- 1IRCCS Centro Neurolesi Bonino Pulejo, Messina, Italy
- 2Clinical Laboratory of Experimental Neurorehabilitation, IRCCS Fondazione Santa Lucia, Rome, Italy
- 3Laboratory of Neuromotor Physiology, IRCCS Fondazione Santa Lucia, Rome, Italy
- 4Institute of Cognitive Sciences and Technologies, Consiglio Nazionale delle Ricerche (CNR), Rome, Italy
- 5Department of Systems Medicine and Center of Space Biomedicine, University of Rome Tor Vergata, Rome, Italy
- 6Department of Biomedical Sciences, Dentistry and Morpho-Functional Imaging, University of Messina, Messina, Italy
- 7Department of Computer, Control and Management Engineering, Sapienza University of Rome, Rome, Italy
- 8Neuroelectrical Imaging and Brain Computer Interface Lab, IRCCS Fondazione Santa Lucia, Rome, Italy
- 9Institute of Robotics and Mechatronics, German Aerospace Center (DLR), Weßling, Germany
- 10NEEEU Spaces GmbH, Berlin, Germany
- 11Assistive Intelligent Robotics Lab, Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Erlangen, Germany
- 12Department of Life, Health and Environmental Sciences, University of L’Aquila, L’Aquila, Italy
- 13Department of Biology, University of Rome Tor Vergata, Rome, Italy
Introduction: Cerebral stroke often results in significant motor deficits, including contralateral hemiparesis of the upper limb. Rehabilitation protocols with high-intensity and task-specific exercises can improve these deficits. Recent technological advancements in virtual reality (VR), myoelectric control, and exergames may be exploited to enhance rehabilitation effectiveness. However, novel rehabilitation approaches combining these novel methodologies have rarely been developed with the active involvement of both therapists and patients.
Methods: An interdisciplinary team developed a novel system, Validation of the Virtual Therapy Arm (VVITA), for post-stroke upper-limb rehabilitation combining VR, myoelectric control, and exergames using a user-centered design (UCD) approach. The VVITA hardware includes a head-mounted VR display, motion tracking devices integrated in the VR system, and wireless armbands to record electromyographic (EMG) signals, providing an interactive virtual environment for immersive rehabilitation exercises implementing a virtual mirror therapy. Assistance and task difficulty are adjusted dynamically based on patient performance, promoting active participation and motor learning.
Results: The development process involved iterative phases, involving focus groups with stroke patients, therapists, and researchers. A pilot study with four stroke survivors assessed the system’s feasibility, demonstrating its potential for personalized and adaptive rehabilitation.
Conclusion: The VVITA system enhances mirror therapy by integrating VR and myoelectric control, providing a tailored approach to upper-limb post-stroke rehabilitation. The UCD approach ensured the system met patient and therapist needs, showing promise for improving motor recovery and rehabilitation outcomes.
1 Introduction
Cerebral stroke is one of the main causes of disability in adults and most frequently involves hemiparesis of the contralateral body. Hemiparesis can cause muscular stiffness and other impairments to the fine and global motor coordination of the upper limbs, including restricted range of motion of the arm’s joints which hinders reaching and grasping movements, essential to perform daily living activities (Hatem et al., 2016).
These motor deficits occur for 80% of patients in the acute phase and for 40% of patients in the chronic phase and can be improved through rehabilitation protocols with high-intensity and/or task-specific exercises (Cramer et al., 1997; Kwakkel et al., 2004; Veerbeek et al., 2014). Technological advances in recent years have provided new methodologies to support and foster the rehabilitation process by increasing its repeatability and intensity (Foley et al., 2012; Cikajlo et al., 2020; Ceradini et al., 2024; De Luca et al., 2024), including but not limited to virtual reality (VR), myoelectric control, and exergames.
Rehabilitation systems integrated into VR systems can provide training scenarios that are more complex and engaging than those employed in conventional therapy (CT) (Maggio et al., 2023). In addition, activities of daily living (ADL) can be simulated in an ecological and controlled manner thanks to the immersivity of head-mounted displays, large projection screens, and VR caves. Thus, VR scenarios effectively increase patient’s active participation and motivation leading to a better adherence to the rehabilitation protocol (Domínguez-Téllez et al., 2020). Immersive VR systems currently available in consumer electronics and gaming markets can also record movement kinematics and allow quantitative monitoring of the motor performance of the rehabilitation exercise (De Pasquale et al., 2024). Affordable prices and ease of use make these systems suitable for home rehabilitation after hospitalization using a telemedicine approach (Piron et al., 2009).
Myoelectric control is a novel and promising approach for rehabilitation. Myoelectric interfaces have been mostly used for the control of actuators such as exoskeletons or prostheses (DiCicco et al., 2004; Liarokapis et al., 2013; Nissler et al., 2019). Thanks to myoelectric interfaces, which decode the patient’s intention through the residual myoelectric activity in the paretic limb, patients may generate voluntary movements through their spared cortico-spinal pathway and receive feedback (e.g., a realistic visual feedback from an embodied limb in VR) thus establishing a closed-loop system that promotes re-learning and encourages active participation, increasing motor coordination, muscle strength and reducing spasticity (Song et al., 2013; Sarasola-Sanz et al., 2018). The use of myoelectric interfaces for rehabilitation also aims at promoting neuroplasticity to reshape neuromuscular activity and to enhance motor learning, and the restoration of motor function. For instance, stroke patients can learn to control, with the more affected upper limb, a multi-degree-of-freedom exoskeleton using a decoder trained with EMG from the healthy limb (Sarasola-Sanz et al., 2022). Moreover, myoelectric control training can reduce abnormal co-activation (i.e., undesired coupling) by training only the desired muscles while leaving other muscles unaffected (Seo et al., 2022).
Virtual Reality and active video games that combine physical activity with interactive serious games, or exergames, have been used for rehabilitation (Maggio et al., 2020; Morone et al., 2024). In addition, a serious game was recently approved by the FDA (Food and Drug Administration) as the first digital drug in children affected by ADHD (Attention Disorder Hyperactivity Disorder) (Laver et al., 2017; Commissioner, 2020; Mubin et al., 2022). Exergames make rehabilitation exercises more enjoyable and engaging. However, exergames commercially released often are not tailored to the specific needs and requirements of patients and cannot easily incorporate feedback and suggestions from therapists. In contrast, recent advancements in assessment methodologies can be exploited to devise personalized rehabilitation approaches, aimed at restoring specific components of the motor deficits. For instance, such approaches may target the motor functionality of the proximal upper limb (shoulder and elbow joints), the distal extremities (wrist and fingers), both simultaneously, or they may focus on restoring balance or reducing treatment time (Henrique, Colussi, and De Marchi, 2019). Customized approaches also allow to take into account patients’ needs and to modify the level of difficulty of the exercise considering the patients’ functional assessment, ability and capability, and the achieved level of motor recovery, which are not usually quantified in commercial game-based applications (Tsekleves et al., 2016; Han et al., 2017; Triandafilou et al., 2018; Zirbel et al., 2018). Customization also allows to directly involve therapists in the development of rehabilitation systems regarding the definition of system requirements including difficulty to use, time to set the system and the required knowledge (Rand et al., 2018).
Indeed, while VR, myoelectric control, and exergames represent promising methodologies that can be exploited to introduce novel rehabilitation therapies, the active involvement of both therapists and patients during the whole development process is critical to improve their usability and effectiveness. In fact, a user-centered design (UCD) approach is increasingly used to develop, improve, and evaluate new rehabilitation systems or procedures. This approach may also facilitate system usage and integration in clinical and domestic settings thus overcoming the limitations of current approaches (Ríos-Hernández et al., 2021; Semprini et al., 2022).
The goal of the present work is to present the development of a system combining VR and myoelectric control to implement a new mirror therapy (MT) approach for post-stroke upper-limb neuromotor rehabilitation. MT is a rehabilitation technique that has significant potential to be enhanced by exploiting new methodologies such as VR, myoelectric control, and personalized exergames through a UCD development process. During conventional MT patients watch their unaffected limb reflection in a mirror placed on a sagittal plane between patients’ limbs. The reflection of the unaffected limb in the mirror creates the illusion that the affected limb moves effectively and painlessly and provides encouragement. MT was first developed to alleviate phantom limb discomfort in persons with amputation (Ramachandran and Rogers-Ramachandran, 1996), and then applied to stroke patients with weaker limbs to improve muscle control (Altschuler et al., 1999). According to a systematic review, MT is recommended as a valuable approach to be integrated in the rehabilitation intervention of stroke patients (Hatem et al., 2016). However, conventional MT has a few limitations that restrict its use in clinical settings including being monotonous, only providing a low-dose therapy, and requiring specialized equipment and a professional on-site (Horne et al., 2015). Moreover, movements are limited by the physical dimension of the “mirror box”. By allowing for more clinically viable use of MT approach, VR-based therapy may overcome these restrictions and motivate patients to perform rehabilitation protocol including meaningful ADL tasks. VR is indeed seen as a potential method for delivering larger therapeutic dosages and enhancing post-stroke arm/hand rehabilitation (Chen et al., 2015).
Arm-hand movement training in VR is a successful technique for increasing the functional motor recovery of stroke patients, thanks to VR granting the possibility to enhance multisensory feedback in a highly controllable and versatile fashion (Laver et al., 2017; Massetti et al., 2018). The mirror visual illusion that appears in VR systems facilitates the multisensory integration by promoting the interaction between bilateral proprioceptive signals and visual input (Giroux et al., 2018). Recent studies show that, combining VR with MT leads to better rehabilitation results (Perez-Marcos et al., 2018), especially when using a contralateral lesion action observation network (Saleh et al., 2017). Although it has been already proven that VR technology positively affects motor functionality delivering highly immersive environments for motor learning, the use of VR with MT protocols have so far shown limited evidence of effectiveness due to the small number of recruited patients, an inadequate research design and/or low-intensity training (Hsu et al., 2022).
Therefore, we developed a novel system to overcome the limitations of a conventional MT by exploiting the potentiality of VR to enhance the effectiveness of rehabilitation. Specifically, the system allows patients suffering from stroke upper-limb hemiparesis to control the related virtual paretic arm thanks to an innovative control algorithm based on input from both limbs whose relative contribution is modulated adaptively by monitoring the progress of patients. Here, we present the UCD of the system, from the initial concept that emerged within our interdisciplinary research team, through a series of development and validation steps, up to the definition of a specific system configuration and training protocols to be evaluated systematically in future studies.
Using the UCD approach, we developed the Validation of the Virtual Therapy Arm (VVITA) system for upper-limb post-stroke rehabilitation starting from the existing Virtual Therapy Arm (VITA) system, designed for treating phantom-limb pain in people with limb-loss and performing prosthetic training (Nissler et al., 2019). The development process included focus group sessions with clinical and motor control experts to ensure the translation of engineering outcomes into clinical practice. Hence, the definition of the new control modality of the system to be used with stroke patients through intermediate assessments and, where needed, the refinement of the design. In summary, although previous studies have explored VR-based mirror therapy and myoelectric interfaces separately or in combination, our system presents distinctive innovations. First, we propose an adaptive bimanual control algorithm that dynamically integrates EMG signals from both upper limbs, modulating their relative contribution according to individual patient progress. This allows a personalized progression of training intensity and supports active engagement of the paretic limb beyond traditional unimanual or fixed-threshold approaches. Furthermore, the system was developed following a rigorous user-centered design process, involving clinicians and therapists throughout all stages of development to ensure clinical relevance, usability, and effective integration into rehabilitation practice. These features represent a novel contribution to the field by addressing key limitations of conventional mirror therapy and previous VR-EMG systems. Finally, we present the results obtained from a pilot study involving four stroke survivors, providing an initial assessment of the feasibility of our novel personalized adaptive mirror therapy approach for upper-limb post-stroke rehabilitation based on virtual reality and myoelectric control.
2 Methods
The VVITA system has been developed as a stroke rehabilitation application of the VR platform originally developed for the treatment of phantom limb pain in upper-limb amputees within the VITA project led by a research group at the Institute of Robotics and Mechatronics of the German Aerospace Center (DLR) in Munich, Germany. The VITA system originally developed for amputees has been modified to be used with post-stroke patients to rehabilitate the neuromotor functionality of the more affected upper limb. This development involved an interdisciplinary and international team composed of several research groups. The DLR group modified the system according to suggestions provided by two groups at Fondazione Santa Lucia (FSL) in Rome, Italy, including motor control and rehabilitation experts. Following an iterative UCD approach, the team specified the requirements for new training protocols for upper-limb post-stroke neurorehabilitation, implemented those protocols in the new software named VVITA, developed methods for their assessment, and performed several evaluations of the new system by testing it in a pilot study with stroke patients.
2.1 The VITA system
The VITA system (Nissler et al., 2019) is a low-cost VR solution developed to treat phantom-limb pain of people with limb-loss or to perform prosthetic training. It uses an HTC Vive Pro VR platform (HTC Europe Co. Ltd., Slough, Berkshire, United Kingdom), including a head-mounted display and two trackers, and two Myo armbands (Thalmic Labs, Ontario, Canada) with EMG sensors. One tracker and one armband are placed on each amputee’s arm. One of the Vive Trackers is placed on the unimpaired hand dorsum and provides the position and orientation of the hand. The second tracker is placed on the remaining portion of the amputated limb to provide its position and orientation. The Myo armband consists of eight bipolar surface EMG sensors measuring the activity of several forearm muscles. The sensors are mechanically connected and arranged in an armband placed on the amputee’s forearms to record most of the muscle controlling opening and closing of the fingers. A representation of the hands is provided in the virtual environment as visual feedback. Virtual hand movements are directly predicted from the kinematic recorded with the trackers while virtual finger movements are predicted from the forearm muscle signals using a model trained through a machine learning procedure. The machine learning method, an iterative variant of Random Fourier Features Ridge Regression (iRR-RFF) (Patel et al., 2017), trains a non-linear decoder mapping features from the EMG signals onto finger gestures and used to control the feedback given by the virtual hands. The acquired signals (kinematic and EMG) are wirelessly transmitted via Bluetooth technology. Data (kinematic and EMG signals) acquisition, model training, and prediction processes are performed by a laptop (Alienware m15, Dell) with a dedicated GPU (GeForce RTX 2060, Nvidia). The virtual scene reproduces a house surrounded by nature (trees, lake, mountains, etc.), in which various activities both inside the house (cooking, playing the drums, stoking up a fireplace, etc.) and external (picking fruit, etc.) can be carried out for rehabilitation purposes. Participants can navigate within the virtual environment by moving to the desired area according to the exercise they choose to perform.
2.2 UCD approach
A UCD approach was implemented in the VVITA project to develop a novel application of the VITA system optimized for stroke rehabilitation, involving a multidisciplinary team of biomedical engineers, motor control neuroscientists, neurologists, therapists, and patients. A focus group, including seven stroke patients, one physiotherapist, one psychologist, one specialist in physical medicine and rehabilitation, one neurologist, five motor control scientists, and five engineers supported the protocol development. This team brought expertise in neurological disorders, VR-based therapies, and neuromotor rehabilitation to ensure the system met both clinical and patient needs. The technical development also involved NEEEU (NEEEU Spaces GmbH, Berlin, Germany), a company focused on applying Human Centered Design processes to new technologies, which collaborated as subcontractor on the design, qualitative research and development of the patient and therapist journeys, the virtual environment and the digital therapy platform for therapists. For these developments, we specifically used a service design–centered approach, leveraging tools such as journey maps and service blueprints to map the user’s experience over time. By combining these methodologies, we ensured that the evolving rehabilitation service was continuously refined in collaboration with end users.
The development activities followed three iterative cycles, each composed of distinct phases (P). The first cycle included four phases: (0) evaluation of the system’s context of use, included only in first the cycle, (1) specification of the rehabilitation protocol based on experts’ requirements, (2) software development to implement the desired protocol, and (3) system evaluation with healthy participants and stroke survivors. The initial phase (P0), focused on assessing the system’s intended context of use, defining scientific questions, and identifying desired outcomes. Inputs from this phase guided the specification of system requirements and training protocols in the subsequent phase (P1). The first version of the software was developed in the third phase (P2) and it was critically tested and evaluated during the fourth phase (P3). In the two additional cycles, the first, second and third phases were repeated to refine the protocol, address updated system requirements, incorporate feedback from therapists and patients, and resolve issues identified during development (Figure 1).
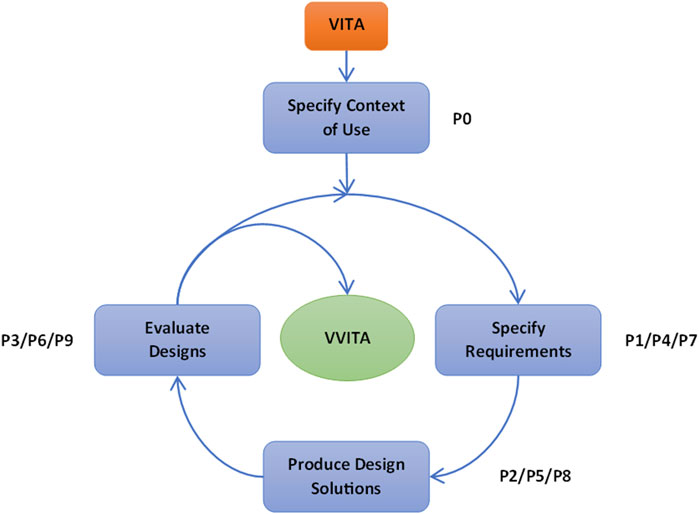
Figure 1. Schematic of the UCD process. The figure illustrates the iterative approach of the UCD methodology, detailing the various phases (P) that transformed the initial VITA into the final VVITA system. The phases specify requirements, produce design solutions and evaluate designs were repeated three times to refine the system and address issues identified during testing.
The first cycle (P0, P1, P2, P3), lasted 30 months and consisted of 40 interactions, the second cycle (P4, P5, P6) lasted 2 months and consisted of 13 interactions, while the last cycle (P7, P8, P9) lasted 4 months and consisted of 12 interactions (See Table 1).
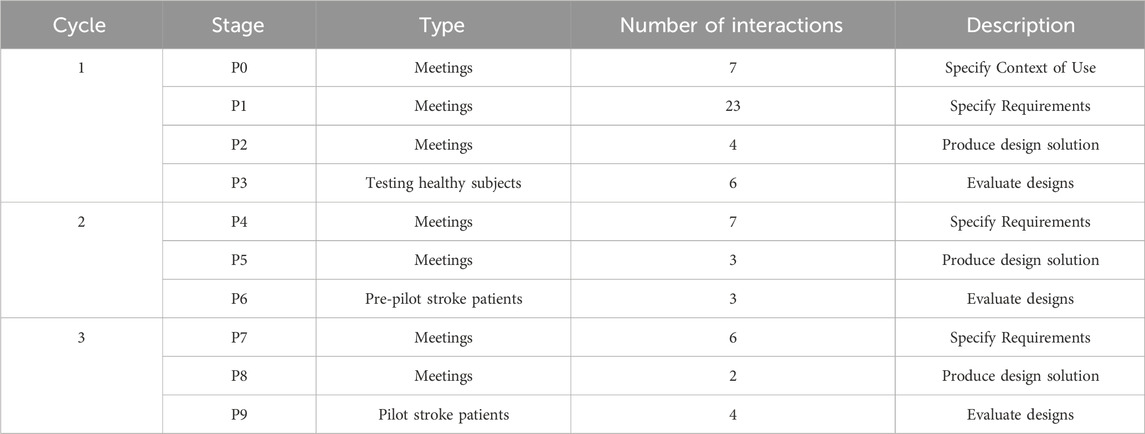
Table 1. Summary of UCD phases and cycles. The phases included in each cycle of the UCD approach: the type of phase, number of iterations conducted within each phase, and a brief description of their objectives and outcomes.
The final software release addressed all the issues that had emerged during the development, and it was tested in a pilot study (P9) involving four stroke patients (two chronic and two sub-acute). This study evaluated the system’s feasibility, functionality, and usability through kinematic analysis and user feedback, demonstrating the system’s potential for enhancing post-stroke rehabilitation.
2.3 Incremental development and testing
The VVITA system was developed following a structured UCD approach. Each phase was designed to iteratively refine the system to meet the specific needs of post-stroke upper-limb rehabilitation. In this section we describe the main activities performed in each phase of development. The outcome of these activities, including the novel VVITA system and the results of the pilot study, are presented in the Results.
2.3.1 P0 – Specification of the context of use of the VVITA system
The first phase defined the context of use, targeting subacute and chronic stroke patients with diverse motor impairments. Inclusion criteria addressed motor deficits, age, and cognitive ability to ensure compatibility with the system. Patients undergoing treatments like botulinum toxin were excluded if such treatments occurred 2 weeks before or during rehabilitation. A comprehensive review of the literature (Eng et al., 2007; Burke et al., 2009; Cheung et al., 2009; Dohle et al., 2009; Bohil et al., 2011; Lee et al., 2011; Thieme et al., 2012; Zhang and Zhou, 2012; Li et al., 2014; Pollock et al., 2014; Ballester et al., 2015; Choi et al., 2016; Hatem et al., 2016; Hoermann et al., 2017; Kim, 2017; Laver et al., 2017; Sadarangani et al., 2017; Darbois et al., 2018; Morone et al., 2019) identified limitations in existing systems, such as inadequate adaptability and underutilization of immersive VR and myoelectric control. These findings guided the conceptual design of the VVITA system, which integrated VR mirror therapy, myoelectric control, and dynamic task adjustments to enhance patients’ engagement. Focus group discussions refined these concepts, emphasizing patient motivation, adaptive protocols, and therapist control. These inputs formed the foundation for subsequent phases.
2.3.2 P1 – Definition of the system requirements and specifications
Building on the outcomes of Phase P0, detailed requirements were established for the hardware, software, and training protocols. The hardware design included motion sensors for hands’ tracking and EMG sensor bracelets for myoelectric control. The software specifications encompassed immersive VR environments, calibration procedures, and assistive control algorithms for proximal (shoulder, elbow) and distal (hand) joints. Training protocols were designed to include bimanual reaching and grasping tasks tailored to individual motor capabilities, with dynamically adjustable difficulty. The aesthetics of the platform were carefully chosen to avoid an uncanny-valley effect, virtual objects and hand representations are stylized rather than hyperrealistic, while still providing an immersive experience. A graphical user interface (GUI) for therapists was found to be essential for enabling real-time customization of parameters and monitoring of patient progress. Notably, therapists can visualize patients’ actions in VR through a dedicated monitor, allowing them to observe kinematics movements and EMG-driven gestures live as part of the feedback mechanism. An initial GUI design underwent a 30-min click dummy evaluation followed by therapist feedback, ensuring alignment with patients’ needs. This phase produced the initial system architecture, outlining the integration of hardware components, software modules, and control algorithms, providing the basis for Phase P2.
2.3.3 P2 – First software release
The first software version was developed based on the specifications defined in Phase P1. Key features included calibration tools, movement control strategies, task design, and real-time feedback. The calibration tools ensured alignment between the virtual and physical setups, enabling precise interaction with the virtual environment and tailoring experimental parameters to the patient’s motor capabilities. The movement control strategies allowed the virtual more affected limb (VMAL) to be driven by the patient’s residual motor capabilities, with dynamic adjustable parameters to mirror the movements of the real less affected limb (RLAL) to amplify the movements of the real more affected limb (RMAL). The task design incorporated bimanual reaching and grasping exercises, carefully designed to offer engaging and challenging experiences while promoting motor recovery. Real-time feedback mechanisms delivered visual and auditory cues to guide task execution and enhance patient engagement. This version served as a proof of concept, demonstrating the feasibility of integrating virtual reality, motion capture, and myoelectric control into a cohesive rehabilitation platform.
2.3.4 P3 – Initial evaluation of the system by healthy participants and therapists
Healthy participants tested the system under therapist supervision to identify usability and functionality issues. The evaluation aimed to highlight limitations and challenges in calibration and target placement procedures, as well as to identify potential compensatory motor strategies exhibited by patients during tasks. Furthermore, the test supported therapists to identify the range of task parameters and type of feedback mechanisms compatible with the intended applications.
2.3.5 P4 – Refinement of the system requirements and specifications
Based on the findings from Phase P3, several refinements were implemented to optimize the system for usability and adaptability across diverse rehabilitation needs. Calibration procedures were enhanced to improve alignment accuracy, while target placement algorithms were revised to accommodate varying ranges of motion. Task parameters were adjusted to balance challenge and fatigue. For example, grasping holding times were shortened. An adjustable table was integrated to enhance comfort and accessibility for patients of different sizes and mobility levels. Additionally, control algorithms were refined to ensure smoother transitions between mirrored and independent movements. These updates optimized the system for usability and adaptability across diverse rehabilitation needs.
2.3.6 P5 – Second software release
The second software release resolved the issues identified in Phase P3 and incorporated updates based on the refined system requirements from Phase P4. Key improvements included a configuration file that enabled therapists to customize experimental parameters prior to sessions, such as spatial tolerance for successful target reach, maximum trial duration (timeout), and trial success time (holding time required to successfully reach and perform a gesture). Visual and auditory feedback mechanisms were enhanced for better clarity and responsiveness. Additionally, adjustments to target orientation and placement were made to address positioning issues, such as interactions with the table or targets placed in physiologically challenging positions, ensuring better accessibility without compromising therapeutic effectiveness. These updates marked a significant advancement, addressing limitations from earlier phases and preparing the system for evaluation with stroke patients.
2.3.7 P6 – Evaluation of the system by therapists and stroke patients
The revised software was tested with three chronic stroke patients during a pre-pilot assessment to evaluate usability and gather additional feedback. Patients with varying levels of impairment participated in multiple sessions (Table 2), during which therapists dynamically adjusted parameters to tailor tasks to each patient’s individual capabilities. Observations and feedback from both therapists and patients were instrumental in informing further refinements in the subsequent development phase.
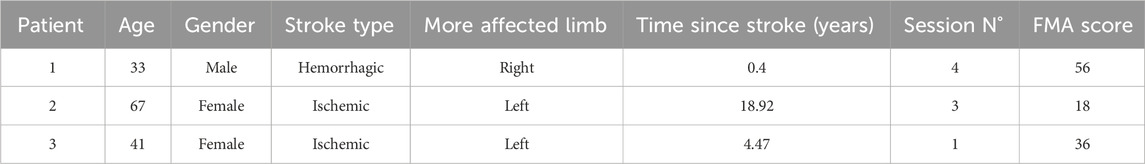
Table 2. Participants included in the pre-pilot usability assessment. Demographic and clinical data of the participants to the pre-pilot assessment.
2.3.8 P7 – Second refinement of the system requirements and specifications
Based on the feedback gathered during Phase P6, several refinements were identified as required to enhance the system’s usability and clinical effectiveness. These included updating the virtual hand design, optimizing the virtual environment, refining target placement algorithms, and addressing issues related to compensatory movement strategies. Updating the virtual hand design involved enhancing its color and appearance to improve patient embodiment and reduce confusion during tasks. Optimizing the virtual environment focused on removing non-interactable objects from the virtual environment to minimize distractions and improve task focus. Refining target placement algorithms aimed to improve accessibility, particularly for lateral targets, while maintaining their therapeutic value. To address the issues identified with the presence of compensatory movements it was decided to optimize the control algorithms and to provide therapists with tools to better manage these behaviors. Additionally, the need to develop a comprehensive operation manual to assist therapists in system calibration, parameter adjustments, and managing compensatory movement strategies effectively was identified. These refinements were deemed critical to delivering a more effective, intuitive, and engaging rehabilitation platform.
2.3.9 P8 – Final software release
The final version of the system successfully addressed all the remaining usability issues, incorporating feedback and improvements to enhance its effectiveness. Key refinements included embodiment improvements, with optimized virtual hand designs for better interaction and engagement, and therapist interface enhancements, featuring improved GUI functionality to allow seamless real-time adjustments of parameters. Calibration refinements ensured consistent accuracy through improved alignment procedures.
While trunk compensation remained a challenge, therapists were equipped with tools and guidelines to manage these behaviors effectively. A comprehensive user manual was also provided to therapists prior to the pilot experiment. The manual detailed the hardware and software components of the system, described operating procedures, outlined the initial calibration process, and explained policies for adjusting assistance parameters during training. This version was deemed ready for clinical evaluation, incorporating critical updates and support materials to facilitate effective use and study of the system in real-world scenarios.
2.3.10 P9 – Evaluation of the system with a pilot study
Phase P9 involved a pilot study designed to evaluate the feasibility, usability, and clinical impact of the final VVITA system. Four stroke patients were recruited for this phase, including two in the chronic phase (at least 1 year post-stroke) and two in the subacute phase (less than 1 year post-stroke) (See Table 3).
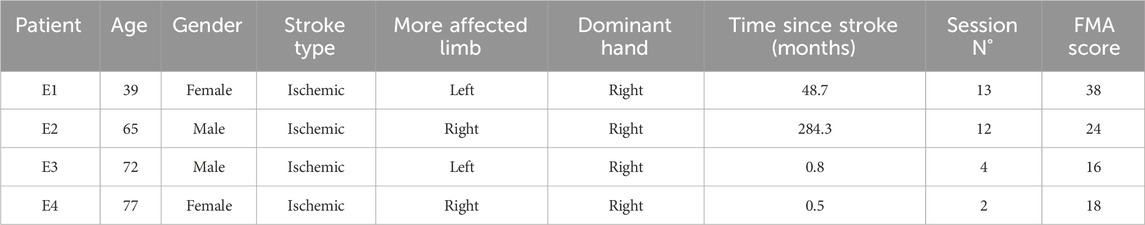
Table 3. Participants included in the pilot study. Demographic and clinical data of the participants to the pilot assessment.
To avoid confounding factors, participants had not received additional treatments, such as botulinum toxin, in the 2 weeks prior to or during the study. The study was conducted in accordance with the Declaration of Helsinki and approved by the ethical review board of FSL (Prot. CE/PROG.790). Written informed consent was obtained from all participants before the experimental sessions began.
The pilot study lasted 1 month, with each participant completing rehabilitation sessions three times per week. Tasks involved bimanual reaching and grasping, calibrated to each patient’s individual movement range. Real-time feedback was provided during the tasks to guide performance, while therapists dynamically adjusted assistance parameters and task difficulty to ensure the optimal balance between challenge and feasibility. The primary focus was to evaluate the system’s ability to support motor recovery and maintain patient engagement throughout the rehabilitation process. Validated questionnaires were used to assess the system’s usability, feasibility and patient experience.
For the evaluation of the usability, the feasibility of the developed rehabilitation system and patient experience, the following questionnaires were administered to the pilot experiment participants: the User Satisfaction Evaluation Questionnaire (USEQ, (Gil-Gómez et al., 2017)), for evaluation of the evaluation of the VR system usability with range from 6 to 30; the Visual Analogical Scale (VAS) with range from 0 to 10 with respect to subjective motivation and satisfaction related to exercise; the Pittsburgh Participation to rehabilitation Scale (PPRS, (Iosa et al., 2021)) compiled by the researcher/therapist to report the patient’s participation levels in the exercise on a Likert scale ranging from 1 to 6 and the National Aeronautics and Space Administration Task Load Index (NASA-TLX, (Hart and Staveland, 1988)) for the multidimensional subjective assessment that rates perceived workload to assess a task, system, or team’s effectiveness or other aspects of performance.
The Fugl-Meyer Assessment (FMA) for the upper limb was used to evaluate motor recovery and functional ability. This evidence-based scale (Deakin et al., 2003) assesses motor recovery across multiple stages, with individual items scored on an ordinal scale from 0 (unable to complete the task) to 2 (successfully completed). The FMA provided a standardized measure of the system’s effectiveness in promoting motor improvements.
Instrumental data were recorded through the VVITA system’s integrated sensors. The recorded kinematic from the hands (3D position and rotation the back of the hand) was tracked at 30 Hz, filtered using a fourth-order Butterworth low-pass filter with a 3 Hz cutoff frequency, and analyzed so to extract assessment variables such as performance scores, assistance parameters, maximum speed, and range of motion (ROM).
Both clinical and instrumental evaluations were conducted at the beginning (T0) and end (T1) of the rehabilitation protocol to assess the system’s impact. Feedback from patients and therapists was also collected after each session to identify any remaining usability challenges and gauge overall satisfaction with the rehabilitation experience.
2.4 Statistical analysis
We evaluated the variation in overall assistance level, Fugl-Meyer index, and maximum speed across sessions (Se) using a linear mixed model (LMM). This model accounted for interindividual variability by including participants as a random effect. The experimental factor (Se) was treated as a fixed effect with categorical (dummy) variables. Data were fitted with the model in Equation 1:
where
3 Results
A UCD approach was employed to develop a novel system for upper-limb post-stroke motor rehabilitation by modifying a system initially created to treat phantom limb pain in amputees. The training activities, assistive algorithms and user interface were developed and refined through 65 interactions among all the members of the development team, achieving a synthesis between technological innovation and rehabilitation principles.
Following an initial analysis of the patients’ needs and rehabilitation goals, a bimanual reaching task was selected as the primary training activity. Virtual mirroring (Saleh et al., 2017; Giroux et al., 2018; Mekbib et al., 2021; Hsu et al., 2022; da Silva Jaques et al., 2023), using both kinematic signals for hand position and EMG signals for hand gesture, was defined as the key assistive approach to enhance functional recovery. Initially, 3 different control algorithms were considered to provide virtual mirroring of the more affected hand. These algorithms were implemented for pilot testing, each affecting the control modality and the guidance of the VMAL differently, based on the RLAL and the RMAL.
The amount of mirrored assistance provided to the VMAL, and the task difficulty were controlled by two parameters: an agency parameter (
3.1 Outcomes of incremental development
3.1.1 VVITA system
The VVITA system is based on the VITA system with which it shares the hardware architecture. Differently from the VITA system, in VVITA both trackers are placed on the dorsum of the patient’s hands (Figure 2). Several software solutions were developed and adopted to provide a virtual mirror therapy assisted by the hand kinematic and gesture prediction of the less affected arm depending on the level of impairment and the functional restoration achieved during the treatment. The system allows a patient to practice rehabilitation exercises that simulate the performance of ADL in an immersive VR environment, providing real-time visual feedback of a bimanual reaching and grasping task in which the movement of a virtual limb reproduces and improves the movement of the paretic limb. The movement of the VMAL is displayed based on the movement and the EMG activity of both the RMAL and the RLAL recorded with the system integrated motion capture sensors and the armbands as in the original VITA system.
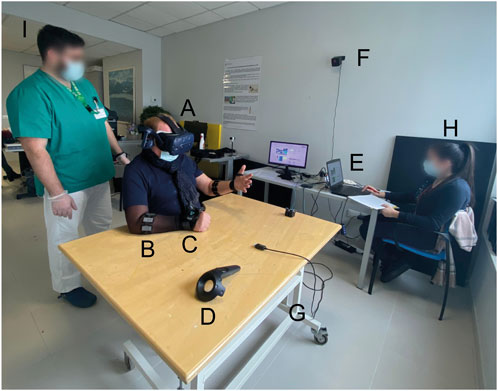
Figure 2. VVITA Setup. (A) Virtual reality headset Vive by HTC. (B) EMG bracelet Myo by Thalmic Labs. (C) HTC tracker. (D) HTC controller. (E) Laptop. (F) One of the two HTC Vive base stations. (G) Adjustable table. (H) Therapist. (I) Physiotherapist.
3.1.2 Assistive and control algorithms
The VVITA system introduces innovative assistive and control algorithms that enable virtual mirror therapy, a functionality not available in the original VITA system. The system supports bimanual rehabilitation tasks by leveraging VR capabilities to provide mirrored assistance for both limb movements (proximal control) and hand gestures (distal control). Proximal control utilizes kinematic data recorded by motion trackers, while distal control uses EMG data acquired from the Myo armband.
To achieve precise control of the VMAL, the system employs an agency parameter (
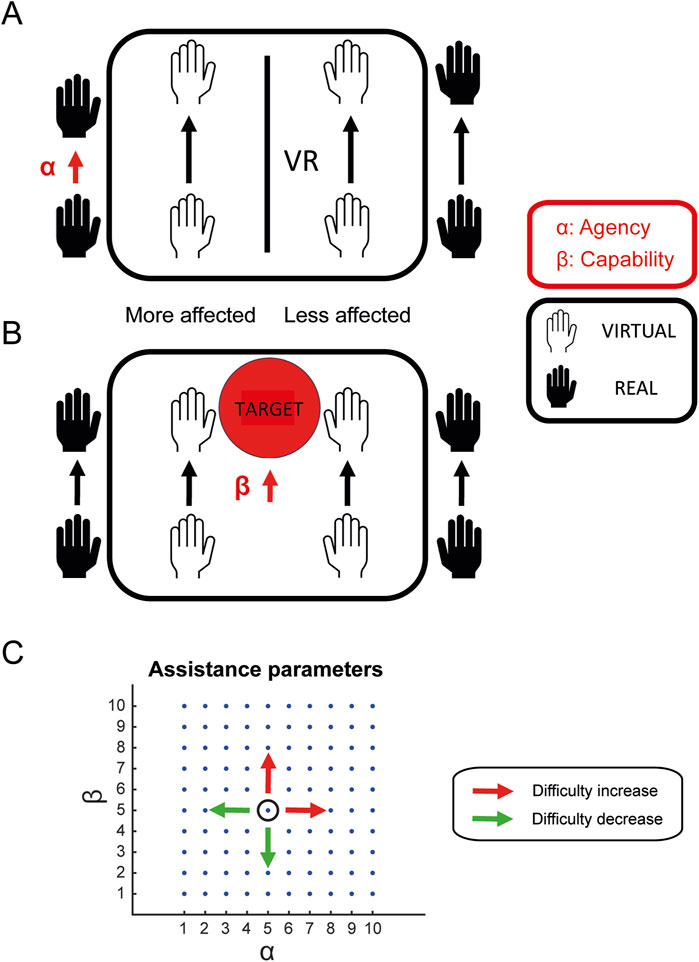
Figure 3. Proximal assistance. (A) The agency parameter
In Equation 2,
For distal control, the VMAL hand gesture is determined by the EMG activity of the RMAL and RLAL:
In Equation 3,
As
The mirroring function
In addition to agency parameters, the system incorporates capability parameters (
The relationship between the assistance parameters and task difficulty is defined such that higher parameter values corresponded to reduced assistance levels, thereby increasing task difficulty. Specifically, as
3.1.3 Virtual environments and GUI
3.1.3.1 Virtual environments
The VVITA software facilitates bimanual upper-limb reaching tasks in an immersive VR environment. The virtual environment consists of three distinct scenarios: a house, a garden near the house, and a green space by a pond, designed to provide engaging and varied settings for rehabilitation based on real life interactions to reduce the cognitive load of learning new mechanisms. One scenario is randomly selected by the software each time it is launched. A table is positioned in front of the patient, and virtual representations of the patient’s hands, along with objects to be reached and grasped bimanually, are displayed. Two types of objects can be presented as targets in the virtual environment: a concertina (Figure 4A) and a ball (Figure 4B). All reaching and grasping tasks are designed to be symmetrical with respect to the target object, to ensure the effectiveness of the RLAL assistive guidance for the VMAL.
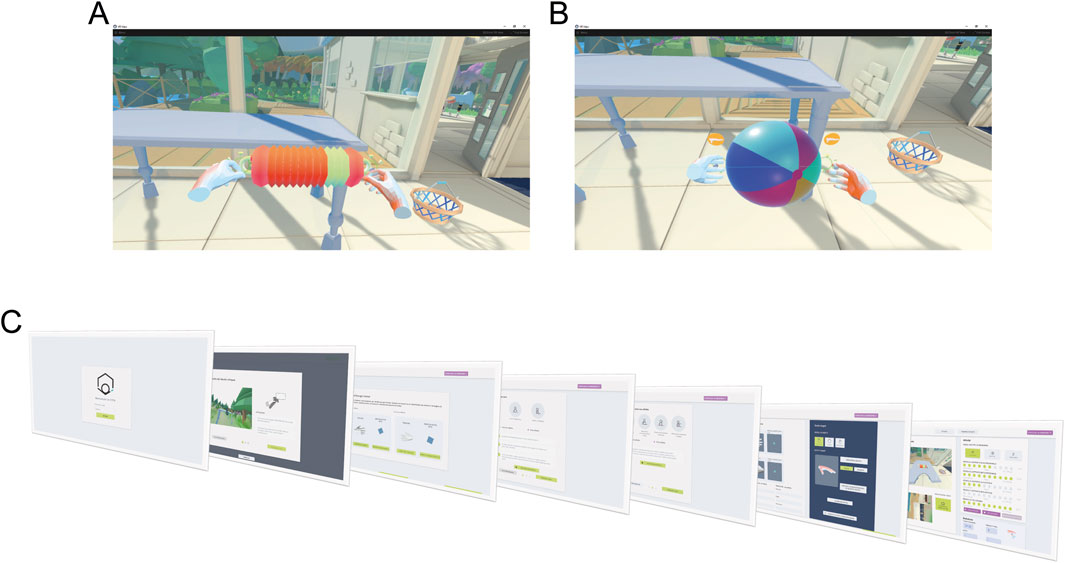
Figure 4. Virtual environment and GUI. The virtual environment consists of a room in a house with a desk and two different objects, a concertina (A) and a beach ball (B). Participants are instructed to reach for these objects with both hands and grab them using the specified gesture—fingers closed for the concertina and fingers open for the beach ball. When the correct position and gesture are maintained for a set duration, the object progressively turns green. (C) Shows the sequential workflow of the therapist’s GUI.
3.1.3.2 GUI: setup of experimental parameters and rehabilitation protocol
To meet the therapists’ specifications and ensure a user experience aligned with their expectations, a dedicated GUI (Figure 4C) was developed to control experimental parameters and guide the execution of the rehabilitation protocol. First, the virtual environment is calibrated to the physical workspace by using HTC Vive controllers. During this step, the operator marks the corners of the real table to ensure precise alignment of the corresponding virtual table. Once this calibration is completed, the Myo armbands and the tracker, worn by the patient, are activated and recognized by the system, enabling it to distinguish between the less affected and more affected limbs. The patient then performs a series of calibrations tasks under the supervision of the operator, aimed at defining motor capabilities and tailoring the experimental parameters accordingly. First, a resting pose calibration is conducted to determine the baseline hand position. This is followed by a proximal calibration to establish maximum range of motion. Finally, during the distal calibration, EMG signals are recorded to train the gesture recognition model. After these preliminary steps, the operator selects the patient’s profile and customizes the rehabilitation approach by choosing one of three assistance modalities: stiff coupling, rubber band, or trajectories. Additionally, the software allows fine-tuning of four key parameters (αp, αd, βp, βd) to adjust task difficulty, provides performance metrics such as the number of successful trials, and securely stores patient-related data.
3.1.4 Rehabilitation protocol and task design
Patients performed rehabilitation exercises with the VVITA system over the course of 1 month, with sessions scheduled three times per week, lasting 30 min each. During each session, the patient performs a series of movements grouped into blocks, with each block consisting of 12 bimanual reaching and grasping movements.
The target to be reached is randomly selected from the predefined set of locations (Figure 5). Target positions vary in height and lateral deviation relative to the initial recorded resting position of the hands on the table. Positions are defined as
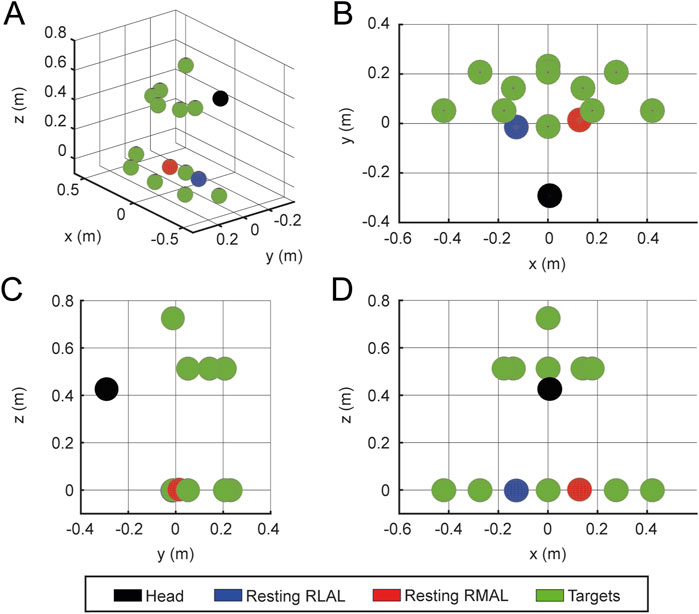
Figure 5. Target placement. Example target placements for
Each trial begins with the patient’s hands resting comfortably on the table, close to the body, with elbows flexed at 90°. Midway through the session, the patients remove the head-mounted display and take a 3-min break. Target positions for each block are randomly selected (without replacement) from the 12 predefined positions. At the beginning of each session, the patients calibrate their range of movement by performing maximal motions to the boundaries of their workspace with both the less-affected and most-affected limbs. The maximum height and forward reach recorded during calibration are used to set the target placement distances for all subsequent trials.
The two target objects, the concertina and ball, are alternated throughout the session to require different hand gestures for each target. The concertina requires a fist gesture (fingers closed), while the ball requires a hand extension gesture (fingers opened). Both objects are equipped with two handles positioned on opposite sides, guiding the required hand placement for successful interaction. A reaching movement is considered successful when both virtual hands are within
Real-time feedback on hand placement and gesture success is provided within the VR environment. When the virtual hands are within the required tolerance, the handles’ color changes from orange to green. Additionally, a visual indicator of the required hand gesture disappears once the gesture is correctly performed. Upon meeting both position and gesture requirements, the object progressively turns green, visually signaling the required holding time (Figure 4A). Two types of auditory feedback are provided: a positive cue for successful completion of the task and a negative cue if the trial exceeds a 20-s time limit. The trial duration is limited to 20 s to prevent fatigue and to maintain task efficiency.
3.1.5 Task adaptation and performance monitoring
At the end of each block, the therapist adjusts the proximal and distal agency (
• >90% Success Rate (≥11 successful trials): Task difficulty is increased.
• 70%–90% Success Rate (9–10 successful trials): Task difficulty remains unchanged.
• <70% Success Rate (≤8 successful trials): Task difficulty is decreased.
This adaptive framework ensures that the task remains within an optimal challenge range, maintaining a success rate of 70%–90% to promote engagement and motor improvement throughout the rehabilitation process (Figure 6). The selection of the parameter to be adjusted, either to increase or decrease task difficulty based on the score, was made by the therapist, guided by clinical expertise and real-time observation of the patient’s needs.
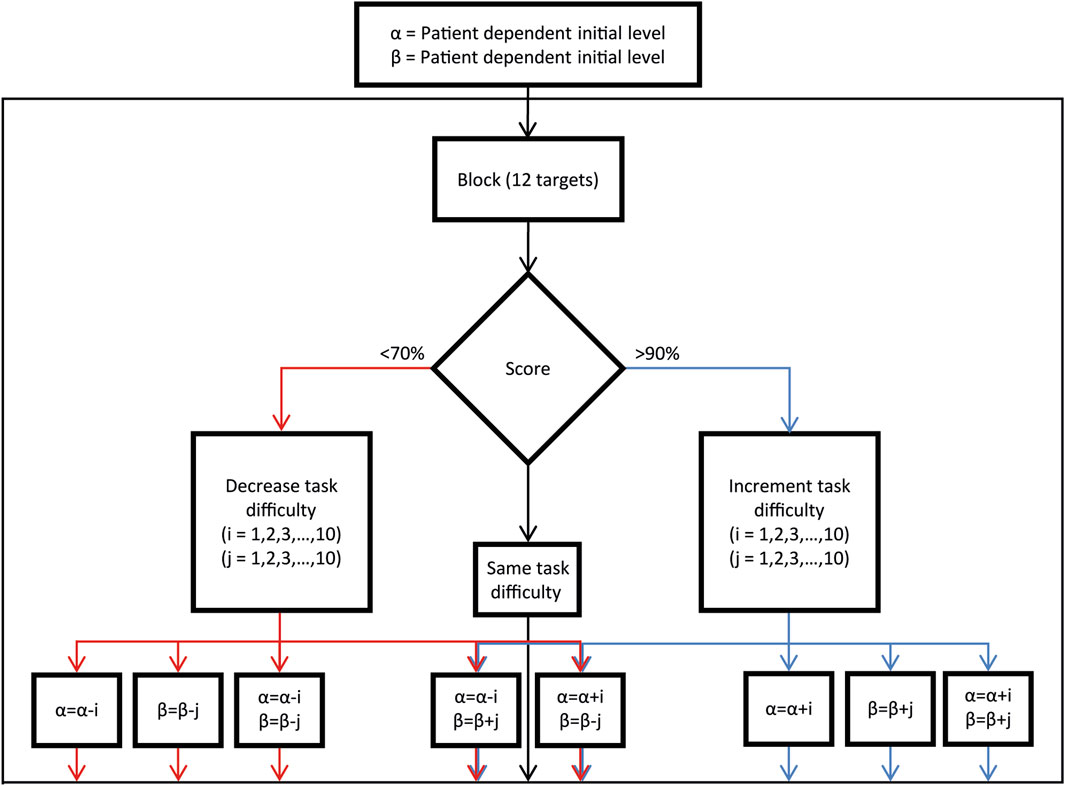
Figure 6. Assistance and difficulty parameters adjustment procedure. Decision block diagram illustrating the process for adjusting difficulty parameters based on the success performance of the previous reaching block (12 trials), as utilized by the operator.
3.1.6 Challenges, limitations and solutions
During the development of the VVITA system, several challenges emerged, which were systematically addressed following the UCD approach. One key issue was the misalignment between the virtual and real tables, causing visuo-proprioceptive mismatches and unreachable targets. This was solved by enhancing the alignment procedure to ensure accurate calibration. Additionally, proximal assistance parameters sometimes led to unnatural movements in “stiff coupling” and “rubber band” modes; the “stiff coupling” method was refined as the default due to its closer resemblance to natural kinematics. Moreover, patients reported visual distractions from non-interactable objects and flickering in the virtual environment, prompting the removal of unnecessary objects and the introduction of a more neutral virtual hand color to improve embodiment. Calibration procedures also required adjustments, as fixed times led to inaccurate motion range estimates, and extended trial durations caused fatigue. These were resolved by introducing operator-controlled calibration and reducing trial durations from 30 to 20 s. Patients with limited functionality often compensated for impaired limb movements with excessive trunk motion, which therapists mitigated through physical interventions rather than more invasive solutions suggested like belts or additional tracking systems. Target placement issues were also addressed by introducing configurable target positions and sequences, along with a threshold to prevent unreachable target positions. These iterative improvements highlight the significance of integrating patient and therapist feedback in the system refinement, ultimately enhancing its usability, adaptability, and effectiveness in post-stroke rehabilitation.
3.2 Outcomes of the pilot study
In this section, clinical and instrumental results from four stroke patients recorded during the pilot study are reported. Outcomes from two chronic patients (E1 and E2) who performed 13 and 12 sessions respectively (including the first familiarization session) are presented in more detail. For the two subacute patients (E3 and E4) who performed 4 and 2 sessions respectively (including the first familiarization session), only feasibility and acceptability questionnaire responses are reported.
3.2.1 Performance assessment
During each session, assistance parameters (α and β, proximal and distal) were adjusted at the end of each block according to the performance in that block, as described above (Figure 6). In Figure 7, the assistance parameters and the performance for each block across all the sessions are reported for patient E1 (A), who had a higher level of residual functionality and patient E2 (B), who had a lower level of residual functionality. As shown in the performance graphs, when performance was below 70% or above 90%, the difficulty was decreased or increased by adjusting the assistance parameters accordingly. Thus, the therapist was successful in keeping the level of performance around 80%. During the first session, a familiarization phase was performed in which the maximum level of
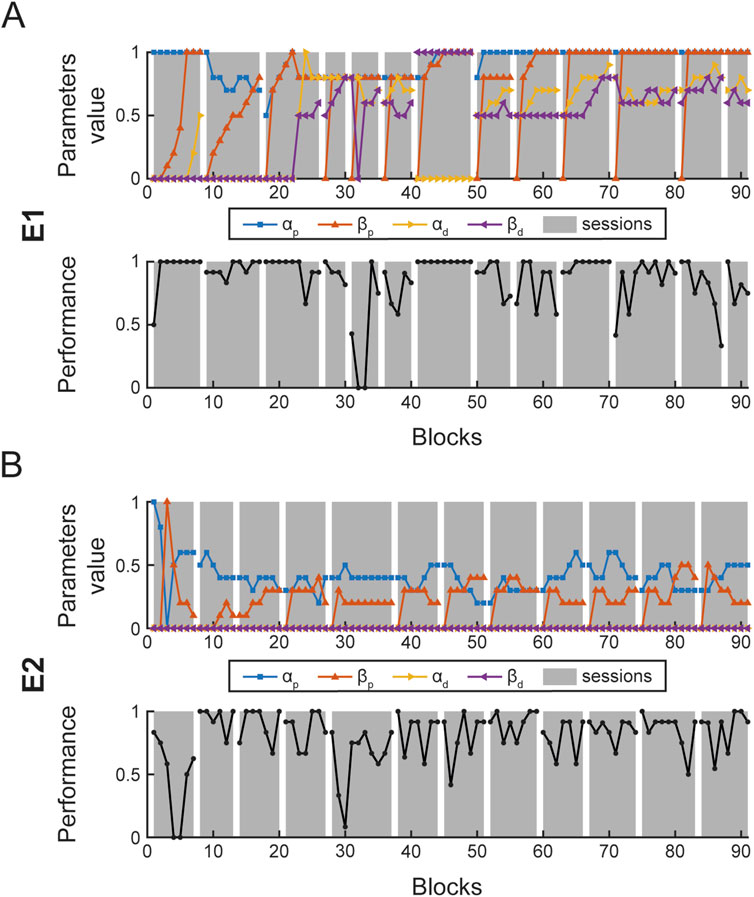
Figure 7. Block parameter selection and performance. Each panel shows the four assistance parameters (
Figure 8 shows the distributions of parameters used during the rehabilitation protocol for E1 (A) and E2 (B). For each patient, the distribution of the proximal (left column) and distal (right column) assistance parameters are shown. The figure highlights a clear difference between E1 and E2. For E1, the proximal parameters are both close to one (mean
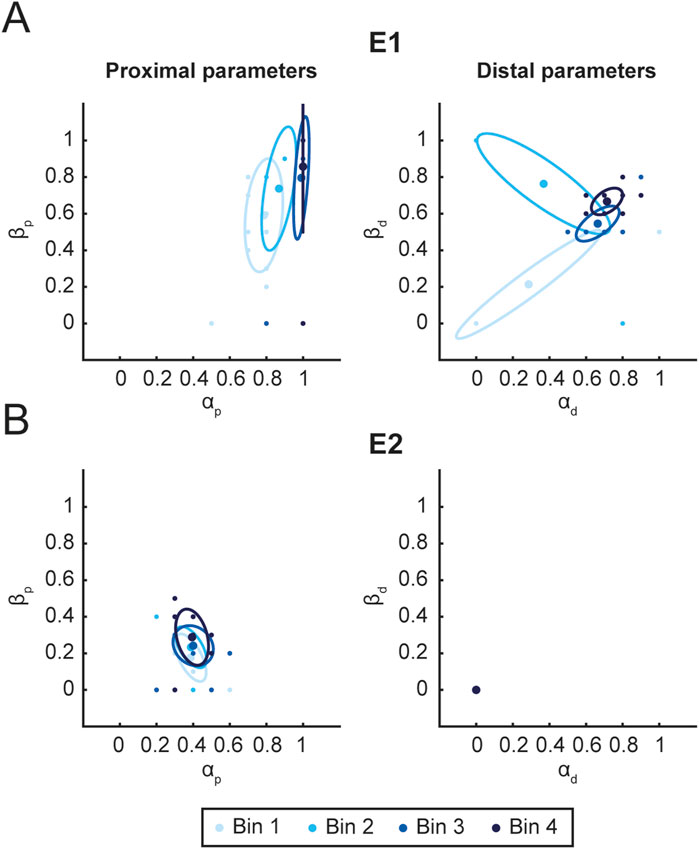
Figure 8. Parameters distribution. Figure shows the distribution of proximal (left column) and distal (right column) assistance parameters for E1 (A) and E2 (B). For each time interval Bin (3 sessions), the mean value (ellipse center) and covariance (95% CI. ellipse) of
The overall assistance level
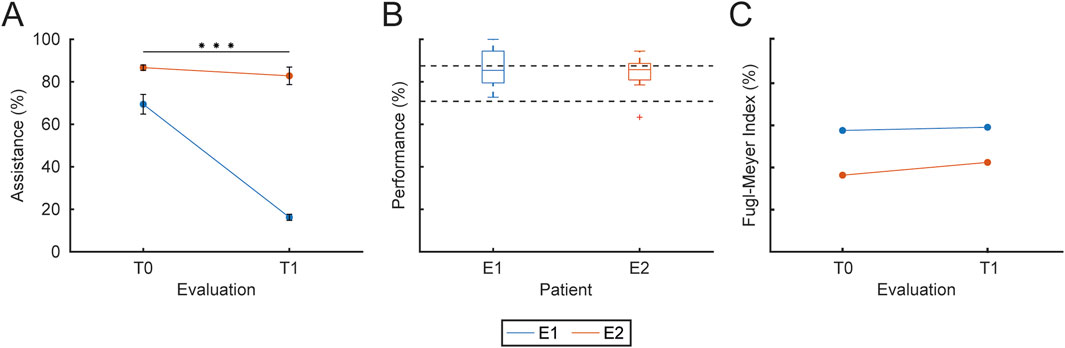
Figure 9. Training assistance, performance and clinical outcomes. The figure presents the assistance level, the overall performance and the clinical evaluation FM index for each patient (different colors). (A) Mean and SD of the assistance level provided to each patient at T0 and T1. (B) Overall performance, expressed as the percentage of successful trials over total trials for each patient. (C) Clinical evaluation of upper-limb motor function at T0 and T1, normalized as a percentage (with 66 corresponding to 100%), for each patient. Statistical significance between sessions is reported as *** for
The therapist was instructed to change the assistance parameters in order to maintain performance at around 80% in each block. Figure 9B shows the overall performance for each patient. Performance was
Participants E1 and E2 were evaluated clinically at the beginning (T0) of the rehabilitation protocol and at the end (T1) using the Fugl-Meyer assessment scale, which has 3 points (index: 0, 1, 2) for each item of the upper-limb motor function assessment.’ with ‘Participants E1 and E2 underwent clinical evaluation at T0 and T1 using the Fugl-Meyer assessment scale, which employs a 3-point scale (0, 1, 2) for each item assessing upper-limb motor function. Figure 9C shows the evolution of the Fugl-Meyer motor function assessment index for each patient (different colors) for each evaluation (
Maximum speed was estimated for RMAL and RLAL in each trial to evaluate its evolution during the rehabilitation protocol. The average maximum speed values for each limb for all patients are shown in Figure 10A for T0 and T1. Maximum speed averaged within each session were fitted with the LMM model of Equation 1. (RMAL
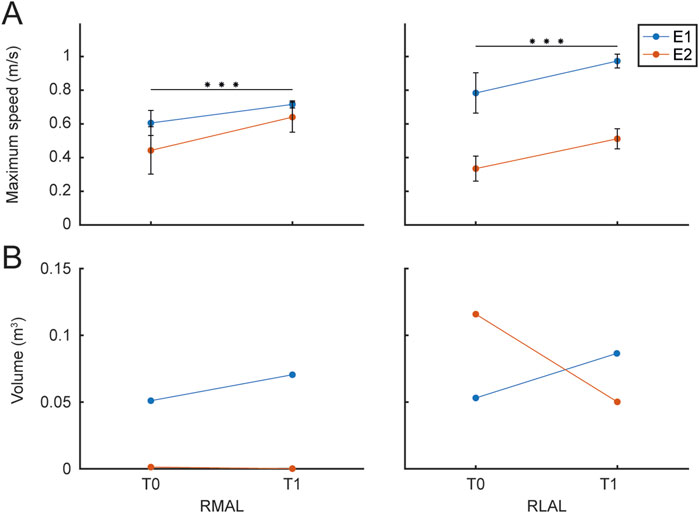
Figure 10. Maximum Speed and Movement Range. The figure presents the maximum speed and the volume of the maximum movement range achieved by each patient (different colors), evaluated during the rehabilitation protocol and the initial calibration procedure at T0 and T1 sessions. (A) The left panel shows the maximum speed for the RMAL, while the right panel shows the maximum speed for the RLAL. (B) The left panel displays the convex hull estimated for RMAL hand paths, while the right panel shows the convex hull for RLAL hand paths, both assessed during the initial calibration of maximum range movements.
Finally, to quantify the range of movement, an estimation of the volume of the convex hull of the trajectories recorded during the calibration procedure, performed at the beginning of each session, was obtained (Matlab, convhull function). Figure 10B shows the average RMAL (A) and RLAL (B) convex hull volume values for each hand path for the two patients for the first session T0 and the last session T1. Convex hull volume data were fitted with the LMM model in Equation 1 (RMAL
To highlight the differences in hand trajectories between the first and last sessions, Figure 11 illustrates the spatial paths recorded during the maximum range of motion calibration at the beginning of the T0 and T1 sessions for both E1 (panel A) and E2 (panel B). Left-hand movements are displayed on the left side of the figure, while right-hand movements are shown on the right. RMAL trajectories are represented in red for T1 and in a lighter shade (orange) for T0, whereas RLAL trajectories are shown in green for T1 and in a lighter shade (yellow-green) for T0.
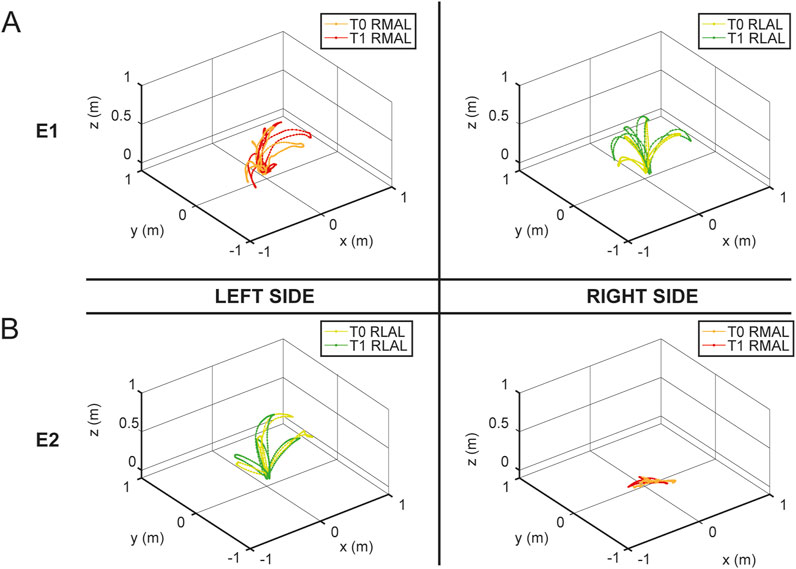
Figure 11. Hand trajectories during maximum range of motion calibration. The figure shows the spatial paths of the hand during the maximum range of motion calibration recorded at the beginning of the T0 and T1 sessions for E1 (panel (A)) and E2 (panel (B)), with both RMAL and RLAL. The left side of the figure represents left-hand movements, while the right side represents right-hand movements. RMAL trajectories are shown in red for T1 and in a lighter shade (orange) for T0. Similarly, RLAL trajectories are shown in green for T1 and in a lighter shade (yellow-green) for T0.
3.2.2 Usability assessment
All participants reported a good level of usability with the USEQ scale, even when motivation and perceived satisfaction were low as in the case of patient E3; further confirmed by the researcher’s evaluation through the administration of the PPRS scale (Figure 12A). Usability assessed by patients every session with NASA-TLX (Figure 12B), reported high levels of cognitive and physical demand; good levels related to the time domain (NASA_T) which indicates a general acceptability of the training, while, there are differences between the two chronic (E1 and E2) and subacute (E3 and E4) patients with respect to frustration (NASA_F) and self-assessment related to perceived success associated with the training (NASA_P).
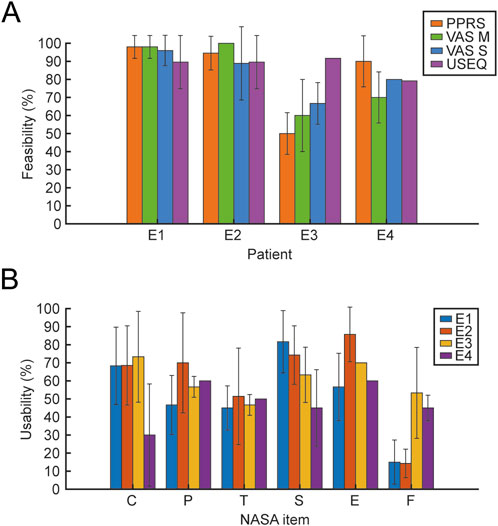
Figure 12. Feasibility and Usability scales. The figure shows the mean and SD of Feasibility (A) and Usability (B) values. For the feasibility outcome, the scales represented in different colors (PPRS, VAS 742 M, VAS S, and USEQ) are reported for the 4 patients. For usability, the 6 items of the NASA scale (C, cognitive demand; P, physical demand; T, temporal demand; S, perceived success; E, effort; F, frustration) are reported for the 4 patients, each represented in different colors.
4 Discussion
The VVITA system represents a significant advancement in stroke rehabilitation approaches based on virtual reality, myoelectric control, and exergames, developed using a rigorous UCD methodology. This approach actively involved patients, therapists, and clinicians, ensuring the system’s features directly address real-world rehabilitation challenges. The system’s integration of immersive VR, adaptive assistance algorithms, and EMG control sets it apart from traditional and earlier VR-based rehabilitation approaches. This discussion provides a critical analysis of the VVITA system’s outcomes, its development process, and its position in the landscape of stroke rehabilitation technologies.
Traditional rehabilitation approaches, such as mirror therapy and conventional VR systems, have demonstrated potential but are constrained by limited adaptability and personalization. Mirror therapy primarily relies on visual feedback to facilitate neural reorganization but cannot dynamically adjust to individual patient needs (Altschuler et al., 1999; Thieme et al., 2012). Similarly, conventional VR systems provide engaging environments but lack advanced control strategies to integrate patient-specific motor capabilities effectively (Bohil et al., 2011; Laver et al., 2017). VVITA addresses these limitations by combining immersive VR environments with dynamic task modulation based on real-time kinematic and EMG data, in which activity from the less and more affected arm are combined to control the virtual representation of the affected arm with adaptive degree of relative contribution. This approach overcomes the “learned non-use” phenomenon (Ballester et al., 2015) by enabling adaptive assistance tailored to the patient’s motor capabilities. Additionally, the use of EMG-driven interfaces promotes targeted motor recovery by integrating muscle activity into rehabilitation tasks (Song et al., 2013). These features position VVITA as a more engaging and effective alternative to traditional and earlier VR systems.
The VVITA system’s design and development were informed by 65 iterative interactions with clinical and technical stakeholders. These interactions highlighted key shortcomings in existing rehabilitation systems, such as insufficient adaptability and a lack of engaging, task-specific scenarios. Informed by these insights, VVITA incorporated gamified environments to sustain patient motivation (Burke et al., 2009). The UCD methodology ensured that every aspect of the system, from its adaptive algorithms to its user interface, was optimized for therapeutic effectiveness and patient usability (Meyer et al., 2019; Ríos-Hernández et al., 2021). Comparatively, VVITA’s UCD methodology builds on established practices in rehabilitation technology design. For instance, it has been demonstrated that the iterative, UCD approach highlights its value in developing effective lower-limb rehabilitation systems (Laffranchi et al., 2021; Semprini et al., 2022). Similarly, haptic robot-based telerehabilitation systems emphasize the importance of user-friendly interfaces and personalized therapy (Ivanova et al., 2017). VVITA extends these principles by integrating advanced EMG control and real-time task modulation, offering a novel approach to stroke rehabilitation.
Preliminary testing with four stroke patients, two chronic and two sub-acute, provided valuable insights into VVITA’s feasibility and effectiveness. Chronic patients exhibited higher engagement and notable improvements in motor performance, thanks to the benefits of tailored interventions (Torrisi et al., 2021; De Luca et al., 2024; De Pasquale et al., 2024). Instrumental assessments revealed significant improvements in maximum movement speed, correlating with clinical outcomes measured by the Fugl-Meyer Assessment. This correlation underscores the system’s potential to integrate objective metrics with subjective clinical evaluations, enhancing the assessment of rehabilitation effectiveness.
Interestingly, the pilot study revealed differences between patients in compliance and outcomes, with chronic patients responding more positively to the training protocol. This aligns with prior research which indicates that gamified VR interventions are particularly effective in maintaining motivation and improving motor function (Domínguez-Téllez et al., 2020). However, sub-acute patients faced challenges such as mood-related issues and medical complications, highlighting the need for further customization and support in this patient subgroup.
Both immersive and non-immersive virtual reality systems are now widespread in research and in clinical practice, however they do not have any type of control and adaptation to the paretic limb limiting the potentiality of the VR-assisted arm sensory motor rehabilitation. An important novelty of the present work is the myoelectric control of the paretic limb in virtual reality. This has two direct clinical positive consequences: i) it enhances the effectiveness of rehabilitation thanks to the greater integration of motor sensors consistent with the task; ii) it makes virtual reality exercise more adaptable to the patient’s motor function both intersession and with respect to the criteria for patient inclusion, allowing in fact to expand the beneficiaries. Moreover, therapists benefit from a real-time monitoring and feedback loop introduced by the system, which displays live kinematic, EMG, and performance metrics, enabling immediate adjustments to assistance levels and task parameters as therapy progresses.
The novelty of the VVITA approach lies in its integration of VR, EMG control, and adaptive assistance, features that distinguish it from existing rehabilitation systems. For example, traditional VR systems lacked the capability to dynamically adjust task difficulty based on real-time performance data (Ballester et al., 2015; Hoermann et al., 2017). Similarly, other systems focused on immersive environments but did not integrate advanced control strategies to modulate assistance levels or enhance task specificity (Lee et al., 2011; Laver et al., 2017; Ceradini et al., 2024; Mani Bharathi et al., 2024).
The VVITA system’s ability to combine kinematic and EMG data for personalized therapy represents significant improvement with respect to previous approaches presented in literature. Indeed, the importance of adaptive systems in achieving meaningful motor recovery aligns with findings in the literature (Sadarangani et al., 2017; Morone et al., 2019). However, further studies are needed to compare VVITA directly with other advanced systems, such as robotic exoskeletons or hybrid VR systems, to assess its clinical efficacy and cost-effectiveness.
Nonetheless, the very small sample size (four patients) is an important limitation of this study. Such sample size is appropriate for a pilot study assessing the feasibility of a novel approach but limits the generalizability of the findings. Therefore, the results should be interpreted with caution, and further research with larger, more diverse cohorts is necessary to validate these preliminary observations. Building on the promising results of the pilot study, a randomized controlled trial will evaluate VVITA’s clinical efficacy compared to conventional rehabilitation methods. Similarly to what is shown here, key outcomes will include clinical indices (e.g., Fugl-Meyer Assessment), kinematic performance metrics, and patient-reported satisfaction.
5 Conclusion
VVITA provides a novel and adaptable approach to stroke rehabilitation, addressing critical gaps in traditional and VR-based systems. By leveraging UCD principles, advanced control mechanisms, and engaging VR environments, the system holds significant potential to improve patient outcomes and set a new standard for adaptive rehabilitation technologies.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethical review board of IRCCS Santa Lucia Foundation (Prot. CE/PROG.790). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
PDP: Writing – original draft, Conceptualization, Writing – review and editing, Investigation, Formal Analysis, Project administration, Validation, Methodology, Visualization, Data curation. DDB: Writing – review and editing, Investigation, Conceptualization, Data curation, Validation, Project administration. MR: Methodology, Validation, Investigation, Conceptualization, Writing – review and editing. DJB: Methodology, Writing – review and editing, Investigation, Validation, Conceptualization. AM: Conceptualization, Writing – review and editing, Methodology. DB: Writing – review and editing, Methodology, Conceptualization. EC: Writing – review and editing, Conceptualization, Methodology. DM: Methodology, Conceptualization, Writing – review and editing. CN: Writing – review and editing, Software, Methodology, Conceptualization. MN: Software, Methodology, Conceptualization, Writing – review and editing. EF: Writing – review and editing, Software, Conceptualization, Methodology. JSM: Conceptualization, Writing – review and editing, Methodology, Software. MRS: Software, Writing – review and editing, Conceptualization, Methodology. CC: Conceptualization, Writing – review and editing, Software, Resources, Funding acquisition, Methodology, Validation. GM: Methodology, Validation, Conceptualization, Supervision, Funding acquisition, Writing – review and editing, Resources. AdA: Funding acquisition, Writing – review and editing, Resources, Validation, Supervision, Methodology, Conceptualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was partially supported by the German Federal Ministry of Education and Research (BMBF) under the Robotics Institute Germany (RIG); by the project (VVITA) awarded to Deutsches Zentrum für Luft-und Raumfahrt e.V. (DLR, Germany) by Helmoltz Association e.V.; by the Next-Generation EU Project (NGEU) National Recovery and Resilience Plan (NRRP), project MNESYS (PE0000006) – A Multiscale integrated approach to the study of the nervous system in health and disease (DN. 1553 11.10.2022) Spoke 1; and by Current Research Funds 2025, Ministry of Health, Italy.
Conflict of interest
Authors EF, JSM, and MRS were employed by NEEEU Spaces GmbH.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
VR, virtual reality; VITA, Virtual Therapy Arm; VVITA, Validation of the Virtual Therapy Arm; UCD, user-centered design; EMG, electromyographic; CT, conventional therapy; ADL, activities of daily living; MT, mirror therapy; GUI, graphical user interface; VMAL, virtual more affected limb; RMAL, real more affected limb; RLAL, real less affected limb; USEQ, User Satisfaction Evaluation Questionnaire; VAS, Visual Analogical Scale; PPRS, Pittsburgh Participation to rehabilitation Scale; NASA-TLX, NASA Task Load Index; NASA-TLX, National Aeronautics and Space Administration Task Load Index; FMA, Fugl-Meyer Assessment; T0, beginning of the rehabilitation protocol (baseline assessment); T1, end of the rehabilitation protocol (post-treatment assessment); LMM, linear mixed model; α, agency parameter; β, capability parameter.
References
Altschuler, E. L., Wisdom, S. B., Stone, L., Foster, C., Galasko, D., Llewellyn, D. M. E., et al. (1999). Rehabilitation of hemiparesis after stroke with a mirror. Lancet 353, 2035–2036. doi:10.1016/S0140-6736(99)00920-4
Ballester, B. R., Nirme, J., Duarte, E., Cuxart, A., Rodriguez, S., Verschure, P., et al. (2015). The visual amplification of goal-oriented movements counteracts acquired non-use in hemiparetic stroke patients. J. NeuroEngineering Rehabil. 12, 50. doi:10.1186/s12984-015-0039-z
Bohil, C. J., Alicea, B., and Biocca, F. A. (2011). Virtual reality in neuroscience research and therapy. Nat. Rev. Neurosci. 12, 752–762. doi:10.1038/nrn3122
Burke, J. W., McNeill, M. D. J., Charles, D. K., Morrow, P. J., Crosbie, J. H., and McDonough, S. M. (2009). Optimising engagement for stroke rehabilitation using serious games. Vis. Comput. 25, 1085–1099. doi:10.1007/s00371-009-0387-4
Ceradini, M., Losanno, E., Micera, S., Bandini, A., and Orlandi, S. (2024). Immersive VR for upper-extremity rehabilitation in patients with neurological disorders: a scoping review. J. NeuroEngineering Rehabilitation 21, 75. doi:10.1186/s12984-024-01367-0
Chen, M.-H., Huang, L.-L., Lee, C.-F., Hsieh, C.-L., Lin, Y.-C., Liu, H., et al. (2015). A controlled pilot trial of two commercial video games for rehabilitation of arm function after stroke. Clin. Rehabil. 29, 674–682. doi:10.1177/0269215514554115
Cheung, V. C. K., Piron, L., Agostini, M., Silvoni, S., Turolla, A., and Bizzi, E. (2009). Stability of muscle synergies for voluntary actions after cortical stroke in humans. Proc. Natl. Acad. Sci. U.S.A. 106, 19563–19568. doi:10.1073/pnas.0910114106
Choi, Y.-H., Ku, J., Lim, H., Kim, Y. H., and Paik, N.-J. (2016). Mobile game-based virtual reality rehabilitation program for upper limb dysfunction after ischemic stroke. RNN 34, 455–463. doi:10.3233/RNN-150626
Cikajlo, I., Rudolf, M., Mainetti, R., and Borghese, N. A. (2020). Multi-exergames to set targets and supplement the intensified conventional balance training in patients with stroke: a randomized pilot trial. Front. Psychol. 11, 572. doi:10.3389/fpsyg.2020.00572
Commissioner, O. (2020). FDA permits marketing of first game-based digital therapeutic to improve attention function in children with ADHD. FDA. Available online at: https://www.fda.gov/news-events/press-announcements/fda-permits-marketing-first-game-based-digital-therapeutic-improve-attention-function-children-adhd (Accessed November 19, 2024).
Cramer, S. C., Nelles, G., Benson, R. R., Kaplan, J. D., Parker, R. A., Kwong, K. K., et al. (1997). A functional MRI study of subjects recovered from hemiparetic stroke. Stroke 28, 2518–2527. doi:10.1161/01.STR.28.12.2518
da Silva Jaques, E., Figueiredo, A. I., Schiavo, A., Loss, B. P., da Silveira, G. H., Sangalli, V. A., et al. (2023). Conventional mirror therapy versus immersive virtual reality mirror therapy: the perceived usability after stroke. Stroke Res. Treat. 2023, 1–8. doi:10.1155/2023/5080699
Darbois, N., Guillaud, A., and Pinsault, N. (2018). Do robotics and virtual reality add real progress to mirror therapy rehabilitation? A scoping review. Rehabilitation Res. Pract. 2018, 1–15. doi:10.1155/2018/6412318
De Luca, R., Gangemi, A., Maggio, M. G., Bonanno, M., Calderone, A., Mazzurco Masi, V. M., et al. (2024). Effects of virtual rehabilitation training on post-stroke executive and praxis skills and depression symptoms: a quasi-randomised clinical trial. Diagnostics 14, 1892. doi:10.3390/diagnostics14171892
De Pasquale, P., Bonanno, M., Mojdehdehbaher, S., Quartarone, A., and Calabrò, R. S. (2024). The use of head-mounted display systems for upper limb kinematic analysis in post-stroke patients: a perspective review on benefits, challenges and other solutions. Bioengineering 11, 538. doi:10.3390/bioengineering11060538
Deakin, A., Hill, H., and Pomeroy, V. M. (2003). Rough guide to the fugl-meyer assessment: upper limb section. Physiotherapy 89, 751–763. doi:10.1016/S0031-9406(05)60502-0
DiCicco, M., Lucas, L., and Matsuoka, Y. (2004). “Comparison of control strategies for an EMG controlled orthotic exoskeleton for the hand,” in IEEE International Conference on Robotics and Automation, 2004. Proceedings. ICRA ’04. 2004, New Orleans, LA, USA, 26 April 2004 - 01 May 2004 (IEEE), 1622–1627.
Dohle, C., Püllen, J., Nakaten, A., Küst, J., Rietz, C., and Karbe, H. (2009). Mirror therapy promotes recovery from severe hemiparesis: a randomized controlled trial. Neurorehabil Neural Repair 23, 209–217. doi:10.1177/1545968308324786
Domínguez-Téllez, P., Moral-Muñoz, J. A., Salazar, A., Casado-Fernández, E., and Lucena-Antón, D. (2020). Game-based virtual reality interventions to improve upper limb motor function and quality of life after stroke: systematic review and meta-analysis. Games Health J. 9, 1–10. doi:10.1089/g4h.2019.0043
Eng, K., Siekierka, E., Pyk, P., Chevrier, E., Hauser, Y., Cameirao, M., et al. (2007). Interactive visuo-motor therapy system for stroke rehabilitation. Med. Bio Eng. Comput. 45, 901–907. doi:10.1007/s11517-007-0239-1
Foley, N., Pereira, S., Salter, K., Meyer, M., Andrew McClure, J., and Teasell, R. (2012). Are recommendations regarding inpatient therapy intensity following acute stroke really evidence-based? Top. Stroke Rehabilitation 19, 96–103. doi:10.1310/tsr1902-96
Gil-Gómez, J.-A., Manzano-Hernández, P., Albiol-Pérez, S., Aula-Valero, C., Gil-Gómez, H., and Lozano-Quilis, J.-A. (2017). USEQ: a short questionnaire for satisfaction evaluation of virtual rehabilitation systems. Sensors (Basel) 17, 1589. doi:10.3390/s17071589
Giroux, M., Barra, J., Zrelli, I.-E., Barraud, P.-A., Cian, C., and Guerraz, M. (2018). The respective contributions of visual and proprioceptive afferents to the mirror illusion in virtual reality. PLOS ONE 13, e0203086. doi:10.1371/journal.pone.0203086
Han, J., Lian, S., Guo, B., Li, X., and You, A. (2017). Active rehabilitation training system for upper limb based on virtual reality. Adv. Mech. Eng. 9, 168781401774338. doi:10.1177/1687814017743388
Hart, S. G., and Staveland, L. E. (1988). “Development of NASA-TLX (task load index): results of empirical and theoretical research,” in Advances in psychology. Editors P. A. Hancock, and N. Meshkati (North-Holland), 139–183. doi:10.1016/S0166-4115(08)62386-9
Hatem, S. M., Saussez, G., della Faille, M., Prist, V., Zhang, X., Dispa, D., et al. (2016). Rehabilitation of motor function after stroke: a multiple systematic review focused on techniques to stimulate upper extremity recovery. Front. Hum. Neurosci. 10, 442. doi:10.3389/fnhum.2016.00442
Henrique, P. P. B., Colussi, E. L., and De Marchi, A. C. B. (2019). Effects of exergame on patients’ balance and upper limb motor function after stroke: a randomized controlled trial. J. Stroke Cerebrovasc. Dis. 28, 2351–2357. doi:10.1016/j.jstrokecerebrovasdis.2019.05.031
Hoermann, S., Ferreira dos Santos, L., Morkisch, N., Jettkowski, K., Sillis, M., Devan, H., et al. (2017). Computerised mirror therapy with augmented reflection technology for early stroke rehabilitation: clinical feasibility and integration as an adjunct therapy. Disabil. Rehabilitation 39, 1503–1514. doi:10.1080/09638288.2017.1291765
Horne, M., Thomas, N., McCabe, C., Selles, R., Vail, A., Tyson, S., et al. (2015). Patient-directed therapy during in-patient stroke rehabilitation: stroke survivors’ views of feasibility and acceptability. Disabil. Rehabilitation 37, 2344–2349. doi:10.3109/09638288.2015.1024341
Hsu, H.-Y., Kuo, L.-C., Lin, Y.-C., Su, F.-C., Yang, T.-H., and Lin, C.-W. (2022). Effects of a virtual reality–based mirror therapy program on improving sensorimotor function of hands in chronic stroke patients: a randomized controlled trial. Neurorehabil Neural Repair 36, 335–345. doi:10.1177/15459683221081430
Iosa, M., Galeoto, G., De Bartolo, D., Russo, V., Ruotolo, I., Spitoni, G. F., et al. (2021). Italian version of the Pittsburgh rehabilitation participation scale: psychometric analysis of validity and reliability. Brain Sci. 11, 626. doi:10.3390/brainsci11050626
Ivanova, E., Minge, M., Schmidt, H., Thüring, M., and Krüger, J. (2017). User-centered design of a patient’s work station for haptic robot-based telerehabilitation after stroke. Curr. Dir. Biomed. Eng. 3, 39–43. doi:10.1515/cdbme-2017-0009
Kim, J.-H. (2017). The effects of training using EMG biofeedback on stroke patients upper extremity functions. J. Phys. Ther. Sci. 29, 1085–1088. doi:10.1589/jpts.29.1085
Kwakkel, G., van Peppen, R., Wagenaar, R. C., Wood Dauphinee, S., Richards, C., Ashburn, A., et al. (2004). Effects of augmented exercise therapy time after stroke. Stroke 35, 2529–2539. doi:10.1161/01.STR.0000143153.76460.7d
Laffranchi, M., D’Angella, S., Vassallo, C., Piezzo, C., Canepa, M., De Giuseppe, S., et al. (2021). User-centered design and development of the modular TWIN lower limb exoskeleton. Front. Neurorobotics 15, 709731. doi:10.3389/fnbot.2021.709731
Laver, K. E., Lange, B., George, S., Deutsch, J. E., Saposnik, G., and Crotty, M. (2017). Virtual reality for stroke rehabilitation. Cochrane Database Syst. Rev. 2018. doi:10.1002/14651858.CD008349.pub4
Lee, S. W., Wilson, K. M., Lock, B. A., and Kamper, D. G. (2011). Subject-specific myoelectric pattern classification of functional hand movements for stroke survivors. IEEE Trans. Neural Syst. Rehabil. Eng. 19, 558–566. doi:10.1109/TNSRE.2010.2079334
Li, S., Liu, J., Bhadane, M., Zhou, P., and Rymer, W. Z. (2014). Activation deficit correlates with weakness in chronic stroke: evidence from evoked and voluntary EMG recordings. Clin. Neurophysiol. 125, 2413–2417. doi:10.1016/j.clinph.2014.03.019
Liarokapis, M. V., Artemiadis, P. K., Kyriakopoulos, K. J., and Manolakos, E. S. (2013). A learning scheme for reach to grasp movements: on EMG-based interfaces using task specific motion decoding models. IEEE J. Biomed. Health Inf. 17, 915–921. doi:10.1109/JBHI.2013.2259594
Maggio, M. G., Torrisi, M., Buda, A., De Luca, R., Piazzitta, D., Cannavò, A., et al. (2020). Effects of robotic neurorehabilitation through lokomat plus virtual reality on cognitive function in patients with traumatic brain injury: a retrospective case-control study. Int. J. Neurosci. 130, 117–123. doi:10.1080/00207454.2019.1664519
Maggio, M. G., Cezar, R. P., Milardi, D., Borzelli, D., De Marchis, C., D’Avella, A., et al. (2023). Do patients with neurological disorders benefit from immersive virtual reality? A scoping review on the emerging use of the computer-assisted rehabilitation environment. Eur. J. Phys. Rehabil. Med. 60, 37–43. doi:10.23736/S1973-9087.23.08025-5
Mani Bharathi, V., Manimegalai, P., George, S. T., Pamela, D., Mohammed, M. A., Abdulkareem, K. H., et al. (2024). A systematic review of techniques and clinical evidence to adopt virtual reality in post-stroke upper limb rehabilitation. Virtual Real. 28, 172. doi:10.1007/s10055-024-01065-1
Massetti, T., da Silva, T. D., Crocetta, T. B., Guarnieri, R., de Freitas, B. L., Bianchi Lopes, P., et al. (2018). The clinical utility of virtual reality in neurorehabilitation: a systematic review. J. Cent. Nerv. Syst. Dis. 10, 1179573518813541. doi:10.1177/1179573518813541
Mekbib, D. B., Debeli, D. K., Zhang, L., Fang, S., Shao, Y., Yang, W., et al. (2021). A novel fully immersive virtual reality environment for upper extremity rehabilitation in patients with stroke. Ann. N. Y. Acad. Sci. 1493, 75–89. doi:10.1111/nyas.14554
Meyer, J. T., Schrade, S. O., Lambercy, O., and Gassert, R. (2019). “User-centered design and evaluation of physical interfaces for an exoskeleton for paraplegic users,” in 2019 IEEE 16th International Conference on Rehabilitation Robotics (ICORR), Toronto, ON, Canada (IEEE), 1159–1166.
Morone, G., Spitoni, G. F., Bartolo, D. D., Ghooshchy, S. G., Iulio, F. D., Paolucci, S., et al. (2019). Rehabilitative devices for a top-down approach. Expert Rev. Med. Devices 16, 187–195. doi:10.1080/17434440.2019.1574567
Morone, G., Papaioannou, F., Alberti, A., Ciancarelli, I., Bonanno, M., and Calabrò, R. S. (2024). Efficacy of sensor-based training using exergaming or virtual reality in patients with chronic low back pain: a systematic review. Sensors 24, 6269. doi:10.3390/s24196269
Mubin, O., Alnajjar, F., Al Mahmud, A., Jishtu, N., and Alsinglawi, B. (2022). Exploring serious games for stroke rehabilitation: a scoping review. Disabil. Rehabil. Assist. Technol. 17, 159–165. doi:10.1080/17483107.2020.1768309
Nissler, C., Nowak, M., Connan, M., Büttner, S., Vogel, J., Kossyk, I., et al. (2019). VITA-an everyday virtual reality setup for prosthetics and upper-limb rehabilitation. J. Neural Eng. 16, 026039. doi:10.1088/1741-2552/aaf35f
Patel, G. K., Nowak, M., and Castellini, C. (2017). Exploiting knowledge composition to improve real-life hand prosthetic control. IEEE Trans. Neural Syst. Rehabil. Eng. 25, 967–975. doi:10.1109/TNSRE.2017.2676467
Perez-Marcos, D., Bieler-Aeschlimann, M., and Serino, A. (2018). Virtual reality as a vehicle to empower motor-cognitive neurorehabilitation. Front. Psychol. 9, 2120. doi:10.3389/fpsyg.2018.02120
Piron, L., Turolla, A., Agostini, M., Zucconi, C., Cortese, F., Zampolini, M., et al. (2009). Exercises for paretic upper limb after stroke: a combined virtual-reality and telemedicine approach. J. Rehabilitation Med. 41, 1016–1102. doi:10.2340/16501977-0459
Pollock, A., Farmer, S. E., Brady, M. C., Langhorne, P., Mead, G. E., Mehrholz, J., et al. (2014). Interventions for improving upper limb function after stroke. Cochrane Database Syst. Rev. 2014, CD010820. doi:10.1002/14651858.CD010820.pub2
Ramachandran, V. S., and Rogers-Ramachandran, D. (1996). Synaesthesia in phantom limbs induced with mirrors. Proc. Biol. Sci. 263, 377–386. doi:10.1098/rspb.1996.0058
Rand, D., Givon, N., and Avrech Bar, M. (2018). A video-game group intervention: experiences and perceptions of adults with chronic stroke and their therapists: intervention de groupe à l’aide de jeux vidéo: expériences et perceptions d’adultes en phase chronique d’un accident vasculaire cérébral et de leurs ergothérapeutes. Can. J. Occup. Ther. 85, 158–168. doi:10.1177/0008417417733274
Ríos-Hernández, M., Jacinto-Villegas, J. M., Portillo-Rodríguez, O., and Vilchis-González, A. H. (2021). User-centered design and evaluation of an upper limb rehabilitation system with a virtual environment. Appl. Sci. 11, 9500. doi:10.3390/app11209500
Sadarangani, G. P., Jiang, X., Simpson, L. A., Eng, J. J., and Menon, C. (2017). Force myography for monitoring grasping in individuals with stroke with mild to moderate upper-extremity impairments: a preliminary investigation in a controlled environment. Front. Bioeng. Biotechnol. 5, 42. doi:10.3389/fbioe.2017.00042
Saleh, S., Yarossi, M., Manuweera, T., Adamovich, S., and Tunik, E. (2017). Network interactions underlying mirror feedback in stroke: a dynamic causal modeling study. Neuroimage Clin. 13, 46–54. doi:10.1016/j.nicl.2016.11.012
Sarasola-Sanz, A., Irastorza-Landa, N., López-Larraz, E., Shiman, F., Spüler, M., Birbaumer, N., et al. (2018). Design and effectiveness evaluation of mirror myoelectric interfaces: a novel method to restore movement in hemiplegic patients. Sci. Rep. 8, 16688. doi:10.1038/s41598-018-34785-x
Sarasola-Sanz, A., López-Larraz, E., Irastorza-Landa, N., Rossi, G., Figueiredo, T., McIntyre, J., et al. (2022). Real-time control of a multi-degree-of-freedom mirror myoelectric interface during functional task training. Front. Neurosci. 16, 764936. doi:10.3389/fnins.2022.764936
Semprini, M., Lencioni, T., Hinterlang, W., Vassallo, C., Scarpetta, S., Maludrottu, S., et al. (2022). User-centered design and development of TWIN-Acta: a novel control suite of the TWIN lower limb exoskeleton for the rehabilitation of persons post-stroke. Front. Neurosci. 16, 915707. doi:10.3389/fnins.2022.915707
Seo, G., Kishta, A., Mugler, E., Slutzky, M. W., and Roh, J. (2022). Myoelectric interface training enables targeted reduction in abnormal muscle co-activation. J. NeuroEngineering Rehabilitation 19, 67. doi:10.1186/s12984-022-01045-z
Song, R., Tong, K., Hu, X., and Zhou, W. (2013). Myoelectrically controlled wrist robot for stroke rehabilitation. J. NeuroEngineering Rehabilitation 10, 52. doi:10.1186/1743-0003-10-52
Thieme, H., Mehrholz, J., Pohl, M., Behrens, J., and Dohle, C. (2012). Mirror therapy for improving motor function after stroke. Cochrane Database Syst. Rev. 2012. doi:10.1002/14651858.CD008449.pub2
Torrisi, M., Maggio, M. G., De Cola, M. C., Zichittella, C., Carmela, C., Porcari, B., et al. (2021). Beyond motor recovery after stroke: the role of hand robotic rehabilitation plus virtual reality in improving cognitive function. J. Clin. Neurosci. 92, 11–16. doi:10.1016/j.jocn.2021.07.053
Triandafilou, K. M., Tsoupikova, D., Barry, A. J., Thielbar, K. N., Stoykov, N., and Kamper, D. G. (2018). Development of a 3D, networked multi-user virtual reality environment for home therapy after stroke. J. NeuroEngineering Rehabilitation 15, 88. doi:10.1186/s12984-018-0429-0
Tsekleves, E., Paraskevopoulos, I. T., Warland, A., and Kilbride, C. (2016). Development and preliminary evaluation of a novel low cost VR-based upper limb stroke rehabilitation platform using wii technology. Disabil. Rehabilitation Assistive Technol. 11, 413–422. doi:10.3109/17483107.2014.981874
Veerbeek, J. M., van Wegen, E., van Peppen, R., van der Wees, P. J., Hendriks, E., Rietberg, M., et al. (2014). What is the evidence for physical therapy poststroke? A systematic review and meta-analysis. PLoS ONE 9, e87987. doi:10.1371/journal.pone.0087987
Zhang, X., and Zhou, P. (2012). High-density myoelectric pattern recognition toward improved stroke rehabilitation. IEEE Trans. Biomed. Eng. 59, 1649–1657. doi:10.1109/TBME.2012.2191551
Zirbel, C., Zhang, X., and Hughes, C. (2018). “The VRehab system: a low-cost Mobile virtual reality system for post-stroke upper limb rehabilitation for medically underserved populations,” in 2018 IEEE Global Humanitarian Technology Conference (GHTC), San Jose, CA, USA, 18-21 October 2018 (IEEE), 1–8.
Keywords: stroke rehabilitation, virtual reality, myoelectric control, user-centered design, mirror therapy
Citation: De Pasquale P, De Bartolo D, Russo M, Berger DJ, Maselli A, Borzelli D, Colamarino E, Mattia D, Nissler C, Nowak M, Falomo E, Soto Morras J, Schiller MR, Castellini C, Morone G and d'Avella A (2025) User-centered development of a personalized adaptive mirror therapy for upper-limb post-stroke rehabilitation using virtual reality and myoelectric control. Front. Bioeng. Biotechnol. 13:1655416. doi: 10.3389/fbioe.2025.1655416
Received: 27 June 2025; Accepted: 31 July 2025;
Published: 28 August 2025.
Edited by:
Yu Cao, University of Leeds, United KingdomReviewed by:
Xin Wang, The University of Leeds, United KingdomJun Huo, Huazhong University of Science and Technology, China
Copyright © 2025 De Pasquale, De Bartolo, Russo, Berger, Maselli, Borzelli, Colamarino, Mattia, Nissler, Nowak, Falomo, Soto Morras, Schiller, Castellini, Morone and d'Avella. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Paolo De Pasquale, cGFvbG8uZGVwYXNxdWFsZUBpcmNjc21lLml0; Andrea d'Avella, YS5kYXZlbGxhQGhzYW50YWx1Y2lhLml0
†These authors have contributed equally to this work to the work
 Paolo De Pasquale
Paolo De Pasquale Daniela De Bartolo
Daniela De Bartolo Marta Russo
Marta Russo Denise J. Berger
Denise J. Berger Antonella Maselli
Antonella Maselli Daniele Borzelli
Daniele Borzelli Emma Colamarino
Emma Colamarino Donatella Mattia
Donatella Mattia Christian Nissler
Christian Nissler Markus Nowak
Markus Nowak Elena Falomo
Elena Falomo Javier Soto Morras10
Javier Soto Morras10 Claudio Castellini
Claudio Castellini Giovanni Morone
Giovanni Morone Andrea d'Avella
Andrea d'Avella