- 1Division of Clinical Anatomy, Laboratory of Tissue Engineering and Regenerative Medicine, Medical University of Gdansk, Gdansk, Poland
- 2The Department of General Surgery, Hospital Copernicus in Gdansk, Gdansk, Poland
- 3Laboratory of Tissue Engineering and Regenerative Medicine, Department of Embryology, Medical University of Gdansk, Gdansk, Poland
- 4Department of Biochemistry, University of Physical Education and Sport, Gdańsk, Poland
Hydrogels as three-dimensional polymer networks capable of reversibly absorbing water are of increasing interest among researchers. Hydrogels, especially those of natural origin such as alginate, chitosan, hyaluronic acid, peptide hydrogels, thanks to properties such as biocompatibility, biodegradability, bioactivity, can serve as an effective protective barrier or drug carrier. Thanks to the possibility of their modification, they can be an innovative platform supporting anticancer treatment. The examples presented in this publication confirm that these products can increase the effectiveness of treatment and reduce the effects of side effects.
1 Introduction
One of the greatest socio-economic problems of the 21st century is cancer. It causes 1 in 6 deaths worldwide. This disease constitutes a significant obstacle to extending life expectancy, but also a serious problem related to social and macroeconomic costs, which vary depending on the type of cancer, geographical location or gender (Bray et al., 2024). The global economic cost of cancer between 2020 and 2050 is estimated to be $25.2 trillion (in international dollars). Cancers with the highest economic costs include: cancer of the trachea, bronchi and lungs, cancer of the colon and rectum, breast cancer, liver cancer, and leukaemia (Chen et al., 2023). Cancer is a disease that originates from normal body tissues. But, due to persistent pathological features, it grows in an uncontrolled manner and is not susceptible to factors regulating cell growth, maturation and function (Brown et al., 2023). In the development of this disease, there is a change in cellular metabolism, which meets the energy and biosynthetic needs of uncontrolled proliferation of cancer cells (Xu et al., 2023).
Radiotherapy, chemotherapy and surgery play an essential role in the treatment of cancer individually or in combination. Cancer treatment is evolving, which is associated with technical progress in surgery, radiotherapy, as well as the discovery of new drugs such as cell cycle checkpoint inhibitors, immune response modifiers: CAR-T immunotherapy, anti-PD-1 and anti-PD-L1 antibody therapy (Mee et al., 2023). The type of cancer, its location and the stage of cancer advancement determined by the TNM classification allow us to choose the best treatment option and its progression (Rosen and Sapra, 2025). One of the methods of early prevention of this disease is prophylaxis. We are unable to determine one specific factor responsible for the development of cancer, because usually a number of factors related to addictions, lifestyle and the surrounding environment are involved in the development of cancer (Vineis and Wild, 2014; Collatuzzo and Boffetta, 2023). It is extremely important to perform preventive tests that will allow for the observation of disturbing changes at their early, non-advanced stage, as well as annual check-ups in people at increased risk of hereditary cancers.
Since any treatment can cause side effects, the treatment of cancer is no different (Figure 1). It is important to remember that each person may respond differently to the treatment given. The purpose of chemotherapy is to inhibit cell proliferation and multiplication to avoid the spread of abnormal cells and prevent metastasis to other organs. Chemotherapy preparations interfere with the synthesis of RNA, DNA or proteins (Sun Y. et al., 2021). When the chemotherapy drug works properly, cell death occurs or programmed cell death is triggered by apoptosis. The effects of chemotherapy are a reflection of their mechanisms of action. Drugs administered during chemotherapy affect rapidly multiplying cells, hair follicles, bone marrow and the digestive tract, therefore undesirable effects may include: excessive hair loss, myelosuppression, vomiting, inflammation of mucous membranes, other adverse effects on the digestive system, and infertility (Amjad et al., 2025; Katta et al., 2023). It should be emphasized that in the case of chemotherapy, due to its non-selectivity, the toxic effect affects not only abnormal cells, but also those that are healthy. The bioavailability of these chemotherapy drugs is poor for cancer tissues, so higher doses of chemotherapeutics are needed, resulting in increased toxicity in healthy cells. Multidrug resistance may also occur in such conditions (Senapati et al., 2018). Side effects affecting healthy tissue are the cause of high mortality among cancer patients. New solutions are being sought that will enable the delivery of an optimal dose of chemotherapy drug so that it has the least possible impact on healthy cells.
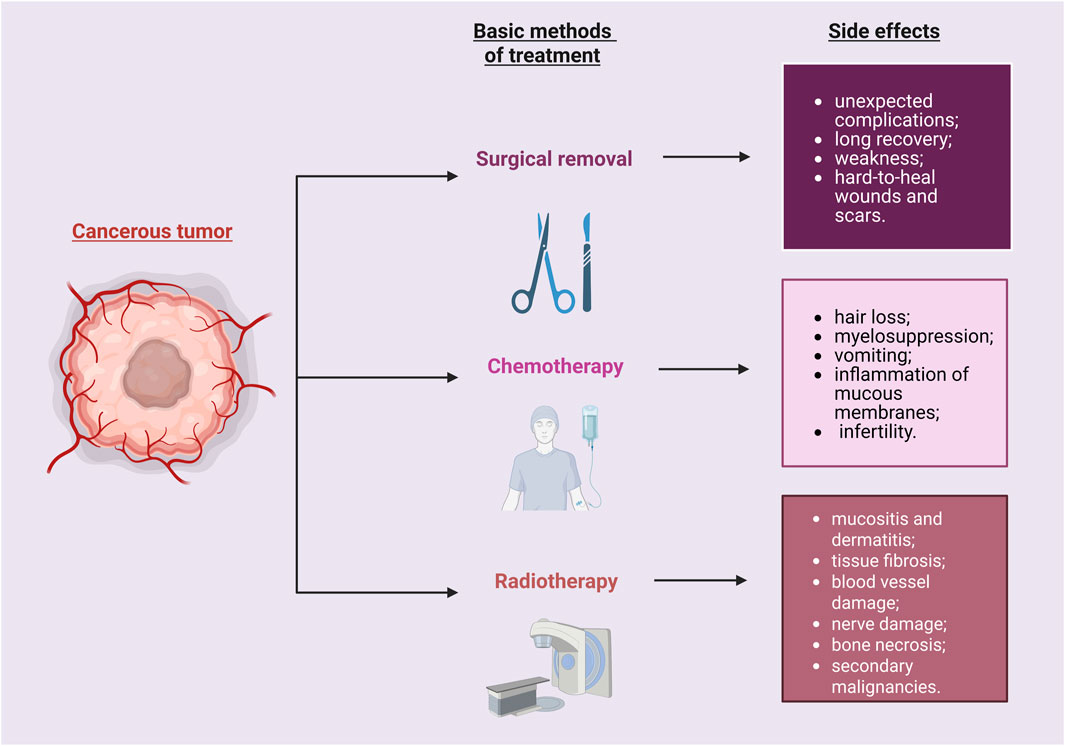
Figure 1. Cancer therapy may cause various side effects. They may impact the patient’s recovery process. Created with BioRender.com.
Radiotherapy is a treatment method that uses high-energy rays or radioactive substances to damage cancer cells and stop them from growing and dividing (Gianfaldoni et al., 2017). High frequency waves cause ionization in the tissue. Ionization occurs as a result of an electron being knocked out of the atomic orbit by an electromagnetic wave. Free electrons cause the formation of free radicals – unstable molecules with high chemical reactivity (Tumilaar et al., 2024). The DNA of the cell nucleus is a structure susceptible to damage as a result of ionization. DNA is damaged when a free electron hits the DNA strand and as a result of the action of free radicals. Ionizing radiation damages cancer cells more effectively than normal cells. Radiotherapy is by nature a conservative treatment – radiation treatments are painless and bloodless, but they last relatively long. The side effects of radiotherapy depend primarily on the location of the tumour and the dose of radiation received (Barazzuol et al., 2020). During radiotherapy, the skin is particularly exposed to the effects. The earliest adverse events of radiotherapy include mucositis and dermatitis (Iacovelli et al., 2020). Over time, tissue fibrosis, blood vessel damage, nerve damage, bone necrosis, and secondary malignancies may occur (Li et al., 2023). From a trivial inconvenience it can develop into a life-threatening situation. Radiation-induced mucositis first begins with acute inflammation of the mucosa, tongue, and throat after exposure to radiation (Rao et al., 2021). During this time, a cascade of immune reactions occurs, including recruitment of immune cells, release of inflammatory cytokines, chemotactic mediators, and growth factors. The described condition can progress to an acute stage, where food and water intake is prevented, which is associated with weight loss, and also a septic complication, which is a consequence of protective epithelial barriers and the basement membrane (Maria et al., 2017).
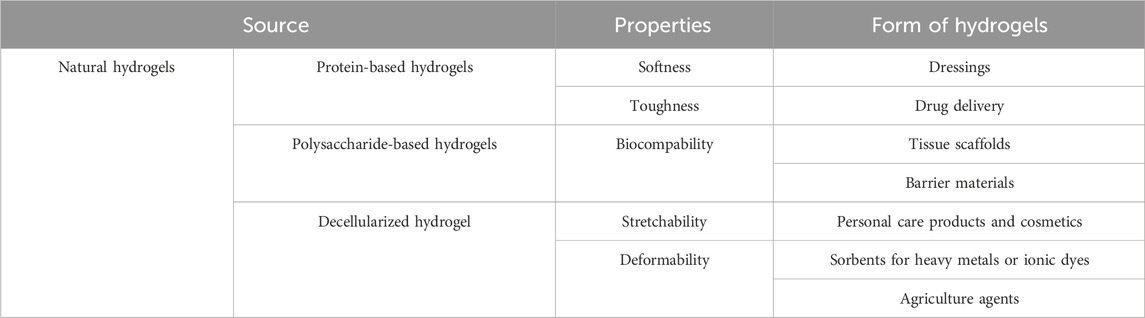
Currently, advanced techniques and products are being sought that reduce the risk of negative effects caused by radiotherapy and chemotherapy. Hydrogel is a three-dimensional network of hydrophilic polymer matrices that are able to retain a large amount of water (>10%) (Ho et al., 2022; Zhao et al., 2023; Lee et al., 2023). Crosslinking, which arises between hydrophilic matrices, maintains a constant, stable structure that is not dissolved in the aquatic environment. The unique property of this group of materials - the ability to absorb large amounts of water is caused by the presence of functional groups in their structure such as: OH, -COOH, -CONH, -NH2, -SO3H (Dodda et al., 2023). There are many classifications of hydrogels due to their different properties: chemical, physical or source of origin (Bashir et al., 2020; Bustamante-Torres et al., 2021; Khan et al., 2024) (Figure 2). One way to classify hydrogels is by cross-linking. Cross-linking is the process by which covalent or ionic bonds are formed between polymer chains. This process creates a network structure responsible for the mechanical properties of the hydrogel. This allows for modifications of polymers and biomolecules, which promote specific hydrogel characteristics. There are two methods of hydrogel cross-linking: chemical and physical (Akhtar et al., 2016). The formation of covalent bonds between polymer chains is commonly described as chemical cross-linking. The reaction requires a cross-linking agent that initiates and forms the bonds (Alavarse et al., 2022). The covalent bond between polymers is characterized by durability (compared to physical cross-linking), and the reaction product itself is characterized by increased stability in a physiological environment and desirable mechanical properties. Several chemical cross-linking methods are distinguished, including the Diels–Alder “click” reaction and Schiff base formation (Hu et al., 2019). Physical cross-linking, on the other hand, relies on reversible intermolecular interactions—ionic, electrostatic, hydrophobic/hydrophilic, and hydrogen bonds. Hydrogels created using physical cross-linking are characterized by the absence of cross-linking agents, which can cause cytotoxicity, self-healing properties, injectability (at room temperature), and stimuli sensitivity (Priya et al., 2024; Bashir et al., 2020). For medical applications, the greatest attention is paid to hydrogels of natural origin. Thanks to the similarity to natural tissues, through softness and elasticity, as well as the ability to retain large amounts of water or biology solutions, it makes them present to candidates for use in regenerative medicine and tissue engineering. New possibilities offered by the modification of hydrogels or combining hydrogels with specific properties allow for the design and creation of new constructs that have the ability to respond to changing conditions that may occur in a living organism or be used for human needs (Ahmed et al., 2025) (Table 1).
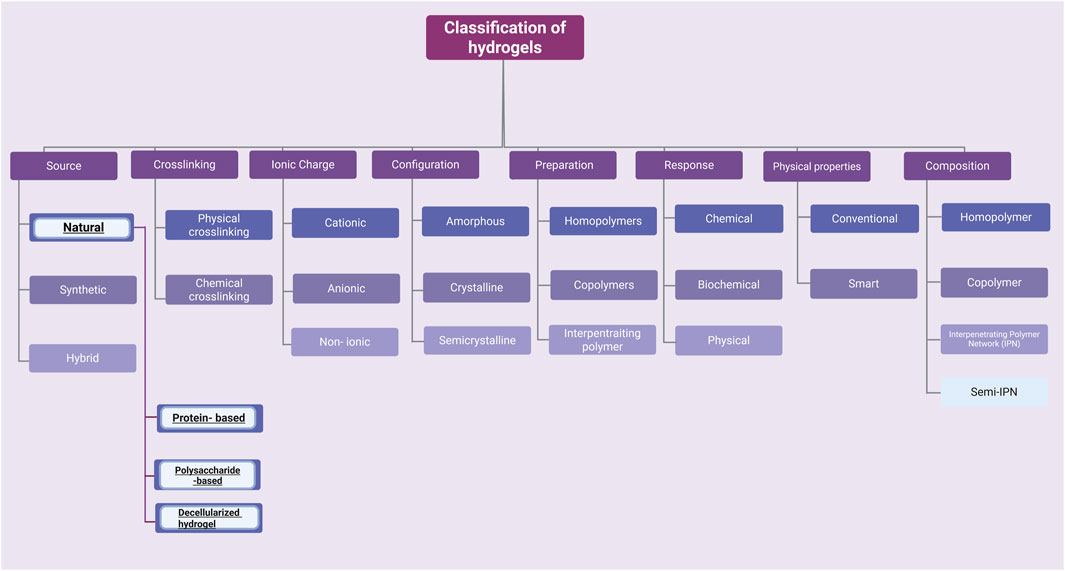
Figure 2. There are many possibilities for classifying hydrogels. This article focuses only on a few examples of naturally derived hydrogels. Created with BioRender.com.
Hydrogels enable prolonged release of chemotherapeutic agents, thereby enhancing treatment effectiveness. Furthermore, in radiotherapy, hydrogels can serve as a carrier to load the right dose of radionuclides that can be disseminated throughout tumour cells (Norouzi et al., 2016). One of the advantages of using hydrogels and nanomaterials is reducing cancer resistance to chemotherapy (Cao et al., 2021). The development of multidrug resistance is the most common cause of death. Hydrogels enable the administration of active substances directly to the tumour (Yadav et al., 2022). Placing the chemotherapy agent in a hydrogel allows for prolonged, controlled release at a lower dose than oral or intravenous administration (Sun et al., 2023). Therefore, there is a significant need for personalized, targeted immunotherapy that allows for the local and controlled release of antibodies, cytokines, and CAR-T cells (Zhu et al., 2024). The greatest barrier to CAR-T cell therapy is ineffective infiltration of solid tumours, which is caused by physical and biological obstacles within the tumour. Hydrogels are effective drug delivery systems that enable a precise and controlled release profile of CAR-T cells (Zhou et al., 2022). Such a carrier can also act as a niche, protecting CAR-T cells from sudden loss of viability after intratumorally administration, or modify the tumour environment by neutralizing hypoxia (Shin et al., 2023). In the field of oncology, research conducted by Gollins et al. (2008) demonstrated the advantages of hydrogel dressings over gentian violet (GV) for patients undergoing radiotherapy treatment on the chest wall or in the head and neck region. However, current recommendations advise against the use of GV, as better healing outcomes are observed with the moist wound bed facilitated by hydrogel dressings. The dry crust formed over the affected area when using GV led to impaired healing (Gollins et al., 2008). Hydrogels appear to be ideal candidates for such smart wound care products (Op ’t Veld et al., 2020).
In this paper, issues related to natural hydrogels: alginates, chitosan and hyaluronic acid, peptide hydrogels, regarding their use in chemo- and radiotherapy were analysed. These biomaterials with a polysaccharide structure are characterized by biocompatibility, bioactivity, and the ability to support the regeneration of damaged tissue. Thanks to the possibility of chemical modification of these biomaterials, it is possible to create solutions with the desired features.
2 Alginate in oncological treatment
Alginate belongs to linear, anionic, acidic polysaccharides of natural origin. Their source is brown algae from the family Phaeophyceae, examples: Ascophyllum nodosum, Laminaria hyperborea, Macrocystis pyrifera, Ecklonia maxima, Laminaria digitata, Laminaria japonica, and Scagassum. Bacteria Azotobacter sp., Pseudomonas sp. Are also used for alginate extraction (Hasnain et al., 2020). It is composed of linear copolymers of mannuronic acid (M) and guluronic acid (G) linked by 1-4 bonds. These alduronic acid residues can form the same types of structures - MM, GG or their combinations MG, GM in different proportions. This is mainly due to the source of alginate. The arrangement of these residues affects the physical and chemical properties of alginate (Hasnain et al., 2020; Shao et al., 2025; Yin et al., 2025).
2.1 Alginate in radiotherapy
One of the key issues is that IR radiation during radiotherapy can affect the process of wound formation and healing. Wound healing during radiotherapy is more complex and complicated than in the case of typical wound healing. It is important to be aware that IR can interact with water, which constitutes 70% in the tissues of the human body, and thus produce reactive oxygen species (ROS), which are responsible for DNA damage. Overproduction of ROS leads to the development of high oxidative stress, which causes the regeneration process during healing to become longer. The presence of open wounds and a weakened immune system during the course of cancer and chemotherapy are prone to skin infections and even to cause deep tissue necrosis (Figure 3).
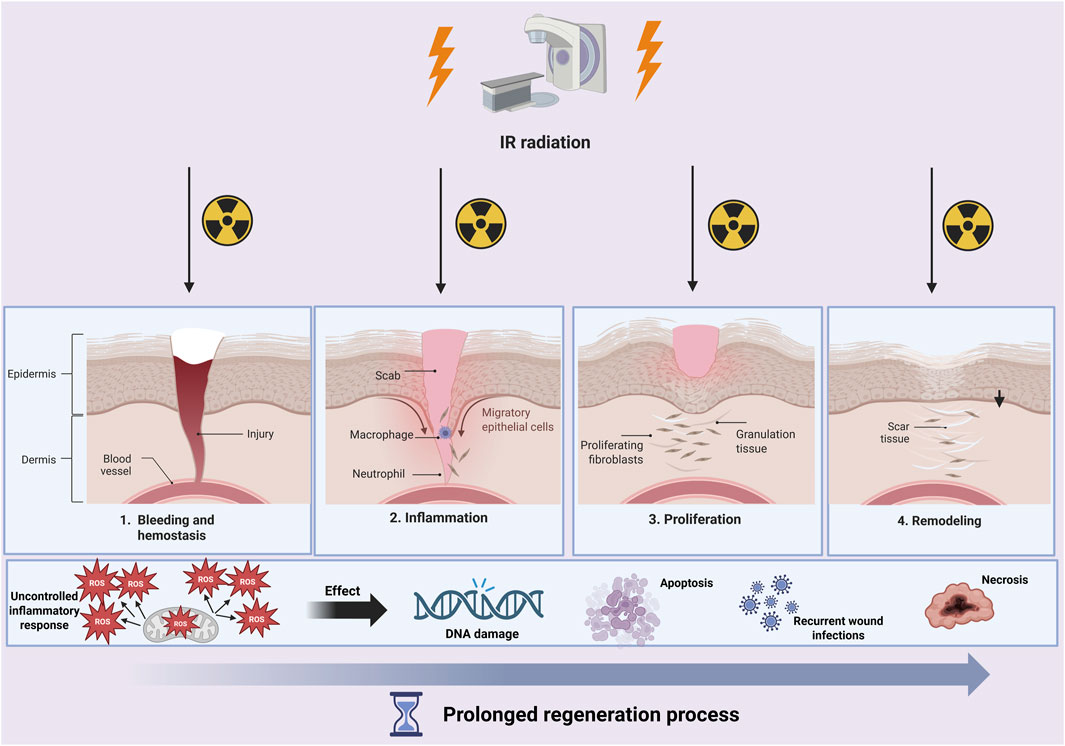
Figure 3. During wound healing and radiotherapy treatment, a cascade of pathological processes can occur. Not only will the healing process be prolonged, but DNA damage, cell apoptosis, or even necrosis may occur. Created with BioRender.com.
Simultaneously, the number of oncologic patients is increasing, in whom the administration of radiotherapy or chemotherapy can result in development of non-healing wounds. Approximately 95% of patients undergoing radiotherapy or radio chemotherapy will develop a skin condition due to the administration of high doses of ionizing radiation, known as radiodermatitis (RD) (Singh et al., 2016). The severity of radiation-induced radiodermatitis (RD) is assessed using the NCI CTCAE [National Cancer Institute-The Common Terminology Criteria for Adverse Events] grading scale and may manifest as erythema, dry desquamation, or moist desquamation. Discomfort, pain, and aesthetic concerns associated with this adverse effect can significantly impair patients’ quality of life, potentially leading to treatment interruptions or even discontinuation (Singh et al., 2016). Therefore, a more advanced wound healing management becomes imperative.
In advanced wound care management, Bonomo et al. (2019) highlight the pivotal role of topical calcium alginate dressings in patients experiencing radiation dermatitis (RD) due to radiotherapy and cetuximab treatment. Their findings suggest that early application of these dressings in cases of grade 2 and 3 RD with presence of moist desquamation significantly improved patients’ compliance, treatment tolerability, and reduced interruptions in radiation treatment. Therefore, authors recommend calcium alginate dressing in management of above-mentioned wounds.
The reports in Cao et al. (2024) indicate the positive effects of hydrogel and alginate dressings in a patient with grade 4 radiation dermatitis with head and neck cancer. The hydrogel dressing maintained a moist environment, supported autolytic debridement of necrotic tissue, and thus the process of phagocytosis, which was responsible for the removal of dead debris and bacteria. The alginate dressing allowed for the removal of excess exudate while providing optimal moisture. An additional advantage was the support of blood clotting and accelerated wound healing. Complete wound healing occurred within 20 days after 17 dressing changes. As the wound healed, the patient’s comfort improved significantly (Cao et al., 2024).
One example of preventing IR-induced skin injury is a dressing created by Zhang J. et al. (2021). Their hydrogel dressing, in which the main components were: alginate, hyaluronic acid, polylysine. Adding curcumin and (−)-epigallocatechin gallate to them allowed for a reduction in inflammation, due to antioxidant and anti-inflammatory properties (Zhang J. et al., 2021).
As another demonstration of the use of alginate-containing hydrogel as a dressing potentially supporting wound healing during radiotherapy is the product developed by Kodous et al. (2024). The researchers synthesized a hydrogel consisting of polyvinyl alcohol and sodium alginate, which was loaded with hesperidin. This flavonoid was subject to controlled release from the hydrogel. The hydrogel treatment reduced the expression of TNF-alpha, NFκB, iNOS and COX2 compared to the BJ-1 cell line stimulated with LPS (Kodous et al., 2024).
Due to the moisturizing properties of sodium alginate researchers (Hao et al., 2022) used it along with interferon-induced protein alpha 6 (IFI6) and graphene oxide to create a hydrogel that would find application in treating radiation-induced skin. Analyses conducted by the team of researchers on an animal model showed that such a designed hydrogel alleviates inflammation in the treatment of radiation-induced skin, and also induces granulation tissue formation, collagen deposition, or angiogenesis. Thanks to these changes, it was possible to close the wound more quickly.
In terms of treating infected wounds during oncological treatment, the Alinezhad et al. (2024) presented a hydrogel containing alginate, hyaluronic acid, polydopamine nanoparticles cross-linked with Zn2+. This type of dressing could potentially be used in photothermal therapy, because the use of near infrared (NIR) caused the generation of heat and the destruction of bacteria - E. coli and S. aureus (Alinezhad et al., 2024).
Alginates have found application in photodynamic therapy (Shu et al., 2021). This method is based on a phototoxic reaction, which occurs through the interaction of a photosensitising substance and light of the appropriate wavelength for the substance. The reaction occurs only in the presence of a photosensitiser, which is characterized by its ability to absorb quanta of energy, transmitted in the form of light. This causes a transition to the excited state and, in turn, a return to the ground state with the radiation of a portion of the energy in the form of the appropriate wavelength. The developed hydrogel is made resistant to luminescence through the incorporation of persistent luminescent materials and an immunoadjuvant (R837) into the Ca2+ alginate hydrogel, allowing for multiple loading of photodynamic cancer immunotherapy. The presented hydrogel showed high biocompatibility and allowed easy injection. The study confirmed a strong immune response and a synergistic effect of photodynamic immunotherapy to inhibit tumorigenesis (Shu et al., 2021).
A hydrogel comprising sodium alginate and catalase, labelled with the radioisotope 131I, was developed to alleviate hypoxia at the tumour site and destroy cancer cells using low doses of radioactivity (Chao et al., 2018). Using an injection, sodium alginate transformed into a hydrogel while binding the radioisotope and catalase at the tumour site.
Detailed information on the studies included in the text can be found in Table 2.
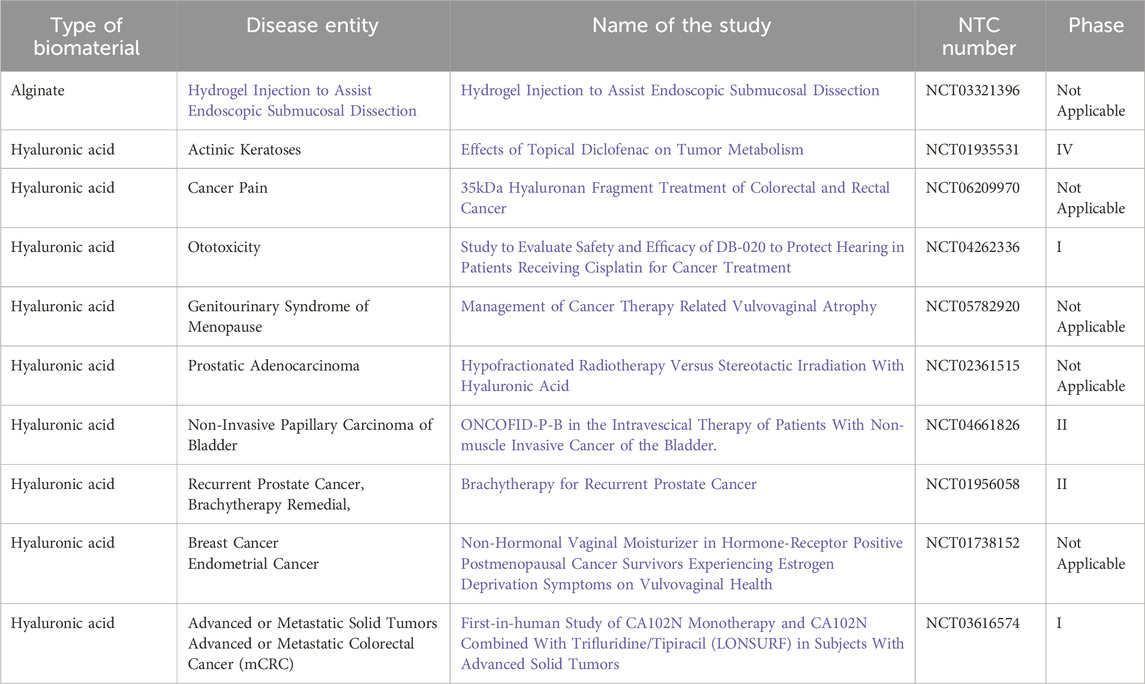
Table 3. List of clinical trials using natural hydrogels with status: completed (clinicaltrials.gov from 02/09/2025).
2.2 Alginate in chemotherapy
In oncological treatment, alginate microparticles combined with paclitaxel have been used as a carrier of cytostatic drugs (Alipour et al., 2010). Researchers used calcium alginate as a mucoadhesive polymer, and its microparticles stick to the mucosa for a long time and are therefore used as vehicles for specific drug delivery to mucosal tissues. The conducted in vitro studies showed that exposure of cells to the paclitaxel alginian carrier and pure paclitaxel inhibited cell growth in a similar way, which depended on concentration and time.
The innovative use of alginate in another study resulted in the release of immunoadjuvants during multiple sessions of chemotherapy/radiotherapy (Sun L. et al., 2021). The component of this hydrogel was sodium alginate, which was conjugated to an ATP-specific aptamer that simultaneously hybridizes to the immunoadjuvant CpG oligonucleotide. After injection, an alginate hydrogel is formed at the tumour site. Its premise was that low doses of oxaliplatin or X-rays would induce immunogenic tumour cell death, which would trigger the release of ATP, which would bind to the ATP-specific aptamer, and this would lead to the release of CpG.
Using the properties of alginate as a carrier, which has the ability to control the release of substances contained in it, Liu Q. et al. (2024) created a type of “in situ vaccine.” The researchers injected a hydrogel that formed near the tumour. It allowed the controlled release of nanoparticle paclitaxel bound to albumin, which shows better efficiency than paclitaxel itself and does not cause allergic conditions, and can also be enriched at the tumour site. Additionally, the hydrogel was attached to the immunostimulant agent R837 – Imiquimod, a TLR9 receptor agonist that promotes dendritic cell maturation, as well as migration, antigen presentation and induction of tumour-specific T lymphocytes. Elements of the immune system such as chemokines and receptors were also significantly involved. Injecting alginate near the tumour allows for a reduced dose of the drug and fewer injections, which reduces toxicity to healthy cells and tissue (Liu Q. et al., 2024).
Carriers for chemotherapeutic drugs can be created using proven and innovative 3D printing technology. Mahdizadeh et al. (2024) used niosome technology, nanocarriers consisting of cholesterol-based vesicles and non-ionic surfactants forming bilayer structures. Their main advantage is low toxicity due to the non-ionic properties of the surfactants (Mahdizadeh et al., 2024).
Detailed information on the studies included in the text can be found in Table 2.
3 Chitosan in oncological treatment
Chitosan is a linear polysaccharide that occurs naturally in the shells of crustaceans, insects and the cell walls of some fungi. It is obtained in the process of deacetylation of chitin. As a biomaterial, it has antibacterial, antifungal and gelling properties (Tymińska et al., 2024). Due to its cationic nature, it is used with negatively charged groups found in proteins, enzymes and other polymers (Li et al., 2020; Sharkawy et al., 2020).
Chitosan, due to its mechanical properties, has been used in the treatment of wounds exposed to radiation as a dressing in the form of a film. In combination with keratin, which has the properties of rapid hemostasis, peripheral nerve repair and supports wound healing, but poor mechanical properties, it resulted in the creation of a product that is easy to use and has high mechanical strength. Studies on a rat model showed an improved healing marker, and a biopsy of the wound site after 7 and 14 days confirmed increased angiogenesis and collagen production (Wang et al., 2025).
3.1 Chitosan in radiotherapy
The current treatment of wounds resulting from radiotherapy involves biological agents such as the administration of epidermal growth factor, synthetic drugs, including corticosteroids, statins, and vitamins. Their use is expensive for the patient and is burdened with side effects. This type of therapy is not precise. Wang et al. (2023) proposed a type of injectable hydrogel based on chitosan and gelatin, among others, to which epigallocatechin gallate, which captures hydroxyl radicals, was added. In cell studies and those conducted on an animal model, the hydrogel constructed in this way demonstrated an angiogenesis-promoting and anti-inflammatory effect (Wang et al., 2023).
In the field of oncology, Su et al. (2021) developed hydrogel, which is near-infrared light-responsive and applicable to peri-tumour injection. This could be used for photothermal therapy, particularly in tongue tumours. Photothermal therapy involves the use of compounds, photothermal agents and local heating of the tissue (Figure 4). The photothermal agent absorbs the laser light, resulting in the excitation of electrons and their transition from the ground state to a higher level. The energy thus supplied is given off in the form of heat instead of by photon emission. This method is characterized by high efficiency and stability, and high stability in time and space. Su et al. used biomaterial, which had a good biocompatibility and strong photothermal effect because of the formed network by negatively charged proteins, chitosan molecules and Ag3AuS2 nanoparticles. The researchers, through the mouse model of tongue cancer used, demonstrated that sublingual cancer cells were eliminated during a single photothermal therapy. The hydrogel system used, thanks to its low immunogenicity, reduced direct biotoxicity and improved therapeutic effects. One of the unique properties of chitosan is that it is a linear polymer with a positive charge. Combination with negatively charged carboxymethylcellulose results in the formation of a polyelectrolyte complex, in which the obtained hydrogel controls the release of substances placed in it. Zhou et al. (2025) presented a hydrogel based on these substrates with the addition of cannabidiol, the effect of which reduces oxidative stress. Studies conducted on mice confirmed and an increase in collagen synthesis.
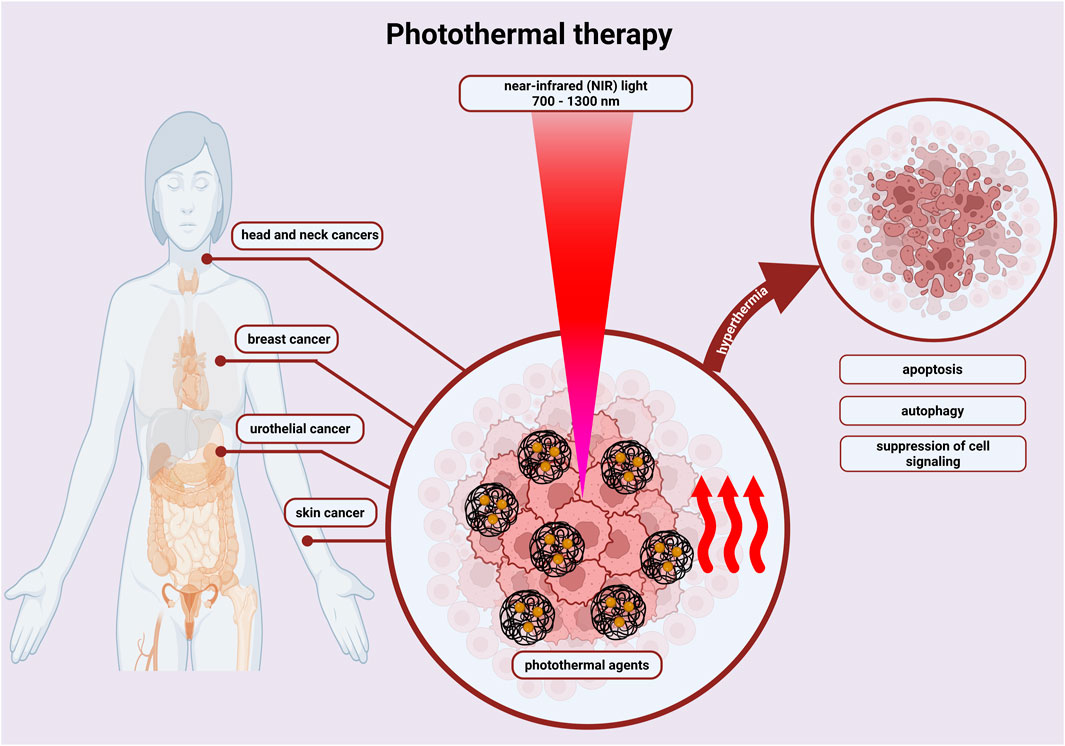
Figure 4. By irradiating photothermal agents with near infrared (NIR) light, hyperthermia is induced, which destroys cancer cells through energy transfer - increasing temperature. Photothermal therapy activates apoptosis, autophagy or suppresses cell signalling, inducing cancer cell death. This is done with a shorter treatment time, reduced pain and fewer side effects. Created with BioRender.com.
The addition of exosomes, which have properties that promote angiogenesis in ischemic tissues and improve the activity of keratinocytes, fibroblasts, endothelial cells and components of the immune system, and their introduction into a hydrogel may bring the desired effects in the case of treatment of radiation injuries. The team of Peng et al. (2024) undertook this task and created a hydrogel based on quaternized chitosan and oxidized sodium alginate with exosomes enclosed in them, which was applied superficially to the wound to obtain a protective barrier. The synthesized hydrogel showed protective properties against microorganisms, promoted rapid re-epithelialization, angiogenesis and collagen deposition (Peng et al., 2024).
The team of Parès et al. (2024) presented a different point of view regarding chitosan hydrogel. Their area of interest was glioblastoma multiforme stage IV. In this aggressive form of cancer, healthy cells surrounding brain tissue are attacked - in them, cancer cells multiply. New reports speak of the attraction and accumulation of glioblastoma multiforme cells in a hydrogel introduced to the site of tumour resection, and then a high dose of radiotherapy is administered. The researchers prepared a macroporous hydrogel consisting of chitosan, sodium alginate, cross-linked with genipin and calcium chloride. Studies conducted on the F98 mCherry cell model distributed, accumulated and were retained throughout the gel volume (Parès et al., 2024).
Detailed information on the studies included in the text can be found in Table 2.
3.2 Chitosan in chemotherapy
Chitosan hydrogels may provide a drug delivery platform for altering antitumor immunity to enhance immunotherapy. Seo et al. (2024) developed a hydrogel based on methacrylated chitosan glycol and a complex of DNA-a sodium salt extracted from salmon testes and doxorubicin. With this combination, controlled release of the DNA/DOX complex was proven to protect against tumour recurrence and metastasis and induce an antitumor response (Seo et al., 2024).
5-Fluorouracil is the most commonly prescribed drug in the treatment of solid tumours. Due to its rapid metabolism by dihydropyrimidine dehydrogenase and short half-life, limited bioavailability and high systemic toxicity, new methods are sought to target it and improve therapeutic efficacy. Chitosan as a nanocarrier has the advantage of creating a uniform particle size distribution, and the release of the substance contained in it is pH dependent. It is important to know that the pH range occurring in tumour tissues differ from the pH range in healthy tissue. Researchers have developed various methods of cross-linking chitosan so that it optimally releases the chemotherapeutic agent contained in it (Wan et al., 2024).
Reducing the side effects of chemotherapeutics is a primary concern in the design of modern drug delivery systems. Bin Jumah et al. (2024) designed a biocomposite with improved physicochemical and biological properties, consisting of a zinc-phosphate/hydroxyapatite hybrid form in core-shell nanostructure and functionalized with both chitosan and β-cyclodextrin as a 5-fluorouracil carrier. Studies on the colon cancer cell line confirmed the antitumor activity and the controlled release profile of 5-fluorouracil (Bin Jumah et al., 2024).
The idea of creating an injectable drug delivery nanosystem that would allow for better bioavailability of the target drug used in chemotherapy allowed the development of the concept by the team of Haider et al. (2024). Erlotinib is ideally suited for this type of system because it has difficulties in dissolving in water, gastrointestinal absorption and low bioavailability. In vivo studies have confirmed that intratumorally injection of the preparation causes a slowdown in tumour growth (Haider et al., 2024).
Similar conclusions were used by Daneshmehr et al. (2024), who studied the attachment of 5-fluorouracil and schionine to hydrogel nanoparticles of chitosan and pectin. They were prepared by two methods: mixing and coating. The studies carried out on cell lines for the treatment of colon cancer indicated that the mixing method showed the appropriate loading power (Daneshmehr et al., 2024).
Hydrogel based on chitosan and tannic acid was created as an antioxidant crosslinker whose goal was to control the activity of photodynamic therapy used in the treatment of cancer (Azadikhah et al., 2021). In this method, it should be mentioned that photosensitizers cause the formation of reactive oxygen species during photochemical reactions. This results in oxidative damage to cancer cells (Figure 5). The research showed that chitosan with the addition of tannic acid formed a three-dimensional structure that had good mechanical strength and antioxidant activity. The designed hydrogel effectively inhibited tumour growth and protected the dental pulp stem cells from phototoxicity.
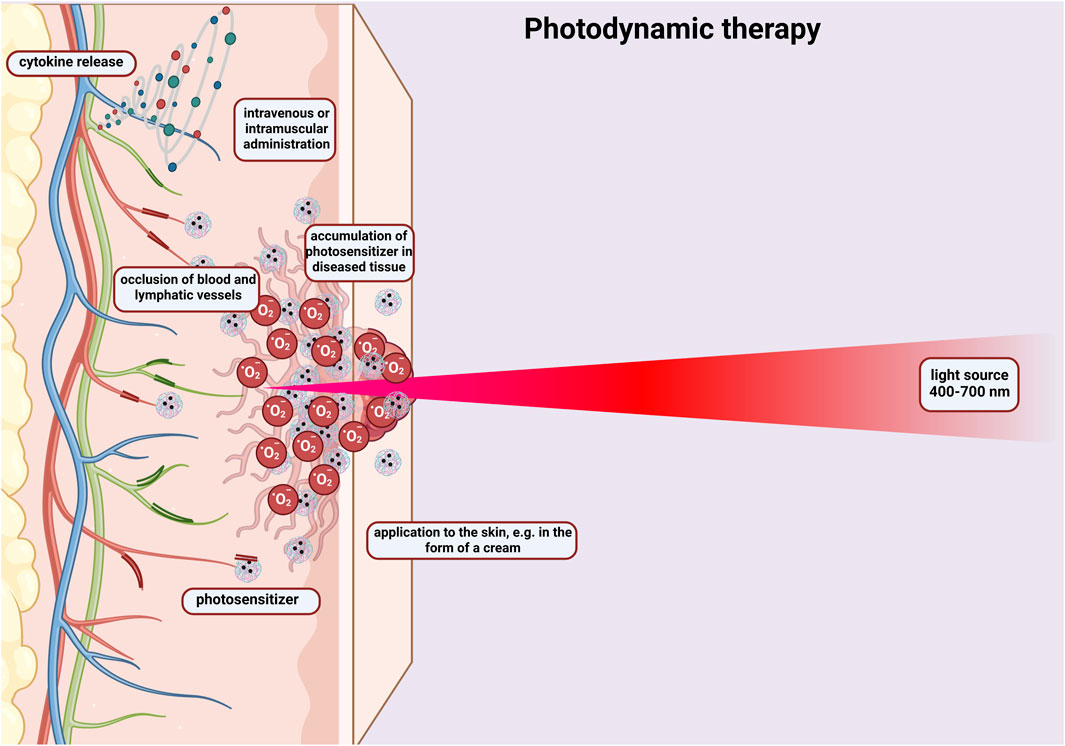
Figure 5. Diagram showing the principle of photodynamic therapy. Activation of a photosensitizer administered intravenously or to the skin in the form of a cream with visible light, which leads to the formation of free oxygen radicals that destroy cancer cells. The photosensitizer penetrates the cells changed by the cancer. The therapy causes occlusion of blood and lymphatic vessels and the release of cytokines. Created with BioRender.com.
Newly developed hydrogels containing N-carboxyethylchitosan has a pH-sensitive property, which were presented as carriers for doxorubicin (Qu et al., 2017). Analyses showed that the pH-sensitive hydrogels released more doxorubicin around/inside the tumour than in normal healthy tissue. With such carriers, it is possible to have a more effective, targeted delivery site for the anti-tumour drug, reduce side effects and protect healthy tissue not occupied by the tumour.
What’s more a biomimetic, thermosensitive hydrogel had ability to incorporate heterodimers of tegafur, protoporphyrin IX (Zhang Z. et al., 2021). The action was to combine chemotherapy with photodynamic therapy. Due to the laser effect, the concentration of reactive oxygen species increased in the tumour, and this phenomenon was important for the release of the drug. The drug injected, and released only with the laser action, reduced the disorderly effects. The researchers opted for chitosan and silk sericin. The thermosensitive properties exhibited by chitosan played an essential role in the choice of carrier.
A related case of the use of chitosan in oncology is chitosan-based hydrogel that contained albumin beads, which as a carrier are characterized by accumulation near cancer cells, and aloe vera juice, which is characterized by antibacterial and anti-inflammatory properties (Kudłacik-Kramarczyk et al., 2021). The hydrogel constructed in this way was to be used for the treatment of skin cancer or burn wounds resulting from radiation therapy. Analyses showed that the tested hydrogels were non-toxic to L929 mouse fibroblasts, and albumin beads, which are a component of the hydrogel.
A hydrogel incorporating chitosan and conjugated with porphyrins was developed to enhance the application of photodynamic therapy (Belali et al., 2018). The hydrogel designed in this way caused the excitation energy to be transferred to the porphyrin unit, and this resulted in an improved release of singlet oxygen. Cytotoxicity and phototoxicity studies of chitosan-based hydrogels highlighted the property to selectively kill cancer cells and protect healthy body cells.
Patients undergoing oncological treatment face various side effects of this therapy, one of them is ulceration of the mucous membranes. Choi et al. (2016) prepared two-layer electrospun fiber sheets that consisted of Eudraugite, chitosan and human growth hormone. Fiber sheets containing growth hormone resulted in better proliferation of human fibroblasts. Studies on an animal model proved that this type of dressing could improve the healing of mucosal ulcers.
Detailed information on the studies included in the text can be found in Table 2.
4 Hyaluronic acid in oncological treatment
Hyaluronic acid (HA) is a linear mucopolysaccharide composed of repeating units of β-1,4-D-glucuronic acid and β-1,3-N-acetylglucosamine. The alternating β-1,3 bonds are responsible for the high elasticity and solubility of hyaluronic acid polymers. It is the main component of the extracellular matrix (Gupta et al., 2019; Buckley et al., 2022; Valachová et al., 2024; Encyclopedia, 2025). HA has some disadvantages, it plays a role in tumour physiology, increased hyaluronan level mostly is associated with poor prognosis. HA and hyaluronidase have both: pro- and antitumor effect, which is concentration and origin dependent (Salwowska et al., 2016). HA has also poor mechanical properties and rapid degradation in vivo, but chemical modification and crosslinking can overcome disadvantages (Chircov et al., 2018).
4.1 Hyaluronic acid in radiotherapy
An example of creating hydrogel dressings using HA is the work of Xia et al. (2025), in which they presented a dressing that protected against radiation and at the same time had antioxidant properties. Additionally, the structure of the patch contained epidermal growth factor (EGF), which supported skin healing. The dressing designed by the researchers uses the property of releasing large amounts of free radicals, then the bonds in the structure of the dressing can be oxidized and transformed into a hydrophilic sulfone. The consequence of this action is the stretching of the hydrogel network and the release of the encapsulated EGF (Xia et al., 2025).
The use of HA cream in patients experiencing radiation dermatitis (RD) shows promise in alleviating symptoms. Preliminary data presented by Gracy et al. (2004) suggest that topical application of HA protected cultured fibroblasts from radiation damage. Chan et al. (2014) advocate for the additional use of HA to provide symptomatic relief, among other topical agents, as in vivo studies have not yet definitively established its benefits in preventing the development of RD.
Another example of using a scheme in which the primary element is the elimination of free radicals formed in wounds during radiotherapy is the creation by Shen et al. (2025) of an injectable hydrogel with Pluronic F127 diacrylate and hyaluronic acid methacryloyl, and nanoparticles of Prussian blue and resveratrol placed in them. It was proven that the hydrogel promoted the migration of fibroblasts to the site of oxidative stress A subsequent exampleis placing proteins and stem cells in hydrogels. Jin et al. (2015) created a hyaluronic acid-based hydrogel loaded with rat mesenchymal stem cells and bone morphogenetic protein-2 (BMP-2). The biomaterial was designed to affect bone healing in osteoradionecrosis of the rat mandible. Anchoring the hydrogel with stem cells and BMP-2 increased bone mineral density and bone volume.
An important aspect is the inhibition of the growth of cancer cells. Tang et al. (2019) developed a hydrogel that included hyaluronic acid, cytarabine and tyramine in its formulation. Combination therapy with radiotherapy significantly reduced 18F-FDG uptake, triggered increased apoptosis and histone H2AX phosphorylation, cell cycle arrest in the G2/M phase, and reduced the proliferation rate in tumour cells compared to monotherapy.
To mitigate the damage caused by radiotherapy, Fang et al. (2023) designed a hydrogel that included cross-linked carbohydrazide-modified gelatin and oxidized HA, polydopamine nanoparticles, and extracellular vesicles secreted by mesenchymal stem cells. In vitro studies showed enhanced cell viability, as evidenced by stimulation of proliferation and migration. Proteomic studies proved that the hydrogel alleviated the radiation-related wound microenvironment by regulating adipocyte and hypoxia-related pathways.
The advantage of hyaluronic acid as a biomaterial is that it can combine with collagen and fibronectin, i.e., matrix structures in tissues, creating a temporary scaffold. Such an organization of materials can effectively promote cell adhesion, migration and proliferation, collagen synthesis. However, its disadvantages are also noticed: poor durability in an aqueous environment, sensitivity to hyaluronidase or free radicals. Therefore, Wang et al. (2024) created a hydrogel based on hyaluronic acid and a self-assembling peptide with attached cordycipin. Hyaluronic acid was to strengthen non-covalent interactions with the peptide. The scaffold skeleton created in this way supported cell adhesion and proliferation.
Detailed information on the studies included in the text can be found in Table 2.
4.2 Hyaluronic acid in chemotherapy
Hyaluronic acid has high affinity for the CD44 receptor, which is overexpressed in cancer cells. Liu et al. (2023) used this property and designed micelles in which modified hyaluronic acid served as a carrier to bind to CD44. In vivo and in vitro studies confirmed inhibition of tumour growth. And the drug delivery vehicle itself was nontoxic.
A very valuable technological achievement is the placement of drugs in hydrogel carriers. In studies by Wang et al. (2021), HA-tyramine was utilized to create a carrier for endostatin, which could be locally injected. The hydrogel prepared in this way showed longer half-life, less systemic toxic side effects. ES/HA-Tyr revealed better anti-angiogenic effect tested on cellular model and anti-tumour effect with radiotherapy tested on animal model. Intra-tumour administration allowed the drug to increase its concentration locally, thereby reducing serum endostatin levels.
Similarly, Li et al. (2016) developed redox-sensitive and intrinsically fluorescent photoclick hyaluronic acid nanogels to deliver cytochrome c to xenografted MCF-7 breast tumours in mice. The study confirmed that the production of the hyaluronic acid nanogel had high specificity. The prepared nanogel allows rapid targeting of tumour cells and rapid release of therapeutic proteins under cytoplasmic reduction conditions while providing potent anti-tumour activity. By exhibiting intrinsic fluorescence, potential use for in vivo tumour monitoring is possible.
Detailed information on the studies included in the text can be found in Table 2.
5 Peptide hydrogels in oncological treatment
Peptide hydrogels are a soft material platform that uses amino acids and peptides as building blocks that can retain water or transform into a hydrogel under physiological conditions. Peptides enable the formation of hydrogels and nanoparticles through self-assembly, which is formed similarly to the beta-arc and alpha-helical structures in peptide networks and globular protein structures (Fu et al., 2021; Mitrovic et al., 2023; Nizam et al., 2025).
5.1 Peptide hydrogels in radiotherapy
Skin changes after radiotherapy are a serious problem that occurs in patients undergoing this therapy. Hao et al. (2023) created a hydrogel with antioxidant properties, which included a heparinomimetic peptide. The created system was intended to be used in the process of repairing skin damage caused by radiation. The researchers used the K16 peptide, which had the ability to self-assemble into a hydrogel that formed a 3D mesh and thus created an environment resembling the extracellular matrix. Studies showed that this peptide also supported cell proliferation, migration, angiogenesis, well as protecting cell DNA against radiation-induced damage. The hydrogel dressing prepared in this way promoted collagen deposition and inhibited early wound degradation.
Researchers have also drawn attention to the problem of radiation-induced ototoxicity. This occurs in head and neck cancers. The mechanism of this phenomenon is not fully understood, probably radiolysis of water, which is induced by radiation, is the hotspot of cochlear cell destruction. Liu J. et al. (2024) proposed an injectable peptide hydrogel based on RADA with attached dexamethasone. Additionally, the developed peptide hydrogel reactivated the mTOR signalling pathway, which allowed protection of neuronal cells. During radiotherapy, this signalling pathway is suppressed and neuronal cells in the cochlea are destroyed.
The property of peptide hydrogels is to prolong drug retention by spatial restriction. Zhu et al. (2023) constructed a nonapeptide hydrogel with doxorubicin that responds to changing pH in the environment. The developed nonapeptide called P1 belongs to the type of self-assembling peptides, similar to surfactants. The proposed model had high efficiency of doxorubicin encapsulation, retained sensitivity in an acidic environment. Detailed information on the studies included in the text can be found in Table 2.
5.2 Peptide hydrogels in chemotherapy
The team of Ye et al. (2024) had a similar assumption. In their work, they presented the IEIIIK peptide, which had the ability to surround doxorubicin and self-assemble into a hydrogel under physiological conditions, and after injection into the acidic environment of the tumour, was able to release doxorubicin and allowed its accumulation within the tumour cells.
An important challenge is to create effective peptide hydrogels as carriers of cytostatic drugs, which, thanks to their properties, can influence the change of the microenvironment inside the tumour. Jin et al. (2018) created a of melittin-RADA32 hybrid peptide hydrogel, which was loaded with doxorubicin. It was designed to affect the immunosuppressive tumour microenvironments in melanoma.
This biomaterial has been shown to have the property of controlled drug release and to affect immune cells. As for subcutaneous and metastatic tumours, this hydrogel showed strong anti-tumour efficacy in their case. Raza et al. (2019) developed a FER-8 peptide hydrogel loaded with paclitaxel, which was also pH-sensitive. Analyses in an animal model showed that the peptide hydrogel, as a carrier for paclitaxel, significantly increased the amount of drug in tumour tissues, and by direct injection into the tumour, showed prolonged retention at the injection site. Due to the drug’s release property in the presence of acidic pH, prolonged delivery of the drug and thus increased tumour inhibition was possible.
In an attempt to overcome the reduced efficacy when administering combination therapy, when two or more drugs are used that would reach the target at the same time, Liu et al. (2022) were designing carriers that could release the active substances at the same time. The study showed that the peptide carrier for both drugs, when injected into the tumour site, results in a prolonged and concentrated release -gemcitabine, which is hydrophilic in nature, is released in large amounts, while paclitaxel, which is hydrophobic in nature, is released slowly, allowing for prolonged drug action and increased efficacy.
Detailed information on the studies included in the text can be found in Table 2.
6 Limitations of hydrogels in oncology therapies
Although hydrogels are a promising strategy for therapeutic applications in oncology, they also have certain limitations (Omidian et al., 2024). The first is the detailed replication of the mechanical properties of natural tissues. In practice, tissues are characterized by significantly more complex mechanics, and simple parameters such as Young’s modulus are not sufficient to achieve a biomimetic environment (Roth et al., 2023; Fatima and Almeida, 2024). Another aspect to consider when designing hydrogels is the excessively rapid degradation of the carrier (Zhu et al., 2025). This can result in a lack of control over the release of the active substance, thus leading to a failure to achieve the intended therapeutic effects (Segneanu et al., 2025). There is also the possibility of uneven distribution of the hydrogel at the site of administration, which can lead to unpredictable body reactions (Almawash et al., 2022). Technological limitations are another obstacle to the use of hydrogels in oncological therapies. The challenge is the reproducible synthesis of hydrogels with specific mechanical and biological properties. It should be noted that there is little data on the long-term effects of hydrogels in oncological therapies. Most reports and publications refer to studies on animals and cell lines. Translating these findings into the human body could yield completely different results. This could result in the termination of promising studies. It should be noted that there is little data on the long-term effects of using hydrogels in cancer therapies. One important factor is financial considerations. Developing high-quality hydrogels on a large scale is expensive. Clinical registration and eventual commercialization of the product are a separate issue. The process can take several years and involve significant costs. Various regulatory aspects, including legal and scientific requirements, must also be considered (Almawash et al., 2022; Roth et al., 2023; Fatima and Almeida, 2024; Omidian et al., 2024).
7 Future perspectives
Hydrogels represent a highly promising class of materials for clinical applications. Future research is expected to focus on the development of intelligent, stimuli-responsive systems (e.g., triggered by enzymes, pH or light/laser stimulation), enabling controlled and localized drug release within tumor tissues. Another important direction involves exploring the role of hydrogels in inhibiting metastatic spread by serving as anti-adhesion and protective barriers. Hybrid hydrogels incorporating functional particles such as liposomes or exosomes, hold great potential for enhancing the targeted delivery of therapeutic agents. Moreover, the integration of mRNA into hydrogel systems opens opportunities for advancing personalized cancer therapies. Efforts should also be directed toward the design of fully biodegradable and immunologically safe hydrogels to ensure clinical safety and efficacy. Finally the incorporation of artificial intelligence, and 3D printing technologies into hydrogel development is likely to accelerate the creation of patient-specific solutions, advancing both regenerative medicine and oncology.
8 Conclusion
Hydrogels based on natural components such as alginate, chitosan, hyaluronic acid or self-assembling peptide hydrogels show great potential in oncological treatment. Thanks to their unique properties such as biocompatibility and biodegradability, the ability to absorb large amounts of water and create 3D structures, they are becoming modern, desirable solutions in the field of drug carriers and dressings (Zielińska et al., 2023; Satchanska et al., 2024; Arif, 2025). Translating the results and innovative solutions from basic research into clinical trials is very tedious. This is evidenced by the number of clinical trials using hydrogels in oncology therapies that have been completed. Only two types of hydrogels have been used in research: alginate and hyaluronic acid. The patient’s wellbeing must be taken into account, because the body is in a weaker condition due to the fight against cancer, additional side effects such as the appearance of wounds and ulcers, and consequently disfigurement, negatively affect the further treatment process. The formation of radiation-induced wounds causes the accumulation of ROS (Liu C. et al., 2024). This phenomenon disrupts the redox balance and triggers an inflammatory response, thus increasing the production of proinflammatory cytokines and activating signalling pathways that lead to cell damage (Pizzino et al., 2017). This causes cell cycle arrest and the production of aging-related factors. Hydrogels and their modifications can act as free radical scavengers and reduce oxidative damage in healthy tissues (Joorabloo and Liu, 2024). The cited studies confirm the healing-promoting properties in the treatment of wounds during radiotherapy treatment. Drugs used in chemotherapy have low specificity, limited solubility and bioavailability, and when taken in large doses, they have a toxic effect on the body (Zhang et al., 2022; Prieložná et al., 2024). It has been proven that hydrogels as drug carriers cause prolonged drug retention at the tumour site, while limiting diffusion to surrounding tissues (Ma et al., 2022; Mikhail et al., 2023; Lu et al., 2024). This action allows for minimizing systemic toxicity and drug access even to poorly vascularized tumour areas.
The last two decades have seen significant advances beyond oncology therapies, including surgical techniques for tumor resection. A milestone was the introduction of robotics (Chatterjee et al., 2024) and the use of techniques to assess tissue perfusion using indocyanine green (Cassinotti et al., 2023). However, the progress that has been made has not significantly changed the problem of leakage in the digestive tract, which is 3%–30% (Chiarello et al., 2022). Research is currently underway into the use of hydrogels as an adjunct method in gastrointestinal anastomoses, although their use is currently experimental. The additional use of hydrogels would benefit from reducing the rate of leaks at the anastomosis site. However, one of the drawbacks of using hydrogels is the difficulty in maintaining this type of preparation at the anastomosis site, which should be applied externally to the anastomosis, where the outer layer is smooth serosa.
The development of hydrogels in oncology in the coming years will dynamically evolve towards personalized therapy and intelligent therapeutic systems (Lee et al., 2025; Liu et al., 2025) (Figure 6). Their further development towards modification may change the perspective on cancer treatment methods. It is important to support interdisciplinary research, especially combining basic science with practical knowledge transfer in clinical practice.
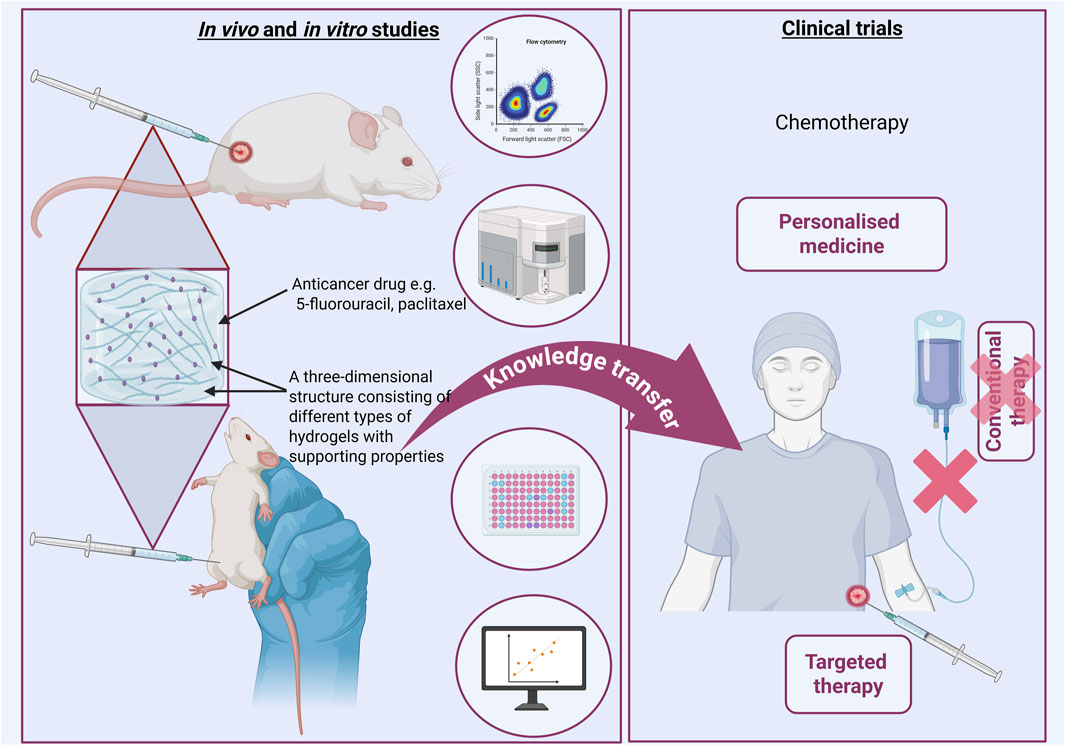
Figure 6. Intensive research in basic sciences is a real foundation for using their achievements in clinical trials. Perhaps in the near future it will be possible to use these achievements in practice. Created with BioRender.com.
Author contributions
KC: Visualization, Writing – original draft, Writing – review and editing, Supervision. WS: Writing – original draft. MP: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Science Centre—Poland [grant number 2019/33/B/NZ7/02676 granted to MP].
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahmed, Md., Shahriar, S. Y., Kim, H.-Y., Ko, S., Islam, M., and Nam, K.-W. (2025). Hydrogels and microgels: driving revolutionary innovations in targeted drug delivery, strengthening infection management, and advancing tissue repair and regeneration. Gels 11, 179. doi:10.3390/gels11030179
Akhtar, M. F., Hanif, M., and Ranjha, N. M. (2016). Methods of synthesis of hydrogels A review. Saudi Pharm. J. 24, 554–559. doi:10.1016/j.jsps.2015.03.022
Alavarse, A. C., Frachini, E. C. G., da Silva, R. L. C. G., Lima, V. H., Shavandi, A., and Petri, D. F. S. (2022). Crosslinkers for polysaccharides and proteins: synthesis conditions, mechanisms, and crosslinking efficiency, a review. Int. J. Biol. Macromol. 202, 558–596. doi:10.1016/j.ijbiomac.2022.01.029
Alinezhad, V., Ghodsi, R., Bagheri, H., Beram, F. M., Zeighami, H., Kalantari-Hesari, A., et al. (2024). Antioxidant, hemostatic, and injectable hydrogels with photothermal antibacterial activity to accelerate full-thickness wound regeneration. New J. Chem. 48, 7761–7778. doi:10.1039/D3NJ05871A
Alipour, S., Montaseri, H., and Tafaghodi, M. (2010). Preparation and characterization of biodegradable paclitaxel loaded alginate microparticles for pulmonary delivery. Colloids Surf. B Biointerfaces 81, 521–529. doi:10.1016/j.colsurfb.2010.07.050
Almawash, S., Osman, S. K., Mustafa, G., and El Hamd, M. A. (2022). Current and future prospective of injectable hydrogels—design challenges and limitations. Pharmaceuticals 15, 371. doi:10.3390/ph15030371
Amjad, M. T., Chidharla, A., and Kasi, A. (2025). “Cancer chemotherapy,” in StatPearls (Treasure Island, FL: StatPearls Publishing). Available online at: http://www.ncbi.nlm.nih.gov/books/NBK564367/(Accessed May 13, 2025).
Arif, Z. U. (2025). The role of polysaccharide-based biodegradable soft polymers in the healthcare sector. Adv. Industrial Eng. Polym. Res. 8, 132–156. doi:10.1016/j.aiepr.2024.05.001
Azadikhah, F., Karimi, A. R., Yousefi, G. H., and Hadizadeh, M. (2021). Dual antioxidant-photosensitizing hydrogel system: cross-linking of chitosan with tannic acid for enhanced photodynamic efficacy. Int. J. Biol. Macromol. 188, 114–125. doi:10.1016/j.ijbiomac.2021.08.006
Barazzuol, L., Coppes, R. P., and van Luijk, P. (2020). Prevention and treatment of radiotherapy-induced side effects. Mol. Oncol. 14, 1538–1554. doi:10.1002/1878-0261.12750
Bashir, S., Hina, M., Iqbal, J., Rajpar, A. H., Mujtaba, M. A., Alghamdi, N. A., et al. (2020). Fundamental concepts of hydrogels: synthesis, properties, and their applications. Polymers 12, 2702. doi:10.3390/polym12112702
Belali, S., Karimi, A. R., and Hadizadeh, M. (2018). Cell-specific and pH-sensitive nanostructure hydrogel based on chitosan as a photosensitizer carrier for selective photodynamic therapy. Int. J. Biol. Macromol. 110, 437–448. doi:10.1016/j.ijbiomac.2017.12.169
Bin Jumah, M. N., Al Othman, S. I., Alomari, A. A., Allam, A. A., Bellucci, S., and Abukhadra, M. R. (2024). Insight into the integration effect of chitosan and β-cyclodextrin on the properties of zinc-phosphate/hydroxyapatite hybrid as delivery structures for 5-fluorouracil: loading and release profiles. Front. Chem. 12, 1456057. doi:10.3389/fchem.2024.1456057
Bonomo, P., Desideri, I., Loi, M., Ciccone, L. P., Lo Russo, M., Becherini, C., et al. (2019). Management of severe bio-radiation dermatitis induced by radiotherapy and cetuximab in patients with head and neck cancer: emphasizing the role of calcium alginate dressings. Support Care Cancer 27, 2957–2967. doi:10.1007/s00520-018-4606-2
Bray, F., Laversanne, M., Sung, H., Ferlay, J., Siegel, R. L., Soerjomataram, I., et al. (2024). Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 74, 229–263. doi:10.3322/caac.21834
Brown, J. S., Amend, S. R., Austin, R. H., Gatenby, R. A., Hammarlund, E. U., and Pienta, K. J. (2023). Updating the definition of cancer. Mol. Cancer Res. 21, 1142–1147. doi:10.1158/1541-7786.MCR-23-0411
Buckley, C., Murphy, E. J., Montgomery, T. R., and Major, I. (2022). Hyaluronic acid: a review of the drug delivery capabilities of this naturally occurring polysaccharide. Polym. (Basel) 14, 3442. doi:10.3390/polym14173442
Bustamante-Torres, M., Romero-Fierro, D., Arcentales-Vera, B., Palomino, K., Magaña, H., and Bucio, E. (2021). Hydrogels classification according to the physical or chemical interactions and as stimuli-sensitive materials. Gels 7, 182. doi:10.3390/gels7040182
Cao, L., Zhu, Y., Wang, W., Wang, G., Zhang, S., and Cheng, H. (2021). Emerging nano-based strategies against drug resistance in tumor chemotherapy. Front. Bioeng. Biotechnol. 9, 798882. doi:10.3389/fbioe.2021.798882
Cao, Y., Zhu, C., Zhu, H., Chen, X., and Luo, H. (2024). Observation of the therapeutic effect of hydrogel combined with alginate dressings for a patient with grade 4 acute radiation dermatitis: a case report. Adv. Skin. Wound Care 37, 1–5. doi:10.1097/ASW.0000000000000198
Cassinotti, E., Al-Taher, M., Antoniou, S. A., Arezzo, A., Baldari, L., Boni, L., et al. (2023). European association for endoscopic surgery (EAES) consensus on indocyanine green (ICG) fluorescence-guided surgery. Surg. Endosc. 37, 1629–1648. doi:10.1007/s00464-023-09928-5
Chan, R. J., Webster, J., Chung, B., Marquart, L., Ahmed, M., and Garantziotis, S. (2014). Prevention and treatment of acute radiation-induced skin reactions: a systematic review and meta-analysis of randomized controlled trials. BMC Cancer 14, 53. doi:10.1186/1471-2407-14-53
Chao, Y., Xu, L., Liang, C., Feng, L., Xu, J., Dong, Z., et al. (2018). Combined local immunostimulatory radioisotope therapy and systemic immune checkpoint blockade imparts potent antitumour responses. Nat. Biomed. Eng. 2, 611–621. doi:10.1038/s41551-018-0262-6
Chatterjee, S., Das, S., Ganguly, K., and Mandal, D. (2024). Advancements in robotic surgery: innovations, challenges and future prospects. J. Robot. Surg. 18, 28. doi:10.1007/s11701-023-01801-w
Chen, S., Cao, Z., Prettner, K., Kuhn, M., Yang, J., Jiao, L., et al. (2023). Estimates and projections of the global economic cost of 29 cancers in 204 countries and territories from 2020 to 2050. JAMA Oncol. 9, 465–472. doi:10.1001/jamaoncol.2022.7826
Chiarello, M. M., Fransvea, P., Cariati, M., Adams, N. J., Bianchi, V., and Brisinda, G. (2022). Anastomotic leakage in colorectal cancer surgery. Surg. Oncol. 40, 101708. doi:10.1016/j.suronc.2022.101708
Chircov, C., Grumezescu, A. M., and Bejenaru, L. E. (2018). Hyaluronic acid-based scaffolds for tissue engineering. Rom. J. Morphol. Embryol. 59, 71–76.
Choi, J. S., Han, S.-H., Hyun, C., and Yoo, H. S. (2016). Buccal adhesive nanofibers containing human growth hormone for oral mucositis. J. Biomed. Mater Res. B Appl. Biomater. 104, 1396–1406. doi:10.1002/jbm.b.33487
Collatuzzo, G., and Boffetta, P. (2023). Cancers attributable to modifiable risk factors: a road map for prevention. Annu. Rev. Public Health 44, 279–300. doi:10.1146/annurev-publhealth-052220-124030
Daneshmehr, M., Pazhang, M., Mollaei, S., Ebadi, M., and Pazhang, Y. (2024). Targeted delivery of 5-fluorouracil and shikonin by blended and coated chitosan/pectin nanoparticles for treatment of colon cancer. Int. J. Biol. Macromol. 270, 132413. doi:10.1016/j.ijbiomac.2024.132413
Dodda, J. M., Deshmukh, K., Bezuidenhout, D., and Yeh, Y.-C. (2023). Hydrogels: definition, history, classifications, formation, constitutive characteristics, and applications. 1, 25. doi:10.1039/BK9781837670055-00001
Encyclopedia (2025). Design challenges and limitations of injectable hydrogels. Available online at: https://encyclopedia.pub/entry/21456 (Accessed September 4, 2025).
Fang, Z., Lv, Y., Zhang, H., He, Y., Gao, H., Chen, C., et al. (2023). A multifunctional hydrogel loaded with two nanoagents improves the pathological microenvironment associated with radiation combined with skin wounds. Acta Biomater. 159, 111–127. doi:10.1016/j.actbio.2023.01.052
Fatima, R., and Almeida, B. (2024). Methods to achieve tissue-mimetic physicochemical properties in hydrogels for regenerative medicine and tissue engineering. J. Mater. Chem. B 12, 8505–8522. doi:10.1039/D4TB00716F
Fu, K., Wu, H., and Su, Z. (2021). Self-assembling peptide-based hydrogels: fabrication, properties, and applications. Biotechnol. Adv. 49, 107752. doi:10.1016/j.biotechadv.2021.107752
Gianfaldoni, S., Gianfaldoni, R., Wollina, U., Lotti, J., Tchernev, G., and Lotti, T. (2017). An overview on radiotherapy: from its history to its current applications in dermatology. Open Access Maced. J. Med. Sci. 5, 521–525. doi:10.3889/oamjms.2017.122
Gollins, S., Gaffney, C., Slade, S., and Swindell, R. (2008). RCT on gentian violet versus a hydrogel dressing for radiotherapy-induced moist skin desquamation. J. Wound Care 17, 268–275. doi:10.12968/jowc.2008.17.6.29589
Gracy, R., Lloyd, K., and Phelps, J. (2004). RadiaPlexRx gel protects cultured skin cells from oxidative free radical damage induced by hydrogen peroxide and by irradiation: a pilot study.
Gupta, R. C., Lall, R., Srivastava, A., and Sinha, A. (2019). Hyaluronic acid: molecular mechanisms and therapeutic trajectory. Front. Vet. Sci. 6, 192. doi:10.3389/fvets.2019.00192
Haider, M., Jagal, J., Ali Alghamdi, M., Haider, Y., Hassan, H. A. F. M., Najm, M. B., et al. (2024). Erlotinib and curcumin-loaded nanoparticles embedded in thermosensitive chitosan hydrogels for enhanced treatment of head and neck cancer. Int. J. Pharm. 666, 124825. doi:10.1016/j.ijpharm.2024.124825
Hao, J., Sun, M., Li, D., Zhang, T., Li, J., and Zhou, D. (2022). An IFI6-based hydrogel promotes the healing of radiation-induced skin injury through regulation of the HSF1 activity. J. Nanobiotechnology 20, 288. doi:10.1186/s12951-022-01466-x
Hao, Y., Li, H., Guo, J., Wang, D., Zhang, J., Liu, J., et al. (2023). Bio-inspired antioxidant heparin-mimetic peptide hydrogel for radiation-induced skin injury repair. Adv. Healthc. Mater 12, e2203387. doi:10.1002/adhm.202203387
Hasnain, M. S., Jameel, E., Mohanta, B., Dhara, A. K., Alkahtani, S., and Nayak, A. K. (2020). “Chapter 1 - alginates: sources, structure, and properties,” in Alginates in drug delivery. Editors A. K. Nayak, and M. S. Hasnain (Academic Press), 1–17. doi:10.1016/B978-0-12-817640-5.00001-7
Ho, T.-C., Chang, C.-C., Chan, H.-P., Chung, T.-W., Shu, C.-W., Chuang, K.-P., et al. (2022). Hydrogels: properties and applications in biomedicine. Molecules 27, 2902. doi:10.3390/molecules27092902
Hu, W., Wang, Z., Xiao, Y., Zhang, S., and Wang, J. (2019). Advances in crosslinking strategies of biomedical hydrogels. Biomater. Sci. 7, 843–855. doi:10.1039/C8BM01246F
Iacovelli, N. A., Torrente, Y., Ciuffreda, A., Guardamagna, V. A., Gentili, M., Giacomelli, L., et al. (2020). Topical treatment of radiation-induced dermatitis: current issues and potential solutions. Drugs Context 9, 1–13. doi:10.7573/dic.2020-4-7
Jin, I. G., Kim, J. H., Wu, H.-G., Kim, S. K., Park, Y., and Hwang, S. J. (2015). Effect of bone marrow-derived stem cells and bone morphogenetic protein-2 on treatment of osteoradionecrosis in a rat model. J. Craniomaxillofac Surg. 43, 1478–1486. doi:10.1016/j.jcms.2015.06.035
Jin, H., Wan, C., Zou, Z., Zhao, G., Zhang, L., Geng, Y., et al. (2018). Tumor ablation and therapeutic immunity induction by an injectable peptide hydrogel. ACS Nano 12, 3295–3310. doi:10.1021/acsnano.7b08148
Joorabloo, A., and Liu, T. (2024). Recent advances in reactive oxygen species scavenging nanomaterials for wound healing. Exploration 4, 20230066. doi:10.1002/EXP.20230066
Katta, B., Vijayakumar, C., Dutta, S., Dubashi, B., and Nelamangala Ramakrishnaiah, V. P. (2023). The incidence and severity of patient-reported side effects of chemotherapy in routine clinical care: a prospective observational study. Cureus 15, e38301. doi:10.7759/cureus.38301
Khan, M. U. A., Aslam, M. A., Abdullah, M. F. B., Al-Arjan, W. S., Stojanovic, G. M., and Hasan, A. (2024). Hydrogels: classifications, fundamental properties, applications, and scopes in recent advances in tissue engineering and regenerative medicine – a comprehensive review. Arabian J. Chem. 17, 105968. doi:10.1016/j.arabjc.2024.105968
Kodous, A. S., Abdel-Maksoud, M. A., El-Tayeb, M. A., Al-Sherif, D. A., Mohamed, S. S. A., Ghobashy, M. M., et al. (2024). Hesperidin - loaded PVA/alginate hydrogel: targeting NFκB/iNOS/COX-2/TNF-α inflammatory signaling pathway. Front. Immunol. 15, 1347420. doi:10.3389/fimmu.2024.1347420
Kudłacik-Kramarczyk, S., Głąb, M., Drabczyk, A., Kordyka, A., Godzierz, M., Wróbel, P. S., et al. (2021). Physicochemical characteristics of chitosan-based hydrogels containing albumin particles and aloe vera juice as transdermal systems functionalized in the viewpoint of potential biomedical applications. Mater. (Basel) 14, 5832. doi:10.3390/ma14195832
Lee, K. Z., Jeon, J., Jiang, B., Subramani, S. V., Li, J., and Zhang, F. (2023). Protein-based hydrogels and their biomedical applications. Molecules 28, 4988. doi:10.3390/molecules28134988
Lee, K. K., Go, K., Lee, E., Kim, H., Kim, S., Kim, J.-H., et al. (2025). Multifunctional hydrogels for advanced cancer treatment: diagnostic imaging and therapeutic modalities. Gels 11, 426. doi:10.3390/gels11060426
Li, S., Zhang, J., Deng, C., Meng, F., Yu, L., and Zhong, Z. (2016). Redox-sensitive and intrinsically fluorescent photoclick hyaluronic acid nanogels for traceable and targeted delivery of cytochrome c to breast tumor in mice. ACS Appl. Mater Interfaces 8, 21155–21162. doi:10.1021/acsami.6b05775
Li, B., Elango, J., and Wu, W. (2020). Recent advancement of molecular structure and biomaterial function of chitosan from marine organisms for pharmaceutical and nutraceutical application. Appl. Sci. 10, 4719. doi:10.3390/app10144719
Li, Y., Liu, H., Ding, Y., Li, W., Zhang, Y., Luo, S., et al. (2023). The use of hydrogel-based materials for radioprotection. Gels 9, 301. doi:10.3390/gels9040301
Liu, Y., Ran, Y., Ge, Y., Raza, F., Li, S., Zafar, H., et al. (2022). pH-sensitive peptide hydrogels as a combination drug delivery system for cancer treatment. Pharmaceutics 14, 652. doi:10.3390/pharmaceutics14030652
Liu, Z., Chen, X., Jin, Q., Li, M., Zhu, S., Zhang, Y., et al. (2023). Dual functionalized hyaluronic acid micelles loading paclitaxel for the therapy of breast cancer. Front. Bioeng. Biotechnol. 11, 1230585. doi:10.3389/fbioe.2023.1230585
Liu, C., Wei, J., Wang, X., Zhao, Q., Lv, J., Tan, Z., et al. (2024a). Radiation-induced skin reactions: oxidative damage mechanism and antioxidant protection. Front. Cell Dev. Biol. 12, 1480571. doi:10.3389/fcell.2024.1480571
Liu, J., Zhu, L., Bao, Y., Du, Z., Shi, L., Hong, X., et al. (2024b). Injectable dexamethasone-loaded peptide hydrogel for therapy of radiation-induced ototoxicity by regulating the mTOR signaling pathway. J. Control. Release 365, 729–743. doi:10.1016/j.jconrel.2023.12.004
Liu, Q., Xu, R., Shen, J., Tao, Y., Shao, J., Ke, Y., et al. (2024c). In situ chemoimmunotherapy hydrogel elicits immunogenic cell death and evokes efficient antitumor immune response. J. Transl. Med. 22, 341. doi:10.1186/s12967-024-05102-0
Liu, X., Zhou, Q., Yang, Y., Wu, X., Chen, J., Wang, R., et al. (2025). Hydrogels in cancer treatment: mapping the future of precision drug delivery. Front. Immunol. 16, 1607240. doi:10.3389/fimmu.2025.1607240
Lu, P., Ruan, D., Huang, M., Tian, M., Zhu, K., Gan, Z., et al. (2024). Harnessing the potential of hydrogels for advanced therapeutic applications: current achievements and future directions. Sig Transduct. Target Ther. 9, 166. doi:10.1038/s41392-024-01852-x
Ma, J., Wang, B., Shao, H., Zhang, S., Chen, X., Li, F., et al. (2022). Hydrogels for localized chemotherapy of liver cancer: a possible strategy for improved and safe liver cancer treatment. Drug Deliv. 29, 1457–1476. doi:10.1080/10717544.2022.2070299
Mahdizadeh, N., Khorshid Shabestari, M., Tafvizi, F., and Khodarahmi, P. (2024). Delivery of letrozole-encapsulated niosomes via a 3D bioprinting gelatin–alginate scaffold for potential breast cancer treatment. Cancer Nanotechnol. 15, 33. doi:10.1186/s12645-024-00271-5
Maria, O. M., Eliopoulos, N., and Muanza, T. (2017). Radiation-induced oral mucositis. Front. Oncol. 7, 89. doi:10.3389/fonc.2017.00089
Mee, T., Kirkby, N. F., Defourny, N. N., Kirkby, K. J., and Burnet, N. G. (2023). The use of radiotherapy, surgery and chemotherapy in the curative treatment of cancer: results from the FORTY (Favourable Outcomes from RadioTherapY) project. Br. J. Radiology 96, 20230334. doi:10.1259/bjr.20230334
Mikhail, A. S., Morhard, R., Mauda-Havakuk, M., Kassin, M., Arrichiello, A., and Wood, B. J. (2023). Hydrogel drug delivery systems for minimally invasive local immunotherapy of cancer. Adv. Drug Deliv. Rev. 202, 115083. doi:10.1016/j.addr.2023.115083
Mitrovic, J., Richey, G., Kim, S., and Guler, M. O. (2023). Peptide hydrogels and nanostructures controlling biological machinery. Langmuir 39, 11935–11945. doi:10.1021/acs.langmuir.3c01269
Nizam, A. A. K., Masri, S., Fadilah, N. I. M., Maarof, M., and Fauzi, M. B. (2025). Current insight of peptide-based hydrogels for chronic wound healing applications: a concise review. Pharmaceuticals 18, 58. doi:10.3390/ph18010058
Norouzi, M., Nazari, B., and Miller, D. W. (2016). Injectable hydrogel-based drug delivery systems for local cancer therapy. Drug Discov. Today 21, 1835–1849. doi:10.1016/j.drudis.2016.07.006
Omidian, H., Chowdhury, S. D., and Wilson, R. L. (2024). Advancements and challenges in hydrogel engineering for regenerative medicine. Gels 10, 238. doi:10.3390/gels10040238
Op ’t Veld, R. C., Walboomers, X. F., Jansen, J. A., and Wagener, F. A. D. T. G. (2020). Design considerations for hydrogel wound dressings: strategic and molecular advances. Tissue Eng. Part B Rev. 26, 230–248. doi:10.1089/ten.TEB.2019.0281
Parès, L., Naasri, S., Delattre, L., Therriault, H., Liberelle, B., Crescenzo, G. D., et al. (2024). Macroporous chitosan/alginate hydrogels crosslinked with genipin accumulate and retain glioblastoma cancer cells. RSC Adv. 14, 35286–35304. doi:10.1039/D4RA06197G
Peng, G., Hu, J., Guo, J., Dong, J., Zhao, Y., Ye, T., et al. (2024). Injectable exosome-loaded quaternized chitosan/oxidized sodium alginate hydrogel with self-healing, bioadhesive, and antibacterial properties for treating combined radiation-wound injury. Chem. Eng. J. 494, 152933. doi:10.1016/j.cej.2024.152933
Pizzino, G., Irrera, N., Cucinotta, M., Pallio, G., Mannino, F., Arcoraci, V., et al. (2017). Oxidative stress: harms and benefits for human health. Oxidative Med. Cell. Longev. 2017, 8416763. doi:10.1155/2017/8416763
Prieložná, J., Mikušová, V., and Mikuš, P. (2024). Advances in the delivery of anticancer drugs by nanoparticles and chitosan-based nanoparticles. Int. J. Pharm. 8, 100281. doi:10.1016/j.ijpx.2024.100281
Priya, A. S., Premanand, R., Ragupathi, I., Bhaviripudi, V. R., Aepuru, R., Kannan, K., et al. (2024). Comprehensive review of hydrogel synthesis, characterization, and emerging applications. J. Compos. Sci. 8, 457. doi:10.3390/jcs8110457
Qu, J., Zhao, X., Ma, P. X., and Guo, B. (2017). pH-responsive self-healing injectable hydrogel based on N-carboxyethyl chitosan for hepatocellular carcinoma therapy. Acta Biomater. 58, 168–180. doi:10.1016/j.actbio.2017.06.001
Rao, D., Behzadi, F., Le, R. T., Dagan, R., and Fiester, P. (2021). Radiation induced mucositis: what the radiologist needs to know. Curr. Problems Diagnostic Radiology 50, 899–904. doi:10.1067/j.cpradiol.2020.10.006
Raza, F., Zhu, Y., Chen, L., You, X., Zhang, J., Khan, A., et al. (2019). Paclitaxel-loaded pH responsive hydrogel based on self-assembled peptides for tumor targeting. Biomater. Sci. 7, 2023–2036. doi:10.1039/C9BM00139E
Rosen, R. D., and Sapra, A. (2025). “TNM classification,” in StatPearls, (Treasure Island, FL: StatPearls Publishing). Available online at: http://www.ncbi.nlm.nih.gov/books/NBK553187/(Accessed July 17, 2025).
Roth, J. G., Huang, M. S., Navarro, R. S., Akram, J. T., LeSavage, B. L., and Heilshorn, S. C. (2023). Tunable hydrogel viscoelasticity modulates human neural maturation. Sci. Adv. 9, eadh8313. doi:10.1126/sciadv.adh8313
Salwowska, N. M., Bebenek, K. A., Żądło, D. A., and Wcisło-Dziadecka, D. L. (2016). Physiochemical properties and application of hyaluronic acid: a systematic review. J. Cosmet. Dermatol 15, 520–526. doi:10.1111/jocd.12237
Satchanska, G., Davidova, S., and Petrov, P. D. (2024). Natural and synthetic polymers for biomedical and environmental applications. Polymers 16, 1159. doi:10.3390/polym16081159
Segneanu, A.-E., Bejenaru, L. E., Bejenaru, C., Blendea, A., Mogoşanu, G. D., Biţă, A., et al. (2025). Advancements in hydrogels: a comprehensive review of natural and synthetic innovations for biomedical applications. Polymers 17, 2026. doi:10.3390/polym17152026
Senapati, S., Mahanta, A. K., Kumar, S., and Maiti, P. (2018). Controlled drug delivery vehicles for cancer treatment and their performance. Sig Transduct. Target Ther. 3, 7–19. doi:10.1038/s41392-017-0004-3
Seo, H. S., Han, J.-H., Lim, J., Bae, G.-H., Byun, M. J., Wang, C.-P. J., et al. (2024). Enhanced postsurgical cancer treatment using methacrylated glycol chitosan hydrogel for sustained DNA/doxorubicin delivery and immunotherapy. Biomater. Res. 28, 0008. doi:10.34133/bmr.0008
Shao, H., Liu, M., Jiang, H., and Zhang, Y. (2025). Polysaccharide-based drug delivery targeted approach for colon cancer treatment: a comprehensive review. Int. J. Biol. Macromol. 302, 139177. doi:10.1016/j.ijbiomac.2024.139177
Sharkawy, A., Barreiro, M. F., and Rodrigues, A. E. (2020). Chitosan-based Pickering emulsions and their applications: a review. Carbohydr. Polym. 250, 116885. doi:10.1016/j.carbpol.2020.116885
Shen, J., Jiao, W., Yang, J., Zhuang, B., Du, S., Wu, Y., et al. (2025). In situ photocrosslinkable hydrogel treats radiation-induced skin injury by ROS elimination and inflammation regulation. Biomaterials 314, 122891. doi:10.1016/j.biomaterials.2024.122891
Shin, M. H., Oh, E., Kim, Y., Nam, D.-H., Jeon, S. Y., Yu, J. H., et al. (2023). Recent advances in CAR-based solid tumor immunotherapy. Cells 12, 1606. doi:10.3390/cells12121606
Shu, G., Zhu, W., Jiang, Y., Li, X., Pan, J., Zhang, X., et al. (2021). Persistent luminescence immune hydrogel for photodynamic-immunotherapy of tumors in vivo. Adv. Funct. Mater. 31, 2104472. doi:10.1002/adfm.202104472
Singh, M., Alavi, A., Wong, R., and Akita, S. (2016). Radiodermatitis: a review of our current understanding. Am. J. Clin. Dermatol 17, 277–292. doi:10.1007/s40257-016-0186-4
Su, J., Lu, S., Jiang, S., Li, B., Liu, B., Sun, Q., et al. (2021). Engineered protein photo-thermal hydrogels for outstanding in situ tongue cancer therapy. Adv. Mater 33, e2100619. doi:10.1002/adma.202100619
Sun, L., Shen, F., Tian, L., Tao, H., Xiong, Z., Xu, J., et al. (2021a). ATP-responsive smart hydrogel releasing immune adjuvant synchronized with repeated chemotherapy or radiotherapy to boost antitumor immunity. Adv. Mater 33, e2007910. doi:10.1002/adma.202007910
Sun, Y., Liu, Y., Ma, X., and Hu, H. (2021b). The influence of cell cycle regulation on chemotherapy. Int. J. Mol. Sci. 22, 6923. doi:10.3390/ijms22136923
Sun, X., Zhao, P., Lin, J., Chen, K., and Shen, J. (2023). Recent advances in access to overcome cancer drug resistance by nanocarrier drug delivery system. Cancer Drug Resist 6, 390–415. doi:10.20517/cdr.2023.16
Tang, J., Wang, N., Wu, J., Ren, P., Li, J., Yang, L., et al. (2019). Synergistic effect and reduced toxicity by intratumoral injection of cytarabine-loaded hyaluronic acid hydrogel conjugates combined with radiotherapy on lung cancer. Invest New Drugs 37, 1146–1157. doi:10.1007/s10637-019-00740-4
Tumilaar, S. G., Hardianto, A., Dohi, H., and Kurnia, D. (2024). A comprehensive review of free radicals, oxidative stress, and antioxidants: overview, clinical applications, global perspectives, future directions, and mechanisms of antioxidant activity of flavonoid compounds. J. Chem. 2024, 1–21. doi:10.1155/2024/5594386
Tymińska, A., Karska, N., Skoniecka, A., Zawrzykraj, M., Banach-Kopeć, A., Mania, S., et al. (2024). A novel chitosan-peptide system for cartilage tissue engineering with adipose-derived stromal cells. Biomed. Pharmacother. 181, 117683. doi:10.1016/j.biopha.2024.117683
Valachová, K., Hassan, M. E., and Šoltés, L. (2024). Hyaluronan: sources, structure, features and applications. Molecules 29, 739. doi:10.3390/molecules29030739
Vineis, P., and Wild, C. P. (2014). Global cancer patterns: causes and prevention. Lancet 383, 549–557. doi:10.1016/S0140-6736(13)62224-2
Wan, T., Zhang, Q., Jin, G., and Xu, S. (2024). Controlled delivery of 5-fluorouracil from monodisperse chitosan microspheres prepared by emulsion crosslinking. RSC Adv. 14, 11311–11321. doi:10.1039/D4RA01377H
Wang, N., Gao, Q., Tang, J., Jiang, Y., Yang, L., Shi, X., et al. (2021). Anti-tumor effect of local injectable hydrogel-loaded endostatin alone and in combination with radiotherapy for lung cancer. Drug Deliv. 28, 183–194. doi:10.1080/10717544.2020.1869864
Wang, J., Gao, L., Song, J., and Li, S. (2023). Study of EGCG composite hydrogel for the treatment of radiation-induced skin injuries. J. Appl. Biomaterials and Funct. Mater. 21, 22808000231218996. doi:10.1177/22808000231218996
Wang, H., Mu, G., Cai, X., Zhang, X., Mao, R., Jia, H., et al. (2024). Glucopeptide superstructure hydrogel promotes surgical wound healing following neoadjuvant radiotherapy by producing NO and anticellular senescence. Adv. Healthc. Mater. 13, 2400406. doi:10.1002/adhm.202400406
Wang, Y., Xin, T., Deng, H., Chen, J., Tang, S., Liu, L., et al. (2025). Keratin/chitosan film promotes wound healing in rats with combined radiation-wound injury. J. Mater Sci. Mater Med. 36, 15. doi:10.1007/s10856-025-06860-z
Xia, Y., Gui, H., Li, X., Wu, Y., Liu, J., and Liu, J. (2025). X-Ray responsive antioxidant drug-free hydrogel for treatment of radiation skin injury. ACS Appl. Mater. Interfaces 17, 5671–5683. doi:10.1021/acsami.4c16810
Xu, X., Peng, Q., Jiang, X., Tan, S., Yang, Y., Yang, W., et al. (2023). Metabolic reprogramming and epigenetic modifications in cancer: from the impacts and mechanisms to the treatment potential. Exp. Mol. Med. 55, 1357–1370. doi:10.1038/s12276-023-01020-1
Yadav, P., Ambudkar, S. V., and Rajendra Prasad, N. (2022). Emerging nanotechnology-based therapeutics to combat multidrug-resistant cancer. J. Nanobiotechnology 20, 423. doi:10.1186/s12951-022-01626-z
Ye, G., Luo, S., Zafar, H., Ge, H., Liu, B., Wang, N., et al. (2024). pH-sensitive supramolecular self-assembled peptide hydrogel for the treatment of esophageal cancer. Front. Pharmacol. 15, 1453422. doi:10.3389/fphar.2024.1453422
Yin, X., Geng, X., Li, W., Che, T., Yan, L., Yuan, B., et al. (2025). Advance of the application of seaweed polysaccharides on antitumor drug delivery systems. Int. J. Pharm. 675, 125502. doi:10.1016/j.ijpharm.2025.125502
Zhang, C., Xu, C., Gao, X., and Yao, Q. (2022). Platinum-based drugs for cancer therapy and anti-tumor strategies. Theranostics 12, 2115–2132. doi:10.7150/thno.69424
Zhang, J., Zhu, Y., Zhang, Y., Lin, W., Ke, J., Liu, J., et al. (2021). A balanced charged hydrogel with anti-biofouling and antioxidant properties for treatment of irradiation-induced skin injury. Mater Sci. Eng. C Mater Biol. Appl. 131, 112538. doi:10.1016/j.msec.2021.112538
Zhang Z., Z., Li, A., Min, X., Zhang, Q., Yang, J., Chen, G., et al. (2021). An ROS-sensitive tegafur-PpIX-heterodimer-loaded in situ injectable thermosensitive hydrogel for photodynamic therapy combined with chemotherapy to enhance the tegafur-based treatment of breast cancer. Biomater. Sci. 9, 221–237. doi:10.1039/D0BM01519A
Zhao, L., Zhou, Y., Zhang, J., Liang, H., Chen, X., and Tan, H. (2023). Natural polymer-based hydrogels: from polymer to biomedical applications. Pharmaceutics 15, 2514. doi:10.3390/pharmaceutics15102514
Zhou, W., Lei, S., Liu, M., Li, D., Huang, Y., Hu, X., et al. (2022). Injectable and photocurable CAR-T cell formulation enhances the anti-tumor activity to melanoma in mice. Biomaterials 291, 121872. doi:10.1016/j.biomaterials.2022.121872
Zhou, W., Zhu, L., Zu, X., Ma, Y., Shen, P., Hu, Y., et al. (2025). A novel antioxidant and anti-inflammatory carboxymethylcellulose/chitosan hydrogel loaded with cannabidiol promotes the healing of radiation-combined wound skin injury in the 60Co γ-irradiated mice. Phytomedicine 142, 156790. doi:10.1016/j.phymed.2025.156790
Zhu, J., Gao, R., Wang, Z., Cheng, Z., Xu, Z., Liu, Z., et al. (2023). Sustained and targeted delivery of self-assembled doxorubicin nonapeptides using pH-responsive hydrogels for osteosarcoma chemotherapy. Pharmaceutics 15, 668. doi:10.3390/pharmaceutics15020668
Zhu, C., Ke, L., Ao, X., Chen, Y., Cheng, H., Xin, H., et al. (2024). Injectable supramolecular hydrogels for in situ programming of car-T cells toward solid tumor immunotherapy. Adv. Mater 36, e2310078. doi:10.1002/adma.202310078
Zhu, B., Chen, Y., Yang, X., Zhu, Y., Zhao, Y., Liu, Q., et al. (2025). Succinamide ester-containing adhesive hydrogels with controllable degradation for biomedical applications. Cell Biomater., 100176. doi:10.1016/j.celbio.2025.100176
Keywords: hydrogels, oncology, alginate, chitosan, hyaluronic acid, peptide hydrogels
Citation: Czerwiec K, Szczecińska W and Pikuła M (2025) Hydrogels as a new platform of therapeutic systems in oncological treatment - use as a protective barrier and drug carriers. Front. Bioeng. Biotechnol. 13:1673253. doi: 10.3389/fbioe.2025.1673253
Received: 25 July 2025; Accepted: 09 September 2025;
Published: 23 September 2025.
Edited by:
Cheng Hu, Sichuan University, ChinaReviewed by:
Vinoy Thomas, University of Kerala, IndiaShuaishuai Zhang, South China Normal University, China
Copyright © 2025 Czerwiec, Szczecińska and Pikuła. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Katarzyna Czerwiec, a2F0YXJ6eW5hLmN6ZXJ3aWVjQGd1bWVkLmVkdS5wbA==
 Katarzyna Czerwiec
Katarzyna Czerwiec Weronika Szczecińska2
Weronika Szczecińska2 Michał Pikuła
Michał Pikuła