- Biological and Environmental Science and Engineering Division, King Abdullah University of Science and Technology, Makkah, Saudi Arabia
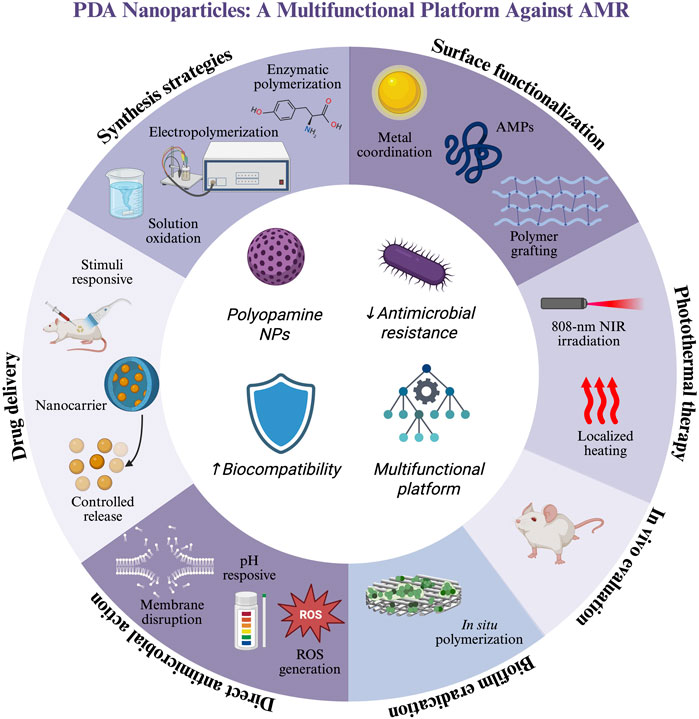
The global rise of antimicrobial resistance has driven the search for novel antimicrobial strategies with higher effectiveness than common antibiotics. Among various solutions, polydopamine nanoparticles (PDA NPs) have gained widespread attention owing to their biocompatibility, functional versatility, and responsiveness to environmental stimuli. This review summarizes recent advances in the synthesis, functionalization, and antimicrobial applications of PDA NPs, highlighting their potential as smart nanomaterials. PDA NPs exhibit intrinsic antimicrobial activity, large drug delivery capabilities, and excellent photothermal properties. Moreover, they can potentially eradicate biofilms; can be synergistically combined with other entities such as metal ions, antimicrobial peptides, and Fenton-like catalysts; and can provide in vivo models of bacterial infection. Despite these advantages, the widespread use of PDA NPs is limited by low synthesis reproducibility, insufficient accurate characterization, and lack of comprehensive biocompatibility assessment. Resolving these challenges is essential for fully comprehending and using the potential of PDA-based antimicrobial platforms. This review aims to explain the current landscape of PDA-based nanoformulations and to inspire future research toward clinically viable PDA-based nanoformulations.
1 Introduction
Drug-resistant bacteria have emerged as a critical threat to global health. As drug-resistance development in bacteria has outpaced the development of new antibiotics, alternative antimicrobial approaches are urgent in demand. Antimicrobial resistance (AMR) is an evolutionary process through which microorganisms, such as bacteria, fungi, parasites, and viruses, resist antimicrobial treatments in humans and animals, thus increasing the difficulty of treating infections (Tang et al., 2023). The driving factors of AMR, dominated by the overuse and misuse of antibiotics, can be classified into four categories: spread of microorganisms owing to environmental problems such as rapid population growth and overcrowding; drug-related issues, such as fake drugs and easy access to over-the-counter medications; patient-related behaviors, such as self-medication; and healthcare-related factors, such as inappropriate prescriptions and overdosage (Salam et al., 2023).
The extent of this threat is reflected in the number of associated deaths and economic losses. In 2019 alone, approximately 1.27 million deaths worldwide were owing to drug-resistant bacterial infections. The highest mortality rates were recorded in sub-Saharan Africa and South Asia, where the impact of resistant infections is aggravated by limited access to healthcare and effective treatments (Murray et al., 2022). Without intervention, the global mortality rate is projected to reach to approximately 10 million deaths per year by 2050 (Chinemerem Nwobodo et al., 2022; Pulingam et al., 2022). Besides threatening public health, the increase in drug-resistant infections imposes substantial economic burdens, either directly (e.g., by increasing healthcare expenses) or indirectly (e.g., by reducing productivity and prolonging hospital stays). By 2050, AMR is projected to reduce annual productivity by 3% worldwide, corresponding to the economic losses of approximately $2.4–$6.9 trillion USD per year and an additional 8–24 million people living in poverty (Hall et al., 2018).
Nanomaterials have revolutionized the biomedical field, offering new possibilities for theranostics, drug delivery, regenerative medicine, and other applications. Physiochemical properties are magnified at the typical size of nanomaterials (0.1–100 nm; Singh et al., 2022; Yusuf et al., 2023). However, the lack of regulatory frameworks has prevented the widespread application of nanomaterials as therapeutic agents in biomedicine (Husen and Siddiqi, 2023). So-called “smart nanomaterials,” arising from the increasing demand for functionalized biomaterials that can mimic the behaviors of living organisms (Aflori, 2021), alter their own physical, chemical, and biological properties in response to environmental changes (Singh et al., 2022). Under external biological stimuli, smart nanomaterials self-modify their shape, surface area, size, and other properties (Aflori, 2021). Many smart nanomaterials are biomimetic and therefore usable in drug delivery systems and self-healing materials (Sharma and Hussain, 2020; Yoshida and Lahann, 2008). Such tools avail new opportunities in biomedical research and development, enabling enhanced drug therapies while reducing side effects. Personalized medicine is an especially exciting outcome of these opportunities (Michalska et al., 2018; Sharma and Hussain, 2020).
Smart nanomaterials include polydopamine nanoparticles (PDA NPs), introduced relatively recently in 2007. A PDA NP is an insoluble biopolymer resulting from the oxidative self-polymerization of dopamine, the PDA monomer (D’Ischia et al., 2014; Schanze et al., 2018). PDA possesses a complex chemical structure with multiple functional groups, such imines, amines, and catechol, that impart reactivity and adhesion properties. Consequently, PDA can adhere to numerous organic and inorganic materials (Tran et al., 2019). Unlike their inorganic counterparts, smart nanomaterials exhibit high biocompatibility, biodegradability, and photothermal activity; therefore, they are suitable for diverse biomedical applications (Battaglini et al., 2024; Gholami Derami et al., 2021).
In the past few years, the antimicrobial properties of polydopamine nanostructures have occupied an increasing share of the scientific literature. This growing trend reflects the increasing interest in PDA within the research community; in particular, PDA is regarded as a promising solution to the urgent global challenge of antimicrobial resistance. Although PDA has been extensively studied for its broad biomedical applications, most existing reviews have focused on general medical applications or hybrid systems that combine PDA with other materials. Few reviews have examined the inherent antimicrobial capabilities of PDA. As shown in Figure 1, the number of publications in this area is increasing each year, highlighting the relevance of such a review.
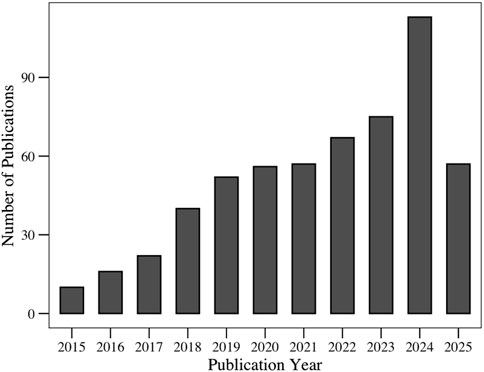
Figure 1. Evolution of number of publications related to the “antimicrobial applications of polydopamine,” retrieved from the Web of Science database.
This review highlights the recent advancements in PDA-based antimicrobial strategies and identifies opportunities for future research, focusing on recent advancements in antimicrobial PDA NP agents and the potential of PDA NPs in combating AMR. This review discusses recent synthetic developments, the mechanisms underlying the antimicrobial activity of PDA NPs, and the different contexts of PDA NP applications. Moreover, it highlights the current limitations of nanomaterials, namely, the challenges of characterization, controlled delivery, and achieving high performance in biological systems. This review concludes with insights into future research directions in this area, aiming to guide the development of fully organic, biocompatible, and clinically viable PDA-based antimicrobial nanoplatforms. By presenting the latest progress, this review also aims to clarify the PDA NP role in designing next-generation strategies against AMR.
2 Synthesis of polydopamine nanoparticles
2.1 Solution oxidation method
PDA NPs are usually synthesized through the oxidative self-polymerization of dopamine hydrochloride under mildly alkaline conditions, with dissolved oxygen acting as the oxidizing agent. This approach has been popularized for its simplicity and versatility (Ren et al., 2024). In general, dopamine hydrochloride is dissolved in an alkaline solution and the pH is adjusted to 7.5 or higher using a buffer (typically tris hydrochloride or sodium bicarbonate) or ammonia solution (Browne et al., 2022; Cicogna et al., 2023; Costa et al., 2024; Cui et al., 2024; 2025; Shumbula et al., 2022; Souza et al., 2025; Zhao B et al., 2024). The reaction proceeds under adjusted conditions at 25 °C or slightly higher (e.g., 37 °C) (Z. P. Li et al., 2022), with constant shaking over a fixed duration (24–48 h) to enable self-polymerization as shown in Figure 2 (Bano et al., 2023; Browne et al., 2022; Costa et al., 2024; Cui et al., 2024; 2025; S. Li et al., 2025; Qi et al., 2022; Shumbula et al., 2022; Souza et al., 2025; Wang H et al., 2024; Wang R et al., 2024; Wang H et al., 2024; Yu et al., 2022; Zhao B et al., 2024).
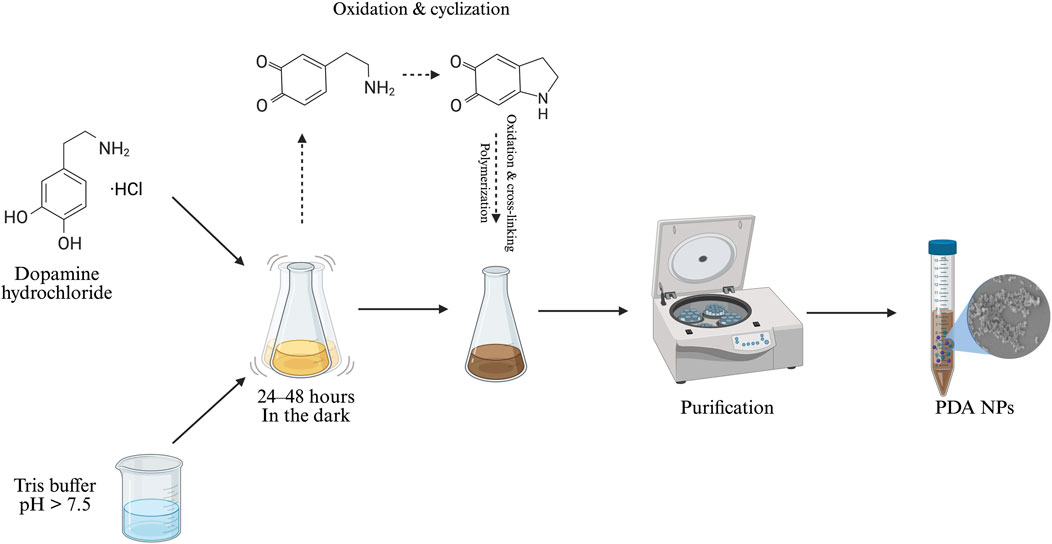
Figure 2. Synthesis of PDA NPs (image prepared using BioRender.com).
Polydopamine formation is initiated under mild alkaline conditions in the presence of dissolved oxygen to encourage the spontaneous oxidation of dopamine. The mechanism begins when dopamine oxidizes to the reactive compound dopamine–quinone. Facilitated by the availability of oxygen, this process is further accelerated at higher pH levels (Batul et al., 2023; Cai et al., 2024; Kensel Rajeev et al., 2024). A series of intramolecular transformations—intramolecular cyclization, oxidation, and crosslinking—lead to the insoluble and melanin-like PDA polymer. However, despite extensive studies, the exact polymerization mechanism remains incompletely understood, as researchers continue to debate the details of its polymerization pathway. The process is complicated by multiple competing reactions, shifts in protonation states influenced by pH, and the coexistence of several reactive species. This complexity not only makes the chemical structure of PDA heterogeneous, but also difficult to characterize with precision (Batul et al., 2023; Cai et al., 2024; Shumbula et al., 2022; Souza et al., 2025; Wang S et al., 2024; N. Xu et al., 2023; Yu et al., 2022).
Variations of this method use water-ethanol mixtures, followed by the addition of ammonium hydroxide to raise the pH. Ethanol affects the nucleation and growth of PDA NPs, allowing precise control over the NP formation (Cui et al., 2024; 2025; Wu et al., 2023). This process can be adapted to the co-formation or encapsulation of other materials and is suitable for biomedical, environmental, and materials science applications.
2.2 Electropolymerization of dopamine
The highly controlled electropolmerization method, creating PDA films on substrates or nanopores in the PDA matrix, offers numerous advantages over the solution oxidation method (Varol et al., 2023). The electropolymerization method oxidizes the dopamine monomer to electropolymerized polydopamine (ePDA) (Wang L et al., 2024), beginning with the removal of electrons from the catechol groups in dopamine when a potential difference is applied to the working electrode. The resulting current leads to the formation of dopamine–quinone. The activated benzoic ring then chemically interacts with the amine group through different reactions, ultimately forming an insoluble precipitate of PDA NPs on the surface of the working electrode (Marchesi D’Alvise et al., 2023).
Unlike traditional solution oxidation, electropolymerization can precisely control the thickness of nanometer-scale polymer coatings by adjusting the time and number of potential cycles (Varol et al., 2023). In addition, this technique produces uniform and high-quality films that seamlessly coat the substrate and deliver high performance (GhavamiNejad et al., 2015). The deposited films are adhesive, biocompatible, and can be functionalized with diverse molecules or efficiently immobilized nanoparticles to increase their antimicrobial properties (Almeida et al., 2021). The film thickness and composition are further influenced by pH, affecting the properties of PDA films during the electropolymerization process. Variations in pH can alter the deposition rate, structural arrangement, and overall characteristics of the resulting material (Marchesi D’Alvise et al., 2023). Alkaline conditions improve the efficiency of this type of synthesis, thus yielding thicker PDA layers. A change in pH directly influences the coating thickness by allowing precise control over the polymerization process of PDA, thereby enabling the tunability of properties such as layer thickness and uniformity. Furthermore, the presence of metal ions can augment the stability of the PDA film, thus strengthening the coating (Polaczek et al., 2024).
Its primary applications include creating antibacterial coatings, serving as an intermediate layer for molecule attachment, and regulating cell-surface interactions (Caniglia et al., 2023; Larrieu et al., 2024; Wang Y et al., 2024). However, this method has specific limitations as it requires conductive substrates, restricting its use compared to dopamine self-polymerization, which can coat nearly any surface. The method is primarily for surface-level modification, is less suitable for bulk material functionalization (Caniglia et al., 2023; 2024; Wang Y et al., 2024).
2.3 Enzymatic oxidation
Enzymatic oxidation catalyzes the polymerization using an oxidative enzyme such as laccase or tyrosinase (Magalhães et al., 2022; H. Zhang et al., 2020). Unlike the self-polymerization approach, requiring high alkalinity and long reaction times, the enzymatic method achieves fast polymerization under mild conditions, which broadens its range of substrates and applications (F. Li et al., 2018; Magalhães et al., 2022). For instance, one study found that laccase-catalyzed polymerization optimally proceeds at pH 5.5 °C and 30 °C with an initial dopamine concentration of 2 mg/mL; however, it remains more efficient than the conventional method over a broader pH range (4.5–7.5). At more alkaline pH, the performance is degraded by hydroxide interference at the active site of the enzyme (Magalhães et al., 2022).
In this approach, dopamine hydrochloride is dissolved in an aqueous buffer such as sodium acetate and the pH is adjusted to favor enzyme activity. After adding laccase, the solution is stirred at a controlled temperature. The polymerization is monitored visually (by observing changes, typically the darkening of the solution) and spectrophotometrically (by tracking the absorbance peaks around 305 and 480 nm denoting the intermediate species involved in PDA formation). Interestingly, the structure of enzymatically oxidized PDA differs from that of traditionally oxidized PDA. For instance, the dopamine units in enzymatically derived polymers are joined only by ether bonds. The resulting PDA films are generally more homogeneous, compact, and stable, with high reactivity and numerous amine-terminated molecules available for functionalization (F. Li et al., 2018; Magalhães et al., 2022). Moreover, dopamine polymerization has been catalyzed by tyrosinase, another oxidative enzyme that forms PDA in hydrogel matrices (for example, magnetic alginate–PDA beads), in situ. Here, PDA performs as a crosslinker and covalently bonds with amino groups to inhibit enzyme leakage (H. Zhang et al., 2020).
The enzymatic polymerization method offers several advantages over the conventional oxidation approach. Spontaneous oxidation in the traditional method is slow, requires alkaline conditions, and does not guarantee the stability of PDA. By contrast, enzymatic polymerization is fast, well-controlled, and proceeds under lower pH conditions, thus rendering it suitable for systems sensitive to high pH. Under optimized conditions, the enzymatic method achieves a much higher polymerization efficiency than the conventional process and is potentially applicable to various applications including surface coatings, biomedical materials, and enzyme immobilization.
3 PDA functionalization strategies
3.1 Metal-ion coordination and deposition
PDA exhibits a rich surface chemistry with catechol, amine, and imine functional groups that can be coordinated with metals. Therefore, PDA NPs can be functionalized with numerous metallic ions (Cu2+, Fe3+, Fe2+, Ti4+, Mn2+, Zn2+, and others) through coordination or deposition (Siciliano et al., 2022; Wang L et al., 2024; Z. Wang et al., 2020a).
The surface catechol groups on PDA NPs can stably coordinate with metal ions through chelation (Meng et al., 2018). Currently, metal ions are integrated into the PDA NPs matrix using the following three main methods:
1. Postdoping/postcoordination: Already synthesized PDA NPs are directly added to a solution containing a considerable excess of metal ions. This process requires a long exposure time for high functionalization (Wang L et al., 2024; Z. Wang et al., 2020b).
2. Predoping/precoordination: Dopamine monomers are copolymerized with metal ions in an alkaline environment. During polymerization, metal ions are continuously added to the growing PDA NPs structure, yielding NPs with a higher metal-ion content than those obtained by the postdoping process (Wang L et al., 2024; Z. Wang et al., 2020b).
3. Metal-ion exchange: PDA NPs are preloaded with specific metal ions using a preloading technique. The loaded ions can be exchanged with other desirable metal ions without causing colloidal instability or aggregation (Wang L et al., 2024; Z. Wang et al., 2020b).
Metal ions (most notably silver and gold) can also be incorporated into PDA NPs via metal deposition. This method exploits the redox capacity and strong binding affinity of PDA toward metal ions (e.g., Ag+, Au3+, and Cu2+). Subsequently, the ions are reduced to their elemental metallic state, thus generating noble metal NPs, metal oxide NPs, or metal sulfide NPs (H. Li et al., 2020; Z. Wang et al., 2020b).
Several studies have suggested that functionalization with metal ions can improve the catalytic activity, DNA adsorption, and controlled release of PDA NPs, promising applications ranging from cancer therapy to catalysis and biosensors. For instance, Wang S et al. (2024) combined the properties of metal ions with the biocompatibility of PDA, obtaining a metal-coordinated PDA structure with improved antimicrobial performance. They added silver nitrate to a preformed PDA solution, forming silver nanoparticles (Ag NPs) in situ through the predoping strategy. They observed that the catechol groups in PDA act as reducing agents, converting Ag+ ions into elemental silver, which binds to nitrogen and oxygen active sites and grows uniformly on the PDA surface. The resulting PDA–Ag-coated surfaces obviously reduced bacterial attachment and generated a strong antibiofilm effect. The antimicrobial performance was attributed to the direct antimicrobial effects of silver. When stabilized within PDA adhered to the antimicrobial surface, silver atoms can directly interact with and disrupt bacterial cells (Wang S et al., 2024).
3.2 Polymer grafting
The main strategies for grafting polymers onto PDA-coated surfaces are grafting-to and grafting-from. The first approach involves the separate synthesis of polymers in solution, which are later attached to the modified PDA surface. This method can be advantageous when the polymers must be thoroughly characterized before grafting because it can precisely control diverse properties such as molecular weight, polydispersity, and functionality. Nevertheless, steric hindrance limits the layer thickness to 10 nm or less because the preattached chains obstruct further polymer grafting (Teunissen et al., 2023).
The grafting-from method involves the direct in situ polymerization of monomers from initiators immobilized on the PDA surface (Nothling et al., 2022). This process typically begins with preparing a thin layer of PDA synthesized via the solution oxidation method. Polymerization initiators are covalently attached to PDA NPs. 2-Bromoisobutyryl bromide (BIBB) is commonly employed as the initiator because it reacts with the catechol and amine groups in PDA through nucleophilic acyl substitution, forming ester and amide bonds, respectively. BIBB-bound PDA provides surface-bound initiators for subsequent polymer growth (Hemmatpour et al., 2023). Subsequently, monomers are directly polymerized from the NP surface (Nothling et al., 2022). Afterward, the growth of polymer chains can be controlled using techniques such as atom transfer radical polymerization (Hemmatpour et al., 2023; Xiong and Ulbricht, 2024).
Polymer grafting onto PDA nanoparticles is a versatile approach for modifying PDA properties, especially in antimicrobial applications. For instance, Larrieu et al. (2024) coated polyurethane foams with PDA and further grafted them with silver NPs, obtaining a filter with effective antimicrobial activity against bacteria such as Staphylococcus aureus and Escherichia coli (Larrieu et al., 2024). PDA-based polymer grafting has also been explored in studies of drug delivery systems; for example, PDA-functionalized nanocarriers grafted with stimuli-responsive polymers exhibit controlled drug-release properties and are promising candidates for targeted therapies (Hemmatpour et al., 2023). PDA NPs can be modified with various functional polymers, highlighting their potential as advanced materials in biomedical and environmental applications.
3.3 PDA as a surface coating material
The strong adhesiveness of PDA is widely exploited in surface coating materials. PDA coatings are typically formed via the oxidative self-polymerization method; however, other methods are also employed. In the common dip-coating method, a substrate is immersed in a solution containing dopamine under alkaline conditions, inducing the self-polymerization of dopamine and the formation of a uniform dark-colored film that strongly adheres to organic and inorganic surfaces (Saikawa et al., 2024). After dipping, the coated material is typically washed to remove any unreacted dopamine. In some cases, the material is heat-treated to boost its durability (Behzadinasab et al., 2023). Electrochemical methods such as cyclic voltammetry or pulsed deposition can tune the properties of PDA, forming the desired material to be deposited on a preformed PDA layer (Caniglia et al., 2023; 2024). Various materials can be coated with PDA, including metals, e.g., titanium (Peng et al., 2025), magnesium alloy (Liu et al., 2025), gold (Caniglia et al., 2023), semiconductors, ceramics, polymers (polyvinylidene fluoride (He et al., 2023), polyurethane (Kan et al., 2025), polypropylene (e.g., Cicogna et al., 2023), and hydroxyapatite (Erdem et al., 2022).
For example, X. Zhao et al. (2023) fabricated nanopillar arrays as a mechanobactericidal nanostructured surface on polypropylene. They immersed the substrate in a dopamine HCl solution at pH 8.5, typically for 24 h in the dark, forming a uniform PDA layer. Air-plasma pretreatment increased the surface reactivity and hydrophilicity of the surface, thus improving the uniformity of the PDA layer. The obtained film was less than ∼10-nm thick and preserved the physical morphology and hence the mechanical bactericidal properties of the nanostructure. However, the introduction of hydroxyl and amino groups altered the surface chemistry, improving the photothermal antibacterial activity of the material.
As another example, Lu et al. (2015) coated PDA NPs on modified silk fibers, which are naturally prone to bacterial contamination. When silk is immersed in dopamine solution, the PDA coating formed on its surface provides a matrix for the in situ synthesis of AgNPs. Owing to its redox and metal-chelating capabilities, PDA readily adsorbs silver ions and directly reduces them on the fiber surface without an external reducing agent. Their approach controls the size and surface density of AgNPs by modulating the reaction time and silver nitrate concentration. Notably, this process improves the antibacterial performance and reduces the silver-ion release rate of the composite without affecting the crystalline structure of the silk fibers (Lu et al., 2015).
4 Antimicrobial applications of PDA NPs
4.1 Direct antimicrobial properties
Polydopamine nanoparticles exhibit intrinsic antimicrobial activity mainly because the redox reactions between the catechol/quinone groups of PDA and molecular oxygen generate reactive oxygen species (ROS) (Jabbar et al., 2023; Smies et al., 2022; Soto-Garcia et al., 2023; Souza et al., 2025). Electron transfer in the catechol and quinone groups yields different ROS; for instance, catechol oxidation produces hydrogen peroxide (H2O2), electron transfer from PDA to molecular oxygen (O2·–) produces superoxide anions, and catechol oxidation forms hydroxyl radicals (·OH) and, rarely, singlet oxygen (1O2). Besides electron transfer, these reactions are sometimes attributed to ion isolation and the synergistic interaction of PDA with other materials (Kan et al., 2025; Kensel Rajeev et al., 2024; Smies et al., 2022; Souza et al., 2025; Wang J et al., 2024; X. Wang et al., 2025). The antibacterial action of PDA–ROS highly depends on the environmental conditions. For example, the pH of the medium influences the polymerization and hence the redox behavior of PDA. Alkaline environments accelerate the polymerization and favor the formation of semiquinone radicals (Souza et al., 2025), whereas acidic solutions tend to inhibit dopamine oxidation and reduce ROS formation (Lv et al., 2025). Overall, ROS causes oxidative damage to bacterial membranes, proteins, and nucleic acids, thus causing structural and functional disruption and ultimately inducing cell death (Cicogna et al., 2023; Jabbar et al., 2023; Smies et al., 2022; Soto-Garcia et al., 2023; Souza et al., 2025).
Furthermore, PDA can physically interact with the bacterial cell membrane, causing structural damage mainly through strong adhesion. This interaction can involve the chelation of essential ions or interaction with membrane proteins that are essential for bacterial function. In specific cases, in situ polymerization of PDA on bacterial surfaces can form a coating that suffocates cellular activity (Costa et al., 2024; Dou et al., 2024; Lv et al., 2025).
However, the reported direct antimicrobial activities of pure PDA NPs vary across studies, and antimicrobial action is influenced by the concentration and form of the nanoparticles, environmental conditions (such as pH and oxygen level), the species and structure of the bacteria, and the application of external stimuli. Most studies have concluded that pristine PDA lacks remarkable antimicrobial activity (Dou et al., 2024; Jabbar et al., 2023; Kensel Rajeev et al., 2024; Shumbula et al., 2022; Souza et al., 2025); however, PDA NPs combined with other antimicrobial strategies can contribute antimicrobial action through mechanisms such as photothermal/photodynamic enhancement, ROS generation, and membrane interaction, enhancing the antimicrobial efficacy of the overall system.
4.2 Drug delivery systems
With their strong adhesiveness, biocompatibility, chemical reactivity, and biodegradability (Bano et al., 2023; Patel et al., 2023), PDA NPs are promising drug delivery systems offering multiple drug-loading strategies. In one strategy, therapeutic agents are encapsulated within the porous structure of PDA. For example, the biocompatible antimicrobial compound curcumin has been effectively encapsulated into a PDA-coated zinc metal–organic framework (MOF), forming a drug delivery system with high bactericidal efficacy (Jabbar et al., 2023). Another method relies on surface adsorption. The surfaces of PDA NPs can be loaded with biomolecules through
An illustrative case is the HPDA@GLA/AMP nanoplatform (HPDA = hollow PDA; GLA = glycyrrhizic acid; AMP = antimicrobial peptide), developed by Wang, Dong, and others (2024) for treating bacterial keratitis. Besides exemplifying the versatility of PDA as a nanocarrier, this system demonstrates engineering of PDA-based platforms to simultaneously inhibit bacterial infection and inflammatory damage. HPDA NPs are synthesized by coating silica NPs with PDA via autooxidation of dopamine, followed by etching of the silica core. The resulting hollow structure largely increases the drug-loading capacity. Moreover, HPDA retains the antioxidant and ROS-scavenging properties of PDA, providing an immunomodulatory function that contrasts with that of passive carriers. To achieve targeted delivery, the authors further functionalized HPDA with 3-aminophenylboronic acid and hyaluronic acid (HPBH), enabling dual-targeting functionality. Finally, the HPBH nanocarrier was loaded with two bioactive agents: AMPs and GLA. The platform showed high antibacterial efficacy against Pseudomonas aeruginosa and S. aureus, inhibiting more than 90% of bacterial growth at therapeutic concentrations in vitro. An in vivo study in a mouse model of bacterial keratitis confirmed the therapeutic efficacy of the HPBH@GLA/AMP system. Wang R et al. (2024) observed bacterial elimination, attenuated inflammation, and intensified wound healing.
In addition, the polymeric network of PDA facilitates controlled and stimuli-responsive drug release, enabling precise control over therapeutic delivery (Bano et al., 2023; Soto-Garcia et al., 2023). For instance, Yu et al. (2022) demonstrated that near-infrared light (NIR) increases levofloxacin (Levo) release from PDA@Levo NPs. NPs respond to the typical acidic pH levels of biofilms and elevated temperatures, rapidly delivering the drug to biofilms under NIR light. Supporting this study, Patel et al. (2023) highlighted the effectiveness of PDA NPs in stimuli-responsive drug delivery systems. They developed a nanocomposite hydrogel system incorporating PDA NPs and thermoresponsive liposomes loaded with antibiotics. Under an NIR laser, the PDA triggered sequential drug release from the liposomes. Many controlled delivery applications use PDA as a coating material that modulates the diffusion of loaded agents. For instance, in various systems designed for antibacterial silver delivery, PDA coatings immobilize silver NPs (AgNPs) on diverse substrates, reducing nanoparticle leakage and enabling gradual Ag+ ion release over extended periods of time (Batul et al., 2023; Lu et al., 2015).
4.3 Photothermal therapy
As confirmed in several studies, PDA inherently possess strong photothermal properties, especially under NIR light with a wavelength of 808 nm, stimulating rapid light conversion into heat (Lin et al., 2025; Lv et al., 2025; Soto-Garcia et al., 2023). This property arises from the structure of PLA. The electron donor–acceptor characteristics of the planar conjugated system enable light absorption over a broad spectrum, including the NIR region (N. Xu et al., 2023). The light energy adsorbed by PDA NPs excites the electrons within the PDA structure. Later, the excited electrons relax and release the absorbed energy as heat (Soto-Garcia et al., 2023). Ultimately, this process raises the local temperature to a level that can damage bacterial membranes (Lin et al., 2025; Lv et al., 2025; Soto-Garcia et al., 2023). NIR radiation easily penetrates tissue, allowing treatment of deeper infections (Z. P. Li et al., 2022). In addition, the generated heat can disrupt cellular metabolism, leading to bacterial death (Lv et al., 2025; Soto-Garcia et al., 2023). PDA nanomaterials exhibit excellent photothermal stability with no substantial efficiency loss after various heating–cooling cycles (Cai et al., 2024; T. Li et al., 2024; Lin et al., 2025; Liu et al., 2025; Qi et al., 2022; Wang J et al., 2024; X. Wang et al., 2025; Wu et al., 2023; N. Xu et al., 2023; Yang et al., 2025; Zeng et al., 2021; X. Zhao et al., 2023).
Several mechanisms drive the antimicrobial effects of heat generation. Elevated temperatures can disrupt bacterial cell membranes, ultimately causing cell lysis (T. Li et al., 2024; Liu et al., 2025). High temperatures also denature and inactivate proteins, nucleic acids, and enzymes within bacteria, thereby suppressing their biological functions. Moreover, photothermal therapy can disrupt the extracellular matrix of bacterial biofilms (T. Li et al., 2024; Wang J et al., 2024; Zeng et al., 2021; Zhao Y et al., 2024). Although elevated temperatures are highly effective antimicrobial treatments, excessively high temperatures can damage the surrounding healthy tissue (X. Zhao et al., 2023). Consequently, current strategies leveraging the photothermal capacity of PDA often rely on synergistic effects that operate at more biocompatible lower temperatures. These mechanisms remain effective when synergized with other mechanisms that increase cell-membrane permeability or promote catalytic activity (T. Li et al., 2024; Wu et al., 2023; N. Xu et al., 2023).
Moreover, the photothermal performance of PDA NPs depends on various additional factors. Increasing the concentration of PDA-containing materials is known to raise the local temperature under the same irradiation power (T. Li et al., 2024; Wang J et al., 2024; Wu et al., 2023; Yang et al., 2025; Zeng et al., 2021; Zhao Y et al., 2024). Similarly, increasing the power of the NIR laser considerably increases the temperature rise rate. The photothermal efficiency of PDA can be improved by incorporating PDA into other materials (T. Li et al., 2024; Qi et al., 2022).
4.4 Integration of PDA NPs with antimicrobial peptides
Antimicrobial peptides (AMPs) are short proteins with broad-spectrum inhibitory activity against various pathogens, including bacteria and fungi. Typical AMPs comprise 10–60 amino acid residues that play critical roles in the natural immune response. AMP molecules are characteristically amphipathic, possessing hydrophilic and hydrophobic areas, and are commonly cationic, facilitating their interaction with microbial membranes (Siddiqui et al., 2024; Talapko et al., 2022). This subsection discusses the combination of PDA NPs with AMPs or peptidomimetics. This innovative strategy is being explored as a bacterial infection treatment, particularly against antimicrobial-resistant organisms.
Owing to its inherent adhesive and surface-reactive properties, PDA is an ideal coating or nanocarrier candidate for immobilizing or encapsulating AMPs (Browne et al., 2022). PDA NPs integrated with AMPs synergistically enhance the therapeutic efficacy against a broad spectrum of bacterial strains, including multidrug-resistant strains. For instance, ZnO NPs, which are known to generate intracellular ROS and disrupt bacterial membranes, have been effectively coated with a PDA coupling interface that immobilizes AMP GL13K, a 13-amino-acid peptide with potent antimicrobial activity against S. aureus and E. coli. The resulting ZnO@PDA/GL13K nanoplatform considerably enhances the bactericidal activity from that of unmodified ZnO, even at low peptide concentrations (Zhang X et al., 2024). Similarly, PDA-coated ZnO NPs have been conjugated with the antimicrobial peptide ε-poly-L-lysine (ε-PL) to form a multifunctional nanocomposite in which ZnO serves as a broad-spectrum antimicrobial agent, PDA serves as a linker layer and performs photothermal conversion, while ε-PL naturally improves bacterial targeting through electrostatic interactions. This composite demonstrated high antibacterial efficacy, eliminating 99.70% of E. coli and 100% of S. aureus at low dosages (<100 μg/mL), highly outperforming its individual components. The performance improvement was attributed to a dual mechanism: ε-PL facilitates initial bacterial capture via electrostatic interactions, whereas PDA provides photothermal heating that disrupts the membrane, eventually leading to cell death. In addition, the platform exhibited strong biofilm disruption, eradicating established S. aureus biofilms (Qi et al., 2022).
Browne et al. (2022) applied PDA as a versatile linking agent in the functionalization of biomaterial surfaces. Their strategy uses three synthetic peptidomimetic AMPs—melimine, Mel4, and RK758—selected for their broad-spectrum efficacy against clinically relevant bacterial pathogens, including drug-resistant strains. The PDA-functionalized surfaces completely eradicated S. aureus and demonstrated substantial activity against Gram-negative bacteria such as E. coli and P. aeruginosa. The antimicrobial mechanism was inferred as interaction and disruption of the cell membrane, consistent with the amphipathic and cationic nature of peptidomimetics. More recently, Peng et al. (2025) synthesized a pH-responsive hydrogel coating comprising chitosan (CS) and PDA, embedded it with the AMP HHC36, and applied it to titanium substrates. They selected titanium because it is used in implantable medical devices, CS because it possesses intrinsic antimicrobial and pH-sensitive properties, PDA because it provides strong wet adhesion and can potentially destroy bacteria through a photothermal mechanism, and the synthetic AMP HHC36 because it effectively eliminates multidrug-resistant bacteria or superbugs. The resulting hydrogel was largely effective against S. aureus and E. coli, with inhibition rates above >90%. Remarkably, the pH-responsiveness of the hydrogel enabled the controlled release of HHC36 in the acidic microenvironments commonly found at bacterial infection sites, thus optimizing the therapeutic efficacy of the composite while minimizing off-target effects.
4.5 Fenton-like reaction improvement via PDA NPs: a synergistic approach
In therapeutic systems integrating PDA with Fenton-like catalysts, PDA with intrinsic photothermal properties induces localized hyperthermia under NIR irradiation, thus improving the catalytic activity of the Fenton-like agents. This synergistic mechanism notably increases the ROS production and strengthens the bactericidal effect (Gao et al., 2024; Zhao Q et al., 2024). Such synergy between the photothermal effect and chemodynamic therapy is commonly known as the photoenhanced Fenton effect or synergetic photothermal–chemodynamic therapy. In this context, PDA is often used as a multifunctional platform that incorporates or coordinates with transition metal ions that catalyze Fenton or Fenton-like reactions, increasing the therapeutic efficacy of the platform (N. Xu et al., 2023; Zhao Q et al., 2024).
In composites such as PDA@FeS, PDA stabilizes the material by promoting uniform dispersion and inhibiting the oxidation of FeS. Meanwhile, sulfur ions facilitate the reduction of ferric (Fe3+) to ferrous (Fe2+) iron, accelerating the Fenton-like conversion of H2O2 to hydroxyl radicals (·OH). This catalytic activity (a key mechanism in chemodynamic therapy) is further potentiated by the photothermal effect of PDA under NIR irradiation, enhancing ROS generation and therapeutic efficacy (N. Xu et al., 2023). Similarly, Wu et al. (2023) designed a copper-based composite with PDA and FDM-23, a 2D metal-organic framework that amplifies peroxidase-like activity to promote antibacterial effects. This composite catalyzes the decomposition of H2O2 to toxic OH radicals while depleting intracellular glutathione and weakening the antioxidant defense mechanisms of bacteria. The resulting increase in cuprous ion (Cu+) levels further amplifies ROS production by improving the efficiency of redox cycling, ultimately contributing to effective bacterial eradication.
Another interesting example is the cuprous oxide (Cu2O)–tin oxide (SnO2)-doped PDA (CSPDA) composite, which integrates a heterostructured nanoenzyme core with a polydopamine shell to achieve synergistic Fenton-like catalytic and photothermal effects. In this design, Cu2O nanocubes are first synthesized and then coated with SnO2 NPs. The P-type Cu2O and N-type SnO2 semiconductros possess similar lattice parameters, facilitating the formation of a P–N junction that improves the interfacial charge transfer and catalytic efficiency. This heterostructure catalyzes the decomposition of H2O2 into hydroxyl radicals (·OH) through a Fenton-like mechanism. Unlike traditional Fenton systems, which typically require weakly acidic conditions, the Cu+/Cu2+ redox cycle in Cu2O achieves effective catalysis over a wide pH range encompassing weakly acidic, neutral, and weakly basic environments. The PDA coating plays triplicate roles as a biocompatible encapsulating matrix, an enhancer of the photothermal response under NIR irradiation, and an additional ROS generator via localized hyperthermia. This combination of chemodynamic and photothermal functionalities enhances the overall catalytic and antibacterial performance of the nanoplatform. CSPDA demonstrated effective peroxidase activity and a high photocatalytic potential for antibacterial therapy and oxidative stress–mediated treatments (Gao et al., 2024).
4.6 PDA NPs for the eradication of established drug-resistant bacterial biofilms
The emergence of multidrug-resistant bacteria is a major public health concern, exacerbated by biofilm formation. Biofilms are dynamic bacterial communities embedded in a protective self-produced extracellular polymeric substance (EPS) matrix that impedes antibiotic penetration, reduces drug efficacy, and promotes persistent infections (Lv et al., 2025; Wang J et al., 2024). PDA-based materials can potentially eradicate mature bacterial biofilms through synergistic mechanisms involving enzymatic disruption, antibiotic delivery, and photothermal activation, often exploiting the adhesive capabilities of PDA as shown in Figure 3. This section highlights recent strategies using PDA NPs for eradicating bacterial biofilms.
Yu et al. (2022) combined enzymatic degradation, antibiotic therapy, and the photothermal effect into a dissolving microneedle patch. The enzyme
Zhang R et al. (2024) designed a PDA with enhanced antibiofilm efficacy for use in photodynamic therapy platforms. Their CaO2/ICG@PDA system (ICG = indocyanine green) generates oxygen to alleviate hypoxia in an acidic biofilm environment. Under NIR activation, ROS generation by ICG enhances the efficacy of photodynamic therapy and PDA heats the local area. This system increases membrane permeability and bacterial mortality, eliminating over 99.9999% of methicillin-resistant S. aureus (MRSA) in vitro as shown in Figure 4 (Zhang H et al., 2024).
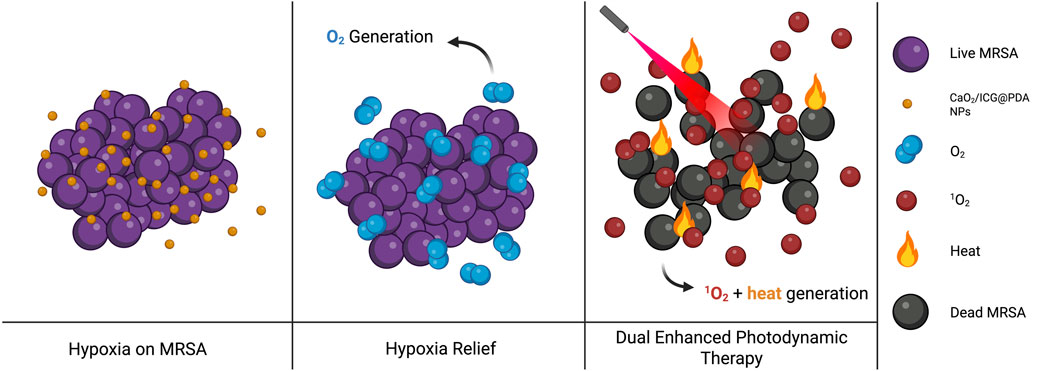
Figure 4. Schematic illustration of the dual-functional CaO2/ICG@PDA system improving PDT outcomes against MRSA biofilms. Image inspired on (Zhang H et al., 2024) (image prepared using BioRender.com).
Another approach polymerizes dopamine in situ within a preformed biofilm. The dopamine monomer of PDA is allowed to diffuse throughout the biofilm structure. Ferrous ions are then externally added to catalyze the polymerization process. The reaction proceeds even under acidic conditions, thus forming PDA within the biofilm. Besides physically restricting microbial movement and activity, this mechanism prepares the system for subsequent photothermal therapy. PDA strongly converts NIR irradiation to heat, causing localized hyperthermia that damages the EPS structure and compromises bacterial membranes. This dual-action mechanism effectively disrupts the biofilm integrity from within, offering a controllable and targeted approach with minimal systemic toxicity (Lv et al., 2025).
In a more complex strategy designed by Wang J et al. (2024), Fe3O4@AgAu@PDA nanospindles are engineered to exploit magnetic and photothermal modes for enhanced biofilm elimination. These nanoparticles comprise a magnetic Fe3O4 core, a layer of AgAu nanorods, and an outer PDA shell with high biocompatibility and photothermal sensitivity. The spindle-like morphology deeply penetrates the biofilm matrix, whereas the Fe3O4 core is externally controlled by a rotating magnetic field that induces rotation and mechanical agitation of the nanospindles, enabling effective disruption of the biofilm structure. Meanwhile, NIR irradiation activates heat production by both AgAu and PDA, accelerating bacterial death. This multimodal attack—mechanical disruption, localized hyperthermia, and adverse chemical effects (Ag+ release and ROS generation)—is remarkably more efficacious than conventional therapies (Wang J et al., 2024).
4.7 In vivo evaluation of PDA NPs for antimicrobial applications
In the past few years, the antimicrobial properties of PDA NPs against diverse bacterial strains have been studied in vivo. These studies have increased our understanding of antimicrobial efficacy under physiological conditions and have revealed the biocompatibility, biodistribution, and potential toxicity of nanomaterials. This section summarizes representative in vivo studies, highlighting the key findings supporting the use of PDA NPs in next-generation antimicrobial therapies.
The delivery and in situ polymerization of dopamine have been investigated in a rat model of severe biofilm-associated wound infection. Lv et al. (2025) created full-thickness skin defects in rats and inoculated them with P. aeruginosa, a pathogen recognized for its biofilm-forming capacity and resistance to antimicrobial treatment. The infected wounds were subjected to different treatments: phosphate-buffered saline as a negative control, NIR light irradiation alone, in situ polymerized PDA, and combined PDA and NIR irradiation. Over a 14-day treatment period, the PDA–NIR combination greatly reduced the bacterial burden and effectively disrupted the biofilm matrix, removing biofilm layers as thick as 120 μm; by contrast, the control, PDA, and NIR-alone groups exhibited minimal antibacterial activity. Histological analysis revealed accelerated wound healing and reduced inflammatory cell infiltration after the PDA-based treatment, indicating antimicrobial efficacy and enhanced tissue regeneration. Furthermore, biosafety assessments based on hemolysis assays showed no remarkable cytotoxic effects at concentrations up to 400 μg/mL, indicating the therapeutic potential and biocompatibility of the system (Lv et al., 2025).
PDA NPs modified with cationic polymers have demonstrated similar potential as the promoters of bacterial wound healing. Cai et al. (2024) engineered an alternating polymeric coating to enhance the penetration of PDA NPs into dense bacterial biofilms. Under NIR irradiation, the photothermal effect induced by PDA disrupts the coating–NP interactions, triggering the controlled and direct release of the cationic antimicrobial polymer into the bacteria. To test the therapeutic efficiency of this in situ antimicrobial delivery for targeted bacterial eradication, the researchers infected a mouse wound model with methicillin-resistant S. aureus and treated it with seven different formulations. The group receiving NIR-irradiated polymer-functionalized PDA NPs exhibited the best therapeutic outcome, with a large reduction in wound size within the first 24 h and near-complete healing within 7 days. Histological analyses, including hematoxylin/eosin (H&E) staining and immunohistochemistry on days 3 and 7, confirmed a remarkable reduction in inflammatory cell infiltration and enhanced tissue regeneration. In vivo biosafety assessments, including body weight monitoring, histological examination of major organs, and serum biochemical and hematological analyses, revealed no remarkable abnormalities, indicating the high biocompatibility and safety of the treatment.
In another recent study, Wang, Ma, and others (2024) functionalized a mesoporous PDA nanocarrier with sodium nitroprusside (SNP) and lecithin (SNP/MPDA + L) for synergistic antibacterial activity and wound-healing promotion. The photothermal properties of MPDA, together with the nitric oxide–releasing capability of SNP, enhanced the therapeutic efficacy. The therapeutic formulation combined with NIR irradiation was administered to an S. aureus–infected rat wound model over a 9-day period. The SNP/MPDA + L + NIR treatment considerably reduced the bacterial colony count from that of the control group and accelerated wound healing. Histological analysis confirmed a marked reduction in the inflammatory cell count and greater collagen deposition at the wound site, indicating a higher degree of tissue regeneration than in the control group. Furthermore, the histological examination of the major organs (heart, liver, spleen, lungs, and kidneys) revealed no pathological abnormalities, suggesting the biocompatibility and safety of the nanotherapeutic platform as shown in Figure 5.
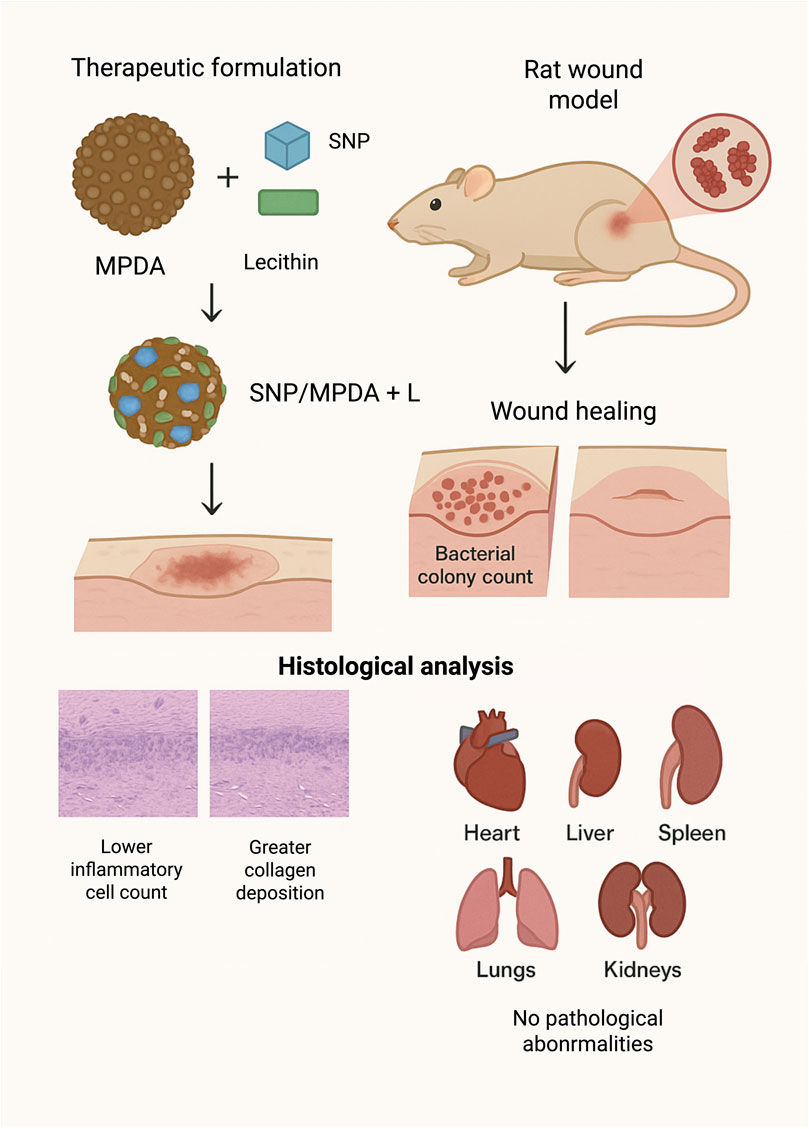
Figure 5. Synergistic Antibacterial and Wound-Healing Effects of SNP/MPDA + L Nanoplatform under NIR Irradiation in an S. aureus–Infected Rat Model (image prepared using BioRender.com).
Alternatively, Xu et al. (2025) functionalized poly (lactic-co-glycolic acid) with PDA and polyethylene glycol–thiol (PEG–SH) (PEG–PDA@PLGA) microspheres as an oral formulation against intestinal bacterial infections. This multifunctional platform exploits the iron-chelating capability of PDA to coordinate iron ions while codelivering rifampicin and FeCl3. The system promotes localized ROS-generating Fenton reactions that efficiently eradicate pathogenic bacteria. The therapeutic efficacy was assessed in a mouse model infected with Salmonella typhimurium. In vitro assays demonstrated a remarkable 99% bacterial clearance within just 4 hours. In vivo, the PEG–PDA@PLGA treatment group achieved a survival rate of 100%, considerably outperforming the control group. Histological analyses (H&E staining) of treated tissues revealed lower inflammatory cell infiltration, less oxidative stress, and lower proinflammatory-factor expression in the treatment group than in the control group. Furthermore, biocompatibility assessments indicated minimal hemolytic activity (less than 5%) and no remarkable major pathological alterations within 48 h after administration, implying that PEG–PDA@PLGA microspheres are safe and effective antimicrobial therapies.
5 Conclusion: research gaps and future directions
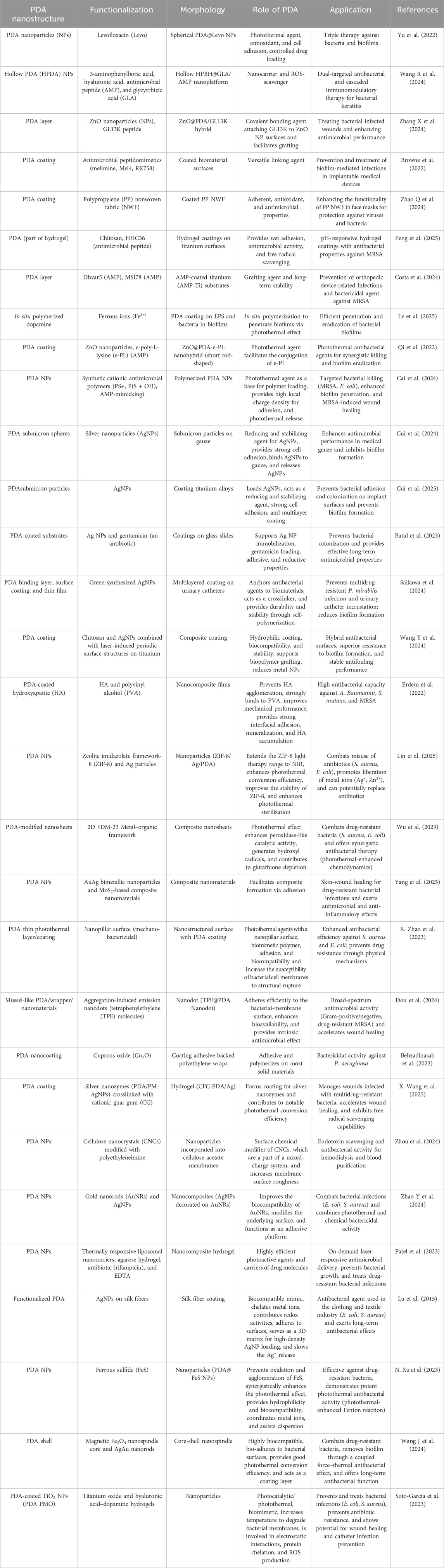
Polydopamine (PDA) nanoparticles have emerged as versatile and promising smart antimicrobial agents owing to their intrinsic biocompatibility, tunable surface chemistry, and robust photothermal properties. Recent advancements have improved synthesis methods, including solution oxidation, electropolymerization, and enzymatic catalysis, that enable control over particle size, morphology, and coating thickness. Diverse functionalization strategies such as metal-ion coordination, polymer grafting, and AMP incorporation have further endowed PDA nanomaterials with synergistic antibacterial, antibiofilm, and stimuli-responsive drug delivery capabilities. These innovations have successfully broadened the antimicrobial and biomedical applications of PDA-based platforms, ranging from biofilm eradication and wound healing to targeted therapies for drug-resistant infections (Table 1 provides an overview of the articles discussed in this review).
Despite these advances, several challenges restrict the clinical translation of PDA nanomaterials. Biocompatibility assessments are often confused by interference in conventional cytotoxicity assays, and incomplete understanding of long-term biodegradation or potential off-target effects raises safety concerns, particularly in applications involving photothermal or combined therapies. Furthermore, limited standardization in evaluating antimicrobial efficacy and insufficient in vivo validation hinder the comparability and reproducibility of many reported results.
To fully realize the potential of PDA-based antimicrobial systems, future research should focus on establishing robust, reproducible synthesis protocols and standardized characterization methodologies that ensure predictable nanoparticle performance. Broad and rigorous evaluations of biocompatibility, biodegradability, and biosafety, including new assay designs and long-term in vivo studies, are crucial for advancing clinical translation. Additionally, interdisciplinary approaches integrating materials science, microbiology, and biomedical engineering will be essential for optimizing functionalization strategies, deciphering antibacterial mechanisms, and developing scalable, clinically viable PDA nanoplatforms.
Through coordinated efforts to address these prevalent weaknesses, PDA nanomaterials are well positioned to shape the next-generation of smart antimicrobial technologies, offering innovative solutions to combat the growing threat of antimicrobial resistance.
Author contributions
SG-S: Writing – review and editing, Writing – original draft. NP: Writing – review and editing. AR: Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was financially supported by KAUST Baseline Grant BAS/1/1096-01-0 (to Prof. A. S. R).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fbioe.2025.1678136/full#supplementary-material
References
Aflori, M. (2021). Smart nanomaterials for biomedical applications—A review. Nanomaterials 11 (2). 396. doi:10.3390/nano11020396
Almeida, L. C., Frade, T., Correia, R. D., Niu, Y., Jin, G., Correia, J. P., et al. (2021). Electrosynthesis of polydopamine-ethanolamine films for the development of immunosensing interfaces. Sci. Rep. 11 (1), 2237. doi:10.1038/s41598-021-81816-1
Bano, S., Hasnain, M., Rehman, K., Kanwal, T., Perveen, S., Yasmeen, S., et al. (2023). Comparative analysis of polydopamine and casein coated Arabic gum stabilized silver nanoparticles for enhanced antimicrobial activity of quercitrin. J. Mol. Struct. 1294, 136515. doi:10.1016/j.molstruc.2023.136515
Battaglini, M., Emanet, M., Carmignani, A., and Ciofani, G. (2024). “Polydopamine-based nanostructures: a new generation of versatile, multi-tasking, and smart theranostic tools,”, 55. Elsevier B.V. doi:10.1016/j.nantod.2024.102151
Batul, R., Bhave, M., and Yu, A. (2023). Investigation of antimicrobial effects of polydopamine-based composite coatings. Molecules 28 (11), 4258. doi:10.3390/molecules28114258
Behzadinasab, S., Williams, M. D., Falkinham, J. O., and Ducker, W. A. (2023). Facile implementation of antimicrobial coatings through adhesive films (Wraps) demonstrated with cuprous oxide coatings. Antibiotics 12 (5), 920. doi:10.3390/antibiotics12050920
Browne, K., Kuppusamy, R., Chen, R., Willcox, M. D. P., Walsh, W. R., Black, D. S., et al. (2022). Bioinspired polydopamine coatings facilitate attachment of antimicrobial peptidomimetics with broad-spectrum antibacterial activity. Int. J. Mol. Sci. 23 (6), 2952. doi:10.3390/ijms23062952
Cai, S., Hao, Y., Wang, X., Hu, Y., Zhao, J., Amier, Y., et al. (2024). Photothermal-mediated in situ delivery of polycations into bacteria via alternating polymer-modified nanoparticles for targeted bacterial killing and enhanced biofilm penetration. Adv. Funct. Mater. 35, 2413036. doi:10.1002/adfm.202413036
Caniglia, G., Teuber, A., Barth, H., Mizaikoff, B., and Kranz, C. (2023). Atomic force and infrared spectroscopic studies on the role of surface charge for the anti-biofouling properties of polydopamine films. Anal. Bioanal. Chem. 415 (11), 2059–2070. doi:10.1007/s00216-022-04431-7
Caniglia, G., Valavanis, D., Tezcan, G., Magiera, J., Barth, H., Bansmann, J., et al. (2024). Antimicrobial effects of silver nanoparticle-microspots on the mechanical properties of single bacteria. Analyst 149 (9), 2637–2646. doi:10.1039/d4an00174e
Chinemerem Nwobodo, D., Ugwu, M. C., Oliseloke Anie, C., Al-Ouqaili, M. T. S., Chinedu Ikem, J., Victor Chigozie, U., et al. (2022). Antibiotic resistance: the challenges and some emerging strategies for tackling a global menace. John Wiley Sons Inc 36 (Issue 9), e24655. doi:10.1002/jcla.24655
Cicogna, F., Passaglia, E., Elainaoui, E., Bramanti, E., Oberhauser, W., Casini, B., et al. (2023). Coating of polypropylene non-woven fabric with layered double hydroxides bearing antioxidant and antibacterial natural compounds. Macromol. Chem. Phys. 224 (23), 2300148. doi:10.1002/macp.202300148
Costa, B., Coelho, J., Silva, V., Shahrour, H., Costa, N. A., Ribeiro, A. R., et al. (2024). Dhvar5-and MSI78-coated titanium are bactericidal against methicillin-resistant Staphylococcus aureus, immunomodulatory and osteogenic. Acta Biomater. 191, 98–112. doi:10.1016/j.actbio.2024.11.016
Cui, J., Shu, H., Zhu, P., Cao, Z., Wang, S., and Cao, P. (2024). Enhancing antimicrobial performance of gauze via modification by Ag-Loaded polydopamine submicron particles. J. Funct. Biomaterials 15 (6), 152. doi:10.3390/jfb15060152
Cui, J., Shu, H., Gu, X., Wu, S., Liu, X., and Cao, P. (2025). Enhancing antibacterial performance and stability of implant materials through surface modification with polydopamine/silver nanoparticles. Colloids Surfaces B Biointerfaces 245, 114327. doi:10.1016/j.colsurfb.2024.114327
Dou, L., Wang, X., Bai, Y., Li, Q., Luo, L., Yu, W., et al. (2024). Mussel-like polydopamine-assisted aggregation-induced emission nanodot for enhanced broad-spectrum antimicrobial activity: in vitro and in vivo validation. Int. J. Biol. Macromol. 282, 136762. doi:10.1016/j.ijbiomac.2024.136762
D’Ischia, M., Napolitano, A., Ball, V., Chen, C. T., and Buehler, M. J. (2014). Polydopamine and eumelanin: from structure-property relationships to a unified tailoring strategy. Accounts Chem. Res. 47 (12), 3541–3550. doi:10.1021/AR500273Y
Erdem, U., Dogan, D., Bozer, B. M., Turkoz, M. B., Yıldırım, G., and Metin, A. U. (2022). Fabrication of mechanically advanced polydopamine decorated hydroxyapatite/polyvinyl alcohol bio-composite for biomedical applications: in-vitro physicochemical and biological evaluation. J. Mech. Behav. Biomed. Mater. 136, 105517. doi:10.1016/j.jmbbm.2022.105517
Gao, J., Yan, Y., Gao, S., Li, H., Lin, X., Cheng, J., et al. (2024). Heterogeneous Cu2O-SnO2 doped polydopamine fenton-like nanoenzymes for synergetic photothermal-chemodynamic antibacterial application. Acta Biomater. 173, 420–431. doi:10.1016/j.actbio.2023.11.009
GhavamiNejad, A., Aguilar, L. E., Ambade, R. B., Lee, S. H., Park, C. H., and Kim, C. S. (2015). Immobilization of silver nanoparticles on electropolymerized polydopamine films for metal implant applications. Colloids Interface Sci. Commun. 6, 5–8. doi:10.1016/j.colcom.2015.08.001
Gholami Derami, H., Gupta, P., Weng, K. C., Seth, A., Gupta, R., Silva, J. R., et al. (2021). Reversible photothermal modulation of electrical activity of excitable cells using polydopamine nanoparticles. Adv. Mater. 33 (32), 2008809. doi:10.1002/adma.202008809
Hall, W., McDonnell, A., and O’Neill, J. (2018). Superbugs: an arms race against bacteria. Harvard University Press.
He, G., Wan, M., Wang, Z., Zhou, X., Zhao, Y., Sun, L., et al. (2023). A simple surface modification method to prepare versatile PVDF electrospun nanofibrous felts for separation, sterilization and degradation. Prog. Org. Coat 182. doi:10.1016/j.porgcoat.2023.107664
Hemmatpour, H., Haddadi-Asl, V., Burgers, T. C. Q., Yan, F., Stuart, M. C. A., Reker-Smit, C., et al. (2023). Temperature-responsive and biocompatible nanocarriers based on clay nanotubes for controlled anti-cancer drug release. Nanoscale 15 (5), 2402–2416. doi:10.1039/d2nr06801j
Husen, A., and Siddiqi, K. S. (2023). Advances in smart nanomaterials and their applications. Elsevier, 23–50.
Jabbar, A., Rehman, K., Jabri, T., Kanwal, T., Perveen, S., Rashid, M. A., et al. (2023). Improving curcumin bactericidal potential against multi-drug resistant bacteria via its loading in polydopamine coated zinc-based metal–organic frameworks. Drug Deliv. 30 (1), 2159587. doi:10.1080/10717544.2022.2159587
Kan, Z., Chen, Y., Zhang, Q., Pan, L., Chen, A., Wang, D., et al. (2025). Covalent bonding coating of quantum-sized TiO2 with polydopamine on catheter surface for synergistically enhanced antimicrobial and anticoagulant performances. Colloids Surfaces B Biointerfaces 245, 114249. doi:10.1016/j.colsurfb.2024.114249
Kensel Rajeev, A., Sathish, N., Elango, H., Sivagnanam, S., Nayak, S., Das, P., et al. (2024). In-situ eco-friendly synthesis of a biomimetic robust antibacterial nanohybrid via catecholamine-induced metallization. Inorg. Chem. Commun. 170, 113172. doi:10.1016/j.inoche.2024.113172
Larrieu, M., Mouniee, D., Agusti, G., Blaha, D., and Edouard, D. (2024). Antimicrobial foam-filter based on commercial support coated with polydopamine and silver nanoparticles for water treatment. Environ. Technol. Innovation 33, 103468. doi:10.1016/j.eti.2023.103468
Li, F., Yu, Y., Wang, Q., Yuan, J., Wang, P., and Fan, X. (2018). Polymerization of dopamine catalyzed by laccase: Comparison of enzymatic and conventional methods. Enzyme Microb. Technol. 119, 58–64. doi:10.1016/j.enzmictec.2018.09.003
Li, H., Xi, J., Donaghue, A. G., Keum, J., Zhao, Y., An, K., et al. (2020). Synthesis and catalytic performance of polydopamine supported metal nanoparticles. Sci. Rep. 10 (1), 10416. doi:10.1038/s41598-020-67458-9
Li, Z. P., You, S., Mao, R., Xiang, Y., Cai, E., Deng, H., et al. (2022). Architecting polyelectrolyte hydrogels with Cu-assisted polydopamine nanoparticles for photothermal antibacterial therapy. Mater. Today Bio 15, 100264. doi:10.1016/j.mtbio.2022.100264
Li, T., Zhang, J., Wen, B., Wu, Y., Che, F., and Guo, Y. (2024). Dual-mode regulation of microbial cell membrane permeability for an enhanced microbial cuproptosis-like death pathway. Mater. Chem. Front. 9, 618–627. doi:10.1039/d4qm00935e
Li, S., Li, J., Xing, J., Li, L., Wang, L., and Wang, C. (2025). Development and characterization of hyaluronic acid graft-modified polydopamine nanoparticles for antibacterial studies. Polymers 17 (2), 162. doi:10.3390/polym17020162
Lin, J., Li, Y., Ge, L., Zhang, Y., Yuan, S., Wang, M., et al. (2025). Photothermal enhanced ion release of ZIF-8/Ag/PDA for boosted antimicrobial performance. Inorg. Chem. Commun. 174, 114001. doi:10.1016/j.inoche.2025.114001
Liu, Y., Liu, R., Nie, W., Yu, L., Lyu, S., Han, Q., et al. (2025). Enhanced corrosion resistance and photothermal antibacterial properties of MAO/PCL-PDA/NG composite coating on Mg alloy. Mater. Today Commun. 42, 111252. doi:10.1016/j.mtcomm.2024.111252
Lu, Z., Xiao, J., Wang, Y., and Meng, M. (2015). In situ synthesis of silver nanoparticles uniformly distributed on polydopamine-coated silk fibers for antibacterial application. J. Colloid Interface Sci. 452, 8–14. doi:10.1016/j.jcis.2015.04.015
Lv, Q., Cai, Y., Yang, R., Zhang, L., Han, Y., Marfavi, Z., et al. (2025). Efficient penetration and in situ polymerization of dopamine in biofilms for the eradication. Chem. Eng. J. 503, 158562. doi:10.1016/j.cej.2024.158562
Magalhães, F. F., Pereira, A. F., Freire, M. G., and Tavares, A. P. M. (2022). New liquid supports in the development of integrated platforms for the reuse of oxidative enzymes and polydopamine production. Front. Bioeng. Biotechnol. 10, 1037322. doi:10.3389/fbioe.2022.1037322
Marchesi D’Alvise, T., Sunder, S., Hasler, R., Moser, J., Knoll, W., Synatschke, C. V., et al. (2023). Preparation of ultrathin and degradable polymeric films by electropolymerization of 3-Amino-l-tyrosine. Macromol. Rapid Commun. 44 (16), 2200332. doi:10.1002/MARC.202200332
Meng, Y., Liu, P., Zhou, W., Ding, J., and Liu, J. (2018). Bioorthogonal DNA adsorption on polydopamine nanoparticles mediated by metal coordination for highly robust sensing in serum and living cells. ACS Nano 12 (9), 9070–9080. doi:10.1021/acsnano.8b03019
Michalska, M., Gambacorta, F., Divan, R., Aranson, I. S., Sokolov, A., Noirot, P., et al. (2018). Tuning antimicrobial properties of biomimetic nanopatterned surfaces. Nanoscale 10 (14), 6639–6650. doi:10.1039/c8nr00439k
Murray, C. J., Ikuta, K. S., Sharara, F., Swetschinski, L., Robles Aguilar, G., Gray, A., et al. (2022). Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 399 (10325), 629–655. doi:10.1016/S0140-6736(21)02724-0
Nothling, M. D., Bailey, C. G., Fillbrook, L. L., Wang, G., Gao, Y., McCamey, D. R., et al. (2022). Polymer grafting to polydopamine free radicals for universal surface functionalization. J. Am. Chem. Soc. 144 (15), 6992–7000. doi:10.1021/jacs.2c02073
Patel, M., Corbett, A. L., Vardhan, A., Jeon, K., Andoy, N. M. O., and Sullan, R. M. A. (2023). Laser-responsive sequential delivery of multiple antimicrobials using nanocomposite hydrogels. Biomaterials Sci. 11 (7), 2330–2335. doi:10.1039/d2bm01471h
Peng, S., Liu, Y., Zhao, W., Liu, X., Yu, R., and Yu, Y. (2025). Construction of pH-responsive hydrogel coatings on titanium surfaces for antibacterial and osteogenic properties. Front. Chem. 13, 1546637. doi:10.3389/fchem.2025.1546637
Polaczek, K., Olejnik, A., Gumieniak, J., Kramek, A., Karczewski, J., and Siuzdak, K. (2024). Lewis acid catalysis of polydopamine electropolymerisation as a tool for shaping its morphology and electrochemical properties. J. Mater. Sci. 59 (20), 9126–9149. doi:10.1007/s10853-024-09722-1
Pulingam, T., Parumasivam, T., Gazzali, A. M., Sulaiman, A. M., Chee, J. Y., Lakshmanan, M., et al. (2022). “Antimicrobial resistance: prevalence, economic burden, mechanisms of resistance and strategies to overcome,”, 170. Elsevier B.V. doi:10.1016/j.ejps.2021.106103
Qi, C., Zhang, Y., and Tu, J. (2022). Facile synthesis of ε-poly- -lysine-conjugated ZnO@PDA as photothermal antibacterial agents for synergistic bacteria killing and biofilm eradication. Biochem. Eng. J. 186, 108569. doi:10.1016/j.bej.2022.108569
Ren, S., Xiao, X., Lv, J., Lv, S., Wang, X., Liu, R., et al. (2024). Advances in the study of polydopamine nanotechnology in central nervous system disorders. Fronti. Mater. 11. doi:10.3389/fmats.2024.1396397
Saikawa, G. I. A., Guidone, G. H. M., Noriler, S. A., Reis, G. F., de Oliveira, A. G., Nakazato, G., et al. (2024). Green-synthesized silver nanoparticles in the prevention of multidrug-resistant Proteus mirabilis infection and incrustation of urinary catheters BioAgNPs against P. mirabilis infection. Curr. Microbiol. 81 (4), 100. doi:10.1007/s00284-024-03616-w
Salam, M. A., Al-Amin, M. Y., Salam, M. T., Pawar, J. S., Akhter, N., Rabaan, A. A., et al. (2023). Antimicrobial resistance: a growing serious threat for global public health. Healthc. Switz. 11 (Issue 13), 1946. doi:10.3390/healthcare11131946
Schanze, K. S., Lee, H., and Messersmith, P. B. (2018). Ten years of polydopamine: current status and future directions. ACS Appl. Mater. Interfaces 10 (9), 7521–7522. doi:10.1021/acsami.8b02929
Sharma, D., and Hussain, C. M. (2020). “Smart nanomaterials in pharmaceutical analysis,”, 13. Elsevier B.V, 3319–3343. doi:10.1016/j.arabjc.2018.11.007
Shumbula, N. P., Nkabinde, S. S., Ndala, Z. B., Mpelane, S., Shumbula, M. P., Mdluli, P. S., et al. (2022). Evaluating the antimicrobial activity and cytotoxicity of polydopamine capped silver and silver/polydopamine core-shell nanocomposites. Arabian J. Chem. 15 (6), 103798. doi:10.1016/j.arabjc.2022.103798
Siciliano, G., Monteduro, A. G., Turco, A., Primiceri, E., Rizzato, S., Depalo, N., et al. (2022). Polydopamine-coated magnetic iron oxide nanoparticles: from design to applications. Nanomaterials 12 (7), 1145. doi:10.3390/nano12071145
Siddiqui, I., Owais, M., and Husain, Q. (2024). Antimicrobial effects of peptides from fenugreek and ginger proteins using Fe3O4@PDA-MWCNT conjugated trypsin by improving enzyme stability and applications. Int. J. Biol. Macromol. 282, 137197. doi:10.1016/j.ijbiomac.2024.137197
Singh, R., Sharma, A., Saji, J., Umapathi, A., Kumar, S., and Daima, H. K. (2022). Smart nanomaterials for cancer diagnosis and treatment. Nano Converg. 9 (1), 21. doi:10.1186/s40580-022-00313-x
Smies, A., Wales, J., Hennenfent, M., Lyons, L., Dunn, C., Robbins, J., et al. (2022). Non-antibiotic antimicrobial polydopamine surface coating to prevent stable biofilm formation on satellite telemetry tags used in cetacean conservation applications. Front. Mar. Sci. 9, 989025. doi:10.3389/fmars.2022.989025
Soto-Garcia, L. F., Guerrero-Rodriguez, I. D., Hoang, L., Laboy-Segarra, S. L., Phan, N. T. K., Villafuerte, E., et al. (2023). Photocatalytic and photothermal antimicrobial mussel-inspired nanocomposites for biomedical applications. Int. J. Mol. Sci. 24 (17), 13272. doi:10.3390/ijms241713272
Souza, A. L. de, Oliveira, A. V. de A., Ribeiro, L. D., Moraes, A. R. F. e., Jesus, M., Santos, J., et al. (2025). Experimental and theoretical analysis of dopamine polymerization on the surface of cellulose nanocrystals and its reinforcing properties in cellulose acetate films. Polymers 17 (3), 345. doi:10.3390/polym17030345
Talapko, J., Meštrović, T., Juzbašić, M., Tomas, M., Erić, S., Horvat Aleksijević, L., et al. (2022). Antimicrobial peptides—Mechanisms of Action, antimicrobial effects and clinical applications. Antibiotics 11 (10), 1417. doi:10.3390/antibiotics11101417
Tang, K. W. K., Millar, B. C., and Moore, J. E. (2023). Antimicrobial resistance (AMR). Br. J. Biomed. Sci. 80, 11387. doi:10.3389/bjbs.2023.11387
Teunissen, L. W., Smulders, M. M. J., and Zuilhof, H. (2023). 19 nm-Thick Grafted-To polymer brushes onto optimized Poly(Dopamine)-Coated surfaces. Adv. Mater. Interfaces 10 (18), 2202503. doi:10.1002/admi.202202503
Tran, H. Q., Batul, R., Bhave, M., and Yu, A. (2019). Current advances in the utilization of polydopamine nanostructures in biomedical therapy. Biotechnol. J. 14 (12), 1900080. doi:10.1002/BIOT.201900080
Varol, H. S., Herberger, T., Kirsch, M., Mikolei, J., Veith, L., Kannan-Sampathkumar, V., et al. (2023). Electropolymerization of polydopamine at electrode-supported insulating mesoporous films. Chem. Mater. 35 (21), 9192–9207. doi:10.1021/acs.chemmater.3c01890
Wang, Z., Zou, Y., Li, Y., and Cheng, Y. (2020a). Metal-containing polydopamine nanomaterials: catalysis, energy, and theranostics. Small 16 (Issue 18), 1907042. doi:10.1002/smll.201907042
Wang, Z., Zou, Y., Li, Y., and Cheng, Y. (2020b). Metal-containing polydopamine nanomaterials: catalysis, energy, and theranostics. Small 16 (Issue 18), 1907042. doi:10.1002/smll.20190042
Wang, H., Ma, J., Meng, S., Zou, H., Wang, H., and Zhou, M. (2024). Functionalized mesoporous polydopamine nanocarrier with near-infrared laser-trigged NO release and photothermal effects for the killing of pathogenic bacteria. ACS Appl. Nano Mater. 7 (9), 10429–10441. doi:10.1021/acsanm.4c00865
Wang, X., Huang, J., Zhao, J., Yue, T., Shenyang, W., Xu, Y., et al. (2025). pH-responsive cationic guar gum-based multifunctional hydrogel with silver nanoenzymes: combined photothermal antibacterial therapy and antioxidant properties for MRSA infected wound healing. Int. J. Biol. Macromol. 292, 139201. doi:10.1016/j.ijbiomac.2024.139201
Wang H, H., Xu, X., Mei, X., Zeng, D., Ying, B., Yu, Z., et al. (2024). 3D-printed porous PEI/TCP composite scaffolds loaded with graphdiyne oxide on the surface for bone defect repair and near-infrared light-responsive antibacterial. Mater. Des. 237. doi:10.1016/j.matdes.2023.112569
Wang J, J., Fang, X., Yu, G., Luo, T., Xu, Y., Xu, C., et al. (2024). Magnetic hybrid nanospindle with an unconventional force-thermal coupling antibacterial effect. Colloids Surfaces A Physicochem. Eng. Aspects 683, 133060. doi:10.1016/j.colsurfa.2023.133060
Wang L, L, chao, B., Li, Z., Ren, , feng, K., jiang, L., et al. (2014). Electropolymerization of dopamine for surface modification of complex-shaped cardiovascular stents. Biomaterials, 35(27), 7679–7689. doi:10.1016/j.biomaterials.2014.05.047
Wang L, L., Song, K., Jiang, C., Liu, S., Huang, S., Yang, H., et al. (2024). “Metal-coordinated polydopamine structures for tumor imaging and therapy,” in Advanced healthcare materials (John Wiley and Sons Inc). doi:10.1002/adhm.202401451
Wang R, R., Dong, Y., Zhang, J., Hao, L., Zhou, L., Sun, L., et al. (2024). A multifunctional nanoplatform with dual-targeted antibacterial and cascaded immunomodulatory strategy for the treatment of bacterial keratitis. Chem. Eng. J. 498, 155323. doi:10.1016/j.cej.2024.155323
Wang S, S., Meng, F., and Cao, Z. (2024). Improving surface antimicrobial performance by coating homogeneous PDA-Ag micro–nano particles. Coatings 14 (7), 887. doi:10.3390/coatings14070887
Wang Y, Y., Dong, Y., Quan, Y., Wackerow, S., Abdolvand, A., Zolotovskaya, S. A., et al. (2024). Hybrid antibacterial surfaces: combining laser-induced periodic surface structures with polydopamine-chitosan-silver nanoparticle nanocomposite coating. Adv. Mater. Interfaces 12, 2400660. doi:10.1002/admi.202400660
Wu, Y., Liu, X., Zhang, X., Zhang, S., Niu, P., and Gao, H. (2023). Photothermal theranostics with glutathione depletion and enhanced reactive oxygen species generation for efficient antibacterial treatment. RSC Adv. 13 (33), 22863–22874. doi:10.1039/d3ra03246a
Xiong, C., and Ulbricht, M. (2024). Thermally responsive porous membranes with both switching wettability and permeability prepared via grafting-to and grafting-from methods. Chem. Eng. Res. Des. 209, 2–11. doi:10.1016/j.cherd.2024.07.025
Xu, N., Huang, Q., Shi, L., Wang, J., Li, X., Guo, W., et al. (2023). A bioinspired polydopamine-FeS nanocomposite with high antimicrobial efficiency via NIR-Mediated fenton reaction. Dalton Trans. 52 (6), 1687–1701. doi:10.1039/d2dt03765c
Xu, Q., Zhao, Y., Yuan, P., Ma, X., Wang, S., Li, L., et al. (2025). Functionalized microsphere platform combining nutrient restriction and combination therapy to combat bacterial infections. ACS Appl. Mater. and Interfaces 17 (2), 2966–2976. doi:10.1021/acsami.4c16610
Yang, Z., You, J., Zhai, S., Zhou, J., Quni, S., Liu, M., et al. (2025). pH-responsive molybdenum disulphide composite nanomaterials for skin wound healing using “ROS leveraging” synergistic immunomodulation. Mater. Today Bio 31, 101481. doi:10.1016/j.mtbio.2025.101481
Yoshida, M., and Lahann, J. (2008). Smart nanomaterials. ACS Nano 2 (6), 1101–1107. doi:10.1021/nn800332g
Yu, X., Zhao, J., and Fan, D. (2022). A dissolving microneedle patch for antibiotic/enzymolysis/photothermal triple therapy against bacteria and their biofilms. Chem. Eng. J. 437, 135475. doi:10.1016/j.cej.2022.135475
Yusuf, A., Almotairy, A. R. Z., Henidi, H., Alshehri, O. Y., and Aldughaim, M. S. (2023). Nanoparticles as drug delivery systems: a review of the implication of nanoparticles’ physicochemical properties on responses in biological systems. Polymers 15 (7), 1596. doi:10.3390/POLYM15071596
Zeng, Q., Qian, Y., Huang, Y., Ding, F., Qi, X., and Shen, J. (2021). Polydopamine nanoparticle-dotted food gum hydrogel with excellent antibacterial activity and rapid shape adaptability for accelerated bacteria-infected wound healing. Bioact. Mater. 6 (9), 2647–2657. doi:10.1016/j.bioactmat.2021.01.035
Zhang, H., Lu, M., Jiang, H., Wu, Z. Y., Zhou, D. D., Li, D. Q., et al. (2020). Tyrosinase-mediated dopamine polymerization modified magnetic alginate beads for dual-enzymes encapsulation: preparation, performance and application. Colloids Surfaces B Biointerfaces 188, 110800. doi:10.1016/j.colsurfb.2020.110800
Zhang H, H., Zou, Y., Lu, K., Wu, Y., Lin, Y., Cheng, J., et al. (2024). A nanoplatform with oxygen self-supplying and heat-sensitizing capabilities enhances the efficacy of photodynamic therapy in eradicating multidrug-resistant biofilms. J. Mater. Sci. Technol. 169, 209–219. doi:10.1016/j.jmst.2023.07.001
Zhang R, R., Zhang, W., Zhu, Q., Nie, Q., Zhang, S., Zhang, Y., et al. (2024). Engineering polydopamine-functionalized NH2-MIL-125 (ti) for tetracycline degradation and antibacterial applications. Surfaces Interfaces 54, 105188. doi:10.1016/j.surfin.2024.105188
Zhang X, X., Liu, Y., Zhang, X., Tang, M., Xi, W., and Wei, J. (2024). Antimicrobial GL13K peptide-decorated ZnO nanoparticles to treat bacterial infections. Langmuir 40, 25042–25050. doi:10.1021/acs.langmuir.4c03206
Zhao B, B., Shi, X., Qiu, H., and Chen, K. (2024). Design and application of polyurethane-polydopamine/Ag double-shell microcapsules for enhanced photothermal conversion and incremental energy storage. Sustain. Mater. Technol. 40, e00895. doi:10.1016/j.susmat.2024.e00895
Zhao, X., Xu, Z., Wei, Z., Sun, Y., and Zhou, Q. (2023). Nature-inspired mechano-bactericidal nanostructured surfaces with photothermally enhanced antibacterial performances. Prog. Org. Coatings 182, 107599. doi:10.1016/j.porgcoat.2023.107599
Zhao Q, Q., Zhou, Y., Zhang, Q., Qu, X., Jiang, Y., Wu, S., et al. (2024). Cobalt doped Prussian blue modified hollow polydopamine for enhanced antibacterial therapy. Nanotechnology 35 (36), 365101. doi:10.1088/1361-6528/ad53d2
Zhao Y, Y., Zhan, K., Geng, P., and Jiang, S. (2024). Polydopamine-assisted decoration of silver nanoparticles on gold nanorods for photothermal and chemical antimicrobial applications. New J. Chem. 49, 624–631. doi:10.1039/d4nj04434g
Keywords: polydopamine nanoparticles, antimicrobial resistance, smart nanomaterials, photothermal therapy, biofilm eradication
Citation: Guzman-Sanchez S, Patel N and Rosado AS (2025) Recent progress of polydopamine nanoparticles as advanced antimicrobial nanomaterials. Front. Bioeng. Biotechnol. 13:1678136. doi: 10.3389/fbioe.2025.1678136
Received: 01 August 2025; Accepted: 02 October 2025;
Published: 17 October 2025.
Edited by:
Hsin-hui Shen, Monash University, AustraliaReviewed by:
Leina Dou, China Agricultural University, ChinaRahila Batul, University of Hail, Saudi Arabia
Copyright © 2025 Guzman-Sanchez, Patel and Rosado. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Niketan Patel, TmlrZXRhbi5wYXRlbEBrYXVzdC5lZHUuc2E=; Alexandre Soares Rosado, QWxleGFuZHJlLnJvc2Fkb0BrYXVzdC5lZHUuc2E=
†ORCID: Niketan Patel, orcid.org/0000-0002-4702-7361; Alexandre Soares Rosado, orcid.org/0000-0001-5135-1394
 Sandra Guzman-Sanchez
Sandra Guzman-Sanchez Niketan Patel
Niketan Patel Alexandre Soares Rosado
Alexandre Soares Rosado