- 1Anti-Aging Bio Cell Factory Regional Leading Research Center, Gyeongsang National University, Jinju, Republic of Korea
- 2Division of Applied Life Science (BK21 Four), Gyeongsang National University, Jinju, Republic of Korea
- 3School of Biology and Basic Medical Sciences, Soochow University, Suzhou, China
- 4Research Institute of Molecular Alchemy (RIMA), Gyeongsang National University, Jinju, Republic of Korea
- 5Plant Molecular Biology and Biotechnology Research Center, Gyeongsang National University, Jinju, Republic of Korea
Bacillus subtilis a Generally Recognized As Safe (GRAS) microorganism, is an attractive chassis for producing high-value compounds in a safe and sustainable way. However, its potential for producing the C40 carotenoid lycopene has been limited by inefficient precursor supply and enzyme incompatibility. This study demonstrates that lycopene production in B. subtilis can be significantly enhanced through systematic metabolic engineering by rewiring the lycopene and methylerythritol phosphate (MEP) pathways. A synthetic lycopene biosynthesis pathway expressing the crtE gene from Pantoea agglomerans, which is commonly used for microbial lycopene production, failed to yield lycopene production in B. subtilis. However, replacing crtE with a multifunctional geranylgeranyl diphosphate synthase (GGPPS) from Archaeoglobus fulgidus successfully enabled lycopene synthesis. The optimization of the fermentation medium demonstrated that a combined carbon supply of glucose and glycerol markedly enhanced both cell growth and lycopene production in comparison with separate carbon sources. To further boost production, the methylerythritol phosphate (MEP) pathway was engineered by overexpressing the rate-limiting enzyme, 1-deoxy-D-xylulose-5-phosphate synthase (dxs), which resulted in a five-fold increase in lycopene titer after 72 h. Screening of various GGPPS enzymes revealed that idsA from Corynebacterium glutamicum was the most efficient, further increasing the yield. The final engineered strain achieved a lycopene titer of 55 mg/L in shake-flask cultivation, a significant improvement over the previously reported level in B. subtilis. These results demonstrate that targeted GGPPS selection and precursor pathway engineering are critical strategies for developing B. subtilis into a robust and sustainable platform for carotenoid production.
1 Introduction
Bacillus subtilis is a Gram-positive microorganism widely utilized in industrial biotechnology. Its GRAS (Generally Recognized As Safe) status and lack of endotoxin facilitate the purification of heterologous proteins and metabolites, making it a preferred host for food and pharmaceutical applications (Schallmey et al., 2004; Liu et al., 2013; Guan et al., 2015). Furthermore, B. subtilis exhibits broad substrate compatibility, resistance to harsh fermentation conditions, and an efficient protein secretion system, positioning it as an ideal chassis for sustainable biomanufacturing (Liu et al., 2023). Consequently, B. subtilis has shown promise as a chassis host for the synthesis of valuable compounds and drugs, such as squalene (Song et al., 2020), amorphadiene (Zhou et al., 2013), menaquinone-7 (Cui et al., 2019), riboflavin (Shi et al., 2009; Shi et al., 2014), and taxadiene (Abdallah et al., 2019).
Terpenoids, a large class of natural products, are synthesized from the universal C5 precursors isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP). While Saccharomyces cerevisiae and Escherichia coli have been the traditional hosts for terpenoid biosynthesis research, the potential of B. subtilis remains comparatively underexplored. Previous work in B. subtilis has focused on modifying the native 2-C-methyl-D-erythritol-4-phosphate (MEP) pathway. For instance, Xue and Ahring (Xue and Ahring, 2011) demonstrated that overexpressing dxs increased isoprene (C5) yield by 40%, whereas dxr overexpression had no effect. Early work demonstrated the feasibility of producing C30 carotenoids in B. subtilis. Yoshida et al. (2009) introduced the crtM and crtN genes from Staphylococcus aureus to synthesize these C30 compounds, which also conferred increased resistance to oxidative stress in the modified B. subtilis host. Building on this, (Xue et al., 2015) significantly enhanced C30 carotenoid yields by systematically overexpressing genes from the native MEP pathway. Strains engineered to co-express different subsets of MEP pathway genes along with the carotenoid genes achieved production levels more than 15-fold higher than strains harboring only crtM and crtN. For example, engineered strains containing gene cassettes such as Group A (dxs, ispD, ispF, and ispH) or Group B (ispC, ispE, ispG, and ispA) reached a C30 carotenoid yield of 9.1 mg/g dry cell weight.
Lycopene, a C40 tetraterpene carotenoid, is a potent antioxidant with widespread use in the food, pharmaceutical, and cosmetic industries (Siwach et al., 2018). It also serves as a key precursor for the synthesis of other valuable carotenoids. To date, the biotechnological production of lycopene has been extensively developed in non-GRAS organisms like E. coli, which raises safety and regulatory concerns for applications requiring high purity, such as in food and nutraceuticals. In this context, B. subtilis emerges as a superior alternative. As a GRAS organism, it provides an inherently safer platform, circumventing the endotoxin issues associated with Gram-negative hosts and simplifying downstream purification processes. Beyond its safety profile, B. subtilis is renowned for its robustness in large-scale industrial fermentations and its natural capacity for high-density growth, making it an industrially relevant chassis. However, despite these advantages, its potential for C40 carotenoid production has been underexplored and hindered by low titers of around 1.12 mg/L to 4.1 mg/L (Liu et al., 2023), often attributed to enzymatic incompatibilities and suboptimal precursor flux.
This study aims to address this gap by systematically engineering B. subtilis for enhanced lycopene production. Our strategy involves constructing a functional heterologous pathway and optimizing precursor supply by targeting bottlenecks in the native MEP pathway (Figure 1). Specifically, we focus on screening for an efficient geranylgeranyl diphosphate synthase (GGPPS) and overexpressing key pathway genes, dxs and idi, to increase the metabolic flux towards lycopene. This work demonstrates the potential of B. subtilis as a viable and effective platform for producing lycopene and other high-value carotenoids.
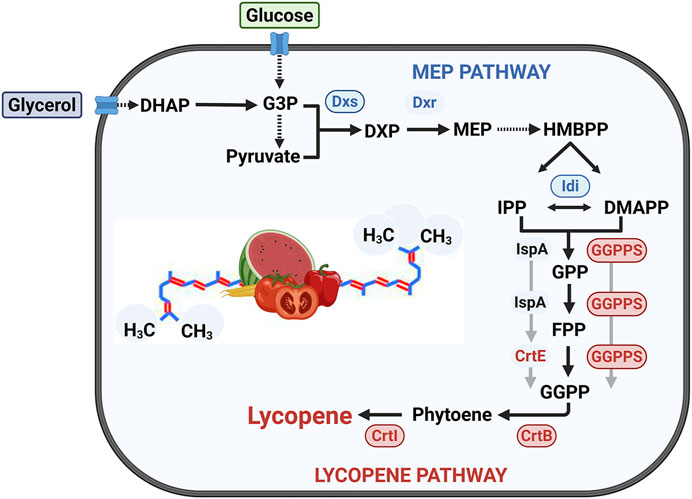
Figure 1. Metabolic pathway for lycopene biosynthesis in B. subtilis and corresponding engineering strategies. The native MEP pathway converts central metabolites derived from glucose and glycerol into the C5 precursors IPP and DMAPP. A heterologous pathway was then introduced to synthesize lycopene from these precursors. This study investigated two distinct routes for producing the key C20 intermediate, GGPP: (1) a route dependent on farnesyl diphosphate (FPP), utilizing the native FPP synthase (IspA) and the heterologous GGPP synthase (CrtE); and (2) a direct route bypassing FPP, which uses a multifunctional GGPP synthase (GGPPS) to convert C5/C10 precursors directly to GGPP. Key enzymes targeted for overexpression are encircled and highlighted. Abbreviations: (Enzymes) Dxs, 1-deoxy-D-xylulose-5-phosphate synthase; Dxr, 1-deoxy-D-xylulose-5-phosphate reductoisomerase; Idi, IPP isomerase; IspA, FPP synthase; CrtE, GGPP synthase; GGPPS, multifunctional GGPP synthase; CrtB, phytoene synthase; CrtI, phytoene desaturase. (Intermediates) G3P, glyceraldehyde-3-phosphate; DHAP, dihydroxyacetone phosphate; F6P, fructose-6-phosphate; DXP, 1-deoxy-D-xylulose 5-phosphate; MEP, 2-C-methyl-D-erythritol 4-phosphate; HMBPP, 1-hydroxy-2-methyl-2(E)-butenyl 4-pyrophosphate; IPP, isopentenyl diphosphate; DMAPP, dimethylallyl diphosphate; GPP, geranyl diphosphate; FPP, farnesyl diphosphate; GGPP, geranylgeranyl diphosphate.
2 Materials and methods
2.1 Bacterial strains, plasmids, and reagents
E. coli DH5α (F−φ80dlacZM15 (lacZYA—argF) U169 deoR recA1 endA1 hsdR17 (rK—, mK+) phoA supE44λ¯ thi¯1 gyrA96 relA1) was used for cloning and plasmid propagation. B. subtilis 168 (ATCC 23857) was used as the host strain for lycopene production. Plasmids used are listed in Supplementary Table S1 and the full plasmid maps and annotated sequences for all key constructs are compiled into Supplementary_Data_Plasmid_Maps_And_Sequences. Restriction enzymes, T4 DNA Ligase (New England Biolabs), and Phusion DNA Polymerase (ThermoScientific, United States) were used for molecular cloning. HPLC-grade methanol was purchased from Daejung Chemical Co., Ltd. (South Korea).
2.2 Plasmids construction
Standard molecular biology techniques were employed. The lycopene biosynthesis operon (crtE, crtB, crtI, ipiHP1) was sourced from the plasmid pT-LYCm4 (Yoon et al., 2006). The genes encoding geranylgeranyl diphosphate synthase (GGPPS) were obtained from various sources: gps and idsA were amplified from the genomic DNA of Archaeoglobus fulgidus and Corynebacterium glutamicum, respectively, while GGPPS1, GGPPS11 and SlG1 were amplified from the cDNA of Oryza sativa, Arabidopsis thaliana and Solanum lycopersicum, respectively. The dxs and dxr genes were amplified from B. subtilis 168 genomic DNA. These genes were cloned into the BamHI/XbaI sites of the IPTG-inducible shuttle vector pHT100 using Gibson assembly. All constructs were verified by colony PCR, restriction mapping, and DNA sequencing (Cosmogenetech Co., Ltd, South Korea). Verified plasmids were transformed into B. subtilis 168 as previously described (Spizizen, 1958).
2.3 Culture media and conditions
Culture media were prepared using the medium composition described in the Difco manual (11th edition, Difco; BD Science, U.S.A). The reagents used for media preparation were purchased from BD Science (United States) and Sigma (United States). E. coli was cultured in 2 YT medium (16 g tryptone, 5 g sodium chloride, and 10 g yeast extract per 1 L) at 37 °C. B. subtilis seed cultures were grown in LB medium (10 g tryptone, 10 g sodium chloride, and 5 g yeast extract per 1 L). For lycopene production, a fermentation medium containing 5% (w/v) glucose, 5% (w/v) glycerol, 5% (w/v) soy peptone, and 0.06% (w/v) KH2PO4 was used. The fermentation medium was successfully used for the production of menaquinone-7 from B. subtilis (Sun et al., 2024). Antibiotics were added as required: ampicillin (100 μg/mL) for E. coli and chloramphenicol (5 μg/mL) for B. subtilis. Gene expression was induced with 1 mM IPTG. Seed cultures were grown at 37 °C, while main fermentations for lycopene production were conducted at 25 °C.
2.4 Shake-flask culture
Engineered B. subtilis strains were pre-cultured in 5 mL LB medium at 37 °C and 250 rpm for 8 h. The seed culture was then inoculated (2% v/v) into 20 mL of fermentation medium in a 300-mL baffled flask. The main culture was incubated at 25 °C and 180 rpm for up to 144 h. Samples were collected every 12 h for analysis. Cell growth was monitored by measuring optical density at 600 nm (OD600) (Beckman DU730, Germany). pH was measured with a pH meter B-212 (HORIBA, Japan).
2.5 Extraction and quantification of lycopene from B. subtilis
Lycopene was extracted from B. subtilis as described previously (Yoshida et al., 2009) with slight modifications. The 200 μL of culture broths were centrifuged at 12,000 rpm for 2 min to harvest cells, and the cell pellets were washed with 1 mL TE (10 mM Tris, 0.1 mM EDTA). The cells were centrifuged again for 1 min at 12,000 rpm, resuspended in 500 μL of TE and 250 μL of lysozyme (10 mg/mL), and incubated at 37 °C with shaking for 1 h. After 1 h of cell lysis, the cell lysates were centrifuged at 12,000 rpm for 1 min, and the pellets were then resuspended in 200 μL of methanol and 300 μL of dichloromethane (DCM) by vortexing and incubated at 55 °C for 15 min. Then, 300 μL of acetone was added, and the mixtures were incubated for an additional 15 min. After this, the samples were centrifuged for 10 min, and the supernatants were collected in HPLC vials. The extracts were used for HPLC analysis. All strains were cultured in triplicate under the same conditions, and each sample was extracted in parallel.
For quantification of Lycopene, lycopene standard (Sigma-Aldrich, United States; ≥90% purity) was used to prepare standard curve. Calibration curves were generated with R2 > 0.998. For the lycopene analysis, standard solutions were prepared by dissolving 1 mg in 1 mL of acetone and 5 mg in 10 mL of acetone, respectively. Calibration curves were obtained using freshly prepared standard solutions in the range of 0.5–50 mg/L.
2.6 HPLC analysis of lycopene and residual carbon sources
Lycopene quantification was performed using a Shimadzu LC-20A HPLC system with Symmetry C18 (250 mm × 4.6 mm, 5 μm) and Sentry Guard C18 (15 mm × 4.6 mm, 5 μm) HPLC columns from Waters (Milford, United States). The mobile phase was a 70:30 mixture of methanol and acetonitrile at a flow rate of 1.5 mL/min, and lycopene was detected at 475 nm. The analysis was conducted at 40 °C. The chromatograms of both the standard and extracted samples are shown in Supplementary Figure S4.
For the analysis of residual glucose and glycerol, culture supernatants were collected by centrifugation and filtered (0.22 μm), and residual sugars were quantified using HPLC with refractive index detection with Aminex HPLC Columns from Bio-Rad.
3 Results
3.1 Construction of a functional lycopene pathway in B. subtilis
To enable lycopene production in B. subtilis, we first constructed the plasmid pHT-LYC4, an E. coli - B. subtilis shuttle vector expressing the lycopene biosynthesis genes (crtE, crtB, crtI) and the IPP isomerase gene (ipiH) (Figure 2A). As a crucial positive control, we first confirmed that this plasmid successfully produced lycopene in E. coli, resulting in a red-colored culture (data not shown). This demonstrated that the crtEBI-containing operon itself was functionally intact. However, when the same plasmid was introduced into B. subtilis, the resulting strain failed to produce any detectable lycopene, as indicated by the absence of the characteristic pink phenotype and further confirmed by HPLC analysis. This host-specific failure indicated that the bottleneck was not due to a faulty gene cassette but rather an issue within the B. subtilis metabolic context. We hypothesized two possibilities: 1) an insufficient supply of the precursor FPP, or 2) poor functionality of the CrtE enzyme within the B. subtilis host. To test the first hypothesis, we overexpressed the native FPP synthase gene, ispA, creating pHT-LYC4A. This modification also failed to yield lycopene, allowing us to largely exclude FPP availability as the primary limiting factor. By this deductive process, we identified the CrtE-catalyzed step as the critical host-specific bottleneck. To circumvent this issue, we pursued a strategy to bypass the FPP-dependent CrtE altogether by replacing it with gps from A. fulgidus, a multifunctional GGPPS. This new construct, pHT-gpsLYC3, successfully restored the pathway, leading to visible lycopene production (Figure 2B). This result confirmed that selecting a functionally compatible GGPPS is critical for establishing C40 carotenoid synthesis in B. subtilis. The potential of the multifunctional GGPP synthase to increase the flux towards lycopene production in B. subtilis was shown. Previous research has also shown that overexpression of A. fulgidus gps in E. coli dramatically boosted the production of astaxanthin (Wang et al., 1999).
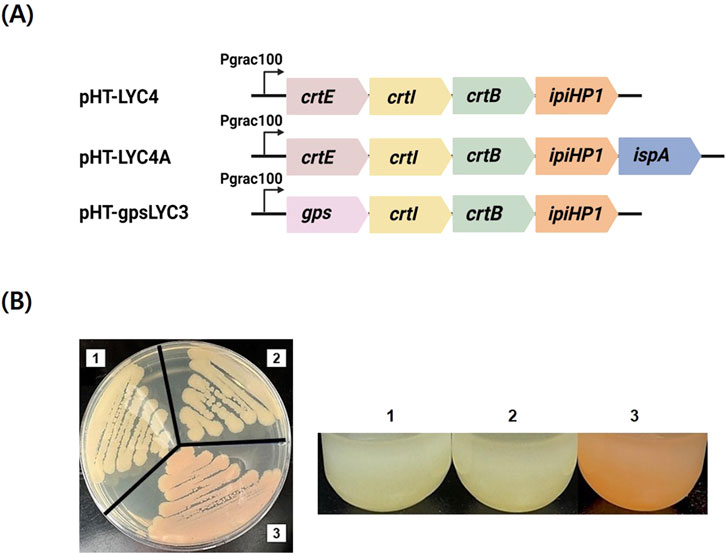
Figure 2. Initial construction of the lycopene biosynthesis pathway and resulting phenotypes. (A) Schematic representations of the key constructs used to establish the pathway. pHT-LYC4 contains the lycopene biosynthesis operon with crtE from P. agglomerans. pHT-LYC4A is a derivative of pHT-LYC4 with the additional overexpression of the native ispA gene. pHT-gpsLYC3 replaces the original crtE gene with the multifunctional gps gene from A. fulgidus. (B) Phenotypic analysis of B. subtilis colonies transformed with the corresponding plasmids. Strains harboring (1) pHT-LYC4 and (2) pHT-LYC4A exhibit no pigmentation, indicating a failure to produce lycopene. In contrast, the strain containing (3) pHT-gpsLYC3 displays a distinct pink-red color, providing visual confirmation of successful lycopene synthesis.
3.2 Optimization of carbon source for lycopene production
To determine the optimal carbon source for lycopene production in B. subtilis, we evaluated cell growth and product synthesis using a fermentation medium containing three different carbon compositions. The base medium, successfully used for menaquinone-7 production in B. subtilis by Sun et al. (2024), contained a mixture of 5% glucose and 5% glycerol. To confirm the optimal carbon source for our lycopene engineered strain, a comparison was conducted against two single-source media containing either 10% glucose or 10% glycerol. Glycerol has been demonstrated to be a more effective carbon source for isoprenoid production than glucose in E. coli. In one study by Yoon et al. (2009) on E. coli, glycerol was found to yield the highest β-carotene production and cell growth among the various carbon sources tested. However, to determine whether same advantages would apply to B. subtilis comparison was conducted. As shown in Figures 3A,B, the carbon source composition significantly influenced both cell growth and lycopene production. In the medium with 10% glucose, cell growth and lycopene production patterns were initially comparable to those in the mixed-carbon medium for the first 36 h, after which both metrics stagnated. Conversely, the culture with 10% glycerol supported a slow but continuous increase in growth and production throughout the fermentation, though its final yields were lower than those of the mixed-carbon source. The medium containing both 5% glucose and 5% glycerol proved to be the most effective for B. subtilis, resulting in the highest final cell density and lycopene titer. The results obtained suggest that using this mixed carbon source is the most beneficial strategy to produce lycopene in our system. Therefore, this mixed-carbon strategy was employed for all subsequent experiments.
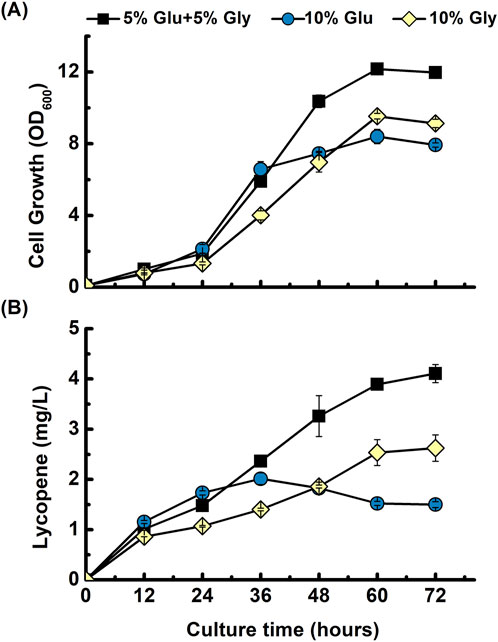
Figure 3. Comparison of different carbon sources for lycopene production. Time-course profiles of (A) cell growth (OD600), and (B) lycopene concentration (mg/L) are shown for the B. subtilis strain harboring the pHT-gpsLYC3 construct. The strain was cultivated in fermentation media containing three different carbon source compositions: a mixture of 5% glucose and 5% glycerol (■), 10% glucose alone (●), or 10% glycerol alone (♦). The data points represent the mean of three independent experiments, and error bars indicate the standard deviation.
3.3 Overexpression of dxs significantly boosts lycopene production
The 1-deoxyxylulose-5-phosphate synthase encoded by dxs catalyzes the condensation of pyruvate and glyceraldehyde 3-phosphate to form 1-deoxyxylulose-5-phosphate (DXP) (Hess et al., 2013). To enhance the metabolic flux towards lycopene, we targeted the first committed step of the MEP pathway by overexpressing the rate-limiting gene dxs. A new plasmid, pHT-gpsLYC3dxs, was constructed and introduced into B. subtilis. Shake-flask cultivation revealed that the overexpression of dxs had no discernible negative effect on cell growth, as the growth profile was nearly identical to that of the control strain (Figure 4A). In contrast, lycopene production was significantly enhanced. The dxs-overexpressing strain exhibited a 2.6-fold increase in lycopene titer at 60 h and a substantial 5-fold increase at 72 h compared to the control (Figure 4B). This result clearly demonstrates that the availability of the precursor DXP is a major bottleneck and that upregulating dxs expression is a highly effective strategy for increasing lycopene yields in B. subtilis.
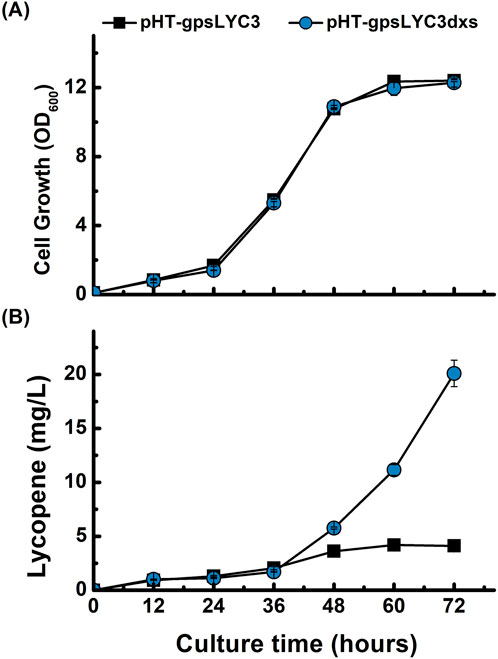
Figure 4. Effect of dxs overexpression on lycopene production and cell growth. Time-course profiles of (A) cell growth (OD600) and (B) lycopene concentration (mg/L). The performance of the control strain harboring pHT-gpsLYC3 (■) is compared with the strain overexpressing dxs from the plasmid pHT-gpsLYC3dxs (●). Data points represent the mean of three independent experiments, and error bars indicate the standard deviation.
3.4 Screening identifies idsA as a superior GGPP synthase for lycopene production
To further optimize the pathway, we sought to identify a more efficient GGPP synthase than the gps from A. fulgidus. We hypothesized that enzymes from different evolutionary backgrounds might exhibit varied compatibility and performance within the B. subtilis metabolic environment. Therefore, we conducted a screening of a functionally and evolutionarily diverse panel of GGPPS enzymes to maximize the probability of identifying a superior candidate. The screening was conducted by replacing gps in the pHT-gpsLYC3dxs construct with four different candidate genes: idsA from C. glutamicum, GGPPS1 from O. sativa, GGPPS11 from A. thaliana, and SlG1 from S. lycopersicum according to their known substrate specificities and varied evolutionary backgrounds (Supplementary Figure S1). Prokaryotic enzymes from C. glutamicum (a Gram-positive bacterium) and A. fulgidus (an archaeon) were chosen due to their established roles in native carotenoid biosynthesis pathways and reported efficiency in bacterial hosts, respectively. In contrast, enzymes from the eukaryotic plant sources O. sativa, A. thaliana, and S. lycopersicum were included to evaluate whether their documented broad substrate promiscuity might offer functional advantages in the B. subtilis. Shake-flask cultivation of these new strains revealed that while all exhibited similar cell growth profiles, their lycopene production levels varied significantly (Figures 5A,B). The strain expressing idsA from C. glutamicum demonstrated markedly superior performance, yielding the highest lycopene titer among all candidates. In contrast, the strain containing SlG1 from S. lycopersicum failed to produce any detectable lycopene. A direct comparison showed that the idsA construct resulted in a ∼2.6-fold higher production than the gps construct at 60 h and a 1.9-fold increase at 72 h. This superior production, reaching approximately 40 mg/L, was visibly apparent from the deep red phenotype of the culture (Supplementary Figure S2). Consequently, the idsA enzyme was selected as the optimal GGPP synthase for all further engineering.
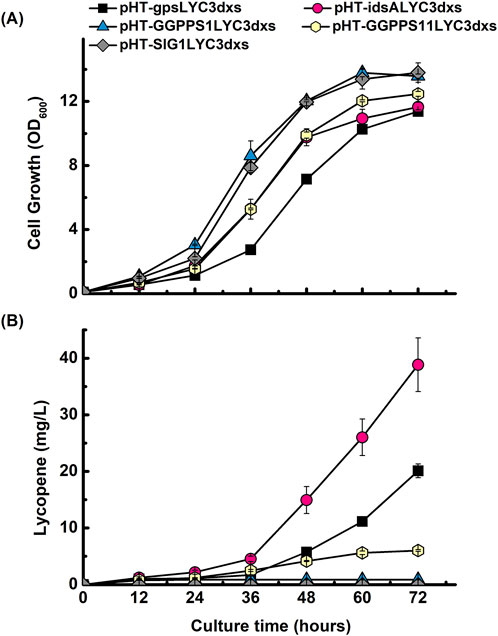
Figure 5. Screening of diverse GGPP synthases to optimize lycopene production. Time-course profiles of (A) cell growth (OD600) and (B) lycopene concentration (mg/L) for B. subtilis strains engineered to express different GGPP synthase enzymes. All constructs were built in the dxs-overexpressing background. The performance of five different synthases was compared: gps from A. fulgidus (■), idsA from C. glutamicum (●), GGPPS1 from O. sativa (▲), GGPPS11 from A. thaliana (●), and SlG1 from S. lycopersicum (♦). Data points represent the mean of three independent experiments, and error bars indicate the standard deviation.
3.5 Overexpression of dxr fails to improve lycopene production
The second step of the MEP pathway involves the reduction and isomerization of DXP to produce MEP, which is carried out by dxr-encoded enzyme. It has been reported in E. coli that the overexpression of dxr along with dxs increased lycopene production (Kim and Keasling, 2001). To investigate the impact of modulating the second step of the MEP pathway, the dxr gene was overexpressed in our best-performing strain, creating the construct pHT-idsALYC3dxs/r. However, contrary to enhancing the pathway, the co-expression of dxr with dxs proved to be detrimental to lycopene synthesis. The final lycopene titer in the dxr-overexpressing strain was approximately 30% lower than that of the control strain lacking the additional dxr gene (Figures 6A,B). This result indicates that DXR is not a limiting factor for lycopene production in this engineered background, and its overexpression creates an imbalance that negatively affects the overall pathway flux.
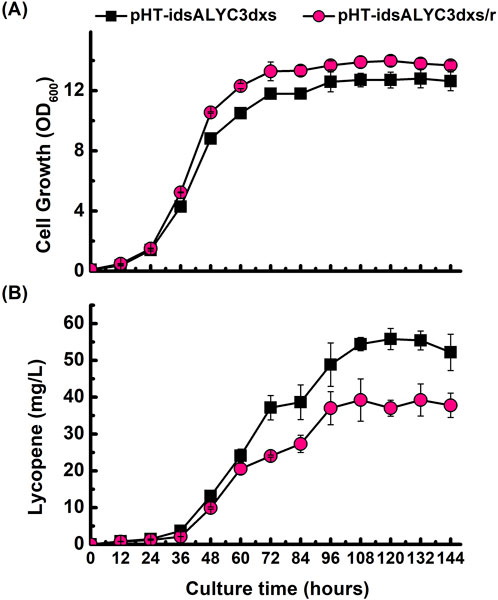
Figure 6. Effect of dxr co-overexpression on lycopene production. Time-course profiles of (A) cell growth (OD600) and (B) lycopene concentration (mg/L). The performance of the optimal strain harboring pHT-idsALYC3dxs (■) is compared with a derivative strain that additionally overexpresses the dxr gene, harboring pHT-idsALYC3dxs/r (●). Data points represent the mean of three independent experiments, and error bars indicate the standard deviation.
Since the optimal strain (pHT-idsALYC3dxs) was still actively producing lycopene at the 72-h mark, the fermentation was extended to 144 h to determine the maximum achievable titer. Cell growth was observed to plateau after 96 h. Despite the cessation of growth, lycopene accumulation continued, increasing by an additional 1.4-fold between 72 and 144 h. This extended cultivation resulted in a final maximum lycopene production of 55 mg/L. Analysis of the culture supernatant at the end of the fermentation revealed the presence of residual glucose and glycerol (Supplementary Figure S3), indicating that the final production limit was not caused by carbon source depletion.
4 Discussion
This study successfully demonstrates the potential of B. subtilis as a robust platform for the enhanced production of lycopene. Through a systematic, multi-step engineering strategy, we achieved a final production of 55 mg/L, which represents an approximate 10-fold improvement over previously reported titers in this organism (Xu et al., 2023). This significant advancement was built on an alleviation of several key metabolic and enzymatic bottlenecks, providing valuable insights for future carotenoid engineering in B. subtilis. A critical finding of our work was the functional failure of the heterologous CrtE enzyme in B. subtilis, despite the same construct being functional in E. coli. This host-specific inactivity, even when the FPP precursor pool was supplemented via ispA overexpression, strongly suggests that the bottleneck is not a simple lack of substrate. Instead, we hypothesize that the CrtE enzyme is unable to effectively compete for the FPP substrate against potent native metabolic pathways in B. subtilis. Notably, B. subtilis is a prominent commercial producer of menaquinone-7 (MK-7), a pathway that also utilizes FPP as a key precursor (Cui et al., 2019). The strong intrinsic activity of this and other native pathways likely creates intense competition at the FPP node, preventing the foreign CrtE from efficiently channeling the flux towards GGPP. In contrast, the success of multifunctional synthases like gps and idsA can be attributed to their ability to bypass this competitive FPP branch point entirely. By directly synthesizing GGPP from earlier, less-contested precursors (IPP, DMAPP, and GPP), these enzymes circumvent the metabolic traffic jam at the FPP node. This highlights a crucial engineering principle for C40 carotenoid production in B. subtilis: the most effective strategy may not be to simply push a linear pathway, but to select enzymes that can navigate around the host’s entrenched metabolic highways.
Our results demonstrated that a mixed-carbon source of glucose and glycerol significantly outperformed single-source media for both cell growth and lycopene production. This synergistic effect can be attributed to several metabolic advantages in B. subtilis. Initially, the cells likely utilize glucose for rapid biomass accumulation, a phenomenon governed by Carbon Catabolite Repression (CCR). Once glucose is depleted, the cells switch to metabolizing glycerol. This metabolic shift is particularly beneficial for lycopene synthesis, as glycerol enters glycolysis as dihydroxyacetone phosphate (DHAP), providing a more direct route to glyceraldehyde-3-phosphate (G3P), a key precursor for the MEP pathway. This sequential utilization effectively separates the growth phase from the production phase, allowing for robust biomass generation followed by sustained product synthesis. This strategy avoids the early growth stagnation often seen with high glucose concentrations while leveraging glycerol’s efficient entry into the precursor pathway for terpenoid production.
Beyond establishing the core pathway, optimizing the supply of C5 precursors was essential. Our results reinforce that dxs, which catalyzes the first committed step of the MEP pathway, is the primary rate-limiting node for terpenoid synthesis in B. subtilis. Overexpression of dxs alone led to a notable five-fold increase in lycopene production without impairing cell growth, confirming that enhancing this initial flux is a highly effective strategy. In sharp contrast, further overexpression of dxr, the second pathway enzyme, was detrimental to production. This finding aligns perfectly with previous reports on isoprene synthesis in B. subtilis (Xue and Ahring, 2011) and clarifies that engineering the MEP pathway requires precise interventions; simply upregulating multiple enzymes can create metabolic imbalances that hinder overall productivity.
Finally, our fermentation analysis revealed that while the mixed-carbon source of glucose and glycerol was beneficial, production began to plateau after 96–120 h, even though the primary carbon sources were not depleted. This suggests that in the late stages of fermentation, lycopene synthesis becomes limited by factors other than carbon availability. These limitations could include the depletion of other essential nutrients (e.g., nitrogen, phosphorus), an insufficient supply of the reducing cofactor NADPH required for the MEP pathway, or potential feedback inhibition. Therefore, future optimization strategies should move beyond precursor supply and focus on these areas. Implementing dynamic gene expression systems, optimizing the supply of key nutrients and cofactors, and balancing the cellular redox state will be essential to extend the productive phase and push titers closer to commercially viable levels.
In conclusion, this study provides valuable strategic insights into the metabolic engineering of B. subtilis for C40 carotenoid production. While the individual methods employed, such as enzyme screening and pathway upregulation, are standard tools in the field, the key contribution of our work lies in elucidating a host-specific, logical engineering sequence for overcoming critical bottlenecks. We have demonstrated that for producing FPP-derived compounds in B. subtilis, a successful strategy involves two key steps: first, diagnosing and circumventing the intense substrate competition at the FPP node by selecting a multifunctional enzyme that bypasses it entirely; and second, after alleviating this primary enzymatic bottleneck, amplifying the precursor flux by targeting the correct rate-limiting step in the MEP pathway, which we confirmed to be dxs. This problem-solving approach, tailored to the unique metabolic context of B. subtilis, offers an effective engineering strategy that can be applied to enhance the production of other high-value terpenoids in this promising industrial chassis.
5 Conclusion
This study successfully engineered B. subtilis for enhanced lycopene production, achieving a final titer of 55 mg/L in shake-flask cultivation. By replacing an incompatible CrtE with a superior multifunctional GGPP synthase (idsA) and amplifying precursor flux via dxs overexpression, we systematically overcame key host-specific bottlenecks, resulting in a ten-fold improvement over previously reported levels in this organism. While the current titer is not yet industrially competitive and was achieved at the laboratory scale, this work establishes a critical foundation and a clear engineering strategy for developing B. subtilis as a safe and promising platform for carotenoid biosynthesis. Future efforts in process optimization and scale-up are required to unlock its full industrial potential.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to Esha Rehman: ZXNoYXJlaG1hbjIwMDBAZ21haWwuY29t, Seon-Won Kim: c3draW1AZ251LmFjLmty.
Author contributions
ER: Conceptualization, Investigation, Methodology, Writing – original draft, Writing – review and editing. HS: Formal Analysis, Writing – review and editing. MN: Formal Analysis, Visualization, Writing – review and editing. CW: Project administration, Writing – review and editing. S-HY: Supervision, Writing – review and editing. MK: Funding acquisition, Resources, Supervision, Writing – review and editing. M-KK: Conceptualization, Supervision, Writing – review and editing. S-WK: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Writing – review and editing, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Research Foundation of Korea (Grant NRF 2021R1A5A8029490, 2022M3A9I3018121, RS-2023-00235511, and RS-2024-00508861); The Technology Development Program (grant number 20014582) funded by the Ministry of Trade, Industry and Energy (MOTIE, Korea).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fbioe.2025.1670015/full#supplementary-material
References
Abdallah, I. I., Pramastya, H., van Merkerk, R., and Quax, W. J. (2019). Metabolic engineering of Bacillus subtilis toward taxadiene biosynthesis as the first committed step for taxol production. Front. Microbiol. 10, 218. doi:10.3389/fmicb.2019.00218
Cui, S., Lv, X., Wu, Y., Li, J., Du, G., Ledesma-Amaro, R., et al. (2019). Engineering a bifunctional Phr60-Rap60-Spo0A quorum-sensing molecular switch for dynamic fine-tuning of menaquinone-7 synthesis in Bacillus subtilis. ACS Synth. Biol. 8 (8), 1826–1837. doi:10.1021/acssynbio.9b00140
Guan, Z., Xue, D., Abdallah, I. I., Dijkshoorn, L., Setroikromo, R., Lv, G., et al. (2015). Metabolic engineering of Bacillus subtilis for terpenoid production. Appl. Microbiol. Biotechnol. 99 (22), 9395–9406. doi:10.1007/s00253-015-6950-1
Hess, B. M., Xue, J., Markillie, L. M., Taylor, R. C., Wiley, H. S., Ahring, B. K., et al. (2013). Coregulation of terpenoid pathway genes and prediction of isoprene production in Bacillus subtilis using transcriptomics. PLoS One 8 (6), e66104. doi:10.1371/journal.pone.0066104
Kim, S.-W., and Keasling, J. D. (2001). Metabolic engineering of the nonmevalonate isopentenyl diphosphate synthesis pathway in Escherichia coli enhances lycopene production. Biotechnol. Bioeng. 72 (4), 408–415. doi:10.1002/1097-0290(20000220)72:4<408::aid-bit1003>3.0.co;2-h
Liu, L., Liu, Y., Shin, H.-d., Chen, R. R., Wang, N. S., Li, J., et al. (2013). Developing bacillus spp. as a cell factory for production of microbial enzymes and industrially important biochemicals in the context of systems and synthetic biology. Appl. Microbiol. Biotechnol. 97 (14), 6113–6127. doi:10.1007/s00253-013-4960-4
Liu, Y., Cheng, H., Li, H., Zhang, Y., and Wang, M. (2023). A programmable CRISPR/Cas9 toolkit improves lycopene production in Bacillus subtilis. Appl. Environ. Microbiol. 89 (6), e00230-23–00223. doi:10.1128/aem.00230-23
Schallmey, M., Singh, A., and Ward, O. P. (2004). Developments in the use of bacillus species for industrial production. Can. J. Microbiol. 50 (1), 1–17. doi:10.1139/w03-076
Shi, S., Chen, T., Zhang, Z., Chen, X., and Zhao, X. (2009). Transcriptome analysis guided metabolic engineering of Bacillus subtilis for riboflavin production. Metab. Eng. 11 (4), 243–252. doi:10.1016/j.ymben.2009.05.002
Shi, T., Wang, Y., Wang, Z., Wang, G., Liu, D., Fu, J., et al. (2014). Deregulation of purine pathway in Bacillus subtilis and its use in riboflavin biosynthesis. Microb. Cell. Fact. 13 (1), 101. doi:10.1186/s12934-014-0101-8
Siwach, R., Tokas, J., and Seth, R. (2018). Lycopene: a natural antioxidant for anhydrous buffalo milk fat. Int. J. Dairy Technol. 71 (1), 164–173. doi:10.1111/1471-0307.12335
Song, Y., Guan, Z., van Merkerk, R., Pramastya, H., Abdallah, I. I., Setroikromo, R., et al. (2020). Production of squalene in Bacillus subtilis by squalene synthase screening and metabolic engineering. J. Agric. Food Chem. 68 (15), 4447–4455. doi:10.1021/acs.jafc.0c00375
Spizizen, J. (1958). Transformation of biochemically deficient strains of Bacillus subtilis by deoxyribonucleate. Proc. Natl. Acad. Sci. U. S. A. 44 (10), 1072–1078. doi:10.1073/pnas.44.10.1072
Sun, X., Bi, X., Li, G., Cui, S., Xu, X., Liu, Y., et al. (2024). Combinatorial metabolic engineering of Bacillus subtilis for menaquinone-7 biosynthesis. Biotechnol. Bioeng. 121 (10), 3338–3350. doi:10.1002/bit.28800
Wang, C.-W., Oh, M.-K., and Liao, J. C. (1999). Engineered isoprenoid pathway enhances astaxanthin production in Escherichia coli. Biotechnol. Bioeng. 62 (2), 235–241. doi:10.1002/(sici)1097-0290(19990120)62:2<235::aid-bit14>3.0.co;2-u
Xu, X., Lv, X., Cui, S., Liu, Y., Xia, H., Li, J., et al. (2023). Remodeling isoprene pyrophosphate metabolism for promoting terpenoids bioproduction. Eng 28, 166–178. doi:10.1016/j.eng.2023.03.019
Xue, J., and Ahring, B. K. (2011). Enhancing isoprene production by genetic modification of the 1-deoxy-d-xylulose-5-phosphate pathway in Bacillus subtilis. Appl. Environ. Microbiol. 77 (7), 2399–2405. doi:10.1128/aem.02341-10
Xue, D., Abdallah, I. I., de Haan, I. E., Sibbald, M. J., and Quax, W. J. (2015). Enhanced C30 carotenoid production in Bacillus subtilis by systematic overexpression of MEP pathway genes. Appl. Microbiol. Biotechnol. 99 (14), 5907–5915. doi:10.1007/s00253-015-6531-3
Yoon, S.-H., Lee, Y.-M., Kim, J.-E., Lee, S.-H., Lee, J.-H., Kim, J.-Y., et al. (2006). Enhanced lycopene production in Escherichia coli engineered to synthesize isopentenyl diphosphate and dimethylallyl diphosphate from mevalonate. Biotechnol. Bioeng. 94 (6), 1025–1032. doi:10.1002/bit.20912
Yoon, S.-H., Lee, S.-H., Das, A., Ryu, H.-K., Jang, H.-J., Kim, J.-Y., et al. (2009). Combinatorial expression of bacterial whole mevalonate pathway for the production of β-carotene in E. coli. J. Biotechnol. 140 (3), 218–226. doi:10.1016/j.jbiotec.2009.01.008
Yoshida, K., Ueda, S., and Maeda, I. (2009). Carotenoid production in Bacillus subtilis achieved by metabolic engineering. Biotechnol. Lett. 31 (11), 1789–1793. doi:10.1007/s10529-009-0082-6
Keywords: metabolic engineering, lycopene, Bacillus subtilis, MEP pathway, GGPP synthase
Citation: Rehman E, Birla Singh H, Nguyen MP, Wang C, Yoon S-H, Kwon M, Kang M-K and Kim S-W (2025) Enhancing lycopene production in Bacillus subtilis by overcoming a critical enzymatic bottleneck. Front. Bioeng. Biotechnol. 13:1670015. doi: 10.3389/fbioe.2025.1670015
Received: 21 July 2025; Accepted: 13 August 2025;
Published: 29 August 2025.
Edited by:
Jung-Kul Lee, Konkuk University, Republic of KoreaReviewed by:
Ian Yunus, Independent Researcher, Elarion, United StatesHyun Gyu Lim, Inha University, Republic of Korea
Copyright © 2025 Rehman, Birla Singh, Nguyen, Wang, Yoon, Kwon, Kang and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chonglong Wang, d2FuZ2Nob25nbG9uZ0BnbWFpbC5jb20=; Min-Kyoung Kang, bWtrYW5nQGdudS5hYy5rcg==; Seon-Won Kim, c3draW1AZ251LmFjLmty
 Esha Rehman
Esha Rehman Hawaibam Birla Singh
Hawaibam Birla Singh Minh Phuong Nguyen
Minh Phuong Nguyen Chonglong Wang
Chonglong Wang Sang-Hwal Yoon1
Sang-Hwal Yoon1 Moonhyuk Kwon
Moonhyuk Kwon Min-Kyoung Kang
Min-Kyoung Kang Seon-Won Kim
Seon-Won Kim