not only enables efficient and safe bacterial elimination but also promotes cell proliferation, angiogenesis, wound healing, and bone regeneration—key factors in periodontal recovery (Zhang et al., 2021). More significantly, it can be integrated with PDT, chemical dynamic therapy (CDT), antibacterial agents, and bioactive materials to construct a material system featuring multi-functional synergy in antibacterial, anti-inflammatory, and tissue-regeneration functions (Shi et al., 2021; Bai et al., 2022; Wang H. et al., 2023; Lin et al., 2024; Wu et al., 2025).
Unlike dental caries or endodontic treatments—which involve heat-tolerant hard tissues—periodontitis affects the thermally sensitive periodontium. Combination therapy—a well-established paradigm in antimicrobial treatment—enhances therapeutic efficacy by integrating distinct therapeutic mechanisms beyond the capabilities of individual monotherapies (Wang N. et al., 2022). This approach enables superior outcomes at reduced thermal dosages. Mild hyperthermia (<45 °C) enhances the bactericidal efficacy of antibiotics by inhibiting enzyme activity, while preserving surrounding tissue integrity. tissues (Gao et al., 2019). The synergy between PTT and antibiotics such as tetracycline (TC) (Zhang et al., 2020) and minocycline (Lin et al., 2020; Wang X. et al., 2023) represents a strategic approach for an efficacious periodontal antibacterial therapy. To enhance drug delivery efficiency and curtail systemic harm, drug delivery systems (DDS) are frequently utilized to administer antibiotics (Lin et al., 2020; Zhang et al., 2020; Shi et al., 2021). Hydrogels, widely employed in DDS, can conform to the irregular morphology of periodontal pockets and enhance the retention rate of the released drugs at the local infection site. The heat of PTT can stimulate and trigger the controlled release of drugs within the DDS, thereby achieving synergistic sterilization through the combined action of antibiotics and photothermal effects (Lin et al., 2020; Zhang et al., 2020). Beyond antibiotics, PTT could synergize with alternative antibacterial strategies—including Ag+ (Wang F. et al., 2023),. PDT (Bai et al., 2022; Gao et al., 2023; Wu et al., 2023), CDT (Chen Q. et al., 2023; Dai et al., 2023; Lin et al., 2024), and gas therapy (Dai et al., 2023; Li Z. et al., 2024)—to enhance periodontal biofilm eradication. The synergistic integration of PDT and PTT, activated by a single 808 nm NIR source, amplifies antibacterial efficacy while reducing both drug dosage and laser energy requirements (Qi et al., 2022). This dual-modal approach enhances bacterial elimination beyond monotherapies: PTT-induced hyperthermia disrupts membrane integrity, facilitating deeper penetration of ROS generated through PDT to inflict lethal oxidative damage (Wang R. et al., 2022; Wu et al., 2023). Highly toxic ·OH generated by CDT exhibits potent destructive effects on bacterial biofilms and cell membranes, demonstrating significant efficacy against bacterial infections (Guo et al., 2020). Notably, these ·OH radicals critically deplete ATP levels, inhibiting heat shock proteins and reducing bacterial heat resistance (Chen et al., 2020; Wang F. et al., 2023). This thereby enhances the efficiency of PTT, highlighting a promising single-material solution for concurrent CDT and PTT. Gas therapy represents a novel, promising strategy for targeting deep infections in periodontal tissues. Nitric oxide (NO) has demonstrated outstanding antimicrobial efficacy and the ability to combat resistance linked to bacterial biofilms (Li Z. et al., 2024). It could increase the sensitivity of the bacteria to heat and promote tissue healing by stimulating angiogenesis and alleviating the damage caused by periodontitis (Yuan et al., 2020; Dai et al., 2023). When combined with PTT, this approach demonstrates significant synergistic efficacy in the treatment of periodontitis (Dai et al., 2023; Li T. et al., 2024).
Bacterial infection might be the primary cause of inflammation’s initial stages, but the host’s immune inflammatory response is responsible for promoting periodontitis (Hajishengallis, 2014). To treat periodontitis thoroughly, regulating host immunity is also crucial in addition to clearing the biofilm in the disease area (Qi et al., 2022; Wang N. et al., 2022; Chen Q. et al., 2023). Proanthocyanidins (PCs), a class of natural phenolic compounds, demonstrate efficacy in impeding the elevation of ROS inhibiting inflammatory factors, and regulating macrophage polarisation in periodontal disease sites (Gil-Cardoso et al., 2019; Kim et al., 2019; Wang H. et al., 2022; Zhang et al., 2023). A nanocomposite named AuAg-PC NPs was synthesized with PCs as a reducing agent. Biofilms can be eradicated through Ag+-synergistic PTT, whereas PCs demonstrate the capacity to eliminate ROS and modulate tissue self-healing via the PI3K/Akt signaling pathway. Hence, the nanocomposites can eradicate periodontal pathogens and restore the immune regulation environment (Figure 5A) (Wang F. et al., 2023). Additionally, baicalein (BA) (Tian et al., 2022), nitric oxide (NO) (Qi et al., 2022), PB nanozymes (Wang P. et al., 2023; Li Z. et al., 2024), ceria (CeO2) (Li T. et al., 2024), rapamycin Xiao et al., 2024) and dimethyl fumarate (DMF) (Li T. et al., 2024) have been employed to modulate the detrimental innate inflammatory responses triggered during persistent infections. Considering that the destruction of periodontal soft tissues and the resorption of alveolar bone induced by periodontitis are irreversible processes, periodontal tissue regeneration is crucial for treating periodontitis (Yao H. et al., 2025). Although PTT can promote osteogenesis, monotherapies are often insufficient to elicit an adequate therapeutic response—and PTT is no exception. Recently, tissue engineering has proffered new prospects for repairing periodontal tissue defects in patients with periodontitis (Hussain et al., 2022; Wang P. et al., 2023). A thermosensitive and injectable hydrogel with a three-dimensional (3D) network architecture was employed as a delivery system for the controlled release of osteoinductive agents (BMP-2) and phototherapy agents (T8IC and H2O2). PTT combined with PDT exhibited excellent bactericidal effects while sustained release of BMP-2 and mild temperature (45 °C) induced osteogenesis (Figure 5B) (Wang P. et al., 2023). An appropriate concentration of Cu2+ promotes the proliferation and osteogenic differentiation of bone marrow mesenchymal stem cells (BMSCs) (Burghardt et al., 2015). Nanomaterials such as copper sulfide (CuS) nanoparticles leverage this biological activity while exhibiting strong NIR absorption and exceptional PCE, enabling their use as potent PTAs (Yang et al., 2025). This dual functionality facilitates simultaneous spatiotemporal antibacterial action and alveolar bone regeneration.
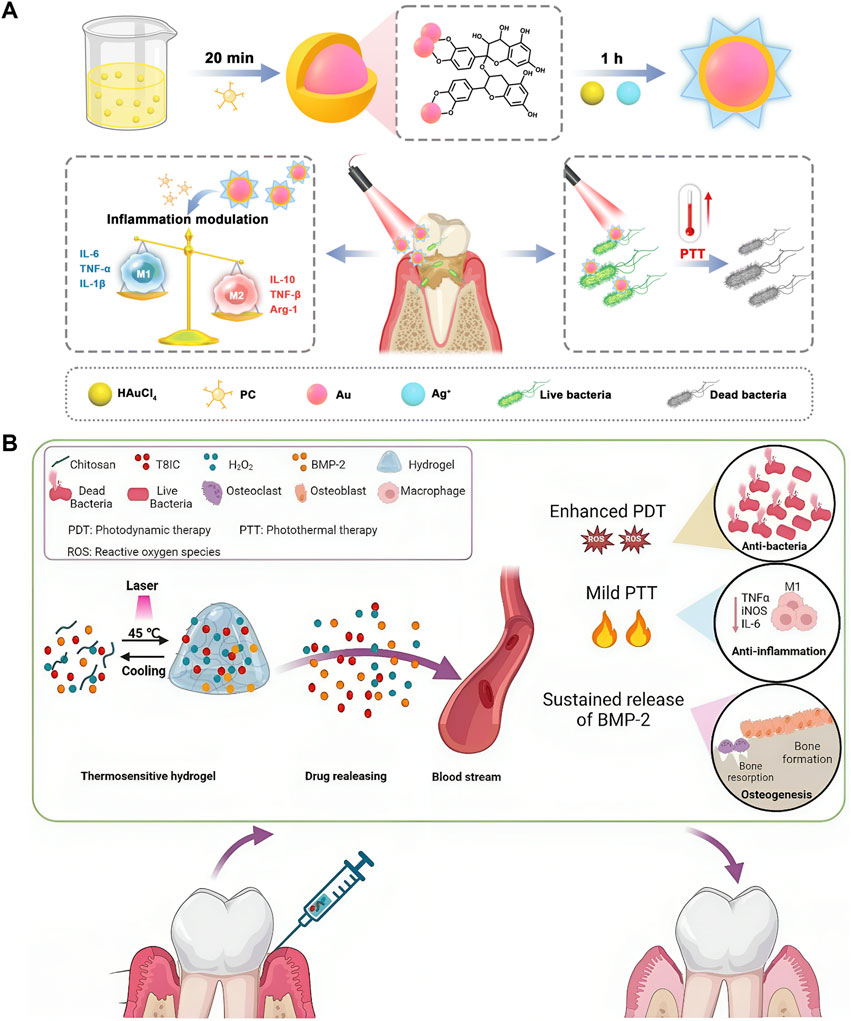
Figure 5. Photothermal therapy (PTT) for periodontitis. (A) Schematic illustration depicting the synthesis principle and therapeutic mechanism of AuAg-PC nanoparticles in treating periodontitis. Reproduced with permission (Wang F. et al., 2023). Copyright 2023, Royal Society of Chemistry. (B) Schematic illustration of thermosensitive and injectable hydrogel with T8IC, H2O2, and bone morphogenetic protein-2 (BMP-2). Reproduced under Creative Commons CC BY license (Wang P. et al., 2023). Copyright 2023, The Author(s), Published by Springer Nature Group.
Notable progress has been achieved in the research on PTT for periodontitis. The research spans three key aspects: antibacterial, anti-inflammatory, and tissue-regeneration. Each function can synergize with the others, yielding favorable outcomes. However, we have not yet seen a multifunctional material or system integrating all of them, and the development of triple-functional materials or systems represents a future research trajectory. Although current photothermal conversion materials, such as gold nanorods, exhibit excellent biocompatibility, their long-term retention in the body may hinder periodontal tissue regeneration and affect overall health. Future research should focus on developing photothermal materials that can be metabolized and cleared by the body to avoid potential adverse effects. Additionally, previous studies predominantly utilize near-infrared region I (NIR-I, 650–1000 nm) lasers; near-infrared region II (NIR-II, 1000–1700 nm) which offers deeper tissue penetration into the periodontal pocket and improved precision in targeting periodontal lesions is a promising direction for future research (Luo et al., 2025).
5 PTT for peri-implantitis
Peri-implantitis constitutes a pathological condition associated with dental plaque that occurs in the tissues surrounding dental implants (Giok et al., 2024). The hallmark of this condition includes inflammation of the peri-implant mucosa and concomitant supporting bone loss (Berglundh et al., 2018; Schwarz et al., 2018). Inflammatory manifestations, bleeding or hyperemia upon probing, an augmentation in probing depth, and radiographic indications of bone resorption constitute the typical clinical features of peri-implantitis (Diaz et al., 2022). Unlike natural teeth, implants lack a periodontal ligament to separate the inflammatory cell infiltrate from the crestal bone (Carcuac et al., 2013). Therefore, peri-implantitis progresses faster than periodontitis around teeth. In the absence of effective intervention, the inflammatory process progressively damages osseointegration and ultimately causing implant mobility and loss (Daubert et al., 2015; Derks et al., 2016). Furthermore, the peri-implant microbiome and biofilm composition differ from those around natural teeth, making peri-implantitis management more challenging and less predictable than periodontitis treatment (Koyanagi et al., 2013; Wu L. et al., 2019). Clinically, the management of peri-implantitis bears resemblance to that of periodontitis, predominantly relying on the mechanical elimination of biofilms and the administration of antibiotics (Giok et al., 2024). However, due to the inaccessibility of infected implant surfaces and the potential for mechanical debridement to damage implant topography, effective biofilm eradication and re-osseointegration remain clinically challenging (Wang et al., 2020; Munakata et al., 2022; Ichioka et al., 2023). Therefore, preventing peri-implantitis is clinically paramount—significantly more critical than treatment.
The development of peri-implantitis begins with planktonic bacterial adhesion to implant surfaces (Osman et al., 2022). While titanium alloys and zirconia are common dental implant materials, neither exhibits inherent antibacterial activity (Pieralli et al., 2017; W. Nicholson, 2020; Chen et al., 2021). Consequently, enhancing the antimicrobial functionality of implants is critical to mitigate peri-implantitis. Surface modifications can profoundly alter the micro/nanotopography and chemical composition of titanium implants, enhancing hydrophilicity, mechanical stability, osseointegration capacity, and antibacterial efficacy (Sun et al., 2023; Gkioka and Rausch-Fan, 2024; Yu Y. M. et al., 2024). When irradiated with NIR light, dental implants coated with graphene oxide (GO) (Park et al., 2023) or PDA nanoparticles (Ren et al., 2020) demonstrate reduced adhesion of S. mutans and Porphyromonas gingivalis (P. gingivalis). Despite their antibacterial efficacy, photothermal coatings risk collateral tissue damage through heat dissipation near infection sites, potentially compromising healthy peri-implant tissue integration (Werner et al., 2009). Ren et al. (2020) devised a model wherein keratinocytes were cultured on a membrane filter within a transwell system while fibroblasts adhered to a titanium surface beneath the membrane. This model could be used to investigate the previously uninvestigated risk of collateral tissue damage from photothermal coatings on implant surfaces (Figure 6A). The use of novel biomaterials represents another strategy. Similar to periodontal therapy, this approach targets bacterial elimination and reduces inflammatory responses through immunoregulation (Xue et al., 2023; Liu et al., 2025). A critical distinction, however, is the requirement for a firm biological seal between the abutment and the gingival epithelium (Mahmoud et al., 2019). This seal is essential to prevent bacterial invasion and subsequent marginal bone loss (Fischer et al., 2022). Additionally, dental implants must achieve osseointegration with alveolar bone post-implantation (Chen et al., 2024). Xue et al. (2023) proposed a multipurpose photothermal strategy that uses Si/P/F-doped TiO2 to address these challenges through dual functionality: exhibiting strong photothermal response and NIR-triggered F− release. The resulting hyperthermia-F- synergy disrupts Staphylococcus aureus (S. aureus) by reducing ATP synthesis, increasing membrane permeability, and generating ROS that oxidize cellular components to cause bacterial death. Concurrently, mild hyperthermia with released ions enhances gingival epithelial hemidesmosome formation and osteoblast activity. Another critical distinction in the field of peri-implantitis is the complexity of establishing animal models. Several in vivo studies of dental peri-implantitis have employed mouse femoral peri-implantitis models (Xiao et al., 2024; Wang et al., 2025); however, these models fail to accurately replicate the clinical condition of dental peri-implantitis within the alveolar bone (Zhang J. et al., 2024). The “ligature model” in alveolar bone mimics naturally occurring peri-implantitis and is suitable for studying the disease (Carcuac et al., 2013). The optimal timing for implant placement in mouse alveolar bone to establish a murine peri-implantitis model remains a contentious issue due to the limited understanding of the anatomical structure and physiological state of the alveolar bone after implant placement (Tzach-Nahman et al., 2017; Wong et al., 2018). Micro-CT and histological sectioning techniques suggested 6 weeks after the extraction of the maxillary first molar might be the appropriate time for implant placement (Figure 6B) (Liu et al., 2025). This finding offers significant data supporting the development of the murine peri-implantitis model.
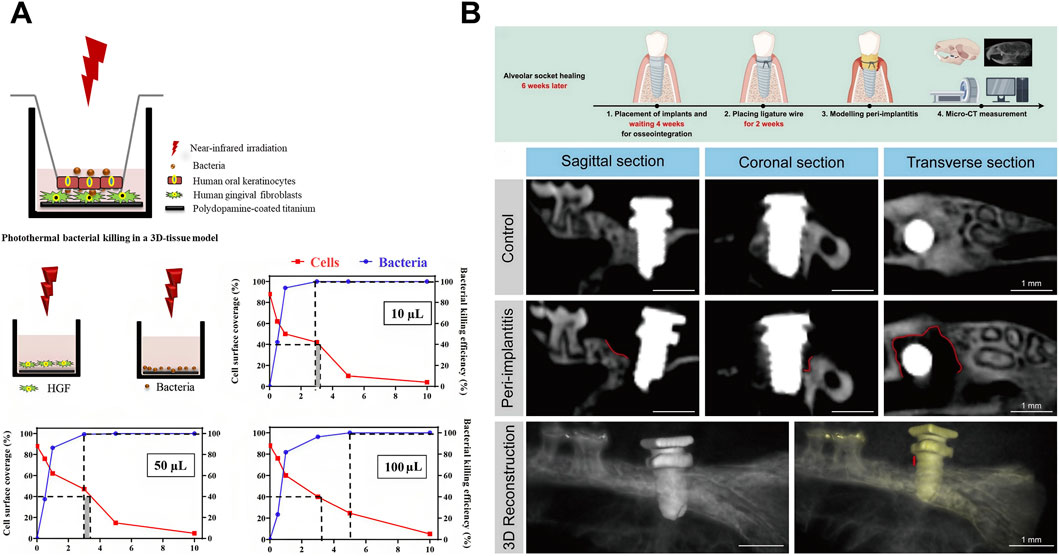
Figure 6. Photothermal therapy (PTT) for peri-implantitis. (A) Schematic illustration demonstrating photothermal bacterial killing in a 3D tissue model and surface coverage of human gingival fibroblasts (HGFs) and the eradication of Staphylococcus aureus (S. aureus) upon NIR irradiation of PDA nanoparticle coated titanium surfaces in monocultures. Samples were immersed in varying volumes of DMEM-HG medium (for HGFs) and PBS (for staphylococci), as illustrated in the schematics. The dotted lines demarcate NIR irradiation times considered acceptable for preserving tissue integration (>40% cell surface coverage; red data) and ensuring significant bacterial killing (>99.9%; blue data). The gray shading indicates the range of acceptable irradiation times that satisfy both criteria. Reproduced under Creative Commons CC BY-NC-ND 4.0 license (Ren et al., 2020). Copyright 2020, American Chemical Society. (B) Schematic diagram, Micro-CT, Hematoxylin-Eosin (HE) staining, and tartrate-resistant acid phosphatase (TRAP) staining of in vivo modeling of peri-implantitis in mice. Reproduced with permission (Liu et al., 2025). Copyright 2025, Elsevier Group.
Given the escalating prevalence of dental implants in dental prosthodontics clinical practice, it is anticipated that peri-implantitis will garner increasing attention. As a non-invasive and non-antibiotic-resistant antibacterial strategy, PTT holds great promise in preventing and treating peri-implantitis. Notably, when integrated with bone regeneration strategies, it can substantially promote the osseointegration process while preventing postoperative infection and enhancing the success rate of implant surgery. The current research bottleneck lies in determining how to minimize or eliminate collateral photothermal damage to healthy tissue cells in the peri-implant region while effectively eradicating bacteria through photothermal action. With the establishment of suitable animal models, future research in this field will accelerate, leading to significant advances.
6 PTT for other oral infectious diseases
Infectious bone defects (IBD) collectively refer to a class of diseases characterized by tenacious infection, persistent inflammation, bone destruction, impaired blood supply, and a protracted course of diseases, making them particularly challenging to manage (Han et al., 2024). It can be caused by jaw osteomyelitis, trauma, postoperative infection of tumors, etc (Dong et al., 2017). Clinical treatment strategies typically encompass antibiotic administration, excision of necrotic bone fragments, debridement procedures, and transplantation of bone grafts (Qian et al., 2023; Han et al., 2024). Nevertheless, antimicrobial overuse drives the evolution of drug-resistant strains (Hu et al., 2024). Moreover, requiring bone graft implantation post-infection eradication significantly prolongs treatment duration. Therefore, developing biomaterials that simultaneously deliver antibacterial functionality and personalized osteogenic capabilities is imperative. The photothermal effect delivers dual benefits: conferring antibacterial activity while using moderate local heating to upregulate key genes (e.g., osteogenesis-related genes) that promote tissue regeneration (Avci et al., 2013; Ma et al., 2020). Wang W. et al. (2023) developed a lamellar heterostructured Mg/PLLA composite periosteum membrane via an accumulative rolling method for application at bone defect sites. A consistent supply of Mg2+ activates key extracellular matrix proteins and transcription factors implicated in bone regeneration and angiogenesis. The photothermal effect of Mg microparticles can eliminate bacteria while further enhancing bone marrow-derived mesenchymal stromal cells (BMSCs) differentiation. Although overheating risks inducing apoptosis in both bacteria and healthy cells, longitudinal analysis revealed converging cell densities between composite membrane treated and control groups over time. This demonstrates that strategically controlled PTT ultimately favors tissue repair over thermal damage. Consequently, the PTT-enhanced composite periosteum achieved on-demand antibacterial efficacy and exceptional endogenous vascularized bone regeneration (Figure 7A). Beyond artificial periosteum, research has extended to 3D-printed hydrogels for tissue regeneration (Nie et al., 2022). These studies indicate that the application of PTT in the field of biomedical engineering holds great promise.
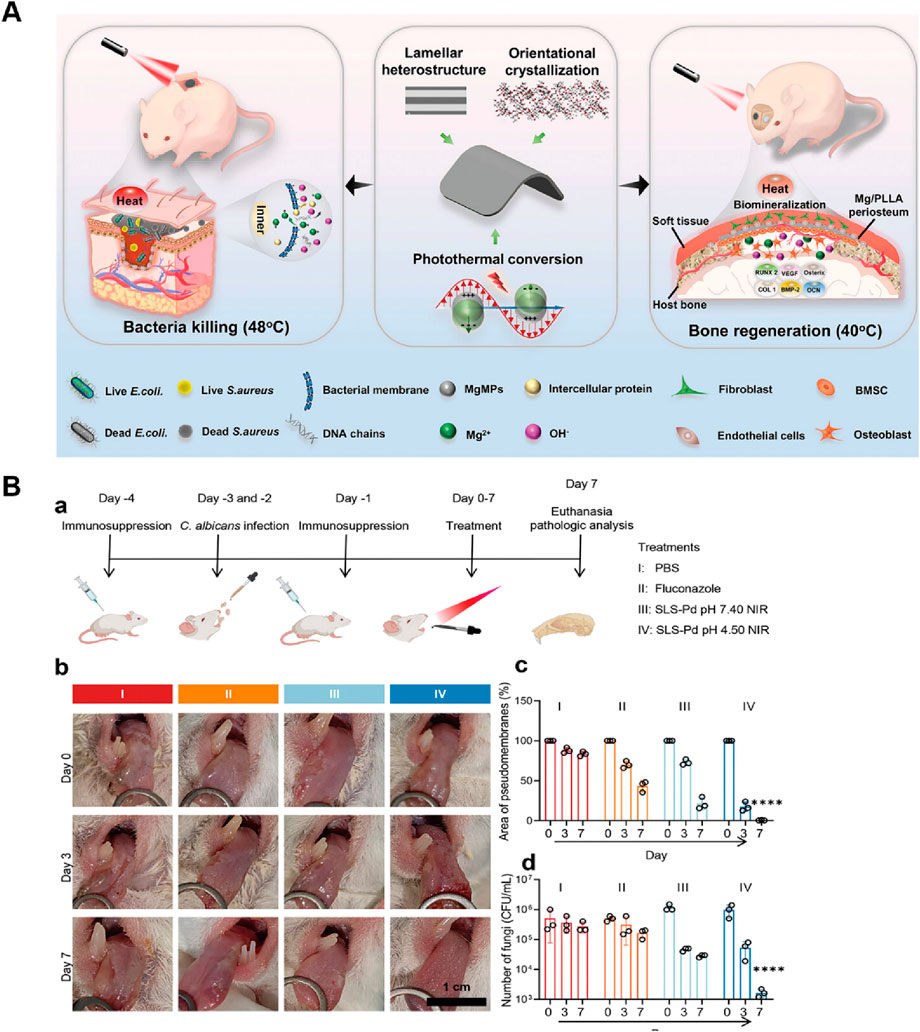
Figure 7. PTT for infectious bone defect and candida infection. (A) Schematic illustration of the self-reinforced Mg/PLLA composite membrane with lamellar heterostructure working as a periosteum for on-demand bacteria inhibition and rapid bone reconstruction. Reproduced with permission (Wang F. et al., 2023). Copyright 2022, John Wiley and Sons Group. (B) MPN-Pd-mediated system for the treatment of oral candidiasis. (a) Workflow of the in vivo experiment. (b) Digital images of oral candidiasis models under different treatments. (c) Quantitative analysis of the pseudomembrane area. (d) Viability of Candida albicans (C. albicans) evaluation. Reproduced with permission (Chen et al., 2023a). Copyright 2023, John Wiley and Sons Group.
Beyond its efficacy against oral bacterial infections, PTT has demonstrated effectiveness against other microbial infections, including fungal infections. Candida albicans (C. albicans) is a primary etiological agent for most nosocomial infections affecting immunocompromised patients, and emerging multidrug resistance has made it an urgent threat (Arendrup and Patterson, 2017). Oropharyngeal candidiasis represents a form of oral candidal infection with a higher prevalence in individuals with conditions such as diabetes mellitus, immunodeficiency, and xerostomia (Stoopler et al., 2024). Similarly, it is associated with C. albicans biofilms on the oral mucosa. The intrinsic resistance of biofilms to antifungal agents has augmented the challenges associated with effective antifungal treatment. Chen et al. (2023a) developed a metal-phenolic network with Pd nanoparticle nodes (MPN-Pd) and found that C. albicans is more sensitive to hyperthermia than bacteria like E. faecalis and S. mutans which might be attributed to the fungal membrane containing dipalmitoylphosphatidylcholine phospholipid molecules that are more sensitive to temperature. The histological evaluation of mouse oral candida infection model indicates that the PTT is effective in the therapeutic goal of treating oropharyngeal candidiasis by eradicating C. albicans in the oral cavity, while showing no sign of collateral damage (Figure 7B). However, the current research on the antifungal application of PTT remains in its nascent stages, with limited experimental and clinical data currently available. Viral infections cause oral infectious like herpetic stomatitis. Theoretically, PTT also has the potential to be used for antiviral therapy, as viral structures and proteins are also prone to denaturation and inactivation at high temperatures (Bai et al., 2023; Li B. et al., 2024). Given PTT’s remarkable antibacterial prowess and compatibility with other treatment modalities or bioactive materials, it is expected to be used to treat a broader spectrum of oral infections.
7 Summary and outlook
PTT heralds a paradigm shift in the prophylaxis and therapeutic strategies for oral infections. It proffers an efficacious, precise, and minimally invasive alternative to antibiotics. By capitalizing on the potency of light and heat, PTT surmounts the limitations inherent in extant therapies, such as the burgeoning issue of antibiotic resistance and the propensity for tissue damage. Simultaneously, it furnishes a platform conducive to innovative and multifarious applications. As research within this domain continues to burgeon, PTT offers significant potential to revolutionize the management of oral infections charting a course towards more efficacious and sustainable solutions in oral healthcare. Critically, PTT demonstrates not only potent antibacterial efficacy but also significant potential for promoting tissue regeneration. Its compatibility with other therapeutic modalities enables synergistic treatment outcomes—particularly valuable for managing periodontitis and infectious bone defects where restoring biological function extends beyond mere antibacterial control.
Despite its considerable potential, PTT’s clinical translation markedly lags behind that of its counterpart, PDT, with scarce clinical trials, and a range of challenges must be surmounted to fully actualize its clinical implementation for treating oral infections. The dual objectives of potent bactericidal effects and minimal collateral tissue damage pose an inherent trade-off, which may be addressed by improving targeting specificity. This underscores the need for advanced intelligent drug delivery systems and highly precise laser irradiation with deep-tissue penetration capability. Furthermore, combining PTT with adjuvant therapies to enhance bacterial photosensitization offers an alternative viable approach. Moreover, long-term biocompatibility and safety of PTAs necessitate comprehensive assessment to attenuate potential risks, such as tissue inflammation or systemic toxicity. The clinical translation of PTAs hinges on their long-term biosafety and effective clearance to mitigate the toxicity risk from bioaccumulation. To address this, key strategies focus on either biodegradability or renal clearance. For metallic PTAs like gold, which are poorly biodegradable, engineering ultrasmall, renally-clearable (<5 nm) nanoparticles offers a promising solution (Hwang et al., 2014; Tang et al., 2014). Alternatively, designing for biodegradability is a major focus. This includes inherently biodegradable inorganic materials like black phosphorus, which degrades into harmless phosphates, and carbon-based materials (e.g., GO) that can be broken down by enzymes (Lalwani et al., 2014). Organic materials often show superior biocompatibility; the FDA-approved dye indocyanine green (ICG) provides a clinical benchmark with its rapid hepatobiliary clearance, while engineered polymers (semiconducting polymer nanoparticles, SPNPs) can be designed with cleavable bonds (Lyu et al., 2018; Della Pelle et al., 2021). Ultimately, this focus on creating intentionally degradable or clearable nanoparticles is the critical step toward bringing PTT from preclinical studies to clinical reality. Finally, a significant barrier to the clinical translation of PTT is the lack of standardized parameters across preclinical studies. This challenge is formidable, extending beyond just light exposure conditions. A review of the literature reveals considerable variability in irradiation, with typical parameters involving an 808 nm laser at a power density of 0.5-3 Wcm-2 for 5–10 min, aiming for temperatures of 55 °C–60 °C for conventional PTT or a milder ∼41 °C–43 °C for mild PTT. Furthermore, given that this research is still largely in the preclinical stage, different laboratories employ unique nanoparticle platforms and diverse infection models. This heterogeneity makes it exceedingly difficult to directly compare the therapeutic efficacy of different photothermal systems. Therefore, establishing standardized protocols that encompass not only irradiation parameters but also the class of nanomaterial and the type of infection being modeled is imperative to accelerate the clinical translation of this promising therapeutic modality.
In light of this, future research endeavors regarding the application of PTT in oral infections, encompassing dental caries, endodontics, periodontitis, and peri-implantitis, should center on the following aspects: ⅰ. Establish experimental models that can duplicate the complexity of biofilms to evaluate the antibacterial efficacy of PTT comprehensively. The mechanism of PTT against dental plaque biofilms also needs to be further studied. ⅱ. Develop photothermal materials that are smart-responsive, degradable, or can be cleared by body metabolism to improve the biosafety of PTT. ⅲ. Probe into applying NIR-II lasers in deep-seated oral tissues to augment the precision of treatment. ⅳ. Fortify interdisciplinary integration, promote the combinatorial utilization of PTT with traditional antibacterial, immunomodulatory, and tissue-regeneration strategies, and engineer multifunctional materials. ⅴ. Facilitate large-scale clinical trials, standardize treatment parameters, evaluate long-term biosafety, and ultimately propel its clinical translation. Future research should also be directed towards elucidating the interplay between PTT and the oral microbiota, especially its implications for non-pathogenic commensal bacteria. Preserving the eco-logical equilibrium of the oral microbiota is pivotal for upholding overall oral health and forestalling diseases associated with dysbiosis. Additionally, developing cost-effective and scalable PTT systems is imperative for its widespread clinical deployment, particularly in resource-constrained settings. In summary, PTT presents a highly promising approach to the treatment of oral infections, replete with substantial potential for clinical translational applications. With the evolution of multi-disciplinary convergence, it may emerge as a novel approach for combating oral-related infections, thereby conferring greater benefits to humanity.
Author contributions
PW: Writing – review and editing, Writing – original draft, Funding acquisition, Conceptualization. JL: Writing – original draft. FL: Writing – original draft, Writing – review and editing, Conceptualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the Technological Plan of Traditional Chinese Medicine Administration of Jiangxi Province of China (2022A329), the Jiangxi Provincial Natural Science Foundation (20212BAB206072, 20224BAB216078), the Jiangxi Province Chinese Medicine Science and Technology Program (2021A385).
Acknowledgements
The authors thank Yanfang Lin, Ao Jia, Yifan Liu, Yuyao Li, Rong Li, Junping Fan, and Dongping Li for their review of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. AI help us polish manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abou-Rass, M., and Oglesby, S. W. (1981). The effects of temperature, concentration, and tissue type on the solvent ability of sodium hypochlorite. J. Endod. 7 (8), 376–377. doi:10.1016/s0099-2399(81)80059-3
Akindele, B. O., Orenuga, O. O., Olatosi, O. O., and Oladele, R. O. (2025). Comparative evaluation of the clinical effectiveness of chemomechanical (papacarie) and conventional mechanical caries removal methods in treatment of carious primary molars: a randomized controlled clinical study. BMC Oral Health 25 (1), 290. doi:10.1186/s12903-025-05654-7
Aksoy, İ., Küçükkeçeci, H., Sevgi, F., Metin, Ö., and Hatay Patir, I. (2020). Photothermal antibacterial and antibiofilm activity of black phosphorus/gold nanocomposites against pathogenic bacteria. ACS Appl. Mat. Interfaces 12 (24), 26822–26831. doi:10.1021/acsami.0c02524
Alawaji, Y. N., Alshammari, A., Mostafa, N., Carvalho, R. M., and Aleksejuniene, J. (2022). Periodontal disease prevalence, extent, and risk associations in untreated individuals. Clin. Exp. Dent. Res. 8 (1), 380–394. doi:10.1002/cre2.526
AlSahafi, R., Wang, X., Mitwalli, H., Alhussein, A., Balhaddad, A. A., Melo, M. A. S., et al. (2022). Novel antibacterial low-shrinkage-stress resin-based cement. Dent. Mat. 38 (11), 1689–1702. doi:10.1016/j.dental.2022.08.005
Altamura, S., Del Pinto, R., Pietropaoli, D., and Ferri, C. (2024). Oral health as a modifiable risk factor for cardiovascular diseases. Trends cardiovasc. Med. 34 (4), 267–275. doi:10.1016/j.tcm.2023.03.003
Arendrup, M. C., and Patterson, T. F. (2017). Multidrug-resistant candida: epidemiology, molecular mechanisms, and treatment. J. Infect. Dis. 216 (Suppl. l_3), S445–S451. doi:10.1093/infdis/jix131
Avci, P., Gupta, A., Sadasivam, M., Vecchio, D., Pam, Z., Pam, N., et al. (2013). Low-level laser (light) therapy (LLLT) in skin: stimulating, healing, restoring. Semin. Cutan. Med. Surg. 32 (1), 41–52. Available online at: https://pubmed.ncbi.nlm.nih.gov/24049929/
Bai, L., Shi, E., Li, Y., Yang, M., Li, C., Li, C., et al. (2022). Oxyhemoglobin-based nanophotosensitizer for specific and synergistic photothermal and photodynamic therapies against Porphyromonas gingivalis oral infection. ACS Biomater. Sci. Eng. 9 (1), 485–497. doi:10.1021/acsbiomaterials.2c01034
Bai, Y., Huang, P., Feng, N., Li, Y., Huang, J., Jin, H., et al. (2023). Treat the “untreatable” by a photothermal agent: triggering heat and immunological responses for rabies virus inactivation. Adv. Sci. 10 (2), 2205461. doi:10.1002/advs.202205461
Baker, J. L., Mark Welch, J. L., Kauffman, K. M., McLean, J. S., and He, X. (2024). The oral microbiome: diversity, biogeography and human health. Nat. Rev. Microbiol. 22 (2), 89–104. doi:10.1038/s41579-023-00963-6
Belibasakis, G. N., Seneviratne, C. J., Jayasinghe, R. D., Vo, P.T.-D., Bostanci, N., and Choi, Y. (2024). Bacteriome and mycobiome dysbiosis in oral mucosal dysplasia and oral cancer. Periodontol 96 (1), 95–111. doi:10.1111/prd.12558
Berglundh, T., Armitage, G., Araujo, M. G., Avila-Ortiz, G., Blanco, J., Camargo, P. M., et al. (2018). Peri-implant diseases and conditions: consensus report of workgroup 4 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. J. Clin. Periodontol. 45 (S20), S286-S291–S291. doi:10.1111/jcpe.12957
Bermúdez-Jiménez, C., Niño-Martínez, N., Patiño-Marín, N., Martínez-Gutiérrez, F., Ruiz, F., Bach, H., et al. (2020). Effective control of biofilms by photothermal therapy using a gold nanorod hydrogel. J. Biomed. Mat. Res. Part B Appl. Biomater. 108 (2), 333–342. doi:10.1002/jbm.b.34392
Bernabe, E., Marcenes, W., Hernandez, C. R., Bailey, J., Abreu, L. G., Alipour, V., et al. (2020). Global, regional, and national levels and trends in burden of oral conditions from 1990 to 2017: a systematic analysis for the global burden of disease 2017 study. J. Dent. Res. 99 (4), 362–373. doi:10.1177/0022034520908533
Bowen, W. H., Burne, R. A., Wu, H., and Koo, H. (2018). Oral biofilms: Pathogens, matrix, and polymicrobial interactions in microenvironments. Trends Microbiol. 26 (3), 229–242. doi:10.1016/j.tim.2017.09.008
Brookes, Z., Teoh, L., Cieplik, F., and Kumar, P. (2023). Mouthwash effects on the oral microbiome: are they good, bad, or balanced? Int. Dent. J. 73 (Suppl. 2), S74–S81. doi:10.1016/j.identj.2023.08.010
Burghardt, I., Luthen, F., Prinz, C., Kreikemeyer, B., Zietz, C., Neumann, H. G., et al. (2015). A dual function of copper in designing regenerative implants. Biomaterials 44, 36–44. doi:10.1016/j.biomaterials.2014.12.022
Burns, L. E., Kim, J., Wu, Y., Alzwaideh, R., McGowan, R., and Sigurdsson, A. (2022). Outcomes of primary root canal therapy: an updated systematic review of longitudinal clinical studies published between 2003 and 2020. Int. Endod. J. 55 (7), 714–731. doi:10.1111/iej.13736
Cao, J., Sun, Q., Shen, A.-G., Fan, B., and Hu, J.-M. (2021). Nano au@ Cu2-xS with near-infrared photothermal and peroxidase catalytic activities redefines efficient antibiofilm-oriented root canal therapy. Chem. Eng. J. 422, 130090. doi:10.1016/j.cej.2021.130090
Cao, J., Song, Z., Du, T., and Du, X. (2024). Antimicrobial materials based on photothermal action and their application in wound treatment. Burns Trauma 12, tkae046. doi:10.1093/burnst/tkae046
Carcuac, O., Abrahamsson, I., Albouy, J. P., Linder, E., Larsson, L., and Berglundh, T. (2013). Experimental periodontitis and peri-implantitis in dogs. Clin. Oral Implant. Res. 24 (4), 363–371. doi:10.1111/clr.12067
Castillo-Martínez, J. C., Martínez-Castañón, G. A., Martínez-Gutierrez, F., Zavala-Alonso, N. V., Patiño-Marín, N., Niño-Martinez, N., et al. (2015). Antibacterial and antibiofilm activities of the photothermal therapy using gold nanorods against seven different bacterial strains. J. Nanomater. 2015, 783671. doi:10.1155/2015/783671
Chen, F., and Wang, D. (2010). Novel technologies for the prevention and treatment of dental caries: a patent survey. Expert. Opin. Ther. Pat. 20 (5), 681–694. doi:10.1517/13543771003720491
Chen, Q. W., Liu, X. H., Fan, J. X., Peng, S. Y., Wang, J. W., Wang, X. N., et al. (2020). Self-mineralized photothermal bacteria hybridizing with mitochondria-targeted metal–organic frameworks for augmenting photothermal tumor therapy. Adv. Funct. Mat. 30 (14), 1909806. doi:10.1002/adfm.201909806
Chen, Z., Wang, Z., Qiu, W., and Fang, F. (2021). Overview of antibacterial strategies of dental implant materials for the prevention of peri-implantitis. Bioconjug. Chem. 32 (4), 627–638. doi:10.1021/acs.bioconjchem.1c00129
Chen, L., Peng, M., Li, H., Zhou, J., He, W., Hu, R., et al. (2023a). Metal-phenolic network with Pd nanoparticle nodes synergizes oxidase-like and photothermal properties to eradicate oral polymicrobial biofilm-associated infections. Adv. Mat. 36 (7), 2306376. doi:10.1002/adma.202306376
Chen, L., Peng, M., Zhou, J., Hu, X., Piao, Y., Li, H., et al. (2023b). Supramolecular photothermal Cascade nano-reactor enables photothermal effect, Cascade reaction, and in situ hydrogelation for biofilm-associated tooth-extraction wound healing. Adv. Mat. 35 (31), 2301664. doi:10.1002/adma.202301664
Chen, Q., Qi, M., Shi, F., Liu, C., Shi, Y., Sun, Y., et al. (2023c). Novel twin-crystal nanosheets with MnO2 modification to combat bacterial biofilm against periodontal infections via multipattern strategies. Adv. Healthc. Mat. 12 (19), 2300313. doi:10.1002/adhm.202300313
Chen, B., Wang, W., Hu, M., Liang, Y., Wang, N., Li, C., et al. (2024). Photo-thermo-electric dental implant for anti-infection and enhanced osteoimmunomodulation. ACS Nano 18 (36), 24968–24983. doi:10.1021/acsnano.4c05859
Chiang, C.-P., Hsieh, O., Tai, W.-C., Chen, Y.-J., and Chang, P.-C. (2020). Clinical outcomes of adjunctive indocyanine green-diode lasers therapy for treating refractory periodontitis: a randomized controlled trial with in vitro assessment. J. Formos. Med. Assoc. 119 (2), 652–659. doi:10.1016/j.jfma.2019.08.021
Cobb, C. M., and Sottosanti, J. S. (2021). A re-evaluation of scaling and root planing. J. Periodontol. 92 (10), 1370–1378. doi:10.1002/jper.20-0839
Collaborators, G. B. D. O. D., Marcenes, W., Abdulkader, R. S., Abreu, L. G., Afzal, S., Alhalaiqa, F. N., et al. (2025). Trends in the global, regional, and national burden of oral conditions from 1990 to 2021: a systematic analysis for the global burden of disease study 2021. Lancet 405 (10482), 897–910. doi:10.1016/S0140-6736(24)02811-3
Dai, X., Liu, Y., Meng, F., Li, Q., Wu, F., Yuan, J., et al. (2023). Amplification of oxidative damage using near-infrared II-mediated photothermal/thermocatalytic effects for periodontitis treatment. Acta Biomater. 171, 519–531. doi:10.1016/j.actbio.2023.09.014
Daubert, D. M., Weinstein, B. F., Bordin, S., Leroux, B. G., and Flemming, T. F. (2015). Prevalence and predictive factors for peri-implant disease and implant failure: a cross-sectional analysis. J. Periodontol. 86 (3), 337–347. doi:10.1902/jop.2014.140438
Della Pelle, G., Delgado Lopez, A., Salord Fiol, M., and Kostevsek, N. (2021). Cyanine dyes for photo-thermal therapy: a comparison of synthetic liposomes and natural erythrocyte-based carriers. Int. J. Mol. Sci. 22 (13), 6914. doi:10.3390/ijms22136914
Deo, P. N., and Deshmukh, R. (2019). Oral microbiome: unveiling the fundamentals. J. Oral Maxillofac. Pathol. 23 (1), 122–128. doi:10.4103/jomfp.JOMFP_304_18
Derks, J., Schaller, D., Håkansson, J., Wennström, J. L., Tomasi, C., and Berglundh, T. (2016). Peri-implantitis - onset and pattern of progression. J. Clin. Periodontol. 43 (4), 383–388. doi:10.1111/jcpe.12535
Diaz, P., Gonzalo, E., Villagra, L. J. G., Miegimolle, B., and Suarez, M. J. (2022). What is the prevalence of peri-implantitis? A systematic review and meta-analysis. BMC Oral Health 22 (1), 449. doi:10.1186/s12903-022-02493-8
Dong, Y., Liu, W., Lei, Y., Wu, T., Zhang, S., Guo, Y., et al. (2017). Effect of gelatin sponge with colloid silver on bone healing in infected cranial defects. Mat. Sci. Eng. c-mater. Biol. Appl. 70 (Pt 1), 371–377. doi:10.1016/j.msec.2016.09.015
Duan, X., Zhang, Q., Jiang, Y., Wu, X., Yue, X., Geng, Y., et al. (2022). Semiconducting polymer nanoparticles with intramolecular motion-induced photothermy for tumor phototheranostics and tooth root canal therapy. Adv. Mat. 34 (17), 2200179. doi:10.1002/adma.202200179
Ertem, E., Gutt, B., Zuber, F., Allegri, S., Le Ouay, B., Mefti, S., et al. (2017). Core-shell silver nanoparticles in endodontic disinfection solutions enable long-term antimicrobial effect on oral biofilms. ACS Appl. Mat. Interfaces 9 (40), 34762–34772. doi:10.1021/acsami.7b13929
Fang, Z., Zhang, S., Wang, W., Xu, Y., Lu, M., Qian, Y., et al. (2025). Aggregation-induced emission-based phototheranostics to combat bacterial infection at wound sites: a review. Biomaterials 315, 122950. doi:10.1016/j.biomaterials.2024.122950
Feng, Y., Sun, Q., Liu, P., Fan, W., and Fan, B. (2024). Antibacterial property and mechanisms of au@ag core-shell nanoparticles with near-infrared absorption against E. faecalis infection of dentin. Int. J. Nanomed. 19, 6981–6997. doi:10.2147/ijn.S468649
Fischer, N. G., Kobe, A. C., Dai, J., He, J., Wang, H., Pizarek, J. A., et al. (2022). Tapping basement membrane motifs: oral junctional epithelium for surface-mediated soft tissue attachment to prevent failure of percutaneous devices. Acta Biomater. 141, 70–88. doi:10.1016/j.actbio.2021.12.030
Fulaz, S., Hiebner, D., Barros, C. H. N., Devlin, H., Vitale, S., Quinn, L., et al. (2019). Ratiometric imaging of the in situ pH distribution of biofilms by use of fluorescent mesoporous silica nanosensors. ACS Appl. Mat. Interfaces 11 (36), 32679–32688. doi:10.1021/acsami.9b09978
Galdámez-Falla, V.-M., Castillo-Martínez, J.-C., de Alba-Montero, I., Patiño-Marín, N., Niño-Martínez, N., Ruiz, F., et al. (2022). Formation of a mature biofilm of Enterococcus faecalis in root canal and its treatment using gold nanorods. J. Mat. Sci. Res. Rev. 5 (1), 31–43. Available online at: https://journaljmsrr.com/index.php/JMSRR/article/view/200.
Gao, G., Jiang, Y. W., Jia, H. R., and Wu, F. G. (2019). Near-infrared light-controllable on-demand antibiotics release using thermo-sensitive hydrogel-based drug reservoir for combating bacterial infection. Biomaterials 188, 83–95. doi:10.1016/j.biomaterials.2018.09.045
Gao, P., Li, G., Wang, Z., Zhang, H., Shan, Y., Yuan, X., et al. (2023). Protease-loaded CuS nanoparticles with synergistic photothermal/dynamic therapy against F. Nucleatum-induced periodontitis. ACS Appl. Mat. Interfaces 15 (27), 32215–32225. doi:10.1021/acsami.3c04534
Gil-Cardoso, K., Comitato, R., Ginés, I., Ardévol, A., Pinent, M., Virgili, F., et al. (2019). Protective effect of proanthocyanidins in a rat model of mild intestinal inflammation and impaired intestinal permeability induced by LPS. Mol. Nutr. Food. Res. 63 (8), 1800720. doi:10.1002/mnfr.201800720
Giok, K. C., Veettil, S. K., and Menon, R. K. (2024). Comparative effectiveness of interventions for the treatment of peri-implantitis: a systematic review with network meta-analysis. J. Prosthet. Dent. doi:10.1016/j.prosdent.2024.03.024
Gkioka, M., and Rausch-Fan, X. (2024). Antimicrobial effects of metal coatings or physical, chemical modifications of titanium dental implant surfaces for prevention of peri-implantitis: a systematic review of in vivo studies. Antibiotics-Basel 13 (9), 908. doi:10.3390/antibiotics13090908
Gondivkar, S., Gadbail, A., Sarode, G. S., Sarode, S. C., Patil, S., and Awan, K. H. (2019). Infectious diseases of oral cavity. Dis. Mon. 65 (6), 164–184. doi:10.1016/j.disamonth.2018.09.008
Guo, G., Zhang, H., Shen, H., Zhu, C., He, R., Tang, J., et al. (2020). Space-selective chemodynamic therapy of CuFe5O8 nanocubes for implant-related infections. ACS Nano 14 (10), 13391–13405. doi:10.1021/acsnano.0c05255
Gupta, A., Saleena, L. M., Kannan, P., and Shivachandran, A. (2024). The impact of oral diseases on respiratory health and the influence of respiratory infections on the oral microbiome. J. Dent. 148, 105213. doi:10.1016/j.jdent.2024.105213
Hajishengallis, G. (2014). Immunomicrobial pathogenesis of periodontitis: keystones, pathobionts, and host response. Trends Immunol. 35 (1), 3–11. doi:10.1016/j.it.2013.09.001
Hajishengallis, G., Lamont, R. J., and Koo, H. (2023). Oral polymicrobial communities: assembly, function, and impact on diseases. Cell Host Microbe 31 (4), 528–538. doi:10.1016/j.chom.2023.02.009
Han, Z., Xiong, J., Jin, X., Dai, Q., Han, M., Wu, H., et al. (2024). Advances in reparative materials for infectious bone defects and their applications in maxillofacial regions. J. Mat. Chem. B 12 (4), 842–871. doi:10.1039/d3tb02069j
He, L., Di, D., Chu, X., Liu, X., Wang, Z., Lu, J., et al. (2023). Photothermal antibacterial materials to promote wound healing. J. Control. Release 363, 180–200. doi:10.1016/j.jconrel.2023.09.035
Heitz-Mayfield, L. J. A. (2024). Conventional diagnostic criteria for periodontal diseases (plaque-induced gingivitis and periodontitis). Periodontol 95 (1), 10–19. doi:10.1111/prd.12579
Hernando-Amado, S., Coque, T. M., Baquero, F., and Martínez, J. L. (2019). Defining and combating antibiotic resistance from one health and global health perspectives. Nat. Microbiol. 4 (9), 1432–1442. doi:10.1038/s41564-019-0503-9
Hu, X., Chen, J., Yang, S., Zhang, Z., Wu, H., He, J., et al. (2024). 3D printed multifunctional biomimetic bone scaffold combined with TP-Mg nanoparticles for the infectious bone defects repair. Small 20 (40), 2403681. doi:10.1002/smll.202403681
Huang, K., Liu, W., Wei, W., Zhao, Y., Zhuang, P., Wang, X., et al. (2022). Photothermal hydrogel encapsulating intelligently bacteria-capturing Bio-MOF for infectious wound healing. ACS Nano 16 (11), 19491–19508. doi:10.1021/acsnano.2c09593
Huang, D., Wang, X., Liang, J., Ling, J., Bian, Z., Yu, Q., et al. (2024). Expert consensus on difficulty assessment of endodontic therapy. Int. J. Oral Sci. 16 (1), 22. doi:10.1038/s41368-024-00285-0
Hussain, A., Tebyaniyan, H., and Khayatan, D. (2022). The role of epigenetic in dental and oral regenerative medicine by different types of dental stem cells: a comprehensive overview. Stem Cells Int. 2022, 1–15. doi:10.1155/2022/5304860
Hwang, S., Nam, J., Jung, S., Song, J., Doh, H., and Kim, S. (2014). Gold nanoparticle-mediated photothermal therapy: current status and future perspective. Nanomedicine 9 (13), 2003–2022. doi:10.2217/nnm.14.147
Ichioka, Y., Derks, J., Larsson, L., and Berglundh, T. (2023). Surface decontamination of explanted peri-implantitis-affected implants. J. Clin. Periodontol. 50 (8), 1113–1122. doi:10.1111/jcpe.13836
Iniesta, M., Chamorro, C., Ambrosio, N., Marín, M. J., Sanz, M., and Herrera, D. (2023). Subgingival microbiome in periodontal health, gingivitis and different stages of periodontitis. J. Clin. Periodontol. 50 (7), 905–920. doi:10.1111/jcpe.13793
Jakubovics, N. S., Goodman, S. D., Mashburn-Warren, L., Stafford, G. P., and Cieplik, F. (2021). The dental plaque biofilm matrix. Periodontol. 2000 86 (1), 32–56. doi:10.1111/prd.12361
Jiang, W., Deng, Z., Dai, X., and Zhao, W. (2021). PANoptosis: a new insight into oral infectious diseases. Front. Immunol. 12, 789610. doi:10.3389/fimmu.2021.789610
Karamifar, K., Tondari, A., and Saghiri, M. A. (2020). Endodontic periapical lesion: an overview on the etiology, diagnosis and current treatment modalities. Eur. Endod. J. 5 (2), 54–67. doi:10.14744/eej.2020.42714
Khantamat, O., Li, C.-H., Yu, F., Jamison, A. C., Shih, W.-C., Cai, C., et al. (2015). Gold nanoshell-decorated silicone surfaces for the near-infrared (NIR) photothermal destruction of the pathogenic bacterium E. faecalis. ACS Appl. Mat. Interfaces 7 (7), 3981–3993. doi:10.1021/am506516r
Kilian, M., Chapple, I. L., Hannig, M., Marsh, P. D., Meuric, V., Pedersen, A. M., et al. (2016). The oral microbiome - an update for oral healthcare professionals. Br. Dent. J. 221 (10), 657–666. doi:10.1038/sj.bdj.2016.865
Kim, J., Kim, H. Y., Song, S. Y., Go, S. H., Sohn, H. S., Baik, S., et al. (2019). Synergistic oxygen generation and reactive oxygen species scavenging by manganese ferrite/ceria Co-decorated nanoparticles for rheumatoid arthritis treatment. ACS Nano 13 (3), 3206–3217. doi:10.1021/acsnano.8b08785
Kong, Q., Qi, M., Li, W., Shi, Y., Su, J., Xiao, S., et al. (2023). A novel Z-Scheme heterostructured Bi2S3/Cu-TCPP nanocomposite with synergistically enhanced therapeutics against bacterial biofilm infections in periodontitis. Small 19 (43), 2302547. doi:10.1002/smll.202302547
Koyanagi, T., Sakamoto, M., Takeuchi, Y., Maruyama, N., Ohkuma, M., and Izumi, Y. (2013). Comprehensive microbiological findings in peri-implantitis and periodontitis. J. Clin. Periodontol. 40 (3), 218–226. doi:10.1111/jcpe.12047
Kuboniwa, M., Houser, J. R., Hendrickson, E. L., Wang, Q., Alghamdi, S. A., Sakanaka, A., et al. (2017). Metabolic crosstalk regulates Porphyromonas gingivalis colonization and virulence during oral polymicrobial infection. Nat. Microbiol. 2 (11), 1493–1499. doi:10.1038/s41564-017-0021-6
Kunath, B. J., De Rudder, C., Laczny, C. C., Letellier, E., and Wilmes, P. (2024). The oral-gut microbiome axis in health and disease. Nat. Rev. Microbiol. 22 (12), 791–805. doi:10.1038/s41579-024-01075-5
Kwon, T., Lamster, I. B., and Levin, L. (2021). Current concepts in the management of periodontitis. Int. Dent. J. 71 (6), 462–476. doi:10.1111/idj.12630
Laforgia, A., Inchingolo, A. D., Piras, F., Colonna, V., Giorgio, R. V., Carone, C., et al. (2024). Therapeutic strategies and genetic implications for periodontal disease management: a systematic review. Int. J. Mol. Sci. 25 (13), 7217. doi:10.3390/ijms25137217
Lalwani, G., Xing, W., and Sitharaman, B. (2014). Enzymatic degradation of oxidized and reduced graphene nanoribbons by lignin peroxidase. J. Mat. Chem. B 2 (37), 6354–6362. doi:10.1039/C4TB00976B
Lamont, R. J., Koo, H., and Hajishengallis, G. (2018). The oral microbiota: dynamic communities and host interactions. Nat. Rev. Microbiol. 16 (12), 745–759. doi:10.1038/s41579-018-0089-x
Li, S. S., Wu, C. Z., and Li, L. J. (2021). Progress on photodynamic therapy in oral diseases. West China J. Stomatol. 39 (2), 215–220. doi:10.7518/hxkq.2021.02.014
Li, B., Liu, F., Ye, J., Cai, X., Qian, R., Zhang, K., et al. (2022). Regulation of macrophage polarization through periodic photo-thermal treatment to facilitate osteogenesis. Small 18 (38), 2202691. doi:10.1002/smll.202202691
Li, B., Wang, W., Zhao, L., Li, M., Yan, D., Li, X., et al. (2024a). Aggregation-induced emission-based macrophage-like nanoparticles for targeted photothermal therapy and virus transmission blockage in monkeypox. Adv. Mat. 36 (9), 2305378. doi:10.1002/adma.202305378
Li, J., Wang, Y., Tang, M., Zhang, C., Fei, Y., Li, M., et al. (2024b). New insights into nanotherapeutics for periodontitis: a triple concerto of antimicrobial activity, immunomodulation and periodontium regeneration. J. Nanobiotechnol. 22 (1), 19. doi:10.1186/s12951-023-02261-y
Li, S., Li, Q., Zhang, H., Li, F., Hu, J., Qian, J., et al. (2024c). Dental caries management with antibacterial silver-doped prussian blue hydrogel by the combined effects of photothermal response and ion discharge. ACS Appl. Mat. Interfaces 16 (22), 28172–28183. doi:10.1021/acsami.4c04302
Li, S., Wang, F., Chen, Y., Shi, W., Liu, D., Lv, M., et al. (2024d). Lysine aggregates-based nanostructured antimicrobial peptides for cariogenic biofilm microenvironment-activated caries treatment. Aggregate 5 (5), e578. doi:10.1002/agt2.578
Li, T., Shu, M., Zhu, C., Liu, Q., Li, Y., Wang, R., et al. (2024e). Triple-combination therapy with a multifunctional yolk-shell nanozyme Au@CeO2 loaded with dimethyl fumarate for periodontitis. Adv. Sci. 12 (7), 2413891. doi:10.1002/advs.202413891
Li, Z., Fan, X., Liu, Y., Yue, M., Wu, T., Wang, X., et al. (2024f). Engineering mild-photothermal responsive and NO donor prussian blue nanozymes using mild synthesis for inflammation regulation and bacterial eradication in periodontal disease. Adv. Mat. 37 (6), 2409840. doi:10.1002/adma.202409840
Li, Y., Wang, P., Liu, Y., Wu, X., Long, G., Chen, Y., et al. (2025). Fe3O4-Based nanospheres with high photothermal conversion efficiency for dual-effect and mild biofilm eradication against periodontitis. ACS Appl. Mat. Interfaces 17 (10), 14832–14845. doi:10.1021/acsami.4c17966
Liang, J., Wang, P., Lin, Y., Jia, A., Tong, F., and Li, Z. (2025). Advances in photothermal therapy for oral cancer. Int. J. Mol. Sci. 26 (9), 4344. doi:10.3390/ijms26094344
Lin, J., He, Z., Liu, F., Feng, J., Huang, C., Sun, X., et al. (2020). Hybrid hydrogels for synergistic periodontal antibacterial treatment with sustained drug release and NIR-Responsive photothermal effect. Int. J. Nanomed. 15, 5377–5387. doi:10.2147/IJN.S248538
Lin, J., Fang, J., Zhou, J., Qi, M., Shi, Y., Li, C., et al. (2024). NIR-II triggered Cu(I) phosphide for chemodynamic and photothermal periodontitis treatment: efficient reduction of bacterial co-aggregation. Acta Biomater. 187, 396–408. doi:10.1016/j.actbio.2024.08.013
Liu, Y., Bhattarai, P., Dai, Z., and Chen, X. (2019). Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem. Soc. Rev. 48 (7), 2053–2108. doi:10.1039/C8CS00618K
Liu, Z., Zhao, X., Yu, B., Zhao, N., Zhang, C., and Xu, F. J. (2021). Rough carbon-iron oxide nanohybrids for Near-Infrared-II light-responsive synergistic antibacterial therapy. ACS Nano 15 (4), 7482–7490. doi:10.1021/acsnano.1c00894
Liu, D., Chen, J., Gao, L., Chen, X., Lin, L., Liu, Y., et al. (2025). Nano Sim@ZIF8@PDA modified injectable temperature sensitive nanocomposite hydrogel for photothermal/drug therapy for peri-implantitis. Carbohydr. Polym. 354, 123327. doi:10.1016/j.carbpol.2025.123327
Lu, B.-Y., Zhu, G.-Y., Yu, C.-H., Chen, G.-Y., Zhang, C.-L., Zeng, X., et al. (2021). Functionalized graphene oxide nanosheets with unique three-in-one properties for efficient and tunable antibacterial applications. Nano Res. 14 (1), 185–190. doi:10.1007/s12274-020-3064-6
Luo, R., Zhang, C., Zhang, Z., Ren, P., Xu, Z., and Liu, Y. (2025). NIR-II upconversion nanomaterials for biomedical applications. Nanoscale 17 (6), 2985–3002. doi:10.1039/d4nr04445b
Lyu, Y., Zeng, J., Jiang, Y., Zhen, X., Wang, T., Qiu, S., et al. (2018). Enhancing both biodegradability and efficacy of semiconducting polymer nanoparticles for photoacoustic imaging and photothermal therapy. ACS Nano 12 (2), 1801–1810. doi:10.1021/acsnano.7b08616
Ma, L., Feng, X., Liang, H., Wang, K., Song, Y., Tan, L., et al. (2020). A novel photothermally controlled multifunctional scaffold for clinical treatment of osteosarcoma and tissue regeneration. Mat. Today 36, 48–62. doi:10.1016/j.mattod.2019.12.005
Madani, M., Berardi, T., and Stoopler, E. T. (2014). Anatomic and examination considerations of the oral cavity. Med. Clin. North Am. 98 (6), 1225–1238. doi:10.1016/j.mcna.2014.08.001
Mahmoud, M. Y., Steinbach-Rankins, J. M., and Demuth, D. R. (2019). Functional assessment of peptide-modified PLGA nanoparticles against oral biofilms in a murine model of periodontitis. J. Control. Release 297, 3–13. doi:10.1016/j.jconrel.2019.01.036
Mammari, N., and Duval, R. E. (2023). Photothermal/photoacoustic therapy combined with metal-based nanomaterials for the treatment of microbial infections. Microorganisms 11 (8), 2084. doi:10.3390/microorganisms11082084
Manoil, D., Cerit, E. E., Fang, H., Durual, S., Brundin, M., and Belibasakis, G. N. (2023). Profiling antibiotic susceptibility among distinct Enterococcus faecalis isolates from dental root canals. Antibiotics-Basel 13 (1), 18. doi:10.3390/antibiotics13010018
Mazurel, D., Brandt, B. W., Boomsma, M., Crielaard, W., Lagerweij, M., Exterkate, R. A. M., et al. (2025). Streptococcus mutans and caries: a systematic review and meta-analysis. J. Dent. Res. 104, 594–603. doi:10.1177/00220345241303880
Mei, J., Xu, D., Wang, L., Kong, L., Liu, Q., Li, Q., et al. (2023). Biofilm microenvironment-responsive self-assembly nanoreactors for all-stage biofilm associated infection through bacterial cuproptosis-like death and macrophage Re-Rousing. Adv. Mat. 35 (36), e2303432. doi:10.1002/adma.202303432
Mombelli, A. (2018). Microbial colonization of the periodontal pocket and its significance for periodontal therapy. Periodontol. 2000 76 (1), 85–96. doi:10.1111/prd.12147
Mondal, S. K., Chakraborty, S., Manna, S., and Mandal, S. M. (2024). Antimicrobial nanoparticles: current landscape and future challenges. RSC Pharm. 1 (3), 388–402. doi:10.1039/d4pm00032c
Moradi Eslami, L., Vatanpour, M., Aminzadeh, N., Mehrvarzfar, P., and Taheri, S. (2019). The comparison of intracanal medicaments, diode laser and photodynamic therapy on removing the biofilm of Enterococcus faecalis and Candida albicans in the root canal system (ex-vivo study). Photodiagn. Photodyn. Ther. 26, 157–161. doi:10.1016/j.pdpdt.2019.01.033
Munakata, M., Suzuki, A., Yamaguchi, K., Kataoka, Y., and Sanda, M. (2022). Effects of implant surface mechanical instrumentation methods on peri-implantitis: an in vitro study using a circumferential bone defect model. J. Dent. Sci. 17 (2), 891–896. doi:10.1016/j.jds.2021.08.018
Muras, A., Mallo, N., Otero-Casal, P., Pose-Rodríguez, J. M., and Otero, A. (2022). Quorum sensing systems as a new target to prevent biofilm-related oral diseases. Oral Dis. 28 (2), 307–313. doi:10.1111/odi.13689
Neelakantan, P., Romero, M., Vera, J., Daood, U., Khan, A. U., Yan, A., et al. (2017). Biofilms in endodontics-current status and future directions. Int. J. Mol. Sci. 18 (8), 1748. doi:10.3390/ijms18081748
Nicholson, W. (2020). Titanium alloys for dental implants: a review. Prosthesis 2 (2), 100–116. doi:10.3390/prosthesis2020011
Nie, R., Sun, Y., Lv, H., Lu, M., Huangfu, H., Li, Y., et al. (2022). 3D printing of MXene composite hydrogel scaffolds for photothermal antibacterial activity and bone regeneration in infected bone defect models. Nanoscale 14 (22), 8112–8129. doi:10.1039/d2nr02176e
Osman, M. A., Kushnerev, E., Alamoush, R. A., Seymour, K. G., and Yates, J. M. (2022). Two gingival cell lines response to different dental implant abutment materials: an in vitro study. Dent. J. 10 (10), 192. doi:10.3390/dj10100192
Overchuk, M., Weersink, R. A., Wilson, B. C., and Zheng, G. (2023). Photodynamic and photothermal therapies: synergy opportunities for nanomedicine. ACS Nano 17 (9), 7979–8003. doi:10.1021/acsnano.3c00891
Pan, G., Zheng, J., Li, Z., Duan, Q., Zhang, M., and Wang, D. (2025). Dual-responsive polydopamine-embellished Zn-MOFs enabling synergistic photothermal and antibacterial metal ion therapy for oral biofilm eradication. J. Mat. Chem. B 13 (11), 3730–3743. doi:10.1039/d4tb02427c
Park, L., Kim, H.-S., Jang, W., Ji, M.-K., Ryu, J.-H., Cho, H., et al. (2023). Antibacterial evaluation of zirconia coated with plasma-based graphene oxide with photothermal properties. Int. J. Mol. Sci. 24 (10), 8888. doi:10.3390/ijms24108888
Peres, M. A., Macpherson, L. M. D., Weyant, R. J., Daly, B., Venturelli, R., Mathur, M. R., et al. (2019). Oral diseases: a global public health challenge. Lancet 394 (10194), 249–260. doi:10.1016/s0140-6736(19)31146-8
Pieralli, S., Kohal, R. J., Jung, R. E., Vach, K., and Spies, B. C. (2017). Clinical outcomes of zirconia dental implants: a systematic review. J. Dent. Res. 96 (1), 38–46. doi:10.1177/0022034516664043
Pinto, R. M., Soares, F. A., Reis, S., Nunes, C., and Van Dijck, P. (2020). Innovative strategies toward the disassembly of the EPS matrix in bacterial biofilms. Front. Microbiol. 11, 952. doi:10.3389/fmicb.2020.00952
Pitts, N. B., and Mayne, C. (2021). Making cavities history: a global policy consensus for achieving a dental cavity–free future. JDR Clin. Transl. Res. 6 (3), 264–267. doi:10.1177/23800844211020298
Pitts, N. B., Zero, D. T., Marsh, P. D., Ekstrand, K., Weintraub, J. A., Ramos-Gomez, F., et al. (2017). Dental caries. Nat. Rev. Dis. Prim. 3, 17030. doi:10.1038/nrdp.2017.30
Pohl, S., Akamp, T., Smeda, M., Uderhardt, S., Besold, D., Krastl, G., et al. (2024). Understanding dental pulp inflammation: from signaling to structure. Front. Immunol. 15, 1474466. doi:10.3389/fimmu.2024.1474466
Popescu, C., Munteanu, C., Anghelescu, A., Ciobanu, V., Spînu, A., Andone, I., et al. (2024). Novelties on neuroinflammation in alzheimer's disease-focus on gut and oral microbiota involvement. Int. J. Mol. Sci. 25 (20), 11272. doi:10.3390/ijms252011272
Pourhajibagher, M., Kazemian, H., Chiniforush, N., Hosseini, N., Pourakbari, B., Azizollahi, A., et al. (2018). Exploring different photosensitizers to optimize elimination of planktonic and biofilm forms of Enterococcus faecalis from infected root canal during antimicrobial photodynamic therapy. Photodiagn. Photodyn. Ther. 24, 206–211. doi:10.1016/j.pdpdt.2018.09.014
Qi, M., Ren, X., Li, W., Sun, Y., Sun, X., Li, C., et al. (2022). NIR responsive nitric oxide nanogenerator for enhanced biofilm eradication and inflammation immunotherapy against periodontal diseases. Nano Today 43, 101447. doi:10.1016/j.nantod.2022.101447
Qi, J., Si, C., Liu, H., Li, H., Kong, C., Wang, Y., et al. (2025). Advances of metal-based nanomaterials in the prevention and treatment of oral infections. Adv. Healthc. Mat. 14 (15), 2500416. doi:10.1002/adhm.202500416
Qian, H., Lei, T., Hua, L., Zhang, Y., Wang, D., Nan, J., et al. (2023). Fabrication, bacteriostasis and osteointegration properties researches of the additively-manufactured porous tantalum scaffolds loading vancomycin. Bioact. Mat. 24, 450–462. doi:10.1016/j.bioactmat.2022.12.013
Rams, T. E., Sautter, J. D., and van Winkelhoff, A. J. (2020). Comparative in vitro resistance of human periodontal bacterial pathogens to tinidazole and four other antibiotics. Antibiotics-Basel 9 (2), 68. doi:10.3390/antibiotics9020068
Ran, Y., Shi, J., Ding, Y., Li, L., Lu, D., Zeng, Y., et al. (2024). Black phosphorus nanosheets-loaded mussel-inspired hydrogel with wet adhesion, photothermal antimicrobial, and in situ remineralization capabilities for caries prevention. Adv. Sci. 11 (45), 2409155. doi:10.1002/advs.202409155
Ren, X., Gao, R., van der Mei, H. C., Ren, Y., Peterson, B. W., and Busscher, H. J. (2020). Eradicating infecting bacteria while maintaining tissue integration on photothermal nanoparticle-coated titanium surfaces. ACS Appl. Mat. Interfaces 12 (31), 34610–34619. doi:10.1021/acsami.0c08592
Sanz, M., Herrera, D., Kebschull, M., Chapple, I., Jepsen, S., Beglundh, T., et al. (2020). Treatment of stage I-III periodontitis-the EFP S3 level clinical practice guideline. J. Clin. Periodontol. 47 (22), 4–60. doi:10.1111/jcpe.13290
Scannapieco, F. A., and Cantos, A. (2016). Oral inflammation and infection, and chronic medical diseases: implications for the elderly. Periodontol 72 (1), 153–175. doi:10.1111/prd.12129
Schwarz, F., Derks, J., Monje, A., and Wang, H.-L. (2018). Peri-implantitis. J. Clin. Periodontol. 45 (S20), S246-S266–S266. doi:10.1111/jcpe.12954
Sczepanik, F. S. C., Grossi, M. L., Casati, M., Goldberg, M., Glogauer, M., Fine, N., et al. (2020). Periodontitis is an inflammatory disease of oxidative stress: we should treat it that way. Periodontol. 2000 84 (1), 45–68. doi:10.1111/prd.12342
Sedghi, L., DiMassa, V., Harrington, A., Lynch, S. V., and Kapila, Y. L. (2021). The oral microbiome: role of key organisms and complex networks in oral health and disease. Periodontol 87 (1), 107–131. doi:10.1111/prd.12393
Shen, L., Sun, F., Wang, Y., Liu, Y., Xin, Q., Zhu, Z., et al. (2024). Caries-prone primary teeth: a hidden reason and prophylactic treatment in the viewpoint of materials science. ACS Appl. Mat. Interfaces 16 (32), 41881–41891. doi:10.1021/acsami.4c07388
Sheng, L., Zhang, Z., Zhang, Y., Wang, E., Ma, B., Xu, Q., et al. (2021). A novel “hot spring”-mimetic hydrogel with excellent angiogenic properties for chronic wound healing. Biomaterials 264, 120414. doi:10.1016/j.biomaterials.2020.120414
Shi, E., Bai, L., Mao, L., Wang, H., Yang, X., Wang, Y., et al. (2021). Self-assembled nanoparticles containing photosensitizer and polycationic brush for synergistic photothermal and photodynamic therapy against periodontitis. J. Nanobiotechnol. 19, 413–415. doi:10.1186/s12951-021-01114-w
Shim, S. H., Lee, S. Y., Lee, J.-B., Chang, B.-S., Lee, J.-K., and Um, H.-S. (2022). Antimicrobial photothermal therapy using diode laser with indocyanine green on Streptococcus gordonii biofilm attached to zirconia surface. Photodiagn. Photodyn. Ther. 38, 102767. doi:10.1016/j.pdpdt.2022.102767
Song, X., Ji, M., Shu, X., and Zou, L. (2025). Drug delivery systems loaded with plant-derived natural products for dental caries prevention and treatment. J. Mat. Chem. B 13 (6), 1920–1934. doi:10.1039/d4tb01924e
Spanemberg, J. C., Cardoso, J. A., Slob, E., and López-López, J. (2019). Quality of life related to oral health and its impact in adults. J. Stomatol. Oral Maxillofac. Surg. 120 (3), 234–239. doi:10.1016/j.jormas.2019.02.004
Stoopler, E. T., Villa, A., Bindakhil, M., Díaz, D. L. O., and Sollecito, T. P. (2024). Common oral conditions: a review. JAMA 331 (12), 1045–1054. doi:10.1001/jama.2024.0953
Sun, X. D., Liu, T. T., Wang, Q. Q., Zhang, J., and Cao, M. S. (2023). Surface modification and functionalities for titanium dental implants. ACS Biomater. Sci. Eng. 9 (8), 4442–4461. doi:10.1021/acsbiomaterials.3c00183
Tan, L., Li, J., Liu, X., Cui, Z., Yang, X., Zhu, S., et al. (2018). Rapid biofilm eradication on bone implants using red phosphorus and near-infrared light. Adv. Mat. 30 (31), e1801808. doi:10.1002/adma.201801808
Tang, S., Chen, M., and Zheng, N. (2014). Sub-10-nm Pd nanosheets with renal clearance for efficient near-infrared photothermal cancer therapy. Small 10 (15), 3139–3144. doi:10.1002/smll.201303631
Tian, Y., Li, Y., Liu, J., Lin, Y., Jiao, J., Chen, B., et al. (2022). Photothermal therapy with regulated Nrf2/NF-κB signaling pathway for treating bacteria-induced periodontitis. Bioact. Mat. 9, 428–445. doi:10.1016/j.bioactmat.2021.07.033
Tosić, G., Miladinović, M., Kovaević, M., and Stojanović, M. (2016). Choice of root canal irrigants by Serbian dental practitioners. Vojnosanit. Pregl. 73 (1), 47–52. doi:10.2298/vsp140909034t
Tóthová, L., and Celec, P. (2017). Oxidative stress and antioxidants in the diagnosis and therapy of periodontitis. Front. Physiol. 8, 1055. doi:10.3389/fphys.2017.01055
Tzach-Nahman, R., Mizraji, G., Shapira, L., Nussbaum, G., and Wilensky, A. (2017). Oral infection with Porphyromonas gingivalis induces peri-implantitis in a murine model: evaluation of bone loss and the local inflammatory response. J. Clin. Periodontol. 44 (7), 739–748. doi:10.1111/jcpe.12735
Umeda, M., Takeuchi, Y., Noguchi, K., Huang, Y., Koshy, G., and Ishikawa, I. (2004). Effects of nonsurgical periodontal therapy on the microbiota. Periodontol 36, 98–120. doi:10.1111/j.1600-0757.2004.03675.x
Villoria, G. E. M., Fischer, R. G., Tinoco, E. M. B., Meyle, J., and Loos, B. G. (2024). Periodontal disease: a systemic condition. Periodontol 96 (1), 7–19. doi:10.1111/prd.12616
Wang, C.-Y., Yang, Y.-H., Li, H., Lin, P.-Y., Su, Y.-T., Kuo, M.Y.-P., et al. (2020). Adjunctive local treatments for patients with residual pockets during supportive periodontal care: a systematic review and network meta-analysis. J. Clin. Periodontol. 47 (12), 1496–1510. doi:10.1111/jcpe.13379
Wang, H., Wang, D., Huangfu, H., Lv, H., Qin, Q., Ren, S., et al. (2022a). Branched AuAg nanoparticles coated by metal–phenolic networks for treating bacteria-induced periodontitis via photothermal antibacterial and immunotherapy. Mat. Des. 224, 111401. doi:10.1016/j.matdes.2022.111401
Wang, N., Luo, J., Deng, F., Huang, Y., and Zhou, H. (2022b). Antibiotic combination therapy: a strategy to overcome bacterial resistance to aminoglycoside antibiotics. Front. Pharmacol. 13, 839808. doi:10.3389/fphar.2022.839808
Wang, R., Kim, D., Yang, M., Li, X., and Yoon, J. (2022c). Phthalocyanine-assembled “one-for-two” nanoparticles for combined photodynamic-photothermal therapy of multidrug-resistant bacteria. ACS Appl. Mat. Interfaces 14 (6), 7609–7616. doi:10.1021/acsami.1c21891
Wang, F., Wu, Q., Jia, G., Kong, L., Zuo, R., Feng, K., et al. (2023a). Black Phosphorus/MnO2 nanocomposite disrupting bacterial thermotolerance for efficient mild-temperature photothermal therapy. Adv. Sci. 10 (30), e2303911. doi:10.1002/advs.202303911
Wang, H., Wang, D., Huangfu, H., Chen, S., Qin, Q., Ren, S., et al. (2023b). Highly efficient photothermal branched Au–Ag nanoparticles containing procyanidins for synergistic antibacterial and anti-inflammatory immunotherapy. Biomater. Sci. 11 (4), 1335–1349. doi:10.1039/D2BM01212J
Wang, P., Wang, L., Zhan, Y., Liu, Y., Chen, Z., Xu, J., et al. (2023c). Versatile hybrid nanoplatforms for treating periodontitis with chemical/photothermal therapy and reactive oxygen species scavenging. Chem. Eng. J. 463, 142293. doi:10.1016/j.cej.2023.142293
Wang, W., Zhang, G., Wang, Y., Ran, J., Chen, L., Wei, Z., et al. (2023d). An injectable and thermosensitive hydrogel with nano-aided NIR-II phototherapeutic and chemical effects for periodontal antibacteria and bone regeneration. J. Nanobiotechnol. 21 (1), 367. doi:10.1186/s12951-023-02124-6
Wang, X., Qian, Y., Wang, S., Wang, M., Sun, K., Cheng, Z., et al. (2023e). Accumulative rolling Mg/PLLA composite membrane with lamellar heterostructure for enhanced bacteria inhibition and rapid bone regeneration. Small 19 (42), 2301638. doi:10.1002/smll.202301638
Wang, Y., Chang, L., Gao, H., Yu, C., Gao, Y., and Peng, Q. (2024). Nanomaterials-based advanced systems for photothermal/photodynamic therapy of oral cancer. Eur. J. Med. Chem. 272, 116508. doi:10.1016/j.ejmech.2024.116508
Wang, X., Shi, W., Jin, Y., Li, Z., Deng, T., Su, T., et al. (2025). Photodynamic and photothermal bacteria targeting nanosystems for synergistically combating bacteria and biofilms. J. Nanobiotechnol. 23 (1), 40. doi:10.1186/s12951-025-03126-2
Werner, S., Huck, O., Frisch, B., Vautier, D., Elkaim, R., Voegel, J.-C., et al. (2009). The effect of microstructured surfaces and laminin-derived peptide coatings on soft tissue interactions with titanium dental implants. Biomaterials 30 (12), 2291–2301. doi:10.1016/j.biomaterials.2009.01.004
Wong, R. L., Hiyari, S., Yaghsezian, A., Davar, M., Casarin, M., Lin, Y. L., et al. (2018). Early intervention of peri-implantitis and periodontitis using a mouse model. J. Periodontol. 89 (6), 669–679. doi:10.1002/jper.17-0541
Wu, D., Duan, X., Guan, Q., Liu, J., Yang, X., Zhang, F., et al. (2019a). Mesoporous polydopamine carrying manganese carbonyl responds to tumor microenvironment for multimodal imaging-guided cancer therapy. Adv. Funct. Mat. 29 (16), 1900095. doi:10.1002/adfm.201900095
Wu, J., Li, F., Hu, X., Lu, J., Sun, X., Gao, J., et al. (2019b). Responsive assembly of silver nanoclusters with a biofilm locally amplified bactericidal effect to enhance treatments against multi-drug-resistant bacterial infections. ACS Cent. Sci. 5 (8), 1366–1376. doi:10.1021/acscentsci.9b00359
Wu, L., Li, B. H., Wang, Y. Y., Wang, C. Y., Zi, H., Weng, H., et al. (2019c). Periodontal disease and risk of benign prostate hyperplasia: a cross-sectional study. Mil. Med. Res. 6 (1), 34. doi:10.1186/s40779-019-0223-8
Wu, Y., Zhang, X., Tan, B., Shan, Y., Zhao, X., and Liao, J. (2022). Near-infrared light control of GelMA/PMMA/PDA hydrogel with mild photothermal therapy for skull regeneration. Biomater. Adv. 133, 112641. doi:10.1016/j.msec.2022.112641
Wu, X., Qi, M., Liu, C., Yang, Q., Li, S., Shi, F., et al. (2023). Near-infrared light-triggered nitric oxide nanocomposites for photodynamic/photothermal complementary therapy against periodontal biofilm in an animal model. Theranostics 13 (7), 2350–2367. doi:10.7150/thno.83745
Wu, H., Li, Y., Shi, L., Liu, Y., and Shen, J. (2025). New advances in periodontal functional materials based on antibacterial, anti-inflammatory, and tissue regeneration strategies. Adv. Healthc. Mat. 14, 2403206. doi:10.1002/adhm.202403206
Xiao, J., Fiscella, K. A., and Gill, S. R. (2020). Oral microbiome: possible harbinger for children's health. Int. J. Oral. Sci. 12 (1), 12. doi:10.1038/s41368-020-0082-x
Xiao, L., Feng, M., Chen, C., Xiao, Q., Cui, Y., and Zhang, Y. (2024). Microenvironment-regulating drug delivery nanoparticles for treating and preventing typical biofilm-induced oral diseases. Adv. Mat. 37, 202304982. doi:10.1002/adma.202304982
Xu, M., Hu, Y., Xiao, Y., Zhang, Y., Sun, K., Wu, T., et al. (2020a). Near-infrared-controlled nanoplatform exploiting photothermal promotion of peroxidase-like and OXD-Like activities for potent antibacterial and anti-biofilm therapies. ACS Appl. Mater Interfaces 12 (45), 50260–50274. doi:10.1021/acsami.0c14451
Xu, Y., Zhao, S., Weng, Z., Zhang, W., Wan, X., Cui, T., et al. (2020b). Jelly-inspired injectable guided tissue regeneration strategy with shape auto-matched and dual-light-defined antibacterial/osteogenic pattern switch properties. ACS Appl. Mat. Interfaces 12 (49), 54497–54506. doi:10.1021/acsami.0c18070
Xu, H., Ye, Z., Zhang, A., Lin, F., Fu, J., and Fok, A. S. L. (2022a). Effects of concentration of sodium hypochlorite as an endodontic irrigant on the mechanical and structural properties of root dentine: a laboratory study. Int. Endod. J. 55 (10), 1091–1102. doi:10.1111/iej.13800
Xu, X., Fan, M., Yu, Z., Zhao, Y., Zhang, H., Wang, J., et al. (2022b). A removable photothermal antibacterial “warm paste” target for cariogenic bacteria. Chem. Eng. J. 429, 132491. doi:10.1016/j.cej.2021.132491
Xu, V. W., Yin, I. X., Niu, J. Y., and Chu, C.-H. (2025). Enhancing caries preventive effects of nanomaterials with phototherapy: a scoping review. J. Func. Biomater. 16 (9), 308. doi:10.3390/jfb16090308
Xue, Y., Zhang, L., Liu, F., Zhao, Y., Zhou, J., Hou, Y., et al. (2022). Surface bandgap engineering of nanostructured implants for rapid photothermal ion therapy of bone defects. Adv. Healthc. Mat. 11 (22), 2200998. doi:10.1002/adhm.202200998
Xue, Y., Zhang, L., Liu, F., Kong, L., Ma, D., and Han, Y. (2023). Fluoride releasing photothermal responsive TiO2 matrices for antibiosis, biosealing and bone regeneration. J. Control. Release 363, 657–669. doi:10.1016/j.jconrel.2023.10.016
Yang, Y., Ma, L., Cheng, C., Deng, Y., Huang, J., Fan, X., et al. (2018). Nonchemotherapic and robust dual-responsive nanoagents with On-Demand bacterial trapping, ablation, and release for efficient wound disinfection. Adv. Funct. Mat. 28 (21), 1705708. doi:10.1002/adfm.201705708
Yang, Y., Xu, C., Xu, S., Li, Y., Chen, K. e., Yang, T., et al. (2025). Injectable hydrogels activated with copper sulfide nanoparticles for enhancing spatiotemporal sterilization and osteogenesis in periodontal therapy. Biomater. Sci. 13, 1434–1448. doi:10.1039/d3bm02134c
Yao, H., Turali Emre, E. S., Fan, Y., Wang, J., Liu, F., and Wei, J. (2025a). L-arginine modified mesoporous bioactive glass with ROS scavenging and NO release for periodontitis treatment. Bioact. Mat. 48, 200–216. doi:10.1016/j.bioactmat.2025.02.015
Yao, W., Wang, T., Sun, W., Zhang, X., Xiong, H., Yin, J., et al. (2025b). Mature biofilm-sensitive lysozyme-grafted Bi-guanidine backbone porphyrin nanorods for deep penetration and double phototherapy. Biomaterials 323, 123431. doi:10.1016/j.biomaterials.2025.123431
Yin, L., Ma, H., Nakayasu, E. S., Payne, S. H., Morris, D. R., and Harwood, C. S. (2019). Bacterial longevity requires protein synthesis and a stringent response. mBio 10 (5), e02189-19. doi:10.1128/mBio.02189-19
You, J., Li, Y., Wang, C., Lv, H., Zhai, S., Liu, M., et al. (2024). Mild thermotherapy-assisted GelMA/HA/MPDA@Roxadustat 3D-Printed scaffolds with combined angiogenesis-osteogenesis functions for bone regeneration. Adv. Healthc. Mat. 13 (22), 2400545. doi:10.1002/adhm.202400545
Yu, Y., Zhang, Y., Cheng, Y., Wang, Y., Chen, Z., Sun, H., et al. (2022). NIR-Activated nanosystems with self-modulated bacteria targeting for enhanced biofilm eradication and caries prevention. Bioact. Mat. 13, 269–285. doi:10.1016/j.bioactmat.2021.10.035
Yu, S., Xia, G., Yang, N., Yuan, L., Li, J., Wang, Q., et al. (2024a). Noble metal nanoparticle-based photothermal therapy: development and application in effective cancer therapy. Int. J. Mol. Sci. 25 (11), 5632. doi:10.3390/ijms25115632
Yu, Y. M., Lu, Y. P., Zhang, T., Zheng, Y. F., Liu, Y. S., and Xia, D. D. (2024b). Biomaterials science and surface engineering strategies for dental peri-implantitis management. Mil. Med. Res. 11 (1), 29. doi:10.1186/s40779-024-00532-9
Yuan, Z., Lin, C., He, Y., Tao, B., Chen, M., Zhang, J., et al. (2020). Near-infrared light-triggered nitric-oxide-enhanced photodynamic therapy and low-temperature photothermal therapy for biofilm elimination. ACS Nano 14 (3), 3546–3562. doi:10.1021/acsnano.9b09871
Zeng, J., Sun, Z., Zeng, F., Gu, C., and Chen, X. (2023). M2 macrophage-derived exosome-encapsulated microneedles with mild photothermal therapy for accelerated diabetic wound healing. Mat. Today Bio 20, 100649. doi:10.1016/j.mtbio.2023.100649
Zhang, C., and Chen, X. (2025). Photothermal-therapy-based targeting thrombolytic therapy. ACS Appl. Bio Mat. 8 (3), 1820–1834. doi:10.1021/acsabm.4c01820
Zhang, X., Cheng, G., Xing, X., Liu, J., Cheng, Y., Ye, T., et al. (2019). Near-infrared light-triggered porous AuPd alloy nanoparticles to produce mild localized heat to accelerate bone regeneration. J. Phys. Chem. Lett. 10 (15), 4185–4191. doi:10.1021/acs.jpclett.9b01735
Zhang, L., Wang, Y., Wang, C., He, M., Wan, J., Wei, Y., et al. (2020). Light-activable On-Demand release of nano-antibiotic platforms for precise synergy of thermochemotherapy on periodontitis. ACS Appl. Mat. Interfaces 12 (3), 3354–3362. doi:10.1021/acsami.9b17335
Zhang, X., Tan, B., Wu, Y., Zhang, M., and Liao, J. (2021). A review on hydrogels with photothermal effect in wound healing and bone tissue engineering. Polymers 13 (13), 2100. doi:10.3390/polym13132100
Zhang, B., Zheng, R., Liu, Y., Lou, X., Zhang, W., Cui, Z., et al. (2023). Perylene-mediated electron leakage in respiratory chain to trigger endogenous ROS burst for hypoxic cancer chemo-immunotherapy. Adv. Sci. 10 (3), e2204498. doi:10.1002/advs.202204498
Zhang, J., Tong, Z., Chen, L., Qian, Y., Lu, Y., Chen, Q., et al. (2024a). Development and applications of peri-implantitis mouse models. Oral Dis. 30 (6), 3788–3798. doi:10.1111/odi.14929
Zhang, S., Kong, N., Wang, Z., Zhang, Y., Ni, C., Li, L., et al. (2024b). Nanochemistry of gold: from surface engineering to dental healthcare applications. Chem. Soc. Rev. 53 (8), 3656–3686. doi:10.1039/d3cs00894k
Zhang, Y., Jiang, Z.-T., Wang, Y., Wang, H.-Y., Hong, S., Li, W., et al. (2024c). A supramolecular nanoformulation with adaptive photothermal/photodynamic transformation for preventing dental caries. ACS Nano 18 (40), 27340–27357. doi:10.1021/acsnano.4c06051
Zhang, H., Yang, M., Wu, Q., Xue, J., and Liu, H. (2025). Engineering two-dimensional nanomaterials for photothermal therapy. Angew. Chem.-Int. Ed. 64 (12), e202424768. doi:10.1002/anie.202424768
Zhao, J., Long, X., and Zhou, M. (2021). “Clearable nanoparticles for cancer photothermal therapy,” in Bio-nanomedicine for cancer therapy (Cham: Springer International Publishing), 121–134.
Zhao, L., Zhu, H., Duo, Y. Y., Wang, Z. G., Pang, D. W., and Liu, S. L. (2024). A cyanine with 83.2% photothermal conversion efficiency and absorption wavelengths over 1200 nm for photothermal therapy. Adv. Healthc. Mat. 13 (20), 2304421. doi:10.1002/adhm.202304421
Zheng, P., Qiu, X., Zhang, L., Liu, P., Peng, Z., and Huang, Z. (2025). Comparative analysis of oral disorder burden in China and globally from 1990 to 2021 based on GBD data. Sci. Rep. 15 (1), 10061. doi:10.1038/s41598-025-94899-x
Zhou, B., Dong, B., Hu, S., Liu, W., Sun, L., Xu, L., et al. (2024). NIR-triggered multifunctional NO nanoplatform for conquering thermal resistance in biofilms. Small 20 (31), e2310706. doi:10.1002/smll.202310706
Zhu, Y., Ramasamy, M., and Yi, D. K. (2014). Antibacterial activity of ordered gold nanorod arrays. ACS Appl. Mat. Interfaces 6 (17), 15078–15085. doi:10.1021/am503153v
Zhu, T., Huang, Z., Shu, X., Zhang, C., Dong, Z., and Peng, Q. (2022). Functional nanomaterials and their potentials in antibacterial treatment of dental caries. Colloid Surf. B-Biointerfaces 218, 112761. doi:10.1016/j.colsurfb.2022.112761
Glossary
3D Three-dimensional
Alg Sodium alginate
ATP Adenosine triphosphate
ATRP Atom transfer radical polymerization
AuNPs Gold nanoparticles
AuNRs Gold nanorods
BA Baicalein
BMP-2 Bone morphogenetic protein-2
BMSCs Bone marrow-derived mesenchymal stromal cells
BP Black phosphorus
C. albicans Candida albicans
CDT Chemical dynamic therapy
DDS Drug delivery systems
E. coli Escherichia coli
E. faecalis Enterococcus faecalis
eNOS Endothelial nitric oxide synthase
EPL Epsilon-polylysine
EPS Extracellular polymeric substances
F. nucleatum Fusobacterium nucleatum
GelMA Gelatin methacrylate
GNC Gold nanocages
GO Graphene oxide
HE Hematoxylin-Eosin
HGFs Human gingival fibroblasts
HSP90 Heat shock protein 90
IBD Infectious bone defects
ICG Indocyanine green
MgMps Mg microparticles
MPB Mesoporous Prussian blue
MPN Metal-phenolic networks
mPTT Mild photothermal therapy
MRSA Methicillin-resistant Staphylococcus aureus
MSC Mesenchymal stem cell
NIR Near-infrared
NIR-I Near-infrared region I
NIR-II Near-infrared region II
NO Nitric oxide
NPs Nanoparticles
NSs Nanosheets
P. gingivalis Porphyromonas gingivalis
PB Prussian blue
PC Proanthocyanidins
PCE Photothermal conversion efficiency
PCM Phase-change materials
PDA Polydopamine
PDT Photodynamic therapy
PEG Polyethylene glycol
PLGA Poly lactic acid-co-glycolic acid
PLLA Polylactic acid
POD Photothermal and peroxidase
PTAs Photothermal agents
PTT Photothermal therapy
rGO Reduced graphene oxide
RCT Root canal therapy
RNS Reactive nitrogen species
ROS Reactive oxygen species
S. aureus Staphylococcus aureus
S. gordonii Streptococcus gordonii
S. mutans Streptococcus mutans
S. oralis Streptococcus oralis
S. sanguinis Streptococcus sanguinis
S. sobrinus Streptococcus sobrinus
SDT Sonodynamic Therapy
SEM Scanning electron microscopy
SNP Sodium nitroprusside
SRP Scaling and root planning
TC Tetracycline
TRAP Tartrate-resistant acid phosphatase
UBI Ubiquicidine
VEGF Vascular endothelial growth factor
Keywords: photothermal nanoparticles, biofilm disruption, antibiotic-free strategies, localized hyperthermia, oral immunotherapy
Citation: Wang P, Liang J and Liu F (2025) Beyond antibiotics: advances in photothermal strategies for oral infections. Front. Bioeng. Biotechnol. 13:1637941. doi: 10.3389/fbioe.2025.1637941
Received: 30 May 2025; Accepted: 29 October 2025;
Published: 12 November 2025.
Edited by:
Bolei Cai, The Fourth Military Medical University, ChinaReviewed by:
Dan Lin, Shanghai University of Medicine and Health Sciences, ChinaKunal Sharma, Swiss Federal Institute of Technology Lausanne, Switzerland
Copyright © 2025 Wang, Liang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pei Wang, bmRmc2txeXk2MjBAbmN1LmVkdS5jbg==; Fen Liu, bmRmc2txeXkzNDdAbmN1LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Pei Wang
Pei Wang Jian Liang
Jian Liang Fen Liu
Fen Liu