- School of Physical Education and Sports, Soochow University, Suzhou, China
Background: Lateral ankle sprains often progress to functional ankle instability (FAI). Obstacle-crossing could pose greater challenges for individuals with FAI due to significant impairments in ankle kinesthesia and joint position sense. While existing studies have focused on level-ground gait characteristics in FAI, the postural control strategies underlying obstacle-crossing remain unclear, and the impact of obstacle height on these strategies has not been investigated.
Purpose: This study is aimed at analyzing the postural control strategies of individuals with FAI during obstacle-crossing at different heights.
Methods: Twenty-three male participants [unilateral FAI group (n = 11) and matched controls (n = 12)] were recruited. FAI was identified using the Cumberland Ankle Instability Tool (CAIT score <24). Obstacle heights were set at 0%, 10%, and 20% of individuals’ leg length (LL). Participants completed crossing tasks in randomized order. The individuals with FAI use their FAI-affected limb as the swing leg and controls use the matched limb.
Results: Compared to the control group, the FAI group exhibited smaller hip flexion angles (P = 0.008), greater trunk lateral flexion (P = 0.033), and reduced medio-lateral margin of stability (ML_MoS) at landing (P = 0.046). As obstacle height increased, the FAI group showed significant differences in ML_MoS at landing (P < 0.001), with notably lower ML_MoS when the obstacle height was set at 20% LL compared to controls (P = 0.001).
Conclusion: Compared to healthy individuals, those with FAI adapt movement patterns through proximal compensation strategies, characterized by compensatory trunk lateral flexion. Increased obstacle height exacerbates instability during landing, particularly at higher heights, where individuals with FAI demonstrate significantly diminished lateral stability. These findings emphasize the critical influence of FAI on balance control and adaptive postural control strategies during obstacle-crossing.
1 Introduction
Lateral ankle sprains, one of the most common musculoskeletal injuries, have a prevalence of 11.88% in the general population and account for approximately 20% of all sports injuries (Doherty et al., 2013; Wagemans et al., 2022). There is consensus that the initial injuries that are not treated properly can lead to chronic musculoskeletal problems. In most cases, individuals with functional ankle instability (FAI) experience difficulty making a full recovery within 3 years (van Rijn et al., 2008). Furthermore, the risk of recurrent injuries is significantly increased (Hung, 2015; Docherty et al., 2006), which ultimately evolves into FAI (Beynnon et al., 2001). FAI-induced damage to mechanoreceptors in the lateral ankle ligaments (e.g., the anterior talofibular ligament) causes abnormal proprioceptive input (Arnold et al., 2011), which has been demonstrated to trigger progressive hyperalgesia of ankle positional, kinesthetic, and force sensations (Liu et al., 2024). As the disease progresses, this neurosensory dysfunction evolves into reduced muscle strength and neuromuscular control (Cruz-Montecinos et al., 2025; Akbari and Yousefi, 2023). Previous studies have revealed that individuals with FAI may adopt compensatory strategies during walking, such as increased hip and knee joint mobility, to maintain dynamic balance (Akbari and Yousefi, 2023). While this abnormal gait pattern partially compensates for ankle function deficits, it may elevate injury risks under certain circumstances (Son et al., 2019). Individuals with FAI have been found to have a higher incidence of falls in the past 12 months and a greater prevalence of fall-related injuries and hospitalizations compared to those without FAI (Al Mahrouqi et al., 2023).
Improper crossing of obstacles during walking is a critical risk factor for falls (Al Mahrouqi et al., 2023). Compared to level walking, obstacle-crossing imposes higher demands on neuromuscular control, requiring not only enhanced activation of specific muscles (e.g., knee flexors) and refined allocation of cognitive-sensory resources but also significant increases in prefrontal cortex activity, reflecting heightened neuromuscular and central regulatory demands (Patla et al., 1996; Patla et al., 1991; MacLellan and McFadyen, 2013; Chen et al., 2017). Consequently, intact proprioception is essential for successful obstacle-crossing (Billington et al., 2013). Ligament injuries in individuals with FAI damage proprioceptive nerve endings and trigger reorganization within the central nervous system’s motor cortex. This impairs multiple facets of proprioception, including position sense, movement sense, force sense, and vibration sensation (Peng et al., 2024; Ward et al., 2015). Research indicates a significant negative correlation between CAIT scores and inversion proprioception (Peng et al., 2024; Xue et al., 2021). Crucially, the concurrent integration deficits in multiple proprioceptive domains compromise precise foot clearance height regulation and landing impact attenuation, ultimately elevating injury risk.
Research further indicates that compared to healthy individuals, those with FAI display reduced ankle dorsiflexion, increased inversion, and elevated knee internal rotation moments during walking and jogging (Lee et al., 2022). Additionally, individuals with FAI frequently exhibit neuromuscular control deficits, such as prolonged reaction times in the peroneus longus muscle (Méndez-Rebolledo et al., 2015). They also demonstrate impaired control of ankle muscle force output, suggesting a potential link between FAI and diminished ability to perceive force generation (Yen et al., 2019). Furthermore, weakness in muscle groups surrounding other lower limb joints may compromise ankle stability in individuals with FAI (Yeum et al., 2024). Thus, analyzing lower limb joint kinematics during obstacle-crossing in FAI populations could help identify potential postural control deficits. Although existing evidence implies that individuals with FAI may adopt abnormal gait patterns during crossing obstacles, no studies have yet investigated the postural control strategies of obstacle-crossing in this population, leaving the impact of obstacle-crossing on individuals with FAI poorly understood.
As obstacle height increases, the complexity of obstacle-crossing rises significantly (Huang et al. 2008). During dynamic locomotion, it is necessary to appropriately maintain the extrapolated center of mass (XCoM) within the base of support (BoS) to enhance stability (Hof et al., 2005). The margin of stability (MoS) serves as an effective metric for analyzing the dynamic relationship between the XCoM and the BoS (AminiAghdam et al., 2019). As previously discussed, given that individuals with FAI exhibit proprioceptive deficits, elevated obstacle heights likely impose greater demands on their neuromuscular control capacity. These individuals must precisely regulate gait parameters (e.g., step length, walking speed, and body posture) to meet the challenge, creating dual pressure on their already compromised motor control abilities (Wang et al., 2007; Galna et al., 2010; Rosker et al., 2024). Additionally, to compensate for distal joint control deficits, individuals with FAI often adopt compensatory strategies by increasing proximal joint (e.g., hip and trunk) movements to maintain dynamic balance (Huang et al., 2020). However, current research has yet to systematically explore the mechanisms by which obstacles of varying heights affect individuals with FAI, leaving a knowledge gap regarding the postural control strategies of their obstacle-crossing strategies across different obstacle heights.
Therefore, to prevent fall-related injuries that may disrupt daily activities and incur economic burdens, it is essential to investigate the postural control strategies of obstacle-crossing in individuals with FAI. This study aims to examine the postural control strategies of walking while crossing obstacles of different heights in individuals with FAI, specifically analyzing the impact of obstacle heights relative to leg length (LL). To the best of our knowledge, this study may be the first to systematically quantify how progressive increases in obstacle height (0%, 10%, 20% LL) exacerbate postural control deficits in individuals with FAI during obstacle-crossing, thereby addressing the critical research gap regarding the dose-response relationship between obstacle height and dynamic stability. The findings will provide theoretical insights to guide the development of healthy behavioural awareness, fall prevention strategies, and injury avoidance in daily activities for individuals with FAI. The specific objectives of this study are:
1. To clarify the postural control strategies of obstacle-crossing in individuals with FAI.
2. To explore how obstacle height influences obstacle-crossing strategies in individuals with FAI compared to healthy controls.
To address the above objectives, this study proposes the following two hypotheses based on existing literature and prior research findings:
Hypothesis 1. The postural control strategies during obstacle-crossing tasks may differ between individuals with FAI and healthy controls. Individuals with FAI may be unable to effectively utilize distal joints and may rely compensatorily on proximal joints to maintain posture during obstacle-crossing.
Hypothesis 2. Increased obstacle height will induce changes in postural control strategies, and individuals with Individuals with FAI will face greater postural control challenges compared to healthy individuals as obstacle height rises, which may exacerbate stability deficits in individuals with FAI during landing.
2 Methods
2.1 Sample size calculation
The required sample size was calculated using G*Power (Version 3.1.9, Heinrich Heine University Disseldorf, Germany). We estimated the sample size for both the group main effect and the group-by-condition interaction effect. Based on our research group’s previous study comparing individuals with FAI and healthy controls (Wang et al., 2024a; Wang et al., 2024b), the η2 values for the group main effect ranged from 0.224 to 0.542, while those for the group-by-condition interaction effect ranged from 0.128 to 0.283. Intermediate η2 values were selected to determine the effect sizes: 0.383 for the group main effect and 0.206 for the interaction effect. Using a significance level of α = 0.05 and a desired statistical power of 1 − β = 0.8, the calculation indicated a minimum requirement of 12 participants for the group main effect size and 10 participants for the interaction effect size. Consequently, the more conservative estimate of 12 participants was adopted. To account for a potential 20% rate of invalid samples, the target sample size was increased to 15 participants.
2.2 Participants
This study recruited 12 males with unilateral FAI as the experimental group through the Cumberland Ankle Instability Tool (CAIT) (Hiller et al., 2006) and anterior drawer test, along with 12 healthy males matched for age, height, weight, and other demographic criteria as the control group. Due to equipment malfunction, data from one participant were excluded, resulting in a final sample of 23 participants. The baseline characteristics of the two groups are summarized in Table 1. All participants provided written informed consent, and the study was approved by the Ethics Committee of Soochow University (Ethics Approval No: SUDA20250327H01).
Inclusion and exclusion criteria were based on previous literature. Inclusion criteria for the FAI group included at least one ankle sprain in the past year with self-reported instability (Ardakani et al., 2019); A CAIT score below 24 (Donahue et al., 2011); No history of severe lower limb injuries (e.g., fractures or major orthopedic trauma), excluding ankle sprains (Wu et al., 2022); and Unilateral FAI. Exclusion criteria for both groups were: History of bilateral ankle sprains (Wang et al., 2022); Acute lower limb pathologies; Prior lower limb surgeries (Kweon et al., 2022); Balance dysfunction (Donahue et al., 2011); Congenital deformities of the feet, ankles, knees, pelvis, or spine; Positive talar tilt test and/or anterior drawer test results in either ankle.
2.3 Experimental equipment
This study utilized an 8-camera infrared motion capture system (Vicon, United Kingdom) synchronized with two three-dimensional force plates (9281, Kistler, Switzerland) to collect kinematic and kinetic data during obstacle-crossing tasks. The Vicon system recorded body movement trajectories at a sampling frequency of 100 Hz, while the force plates captured ground reaction force parameters at 1,000 Hz. An adjustable-height obstacle frame assembly (AOTII, China) was employed to create obstacle heights at 0%, 10%, and 20% of each participant’s LL (Figure 1).
2.4 Experimental protocol
2.4.1 Pre-test preparation
First, participants’ anthropometric measurements (height, weight, and LL) were recorded. Marker placement (39 markers in total) adhered to the Plug-in Gait full-body model (Vicon, Oxford, United Kingdom), with reflective markers positioned at key anatomical landmarks including the head, trunk (C7/T10/sternum/clavicle), pelvis (anterior/posterior superior iliac spines), upper extremities (shoulders/elbows/wrists), and lower extremities (thighs/knees/shanks/ankles/heels/toes). Marker placements are detailed in Figure 2. To minimize variability, all markers were positioned by the same researcher, who also collected morphological data prior to testing.
Figure 2. Marker placement configuration for the Plug-in Gait full-body model: (a) Anterior view; (b) Posterior view.
2.4.2 Obstacle-crossing task
Before dynamic data collection, static calibration trials were performed following standardized procedures. Participants then completed obstacle-crossing trials under three height conditions: 0%, 10%, and 20% of their LL. The order of obstacle heights was randomized. Each condition was repeated multiple times (with a minimum 30-s rest between trials) until three valid trials per condition were obtained (Chardon et al., 2024; Lee et al., 2022). A valid trial was defined as one where: (1) the participant successfully crossed the obstacle during the task, (2) motion capture data from all markers was successfully collected, enabling the generation of a complete biomechanical model, (3) at the moment of swing leg touchdown, the foot remained completely within the boundaries of the force plate, and (4) no abnormal signals were contained in the data during preprocessing. Based on this procedure, we prevented the inclusion of potential outliers, as any aberrant data points were excluded during our experimental process.
The task protocol was as follows: Participants stood 3 m in front of the Kistler force plates in a natural upright posture, gazing forward. Upon the “start” command, they walked forward at a self-selected comfortable speed, crossed the obstacle, and continued walking 5 m beyond the force plates. During the crossing, the support leg (FAI-affected limb in the experimental group or matched limb in controls) contacted the proximal force plate, while the swing leg landed on the distal force plate (Figure 3). Three successful trials were collected for each obstacle height, with the FAI-affected limb (or matched limb in controls) consistently used as the swing leg during the crossing.
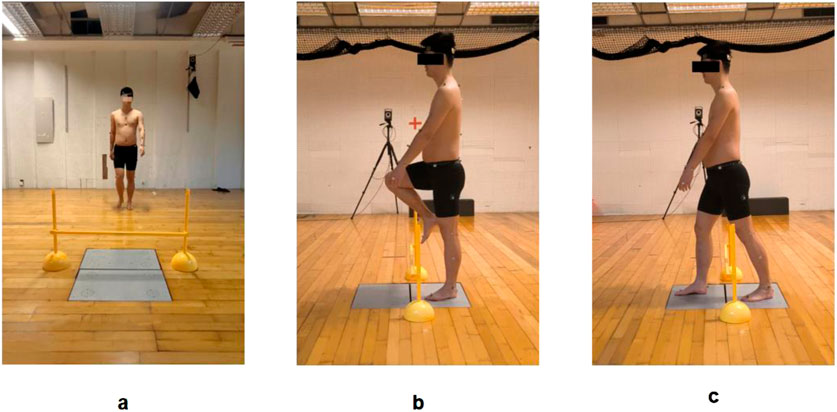
Figure 3. Obstacle-crossing test during walking: (a) Starting position; (b) Mid-crossing phase; (c) Completion phase.
2.5 Data analysis
The full-body skeletal model was defined in Vicon Nexus software, and the Plug-in Gait Full Body model (15 rigid segments: head, trunk, pelvis, bilateral thighs, shanks, feet, upper arms, forearms, and hands) was reconstructed. Motion capture data and ground reaction forces were processed with a Butterworth low-pass filter (cutoff frequencies: 6 Hz for kinematics and 50 Hz for kinetics) and analyzed using custom MATLAB scripts to extract relevant parameters (Zhang and Zhang, 2023).
2.5.1 Spatiotemporal gait parameters
The obstacle-crossing task was defined as the period from the moment the swing leg leaves the ground to when it makes contact with the ground again after crossing the obstacle. This period was further divided into three phases: (1) Preparation phase, defined as the interval from when the swing leg toe lifts more than 3 cm above the ground (while the supporting leg remains in contact with the proximal force plate) to when the swing leg reaches a position within ±1 cm in front of the obstacle; (2) Execution phase, spanning from the instant the swing leg reaches ±1 cm anterior to the obstacle to the point the heel passes at least 1 cm beyond the obstacle; (3) Completion phase, which is from the heel extending 1 cm beyond the obstacle until the swing leg contacts the distal force plate (ground reaction force >10 N).
For each phase and the entire obstacle-crossing period, both phase-specific and total crossing velocities were calculated, with the overall velocity determined as the obstacle length divided by total crossing time. The proportion of time spent in each phase relative to the entire swing phase was also analyzed. Additionally, spatial parameters were assessed, including step width and length (measured as the mediolateral and anteroposterior distances between heel markers at initial contact), vertical clearance (the vertical distance between the swing foot’s toe and heel markers and the obstacle during the execution phase), and horizontal distance (the anteroposterior distance between the support leg’s toe marker and the swing leg’s heel marker relative to the obstacle at task completion).
2.5.2 Joint kinematic parameters
Joint angles were calculated in local anatomical coordinate systems according to the Plug-in Gait standards. The swing phase of the swinging leg was rescaled to a normalized 0%–100% time base, and the mean joint angle across this normalized swing phase was extracted for group comparisons. For the ankle, dorsiflexion (sagittal plane) and inversion (frontal plane) were defined as positive, while plantarflexion (sagittal plane) and eversion (frontal plane) were defined as negative values. For the hip, extension (sagittal) and adduction (frontal) were negative, while flexion (sagittal) and abduction (frontal) were positive. For the knee, extension was negative, and flexion was positive in the sagittal plane. For the trunk, flexion was positive and extension negative in the sagittal plane, while medio flexion was negative and lateral flexion positive in the frontal plane.
2.5.3 Dynamic stability metrics
To quantitatively reflect the participants’ ability to maintain dynamic balance upon landing after obstacle crossing, the MoS at the moment when the swing leg lands after crossing the obstacle was used (AminiAghdam et al., 2019). By Equation 1 the MoS represents the shortest distance from the horizontal projection of the body’s XCoM to the nearest boundary of the BoS. The calculation of XCoM involves determining the dynamic position of the center of mass (CoM). And we computed the Medio-lateral Margin of Stability (ML_MoS) and the Anterior-posterior Margin of Stability (AP_MoS) are defined, see Equations 2, 3. These spatial and postural stability variables are illustrated in Figure 4. Following prior literature (Qu et al., 2021), XCoM, ML_MoS and AP_MoS were calculated as:
where: CoM represents the whole-body CoM displacement and was obtained from the Plug in Gait model output, VELCoM is the velocity of the CoM, l is the vertical distance from the CoM to the ground, and g = 9.81 m/s2 is the acceleration due to gravity. When calculating the ML_MoS and AP_MoS, the edge of the BoS can be judged by the marker at the heel. Positive MoS values indicate CoM position within the BoS, while negative values indicate instability (CoM outside BoS). Positive MoS values indicate CoM position within the BoS, while negative values indicate instability (CoM outside BoS) (Hof et al., 2005).
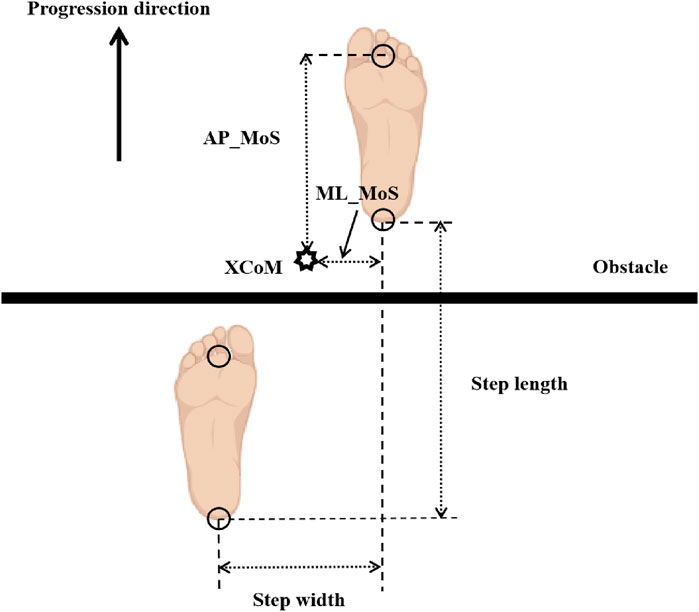
Figure 4. Definitions of spatial gait parameters and margin of stability (MoS). Foot markers indicate the position of each foot at the instant of swing limb heel contact. The arrow denotes the direction of walking progression. Step length = anterior-posterior (AP) distance between the two heel markers; step width = medial-lateral (ML) distance between the two heel markers. AP_MoS = AP distance between the XCoM and the toe marker of the support leg (Preparation phase/Execution phase) or the toe marker of the swing leg (Completion phase); ML_MoS = lateral distance between the XCoM and the toe marker of the support leg (Preparation phase/Execution phase) or the heel marker of the swing leg (Completion phase).
2.5.4 Statistical analysis
Data analysis was performed using SPSS 27.0. Continuous variables are presented as mean ± standard deviation (SD). A mixed ANOVA was conducted to evaluate the main effects of obstacle height (within-subject factor: 0% LL, 10% LL, 20% LL), the main effects of group (between-subject factor: FAI vs. control), and interaction effects between group and obstacle height. If a significant interaction was detected, a simple effects analysis was performed to compare differences between groups at each obstacle height. Statistical significance was set at α = 0.05. Post hoc comparisons were adjusted using the Bonferroni correction.
3 Results
Table 2 presents the spatiotemporal parameters. Significant main effects of obstacle height were observed for step length (F = 5.156, P = 0.022, η2 = 0.197), swing total speed (F = 130.267, P < 0.001, η2 = 0.861), swing pre speed (F = 95.016, P < 0.001, η2 = 0.819), swing obstacle speed (F = 158.379, P < 0.001, η2 = 0.883), swing post speed (F = 158.508, P < 0.001, η2 = 0.883), the proportion of the preparation phase (F = 47.061, P < 0.001, η2 = 0.691), the proportion of the execution phase (F = 20.946, P < 0.001, η2 = 0.499), and the proportion of the completion phase (F = 44.987, P < 0.001, η2 = 0.682). Post hoc comparisons revealed the following trends as obstacle height increased: From 0% to 10% and 20% LL, swing total speed decreased by 0.215 m/s (P < 0.001) and 0.264 m/s (P < 0.001). Swing pre-speed decreased by 0.175 m/s (P < 0.001) and 0.218 m/s (P < 0.001). Swing obstacle speed decreased by 0.259 m/s (P < 0.001) and 0.314 m/s (P < 0.001). Swing post speed decreased by 0.250 m/s (P < 0.001) and 0.301 m/s (P < 0.001). The proportion of the preparation phase decreased by 7.794% (P < 0.001) and 9.760% (P < 0.001). The proportion of the execution phase decreased by 2.863% (P < 0.001) and 3.524% (P < 0.001). The proportion of the completion phase increased by 10.657% (P < 0.001) and 13.283% (P < 0.001). From 10% to 20% LL, Swing total speed decreased by 0.049 m/s (P < 0.001). Swing pre speed decreased by 0.042 m/s (P = 0.002). Swing obstacle speed decreased by 0.054 m/s (P < 0.001). Swing post speed decreased by 0.051 m/s (P < 0.001). The proportion of the preparation phase decreased by 1.965% (P = 0.011). The proportion of the completion phase increased by 2.626% (P < 0.001). No significance was found in the other metrics. No significant between-group differences or interaction effects were observed in the obstacle distance-related parameters during obstacle-crossing.
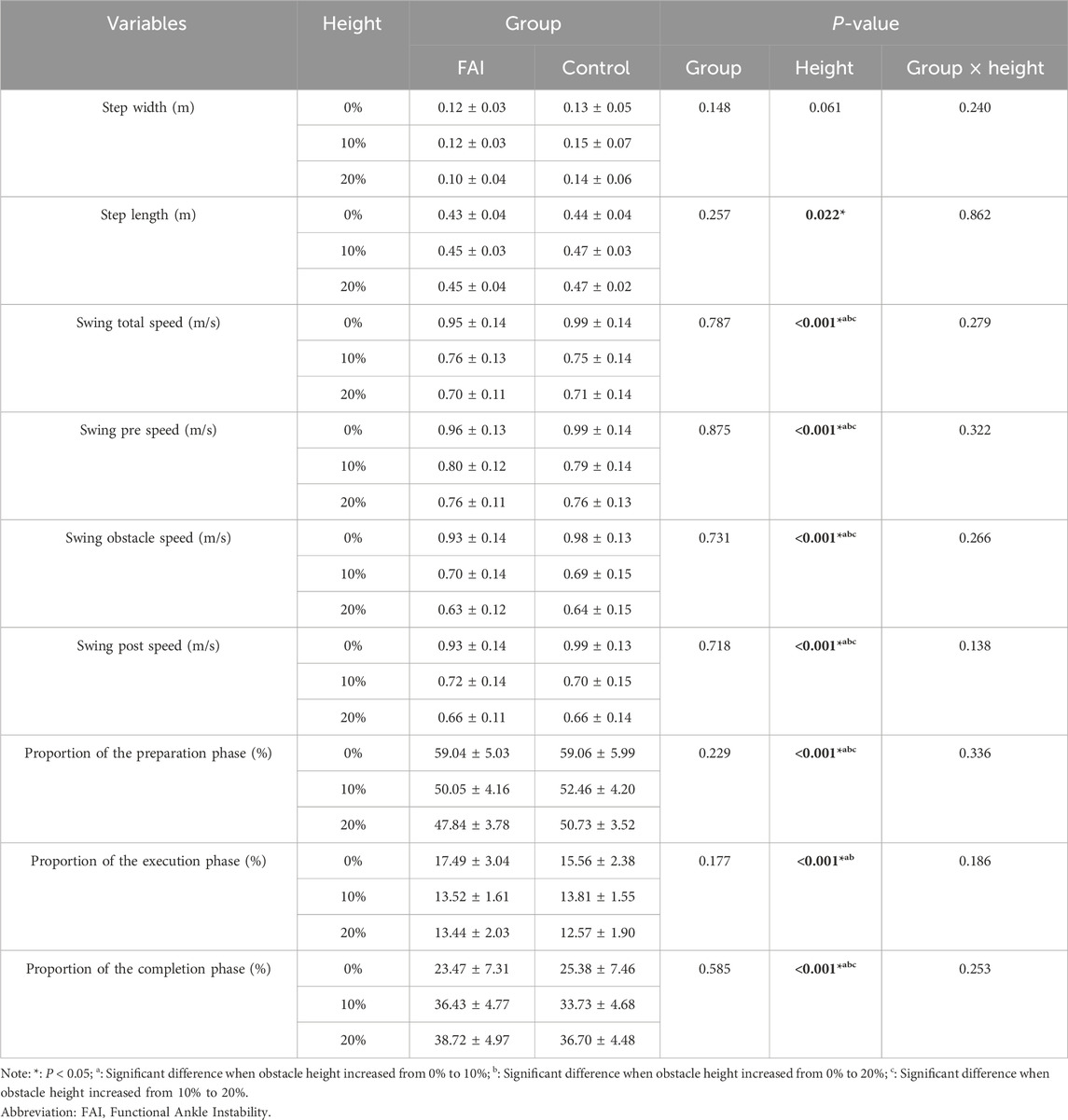
Table 2. Comparison of step length, step width, velocity, and phase proportions between the FAI group and control group during walking while crossing obstacles of different heights (mean ± SD).
Table 3 displays foot-to-obstacle distance metrics during obstacle-crossing. Significant main effects of obstacle height were observed for vertical clearance of right toe (F = 233.764, P < 0.001, η2 = 0.918), vertical clearance of right heel (F = 178.532, P < 0.001, η2 = 0.895), vertical clearance of left toe (F = 220.062, P < 0.001, η2 = 0.913), vertical clearance of left heel (F = 224.172, P < 0.001, η2 = 0.914), horizontal distance of right heel (F = 5.760, P = 0.011, η2 = 0.215), horizontal distance of left toe (F = 3.667, P = 0.049, η2 = 0.149). Post hoc comparisons revealed the following trends as obstacle height increased: From 0% to 10% and 20% LL, Vertical clearance of right toe increased by 0.204 m (P < 0.001) and 0.165 m (P < 0.001). Vertical clearance of right heel increased by 0.192 m (P < 0.001) and 0.155 m (P < 0.001). Vertical clearance of left toe increased by 0.236 m (P < 0.001) and 0.200 m (P < 0.001). Vertical clearance of left heel increased by 0.246 m (P < 0.001) and 0.208 m (P < 0.001). Horizontal distance of right heel increased by 0.023 m (P = 0.036) and 0.021 m (P = 0.067). From 10% to 20% LL, Vertical clearance of right toe increased by 0.039 m (P = 0.001). Vertical clearance of right heel decreased by 0.037 m (P = 0.001). Vertical clearance of left toe decreased by 0.036 m (P = 0.001). Vertical clearance of left heel decreased by 0.038 m (P = 0.004). No significance was found in the other metrics. And no significant between-group differences or interaction effects were observed in the obstacle distance-related parameters during obstacle-crossing.
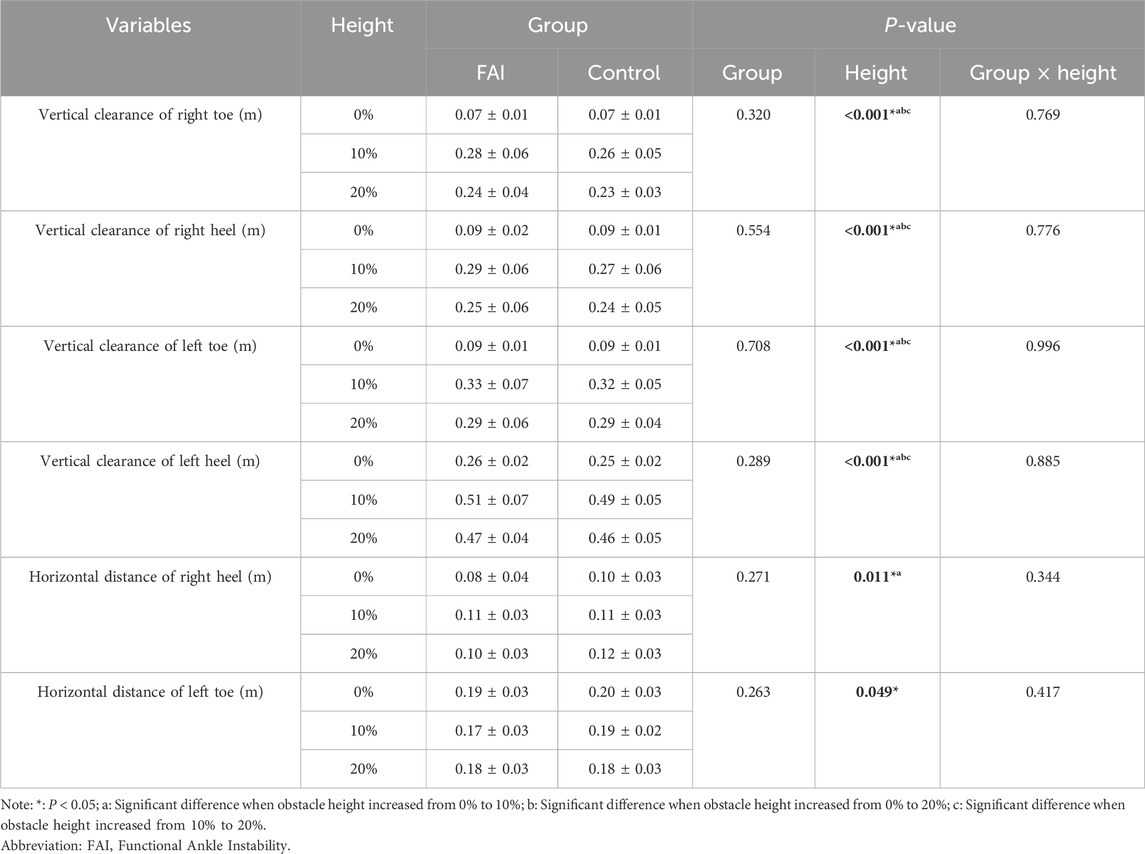
Table 3. Comparison of foot-to-obstacle distances between the FAI group and control group during walking while crossing obstacles of different heights (mean ± SD).
Table 4 presents the results regarding joint angles. Main effects of obstacle height were found in ankle sagittal plane angle (F = 6.781, P = 0.010, η2 = 0.224), ankle frontal plane angle (F = 18.179, P < 0.001, η2 = 0.464), hip sagittal plane angle (F = 660.805, P < 0.001, η2 = 0.969), hip frontal plane angle (F = 10.041, P = 0.002, η2 = 0.323), knee sagittal plane angle (F = 601.895, P < 0.001, η2 = 0.167), trunk sagittal plane angle (F = 14.976, P < 0.001, η2 = 0.416), and trunk frontal plane angle (F = 4.638, P = 0.015, η2 = 0.181). Post-hoc comparisons revealed that as obstacle height increased from 0% to 10% and 20%, ankle plantar flexion decreased by 2.4° (P = 0.025) and 1.8° (P = 0.088), ankle inversion increased by 0.9° (P < 0.001) and 0.8° (P = 0.001), hip flexion increased by 25.1° (P < 0.001) and 27.8° (P < 0.001), hip adduction increased by 1.9° (P = 0.027) and 2.6° (P = 0.005), knee flexion increased by 40.1° (P < 0.001) and 43.2° (P < 0.001), trunk extension decreased by 2.0° (P < 0.001) and 2.3° (P = 0.002), and trunk lateral flexion increased by 0.1° (P = 1.000) and 0.8° (P = 0.023). When obstacle height increased from 10% to 20%, hip flexion increased by 2.8° (P = 0.002), and knee flexion increased by 3.1° (P = 0.021), while no significant differences were found in other parameters. Group differences were observed in hip sagittal plane angle (F = 8.642, P = 0.008, η2 = 0.292) and trunk frontal plane angle (F = 5.239, P = 0.033, η2 = 0.200), with post-hoc analysis showing the FAI group had significantly smaller hip flexion angles (7.3°, P = 0.008) and greater trunk lateral flexion angles (1.5°, P = 0.033) than the control group. No interaction effects were observed in any joint angle measures.
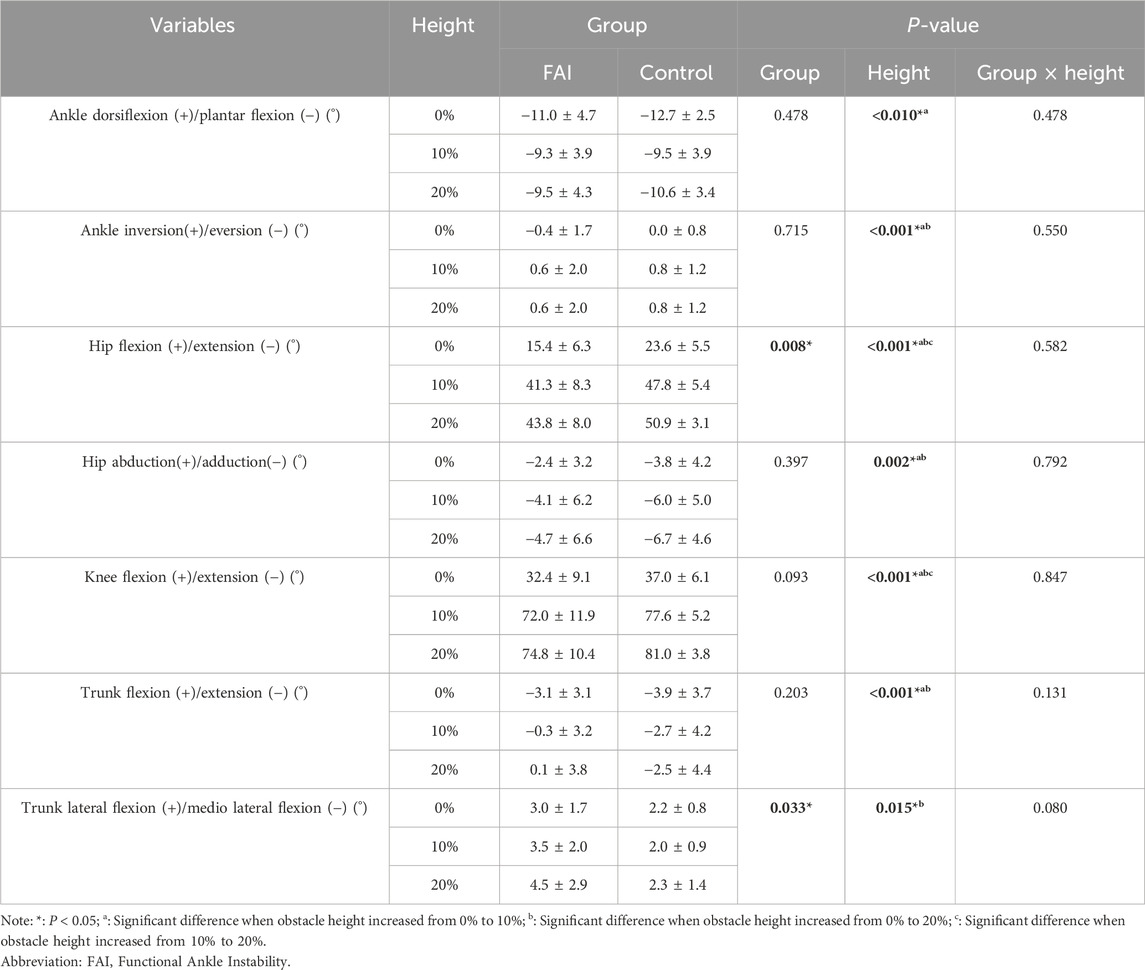
Table 4. Comparison of ankle, hip, knee, and trunk joint angles between the FAI group and control group during walking over obstacles of different heights (mean ± SD).
Table 5 presents the results for MoS. The main effects of obstacle height were observed in the preparation phase for ML_MoS (F = 44.158, P < 0.001, η2 = 0.678) and AP_MoS (F = 109.868, P < 0.001, η2 = 0.840); in the Execution Phase - Toe Clearance Over Obstacle for ML_MoS (F = 28.783, P < 0.001, η2 = 0.578) and AP_MoS (F = 166.504, P < 0.001, η2 = 0.888); in the Execution Phase - Heel Clearance Over Obstacle for ML_MoS (F = 34.515, P < 0.001, η2 = 0.622) and AP_MoS (F = 175.546, P < 0.001, η2 = 0.893); and in the completion phase for ML_MoS (F = 9.602, P < 0.001, η2 = 0.314) and AP_MoS (F = 141.731, P < 0.001, η2 = 0.871). Post-hoc comparisons revealed that as obstacle height increased from 0% to 10% and 20%, ML_MoS significantly increased by 0.013 m (P < 0.001) and 0.022 m (P < 0.001) in the preparation phase, while AP_MoS increased by 0.073 m (P < 0.001) and 0.084 m (P < 0.001). During the Execution Phase - Heel Clearance Over Obstacle, ML_MoS increased by 0.017 m (P = 0.002) and 0.031 m (P < 0.001), and AP_MoS increased by 0.120 m (P < 0.001) and 0.144 m (P < 0.001). In the Execution Phase - Heel Clearance Over Obstacle, ML_MoS increased by 0.022 m (P < 0.001) and 0.038 m (P < 0.001), and AP_MoS increased by 0.135 m (P < 0.001) and 0.162 m (P < 0.001). In the completion phase, ML_MoS increased by 0.011 m (P = 0.014) and 0.013 m (P = 0.004), and AP_MoS increased by 0.084 m (P < 0.001) and 0.097 m (P < 0.001).
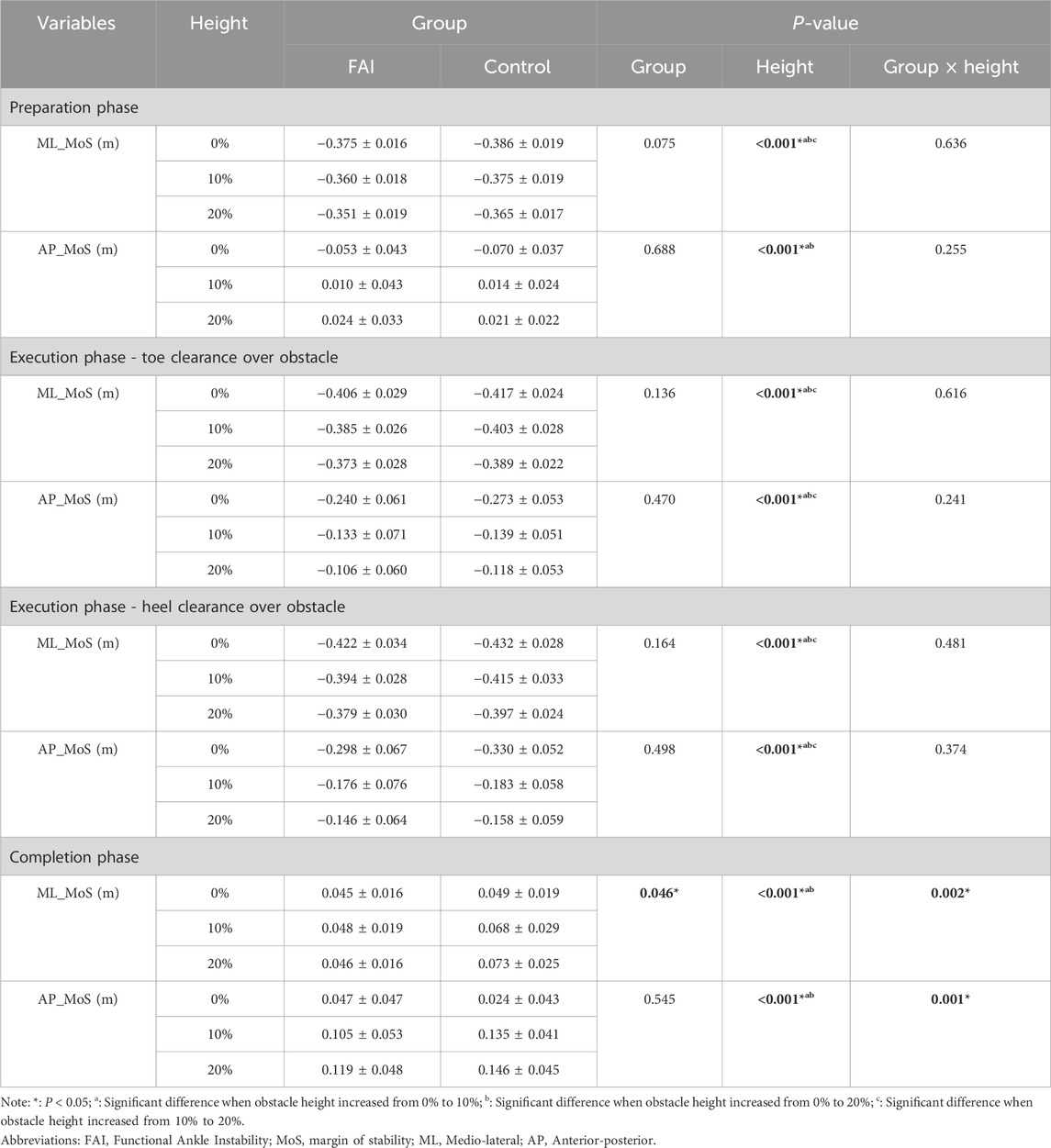
Table 5. Comparison of stability margin between the FAI group and the control group during walking over obstacles of different heights (mean ± SD).
Furthermore, as obstacle height increased from 10% to 20%, ML_MoS in the preparation phase significantly increased by 0.009 m (P < 0.001); during the execution phase (toe clearance), ML_MoS increased by 0.013 m (P < 0.001) and AP_MoS increased by 0.024 m (P = 0.010); and during the execution phase (heel clearance), ML_MoS increased by 0.016 m (P < 0.001) and AP_MoS increased by 0.028 m (P = 0.005). Additionally, a significant group difference was observed in ML_MoS during the completion phase (F = 4.497, P = 0.046, η2 = 0.176), with post hoc analysis showing the FAI group had a 0.017 m lower MoS than the control group (P = 0.046).
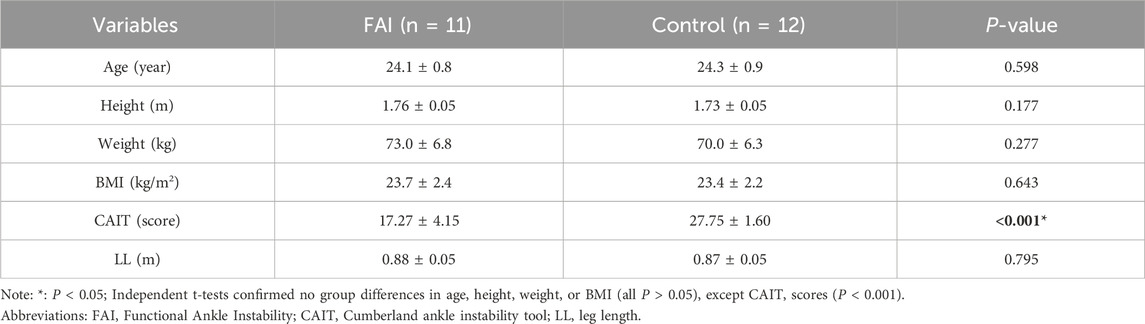
Interaction effects between obstacle height and group were found for ML_MoS (F = 7.073, P = 0.002, η2 = 0.252) and AP_MoS (F = 141.731, P < 0.001, η2 = 0.871) in the completion phase. Simple effects analysis revealed that at the 20% obstacle height, the FAI group exhibited significantly lower ML_MoS than the control group (0.027 ± 0.09 m, P = 0.005) (Figure 5), indicating reduced ML_MoS in individuals with FAI when negotiating higher obstacles.
4 Discussion
4.1 The differences of postural control strategies in obstacle-crossing strategies between FAI and healthy individuals
4.1.1 Postural control challenges during obstacle-crossing in individuals with FAI
This study examined the postural control strategies of individuals with FAI during obstacle-crossing tasks at various heights. Consistent with our Hypothesis 1, the biomechanical characteristics observed during obstacle-crossing were significantly different between individuals with FAI and healthy controls. The kinetic chain theory posits that human joints do not function in isolation but rather operate through a “proximal-distal” linkage to maintain overall mechanical efficiency and stability. When distal segments (e.g., the ankle joint) exhibit functional decline, the kinetic chain triggers compensatory synergies in proximal joints (knee, hip, pelvis, trunk) to redistribute moments, adjust center-of-mass trajectories, and ensure task completion (Cole et al., 1995; Kang and Kim, 2020). Previous studies have demonstrated that FAI induces adaptive alterations in the lower limb kinetic chain, which extends beyond the ankle to affect the knee and hip joints (Moisan et al., 2021; Son et al., 2019; Lee et al., 2022). For instance, Son et al. revealed that during level walking, individuals with FAI adopt a hip-dominant gait strategy by restricting ankle propulsive force while increasing hip power and hip flexion angles. These individuals exhibited sagittal-plane compensatory movements, marked by a 2.0° increase in hip flexion and a 22% elevation in hip extension moment (Son et al., 2019). Similarly, Kim et al. found that individuals with FAI employ analogous hip-dominant strategies during landing and jumping tasks, characterized by heightened hip extension moments, stiffness, and eccentric/concentric power (Kim et al., 2018). However, in contrast to the hip-dominant gait strategy observed by Son et al. during level walking in individuals with FAI, the results of the present study indicate that during obstacle-crossing, individuals with FAI exhibited reduced hip flexion, while the knee and ankle joints showed no significant differences compared to healthy controls. This discrepancy in findings may suggest that individuals with FAI adopt distinct postural control strategies when negotiating obstacles.
The act of crossing an obstacle, particularly as its height increases, is fundamentally a demanding proprioceptive-motor task. The requirement for precise ankle positioning, controlled weight transfer, and coordinated muscle force generation escalates with obstacle height (Yamagata et al., 2023). Concurrently, attention must be allocated to maintain continuous environmental monitoring and precise motor control (Friesen et al., 2022). Consequently, obstacle-crossing performance is critically dependent on proprioceptive integrity, with individuals exhibiting severe proprioceptive deficits often demonstrating impaired motor control during obstacle negotiation (Lu et al., 2022; Lee and Lee, 2023). In individuals with FAI, proprioceptive decline manifests as a key pathological feature. During ankle sprains, the lateral ligaments–the most frequently injured structures–sustain damage to their embedded proprioceptors, resulting in diminished or distorted proprioceptive feedback to the central nervous system (Kawabata et al., 2024). This proprioceptive information disruption directly compromises neuromuscular control efficiency, leading to reduced balance capacity (Sagnard et al., 2025), impaired joint position sense (Marinho et al., 2017), and increased postural control challenges during obstacle-crossing. Simultaneously, obstacle-crossing requires anterior flexor contraction to drive hip flexion. However, in individuals with FAI, compromised proprioception and pre-existing compensatory impairments in periarticular hip musculature (Son et al., 2019) create a mismatch: proximal muscle strength and neural drive fail to proportionally enhance to counteract distal control deficiencies. This renders the hip-dominant anti-perturbation strategy ineffective, manifesting as incomplete activation and progressive weakening of periarticular hip muscles. Consequently, an “insufficient compensation phenomenon” emerges: although hip flexion shows marginal increases with obstacle height, it remains markedly lower than in controls. Although the kinetic chain attempts compensatory adaptations, proprioceptive-motor task competition induced by FAI prevent full proximal compensation for distal deficits, ultimately sustaining stability impairments.
4.1.2 Trunk compensatory mechanisms in restricted hip mobility
Notably, despite limited hip joint mobility in individuals with FAI, their vertical clearance between toes/heels and obstacles showed no significant reduction. This phenomenon may be achieved through compensatory mechanisms. The study revealed that compared to healthy controls, individuals with FAI exhibited increased trunk lateral flexion, adjusting their center of mass trajectory through lateral trunk tilting to indirectly reduce dependence on hip joint mobility. This compensation might be associated with impaired ankle proprioception resulting from previous ankle sprains in individuals with FAI. Studies have shown that individuals with compromised proprioception and diminished balance capacity tend to actively increase trunk lateral flexion and medial-lateral center of mass displacement during obstacle-crossing as compensatory adaptations to avoid collisions (Shin et al., 2015; Chou et al., 2003).
However, this compensatory strategy may initiate a dual vicious cycle: On one hand, proximal compensatory inhibition prevents adequate activation of hip muscle strength, leading to weakening of periarticular hip musculature. On the other hand, to compensate for restricted hip mobility, individuals with FAI increase trunk lateral flexion angles to modify center of mass trajectory, thereby reducing medial-lateral stability margins. This compensatory approach further hinders proper hip flexion and ultimately increases postural control difficulty when confronting unexpected external perturbations. Additionally, compensatory trunk tilting in individuals with FAI elevates energy expenditure (Takacs et al., 2014) and exacerbates dynamic instability through altered center of mass trajectory (Shin et al., 2015; Chou et al., 2003). Consequently, while this compensatory strategy preserves basic obstacle-crossing functionality, it increases movement economy costs (e.g., energy consumption) and dynamic stability risks.
4.2 Unique impact of increased obstacle height on individuals with FAI
4.2.1 Changes in postural control due to increased obstacle height
The results demonstrate that as obstacle height increases, significant alterations occur in step length, swing velocity, phase duration distribution, joint kinematics, and MoS, indicating that obstacle height profoundly influences gait patterns, spatiotemporal parameters, joint mechanics, and stability control. These findings align with prior studies by Austin et al., who reported that higher obstacles necessitate greater postural adjustments (Austin et al., 1999), including modifications to gait (Park et al., 2012), spatiotemporal parameters (Simieli et al., 2018), joint angles (Wang et al., 2025), and stability strategies (Wang et al., 2007), to ensure successful obstacle negotiation and balance maintenance.
4.2.2 Specific challenges posed by increased obstacle height for FAI individuals
Furthermore, we observed in this study that with increasing obstacle height, distinct differences in ML_MoS were observed between the FAI group and controls at the moment of obstacle-crossing completion. This result supports our Hypothesis 2, indicating that increased obstacle height leads to alterations in postural control strategies and that individuals with FAI experience greater challenges in maintaining postural stability compared to healthy controls as obstacle height increases. Notably, the FAI group exhibited significantly lower ML_MoS at 20% LL compared to controls. At lower heights (0% and 10% LL), minimal between-group differences in stability margins were observed, likely due to reduced postural challenges at these levels, which diminished the manifestation of compensatory strategies in individuals with FAI. However, at 20% LL, particularly during completion phase, ankle joint loads increased abruptly. This heightened load directly affected ankle stability and overall biomechanical characteristics, with more pronounced effects in individuals with FAI (Watanabe et al., 2021; Jang et al., 2024; Liu et al., 2025). Furthermore, impaired proprioception in individuals with FAI (Marinho et al., 2017) compromised their ability to perceive and adjust to foot loading during landing, exacerbating ankle instability (Kang et al., 2022). Given the critical role of ankle stability in maintaining dynamic stability (Simpson et al., 2019), these findings collectively suggest that FAI significantly reduces overall stability during obstacle landing phases. This height-dependent instability highlights the unique challenges faced by individuals with FAI in high-obstacle environments. While healthy controls adapt effectively to heightened demands, individuals with FAI struggle to maintain stability under increased mechanical and sensorimotor stress.
5 Clinical recommendations
By systematically exploring the dose-response relationship between obstacle height and obstacle height and stability deficits in FAI, which provides valuable insights into the challenging stability issues faced by individuals with FAI during obstacle-crossing. In contrast, healthy controls demonstrated superior adaptability to these environments.
Current rehabilitation for individuals with FAI primarily utilizes balance and proprioceptive training. While these approaches demonstrably improve dynamic stability and subjective outcomes (Yekdaneh and Mutlu, 2024), their benefits remain limited, and no single intervention has emerged as clearly optimal (Tedeschi et al., 2024). Moreover, the adaptive responses of the central nervous system to ankle injury in individuals with FAI alter postural control strategies, thereby increasing their risk of subsequent injury during complex movements (Wang et al., 2024a). This study found that FAI involves deficits in proprioception and balance control, significantly increasing the difficulty of maintaining postural stability during obstacle-crossing. Consequently, obstacle-crossing training offers a valuable functional adjunct to rehabilitation. As a targeted functional activity, this training simulates real-world environmental demands, effectively enhancing an individual’s obstacle avoidance capability (Weerdesteyn et al., 2008), spatial awareness (Pramodhyakul et al., 2013; Barral, 2023), and overall postural control (Pramodhyakul et al., 2013). Importantly, the objective performance feedback inherent in obstacle-crossing tasks (e.g., success/failure, clearance height) may enhance individuals with FAI’s motivation and rehabilitation adherence by providing tangible evidence of functional improvement. Incorporating obstacle-crossing training into rehabilitation protocols, integrated with sensorimotor and motor control strategies, is essential in fostering neural remodeling associated with ankle ligament injuries (Maricot et al., 2023), thus also mitigating fall risks during complex daily activities for individuals with FAI.
6 Limitations
This study still has some limitations: (1) To avoid potential confounding effects of sex and age differences, the current cohort was restricted to young male participants only. Future research should include female participants and more diverse age groups to improve generalizability and reliability; (2) The experimental design required participants to perform the task with the affected limb crossing the obstacle and the unaffected limb supporting the body. Differences in strategy when reversing this pattern (unaffected limb crossing, affected limb supporting) remain unexplored; (3) The study only tested obstacle heights of 0%, 10%, and 20% of LL. The effects of higher obstacles on individuals with FAI remain unclear.
7 Conclusion
Individuals with FAI employed unique compensatory mechanisms during obstacle-crossing with the affected limb. Compared to healthy controls, their hip joints could not be sufficiently activated, thus adopting a trunk compensatory strategy, characterized by significantly reduced hip flexion angles and compensatory increases in trunk lateral flexion. As obstacle height increased, individuals with FAI demonstrated decreased stability during landing phases, particularly showing a significant reduction in lateral stability at higher obstacle heights. These findings emphasize the impact of FAI on balance control and motor strategy adaptation during obstacle negotiation, providing insights into mitigating reinjury risks in this population.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of Soochow University (Ethics Approval No: SUDA20250327H01). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
KM: Project administration, Validation, Software, Conceptualization, Resources, Supervision, Writing – review and editing, Writing – original draft, Methodology, Formal Analysis, Data curation, Investigation, Visualization. WZ: Writing – original draft, Supervision, Writing – review and editing, Resources, Formal Analysis, Software, Investigation, Data curation, Project administration, Visualization, Methodology, Conceptualization, Validation. XS: Investigation, Formal Analysis, Writing – review and editing, Software, Writing – original draft, Resources, Validation, Data curation, Conceptualization, Visualization, Methodology. GW: Supervision, Resources, Validation, Writing – review and editing, Project administration, Writing – original draft, Methodology, Formal Analysis, Visualization, Software, Investigation. XM: Supervision, Writing – review and editing, Methodology, Investigation, Software, Project administration, Writing – original draft, Resources, Formal Analysis, Validation, Visualization. LK: Data curation, Investigation, Methodology, Software, Conceptualization, Validation, Writing – review and editing, Supervision, Resources, Formal Analysis, Visualization, Writing – original draft, Funding acquisition, Project administration. QZ: Software, Funding acquisition, Supervision, Resources, Writing – review and editing, Writing – original draft, Formal Analysis, Methodology, Visualization, Data curation, Investigation, Conceptualization, Validation, Project administration.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors are grateful to all the young adults who participated in this study.
Conflict of interest
The authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Akbari, N. J., and Yousefi, M. (2023). Which strategy is dominant in functional ankle instability individuals during gait walking? Gait Posture 106, S89. doi:10.1016/j.gaitpost.2023.07.109
Al Mahrouqi, M. M., Vicenzino, B., MacDonald, D. A., and Smith, M. D. (2023). Falls and falls-related injuries in individuals with chronic ankle symptoms: a cross-sectional study. J. Foot Ankle Res. 16 (1), 49. doi:10.1186/s13047-023-00649-5
AminiAghdam, S., Griessbach, E., Vielemeyer, J., and Müller, R. (2019). Dynamic postural control during (in)visible curb descent at fast versus comfortable walking velocity. Gait Posture 71, 38–43. doi:10.1016/j.gaitpost.2019.04.014
Ardakani, M. K., Wikstrom, E. A., Minoonejad, H., Rajabi, R., and Sharifnezhad, A. (2019). Hop-stabilization training and landing biomechanics in athletes with chronic ankle instability: a randomized controlled trial. J. Athl. Train. 54 (12), 1296–1303. doi:10.4085/1062-6050-550-17
Arnold, B. L., Wright, C. J., and Ross, S. E. (2011). Functional ankle instability and health-related quality of life. J. Athl. Train. 46 (6), 634–641. doi:10.4085/1062-6050-46.6.634
Austin, G. P., Garrett, G. E., and Bohannon, R. W. (1999). Kinematic analysis of obstacle clearance during locomotion. Gait Posture 10 (2), 109–120. doi:10.1016/s0966-6362(99)00022-3
Barral, J.-P. (2023). Gleichgewicht und Propriozeption. Osteopat. Med. 24 (3), 24–28. doi:10.1016/s1615-9071(23)00077-1
Beynnon, B. D., Renström, P. A., Alosa, D. M., Baumhauer, J. F., and Vacek, P. M. (2001). Ankle ligament injury risk factors: a prospective study of college athletes. J. Orthop. Res. 19 (2), 213–220. doi:10.1016/s0736-0266(00)90004-4
Billington, J., Wilkie, R. M., and Wann, J. P. (2013). Obstacle avoidance and smooth trajectory control: neural areas highlighted during improved locomotor performance. Front. Behav. Neurosci. 7, 9. doi:10.3389/fnbeh.2013.00009
Chardon, M., Barbieri, F. A., Hansen, C., Petit, P., and Vuillerme, N. (2024). Impact of overweight on spatial–temporal gait parameters during obstacle-crossing in young adults: a cross-sectional study. Sensors 24 (23), 7867. doi:10.3390/s24237867
Chen, M., Pillemer, S., England, S., Izzetoglu, M., Mahoney, J. R., and Holtzer, R. (2017). Neural correlates of obstacle negotiation in older adults: an fNIRS study. Gait Posture 58, 130–135. doi:10.1016/j.gaitpost.2017.07.043
Chou, L. S., Kaufman, K. R., Hahn, M. E., and Brey, R. H. (2003). Medio-lateral motion of the center of mass during obstacle-crossing distinguishes elderly individuals with imbalance. Gait Posture 18 (3), 125–133. doi:10.1016/s0966-6362(02)00067-x
Cole, A. J., Herring, S. A., Stratton, S. A., and Narvaez, J. (1995). Spine injuries in runners: a functional approach. J. Back Musculoskelet. Rehabilitation 5 (4), 317–339. doi:10.3233/bmr-1995-5408
Cruz-Montecinos, C., Sanzana-Cuche, R., and Mendez-Rebolledo, G. (2025). Regional muscle fiber conduction velocity of the fibularis longus in individuals with chronic ankle instability. J. Anat. 247 (2), 375–384. doi:10.1111/joa.14244
Docherty, C. L., Valovich McLeod, T. C., and Shultz, S. J. (2006). Postural control deficits in participants with functional ankle instability as measured by the balance error scoring system. Clin. J. Sport Med. 16 (3), 203–208. doi:10.1097/00042752-200605000-00003
Doherty, C., Delahunt, E., Caulfield, B., Hertel, J., Ryan, J., and Bleakley, C. (2013). The incidence and prevalence of ankle sprain injury: a systematic review and meta-analysis of prospective epidemiological studies. Sports Med. 44 (1), 123–140. doi:10.1007/s40279-013-0102-5
Donahue, M., Simon, J., and Docherty, C. L. (2011). Critical review of self-reported functional ankle instability measures. Foot Ankle Int. 32 (12), 1140–1146. doi:10.3113/FAI.2011.1140
Friesen, K. B., Lanovaz, J. L., Moraes, R., and Oates, A. R. (2022). Don’t get tripped up: haptic modalities alter gait characteristics during obstacle-crossing. Hum. Mov. Sci. 82, 102935. doi:10.1016/j.humov.2022.102935
Galna, B., Murphy, A. T., and Morris, M. E. (2010). obstacle-crossing in people with Parkinson’s disease: foot clearance and spatiotemporal deficits. Hum. Mov. Sci. 29 (5), 843–852. doi:10.1016/j.humov.2009.09.006
Hiller, C. E., Refshauge, K. M., Bundy, A. C., Herbert, R. D., and Kilbreath, S. L. (2006). The cumberland ankle instability tool: a report of validity and reliability testing. Archives Phys. Med. Rehabilitation 87 (9), 1235–1241. doi:10.1016/j.apmr.2006.05.022
Hof, A. L., Gazendam, M. G. J., and Sinke, W. E. (2005). The condition for dynamic stability. J. Biomechanics 38 (1), 1–8. doi:10.1016/j.jbiomech.2004.03.025
Huang, S. C., Lu, T. W., Chen, H. L., Wang, T. M., and Chou, L. S. (2008). Age and height effects on the center of mass and center of pressure inclination angles during obstacle-crossing. Med. Eng. Phys. 30 (8), 968–975. doi:10.1016/j.medengphy.2007.12.005
Huang, S.-J., Yu, X.-M., Wang, K., Wang, L.-J., Wu, X.-B., Wu, X., et al. (2020). Short-step adjustment and proximal compensatory strategies adopted by stroke survivors with knee extensor spasticity for obstacle-crossing. Front. Bioeng. Biotechnol. 8, 939. doi:10.3389/fbioe.2020.00939
Hung, Y. (2015). Neuromuscular control and rehabilitation of the unstable ankle. World J. Orthop. 6 (5), 434. doi:10.5312/wjo.v6.i5.434
Jang, J., Franz, J. R., Pietrosimone, B. G., and Wikstrom, E. A. (2024). Muscle contributions to reduced ankle joint contact force during drop vertical jumps in patients with chronic ankle instability. J. Biomechanics 163, 111926. doi:10.1016/j.jbiomech.2024.111926
Kang, S., and Kim, K. (2020). Comparison of shoulder neuromuscular control in overhead athletes with and without shoulder hypermobility. Exerc. Sci. 29 (3), 316–323. doi:10.15857/ksep.2020.29.3.316
Kang, M., Zhang, T., Yu, R., Ganderton, C., Adams, R., and Han, J. (2022). Effect of different landing heights and loads on ankle inversion proprioception during landing in individuals with and without chronic ankle instability. Bioengineering 9 (12), 743. doi:10.3390/bioengineering9120743
Kawabata, S., Ozone, K., Minegishi, Y., Oka, Y., Terada, H., Takasu, C., et al. (2024). Chronic ankle joint instability induces ankle sensorimotor dysfunction: a controlled laboratory study. Am. J. Sports Med. 52 (3), 739–749. doi:10.1177/03635465231217490
Kim, H., Son, S. J., Seeley, M. K., and Hopkins, J. T. (2018). Kinetic compensations due to chronic ankle instability during landing and jumping. Med. Sci. Sports Exerc. 50 (2), 308–317. doi:10.1249/MSS.0000000000001442
Kweon, S. J., Harrison, K., Williams, D. S. B., and Kwon, Y. U. (2022). Foot and shank coordination during walking in copers compared with patients with chronic ankle instability and controls. Orthop. J. Sports Med. 10 (12), 23259671221139482. doi:10.1177/23259671221139482
Lee, S. M., and Lee, H. S. (2023). Correlation between executive function and walk while crossing over an obstacle under different gait phases. Dementia Neurocognitive Disord. 22 (4), 139. doi:10.12779/dnd.2023.22.4.139
Lee, I., Ha, S., Chae, S., Jeong, H. S., and Lee, S. Y. (2022). Altered biomechanics in individuals with chronic ankle instability compared with copers and controls during gait. J. Athl. Train. 57 (8), 760–770. doi:10.4085/1062-6050-0605.20
Liu, Y., Dong, S., Wang, Q., Liu, Z., Song, Q., and Shen, P. (2024). Deficits in proprioception and strength may contribute to the impaired postural stability among individuals with functional ankle instability. Front. Physiology 15, 1342636. doi:10.3389/fphys.2024.1342636
Liu, W., Xu, L., Wu, H., Wang, Y., Jiang, H., Gao, Z., et al. (2025). Bilateral asymmetries of plantar pressure and foot balance during walking, running, and turning gait in typically developing children. Bioengineering 12 (2), 151. doi:10.3390/bioengineering12020151
Lu, S.-H., Kuan, Y.-C., Wu, K.-W., Lu, H.-Y., Tsai, Y.-L., Chen, H.-H., et al. (2022). Kinematic strategies for obstacle-crossing in older adults with mild cognitive impairment. Front. Aging Neurosci. 14, 950411. doi:10.3389/fnagi.2022.950411
MacLellan, M. J., and McFadyen, B. J. (2013). Proximal lower limb muscle energetics and the adaptation of segment elevation angle phasing for obstacle avoidance. Gait Posture 37 (2), 274–279. doi:10.1016/j.gaitpost.2012.07.019
Maricot, A., Dick, E., Walravens, A., Pluym, B., Lathouwers, E., De Pauw, K., et al. (2023). Brain neuroplasticity related to lateral ankle ligamentous injuries: a systematic review. Sports Med. 53 (7), 1423–1443. doi:10.1007/s40279-023-01834-z
Marinho, H. V. R., Amaral, G. M., de Souza Moreira, B., Araújo, V. L., Souza, T. R., Ocarino, J. M., et al. (2017). Influence of passive joint stiffness on proprioceptive acuity in individuals with functional instability of the ankle. J. Orthop. Sports Phys. Ther. 47 (12), 899–905. doi:10.2519/jospt.2017.7030
Méndez-Rebolledo, G., Guzmán-Muñoz, E., Gatica-Rojas, V., and Zbinden-Foncea, H. (2015). Longer reaction time of the fibularis longus muscle and reduced postural control in basketball players with functional ankle instability: a pilot study. Phys. Ther. Sport 16 (3), 242–247. doi:10.1016/j.ptsp.2014.10.008
Moisan, G., Mainville, C., Descarreaux, M., and Cantin, V. (2021). Lower limb biomechanics in individuals with chronic ankle instability during gait: a case-control study. J. Foot Ankle Res. 14 (1), 36. doi:10.1186/s13047-021-00476-6
Park, S.-J., Otaka, Y., Okada, S., Kamioka, H., Okuizumi, H., Komatu, T., et al. (2012). The effect of obstacle height and maximum step length (MSL) on obstacle-crossing in healthy adults. Jpn. J. Phys. Fit. Sports Med. 61 (1), 103–109. doi:10.7600/jspfsm.61.103
Patla, A. E., Prentice, S. D., Robinson, C., and Neufeld, J. (1991). Visual control of locomotion: strategies for changing direction and for going over obstacles. J. Exp. Psychol. 17 (3), 603–634. doi:10.1037//0096-1523.17.3.603
Patla, A. E., Rietdyk, S., Martin, C., and Prentice, S. (1996). Locomotor patterns of the leading and the trailing limbs as solid and fragile obstacles are stepped over: some insights into the role of vision during locomotion. J. Mot. Behav. 28 (1), 35–47. doi:10.1080/00222895.1996.9941731
Peng, D., Tang, H., Mao, M., Song, Q., Mao, D., Wang, J., et al. (2024). Correlations of strength, proprioception, and dynamic balance to the Cumberland Ankle Instability Tool Score among patients with chronic ankle instability: a cross-sectional study. BMC Musculoskelet. Disord. 25 (1), 970. doi:10.1186/s12891-024-08092-8
Pramodhyakul, W., Wattanapan, P., Siritaratiwat, W., Eungpinichpong, W., and Amatachaya, S. (2013). Immediate effects of obstacle-crossing training in independent ambulatory patients with spinal cord injury. Spinal Cord. 51 (5), 379–383. doi:10.1038/sc.2012.178
Qu, X., Hu, X., and Tao, D. (2021). Gait initiation differences between overweight and normal weight individuals. Ergonomics 64 (8), 995–1001. doi:10.1080/00140139.2021.1896788
Rosker, Z. M., Mesaric, L., and Rosker, J. (2024). Training motor control in the cervical spine improves reliability of gait parameters in individuals with traumatic brain injury. Archives Phys. Med. Rehabilitation 105 (4), e186. doi:10.1016/j.apmr.2024.02.640
Sagnard, T., Picot, B., and Forestier, N. (2025). Proprioceptive acuity, proprioceptive weighting and balance in individuals with chronic ankle instability. Gait Posture 119, 178–184. doi:10.1016/j.gaitpost.2025.03.006
Shin, S., Demura, S., Watanabe, T., Yabumoto, T., Shi, B., Sakakibara, N., et al. (2015). Age-related and obstacle height-related differences in movements while stepping over obstacles. J. Physiol. Anthropol. 34 (1), 15. doi:10.1186/s40101-015-0052-8
Simieli, L., Barbieri, F. A., Orcioli-Silva, D., Lirani-Silva, E., Beretta, V. S., Santos, P. C. R. D., et al. (2018). Variability of crossing phase in older people with Parkinson’s disease is dependent of obstacle height. Sci. Rep. 8 (1), 14852. doi:10.1038/s41598-018-33312-2
Simpson, J. D., Stewart, E. M., Macias, D. M., Chander, H., and Knight, A. C. (2019). Individuals with chronic ankle instability exhibit dynamic postural stability deficits and altered unilateral landing biomechanics: a systematic review. Phys. Ther. Sport 37, 210–219. doi:10.1016/j.ptsp.2018.06.003
Son, S. J., Kim, H., Seeley, M. K., and Hopkins, J. T. (2019). Altered walking neuromechanics in patients with chronic ankle instability. J. Athl. Train. 54 (6), 684–697. doi:10.4085/1062-6050-478-17
Takacs, J., Kirkham, A. A., Perry, F., Brown, J., Marriott, E., Monkman, D., et al. (2014). Lateral trunk lean gait modification increases the energy cost of treadmill walking in those with knee osteoarthritis. Osteoarthr. Cartil. 22 (2), 203–209. doi:10.1016/j.joca.2013.12.003
Tedeschi, R., Ricci, V., Tarantino, D., Tarallo, L., Catani, F., and Donati, D. (2024). Rebuilding stability: exploring the best rehabilitation methods for chronic ankle instability. Sports 12 (10), 282. doi:10.3390/sports12100282
van Rijn, R. M., van Os, A. G., Bernsen, R. M., Luijsterburg, P. A., Koes, B. W., and Bierma-Zeinstra, S. M. (2008). What is the clinical course of acute ankle sprains? A systematic literature review. Am. J. Med. 121 (4), 324–331.e7. doi:10.1016/j.amjmed.2007.11.018
Wagemans, J., Bleakley, C., Taeymans, J., Schurz, A. P., Kuppens, K., Baur, H., et al. (2022). Exercise-based rehabilitation reduces reinjury following acute lateral ankle sprain: a systematic review update with mη2-analysis. PLoS One 17 (2), e0262023. doi:10.1371/journal.pone.0262023
Wang, T., Chen, H., and Lu, T. (2007). Effects of obstacle height on the control of the body center of mass motion during obstructed gait. J. Chin. Inst. Eng. 30 (3), 471–479. doi:10.1080/02533839.2007.9671275
Wang, B., Zhang, X., Zhu, F., Zhu, W., Wang, X., Jia, F., et al. (2022). A randomized controlled trial comparing rehabilitation with isokinetic exercises and Thera-Band strength training in patients with functional ankle instability. PLoS One 17 (12), e0278284. doi:10.1371/journal.pone.0278284
Wang, Z., Lu, M., Kong, L., Meng, L., Xue, J., Zheng, Y., et al. (2024a). Impact of cognitive tasks on biomechanical adjustments during single-leg drop landings in individuals with functional ankle instability. Appl. Sci. 14 (22), 10297. doi:10.3390/app142210297
Wang, Z., Meng, L., Lu, M., Kong, L., Xue, J., Zhang, Z., et al. (2024b). Effects of attentional focus strategies in drop landing biomechanics of individuals with unilateral functional ankle instability. Front. Physiology 15, 1444782. doi:10.3389/fphys.2024.1444782
Wang, C., Guo, Y., Du, W., Li, Z., and Chen, W. (2025). Gender differences in joint biomechanics during obstacle-crossing with different heights. Bioengineering 12 (2), 189. doi:10.3390/bioengineering12020189
Ward, S., Pearce, A. J., Pietrosimone, B., Bennell, K., Clark, R., and Bryant, A. L. (2015). Neuromuscular deficits after peripheral joint injury: a neurophysiological hypothesis. Muscle Nerve 51 (3), 327–332. doi:10.1002/mus.24463
Watanabe, K., Koshino, Y., Ishida, T., Samukawa, M., and Tohyama, H. (2021). Energy dissipation during single-leg landing from three heights in individuals with and without chronic ankle instability. Sports Biomech. 21 (4), 408–427. doi:10.1080/14763141.2021.2009549
Weerdesteyn, V., Nienhuis, B., and Duysens, J. (2008). Exercise training can improve spatial characteristics of time-critical obstacle avoidance in elderly people. Hum. Mov. Sci. 27 (5), 738–748. doi:10.1016/j.humov.2008.03.003
Wu, H. W., Chang, Y. S., Arefin, M. S., You, Y. L., Su, F. C., and Lin, C. F. (2022). Six-week remodeled bike pedal training improves dynamic control of lateral shuffling in athletes with functional ankle instability. Sports Health 14 (3), 348–357. doi:10.1177/19417381211035781
Xue, X., Ma, T., Li, Q., Song, Y., and Hua, Y. (2021). Chronic ankle instability is associated with proprioception deficits: a systematic review and meta-analysis. J. Sport Health Sci. 10 (2), 182–191. doi:10.1016/j.jshs.2020.09.014
Yamagata, M., Nagai, R., Morihiro, K., and Nonaka, T. (2023). Relation between the kinematic synergy controlling swing foot and visual exploration during obstacle-crossing. J. Biomechanics 157, 111702. doi:10.1016/j.jbiomech.2023.111702
Yekdaneh, A., and Mutlu, Ç. Y. (2024). Effects of balance and strength training for ankle proprioception in people with chronic ankle instability: a randomized controlled study. J. Am. Podiatric Med. Assoc. 114 (3), 23-008. doi:10.7547/23-008
Yen, S.-C., Chui, K. K., Wang, Y.-C., Corkery, M. B., Nabian, M., and Farjadian, A. B. (2019). An examination of muscle force control in individuals with a functionally unstable ankle. Hum. Mov. Sci. 64, 221–229. doi:10.1016/j.humov.2019.02.005
Yeum, W. J., Lee, M. Y., and Lee, B. H. (2024). The influence of hip-strengthening program on patients with chronic ankle instability. Med. Kaunas. Lith. 60 (8), 1199. doi:10.3390/medicina60081199
Keywords: FAI, obstacle height, biomechanics, musculoskeletal injury, margin of stability, postural control strategies
Citation: Ma K, Zhou W, Shi X, Wang G, Mao X, Kong L and Zhang Q (2025) Impacts of obstacle-crossing during walking on postural control strategies in individuals with functional ankle instability. Front. Bioeng. Biotechnol. 13:1650015. doi: 10.3389/fbioe.2025.1650015
Received: 19 June 2025; Accepted: 28 July 2025;
Published: 15 August 2025.
Edited by:
Jia Han, Shanghai University of Medicine and Health Sciences, ChinaReviewed by:
Wenxin Niu, Tongji University, ChinaYuki Suda, National Institute of Information and Communications Technology, Japan
Copyright © 2025 Ma, Zhou, Shi, Wang, Mao, Kong and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiuxia Zhang, cXh6aGFuZ0BzdWRhLmVkdS5jbg== Lingyu Kong, a2x5OTcxMEAxNjMuY29t
†Present address: Wenlong Zhou, Renmin University of China Affiliated Middle School, Suzhou, China
‡These authors have contributed equally to this work
 Ke Ma
Ke Ma Wenlong Zhou
Wenlong Zhou Xiangwei Shi
Xiangwei Shi Guodong Wang
Guodong Wang Xiaokun Mao
Xiaokun Mao Lingyu Kong
Lingyu Kong Qiuxia Zhang
Qiuxia Zhang