- 1Nanjing University of Chinese Medicine, Nanjing, China
- 2Jiangsu CM Clinical Medical Innovation Center of Degenerative Bone and Joint Disease, Wuxi Affiliated Hospital of Nanjing University of Chinese Medicine, Wuxi, China
- 3Orthopedics Department, Shanghai Minhang Hospital of Integrated Traditional Chinese and Western Medicine, Shanghai, China
Osteomyelitis is an inflammatory bone disease caused by bacterial infection, often leading to bone destruction and functional impairment. Traditional treatments face significant limitations, including substantial surgical trauma, low drug delivery efficiency, and a high risk of recurrence. Nanomaterial-mediated antibiotic delivery has emerged as an innovative strategy, enabling localized, targeted and controlled antibiotic release. Representative platforms include nanohydroxyapatite (nHA), mesoporous bioactive glass (MBG), poly (lactic-co-glycolic acid) (PLGA), metal–organic frameworks (MOFs), silver nanoparticles (AgNPs), and multifunctional hybrid composites. This approach can enhance therapeutic efficacy, reduces systemic side effects, and promotes bone regeneration. This review summarizes the pathogenesis and therapeutic challenges of osteomyelitis, explores the construction and delivery mechanisms of nanocarriers, and discusses recent advances from in vitro studies to animal models and clinical research. Current evidence indicates that nanocarrier-based drug delivery systems can effectively inhibit bacterial growth, modulate inflammatory responses, and facilitate bone regeneration. However, their large-scale clinical application remains limited by concerns regarding safety, manufacturing complexity, regulatory standardization, and cost. Future directions include the development of intelligent nanocarriers, integration with multimodal therapeutic strategies (e.g., photothermal, immunomodulatory, and stem cell-assisted therapies), establishment of standardized multi-tier toxicity evaluation frameworks, and progression toward large-animal validation and early phase clinical trials, which are expected to drive further progress and provide more effective and safer treatment options for osteomyelitis.
1 Introduction
Osteomyelitis is a bone infection resulting from bacterial invasion of the bone marrow, cortical bone, and periosteum, often accompanied by sequestrum formation and it frequently leads to bone destruction and necrosis, as well as functional impairment, and may ultimately result in permanent disability in affected patients. When the infection occurs in proximity to a joint, it can cause joint contracture, stiffness, and restricted mobility (Lew and Waldvogel, 2004; Kavanagh et al., 2018). Recent population-based data show an overall incidence of 21.8 per 100,000 persons, higher in males; approximately 13 per 100,000 in children versus 90 per 100,000 in adults (Kremers et al., 2015). In diabetes, 19%–34% of patients develop a lifetime diabetic foot ulcer and a substantial proportion progress to osteomyelitis (McDermott et al., 2023). Each complex episode imposes considerable clinical and economic burden: the median hospital stay is about 22 days, and the median inpatient cost for post-traumatic osteomyelitis (USD 10,504) is 4.8-fold that of non-traumatic cases (USD 2,189) (Jiang et al., 2020). These epidemiological and economic indicators underscore the need for more effective, cost-efficient strategies.
The primary treatment for osteomyelitis involves surgical debridement combined with antibiotic therapy. However, conventional therapeutic approaches present several limitations. Surgical intervention is highly invasive, requires a prolonged recovery period, and may result in bone defects or disease recurrence (Zhang H. et al., 2025). Systemic antibiotic therapy frequently fails to achieve and maintain effective drug concentrations at the infection site due to poor vascularization, thereby leading to suboptimal therapeutic outcomes (Abdulrehman et al., 2024). Although local administration can deliver higher drug concentrations to the infected area, commonly used carriers (such as bone cement or calcium sulfate) typically involve simple physical mixing with antibiotics. This approach often results in uneven drug distribution and burst release, thereby making it challenging to achieve sustained and controlled antibiotic delivery (Štícha et al., 2023; Birk et al., 2020).
Nanomaterials, defined as materials with structural dimensions ranging from 1 to 100 nm, exhibit a high surface-to-volume ratio, advantageous mechanical properties, and reactive surface chemistry (Hammond, 2011; Zapata et al., 2022; Eivazzadeh-Keihan et al., 2024). These features enhance their capacity for drug adsorption and controlled release, thereby demonstrating significant potential for the treatment of osteomyelitis. As an emerging therapeutic strategy, nanomaterial-mediated antibiotic delivery offers new prospects by enabling localized drug delivery, minimizing systemic side effects, and providing controlled and sustained antibiotic release. Moreover, this approach reduces toxicity to surrounding tissues and promotes bone regeneration (Geurts et al., 2021; Liu et al., 2022). Therefore, comprehensive research into nanomaterial-mediated antibiotic delivery is essential for improving therapeutic outcomes in osteomyelitis.
Although some excellent reviews summarize multifunctional strategies for nanocarriers and bone infection (Geurts et al., 2021; Zapata et al., 2022), this review shows differences from current research progress. First, we highlight recent advances in intelligent (stimulus-responsive) nanocarriers and multifunctional composite scaffolds that have not been previously covered. Second, we propose a cross-mapping framework that links material types to therapeutic functions (infection control, immunomodulation, and bone regeneration) to reduce redundancy and facilitate translation-oriented vector selection. Finally, we have dedicated discussions on clinical translation, regulatory considerations, and industrial scalability, topics that are critical to moving nanomedicines from the lab to the bedside but were not fully addressed in earlier reviews.
2 Overview of osteomyelitis
Osteomyelitis is an inflammatory disease of bone tissue caused by infection with bacteria, fungi, or other pathogens (Yang et al., 2024). The condition involves not only the bone marrow but also the cortical bone and periosteum. According to etiology, clinical course, and route of infection, osteomyelitis can be categorized into several types, among which hematogenous osteomyelitis, post-traumatic osteomyelitis, and contiguous spread osteomyelitis are the most prevalent (Ziran, 2007). Pediatric and adult osteomyelitis differ in vascular anatomy, sites, pathogens and presentation: children typically develop acute hematogenous infection in long-bone metaphyses (rich but slow metaphyseal flow across an open growth plate), with abrupt fever and pain; adults more often have vertebral, post-traumatic or diabetic foot involvement, insidious onset, frequent biofilm and polymicrobial (including Gram-negative) infection, and higher chronicity and comorbidity burden (e.g., diabetes, peripheral vascular disease) (Birt et al., 2017).
The pathogenesis of osteomyelitis is highly complex, involving multiple interconnected processes such as microbial infection, inflammatory responses, bone destruction, and host immune reactions (Masters et al., 2019). S. aureus is the most prevalent bacterial pathogen responsible for osteomyelitis (Kavanagh et al., 2018; Urish and Cassat, 2020; Chen Y. et al., 2023). When bacteria invade bone tissue via the bloodstream, trauma, or contiguous spread from adjacent tissues, they initially colonize the bone marrow cavity and proliferate extensively. The toxins and enzymes released by these bacteria subsequently destroy surrounding osteoblasts and the bone marrow stroma, thereby triggering inflammatory responses (Granata et al., 2022; Johnson et al., 2023). Inflammatory cells, including neutrophils and macrophages, rapidly accumulate at the site of infection and secrete a variety of proinflammatory mediators—such as tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), and interleukin-6 (IL-6)—which amplify the inflammatory response and resulting in local hyperemia, edema, and pain. As inflammation progresses, bone destruction is further exacerbated (Kang et al., 2018). With persistence, an immune-evasive milieu emerges. Biofilm matrix (PNAG, extracellular DNA) and agr down-regulation reduce PAMP exposure, while small-colony variants shift metabolism and dampen ROS triggers. S. aureus engages TLR2-PI3K/Akt-STAT3 and MAPK pathways to elevate IL-10, and induces TGF-β, skewing macrophages toward an M2/repair phenotype (Arg1, SOCS3, reduced iNOS) (Branquist and Kielian, 2025; Rasquel-Oliveira et al., 2025). These cytokines expand FOXP3+ regulatory T cells and upregulate PD-1/PD-L1, restraining Th1/Th17 effector activity. Concomitantly, MDSCs accumulate, secreting IL-10 and depleting L-arginine, further impairing neutrophil and T-cell function. Collectively, spatial sequestration (deep foci, biofilm) and these immunosuppressive circuits reduce antibiotic and immune penetration, rendering eradication difficult (Lindsay et al., 2021).
Current treatment strategies for osteomyelitis mainly comprise surgical debridement, systemic antibiotic therapy, and local drug delivery (Masters et al., 2022), as illustrated in Figure 1. Nevertheless, each of these modalities is associated with significant clinical challenges. Surgical debridement is a cornerstone in managing osteomyelitis, aiming to remove necrotic tissue, sequestra, and foreign bodies to reduce bacterial load and establish a favorable environment for subsequent therapies (Zhou et al., 2021). However, this procedure is invasive and may inadvertently damage adjacent healthy bone and soft tissue, thereby adversely affecting bone healing and limb function. Systemic antibiotic therapy, delivered orally or intravenously, remains fundamental to treatment. However, owing to poor vascularization at infected bone sites, achieving and maintaining effective drug concentrations is often challenging, resulting in suboptimal therapeutic outcomes (Mills et al., 2018). Moreover, the widespread use of antibiotics has led to the emergence of resistant bacterial strains, further diminishing the efficacy of conventional antimicrobial agents. Local antibiotic delivery aims to achieve high drug concentrations at the infection site while minimizing systemic side effects. Although elevated local antibiotic concentrations can effectively control infection, they may also exert cytotoxic effects on surrounding tissues, interfere with new bone formation, and ultimately impair bone repair and regeneration (Mulazzi et al., 2021; Mills et al., 2024).
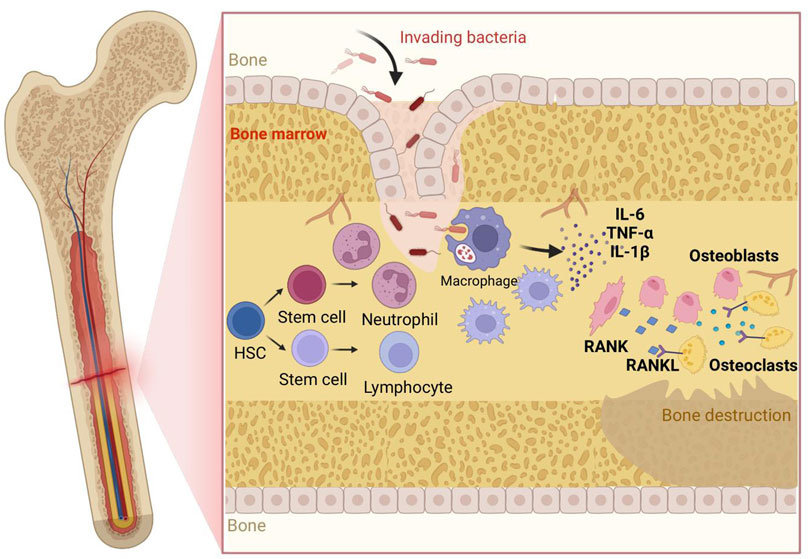
Figure 1. Pathogenesis of osteomyelitis:Bacteria invade bone/marrow, adhere and initiate (pre-)biofilm formation, driving rapid neutrophil influx; macrophages polarize to M1 releasing IL-1β, IL-6, TNF-α, amplifying inflammation and—together with lymphocyte/stromal cues—upregulating RANKL, which engages RANK on hematopoietic precursors to generate active osteoclasts; heightened resorption plus impaired osteoblast function yields bone destruction; persistent inflammatory/biofilm signals suppress MSC osteogenesis and prolong immune activation, worsening loss. Abbreviations: IL-6, Interleukin-6; TNF-α, Tumor Necrosis Factor-α; IL-1β, Interleukin-1β; RANK, Receptor Activator of Nuclear Factor κB; RANKL, Receptor Activator of Nuclear Factor κB Ligand; HSC, Hematopoietic Stem Cell; OC, Osteoclast; OB, Osteoblast; MSC, Mesenchymal Stem Cell; BM, Bone Marrow; Neu, Neutrophil; Mac (Mφ), Macrophage; Lym, Lymphocyte; EPS (optional), Extracellular Polymeric Substance; ROS (optional), Reactive Oxygen Species.
3 Strategies for nanomaterial mediated antibiotics
3.1 Types of nanocarriers
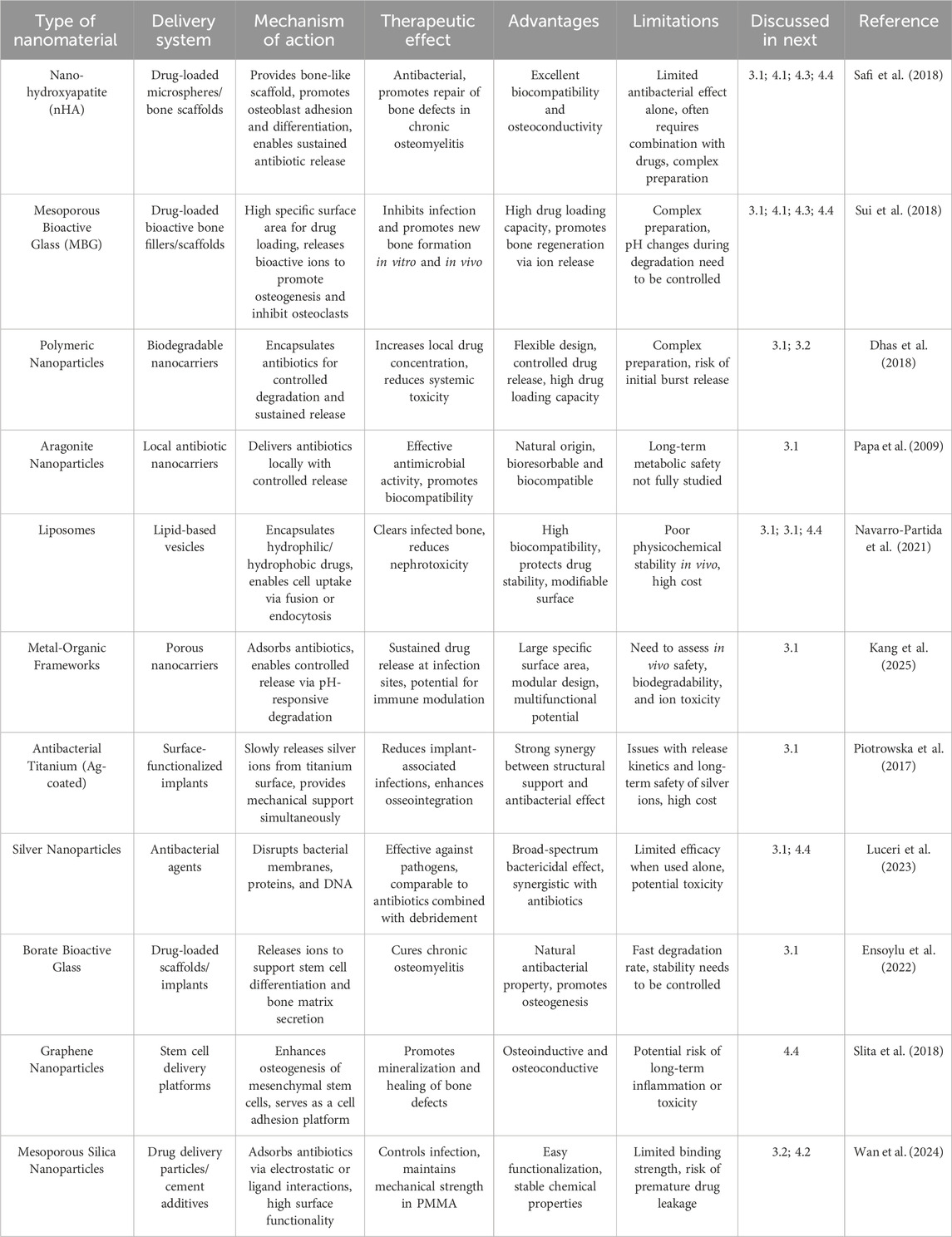
Types of Nanomaterials and Their Applications in Osteomyelitis Therapy. In the context of osteomyelitis treatment, a variety of nanomaterials have emerged as promising antibiotic carriers owing to their unique physicochemical properties and biological activities, thereby offering new opportunities to enhance therapeutic efficacy. Common nanomaterials are shown in Figure 2.
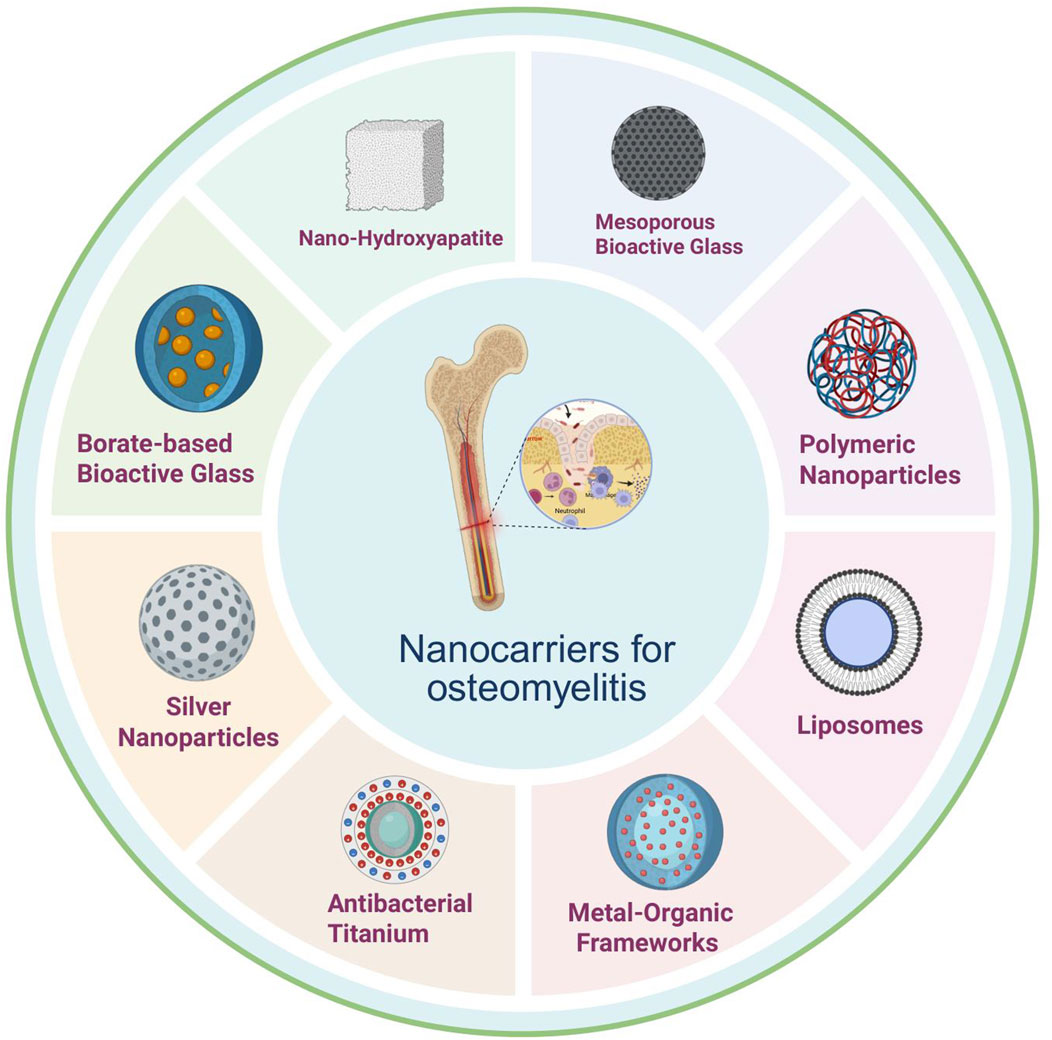
Figure 2. Nanodelivery materials in osteomyelitis: principal classes include nano-hydroxyapatite, silver nanoparticles, metal–organic frameworks, liposomes, antibacterial titanium, borate-based bioactive glass, mesoporous bioactive glass, and polymeric nanoparticles, offering combinable advantages in localized high loading and controlled release, antimicrobial/biofilm disruption, osteoconduction/osteogenesis support, structural reinforcement, and surface functionalization capacity. Abbreviations: nHA, nano-hydroxyapatite; AgNPs, silver nanoparticles; MOFs, metal-organic frameworks; Lipo, liposomes; Ti (antibacterial Ti), antibacterial titanium (surface-modified titanium); BBG (or B-BG), borate-based bioactive glass; MBG, mesoporous bioactive glass; PNPs, polymeric nanoparticles. (Optional if used elsewhere: EPS, extracellular polymeric substance; ROS, reactive oxygen species).
Nano-hydroxyapatite (nHA) is a nanomaterial that closely resembles the inorganic component of human bone in chemical composition. It exhibits excellent biocompatibility and osteoconductivity, promoting the adhesion, proliferation, and differentiation of osteoblasts, thereby providing an ideal scaffold for bone repair and regeneration (Hassan et al., 2016). Studies have demonstrated that vancomycin-loaded nHA, when combined with polylactic acid, is effective in the treatment of chronic osteomyelitis. This approach not only eradicates bacteria but also promotes the repair of bone defects and improves treatment success rates (Lv et al., 2022). To avoid rapid degradation of growth factors and minimize antibiotic-related side effects, a dual-controlled release microsphere scaffold system based on nHA has been developed, which further facilitates bone regeneration (Zhan et al., 2024).
Mesoporous bioactive glass (MBG) is a novel bioactive material distinguished by a highly ordered mesoporous structure and large specific surface area, features that enable efficient antibiotic loading (Guduric et al., 2021). Upon degradation in vivo, MBG releases bioactive ions that significantly stimulate bone regeneration by promoting the proliferation and differentiation of osteoblasts while simultaneously inhibiting osteoclast activity (Jia et al., 2017; Zhao et al., 2023). Studies have demonstrated that antibiotic-loaded MBG can effectively suppress infection, promote bone tissue regeneration, and attenuate inflammation in the treatment of osteomyelitis (Rahaman et al., 2014; Aurégan and Bégué, 2015; Kojima et al., 2021). Among the various types of MBG, S53P4 is the most widely utilized and has achieved excellent clinical outcomes in multiple studies (Romanò et al., 2014; Ferrando et al., 2017), exhibiting both effective local infection control and enhanced bone regeneration. In a rat tibial defect osteomyelitis model, vancomycin-loaded MBG granules (S53P4 base) reduced S. aureus counts by approximately 2 log compared with systemic therapy alone and enhanced new bone volume (Zhang P. et al., 2023). Clinically, S53P4 bioactive glass achieved infection eradication rates of 85%–92% in chronic osteomyelitis cohorts after debridement, comparable or superior to antibiotic PMMA beads while supporting bone regeneration (Lindfors et al., 2010).
Polymeric nanoparticles, synthesized from either natural or synthetic polymers, offer considerable design flexibility and a variety of drug loading mechanisms. These nanoparticles can incorporate antibiotics through physical encapsulation or chemical conjugation, thereby enabling stable drug loading and sustained release (Chopra et al., 2021). For example, poly (lactic-co-glycolic acid) (PLGA) copolymer nanoparticles exhibit excellent biodegradability and controlled drug release properties, allowing for slow degradation and sustained antibiotic release in vivo. This helps maintain therapeutic drug concentrations at the infection site and reduces the frequency of drug administration (Shakya et al., 2023; Lehner et al., 2025). Vancomycin-loaded aragonite nanoparticles (VANP) have also been investigated for osteomyelitis treatment (Saidykhan et al., 2016). These studies validate VANP as a promising candidate for the development of topical antibiotic delivery systems against osteomyelitis, demonstrating optimal antimicrobial efficacy, favorable bone resorption characteristics, and good biocompatibility.
Liposomes are vesicular structures composed of phospholipid bilayers, exhibiting excellent biocompatibility and biodegradability. They are capable of encapsulating both hydrophilic and hydrophobic antibiotics, thereby protecting the drugs from environmental degradation and enhancing its stability (Wang et al., 2019; Dymek and Sikora, 2022). The surfaces of liposomes can be functionalized to achieve active targeting, thereby increasing antibiotic accumulation at infection sites. Furthermore, liposomes can enter cells via membrane fusion or endocytosis, facilitating intracellular drug delivery and enhancing the eradication of intracellular pathogens (Zhang et al., 2018; Jose et al., 2019; Chen et al., 2021).
Metal-organic frameworks (MOFs) are porous materials through the self-assembly of metal ions or clusters with organic ligands. They are characterized by high tunability, large porosity, and high specific surface area, providing an ideal platform for efficient loading and controlled release of antibiotics (Qin et al., 2023). ZIF-8, a representative MOF, can encapsulate various antibiotics through coordination or physical adsorption (Zhu et al., 2024). In the treatment of osteomyelitis, antibiotic-loaded ZIF-8 can be slowly degraded in the micro-acidic environment of the infected site, enabling continuous antibiotic release for precise bacterial eradication (Shao et al., 2024). Additionally, MOF materials can be further enhanced by functional modification of organic ligands to confer additional functions, such as immunomodulation, thereby further improving therapeutic effect (Ge et al., 2022). In a murine S. aureus femoral osteomyelitis model, vancomycin@ZIF-8 exhibited pH-triggered release, lowering bacterial burden and preserving trabecular architecture versus free vancomycin (Liang et al., 2025).
In addition to the aforementioned nanocarrier systems, several specialized materials and technologies have also demonstrated significant therapeutic potential. Antibacterial titanium materials, for example, can be functionalized with silver ions via surface modification techniques, enabling the slow release of silver ions to inhibit bacterial growth (Macwan et al., 2017). These materials can exert antibacterial effects independently or be combined with antibiotics to achieve synergistic antibacterial activity, thereby significantly reducing the incidence of implant-associated osteomyelitis. Additionally, silver nanoparticles, exhibit strong inhibitory effects against pathogens. In vivo biodistribution and safety: Systemically or locally released AgNPs display size- and surface chemistry-dependent accumulation primarily in liver and spleen, with secondary deposition in lung and bone marrow (Jiang et al., 2020; Kumar et al., 2023). Partial oxidative dissolution liberates Ag+, which binds thiol-rich proteins and is progressively transformed into Ag2S or Ag2Se, decreasing acute ionic reactivity (Zhang et al., 2022). Therapeutic local concentrations generally spare osteoblast viability, whereas higher or repeated exposures induce mitochondrial ROS, inflammatory mediator upregulation, and delayed bone remodeling (Castiglioni et al., 2017). Monitoring cumulative silver burden and oxidative stress biomarkers is advisable for prolonged or repeated applications. However, when used alone, their therapeutic efficacy is comparable to that of classical antibiotics combined with local surgical debridement, suggesting that their synergistic effects with other therapeutic modalities should be comprehensively considered in clinical applications (Ip et al., 2022). AgNP-coated titanium or chitosan-stabilized AgNP composites curtailed S. aureus biofilm formation and reduced bacterial load in rabbit or rat long-bone infection models while maintaining acceptable local tissue responses (Chen X. et al., 2023). Borate glass, as a novel bioactive material, is capable of releasing ions to support proliferation, differentiation, osteogenic gene expression, and protein secretion of human bone marrow mesenchymal stem cells (Kędziora et al., 2020). In a rabbit infected bone defect model, antibiotic-loaded borate bioactive glass scaffolds facilitated rapid ionic (B, Ca) release, suppressed S. aureus colonization, and promoted vascularized new bone formation relative to inert carriers (Zhang et al., 2010; Jia et al., 2015). The ongoing development and application of these materials and technologies provide more options and possibilities for the treatment of osteomyelitis.
3.2 Construction of nano-antibiotic carriers
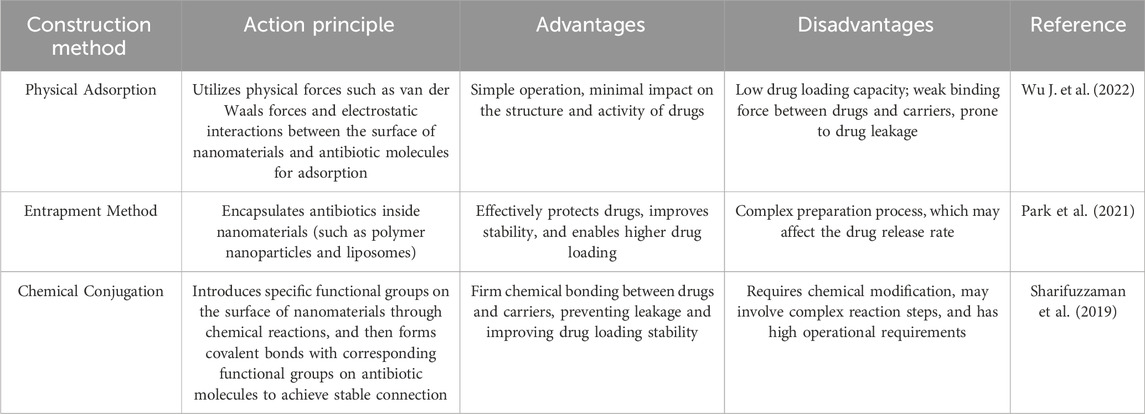
The construction of efficient nano-antibiotic carrier systems is a crucial step toward achieving nanomaterial-mediated antibiotic delivery. This process involves a variety of physical and chemical methods, as summarized in Table 1. The goal is to achieve stable binding of antibiotics to nanomaterials while ensuring that the carrier systems demonstrate optimal performance.
Physical adsorption is one of the simplest approaches, in which antibiotics are attached to the surface of nanomaterials through non-covalent interactions such as van der Waals forces and electrostatic attraction. For instance, mesoporous silica nanoparticles possess abundant surface silanol groups (Saeting et al., 2022), which confer hydrophilicity and surface charge, enabling the adsorption of oppositely charged antibiotic molecules via electrostatic interactions. Surface functionalization can markedly enhance adsorption: acid-induced carboxylation of carbon nanotubes adds-COOH groups to bind cationic aminoglycosides; amination of silica with APTES introduces-NH3+ sites favoring anionic β-lactams; PEI modification of graphene oxide increases positive charge and available area, often raising loading 2-3 fold (Alosime, 2023). In one study, silica nanoparticles were used to adsorb interleukin-13 (IL-13), which successfully directed macrophage polarization both in vitro and in vivo, and alleviated experimental autoimmune encephalomyelitis (Zhang H. et al., 2023). Physical adsorption is easy to implement and minimally affects the structure and bioactivity of the drug. However, it typically results in relatively low drug loading and weak interactions between the drug and carrier, thereby increasing the risk of drug leakage during transport.
Encapsulation, or entrapment, is a method in which antibiotics are enclosed within the core of nanomaterials and is commonly used in the preparation of polymeric nanoparticles and liposomes. For example, polymeric nanoparticles made from poly (lactic-co-glycolic acid) (PLGA) are typically synthesized using an emulsion-solvent evaporation or nanoprecipitation technique (Gonzalez Gomez et al., 2019). In the emulsion-solvent evaporation method, PLGA is dissolved in an organic solvent and mixed with an aqueous solution of the antibiotic to form an oil-in-water (O/W) emulsion through vigorous stirring or ultrasonication. As the organic solvent evaporates, PLGA solidifies within the aqueous phase, thereby entrapping the antibiotic and forming nanoparticles. This strategy offers effective protection for the drug and improves its stability, while also allowing for relatively high drug loading efficiency. Nonetheless, the preparation process is more complex and may affect the drug release profile (Wang et al., 2025).
Chemical conjugation refers to the formation of covalent bonds between antibiotics and surface-functionalized nanomaterials. Representative reactions include EDC/NHS-mediated amide coupling (generally retaining 80%–95% activity), glutaraldehyde Schiff base cross-linking (possible 20%–40% activity loss), and bioorthogonal click chemistry (CuAAC) for high specificity. Cleavable linkers (disulfide, hydrazone) can be introduced to preserve function and enable triggered release (Girardot and Girardot, 1996; Sionkowska et al., 2024). Functional groups, such as amines or carboxyls, are incorporated onto nanoparticle surfaces, and cross-linking agents are used to covalently attach antibiotics to the carriers (Gonzalez Gomez et al., 2019). Choice of chemistry balances conjugation stability with preservation of antibiotic active sites. This strategy establishes robust chemical linkages between the drug and the carrier, effectively preventing premature drug release and markedly improving drug retention and delivery stability.
3.3 Principles of nanomaterial-mediated antibiotic delivery
Nanomaterials exhibit unique physicochemical properties that distinguish them fundamentally from conventional bulk materials, largely owing to their size-dependent and surface-dominated effects (Yi et al., 2024). These attributes profoundly influence drug adsorption, release, and targeting efficiency (Biesold et al., 2021). At the nanoscale, quantum confinement leads to changes in electronic energy states, which in turn modulate the optical, electrical, and magnetic behaviors of the materials. In drug delivery applications, small-sized nanoparticles can more easily penetrate biological barriers such as capillary walls and cellular membranes, thereby facilitating enhanced cellular uptake of therapeutic agents. Research indicates that nanoparticles with diameters smaller than 100 nm can enter cells via endocytosis, enabling intracellular drug delivery (Naruphontjirakul et al., 2018). Moreover, the surface properties of nanomaterials play a crucial role in drug delivery. Surface charge, hydrophilicity or hydrophobicity, and other physicochemical attributes can be precisely engineered through surface modification to achieve active targeting of specific cells or tissues. For instance, nanoparticles decorated with tumor-specific antibodies can selectively recognize and bind to cancer cells, thereby promoting preferential drug accumulation at tumor sites (Torrano et al., 2016; Maparu et al., 2022). Extending this principle to infectious/inflammatory niches, folate-decorated nanoparticles exploit overexpressed folate receptor-β (FRβ) on activated inflammatory macrophages, enhancing macrophage uptake and enabling localized delivery of antibiotics (e.g., vancomycin, rifampicin) within osteomyelitic lesions (Thomas et al., 2011). Likewise, anti-Staphylococcus aureus antibody-functionalized polymer nanoparticles exhibit selective adhesion to S. aureus biofilms and improve antibiotic concentration and biofilm disruption compared with non-targeted carriers, illustrating the utility of pathogen- or biofilm-specific immunoligands in augmenting local therapeutic efficacy (Aflakian et al., 2023).
Nanomaterial-mediated antibiotic delivery is a sophisticated and multifaceted process, the core of which lies in the precise encapsulation, transport, and targeted release of antibiotics at osteomyelitis infection sites (Hassani Besheli et al., 2017). Following encapsulation, nanocarriers can be administered either systemically or locally. To improve transport efficiency and stability in vivo, surface engineering strategies are frequently utilized (Cevc, 2004). Once the nanocarriers reach the infection site, targeted antibiotic delivery is accomplished through a combination of passive and active targeting mechanisms.
The advantages of nanomaterial-mediated antibiotic delivery are considerable. Conventional systemic antibiotic therapies often struggle to achieve therapeutic drug concentrations at osteomyelitic lesions and may result in unintended harm to healthy tissues. In contrast, nanocarriers can specifically direct antibiotics to infection site, enabling localized therapy, reducing systemic toxicity, and sparing adjacent healthy tissues from damage. In addition, nanocarriers provide controlled and sustained drug release, thereby overcoming the burst release issues associated with conventional carriers (Gounani et al., 2019; Birk et al., 2021). This capability allows for precise modulation of drug release kinetics and duration, ultimately improving therapeutic efficacy, minimizing drug wastage, and lowering overall treatment costs.
4 Delivery systems in osteomyelitis
This section focuses on functional strategies for the treatment of osteomyelitis–single antibiotic delivery, synergistic/multimodal therapy, immunomodulation, and bone repair. For each functional strategy, we summarize the most commonly applied material categories, compare their performance, and highlight conversion potential. The nano-delivery system has been applied in various ways in osteomyelitis, mainly through a single delivery method and synergistic delivery to regulate the immune system and promote bone repair, as shown in Figure 3.
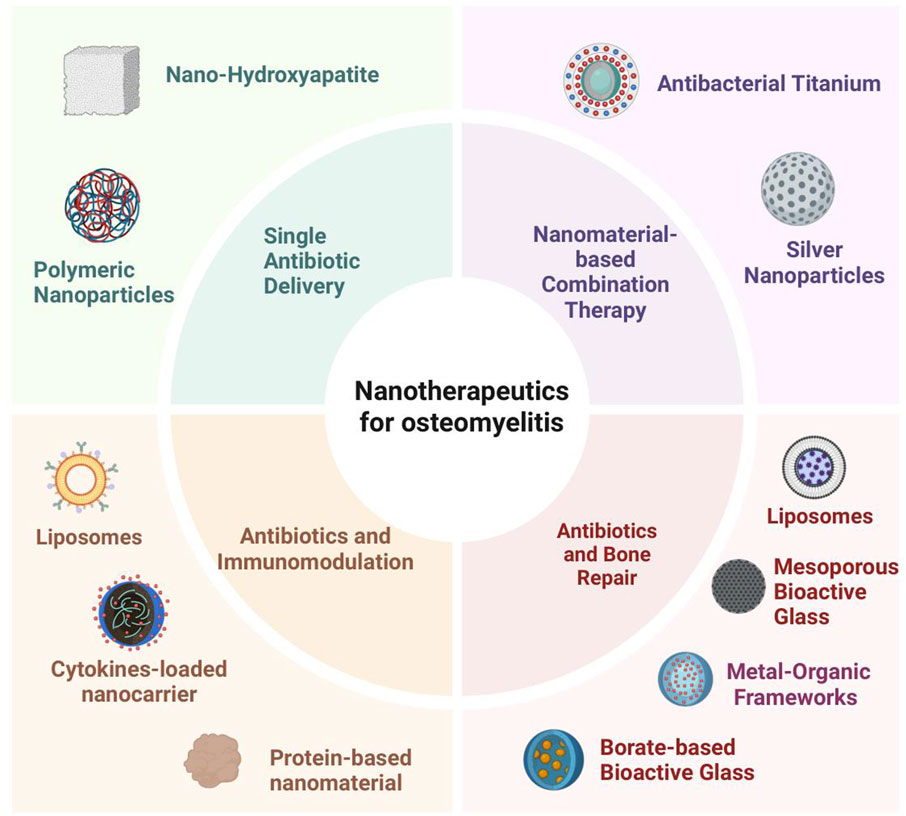
Figure 3. Application of nanodelivery systems in osteomyelitis: single antibiotic delivery, combination therapy, bone repair adjunct, and immunomodulation. Carriers include nHA, AgNPs, MOFs, liposomes, Ti, BBG, MBG, PNPs, protein- and cytokine-based systems.
4.1 Single antibiotic delivery
Conventional antibiotic delivery methods, such as oral and intravenous administration, face significant challenges in overcoming the physiological barriers of bone tissue during osteomyelitis treatment. Research indicates that, following traditional administration, antibiotic concentrations in bone tissue reach only 20%–30% of those in the bloodstream, which is insufficient to effectively eradicate bacteria embedded within biofilms. Moreover, frequent dosing increases the risk of drug accumulation in the body, potentially leading to adverse effects such as hepatic and renal toxicity (Thabit et al., 2019).
Degradable polymer carriers excel in the delivery of single antibiotics. Nanoparticles prepared from polylactic acid-hydroxyacetic acid copolymer (PLGA) can encapsulate antibiotics to achieve slow release lasting for weeks, such as vancomycin encapsulated in PLGA nanoparticles, which significantly increased the drug concentration at the site of infection and reduced systemic toxicity in animal experiments (Zirak et al., 2022).
Studies have shown that nano-antibiotic carrier systems are highly effective at suppressing bacterial proliferation. For example, Mohd Zaffarin et al. (2021), Padrão et al. (2021) a nanocarrier platform constructed by incorporating vancomycin into nanohydroxyapatite (nHA) exhibited pronounced antibacterial activity against major osteomyelitis pathogens, including S. aureus and E. coli.
Cytotoxicity assays serve as critical metrics for evaluating the safety of nano-antibiotic carrier systems. Evidence from various studies indicates that many nanomaterials exhibit excellent biocompatibility and minimal cytotoxicity. For example, copper-doped MBG nanoparticles loaded with antibiotics have been shown to exert negligible effects on the viability of normal cells, including fibroblasts and osteoblasts (Wang et al., 2016).
Comparative pharmacokinetic data show that nano/local systems markedly improve bone exposure versus free systemic drug: free vancomycin reaches only ≈20–30% of plasma levels in bone and falls below MIC within 6–8 h, whereas PLGA nanoparticles raise bone Cmax ≈2–3-fold and extend t1/2 to >24 h (Pisani et al., 2024); thermosensitive PLGA-PEG-PLGA gels maintain local concentrations >4 × MIC for 5–7 days (Yuan et al., 2022); PMMA or mesoporous silica-PMMA composites give an initial high local burst (>1,000 μg/mL eluate, first 24 h) followed by ≥ 10–20 × MIC for 1–3 weeks with low plasma levels; nHA or liposomal gentamicin similarly increases bone AUC (≈1.5–2×) while reducing renal accumulation (Rocha et al., 2022). These profiles collectively improve bone/plasma ratios, sustain antibacterial levels in biofilm niches, and lower systemic toxicity.
4.2 Nanomaterial synergistic therapy
Antimicrobial titanium materials achieve bactericidal functionality in titanium alloys via advanced surface modification techniques. Incorporating silver ions onto the titanium surface enables their gradual release, thereby suppressing bacterial proliferation. Combining antibiotics with antimicrobial titanium provides a dual antimicrobial effect. For example, Akshaya et al. (2022) coating the surface of titanium implants with antibiotic-loaded coatings effectively reduces the incidence of implant-associated osteomyelitis by both utilizing the mechanical properties of titanium to support bone tissue and by synergistically sterilizing the bone with antibiotics and silver ions.
Photothermal/photoacoustic nanoplatforms (e.g., polydopamine or black phosphorus coatings with vancomycin) reach 50 °C–55 °C under 808 nm irradiation, disrupt biofilm matrix, achieve additional 2-3 log10 CFU reductions versus antibiotic alone, and permit imaging-guided, on-demand release using gold nanorods or carbon dots, while avoiding collateral thermal damage (Mammari and Duval, 2023).
Several clinical trials investigating nanomaterial-mediated antibiotic delivery for osteomyelitis treatment are currently in progress. One research group assessed the efficacy of drug-loaded microporous nanohydroxyapatite beads in managing chronic osteomyelitis. In their study, vancomycin, gentamicin, and ceftriaxone were incorporated into the microporous nanohydroxyapatite beads at a 1:1:1 ratio to fill bone defects. This localized delivery approach proved effective in controlling infection and maintaining elevated local antibiotic concentrations for one to 6 weeks postoperatively (Jiamton et al., 2023). Evidence suggests that composite multifunctional antibiotic carriers based on nanoparticles can reduce or even eliminate the need for prolonged, repeated, and systemic antibiotic administration, as well as minimize or obviate surgical debridement of necrotic tissue (Uskokovic, 2015).
To facilitate antibiotic release, a novel micro-composite implant has been engineered for osteomyelitis management following total joint replacement. This approach involves incorporating drug-loaded mesoporous silica nanoparticles into polymethylmethacrylate (PMMA) bone cement, thereby enabling controlled antibiotic release. Such implants are capable of effectively eradicating infections and reducing the emergence of new drug-resistant bacterial strains, while preserving the spatial integrity and mechanical strength essential for joint fixation (Inceoglu et al., 2021). Similarly, PMMA bone cement is the clinical gold standard biomaterial for localized antibiotic therapy in osteomyelitis, and there are studies for slow and slow release of antibiotics and the release kinetics of antibiotics (Rajendran et al., 2021). Antibiotic-loaded PMMA also reduces the incidence of osteomyelitis infections and can be a useful option for long-term pharmacologic treatment of osteomyelitis (Wentao et al., 2017).
In order to address the side effects and resistance of antibiotics and to explore some local drug delivery systems for the treatment of osteomyelitis, some studies have prepared PLGA-PEG-PLGA copolymers, which are capable of slow-releasing antibiotics and promoting bone tissue repair, and have a wide range of potential for clinical applications (Yuan et al., 2022).
Immunomodulatory synergy further enhances outcomes: vancomycin-loaded mesoporous bioactive glass releasing Si/Ca ions shifts macrophages toward M2, lowers TNF-α/IL-6, and elevates osteogenic markers (ALP, RUNX2); antibiotic + IL-4 microsphere or folate-targeted M2-polarizing systems add ∼1–1.5 log10 extra CFU reduction and improve bone volume fraction compared with antibiotic alone (Rahaman et al., 2023).
4.3 Antibiotics and immunomodulation
The development of osteomyelitis is intimately linked to the host immune response. The inflammatory response triggered by bacterial infection activates the immune system, but an excessive immune response can also lead to bone tissue damage, while the presence of a bacterial biofilm evades recognition and clearance by the immune system, making the infection difficult to control (Wu S. et al., 2022). The pathogenesis of osteomyelitis is complex, and bacterial invasion of bone triggers an immune response in the body. Macrophages, as important members of the immune system, play a key role in the osteomyelitis process. Consequently, modulating the immune response is essential for effective osteomyelitis treatment.
The combination of immunomodulators and antibiotics offers a promising strategy to regulate immune homeostasis and enhance antimicrobial efficacy. For example, interferon-γ can enhance the phagocytosis of macrophages and promote their bacterial killing effect, which can improve the therapeutic effect when used in combination with antibiotics (Pomposello et al., 2020). In addition, anti-inflammatory cytokines such as interleukin-10 can mitigate excessive inflammatory responses and minimize bone tissue damage, demonstrating therapeutic potential for both bone regeneration and bone repair (Chen et al., 2025).
Nanomaterial-loaded antibiotics for the treatment of osteomyelitis offer distinct advantages. Nanomaterials can enhance the accumulation of antibiotics at infection sites, enable sustained and controlled drug release, maintain effective local drug concentrations, and thereby improve antimicrobial efficacy. Additionally, nanomaterials themselves can participate in immune modulation, influencing macrophage polarization. For example, nHA particles are capable of M2-type polarization of macrophages (Mahon et al., 2020). M2 macrophages secrete anti-inflammatory cytokines, such as IL-10, which help attenuate inflammation and facilitate tissue repair. The successful repair of bone defects by this functionalized scaffold in a rat femoral defect model confirms the role of nanomaterials in improving the local immune microenvironment of osteomyelitis and promoting bone healing by modulating macrophage polarization.
In terms of immunomodulation, nanomaterial-loaded antibiotics can also influence the crosstalk between osteoblasts and immune cells. For instance, one study developed a nanodrug delivery system incorporating silicon ions, in which MBG was coated onto nanohydroxyapatite carriers and loaded with vancomycin. In a chronic osteomyelitis animal model, this system not only effectively inhibited infection, but the presence of silicon ions also attenuated the cytotoxic side effects of vancomycin and enhanced bone regeneration at the infection site. These findings suggest that nanomaterial-based antibiotic delivery systems can modulate the immune microenvironment, thereby creating favorable conditions for osteoblast function and promoting bone tissue repair (Xu et al., 2021).
Nanomaterial-loaded antibiotics can positively affect the immune regulation by regulating macrophage polarization and influencing the interaction between osteoblasts and immune cells in the treatment of osteomyelitis, which opens up a new path for osteomyelitis treatment, and is expected to achieve better therapeutic effects in clinical applications.
4.4 Antibiotics and bone repair
The treatment of osteomyelitis requires addressing two major challenges simultaneously: effective infection control and bone repair. Traditional therapies often struggle to balance both aspects, leading to prolonged treatment durations and suboptimal prognoses (Ismat et al., 2021). Recently, integrated strategies combining antibiotics with bone regeneration have become a research focus, overcoming the limitations of conventional approaches. By leveraging innovative materials and technologies, these strategies seamlessly unite infection control with bone tissue regeneration, offering new perspectives and hope for the management of osteomyelitis and other complex bone diseases. This integrated approach is poised to transform the landscape of bone disease treatment.
In terms of infection control, the nano-antibiotic carrier system has demonstrated strong bactericidal ability. For example, borate glass has been developed as a local drug delivery vehicle for osteomyelitis treatment. In a rabbit tibial chronic osteomyelitis model, the ions released from borate glass were shown to support the proliferation, differentiation, osteogenic gene expression, and protein secretion of human bone marrow mesenchymal stem cells in vitro. Furthermore, after implantation into a rabbit tibia osteomyelitis model, the infection was effectively eradicated, and significant bone regeneration was observed after 12 weeks Nanomaterial-mediated antibiotic delivery (Egawa et al., 2020). This illustrates the dual functionality of such systems, providing both robust infection control and enhanced bone repair.
Nanomaterial-mediated delivery of antibiotics also promotes bone repair. At the cellular/molecular level, antibiotic-loaded nanoscaffolds increase early ALP activity, mineralized nodule formation, and upregulate RUNX2, COL1A1, OPN, OCN; ion-releasing (Si/Ca/B/Cu) platforms additionally elevate VEGF, linking angiogenesis with bone regeneration (Dousti et al., 2024; Lin et al., 2024).
For instance, one study developed an antibiotic-eluting collagen-hydroxyapatite scaffold, forming a layered dual-release system that could both eliminate infection and facilitate bone healing. In a mouse osteomyelitis model, vancomycin-eluting scaffolds significantly reduced S. aureus colonization in the tibia. Similarly, in a rabbit model of chronic osteomyelitis, these scaffolds promoted the healing of radial bone defects while eradicating S. aureus infection (Sheehy et al., 2025). In addition, graphene nanoparticles combined with mesenchymal stem cells have been shown to enhance cell differentiation, stimulate active bone formation, increase mineralization, and improve the repair of tibial bone defects in rats. These findings underscore the potential of nanomaterial-based delivery systems to integrate infection control with effective bone regeneration (Elkhenany et al., 2017).
There are also studies using liposome-encapsulated antibiotics to treat rabbit osteomyelitis models (Kadry et al., 2004). The results showed that co-treatment of bone tissue in the form of liposomes completely sterilized the bone tissue, and the liposomal formulation showed a lower incidence of nephrotoxicity and severe diarrhea than free drug treatment. There have also been studies using silver nanoparticles to treat a model of osteomyelitis in the tibia of rats and found that silver nanoparticles were effective against the pathogen, but that the use of nanosilver was close to the effectiveness of osteomyelitis treatment with a combination of classical antibiotics and local surgical debridement (Kemah et al., 2018).
Bioactive glass has natural antimicrobial properties and can inhibit the growth of osteomyelitis-causing bacteria even without the addition of antibiotics, in addition, drug-carrying bioactive glass showed good antimicrobial effects and bone regeneration in animal models (Zapata et al., 2022).
The effect of nano-antibiotic carrier system on osteoblast differentiation is also one of the focuses of research, certain nanomaterials can not only load antibiotics, but also promote the differentiation and proliferation of osteoblasts, for example, Xu et al. (2021) the nano-loading system constructed by wrapping MBG containing silica ions around nHA-loaded vancomycin can significantly increase the activity of alkaline phosphatase (ALP) in osteoblasts, and promote the calcium nodule Formation. Collectively, a cascade emerges: localized antibiotic release clears infection, attenuates excessive inflammation, re-polarizes macrophages, and permits sustained activation of osteogenic programs culminating in accelerated matrix mineralization and structural bone regeneration (Gou et al., 2024).
5 Challenges and prospects
Nanomaterial-mediated antibiotic delivery has brought about revolutionary changes in the treatment of osteomyelitis, demonstrating significant research significance and enormous application potential. Common nanomaterials for antibiotic delivery are shown in Table 2. Nanomaterial-mediated antibiotic delivery for osteomyelitis treatment faces many challenges and solutions.
The safety of nanomaterials is a primary concern, including potential risks in terms of cytotoxicity, immunogenicity, and long-term in vivo metabolism, which require in-depth studies to ensure their safety for human applications (Lee et al., 2019; Lu and Fan, 2024; Wang et al., 2024). Current toxicological evaluation incorporates: (1) acute/sub-chronic toxicity (MTD, NOAEL) in rodent models (Guo et al., 2022); (2) organ biodistribution/accumulation via ICP-MS or fluorescence (liver-spleen transient uptake with PLGA or black phosphorus clearing within 14–28 days; persistent silver >10 mg/kg may induce mitochondrial swelling) (Mohammadpour et al., 2019); (3) histopathology (H&E), serum biochemistry (ALT, AST, BUN, creatinine), hematology, hemolysis, complement activation (CH50), cytokine panels (IL-6, TNF-α, IL-1β), macrophage polarization profiling (CD86/CD206), genotoxicity (Ames, micronucleus), and immunogenicity (anti-carrier antibody ELISA); representative vancomycin–PLGA or nHA systems show no significant elevation of hepatic/renal markers and reversible mild splenic macrophage loading (Parisi et al., 2018).
Preparation process and cost issues are also important factors limiting their wide application. Currently, the large-scale preparation technology of nanomaterials is still not perfect and the cost is high, which hinders their clinical promotion (Zhang X. et al., 2023). Mainstream scalable fabrication routes include nanoprecipitation, emulsification-solvent evaporation, spray drying, microfluidic continuous flow (parallelized chips), supercritical fluid processing, melt extrusion (polymer-drug solid dispersions), sol-gel (bioactive glass), layer-by-layer assembly, and 3D printing of composite scaffolds; approximate pilot costs: PLGA or lipid nanoparticles ≈ USD 5–20/g (carrier) excluding API(Kumar et al., 2023), mesoporous silica ≈ USD 10–30/g, while functional (multi-responsive) constructs can exceed USD 50/g (Tenchov et al., 2025); key scale-up bottlenecks involve batch reproducibility (size/PDI drift), solvent residual compliance (ICH Q3C), sterilization-induced degradation (γ, e-beam), lyophilization stability (cryoprotectant selection), continuous in-line PAT (NIR/DLS analytics), and regulatory CMC definition of complex multi-component systems.
In addition, the effect of nanomaterial-mediated antibiotic delivery on antibiotic resistance is still unclear, and further research is needed to avoid or reduce the emergence of resistance (Wang and Vikesland, 2023). While high local concentrations typically suppress resistance selection, potential new mechanisms include: (a) sub-MIC “tail” release promoting tolerance/persister enrichment; (b) biofilm penetration gradients inducing efflux pump upregulation; (c) co-delivered metal ions (Ag, Cu) co-selecting metal-antibiotic resistance genes; (d) surface-adaptive mutations to persistent nano-bio interfaces; mitigation strategies involve PK/PD modeling to eliminate sub-MIC tails, combinational stimuli (photothermal + antibiotic), periodic triggerable pulses, and longitudinal metagenomic surveillance (mecA, van, bla, efflux operons) over serial passages—early studies show slower MIC shift trajectories versus free-drug regimens when burst + sustained profiles are optimized (Soto, 2013; Hajiagha and Kafil, 2023).
In the future, the research on nanomaterial-mediated antibiotic delivery for osteomyelitis will primarily focus on the development of intelligent nanocarriers, the integration of multimodal therapeutic strategies, and advancing clinical translation (Sadowska et al., 2021; Zarepour et al., 2024; Nguyen Ngoc et al., 2025). The design of intelligent nanocarriers aims to achieve precise regulation and active targeting of drug release, thereby enhancing therapeutic efficacy (Agiba et al., 2024). Representative “smart” systems include pH-responsive ZIF-8/polymer hybrids releasing cargo in acidic infection niches (pH ≈ 6.5), enzyme-responsive gelatin/MMP- or bacterial β-lactamase-cleavable linkers for on-demand gentamicin or vancomycin liberation, ROS-triggered thioketal or boronic ester platforms accelerating release under oxidative bursts, NIR photothermal/photodynamic dual-responsive black phosphorus or polydopamine composites, magnetic or ultrasound-triggered phase-change liposomes enabling spatiotemporal pulses, and multi-responsive injectable hydrogels (recent patents integrating pH + enzyme + photothermal modules) that couple theranostic imaging (photoacoustic/fluorescence) with controlled antibiotic dosing (Gade et al., 2024; Su et al., 2025; Zhang R. et al., 2025). Moreover, combining nanomaterial-based antibiotic delivery with multimodal therapies—such as photothermal therapy, gene therapy, and immunotherapy—can exert synergistic effects, further improving treatment outcomes and reducing the risk of drug resistance (Mammari and Duval, 2023; Sharma et al., 2024). Clinical translation will depend on harmonized characterization standards (size, surface chemistry, release kinetics), validated immunotoxicity panels, accelerated stability protocols, cost-reduction via continuous manufacturing, and early phase adaptive trial designs capturing both infection eradication and bone regeneration endpoints. In parallel, intensified efforts in clinical translational research will address issues related to the safety, quality control, and cost of nanomaterials, facilitating the transition of these technologies from laboratory research to clinical application. Ultimately, these advancements are expected to provide more effective therapeutic options for patients with osteomyelitis (Csóka et al., 2021). Scalable production requires processes that ensure batch-to-batch reproducibility, sterility, and cost-effectiveness. Techniques operating at benchtop scale must be validated for use in large reactors, with attention to solvent removal, residues, and downstream purification, and economic analysis should be considered early to assess the feasibility of widespread clinical deployment.
Nanomaterial-mediated delivery of antibiotics brings new hope for the treatment of osteomyelitis. Despite the challenges, with the deepening of research and the continuous progress of technology, it is expected to make greater breakthroughs in the field of osteomyelitis treatment, improve the quality of life of patients, and reduce the burden on society.
6 Conclusion
Nanomaterial-mediated antibiotic delivery has fundamentally transformed the treatment landscape for osteomyelitis, demonstrating significant research value and tremendous application potential. The traditional treatment of osteomyelitis has many limitations, such as large surgical trauma, numerous side effects of systemic antibiotic therapy, and poor local drug delivery effects. Nanomaterials, owing to their unique physical and chemical properties, offer innovative solutions to these challenges.
The antibiotic delivery strategy mediated by nanomaterials, leveraging its unique physical-chemical characteristics and biological activities, has opened up a brand-new path for osteomyelitis treatment. In terms of therapeutic advantages, nanocarriers can achieve local targeted delivery and controlled release of antibiotics. This not only significantly increases the drug concentration at the infection site, effectively inhibiting bacterial growth and inflammatory responses, but also reduces side effects caused by systemic medication. Meanwhile, the material properties can promote bone tissue repair and regeneration.
Although challenges remain, nanomaterial-mediated antibiotic delivery brings renewed hope to osteomyelitis management. With ongoing research and technological progress, this approach is expected to achieve even greater breakthroughs, improving patient outcomes and quality of life while reducing the societal burden of the disease. Future research priorities: targeted ligands; standardized safety frameworks; PK/PD-guided smart multi-responsive delivery; robust large-animal and adaptive clinical studies with dual endpoints; staged combinational (antimicrobial + immune + pro-angiogenic) strategies; scalable continuous manufacturing plus open data and long-term host/microbiome monitoring.
Author contributions
SC: Writing – original draft, Writing – review and editing. X-HM: Writing – review and editing. ZL: Investigation, Writing – review and editing, Methodology, Project administration. H-HH: Investigation, Writing – review and editing, Methodology, Supervision. Y-FZ: Investigation, Writing – review and editing, Conceptualization, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was financially supported by Nanjing University of Chinese Medicine Natural Science Foundation Project (XZR2024078), China Postdoctoral Science Foundation (2025M773943), Wuxi Science and Technology Innovation and Entrepreneurship Fund Ninth Batch Science and Technology Development Plan Project (K20241024), Wuxi Health Commission Research Project (Q202423), Jiangsu Provincial Clinical Medical Innovation Center for Traditional Chinese Medicine Degenerative Osteoarthritis (2021040501).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdulrehman, T., Qadri, S., Haik, Y., Sultan, A., Skariah, S., Kumar, S., et al. (2024). Advances in the targeted theragnostics of osteomyelitis caused by Staphylococcus aureus. Arch. Microbiol. 206, 288. doi:10.1007/s00203-024-04015-2
Aflakian, F., Mirzavi, F., Aiyelabegan, H. T., Soleimani, A., Gholizadeh Navashenaq, J., Karimi-Sani, I., et al. (2023). Nanoparticles-based therapeutics for the management of bacterial infections: a special emphasis on FDA approved products and clinical trials. Eur. J. Pharm. Sci. 188, 106515. doi:10.1016/j.ejps.2023.106515
Agiba, A. M., Arreola-Ramírez, J. L., Carbajal, V., and Segura-Medina, P. (2024). Light-responsive and dual-targeting liposomes: from mechanisms to targeting strategies. Molecules 29, 636. doi:10.3390/molecules29030636
Akshaya, S., Rowlo, P. K., Dukle, A., and Nathanael, A. J. (2022). Antibacterial coatings for titanium implants: recent trends and future perspectives. Antibiot. (Basel) 11, 1719. doi:10.3390/antibiotics11121719
Alosime, E. M. (2023). A review on surface functionalization of carbon nanotubes: methods and applications. Discov. Nano 18, 12. doi:10.1186/s11671-023-03789-6
Aurégan, J.-C., and Bégué, T. (2015). Bioactive glass for long bone infection: a systematic review. Injury 46 (Suppl. 8), S3–S7. doi:10.1016/S0020-1383(15)30048-6
Biesold, G. M., Liang, S., Brettmann, B., Thadhani, N., Kang, Z., and Lin, Z. (2021). Tailoring optical properties of luminescent semiconducting nanocrystals through hydrostatic, anisotropic static, and dynamic pressures. Angew. Chem. Int. Ed. Engl. 60, 9772–9788. doi:10.1002/anie.202008395
Birk, S. E., Haagensen, J. A. J., Johansen, H. K., Molin, S., Nielsen, L. H., and Boisen, A. (2020). Microcontainer delivery of antibiotic improves treatment of Pseudomonas aeruginosa biofilms. Adv. Healthc. Mater 9, 1901779. doi:10.1002/adhm.201901779
Birk, S. E., Boisen, A., and Nielsen, L. H. (2021). Polymeric nano- and microparticulate drug delivery systems for treatment of biofilms. Adv. Drug Deliv. Rev. 174, 30–52. doi:10.1016/j.addr.2021.04.005
Birt, M. C., Anderson, D. W., Bruce Toby, E., and Wang, J. (2017). Osteomyelitis: recent advances in pathophysiology and therapeutic strategies. J. Orthop. 14, 45–52. doi:10.1016/j.jor.2016.10.004
Branquist, N. D., and Kielian, T. (2025). Immune dysfunction during S. aureus biofilm-associated implant infections: opportunities for novel therapeutic strategies. NPJ Biofilms Microbiomes 11, 144. doi:10.1038/s41522-025-00782-y
Castiglioni, S., Cazzaniga, A., Locatelli, L., and Maier, J. A. M. (2017). Silver nanoparticles in orthopedic applications: new insights on their effects on osteogenic cells. Nanomater. (Basel) 7, 124. doi:10.3390/nano7060124
Cevc, G. (2004). Lipid vesicles and other colloids as drug carriers on the skin. Adv. Drug Deliv. Rev. 56, 675–711. doi:10.1016/j.addr.2003.10.028
Chen, F., Bian, M., Nahmou, M., Myung, D., and Goldberg, J. L. (2021). Fusogenic liposome-enhanced cytosolic delivery of magnetic nanoparticles. RSC Adv. 11, 35796–35805. doi:10.1039/d1ra03094a
Chen, L., Zhu, J., Ge, N., Liu, Y., Yan, Z., Liu, G., et al. (2025). A biodegradable magnesium alloy promotes subperiosteal osteogenesis via interleukin-10-dependent macrophage immunomodulation. Biomaterials 318, 122992. doi:10.1016/j.biomaterials.2024.122992
Chen, X., Zhou, J., Qian, Y., and Zhao, L. (2023). Antibacterial coatings on orthopedic implants. Mater Today Bio 19, 100586. doi:10.1016/j.mtbio.2023.100586
Chen, Y., Liu, Z., Lin, Z., Lu, M., Fu, Y., Liu, G., et al. (2023). The effect of Staphylococcus aureus on innate and adaptive immunity and potential immunotherapy for S. aureus-induced osteomyelitis. Front. Immunol. 14, 1219895. doi:10.3389/fimmu.2023.1219895
Chopra, H., Kumar, S., and Singh, I. (2021). Biopolymer-based scaffolds for tissue engineering applications. Curr. Drug Targets 22, 282–295. doi:10.2174/1389450121999201102140408
Csóka, I., Ismail, R., Jójárt-Laczkovich, O., and Pallagi, E. (2021). Regulatory considerations, challenges and risk-based approach in nanomedicine development. Curr. Med. Chem. 28, 7461–7476. doi:10.2174/0929867328666210406115529
Dhas, N. L., Kudarha, R. R., Acharya, N. S., and Acharya, S. R. (2018). Polymeric immunonanoparticles mediated cancer therapy: versatile nanocarriers for cell-specific cargo delivery. Crit. Rev. Ther. Drug Carr. Syst. 35, 1–64. doi:10.1615/CritRevTherDrugCarrierSyst.2017018714
Dousti, M., Parsa, S., Sani, F., Bagherzadeh, E., Zamanzadeh, Z., Dara, M., et al. (2024). Enhancing bone regeneration: unleashing the potential of magnetic nanoparticles in a microtissue model. J. Cell Mol. Med. 28, e70040. doi:10.1111/jcmm.70040
Dymek, M., and Sikora, E. (2022). Liposomes as biocompatible and smart delivery systems - the current state. Adv. Colloid Interface Sci. 309, 102757. doi:10.1016/j.cis.2022.102757
Egawa, S., Hirai, K., Matsumoto, R., Yoshii, T., Yuasa, M., Okawa, A., et al. (2020). Efficacy of antibiotic-loaded hydroxyapatite/collagen composites is dependent on adsorbability for treating Staphylococcus aureus osteomyelitis in rats. J. Orthop. Res. 38, 843–851. doi:10.1002/jor.24507
Eivazzadeh-Keihan, R., Saadatidizaji, Z., Mahdavi, M., Maleki, A., Irani, M., and Zare, I. (2024). Recent advances in gold nanoparticles-based biosensors for tuberculosis determination. Talanta 275, 126099. doi:10.1016/j.talanta.2024.126099
Elkhenany, H., Bourdo, S., Hecht, S., Donnell, R., Gerard, D., Abdelwahed, R., et al. (2017). Graphene nanoparticles as osteoinductive and osteoconductive platform for stem cell and bone regeneration. Nanomedicine 13, 2117–2126. doi:10.1016/j.nano.2017.05.009
Ensoylu, M., Deliormanlı, A. M., and Atmaca, H. (2022). Preparation, characterization, and drug delivery of hexagonal boron nitride-borate bioactive glass biomimetic scaffolds for bone tissue engineering. Biomimetics (Basel) 8, 10. doi:10.3390/biomimetics8010010
Ferrando, A., Part, J., and Baeza, J. (2017). Treatment of cavitary bone defects in chronic osteomyelitis: bioactive glass S53P4 vs. Calcium sulphate antibiotic beads. J. Bone Jt. Infect. 2, 194–201. doi:10.7150/jbji.20404
Gade, L., Boyd, B. J., Malmsten, M., and Heinz, A. (2024). Stimuli-responsive drug delivery systems for inflammatory skin conditions. Acta Biomater. 187, 1–19. doi:10.1016/j.actbio.2024.08.037
Ge, Y., Wang, K., Liu, J., Tian, Y., Li, H., Wang, H., et al. (2022). A ZIF-8-based multifunctional intelligent drug release system for chronic osteomyelitis. Colloids Surf. B Biointerfaces 212, 112354. doi:10.1016/j.colsurfb.2022.112354
Geurts, J. A. P., van Vugt, T. A. G., and Arts, J. J. C. (2021). Use of contemporary biomaterials in chronic osteomyelitis treatment: clinical lessons learned and literature review. J. Orthop. Res. 39, 258–264. doi:10.1002/jor.24896
Girardot, J. M., and Girardot, M. N. (1996). Amide cross-linking: an alternative to glutaraldehyde fixation. J. Heart Valve Dis. 5, 518–525.
Gonzalez Gomez, A., Xu, C., and Hosseinidoust, Z. (2019). Preserving the efficacy of glycopeptide antibiotics during nanoencapsulation in liposomes. ACS Infect. Dis. 5, 1794–1801. doi:10.1021/acsinfecdis.9b00232
Gou, M., Wang, H., Xie, H., and Song, H. (2024). Macrophages in guided bone regeneration: potential roles and future directions. Front. Immunol. 15, 1396759. doi:10.3389/fimmu.2024.1396759
Gounani, Z., Asadollahi, M. A., Pedersen, J. N., Lyngsø, J., Skov Pedersen, J., Arpanaei, A., et al. (2019). Mesoporous silica nanoparticles carrying multiple antibiotics provide enhanced synergistic effect and improved biocompatibility. Colloids Surf. B Biointerfaces 175, 498–508. doi:10.1016/j.colsurfb.2018.12.035
Granata, V., Possetti, V., Parente, R., Bottazzi, B., Inforzato, A., and Sobacchi, C. (2022). The osteoblast secretome in Staphylococcus aureus osteomyelitis. Front. Immunol. 13, 1048505. doi:10.3389/fimmu.2022.1048505
Guduric, V., Wieckhusen, J., Bernhardt, A., Ahlfeld, T., Lode, A., Wu, C., et al. (2021). Composite bioinks with mesoporous bioactive glasses-A critical evaluation of results obtained by in vitro experiments. Front. Bioeng. Biotechnol. 9, 767256. doi:10.3389/fbioe.2021.767256
Guo, X., Weng, L., Yi, L., and Geng, D. (2022). Toxicological safety evaluation in acute and 21-day studies of ethanol extract from solanum lyratum thunb. Evid. Based Complement. Altern. Med. 2022, 1–9. doi:10.1155/2022/8518324
Hajiagha, M. N., and Kafil, H. S. (2023). Efflux pumps and microbial biofilm formation. Infect. Genet. Evol. 112, 105459. doi:10.1016/j.meegid.2023.105459
Hammond, P. T. (2011). Virtual issue on nanomaterials for drug delivery. ACS Nano 5, 681–684. doi:10.1021/nn2003508
Hassan, M. N., Mahmoud, M. M., El-Fattah, A. A., and Kandil, S. (2016). Microwave-assisted preparation of Nano-hydroxyapatite for bone substitutes. Ceram. Int. 42, 3725–3744. doi:10.1016/j.ceramint.2015.11.044
Hassani Besheli, N., Mottaghitalab, F., Eslami, M., Gholami, M., Kundu, S. C., Kaplan, D. L., et al. (2017). Sustainable release of vancomycin from silk fibroin nanoparticles for treating severe bone infection in rat tibia osteomyelitis model. ACS Appl. Mater Interfaces 9, 5128–5138. doi:10.1021/acsami.6b14912
Inceoglu, S., Botimer, G., and Maskiewicz, V. K. (2021). Novel microcomposite implant for the controlled delivery of antibiotics in the treatment of osteomyelitis following total joint replacement. J. Orthop. Res. 39, 365–375. doi:10.1002/jor.24919
Ip, H. T., Liu, L., Hong, L., and Ngai, T. (2022). Synthesis of polystyrene/silica and poly(styrene-co-butyl acrylate)/silica nanocomposite particles by Pickering emulsion polymerization with non-functionalized silica nanoparticles. Colloids Surfaces A Physicochem. Eng. Aspects 654, 130104. doi:10.1016/j.colsurfa.2022.130104
Ismat, A., Walter, N., Baertl, S., Mika, J., Lang, S., Kerschbaum, M., et al. (2021). Antibiotic cement coating in orthopedic surgery: a systematic review of reported clinical techniques. J. Orthop. Traumatol. 22, 56. doi:10.1186/s10195-021-00614-7
Jia, W.-T., Fu, Q., Huang, W.-H., Zhang, C.-Q., and Rahaman, M. N. (2015). Comparison of borate bioactive glass and calcium sulfate as implants for the local delivery of teicoplanin in the treatment of methicillin-resistant Staphylococcus aureus-induced osteomyelitis in a rabbit model. Antimicrob. Agents Chemother. 59, 7571–7580. doi:10.1128/AAC.00196-15
Jia, X., Long, Q., Miron, R. J., Yin, C., Wei, Y., Zhang, Y., et al. (2017). Setd2 is associated with strontium-induced bone regeneration. Acta Biomater. 53, 495–505. doi:10.1016/j.actbio.2017.02.025
Jiamton, C., Apivatgaroon, A., Aunaramwat, S., Chawalitrujiwong, B., Chuaychoosakoon, C., Suwannaphisit, S., et al. (2023). Efficacy and safety of antibiotic impregnated microporous nanohydroxyapatite beads for chronic osteomyelitis treatment: a multicenter, open-label, prospective cohort study. Antibiot. (Basel) 12, 1049. doi:10.3390/antibiotics12061049
Jiang, N., Wu, H.-T., Lin, Q.-R., Hu, Y.-J., and Yu, B. (2020). Health care costs of post-traumatic osteomyelitis in China: current situation and influencing factors. J. Surg. Res. 247, 356–363. doi:10.1016/j.jss.2019.10.008
Johnson, M. B., Suptela, S. R., Sipprell, S. E., and Marriott, I. (2023). Substance P exacerbates the inflammatory and pro-osteoclastogenic responses of murine osteoclasts and osteoblasts to Staphylococcus aureus. Inflammation 46, 256–269. doi:10.1007/s10753-022-01731-z
Jose, G., Lu, Y.-J., Chen, H.-A., Hsu, H.-L., Hung, J.-T., Anilkumar, T. S., et al. (2019). Hyaluronic acid modified bubble-generating magnetic liposomes for targeted delivery of doxorubicin. J. Magnetism Magnetic Mater. 474, 355–364. doi:10.1016/j.jmmm.2018.11.019
Kadry, A. A., Al-Suwayeh, S. A., Abd-Allah, A. R. A., and Bayomi, M. A. (2004). Treatment of experimental osteomyelitis by liposomal antibiotics. J. Antimicrob. Chemother. 54, 1103–1108. doi:10.1093/jac/dkh465
Kang, S.-S., Kim, A. R., Yun, C.-H., and Han, S. H. (2018). Staphylococcus aureus lipoproteins augment inflammatory responses in poly I:C-primed macrophages. Cytokine 111, 154–161. doi:10.1016/j.cyto.2018.08.020
Kang, M.-J., Cho, Y.-W., and Kim, T.-H. (2025). Metal- and covalent-organic framework-based drug delivery systems: applications to control cell functions. Coord. Chem. Rev. 527, 216400. doi:10.1016/j.ccr.2024.216400
Kavanagh, N., Ryan, E. J., Widaa, A., Sexton, G., Fennell, J., O’Rourke, S., et al. (2018). Staphylococcal osteomyelitis: disease progression, treatment challenges, and future directions. Clin. Microbiol. Rev. 31, e00084-17. doi:10.1128/cmr.00084-17
Kędziora, A., Wernecki, M., Korzekwa, K., Speruda, M., Gerasymchuk, Y., Łukowiak, A., et al. (2020). Consequences of long-term bacteria’s exposure to silver nanoformulations with different PhysicoChemical properties. Int. J. Nanomedicine 15, 199–213. doi:10.2147/IJN.S208838
Kemah, B., Uzer, G., Turhan, Y., Özturan, B., Kılıç, B., Gültepe, B. S., et al. (2018). Effects of local application of nano-silver on osteomyelitis and soft tissue infections: an experimental study in rats. J. Bone Jt. Infect. 3, 43–49. doi:10.7150/jbji.22121
Kojima, K. E., de Andrade E Silva, F. B., Leonhardt, M. C., de Carvalho, V. C., de Oliveira, P. R. D., Lima, A. L. L. M., et al. (2021). Bioactive glass S53P4 to fill-up large cavitary bone defect after acute and chronic osteomyelitis treated with antibiotic-loaded cement beads: a prospective case series with a minimum 2-year follow-up. Injury 52 (Suppl. 3), S23–S28. doi:10.1016/j.injury.2021.05.030
Kremers, H. M., Nwojo, M. E., Ransom, J. E., Wood-Wentz, C. M., Melton, L. J., and Huddleston, P. M. (2015). Trends in the epidemiology of osteomyelitis: a population-based study, 1969 to 2009. J. Bone Jt. Surg. Am. 97, 837–845. doi:10.2106/JBJS.N.01350
Kumar, M., Kulkarni, P., Liu, S., Chemuturi, N., and Shah, D. K. (2023). Nanoparticle biodistribution coefficients: a quantitative approach for understanding the tissue distribution of nanoparticles. Adv. Drug Deliv. Rev. 194, 114708. doi:10.1016/j.addr.2023.114708
Lee, K., Lee, J., Kwak, M., Cho, Y.-L., Hwang, B., Cho, M. J., et al. (2019). Two distinct cellular pathways leading to endothelial cell cytotoxicity by silica nanoparticle size. J. Nanobiotechnology 17, 24. doi:10.1186/s12951-019-0456-4
Lehner, E., Trutschel, M.-L., Menzel, M., Jacobs, J., Kunert, J., Scheffler, J., et al. (2025). Enhancing drug release from PEG-PLGA implants: the role of Hydrophilic Dexamethasone Phosphate in modulating release kinetics and degradation behavior. Eur. J. Pharm. Sci. 209, 107067. doi:10.1016/j.ejps.2025.107067
Lew, D. P., and Waldvogel, F. A. (2004). Osteomyelitis. Lancet 364, 369–379. doi:10.1016/S0140-6736(04)16727-5
Liang, S., Pan, Y., Wang, J., Jiang, Z., Ma, T., Chen, S., et al. (2025). Bone-targeting ZIF-8 based nanoparticles loaded with vancomycin for the treatment of MRSA-induced periprosthetic joint infection. J. Control Release 385, 113965. doi:10.1016/j.jconrel.2025.113965
Lin, H., Li, Z., Xie, Z., Tang, S., Huang, M., Feng, J., et al. (2024). An anti-infection and biodegradable TFRD-loaded porous scaffold promotes bone regeneration in segmental bone defects: experimental studies. Int. J. Surg. 110, 3269–3284. doi:10.1097/JS9.0000000000001291
Lindfors, N. C., Hyvönen, P., Nyyssönen, M., Kirjavainen, M., Kankare, J., Gullichsen, E., et al. (2010). Bioactive glass S53P4 as bone graft substitute in treatment of osteomyelitis. Bone 47, 212–218. doi:10.1016/j.bone.2010.05.030
Lindsay, S. E., Lindsay, H. G., Kallet, J., Weaver, M. R., Curran-Everett, D., Crapo, J. D., et al. (2021). MnTE-2-PyP disrupts Staphylococcus aureus biofilms in a novel fracture model. J. Orthop. Res. 39, 2439–2445. doi:10.1002/jor.24967
Liu, Y., Li, X., and Liang, A. (2022). Current research progress of local drug delivery systems based on biodegradable polymers in treating chronic osteomyelitis. Front. Bioeng. Biotechnol. 10, 1042128. doi:10.3389/fbioe.2022.1042128
Lu, D., and Fan, X. (2024). Insights into the prospects of nanobiomaterials in the treatment of cardiac arrhythmia. J. Nanobiotechnology 22, 523. doi:10.1186/s12951-024-02805-w
Luceri, A., Francese, R., Lembo, D., Ferraris, M., and Balagna, C. (2023). Silver nanoparticles: review of antiviral properties, mechanism of action and applications. Microorganisms 11, 629. doi:10.3390/microorganisms11030629
Lv, X.-F., Zhou, D.-M., Sun, X.-H., and Zhao, Z. (2022). Nano sized hydroxyapatite-polylactic acid-vancomycin in alleviation of chronic osteomyelitis. Drug Des. Devel Ther. 16, 1983–1993. doi:10.2147/DDDT.S356257
Macwan, I., Khan, M. D. H., Aphale, A., Singh, S., Liu, J., Hingorani, M., et al. (2017). Interactions between avidin and graphene for development of a biosensing platform. Biosens. Bioelectron. 89, 326–333. doi:10.1016/j.bios.2016.07.024
Mahon, O. R., Browe, D. C., Gonzalez-Fernandez, T., Pitacco, P., Whelan, I. T., Von Euw, S., et al. (2020). Nano-particle mediated M2 macrophage polarization enhances bone formation and MSC osteogenesis in an IL-10 dependent manner. Biomaterials 239, 119833. doi:10.1016/j.biomaterials.2020.119833
Mammari, N., and Duval, R. E. (2023). Photothermal/photoacoustic therapy combined with metal-based nanomaterials for the treatment of microbial infections. Microorganisms 11, 2084. doi:10.3390/microorganisms11082084
Maparu, A. K., Singh, P., Rai, B., Sharma, A., and Sivakumar, S. (2022). A simple, robust and scalable route to prepare sub-50 nm soft PDMS nanoparticles for intracellular delivery of anticancer drugs. Nanotechnology 33, 495102. doi:10.1088/1361-6528/ac8d99
Masters, E. A., Trombetta, R. P., de Mesy Bentley, K. L., Boyce, B. F., Gill, A. L., Gill, S. R., et al. (2019). Evolving concepts in bone infection: redefining “biofilm”, “acute vs. chronic osteomyelitis”, “the immune proteome” and “local antibiotic therapy”. Bone Res. 7, 20. doi:10.1038/s41413-019-0061-z
Masters, E. A., Ricciardi, B. F., Bentley, K. L. M., Moriarty, T. F., Schwarz, E. M., and Muthukrishnan, G. (2022). Skeletal infections: microbial pathogenesis, immunity and clinical management. Nat. Rev. Microbiol. 20, 385–400. doi:10.1038/s41579-022-00686-0
McDermott, K., Fang, M., Boulton, A. J. M., Selvin, E., and Hicks, C. W. (2023). Etiology, epidemiology, and disparities in the burden of diabetic foot ulcers. Diabetes Care 46, 209–221. doi:10.2337/dci22-0043
Mills, D. K., Jammalamadaka, U., Tappa, K., and Weisman, J. (2018). Studies on the cytocompatibility, mechanical and antimicrobial properties of 3D printed poly(methyl methacrylate) beads. Bioact. Mater 3, 157–166. doi:10.1016/j.bioactmat.2018.01.006
Mills, H., Donnelly, L., and Platt, S. (2024). Locally delivered antibiotics in fracture-related infection. Cureus 16, e73210. doi:10.7759/cureus.73210
Mohammadpour, R., Dobrovolskaia, M. A., Cheney, D. L., Greish, K. F., and Ghandehari, H. (2019). Subchronic and chronic toxicity evaluation of inorganic nanoparticles for delivery applications. Adv. drug Deliv. Rev. 144, 112–132. doi:10.1016/j.addr.2019.07.006
Mohd Zaffarin, A. S., Ng, S.-F., Ng, M. H., Hassan, H., and Alias, E. (2021). Nano-hydroxyapatite as a delivery system for promoting bone regeneration in vivo: a systematic review. Nanomater. (Basel) 11, 2569. doi:10.3390/nano11102569
Mulazzi, M., Campodoni, E., Bassi, G., Montesi, M., Panseri, S., Bonvicini, F., et al. (2021). Medicated hydroxyapatite/collagen hybrid scaffolds for bone regeneration and local antimicrobial therapy to prevent bone infections. Pharmaceutics 13, 1090. doi:10.3390/pharmaceutics13071090
Naruphontjirakul, P., Porter, A. E., and Jones, J. R. (2018). In vitro osteogenesis by intracellular uptake of strontium containing bioactive glass nanoparticles. Acta Biomater. 66, 67–80. doi:10.1016/j.actbio.2017.11.008
Navarro-Partida, J., Castro-Castaneda, C. R., Santa Cruz-Pavlovich, F. J., Aceves-Franco, L. A., Guy, T. O., and Santos, A. (2021). Lipid-based nanocarriers as topical drug delivery systems for intraocular diseases. Pharmaceutics 13, 678. doi:10.3390/pharmaceutics13050678
Nguyen Ngoc, D., Latalski, M., Danielewicz, A., Szponder, T., Wessely-Szponder, J., and Mazur, E. (2025). Application of antimicrobial peptides (AMPs) in treatment of osteomyelitis in human and veterinary orthopedics. J. Funct. Biomater. 16, 90. doi:10.3390/jfb16030090
Padrão, T., Coelho, C. C., Costa, P., Alegrete, N., Monteiro, F. J., and Sousa, S. R. (2021). Combining local antibiotic delivery with heparinized nanohydroxyapatite/collagen bone substitute: a novel strategy for osteomyelitis treatment. Mater Sci. Eng. C Mater Biol. Appl. 119, 111329. doi:10.1016/j.msec.2020.111329
Papa, F., Cortese, A., Sagliocco, R., Farella, M., Banzi, C., Maltarello, M. C., et al. (2009). Outcome of 47 consecutive sinus lift operations using aragonitic calcium carbonate associated with autologous platelet-rich plasma: clinical, histologic, and histomorphometrical evaluations. J. Craniofac Surg. 20, 2067–2074. doi:10.1097/SCS.0b013e3181be88ab
Parisi, L., Gini, E., Baci, D., Tremolati, M., Fanuli, M., Bassani, B., et al. (2018). Macrophage polarization in chronic inflammatory diseases: killers or builders? J. Immunol. Res. 2018, 1–25. doi:10.1155/2018/8917804
Park, J., Choi, S. W., Cha, B. G., Kim, J., and Kang, S.-J. (2021). Alternative activation of macrophages through interleukin-13-loaded extra-large-pore mesoporous silica nanoparticles suppresses experimental autoimmune encephalomyelitis. ACS Biomater. Sci. Eng. 7, 4446–4453. doi:10.1021/acsbiomaterials.1c00946
Piotrowska, U., Sobczak, M., and Oledzka, E. (2017). Current state of a dual behaviour of antimicrobial peptides-Therapeutic agents and promising delivery vectors. Chem. Biol. Drug Des. 90, 1079–1093. doi:10.1111/cbdd.13031
Pisani, S., Tufail, S., Rosalia, M., Dorati, R., Genta, I., Chiesa, E., et al. (2024). Antibiotic-loaded nano-sized delivery systems: an insight into gentamicin and vancomycin. J. Funct. Biomater. 15, 194. doi:10.3390/jfb15070194
Pomposello, M. M., Nemes, K., and Mosovsky, K. (2020). Dietary antioxidant seleno-L-methionine protects macrophages infected with Burkholderia thailandensis. PLoS One 15, e0238174. doi:10.1371/journal.pone.0238174
Qin, H., Zhao, S., Gong, H., Yu, Z., Chen, Q., Liang, P., et al. (2023). Recent progress in the application of metal organic frameworks in surface-enhanced Raman scattering detection. Biosens. (Basel) 13, 479. doi:10.3390/bios13040479
Rahaman, M. N., Bal, B. S., and Huang, W. (2014). Review: emerging developments in the use of bioactive glasses for treating infected prosthetic joints. Mater Sci. Eng. C Mater Biol. Appl. 41, 224–231. doi:10.1016/j.msec.2014.04.055
Rahaman, S. N., Pathmanapan, S., Sidharthan, A., and Anandasadagopan, S. K. (2023). Vancomycin loaded amino-functionalized MCM-48 mesoporous silica nanoparticles as a promising drug carrier in bone substitutes for bacterial infection management. Appl. Biochem. Biotechnol. 195, 6607–6632. doi:10.1007/s12010-023-04406-z
Rajendran, M., Iraivan, G., Ghayathri, B. L., Mohan, P., Chandran, K. R., Nagaiah, H. P., et al. (2021). Antibiotic loaded nano rod bone cement for the treatment of osteomyelitis. Recent Pat. Nanotechnol. 15, 70–89. doi:10.2174/1872210514666200811103724
Rasquel-Oliveira, F. S., Ribeiro, J. M., Martelossi-Cebinelli, G., Costa, F. B., Nakazato, G., Casagrande, R., et al. (2025). Staphylococcus aureus in inflammation and pain: update on pathologic mechanisms. Pathogens 14, 185. doi:10.3390/pathogens14020185
Rocha, C. V., Gonçalves, V., da Silva, M. C., Bañobre-López, M., and Gallo, J. (2022). PLGA-based composites for various biomedical applications. Int. J. Mol. Sci. 23, 2034. doi:10.3390/ijms23042034
Romanò, C. L., Logoluso, N., Meani, E., Romanò, D., De Vecchi, E., Vassena, C., et al. (2014). A comparative study of the use of bioactive glass S53P4 and antibiotic-loaded calcium-based bone substitutes in the treatment of chronic osteomyelitis: a retrospective comparative study. Bone Jt. J. 96-B, 845–850. doi:10.1302/0301-620X.96B6.33014
Sadowska, J. M., Genoud, K. J., Kelly, D. J., and O’Brien, F. J. (2021). Bone biomaterials for overcoming antimicrobial resistance: advances in non-antibiotic antimicrobial approaches for regeneration of infected osseous tissue. Mater. Today 46, 136–154. doi:10.1016/j.mattod.2020.12.018
Saeting, K., Mitrevej, A., Leuenberger, H., and Sinchaipanid, N. (2022). Development of alendronate niosomal delivery system for gastrointestinal permeability improvement. J. Drug Deliv. Sci. Technol. 67, 102885. doi:10.1016/j.jddst.2021.102885
Safi, S., Karimzadeh, F., and Labbaf, S. (2018). Mesoporous and hollow hydroxyapatite nanostructured particles as a drug delivery vehicle for the local release of ibuprofen. Mater Sci. Eng. C Mater Biol. Appl. 92, 712–719. doi:10.1016/j.msec.2018.07.004
Saidykhan, L., Abu Bakar, M. Z. B., Rukayadi, Y., Kura, A. U., and Latifah, S. Y. (2016). Development of nanoantibiotic delivery system using cockle shell-derived aragonite nanoparticles for treatment of osteomyelitis. Int. J. Nanomedicine 11, 661–673. doi:10.2147/IJN.S95885
Shakya, A. K., Al-Sulaibi, M., Naik, R. R., Nsairat, H., Suboh, S., and Abulaila, A. (2023). Review on PLGA polymer based nanoparticles with antimicrobial properties and their application in various medical conditions or infections. Polym. (Basel) 15, 3597. doi:10.3390/polym15173597
Shao, Y., Suo, H., Wang, S., Peng, Y., Chu, X., Long, Z., et al. (2024). A facile method to construct ZIF-8 MOFs on contact lens for high antibiotics loading and self-defensive release. Chem. Eng. J. 481, 148576. doi:10.1016/j.cej.2024.148576
Sharifuzzaman, M., Barman, S. C., Rahman, M. T., Zahed, M. A., Xuan, X., and Park, J. Y. (2019). Green synthesis and layer-by-layer assembly of amino-functionalized graphene oxide/carboxylic surface modified trimetallic nanoparticles nanocomposite for label-free electrochemical biosensing. J. Electrochem. Soc. 166, B983–B993. doi:10.1149/2.0821912jes
Sharma, D., Gautam, S., Singh, S., Srivastava, N., Khan, A. M., and Bisht, D. (2024). Unveiling the nanoworld of antimicrobial resistance: integrating nature and nanotechnology. Front. Microbiol. 15, 1391345. doi:10.3389/fmicb.2024.1391345
Sheehy, E. J., von Diemling, C., Ryan, E., Widaa, A., O’Donnell, P., Ryan, A., et al. (2025). Antibiotic-eluting scaffolds with responsive dual-release kinetics facilitate bone healing and eliminate S. aureus infection. Biomaterials 313, 122774. doi:10.1016/j.biomaterials.2024.122774
Sionkowska, A., Kulka-Kamińska, K., Brudzyńska, P., Lewandowska, K., and Piwowarski, Ł. (2024). The influence of various crosslinking conditions of EDC/NHS on the properties of fish collagen film. Mar. Drugs 22, 194. doi:10.3390/md22050194
Slita, A., Egorova, A., Casals, E., Kiselev, A., and Rosenholm, J. M. (2018). Characterization of modified mesoporous silica nanoparticles as vectors for siRNA delivery. Asian J. Pharm. Sci. 13, 592–599. doi:10.1016/j.ajps.2018.01.006
Soto, S. M. (2013). Role of efflux pumps in the antibiotic resistance of bacteria embedded in a biofilm. Virulence 4, 223–229. doi:10.4161/viru.23724
Štícha, R., Fulín, P., Nyč, O., Gajdošová, V., Pokorný, D., and Šlouf, M. (2023). Antimicrobial activity of the most common antibiotic-releasing systems employed in current orthopedic surgery: in vitro study. Acta Chir. Orthop. Traumatol. Cech 90, 188–197. doi:10.55095/achot2023/027
Su, G.-L., Peng, Y.-J., Ruan, H.-Z., Cheng, J., Deng, T., and Zhang, Y.-F. (2025). Regulating periodontal disease with smart stimuli-responsive systems: antimicrobial activity, immunomodulation, periodontium regeneration. Mater. today. Bio 32, 101863. doi:10.1016/j.mtbio.2025.101863
Sui, B., Liu, X., and Sun, J. (2018). Dual-functional dendritic mesoporous bioactive glass nanospheres for calcium influx-mediated specific tumor suppression and controlled drug delivery in vivo. ACS Appl. Mater Interfaces 10, 23548–23559. doi:10.1021/acsami.8b05616
Tenchov, R., Hughes, K. J., Ganesan, M., Iyer, K. A., Ralhan, K., Lotti Diaz, L. M., et al. (2025). Transforming medicine: cutting-edge applications of nanoscale materials in drug delivery. ACS Nano 19, 4011–4038. doi:10.1021/acsnano.4c09566
Thabit, A. K., Fatani, D. F., Bamakhrama, M. S., Barnawi, O. A., Basudan, L. O., and Alhejaili, S. F. (2019). Antibiotic penetration into bone and joints: an updated review. Int. J. Infect. Dis. 81, 128–136. doi:10.1016/j.ijid.2019.02.005
Thomas, T. P., Goonewardena, S. N., Majoros, I. J., Kotlyar, A., Cao, Z., Leroueil, P. R., et al. (2011). Folate-targeted nanoparticles show efficacy in the treatment of inflammatory arthritis. Arthritis Rheum. 63, 2671–2680. doi:10.1002/art.30459
Torrano, A. A., Herrmann, R., Strobel, C., Rennhak, M., Engelke, H., Reller, A., et al. (2016). Cell membrane penetration and mitochondrial targeting by platinum-decorated ceria nanoparticles. Nanoscale 8, 13352–13367. doi:10.1039/c5nr08419a
Urish, K. L., and Cassat, J. E. (2020). Staphylococcus aureus osteomyelitis: bone, bugs, and surgery. Infect. Immun. 88, e00932-19. doi:10.1128/IAI.00932-19
Uskokovic, V. (2015). Nanostructured platforms for the sustained and local delivery of antibiotics in the treatment of osteomyelitis. Crit. Rev. Ther. Drug Carr. Syst. 32, 1–59. doi:10.1615/critrevtherdrugcarriersyst.2014010920
Wan, R., Luo, Z., Nie, X., Feng, X., He, Y., Li, F., et al. (2024). A mesoporous silica-loaded multi-functional hydrogel enhanced tendon healing via immunomodulatory and pro-regenerative effects. Adv. Healthc. Mater 13, e2400968. doi:10.1002/adhm.202400968
Wang, W., and Vikesland, P. J. (2023). Metabolite-mediated bacterial antibiotic resistance revealed by surface-enhanced Raman spectroscopy. Environ. Sci. Technol. 57, 13375–13383. doi:10.1021/acs.est.3c04001
Wang, X., Cheng, F., Liu, J., Smått, J.-H., Gepperth, D., Lastusaari, M., et al. (2016). Biocomposites of copper-containing mesoporous bioactive glass and nanofibrillated cellulose: biocompatibility and angiogenic promotion in chronic wound healing application. Acta Biomater. 46, 286–298. doi:10.1016/j.actbio.2016.09.021
Wang, D.-Y., van der Mei, H. C., Ren, Y., Busscher, H. J., and Shi, L. (2019). Lipid-based antimicrobial delivery-systems for the treatment of bacterial infections. Front. Chem. 7, 872. doi:10.3389/fchem.2019.00872
Wang, J., Ding, Y., Chong, K., Cui, M., Cao, Z., Tang, C., et al. (2024). Recent advances in lipid nanoparticles and their safety concerns for mRNA delivery. Vaccines (Basel) 12, 1148. doi:10.3390/vaccines12101148
Wang, X., Li, X., Li, S., Du, W., Yan, W., and Xu, H. (2025). Degradation of antibiotics by electrochemical oxidation: current issues, environmental risks, and future strategies. Chem. Eng. J. 516, 163941. doi:10.1016/j.cej.2025.163941
Wentao, Z., Lei, G., Liu, Y., Wang, W., Song, T., and Fan, J. (2017). Approach to osteomyelitis treatment with antibiotic loaded PMMA. Microb. Pathog. 102, 42–44. doi:10.1016/j.micpath.2016.11.016
Wu, J., Wang, R., Zhang, Y., Chen, B., and Zhu, X. (2022). In situ scrutinize the adsorption of sulfamethoxazole in water using AFM force spectroscopy: molecular adhesion force determination and fractionation. J. Hazard Mater 426, 128128. doi:10.1016/j.jhazmat.2021.128128
Wu, S., Wu, B., Liu, Y., Deng, S., Lei, L., and Zhang, H. (2022). Mini review therapeutic strategies targeting for biofilm and bone infections. Front. Microbiol. 13, 936285. doi:10.3389/fmicb.2022.936285
Xu, Z., Xia, Y., Zhou, P., Li, J. J., Yang, M., Zhang, Y., et al. (2021). Silicon incorporation into hydroxyapatite nanocarrier counteracts the side effects of vancomycin for efficient chronic osteomyelitis treatment. Chem. Eng. J. 406, 126821. doi:10.1016/j.cej.2020.126821
Yang, M., Tan, Q., and Tang, Z. (2024). Bones on fire: illuminating osteomyelitis through the radiant lens of 18F-FDG PET/CT. Front. Immunol. 15, 1378409. doi:10.3389/fimmu.2024.1378409
Yi, X., Leng, P., Wang, S., Liu, L., and Xie, B. (2024). Functional nanomaterials for the treatment of osteoarthritis. Int. J. Nanomedicine 19, 6731–6756. doi:10.2147/IJN.S465243
Yuan, B., Zhang, Y., Wang, Q., Ren, G., Wang, Y., Zhou, S., et al. (2022). Thermosensitive vancomycin@PLGA-PEG-PLGA/HA hydrogel as an all-in-one treatment for osteomyelitis. Int. J. Pharm. 627, 122225. doi:10.1016/j.ijpharm.2022.122225
Zapata, D., Higgs, J., Wittholt, H., Chittimalli, K., Brooks, A. E., and Mulinti, P. (2022). Nanotechnology in the diagnosis and treatment of osteomyelitis. Pharmaceutics 14, 1563. doi:10.3390/pharmaceutics14081563
Zarepour, A., Venkateswaran, M. R., Khosravi, A., Iravani, S., and Zarrabi, A. (2024). Bioinspired nanomaterials to combat microbial biofilm and pathogen challenges: a review. ACS Appl. Nano Mater. 7, 25287–25313. doi:10.1021/acsanm.4c04806
Zhan, Y., Hong, Y., and Wang, Y. (2024). Sequential release of vancomycin and BMP-2 from chitosan/nano-hydroxyapatite thermosensitive hydrogel for the treatment of chronic osteomyelitis. J. Orthop. Surg. Res. 19, 602. doi:10.1186/s13018-024-05097-w
Zhang, X., Jia, W., Gu, Y., Xiao, W., Liu, X., Wang, D., et al. (2010). Teicoplanin-loaded borate bioactive glass implants for treating chronic bone infection in a rabbit tibia osteomyelitis model. Biomaterials 31, 5865–5874. doi:10.1016/j.biomaterials.2010.04.005
Zhang, Y., Zhang, L., Hu, Y., Jiang, K., Li, Z., Lin, Y.-Z., et al. (2018). Cell-permeable NF-κB inhibitor-conjugated liposomes for treatment of glioma. J. Control Release 289, 102–113. doi:10.1016/j.jconrel.2018.09.016
Zhang, Q., Hu, Y., Masterson, C. M., Jang, W., Xiao, Z., Bohloul, A., et al. (2022). When function is biological: discerning how silver nanoparticle structure dictates antimicrobial activity. iScience 25, 104475. doi:10.1016/j.isci.2022.104475
Zhang, H., Li, L., Chu, L., Huang, J., Chen, X., Zhuo, X., et al. (2023). Dual-functional borosilicate glass (BSG) delivery implant for osteomyelitis treatment and bone regeneration. Compos. Part B Eng. 259, 110749. doi:10.1016/j.compositesb.2023.110749
Zhang, H., Qiao, W., Liu, Y., Yao, X., Zhai, Y., and Du, L. (2025). Addressing the challenges of infectious bone defects: a review of recent advances in bifunctional biomaterials. J. Nanobiotechnology 23, 257. doi:10.1186/s12951-025-03295-0
Zhang, P., Sun, Y., Yang, H., Liu, D., Zhang, F., Zhang, Y., et al. (2023). Vancomycin-loaded silk fibroin microspheres in an injectable hydrogel for chronic osteomyelitis therapy. Front. Bioeng. Biotechnol. 11, 1163933. doi:10.3389/fbioe.2023.1163933
Zhang, R., Stehle, Y., Chen, L., Zeng, K., Lin, M., Wang, C., et al. (2025). Multifunctional, enzyme/pH-responsive gelatin microspheres with aptamer-targeted antibacterial and ionic-mediated dual therapy for infected bone defects. Biomaterials 326, 123642. doi:10.1016/j.biomaterials.2025.123642
Zhang, X., Hou, Q., Cao, S., Lin, X., Chen, X., Wang, Z., et al. (2023). Research status, opportunities, and challenges of cobalt phosphate based materials as OER electrocatalysts. Green Chem. 25, 7883–7903. doi:10.1039/d3gc02416d
Zhao, M., Zhang, C., Gao, C., and Wu, Z. (2023). Alendronate-decorated strontium-containing bioactive glass nanoparticles with dual osteogenic and anti-osteoclastic effects for osteoporosis. J. Non-Crystalline Solids 622, 122681. doi:10.1016/j.jnoncrysol.2023.122681
Zhou, C.-H., Ren, Y., Song, H.-J., Ali, A. A., Meng, X.-Q., Xu, L., et al. (2021). One-stage debridement and bone transport versus first-stage debridement and second-stage bone transport for the management of lower limb post-traumatic osteomyelitis. J. Orthop. Transl. 28, 21–27. doi:10.1016/j.jot.2020.12.004
Zhu, Z., Huang, C., Liu, L., Wang, J., and Gou, X. (2024). Magnetically actuated pandanus fruit-like nanorobots for enhanced pH-stimulated drug release and targeted biofilm elimination in wound healing. J. Colloid Interface Sci. 661, 374–388. doi:10.1016/j.jcis.2024.01.197
Zirak, N., Maadani, A. M., Salahinejad, E., Abbasnezhad, N., and Shirinbayan, M. (2022). Fabrication, drug delivery kinetics and cell viability assay of PLGA-coated vancomycin-loaded silicate porous microspheres. Ceram. Int. 48, 48–54. doi:10.1016/j.ceramint.2021.08.298
Keywords: osteomyelitis, nanomaterials, antibiotic delivery, targeted therapy, bone regeneration
Citation: Cheng S, Meng X-H, Li Z, Han H-H and Zhang Y-F (2025) Nanomaterial-mediated antibiotic delivery: a novel strategy for osteomyelitis therapy. Front. Bioeng. Biotechnol. 13:1671151. doi: 10.3389/fbioe.2025.1671151
Received: 22 July 2025; Accepted: 15 September 2025;
Published: 30 September 2025.
Edited by:
Xu Zhang, Peking University Hospital of Stomatology, ChinaReviewed by:
Dae Hyeok Yang, Catholic University of Korea, Republic of KoreaPing Jiang, Shanghai Jiao Tong University, China
Copyright © 2025 Cheng, Meng, Li, Han and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hai-Hui Han, aGFuaGFpaHVpQGhvdG1haWwuY29t; Ya-Feng Zhang, d3h6eTAwN0BuanVjbS5lZHUuY24=
†These authors have contributed equally to this work
 Shuang Cheng1†
Shuang Cheng1† Xiao-Hui Meng
Xiao-Hui Meng Ya-Feng Zhang
Ya-Feng Zhang