- 1Key Laboratory of Biotechnology and Bioengineering of State Ethnic Affairs Commission, Biomedical Research Center, Northwest Minzu University, Lanzhou, China
- 2College of Life Sciences and Engineering, Northwest Minzu University, Lanzhou, China
Introduction: Potentilla anserina L., a traditional Chinese medicinal herb, is valued for its edible, medicinal, and ornamental properties.
Methods: In this study, flavonoids were extracted from Potentilla anserina L. using ultrasonic-assisted extraction. The extraction process was optimized through response surface methodology, followed by separation and purification using Sephadex G-100 gel chromatography. Finally, the antioxidant activity of the extracted flavonoids was evaluated.
Results: The results showed that the optimal extraction conditions were 60% ethanol concentration, an ultrasonic temperature of 50°C, ultrasonic power of 400 W, and ultrasonic time of 180 min. Under these conditions, the average extraction yield of flavonoids from Potentilla anserina L. was 3.74 ± 0.06 mg/g. The crude flavonoid extract was purified by Sephadex G-100 gel chromatography, yielding two fractions, LF-1 and LF-2, which accounted for 63.34% and 25.79% of the crude extract, respectively.
Discussion: The results of in vitro antioxidant activity experiments demonstrated that both fractions (LF-1 and LF-2) exhibited significant antioxidant activity, showing a dose-dependent capacity. These findings provide a theoretical basis for the further development and utilization of flavonoids from Potentilla anserina L..
Introduction
Potentilla anserina L., a perennial herbaceous plant in the genus “Potentilla” of the family Rosaceae, is also known as “poke ma” in Tibetan (Guo et al., 2023). This species is not only found in alpine meadows, but also inhabits riverbanks, roadsides, and hillside grasslands, occurring at altitudes ranging from 500 to 4,100 m (Luan et al., 2022). Widely distributed across North America, Asia, and Europe. Potentilla anserina L. is primarily found within China’s territorial boundaries in Qinghai Province, the Tibet Autonomous Region, Gansu Province, and Sichuan Province (Yang et al., 2021). Studies have shown that this plant possesses considerable value in terms of edibility, medicinal properties, and ornamental use, indicating significant potential for further development and application (Tang et al., 2022).
Potentilla anserina L. is rich in nutritional and bioactive components, including polysaccharides, triterpenoids, and flavonoids. These compounds not only provided with abundant nutritional value but also exhibit a range of biological activities, such as antioxidant, anti-myocardial ischemia, antiviral, and immunomodulatory effects, thereby offering protective benefits to human organs (Čižmárová et al., 2023; Kim et al., 2020). To date, extensive studies have reported on the extraction of, polysaccharides, polyphenols (Huang et al., 2020), and other bioactive substances from Potentilla anserina L. Flavonoids, in particular, are widely recognized for their diverse functionalities, including antihypertensive, antibacterial, anti-inflammatory, and antioxidant properties (Binkowska, 2020). Therefore, this study is dedicated to optimizing the ultrasound-assisted extraction process of flavonoids from Potentilla anserina L. and characterizing the in vitro antioxidant activity of the resulting flavonoid extracts.
Flavonoids are traditionally extracted from natural plants using solvent extraction, a technique that exploits differences in the solubility of target compounds across various solvents to achieve separation (Tang et al., 2023). This method is known for its simple equipment requirements, ease of operation, and relatively low cost. However, it also has drawbacks, such as potential environmental pollution caused by solvent usage and the need for multiple extraction cycles. To improve the efficiency of natural product extraction, several emerging techniques have been developed and applied, including silica gel column chromatography, methanol Soxhlet extraction, and ultrasonic-assisted extraction and supercritical carbon dioxide extraction. Despite being recognized for its green and safe profile along with high extraction efficiency, supercritical CO2 extraction is characterized by high equipment costs, elevated energy consumption, and a requirement for stringent raw material pretreatment. Among these, ultrasonic-assisted extraction stands out due to its short extraction time, cost-effectiveness, and environmental friendliness, which have contributed to its widespread use in the extraction of bioactive compounds from plant materials. The mechanism of ultrasonic-assisted extraction relies on the mechanical effects, cavitation effects, thermal effects, and secondary effects induced by ultrasonic waves. These effects enhance the penetration ability of the solvent and accelerate the release and diffusion of target molecules into the solvent, thereby improving overall extraction efficiency (Zhang et al., 2018).
In this study, the ultrasonic-assisted extraction method was employed to extract flavonoids from Potentilla anserina L. single-factor experiments and response surface methodology were utilized to optimize the extraction conditions. Subsequently, Sephadex gel chromatography was used to separate and purify the flavonoids from the extracted solution. Finally, the in vitro antioxidative activity of the purified flavonoids was evaluated by assessing their DPPH radical scavenging capacity, hydroxyl radical (·OH) scavenging activity, superoxide anion (·O2−) scavenging ability, and total reducing power. These assays provide a comprehensive understanding of the potential antioxidant properties of Potentilla anserina L. flavonoids, which may contribute to enhancing the utilization value of this plant in pharmaceutical, nutraceutical, and functional food applications.
Materials and methods
Materials and reagents
Analytical grade ethanol, vitamin C (VC), and sodium nitrite (NaNO2) were purchased from Chengdu Cologne Chemical Co., Ltd. (Chengdu, China). Rutin (>98%) and 1,1-diphenyl-2-picrylhydrazyl (DPPH) were obtained from Aladdin Reagent Co., Ltd. (Shanghai, China). Aluminum nitrate (Al(NO3)3) and sodium hydroxide (NaOH) were analytical grade and provided by the Beijing Soleibao Technology Co., Ltd. (Beijing, China).
Potentilla anserina L. was purchased from a local market in Lanzhou, Gansu Province, China. The original collection site was Hezuo City, Gannan Tibetan Autonomous Prefecture (102°53′E, 34°57′N), with samples collected in 2025. It was cleaned and then freeze-dried using a vacuum freeze-dryer (LGJ-100F, Thermo, United States). The dried Potentilla anserine L. was ground into powder, which was collected through an 80-mesh sieve. The powder was subsequently degreased twice with n-hexane (M: V = 1:3 g/mL) for 6 h each time was conducted under room temperature conditions, dried, and stored for later use.
Ultrasound-assisted extraction of flavonoids
A total of 3.00 g of defatted Potentilla anserina L powder was accurately weighed, and 60 mL of ethanol was added according to a solid–liquid ratio of 1: 20 (g/mL). The extraction was performed using an ultrasonic cleaner (SB-500DTY, Ningbo Xinzhi Biotechnology Co., China), and the procedure was repeated three times. The combined extracts were centrifuged at 5,000 rpm for 10 min (Heraeus Multifuge X1R, Thermo, United States). Following concentration under vacuum rotary evaporation, the crude flavonoids obtained must be stored at 4 °C in complete darkness within amber glass containers to prevent ultraviolet light from causing oxidation and degradation of the flavonoid structure (Yu et al., 2018).
Determination of flavonoid extraction rate
The yield of flavonoids was determined using the colorimetric method with aluminium nitrate (Liao et al., 2021). A 0.5 mL aliquot of the sample was diluted with 70% ethanol to a final volume of 5 mL. Subsequently, 0.3 mL of 5% NaNO2 solution was added, and the mixture was allowed to react for 6 min. Then, 1 mL of 10% Al(NO3)3 solution was introduced, followed by a further 6 min reaction period. Finally, 10 mL of 4% NaOH solution was added, and the mixture was thoroughly mixed and left to react completely. The solution was then left at room temperature for 15 min, after which the absorbance was measured at 510 nm using a UV spectrophotometer (Nie et al., 2019). The flavonoid yield was expressed as rutin equivalent (RE) per gram of Potentilla anserina L. extract and calculated using the following Equation 1:
The effect of ethanol concentration, ultrasonic temperature, ultrasonic power, and extraction time on flavonoid extraction rate
The effects of four factors on the efficiency of ultrasonic-assisted extraction were investigated to optimize the extraction yield of Potentilla anserina L. flavonoids. The four independent variables included ethanol concentration (50%, 60%, 70%, 80%, and 90%), ultrasonic temperature (40, 50, 60, 70, and 80 °C), ultrasonic power (250, 300, 350, 400, and 450 W), and ultrasonic time (1, 2, 3, 4, and 5 h).
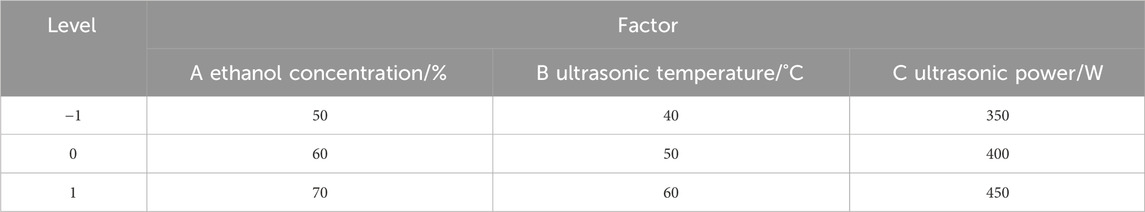
Optimization of the extraction conditions
Based on the results of single -factor experiments, the response surface methodology was employed to optimize the ultrasound-assisted extraction conditions of Potentilla anserina L. flavonoids. The extraction yield (Y) was selected as the response variable, while ethanol concentration (X1), ultrasonic power (X2), and ultrasonic temperature (X3) were chosen as the three independent variables. A total of 17 experimental runs were performed using a three-factor, three-level Box-Behnken Design (BBD), including 5 center points and 12 factorial points (−1, 0, and 1). The test factors and their levels are presented in Table 1. The experimental data were fitted to a second-order polynomial regression model described by the following Equation 2:
Where Y is the response variable representing the extraction yield of Potentilla anserina L. flavonoids (%); Z0, Zi, Zii, and Zij are the regression coefficients for the intercept, linear terms, quadratic terms, and interaction terms, respectively; and Xi and Xj are the coded independent variables (i ≠ j).
Sephadex G-100 gel chromatography
The Sephadex G-100 gel chromatography system used for the separation and purification of Potentilla anserina L. flavonoids consists primarily of a chromatographic column unit and a detection unit. The separation unit includes a 1.0 cm × 100 cm chromatographic column (Shanghai Huxi Analytical Instrument Factory Co., Ltd.), a CXG-1 Computer-Controlled Thermostatic Chromatography Cabinet (Shanghai Jiapeng Technology Co., Ltd.), and an HL-2S Constant Flow Pump (Shanghai Huxi Analytical Instrument Factory Co., Ltd.). The detection unit comprises an HD-3 UV Detector, an HD-A Computer-Controlled Chromatography Data Acquisition System, and an SBS-100 Automatic Fraction Collector, all supplied by Shanghai Jiapeng Technology Co., Ltd.
A 10 mg/mL solution of Potentilla anserina L. flavonoids was filtered through a 0.45 μm microporous membrane to prevent potential clogging of the gel matrix and then loaded onto the column. Equilibration was performed using a column (1.6 cm inner diameter × 50 cm length, i. d. × L) and 8 column volumes of buffer. A loading volume of 3 mL was used, followed by isocratic elution with 0.02 mol/L phosphate buffer (pH 6.8) at a flow rate of 0.4 mL/min. The eluate was monitored at 510 nm using a UV detector, and fractions were automatically collected at intervals of 7.5 min per tube. Following multiple cycles, distinct fractions were aliquoted, lyophilized, and stored as dry powders for further analysis.
In vitro antioxidant activity assay
Determination of DPPH radical scavenging activity
The DPPH radical scavenging capacity was evaluated using the method described by Franco et al. (2019). Various concentrations (0.1, 0.2, 0.3, 0.4, and 0.5 mg/mL) of Potentilla anserina L. flavonoids (2 mL each) were mixed with 2 mL of DPPH solution (0.2 mM), and the mixture was allowed to react for 30 min at room temperature in the dark. The absorbance of the resulting solution was measured at 517 nm. Control 1 consisted of 2.0 mL DPPH solution and 2.0 mL distilled water, while Control 2 consisted 2.0 mL sample solution and 2.0 mL distilled water, The Vitamin C (VC) group was used as the positive control, and the procedure was repeated three times. The DPPH radical scavenging activity was calculated using the following Equation 3:
Where E represents the DPPH radical scavenging activity (%); Ax is the absorbance of the sample group; A1 denotes the absorbance of the control 1, A2 refers to the absorbance of control 2.
Determination of hydroxyl (·OH) radical scavenging activity
The hydroxyl radical (·OH) scavenging activity of flavonoids from Potentilla anserina L. was evaluated using the methods described by Zhou et al. (Zhou et al., 2021) and Liang et al. (Liang et al., 2018). Solutions of Potentilla anserina L. flavonoids were prepared at concentrations of 0.1, 0.2, 0.3, 0.4, and 0.5 mg/mL. Each sample solution (1.0 mL) was mixed with o-phenanthroline ethanol solution (0.75 mM) and FeSO4 solution (0.75 mM), and the mixture was incubated in a water bath at 37 °C for 30 min. Subsequently, 1.0 mL of H2O2 (0.01%) and 2.0 mL of PBS (pH 7.4) were added, followed by thorough mixing and further incubation under the same conditions for 15 min. After cooling to room temperature, the absorbance (Ax) of each sample solution was measured at 510 nm against a blank control consisting of distilled water. Vitamin C (VC) solutions at the same concentrations served as a positive control, and the procedure was repeated three times. The hydroxyl radical scavenging activity was calculated using the following Equation 4:
Where A0 is the absorbance of the reaction system without the hydroxyl radical (OH), Ax represents the absorbance of the sample group, and Aj denotes the absorbance of the blank control.
Determination of hydroxyl (O2−) radical scavenging activity
The superoxide anion (O2−) radical scavenging activity of Potentilla anserina L. flavonoids was evaluated using a previously described method. Solutions of Potentilla anserina L. flavonoids and vitamin C (VC) were prepared at concentrations of 0.1, 0.2, 0.3, 0.4 and 0.5 mg/mL, respectively. A 1.0 mL aliquot of each flavonoid solution was mixed with 4.5 mL of Tris-HCl buffer (pH 8.2), and the mixture was incubated in a water bath at 25 °C for 10 min. Subsequently, 0.4 mL of 25 mM catechol solution was added, the mixture was thoroughly mixed and incubated again at 25 °C for 5 min. Finally, 1 mL of 8 M hydrochloric acid was introduced to terminate the reaction, and the absorbance was measured at 320 nm. Distilled water substituted for the flavonoid solution served as the blank control, while the VC group was used as the positive control, and the procedure was repeated three times. The scavenging activity was calculated using the following Equation 5:
Where Ac represents the absorbance of the blank control; As is the absorbance of the sample, and A0 denotes the absorbance of the sample solution mixed with Tris-HCl buffer after incubation.
Assay of total reducing ability
The total reducing capacity of Potentilla anserina L. flavonoids was assessed using the method described in a previous study (Goswami et al., 2020). Solutions of Potentilla anserina L. flavonoids and vitamin C (VC) were prepared at concentration gradients of 2, 4, 6, 8, and 10 mg/mL, respectively. A 1 mL aliquot of each sample solution was mixed with 2.5 mL of phosphate buffer (pH 6.6) and 2.5 mL of 1% potassium ferricyanide solution, followed by incubation in a water bath at 50 °C for 20 min. Afterward, 2.5 mL of 10% trichloroacetic acid solution was added, and the mixture was centrifuged at 5,000 rpm for 10 min. Then, 5 mL of the resulting supernatant was combined with 5 mL of deionized water and 1 mL of 0.1% ferric chloride solution, and the reaction was allowed to proceed for 10 min. Absorbance was measured at 700 nm using deionized water as a blank reference, with VC used as the positive control.
Statistical analysis
SPSS 25.0 software was employed for data analysis, with results presented as mean ± standard deviation. A one-way analysis of variance (ANOVA) was conducted to assess statistical significance. Design-Expert software (Version 8.0.6, Stat-Ease, Inc., Minneapolis, MN, United States) was utilized for experimental design. All experiments and analyses were performed in triplicate to ensure reproducibility. The 50% inhibitory concentrations (IC50 values) were calculated using the probit analysis method with SPSS software.
Results
The effect of ethanol concentration, ultrasonic temperature, ultrasonic power, and extraction time on flavonoid extraction rate
Ethanol was selected as the extraction solvent due to its high safety profile and environmental compatibility. The concentration of ethanol plays a crucial role in the extraction efficiency plant flavonoids. Flavonoids with higher polarity are more effectively extracted using low-concentration ethanol, whereas nonpolar free flavonoids are better dissolved and extracted by high-concentration ethanol. As illustrated in Figure 1A, in the single-factor experiments, all other conditions were fixed as follows: ultrasonic power of 350 W, ultrasonic temperature of 50 °C, and ultrasonic time of 3 h. The maximum flavonoid yield of 2.67 ± 0.06 mg/g was achieved when the ethanol concentration was 60%. According to the principle of like dissolves like, the solubility of flavonoid compounds in ethanol is closely related to their respective polarities. The polarity of Potentilla anserina L. flavonoids is comparable to that of a 60% ethanol solution, which explains the highest yield observed at this concentration. When the ethanol concentration exceeds 60%, the extraction yield decreases. A possible explanation for this decline is that excessively high ethanol concentrations may dissolve other alcohol-soluble impurities, thereby interfering with the accurate determination of flavonoid content (Michalaki et al., 2023).
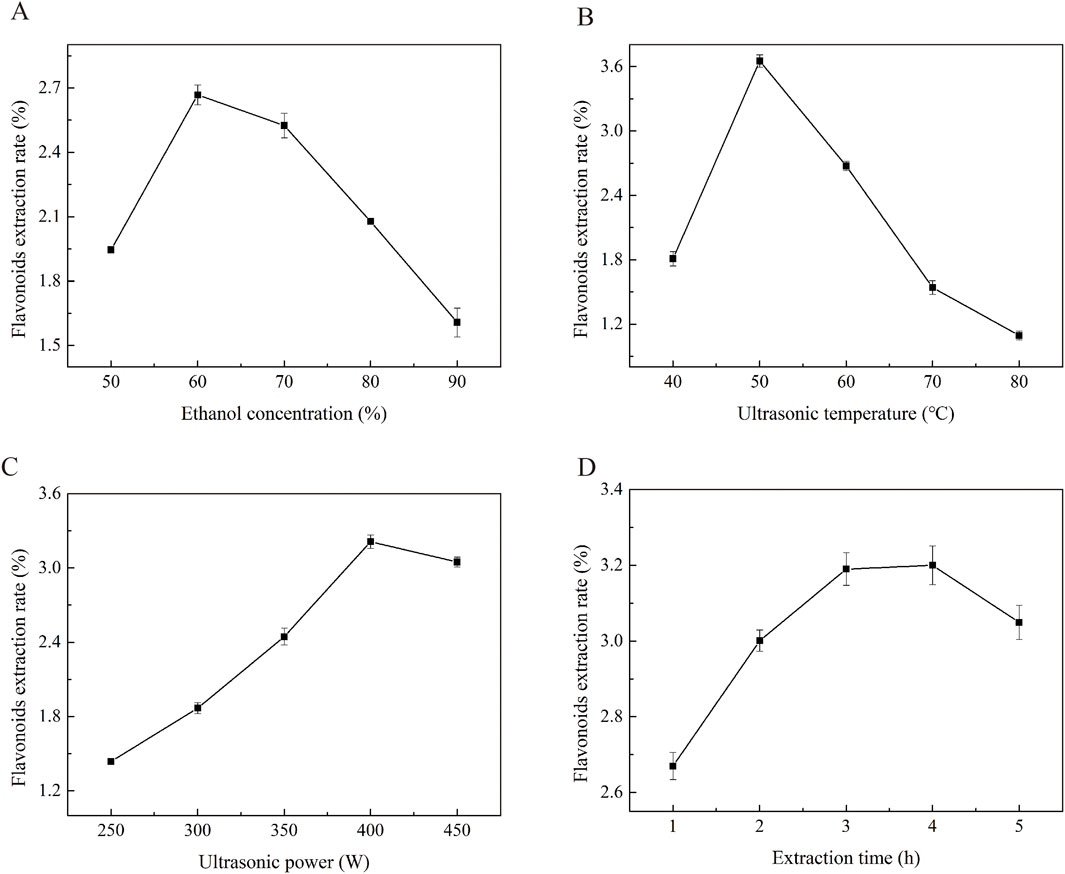
Figure 1. The effects of ethanol concentration (A), Ultrasound temperature (B), and Ultrasonic power (C), Ultrasonic time (D) on the yield of Potentilla anserine L. flavonoids.
The diffusion coefficient and solubility of flavonoids in the extraction solvent are improved at higher temperatures, which may contribute to an increased extraction rate (Cui et al., 2022). To evaluate the effect of ultrasonic temperature (40, 50, 60, 70, and 80 °C) on the extraction yield, experiments were conducted under fixed conditions: ultrasonic power of 350 W, ethanol concentration of 60%, and ultrasonic time of 3 h. As shown in Figure 1B, the extraction yield of Potentilla anserina L. flavonoids increased from 1.81% to 3.65% as the ultrasonic temperature rose from 40 °C to 50 °C, followed by a gradual decrease when the temperature was further increased to 80 °C. This trend can be explained by the fact that increasing temperature enhances the dissolution rate of flavonoids, thereby improving the extraction yield (Wu et al., 2021). However, beyond a certain threshold, excessive heat may compromise the molecular structure of flavonoids, reduce their thermal stability, and lead to degradation, ultimately resulting in a decline in yield.
The extraction of flavonoids is strongly influenced by the ultrasonic power, a critical parameter. As ultrasonic power increase, flavonoids are released and extracted through the combined effects of the mechanical forces and cavitation generated by ultrasound, which facilitates the extraction process (Chen et al., 2022). To investigate the effect of ultrasonic power (250, 300, 350, 400, and 450 W) on extraction yield, experiments were conducted under fixed conditions: an ultrasonic temperature of 50 °C, extraction time of 3, hours and ethanol concentration of 60%. As shown in Figure 1C, the flavonoid yield increased with rising power within the range of 250–400 W, reaching a maximum of 3.21 ± 0.08 mg/g at 400 W. Beyond this level, the yield decreased. This decline can be attributed to the fact that excessive ultrasonic power may induce intense cavitation activity, leading to structural degradation of flavonoids and consequently reducing the extraction yield (Xue Y. et al., 2022).
Ultrasonic treatment enhances the extraction efficiency of flavonoids by disrupting the chemical bonds of polysaccharides in cell walls through high pressure, elevated temperature, and shear forces generated during sonication (Chen et al., 2017). To evaluate the effect of ultrasonic time (1, 2, 3, 4, and 5 h) on extraction yield, experiments were conducted under fixed conditions: an ultrasonic temperature of 50 °C, ultrasonic power of 350 W, and ethanol concentration of 60%. As shown in Figure 1D, the maximum flavonoid yield of 3.20 ± 0.02 mg/g was achieved when the ultrasonic time was set to 4 h. Beyond this point, the extraction yield decreased significantly. This decline can be attributed to the fact that although the amount of dissolved flavonoids increases gradually with extraction time (Xue H. et al., 2022), the rate of increase becomes negligible after 3 h, as most flavonoids have already been extracted. Prolonged exposure beyond 4 h may lead to structure degradation of the flavonoids, resulting in a reduced yield.
Optimization of flavonoid extraction conditions by response surface methodology
Statistical analysis and the model fitting
According to the principles of the Box-Behnken Design (BBD) in central composite experimentation, a total of 17 experimental groups were designed using Design-expert 8.0.6 software. The levels and corresponding results of the independent variables are summarized in Table 2. The extraction yield ranged from 2.58% to 3.81%, with the maximum yield achieved under the conditions of 60% ethanol concentration, 50 °C ultrasonic temperature, and 400 W ultrasonic power. A second-order polynomial regression equation was established to describe the relationship between the three independent variables and the response variable as follows:
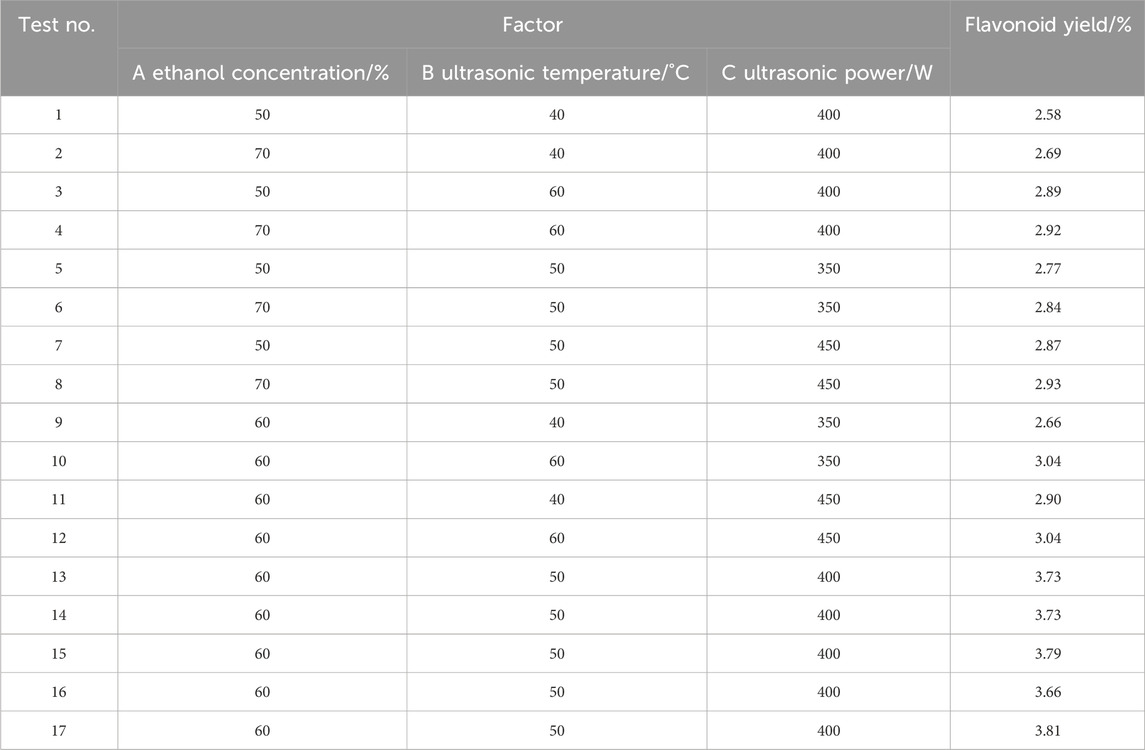
Table 2. Box-Behnken experimental design and results of flavonoids extracted from Potentilla anserine L.
Where Y represents the flavonoid yield (%) from Potentilla anserine L.; X1, X2 and X3 denote the coded values of ethanol concentration (%), ultrasonic temperature (°C), and ultrasonic power (W), respectively.
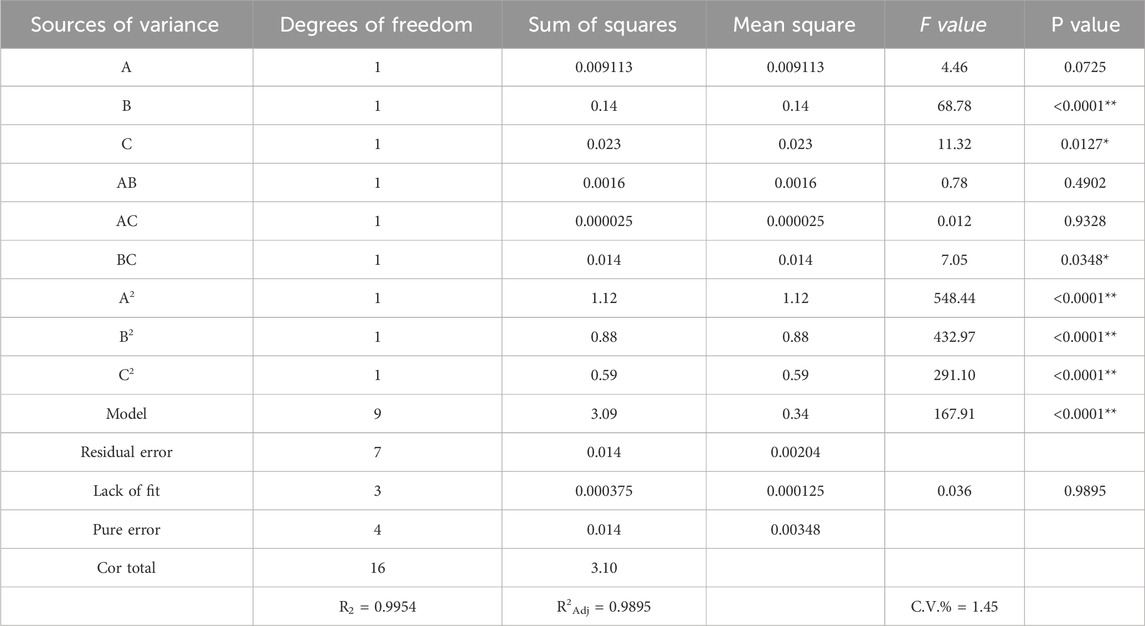
The results of the analysis of variance (ANONA) are presented in Table 3. The model exhibited a high F-value (167.91) and a low P-value (<0.0001), indicating that it is statistically significant and reliable. The coefficient of determination (R2) was 0.9954, meaning that 99.54% of the variation in the response can be explained by the model, while only 0.46% remains unexplained. The adjusted R2 value (R2Adj = 0.9895), which is close to R2, further confirms that the model fits the experimental data well and demonstrates a strong correlation between the observed and predicted values (Li et al., 2020). Additionally, the low coefficient of variation (C.V. = 1.45%) indicates high precision and reliability of the model. As shown in Table 3, among the factors included in the regression equation, the linear term X2 showed an extremely significant effect, X3 was significant, followed by the interaction term X2X3, which was also significant. The quadratic terms X12, X22, and X32 were all extremely significant. The order of influence on the flavonoid extraction yield from Potentilla anserina L. was determined as X2 > X3 > X1, indicating that ultrasonic temperature had the greatest impact on the extraction rate., followed by ultrasonic power, and finally ethanol concentration (Liu et al., 2019).
Response surface and contour plot analyses of the extracted Potentilla anserina L. flavonoids
The three-dimensional response surfaces and corresponding contour plots are presented in Figure 2. Each subplot illustrates the effects interaction of two variables on the extraction yield of Potentilla anserina L. flavonoids by displaying both the response surface and the contour plot. The influence of various factors on the response is hierarchical, with the intensity of the contour lines in the plots and the steepness of the response surfaces clearly reflecting the degree of impact. Closely spaced isoclines indicate a steeper response surface and a more significant effect, whereas wider intervals between isoclines suggests a relatively minor influence (Yuan et al., 2020). Among all the tested factors, ultrasonic temperature exhibited the most pronounced effect on the extraction yield of flavonoids, as evidenced by the steepest gradient observed in its 3D curve, followed by ultrasonic power and then ethanol concentration. This finding is consistent with the results of the Analysis of Variance (ANOVA).
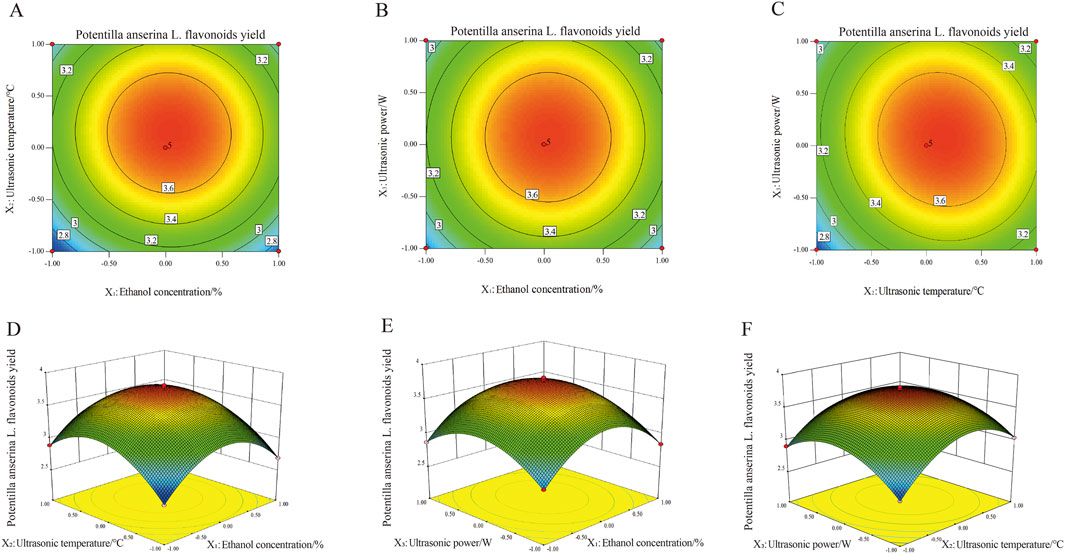
Figure 2. Effect of mutual interaction of independent variables on Potentilla anserine L. flavonoids yield ((A–C) represent contour maps. (D–F) represent 3D response surface plots).
Validation of the predictive model
Based on Response Surface Methodology (RSM), the optimal extraction conditions for flavonoids from Potentilla anserina L. were determined as follows: ethanol concentration of 60.29%, ultrasonic power of 403.02 W, ultrasonic temperature of 51.4 °C, and ultrasonic time of 3 h. The theoretical yield was predicted to be 3.7554 mg/g, and the experimental obtained yield was 3.74 ± 0.06 mg/g. These results indicate that the model exhibits both good fit and predictive accuracy.
Sephadex G-100 gel chromatography analysis
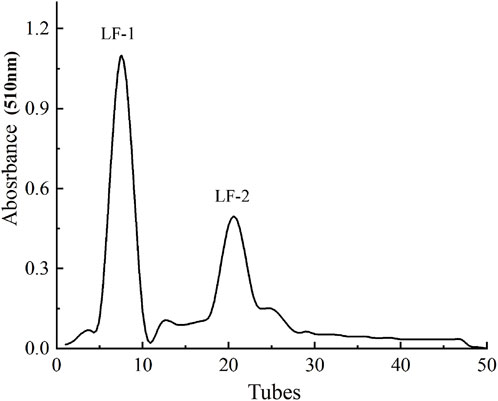
As shown in Figure 3, after Sephadex G-100 gel chromatography, the flavonoids extracted from Potentilla anserina L. were preliminarily separated into two fractions based on molecular weight, designated as LF-1 and LF-2, with relative proportions of 63.34% and 25.79%, respectively. The yields of the collected fractions LF-1 and LF-2 were 41.32% and 34.56%, respectively, which represent a significant increase compared to the yields before purification.
Antioxidant activity analysis
Analysis of DPPH radical scavenging activity
Flavonoids contain multiple hydroxyl groups, most of which are capable of donating hydrogen to reduce the DPPH radical (Xu et al., 2020). The DPPH radical scavenging assay is a widely used method for the quantitative evaluation of antioxidant capacity in vitro. As shown in Figure 4A, the DPPH scavenging activity of LF-1 increased from 47.85% to 83.04%, and that of LF-2 increased from 33.98% to 75.55%, as the concentration rose from 0.1 to 0.5 mg/mL. This indicates that the DPPH free radical scavenging ability of flavonoids from Potentilla anserina L. is concentration-dependent. The reduction of the DPPH radical occurs through either hydrogen atom transfer (HAT) or single electron transfer (SET) mechanisms when antioxidants interact with it, leading to its transformation into a stable diamagnetic species (Tunnisa et al., 2022). This redox process results in a distinct hypsochromic shift in the visible spectrum of the solution, manifested as a color change from characteristic purple to pale yellow. The intensity of this color transition is quantitatively correlated with the antioxidant’s radical-neutralizing capacity of the antioxidant (Mesmar et al., 2022). Furthermore, the half-maximal inhibitory concentrations (IC50) were determined as 0.120 mg/mL for LF-1, 0.400 mg/mL for LF-2, and 0.11 mg/mL for the positive control ascorbic acid (VC). These results demonstrate that LF-1 exhibits a significant scavenging effect on DPPH free radicals.
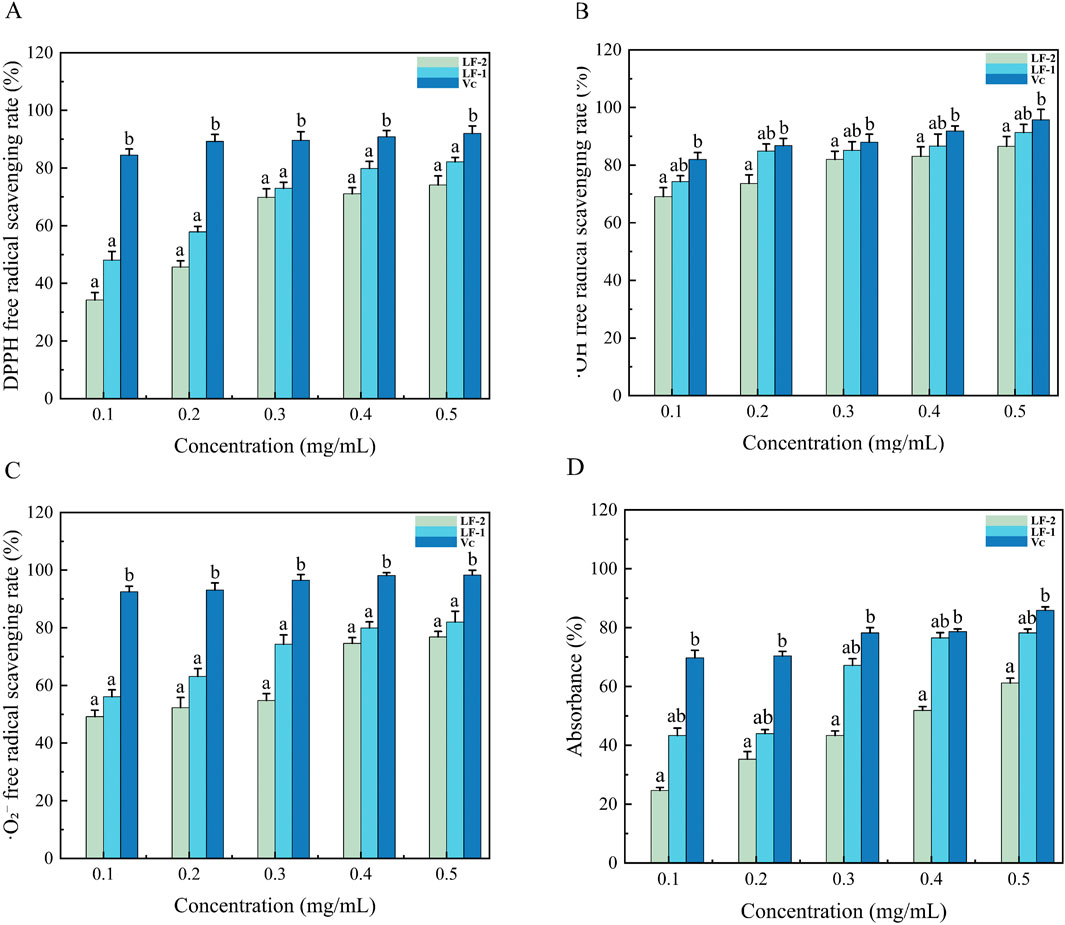
Figure 4. (A) Scavenging effect of Potentilla anserine L. on DPPH radicals compared with VC, (B) ·OH radicals compared with VC, (C) O2- radicals compared with VC, and (D)total reducing ability compared with VC.
Analysis of hydroxyl (OH) radical scavenging activity
Hydroxyl radicals are highly reactive species that are detrimental to biological systems and capable of attacking and damaging living cells (Wang et al., 2020). Flavonoids can act as electron or hydrogen donors to neutralize hydroxyl radicals. They not only inhibit the formation of OH radicals but also scavenge existing OH radicals, demonstrating significant in vitro antioxidant activity (Che et al., 2023). The OH radical scavenging capacity of flavonoids from Potentilla anserina L. is presented in Figure 4B. Within the concentration the range of 0.1–0.5 mg/mL, the scavenging ability increased progressively. Notably, at a concentration of 0.5 mg/mL, LF-1 reached a maximum scavenging rate of 90.60% ± 0.57%, while LF-2 reached 86.15 ± 0.84%, indicating a concentration-dependent scavenging effect of Potentilla anserina L. flavonoids against hydroxyl radicals. The half-maximal inhibitory concentrations (IC50) were determined as 0.019 mg/mL for LF-1 and 0.032 mg/mL for LF-2, compared to 0.013 mg/mL for the positive control ascorbic acid (VC). The OH group scavenging ability of these flavonoids may be attributed to either the presence of hydroxyl groups in their molecular structures or the influence of electron density around heterocyclic carbon atoms.
Analysis of superoxide anion (O2−) radical scavenging activity
The superoxide anion radical, a precursor of more reactive oxygen species (ROS), plays a key role in the pathogenesis of various oxidative stress-related disorders (Du et al., 2022; Lai et al., 2009). As shown in Figure 4C, flavonoids isolated from Potentilla anserina L. exhibited significant scavenging activity against the superoxide anion radical (·O2−). The scavenging capacity of these flavonoids increased in a concentration-dependent manner within the range of 0.1–0.5 mg/mL range. At a concentration of 0.4 mg/mL, the inhibition rates of flavonoid fractions LF-1 and LF-2 were comparable to that of the ascorbic acid (VC) positive control. At the maximal scavenging efficiency observed at 0.5 mg/mL, the half-maximal inhibitory concentration (IC50) values were determined to be 0.081 mg/mL for LF-1, 0.012 mg/mL for ascorbic acid (VC), and 0.139 mg/mL for LF-2. Although HF1 demonstrated stronger in vitro antioxidant activity than LF-2, its efficacy was still lower than that of VC. Nevertheless, LF-1 exhibited potent superoxide radical scavenging activity, indicating its potential as a functional antioxidant agent for preventing oxidative stress-induced cellular damage (Zhang et al., 2022).
Analysis of total reducing ability
The total reducing power serves as a key indicator for assessing the redox activity of flavonoids. The reducing agent can scavenges free radicals through its inherent reductive capacity, reduce Fe3+ to Fe2+, and subsequently reacts with FeCl3 (Yuan et al., 2022). As shown in Figure 4D, a clear dose-dependent relationship exists between the reducing capacity and the concentration of the total flavonoid extract. Higher flavonoid concentrations correspond to stronger reducing abilities. When the concentration reaches 5 mg/mL, the reducing ability of LF-2 is most comparable to that of ascorbic acid, and the absorbance attains its maximum.
Discussion
With the continuous improvement of modern consumers’ health awareness and ongoing advancements in food science research, concerns regarding potential health risks associated with synthetic food additives have gained increasing attention. In this context, the development of biologically safe, environmentally friendly, and highly efficacious natural-source antioxidants has become a key research focus in the field of food science and technology. This study investigates the feasibility of extracting flavonoids from Potentilla anserina L. for use as antioxidants, offering novel insights into the application of natural products in functional foods.
Natural products are secondary metabolites produced by organisms during evolution as adaptive responses to environmental pressures, resulting in diverse chemical structures and distinct biological functions. Flavonoids exhibit pharmacological activities—including antioxidant activity, immunomodulatory effects, anti-inflammatory properties, and cardiovascular protection—mediated by their characteristic benzene ring structures and specific hydroxyl substitution patterns. These structural features allow flavonoids to directly scavenge free radicals by donating hydrogen atoms or electrons, as well as inhibit oxidative chain reactions through metal ion chelation (Park and Han, 2022; Zhang et al., 2017). Compared with conventional extraction methods, ultrasound-assisted extraction has demonstrated advantages such as operational simplicity, shorter extraction time, higher efficiency, and the absence of a heating requirement, indicating significant potential for the extraction of bioactive components from plants materials. The cavitation effect induced by ultrasound disrupts plant cell walls, facilitates the release of flavonoids, and concurrently prevents thermal destruction of active constituents (Guo et al., 2022; Liu et al., 2022; Evary et al., 2024). In this study, the yield of flavonoids extracted from Potentilla anserina L. using ultrasound assistance was determined to be 3.74 ± 0.06 mg/g. This value was observed to be higher than that 2.20 ± 0.257 mg/g reported by Yan et al. for the stems and leaves of Astragalus membranaceus (Cui et al., 2022).
The main factors influencing the extraction yield of flavonoids include the concentration of the extraction solvent, ultrasonic temperature, ultrasonic power, and ultrasonic time. Ethanol is considered an ideal solvent for flavonoid extraction due to its dual polar and non-polar solubilization properties: its molecules can form hydrogen bonds with the polar groups of flavonoids while simultaneously dissolving non-polar structures, thereby aligning with the “like dissolves like” principle (Cui et al., 2022). Ultrasonic temperature affects extraction yield by altering solvent properties, influencing the cavitation effect, and impacting component stability. Within the range of 40 °C–60 °C, increasing temperature enhances molecular mobility; however, temperatures above 60 °C accelerate solvent volatilization and reduce the stability of cavitation bubbles, ultimately decreasing extraction efficiency (Liu et al., 2016). The impact of ultrasonic power on extraction yield follows a trend of initial increase followed by a decrease: low power facilitates cell disruption through the cavitation effect, whereas high power may lead to flavonoid degradation due to excessive heat generation. Regarding ultrasonic time, the extraction yield increases with time up to 180 min; beyond this point, the degradation rate of flavonoids surpasses the dissolution rate, resulting in no further improvement in yield (Liao et al., 2022). Response surface methodology (RSM) is utilized to model the nonlinear relationship between extraction yield and key variables such as ethanol concentration, ultrasonic time, and temperature using a multiple regression approach. This method enables efficient exploration of multi-factor combinations with fewer experimental trials, allowing precise identification of optimal conditions. The optimized extraction process determined in this study comprises an ethanol concentration of 60%, ultrasonic temperature of 50 °C, power of 400 W, and time of 180 min. The strong agreement between the model’s predicted values and the experimentally measured results confirms the effectiveness of this process optimization (Kocer et al., 2024; Md Yusof et al., 2019; Zhang et al., 2019).
High-resolution purification of flavonoids from Potentilla anserina L. was achieved through stepwise optimization of sample loading amount, elution gradient, and column parameters using Sephadex Gel Chromatography. Following fractionation on a Sephadex G-100 chromatography column, two distinct fractions LF-1 and LF-2 were obtained, representing 63.34% and 25.79% of the crude flavonoid content, respectively. Compared to the crude extract, the in vitro antioxidant activities of the purified fractions were markedly enhanced, likely due to the enrichment of active components and elimination of impurities (Zhang et al., 2024; Wang et al., 2023; Oteef, 2022). Excessive accumulation of free radicals can initiate oxidative stress cascade reactions, resulting in lipid peroxidation, organ senescence, and the development of various diseases. Supplementation with exogenous antioxidants may synergistically eliminate excessive free radicals and help restore redox homeostasis. In this study, both LF-1 and LF-2 exhibited concentration-dependent scavenging capacities against DPPH, hydroxyl, and superoxide anion radicals, along with significant total reducing power. Notably, LF-1 consistently exhibited higher in vitro antioxidant activity than LF-2, likely due to a higher proportion of flavonoid aglycones with ortho-diphenolic hydroxyl groups or C-ring unsaturated double bonds. Subsequently, we will perform in-depth compositional analysis (HPLC, LC-MS) to verify this for LF-1 and LF-2.
This study has demonstrated that flavonoids extracted from Potentilla anserina L., particularly the LF-1 fraction, possess potent and broad-spectrum in vitro antioxidant activities, thereby providing a scientific basis for the high-value utilization of Potentilla anserina L. resources. Nevertheless, further research is required to clarify the structural characteristics of the specific active components, their in vivo metabolic pathways, and their stability under food industry application conditions. In future studies, flavonoid research can be conducted by using metabolomics to track their metabolic pathways in organisms, through which the patterns of active metabolites can be elucidated. This can be followed by molecular docking to analyze the binding mechanisms between metabolites and target proteins, thereby clarifying the structure-activity relationships and overcoming the limitations of focusing solely on the activity of native components.
This study is entirely based on in vitro experiments and has not undergone in vivo validation. The flavonoid components have not been subjected to in-depth characterisation via methods such as high-performance liquid chromatography (HPLC) or liquid chromatography-mass spectrometry (LC-MS). Subsequent experiments will undertake detailed chemical characterisation of the two components, LF-1 and LF-2. Findings from in vitro antioxidant studies cannot be directly extrapolated to physiological efficacy; animal studies will be conducted subsequently to provide in vivo validation.
Conclusion
Potentilla anserina L. flavonoids were extracted by ultrasonic-assisted extraction. A Potentilla anserina L. flavonoids yield of 3.74 ± 0.06 mg/g was obtained at the optimized conditions: ethanol concentration 60%, ultrasonic power 400 W, ultrasonic temperature 50 °C, ultrasonic time 3 h. The Potentilla anserine L. flavonoids were preliminarily separated Sephadex G-100 chromatography, and two flavonoids’ components LF-1 and LF-2 were obtained. Between these two fractions, LF-1 was found to exhibit stronger in vitro antioxidant activity and significant total reducing capacity. The results suggested that Potentilla anserine L. flavonoids have potential application as natural antioxidant and food ingredients in functional food.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
DG: Methodology, Writing – original draft. XY: Data curation, Formal Analysis, Investigation, Writing – original draft. DL: Data curation, Writing – review and editing. CW: Formal Analysis, Writing – review and editing. JW: Investigation, Writing – review and editing. HL: Writing – review and editing. XG: Writing – review and editing.
Funding
The authors declare that financial support was received for the research and/or publication of this article. This research was supported by the Fundamental Research Funds for the Central Universities of Northwest Minzu University (31920250026), the Key Talent Project of Gansu Province (2024RCXM45), the science and technology talent innovation project of Lanzhou (2025-2-74), Gansu Provincial Key Research and Development Program Project (24YFFA026), the Longyuan young Talents Project, and the “Innovative Star” Project for Postgraduates in Gansu Provincial Universities (2025CXZX-239).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Binkowska, I. (2020). Hesperidin: synthesis and characterization of bioflavonoid complex. SN Appl. Sci. 2 (3), 445. doi:10.1007/s42452-020-2256-8
Chen, L., Sun, J., Pan, Z., Lu, Y., Wang, Z., Yang, L., et al. (2023). Analysis of chemical constituents of Chrysanthemum morifolium extract and its effect on postprandial lipid metabolism in healthy adults. Molecules 28 (2), 579. doi:10.3390/molecules28020579
Chen, Y., Du, X.-J., Zhang, Y., Liu, X.-H., and Wang, X.-D. (2017). Ultrasound extraction optimization, structural features, and antioxidant activity of polysaccharides from Tricholoma matsutake. J. Zhejiang University-SCIENCE B 18 (8), 674–684. doi:10.1631/jzus.B1600239
Chen, H., Shi, X., Zhang, L., Yao, L., Cen, L., Li, L., et al. (2022). Ultrasonic extraction process of polysaccharides from Dendrobium nobile Lindl.: optimization, physicochemical properties and anti-inflammatory activity. Foods 11 (19), 2957. doi:10.3390/foods11192957
Čižmárová, B., Hubková, B., Tomečková, V., and Birková, A. (2023). Flavonoids as promising natural compounds in the prevention and treatment of selected skin diseases. Int. J. Mol. Sci. 24 (7), 6324. doi:10.3390/ijms24076324
Cui, L., Ma, Z., Wang, D., and Niu, Y. (2022). Ultrasound-assisted extraction, optimization, isolation, and antioxidant activity analysis of flavonoids from Astragalus membranaceus stems and leaves. Ultrason. Sonochemistry 90, 106190. doi:10.1016/j.ultsonch.2022.106190
Du, T., Xu, J., Zhu, S., Yao, X., Guo, J., and Lv, W. (2022). Effects of spray drying, freeze drying, and vacuum drying on physicochemical and nutritional properties of protein peptide powder from salted duck egg white. Front. Nutr. 9, 1026903. doi:10.3389/fnut.2022.1026903
Evary, Y. M., Alam, G., Raihan, M., and Khotimah, K. (2024). Extraction of flavonoids from parasitic plant Macrosolen cochinchinensis using ultrasound-assisted extraction: an optimization approach. J. Exp. Biol. Agric. Sci. 12 (4), 616–624. doi:10.18006/2024.12(4).616.624
Franco, E. P. D. d., Contesini, F. J., Lima da Silva, B., Alves de Piloto Fernandes, A. M., Wielewski Leme, C., Gonçalves Cirino, J. P., et al. (2019). Enzyme-assisted modification of flavonoids from matricaria chamomilla: antioxidant activity and inhibitory effect on digestive enzymes. J. Enzyme Inhibition Med. Chem. 35 (1), 42–49. doi:10.1080/14756366.2019.1681989
Goswami, S., Das, R., Ghosh, P., Chakraborty, T., Barman, A., and Ray, S. (2020). Comparative antioxidant and antimicrobial potentials of leaf successive extract fractions of poison bulb, Crinum asiaticum L. Industrial Crops Prod. 154, 112667. doi:10.1016/j.indcrop.2020.112667
Guo, L., Kong, N., Zhang, X., and Ma, H. (2022). Multimode ultrasonic extraction of polysaccharides from maca (lepidium meyenii): optimization, purification, and in vitro immunoregulatory activity. Ultrason. Sonochemistry 88, 106062. doi:10.1016/j.ultsonch.2022.106062
Guo, P., Chen, H., Ma, J., Zhang, Y., Chen, H., Wei, T., et al. (2023). Enzyme-assisted extraction, characterization, and in vitro antioxidant activity of polysaccharides from Potentilla anserina L. Front. Nutr. 10, 1216572. doi:10.3389/fnut.2023.1216572
Huang, Y., Guo, J., and Zhang, J. (2020). Physicochemical and antioxidant properties of Potentilla anserina L. polysaccharides affected by ultrasonication. Appl. Sci. 10 (13), 4510. doi:10.3390/app10134510
Kim, T. Y., Leem, E., Lee, J. M., and Kim, S. R. (2020). Control of reactive oxygen species for the prevention of parkinson’s disease: the possible application of flavonoids. Antioxidants 9 (7), 583. doi:10.3390/antiox9070583
Kocer, S., Utku Copur, O., Ece Tamer, C., Suna, S., Kayahan, S., Uysal, E., et al. (2024). Optimization and characterization of chestnut shell pigment extract obtained microwave assisted extraction by response surface methodology. Food Chem. 443, 138424. doi:10.1016/j.foodchem.2024.138424
Lai, J.-T., Wang, W.-C., Liao, C.-M., Huang, T.-C., and Liao, C.-C. (2009). Cultivation of Aspergillus phoenicis with high superoxide dismutase-like activity. J. Taiwan Inst. Chem. Eng. 40 (5), 485–490. doi:10.1016/j.jtice.2009.02.005
Li, W., Wang, Y., Wei, H., Zhang, Y., Guo, Z., Qiu, Y., et al. (2020). Structural characterization of Lanzhou lily (Lilium davidii var. unicolor) polysaccharides and determination of their associated antioxidant activity. J. Sci. Food Agric. 100 (15), 5603–5616. doi:10.1002/jsfa.10613
Liang, Y., Farooq, M. U., Hu, Y., Tang, Z., Zhang, Y., Zeng, R., et al. (2018). Study on stability and antioxidant activity of red anthocyanidin glucoside rich hybrid rice, its nutritional and physicochemical characteristics. Food Sci. Technol. Res. 24 (4), 687–696. doi:10.3136/fstr.24.687
Liao, J., Guo, Z., and Yu, G. (2021). Process intensification and kinetic studies of ultrasound-assisted extraction of flavonoids from peanut shells. Ultrason. Sonochemistry 76, 105661. doi:10.1016/j.ultsonch.2021.105661
Liao, J., Xue, H., and Li, J. (2022). Extraction of phenolics and anthocyanins from purple eggplant peels by multi-frequency ultrasound: effects of different extraction factors and optimization using uniform design. Ultrason. Sonochemistry 90, 106174. doi:10.1016/j.ultsonch.2022.106174
Liu, J.-L., Li, L.-Y., and He, G.-H. (2016). Optimization of microwave-assisted extraction conditions for five major bioactive compounds from Flos sophorae immaturus (cultivars of Sophora japonica L.) using response surface methodology. Molecules 21 (3), 296. doi:10.3390/molecules21030296
Liu, L., Luo, P., and Xiao, J. (2019). Effect of carboxymethylation and phosphorylation on the properties of polysaccharides from Sepia esculenta ink: antioxidation and anticoagulation in vitro. Mar. Drugs 17 (11), 626. doi:10.3390/md17110626
Liu, Y., Zhang, D., Li, X., Xiao, J., and Guo, L. (2022). Enhancement of ultrasound-assisted extraction of sulforaphane from broccoli seeds via the application of microwave pretreatment. Ultrason. Sonochemistry 87, 106061. doi:10.1016/j.ultsonch.2022.106061
Luan, G., Yang, M., Nan, X., Lv, H., Liu, Q., Wang, Y., et al. (2022). Optimization and comparative study of different extraction methods of sixteen fatty acids of Potentilla anserina L. from twelve different producing areas of the Qinghai-Tibetan Plateau. Molecules 27 (17), 5443. doi:10.3390/molecules27175443
Md Yusof, A. H., Abd Gani, S. S., Zaidan, U. H., Halmi, M. I. E., and Zainudin, B. H. (2019). Optimization of an ultrasound-assisted extraction condition for flavonoid compounds from cocoa shells (Theobroma cacao) using response surface methodology. Molecules 24 (4), 711. doi:10.3390/molecules24040711
Mesmar, J., Abdallah, R., Hamade, K., Baydoun, S., Al-Thani, N., Shaito, A., et al. (2022). Ethanolic extract of Origanum syriacum L. leaves exhibits potent anti-breast cancer potential and robust antioxidant properties. Front. Pharmacol. 13, 994025. doi:10.3389/fphar.2022.994025
Michalaki, A., Karantonis, H. C., Kritikou, A. S., Thomaidis, N. S., and Dasenaki, M. E. (2023). Ultrasound-assisted extraction of total phenolic compounds and antioxidant activity evaluation from oregano (Origanum vulgare ssp. hirtum) using response surface methodology and identification of specific phenolic compounds with HPLC-PDA and Q-TOF-MS/MS. Molecules 28 (5), 2033. doi:10.3390/molecules28052033
Nie, Z., Wan, C., Chen, C., and Chen, J. (2019). Comprehensive evaluation of the postharvest antioxidant capacity of Majiayou Pomelo harvested at different maturities based on PCA. Antioxidants 8 (5), 136. doi:10.3390/antiox8050136
Oteef, M. D. Y. (2022). Comparison of different extraction techniques and conditions for optimizing an HPLC-DAD method for the routine determination of the content of chlorogenic acids in green coffee beans. Separations 9 (12), 396. doi:10.3390/separations9120396
Park, J.-E., and Han, J.-S. (2022). HM-Chromanone, a major homoisoflavonoid in Portulaca oleracea L., improves palmitate-induced insulin resistance by regulating phosphorylation of IRS-1 residues in L6 skeletal muscle cells. Nutrients 14 (18), 3815. doi:10.3390/nu14183815
Tang, C., Li, X., Chen, J., Liang, J., Wang, T., and Li, Y. (2022). Characterization and phylogenetic relationship of the complete chloroplast genome of a Chinese traditional medicinal plant Potentilla anserina L. Mitochondrial DNA Part B 7 (9), 1653–1655. doi:10.1080/23802359.2022.2119817
Tang, Z., Wang, Y., Huang, G., and Huang, H. (2023). Ultrasound-assisted extraction, analysis and antioxidant activity of polysaccharide from the rinds of Garcinia mangostana L. Ultrason. Sonochemistry 97, 106474. doi:10.1016/j.ultsonch.2023.106474
Tunnisa, F., Nur Faridah, D., Afriyanti, A., Rosalina, D., Ana Syabana, M., Darmawan, N., et al. (2022). Antioxidant and antidiabetic compounds identification in several Indonesian underutilized Zingiberaceae spices using SPME-GC/MS-based volatilomics and in silico methods. Food Chem. X 14, 100285. doi:10.1016/j.fochx.2022.100285
Wang, M., Lei, M., Samina, N., Chen, L., Liu, C., Yin, T., et al. (2020). Impact of Lactobacillus plantarum 423 fermentation on the antioxidant activity and flavor properties of rice bran and wheat bran. Food Chem. 330, 127156. doi:10.1016/j.foodchem.2020.127156
Wang, Y.-F., Lin, P., Huang, Y.-L., He, R.-J., Yang, B.-Y., and Liu, Z.-B. (2023). Isolation of two new phenolic glycosides from Castanopsis chinensis hance by combined multistep CC and HSCCC separation and evaluation of their antioxidant activity. Molecules 28 (8), 3331. doi:10.3390/molecules28083331
Wu, M., Liu, P., Wang, S., Zhong, C., and Zhao, X. (2021). Ultrasonic microwave-assisted micelle combined with fungal pretreatment of Eucommia ulmoides leaves significantly improved the extraction efficiency of total flavonoids and gutta-percha. Foods 10 (10), 2399. doi:10.3390/foods10102399
Xu, M., Shen, C., Zheng, H., Xu, Y., Xue, C., Zhu, B., et al. (2020). Metabolomic analysis of acerola cherry (Malpighia emarginata) fruit during ripening development via UPLC-Q-TOF and contribution to the antioxidant activity. Food Res. Int. 130, 108915. doi:10.1016/j.foodres.2019.108915
Xue, Y., Wang, F., and Zhou, C. (2022). Optimization of ultrasonic extraction of triterpenes from loquat peel and pulp and determination of antioxidant activity and triterpenoid components. Foods 11 (17), 2563. doi:10.3390/foods11172563
Xue, H., Li, J., Wang, G., Zuo, W., Zeng, Y., and Liu, L. (2022). Ultrasound-assisted extraction of flavonoids from Potentilla fruticosa L. using natural deep eutectic solvents. Molecules 27 (18), 5794. doi:10.3390/molecules27185794
Yang, D., Wang, L., Zhai, J., Han, N., Liu, Z., Li, S., et al. (2021). Characterization of antioxidant, α-glucosidase and tyrosinase inhibitors from the rhizomes of Potentilla anserina L. and their structure–activity relationship. Food Chem. 336, 127714. doi:10.1016/j.foodchem.2020.127714
Yu, G., Yu, X., Yang, G., Tang, Y., and Diao, Y. (2018). A novel diagnostic method to detect duck tembusu virus: a colloidal gold-based immunochromatographic assay. Front. Microbiol. 9, 1001. doi:10.3389/fmicb.2018.01001
Yuan, S., Xu, C. Y., Xia, J., Feng, Y. N., Zhang, X. F., and Yan, Y. Y. (2020). Extraction of polysaccharides from Codonopsis pilosula by fermentation with response surface methodology. Food Sci. and Nutr. 8 (12), 6660–6669. doi:10.1002/fsn3.1958
Yuan, T., Huang, J., Gan, L., Chen, L., Zhong, J., Liu, Z., et al. (2022). Ultrasonic enhancement of aqueous two-phase extraction and acid hydrolysis of flavonoids from Malvaviscus arboreus cav. Flower for evaluation of antioxidant activity. Antioxidants 11 (10), 2039. doi:10.3390/antiox11102039
Zhang, X., Li, H., Zhang, H., Liu, Y., Huo, L., Jia, Z., et al. (2017). Inhibition of transmembrane member 16A calcium-activated chloride channels by natural flavonoids contributes to flavonoid anticancer effects. Br. J. Pharmacol. 174 (14), 2334–2345. doi:10.1111/bph.13841
Zhang, Q.-W., Lin, L.-G., and Ye, W.-C. (2018). Techniques for extraction and isolation of natural products: a comprehensive review. Chin. Med. 13 (1), 20. doi:10.1186/s13020-018-0177-x
Zhang, L., Jiang, Y., Pang, X., Hua, P., Gao, X., Li, Q., et al. (2019). Simultaneous optimization of ultrasound-assisted extraction for flavonoids and antioxidant activity of Angelica keiskei using response surface methodology (RSM). Molecules 24 (19), 3461. doi:10.3390/molecules24193461
Zhang, Y., Bian, S., Hu, J., Liu, G., Peng, S., Chen, H., et al. (2022). Natural deep eutectic solvent-based microwave-assisted extraction of total flavonoid compounds from spent sweet potato (Ipomoea batatas L.) leaves: optimization and antioxidant and bacteriostatic activity. Molecules 27 (18), 5985. doi:10.3390/molecules27185985
Zhang, D., Xiao, D., Yin, T., Zhao, S., Zhur, O., Xiao, X., et al. (2024). The extraction of effective components and an antioxidant activity study of Tulipa edulis. Food Sci. Hum. Wellness 13 (1), 276–286. doi:10.26599/fshw.2022.9250023
Keywords: Potentilla anserina L., ultrasound-assisted extraction, flavonoids, purification, antioxidant activity
Citation: Gao D, Yang X, Lu D, Wang C, Wang J, Li H and Guo X (2025) Extraction, purification and antioxidant activity of flavonoids from Potentilla anserina L. Front. Bioeng. Biotechnol. 13:1691606. doi: 10.3389/fbioe.2025.1691606
Received: 24 August 2025; Accepted: 06 November 2025;
Published: 19 November 2025.
Edited by:
Krist V. Gernaey, Technical University of Denmark, DenmarkReviewed by:
Sen Li, Shanxi Agricultural University, ChinaHasin Hasnat, North Carolina State University, United States
Copyright © 2025 Gao, Yang, Lu, Wang, Wang, Li and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dandan Gao, Z2FvZGFuMDMyMkAxNjMuY29t
†These authors have contributed equally to this work
 Dandan Gao
Dandan Gao Xuankang Yang1,2†
Xuankang Yang1,2† Xingchen Guo
Xingchen Guo