- Institute of Orthopaedic Research and Biomechanics, Trauma Research Centre Ulm, Ulm University Medical Centre, Ulm, Germany
Introduction: Coupled motions are defined as motions outside the primary motion plane and are used for in vivo kinematic measurements as well as validation of experimental and numerical models of the spine. Owing to differences in the individual movement comforts of participants and imprecise measurement methods, previous in vivo studies have been unable to determine distinct patterns of coupled motions. The aim of this meta-analysis was to identify reproducible coupled motion patterns from in vitro studies with standardized loading conditions for each section of the spine.
Methods: A systematic literature search was conducted in the PubMed, Web of Science, and Embase databases in accordance with the PRISMA guidelines to identify in vitro studies on the coupled motions of human specimens (n = 120). In a three-stage procedure, we excluded all studies except those that allowed quantitative comparability of coupled motions in the individual loading directions (n = 20). The inclusion criteria were testing the intact state, quasistatic flexibility measurements using pure moments, and specifications of the analyzed levels. The coupled motions were calculated as values relative to the primary range of motions and quantitatively evaluated via meta-analysis. The one-sample Wilcoxon signed-rank test was performed in SPSS to determine the reproducibility of the coupled motions for each segmental level.
Results: Overall, no relevant coupled motions were identified for primary flexion and extension (p > 0.05). For primary lateral bending, there was evidence for low extension and moderate-to-high ipsilateral axial rotation in the thoracic spine as well as moderate ipsilateral axial rotation in the subaxial cervical spine (p < 0.05). For primary axial rotation, there were reports on low-to-moderate contralateral lateral bending in the thoracic spine and high-to-dominant ipsilateral lateral bending in the subaxial cervical spine (p < 0.05).
Discussion: This meta-analysis of in vitro studies identified some characteristic coupled motion patterns of the spine, specifically a strong motion coupling interrelationship between lateral bending and axial rotation. More studies are required to extend and substantiate the findings of this meta-analysis. Nevertheless, this dataset is valuable for validating experimental and numerical studies of the spine as well as interpreting the coupled motion behaviors of the passive spinal structures.
1 Introduction
Coupled motions are also referred to as secondary or out-of-plane motions and are defined as motions about axes that are associated with a simultaneous primary motion about another axis. With regard to the spine, the causes and effects of coupled motions have been investigated extensively and discussed widely in the past. Early in vivo and in vitro investigations by the orthopedic surgeon Robert W. Lovett in 1900 already suggested strong motion coupling behaviors between lateral bending and axial rotation (Lovett, 1900). While Lovett hypothesized that spinal coupled motions play a role in the etiology of idiopathic scoliosis (Lovett, 1905), further in vivo studies reported abnormally increased coupled motions in patients with degenerative lumbar scoliosis (Xu et al., 2024) as well as in patients suffering from lower-back pain (Weitz, 1981; Pearcy et al., 1985; Parnianpour et al., 1988; Stokes et al., 1981; Ochia et al., 2006). Coupled motions have been used to characterize the in vivo three-dimensional motion behaviors in patients receiving surgical treatment on the cervical spine (Lee et al., 2014). However, in vivo studies have limitations for the evaluation of coupled motions owing to the individual movement comforts of the participants and potential imprecisions in the measurement techniques used, which have consequently resulted in conflicting findings. Thus, previous literature reviews of in vivo studies were unable to determine consistent coupled motion characteristics of specific spinal sections (Harrison et al., 1998; Legaspi and Edmond, 2007; Sizer et al., 2007). Another drawback of in vivo studies is that the roles of the passive spinal structures in coupled motions cannot be determined clearly as spinal loading is generated by complex muscle forces. In vitro studies using standardized loading conditions can provide more reproducible coupled motion data, which are required for distinct interpretations of the in vivo findings as well as validation of the experimental and numerical spine models regarding three-dimensional kinematics. Since the extant in vitro studies have predominantly investigated single spinal sections or segment levels, a comprehensive overview of the coupled motion characteristics of the entire spine is yet to be developed. Hence, the aim of the present systematic literature review and meta-analysis is to collate and compare coupled motion data of the spine from in vitro studies performed under standardized loading conditions.
2 Methods
2.1 Systematic literature review
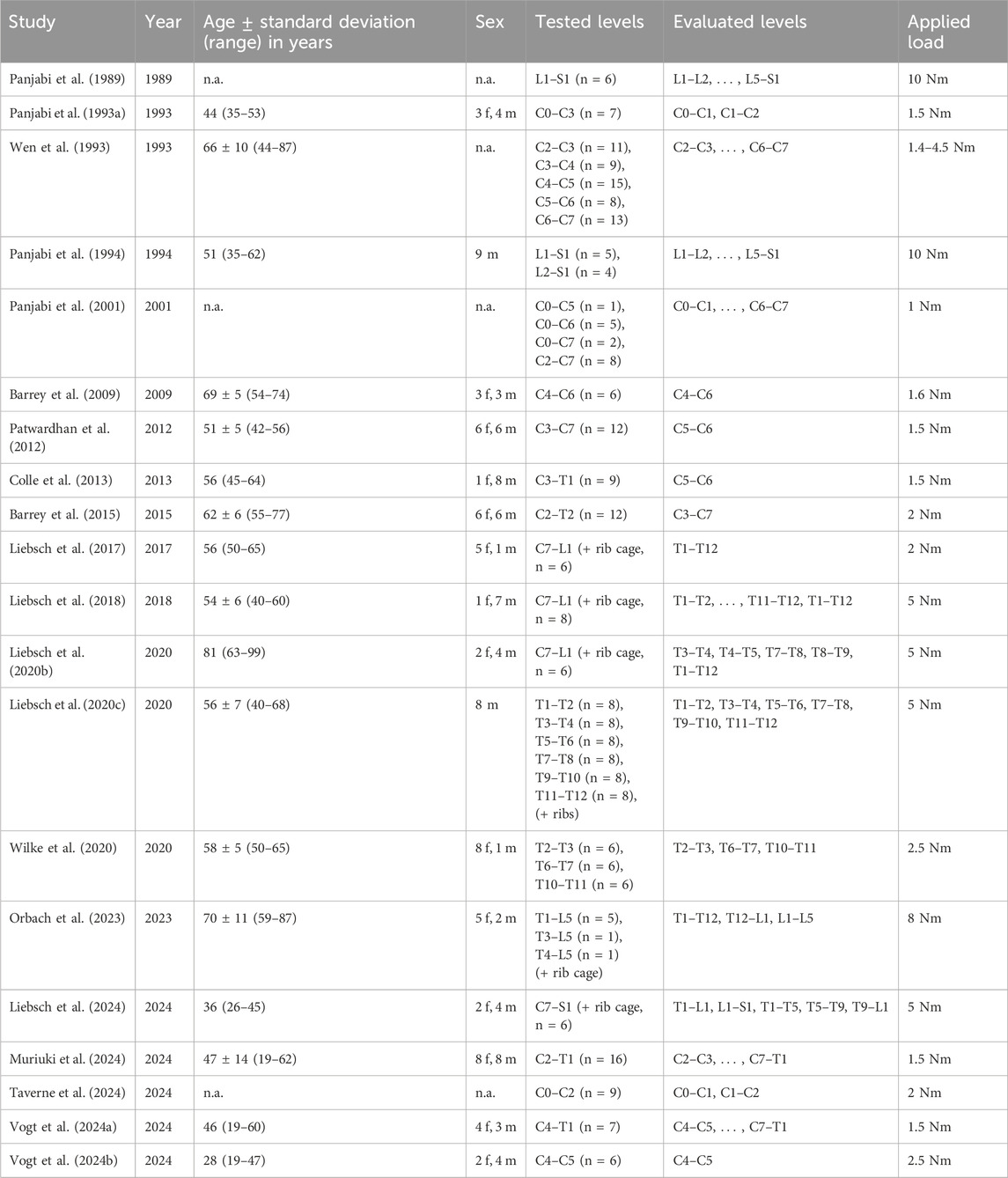
Based on the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines (Page et al., 2021), we conducted a keyword-based literature search on the PubMed, Web of Science, and Embase medical research databases in April 2025 to identify in vitro studies reported on the coupled motions of the human spine (Figure 1). After removing duplicates using EndNote 21.5 (Clarivate Analytics, Philadelphia, PA, United States), n = 328 studies were collected. In the first selection step, we included all articles that reported (1) original data; (2) an in vitro study design; (3) the use of human spine specimens and (4) were written in English language, which resulted in n = 120 articles for closer examination. In the second selection step, the inclusion criteria were defined such that (5) biomechanical data on the intact specimen condition had to be reported (i.e., a testing condition without any implants, resections other than those related to specimen preparation, etc.); (6) quasistatic flexibility testing had to be performed via pure moments (i.e., no eccentric loading, loading without any additional compressive or follower loading, no constraints or shear forces, etc.) according to well-accepted recommendations for spinal in vitro testing (Wilke et al., 1998), and (7) the segmental levels or spinal sections on which the coupled motion measurements were performed had to be specified. On the resulting n = 30 articles from this step, we checked the reference sections for potential publications to be included and data on the specimens (sample size, donor age and sex, etc.), in addition to acquiring information on the tested and evaluated segmental level(s), applied moments, as well as motion directions and range of motion (ROM) of the primary and coupled motions. As some of the articles reported only the ranges of coupled motions but not the primary motions (Oxland et al., 1992), ROM data of intact specimens that were already published in prior studies (Cholewicki et al., 1996; Liebsch et al., 2020a), ratios but not directions of the coupled motions (Puttlitz et al., 2004; Yoganandan et al., 2008; Mannen et al., 2015a; Mannen et al., 2015b), ROM data of non-specified segmental levels (Winkelstein and Myers, 2002; Brasiliense et al., 2011), or combined primary and coupled motions (Daniels et al., 2012), such works were excluded to obtain n = 20 studies for the final data evaluations.
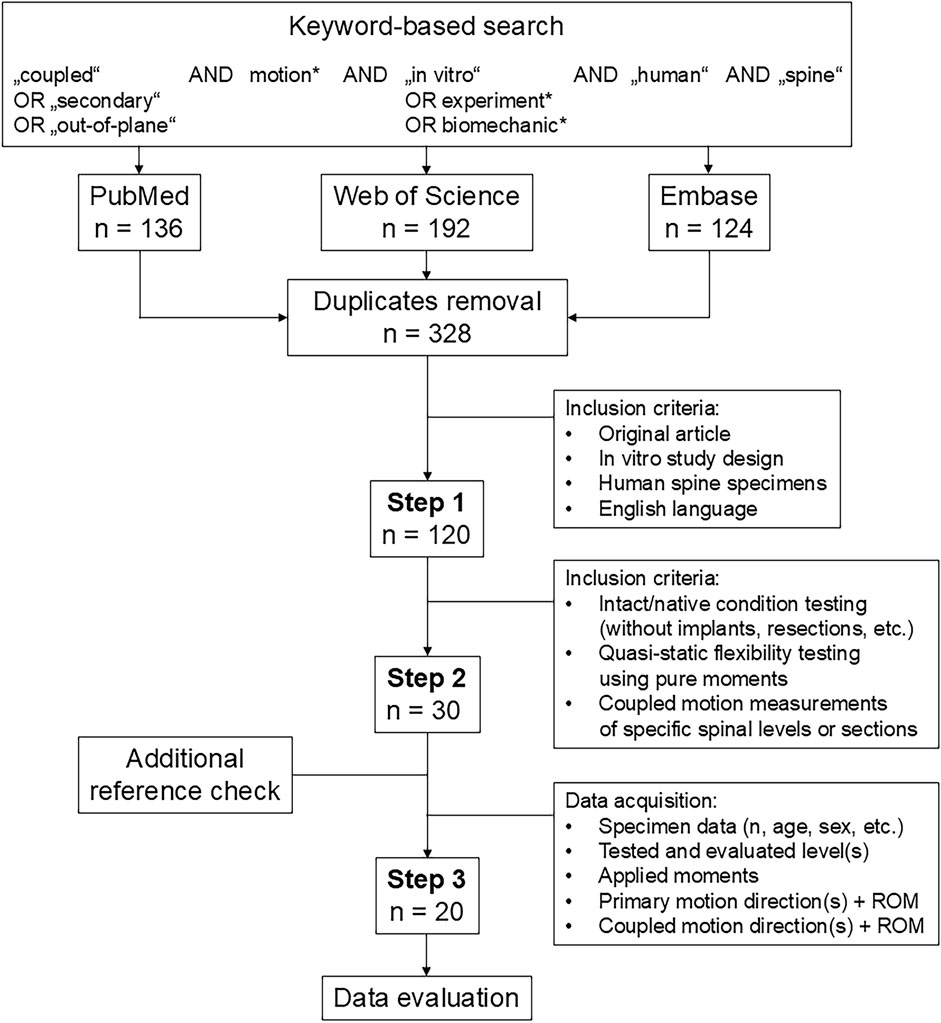
Figure 1. Flow diagram illustrating the systematic literature search according to the PRISMA guidelines (Page et al., 2021).
2.2 Data evaluation
The ROM data of the coupled motions were acquired either directly as percentage values of the respective primary ROMs from the published text (if reported) or via calculations from the absolute mean or median values of the primary and coupled ROMs. If the data were illustrated as diagrams, the single data points were digitized using an open-source software for image analysis (Engauge Digitizer 12.1). All obtained data were then collated and postprocessed using Excel version 2504 (Microsoft Corp., Redmond, WA, United States). For our meta-analysis, the coupled motion data were divided into individual secondary motion planes for each primary motion direction, which resulted in six sub-meta-analyses, namely, coupled lateral bending and coupled axial rotation during primary flexion/extension, coupled flexion/extension and coupled axial rotation during primary lateral bending, as well as coupled flexion/extension and coupled lateral bending during primary axial rotation. To classify the magnitudes of the coupled motions relative to their respective primary motions, percentage values below 20% were defined by the authors as low coupled motions, values between 20% and 50% were defined as moderate coupled motions, values between 50% and 100% were designated as high coupled motions, and values over 100% were assigned as dominant coupled motions. Additionally, to determine the reproducibility of the findings, the non-parametric one-sample Wilcoxon signed-rank test was performed on the percentage data of each segmental level using SPSS version 29 (IBM Corp., Armonk, NY, United States) to determine the statistical difference of the relative coupled ROM values from zero for each segmental level and primary motion direction; p-values greater than or equal to 0.1 were defined as non-reproducible, while values between 0.5 and less than 0.1 were deemed to have a tendency toward reproducibility, and those below 0.05 were considered reproducible, following the recommendations of Bland (1986).
3 Results
3.1 Overall collective
Of the 20 in vitro studies included in this meta-analysis (Table 1), eleven reported the coupled motions of cervical, seven reported the coupled motions of thoracic, and four reported the coupled motions of lumbar spinal sections or segmental levels. The first in vitro study on coupled motions was published by Panjabi et al. (1989) from Yale University on the lumbar spine. This and further investigations were carried out at a total of six institutions: Yale University, New Haven, CT, United States (Panjabi et al., 1993a; Panjabi et al., 1994; Panjabi et al., 2001); ENSAM/Arts et Métiers ParisTech, Paris, France (Wen et al., 1993; Barrey et al., 2009; Barrey et al., 2015; Taverne et al., 2024); Edward Hines Jr VA Hospital, Hines, IL, United States (Patwardhan et al., 2012; Muriuki et al., 2024); St. Joseph’s Hospital and Medical Center, Phoenix, AZ, United States (Colle et al., 2013); Ulm University, Ulm, Germany (Liebsch et al., 2017; Liebsch et al., 2018; Liebsch et al., 2020b; Liebsch et al., 2020c; Wilke et al., 2020; Liebsch et al., 2024; Vogt et al., 2024a; Vogt et al., 2024b); Drexel University, Philadelphia, PA, United States (Orbach et al., 2023). Every study included in this meta-analysis reported the testing of at least n = 6 specimens. The mean donor age of the tested specimens (reported in 17 studies) ranged between 28 and 81 years; the donor sex (reported in 16 studies) was roughly equally distributed in nine studies, while more than two-thirds of the specimens originated from male donors in four studies and more than two-thirds of the specimens originated from female donors in three studies. The applied loads ranged from 1 Nm to 4.5 Nm for the cervical, 2.5 Nm to 8 Nm for the thoracic, and 5 Nm to 10 Nm for the lumbar spinal specimens.
3.2 Coupled lateral bending during primary flexion/extension
There were no reported in vitro studies on coupled lateral bending during primary flexion/extension of the cervical spine (C0–T1). In the thoracic (T1–L1) and lumbar (L1–S1) spine, generally low and non-reproducible (p ≥ 0.1) lateral bending was found during primary flexion and primary extension (Figure 2).
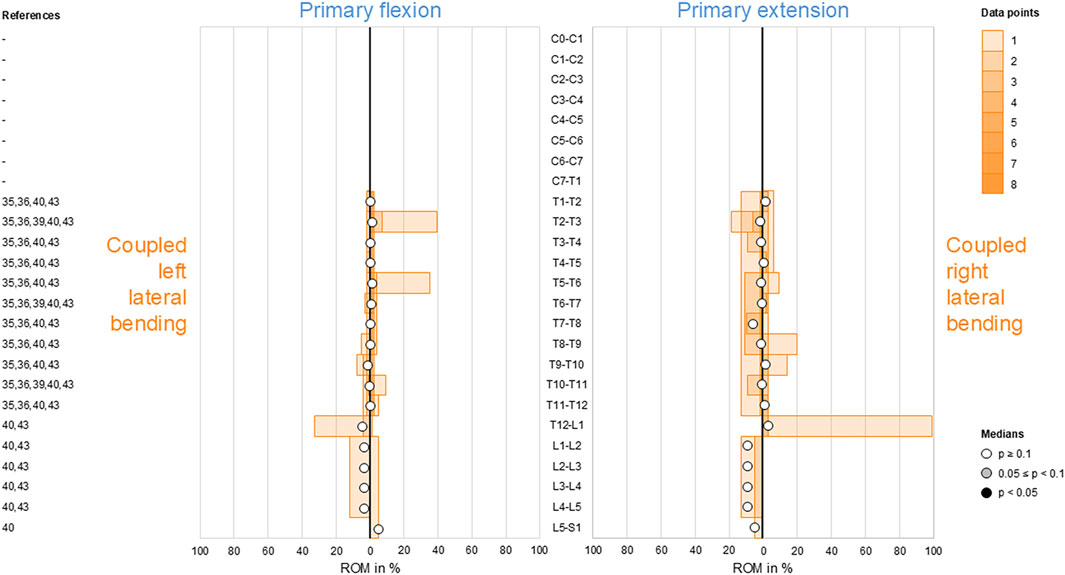
Figure 2. Bar diagrams illustrating segmental coupled lateral bending range of motion (ROM) relative to primary flexion/extension ROM acquired from in vitro studies (Liebsch et al., 2017; Liebsch et al., 2018; Wilke et al., 2020; Liebsch et al., 2024; Orbach et al., 2023).
3.3 Coupled axial rotation during primary flexion/extension
For coupled lateral bending, there was no reported data on coupled axial rotation during primary flexion/extension for the cervical spine (C0–T1). Although coupled axial rotation was overall found to be low and non-reproducible (p ≥ 0.1) in the thoracic (T1–L1) and lumbar (L1–S1) spine, there were tendencies (0.05 ≤ p < 0.1) toward reproducible secondary right axial rotation during primary flexion (T2–T5) and secondary left axial rotation during primary extension (T1–T2, T4–T5) in the upper thoracic spine, with the latter even being reproducible (p < 0.05) at T2–T3 (Figure 3). Moreover, slightly moderate secondary right axial rotation during primary extension was noted in the lumbar spine (L1–L5), although it was non-reproducible (p ≥ 0.1).
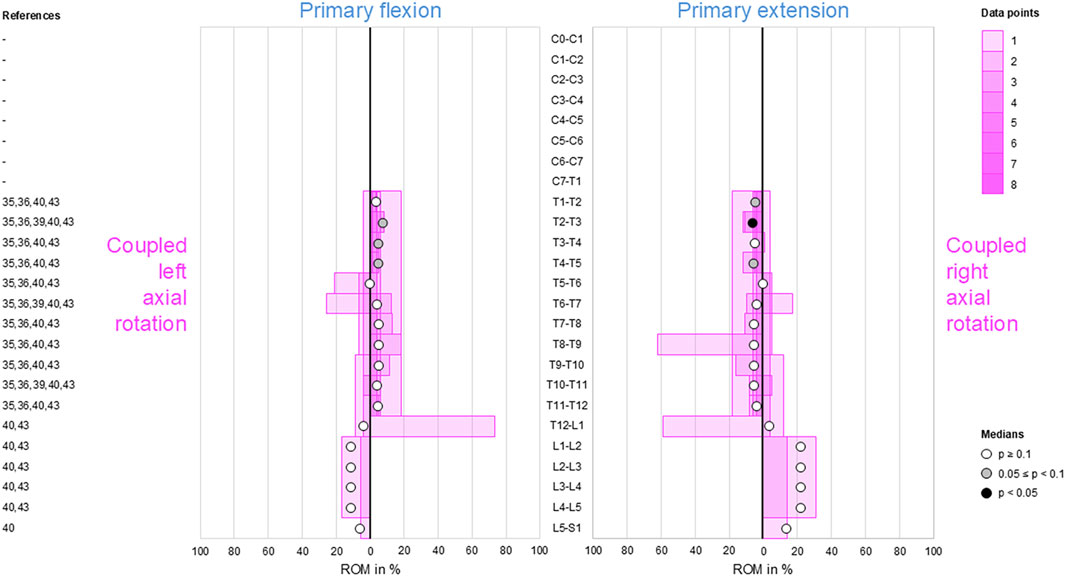
Figure 3. Bar diagrams illustrating segmental coupled axial rotation ROM relative to primary flexion/extension ROM acquired from in vitro studies (Liebsch et al., 2017; Liebsch et al., 2018; Wilke et al., 2020; Liebsch et al., 2024; Orbach et al., 2023).
3.4 Coupled flexion/extension during primary lateral bending
Secondary flexion and extension were found to be low in the thoracic spine (T1–L1) as well as low to moderate in the cervical (C0–T1) and lumbar (L1–S1) spine (Figure 4). However, there was a tendency (0.05 ≤ p < 0.1) toward reproducible coupled flexion during bilateral primary lateral bending in the lumbar spine (L1–L5). Moreover, reproducible (p < 0.05) coupled extension during primary right lateral bending was identified in the upper-thoracic (T1–T6) and mid-thoracic (T7–T9) spine.
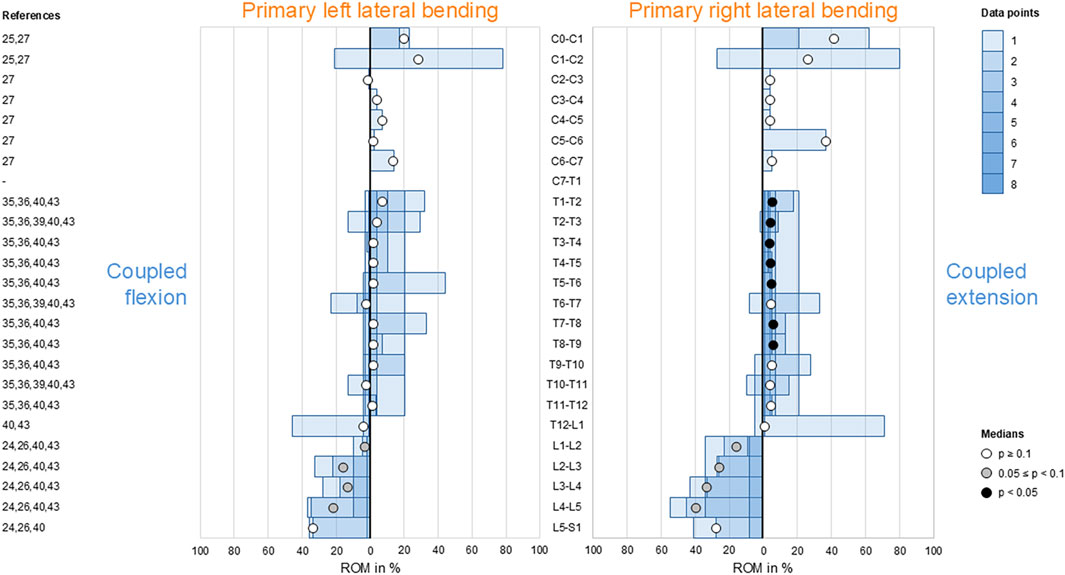
Figure 4. Bar diagrams illustrating segmental coupled flexion/extension ROM relative to primary lateral bending ROM acquired from in vitro studies (Panjabi et al., 1989; Panjabi et al., 1993a; Panjabi et al., 1994; Panjabi et al., 2001; Liebsch et al., 2017; Liebsch et al., 2018; Wilke et al., 2020; Liebsch et al., 2024; Orbach et al., 2023).
3.5 Coupled axial rotation during primary lateral bending
Moderate ipsilateral axial rotations were found during primary lateral bending at the atlanto-occipital joint (C0–C1), in the subaxial cervical spine (C2–C7), and in the thoracic spine (T1–T12), indicating that primary left lateral bending was associated with moderate left axial rotation and primary right lateral bending was linked with moderate right axial rotation in these spinal sections (Figure 5). This coupled motion pattern was reproducible (p < 0.05) in the mid-cervical (C4–C6) and thoracic (T1–T12) spine. At the atlanto-axial joint (C1–C2), high to dominant contralateral axial rotation was detected during primary lateral bending but was non-reproducible (p > 0.1) as there were only two available data points, meaning that primary left lateral bending resulted in high-to-dominant right axial rotation and primary right lateral bending resulted in high-to-dominant left axial rotation in these spinal sections. The same pattern was found in the lumbar spine (L1–S1) with low (L1–L4) to moderate (L4–S1) coupled motions and a tendency (0.05 ≤ p < 0.1) toward reproducibility for the mid-lumbar segment motions (L2–L5).
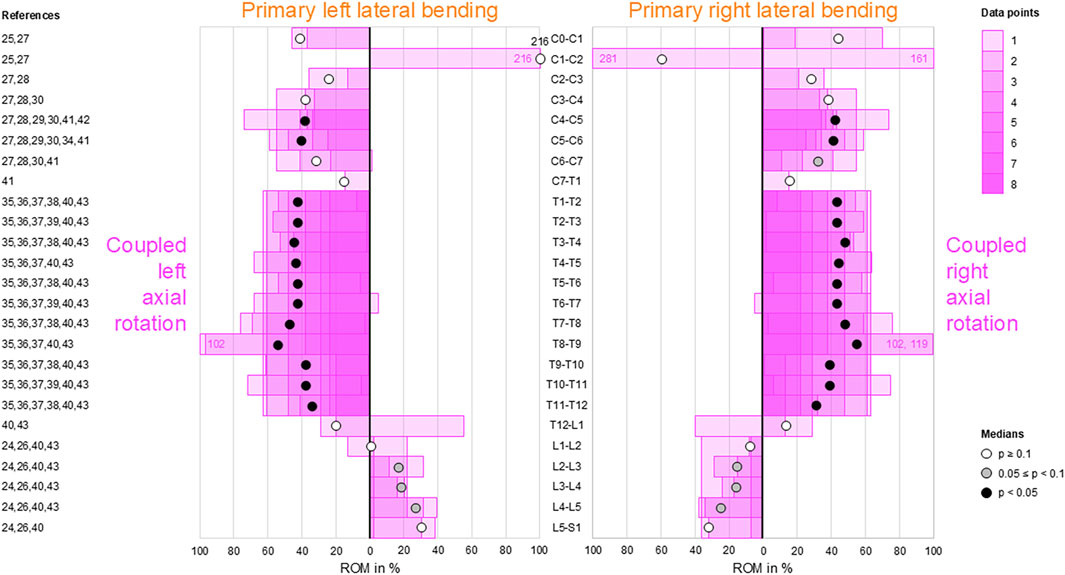
Figure 5. Bar diagrams illustrating segmental coupled axial rotation ROM relative to primary lateral bending ROM acquired from in vitro studies (Panjabi et al., 1989; Panjabi et al., 1993a; Panjabi et al., 1994; Panjabi et al., 2001; Wen et al., 1993; Barrey et al., 2009; Barrey et al., 2015; Colle et al., 2013; Liebsch et al., 2017; Liebsch et al., 2018; Liebsch et al., 2020b; Liebsch et al., 2020c; Wilke et al., 2020; Liebsch et al., 2024; Vogt et al., 2024a; Vogt et al., 2024b; Orbach et al., 2023).
3.6 Coupled flexion/extension during primary axial rotation
During primary axial rotation, secondary flexion and extension were low in the thoracic spine (T1–L1) and low to moderate in the cervical (C1–C2) and lumbar (L1–L5) spine. For these spinal sections, coupled flexion/extension was overall non-reproducible (p > 0.1) except for the lumbar spine (L1–L5), where there was a tendency (0.05 ≤ p < 0.1) toward secondary flexion during primary left axial rotation (Figure 6). High coupled flexion at the lumbosacral joint (L5–S1) and dominant coupled extension at the atlanto-occipital joint (C0–C1) were detected during bilateral primary axial rotation, both of which were non-reproducible (p > 0.1) owing to the low numbers of data points.
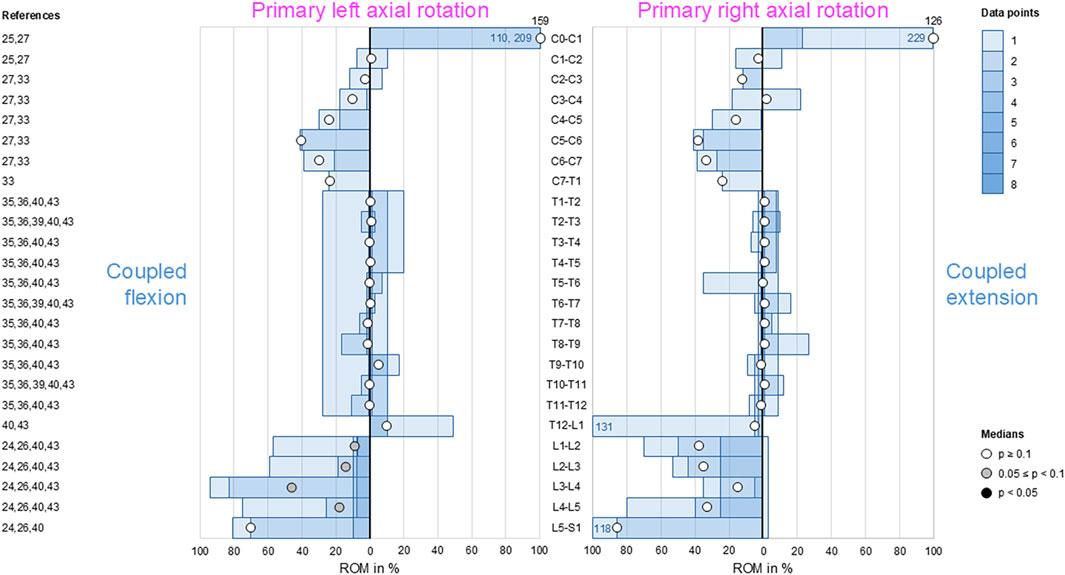
Figure 6. Bar diagrams illustrating segmental coupled flexion/extension ROM relative to primary axial rotation ROM acquired from in vitro studies (Panjabi et al., 1989; Panjabi et al., 1993a; Panjabi et al., 1994; Panjabi et al., 2001; Muriuki et al., 2024; Liebsch et al., 2017; Liebsch et al., 2018; Wilke et al., 2020; Liebsch et al., 2024; Orbach et al., 2023).
3.7 Coupled lateral bending during primary axial rotation
Low-to-moderate contralateral lateral bending during primary axial rotation was detected in the upper (C0–C2) and lower (C7–T1) cervical, thoracic (T1–L1), and upper lumbar (L1–L4) spine, whereas high contralateral bending was found at the atlanto-occipital joint (C0–C1) and upper lumbar (L1–L3) segments during primary right axial rotation (Figure 7); these indicate that primary left axial rotation was associated with low-to-moderate right lateral bending and primary right axial rotation was linked with low-to-high left lateral bending in these spinal sections. However, reproducible (p < 0.05) contralateral lateral bending and tendency toward reproducible (0.05 ≤ p < 0.1) contralateral lateral bending were primarily determined for primary left axial rotations in the thoracic spine (T1–T12). High ipsilateral lateral bending during primary axial rotation was identified in the subaxial cervical spine (C2–C7) and dominant ipsilateral lateral bending was noted during primary axial rotation in the lower lumbar spine (L4–S1), meaning that primary left axial rotation was associated with high-to-dominant left lateral bending and primary right axial rotation was linked with high-to-dominant right lateral bending in these spinal sections. However, reproducible (p < 0.05) ipsilateral lateral bending during primary axial rotation was detected only in the lower cervical spine (C4–C7), and a tendency toward reproducible (0.05 ≤ p < 0.1) contralateral bending during primary axial rotation was noted only in the mid-cervical spinal segments (C3–C4).
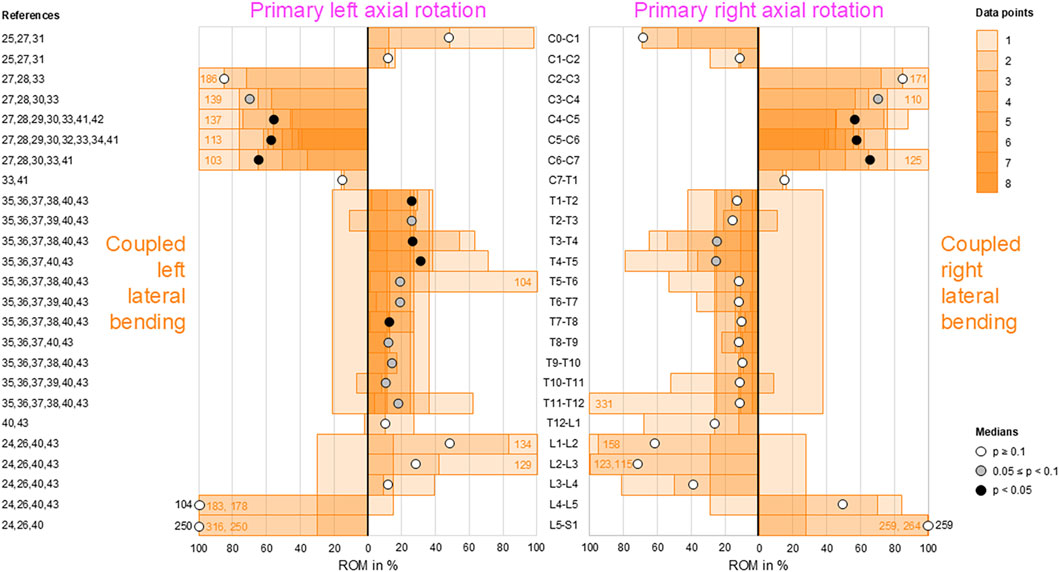
Figure 7. Bar diagrams illustrating segmental coupled lateral bending ROM relative to primary axial rotation ROM acquired from in vitro studies (Panjabi et al., 1989; Panjabi et al., 1993a; Panjabi et al., 1994; Panjabi et al., 2001; Wen et al., 1993; Barrey et al., 2009; Barrey et al., 2015; Taverne et al., 2024; Patwardhan et al., 2012; Muriuki et al., 2024; Colle et al., 2013; Liebsch et al., 2017; Liebsch et al., 2018; Liebsch et al., 2020b; Liebsch et al., 2020c; Wilke et al., 2020; Liebsch et al., 2024; Vogt et al., 2024a; Vogt et al., 2024b; Orbach et al., 2023).
4 Discussion
Coupled motions represent important biomechanical parameters as they can be used to interpret the three-dimensional in vivo kinematics as well as validate the experimental and numerical models of the spine. Moreover, knowledge on coupled motions could support the diagnosis of and assessment of treatment success in pathologies related to three-dimensional spinal mobility, such as degenerative disc disease, ankylosing spondylitis, and spinal deformities. In the specific case of scoliosis, better understanding of the coupled motions would be useful for guiding corrective movements through the use of braces and physical therapy. Although in vivo studies are limited in their extent of reproducibility owing to individual movement comforts of the participants and measurement imprecisions, in vitro studies under clearly defined and standardized loading conditions could provide more reproducible measurements to determine the segment- and section-specific coupled motions, even if only regarding the passive structures of the spine. Therefore, this meta-analysis of a large set of in vitro data from literature was able to identify characteristic coupled motion patterns of the entire spine in the main anatomical motion directions.
Although complex and section-specific relationships were found between the different primary and secondary motions in this meta-analysis, the overall symmetrical coupled motion characteristics with regard to the sagittal plane were determined for individual primary motion planes. These findings may also explain the low and non-reproducible coupled motions noted during primary flexion/extension as the normal non-deformed spine is almost symmetrical with regard to the sagittal plane. However, this meta-analysis also revealed direction-specific differences in the reproducibility of coupled motions, particularly for the more reproducible coupled extension during primary right lateral bending (Figure 4) and more reproducible contralateral right lateral bending during primary left axial rotation (Figure 7) in the thoracic spine. As these observations are contrary to the expected symmetric coupled motions owing to the (ideal) anatomical symmetry of the spine, future studies should additionally evaluate the effects of three-dimensional spinal curvature when determining spinal coupled motions. While the in vitro studies evaluated in this systematic review explicitly excluded spinal specimens exhibiting major deformities, the findings may nevertheless be relevant for the clinical interpretation of early scoliosis. Although some of the results do not completely correspond with common clinical findings, such as the more reproducible coupled extension during primary right lateral bending in contrast to the frequently observed trend toward a flat back for both left and right thoracic curvatures, many of the findings show high correspondence, such as the low coupled sagittal plane motions and ipsilateral axial rotation in the thoracic spine or progressive loss of lordosis in the lumbar spine during primary lateral bending, as well as the characteristic associations between contralateral bending, apical derotation of thoracic scoliosis toward the concavity, and reversed biomechanical effects at the lumbar level. Thus, our meta-analysis confirmed the strong motion coupling relationships between lateral bending and axial rotation in the healthy spine that were previously reported in early investigations by Lovett (1900); Lovett, (1905) and have been confirmed in numerous subsequent in vivo, in vitro, and in silico studies. However, the previous works often report conflicting findings regarding the extent and quality of coupled motions for individual spinal sections or segment levels, whereas our meta-analysis summarizes specific coupled motion patterns of the entire spine in a quantitative (Figures 2–7) as well as qualitative (Figure 8) manner based on a large dataset.
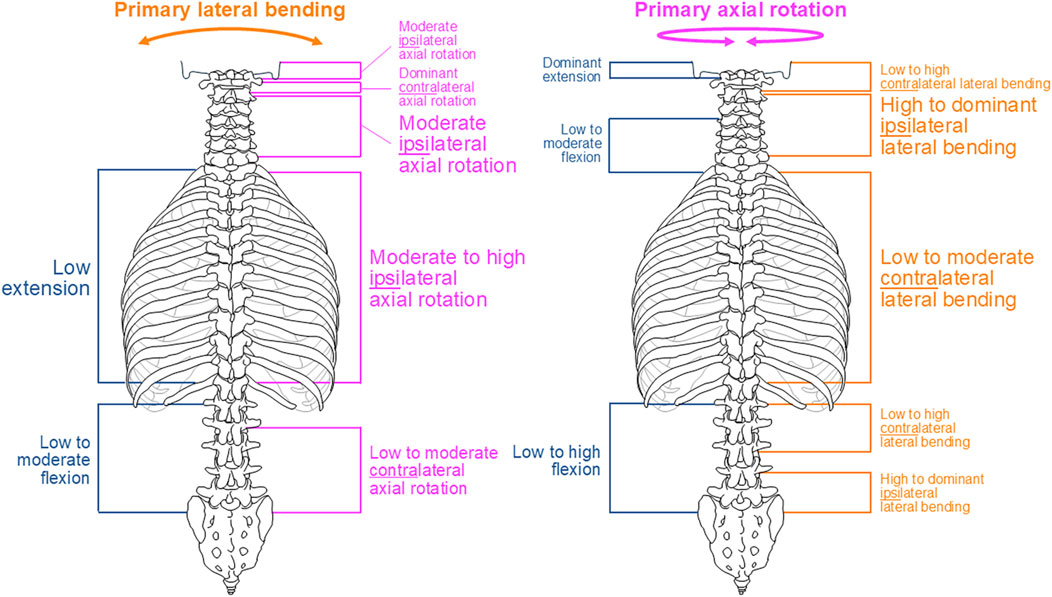
Figure 8. Overview of the main findings of this meta-analysis regarding coupled motion patterns during primary lateral bending (left) and primary axial rotation (right). Consistent but non-significant findings are depicted in small font, findings tending overall toward reproducibility are shown in medium font, and overall reproducible findings are depicted in large font.
This meta-analysis reveals shifts in the coupled motion directions at specific anatomical points, especially the thoracolumbar transition (T12–L2) during primary lateral bending (Figures 4, 5) and cervicothoracic transition (C7–T2) during primary axial rotation (Figures 6, 7); furthermore, transitions are observed in the atlanto-occipital joint (C0–C1), atlanto-axial joint (C1–C2), and upper subaxial spine (C2–C3) during primary lateral bending and primary axial rotation (Figures 4–7). These shifts in the directions of the coupled motions may be explained by the specific changes in the anatomical features among these spinal areas, particularly the morphology and orientation of the facets as well as spinal curvature within the sagittal plane in the case of the subaxial cervical, thoracic, and lumbar spine. Using a computational model of the thoracic and lumbar spine, Schultz et al. (1973) showed in an early work that spinal motion coupling is a function of segment orientation; later in silico investigations reported by Scholten and Veldhuizen (1985) confirmed the impact of sagittal plane curvature on the coupled motion behaviors of the spine, further revealing the effects of the facet angles in the transverse and sagittal planes. Indeed, an anatomical study of the three-dimensional orientations of the facets by Panjabi et al. (1993b) showed that the facet width, height, and width/height ratio as well as the transverse and sagittal plane angles of the facets change abruptly at the cervicothoracic and thoracolumbar transitions, matching the findings of this meta-analysis well. Previous in vitro studies on the thoracic spine did not report any significant coupled motions in monosegmental and bisegmental specimens (Liebsch et al., 2020c; Wilke et al., 2020; Greiner-Perth et al., 2025), substantiating the effect of the sagittal curvature on the emergence of coupled motions. Furthermore, other anatomical structures like the ligaments (Wilke et al., 2020) and rib cage (Brasiliense et al., 2011; Liebsch et al., 2017; Liebsch et al., 2020c; Liebsch and Wilke, 2022) were shown to not cause alterations in the coupled motion behaviors of the thoracic spine during resection or like the intervertebral disc during isolation (Wilke et al., 2020).
Coupled motions of the spine are often equated with and used synonymously for coupled rotations of the spine in literature because coupled translations of the spine are often neglected when considering secondary motions. Indeed, coupled translations were only investigated in two of the 20 in vitro studies evaluated herein (Panjabi et al., 1994; Panjabi et al., 2001). For the cervical spine, Panjabi et al. (2001) applied pure moments of 1 Nm and found a highly coupled anterior translation of about 10 mm during primary flexion and a highly coupled posterior translation of about 10 mm during primary extension at the atlanto-axial joint (C1–C2), a moderate ipsilateral translation of up to 4 mm during bilateral lateral bending at the atlanto-axial joint (C1–C2) and upper subaxial spine (C2–C3), and a highly coupled posterior translation of up to 12 mm at the atlanto-occipital (C0–C1) and atlanto-axial (C1–C2) joints during bilateral axial rotation. To determine the single motion segments of the lumbar spine (L1–S1), Panjabi et al. (1994) applied pure moments of 10 Nm and detected moderately coupled superior translations of up to 4 mm and low-coupled anterior translations of up to 2 mm during primary flexion, along with low-coupled inferior and posterior translations of up to 2 mm during primary extension and low ipsilateral translations of up to 1 mm during left and right lateral bending. Although extensive investigations were performed in these two studies, the available data on coupled translations is still limited, necessitating additional studies in the future.
Despite our attempt to present a comprehensive meta-analysis, this work has several limitations. For the data analyses, coupled motion data of single segmental levels were combined with coupled motion data spanning multiple segmental levels, potentially resulting in underestimation of the coupled motions compared to evaluation of only polysegmental data owing to the enhancing effect of the increasing sagittal plane curvature. This approach was inevitable for increasing the sample size per segmental level and minimizing the effects of outliers to create as statistically significant results as possible. In fact, even with our approach, there were few or even no available data points for some segmental levels and primary motion directions, particularly for the entire cervical spine with regard to primary flexion/extension. As larger sample sizes per segmental level may be assumed to entail higher statistical power of the findings, more in vitro data from different investigations are required to substantiate the findings of this meta-analysis, especially for the cervical and lumbar spinal regions for which moderate-to-dominant but non-reproducible coupled motions were found. Moreover, combining coupled motion data from studies with different numbers of tested segmental levels may result in distortions of the extent and quality of the coupled motions, such as those found for coupled axial rotation during primary lateral bending at the atlanto-axial joint (C1–C2) (Figure 5); these observations were based on one investigation with C0–C3 specimens (Panjabi et al., 1993a) and another investigation with C0–C7 specimens (Panjabi et al., 2001) reported by the same research group using the same loading conditions. Similarly, one study reported the opposite coupled lateral bending during primary axial rotation for the thoracic spine (T1–T12) compared to most of the prior studies (Figure 7) when using the entire thoracic to lumbar spine segments (T1–L5) (Orbach et al., 2023). Although the application of pure moments should theoretically create the same primary and coupled motions in monosegmental and polysegmental specimens, the overall coupled motion behaviors may be affected by the number of contiguous segmental levels when different segmental coupled motion characteristics are tested together. Moreover, we only included in vitro studies that used pure moments in this meta-analysis to ensure the highest possible data comparability. Owing to this approach, in vitro studies that used additional axial compressive or follower loads to simulate bodyweights and muscle forces were not included, even though it was shown that follower loading can increase the extent of coupled motions (Liebsch et al., 2018). This phenomenon might be explained by the loss of intervertebral disc flexibility, which could potentially increase the effect of sagittal plane curvature when assuming the spine as a torsionally stiff and sagittally curved rod, and the enhanced facet surface contact, which would support the facets in acting as three-dimensionally inclined guide rails. Consequently, the non-consideration of axial compressive or follower loading might entail underestimation of the coupled motions compared to pure-moment loading. Moreover, active muscle control affects the in vivo coupled motions that cannot be replicated by in vitro studies using pure moments, as summarized in this meta-analysis on the coupled motions of passive spinal structures. Another limitation of this meta-analysis is that the primary and secondary ROMs of single motion segments are often low, which could potentially cause large variations in the relative coupled motion data. Lastly, the standard deviations and ranges of the data in the original studies were not included in this meta-analysis as these values were often not reported or exhibited high variations. Nevertheless, the strength of this meta-analysis in aggregating quantitative data acquired from in vitro studies using standardized loading conditions might outweigh these limitations with regard to the high comparability of spinal coupled motion data.
5 Conclusion
This meta-analysis of in vitro studies identifies characteristic coupled motion patterns of the spine. Although we did not determine considerable coupled motions for primary flexion/extension, there was evidence for motion coupling relationships between lateral bending and axial rotation. Specifically, primary lateral bending inter alia resulted in moderate-to-high ipsilateral axial rotation of the subaxial cervical and thoracic spine, whereas primary axial rotation inter alia caused low-to-moderate contralateral lateral bending at the thoracic spine and high-to-dominant ipsilateral lateral bending at the subaxial cervical spine. Despite the limited availability of data points that would require additional studies to extend and substantiate the findings of this meta-analysis, the collection of inhomogeneous data containing both monosegmental and polysegmental data as well as the inclusion of only studies using standardized but non-physiological loading conditions in our dataset will be valuable for validating both experimental and numerical studies of the spine as well as interpreting the coupled motion behaviors of passive spinal structures.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, and any further inquiries may be directed to the corresponding author.
Author contributions
CL: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft. H-JW: Conceptualization, Project administration, Supervision, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research publication of this article. This study was funded by the German Research Foundation (DFG), project no. 422439870, reference no. WI 1352/23-2.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Barrey, C., Rousseau, M. A., Persohn, S., Campana, S., Perrin, G., and Skalli, W. (2015). Relevance of using a compressive preload in the cervical spine: an experimental and numerical simulating investigation. Eur. J. Orthop. Surg. Traumatol. 25, S155–S165. doi:10.1007/s00590-015-1625-2
Barrey, C., Mosnier, T., Jund, J., Perrin, G., and Skalli, W. (2009). In vitro evaluation of a ball-and-socket cervical disc prosthesis with cranial geometric center. J. Neurosurg. Spine 11 (5), 538–546. doi:10.3171/2009.6.spine0949
Brasiliense, L. B., Lazaro, B. C., Reyes, P. M., Dogan, S., Theodore, N., and Crawford, N. R. (2011). Biomechanical contribution of the rib cage to thoracic stability. Spine 36 (26), E1686–E1693. doi:10.1097/brs.0b013e318219ce84
Cholewicki, J., Crisco, J. J., Oxland, T. R., Yamamoto, I., and Panjabi, M. M. (1996). Effects of posture and structure on three-dimensional coupled rotations in the lumbar spine. A biomechanical analysis. Spine 21 (21), 2421–2428. doi:10.1097/00007632-199611010-00003
Colle, K. O., Butler, J. B., Reyes, P. M., Newcomb, A. G., Theodore, N., and Crawford, N. R. (2013). Biomechanical evaluation of a metal-on-metal cervical intervertebral disc prosthesis. Spine J. 13 (11), 1640–1649. doi:10.1016/j.spinee.2013.06.026
Daniels, A. H., Paller, D. J., Feller, R. J., Thakur, N. A., Biercevicz, A. M., Palumbo, M. A., et al. (2012). Examination of cervical spine kinematics in complex, multiplanar motions after anterior cervical discectomy and fusion and total disc replacement. Int. J. Spine Surg. 6, 190–194. doi:10.1016/j.ijsp.2012.07.002
Greiner-Perth, A.-K., Wilke, H.-J., and Liebsch, C. (2025). How do compression and flexion-compression injuries destabilize the spine? A novel in vitro protocol for analyzing three-dimensional biomechanical instability. Front. Bioeng. Biotechnol. 13, 1576720. doi:10.3389/fbioe.2025.1576720
Harrison, D. E., Harrison, D. D., and Troyanovich, S. J. (1998). Three-dimensional spinal coupling mechanics: part I. A review of the literature. J. Manip. Physiol. Ther. 21 (2), 101–113.
Lee, J. H., Kim, J. S., Lee, J. H., Chung, E. R., Shim, C. S., and Lee, S. H. (2014). Comparison of cervical kinematics between patients with cervical artificial disc replacement and anterior cervical discectomy and fusion for cervical disc herniation. Spine J. 14 (7), 1199–1204. doi:10.1016/j.spinee.2013.08.010
Legaspi, O., and Edmond, S. L. (2007). Does the evidence support the existence of lumbar spine coupled motion? A critical review of the literature. J. Orthop. Sports Phys. Ther. 37 (4), 169–178. doi:10.2519/jospt.2007.2300
Liebsch, C., and Wilke, H.-J. (2022). How does the rib cage affect the biomechanical properties of the thoracic spine? A systematic literature review. Front. Bioeng. Biotechnol. 10, 904539. doi:10.3389/fbioe.2022.904539
Liebsch, C., Graf, N., Appelt, K., and Wilke, H.-J. (2017). The rib cage stabilizes the human thoracic spine: an in vitro study using stepwise reduction of rib cage structures. PLoS One 12 (6), e0178733. doi:10.1371/journal.pone.0178733
Liebsch, C., Graf, N., and Wilke, H.-J. (2018). The effect of follower load on the intersegmental coupled motion characteristics of the human thoracic spine: an in vitro study using entire rib cage specimens. J. Biomech. 78, 36–44. doi:10.1016/j.jbiomech.2018.06.025
Liebsch, C., Kocak, T., Aleinikov, V., Kerimbayev, T., Akshulakov, S., Jansen, J. U., et al. (2020a). Thoracic spinal stability and motion behavior are affected by the length of posterior instrumentation after vertebral body replacement, but not by the surgical approach type: an in vitro study with entire rib cage specimens. Front. Bioeng. Biotechnol. 8, 572. doi:10.3389/fbioe.2020.00572
Liebsch, C., Aleinikov, V., Kerimbayev, T., Akshulakov, S., Kocak, T., Vogt, M., et al. (2020b). In vitro comparison of personalized 3D printed versus standard expandable titanium vertebral body replacement implants in the mid-thoracic spine using entire rib cage specimens. Clin. Biomech. 78, 105070. doi:10.1016/j.clinbiomech.2020.105070
Liebsch, C., Jonas, R., and Wilke, H.-J. (2020c). Thoracic spinal kinematics is affected by the grade of intervertebral disc degeneration, but not by the presence of the ribs: an in vitro study. Spine J. 20 (3), 488–498. doi:10.1016/j.spinee.2019.10.006
Liebsch, C., Obid, P., Vogt, M., Schlager, B., and Wilke, H.-J. (2024). Scoliosis instrumentation alters primary and coupled motions of the spine: an in vitro study using entire thoracolumbar spine and rib cage specimens. JOR Spine 7 (4), e70028. doi:10.1002/jsp2.70028
Lovett, R. W. (1900). The mechanics of lateral curvature of the spine. Boston Med. Surg. J. 142 (24), 622–627. doi:10.1056/nejm190006141422403
Lovett, R. W. (1905). The mechanism of the normal spine and its relation to scoliosis. Boston Med. Surg. J. 153 (13), 349–358. doi:10.1056/nejm190509281531301
Mannen, E. M., Anderson, J. T., Arnold, P. M., and Friis, E. A. (2015a). Mechanical contribution of the rib cage in the human cadaveric thoracic spine. Spine 40 (13), E760–E766. doi:10.1097/brs.0000000000000879
Mannen, E. M., Anderson, J. T., Arnold, P. M., and Friis, E. A. (2015b). Mechanical analysis of the human cadaveric thoracic spine with intact rib cage. J. Biomech. 48 (10), 2060–2066. doi:10.1016/j.jbiomech.2015.03.021
Muriuki, M. G., Havey, R. M., Blank, K. R., and Patwardhan, A. G. (2024). Coupled rotation patterns in cervical spine axial rotation can change when the head is kept level. J. Biomech. 163, 111924. doi:10.1016/j.jbiomech.2024.111924
Ochia, R. S., Inoue, N., Renner, S. M., Lorenz, E. P., Lim, T. H., Andersson, G. B., et al. (2006). Three-dimensional in vivo measurement of lumbar spine segmental motion. Spine 31 (18), 2073–2078. doi:10.1097/01.brs.0000231435.55842.9e
Orbach, M. R., Mahoney, J., Bucklen, B. S., and Balasubramanian, S. (2023). In vitro coupled motions of the whole human thoracic and lumbar spine with rib cage. JOR Spine 6 (3), e1257. doi:10.1002/jsp2.1257
Oxland, T. R., Crisco, J. J., Panjabi, M. M., and Yamamoto, I. (1992). The effect of injury on rotational coupling at the lumbosacral joint. A biomechanical investigation. Spine 17 (1), 74–80. doi:10.1097/00007632-199201000-00012
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372, n71. doi:10.1136/bmj.n71
Panjabi, M., Yamamoto, I., Oxland, T., and Crisco, J. (1989). How does posture affect coupling in the lumbar spine? Spine 14 (9), 1002–1011. doi:10.1097/00007632-198909000-00015
Panjabi, M. M., Oda, T., Crisco, J. J., Dvorak, J., and Grob, D. (1993a). Posture affects motion coupling patterns of the upper cervical spine. J. Orthop. Res. 11 (4), 525–536. doi:10.1002/jor.1100110407
Panjabi, M. M., Oxland, T., Takata, K., Goel, V., Duranceau, J., and Krag, M. (1993b). Articular facets of the human spine. Quantitative three-dimensional anatomy. Spine 18 (10), 1298–1310. doi:10.1097/00007632-199308000-00009
Panjabi, M. M., Oxland, T. R., Yamamoto, I., and Crisco, J. J. (1994). Mechanical behavior of the human lumbar and lumbosacral spine as shown by three-dimensional load-displacement curves. J. Bone Jt. Surg. Am. 76 (3), 413–424. doi:10.2106/00004623-199403000-00012
Panjabi, M. M., Crisco, J. J., Vasavada, A., Oda, T., Cholewicki, J., Nibu, K., et al. (2001). Mechanical properties of the human cervical spine as shown by three-dimensional load-displacement curves. Spine 26 (24), 2692–2700. doi:10.1097/00007632-200112150-00012
Parnianpour, M., Nordin, M., Kahanovitz, N., and Frankel, V. (1988). The triaxial coupling of torque generation of trunk muscles during isometric exertions and the effect of fatiguing isoinertial movements on the motor output and movement patterns. Spine 13 (9), 982–992. doi:10.1097/00007632-198809000-00004
Patwardhan, A. G., Tzermiadianos, M. N., Tsitsopoulos, P. P., Voronov, L. I., Renner, S. M., Reo, M. L., et al. (2012). Primary and coupled motions after cervical total disc replacement using a compressible six-degree-of-freedom prosthesis. Eur. Spine J. 21, S618–S629. doi:10.1007/s00586-010-1575-7
Pearcy, M., Portek, I., and Shepherd, J. (1985). The effect of low-back pain on lumbar spinal movements measured by three-dimensional x-ray analysis. Spine 10 (2), 150–153. doi:10.1097/00007632-198503000-00007
Puttlitz, C. M., Rousseau, M. A., Xu, Z., Hu, S., Tay, B. K., and Lotz, J. C. (2004). Intervertebral disc replacement maintains cervical spine kinetics. Spine 29 (24), 2809–2814. doi:10.1097/01.brs.0000147739.42354.a9
Scholten, P. J., and Veldhuizen, A. G. (1985). The influence of spine geometry on the coupling between lateral bending and axial rotation. Eng. Med. 14 (4), 167–171. doi:10.1243/emed_jour_1985_014_041_02
Schultz, A. B., Belytschko, T. B., Andriacchi, T. P., and Galante, J. O. (1973). Analog studies of forces in the human spine: mechanical properties and motion segment behavior. J. Biomech. 6 (4), 373–383. doi:10.1016/0021-9290(73)90097-3
Sizer, P. S., Brismee, J. M., and Cook, C. (2007). Coupling behavior of the thoracic spine: a systematic review of the literature. J. Manip. Physiol. Ther. 30 (5), 390–399. doi:10.1016/j.jmpt.2007.04.009
Stokes, I. A., Wilder, D. G., Frymoyer, J. W., and Pope, M. H. (1981). Assessment of patients with low-back pain by biplanar radiographic measurement of intervertebral motion. Spine 6 (3), 233–240. doi:10.1097/00007632-198105000-00005
Taverne, M., Lalieve, L., Persohn, S., Khonsari, R. H., Paternoster, G., James, S., et al. (2024). Anatomy and mobility in the adult cadaveric craniocervical junction. J. Morphol. 285 (7), e21748. doi:10.1002/jmor.21748
Vogt, M., Mehren, C., Hackenbroch, C., and Wilke, H.-J. (2024a). Influence of cervical total disc replacement on motion in the target and adjacent segments. Spine J. 24 (7), 1313–1322. doi:10.1016/j.spinee.2024.01.018
Vogt, M., Zengerle, L., Jonas, R., and Wilke, H.-J. (2024b). The move-C cervical artificial disc can restore intact range of motion and 3-D kinematics. Spine J. 24 (2), 340–351. doi:10.1016/j.spinee.2023.08.020
Weitz, E. M. (1981). The lateral bending sign. Spine 6 (4), 388–397. doi:10.1097/00007632-198107000-00010
Wen, N., Lavaste, F., Santin, J. J., and Lassau, J. P. (1993). Three-dimensional biomechanical properties of the human cervical spine in vitro. I. Analysis of normal motion. Eur. Spine J. 2 (1), 2–11. doi:10.1007/bf00301048
Wilke, H.-J., Wenger, K., and Claes, L. (1998). Testing criteria for spinal implants: recommendations for the standardization of in vitro stability testing of spinal implants. Eur. Spine J. 7 (2), 148–154. doi:10.1007/s005860050045
Wilke, H.-J., Grundler, S., Ottardi, C., Mathew, C. E., Schlager, B., and Liebsch, C. (2020). In vitro analysis of thoracic spinal motion segment flexibility during stepwise reduction of all functional structures. Eur. Spine 29 (1), 179–185. doi:10.1007/s00586-019-06196-7
Winkelstein, B. A., and Myers, B. S. (2002). Importance of nonlinear and multivariable flexibility coefficients in the prediction of human cervical spine motion. J. Biomech. Eng. 124 (5), 504–511. doi:10.1115/1.1504098
Xu, F., Lin, J., Jiang, S., Sun, Z., Zhou, S., Li, Z., et al. (2024). In vivo segmental vertebral kinematics in patients with degenerative lumbar scoliosis. Eur. Spine J. 33 (2), 571–581. doi:10.1007/s00586-023-07974-0
Keywords: spine, coupled motions, in vitro, biomechanics, systematic review, meta-analysis
Citation: Liebsch C and Wilke H-J (2025) Coupled motions of the spine under standardized in vitro conditions: a systematic review and meta-analysis. Front. Bioeng. Biotechnol. 13:1686524. doi: 10.3389/fbioe.2025.1686524
Received: 15 August 2025; Accepted: 06 October 2025;
Published: 12 November 2025.
Edited by:
Marwan El-Rich, Khalifa University, United Arab EmiratesReviewed by:
Jean Claude De Mauroy, Independent Researcher, FranceBin Zheng, Peking University People’s Hospital, China
Copyright © 2025 Liebsch and Wilke. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christian Liebsch, Y2hyaXN0aWFuLmxpZWJzY2hAdW5pLXVsbS5kZQ==
 Christian Liebsch
Christian Liebsch Hans-Joachim Wilke
Hans-Joachim Wilke