- 1Rehabilitation Engineering Laboratory, ETH Zurich, Zurich, Switzerland
- 2GIPSA-Lab Grenoble Images Parole Signal Automatique, University Grenoble Alpes, Centre National de la Recherche Scientifique (CNRS), Grenoble INP, Grenoble, France
- 3Data Analytics & Rehabilitation Technology (DART), Lake Lucerne Institute, Vitznau, Switzerland
- 4Cereneo - Center for Neurorehabilitation, Vitznau, Switzerland
- 5Vascular Neurology and Neurorehabilitation, Department of Neurology, University Hospital of Zurich, Zurich, Switzerland
- 6Future Health Technologies Programme, Singapore - ETH Centre, Campus for Research Excellence and Technological Enterprise (CREATE), Singapore, Singapore
- 7The LOOP Zurich – Medical Research Center, Zurich, Switzerland
Background: Augmented visual feedback (AVF) is a promising approach for gait rehabilitation after stroke. However, we still lack crucial knowledge about how to most efficiently use it.
Research Question: How does the selection of the gait parameter targeted by the AVF signal influence the global motor response (i.e., overall gait pattern)?
Methods: 24 healthy young participants (mean age 25.3
Results: When using ST and POF feedback targets, participants successfully modified their local asymmetry by an average of 10%. Correlation analysis (Spearman) indicated that the modulated gait propagated across parameters, with a fair correlation
Significance: Our results show that the efficacy of AVF is dependent on the selected target parameter. This choice also seems to affect how local symmetry changes affect global motion patterns. This work is a first step towards a more comprehensive understanding of the direct and indirect impact of AVF on gait response, which is crucial before using AVF for clinical applications.
1 Introduction
Healthy gait largely contributes to mobility, functional independence and good quality of life (Park and Kim, 2019; Cohen et al., 2018). When gait is impaired after a neurological injury, one major priority is to recover the associated motor functions through rehabilitation. After stroke, for instance, gait impairment can affect between 30% and 50% of patients (Friedman, 1990; Jørgensen et al., 1995; Katan and Luft, 2018; Feigin et al., 2021). Current rehabilitation interventions, such as intensive physical therapy, rely on motor learning and motor control processes (Richards et al., 2015). While these processes are driven by therapists in conventional approaches, through their observations and oral comments, technology-based augmented feedback is a promising method that could complement conventional therapy in a more persistent, objective and precise manner (Schmidt et al., 2018; Sigrist et al., 2013a). In addition to intrinsic feedback (via sensory inferences), augmented feedback can provide information on a physiological or motor performance (e.g., kinematics or electromyography), through an external stimulus (Sigrist et al., 2013a). This stimulus can be shaped in many different ways via the motion parameter to target with the augmented feedback, the modality (visual, haptic and/or auditory), the mapping (e.g., which sound or visualization conveys the information), the duration or the frequency (Sigrist et al., 2013a).
Many studies focused on the impact of augmented feedback on motor learning for healthy participants, looking at the influence of feedback modality, duration, frequency or its interaction with the task difficulty (Sigrist et al., 2013a; Dozza et al., 2006; Sigrist et al., 2013b). The positive conclusions encourage to explore the use of augmented feedback for motor rehabilitation, and in particular for gait rehabilitation, with the hypothesis that feedback may improve or speed up recovery. Several pilot studies thus investigated the motor response of individuals with stroke in reaction to a visual, auditory, or haptic feedback signal (Afzal et al., 2019; Cha et al., 2018; Liu et al., 2020; Genthe et al., 2018; Jung et al., 2020). These studies tend to show the benefits of augmented feedback on gait recovery, calling now for longitudinal studies and high-quality clinical trials to confirm these findings at a bigger scale (Spencer et al., 2021; Angelis et al., 2021). However, while the influence of the modality or the influence of the mapping are often a topic of interest (Afzal et al., 2019; Sigrist et al., 2013b; Kinnaird et al., 2016; Chamorro-Moriana et al., 2018), the crucial role of the selection of the parameter to be targeted by augmented feedback is often neglected. The effects of selecting different target parameters for augmented feedback have so far not been systematically evaluated, despite being a crucial design criteria.
Augmented feedback usually targets one parameter only, while the different gait parameters can be interdependent (Kim and Eng, 2003; Balasubramanian et al., 2007). It is particularly the case when considering gait asymmetry, a common characteristic of post-stroke gait (Patterson et al., 2010; Patterson et al., 2008; Ogihara et al., 2020), which is a product of changes in multiple movement parameters. For instance, previous studies indicate correlations between temporal symmetry and ground reaction force and between spatial symmetry and push-off force symmetry (Kim and Eng, 2003; Balasubramanian et al., 2007). When tackling gait asymmetry with augmented feedback, two important questions arise: (i) does the choice of the target parameter influence the effects of augmented feedback on local modifications, i.e., on the modifications of the target parameter itself? and (ii) does the choice of the target parameter influence the augmented feedback-induced gait pattern modification on a global level?
In this study, involving healthy participants, we compared the impact of augmented visual feedback (AVF) when targeting three different gait parameters individually: stance time, push-off force and ankle plantarflexion. These parameters were selected as they are commonly affected in stroke patients (Olney and Richards, 1996; Li et al., 2018). With a feedback scenario that enhanced asymmetry (i.e., enforced asymmetric gait in healthy subjects) and simulated stroke-like conditions, we analyzed the impact of the AVF through the evolution of each target parameter during 10 min of treadmill walking, and through a comprehensive analysis of the whole gait pattern. We hypothesize that healthy adults would be more responsive to AVF on the spatiotemporal parameter (stance time), adopting a significant asymmetry, since the focus is more external (effect of one’s movement) than for kinetics and kinematics (that are directly one’s own movement) (Magill and Anderson, 2014; Sigrist et al., 2013a; Durham et al., 2009). We also hypothesize that, for each target parameter, the change induced by the AVF would influence other key gait parameters. This work builds on the results of a pilot study that investigated the impact of AVF on stance time on the whole gait (Legrand et al., 2024), where we showed that AVF on temporal symmetry affects both kinetic symmetry and the kinematic pattern of gait. Here we extend this by comparing AVF on stance time with AVF on push-off force and on ankle plantarflexion, with a higher number of participants.
2 Materials and methods
2.1 Experimental protocol
Twenty-four healthy adults (15 women and 9 men, mean age 25.3
Each participant performed one session of treadmill walking, composed of a baseline walking followed by walking with AVF. During baseline, participants were asked to walk naturally on the treadmill, at their preferred gait speed, for 5 minutes. This phase allowed participants to get used to treadmill walking and to determine each participant’s preferred gait speed (Meyer et al., 2019). The walking speed was then kept constant during the AVF phase: it was equal to 80% of the preferred gait speed of the participant, to make asymmetry modulation easier to manage. The AVF phase was divided into three conditions, each targeting a different gait parameter: stance time (ST - spatiotemporal), antero-posterior force at toe-off or push-off force (POF - kinetics) and ankle plantarflexion (APL - kinematics). The order of the conditions was randomized between participants. Each condition comprised 1 min of natural walking (NW), followed by two periods of intermittent AVF: (1) 3 mins with AVF (AVF-1), 1 min without (no AVF-1) and (2) 3 mins with AVF (AVF-2) and 3 mins without (no AVF-2, see Figure 1a). During the two periods without AVF, participants were instructed to try to retain the same gait pattern as the one learnt during the periods with feedback. The last “no feedback” period (no AVF-2) lasted 3 mins in order to be able to observe any retention or decay of the learning at the end of the training session. We chose to provide intermittent feedback (i.e., alternating periods with and without AVF) to study retention effects and avoid over-reliance on the visual feedback. A break of few minutes (ended by the participants) was provided between each condition.
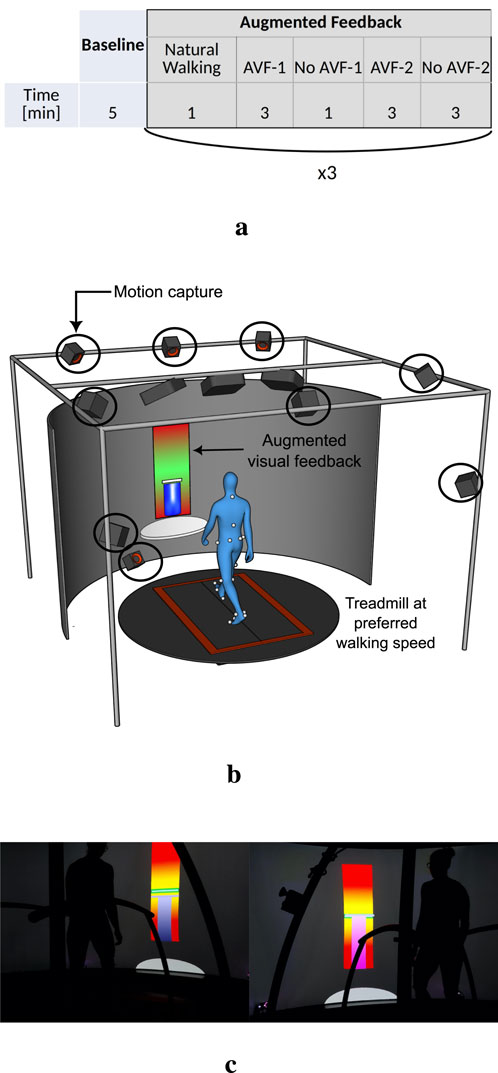
Figure 1. Timing and experimental environment of the treadmill walking session (a) Phases of the experiment, with the corresponding duration (b) CAREN environment (c) Visual AVF.
The entire experiment was performed in a Computer Assisted Rehabilitation Environment (CAREN, Motek), which includes a dual-belt treadmill with integrated force plates, a motion capture system (Vicon Motion Systems Ltd.), and a 180
2.2 Augmented visual feedback
In this study, the objective of the AVF was to induce asymmetric walking in healthy participants. Three different targets were chosen, based on common observations from stroke patients (Olney and Richards, 1996; Li et al., 2018): a shorter ST, a smaller POF or a smaller APL at toe-off, on the left side. With stroke patients, the same type of AVF would be used to improve towards gait symmetry. The characteristics of the visual feedback were inspired from other studies (Genthe et al., 2018; van den Noort et al., 2014; Colborne et al., 1993) and adapted following the outcomes of our pilot study (Legrand et al., 2024). Continuous feedback was selected rather than terminal, since this has shown better efficacy for learning of novel, complex motor tasks (Sigrist et al., 2013a). The AVF was displayed on the 180
Before starting the visual feedback phase, few explanations were given to the participants: (i) it was specified that the side to modify with the feedback was the left side; (ii) the parameter targeted by the feedback was explained (i.e., what is ST/POF/APL); and (iii) participants were instructed to reach the target in the middle of the green zone. We also instructed the participants to maintain their new walking pattern during the no AVF periods. We, however, did not tell them the gait pattern they should achieve (i.e., no information about symmetry) and did not answer if they asked about their performance during the experiment. We deliberately gave limited and standardized guidelines, to let participants explore by themselves and evaluate the ease of understanding of the AVF.
2.3 Data analysis
Three complementary analyses were conducted: (i) evolution of the target symmetry ratio to assess local effects of AVF, (ii) Spearman correlation coefficient between the target parameter and other gait parameters to assess the propagation of the modulated gait across parameters and (iii) Gait Deviation Index (GDI) to assess overall gait pattern changes.
Motion capture data were processed with Vicon
To evaluate whether the local changes due to AVF depended on the target parameter, we looked at the evolution of the target symmetry ratio along each condition: we looked at
We then analyzed the impact of the AVF on the entire gait with
1. The evolution of
2. The Spearman correlation coefficients, between
3. The change of the scaled (GDI) (Schwartz and Rozumalski, 2008), a multivariate measure of overall gait deficit, between NW and AVF-2.
For the last two analyses, only the AVF-2 phase was evaluated, since participants had the longest time to get used to the feedback and to learn an asymmetric gait pattern. No statistical tests were performed with the Spearman correlation coefficient, due to the non-independence between gait cycles. Wilcoxon tests (
GDI computation: The gait features required for the GDI, as described in (Schwartz and Rozumalski, 2008), must include a large scope of gait patterns, healthy but also pathological. In this study, they were obtained by taking the gait vectors from the stride in the middle of each phase (NW, AVF and no-AVF), for left and right sides, for all participants. The total number of gait vectors used to compute the gait features was thus equal to 1 stride × 2 sides × 3 phases × n participants = 6 × n, with n = [19, 17, 20] for ST, POF and APL respectively. The middle-phase stride was selected in order to remove any transition effects. We checked that the obtained features allowed to reconstruct the gait of the participants. The control dataset included one left and one right strides of NW from all the participants. Its feature components were averaged to describe the average control gait to compare against, as described in (Schwartz and Rozumalski, 2008). Finally, using the gait features and the average control feature components described above, we computed the GDI, averaged over ten gait cycles, (i) during NW and (ii) at the end of AVF-2, when participants were expected to exhibit best overall performance. As explained in (Schwartz and Rozumalski, 2008), scores above 100 represent the absence of gait deficit (i.e., no difference with the control gait, here NW), while a decrease of 10 points represents a shift of one standard deviation away from the control mean. A GDI below 90 can typically be considered indicative of mild gait impairment (Mar et al., 2020).
3 Results
3.1 Local impact of the augmented visual feedback
The order of conditions did not affect the results, with no learning or fatigue effect. The evolution of the symmetry ratios,
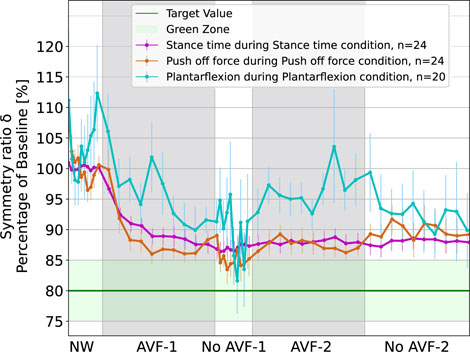
Figure 2. Evolution of symmetry ratios
Regarding the APL condition (in blue), the response is not as clear as for ST and POF. The motor response at group-level was highly variable: the symmetry ratio fluctuated from
3.2 Propagation of asymmetry across gait parameters
Figure 3 shows
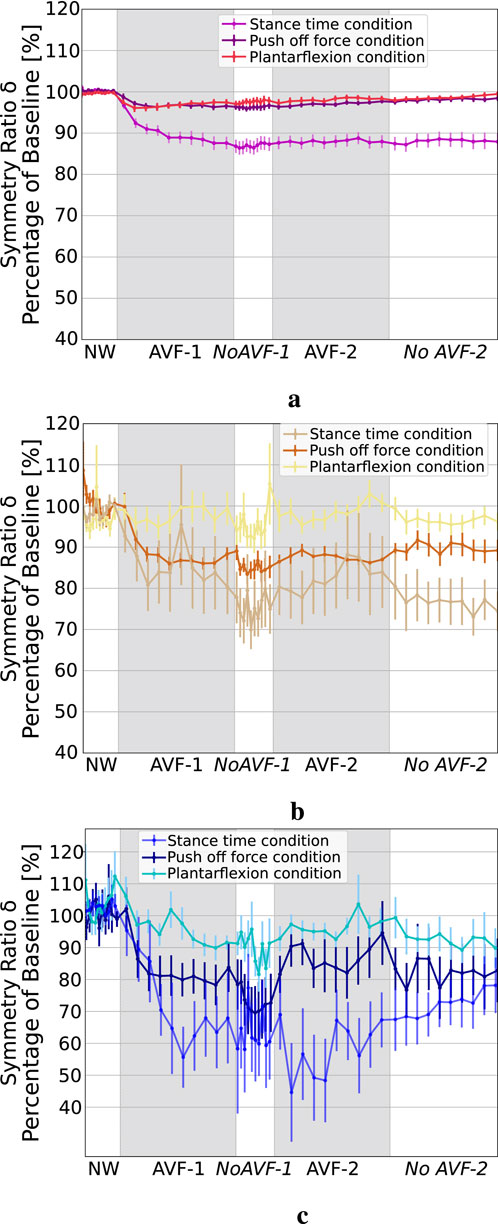
Figure 3. Symmetry ratio of ST, POF and APL during the three AVF conditions. (a) ST symmetry ratio. (b) POF symmetry ratio. (c) APL symmetry ratio.
In addition to the three parameters targeted by the AVF, we analyzed how AVF impacted the symmetry of other relevant and commonly reported gait parameters. Table 1 gathers the Spearman correlation coefficients between the symmetry ratio of the parameter targeted by the feedback and the other ones. The ranking fair
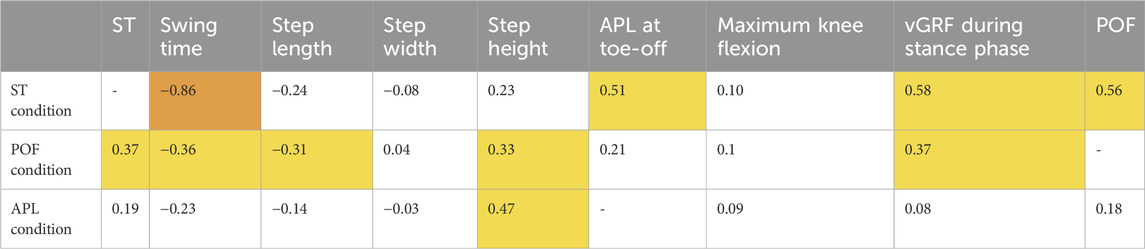
Table 1. Spearman correlation coefficients between the symmetry ratio of the parameter targeted by the AVF and the one of the other gait parameters. Highlighted in yellow are the correlations defined as fair (
3.3 Impact of the augmented visual feedback on the Gait Deviation Index
The scaled GDI, calculated for NW and for AVF-2 phases for each participant, are presented Figure 4. During the ST condition, all but one participant decreased their GDI when walking with the AVF (Figure 4a). The GDI decreased by 7.9±5.1 points on average between NW and AVF-2. The GDI of NW and the GDI of AVF-2 were significantly different
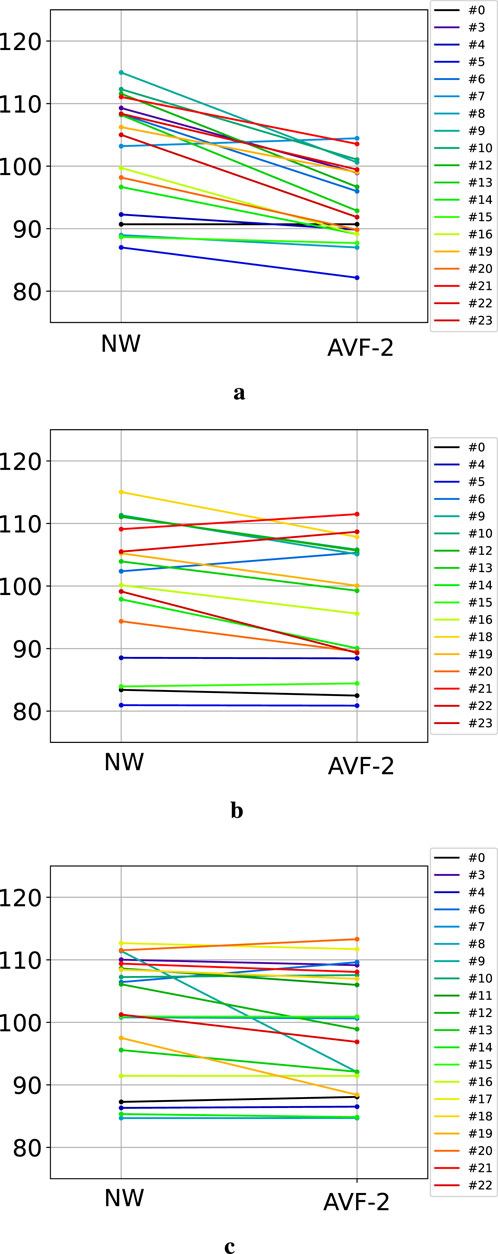
Figure 4. GDI evolution between natural walking and the end of AVF-2, the last feedback phase. Each color represents one participant. (a) GDI during ST condition. (b) GDI during POF condition. (c) GDI during APL condition.
4 Discussion
The objective of this study was to investigate the local and global motor responses to real-time AVF, and to quantify these across different target parameters. With healthy adults, targeting different parameters with visual feedback led to significantly different local motor response patterns, which in turn differentially affected global motion. The AVF drove participants towards gait asymmetry on three separate gait parameters: ST, POF and APL. Results were mixed for APL: participants achieved 5% of asymmetry, which can already be considered as pathological (Patterson et al., 2010), but with large fluctuations, a lot of variability between participants and no significant difference between the last feedback phase and natural walking. This differs with (Colborne et al., 1993), where Colborne et al. presented an AVF targeting ankle kinematics for stroke patients, that had a significant local and global effects (e.g., stride time, knee kinematics) on gait. The AVF design was close to the one of this study (targets to reach at a specific time) but several reasons could explain the distinct results: in Colborne et al. (1993), information on both dorsi and plantarflexion was provided and the AVF was supported by verbal feedback and coupled with auditory feedback (it has been shown that multi-modal feedback can have a higher impact on motor response (Sigrist et al., 2013a)). For ST and POF, participants managed to quickly reach more than 10% of asymmetry and to retain the newly learned gait pattern without the AVF. It thus seems easier to modify gait symmetry when the AVF targets a spatiotemporal or a kinetic parameter. This is aligned with van den Noort et al., who showed that, with healthy subjects, AVF worked better when targeting a kinetic than when targeting a kinematic parameter (van den Noort et al., 2014). With ST or POF, participants focus on the endpoint motion (i.e., the foot), as opposed to the individual joint motion patterns of APL. It may be easier to find how to modify the gait pattern in the first case, compared to when the focus is on one joint. The gait parameter targeted by AVF, with a given mapping, is thus of crucial importance and influences the impact of the AVF on the motor response. Nevertheless, while ST is a discrete parameter and a simple duration to wait until lifting the foot, APL and POF are continuous measures and depend on a whole trajectory. With APL and POF, subjects must be attentive to both amplitude and time and have to find the full trajectory that can lead to the toe-off value targeted by the AVF. The AVF design may not generalize well across the different parameter types, and may require the development of bespoke AVF. Conversely, bespoke AVF would have added a confounder to the experimental design of our study, making it difficult to disentangle the effect of parameter choice from the feedback design. Providing AVF on ST also favors an external focus of attention (i.e., a focus on the effects of one’s movement) compared to providing AVF on POF or APL, which are internal components of one’s movement. Several previous studies showed that augmented feedback with an external focus of attention gave better results than augmented feedback with an internal focus (Magill and Anderson, 2014; Sigrist et al., 2013a; Shafizadeh et al., 2013), which could explain our result on the larger local effect of AVF on ST.
Our work also includes a comprehensive analysis, looking at the impact of the AVF on other components of gait symmetry. We could see that AVF on ST symmetry not only induced local motor adaptations (modifications of
This study highlights that AVF on gait symmetry induces gait changes that exceed the target parameter and that these changes depend on the latter. In this work, we used the symmetry ratio to measure gait symmetry, which is one of the most basic metrics. It is widely used for spatiotemporal parameters (Viteckova et al., 2018; Patterson et al., 2010) but is maybe not the most adequate for kinetics and kinematics, due to the range of their values. Metrics such as the Robinson index (Robinson et al., 1987) or the symmetry angle (Zifchock et al., 2008) could be more suitable, since they were specifically defined for kinetic and kinematic variables and have less dependence on small values. Moreover, we explored one type of AVF only (continuous and visual) and it is still to be explored whether our results are still valid with other modalities (haptic, auditory) or other characteristics (e.g., terminal feedback). The symmetry ratio was encoded in one visualization with one single bar, to keep the AVF signal simple. We yet observed that some participants who did not reach the target symmetry ratio indeed modified their gait but on their left and right sides simultaneously. AVF with more explicit information (left and right sides separately for instance) could have led to more efficient motor response with important gait alteration. Leg dominance may also influence the participant’s response and could be a factor to examine in future works. Finally, further studies should be conducted, first to investigate if the impact of the AVF depends on each parameter individually or if there is a common tendency for the parameters of a same group (spatiotemporal, kinetics and kinematics); second, to evaluate whether results from healthy participants driven towards asymmetry can be transposed to patients driven towards symmetry.
5 Conclusion
This study investigated the role played by the target gait parameter on the local and global impacts of AVF. 24 healthy young adults participated in one session of treadmill walking during which they were driven towards gait asymmetry via real-time AVF. This AVF successively gave information on the symmetry ratio of three gait parameters: ST, POF and APL. We showed (i) that the motor response induced by the AVF depended on the target parameter, with a successful lasting alteration for ST and POF and a less clear effect for APL, and (ii) that the successful alterations of ST and POF also resulted in global gait pattern modifications, with distinct effects depending on the target parameter. AVF on ST impacted POF, APL and vertical ground reaction force symmetry, and disturbed the entire gait as illustrated by a significant decrease of GDI. AVF on POF impacted APL, swing time, step length, step height and vertical ground reaction force. Decrease in GDI due to POF asymmetry was less important than the one due to ST asymmetry but still significant. This work highlights the importance of a careful choice of the target parameter used for the AVF as well as the importance of a comprehensive evaluation of the gait patterns when studying the effect of AVF on gait symmetry. A deeper understanding of the indirect impact of AVF on non-targeted gait parameters is crucial to design personalized feedback scenarios, that target an adequate parameter while minimizing compensatory movements.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by ETH Zürich ethics committee, ETH Zürich. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
ML: Conceptualization, Software, Writing – original draft, Visualization, Supervision, Methodology. FG: Validation, Writing – review and editing, Formal Analysis, Software, Investigation. OH: Writing – review and editing, Formal Analysis, Software, Investigation, Validation. AL: Supervision, Writing – review and editing, Funding acquisition. RG: Funding acquisition, Supervision, Writing – review and editing. OL: Funding acquisition, Writing – review and editing, Supervision, Conceptualization. CA: Writing – review and editing, Funding acquisition, Supervision, Conceptualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the medical research center “The LOOP Zurich”, the Vontobel Foundation, the P&K Foundation, and the ETH Zurich Postdoctoral Fellowship program.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Afzal, M. R., Lee, H., Eizad, A., Lee, C. H., Oh, M. K., and Yoon, J. (2019). Effects of vibrotactile biofeedback coding schemes on gait symmetry training of individuals with stroke. IEEE Trans. Neural Syst. Rehabilitation Eng. 27, 1617–1625. doi:10.1109/tnsre.2019.2924682
Akoglu, H. (2018). User’s guide to correlation coefficients. Turkish J. Emerg. Med. 18, 91–93. doi:10.1016/j.tjem.2018.08.001
Angelis, S. D., Princi, A. A., Farra, F. D., Morone, G., Caltagirone, C., and Tramontano, M. (2021). Vibrotactile-based rehabilitation on balance and gait in patients with neurological diseases: a systematic review and metanalysis. Brain Sci. 11, 518. doi:10.3390/brainsci11040518
Balasubramanian, C. K., Bowden, M. G., Neptune, R. R., and Kautz, S. A. (2007). Relationship between step length asymmetry and walking performance in subjects with chronic hemiparesis. Archives Phys. Med. Rehabilitation 88, 43–49. doi:10.1016/j.apmr.2006.10.004
Cha, Y.-J., Kim, J.-D., Choi, Y.-R., Kim, N.-H., and Son, S.-M. (2018). Effects of gait training with auditory feedback on walking and balancing ability in adults after hemiplegic stroke: a preliminary, randomized, controlled study. Int. J. Rehabilitation Res. 41, 239–243. doi:10.1097/mrr.0000000000000295
Chamorro-Moriana, G., Moreno, A. J., and Sevillano, J. L. (2018). Technology-based feedback and its efficacy in improving gait parameters in patients with abnormal gait: a systematic review. Sensors 18, 142. doi:10.3390/s18010142
Cohen, J. W., Ivanova, T. D., Brouwer, B., Miller, K. J., Bryant, D., and Garland, S. J. (2018). Do performance measures of strength, balance, and mobility predict quality of life and community reintegration after stroke? Archives Phys. Med. Rehabilitation 99, 713–719. doi:10.1016/j.apmr.2017.12.007
Colborne, G., Olney, S. J., and Griffin, M. P. (1993). Feedback of ankle joint angle and soleus electromyography in the rehabilitation of hemiplegic gait. Archives Phys. Med. Rehabilitation 74, 1100–1106. doi:10.1016/0003-9993(93)90069-m
Dozza, M., Chiari, L., Hlavacka, F., Cappello, A., and Horak, F. B. (2006). Effects of linear versus sigmoid coding of visual or audio biofeedback for the control of upright stance. IEEE Trans. Neural Syst. Rehabilitation Eng. 14, 505–512. doi:10.1109/tnsre.2006.886732
Durham, K., Vliet, P. M. V., Badger, F., and Sackley, C. (2009). Use of information feedback and attentional focus of feedback in treating the person with a hemiplegic arm. Physiother. Res. Int. 14, 77–90. doi:10.1002/pri.431
Feigin, V. L., Stark, B. A., Johnson, C. O., Roth, G. A., Bisignano, C., Abady, G. G., et al. (2021). Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet Neurology 20, 795–820. doi:10.1016/s1474-4422(21)00252-0
Friedman, P. J. (1990). Gait recovery after hemiplegic stroke. Int. Disabil. Stud. 12, 119–122. doi:10.3109/03790799009166265
Geijtenbeek, T., Steenbrink, F., Otten, B., and Even-Zohar, O. (2011). “D-flow: immersive virtual reality and real-time feedback for rehabilitation,” in Proceedings of the 10th International Conference on Virtual Reality Continuum and Its Applications in Industry, Hong Kong, China, December 11 - 12, 2011 (ACM). doi:10.1080/10749357.2018.1436384
Genthe, K., Schenck, C., Eicholtz, S., Zajac-Cox, L., Wolf, S., and Kesar, T. M. (2018). Effects of real-time gait biofeedback on paretic propulsion and gait biomechanics in individuals post-stroke. Top. Stroke Rehabilitation 25, 186–193. doi:10.1080/10749357.2018.1436384
Jørgensen, H. S., Nakayama, H., Raaschou, H. O., and Olsen, T. S. (1995). Recovery of walking function in stroke patients: the copenhagen stroke study. Archives Phys. Med. Rehabilitation 76, 27–32. doi:10.1016/S0003-9993(95)80038-7
Jung, K.-S., Bang, H., In, T.-S., and Cho, H.-Y. (2020). Gait training with auditory feedback improves trunk control, muscle activation and dynamic balance in patients with hemiparetic stroke: a randomized controlled pilot study. J. Back Musculoskelet. Rehabilitation 33, 1–6. doi:10.3233/bmr-170852
Katan, M., and Luft, A. (2018). Global burden of stroke. Seminars Neurology 38, 208–211. doi:10.1055/s-0038-1649503
Kim, C., and Eng, J. J. (2003). Symmetry in vertical ground reaction force is accompanied by symmetry in temporal but not distance variables of gait in persons with stroke. Gait and Posture 18, 23–28. doi:10.1016/s0966-6362(02)00122-4
Kinnaird, C., Lee, J., Carender, W. J., Kabeto, M., Martin, B., and Sienko, K. H. (2016). The effects of attractive vs. repulsive instructional cuing on balance performance. J. NeuroEngineering Rehabilitation 13, 29. doi:10.1186/s12984-016-0131-z
Koritnik, T., Koenig, A., Bajd, T., Riener, R., and Munih, M. (2010). Comparison of visual and haptic feedback during training of lower extremities. Gait and Posture 32, 540–546. doi:10.1016/j.gaitpost.2010.07.017
Legrand, M. L., Magrini, C., Branscheidt, M., Luft, A., Gassert, R., Lambercy, O., et al. (2024). “Augmented feedback for gait symmetry: a comprehensive evaluation of gait modification,” in 2024 IEEE international conference on biomedical robotics and biomechatronics (BioRob). doi:10.1109/BioRob60516.2024.10719727
Li, S., Francisco, G. E., and Zhou, P. (2018). Post-stroke hemiplegic gait: new perspective and insights. Front. Physiology 9, 1021. doi:10.3389/fphys.2018.01021
Liu, L. Y., Sangani, S., Patterson, K. K., Fung, J., and Lamontagne, A. (2020). Real-time avatar-based feedback to enhance the symmetry of spatiotemporal parameters after stroke: instantaneous effects of different avatar views. IEEE Trans. Neural Syst. Rehabilitation Eng. 28, 878–887. doi:10.1109/tnsre.2020.2979830
Magill, R. A., and Anderson, D. (2014). Motor learning and control: concepts and applications. 10th edn. New York: McGraw Hill, Connect Learn Suceed.
Mar, D., Lieberman, I., and Haddas, R. (2020). The gait deviation index as an indicator of gait abnormality among degenerative spinal pathologies. Eur. Spine J. 29, 2591–2599. doi:10.1007/s00586-019-06252-2
Meyer, C., Killeen, T., Easthope, C. S., Curt, A., Bolliger, M., Linnebank, M., et al. (2019). Familiarization with treadmill walking: how much is enough? Sci. Rep. 9, 5232. doi:10.1038/s41598-019-41721-0
Ogihara, H., Tsushima, E., Kamo, T., Sato, T., Matsushima, A., Niioka, Y., et al. (2020). Kinematic gait asymmetry assessment using joint angle data in patients with chronic Stroke—A normalized cross-correlation approach. Gait and Posture 80, 168–173. doi:10.1016/j.gaitpost.2020.05.042
Olney, S. J., and Richards, C. (1996). Hemiparetic gait following stroke. Part I: characteristics. Gait and Posture 4, 136–148. doi:10.1016/0966-6362(96)01063-6
Park, J., and Kim, T.-H. (2019). The effects of balance and gait function on quality of life of stroke patients. NeuroRehabilitation 44, 37–41. doi:10.3233/NRE-182467
Patterson, K. K., Parafianowicz, I., Danells, C. J., Closson, V., Verrier, M. C., Staines, W. R., et al. (2008). Gait asymmetry in community-ambulating stroke survivors. Archives Phys. Med. Rehabilitation 89, 304–310. doi:10.1016/j.apmr.2007.08.142
Patterson, K. K., Gage, W. H., Brooks, D., Black, S. E., and McIlroy, W. E. (2010). Evaluation of gait symmetry after stroke: a comparison of current methods and recommendations for standardization. Gait and Posture 31, 241–246. doi:10.1016/j.gaitpost.2009.10.014
Python library (2023). Gaitalytics. Available online at: https://libraries.io/pypi/gaitalytics.
Reh, J., Schmitz, G., Hwang, T.-H., and Effenberg, A. O. (2022). Loudness affects motion: asymmetric volume of auditory feedback results in asymmetric gait in healthy young adults. BMC Musculoskelet. Disord. 23, 586. doi:10.1186/s12891-022-05503-6
Richards, C. L., Malouin, F., and Nadeau, S. (2015). “Stroke rehabilitation,” in Sensorimotor rehabilitation - at the crossroads of basic and clinical sciences (Elsevier), 253–280.
Robinson, R., Herzog, W., and Nigg, B. M. (1987). Use of force platform variables to quantify the effects of chiropractic manipulation on gait symmetry. J. Manip. physiological Ther. 10, 172–176.
Rozumalski, A., and Schwartz, M. H. (2011). The GDI-Kinetic: a new index for quantifying kinetic deviations from normal gait. Gait and Posture 33, 730–732. doi:10.1016/j.gaitpost.2011.02.014
Schmidt, R. A., Zelaznik, H. N., Winstein, C., Wulf, G., and Lee, T. D. (2018). Motor control and learning. Champaign, IL: Human Kinetics Publishers.
Schwartz, M. H., and Rozumalski, A. (2008). The gait deviation index: a new comprehensive index of gait pathology. Gait and Posture 28, 351–357. doi:10.1016/j.gaitpost.2008.05.001
Shafizadeh, M., Platt, G. K., and Mohammadi, B. (2013). Effects of different focus of attention rehabilitative training on gait performance in multiple sclerosis patients. J. Bodyw. Mov. Ther. 17, 28–34. doi:10.1016/j.jbmt.2012.04.005
Sigrist, R., Rauter, G., Riener, R., and Wolf, P. (2013a). Augmented visual, auditory, haptic, and multimodal feedback in motor learning: a review. Psychonomic Bull. and Rev. 20, 21–53. doi:10.3758/s13423-012-0333-8
Sigrist, R., Rauter, G., Riener, R., and Wolf, P. (2013b). Terminal feedback outperforms concurrent visual, auditory, and haptic feedback in learning a complex rowing-type task. J. Mot. Behav. 45, 455–472. doi:10.1080/00222895.2013.826169
Spencer, J., Wolf, S. L., and Kesar, T. M. (2021). Biofeedback for post-stroke gait retraining: a review of current evidence and future research directions in the context of emerging technologies. Front. Neurology 12, 637199. doi:10.3389/fneur.2021.637199
Tomita, Y., Sekiguchi, Y., and Mayo, N. E. (2024). Efficacy of a single-bout of auditory feedback training on gait performance and kinematics in healthy young adults. Sensors 24, 3206. doi:10.3390/s24103206
van den Bogert, A. J., Geijtenbeek, T., Even-Zohar, O., Steenbrink, F., and Hardin, E. C. (2013). A real-time system for biomechanical analysis of human movement and muscle function. Med. and Biol. Eng. and Comput. 51, 1069–1077. doi:10.1007/s11517-013-1076-z
van den Noort, J. C., Steenbrink, F., Roeles, S., and Harlaar, J. (2014). Real-time visual feedback for gait retraining: toward application in knee osteoarthritis. Med. and Biol. Eng. and Comput. 53, 275–286. doi:10.1007/s11517-014-1233-z
Viteckova, S., Kutilek, P., Svoboda, Z., Krupicka, R., Kauler, J., and Szabo, Z. (2018). Gait symmetry measures: a review of current and prospective methods. Biomed. Signal Process. Control 42, 89–100. doi:10.1016/j.bspc.2018.01.013
Keywords: augmented feedback, gait symmetry, gait training, motor adaptation, therapy personalization
Citation: Legrand M, Grenet F, Hochstrasser O, Luft A, Gassert R, Lambercy O and Awai CE (2025) Real-time augmented feedback for gait training: are gait responses affected by the choice of target parameter?. Front. Bioeng. Biotechnol. 13:1645390. doi: 10.3389/fbioe.2025.1645390
Received: 11 June 2025; Accepted: 25 July 2025;
Published: 08 August 2025.
Edited by:
Philip Requejo, Rancho Research Institute, United StatesReviewed by:
Fu-Lien Wu, University of Nevada, Las Vegas, United StatesInes Khiyara, University of Maine, United States
Copyright © 2025 Legrand, Grenet, Hochstrasser, Luft, Gassert, Lambercy and Awai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chris Easthope Awai, Y2hyaXMuYXdhaUBsbHVpLm9yZw==
 Mathilde Legrand
Mathilde Legrand Florette Grenet3
Florette Grenet3 Roger Gassert
Roger Gassert Olivier Lambercy
Olivier Lambercy Chris Easthope Awai
Chris Easthope Awai