- 1CSIRO Energy, Lindfield, NSW, Australia
- 2Indian Ocean Marine Centre, Crawley, WA, Australia
- 3CSIRO Energy, Kensington, WA, Australia
- 4CSIRO Energy, Pullenvale, QLD, Australia
- 5Centre for Bioinnovation, University of the Sunshine Coast, Sippy Downs, QLD, Australia
Microbially induced carbonate precipitation (MICP) offers a promising biological approach to sequester atmospheric CO2 as stable mineral carbonates, mitigating climate change impacts. This perspective highlights the complexity underpinning prokaryote-driven biomineralization processes, emphasizing the necessity for holistic evaluation beyond simple carbonate formation. Key metabolic pathways such as carbonic anhydrase-mediated CO2 hydration, ureolysis, photosynthesis, and sulfate reduction contribute variably to mineral precipitation and the carbon footprint. Furthermore, calcium carbonate polymorphs with varying stability forms can affect carbon storage durability, while net carbon sequestration estimates often overlook critical factors including respiratory CO2 release, growth phases, and embodied emissions in microbial nutrient substrates. Finally, differentiating between transient microbial organic carbon and long-term mineral carbon storage is essential for accurate carbon accounting. Lifecycle carbon footprints vary significantly with metabolic strategies and substrate choices, impacting sustainable application prospects. Advancing MICP as an effective carbon removal technology requires integrated assessment of microbial physiology, environmental interactions, and process lifecycle emissions to optimize CO2 drawdown with environmental and economic viability.
Introduction
The escalating challenges of climate change to a warming planet highlight the critical need for a diverse array of carbon removal technologies (Schweitzer et al., 2021), with microbially induced carbonate precipitation (MICP) increasingly recognized as a promising candidate (e.g., Mitchell et al., 2010; Okyay and Rodrigues, 2015; Gilmour et al., 2024; Wilcox et al., 2025). MICP harnesses the metabolic versatility of prokaryotes to drive the precipitation of stable carbonate minerals, notably calcium carbonate (CaCO3), thereby locking away atmospheric CO2 into solid form (e.g., Zhu and Dittrich, 2016).
A range of biotic and abiotic factors have been shown to contribute to MICP and often act in combination to achieve biomineralization. Central to many biological processes is the enzyme carbonic anhydrase, which catalyzes the hydration of CO2 to form bicarbonate (e.g., Meldrum and Roughton, 1933; Douglas and Beveridge, 1998; Smith and Ferry, 2000; Fu et al., 2021). Other common metabolic strategies relevant to MICP include various ammonia-producing strategies (e.g., via urease or deamination of amino acids during catabolism) as these elevate pH (e.g., Kamennaya et al., 2012; Clarà Saracho and Marek, 2024), photosynthetic uptake of CO2 to shift carbonate equilibrium (e.g., Riding, 2006), and dissimilatory sulfate reduction (e.g., Lin et al., 2018; Castro-Alonso et al., 2019) where H2S production is the mechanism by which pH is increased. Additionally, microbial cell walls and extracellular polymeric substances (EPS) present in biofilms, further assist by trapping divalent cations and providing additional nucleation surfaces, ultimately promoting or modulating the formation and stability of carbonate minerals (e.g., Zhu and Dittrich, 2016). The rate of prokaryote-driven carbonate precipitation is dependent on pH, availability of nucleation sites, concentration of dissolved inorganic carbon (DIC) and saturation of divalent Ca2+ and Mg2+ ions (e.g., Castanier et al., 2000; Zhu and Dittrich, 2016).
Advocates of CO2 sequestration via MICP emphasize its wide-ranging potential for stable carbon storage, citing laboratory successes where bacterial activity accelerated carbonate mineralization rates by orders of magnitude (e.g., Power et al., 2016; Abdelsamad et al., 2022). Unlike many conventional carbon sequestration methods, MICP can proceed under ambient conditions, reducing energy inputs and offering the promise of long-term CO2 storage through the formation of stable minerals (e.g., Wilcox et al., 2025).
Despite these promising attributes, much of the current literature does not fully consider limitations. First, whole-system carbon accounting is often incomplete. Second, the physiological constraints of the microorganisms themselves — respiration, growth phase and tolerance to elevated CO2 — are insufficiently understood in MICP systems. Third, the geochemical stability of the precipitated carbonates is highly dependent on the mineral type, local pH and environmental conditions, raising concerns about the potential for carbonate dissolution and the re-release of sequestered CO2.
The above-mentioned issues are not only critical for scientific accuracy but also for informing policy, monitoring, verification and accounting (MVA) costs and carbon trading frameworks. Carbon markets and regulatory bodies increasingly demand evidence of permanence in sequestration projects (e.g., Meitner, 2024), requiring mechanisms like MICP that offer long-term storage to be heavily scrutinised. These issues are explored in this perspective (Figure 1) with the aim of providing a pathway to progress these technologies out of laboratories and into broader use. Table 1 provides a concise summary of the key quantitative data discussed throughout this article.
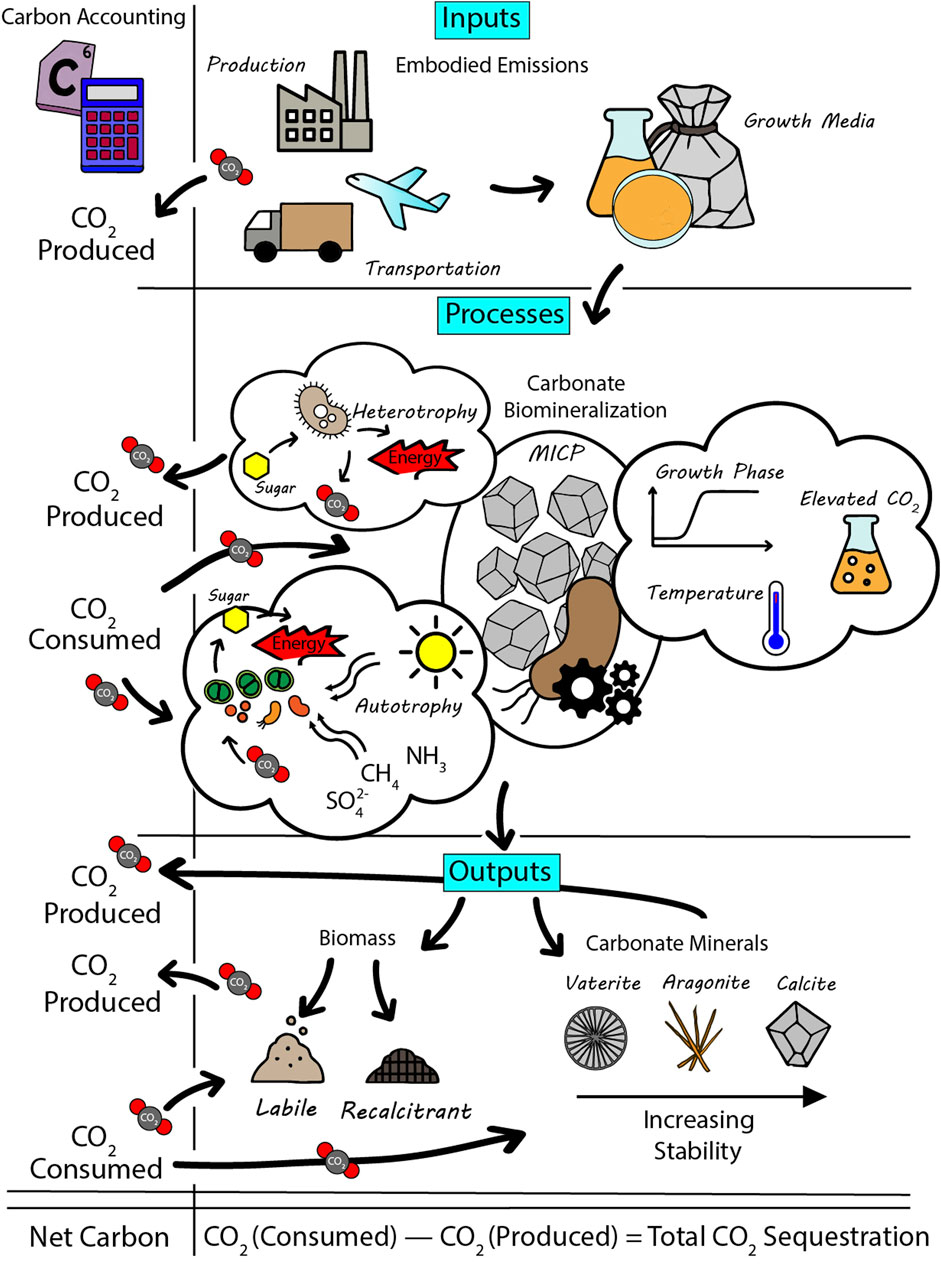
Figure 1. Integrated overview of carbon flows in microbially induced carbonate precipitation (MICP) systems, highlighting the full carbon accounting from inputs to outputs.
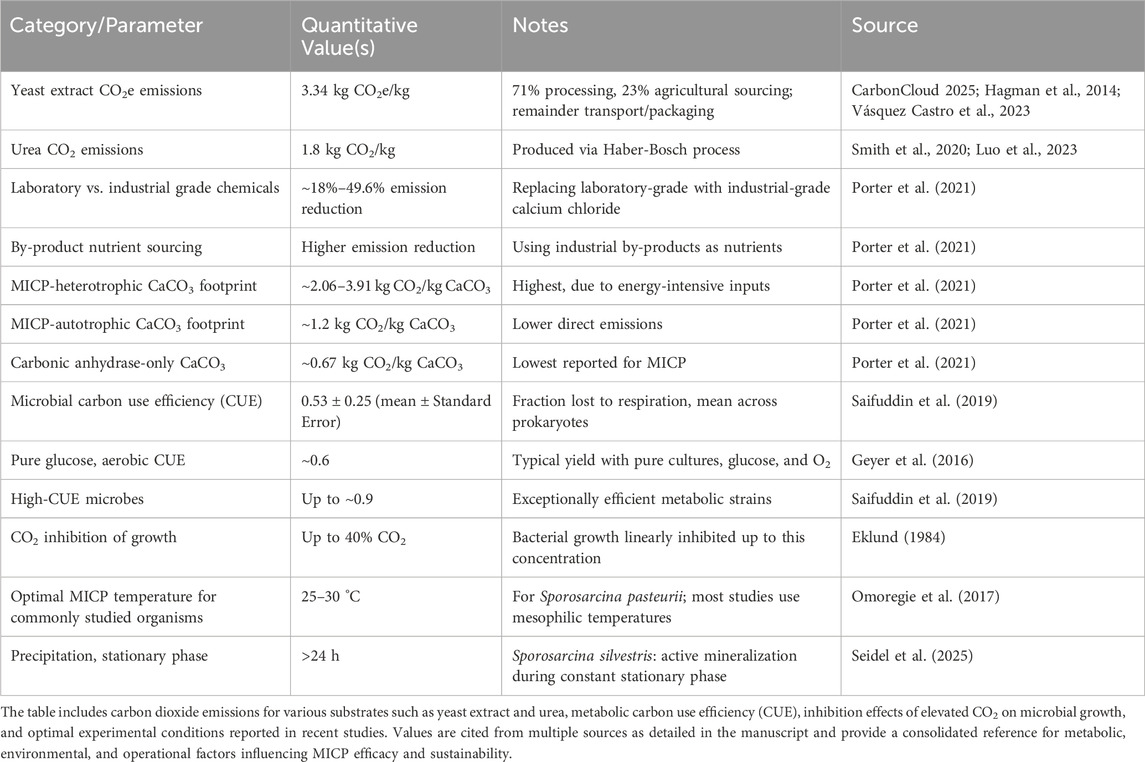
Table 1. Summary of key published quantitative parameters related to microbially induced carbonate precipitation (MICP) processes and associated carbon footprints.
Carbon accounting
The apparent simplicity of MICP as a carbon sequestration strategy belies a relatively complex carbon footprint. A critical oversight in current literature is the frequent absence of a holistic understanding of carbon balances, which requires evaluating all carbon inputs and outputs across the entire process lifecycle. This omission risks significantly overestimating net CO2 sequestration by ignoring critical emissions associated with processes such as nutrient production, metabolism, and other operational conditions. Currently, existing studies rarely validate CO2 sequestration when accounting for these factors, often failing to provide explicit, quantitative carbon footprint values for essential substrates like yeast extract or glucose that would be needed for the cultivation of MICP-associated microbes at an industrial scale.
Embodied emissions in materials for MICP
Recent industrial life-cycle assessments have begun quantifying these substrate-related emissions in carbon dioxide equivalent, or CO2e, expressing the total greenhouse gas emissions as an amount of CO2 equivalent. For example, producing 1 kg of yeast extract powder in the European Union generates 3.34 kg of CO2e emissions, with processing accounting for 71% of the total, agricultural sourcing making up 23%, and transport and packaging comprising the remaining portion (CarbonCloud, 2025). Indeed, factors such as the production and sourcing of nutrients for yeast cultivation, the energy required to maintain optimal growth conditions, subsequent processing steps, and losses through respiration and fermentation all contribute to making yeast extract a relatively high CO2 emitter (Hagman et al., 2014; Vásquez Castro et al., 2023). Urea, which is often cited as an important component of some MICP approaches, is a highly energy-intensive product, emitting approximately 1.8 kg of CO2 per kg of urea primarily due to fossil fuel consumption during the Haber-Bosch process (e.g., Smith et al., 2020; Luo et al., 2023). The use of urea together with yeast extract would therefore compound the emission profile of any MICP process conducted on such media. To better understand how changes in input materials affect emissions in MICP, readers are directed to Porter et al. (2021), who highlight that carefully selecting and optimizing input sources can significantly lower emissions. Notably, they found that replacing laboratory-grade calcium chloride with industrial-grade alternatives reduced emissions by ∼18%–49.62%. Even greater reductions were observed when industrial by-products were used as nutrient sources. Other options, such as making a judicious choice of less carbon-intensive nitrogen sources, can help reduce the overall environmental impact of microbial cultivation. Further work to quantify and subsequently reduce embodied emissions would be of significant assistance in progressing these technologies in terms of economics and sustainability.
MICP metabolic processes affect emissions
The rate of MICP can vary depending on which metabolic pathway predominates, environmental factors, and microbial activity levels (e.g., Fahimizadeh et al., 2022). Likewise, the carbon footprint of MICP also varies depending on the specific biochemical process employed. According to the life cycle assessment by Porter et al. (2021), heterotrophic processes, such as ureolysis, are the most carbon-intensive, producing 2.06–3.91 kg CO2 per kilogram of precipitated calcium carbonate. This high footprint is primarily attributed to the use of energy-intensive inputs like purified urea. By contrast, autotrophic processes generate lower direct emissions, ranging from ∼1.2 kg CO2 per kilogram of CaCO3. Notably, the use of only the carbonic anhydrase enzyme results in the lowest carbon footprint, at ∼0.67 kg CO2 per kilogram of CaCO3. However, the relatively high monetary costs of purified enzymes can be prohibitive for use in many industries (e.g., Jo et al., 2013).
Biomass vs. mineral material for carbon locking
Microbial communities, especially autotrophs, drive carbon draw down both via MICP and the formation of biomolecules. The latter ranges in the degree of permanence from labile (LOM) to refractory organic matter, each with different stability profiles (e.g., Dranseike et al., 2025). LOM, produced through microbial carbon assimilation, is unstable and typically returns to the atmosphere within years via respiration and decomposition (e.g., Visser et al., 2016; Hoikkala et al., 2016), limiting its sequestration potential. In contrast, mineral carbonates can provide long-term carbon storage, emphasizing the importance of distinguishing between these forms in carbon accounting. This duality is especially evident for CO2 uptake by cyanobacteria, where the balance between organic matter formation and carbonate mineralization varies by species and environment (e.g., Kamennaya et al., 2012; Jung et al., 2024). Therefore, robust accounting methods are essential, as failing to distinguish between different forms of sequestered carbon can result in a significant overestimation of net CO2 removal. Mineral carbonates are typically measured using mineralogical analyses (e.g., X-ray diffractometry, scanning electron microscopy), while transient LOM requires dynamic methods such as isotopic tracers (δ13C) and decomposition studies (e.g., Preston et al., 2006). Without clear differentiation, net CO2 removal can be overestimated if transient LOM is credited as stable mineral carbon. Measurements of total inorganic carbon (TIC) versus total organic carbon (TOC) can be used to distinguish and quantify sequestration by analysing the difference between the two forms of carbon (e.g., Jones et al., 2023).
Other physiological considerations
Respiration
Microbial respiration represents a key process in MICP, yet this factor receives limited attention in most experimental studies for CO2 sequestration. Many investigations emphasize the ability of microbes to convert carbon into mineral forms, often neglecting comprehensive accounting of respiration within the same system.
Respiration always results in less carbon loss than the total carbon input, since net growth cannot occur if all consumed carbon is respired. The relationship between carbon consumption and retention in microbial biomass is described by carbon use efficiency (CUE; e.g., Geyer et al., 2016; Mendonça et al., 2024). For heterotrophic microbes, this metric expresses the proportion of carbon retained in biomass relative to total carbon consumed, while for autotrophic organisms, CUE is defined as the ratio of carbon retained in biomass to carbon fixed. For example, a heterotrophic microbe with a CUE of 0.5 loses approximately half of consumed carbon to respiration, whereas a CUE of 0.8 indicates a more efficient mode of growth, with only 20% of carbon lost to respiration.
Variation in CUE among prokaryotes is substantial (Saifuddin et al., 2019), making it essential to determine CUE values for target microbial strains used in MICP applications. Saifuddin et al. (2019) indicate that, on average, about half of consumed carbon (mean = 0.53 ± 0.25 Standard Error) is lost to respiration across prokaryotes, though considerable variability exists among species. Closely related taxa tend to exhibit broadly similar CUE values, yet some species demonstrate notably higher efficiency. In addition, variations in CUE reflect differences in energy conservation and biomass formation efficiency, which are influenced by both the nature of the substrate and the organism’s metabolic capabilities.
Under aerobic conditions with ample glucose availability, pure cultures often exhibit a yield of approximately ∼0.6 (Geyer et al., 2016). In certain cases, particularly when utilizing more reduced substrates or when the organisms display exceptionally efficient metabolic performance, yields can approach ∼0.9 (Saifuddin et al. 2019). Therefore, selection of substrates and microbial strains with elevated CUE values may enhance the overall efficiency of MICP processes.
Growth phase
Most MICP studies are performed entirely during the exponential growth phase, when microbial populations are rapidly increasing. It is during this period that both calcium carbonate precipitation and CO2 uptake typically reach completion, and as a result, most experimental findings are based on observations made within this limited window of microbial activity. Most experiments investigating MICP do not consider the distinct growth phases of the microbes involved, such as the lag phase, exponential growth phase, or stationary phase.
This emphasis on the exponential phase has important implications for the interpretation and practical application of MICP. For instance, if one assumes that only the enzyme carbonic anhydrase is relevant and that its expression remains constant, then the number of enzyme molecules will increase exponentially alongside microbial growth. However, as nutrient availability declines and the culture transitions into the stationary phase, the number of enzyme molecules plateaus. At this point, the process likely achieves its maximum potential, but this peak is generally short-lived, and some form of media renewal will be required to maintain a healthy population of target microbes.
In a recent study by Seidel et al. (2025), a strain of Sporosarcina silvestris showed steady calcium carbonate precipitation during the stationary phase. While cell counts remained stable in that study, calcite precipitation continued over 24 h, demonstrating active mineralization beyond the exponential growth phase. To date, however, there has been limited research focused on the kinetics and biomineralization conditions necessary for efficient MICP beyond the initial growth phase. Even fewer studies (e.g., Murugan et al., 2021) have addressed strategies for maintaining maximum MICP performance in industrial systems over extended periods. Closing these knowledge gaps is essential for advancing MICP technology from controlled laboratory settings to practical, large-scale applications.
Elevated CO2
In addition to these considerations, the effects of elevated CO2 on microbes and their gene expression have received only limited attention in most studies. While some studies suggest fewer profound impacts of elevated CO2 levels on microbial diversity (e.g., Ahrendt et al., 2014), the gene expression of key MICP enzymes under such conditions remains poorly understood (e.g., Xiao et al., 2015; Clarà Saracho and Marek, 2024). Indeed, Eklund (1984) showed that growth rates of MICP-capable bacteria like Bacillus subtilis, Pseudomonas aeruginosa, and Bacillus cereus were linearly inhibited with increasing CO2 concentrations by up to 40%. Elevated CO2 concentrations can have significant negative effects on microbial growth (Yu and Chen, 2019; Wan et al., 2018; Ennaciri et al., 2022) and, to some extent, on the gene expression of carbonic anhydrase (e.g., Xiao et al., 2015), presenting further scalability challenges for MICP. These adverse impacts are likely more pronounced in aerobic taxa, although concomitant acidification of the medium may also affect other microbial groups. Efforts to either maintain lower CO2 concentrations or employ taxa tolerant of elevated CO2 or pH instability, would be highly beneficial. In addition, use of anaerobes for MICP are largely unexplored, these organisms may have some advantages in settings where elevated CO2 atmospheres are being considered.
Temperature
With a few exceptions (e.g., Okwadha and Jin, 2010; Omoregie et al., 2017; Peng and Liu, 2019; Zhang et al., 2021), temperature is often insufficiently addressed in MICP studies. Most research considers it only as necessary for supporting the growth of the target biomineralizing organism, despite its significant influence on both microbial activity and carbonate precipitation rates. Optimal MICP temperatures for many of the well-studied organisms, such as Sporosarcina pasteurii, are usually at relatively low temperatures of up to 30 °C (e.g., Omoregie et al., 2017). While elevated temperatures can accelerate reaction kinetics and enhance mineralization, enzyme activity lifespans may vary (e.g., Peng and Liu, 2019 and references therein). More research is thus warranted to explore organisms adapted to elevated temperatures. Furthermore, while these more efficient enzyme kinetics can be advantageous, they may require substantially higher process temperatures. This, in turn, necessitates thermotolerant microbial strains and greater energy input for heating, which undermines one of MICP’s main benefits, its potential for low energy demand and environmental sustainability. Further research is thus needed to explore this trade-off. If higher temperature processes are determined (through carbon accounting) to be preferable, work to develop or select robust, thermotolerant strains or enzymes will be crucial for advancing MICP technologies toward scalable and economically viable applications.
Carbonate mineral stability
MICP produces a range of carbonate minerals of varying thermodynamic stabilities, most commonly calcite, aragonite, and vaterite (e.g., Anbu et al., 2016; Chang et al., 2017; Zehner et al., 2020), representing different carbonate mineral phases or polymorphs of the same chemical composition but different crystal structures. Metastable vaterite and aragonite are more susceptible to dissolution than calcite, but no single precise pH value universally defines the onset of dissolution for these minerals, since temperature, ionic strength and the degree of saturation of the solution also play a role (e.g., Cooke and Kepkay, 1980; Plummer and Busenberg, 1982; Svenskaya and Pallaeva, 2023 and references therein). Other phases important in MICP include amorphous carbonate as well as Mg-calcite (e.g., Défarge et al., 1994; Jones and Peng, 2014).
The optimal outcome for durable CO2 storage is the exclusive formation of calcite, the most stable and least soluble polymorph of calcium carbonate, which ensures long-term immobilization of carbon. However, achieving the exclusive formation of the calcite phase during MICP is a significant scientific and engineering challenge due to the complex interplay of biochemical and environmental parameters that govern carbonate polymorph selection. The MICP process can foster the nucleation of metastable phases such as vaterite and aragonite, which often appear either concurrently with or prior to calcite precipitation due to kinetic and local saturation effects (e.g., Khanjani et al., 2021). One core difficulty lies in the sensitivity of phase outcomes to subtle fluctuations in parameters such as pH, temperature, calcium and urea concentrations, bacterial species and activity levels, and the specific nature of additives or impurities present in the system (e.g., Dhami et al., 2013; Anbu et al., 2016 and references therein; Khanjani et al., 2021; Haystead et al., 2024). For example, high supersaturation or rapid mixing can promote the nucleation of vaterite or aragonite (e.g., Sun et al., 2015), while the presence of magnesium ions can stabilize these less stable forms and inhibit their transformation to calcite (e.g., Boon et al., 2020).
The cumulative effect of these interacting factors means that establishing a robust, reproducible method for suppressing metastable phases and reliably producing phase-pure calcite remains a persistent obstacle for both laboratory-scale and field-scale MICP applications. This challenge is further compounded by the inherent variability of biological systems and the natural progression of polymorphic transitions over time (e.g., Dhami et al., 2013; Khanjani et al., 2021).
Addressing these challenges through targeted process optimization and environmental monitoring will be essential. Placing a strong emphasis on developing and validating reproducible methods is paramount. Systematic approaches that include rigorous standardization of microbial inoculum, substrate concentrations, and environmental parameters, coupled with comprehensive reporting of experimental details, will significantly enhance cross-laboratory comparability. The adoption of reproducibility-focused protocols not only facilitates more reliable suppression of metastable phases but also enables consistent generation of phase-pure calcite, ultimately advancing the scalability and practical deployment of MICP technologies. This focus will help bridge current gaps between experimental success under controlled conditions and reliable performance in complex, real-world environments.
Conclusion
While MICP holds potential as a biologically driven method for durable CO2 sequestration, realizing its full impact requires a more holistic and integrated evaluation. Current research focused on carbon sequestration often overlooks key factors such as whole-system carbon accounting, physiological constraints and the stability of the resulting carbonate minerals. Effective deployment of MICP must distinguish between transient organic biomass and stable mineral carbon, accurately track emissions from inputs, and account for microbial growth dynamics, carbon use efficiency, and tolerance to elevated CO2 and temperature. Additionally, reproducible control over polymorph formation, with an emphasis on generating stable calcite, is essential to ensure long-term carbon immobilization. Future progress depends on developing standardized methodologies, selecting robust microbial strains, optimizing nutrient sourcing, and validating mineral stability under field conditions. Addressing these challenges will be critical to transitioning MICP from a promising laboratory technique to a reliable and scalable solution for climate change mitigation.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
RS: Conceptualization, Formal Analysis, Funding acquisition, Visualization, Writing – original draft. VN: Writing – review and editing. LS: Writing – review and editing. NT-D: Writing – review and editing. DM: Conceptualization, Formal Analysis, Visualization, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The CSIRO CarbonLock Future Science Platform, and the CSIRO Energy Research Unit.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. Generative AI was used to assist in the creation of template ideas for the figure image.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdelsamad, R., Al Disi, Z., Abu-Dieyeh, M., Al-Ghouti, M. A., and Zouari, N. (2022). Evidencing the role of carbonic anhydrase in the formation of carbonate minerals by bacterial strains isolated from extreme environments in Qatar. Heliyon 8, e11151. doi:10.1016/j.heliyon.2022.e11151
Ahrendt, S. R., Mobberley, J. M., Visscher, P. T., Koss, L. L., and Foster, J. S. (2014). Effects of elevated carbon dioxide and salinity on the microbial diversity in lithifying microbial mats. Minerals 4, 145–169. doi:10.3390/min4010145
Anbu, P., Kang, C. H., Shin, Y. J., and So, J. S. (2016). Formations of calcium carbonate minerals by bacteria and its multiple applications. SpringerPlus 5, 250. doi:10.1186/s40064-016-1869-2
Boon, M., Rickard, W. D. A., Rohl, A. L., and Jones, F. (2020). Stabilization of aragonite: role of Mg2+ and other impurity ions. Cryst. Growth Des. 20, 5006–5017. doi:10.1021/acs.cgd.0c00152
CarbonCloud (2025). Technical report – climate footprint of yeast extract (powder). Available online at: https://apps.carboncloud.com/climatehub/product-reports/id/40832581969 (Accessed June 24, 2025).
Castanier, S., Métayer-Levrel, G. L., and Perthuisot, J. P. (2000). “Bacterial roles in the precipitation of carbonate minerals,” in Microbial sediments. Editors R. E. Riding, and S. M. Awramik (Berlin, Heidelberg: Springer), 32–39. doi:10.1007/978-3-662-04036-2_5
Castro-Alonso, M. J., Montañez-Hernandez, L. E., Sanchez-Muñoz, M. A., Macias Franco, M. R., Narayanasamy, R., and Balagurusamy, N. (2019). Microbially induced calcium carbonate precipitation (MICP) and its potential in bioconcrete: microbiological and molecular concepts. Front. Mat. 6, 126. doi:10.3389/fmats.2019.00126
Chang, R., Kim, S., Lee, S., Choi, S., Kim, M., and Park, Y. (2017). Calcium carbonate precipitation for CO2 storage and utilization: a review of the carbonate crystallization and polymorphism. Front. Energy Res. 5, 17. doi:10.3389/fenrg.2017.00017
Clarà Saracho, A., and Marek, E. J. (2024). Uncovering the dynamics of urease and carbonic anhydrase genes in ureolysis, carbon dioxide hydration, and calcium carbonate precipitation. Environ. Sci. Technol. 58, 1199–1210. doi:10.1021/acs.est.3c06617
Cooke, R. C., and Kepkay, P. E. (1980). The solubility of aragonite in seawater—I. Effect of pH and water chemistry at one atmosphere. Geochim. Cosmochim. Acta 44 (8), 1071–1075. doi:10.1016/0016-7037(80)90060-5
Défarge, C., Trichet, J., and Coute, A. (1994). On the appearance of cyanobacterial calcification in modern stromatolites. Sediment. Geol. 94, 11–19. doi:10.1016/0037-0738(94)90144-9
Dhami, N. K., Reddy, M. S., and Mukherjee, A. (2013). Biomineralization of calcium carbonate polymorphs by the bacterial strains isolated from calcareous sites. J. Microbiol. Biotechnol. 23, 707–714. doi:10.4014/jmb.1212.11087
Douglas, S., and Beveridge, T. J. (1998). Mineral formation by bacteria in natural microbial communities. FEMS Microbiol. Ecol. 26, 79–88. doi:10.1111/j.1574-6941.1998.tb00494.x
Dranseike, D., Cui, Y., Ling, A. S., Donat, F., Bernhard, S., Bernero, M., et al. (2025). Dual carbon sequestration with photosynthetic living materials. Nat. Commun. 16, 3832. doi:10.1038/s41467-025-58761-y
Eklund, T. (1984). The effect of carbon dioxide on bacterial growth and on uptake processes in bacterial membrane vesicles. Int. J. Food Microbiol. 1, 179–185. doi:10.1016/0168-1605(84)90014-X
Ennaciri, A., Clarà Saracho, A., and Marek, E. (2022). “Bacteria-induced mineralisation for enhanced removal of CO2,” in Resented at: 2nd international conference on negative CO2 emissions; 2022 Jun 14–17 (Göteborg, Sweden).
Fahimizadeh, M., Pasbakhsh, P., Mae, L. S., Tan, J. B. L., and Raman, R. K. S. (2022). Multifunctional, sustainable, and biological non-ureolytic self-healing systems for cement-based materials. Engineering 13, 217–237. doi:10.1016/j.eng.2021.11.016
Fu, Y., Fan, F., Zhang, Y., Wang, B., and Cao, Z. (2021). Conformational change of H64 and substrate transportation: insight into a full picture of enzymatic hydration of CO2 by carbonic anhydrase. Front. Chem. 9, 706959. doi:10.3389/fchem.2021.706959
Geyer, K. M., Kyker-Snowman, E., Grandy, A. S., and Frey, S. D. (2016). Microbial carbon use efficiency: accounting for population, community, and ecosystem-scale controls over the fate of metabolized organic matter. Biogeochemistry 127, 173–188. doi:10.1007/s10533-016-0191-y
Gilmour, K. A., Ghimire, P. S., Wright, J., Haystead, J., Dade-Robertson, M., Zhang, M., et al. (2024). Microbially induced calcium carbonate precipitation through CO2 sequestration via an engineered Bacillus subtilis. Microb. Cell Fact. 23, 168. doi:10.1186/s12934-024-02437-7
Hagman, A., Sall, T., and Piskur, J. (2014). Analysis of the yeast short-term Crabtree effect and its origin. FEBS J. 281, 4805–4814. doi:10.1111/febs.13019
Haystead, J., Gilmour, K., Sherry, A., Dade-Robertson, M., and Zhang, M. (2024). Effect of (in)organic additives on microbially induced calcium carbonate precipitation. J. Appl. Microbiol. 135 (1), lxad309. doi:10.1093/jambio/lxad309
Hoikkala, L., Tammert, H., Lignell, R., Eronen-Rasimus, E., Spilling, K., and Kisand, V. (2016). Autochthonous dissolved organic matter drives bacterial community composition during a bloom of filamentous Cyanobacteria. Front. Mar. Sci. 3, 111. doi:10.3389/fmars.2016.00111
Jo, B. H., Kim, I. G., Seo, J. H., Kang, D. G., and Cha, H. J. (2013). Engineered Escherichia coli with periplasmic carbonic anhydrase as a biocatalyst for CO2 sequestration. Appl. Environ. Microbiol. 79, 6697–6705. doi:10.1128/AEM.02400-13
Jones, B., and Peng, X. (2014). Multiphase calcification associated with the atmophytic cyanobacterium Scytonema julianum. Sediment. Geol. 313, 91–104. doi:10.1016/j.sedgeo.2014.09.002
Jones, T. R., Poitras, J., Gagen, E., Paterson, D. J., and Southam, G. (2023). Accelerated mineral bio-carbonation of coarse residue kimberlite material by inoculation with photosynthetic microbial mats. Geochem. Trans. 24, 1. doi:10.1186/s12932-023-00082-4
Jung, P., Briegel-Williams, L., Dultz, S., Neff, C., Heibrock, G., Monger, C., et al. (2024). Hard shell, soft blue-green core: ecology, processes, and modern applications of calcification in terrestrial Cyanobacteria. iScience 27, 111280. doi:10.1016/j.isci.2024.111280
Kamennaya, N. A., Ajo-Franklin, C. M., Northen, T., and Jansson, C. (2012). Cyanobacteria as biocatalysts for carbonate mineralization. Minerals 2, 338–364. doi:10.3390/min2040338
Khanjani, M., Westenberg, D. J., Kumar, A., and Ma, H. (2021). Tuning polymorphs and morphology of microbially induced calcium carbonate: controlling factors and underlying mechanisms. ACS Omega 6, 11988–12003. doi:10.1021/acsomega.1c00559
Lin, C. Y., Turchyn, A. V., Steiner, Z., Bots, P., Lampronti, G. I., and Tosca, N. J. (2018). The role of microbial sulfate reduction in calcium carbonate polymorph selection. Geochim. Cosmochim. Acta 237, 184–204. doi:10.1016/j.gca.2018.06.019
Luo, Y., Xie, K., Ou, P., Lavallais, C., Peng, T., Chen, Z., et al. (2023). Selective electrochemical synthesis of urea from nitrate and CO2via relay catalysis on hybrid catalysts. Nat. Catal. 6, 939–948. doi:10.1038/s41929-023-01020-4
Meitner, L. (2024). “Voluntary carbon markets: a critical assessment,” in Hochschule für Wirtschaft und Recht Berlin (Berlin: Institute for International Political Economy IPE).
Meldrum, N. U., and Roughton, F. J. W. (1933). Carbonic anhydrase. Its preparation and properties. J. Physiol. 80, 113–142. doi:10.1113/jphysiol.1933.sp003077
Mendonça, C. M., Zhang, L., Waldbauer, J. R., and Aristilde, L. (2024). Disproportionate carbon dioxide efflux in bacterial metabolic pathways for different organic substrates leads to variable contribution to carbon-use efficiency. Environ. Sci. Technol. 58, 11041–11052. doi:10.1021/acs.est.4c01328
Mitchell, A. C., Dideriksen, K., Spangler, L. H., Cunningham, A. B., and Gerlach, R. (2010). Microbially enhanced carbon capture and storage by mineral-trapping and solubility-trapping. Environ. Sci. Technol. 44, 5270–5276. doi:10.1021/es903270w
Murugan, R., Suraishkumar, G. K., Mukherjee, A., and Dhami, N. K. (2021). Insights into the influence of cell concentration in design and development of microbially induced calcium carbonate precipitation (MICP) process. PLoS One 16, e0254536. doi:10.1371/journal.pone.0254536
Okwadha, G. D. O., and Jin, L. (2010). Optimum conditions for microbial carbonate precipitation. Chemosphere 81, 1143–1148. doi:10.1016/j.chemosphere.2010.09.066
Okyay, T. O., and Rodrigues, D. F. (2015). Biotic and abiotic effects on CO2 sequestration during microbially-induced calcium carbonate precipitation. FEMS Microbiol. Ecol. 91 (3), fiv017. doi:10.1093/femsec/fiv017
Omoregie, A. I., Khoshdelnezamiha, G., Senian, N., Ong, D. E. L., and Nissom, P. M. (2017). Experimental optimisation of various cultural conditions on urease activity for isolated Sporosarcina pasteurii strains and evaluation of their biocement potentials. Ecol. Eng. 109 (Part A), 65–75. doi:10.1016/j.ecoleng.2017.09.012
Peng, J., and Liu, Z. (2019). Influence of temperature on microbially induced calcium carbonate precipitation for soil treatment. PLoS ONE 14, e0218396. doi:10.1371/journal.pone.0218396
Plummer, L. N., and Busenberg, E. (1982). The solubilities of calcite, aragonite and vaterite in CO2-H2O solutions between 0 and 90°C, and an evaluation of the aqueous model for the system CaCO3-CO2-H2O. Geochim. Cosmochim. Acta 46, 1011–1040. doi:10.1016/0016-7037(82)90056-4
Porter, H., Mukherjee, A., Tuladhar, R., and Dhami, N. K. (2021). Life cycle assessment of biocement: an emerging sustainable solution? Sustainability 13, 13878. doi:10.3390/su132413878
Power, I. M., Harrison, A. L., and Dipple, G. M. (2016). Accelerating mineral carbonation using carbonic anhydrase. Environ. Sci. Technol. 50, 2610–2618. doi:10.1021/acs.est.5b04779
Preston, C. M., Trofymow, J. A., and Flanagan, L. B. (2006). Decomposition, δ13C, and the “lignin paradox”. Can. J. Soil Sci. 86, 235–245. doi:10.4141/s05-090
Riding, R. (2006). Cyanobacterial calcification, carbon dioxide concentrating mechanisms, and Proterozoic–Cambrian changes in atmospheric composition. Geobiology 4, 299–316. doi:10.1111/j.1472-4669.2006.00087.x
Saifuddin, M., Bhatnagar, J. M., Segrè, D., and Finzi, A. C. (2019). Microbial carbon use efficiency predicted from genome-scale metabolic models. Nat. Commun. 10 (1), 3568. doi:10.1038/s41467-019-11488-z
Schweitzer, H., Aalto, N. J., Busch, W., Chan, D. T. C., Chiesa, M., Elvevoll, E. O., et al. (2021). Innovating carbon-capture biotechnologies through ecosystem-inspired solutions. One Earth 4, 49–59. doi:10.1016/j.oneear.2020.12.006
Seidel, M., Hamley-Bennett, C., Reeksting, B. J., Bagga, M., Hellmann, L., Hoffmann, T. D., et al. (2025). Metabolic insights into microbially induced calcite formation by Bacillaceae for application in bio-based construction materials. Environ. Microbiol. 27, e70093. doi:10.1111/1462-2920.70093
Smith, K. S., and Ferry, J. G. (2000). Prokaryotic carbonic anhydrases. FEMS Microbiol. Rev. 24, 335–366. doi:10.1111/j.1574-6976.2000.tb00546.x
Smith, C., Hill, A. K., and Torrente-Murciano, L. (2020). Current and future role of Haber–Bosch ammonia in a carbon-free energy landscape. Energy Environ. Sci. 13, 331–344. doi:10.1039/C9EE02873K
Sun, W., Jayaraman, S., Chen, W., Persson, K. A., and Ceder, G. (2015). Nucleation of metastable aragonite CaCO3 in seawater. Proc. Natl. Acad. Sci. U.S.A. 112 (11), 3199–3204. doi:10.1073/pnas.1423898112
Svenskaya, Y., and Pallaeva, T. (2023). Exploiting benefits of vaterite metastability to design degradable systems for biomedical applications. Pharmaceutics 15 (11), 2574. doi:10.3390/pharmaceutics15112574
Vásquez Castro, E., Memari, G., Ata, Ö., and Mattanovich, D. (2023). Carbon efficient production of chemicals with yeasts. Yeast 40 (12), 583–593. doi:10.1002/yea.3909
Visser, P. M., Verspagen, J. M. H., Sandrini, G., Stal, L. J., Matthijs, H. C. P., Davis, T. W., et al. (2016). How rising CO2 and global warming may stimulate harmful cyanobacterial blooms. Harmful Algae 54, 145–159. doi:10.1016/j.hal.2015.12.006
Wan, R., Wang, L., Chen, Y., Zheng, X., Su, Y., and Tao, X. (2018). Insight into a direct carbon dioxide effect on denitrification and denitrifying bacterial communities in estuarine sediment. Sci. Total Environ. 643, 1074–1083. doi:10.1016/j.scitotenv.2018.06.279
Wilcox, S. M., Mulligan, C. N., and Neculita, C. M. (2025). Mineral carbonation for carbon sequestration: a case for MCP and MICP. Int. J. Mol. Sci. 26 (5), 2230. doi:10.3390/ijms26052230
Xiao, L., Lian, B., Hao, J., Liu, C., and Wang, S. (2015). Effect of carbonic anhydrase on silicate weathering and carbonate formation at present-day CO2 concentrations compared to primordial values. Sci. Rep. 5, 7733. doi:10.1038/srep07733
Yu, T., and Chen, Y. (2019). Effects of elevated carbon dioxide on environmental microbes and its mechanisms: a review. Sci. Total Environ. 655, 865–879. doi:10.1016/j.scitotenv.2018.11.301
Zehner, J., Røyne, A., Wentzel, A., and Sikorski, P. (2020). Microbial-induced calcium carbonate precipitation: an experimental toolbox for in situ and real-time investigation of micro-scale pH evolution. RSC Adv. 10, 20485–20493. doi:10.1039/d0ra03897k
Zhang, J., Shi, X., Chen, X., Huo, X., and Yu, Z. (2021). Microbial-induced carbonate precipitation: a review on influencing factors and applications. Adv. Civ. Eng. 2021, 9974027. doi:10.1155/2021/9974027
Keywords: microbially induced carbonate precipitation (MICP), carbon dioxide (CO2), carbon sequestration, biomineralization, respiration, metabolism, carbonate
Citation: Schinteie R, Nagaraj V, Stalker L, Tran-Dinh N and Midgley DJ (2025) Beyond carbonate biomineralization: why prokaryote-driven CO2 sequestration demands holistic evaluation. Front. Bioeng. Biotechnol. 13:1690042. doi: 10.3389/fbioe.2025.1690042
Received: 21 August 2025; Accepted: 27 October 2025;
Published: 17 November 2025.
Edited by:
Noha M. Mesbah, Suez Canal University, EgyptReviewed by:
Michael Lakatos, Hochschule Kaiserslautern University of Applied Sciences, GermanySasha Wilson, University of Alberta, Canada
Copyright © 2025 Schinteie, Nagaraj, Stalker, Tran-Dinh and Midgley. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Richard Schinteie, cmljaGFyZC5zY2hpbnRlaWVAY3Npcm8uYXU=
 Richard Schinteie
Richard Schinteie Veena Nagaraj
Veena Nagaraj Linda Stalker
Linda Stalker Nai Tran-Dinh
Nai Tran-Dinh David J. Midgley
David J. Midgley