- 1Department of Orthopedics, The Second Affiliated Hospital, Jiangxi Medical College, Nanchang University, Nanchang, Jiangxi, China
- 2Institute of Orthopedics of Jiangxi Province, Nanchang, Jiangxi, China
- 3Jiangxi Provincial Key Laboratory of Spine and Spinal Cord Disease, Nanchang, Jiangxi, China
- 4Institute of Minimally Invasive Orthopedics, Nanchang University, Nanchang, Jiangxi, China
- 5Department of Orthopedics, Tenth People’s Hospital of Tongji University, Shanghai, China
- 6Department of Joint Surgery, Ganzhou People’s Hospital, Ganzhou, China
Infectious bone defects frequently encounter challenges related to bacterial infection and bone integrity. Neutrophils, being the initial responders to sites of inflammation, employ multiple mechanisms to eradicate bacteria, including phagocytosis, degranulation, the formation of neutrophil extracellular traps (NETs), and the oxidative respiratory burst. As a critical component of the human immune system, neutrophils play a pivotal role in modulating the inflammatory response, influencing the processes of osteogenesis and osteoclastogenesis, and impacting fracture healing. In the field of bone tissue engineering, the optimization of the chemical composition and morphology of scaffold materials can effectively modulate neutrophil behavior, thereby enhancing the antibacterial properties and osteogenic potential of the scaffolds. These approaches offer innovative strategies for designing bone tissue engineering scaffolds capable of regulating immune responses, with the potential to achieve improved clinical outcomes in future therapeutic applications.
1 Introduction
In recent years, bone tissue engineering (BTE) has emerged as a significant area of research due to its potential applications in the treatment of bone defects. The primary objective of BTE is to repair or regenerate damaged bone tissue by leveraging the synergistic interactions among biological scaffolds, cells, and bioactive factors (Du et al., 2019). In this methodology, scaffolds function as substitutes for the extracellular matrix (ECM), offering a three-dimensional support structure for osteocytes. They facilitate cell migration, proliferation, and differentiation through specific physical and chemical cues, thereby promoting new bone formation (Zeng et al., 2023; Wei et al., 2024; Xiong et al., 2024). In practical applications, the design of bone tissue engineering scaffolds is progressively advancing towards functionalization. Specifically, the functionality of the scaffold can be enhanced through two primary approaches: first, by altering the chemical composition of the scaffold to achieve targeted functionality; and second, by optimizing the morphology and structure of the scaffold to regulate cellular behavior, thereby promoting cell adhesion and proliferation. Currently, the main materials used for bone scaffolds predominantly include ceramics, polymers, metals, and composite materials. These materials are extensively utilized in bone tissue engineering due to their distinct mechanical properties, biocompatibility, and degradation characteristics (Koons et al., 2020).
As the global population ages rapidly, the proportion of elderly individuals is rising (de Magalhães, 2025). They are more susceptible to falls and fractures due to lower bone density, weaker muscles, and reduced balance (Coughlan and Dockery, 2014; Huang and Huang, 2024). Infectious bone defects pose a challenge in fracture treatment due to their prolonged duration and poor outcomes. Factors like open bone defects, insufficient debridement during graft surgery, and improper aseptic techniques can lead to postoperative infections (Yu et al., 2024). Specifically, the incidence of infection following internal fixation of closed fractures is approximately 1%, whereas for open fractures, it can exceed 15% (Morgenstern et al., 2018). Furthermore, the inherent porous structure of bone implants predisposes them to bacterial colonization and subsequent infection. This is particularly critical in the early stages post-implantation, where pathogenic bacteria can adhere to the surface of the implants prior to the arrival of the body’s immune cells, thereby exerting a head start effect (Chu et al., 2024; Pettygrove et al., 2021). This initial colonization facilitates the formation of a biofilm, which significantly impedes the efficacy of antimicrobial agents in penetrating the barrier. Ultimately, uncontrolled infections may lead to chronic osteomyelitis, characterized by prolonged infection, a high recurrence rate, and a significant disability rate (Spiegel et al., 2010).
Therefore, integrating antimicrobial properties into bone scaffolds holds substantial clinical and scientific importance (Feng et al., 2024; Wang Z. et al., 2025). Currently, a prevalent antibacterial strategy involves utilizing the scaffold as a carrier to deliver antibacterial agents, thereby eradicating bacteria (Zhou et al., 2018; De Silva et al., 2018). However, these biomaterials often prioritize their direct bactericidal effects, potentially at the expense of their role in supporting immune cell defense functions. This oversight may inadvertently compromise the active antibacterial response and timely repair mechanisms of immune cells, potentially resulting in chronic inflammation (Li et al., 2017). Additionally, as foreign implants, scaffolds frequently induce adaptive changes within the host post-implantation in vivo. Upon implantation, the scaffold interacts with blood components such as platelets and fibronectin, rapidly initiating an immune response. This immune response subsequently influences tissue repair and the biodegradation process of the scaffold. The interaction of the scaffold with blood components like platelets and fibronectin further prompts the recruitment of immune cells (Scherlinger et al., 2023; Anders and Schaefer, 2014). This process, termed the foreign body reaction, is typically characterized by the recruitment and activation of immune cells, with a particular emphasis on the involvement of neutrophils. Neutrophils serve as a critical “first line of defense” in the initial immune response. Their swift aggregation not only facilitates antibacterial actions but also influences subsequent tissue repair processes. As the main effector cells of an inflammatory response, they can quickly gather at the injured/infected site to remove pathogens and trigger a strong inflammatory response (Kraus and Gruber, 2021). After the inflammatory period, the neutrophils in the injured site gradually subside and initiate the process of tissue repair, and the bone tissue gradually develops in the direction of healing. The implantation of biomaterials to modulate the immune response for tissue repair represents a significant strategy in bone tissue engineering research. Controllable regulation of neutrophil activity via biomaterials is an effective approach to initiating an early immune response, thereby establishing favorable conditions for subsequent osteogenic repair.
This paper provides a comprehensive review of the mechanisms by which neutrophils clear bacteria, including cell phagocytosis, degranulation, the formation of neutrophil extracellular traps (NETs), and the oxidative respiratory burst. Additionally, it explores the role of neutrophils in osteogenic repair. The strategies for regulating neutrophil antibacterial activity and osteogenic repair through scaffolds are introduced, encompassing the loading of metal ions or bioactive factors, the construction of oxygen supply platforms, and the optimization of scaffold morphology and structure. Finally, this review summarizes the related challenges faced by current bone scaffolds in the antibacterial and bone repair process by regulating neutrophils.
2 Antibacterial function of neutrophils
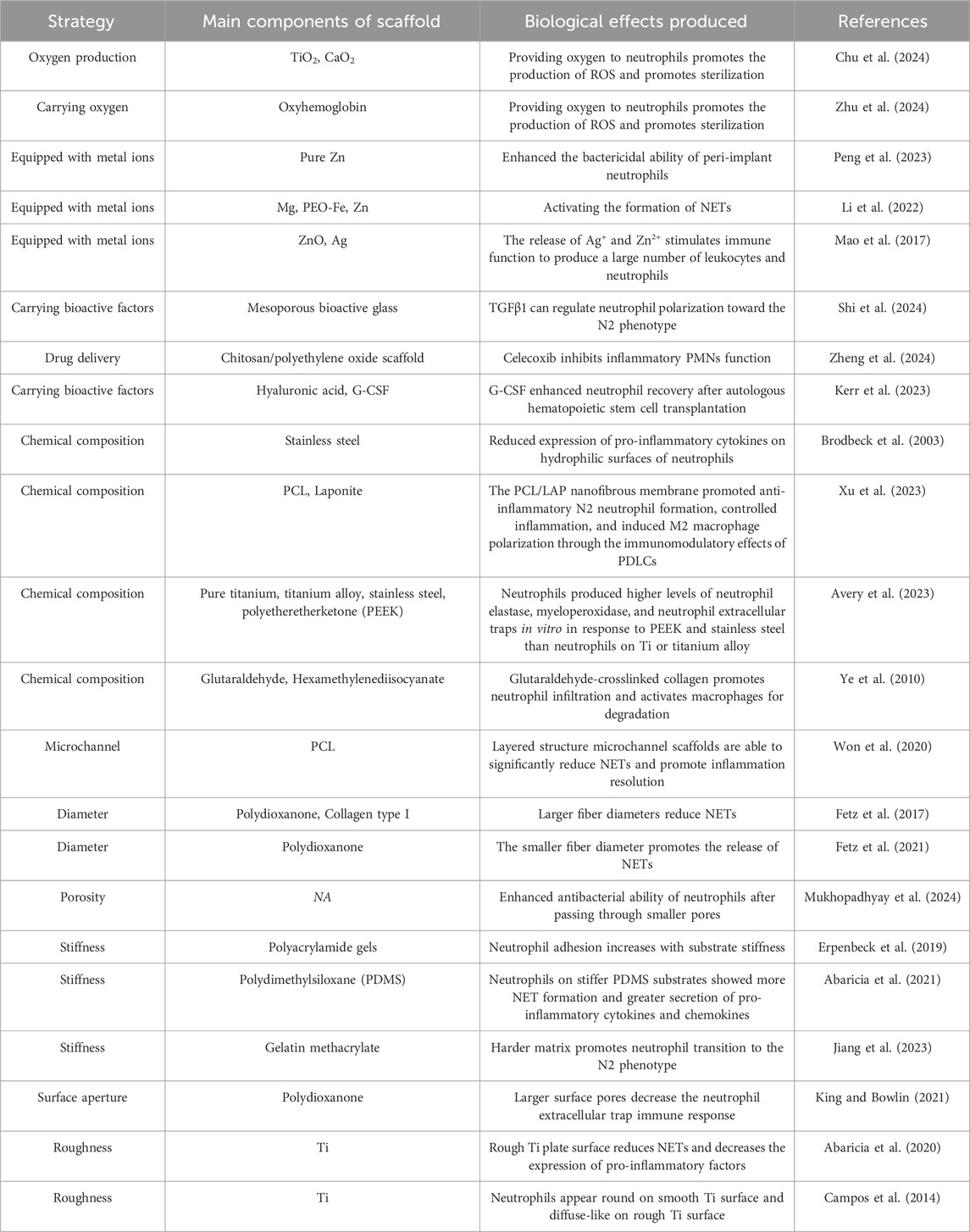
Neutrophils originate from myeloid progenitor cells and undergo maturation in the bone marrow, during which their surface receptors transition from CXCR4 to CXCR2. Subsequently, they are released into the bloodstream in response to IL-8-mediated chemotaxis (Németh et al., 2020). Within the blood vessels, neutrophils must extravasate from the vascular bed and migrate to sites of inflammation. This extravasation process comprises four distinct steps: rolling, adhesion, crawling, and transmigration (Li et al., 2024) (Figure 1). When tissues incur damage due to physical, chemical, or biological factors in vivo, the affected or necrotic cells release damage-associated molecular patterns (DAMPs) (Huang et al., 2024). These DAMPs activate innate immune cells, prompting the production and release of various chemokines, including CXCL8, CCL2, and CCL5. These chemokines establish concentration gradients around the damaged tissue. Neutrophils, through chemokine receptors on their surfaces such as CXCR1 and CXCR2, detect these gradients and migrate towards the site of damage. Subsequently, neutrophils identify damaged cells or invading microorganisms within tissues and initiate the secretion of signaling molecules, including LTB4 and CXCL2. These signals interact with G protein-coupled receptors on neighboring neutrophils, thereby facilitating the recruitment of additional neutrophil populations (Lämmermann et al., 2013).
Figure 1. Neutrophil production process and antibacterial function in vivo. Neutrophils originate from bone marrow HSCs, progenitors, myeloblasts, promyelocytes, myelocytes, metamyelocytes, band neutrophils, and eventually develop into mature neutrophils in the bone marrow. Neutrophils are chemotaxised by IL-8 in the blood and migrate from the bone marrow to the blood vessels, then “roll,” “adhere” and “crawl,” and finally pass through the blood vessels and enter the outside of the blood vessels. Cytokine chemotaxis subjected to concentration gradient eventually reaches the site of pathogen infection. Neutrophils mainly rely on oxidative respiratory bursts, cell phagocytosis, degranulation, and NETs to fight bacteria. Created with BioRender.com.
Neutrophils are the first immune cells to infiltrate the site of injury during the initial phase of fracture healing, where they promptly migrate to the affected area to initiate the primary inflammatory response. This inflammatory reaction is crucial for the clearance of damaged tissue, the release of growth factors, and the recruitment of additional immune cell types. The process facilitates the dilation of local blood vessels and enhances blood flow, thereby delivering essential immune cells, nutrients, and oxygen to the site of injury. This initial phase of inflammation is crucial for the removal of damaged tissue, the recruitment of stem cells, and the activation of osteoblasts, all of which are indispensable for fracture repair (Suliman et al., 2022). Nonetheless, excessive or prolonged inflammation can adversely impact the healing process. Excessive inflammation can result in the release of an overabundance of cytokines and enzymes, potentially causing the destruction of surrounding healthy tissue, exacerbating pain and swelling, and disrupting the normal bone healing process. A chronic inflammatory state may lead to delayed or non-union bone healing by persistently activating osteoclasts, thereby increasing bone resorption and weakening newly formed bone tissue. Neutrophil activity plays a crucial role in regulating the balance between inflammation, antibacterial defense, and tissue repair (Figure 2).
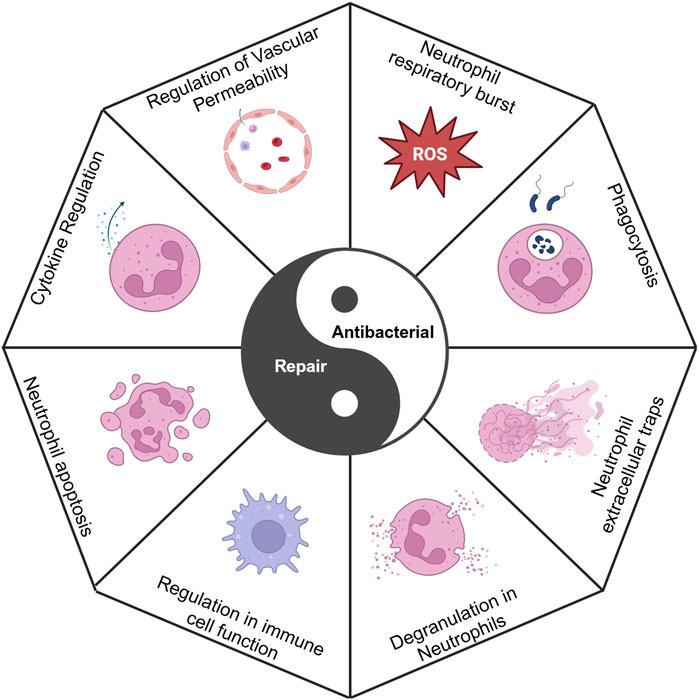
Figure 2. Antibacterial activity and repair maintain a yin-yang balance, in which neutrophils regulate this balance. Created with BioRender.com.
2.1 Cell phagocytosis
Neutrophils exhibit a phagocytic function, enabling them to directly engulf and eliminate bacterial particles and cellular debris. The neutrophil phagosome, a specialized organelle, is formed through the invagination of the plasma membrane, thereby encapsulating the phagocytosed material. The primary energy source for neutrophils is glycolysis (Toller-Kawahisa et al., 2023), with glucose-driven glycolysis specifically supplying the energy required for neutrophil phagocytosis (Borregaard and Herlin, 1982). Subsequently, intracellular lysosomes fuse with phagosomes to form phagolysosomes. Within these phagolysosomes, microorganisms are eradicated through a combination of oxidative and non-oxidative mechanisms. In the oxidative mechanism, neutrophils undergo a respiratory burst, producing substantial quantities of hydrogen peroxide (H2O2) (Iyer et al., 1961). Myeloperoxidase (MPO) then utilizes H2O2 to oxidize chloride ions, generating hypochlorous acid (HOCl), which subsequently damages the bacterial cell membrane and cell wall (Pattison et al., 2012). In the non-oxidative mechanism, neutrophils eliminate microorganisms within the phagolysosome through the release of granule proteins, including MPO, lysozyme, and defensins (Aratani, 2018).
2.2 Neutrophil extracellular traps
NETs are structures formed by activated neutrophils that capture and neutralize extracellular pathogens through the release of extracellular trapping components. These components include nuclear materials such as DNA and histones, as well as intracellular granule proteins like neutrophil elastase (NE) and MPO, along with antimicrobial peptides (Wang H. et al., 2024). NETs consist of a DNA backbone intertwined with various proteins, creating a network structure that entraps and restricts the mobility of pathogens. Following entrapment, antibacterial proteins such as MPO and elastase within the NETs can degrade cell walls or cell membranes of the captured pathogens. NETs are categorized into NADPH oxidase (NOX)-dependent and NOX-independent types based on their formation mechanisms. NOX-dependent NETs primarily depend on the activation of NOX to enhance the production of superoxide and reactive oxygen species (ROS). These active substances can induce the degranulation of neutrophil granule proteins, such as MPO and NE, which subsequently cause cytoskeletal rearrangement and nuclear membrane rupture, ultimately resulting in the release of NETs (Feitz et al., 2021). Factors such as lipopolysaccharide (LPS), IL-6, IL-8, TNF-α, various pathogens, and chemicals have been shown to trigger NOX-dependent NETs (Fousert et al., 2020). In contrast, during the formation of NOX-independent NETs, NETs are predominantly released through nuclear membrane blistering and vesicle transport mechanisms (Kaplan and Radic, 2012). Certain bacteria are capable of inducing neutrophils to undergo NOX-dependent NETs. In contrast, Candida albicans can stimulate neutrophils to inhibit fungal dissemination through the formation of Dectin-2-mediated, NOX-independent NETs. Additionally, LPS from Gram-negative intracellular bacteria activates a caspase-11-dependent pathway that cleaves the gasdermin D protein, compromising plasma membrane integrity and facilitating the release of NETs (Liang et al., 2021).
2.3 Degranulation
Neutrophils encompass a diverse array of granules, which are categorized into primary granules, specific granules, tertiary granules, and secretory vesicles based on their distinct characteristics and constituent substances. Primary granules, in particular, are enriched with the most toxic mediators within the cells, including key components such as MPO, NE, cathepsin G, and defensins. Specific granules contain elements like lactoferrin and lysosomes. Tertiary granules primarily serve as storage sites for metalloproteinases, including gelatinase and leukolysin (Teng et al., 2017). The release of proteases and peptidases into phagolysosomes or through exocytosis to exert antibacterial effects is termed the degranulation process, which is crucial for the non-oxidative sterilization function of neutrophils. NE can directly disrupt the membrane structure of Gram-negative bacteria, such as Escherichia coli, leading to bacterial death (Teng et al., 2017). Additionally, lactoferrin not only binds iron, thereby reducing bacterial iron absorption and inhibiting bacterial growth, but also binds to the lipopolysaccharide of the bacterial cell wall, resulting in bacterial oxidation and cleavage (Telang, 2018).
2.4 Neutrophil respiratory burst
ROS are predominantly generated as byproducts within the electron transport chain of mitochondria, particularly during the oxidative phosphorylation processes at complex I and complex III (Zhao et al., 2019). In the endoplasmic reticulum, the presence of misfolded proteins or the accumulation of unfolded proteins can disrupt the redox balance and alter Ca2+ levels, subsequently inducing ROS production (Ong and Logue, 2023). Ionizing radiation has the capacity to generate ROS, which subsequently target biological macromolecules such as DNA and proteins, leading to cellular damage (Yang et al., 2021) (Figure 3A). Typically, these ROS are intracellularly converted to hydrogen peroxide (H2O2) by superoxide dismutase and ultimately rendered harmless as H2O by catalase (Alfonso-Prieto et al., 2009). However, in neutrophils, the majority of ROS are actively produced through the activation of the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase complex. ROS exhibit significant oxidative potential, capable of inflicting damage on bacterial cell membranes, proteins, and nucleic acids, thereby leading to bacterial death or inactivity (Vatansever et al., 2013).
Figure 3. ROS regulates neutrophil activity to regulate inflammatory processes. (A) ROS mainly include Peroxide, hydrogen peroxide, Hydroxyl radical, and Superoxide, and these ROS can be produced mainly by ionizing radiation, endoplasmic reticulum stress, NOX, and mitochondria. (B) When the pathogen is phagocytosed by neutrophils, phagosomes are formed, and then phagosomes fuse with intracellular lysosomes to form phagolysosomes. In phagolysosomes, ROS produced by NOX2 is converted into H2O2, and under the action of MPO, it is converted into HOCl with strong antibacterial ability with Cl−. (C) ROS plays an important role in inducing apoptosis. ROS can activate the MAPK pathway or promote the release of cytochrome c from mitochondria, and then activate Caspase-9 and Caspase-3 and induce apoptosis. ROS is able to activate the PERK-eIF2α-ATF4 signaling pathway and induce apoptosis. (D) ROS can cause DNA damage in the nucleus, and can induce the formation of NETs in subsequent DNA repair. Created with BioRender.com.
ROS not only directly eliminates bacteria but also modulates various biological functions of neutrophils, thereby influencing inflammatory processes (Lennicke and Cochemé, 2021). Phagolysosomes, organelles characterized by their oxidative and lytic properties, rely on ROS generated by NADPH oxidase for bacterial eradication (Figure 3B). Furthermore, neutrophil apoptosis plays a crucial role in maintaining homeostasis in vivo and facilitates the resolution of inflammation (Karmakar et al., 2021). Excessive production of ROS can result in mitochondrial damage and alterations in mitochondrial membrane potential, subsequently facilitating the release of cytochrome C. This release interacts with apoptotic protease-activating factor 1 to form apoptotic bodies, which then activate cysteine proteases, including caspase-9, thereby initiating the apoptotic pathway (Geering and Simon, 2011). ROS have been demonstrated to activate c-Jun N-terminal kinase and p38 mitogen-activated protein kinase (MAPK), further promoting apoptosis (Zhang et al., 2015). Furthermore, the ROS-mediated PERK-eIF2α-ATF4 pathway is crucial in modulating CHOP-DR5 signaling and subsequent cell apoptosis (Liu and Zhang, 2020) (Figure 3C). Additionally, neutrophil oxidative respiratory bursts are implicated in the formation of NETs. The excessive ROS produced during neutrophil activation leads to significant DNA damage, which subsequently triggers NETs through the DNA repair pathway (Azzouz et al., 2021) (Figure 3D).
3 Pro-inflammatory effects of neutrophils
Neutrophils play a crucial role in mediating inflammatory responses and possess antibacterial capabilities to combat foreign pathogens. However, this inflammatory response can act as a double-edged sword; if not properly regulated, it can result in detrimental effects on host tissues. While an appropriate inflammatory response is beneficial for bacterial resistance and tissue repair, excessive or prolonged inflammation can inflict damage on normal tissues and impede subsequent repair processes (Figure 4A).
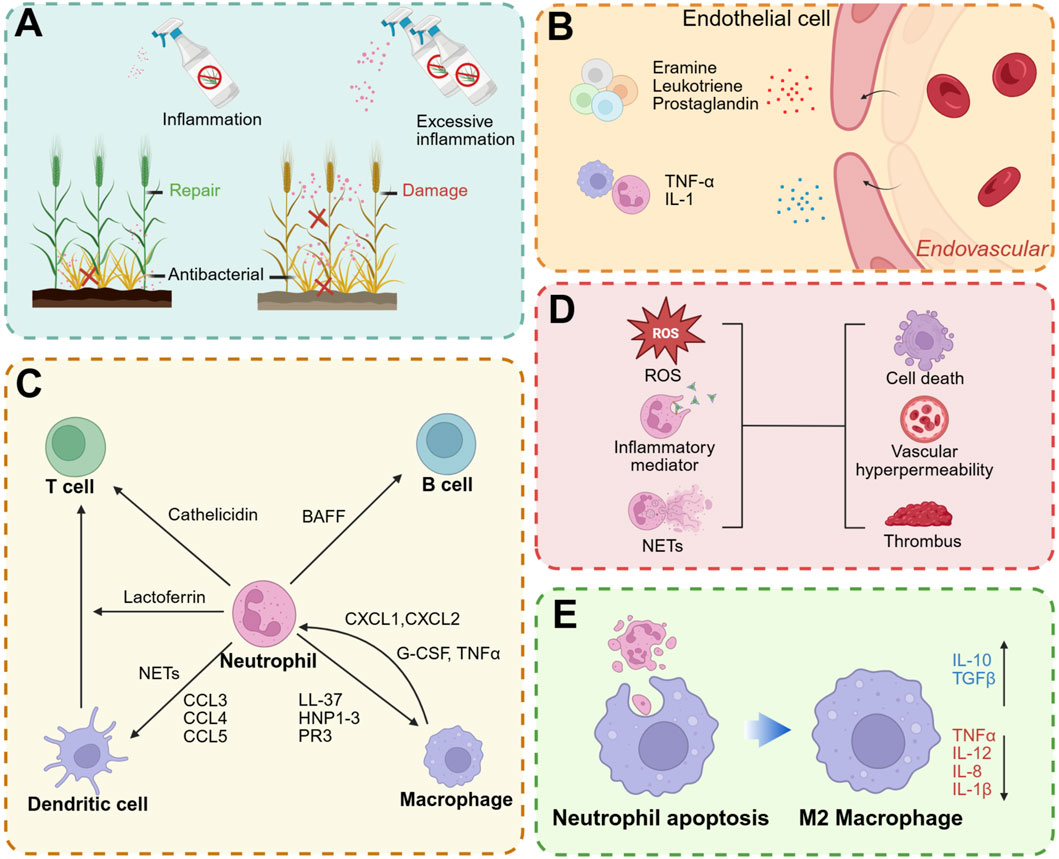
Figure 4. Effect of neutrophils on inflammation. (A) An appropriate inflammatory response is beneficial to fight pathogens and promote tissue repair, while an excessive immune response can lead to tissue damage. (B) Neutrophils can release inflammatory mediators to promote vascular permeability. (C) Interaction between neutrophils and other immune cells. (D) Overactivated neutrophils can also cause damage to normal tissues through ROS, inflammatory mediators and NETs. (E) After uptake of neutrophil apoptotic bodies, it can promote macrophage polarization to M2 type: anti-inflammatory factors such as IL-10 and TGFβ are upregulated, while pro-inflammatory factors such as TNFα, IL-12, IL-1β and IL-8 are downregulated. Created with BioRender.com.
3.1 Increased vascular permeability and chemotaxis
The initial phase of inflammation is characterized by vasodilation, enhanced blood flow, and increased vascular permeability. The release of histamine and leukotrienes from mast cells induces vasodilation and tissue fluid exudation. Concurrently, neutrophils release inflammatory mediators, including IL-1, IL-6, and TNF-α, which further promote vasodilation (Anzai et al., 2015) and facilitate the extravasation of leukocytes and plasma to the site of injury or infection (Figure 4B). Furthermore, neutrophils generate LTB4 and chemokines, which facilitate the recruitment of additional neutrophils to the site of inflammation (Ng et al., 2011). Upon arrival at the inflammatory site, neutrophils further recruit monocytes by secreting LL-37, cathepsin G, human neutrophil peptides 1-3 (HNP1-3), and protease 3 (PR3), thereby amplifying the inflammatory response (Soehnlein et al., 2008; McDonald et al., 2010).
3.2 Affecting immune cells
The immune system comprises a diverse array of immune cells that collaborate to achieve precise mutual regulation through a complex signaling network (Lokwani et al., 2024). As pivotal effector cells within the innate immune system, neutrophils not only directly engage in pathogen clearance but also modulate the function and activity of other immune cells by releasing cytokines, thereby further orchestrating the adaptive immune response (Herrero-Cervera et al., 2022; Scapini and Cassatella, 2014). Neutrophils, lymphocytes, macrophages, dendritic cells (DCs), and other immune cells dynamically interact during the immune response to collectively maintain immune equilibrium and homeostasis (Figure 4C). B cell helper neutrophils localize to the peri-follicular regions of the human spleen under homeostatic conditions, where they express B-cell activating factor. These neutrophils actively contribute to the survival, maturation, and differentiation of B cells, thereby facilitating the development of humoral immunity (Costa et al., 2019; Puga et al., 2011). Neutrophil-derived cathelicidin facilitates the differentiation of T cells towards the Th17 phenotype and enhances their survival (Minns et al., 2021). Soluble mediators secreted by memory T cells, along with direct cell-cell interactions between neutrophils and T cells, collectively promote the acquisition of antigen-presenting capabilities by neutrophils, thereby augmenting their role in adaptive immunity (Lin and Loré, 2017). Furthermore, neutrophils facilitate the recruitment of DCs through the secretion of chemokines such as CCL3, CCL4, CCL5, and CCL20, while the release of NETs stimulates plasmacytoid DCs to secrete inflammatory cytokines (Schuster et al., 2013). The activation of neutrophils to release their granular contents can modulate DC activity and consequently influence T cell function (Hafkamp et al., 2021). Additionally, the secretion of lactoferrin by neutrophils enhances the T cell stimulatory capacity of DCs (de la Rosa et al., 2008). Neutrophils collaborate with macrophages to augment their antibacterial activity (Ladero-Auñon et al., 2021). Macrophages facilitate the recruitment of neutrophils to inflamed sites through the secretion of chemoattractants such as CXCL1 and CXCL2, and they release granulocyte-macrophage colony-stimulating factors (GM-CSF, G-CSF) and TNFα to inhibit apoptosis (Prame Kumar et al., 2018). Simultaneously, neutrophils are capable of releasing LL-37, HNP1-3, and PR3, which serve to attract monocytes and subsequently amplify the inflammatory response. In response to pathogenic challenges, neutrophil-released NETs promote the polarization of macrophages towards the pro-inflammatory M1 phenotype (Tan et al., 2024). Conversely, following neutrophil apoptosis, the resultant apoptotic bodies facilitate the polarization of macrophages towards the anti-inflammatory M2 phenotype (Kraynak et al., 2020; Kraynak et al., 2022).
3.3 Damaged tissue
Neutrophils are capable of releasing a diverse array of inflammatory mediators, including proteases, ROS, cytokines, and chemokines. While these mediators play a crucial role in the body’s defense against pathogenic invasion, their excessive release can result in detrimental effects, such as the disruption of tissue architecture and increased vascular permeability (Saffarzadeh et al., 2012). This hyperactivity can further recruit additional immune cells, thereby exacerbating the inflammatory response. Specifically, neutrophil-derived proteases have the potential to degrade the extracellular matrix and compromise cellular structures, culminating in tissue damage (Cartwright et al., 2024). Furthermore, ROS produced by neutrophils can inflict damage on lipids, proteins, and DNA of normal cells, leading to cellular dysfunction and death (Lennicke and Cochemé, 2021; Ma et al., 2024). While NETs are capable of capturing and eliminating pathogens, they also play a role in the pathogenesis of various diseases (Chamardani and Amiritavassoli, 2022; He et al., 2023; Katsoulis et al., 2024; Leffler et al., 2012). Owing to their unique network structure, NETs provide an effective scaffold for thrombosis (Fuchs et al., 2010). The resultant thrombi can exacerbate tissue damage (Le et al., 2022; Thakur et al., 2023; Mereweather et al., 2023) (Figure 4D).
3.4 Prolonging the duration of inflammation
The regression of neutrophils from the site of inflammation is primarily mediated through macrophage endocytosis or their return to vascular circulation via reverse migration (de Oliveira et al., 2016; Greenlee-Wacker, 2016). Upon neutrophil apoptosis, low concentrations of nucleotides such as ATP and UTP, which can be released by the cells, act as “Find-me” signals. These nucleotides bind to P2Y2 purinergic receptors on macrophages, facilitating their recognition and subsequent phagocytosis by macrophages (Leist et al., 1997; Elliott et al., 2009). Following apoptosis, the formation of granular shedding or autophagosomes leads to the creation of apoptotic bodies. These apoptotic bodies are primarily composed of extracellular vesicles containing cytoplasm, organelles, and nuclear debris. Subsequently, macrophages phagocytose these apoptotic bodies, a process that promotes macrophage polarization towards the M2 phenotype (Liu et al., 2024). This polarization promotes the production of anti-inflammatory cytokines such as IL-10 and TGFβ, while simultaneously reducing the levels of pro-inflammatory cytokines including TNFα, IL-1β, IL-8 and IL-12 (Fadok et al., 1998; Xu et al., 2025) (Figure 4E). Engineered neutrophil apoptotic bodies mitigate myocardial infarction by facilitating macrophage endocytosis and promoting the resolution of inflammation (Bao et al., 2022). In the absence of rapid clearance, apoptotic neutrophils have the potential to form Gasdermin E pores, activate Peptidylarginine deiminase 4, and induce the release of NETs from apoptotic cells (Zhu et al., 2023).
Neutrophil apoptosis constitutes a fundamental physiological mechanism that facilitates the resolution of inflammation and promotes tissue repair (Karmakar et al., 2021). Nonetheless, the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF) (Yamasawa et al., 2004), Type-1 interferons (Aga et al., 2018), IL-8 (Porter et al., 2017), bacterial components (Miralda et al., 2022), hypoxic conditions (Porter et al., 2017; Dölling et al., 2022), and various other pro-inflammatory mediators can extend neutrophil lifespan, thereby contributing to a sustained inflammatory response. For instance, both Anaplasma phagocytophilum and Chlamydia pneumoniae have been shown to enhance the phosphorylation of p38 MAPK, activate the PI3K/Akt signaling pathway, and sustain the expression of the anti-apoptotic protein Mcl-1, thereby leading to delayed neutrophil apoptosis (Sarkar et al., 2012; Sarkar et al., 2015). Conversely, hypoxia-inducible factors HIF-1α and HIF-2α can extend the lifespan of neutrophils in vivo under hypoxic conditions (Thompso et al., 2014; Walmsley et al., 2005). The inhibition of neutrophil apoptosis consequently promotes the inflammatory response (Wang et al., 2021). The sustained presence and activation of neutrophils in chronic inflammation can perpetuate the inflammatory response, disrupt the equilibrium of tissue repair and regeneration processes, and ultimately result in tissue fibrosis and the onset of other chronic diseases.
4 Effect of neutrophils on osteogenesis
Fracture repair is a multifaceted and systematically regulated dynamic process. Typically, fracture healing is categorized into three distinct stages: the hematoma formation stage, the callus formation stage, and the callus remodeling stage (Figure 5A). Upon the occurrence of a fracture, blood vessels within the bone tissue and bone marrow are disrupted, leading to hemorrhage and the subsequent formation of a hematoma at the fracture site. This hematoma serves as a scaffold for subsequent tissue growth and repair, facilitated by the formation of a fibrin network within the hematoma. The initial phase of local inflammation is characterized by the rapid formation of a hematoma, which serves as a provisional scaffold and actively recruits immune cells, including neutrophils and monocytes, among others (Kolar et al., 2010). The early recruitment of neutrophils is essential for effective fracture repair (Eming et al., 2007; Xing et al., 2010; Gru et al., 1993). Within 12 h following bone injury, neutrophils are recruited to the hematoma site, where they secrete cytokines to attract additional inflammatory cells and initiate the healing cascade (Schmidt-Bleek et al., 2012). Cai et al. (Cai et al., 2021) investigated the effects of IL-8 loaded onto a gelatin sponge, which was subsequently implanted into the thigh muscle pocket of mice. Flow cytometry analysis revealed that neutrophils were the initial cell type recruited to the implantation site, with their numbers peaking on day 1. Recruitment of bone marrow-derived mesenchymal stem cells (BMSCs) commenced on day 2, reaching a maximum between days 4 and 5. Notably, as neutrophil numbers declined, the accelerated recruitment of BMSCs contributed to the enhancement of the bone healing process (Figure 5B).
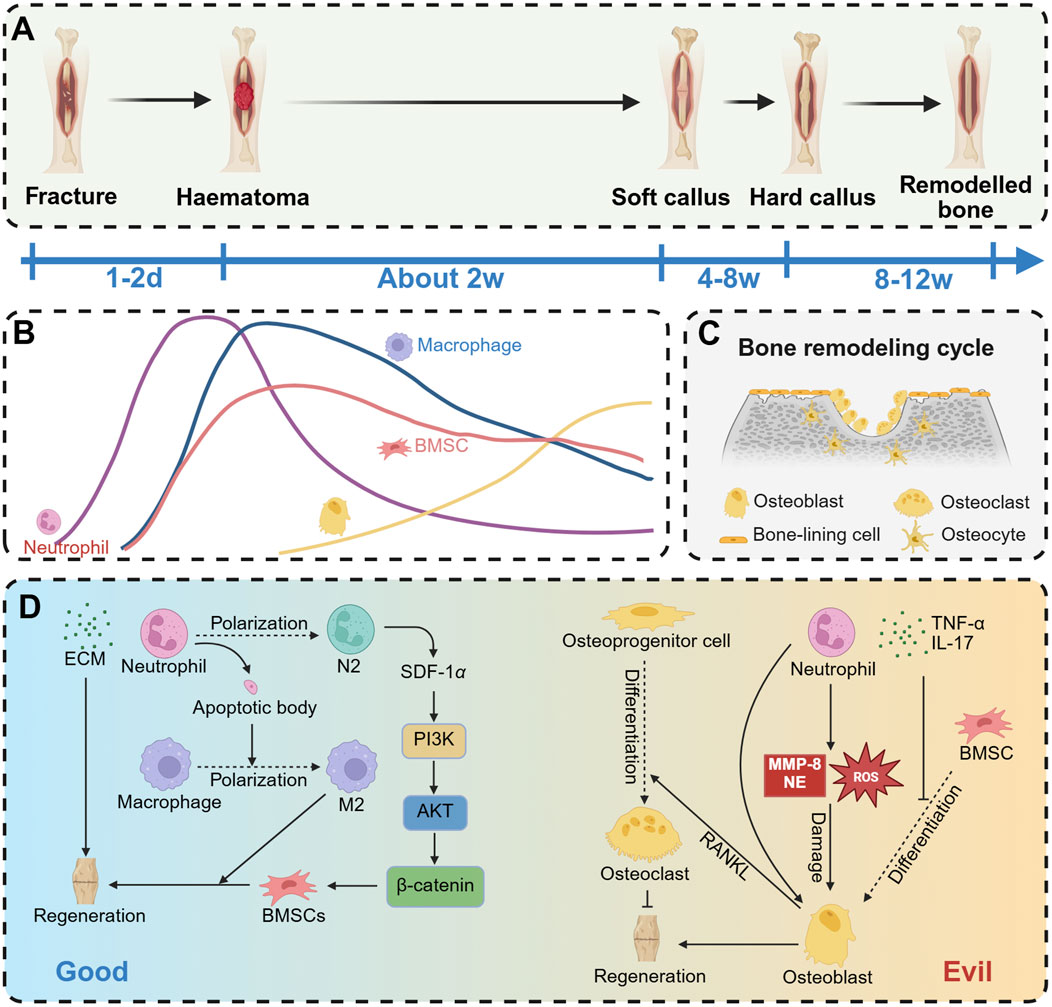
Figure 5. Effect of neutrophils on osteogenesis. (A) The process of fracture repair mainly includes hematoma organization phase, callus formation phase and bone remodeling phase. (B) Aseptic inflammation is caused by fracture occurrence. Early recruitment of neutrophils to the inflammatory site reaches its peak, followed by a decrease in neutrophil numbers within 1–2 days, and then osteoblasts begin to appear. (C) In the late stage of fracture healing, bone remodeling mainly dominates, with osteoblasts and osteoclasts maintaining a delicate balance. (D) Neutrophils have two opposing effects on osteogenesis. On the one hand, they can promote ECM secretion to enhance osteogenesis, or facilitate osteogenesis by polarizing into N2 neutrophils or inducing macrophage polarization to M2. On the other hand, neutrophils can promote the differentiation of osteoprogenitor cells into osteoclasts, release inflammatory mediators such as TNFα and IL-17 (which inhibit BMSC differentiation into osteoblasts), or directly damage osteoblasts by releasing MMP-8, NE, or ROS. Created with BioRender.com.
The bone remodeling cycle comprises three sequential phases: osteoclast-dominated bone resorption, bone reversal mediated by osteoclast-osteoblast interaction, and osteoblast-dominated bone formation and mineralization (Bolamperti et al., 2022) (Figure 5C). Osteoclasts, which originate from myeloid progenitors in the bone marrow, are multinucleated giant cells formed through the fusion of mononuclear macrophages (Takegahara et al., 2024). Under conditions of chronic inflammation, neutrophils have the capacity to induce osteoclast formation (Moonen et al., 2019). NETs facilitate bone erosion in rheumatoid arthritis by augmenting RANKL-induced osteoclastogenesis (Schneider et al., 2024; Guilherme Neto et al., 2023). Neutrophils contribute to the upregulation of RANKL expression by osteoblasts and promote the differentiation of osteoclast progenitor cells into mature osteoclasts (Schneider et al., 2024). This chronic inflammatory stimulus disrupts the homeostasis between osteoblasts and osteoclasts, resulting in bone loss (Kim et al., 2020).
Neutrophils exhibit a dual role in bone repair, encompassing both beneficial and detrimental aspects (Figure 5D). They are crucial in the initial stages of bone healing and regeneration. During the early inflammatory phase, neutrophils secrete pro-inflammatory mediators, pro-angiogenic growth factors, and osteogenic factors, thereby initiating and triggering the bone regeneration cascade (Chung et al., 2006; Shu et al., 2024; Wang L. et al., 2024). Additionally, neutrophils rapidly synthesize fibronectin and extracellular matrix components to promote fracture healing (Bastian et al., 2016). Neutrophils release cytokines, including SDF-1 (Cai et al., 2021; Kitaori et al., 2009), TNF-α (Böcker et al., 2008), and CXCL7 (Almeida et al., 2016), which facilitate the homing of mesenchymal stem cells (MSCs) to fracture healing sites (Wang L. et al., 2024). Additionally, neutrophils upregulate angiogenic markers such as vascular endothelial growth factor (VEGF), CD34, and FGF-2 to promote angiogenesis (Ardi et al., 2009; Herath et al., 2018), a critical process for subsequent bone repair (Zhu et al., 2020; Saberi et al., 2023). Furthermore, neutrophils modulate immune responses to support bone repair processes. Neutrophil apoptotic bodies have been shown to induce macrophage polarization towards the M2 phenotype, thereby facilitating tissue repair (Bao et al., 2022; Martin et al., 2015). Additionally, N2-type neutrophils play a crucial role in directing the recruitment of bone mesenchymal stem cells and initiating bone regeneration (Cai et al., 2021).
Although neutrophils are indispensable defenders during the initial stages of immune response, their prolonged presence can result in persistent inflammation, delayed fracture healing, and additional damage to the organism (Kovach et al., 2015; Ando et al., 2024). Neutrophils exert antibacterial effects by releasing collagenase, elastase, hydrogen peroxide, and hypochlorous acid, which are effective in bacterial eradication. However, these active substances can also inflict damage on tissues and impede the repair process (Bastian et al., 2018). ROS produced by neutrophils have been shown to induce apoptosis in MSCs and osteoblasts (Singh et al., 2012). Additionally, senescent neutrophils secrete significant amounts of granulocalcin, which disrupts the balance between osteogenesis and adipogenesis in BMSCs (Li et al., 2021). Neutrophils also inhibit the synthesis of mineralized extracellular matrix by human bone marrow-derived stromal cells in vitro (Bastian et al., 2018). Furthermore, activated neutrophils can both directly and indirectly promote osteoclastogenesis, thereby disrupting bone homeostasis (Ponzetti and Rucci, 2019).
5 Implant-driven neutrophil modulation promotes antibacterial activity and bone healing
Although bone scaffolds are biocompatible, their implantation induces an in vivo immune response, typically manifesting as an aseptic inflammatory reaction unless subsequently compromised by pathogens (Jhunjhunwala et al., 2015). Neutrophils are pivotal in mediating the aseptic inflammatory response elicited by biomaterials. They act swiftly to establish an acute inflammatory state through mechanisms such as degranulation, chemokine release, and phagocytosis, which are integral to the immediate immune response following biomaterial implantation. Given the pivotal role of neutrophils in aseptic inflammation induced by biomaterials, researchers have initiated investigations into optimizing scaffold design by modulating neutrophil activity. An emerging strategy involves the development of biomaterial scaffolds that can either enhance neutrophil antibacterial functions or regulate immune responses. By employing specific surface modifications, the controlled release of biologically active molecules, or the improvement of morphology and structure, these scaffolds can augment the phagocytic capacity and secretory functions of neutrophils, thereby fostering a more favorable environment for the host to combat infection and promote bone repair at the implantation site (Table 1).
5.1 Bioactive factors
Bone scaffolds serve as carriers capable of incorporating various components, including pharmaceuticals, cytokines, genetic material, and cells, which can be subsequently released at the site of bone defects to fulfill diverse functional roles (Hu et al., 2024) (Figure 6A). The phenotypic switching of neutrophils is regulated by signaling pathways involving TGF-β and IFN-β. Specifically, TGF-β can mediate the conversion of N1 neutrophils to the N2 phenotype (Bu et al., 2022). Exposure of neutrophils to transforming TGF-β or G-CSF results in their polarization towards the N2 phenotype. Conversely, IFN-β has been identified as a pivotal factor in the polarization of neutrophils towards the N1 phenotype in both cancer patients and tumor-bearing mice (Andzinski et al., 2016). N1 neutrophils are capable of upregulating pro-inflammatory mediators such as TNFα, IL-1β, Ccrl2, and Cxcr5. In contrast, N2 neutrophils upregulate anti-inflammatory mediators including IL-4, IL-10, and Cxcr2 (Figure 6B). In a related study, Shi et al. (Shi et al., 2024) developed a genetically engineered composite scaffold by incorporating a hyaluronic acid methacryloyl hydrogel, loaded with a TGF-β1 adenovirus, into a mesoporous bioactive glass scaffold (MBG). Among these, TGF-β1 has been shown to regulate the polarization of neutrophils towards the N2 phenotype and macrophages towards the M2 phenotype through a “relay” mechanism, thereby exerting a reparative effect. Xu et al. (2023) utilized the electrospinning technique to prepare PCL/LAP composites, employing polycaprolactone (PCL) and laponite (LAP) as the constituent materials. The supernatant derived from the co-culture of periodontal ligament cells and a nanofiber membrane has been shown to downregulate the expression of classical pro-inflammatory genes while upregulating the expression of anti-inflammatory and pro-remodeling genes in neutrophils. This suggests a potential role in inducing the polarization of neutrophils towards an anti-inflammatory and pro-remodeling N2 phenotype.
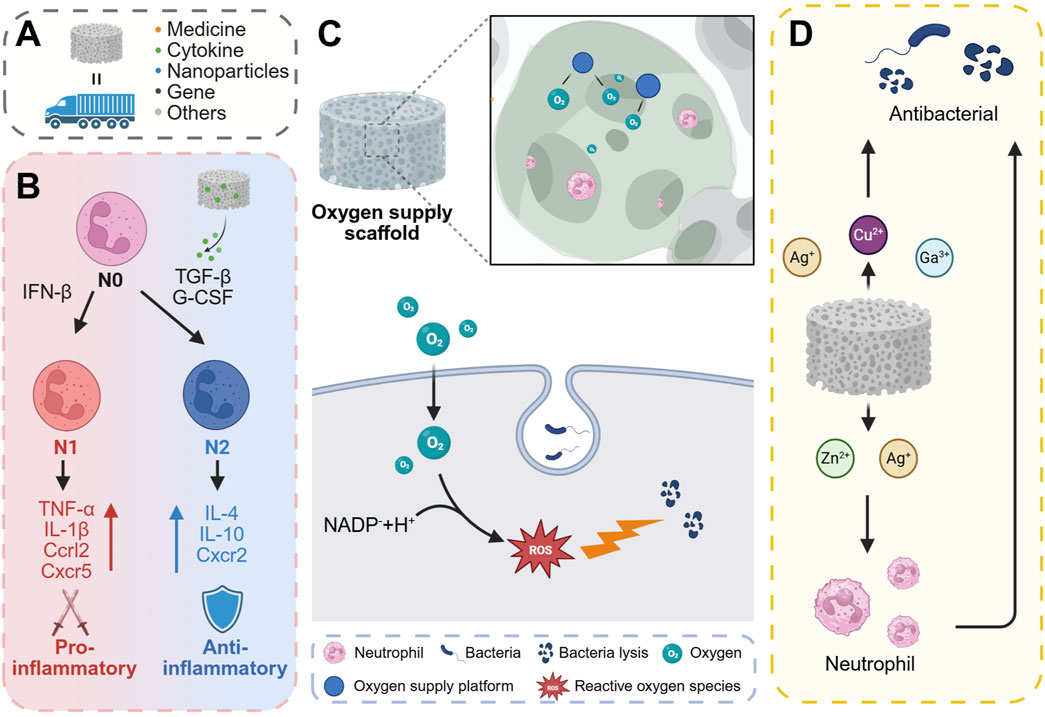
Figure 6. Regulation of neutrophil antibacterial activity or osteogenic function by changing the chemical composition of scaffolds. (A) The scaffold can carry drugs, active factors, nanoparticles, genes, and the like as a carrier. (B) N0-type neutrophils can be classified as N1 and N2, where N1 is predominantly pro-inflammatory and N2 is predominantly anti-inflammatory. (C) The oxygen supply platform in the scaffold can provide oxygen for neutrophil oxidative respiration to promote the antibacterial effect of neutrophils. (D) Certain metal ions in the scaffold, such as Ag+, Cu2+ and Ga3+, can directly kill bacteria. At the same time, some metal ions such as Zn2+ and Ag+ can indirectly kill bacteria by enhancing the bactericidal effect of neutrophils. Created with BioRender.com.
5.2 Building oxygen generation and carrying systems
The neutrophil oxidative respiratory burst is crucial for inflammation and antimicrobial activity, consuming large amounts of oxygen to produce superoxide radicals. Activated neutrophils experience increased oxygen consumption during an “oxygen burst” facilitated by NOX2, generating two superoxide radicals per NADPH molecule and two oxygen molecules (Figure 6C). Fractures often damage bone blood vessels, creating a hypoxic microenvironment around the fracture site. Hypoxic conditions can impair ROS synthesis in neutrophils, thereby diminishing their bactericidal capabilities (Beebout et al., 2022). Additionally, hypoxia can inhibit neutrophil apoptosis and extend the inflammatory phase in vivo (Rani Talla et al., 2016), which may result in delayed fracture healing (Claes et al., 2012). Consequently, early oxygen supplementation is crucial for both bacterial defense and tissue repair. Enhancing neutrophil antibacterial activity through oxygen delivery has emerged as a strategic approach in the design of effective antibacterial materials. Currently, materials that produce oxygen are utilized to construct oxygen supply platforms. These include the use of solid or liquid peroxides (e.g., CaO2, H2O2) to generate oxygen, or the transportation and release of oxygen via carriers such as fluorinated compounds and erythrocyte membranes. Chu et al. (Chu et al., 2024) introduced an innovative approach by employing in-situ deposition of nano-CaO2 on the surface of implants to provide localized oxygen support. This method demonstrated a peak oxygen release at 4 h, with sustained release lasting up to 24 h. The CaO2/PA-Zn@TiNPs scaffolds were fabricated using a layer-by-layer deposition technique on the surface of TiO2 nanopillars, which were pre-coated with a PA-Zn2+ coordination complex, followed by the in-situ deposition of nano CaO2. These scaffolds demonstrated the capability to restore oxygen-dependent neutrophil bactericidal function and support neutrophil ROS production under hypoxic conditions. Additionally, they promoted neutrophil apoptosis, thereby mitigating the subsequent inflammatory response. Zhu et al. (2024) developed a nanomedicine, designated as Hb-Naf@RBCM NPs, by encapsulating red blood cell membrane (RBCM) with nanoparticles formed through the self-assembly of naftifen (Naf) and oxygenated hemoglobin (Hb). The Hb-Naf@RBCM NPs demonstrated the capability to stimulate neutrophils to release superoxide anions and enhance intracellular ROS production, thereby augmenting the neutrophils' response to Staphylococcus aureus, multidrug-resistant S. aureus, and methicillin-resistant S. aureus.
5.3 Addition of metal element
Metal ions, including strontium, copper, zinc, silver, magnesium, and iron, are extensively utilized in the design of bone scaffolds due to their ability to regulate the functional balance of osteoblasts and osteoclasts, as well as to promote bone angiogenesis (Luo et al., 2023; Bosch-Rué et al., 2023; O'Neill et al., 2018; Tao et al., 2024; Qian et al., 2021a; Liu et al., 2023; Wang N. et al., 2025). Additionally, these metal ions can influence bacterial metabolic activity and exhibit bactericidal properties (Lemire et al., 2013; Qian et al., 2021b). Specifically, zinc and copper ions have been shown to interfere with bacterial metabolic processes and DNA replication (Wei et al., 2022). Gallium ions (Ga3+) interfere with bacterial iron metabolism due to their chemical similarity to iron ions (Fe3+), thereby exerting antibacterial and antibiofilm effects (Kaneko et al., 2007). Furthermore, metal nanoparticles, such as those composed of silver and copper, can directly damage bacterial cell walls. These nanoparticles induce the production of ROS, which attack bacterial cell membranes and proteins, ultimately leading to cell death. Additionally, metal ions are crucial components of the body’s immune system (Wang et al., 2020). Metal ions play a pivotal role in regulating various aspects of the immune response and are intricately linked to the pathogenesis and progression of numerous diseases. Specifically, ions such as calcium (Ca), zinc (Zn), manganese (Mn), and magnesium (Mg) are integral to immune signal transduction processes, functioning as second messengers to activate immune cells (Chaigne-Delalande and Lenardo, 2014; Subramanian Vignesh and Deepe, 2016; Krzywoszyńska et al., 2020). Additionally, iron (Fe) and copper (Cu) serve as essential cofactors in the active center of NADPH oxidase, which is critical for the generation of reactive oxygen species (Wu et al., 2020). Iron and zinc chelators have been shown to modulate NETs release (Kuźmicka et al., 2021). Furthermore, an excess of iron, whether due to congenital factors or a high-iron diet, has been found to reduce the release of extracellular traps and reactive oxygen species (Kuźmicka et al., 2022). The release of silver ions (Ag+) and zinc ions (Zn2+) has been observed to stimulate immune function, leading to an increased production of leukocytes and neutrophils, thereby enhancing antibacterial activity (Mao et al., 2017) (Figure 6D). Pure Zn augmented the bactericidal capacity of peri-implant neutrophils, as evidenced by (Peng et al., 2023). Additionally, Zn plays a pivotal role in mediating the formation of NETs (Hasan et al., 2012). Li et al. (2022) conducted a study where they synthesized Zn-doped FeOOH nanolayers via plasma electrolytic oxidation (PEO) on the surface of a Mg alloy and subsequently coated the alloy with these nanolayers. The incorporation of zinc into the PEO-Fe coating markedly augmented the formation of NETs by diminishing the expression of immune evasion factors and promoting the citrullination of histones and intracellular chromatin depolymerization in neutrophils at the infection site, thereby facilitating NETs formation.
5.4 Scaffold structure
Upon in vivo implantation, the scaffold, functioning as an integral component of the extracellular matrix, has the potential to replicate the characteristics of the physiological milieu, thereby influencing cellular behavior (Zhou et al., 2023). The scaffold’s topology, stiffness, and surface topography are critical factors that can modulate neutrophil activity and assume various functional roles. In a study by Won et al. (Won et al., 2020), a hierarchical scaffold incorporating microchannels was fabricated using camphene in a polycaprolactone solution through three-dimensional (3D) printing technology. The internal pore diameter of the scaffold is measured at 12.9 ± 7.69 μm, while the surface pore diameter is 21.1 ± 16.6 μm. Microchannels constitute approximately 28% of the scaffold’s volume. In comparison to 3D printed PCL scaffolds of identical chemical composition but lacking microchannels, the microchannel-incorporated scaffolds demonstrated a significant reduction in NETs, facilitated the resolution of inflammation, and ultimately promoted angiogenesis. Additionally, these scaffolds enhanced stem cell recruitment and chemotaxis, thereby fostering osteogenic differentiation in vivo. Allison et al. (Fetz et al., 2017) fabricated electrospun polydioxanone (PDO), type I collagen (COL), and PDO-COL hybrid scaffolds utilizing fibers of small (0.25–0.35 µm) and large (1–2 µm) diameters, observing that larger fiber diameters mitigated the formation of NETs on PDO templates. Furthermore, the pore size of the scaffold can influence the antibacterial efficacy of neutrophils. Mukhopadhyay et al. (2024) demonstrated using a microfluidic device that neutrophils exhibited a significantly enhanced bacterial clearance capability after traversing 5 μm pores compared to 200 μm pores. As neutrophils traverse the pores, their cell membranes experience mechanical stress, which activates the mechanosensor Piezo1. This activation leads to the upregulation of NADPH oxidase 4, thereby augmenting the antibacterial efficacy of polymorphonuclear leukocytes (Figure 7A).
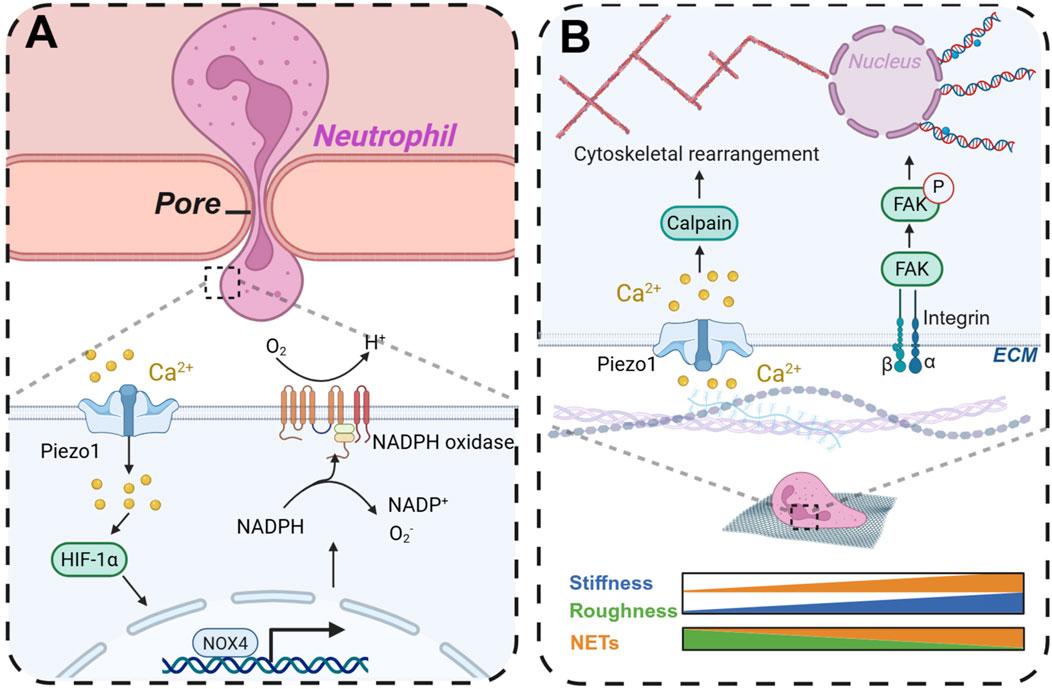
Figure 7. By changing the morphology and structure of scaffolds to regulate neutrophil immunity and thereby modulate antibacterial activity and osteogenic repair. (A) When neutrophils cross the pore, they can promote the production of ROS in neutrophils to enhance antibacterial activity. (B) The scaffold stiffness and surface morphology affect the production of neutrophil NETs. Created with BioRender.com.
5.5 Stiffness
Stiffness denotes the capacity of a material or structure to withstand deformation under the influence of external forces. The stiffness of the ECM is crucial in modulating cellular functions, including viability, communication, migration, and differentiation (Fletcher and Mullins, 2010). Cells can sense ECM stiffness through cell membrane surface receptors and respond accordingly. Additionally, the migration of neutrophils through vascular endothelial cells is influenced by the stiffness of the endothelial cell matrix (Stroka and Aranda-Espinoza, 2011). Neutrophils exhibit reduced migration rates on stiffer substrates but demonstrate enhanced adhesion to rigid surfaces, facilitating their ability to traverse longer distances (Oakes et al., 2009). Abaricia et al. (2021) observed that the formation of NETs was augmented in a stiffness-dependent manner on polydimethylsiloxane (PDMS) substrates with varying physiologically relevant stiffnesses (0.2–32 kPa). Furthermore, the expression of neutrophil chemoattractants and pro-inflammatory factors was elevated on stiffer matrices, a process regulated by integrin/FAK signaling pathways. In a similar vein, Erpenbeck et al. (2019) observed a significant increase in NETs on stiffer polyacrylamide gels following stimulation with LPS, a phenomenon that was attenuated by PI3K inhibition (Figure 7B). Conversely, Jiang et al. (2023) reported that neutrophils exhibited reduced production of ROS and anti-inflammatory cytokines in three-dimensional hydrogel culture systems with higher stiffness. Gelatin methacrylate hydrogels were synthesized with varying degrees of stiffness, revealing that the secretion of pro-inflammatory factors diminished as matrix stiffness increased. Furthermore, the stiffer matrices facilitated the transition of neutrophils to the N2 phenotype, a process regulated by the JAK1/STAT3 signaling pathway.
5.6 Surface topography
The interaction between scaffolds and cells is predominantly influenced by the surface topography, which plays a critical role in cell and tissue organization. Surface topography can be engineered on the scaffold through various techniques such as photolithography (Kurland et al., 2014), 3D printing (Liu et al., 2022; Kim et al., 2021), and deposition methods. Upon in vivo implantation, the scaffold’s surface topography interfaces with cells, thereby modulating cellular behavior and fate (Rabel et al., 2020; Wang et al., 2012). When cells adhere to various topographical features, they undergo deformation, leading to the activation of mechanoreceptors on the cell membrane. These signals are subsequently transmitted to the nucleus via signaling pathways, ultimately resulting in diverse biological responses (Könnig et al., 2018). Specifically, on smooth titanium (Ti) surfaces, neutrophils adopt a rounded morphology, whereas on rough Ti surfaces, they display a spread-out, flattened morphology (Campos et al., 2014). Modulating the surface topography of the scaffold can influence the cell phenotype, transitioning it from a pro-inflammatory to an anti-inflammatory state. The unique morphological structure of the surface, in response to the shear stress generated by cell membrane contact, can mediate calpain activity and cytoskeletal remodeling through the activation of Piezo1, thereby inducing the production of NETs (Baratchi et al., 2024) (Figure 7B). Neutrophils cultured on rough Ti surfaces exhibited a reduction in the production of pro-inflammatory cytokines and enzymes, as well as a decreased formation of NETs, in comparison to neutrophils on smooth Ti surfaces (Abaricia et al., 2020). This attenuation in NET formation is associated with an accelerated resolution of inflammation and enhanced bone formation on Ti implants (Morandini et al., 2023).
6 Conclusion and outlook
In summary, this review primarily focuses on the strategy of neutrophil regulation within bone tissue engineering scaffolds. It aims to modulate non-specific immunity of neutrophils through scaffolds, thereby fostering an immune environment that is favorable for antibacterial activity and tissue repair. In biological organisms, the inflammatory process is typically perceived as a defensive response, whereas the anti-inflammatory process is viewed as a reparative mechanism. Neutrophils are pivotal in regulating both of these processes. Modulating the immune function of neutrophils presents a promising strategy for managing early bacterial infections. Concurrently, neutrophils are also integral to subsequent tissue repair.
Optimizing the physical and chemical properties of scaffolds, regulating the function of neutrophils in antibacterial and osteogenic processes, and achieving a balance between these factors have emerged as significant challenges in scaffold design. While the majority of current tissue engineering research concentrates on either antibacterial efficacy or tissue regeneration, there is a tendency to overlook or inhibit neutrophil activation, or to focus exclusively on eliciting specific macrophage responses. In recent years, the biological function of neutrophils has been progressively elucidated, positioning them as a burgeoning topic in tissue engineering and biomaterials research. Consequently, future investigations into the mechanisms of neutrophils within the biomaterials domain are anticipated to offer novel research avenues for tissue regeneration and immune regulation.
Author contributions
JZ: Conceptualization, Visualization, Writing – original draft. SX: Writing – original draft, Validation. SL: Writing – original draft, Investigation. ZZ: Methodology, Writing – original draft. ML: Project administration, Writing – original draft. HX: Writing – original draft, Software. WK: Software, Writing – original draft. JZ: Writing – review and editing. LX: Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Natural Science Foundation of Jiangxi Province (Nos 20224ACB206012, 20242BAB25453, and 20232BAB216052). The National Science Foundation of China under Grant (No. 32360232), the Ganzhou Science and Technology Innovation Talent Plan (No. 2022-RC1328), the Ganzhou science and technology plan project (2022-YB1395), the Health Commission science and Technology Plan Project of Jiangxi Provincial (202311845), the Jiangxi Graduate Innovation Special Fund (No. YC2025-B186).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abaricia, J. O., Shah, A. H., Musselman, R. M., and Olivares-Navarrete, R. (2020). Hydrophilic titanium surfaces reduce neutrophil inflammatory response and NETosis. Biomater. Sci. 8 (8), 2289–2299. doi:10.1039/c9bm01474h
Abaricia, J. O., Shah, A. H., and Olivares-Navarrete, R. (2021). Substrate stiffness induces neutrophil extracellular trap (NET) formation through focal adhesion kinase activation. Biomaterials 271, 120715. doi:10.1016/j.biomaterials.2021.120715
Aga, E., Mukherjee, A., Rane, D., More, V., Patil, T., van Zandbergen, G., et al. (2018). Type-1 interferons prolong the lifespan of neutrophils by interfering with members of the apoptotic Cascade. Cytokine 112, 21–26. doi:10.1016/j.cyto.2018.06.027
Alfonso-Prieto, M., Biarnés, X., Vidossich, P., and Rovira, C. (2009). The molecular mechanism of the catalase reaction. J. Am. Chem. Soc. 131 (33), 11751–11761. doi:10.1021/ja9018572
Almeida, C. R., Caires, H. R., Vasconcelos, D. P., and Barbosa, M. A. (2016). NAP-2 secreted by human NK cells can stimulate mesenchymal stem/stromal cell recruitment. Stem Cell Rep. 6 (4), 466–473. doi:10.1016/j.stemcr.2016.02.012
Anders, H. J., and Schaefer, L. (2014). Beyond tissue injury-damage-associated molecular patterns, toll-like receptors, and inflammasomes also drive regeneration and fibrosis. J. Am. Soc. Nephrol. 25 (7), 1387–1400. doi:10.1681/asn.2014010117
Ando, Y., Tsukasaki, M., Huynh, N.C.-N., Zang, S., Yan, M., Muro, R., et al. (2024). The neutrophil-osteogenic cell axis promotes bone destruction in periodontitis. Int. J. Oral Sci. 16 (1), 18. doi:10.1038/s41368-023-00275-8
Andzinski, L., Kasnitz, N., Stahnke, S., Wu, C.-F., Gereke, M., von Köckritz-Blickwede, M., et al. (2016). Type I IFNs induce anti-tumor polarization of tumor associated neutrophils in mice and human. Int. J. Cancer 138 (8), 1982–1993. doi:10.1002/ijc.29945
Anzai, A., Shimoda, M., Endo, J., Kohno, T., Katsumata, Y., Matsuhashi, T., et al. (2015). Adventitial CXCL1/G-CSF expression in response to acute aortic dissection triggers local neutrophil recruitment and activation leading to aortic rupture. Circ. Res. 116 (4), 612–623. doi:10.1161/circresaha.116.304918
Aratani, Y. (2018). Myeloperoxidase: its role for host defense, inflammation, and neutrophil function. Arch. Biochem. Biophys. 640, 47–52. doi:10.1016/j.abb.2018.01.004
Ardi, V. C., Van den Steen, P. E., Opdenakker, G., Schweighofer, B., Deryugina, E. I., and Quigley, J. P. (2009). Neutrophil MMP-9 proenzyme, unencumbered by TIMP-1, undergoes efficient activation in vivo and catalytically induces angiogenesis via a basic fibroblast growth factor (FGF-2)/FGFR-2 pathway. J. Biol. Chem. 284 (38), 25854–25866. doi:10.1074/jbc.m109.033472
Avery, D., Morandini, L., Celt, N., Bergey, L., Simmons, J., Martin, R. K., et al. (2023). Immune cell response to orthopedic and craniofacial biomaterials depends on biomaterial composition. Acta Biomater. 161, 285–297. doi:10.1016/j.actbio.2023.03.007
Azzouz, D., Khan, M. A., and Palaniyar, N. (2021). ROS induces NETosis by oxidizing DNA and initiating DNA repair. Cell Death Discov. 7 (1), 113. doi:10.1038/s41420-021-00491-3
Bao, L., Dou, G., Tian, R., Lv, Y., Ding, F., Liu, S., et al. (2022). Engineered neutrophil apoptotic bodies ameliorate myocardial infarction by promoting macrophage efferocytosis and inflammation resolution. Bioact. Mater 9, 183–197. doi:10.1016/j.bioactmat.2021.08.008
Baratchi, S., Danish, H., Chheang, C., Zhou, Y., Huang, A., Lai, A., et al. (2024). Piezo1 expression in neutrophils regulates shear-induced NETosis. Nat. Commun. 15 (1), 7023. doi:10.1038/s41467-024-51211-1
Bastian, O. W., Koenderman, L., Alblas, J., Leenen, L. P. H., and Blokhuis, T. J. (2016). Neutrophils contribute to fracture healing by synthesizing fibronectin+ extracellular matrix rapidly after injury. Clin. Immunol. 164, 78–84. doi:10.1016/j.clim.2016.02.001
Bastian, O. W., Croes, M., Alblas, J., Koenderman, L., Leenen, L. P. H., and Blokhuis, T. J. (2018). Neutrophils inhibit synthesis of mineralized extracellular matrix by human bone marrow-derived stromal cells in vitro. Front. Immunol. 9, 945. doi:10.3389/fimmu.2018.00945
Beebout, C. J., Robertson, G. L., Reinfeld, B. I., Blee, A. M., Morales, G. H., Brannon, J. R., et al. (2022). Uropathogenic Escherichia coli subverts mitochondrial metabolism to enable intracellular bacterial pathogenesis in urinary tract infection. Nat. Microbiol. 7 (9), 1348–1360. doi:10.1038/s41564-022-01205-w
Böcker, W., Docheva, D., Prall, W. C., Egea, V., Pappou, E., Rossmann, O., et al. (2008). IKK-2 is required for TNF-alpha-induced invasion and proliferation of human mesenchymal stem cells. J. Mol. Med. Berl. 86 (10), 1183–1192. doi:10.1007/s00109-008-0378-3
Bolamperti, S., Villa, I., and Rubinacci, A. (2022). Bone remodeling: an operational process ensuring survival and bone mechanical competence. Bone Res. 10 (1), 48. doi:10.1038/s41413-022-00219-8
Borregaard, N., and Herlin, T. (1982). Energy metabolism of human neutrophils during phagocytosis. J. Clin. Invest 70 (3), 550–557. doi:10.1172/jci110647
Bosch-Rué, È., Díez-Tercero, L., Buitrago, J. O., Castro, E., and Pérez, R. A. (2023). Angiogenic and immunomodulation role of ions for initial stages of bone tissue regeneration. Acta Biomater. 166, 14–41. doi:10.1016/j.actbio.2023.06.001
Brodbeck, W. G., Voskerician, G., Ziats, N. P., Nakayama, Y., Matsuda, T., and Anderson, J. M. (2003). In vivo leukocyte cytokine mRNA responses to biomaterials are dependent on surface chemistry. J. Biomed. Mater Res. A 64 (2), 320–329. doi:10.1002/jbm.a.10425
Bu, M. T., Chandrasekhar, P., Ding, L., and Hugo, W. (2022). The roles of TGF-β and VEGF pathways in the suppression of antitumor immunity in melanoma and other solid tumors. Pharmacol. Ther. 240, 108211. doi:10.1016/j.pharmthera.2022.108211
Cai, B., Lin, D., Li, Y., Wang, L., Xie, J., Dai, T., et al. (2021). N2-Polarized neutrophils guide bone mesenchymal stem cell recruitment and initiate bone regeneration: a missing piece of the bone regeneration puzzle. Adv. Sci. (Weinh) 8 (19), e2100584. doi:10.1002/advs.202100584
Campos, V., Melo, R. C. N., Silva, L. P., Aquino, E. N., Castro, M. S., and Fontes, W. (2014). Characterization of neutrophil adhesion to different titanium surfaces. Bull. Mater. Sci. 37 (1), 157–166. doi:10.1007/s12034-014-0611-3
Cartwright, I. M., Zhou, L., Koch, S. D., Welch, N., Zakharov, D., Callahan, R., et al. (2024). Chlorination of epithelial tight junction proteins by neutrophil myeloperoxidase promotes barrier dysfunction and mucosal inflammation. JCI Insight 9 (14), e178525. doi:10.1172/jci.insight.178525
Chaigne-Delalande, B., and Lenardo, M. J. (2014). Divalent cation signaling in immune cells. Trends Immunol. 35 (7), 332–344. doi:10.1016/j.it.2014.05.001
Chamardani, T. M., and Amiritavassoli, S. (2022). Inhibition of NETosis for treatment purposes: friend or foe? Mol. Cell Biochem. 477 (3), 673–688. doi:10.1007/s11010-021-04315-x
Chu, G., Guan, M., Jin, J., Luo, Y., Luo, Z., Shi, T., et al. (2024). Mechanochemically reprogrammed interface orchestrates neutrophil bactericidal activity and apoptosis for preventing implant-associated infection. Adv. Mater 36 (16), e2311855. doi:10.1002/adma.202311855
Chung, R., Cool, J. C., Scherer, M. A., Foster, B. K., and Xian, C. J. (2006). Roles of neutrophil-mediated inflammatory response in the bony repair of injured growth plate cartilage in young rats. J. Leukoc. Biol. 80 (6), 1272–1280. doi:10.1189/jlb.0606365
Claes, L., Recknagel, S., and Ignatius, A. (2012). Fracture healing under healthy and inflammatory conditions. Nat. Rev. Rheumatol. 8 (3), 133–143. doi:10.1038/nrrheum.2012.1
Costa, S., Bevilacqua, D., Cassatella, M. A., and Scapini, P. (2019). Recent advances on the crosstalk between neutrophils and B or T lymphocytes. Immunology 156 (1), 23–32. doi:10.1111/imm.13005
Coughlan, T., and Dockery, F. (2014). Osteoporosis and fracture risk in older people. Clin. Med. (Lond) 14 (2), 187–191. doi:10.7861/clinmedicine.14-2-187
de la Rosa, G., Yang, D., Tewary, P., Varadhachary, A., and Oppenheim, J. J. (2008). Lactoferrin acts as an alarmin to promote the recruitment and activation of APCs and antigen-specific immune responses. J. Immunol. 180 (10), 6868–6876. doi:10.4049/jimmunol.180.10.6868
de Magalhães, J. P. (2025). An overview of contemporary theories of ageing. Nat. Cell Biol. 27 (7), 1074–1082. doi:10.1038/s41556-025-01698-7
de Oliveira, S., Rosowski, E. E., and Huttenlocher, A. (2016). Neutrophil migration in infection and wound repair: going forward in reverse. Nat. Rev. Immunol. 16 (6), 378–391. doi:10.1038/nri.2016.49
De Silva, R. T., Dissanayake, R. K., Mantilaka, M. M. M. G. P. G., Wijesinghe, W. P. S. L., Kaleel, S. S., Premachandra, T. N., et al. (2018). Drug-loaded halloysite nanotube-reinforced electrospun alginate-based nanofibrous scaffolds with sustained antimicrobial protection. ACS Appl. Mater Interfaces 10 (40), 33913–33922. doi:10.1021/acsami.8b11013
Dölling, M., Eckstein, M., Singh, J., Schauer, C., Schoen, J., Shan, X., et al. (2022). Hypoxia promotes neutrophil survival after acute myocardial infarction. Front. Immunol. 13, 726153. doi:10.3389/fimmu.2022.726153
Du, Y., Guo, J. L., Wang, J., Mikos, A. G., and Zhang, S. (2019). Hierarchically designed bone scaffolds: from internal cues to external stimuli. Biomaterials 218, 119334. doi:10.1016/j.biomaterials.2019.119334
Elliott, M. R., Chekeni, F. B., Trampont, P. C., Lazarowski, E. R., Kadl, A., Walk, S. F., et al. (2009). Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 461 (7261), 282–286. doi:10.1038/nature08296
Eming, S. A., Krieg, T., and Davidson, J. M. (2007). Inflammation in wound repair: molecular and cellular mechanisms. J. Invest Dermatol 127 (3), 514–525. doi:10.1038/sj.jid.5700701
Erpenbeck, L., Gruhn, A. L., Kudryasheva, G., Günay, G., Meyer, D., Busse, J., et al. (2019). Effect of adhesion and substrate elasticity on neutrophil extracellular trap formation. Front. Immunol. 10, 2320. doi:10.3389/fimmu.2019.02320
Fadok, V. A., Bratton, D. L., Konowal, A., Freed, P. W., Westcott, J. Y., and Henson, P. M. (1998). Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J. Clin. Invest 101 (4), 890–898. doi:10.1172/jci1112
Feitz, W. J. C., Suntharalingham, S., Khan, M., Ortiz-Sandoval, C. G., Palaniyar, N., van den Heuvel, L. P., et al. (2021). Shiga toxin 2a induces NETosis via NOX-dependent pathway. Biomedicines 9 (12), 1807. doi:10.3390/biomedicines9121807
Feng, P., He, R., Gu, Y., Yang, F., Pan, H., and Shuai, C. (2024). Construction of antibacterial bone implants and their application in bone regeneration. Mater. Horizons 11 (3), 590–625. doi:10.1039/d3mh01298k
Fetz, A. E., Neeli, I., Rodriguez, I. A., Radic, M. Z., and Bowlin, G. L. (2017). Electrospun template architecture and composition regulate neutrophil NETosis in vitro and in vivo. Tissue Eng. Part A 23 (19-20), 1054–1063. doi:10.1089/ten.tea.2016.0452
Fetz, A. E., Radic, M. Z., and Bowlin, G. L. (2021). Human neutrophil FcγRIIIb regulates neutrophil extracellular trap release in response to electrospun polydioxanone biomaterials. Acta Biomater. 130, 281–290. doi:10.1016/j.actbio.2021.06.007
Fletcher, D. A., and Mullins, R. D. (2010). Cell mechanics and the cytoskeleton. Nature 463 (7280), 485–492. doi:10.1038/nature08908
Fousert, E., Toes, R., and Desai, J. (2020). Neutrophil extracellular traps (NETs) take the central stage in driving autoimmune responses. Cells 9 (4), 915. doi:10.3390/cells9040915
Fuchs, T. A., Brill, A., Duerschmied, D., Schatzberg, D., Monestier, M., Myers, D. D., et al. (2010). Extracellular DNA traps promote thrombosis. Proc. Natl. Acad. Sci. U. S. A. 107 (36), 15880–15885. doi:10.1073/pnas.1005743107
Geering, B., and Simon, H. U. (2011). Peculiarities of cell death mechanisms in neutrophils. Cell Death Differ. 18 (9), 1457–1469. doi:10.1038/cdd.2011.75
Greenlee-Wacker, M. C. (2016). Clearance of apoptotic neutrophils and resolution of inflammation. Immunol. Rev. 273 (1), 357–370. doi:10.1111/imr.12453
Grundnes, O., and Reikerås, O. (1993). The importance of the hematoma for fracture healing in rats. Acta Orthop. Scand. 64 (3), 340–342. doi:10.3109/17453679308993640
Guilherme Neto, J. L., Rodrigues Venturini, L. G., Schneider, A. H., Taira, T. M., Duffles Rodrigues, L. F., Veras, F. P., et al. (2023). Neutrophil extracellular traps aggravate apical periodontitis by stimulating osteoclast formation. J. Endod. 49 (11), 1514–1521. doi:10.1016/j.joen.2023.07.027
Hafkamp, F. M. J., Groot Kormelink, T., and de Jong, E. C. (2021). Targeting DCs for tolerance induction: don't lose sight of the neutrophils. Front. Immunol. 12, 732992. doi:10.3389/fimmu.2021.732992
Hasan, R., Rink, L., and Haase, H. (2012). Zinc signals in neutrophil granulocytes are required for the formation of neutrophil extracellular traps. Innate Immun. 19 (3), 253–264. doi:10.1177/1753425912458815
He, L., Liu, R., Yue, H., Zhang, X., Pan, X., Sun, Y., et al. (2023). Interaction between neutrophil extracellular traps and cardiomyocytes contributes to atrial fibrillation progression. Signal Transduct. Target Ther. 8 (1), 279. doi:10.1038/s41392-023-01497-2
Herath, T. D. K., Larbi, A., Teoh, S. H., Kirkpatrick, C. J., and Goh, B. T. (2018). Neutrophil-mediated enhancement of angiogenesis and osteogenesis in a novel triple cell co-culture model with endothelial cells and osteoblasts. J. Tissue Eng. Regen. Med. 12 (2), e1221–e1236. doi:10.1002/term.2521
Herrero-Cervera, A., Soehnlein, O., and Kenne, E. (2022). Neutrophils in chronic inflammatory diseases. Cell Mol. Immunol. 19 (2), 177–191. doi:10.1038/s41423-021-00832-3
Hu, Y., Tang, L., Wang, Z., Yan, H., Yi, X., Wang, H., et al. (2024). Inducing in situ M2 macrophage polarization to promote the repair of bone defects via scaffold-mediated sustained delivery of luteolin. J. Control Release 365, 889–904. doi:10.1016/j.jconrel.2023.11.015
Huang, A. A., and Huang, S. Y. (2024). The impact of aging on outcomes in acute respiratory distress syndrome: a multicenter cohort study. Aging Adv. 1 (2), 61–68. doi:10.4103/agingadv.agingadv-d-24-00024
Huang, Y., Jiang, W., and Zhou, R. (2024). DAMP sensing and Sterile inflammation: intracellular, intercellular and inter-organ pathways. Nat. Rev. Immunol. 24, 703–719. doi:10.1038/s41577-024-01027-3
Iyer, G. Y. N., Islam, M. F., and Quastel, J. H. (1961). Biochemical aspects of phagocytosis. Nature 192 (4802), 535–541. doi:10.1038/192535a0
Jhunjhunwala, S., Aresta-DaSilva, S., Tang, K., Alvarez, D., Webber, M. J., Tang, B. C., et al. (2015). Neutrophil responses to sterile implant materials. PLoS One 10 (9), e0137550. doi:10.1371/journal.pone.0137550
Jiang, T., Tang, X.-Y., Mao, Y., Zhou, Y.-Q., Wang, J.-J., Li, R.-M., et al. (2023). Matrix mechanics regulate the polarization state of bone marrow-derived neutrophils through the JAK1/STAT3 signaling pathway. Acta Biomater. 168, 159–173. doi:10.1016/j.actbio.2023.07.012
Kaneko, Y., Thoendel, M., Olakanmi, O., Britigan, B. E., and Singh, P. K. (2007). The transition metal gallium disrupts Pseudomonas aeruginosa iron metabolism and has antimicrobial and antibiofilm activity. J. Clin. Invest 117 (4), 877–888. doi:10.1172/jci30783
Kaplan, M. J., and Radic, M. (2012). Neutrophil extracellular traps: double-edged swords of innate immunity. J. Immunol. 189 (6), 2689–2695. doi:10.4049/jimmunol.1201719
Karmakar, U., Chu, J. Y., Sundaram, K., Astier, A. L., Garside, H., Hansen, C. G., et al. (2021). Immune complex-induced apoptosis and concurrent immune complex clearance are anti-inflammatory neutrophil functions. Cell Death Dis. 12 (4), 296. doi:10.1038/s41419-021-03528-8
Katsoulis, O., Toussaint, M., Jackson, M. M., Mallia, P., Footitt, J., Mincham, K. T., et al. (2024). Neutrophil extracellular traps promote immunopathogenesis of virus-induced COPD exacerbations. Nat. Commun. 15 (1), 5766. doi:10.1038/s41467-024-50197-0
Kerr, M. D., McBride, D. A., Johnson, W. T., Chumber, A. K., Najibi, A. J., Seo, B. R., et al. (2023). Immune-responsive biodegradable scaffolds for enhancing neutrophil regeneration. Bioeng. Transl. Med. 8 (1), e10309. doi:10.1002/btm2.10309
Kim, J.-M., Lin, C., Stavre, Z., Greenblatt, M. B., and Shim, J.-H. (2020). Osteoblast-osteoclast communication and bone homeostasis. Cells 9 (9), 2073. doi:10.3390/cells9092073
Kim, H.-S., Lee, J.-H., Mandakhbayar, N., Jin, G.-Z., Kim, S.-J., Yoon, J.-Y., et al. (2021). Therapeutic tissue regenerative nanohybrids self-assembled from bioactive inorganic core/chitosan shell nanounits. Biomaterials 274, 120857. doi:10.1016/j.biomaterials.2021.120857
King, W. E., and Bowlin, G. L. (2021). Mechanical characterization and neutrophil NETs response of a novel hybrid geometry polydioxanone near-field electrospun scaffold. Biomed. Mater 16 (6), 065002. doi:10.1088/1748-605x/ac1e43
Kitaori, T., Ito, H., Schwarz, E. M., Tsutsumi, R., Yoshitomi, H., Oishi, S., et al. (2009). Stromal cell-derived factor 1/CXCR4 signaling is critical for the recruitment of mesenchymal stem cells to the fracture site during skeletal repair in a mouse model. Arthritis Rheum. 60 (3), 813–823. doi:10.1002/art.24330
Kolar, P., Schmidt-Bleek, K., Schell, H., Gaber, T., Toben, D., Schmidmaier, G., et al. (2010). The early fracture hematoma and its potential role in fracture healing. Tissue Eng. Part B Rev. 16 (4), 427–434. doi:10.1089/ten.teb.2009.0687
Könnig, D., Herrera, A., Duda, G. N., and Petersen, A. (2018). Mechanosensation across borders: fibroblasts inside a macroporous scaffold sense and respond to the mechanical environment beyond the scaffold walls. J. Tissue Eng. Regen. Med. 12 (1), 265–275. doi:10.1002/term.2410
Koons, G. L., Diba, M., and Mikos, A. G. (2020). Materials design for bone-tissue engineering. Nat. Rev. Mater. 5 (8), 584–603. doi:10.1038/s41578-020-0204-2
Kovach, T. K., Dighe, A. S., Lobo, P. I., and Cui, Q. (2015). Interactions between MSCs and immune cells: implications for bone healing. J. Immunol. Res. 2015, 1–17. doi:10.1155/2015/752510
Kraus, R. F., and Gruber, M. A. (2021). Neutrophils-from bone marrow to first-line defense of the innate immune system. Front. Immunol. 12, 767175. doi:10.3389/fimmu.2021.767175
Kraynak, C. A., Yan, D. J., and Suggs, L. J. (2020). Modulating inflammatory macrophages with an apoptotic body-inspired nanoparticle. Acta Biomater. 108, 250–260. doi:10.1016/j.actbio.2020.03.041
Kraynak, C. A., Huang, W., Bender, E. C., Wang, J.-L., Hanafy, M. S., Cui, Z., et al. (2022). Apoptotic body-inspired nanoparticles target macrophages at sites of inflammation to support an anti-inflammatory phenotype shift. Int. J. Pharm. 618, 121634. doi:10.1016/j.ijpharm.2022.121634
Krzywoszyńska, K., Witkowska, D., Swiatek-Kozlowska, J., Szebesczyk, A., and Kozłowski, H. (2020). General aspects of metal ions as signaling agents in health and disease. Biomolecules 10 (10), 1417. doi:10.3390/biom10101417
Kurland, N. E., Dey, T., Wang, C., Kundu, S. C., and Yadavalli, V. K. (2014). Silk protein lithography as a route to fabricate sericin microarchitectures. Adv. Mater 26 (26), 4431–4437. doi:10.1002/adma.201400777
Kuźmicka, W., Moskalik, A., Manda-Handzlik, A., Demkow, U., Wachowska, M., and Ciepiela, O. (2021). Influence of iron- and zinc-chelating agents on neutrophil extracellular trap formation. Cent. Eur. J. Immunol. 46 (2), 135–139. doi:10.5114/ceji.2021.106985
Kuźmicka, W., Manda-Handzlik, A., Mroczek, A., Cieloch, A., Moskalik, A., Demkow, U., et al. (2022). Iron excess affects release of neutrophil extracellular traps and reactive oxygen species but does not influence other functions of neutrophils. Immunol. Cell Biol. 100 (2), 87–100. doi:10.1111/imcb.12509
Ladero-Auñon, I., Molina, E., Holder, A., Kolakowski, J., Harris, H., Urkitza, A., et al. (2021). Bovine neutrophils release extracellular traps and cooperate with macrophages in Mycobacterium avium subsp. paratuberculosis clearance in vitro. Front. Immunol. 12, 645304. doi:10.3389/fimmu.2021.645304
Lämmermann, T., Afonso, P. V., Angermann, B. R., Wang, J. M., Kastenmüller, W., Parent, C. A., et al. (2013). Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature 498 (7454), 371–375. doi:10.1038/nature12175
Leung, H. H. L., Perdomo, J., Ahmadi, Z., Zheng, S. S., Rashid, F. N., Enjeti, A., et al. (2022). NETosis and thrombosis in vaccine-induced immune thrombotic thrombocytopenia. Nat. Commun. 13 (1), 5206. doi:10.1038/s41467-022-32946-1
Leffler, J., Martin, M., Gullstrand, B., Tydén, H., Lood, C., Truedsson, L., et al. (2012). Neutrophil extracellular traps that are not degraded in systemic lupus erythematosus activate complement exacerbating the disease. J. Immunol. 188 (7), 3522–3531. doi:10.4049/jimmunol.1102404
Leist, M., Single, B., Castoldi, A. F., Kühnle, S., and Nicotera, P. (1997). Intracellular adenosine triphosphate (ATP) concentration: a switch in the decision between apoptosis and necrosis. J. Exp. Med. 185 (8), 1481–1486. doi:10.1084/jem.185.8.1481
Lemire, J. A., Harrison, J. J., and Turner, R. J. (2013). Antimicrobial activity of metals: mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 11 (6), 371–384. doi:10.1038/nrmicro3028
Lennicke, C., and Cochemé, H. M. (2021). Redox metabolism: ROS as specific molecular regulators of cell signaling and function. Mol. Cell 81 (18), 3691–3707. doi:10.1016/j.molcel.2021.08.018
Li, J., Tan, L., Liu, X., Cui, Z., Yang, X., Yeung, K. W. K., et al. (2017). Balancing bacteria-osteoblast competition through selective physical puncture and biofunctionalization of ZnO/Polydopamine/arginine-glycine-aspartic acid-cysteine nanorods. ACS Nano 11 (11), 11250–11263. doi:10.1021/acsnano.7b05620
Li, C.-J., Xiao, Y., Sun, Y.-C., He, W.-Z., Liu, L., Huang, M., et al. (2021). Senescent immune cells release grancalcin to promote skeletal aging. Cell Metab. 33 (10), 1957–1973.e6. doi:10.1016/j.cmet.2021.08.009
Li, M., Zhang, D., Peng, F., Xie, J., Zhang, X., Qian, S., et al. (2022). Zinc-doped ferric oxyhydroxide nano-layer enhances the bactericidal activity and osseointegration of a magnesium alloy through augmenting the formation of neutrophil extracellular traps. Acta Biomater. 152, 575–592. doi:10.1016/j.actbio.2022.08.066
Li, Y., Xu, X., Wang, H. J., Chen, Y. C., Chen, Y., Chiu, J., et al. (2024). Endoplasmic reticulum protein 72 regulates integrin Mac-1 activity to influence neutrophil recruitment. Arterioscler. Thromb. Vasc. Biol. 44 (3), e82–e98. doi:10.1161/atvbaha.123.319771
Liang, H., Huang, Y., and Gao, Q. (2021). Role of non-canonical pyroptosis in sepsis and other inflammatory diseases. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 46 (11), 1276–1284. doi:10.11817/j.issn.1672-7347.2021.210174
Lin, A., and Loré, K. (2017). Granulocytes: new members of the antigen-presenting cell family. Front. Immunol. 8, 1781. doi:10.3389/fimmu.2017.01781
Liu, C., and Zhang, A. (2020). ROS-Mediated PERK-eIF2α-ATF4 pathway plays an important role in arsenite-induced L-02 cells apoptosis via regulating CHOP-DR5 signaling. Environ. Toxicol. 35 (10), 1100–1113. doi:10.1002/tox.22946
Liu, X., Miao, Y., Liang, H., Diao, J., Hao, L., Shi, Z., et al. (2022). 3D-printed bioactive ceramic scaffolds with biomimetic Micro/nano-HAp surfaces mediated cell fate and promoted bone augmentation of the bone-implant interface in vivo. Bioact. Mater 12, 120–132. doi:10.1016/j.bioactmat.2021.10.016
Liu, Q., Yu, Y., Liu, C., Liu, Y., Yuan, L., Wang, Z., et al. (2023). Effect of La3+ and Mg2+ combined system on bioactivity and osteogenesis of bioinspired La-doped magnesium phosphate composites prepared utilizing the precursor method. J. Mater. Res. Technol. 24, 9523–9536. doi:10.1016/j.jmrt.2023.05.133
Liu, X., Ou, X., Zhang, T., Li, X., Qiao, Q., Jia, L., et al. (2024). In situ neutrophil apoptosis and macrophage efferocytosis mediated by glycyrrhiza protein nanoparticles for acute inflammation therapy. J. Control Release 369, 215–230. doi:10.1016/j.jconrel.2024.03.029
Lokwani, R., Josyula, A., Ngo, T. B., DeStefano, S., Fertil, D., Faust, M., et al. (2024). Pro-regenerative biomaterials recruit immunoregulatory dendritic cells after traumatic injury. Nat. Mater 23 (1), 147–157. doi:10.1038/s41563-023-01689-9
Luo, Y., Liu, H., Zhang, Y., Liu, Y., Liu, S., Liu, X., et al. (2023). Metal ions: the unfading stars of bone regeneration-from bone metabolism regulation to biomaterial applications. Biomater. Sci. 11 (22), 7268–7295. doi:10.1039/d3bm01146a
Ma, S., Xiao, Y., Zhang, X., Xu, Y., Zhu, K., Zhang, K., et al. (2024). Dietary exposure to polystyrene microplastics exacerbates liver damage in fulminant hepatic failure via ROS production and neutrophil extracellular trap formation. Sci. Total Environ. 907, 167403. doi:10.1016/j.scitotenv.2023.167403
Mao, C., Xiang, Y., Liu, X., Cui, Z., Yang, X., Yeung, K. W. K., et al. (2017). Photo-inspired antibacterial activity and wound healing acceleration by hydrogel embedded with ag/Ag@AgCl/ZnO nanostructures. ACS Nano 11 (9), 9010–9021. doi:10.1021/acsnano.7b03513
Martin, K. R., Ohayon, D., and Witko-Sarsat, V. (2015). Promoting apoptosis of neutrophils and phagocytosis by macrophages: novel strategies in the resolution of inflammation. Swiss Med. Wkly. 145, w14056. doi:10.4414/smw.2015.14056
McDonald, B., Pittman, K., Menezes, G. B., Hirota, S. A., Slaba, I., Waterhouse, C. C. M., et al. (2010). Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science 330 (6002), 362–366. doi:10.1126/science.1195491
Mereweather, L. J., Constantinescu-Bercu, A., Crawley, J. T. B., and Salles-Crawley, I. I. (2023). Platelet-neutrophil crosstalk in thrombosis. Int. J. Mol. Sci. 24 (2), 1266. doi:10.3390/ijms24021266
Minns, D., Smith, K. J., Alessandrini, V., Hardisty, G., Melrose, L., Jackson-Jones, L., et al. (2021). The neutrophil antimicrobial peptide cathelicidin promotes Th17 differentiation. Nat. Commun. 12 (1), 1285. doi:10.1038/s41467-021-21533-5
Miralda, I., Vashishta, A., Rogers, M. N., Lamont, R. J., and Uriarte, S. M. (2022). The emerging oral pathogen, Filifactor alocis, extends the functional lifespan of human neutrophils. Mol. Microbiol. 117 (6), 1340–1351. doi:10.1111/mmi.14911
Moonen, C. G. J., de Vries, T. J., Rijkschroeff, P., Poubelle, P. E., Nicu, E. A., and Loos, B. G. (2019). The possible role of neutrophils in the induction of osteoclastogenesis. J. Immunol. Res. 2019, 1–14. doi:10.1155/2019/8672604
Morandini, L., Avery, D., Angeles, B., Winston, P., Martin, R. K., Donahue, H. J., et al. (2023). Reduction of neutrophil extracellular traps accelerates inflammatory resolution and increases bone formation on titanium implants. Acta Biomater. 166, 670–684. doi:10.1016/j.actbio.2023.05.016
Morgenstern, M., Kühl, R., Eckardt, H., Acklin, Y., Stanic, B., Garcia, M., et al. (2018). Diagnostic challenges and future perspectives in fracture-related infection. Injury 49 (Suppl. 1), S83–S90. doi:10.1016/s0020-1383(18)30310-3
Mukhopadhyay, A., Tsukasaki, Y., Chan, W. C., Le, J. P., Kwok, M. L., Zhou, J., et al. (2024). trans-Endothelial neutrophil migration activates bactericidal function via Piezo1 mechanosensing. Immunity 57 (1), 52–67.e10. doi:10.1016/j.immuni.2023.11.007
Németh, T., Sperandio, M., and Mócsai, A. (2020). Neutrophils as emerging therapeutic targets. Nat. Rev. Drug Discov. 19 (4), 253–275. doi:10.1038/s41573-019-0054-z
Ng, L. G., Qin, J. S., Roediger, B., Wang, Y., Jain, R., Cavanagh, L. L., et al. (2011). Visualizing the neutrophil response to sterile tissue injury in mouse dermis reveals a three-phase cascade of events. J. Invest Dermatol 131 (10), 2058–2068. doi:10.1038/jid.2011.179
O'Neill, E., Awale, G., Daneshmandi, L., Umerah, O., and Lo, K. W. H. (2018). The roles of ions on bone regeneration. Drug Discov. Today 23 (4), 879–890. doi:10.1016/j.drudis.2018.01.049
Oakes, P. W., Patel, D. C., Morin, N. A., Zitterbart, D. P., Fabry, B., Reichner, J. S., et al. (2009). Neutrophil morphology and migration are affected by substrate elasticity. Blood 114 (7), 1387–1395. doi:10.1182/blood-2008-11-191445
Ong, G., and Logue, S. E. (2023). Unfolding the interactions between endoplasmic reticulum stress and oxidative stress. Antioxidants (Basel) 12 (5), 981. doi:10.3390/antiox12050981
Pattison, D. I., Davies, M. J., and Hawkins, C. L. (2012). Reactions and reactivity of myeloperoxidase-derived oxidants: differential biological effects of hypochlorous and hypothiocyanous acids. Free Radic. Res. 46 (8), 975–995. doi:10.3109/10715762.2012.667566
Peng, F., Xie, J., Liu, H., Zheng, Y., Qian, X., Zhou, R., et al. (2023). Shifting focus from bacteria to host neutrophil extracellular traps of biodegradable pure Zn to combat implant centered infection. Bioact. Mater 21, 436–449. doi:10.1016/j.bioactmat.2022.09.004
Pettygrove, B. A., Kratofil, R. M., Alhede, M., Jensen, P. Ø., Newton, M., Qvortrup, K., et al. (2021). Delayed neutrophil recruitment allows nascent Staphylococcus aureus biofilm formation and immune evasion. Biomaterials 275, 120775. doi:10.1016/j.biomaterials.2021.120775
Ponzetti, M., and Rucci, N. (2019). Updates on osteoimmunology: what's new on the cross-talk between bone and immune system. Front. Endocrinol. (Lausanne) 10, 236. doi:10.3389/fendo.2019.00236
Porter, L. M., Cowburn, A. S., Farahi, N., Deighton, J., Farrow, S. N., Fiddler, C. A., et al. (2017). Hypoxia causes IL-8 secretion, charcot leyden crystal formation, and suppression of corticosteroid-induced apoptosis in human eosinophils. Clin. Exp. Allergy 47 (6), 770–784. doi:10.1111/cea.12877
Prame Kumar, K., Nicholls, A. J., and Wong, C. H. Y. (2018). Partners in crime: neutrophils and monocytes/macrophages in inflammation and disease. Cell Tissue Res. 371 (3), 551–565. doi:10.1007/s00441-017-2753-2
Puga, I., Cols, M., Barra, C. M., He, B., Cassis, L., Gentile, M., et al. (2011). B cell-helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nat. Immunol. 13 (2), 170–180. doi:10.1038/ni.2194
Qian, G., Zhang, L., Wang, G., Zhao, Z., Peng, S., and Shuai, C. (2021a). 3D printed Zn-doped mesoporous silica-incorporated Poly-L-lactic acid scaffolds for bone repair. Int. J. Bioprint 7 (2), 346. doi:10.18063/ijb.v7i2.346
Qian, G., Zhang, L., Liu, X., Wu, S., Peng, S., and Shuai, C. (2021b). Silver-doped bioglass modified scaffolds: a sustained antibacterial efficacy. Mater. Sci. Eng. C 129, 112425. doi:10.1016/j.msec.2021.112425
Rabel, K., Kohal, R.-J., Steinberg, T., Tomakidi, P., Rolauffs, B., Adolfsson, E., et al. (2020). Controlling osteoblast morphology and proliferation via surface micro-topographies of implant biomaterials. Sci. Rep. 10 (1), 12810. doi:10.1038/s41598-020-69685-6
Rani Talla, U., Bozonet, S., Parker, H., Hampton, M., and Vissers, M. (2016). Inhibition of neutrophil apoptosis and initiation of an autophagy-like process in hypoxia and effects on neutrophil function. Free Radic. Biol. Med. 100, S63. doi:10.1016/j.freeradbiomed.2016.10.166
Saberi, A., Kouhjani, M., Mohammadi, M., and Hosta-Rigau, L. (2023). Novel scaffold platforms for simultaneous induction osteogenesis and angiogenesis in bone tissue engineering: a cutting-edge approach. J. Nanobiotechnology 21 (1), 351. doi:10.1186/s12951-023-02115-7
Saffarzadeh, M., Juenemann, C., Queisser, M. A., Lochnit, G., Barreto, G., Galuska, S. P., et al. (2012). Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS One 7 (2), e32366. doi:10.1371/journal.pone.0032366
Sarkar, A., Hellberg, L., Bhattacharyya, A., Behnen, M., Wang, K., Lord, J. M., et al. (2012). Infection with Anaplasma phagocytophilum activates the phosphatidylinositol 3-Kinase/Akt and NF-κB survival pathways in neutrophil granulocytes. Infect. Immun. 80 (4), 1615–1623. doi:10.1128/iai.05219-11
Sarkar, A., Möller, S., Bhattacharyya, A., Behnen, M., Rupp, J., van Zandbergen, G., et al. (2015). Mechanisms of apoptosis inhibition in chlamydia pneumoniae-infected neutrophils. Int. J. Med. Microbiol. 305 (6), 493–500. doi:10.1016/j.ijmm.2015.04.006
Scapini, P., and Cassatella, M. A. (2014). Social networking of human neutrophils within the immune system. Blood 124 (5), 710–719. doi:10.1182/blood-2014-03-453217
Scherlinger, M., Richez, C., Tsokos, G. C., Boilard, E., and Blanco, P. (2023). The role of platelets in immune-mediated inflammatory diseases. Nat. Rev. Immunol. 23 (8), 495–510. doi:10.1038/s41577-023-00834-4
Schmidt-Bleek, K., Schell, H., Schulz, N., Hoff, P., Perka, C., Buttgereit, F., et al. (2012). Inflammatory phase of bone healing initiates the regenerative healing Cascade. Cell Tissue Res. 347 (3), 567–573. doi:10.1007/s00441-011-1205-7
Schneider, A. H., Taira, T. M., Públio, G. A., da Silva Prado, D., Donate Yabuta, P. B., Dos Santos, J. C., et al. (2024). Neutrophil extracellular traps mediate bone erosion in rheumatoid arthritis by enhancing RANKL-induced osteoclastogenesis. Br. J. Pharmacol. 181 (3), 429–446. doi:10.1111/bph.16227
Schuster, S., Hurrell, B., and Tacchini-Cottier, F. (2013). Crosstalk between neutrophils and dendritic cells: a context-dependent process. J. Leukoc. Biol. 94 (4), 671–675. doi:10.1189/jlb.1012540
Shi, W., Feng, Y., Tang, J., Xu, Y., Wang, W., Zhang, L., et al. (2024). A genetically engineered “Reinforced Concrete” scaffold regulates the N2 neutrophil Innate immune Cascade to repair bone defects. Adv. Healthc. Mater 13 (18), e2304585. doi:10.1002/adhm.202304585
Shu, L.-Z., Zhang, X.-L., Ding, Y.-D., and Lin, H. (2024). From inflammation to bone formation: the intricate role of neutrophils in skeletal muscle injury and traumatic heterotopic ossification. Exp. Mol. Med. 56 (7), 1523–1530. doi:10.1038/s12276-024-01270-7
Singh, P., Hu, P., Hoggatt, J., Moh, A., and Pelus, L. M. (2012). Expansion of bone marrow neutrophils following G-CSF administration in mice results in osteolineage cell apoptosis and mobilization of hematopoietic stem and progenitor cells. Leukemia 26 (11), 2375–2383. doi:10.1038/leu.2012.117
Soehnlein, O., Zernecke, A., Eriksson, E. E., Rothfuchs, A. G., Pham, C. T., Herwald, H., et al. (2008). Neutrophil secretion products pave the way for inflammatory monocytes. Blood 112 (4), 1461–1471. doi:10.1182/blood-2008-02-139634
Spiegel, D. A., Shrestha, O. P., Rajbhandary, T., Bijukachhe, B., Sitoula, P., Banskota, B., et al. (2010). Epidemiology of surgical admissions to a children's disability hospital in Nepal. World J. Surg. 34 (5), 954–962. doi:10.1007/s00268-010-0487-3
Stroka, K. M., and Aranda-Espinoza, H. (2011). Endothelial cell substrate stiffness influences neutrophil transmigration via myosin light chain kinase-dependent cell contraction. Blood 118 (6), 1632–1640. doi:10.1182/blood-2010-11-321125
Subramanian Vignesh, K., and Deepe, G. S. (2016). Immunological orchestration of zinc homeostasis: the battle between host mechanisms and pathogen defenses. Arch. Biochem. Biophys. 611, 66–78. doi:10.1016/j.abb.2016.02.020
Suliman, S., Mieszkowska, A., Folkert, J., Rana, N., Mohamed-Ahmed, S., Fuoco, T., et al. (2022). Immune-instructive copolymer scaffolds using plant-derived nanoparticles to promote bone regeneration. Inflamm. Regen. 42 (1), 12. doi:10.1186/s41232-022-00196-9
Takegahara, N., Kim, H., and Choi, Y. (2024). Unraveling the intricacies of osteoclast differentiation and maturation: insight into novel therapeutic strategies for bone-destructive diseases. Exp. Mol. Med. 56 (2), 264–272. doi:10.1038/s12276-024-01157-7
Tan, H., Zhang, S., Zhang, Z., Zhang, J., Wang, Z., Liao, J., et al. (2024). Neutrophil extracellular traps promote M1 macrophage polarization in gouty inflammation via targeting hexokinase-2. Free Radic. Biol. Med. 224, 540–553. doi:10.1016/j.freeradbiomed.2024.09.009
Tao, H., Wang, Q., Chen, K., Zhu, P., Gu, Y., and Geng, D. (2024). Metal ion metabolism and osteoporosis: possible implications for pharmaceutical biotechnology and tissue engineering. Sci. China Life Sci. 67 (8), 1763–1765. doi:10.1007/s11427-023-2541-x
Telang, S. (2018). Lactoferrin: a critical player in neonatal host defense. Nutrients 10 (9), 1228. doi:10.3390/nu10091228
Teng, T.-S., Ji, A.-L., Ji, X.-Y., and Li, Y.-Z. (2017). Neutrophils and immunity: from bactericidal action to being conquered. J. Immunol. Res. 2017, 1–14. doi:10.1155/2017/9671604
Thakur, M., Junho, C. V. C., Bernhard, S. M., Schindewolf, M., Noels, H., and Döring, Y. (2023). NETs-Induced thrombosis impacts on cardiovascular and chronic kidney disease. Circ. Res. 132 (8), 933–949. doi:10.1161/circresaha.123.321750
Thompson, A. A. R., Elks, P. M., Marriott, H. M., Eamsamarng, S., Higgins, K. R., Lewis, A., et al. (2014). Hypoxia-inducible factor 2α regulates key neutrophil functions in humans, mice, and zebrafish. Blood 123 (3), 366–376. doi:10.1182/blood-2013-05-500207
Toller-Kawahisa, J. E., Hiroki, C. H., Silva, C. M. d.S., Nascimento, D. C., Públio, G. A., Martins, T. V., et al. (2023). The metabolic function of pyruvate kinase M2 regulates reactive oxygen species production and microbial killing by neutrophils. Nat. Commun. 14 (1), 4280. doi:10.1038/s41467-023-40021-6
Vatansever, F., de Melo, W. C. M. A., Avci, P., Vecchio, D., Sadasivam, M., Gupta, A., et al. (2013). Antimicrobial strategies centered around reactive oxygen species--bactericidal antibiotics, photodynamic therapy, and beyond. FEMS Microbiol. Rev. 37 (6), 955–989. doi:10.1111/1574-6976.12026
Walmsley, S. R., Print, C., Farahi, N., Peyssonnaux, C., Johnson, R. S., Cramer, T., et al. (2005). Hypoxia-induced neutrophil survival is mediated by HIF-1α–dependent NF-κB activity. J. Exp. Med. 201 (1), 105–115. doi:10.1084/jem.20040624
Wang, P.-Y., Li, W.-T., Yu, J., and Tsai, W.-B. (2012). Modulation of osteogenic, adipogenic and myogenic differentiation of mesenchymal stem cells by submicron grooved topography. J. Mater Sci. Mater Med. 23 (12), 3015–3028. doi:10.1007/s10856-012-4748-6
Wang, C., Zhang, R., Wei, X., Lv, M., and Jiang, Z. (2020). Metalloimmunology: the metal ion-controlled immunity. Adv. Immunol. 145, 187–241. doi:10.1016/bs.ai.2019.11.007
Wang, J.-F., Wang, Y.-P., Xie, J., Zhao, Z.-Z., Gupta, S., Guo, Y., et al. (2021). Upregulated PD-L1 delays human neutrophil apoptosis and promotes lung injury in an experimental mouse model of sepsis. Blood 138 (9), 806–810. doi:10.1182/blood.2020009417
Wang, H., Kim, S. J., Lei, Y., Wang, S., Wang, H., Huang, H., et al. (2024a). Neutrophil extracellular traps in homeostasis and disease. Signal Transduct. Target Ther. 9 (1), 235. doi:10.1038/s41392-024-01933-x
Wang, L., Zhang, G., Gao, Y., Dai, T., Yu, J., Liu, Y., et al. (2024b). Extracellular vesicles derived from neutrophils accelerate bone regeneration by promoting osteogenic differentiation of BMSCs. ACS Biomater. Sci. Eng. 10 (6), 3868–3882. doi:10.1021/acsbiomaterials.4c00106
Wang, Z., Chu, Y., Du, J., Hu, Y., Wang, H., Liu, H., et al. (2025a). Accelerating repair of infected bone defects through post-reinforced injectable hydrogel mediated antibacterial/immunoregulatory microenvironment at bone-hydrogel interface. Carbohydr. Polym. 351, 123082. doi:10.1016/j.carbpol.2024.123082
Wang, N., Tao, Y., Yang, Y., Jin, Y., Zhang, H., Li, C., et al. (2025b). Disrupting the activity of endogenous gas neurotransmitters: a therapeutic strategy using engineered metal-organic frameworks for cancer. Med. Gas. Res. 15 (1), 142–144. doi:10.4103/mgr.medgasres-d-24-00046
Wei, Y., Wang, J., Wu, S., Zhou, R., Zhang, K., Zhang, Z., et al. (2022). Nanomaterial-based zinc ion interference therapy to combat bacterial infections. Front. Immunol. 13, 899992. doi:10.3389/fimmu.2022.899992
Wei, P., Zhou, J., Xiong, S., Yi, F., Xu, K., Liu, M., et al. (2024). Chestnut-inspired hollow hydroxyapatite 3D printing scaffolds accelerate bone regeneration by recruiting calcium ions and regulating inflammation. ACS Appl. Mater Interfaces 16 (8), 9768–9786. doi:10.1021/acsami.3c17087
Won, J.-E., Lee, Y. S., Park, J.-H., Lee, J.-H., Shin, Y. S., Kim, C.-H., et al. (2020). Hierarchical microchanneled scaffolds modulate multiple tissue-regenerative processes of immune-responses, angiogenesis, and stem cell homing. Biomaterials 227, 119548. doi:10.1016/j.biomaterials.2019.119548
Wu, D., Li, J., Xu, S., Xie, Q., Pan, Y., Liu, X., et al. (2020). Engineering Fe-N doped graphene to mimic biological functions of NADPH oxidase in cells. J. Am. Chem. Soc. 142 (46), 19602–19610. doi:10.1021/jacs.0c08360
Xing, Z., Lu, C., Hu, D., Miclau, T., and Marcucio, R. S. (2010). Rejuvenation of the inflammatory system stimulates fracture repair in aged mice. J. Orthop. Res. 28 (8), 1000–1006. doi:10.1002/jor.21087
Xiong, S., Zhang, Y., Zeng, J., Zhou, J., Liu, S., Wei, P., et al. (2024). DLP fabrication of HA scaffold with customized porous structures to regulate immune microenvironment and macrophage polarization for enhancing bone regeneration. Mater Today Bio 24, 100929. doi:10.1016/j.mtbio.2023.100929
Xu, X., Chen, Z., Xiao, L., Xu, Y., Xiao, N., Jin, W., et al. (2023). Nanosilicate-functionalized nanofibrous membrane facilitated periodontal regeneration potential by harnessing periodontal ligament cell-mediated osteogenesis and immunomodulation. J. Nanobiotechnology 21 (1), 223. doi:10.1186/s12951-023-01982-4
Xu, Y., Wang, D., Zhou, S., Yu, D., Deng, A., Lu, X., et al. (2025). Apoptotic bodies from neutrophil-like cells in situ regulating macrophage polarization for autoimmune disease treatment. Nano Res. 18 (9), 94907775. doi:10.26599/nr.2025.94907775
Yamasawa, H., Oshikawa, K., Ohno, S., and Sugiyama, Y. (2004). Macrolides inhibit epithelial cell-mediated neutrophil survival by modulating granulocyte macrophage colony-stimulating factor release. Am. J. Respir. Cell Mol. Biol. 30 (4), 569–575. doi:10.1165/rcmb.2003-0105oc
Yang, P., Luo, X., Li, J., Zhang, T., Gao, X., Hua, J., et al. (2021). Ionizing radiation upregulates glutamine metabolism and induces cell death via accumulation of reactive oxygen species. Oxid. Med. Cell Longev. 2021, 5826932. doi:10.1155/2021/5826932
Ye, Q., Harmsen, M. C., van Luyn, M. J. A., and Bank, R. A. (2010). The relationship between collagen scaffold cross-linking agents and neutrophils in the foreign body reaction. Biomaterials 31 (35), 9192–9201. doi:10.1016/j.biomaterials.2010.08.049
Yu, Y.-M., Lu, Y.-P., Zhang, T., Zheng, Y.-F., Liu, Y.-S., and Xia, D.-D. (2024). Biomaterials science and surface engineering strategies for dental peri-implantitis management. Mil. Med. Res. 11 (1), 29. doi:10.1186/s40779-024-00532-9
Zeng, J., Xiong, S., Zhou, J., Wei, P., Guo, K., Wang, F., et al. (2023). Hollow hydroxyapatite microspheres loaded with rhCXCL13 to recruit BMSC for osteogenesis and synergetic angiogenesis to promote bone regeneration in bone defects. Int. J. Nanomedicine 18, 3509–3534. doi:10.2147/ijn.s408905
Zhang, X., Wang, X., Wu, T., Li, B., Liu, T., Wang, R., et al. (2015). Isoliensinine induces apoptosis in triple-negative human breast cancer cells through ROS generation and p38 MAPK/JNK activation. Sci. Rep. 5, 12579. doi:10.1038/srep12579
Zhao, R.-Z., Jiang, S., Zhang, L., and Yu, Z.-B. (2019). Mitochondrial electron transport chain, ROS generation and uncoupling. Int. J. Mol. Med. 44 (1). doi:10.3892/ijmm.2019.4188
Zheng, W., Li, J., Li, J., Bie, N., Wei, Z., Qin, J., et al. (2024). In-situ nanoplatform with synergistic neutrophil intervention and chemotherapy to prevent postoperative tumor recurrence and metastasis. J. Control Release 375, 316–330. doi:10.1016/j.jconrel.2024.09.011
Zhou, C.-X., Li, L., Ma, Y.-G., Li, B.-N., Li, G., Zhou, Z., et al. (2018). A bioactive implant in situ and long-term releases combined drugs for treatment of osteoarticular tuberculosis. Biomaterials 176, 50–59. doi:10.1016/j.biomaterials.2018.05.039
Zhou, J., Xiong, S., Liu, M., Yang, H., Wei, P., Yi, F., et al. (2023). Study on the influence of scaffold morphology and structure on osteogenic performance. Front. Bioeng. Biotechnol. 11, 1127162. doi:10.3389/fbioe.2023.1127162
Zhu, S., Bennett, S., Kuek, V., Xiang, C., Xu, H., Rosen, V., et al. (2020). Endothelial cells produce angiocrine factors to regulate bone and cartilage via versatile mechanisms. Theranostics 10 (13), 5957–5965. doi:10.7150/thno.45422
Zhu, Y. P., Speir, M., Tan, Z., Lee, J. C., Nowell, C. J., Chen, A. A., et al. (2023). NET formation is a default epigenetic program controlled by PAD4 in apoptotic neutrophils. Sci. Adv. 9 (51), eadj1397. doi:10.1126/sciadv.adj1397
Keywords: neutrophils, scaffolds, bone tissue engineering, antibacterial, osteogenic
Citation: Zhou J, Xiong S, Liu S, Zhou Z, Liu M, Xi H, Kong W, Zhou J and Xiong L (2025) Bioscaffold materials resist infection and promote bone defect repair by regulating neutrophil function. Front. Bioeng. Biotechnol. 13:1644625. doi: 10.3389/fbioe.2025.1644625
Received: 10 June 2025; Accepted: 30 September 2025;
Published: 16 October 2025.
Edited by:
Denghui Xie, Southern Medical University, ChinaReviewed by:
Jiabing Ran, China Three Gorges University, ChinaJorge Masso-Silva, University of California, San Diego, CA, United States
Copyright © 2025 Zhou, Xiong, Liu, Zhou, Liu, Xi, Kong, Zhou and Xiong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Long Xiong, bmN4aW9uZ2xvbmcyQDEyNi5jb20=; Jianguo Zhou, empnODQwODE4QDE2My5jb20=
†These authors have contributed equally to this work
 Jingyu Zhou
Jingyu Zhou Shilang Xiong
Shilang Xiong Shiwei Liu6†
Shiwei Liu6† Jianguo Zhou
Jianguo Zhou Long Xiong
Long Xiong