- 1Institute of Translational Medicine, Jining Medical University, Rizhao, China
- 2Institute of Translational Medicine, Shanghai Jiao Tong University, Shanghai, China
- 3Department of Orthopaedics, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 4School of Rehabilitation Science, Shanghai University of Traditional Chinese Medicine, Shanghai, China
- 5Department of Orthopaedics, The Second Xiangya Hospital of Central South University, Changsha, China
- 6Department of Nursing, The First Affiliated Hospital of Dalian Medical University, Dalian, China
- 7Operating room, The First Affiliated Hospital of Dalian Medical University, Dalian, China
- 8Department of Orthopaedics, The First Affiliated Hospital of Dalian Medical University, Dalian, China
- 9Department of Orthopedics, Affiliated Hospital of Jining Medical University, Jining, China
- 10Department of Orthopaedic Surgery, Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
Biomechanical principles are crucial for spinal research, enabling precise analysis of spinal behavior under various conditions. Through quantitative analysis and simulation of mechanical information, spinal function can be optimized, posture improved, and injury risk reduced. However, current methodologies in spine biomechanics remain incomplete and challenging for researchers to apply effectively. This paper reviews recent advances in experimental models, loading methods, instrumentation, and test data used in spine biomechanics research, aiming to provide a reference for research design and future developments in the field. Two primary types of research objects are highlighted: measurement models and digital models. Common loading modes include dynamic, quasi-static, and static approaches. Various experimental setups, each with distinct characteristics, play essential roles in simulating motion and collecting data. The most frequently used biomechanical indicators are also discussed. In summary, this work redefines, categorizes, and synthesizes the typical research workflow in spinal biomechanics. It is foreseeable that more unified, accurate, and innovative studies will enhance the application of spinal biomechanics in rehabilitation, treatment, auxiliary materials, and clinical evaluation.
Introduction
Biomechanics is a branch of biophysics that applies the principles and methods of mechanics to carry out quantitative research on mechanical problems in organisms. The early studies of anatomy and physicists laid the foundation for modern science and biomechanics. At first, people were obsessed with the study of bone properties. Andreas Vesalius is the first scholar who described the biomechanics of spinal structure in detail from the anatomical level. Giovanni Alfonso Borelli applied the principles of “rotational balance” and “translational balance” to the analysis of spine biomechanics (Pope, 2005). In 1680, he published The Animal Movement, which is considered the pioneering work of biomechanics. It introduced many valuable calculation methods for spinal biomechanics research. Additionally, Borelli was the first to recognize the importance of the intervertebral disc structure, which supports most of the spinal load (Borelli, 1989). Leonhard Euler found that the human spine like a cylinder bears the compression load through the study of digital model, which would lead to spinal instability (Gribbin, 2010; Sanan and Rengachary, 1996).
Spine biomechanics showed a vigorous development trend from the mid-19th century to the early 20th century (Oxland, 2015). At that time, the research of spine biomechanics mostly focused on the military and sports fields (Guillemin 1950; Hirt, 1955). In the mid-19th century, Hirsch, together with Nachemson, pioneered the direct measurement of spinal load. They started with cadaveric intervertebral discs and gradually extended it to in vivo measurement of human body (Nachemson and Morris, 1964; Nachemson, 1966). The effects of these methods were also verified in later studies (Sato et al., 1999; Wilke et al., 1999). Lissner studied spinal biomechanics in the early 1950s (Gurdjian et al., 1949; Hardy et al., 1958; Evans and Lissner, 1959). Then, he conducted a study on the effect of axial compression and lateral bending on lumbar disc herniation. This was also considered to be the first truly modern spinal biomechanical experiment. Lysell had also made a great contribution to the in vitro study of cervical motion and movement patterns (Lysell, 1969). He used fresh cadaveric cervical spine specimens (C2-T1), inserted four steel balls into each vertebral body, and measured the relative three-dimensional motion of each vertebral body by taking quantitative stereogram. In 28 specimens, he found that age or the degree of degeneration of tissue structure had little effect on the range of motion (ROM) (Lysell, 1969). In the following decades, with the development of modern science and technology, the spine biomechanics with animal models and human models as the main research materials had made continuous progress, and gradually refined the four elements needed for these research: experimental model, loading method, experimental installation, test data. In the middle of the 20th century, the emergence of computers provided the most important technical basis for the digital development of models. Subsequently, the rapid progress of biomechanical digital technology continued to update iteration, the emergence of a finite element model as the representative of the digital model. Brekelmans et al. introduced the finite element method into the field of orthopedics for the first time in 1972 (Brekelmans et al., 1972). Then Schultz, Belytschko and Ha-Kim tried to apply the engineering research method of finite element analysis technology to spine modeling (Belytschko et al., 1974; Hakim and King, 1979). After the 1970s, spinal biomechanics underwent significant development, with key contributions from Panjabi and White in understanding spinal stability and mechanical behavior (Panjabi et al., 1976). Panjabi proposed the theory of spinal stability, emphasizing the role of ligaments and the three-joint complex in maintaining spinal stability, which laid the foundation for understanding spinal injury and instability mechanisms. White and Panjabi’s book Clinical Biomechanics of the Spine provided a comprehensive theoretical framework for the mechanical behavior of the spine. With advancements in computer technology, finite element analysis was applied to spinal modeling and simulation, enabling more accurate representations of spinal dynamics, injury mechanisms, and treatment outcomes. In the 21st century, spinal biomechanics research further progressed with the rise of patient-specific modeling and multiscale modeling, incorporating CT, MRI, and other medical imaging technologies to create precise models based on individual differences. Additionally, the integration of artificial intelligence and machine learning has opened new possibilities for spinal surgery planning, rehabilitation, and pathological analysis, further advancing both clinical applications and research in spinal biomechanics.
However, as the field has expanded, the issue of lacking standardization has become more pronounced. The absence of universally accepted standards has resulted in considerable inconsistencies in experimental designs, data collection methods, and model simulations, leading to difficulty in comparing results across different studies. Additionally, the reproducibility and reliability of spinal biomechanical models are compromised, affecting the clinical translation of research findings. Therefore, we hope to provide more diversified design ideas and better element combination schemes for the research in this field.
Experimental model
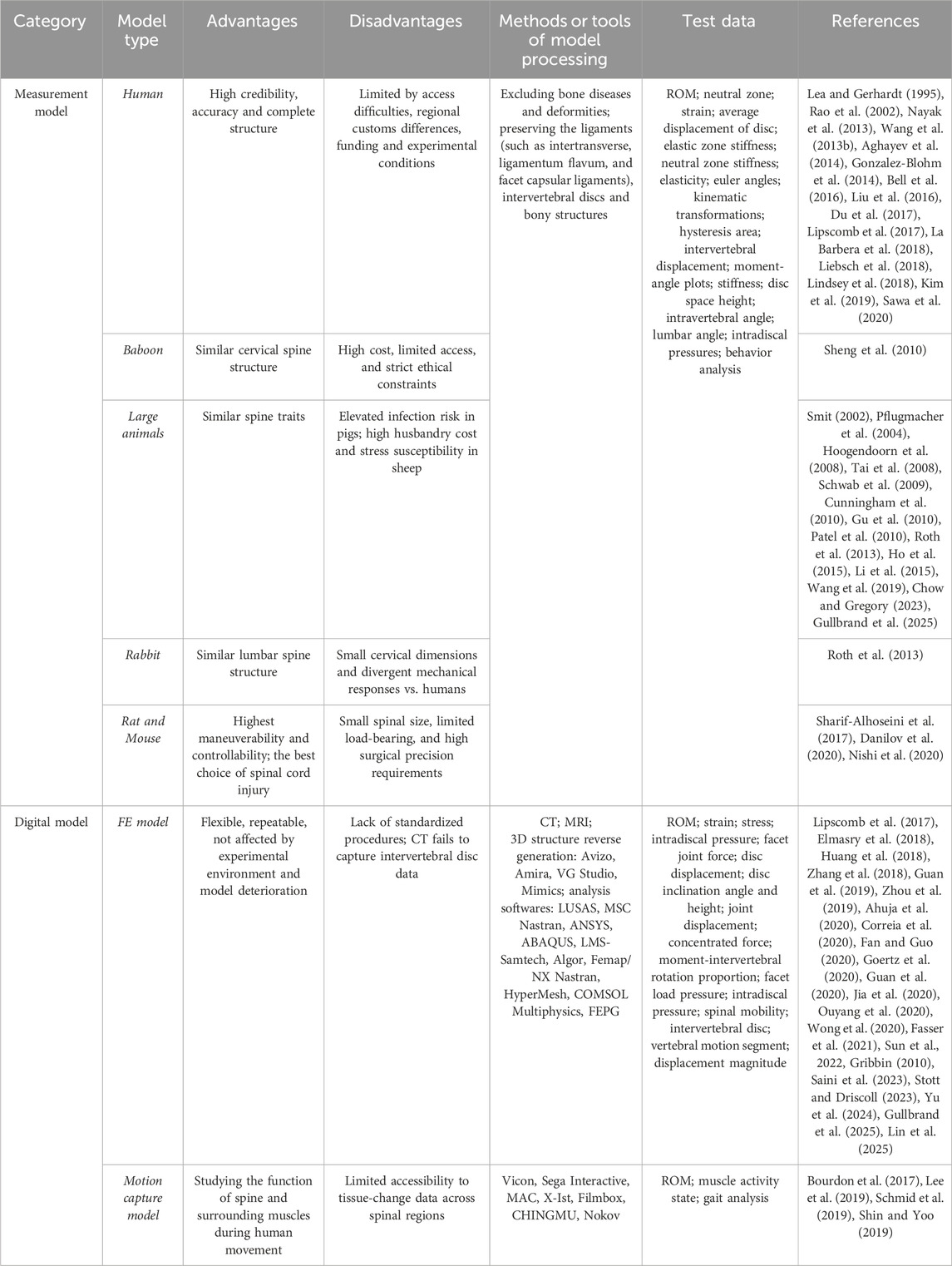
Measurement model
The origin and development of spine biomechanics is based on the initial exploration of human body model. The experimental results obtained by using human model for functional research are more convincing. Both the most widely used animal models and the emerging finite element models are based on human models. All measurement models were summarized in Table 1.
Human models have been used in various research fields of spinal biomechanics, such as physiological characteristics of spine (Sawa et al., 2020), surgical prediction of spinal diseases (Chen et al., 2020), and surgical implants (Ludwig et al., 2000; Wang T. et al., 2013). The advantage of human body model lies in high credibility, high accuracy and complete structure. Therefore, when the model structure selected in the study is closer to the human’s, the reliability of the experimental results will be more convincing (Bozkus et al., 2001). But the application of human body model is more and more limited. For example, it is difficult to obtain the model, ethical Limitations, regional customs differences, funding and experimental conditions.
After many years of screening and trying, mammals have been the main species that can be used for spine biomechanics research, including monkeys, dogs, pigs, sheep, cattle, rabbits and mice. The advantages of animal models are abundant in quantity and easy to obtain. However, the spinal structure of animals is different from that of human beings. For example, TES-induced electric field strength strongly decreases from smaller to larger specimen with up to 100-fold differences across species (Alekseichuk et al., 2019). And the differences in weights, sizes, and characteristics can lead to significant inter-animal variation (Schimandle and Boden, 1994). Therefore, animal models should be selected according to the needs and characteristics of biomechanical research.
Primates have closely evolution relationship with human beings. Monkeys, orangutans, baboons and so on are available for selection in biomechanical research. For example, the spine structure of baboon, especially the cervical spine structure, is very similar to that of human (Sheng et al., 2010). Secondly, the vertebrae anatomical structure of large animals such as pigs, cattle, sheep and dogs in order of perissodactyla and artiodactyla is close to that of human beings. And the stress of spine in physiological state which loaded along the long axis is similar to that of human spine, so it can be the first choice for most spine biomechanical studies (Smit, 2002). Pigs were selected most in scoliosis correction studies (Schwab et al., 2009; Patel et al., 2010; Roth et al., 2013). The biomechanical properties of sheep spinal model are also outstanding, which is also a good choice for spinal biomechanical research (Hoogendoorn et al., 2008; Wang et al., 2019). In addition, some studies have found that the anatomical structure of rabbit and human lumbar spine is similar, and there is enough iliac bone as a support, which is suitable for some studies with low load and stress requirements (Roth et al., 2013). Although the size of rats is small, they have the highest maneuverability and controllability, which is the best choice for the study of spinal cord injury (Sharif-Alhoseini et al., 2017; Danilov et al., 2020; Nishi et al., 2020).
In addition to the above common animal models, some new species have also gradually attracted people’s attention. Kangaroo is an upright animal, which makes the mechanical properties of its spine more like the spine of human beings (Balasubramanian et al., 2016). Some scholars use Japanese small game fowls to study cervical kyphosis (Shimizu et al., 2005). In addition, researchers are also committed to developing new model species through artificial intervention, such as Ao et al., who successfully established a bipedal standing mouse model for the study of intervertebral disc degeneration (Ao et al., 2019).
Digital model
In recent years, due to the rapid development of computer technology, a variety of digital spine research models emerge in endlessly, among which the finite element model and motion capture model are the most widely used. All digital models were summarized in Table 1.
Three-dimensional finite element analysis technology uses the principle of mathematical approximation to simulate the real physical system by setting geometry and load conditions. The birth of finite element model depended on the continuous progress of digital model. The early digital model come from three-dimensional reconstruction technology, such as VOXEL-MAN system (Schiemann et al., 2000), but which has great limitations in the use value and application scope (Jager, 1996). Until the mid-20th century, the finite element model began to rise and was gradually used in various fields of medicine, and emerged in the field of spine biomechanics (Tien and Huston, 1985; Zhai et al., 2019). Researchers use the detection instrument to scan the spine of subjects, and get the required finite element model after software processing. Finite element models of spine often appear in four forms, including whole spine model, local segment model, normal model and pathological model (Liu et al., 2017). Among them, the whole spine model is mostly simplified, which is composed of several segments and the interrelation among the segments (Lalonde et al., 2013). Common reconstruction software could reverse generate 3D model from scanning data, such as Myrian XP-Liver, HexaUnion3D and Mimics. The establishment methods of gender spinal models have also made initial attempts. (Hindman et al., 2020; Stott and Driscoll, 2024). Preparation of personalized model using 3D printing from MRI, CT data is a technique which is cost effective, time efficient, high fidelity, biomechanically accurate and enables proof of concept for simulating finite element models of the lumbar spine (Bohl et al., 2020; Dukkipati and Driscoll, 2025). Moreover, 3D printing technology can make the new type of model, which contains the advantages of both human model and digital model (Clifton et al., 2019). Finite element model is flexible, repeatable, and not affected by experimental environment and model deterioration. Therefore, it is often used in some destructive experiments to collect model change data at multiple time points, such as impact test (Huang et al., 2018; Correia et al., 2020). At the same time, the construction of finite element model has strong pertinence and can realize individual service and research, such as stress state of spinal tissue and artificial implant (Elmasry et al., 2018; Huang et al., 2018; Zhang et al., 2018; Zhou et al., 2019; Guan et al., 2020; Jia et al., 2020; Ouyang et al., 2020; Wong et al., 2020). In addition, traditional finite element models of the spine require laborious segmentation of medical images and extensive computation, but AI methods now automate and accelerate this process. For example, deep learning can generate patient-specific finite element models from MRI scans by creating synthetic CT images and automatically segmenting vertebrae and discs, achieving high geometric accuracy without exposing patients to radiation. This individualized modeling overcomes the time-consuming manual workflows and ensures that simulations reflect a specific patient’s anatomy and tissue properties.
The establishment of motion capture model is different from the finite element model. It is to set up a tracker in the key parts of the moving object, then by monitoring the position of the tracker, the three-dimensional coordinate data can be obtained after computer processing. When the data is recognized by computer, the motion capture model is formed in the software, which can be applied to gait analysis, biomechanics, ergonomics and other fields. At present, a variety of commercial motion capture devices have been developed, among which the optical motion capture system is widely used in the field of spine biomechanics. The advantage of motion capture model is not only its authenticity and non-destructive, but also its real-time monitoring, which is convenient for dynamic research. Combined with AI technology, it allows for the estimation of 3D body shape and spinal kinematic postures with just one camera (Ghezelbash et al., 2024). Therefore, it is widely used in the field of spinal biomechanics, such as sports and rehabilitation exercise.
Model preprocessing
After selecting the appropriate model type, we should choose a reasonable method to preprocess the model. The purpose is to remove the redundant tissue in the model to optimize the experimental scheme, so as to obtain the experimental data more directly.
Firstly, one or more spinal segments were selected according to the needs of the study. Panjabi et al. have pointed out that the functional spinal unit (FSU), defined as two adjacent vertebrae with interconnecting connective tissue, was the basic anatomic unit of study requires dividing the specimen into interest segment (Panjabi et al., 1976). The more FSUs involved in the study, the more factors need to be considered, such as the physiological curvature of the spine and the interaction between the structures, which will increase the difficulty of the experiment and affect the accuracy of the results. Secondly, muscle and fat were removed from the study subjects for pre-processing. It should be noted that ligaments, intervertebral disc and bony structure of spine should be retained (Chazal et al., 1985; Pintar et al., 1992; Han et al., 2012; Bell et al., 2016; Lipscomb et al., 2017; La Barbera et al., 2018). After pretreatment, the spinal structure is relatively complete, which can effectively separate the spinal part from other parts. It is worth noting that the influence of ribs on the stability of thoracic vertebrae model should be fully considered when the thoracic vertebrae model is pretreated. A large number of studies have confirmed that the thoracic cage could provide passive stability for the spine to a certain extent (Sham et al., 2005; Ignasiak et al., 2016; Liebsch et al., 2018). Thirdly, in order to fully ensure the representativeness of the model selected, it is necessary to use detection instruments to assist researchers in excluding unqualified model individuals. Before most spine biomechanical studies, X-ray examination is needed for the subjects to exclude bone diseases and deformities of the model (Xue et al., 2013; Li et al., 2015; Sturges et al., 2016; Liebsch et al., 2018). And bone mineral density examination instrument can also be used to check osteoporosis of the model (Nayak et al., 2013; Aghayev et al., 2014). Finally, animal models and human models are in vitro tissue, and the structure of soft tissues such as ligaments will change with time. Therefore, formal experiments should be started after 2–5 pre-experiments to reduce the impact caused by the viscoelastic effect of the model itself (Wilke et al., 1998).
Experimental loading mode
Dynamic experiment
There are two kinds of dynamic tests. One is the destructive tests with the change of load rate or deformation rate with time, such as fatigue test, impact test and dynamic simulation test of products. The other one is the non-destructive test to study the properties of materials by periodic stress or deformation. Dynamic loading method is mostly used for the study of spinal joint damage (Liu et al., 1983; Tsai et al., 1998) and strength of artificial implants (Burval et al., 2007).
Quasi static experiment
Quasi static process is the sum of all the states that the system experiences when it changes from one equilibrium state to another. Quasi static loading is the most common loading method in spine biomechanics. In the process of slow loading, the related structures of the spine will not be damaged, which can better display the functions of the spine and related structures in daily state. The research of the complex mechanical behavior of a certain segment of the spine (Liebsch et al., 2018; Yamamoto et al., 2022) and the stability of artificial implants after spinal surgery (Pflugmacher et al., 2004; Gu et al., 2010; Aghayev et al., 2014; Gonzalez-Blohm et al., 2014) are the typical examples.
Static experiment
Static test is a kind of strength test and different with dynamic test. Its characteristic is small load, slow deformation speed and short measuring time. Tensile test and hardness test are often adopted static test. In the research of spine biomechanics, the pure static loading is rare, and the static loading and quasi-static loading are usually carried out alternately.
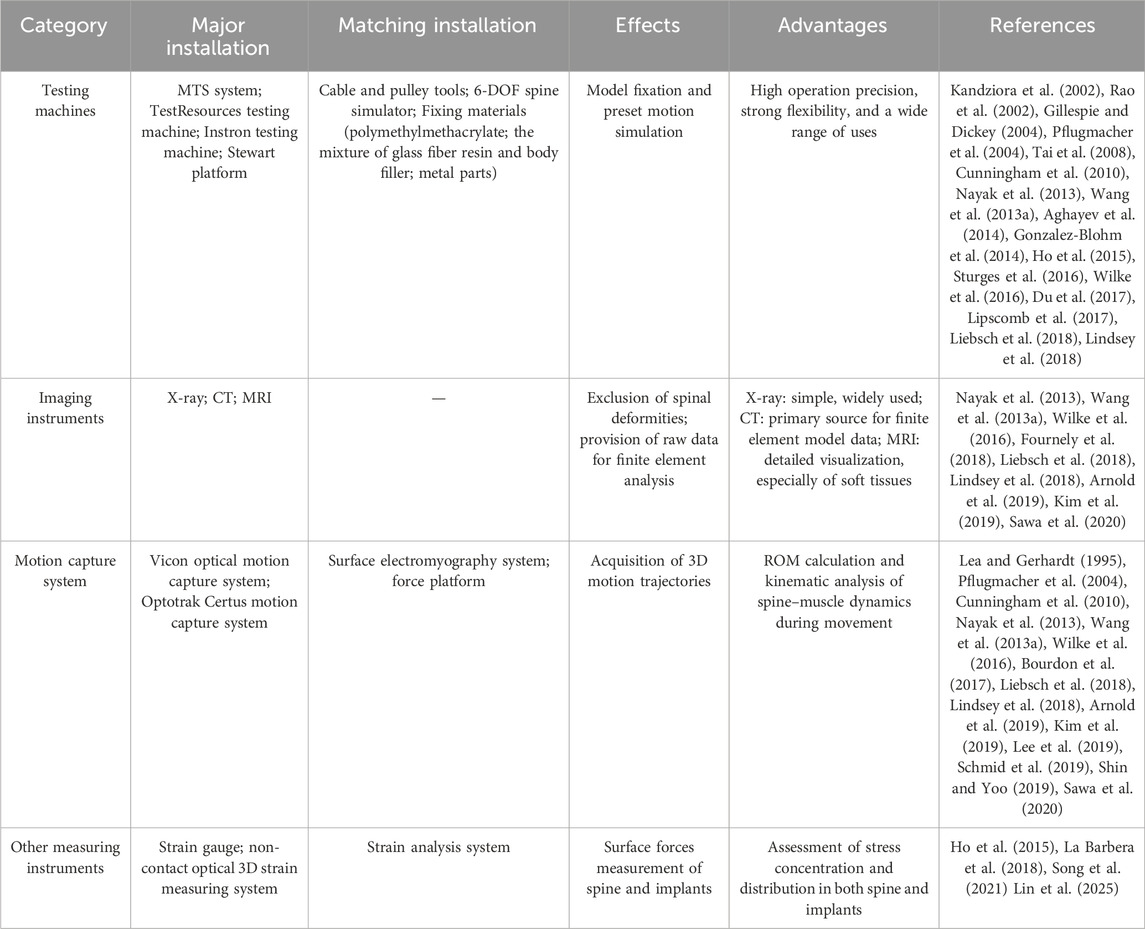
Experimental installation
Installation for physical model testing
The main function of the platform is to drive the model to make various preset movements, such as flexion, extension, lateral bending and rotation. The applicability and operability of the test platform will directly affect the effect of the spinal model to complete the related movements. Installation of spine biomechanics for physical model testing was summarized in Table 2.
The most widely used testing platform in spine biomechanics research is the commercialized testing machines. And MTS system company’s testing machine is the most commonly used testing platform characterized by its high operation precision and strong flexibility, such as 858 Mini bionix type II MTS testing machine. This kind of platform had been used in the research of the stability of sacroiliac joint, the related performance test of intervertebral fusion cage and other research of spine biomechanics (Aghayev et al., 2014; Gonzalez-Blohm et al., 2014; Liu et al., 2016; Lindsey et al., 2018). Du et al. also used the MTS system of Model 608.33. in the research on the influence of intervertebral cage height on lumbar spine (Du et al., 2017), which has stable testing effect and has become the preferred platform for most spinal biomechanical studies (Pflugmacher et al., 2004; Tai et al., 2008; Cunningham et al., 2010). Besides, TestResources testing machine (Nayak et al., 2013), Instron testing machine (Ho et al., 2015; Lipscomb et al., 2017) and Stewart platform (Gillespie and Dickey, 2004) can also be good choices for spine biomechanical research.
Although the commercialized testing machine is a mature experimental platform, its function is still limited. Therefore, in the research with special needs, some auxiliary devices used with the testing machine also play a vital role. The most common auxiliary device is the cable and pulley tools (Kandziora et al., 2002; Rao et al., 2002; Pflugmacher et al., 2004; Gu et al., 2010; Nayak et al., 2013). The testing machine is used to drive the cable and pulley tools to apply pure bending moment, so as to induce bending, extension, left and right lateral bending, left and right axial rotation and other motion states. This system can make the model better simulate various actions under physiological state, so it is widely used. Gillespie et al. had used the improved Stewart platform to assist the model to produce different actions, which also achieved the ideal effect (Gillespie and Dickey, 2004). In addition, some researchers had developed a special six degrees of freedom (6-DOF) spine simulator, which could be used with the testing machine to simulate different spinal movements more conveniently and efficiently, and was expected to become the ideal measurement platform in future spinal biomechanics research (Xue et al., 2013).
After choosing the most suitable testing machine, the next key problem is how to fix the model on the test platform. There are two key points in the selection of fixation method: the fixation device should be able to adapt to the tested models; and under the condition of meeting the experimental requirements, the fixation device should give the model enough stability to prevent the model from fretting during the experiment and affect the research results. These two requirements make the fixture must have strong adaptability and stability.
In the past, a variety of materials have been used to fix the spinal model, the most commonly used are resin materials and metal materials. Resin materials have strong shaping, simple fabrication and high fit with the model, but poor fixation performance. Although the manufacturing process of metal materials is complex and the plasticity is poor, their firmness is often better than other common materials. Metal materials are generally used in the form of fixtures or tooling. Many studies choose metal parts combined with resin materials to fix the model. In Barbera’s study, for exploring the application effect of anterior interbody fusion cage and auxiliary rod, they embed the head end and tail end of the spine with polymethylmethacrylate (PMMA), and then PMMA is connected with the endplate structure at both ends of the model through screws, so as to achieve better reinforcement effect (La Barbera et al., 2018). The similar fixation method was used by Liebsch, Wilke and others (Pflugmacher et al., 2004; Nayak et al., 2013; Wang M. et al., 2013; Wilke et al., 2016; Liebsch et al., 2018). In addition to PMMA, Aghayev and Sabrina used the mixture of glass fiber resin and body filler to fix the spinal model in the in vitro study of spinal biomechanics (Aghayev et al., 2014; Gonzalez-Blohm et al., 2014).
Although a variety of fixation devices and methods for spinal models have been reported, there is still no unified fixation scheme. The reason may be related to the variety of spinal models and individual differences, which easily leads to the fixation device cannot be applied to all spinal biomechanical experiments. From the current research situation, resin material combined with metal parts for model fixation is a method with the strongest applicability. Moreover, other special fixation devices have complex process and small scope of application, but its advantages of reusability can also ensure the stability of the fixation and improve the experimental efficiency.
Clinically, physical model testing combined with cadaveric experiments provides biomechanical references for precision treatment in spinal surgery. As demonstrated by O'Hehir et al. using cadaveric biomechanical techniques, they proved that tethers can generally delay the onset of proximal junctional kyphosis and reduce angular changes (O'Hehir et al., 2024).Rudy et al. used either 2 or 4 screws and 2 or 4 rods for pelvic fixation on cadaveric models (Rudy et al., 2025). This approach showed no significant changes in strain in proximal connecting screws or rods in the cadavers, demonstrating that robust pelvic fixation can prevent distal failure and does not impose harmful effects on the proximal junction.
Installation for finite element model testing
X-ray is mainly used to exclude bone abnormality and deformity of spinal model before finite element study (Zhang et al., 2018). CT is often used for spiral scanning and tomography of the spine in research. The thinner the slice, the finer the three-dimensional image, which provides accurate original data for the later finite element analysis of neck and chest. At the same time, CT scan can also check whether the spinal model is normal (Elmasry et al., 2018; Guan et al., 2019; Ahuja et al., 2020). The data obtained from CT scanning can be used for 3D reconstruction of finite element model. MRI scans are the preferred choice for soft tissue reconstruction, but they also have issues such as high cost, difficulty in identifying tissue structures, and an immature unified coordinate system. Therefore, in the future, we should try to use different sequences of MRI scanning data to establish intervertebral disc, muscle, ligament and other structures, and optimize the calculation method, in order to provide more help for the soft tissue research in spine biomechanics. Installation of spine biomechanics for finite element model testing were summarized in Table 2.
The commonly used finite element analysis softwares for 3D structure reverse generation are Avizo, Amira, VG Studio, Mimics, etc. And common finite element analysis softwares include LUSAS, MSC Nastran, ANSYS, ABAQUS, LMS-Samtech, Algor, Femap/NX Nastran, HyperMesh, COMSOL Multiphysics, FEPG, etc.
Currently, the application of finite element modeling in spinal research is advancing toward personalization, complex load simulation, and multi-scale integration. The development of multi-scale modeling urgently requires establishing reliable connections across tissue-cell-molecular levels to elucidate the mechanisms by which mechanical loading influences cellular metabolism (e.g., inflammatory responses, matrix degradation) (Vergroesen et al., 2015; Cheng et al., 2018). Furthermore, existing models are predominantly limited to quasi-static analyses. Future efforts should integrate dynamic complex loads (e.g., combined motions, high-velocity impacts) and leverage artificial intelligence algorithms to optimize real-time response predictions (Li et al., 2020). Landinez et al. have developed a patient-specific spine digital twin technology without requiring full 3D reconstruction (Landinez et al., 2025). This approach, based on conventional X-ray image segmentation algorithms and mathematical models of disc degeneration, enables quantitative calculation of intervertebral disc strain through finite element analysis. Multi-physics coupling also represents a critical future direction. By integrating electrophysiological signals to predict nerve root compression (e.g., triggering automatic pain simulation when displacement exceeds 1.2 mm), this strategy can ultimately realize the construction of nerve root compression models for degenerative spinal pathologies (Sun et al., 2025).
Installation for motion capture model testing
The optical marker-based motion capture system is the gold standard for spinal biomechanics research, enabling precise quantification of kinematic parameters through non-invasive skin markers. The core components of the testing apparatus include an optical tracking system (e.g., infrared camera arrays), passive markers or active sensors, a data processing unit, and specialized analysis software (e.g., Vicon, Optotrak Certus, MotionAnalysis). The Plug-in-Gait marker set is the most common configuration, typically combined with lumbar marker optimization (e.g., replacing T10 with T12) to enhance accuracy (Hays et al., 2021). Multi-segment analysis requires dense marker strategies, such as the mesh grid protocol (8-row × 5-column layout) or anatomical landmark-derived models (Eldar, 2020).
During data acquisition, software (e.g., Vicon Nexus, Visual3D) converts marker coordinates into skeletal models to calculate kinematic parameters, including the ROM, angular velocity, acceleration, and instantaneous center of rotation (ICR) between spinal segments. Synchronization with surface electromyography or inertial measurement units allows simultaneous recording of paraspinal muscle activation timing and intensity, facilitating biomechanical analysis of “muscle-skeletal” synergistic motion. Studies indicate that the lumbar spine is a primary focus (56.25% of studies) due to its high mobility and load-bearing function, whereas cervical spine research is limited (Hesby et al., 2019). Common test activities include walking (37.5%), standing (25%), and flexion-extension ROM (26.79%), with fewer studies on sports or occupational movements (Glover et al., 2021). Multi-segment protocols require specific designs, such as adding thoracic markers (T3/T4) to track T10 vertebral motion (Rahmatalla et al., 2008).
Analytical methods focus on kinematic and kinetic parameters. Angular parameters (flexion/extension, lateral bending, axial rotation) are reported in 71.43% of studies, with flexion-extension being the most common (Mousavi et al., 2018). Kinetic parameters are involved in only 10.71% of studies and require estimation via musculoskeletal models (Dehghan and Arjmand, 2024). Mathematical models significantly influence results, such as coordinate system establishment methods and rotation sequence selection (Crawford et al., 1996; Anderst and Aucie, 2017). Direct vertebral motion measurement is only achievable through bone-pin marker techniques (MacWilliams et al., 2013). Recently, AI-assisted single-camera, markerless motion capture technology has enabled low-cost kinematic parameter extraction via deep learning and provide reliable gait parameters for certain applications, but large-scale training datas for generalizability are still necessary (Dinh et al., 2025; Usami et al., 2025). Commonly used motion capture software includes MotionAnalysis, Vicon, Sega Interactive, MAC, X-Ist, Filmbox, CHINGMU, and Nokov. AI and machine learning applications are transforming how spinal kinematics and kinetics are captured, moving beyond the confines of laboratory instrumentation. Traditional motion capture systems provide high fidelity data but are expensive, require dedicated lab spaces, and rely on skilled operators–factors that limit their accessibility. AI-driven approaches are overcoming these barriers by leveraging computer vision and wearable sensors for spinal motion tracking. One major advance is the use of markerless pose estimation. Modern deep learning algorithms can reconstruct 3D body shape and spinal posture from ordinary video, even a single camera feed. These methods, combining computer vision with learned biomechanical models, achieve accurate estimation of spinal alignment and joint angles without any wearable markers. As a result, capturing spine movements has become more practical and affordable. A single smartphone or camera can now yield rich kinematic data, making biomechanical analysis “field-ready” and not restricted to motion labs.
Test data
In the field of spine biomechanics, researchers rely on many types of experimental measurements to characterize how the spine behaves under various conditions. In summary, test data serve as the foundation for advancing both basic and applied research in spinal biomechanics. On one level, they provide benchmarks for validating computational models, ensuring that finite element analyses and simulations remain consistent with physiological reality. On another, they act as diagnostic indicators of spinal stability and pathology, allowing researchers to identify abnormal loading patterns, instability, or degeneration. Test data are also indispensable for the design and evaluation of implants, as they reveal how different devices redistribute stresses, alter kinematics, or affect adjacent segments. This section focuses on the data types most frequently reported in prior studies and highlights their special significance to biomechanical analysis and clinical outcomes.
General data
ROM is the most important parameter in spinal kinematics. ROM describes the sum of neutral zone(NZ) and elastic zone(EZ) in one direction of motion. By comparing the ROM data, we can study the stability of the spinal model joint, the factors hindering joint activity and therapeutic effect, such as the evaluation of the curative effect of anterior cervical surgery (Cunningham et al., 2010), the biomechanical comparison of artificial implants (Pflugmacher et al., 2004), the research of joint stability (Lindsey et al., 2018). In the past, the ROM measurement methods were completed by visual measurement or a measuring tool (Lea and Gerhardt, 1995). In recent years, a large number of spine biomechanics research use high-speed dynamic tracking system to obtain real-time ROM data(Nayak et al., 2013; Wang M. et al., 2013; Wilke et al., 2016; Liebsch et al., 2018; Lindsey et al., 2018; Arnold et al., 2019; Kim et al., 2019; Sawa et al., 2020). For example, Bell et al. used Vicon optical motion capture system in the biomechanical study of cervical spine (Bell et al., 2016). In the finite element model, ROM can be directly obtained by computer software measurement, and the results are more accurate (Sun et al., 2022; Gribbin, 2010; Stott and Driscoll, 2023; Gullbrand et al., 2025; Lin et al., 2025).
Wilke et al. proposed in the study published in 1998 that the NZ refers to the angle difference between the two stages of exercise at zero load. The deformation from the end of the NZ to the maximum load point is defined as the EZ (Wilke et al., 1998). The main manifestation of NZ is the activity of ligaments around the spine and other soft tissue under relaxation, while EZ is the activity of ligaments under tension. Therefore, NZ and EZ can show the movement ability and stability of spine. NZ and EZ are very common in the experimental measurement of spinal biomechanics, especially in the evaluation of spinal joint stability (Pflugmacher et al., 2004; Gonzalez-Blohm et al., 2014; Sawa et al., 2020; Lin et al., 2025). The concept of NZ stiffness was also mentioned in Wilke et al. The NZ stiffness was defined as the quotient of load and deformation (Wilke et al., 1998; Aghayev et al., 2014).
Strain can be captured by strain gauge and computer software measurement. Resistance strain gauges can sense mechanical deformation on the surface of an object and reflect the magnitude of the strain force through changes in resistance values. In Ho’s study on the efficacy of minimally invasive lumbar decompression, strain gauges were placed on the surface of L3 and L4 vertebrae to measure the strain of different segments, which can be used to study the stability of lumbar spine after laminectomy (Ho et al., 2015). Song et al. inferred the values of the stress concentration areas by obtaining the strain values of the internal fixation system (Song et al., 2021). Lin et al. achieved high-precision multidimensional strain analysis in cervical spine specimen studies by improving specimen preparation and applying a non-contact optical 3D strain measuring system, revealing the strain concentration of soft tissues under tensile loading and the consistency of deformation of the C4-C7 segments (Lin et al., 2025). In addition, the measurement of stress-strain under the finite element model is not limited by the artificial implant site and the number of stress distribution measurement points, enabling individualized service and research (Sun et al., 2022; Kassab-Bachi et al., 2023; Gullbrand et al., 2025).
Other data
In addition to common indicators, there are many other indicators also used to catering to some special needs of spine biomechanics research. In addition to common indicators, many specialized measures are used in spine biomechanics research to address specific needs. For example, intervertebral disc height, intervertebral angle, and lordosis angle have been measured to evaluate the biomechanical stability of a cap-type cervical interbody fusion cage (Gu et al., 2010). The helical axis of motion (HAM) is another important parameter. Its position and orientation in the cervical spine were first identified in 1993 (Milne, 1993). Energy loss has also been utilized as an evaluation metric for spinal stability after injury, with in vitro studies reporting satisfactory results (Doulgeris et al., 2013; Gonzalez-Blohm et al., 2014). Another kinematic indicator is the instant center of rotation (ICR), defined for each motion segment as the point where its axis of rotation intersects the inferior vertebra’s sagittal plane (Wawrose et al., 2021). Leveraging this concept, a follower load technique can apply compressive force through each segment’s ICR to overcome limitations in axial loading (Patwardhan et al., 1999). Furthermore, anatomical factors such as intervertebral disc height and bone mineral have been used to gauge the physiological status of spinal models (Nayak et al., 2013; Aghayev et al., 2014; Kim et al., 2019; Sawa et al., 2020).
Besides the ROM and the strain force on the surface of the object, the finite element analysis can also make more precise measurement (Elmasry et al., 2018; Zhang et al., 2018). For example, in the impact test, the finite element analysis can calculate the displacement of the vertebral body or the center of gravity through the established coordinate system to understand the structural changes after the impact (Correia et al., 2020). Finite element analysis also facilitates forecast of internal spinal responses: measuring intradiscal pressure (IDP) can demonstrate the effects of decompression procedures (Zhou et al., 2019). With the aid of computer technology, the center of rotation can also be measured and used in the finite element analysis for dynamic experiments (Milne, 1993; Jager, 1996). In the motion capture model, in addition to the commonly used ROM, the motion trajectory of the subject in the spatial coordinate system was monitored, and the data of muscle activity state and gait analysis were obtained through the additional device (Bourdon et al., 2017; Lee et al., 2019; Schmid et al., 2019; Shin and Yoo, 2019).
Despite methodological limitations and variability across studies, continuous improvements in measurement techniques, such as high-resolution imaging, telemetric sensors, and motion capture, are enhancing the reliability and clinical relevance of biomechanical datasets. Collectively, these data not only deepen our understanding of spinal biomechanics but also form the critical link between experimental research, computational modeling, implant innovation, and patient-centered care.
Discussion
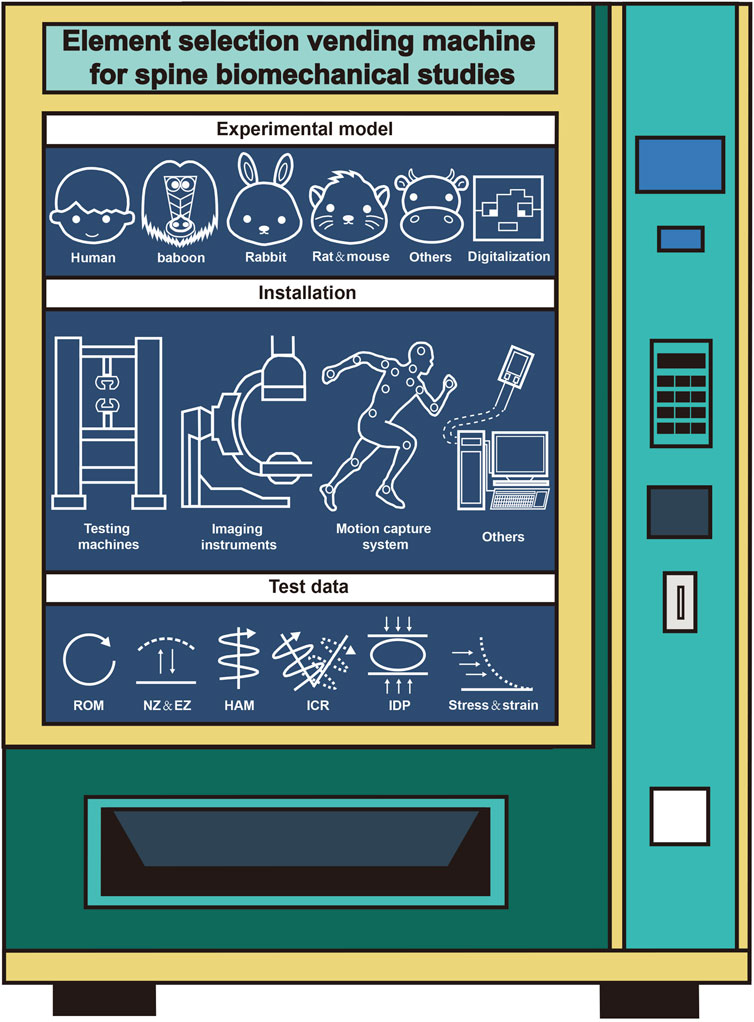
Spinal biomechanics, as an interdisciplinary field, investigates the mechanical behavior of the spine through the integration of anatomy, engineering, and computational science. While significant advancements have been made over the past century, particularly with the rise of digital technologies like finite element analysis and motion capture, the field grapples with persistent challenges. Foremost among these is the lack of standardized experimental protocols and unified research guidelines. This absence leads to inconsistencies in model selection, experimental design, data acquisition, and analysis, hindering result comparability, reproducibility, and ultimately, the clinical translation of findings. This review has systematically reclassified and summarized the fundamental elements of spinal biomechanics research including experimental models, installations, and test data. Our goal of providing clearer guidance and diversified design frameworks for researchers navigating these complexities. Here, we redivided the research process of literature, reclassified and summarized systematically according to certain methods, mainly including experimental model, installation and test data (Figure 1). The ultimate goal is to provide a systematic theoretical framework for researchers of spinal biomechanics.
Three primary types of models commonly used in spinal biomechanics research are identified: human models, animal models, and digital models. Human models are highly valued for their anatomical accuracy and physiological relevance, making them the gold standard for studying spinal behavior. However, their use is often restricted due to ethical concerns, logistical challenges, and limited availability. Animal models, such as those based on primates, pigs, and rabbits, offer a practical alternative but present difficulties in terms of anatomical differences that may limit their direct applicability to human spinal mechanics.
Digital models, particularly finite element models, have become essential tools in the field, providing flexibility, repeatability, and the ability to simulate a wide range of loading conditions and spinal pathologies. While these models are increasingly popular due to their ability to provide personalized simulations, challenges remain in achieving the necessary standardization for their construction and validation. The development of digital models, including the integration of AI and machine learning, holds great promise for enhancing the accuracy and efficiency of spinal biomechanics research by automating model generation and analysis. In terms of installation, the review emphasizes the importance of high-precision testing machines such as MTS systems, instron testing machine and stewart platform. These platforms are used to simulate various spinal movements, such as flexion, extension, and rotation, under different loading conditions. The use of imaging technologies like X-ray, CT, and MRI scans is crucial for obtaining baseline data for model construction and validation, while motion capture systems and strain gauges are vital for capturing real-time kinematic data and stress distribution. In addition, the integration of AI and machine learning in testing has pushed forward the development of more efficient and accessible methods for data collection.
Test data are used to validate computational models and to understand the mechanical behavior of the spine under different conditions. This review identifies several key types of data frequently used in spinal biomechanics research, including ROM, strain, stress, and IDP. In addition, some other data were also mentioned such as HAM, ICR, intervertebral disc height, lordosis angle, and muscle activity, which are increasingly used to assess spinal stability and the effects of specific interventions. The use of finite element analysis allows for more precise measurements of internal spinal responses, such as the effect of decompression procedures on intradiscal pressure.
There are still some issues that researchers should pay attention to when designing spinal biomechanics research:
i. Although computer simulation experiment has become an important part in the field of spine biomechanics, the combined use of digital model and measurement model is expected to be more scientific and accurate. The combination can realize the mutual verification and complementary advantages. Before the mechanical testing of medical devices, finite element modeling can be used to optimize the design and predict performance. Following the evaluation of digital model, the function, histology and biomechanical characteristics of the device can be evaluated with use of measurement model (Goel et al., 2006).
ii. Although spinal biomechanics research has established a certain framework, spinal models still face issues such as large volume, inconvenient experimental operations, and the need for further improvement in data accuracy. Therefore, selecting the most representative model during the research preparation phase is a critical task:
a. For measurement models, pre-processing operations are a crucial step. Excessively reducing the amount of tissue around the spine may lead to decreased structural integrity, compromised spinal stability, and inaccurate experimental results (Bell et al., 2018; Arnold et al., 2019).
b. When using digital models in research, certain special models are difficult to obtain conventional experimental data for, such as burst fracture models.
c. Additionally, due to the iatrogenic damage caused by surgery to the vertebral body, parameter measurements for surgical models cannot be standardized (Elmasry et al., 2018).
d. Establishing uniform and appropriate operational standards for each part of the finite element model is also an urgent issue that needs to be addressed.
iii. The fusion of AI, machine learning, and multiscale modeling represents the next frontier in spinal biomechanics, offering the potential to enhance the precision of simulations and improve the effectiveness of treatments for spinal disorders. However, achieving these advancements will require continued efforts to address the existing challenges related to model validation, data consistency, and clinical translation.
iv. The purpose of this review is to summarize the experimental elements in spinal biomechanics research and provide researchers with standardized experimental design guidelines. However, it lacks the application of systematic review methodologies (such as PRISMA). This review provides an overview of the overall biomechanics research plan, but further discussion is needed on the design of plans for specific topics, such as a more in-depth exploration of spinal in vitro testing or computer simulations, to provide more targeted and valuable insights.
Author contributions
BX: Data curation, Writing – original draft, Investigation, Methodology, Formal Analysis. GL: Data curation, Formal Analysis, Investigation, Writing – review and editing, Methodology. XS: Data curation, Investigation, Writing – original draft, Methodology. SY: Data curation, Investigation, Methodology, Writing – original draft, Formal Analysis. RS: Formal Analysis, Investigation, Methodology, Supervision, Writing – review and editing, Data curation, Validation. YG: Formal Analysis, Investigation, Methodology, Project administration, Supervision, Writing – review and editing. MS: Data curation, Supervision, Validation, Writing – review and editing, Conceptualization, Resources, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aghayev, K., Gonzalez-Blohm, S. A., Doulgeris, J. J., Lee, W. E., Waddell, J. K., and Vrionis, F. D. (2014). Feasibility and biomechanical performance of a novel transdiscal screw system for one level in non-spondylolisthetic lumbar fusion: an in vitro investigation. Spine J. 14 (4), 705–713. doi:10.1016/j.spinee.2013.08.033
Ahuja, S., Moideen, A. N., Dudhniwala, A. G., Karatsis, E., Papadakis, L., and Varitis, E. (2020). Lumbar stability following graded unilateral and bilateral facetectomy: a finite element model study. Clin. Biomech. (Bristol). 75, 105011. doi:10.1016/j.clinbiomech.2020.105011
Alekseichuk, I., Mantell, K., Shirinpour, S., and Opitz, A. (2019). Comparative modeling of transcranial magnetic and electric stimulation in mouse, monkey, and human. Neuroimage 194, 136–148. doi:10.1016/j.neuroimage.2019.03.044
Anderst, W. J., and Aucie, Y. (2017). Three-dimensional intervertebral range of motion in the cervical spine: does the method of calculation matter? Med. Eng. Phys. 41, 109–115. doi:10.1016/j.medengphy.2017.01.009
Ao, X., Wang, L., Shao, Y., Chen, X., Zhang, J., Chu, J., et al. (2019). Development and characterization of a novel bipedal standing mouse model of intervertebral disc and facet joint degeneration. Clin. Orthop. Relat. Res. 477 (6), 1492–1504. doi:10.1097/corr.0000000000000712
Arnold, P. M., Cheng, I., Harris, J. A., Hussain, M. M., Zhang, C., Karamian, B., et al. (2019). Single-level in vitro kinematic comparison of novel inline cervical interbody devices with intervertebral screw, Anchor, or Blade. Glob. Spine J. 9 (7), 697–707. doi:10.1177/2192568219833055
Balasubramanian, S., Peters, J. R., Robinson, L. F., Singh, A., and Kent, R. W. (2016). Thoracic spine morphology of a pseudo-biped animal model (kangaroo) and comparisons with human and quadruped animals. Eur. Spine J. 25 (12), 4140–4154. doi:10.1007/s00586-016-4776-x
Bell, K. M., Yan, Y., Debski, R. E., Sowa, G. A., Kang, J. D., and Tashman, S. (2016). Influence of varying compressive loading methods on physiologic motion patterns in the cervical spine. J. Biomech. 49 (2), 167–172. doi:10.1016/j.jbiomech.2015.11.045
Bell, K. M., Oh, A., Cook, H. A., Yan, Y., and Lee, J. Y. (2018). Adaptation of a clinical fixation device for biomechanical testing of the lumbar spine. J. Biomech. 69, 164–168. doi:10.1016/j.jbiomech.2017.12.029
Belytschko, T., Kulak, R. F., Schultz, A. B., and Galante, J. O. (1974). Finite element stress analysis of an intervertebral disc. J. Biomech. 7 (3), 277–285. doi:10.1016/0021-9290(74)90019-0
Bohl, M. A., McBryan, S., Newcomb, A., Lehrman, J. N., Kelly, B. P., Nakaji, P., et al. (2020). Range of motion testing of a novel 3D-printed synthetic spine model. Glob. Spine J. 10 (4), 419–424. doi:10.1177/2192568219858981
Bourdon, E., Mavor, M., and Hay, D. C. (2017). Assessment of three-dimensional Trunk kinematics and muscle activation during Cycling with Independent Cranks. J. Sports Sci. Med. 16 (4), 536–542. Available online at: https://pmc.ncbi.nlm.nih.gov/articles/PMC5721184/
Bozkus, H., Karakas, A., Hanci, M., Uzan, M., Bozdag, E., and Sarioglu, A. C. (2001). Finite element model of the Jefferson fracture: comparison with a cadaver model. Eur. Spine J. 10 (3), 257–263. doi:10.1007/s005860100256
Brekelmans, W. A., Poort, H. W., and Slooff, T. J. (1972). A new method to analyse the mechanical behaviour of skeletal parts. Acta Orthop. Scand. 43 (5), 301–317. doi:10.3109/17453677208998949
Burval, D. J., McLain, R. F., Milks, R., and Inceoglu, S. (2007). Primary pedicle screw augmentation in osteoporotic lumbar vertebrae: biomechanical analysis of pedicle fixation strength. Spine (Phila Pa 1976) 32 (10), 1077–1083. doi:10.1097/01.brs.0000261566.38422.40
Chazal, J., Tanguy, A., Bourges, M., Gaurel, G., Escande, G., Guillot, M., et al. (1985). Biomechanical properties of spinal ligaments and a histological study of the supraspinal ligament in traction. J. Biomech. 18 (3), 167–176. doi:10.1016/0021-9290(85)90202-7
Chen, T. Y., Chen, W. H., Tzeng, C. Y., Huang, C. W., Yang, C. C., Chen, H. T., et al. (2020). Anterior bone loss after cervical Bryan disc arthroplasty: insight into the biomechanics following total disc replacement. Spine J. 20 (8), 1211–1218. doi:10.1016/j.spinee.2020.04.017
Cheng, X., Zhang, L., Zhang, K., Zhang, G., Hu, Y., Sun, X., et al. (2018). Circular RNA VMA21 protects against intervertebral disc degeneration through targeting miR-200c and X linked inhibitor-of-apoptosis protein. Ann. Rheum. Dis. 77 (5), 770–779. doi:10.1136/annrheumdis-2017-212056
Chow, N., and Gregory, D. E. (2023). The effect of intervertebral disc damage on the mechanical strength of the annulus fibrosus in the adjacent segment. Spine J. 23 (12), 1935–1940. doi:10.1016/j.spinee.2023.07.013
Clifton, W., Nottmeier, E., Damon, A., Dove, C., Chen, S. G., and Pichelmann, M. (2019). A Feasibility study for the production of three-dimensional-printed spine models using simultaneously Extruded Thermoplastic Polymers. Cureus 11 (4), e4440. doi:10.7759/cureus.4440
Correia, M. A., McLachlin, S. D., and Cronin, D. S. (2020). Optimization of muscle activation schemes in a finite element neck model simulating volunteer frontal impact scenarios. J. Biomech. 104, 109754. doi:10.1016/j.jbiomech.2020.109754
Crawford, N. R., Yamaguchi, G. T., and Dickman, C. A. (1996). Methods for determining spinal flexion/extension, lateral bending, and axial rotation from marker coordinate data: analysis and refinement. Hum. Mov. Sci. 15 (1), 55–78. doi:10.1016/0167-9457(95)00049-6
Cunningham, B. W., Sefter, J. C., Hu, N., and McAfee, P. C. (2010). Autologous growth factors versus autogenous graft for anterior cervical interbody fusion: an in vivo caprine model. J. Neurosurg. Spine 13 (2), 216–223. doi:10.3171/2010.3.Spine09512
Danilov, C. A., Gu, Y., Punj, V., Wu, Z., Steward, O., Schönthal, A. H., et al. (2020). Intravenous delivery of microRNA-133b along with Argonaute-2 enhances spinal cord recovery following cervical contusion in mice. Spine J. 20 (7), 1138–1151. doi:10.1016/j.spinee.2020.02.019
Dehghan, P., and Arjmand, N. (2024). The National Institute for occupational Safety and Health (NIOSH) Recommended weight generates different spine loads in load-Handling activity performed using Stoop, Semi-squat and full-Squat techniques; a full-body musculoskeletal model study. Hum. Factors 66 (5), 1387–1398. doi:10.1177/00187208221141652
Dinh, H. G., Zhou, J. Y., Benmira, A., Kenney, D. E., and Ladd, A. L. (2025). Proof of concept and validation of single-camera AI-assisted live Thumb motion capture. Sensors (Basel) 25 (15), 4633. doi:10.3390/s25154633
Doulgeris, J. J., Aghayev, K., Gonzalez-Blohm, S. A., Del Valle, M., Waddell, J., Lee, W. E., et al. (2013). Comparative analysis of posterior fusion constructs as treatments for middle and posterior column injuries: an in vitro biomechanical investigation. Clin. Biomech. (Bristol) 28 (5), 483–489. doi:10.1016/j.clinbiomech.2013.05.001
Du, L., Sun, X. J., Zhou, T. J., Li, Y. C., Chen, C., Zhao, C. Q., et al. (2017). The role of cage height on the flexibility and load sharing of lumbar spine after lumbar interbody fusion with unilateral and bilateral instrumentation: a biomechanical study. BMC Musculoskelet. Disord. 18 (1), 474. doi:10.1186/s12891-017-1845-1
Dukkipati, S. T., and Driscoll, M. (2025). Development and biomechanical evaluation of a 3D printed analogue of the human lumbar spine. 3D Print. Med. 11 (1), 3. doi:10.1186/s41205-025-00249-y
Eldar, R. (2020). A quantitative analysis model of thoracic flexibility for wearable personal protection equipment. Int. J. Interact. Des. Manuf. 14 (3), 887–898. doi:10.1007/s12008-020-00677-6
Elmasry, S. S., Asfour, S. S., and Travascio, F. (2018). Finite element study to evaluate the biomechanical performance of the spine after Augmenting Percutaneous pedicle screw fixation with Kyphoplasty in the treatment of burst fractures. J. Biomech. Eng. 140 (6), 061005. doi:10.1115/1.4039174
Evans, F. G., and Lissner, H. R. (1959). Biomechanical studies on the lumbar spine and pelvis. J. Bone Jt. Surg. Am. 41-a (2), 278–290. doi:10.2106/00004623-195941020-00010
Fan, W., and Guo, L. X. (2020). The effect of non-fusion dynamic stabilization on biomechanical responses of the implanted lumbar spine during whole-body vibration. Comput Methods Programs Biomed.192, 105441. doi:10.1016/j.cmpb.2020.105441
Fasser, M.-R., Jokeit, M., Kalthoff, M., Gomez Romero, D. A., Trache, T., Snedeker, J. G., et al. (2021). Subject-specific alignment and Mass distribution in musculoskeletal models of the lumbar spine. Front. Bioeng. Biotechnol. 9, 721042. doi:10.3389/fbioe.2021.721042
Fournely, M., Petit, Y., Wagnac, É., Laurin, J., Callot, V., and Arnoux, P. J. (2018). High-speed video analysis improves the accuracy of spinal cord compression measurement in a mouse contusion model. J. Neurosci. Methods 293, 1–5. doi:10.1016/j.jneumeth.2017.09.007
Ghezelbash, F., Hossein Eskandari, A., Robert-Lachaine, X., Cao, S., Pesteie, M., Qiao, Z., et al. (2024). Machine learning applications in spine biomechanics. J. Biomech. 166, 111967. doi:10.1016/j.jbiomech.2024.111967
Gillespie, K. A., and Dickey, J. P. (2004). Biomechanical role of lumbar spine ligaments in flexion and extension: determination using a parallel linkage robot and a porcine model. Spine (Phila Pa 1976) 29 (11), 1208–1216. doi:10.1097/00007632-200406010-00010
Glover, N. A., Kakar, R. S., and Chaudhari, A. M. W. (2021). Effects of spinal coupling and marker set on tracking of spine models during running. J. Biomech. 116, 110217. doi:10.1016/j.jbiomech.2020.110217
Goel, V. K., Panjabi, M. M., Patwardhan, A. G., Dooris, A. P., and Serhan, H. (2006). Test protocols for evaluation of spinal implants. J. Bone Jt. Surg. Am. 88 (Suppl. 2), 103–109. doi:10.2106/jbjs.E.01363
Goertz, A. R., Yang, K. H., and Viano, D. C. (2020). Development of a finite element biomechanical whole spine model for analyzing lumbar spine loads under caudocephalad acceleration. Biomed. Phys. Eng. Express 7 (1), 015009. doi:10.1088/2057-1976/abc89a
Gonzalez-Blohm, S. A., Doulgeris, J. J., Aghayev, K., Lee, W. E., Volkov, A., and Vrionis, F. D. (2014). Biomechanical analysis of an interspinous fusion device as a stand-alone and as supplemental fixation to posterior expandable interbody cages in the lumbar spine. J. Neurosurg. Spine 20 (2), 209–219. doi:10.3171/2013.10.Spine13612
Gu, Y. T., Yao, Z. J., Jia, L. S., Qi, J., and Wang, J. (2010). In vivo experimental study of hat type cervical intervertebral fusion cage (HCIFC). Int. Orthop. 34 (8), 1251–1259. doi:10.1007/s00264-010-0978-8
Guan, W., Sun, Y., Qi, X., Hu, Y., Duan, C., Tao, H., et al. (2019). Spinal biomechanics modeling and finite element analysis of surgical instrument interaction. Comput. Assist. Surg. (Abingdon) 24 (Suppl. 1), 151–159. doi:10.1080/24699322.2018.1560086
Guan, T., Zhang, Y., Anwar, A., Zhang, Y., and Wang, L. (2020). Determination of three-dimensional corrective force in Adolescent idiopathic scoliosis and biomechanical finite element analysis. Front. Bioeng. Biotechnol. 8, 963. doi:10.3389/fbioe.2020.00963
Guillemin, G., Jr. (1950). German aviation medicine, World War II Prepared under the auspices of the Surgeon General, U. S. Air Force. (Washington, DC: Government Printing Office), 112, 762–763. doi:10.1126/science.112.2921.762
Gullbrand, S. E., Kiapour, A., Barrett, C., Fainor, M., Orozco, B. S., Hilliard, R., et al. (2025). Restoration of physiologic loading after engineered disc implantation mitigates immobilization-induced facet joint and paraspinal muscle degeneration. Acta Biomater. 192, 128–139. doi:10.1016/j.actbio.2024.12.014
Gurdjian, E. S., Webster, J. E., and Lissner, H. R. (1949). Studies on skull fracture with particular reference to engineering factors. Am. J. Surg. 78 (5), 736–742. doi:10.1016/0002-9610(49)90315-3
Hakim, N. S., and King, A. I. (1979). A three dimensional finite element dynamic response analysis of a vertebra with experimental verification. J. Biomech. 12 (4), 277–292. doi:10.1016/0021-9290(79)90070-8
Han, K. S., Zander, T., Taylor, W. R., and Rohlmann, A. (2012). An enhanced and validated generic thoraco-lumbar spine model for prediction of muscle forces. Med. Eng. Phys. 34 (6), 709–716. doi:10.1016/j.medengphy.2011.09.014
Hardy, W. G., Lissner, H. R., Webster, J. E., and Gurdjian, E. S. (1958). Repeated loading tests of the lumbar spine; a preliminary report. Surg. Forum 9, 690–695.
Hays, C., Fehr, S., Liu, X. C., and Haddas, R. (2021). Impact of corset bracing on 3D spine kinematics during ADL in children with Spondylolysis. Stud. Health Technol. Inf. 280, 126–130. doi:10.3233/shti210450
Hesby, B. B., Hartvigsen, J., Rasmussen, H., and Kjaer, P. (2019). Electronic measures of movement impairment, repositioning, and posture in people with and without neck pain-a systematic review. Syst. Rev. 8 (1), 220. doi:10.1186/s13643-019-1125-2
Hindman, B. J., Dexter, F., Gadomski, B. C., and Bucx, M. J. (2020). Sex-specific Intubation biomechanics: Intubation forces are greater in Male than in Female patients, Independent of body weight. Cureus 12 (6), e8749. doi:10.7759/cureus.8749
Hirt, S. (1955). What is kinesiology? A historical review. Phys. Ther. Rev. 35 (8), 419–426. doi:10.1093/ptj/35.8.419
Ho, Y. H., Tu, Y. K., Hsiao, C. K., and Chang, C. H. (2015). Outcomes after minimally invasive lumbar decompression: a biomechanical comparison of unilateral and bilateral laminotomies. BMC Musculoskelet. Disord. 16, 208. doi:10.1186/s12891-015-0659-2
Hoogendoorn, R. J., Helder, M. N., Kroeze, R. J., Bank, R. A., Smit, T. H., and Wuisman, P. I. (2008). Reproducible long-term disc degeneration in a large animal model. Spine (Phila Pa 1976) 33 (9), 949–954. doi:10.1097/BRS.0b013e31816c90f0
Huang, H., Nightingale, R. W., and Dang, A. B. C. (2018). Biomechanics of coupled motion in the cervical spine during simulated whiplash in patients with pre-existing cervical or lumbar spinal fusion: a Finite Element Study. Bone Jt. Res. 7 (1), 28–35. doi:10.1302/2046-3758.71.Bjr-2017-0100.R1
Ignasiak, D., Dendorfer, S., and Ferguson, S. J. (2016). Thoracolumbar spine model with articulated ribcage for the prediction of dynamic spinal loading. J. Biomech. 49 (6), 959–966. doi:10.1016/j.jbiomech.2015.10.010
Jager, D. (1996). Mathematical head-neck models for acceleration impacts. [Phd Thesis 1 (Research TU/e / Graduation TU/e), Mechanical Engineering]. Eindhoven: Eindhoven University of Technology. doi:10.6100/IR460661
Jia, S., Lin, L., Yang, H., Fan, J., Zhang, S., and Han, L. (2020). The influence of the rib cage on the static and dynamic stability responses of the scoliotic spine. Sci. Rep. 10 (1), 16916. doi:10.1038/s41598-020-73881-9
Kandziora, F., Pflugmacher, R., Kleemann, R., Duda, G., Wise, D. L., Trantolo, D. J., et al. (2002). Biomechanical analysis of biodegradable interbody fusion cages augmented with poly(propylene glycol-co-fumaric acid). Spine (Phila Pa 1976) 27 (15), 1644–1651. doi:10.1097/00007632-200208010-00010
Kassab-Bachi, A., Ravikumar, N., Wilcox, R. K., Frangi, A. F., and Taylor, Z. A. (2023). Contribution of shape Features to intradiscal pressure and Facets contact pressure in L4/L5 FSUs: an in-Silico study. Ann. Biomed. Eng. 51 (1), 174–188. doi:10.1007/s10439-022-03072-2
Kim, J. S., Cheung, Z. B., Arvind, V., Caridi, J., and Cho, S. K. (2019). Role of posterior ligamentous reinforcement in proximal junctional kyphosis: a cadaveric biomechanical study. Asian Spine J. 13 (1), 68–76. doi:10.31616/asj.2018.0102
La Barbera, L., Brayda-Bruno, M., Liebsch, C., Villa, T., Luca, A., Galbusera, F., et al. (2018). Biomechanical advantages of supplemental accessory and satellite rods with and without interbody cages implantation for the stabilization of pedicle subtraction osteotomy. Eur. Spine J. 27 (9), 2357–2366. doi:10.1007/s00586-018-5623-z
Lalonde, N. M., Petit, Y., Aubin, C. E., Wagnac, E., and Arnoux, P. J. (2013). Method to geometrically personalize a detailed finite-element model of the spine. IEEE Trans. Biomed. Eng. 60 (7), 2014–2021. doi:10.1109/tbme.2013.2246865
Landinez, D., Rodríguez, C. F., and Cifuentes-De la Portilla, C. (2025). Patient-specific spine digital twins: a computational characterization of the idiopathic scoliosis. J. Orthop. Surg. Res. 20 (1), 39. doi:10.1186/s13018-024-05417-0
Lea, R. D., and Gerhardt, J. J. (1995). Range-of-motion measurements. J. Bone Jt. Surg. Am. 77 (5), 784–798. doi:10.2106/00004623-199505000-00017
Lee, S. P., Gillis, C. B., Ibarra, J. J., Oldroyd, D. F., and Zane, R. S. (2019). Heel-raised Foot posture does not affect Trunk and lower Extremity biomechanics during a Barbell back squat in Recreational weight lifters. J. Strength Cond. Res 33 (3), 606–614. doi:10.1519/jsc.0000000000001938
Li, X. H., Song, Y. M., and Duan, H. (2015). Reconstruction of segmental stability of Goat cervical spine with poly (D, L-lactic acid) cage. Orthop. Surg. 7 (3), 266–272. doi:10.1111/os.12192
Li, K., Zhang, S. J., Du, C. F., Zhao, J. Z., Liu, Q., Zhang, C. Q., et al. (2020). Effect of strain rates on failure of mechanical properties of lumbar intervertebral disc under flexion. Orthop. Surg. 12 (6), 1980–1989. doi:10.1111/os.12847
Liebsch, C., Graf, N., and Wilke, H. J. (2018). The effect of follower load on the intersegmental coupled motion characteristics of the human thoracic spine: an in vitro study using entire rib cage specimens. J. Biomech. 78, 36–44. doi:10.1016/j.jbiomech.2018.06.025
Lin, F., Cai, Y., Li, J., Zhan, J., Gao, Z., Zeng, X., et al. (2025). Noncontact optical 3D strain measurements in cervical soft tissues biomechanics by digital image correlation under tensile test: an experimental approach. Front. Bioeng. Biotechnol. 13, 1493476. doi:10.3389/fbioe.2025.1493476
Lindsey, D. P., Parrish, R., Gundanna, M., Leasure, J., Yerby, S. A., and Kondrashov, D. (2018). Biomechanics of unilateral and bilateral sacroiliac joint stabilization: laboratory investigation. J. Neurosurg. Spine 28 (3), 326–332. doi:10.3171/2017.7.Spine17499
Lipscomb, K. E., Sarigul-Klijn, N., Klineberg, E., and Mohan, V. (2017). Biomechanical effects of human lumbar Discography: in vitro experiments and their finite element validation. Clin. Spine Surg. 30 (3), E219–e225. doi:10.1097/bsd.0000000000000077
Liu, Y. K., Njus, G., Buckwalter, J., and Wakano, K. (1983). Fatigue response of lumbar intervertebral joints under axial cyclic loading. Spine (Phila Pa 1976) 8 (8), 857–865. doi:10.1097/00007632-198311000-00008
Liu, F., Feng, Z., Liu, T., Fei, Q., Jiang, C., Li, Y., et al. (2016). A biomechanical comparison of 3 different posterior fixation techniques for 2-level lumbar spinal disorders. J. Neurosurg. Spine 24 (3), 375–380. doi:10.3171/2015.7.Spine1534
Liu, Q., Zhang, J., Sun, S. C., and Wang, F. (2017). Application of finite element method in spinal biomechanics. Zhongguo Gu Shang 30 (2), 190–194. doi:10.3969/j.issn.1003-0034.2017.02.020
Ludwig, S. C., Kramer, D. L., Balderston, R. A., Vaccaro, A. R., Foley, K. F., and Albert, T. J. (2000). Placement of pedicle screws in the human cadaveric cervical spine: comparative accuracy of three techniques. Spine (Phila Pa 1976) 25 (13), 1655–1667. doi:10.1097/00007632-200007010-00009
Lysell, E. (1969). Motion in the cervical spine. An experimental study on autopsy specimens. Acta Orthop. Scand. 123, 1–61. doi:10.3109/ort.1969.40.suppl-123.01
MacWilliams, B. A., Rozumalski, A., Swanson, A. N., Wervey, R. A., Dykes, D. C., Novacheck, T. F., et al. (2013). Assessment of three-dimensional lumbar spine vertebral motion during gait with use of indwelling bone pins. J. Bone Jt. Surg. Am. 95 (23), e184–e1848. doi:10.2106/jbjs.L.01469
Milne, N. (1993). Composite motion in cervical disc segments. Clin. Biomech. (Bristol) 8 (4), 193–202. doi:10.1016/0268-0033(93)90014-9
Mousavi, S. J., Tromp, R., Swann, M. C., White, A. P., and Anderson, D. E. (2018). Between-session reliability of opto-electronic motion capture in measuring sagittal posture and 3-D ranges of motion of the thoracolumbar spine. J. Biomech. 79, 248–252. doi:10.1016/j.jbiomech.2018.08.033
Nachemson, A. (1966). The load on lumbar disks in different positions of the body. Clin. Orthop. Relat. Res. 45, 107–122. doi:10.1097/00003086-196600450-00014
Nachemson, A., and Morris, J. M. (1964). In vivo measurements of intradiscal pressure: discometry, a method for the determination of pressure in the lower lumbar discs. J. Bone Jt. Surg. Am. 46, 1077–1092. doi:10.2106/00004623-196446050-00012
Nayak, A. N., Gutierrez, S., Billys, J. B., Santoni, B. G., and Castellvi, A. E. (2013). Biomechanics of lateral plate and pedicle screw constructs in lumbar spines instrumented at two levels with laterally placed interbody cages. Spine J. 13 (10), 1331–1338. doi:10.1016/j.spinee.2013.03.048
Nishi, R. A., Badner, A., Hooshmand, M. J., Creasman, D. A., Liu, H., and Anderson, A. J. (2020). The effects of mouse strain and age on a model of unilateral cervical contusion spinal cord injury. PLoS One 15 (6), e0234245. doi:10.1371/journal.pone.0234245
O'Hehir, M. M., O'Connor, T. E., Mariotti, B. L., Soliman, M. A. R., Quiceno, E., Gupta, M. C., et al. (2024). Tension parameters of junctional tethers in proximal junction kyphosis: a cadaveric biomechanical study. World Neurosurg. 182, e798–e806. doi:10.1016/j.wneu.2023.12.041
Ouyang, P., Li, J., He, X., Dong, H., Zang, Q., Li, H., et al. (2020). Biomechanical comparison of 1-level Corpectomy and 2-level Discectomy for cervical Spondylotic Myelopathy: a finite element analysis. Med. Sci. Monit. 26, e919270. doi:10.12659/msm.919270
Oxland, T. R. (2015). A history of spine biomechanics. Focus on 20th century progress. Unfallchirurg 118 (Suppl. 1), 80–92. doi:10.1007/s00113-015-0087-7
Panjabi, M. M., Brand, R. A., and White, A. A. (1976). Three-dimensional flexibility and stiffness properties of the human thoracic spine. J. Biomech. 9 (4), 185–192. doi:10.1016/0021-9290(76)90003-8
Patel, A., Schwab, F., Lafage, V., Patel, A., Obeidat, M. M., and Farcy, J. P. (2010). Computed tomographic validation of the porcine model for thoracic scoliosis. Spine (Phila Pa 1976) 35 (1), 18–25. doi:10.1097/BRS.0b013e3181b79169
Patwardhan, A. G., Havey, R. M., Meade, K. P., Lee, B., and Dunlap, B. (1999). A follower load increases the load-carrying capacity of the lumbar spine in compression. Spine (Phila Pa 1976) 24 (10), 1003–1009. doi:10.1097/00007632-199905150-00014
Pflugmacher, R., Schleicher, P., Gumnior, S., Turan, O., Scholz, M., Eindorf, T., et al. (2004). Biomechanical comparison of bioabsorbable cervical spine interbody fusion cages. Spine (Phila Pa 1976) 29 (16), 1717–1722. doi:10.1097/01.brs.0000134565.17078.4c
Pintar, F. A., Yoganandan, N., Myers, T., Elhagediab, A., and Sances, A. (1992). Biomechanical properties of human lumbar spine ligaments. J. Biomech. 25 (11), 1351–1356. doi:10.1016/0021-9290(92)90290-h
Pope, M. H. (2005). Giovanni Alfonso Borelli--the father of biomechanics. Spine (Phila Pa 1976) 30 (20), 2350–2355. doi:10.1097/01.brs.0000182314.49515.d8
Rahmatalla, S., Xia, T., Contratto, M., Kopp, G., Wilder, D., Frey Law, L., et al. (2008). Three-dimensional motion capture protocol for seated operator in whole body vibration. Int. J. Ind. Ergon. 38 (5), 425–433. doi:10.1016/j.ergon.2007.08.015
Rao, R. D., Wang, M., Singhal, P., McGrady, L. M., and Rao, S. (2002). Intradiscal pressure and kinematic behavior of lumbar spine after bilateral laminotomy and laminectomy. Spine J. 2 (5), 320–326. doi:10.1016/s1529-9430(02)00402-3
Roth, A. K., Bogie, R., Jacobs, E., Arts, J. J., and van Rhijn, L. W. (2013). Large animal models in fusionless scoliosis correction research: a literature review. Spine J. 13 (6), 675–688. doi:10.1016/j.spinee.2013.02.043
Rudy, R. F., Sawa, A. G. U., McBryan, S., Mugge, L. A., Thielen, K., Assefa, T. G., et al. (2025). Impact of multipoint pelvic fixation and multirod distal constructs on proximal junction biomechanics in cadaveric specimens. J. Neurosurg. Spine, 1–7. doi:10.3171/2025.4.Spine25263
Saini, S., Moger, N. M., Kumar, M., Sarkar, S., Mittal, S., Ifthekar, S., et al. (2023). Biomechanical analysis of Instrumented decompression and Interbody fusion procedures in Lumbar spine: a finite element analysis study. Med. Biol. Eng. Comput. 61 (7), 1875–1886. doi:10.1007/s11517-023-02825-y
Sanan, A., and Rengachary, S. S. (1996). The history of spinal biomechanics. Neurosurgery 39 (4), 657–668. doi:10.1097/00006123-199610000-00001
Sato, K., Kikuchi, S., and Yonezawa, T. (1999). In vivo intradiscal pressure measurement in healthy individuals and in patients with ongoing back problems. Spine (Phila Pa 1976) 24 (23), 2468–2474. doi:10.1097/00007632-199912010-00008
Sawa, A. G. U., Lehrman, J. N., Crawford, N. R., and Kelly, B. P. (2020). Variations among human lumbar spine segments and their relationships to in vitro biomechanics: a Retrospective analysis of 281 motion segments from 85 cadaveric spines. Int. J. Spine Surg. 14 (2), 140–150. doi:10.14444/7021
Schiemann, T., Freudenberg, J., Pflesser, B., Pommert, A., Priesmeyer, K., Riemer, M., et al. (2000). Exploring the visible human using the VOXEL-MAN framework. Comput. Med. Imaging Graph. 24 (3), 127–132. doi:10.1016/s0895-6111(00)00013-6
Schimandle, J. H., and Boden, S. D. (1994). Spine update. The use of animal models to study spinal fusion. Spine (Phila Pa 1976) 19 (17), 1998–2006. doi:10.1097/00007632-199409000-00023
Schmid, S., Stauffer, M., Jäger, J., List, R., and Lorenzetti, S. (2019). Sling-based infant carrying affects lumbar and thoracic spine neuromechanics during standing and walking. Gait Posture 67, 172–180. doi:10.1016/j.gaitpost.2018.10.013
Schwab, F., Patel, A., Lafage, V., and Farcy, J. P. (2009). A porcine model for progressive thoracic scoliosis. Spine (Phila Pa 1976) 34 (11), E397–E404. doi:10.1097/BRS.0b013e3181a27156
Sham, M. L., Zander, T., Rohlmann, A., and Bergmann, G. (2005). Effects of the Rib Cage on Thoracic Spine Flexibility/Einfluss des Brustkorbs auf die Flexibilität der Brustwirbelsäule. Biomed. Tech. (Berl.) 50 (11), 361–365. doi:10.1515/bmt.2005.051
Sharif-Alhoseini, M., Khormali, M., Rezaei, M., Safdarian, M., Hajighadery, A., Khalatbari, M. M., et al. (2017). Animal models of spinal cord injury: a systematic review. Spinal Cord. 55 (8), 714–721. doi:10.1038/sc.2016.187
Sheng, S. R., Wang, X. Y., Xu, H. Z., Zhu, G. Q., and Zhou, Y. F. (2010). Anatomy of large animal spines and its comparison to the human spine: a systematic review. Eur. Spine J. 19 (1), 46–56. doi:10.1007/s00586-009-1192-5
Shimizu, K., Nakamura, M., Nishikawa, Y., Hijikata, S., Chiba, K., and Toyama, Y. (2005). Spinal kyphosis causes demyelination and neuronal loss in the spinal cord: a new model of kyphotic deformity using juvenile Japanese small game fowls. Spine (Phila Pa 1976) 30 (21), 2388–2392. doi:10.1097/01.brs.0000184378.67465.5c
Shin, S. S., and Yoo, W. G. (2019). Stepping over an obstacle in patients with lumbar spinal stenosis: Trunk and lower extremities of kinematic and muscle activation normalized by double limb support. A preliminary study. Technol. Health Care 27 (1), 1–11. doi:10.3233/thc-171082
Smit, T. H. (2002). The use of a quadruped as an in vivo model for the study of the spine - biomechanical considerations. Eur. Spine J. 11 (2), 137–144. doi:10.1007/s005860100346
Song, M., Sun, K., Li, Z., Zong, J., Tian, X., Ma, K., et al. (2021). Stress distribution of different lumbar posterior pedicle screw insertion techniques: a combination study of finite element analysis and biomechanical test. Scientific reports.11 (01), 12968. doi:10.1038/s41598-021-90686-6
Stott, B., and Driscoll, M. (2023). Biomechanical evaluation of the thoracolumbar spine comparing healthy and irregular thoracic and lumbar curvatures. Comput. Biol. Med. 160, 106982. doi:10.1016/j.compbiomed.2023.106982
Stott, B., and Driscoll, M. (2024). Development and evaluation of sex-specific thoracolumbar spine finite element models to study spine biomechanics. Med. Biol. Eng. Comput. 62 (4), 1191–1199. doi:10.1007/s11517-023-03003-w
Sturges, B. K., Kapatkin, A. S., Garcia, T. C., Anwer, C., Fukuda, S., Hitchens, P. L., et al. (2016). Biomechanical comparison of Locking compression plate versus positive Profile pins and polymethylmethacrylate for stabilization of the canine lumbar vertebrae. Vet. Surg. 45 (3), 309–318. doi:10.1111/vsu.12459
Sun, Z., Lu, T., Li, J., Liu, J., Hu, Y., and Mi, C. (2022). A finite element study on the effects of follower load on the continuous biomechanical responses of subaxial cervical spine. Comput. Biol. Med. 145, 105475. doi:10.1016/j.compbiomed.2022.105475
Sun, T., Wang, J., Liu, X., Huang, H., Wang, J., Suo, M., et al. (2025). Finite element models of intervertebral disc: recent advances and prospects. Ann. Med. 57 (1), 2453089. doi:10.1080/07853890.2025.2453089
Tai, C. L., Hsieh, P. H., Chen, W. P., Chen, L. H., Chen, W. J., and Lai, P. L. (2008). Biomechanical comparison of lumbar spine instability between laminectomy and bilateral laminotomy for spinal stenosis syndrome - an experimental study in porcine model. BMC Musculoskelet. Disord. 9, 84. doi:10.1186/1471-2474-9-84
Tien, C. S., and Huston, R. L. (1985). Biodynamic Modelling of the head/neck system. SAE Tech. Pap. 850438,, 850438. doi:10.4271/850438
Tsai, K. H., Lin, R. M., and Chang, G. L. (1998). Rate-related fatigue injury of vertebral disc under axial cyclic loading in a porcine body-disc-body unit. Clin. Biomech. (Bristol) 13 (1 Suppl. 1), S32–s39. doi:10.1016/s0268-0033(98)80134-4
Usami, T., Kisohara, M., Nishida, K., Koboyashi, D., Ida, R., Matsubara, K., et al. (2025). Gait analysis using an artificial intelligence-based motion capture system with a single smartphone camera. Cureus 17 (7), e87837. doi:10.7759/cureus.87837
Vergroesen, P. P., Kingma, I., Emanuel, K. S., Hoogendoorn, R. J., Welting, T. J., van Royen, B. J., et al. (2015). Mechanics and biology in intervertebral disc degeneration: a vicious circle. Osteoarthr. Cartil. 23 (7), 1057–1070. doi:10.1016/j.joca.2015.03.028
Wang, M., Tang, S. J., McGrady, L. M., and Rao, R. D. (2013a). Biomechanical comparison of supplemental posterior fixations for two-level anterior lumbar interbody fusion. Proc. Inst. Mech. Eng. H. 227 (3), 245–250. doi:10.1177/0954411912465057
Wang, T., Liu, H., Zheng, Z., Li, Z., Wang, J., Shrivastava, S. S., et al. (2013b). Biomechanical effect of 4-rod technique on lumbosacral fixation: an in vitro human cadaveric investigation. Spine (Phila Pa 1976) 38 (15), E925–E929. doi:10.1097/BRS.0b013e3182967968
Wang, L., Wang, Y., Shi, L., Liu, P., Kang, J., He, J., et al. (2019). Can the sheep model fully represent the human model for the functional evaluation of cervical interbody fusion cages? Biomech. Model. Mechanobiol. 18 (3), 607–616. doi:10.1007/s10237-018-1104-x
Wawrose, R. A., Howington, F. E., LeVasseur, C. M., Smith, C. N., Couch, B. K., Shaw, J. D., et al. (2021). Assessing the biofidelity of in vitro biomechanical testing of the human cervical spine. J. Orthop. Res. 39 (6), 1217–1226. doi:10.1002/jor.24702
Wilke, H. J., Wenger, K., and Claes, L. (1998). Testing criteria for spinal implants: recommendations for the standardization of in vitro stability testing of spinal implants. Eur. Spine J. 7 (2), 148–154. doi:10.1007/s005860050045
Wilke, H. J., Neef, P., Caimi, M., Hoogland, T., and Claes, L. E. (1999). New in vivo measurements of pressures in the intervertebral disc in daily life. Spine (Phila Pa 1976) 24 (8), 755–762. doi:10.1097/00007632-199904150-00005
Wilke, H. J., Kaiser, D., Volkheimer, D., Hackenbroch, C., Püschel, K., and Rauschmann, M. (2016). A pedicle screw system and a lamina hook system provide similar primary and long-term stability: a biomechanical in vitro study with quasi-static and dynamic loading conditions. Eur. Spine J. 25 (9), 2919–2928. doi:10.1007/s00586-016-4679-x
Wong, C. E., Hu, H. T., Hsieh, M. P., and Huang, K. Y. (2020). Optimization of three-level cervical Hybrid surgery to prevent adjacent segment disease: a finite element study. Front. Bioeng. Biotechnol. 8, 154. doi:10.3389/fbioe.2020.00154
Xue, Z. L., Chen, Z. X., Fu, C. H., Lei, H. J., and Yuan, X. W. (2013). Biomechanical assessment of unilateral pedicle screws plus contralateral transfacetopedicular screws after transforaminal lumbar interbody fusion with two cages. Orthop. Surg. 5 (4), 274–279. doi:10.1111/os.12075
Yamamoto, S., Dias, L., Street, J., Cripton, P. A., and Oxland, T. R. (2022). Anteroposterior shear stiffness of the upper thoracic spine at quasi-static and dynamic loading rates-An in vitro biomechanical study. J. Orthop. Res. 40 (7), 1687–1694. doi:10.1002/jor.25196
Yu, S., Zheng, S., Gao, Y., Liu, Y., Zhang, K., and Dong, R. (2024). Impact of sacroiliac interosseous ligament tension and laxity on lumbar spine biomechanics under vertical vibration: a finite element study. Comput. Methods Biomech. Biomed. Engin., 1–9. doi:10.1080/10255842.2024.2437661
Zhai, X., Kang, J., Chen, X., Dong, J., Qiu, X. W., Ding, X. A., et al. (2019). In vivo measurement of three-dimensional motion of the upper cervical spine using CT three-dimensional reconstruction. Zhongguo Gu Shang 32 (7), 658–665. doi:10.3969/j.issn.1003-0034.2019.07.014
Zhang, Z., Fogel, G. R., Liao, Z., Sun, Y., and Liu, W. (2018). Biomechanical analysis of lumbar interbody fusion cages with various lordotic angles: a finite element study. Comput. Methods Biomech. Biomed. Engin. 21 (3), 247–254. doi:10.1080/10255842.2018.1442443
Keywords: spine biomechanics, experimental models, digital modeling, loading methods, measurement techniques
Citation: Xu B, Li G, Sun X, Yang S, Sun R, Gong Y and Song M (2025) Main elements of current spine biomechanics research: model, installation and test data. Front. Bioeng. Biotechnol. 13:1646046. doi: 10.3389/fbioe.2025.1646046
Received: 12 June 2025; Accepted: 17 September 2025;
Published: 20 October 2025.
Edited by:
Qiang Chen, Southeast University, ChinaReviewed by:
Shaobai Wang, Shanghai University of Sport, ChinaJing Lyu, University College London, United Kingdom
Copyright © 2025 Xu, Li, Sun, Yang, Sun, Gong and Song. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Gong, Z29uZ3lpbmdAZW1haWwuY24=; Ran Sun, cmFucmFuLW5vLjFAMTYzLmNvbQ==; Mingzhi Song, c29uZ21pbmd6aGlAc2p0dS5lZHUuY24=
†These authors have contributed equally to this work
 Bide Xu1,2†
Bide Xu1,2† Gang Li
Gang Li Xueheng Sun
Xueheng Sun Mingzhi Song
Mingzhi Song