- 1School of Stomatology, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2Hospital of Stomatology, Guanghua School of Stomatology, Sun Yat-sen University, Guangzhou, China
- 3Department of Stomatology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 4Hubei Province Key Laboratory of Oral and Maxillofacial Development and Regeneration, Wuhan, China
- 5Department of Oral Biology and Diagnostic Services, Dental College of Georgia, Augusta University, Augusta, GA, United States
Objectives: To evaluate the clinical effect of immunomodulatory therapy in periodontitis, and to identify the possible key inflammatory factors to intervene to modulate the immune status and improve periodontal conditions.
Materials and methods: An electronic search was conducted for human-based studies published until October 2025 on MEDLINE (PubMed), ISI Web of Science, EMBASE, and the Cochrane Database. Randomized controlled trials (RCTs) comparing the effectiveness of immunotherapy and placebo were included. We also compared cytokine levels between the immunotherapy group and the non-immunotherapy group to identify the specific inflammatory mediators influenced by immunotherapy but not by SRP (Scaling and Root Planning). Meta-analyses with fixed and random effects models were performed. Risk of bias assessment was also performed for randomized controlled trials.
Results: Of the 34 articles selected, 22 were included in the meta-analysis (n = 991). It was found that immunomodulatory therapy improved clinical attachment level (CAL), bleeding from probing (BOP), and depth of probing (PD) in patients with periodontitis. A three-group meta-analysis showed that immunotherapy affected periodontal disease progression by modulating local immune factors IL-1β, IL-17, IL-6, IL-8 and TNF-α, thus providing a potential statistically significant benefit.
Conclusion: Immunotherapy influenced periodontal disease progression through the modulation of local immune factors. The data support the use of immunotherapy as an adjunct to conventional mechanical therapy. Further investigations are warranted to elucidate sources of heterogeneity of the results and examine the potentiality of using inflammatory cytokines as novel targets for the treatment of periodontal disease.
1 Introduction
Periodontitis is a common chronic multifactorial inflammatory disease (Jepsen et al., 2000; Basic and Dahlen, 2023). It is estimated that between 2011 and 2020, periodontitis in dentate adults was estimated to be around 62%, and severe periodontitis 23.6% (Kassebaum et al., 2014; Trindade et al., 2023). And with the rapid growth of the elderly population, periodontal health is becoming increasingly important (Eke et al., 2000). The etiology and mechanism of periodontitis is extremely complex. Periodontitis initiation and progression are related to multiple etiologic and risk factors. However, the most critical in periodontal disease pathogenesis is a reciprocally reinforced interplay between microbial dysbiosis and destructive inflammation. The occurrence and development of periodontitis is the result of the interaction between bacteria and the host. The aim of the treatment of periodontitis is to re-establish and maintain periodontal health and function by limiting the inflammatory process. Currently, the main treatment is mechanical debridement to remove calculus and plaque combined with anti-inflammatory therapy (Slots, 2000a; Albandar, 2000). However, the treatment result is not always satisfactory and stable maintenance (Graziani et al., 2000), periodontitis continues to break down periodontal apparatus and leads to tooth loss in some patients (Grover et al., 2016). How to promote the intrinsic repairing power of the local compromised tissue and re-establish the balance of inflammatory breakdown and regeneration is a challenging issue for both researchers and clinicians.
Local plaque and other stimulating factors affect periodontal homeostasis, and the local immune microenvironment changes (Hajishengallis et al., 2000; Mysak et al., 2014; Demkovych et al., 2023). The dysregulation of local homeostasis is mainly manifested by the intensification of pro-inflammatory processes and the inhibition of repair and regeneration processes. This dysbiosis eventually leads to destructive inflammation and bone resorption. Therefore, the progression of periodontitis can be divided into sequential stages of which periodontitis featuring advanced lesion bone loss (Qin et al., 2017a). Each stage has different immunological characteristics, including distinct distributions of immune cells and cytokines (Qin et al., 2017b; Bostanci et al., 2017). For example, inflammatory stimulation causes local periodontal Th17 cell infiltration and increased IL-17 secretion. After removal of the influence of external factors, implementing immunomodulatory interventions according to the characteristics of the immune microenvironment at different stages may help to maintain tissue homeostasis.
Recently, the applications of immunotherapies in the treatment of periodontitis have been noticed.
Immunotherapy is a treatment method that activates the body’s own immune system through various means to defend against diseases. These methods, including stem cell therapy, targeted drug therapy, microbial therapy, gene therapy, and other therapies, generally used as adjuvant therapy for classical mechanical debridement (Hajishengallis et al., 2000; Nile et al., 2016). For example, Omega-3 fatty acids adjuvant therapy can improve periodontal outcomes, not only by reducing inflammation, but also by limiting bone resorption and antibacterial effects (Chee et al., 2016; Azuma et al., 2022). Although various immune agents have been reported for the treatment of periodontitis, the results were not in exact agreement. Generally, the intervention of the immune factors can further facilitate the effectiveness of classic periodontal therapies, however the specific responding markers and the potential mechanism of immune regulation are still unclear.
In this study, clinical data were analyzed to find the potential targeting cytokines that characterize the local immune microenvironment in periodontitis. A systematic review and meta-analysis of all published clinical data was carried out to illustrate the function and work path of immunomodulatory therapy in the treatment of periodontitis for human application.
2 Materials and methods
The review protocol was specified before the implement of the study and registered in an international database (PROSPERO, registration number CRD42023413355). The protocol was compliant with the Cochrane Handbook (Higgins et al., 2019) and the results were presented following the instructions of the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) statement (Page et al., 2021).
2.1 Population, intervention, comparison, outcome (PICO) question
PICO: In human subjects with any form of periodontitis, does immunotherapy increase the clinical efficacy of periodontal treatment (P: humans with periodontitis; I: immunotherapy; C: not undergoing immunotherapy; O: clinical outcomes (probing depth (PD); clinical attachment level (CAL); bleeding on probing (BOP)) and immunological indices (relevant cytokines’ levels).
2.2 Search strategy
MeSH terms and Boolean operators see Supplementary Material 5 for complete search strategy.
The following electronic databases were searched for pertinent papers: EMBASE, MEDLINE/PubMed, Web of Science, and the Cochrane Database (including the Central Register of Controlled Trials (CENTER)) using a search strategy presented in Appendix 1. A manual search of the lists of the included references and of the table of contents (since 1990) of the Journal of Clinical Periodontology, Journal of Periodontal Research, Journal of Periodontology, Journal of Dental Research, Periodontal 2000 and Journal of Dentistry was also performed. Grey literature was searched interrogating OpenGrey and Greylit. ClinicalTrials.gov and the World Health Organization International Clinical Trials Registry Platform were also evaluated to explore ongoing or completed RCTs meeting the inclusion and exclusion criteria. The ambiguous or incomplete data were obtained by contacting the corresponding researchers. Only manuscripts in English were considered. Conference abstracts were excluded. The last electronic search was performed on 15 October 2025.
2.3 Study selection
Two reviewers (SW.T. and X.Q.) screened all titles and abstracts to remove duplicates. The full texts were further obtained and screened when studies were deemed eligible. Disagreements were resolved by discussion with a third reviewer (B.B.) to achieve a consensus.
2.4 Selection criteria
a. Types of studies: randomized controlled clinical trials with at least a 2-month follow-up calculated from the beginning of the treatment protocol. A shorter follow-up was not considered as it would be unlike to reflect a meaningful difference in treatment response between test and control groups.
b. Participants Types: 1) Studies included more than 10 adults (older than 18-year-old) patients diagnosed with periodontitis; 2) The patients affected with periodontitis were either systemically healthy or systemically compromised.
c. Intervention Types: Test group: with immunotherapy (IgY against P. gingivalis gingipains, hyaluronan gels, melatonin, photodynamic therapy (PDT), probiotics, sub-antimicrobial dose of doxycycline, OZONE, unsaturated fatty acids, etc.). Control group: without immunotherapy.
d. Outcomes: 1) Primary outcome: probing depth (PD), identified as the distance between the gingival margin and the periodontal pocket base. 2) Secondary outcomes: ①quantity of inflammatory biomarkers; ②clinical attachment level (CAL), defined as the distance from the cementoenamel junction to the tip of the periodontal probe; and ③ bleeding on probing (BOP). To be as inclusive as possible, the meta-analysis included data available for the closest time point up to 3 months (Martin-Cabezas et al., 2016).
2.5 Data extraction
Two authors (YB.Z. and X.Q.) independently extracted data. Any disagreements were resolved by discussion with a third reviewer (B.B.) until a consensus was reached. A standardized template developed from the Cochrane Collaboration was used to conduct data extraction. Overall, all the following information was extracted: 1) general information of the studies (including the authors names, year of publication, the region/country where the study was conducted, study period, and study design); 2) characteristics of participants (including ① the total participants’ numbers, age, gender, and numbers of included teeth or sites, ② the included periodontal disease stage and periodontal status inclusion criteria, ③ systemic conditions of the participants (including but not limited to smoking habit, systemic diseases, and long term medication situation), ④ the study groups (treatment of study and control groups \ number of participants or sites per group), and ⑤ outcome measures); 3) treatment modalities (including intervention measures, use of a placebo, and other relevant procedures (pre-treatment administrations and oral hygiene instructions, scaling and root planning (SRP) (Cobb, 2002), and supportive periodontal therapy (SPT) (Manresa et al., 2018))); 4) outcomes of the studies (at baseline, regular follow-up, and the end-of-trial).
In case of missing or blur information, attempts were made to contact the first or corresponding authors. Data was excluded from calculation until definite clarification was available. When the results of a study were published along its follow-up periods, only the longest follow-up was included. If a study was comparing more than two arms, the data from the test group was extracted for meta-analysis (Ramanauskaite et al., 2021).
2.6 Risk of bias (RoB) assessment
The assessment of methodological quality and risk of bias of each included research was conducted in duplicate (YB.Z. and SW.T.). The criteria for risk of bias evaluation were derived from the Cochrane Handbook for Systematic Reviews (Higgins et al., 2019). Each study was judged as at different level of bias (low, moderate, high, or unclear risk) based on the following aspects: 1) sequence generation (selection bias); 2) allocation concealment (selection bias); 3) masking of participants and personnel (performance bias); 4) blinding of outcome assessment (detection bias); 5) incomplete outcome data (attrition bias); 6) selective outcome reporting (reporting bias); and 7) other bias. The risk of bias was categorized as follows:
Low risk of bias (plausible bias unlikely to seriously alter the results) if all domains were at low risk of bias;
Unclear risk of bias (plausible bias that raises some doubt about the results) if one or more domains were at unclear risk of bias;
High risk of bias (plausible bias that seriously weakens confidence in the results) if ≥ 1 domains were at high risk of bias.
The RoB assessment results were presented graphically using Review Manager (Version 5.4 The Cochrane Collaboration).
2.7 Data synthesis
Differences between the immunotherapy and control groups were shown as weighted mean differences (WMD) and 95% confidence interval (CI) for continuous data. Mean differences and standard errors (SD) were inputted for each study. Forest plots were generated to represent WMD and 95% CI of primary and secondary outcomes from all included studies. The number of patients was identified as the unit of analysis. Heterogeneity ranged between 0% and 100% was assessed with χ2 test and I2 test with the lower values representing less heterogeneity. The analyses were performed using Review Manager 5.4 and reported adhered to the Preferred Reporting Items for Systematic Review and Meta-analyses (PRISMA) statement (Moher et al., 2009).
2.8 Assessment of heterogeneity
Statistical heterogeneity of the included studies was assessed with Cochran’s test with a significance threshold of p < 0.1. The quantification of the heterogeneity was calculated with I2 statistic. It represents the percentage of variation attributable to statistical heterogeneity and is categorized as low (25%–50%), moderate (51%–75%), or high (>75%) (Higgins et al., 2003).
2.9 Assessment of reporting biases
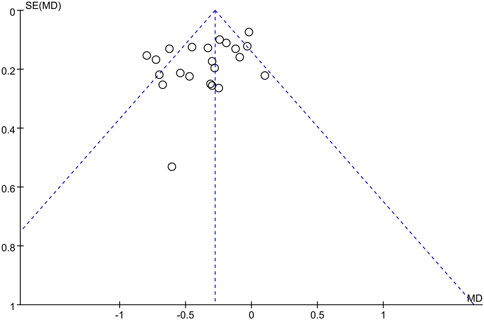
Small-study effects were assessed by testing for funnel plot asymmetry and by calculating Egger’s bias, for publication bias (Higgins et al., 2019). If asymmetry was evident, this was investigated and the possible causes were described.
3 Results
3.1 Study selection
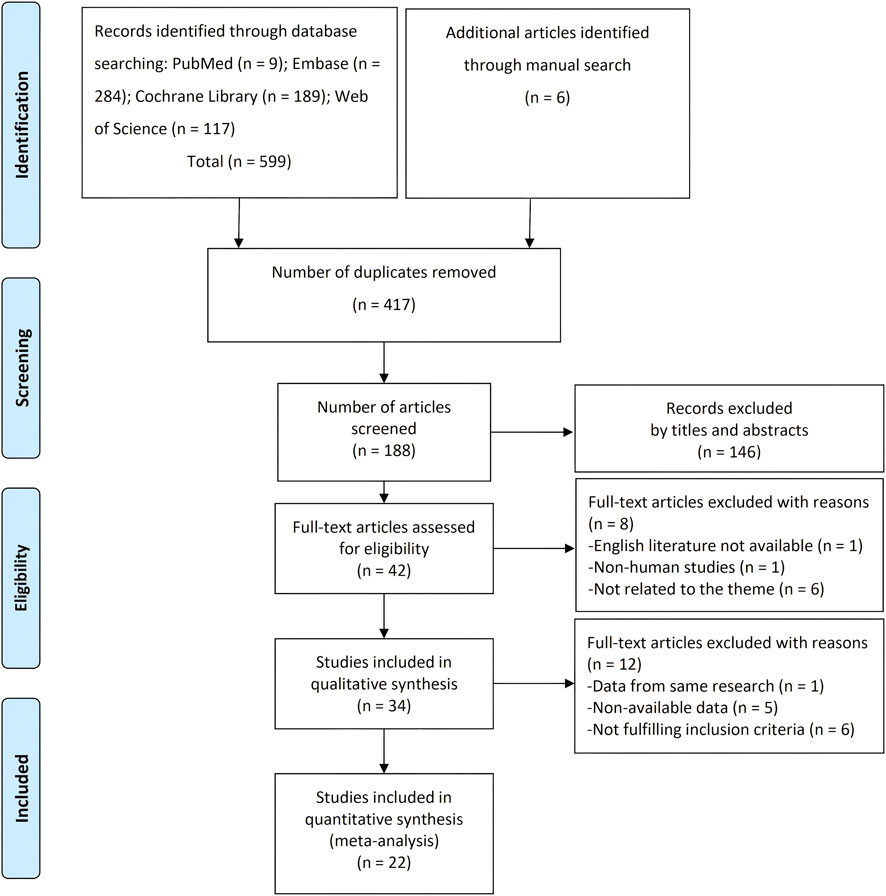
A total of 599 articles were obtained from the database searching, and six additional studies were identified by references. After duplicate removal, 188 records were reviewed. After screening titles and abstracts, 146 records were further excluded. The left 42 articles were assessed by reading the full text. Finally, a total of 34 studies were included in the qualitative synthesis and 22 in the meta-analysis (Booth et al., 1996; Choi et al., 2004; Yokoyama et al., 2007; Bevilacq et al., 2012; Eick et al., 2013; Chitsazi et al., 2014; Deore et al., 2014a; Deore et al., 2014b; El-Sharkawy et al., 2019; El-Sharkawy et al., 2016; Iwasaki et al., 2016; Pradeep et al., 2016; Ramos et al., 2016; Rashidi et al., 2016; Capv et al., 2017; Prakash et al., 2017; AlAhmari et al., 2019; Nędzi-Góra et al., 2020; Zhang et al., 2021; Minić et al., 2022; Talmac et al., 2022; Penala et al., 2016; Pelekos et al., 2019; Keceli et al., 2020; Lecio et al., 2020; Niazi et al., 2020; Qamar et al., 2021; Stańdo et al., 2020; Nguyen et al., 2018; Meghil et al., 2019; Al-Momani, 2021; Gur et al., 2022; Rapone et al., 2022; Sanjay et al., 2025). The 12 excluded studies from the meta-analysis were because of data duplication, non-available data, and not fulfilling inclusion criteria (Figure 1).
3.2 Study characteristics
The characteristics of the included studies are summarized in Table 1. Among them, twelve studies were double-blind, placebo-controlled RCT (Deore et al., 2014a; Deore et al., 2014b; El-Sharkawy et al., 2019; Iwasaki et al., 2016; Pradeep et al., 2016; Prakash et al., 2017; Pelekos et al., 2019; Keceli et al., 2020; Lecio et al., 2020; Nguyen et al., 2018; Meghil et al., 2019; Al-Momani, 2021) and 10 studies were placebo-controlled RCT (Booth et al., 1996; Choi et al., 2004; Bevilacq et al., 2012; Eick et al., 2013; Chitsazi et al., 2014; El-Sharkawy et al., 2016; Talmac et al., 2022; Stańdo et al., 2020; Gur et al., 2022; Rapone et al., 2022). Publication years ranged from 1996 to 2022. The sample sizes ranged from 14 participants to 90 participants. The follow-up period was from 4 weeks to 12 months. Treatments include doxycycline (Choi et al., 2004; Lecio et al., 2020), PDT (Chitsazi et al., 2014; Al-Momani, 2021), omega-3 polyunsaturated fatty acids (Deore et al., 2014b; Stańdo et al., 2020), ozone (Rapone et al., 2022), melatonin (El-Sharkawy et al., 2019), propolis (El-Sharkawy et al., 2016), hyaluronic acid (Bevilacq et al., 2012; Eick et al., 2013), herbal medicine (Deore et al., 2014a; Pradeep et al., 2016; Prakash et al., 2017), folic acid (Keceli et al., 2020), vitamin D (Meghil et al., 2019), laser (Talmac et al., 2022; Gur et al., 2022), probiotics (Iwasaki et al., 2016; Pelekos et al., 2019), antibodies against P. gingivalis (Booth et al., 1996; Nguyen et al., 2018). Twenty studies were controlled against SRP alone or SRP with a placebo, and two studies only applied a placebo without SRP (Iwasaki et al., 2016; Prakash et al., 2017). All studies examined PD, and seventeen examined CAL (Choi et al., 2004; Bevilacq et al., 2012; Eick et al., 2013; Chitsazi et al., 2014; Deore et al., 2014a; Deore et al., 2014b; El-Sharkawy et al., 2019; El-Sharkawy et al., 2016; Pradeep et al., 2016; Talmac et al., 2022; Pelekos et al., 2019; Keceli et al., 2020; Lecio et al., 2020; Stańdo et al., 2020; Al-Momani, 2021; Gur et al., 2022; Rapone et al., 2022), eleven examined BOP (Choi et al., 2004; El-Sharkawy et al., 2019; Iwasaki et al., 2016; Prakash et al., 2017; Talmac et al., 2022; Pelekos et al., 2019; Lecio et al., 2020; Stańdo et al., 2020; Nguyen et al., 2018; Al-Momani, 2021; Rapone et al., 2022). Thirteen studies reported the immune outcomes (Choi et al., 2004; Deore et al., 2014a; Deore et al., 2014b; El-Sharkawy et al., 2019; Prakash et al., 2017; Talmac et al., 2022; Keceli et al., 2020; Lecio et al., 2020; Meghil et al., 2019; Al-Momani, 2021; Gur et al., 2022). No adverse events occurred in all the included studies. Most studies demonstrated that the clinical parameters were notably improved in both groups, while immunotherapy offers a more significant clinical benefit compared to placebo. Nevertheless, some studies suggested that adjunctive immunotherapies only brought similar benefits relative to placebo (Booth et al., 1996; Chitsazi et al., 2014; Pelekos et al., 2019; Meghil et al., 2019).
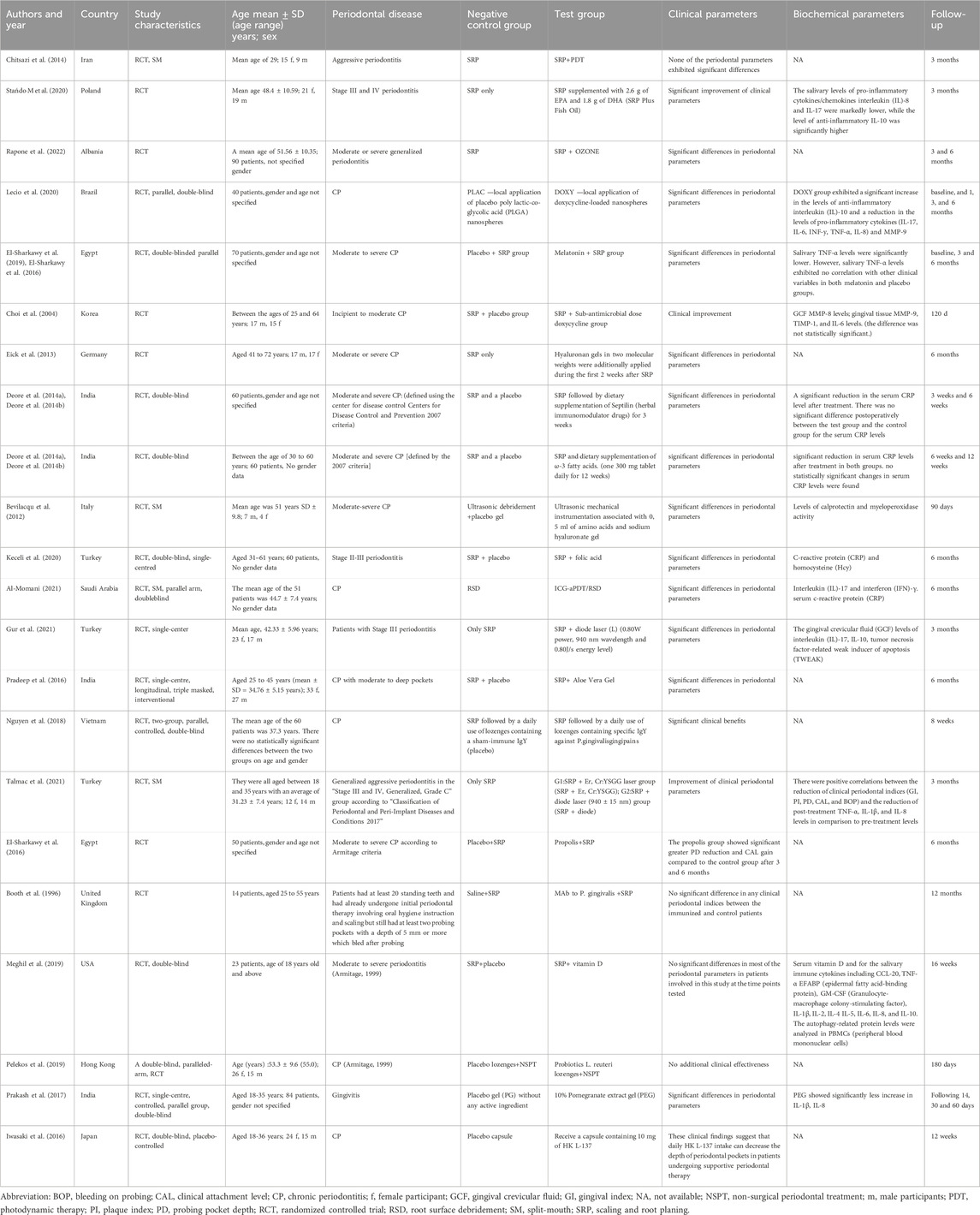
Table 1. The main characteristics of clinical studies related to immunotherapy of periodontital disease.
3.3 Risk of bias
The RoB rating for each study is presented in Figure 2. Overall, 3 studies were classified with high level of RoB due to the absent description of allocation concealment, 12 with unclear RoB because of insufficient method description, and 7 studies were ranked as low RoB (Supplementary Figure S1).
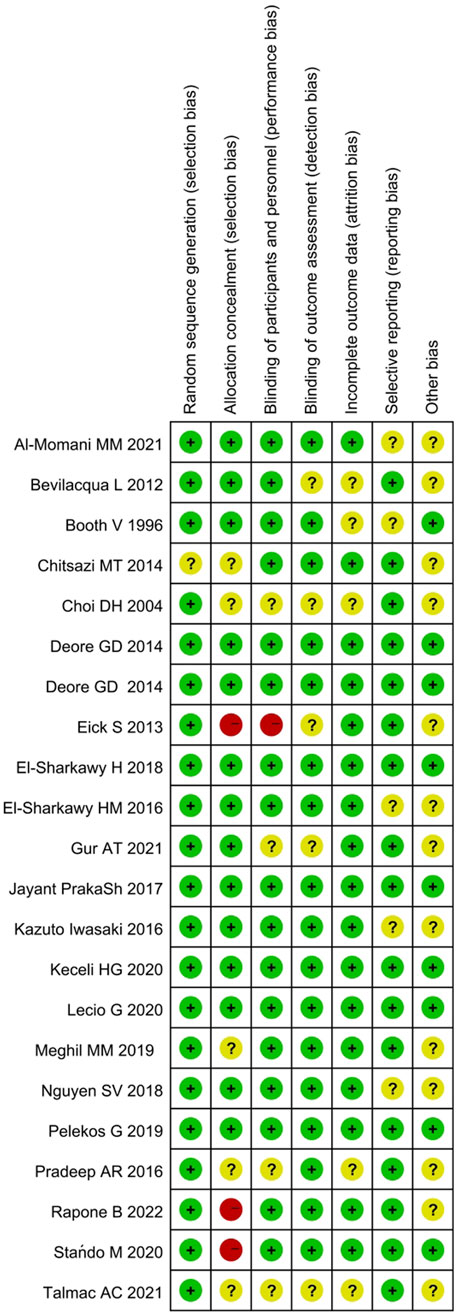
Figure 2. Summary of risk of bias analysis: review authors’ judgment about the different domains for each included study. A green circle (+) indicates a low risk of bias, a yellow circle (?) an unclear risk of bias, and a red circle (−) a high risk of bias in the respective domain.
3.4 Effectiveness
3.4.1 Periodontal pocket depth
Twenty-two studies provided data on the efficacy of immunotherapy on our primary outcome (PD reduction) at 3 months. Substantial statistical heterogeneity across the included studies was identified (I2 = 64%). Meta-analysis showed that the reduction of PD was associated with immunotherapies when compared with placebo therapy (95% CI = −0.45 to 0.22 mm,χ2 = 57.90) (Figure 3). There was no significant publication bias (Figure 4). Subgroup analyses were thus performed to assess whether the combination of immunotherapies with SRP lead to a difference in results compared to placebo treatment (Iwasaki et al., 2016; Prakash et al., 2017). The result reflected that the control groups shown significantly higher PD compared to the immunotherapies with SRP (MD – 0.37 [−0.49, −0.25],p < 0.00001, 95% CI), as well as compared to immunotherapies alone (MD − 0.07 [−0.25, 0.10], p = 0.42, 95% CI). In addition, the immunotherapies combined with SRP could produce a significantly greater effect (lower PD) relative to the undergoing immunotherapies only without SRP (p = 0.006; I2 = 86.7%) (Figure 5).
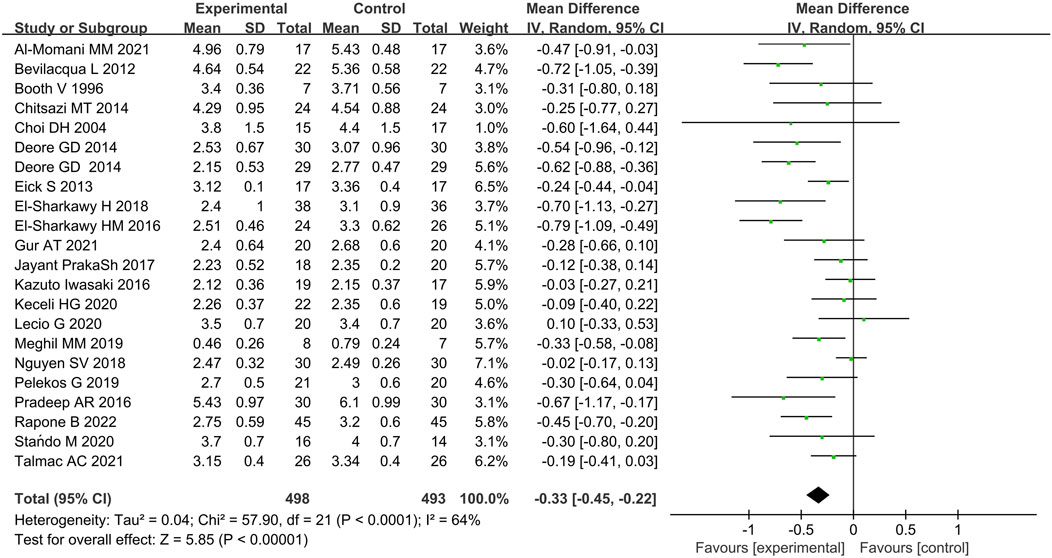
Figure 3. Forest plot of overall PD reduction at 3 months follow-up. WMD, weighted mean difference; CI = confidence interval.
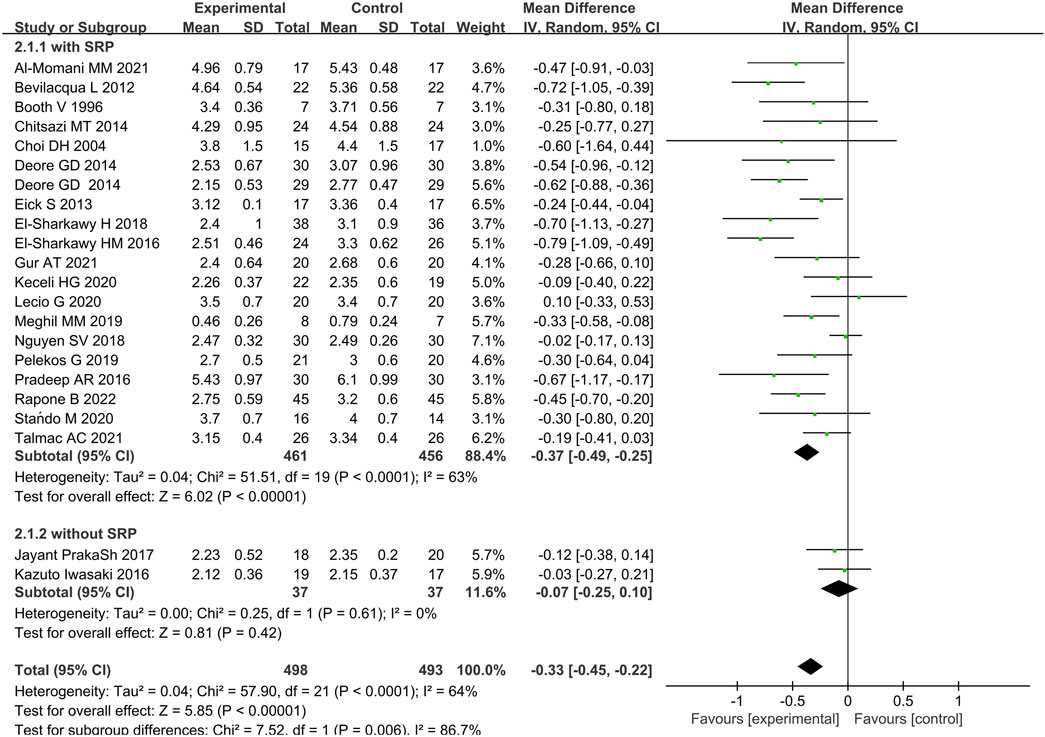
Figure 5. Forest plot of between the immunotherapy group and the non-immunotherapy group subgrouped according to the presence or absence of SRP (immunotherapy + SRP or immunotherapy vs. no immunotherapy).
3.4.2 Clinical attachment level
Seventeen studies included data on the effects of immunotherapies on CAL at 3 months (Choi et al., 2004; Bevilacq et al., 2012; Eick et al., 2013; Chitsazi et al., 2014; Deore et al., 2014a; Deore et al., 2014b; El-Sharkawy et al., 2019; El-Sharkawy et al., 2016; Pradeep et al., 2016; Talmac et al., 2022; Pelekos et al., 2019; Keceli et al., 2020; Lecio et al., 2020; Stańdo et al., 2020; Al-Momani, 2021; Gur et al., 2022; Rapone et al., 2022). Meta-analysis demonstrated that immunotherapies with SRP were associated with significantly improved CAL value when compared with placebo with considerable heterogeneity (p < 0.00001, χ2 = 37.41, I2 = 57%) (Figure 6; Supplementary Figure S2).
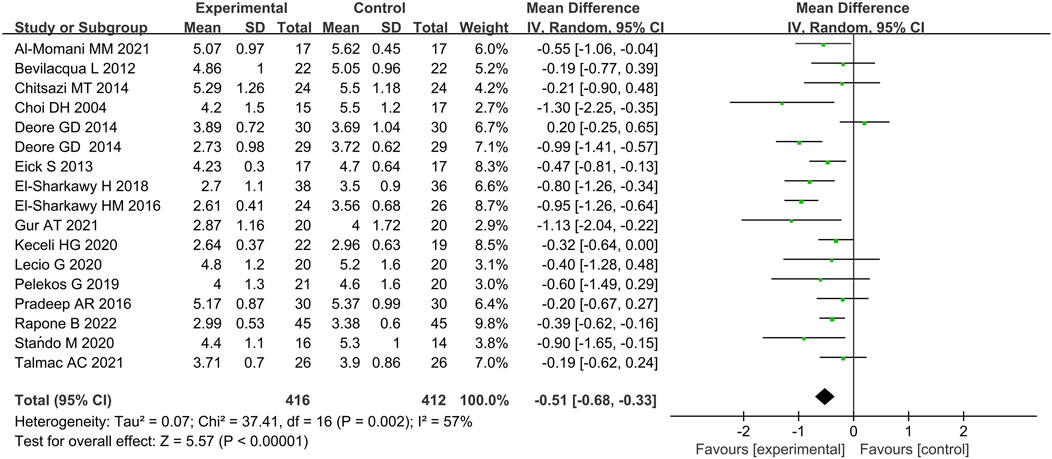
Figure 6. Forest plot of CAL gain at 3 months. WMD, weighted mean difference; CI = confidence interval.
3.4.3 BOP
In terms of BOP, eleven articles were analyzed (Choi et al., 2004; El-Sharkawy et al., 2019; Iwasaki et al., 2016; Prakash et al., 2017; Talmac et al., 2022; Pelekos et al., 2019; Lecio et al., 2020; Stańdo et al., 2020; Nguyen et al., 2018; Al-Momani, 2021; Rapone et al., 2022). The results presented WMD of −11.03% (95% CI = −15.37% to −6.68%, p < 0.00001, nine studies (Choi et al., 2004; El-Sharkawy et al., 2019; Talmac et al., 2022; Pelekos et al., 2019; Lecio et al., 2020; Stańdo et al., 2020; Nguyen et al., 2018; Al-Momani, 2021; Rapone et al., 2022)), 0.80% (95% CI = −4.63%–6.23%, p = 0.77, two study (Iwasaki et al., 2016; Prakash et al., 2017)), and −9.60% (95% CI = −13.68% to −5.52%, p < 0.00001) for immunomodulatory therapy with SRP, immunomodulatory therapy only, and overall comparison, respectively. Statistical significance was found, favoring the periodontal immunomodulatory therapy treatment group (Figure 7; Supplementary Figure S3). However, the comparison demonstrated a high heterogeneity for overall comparison (p < 0.00001, I2 = 87%).
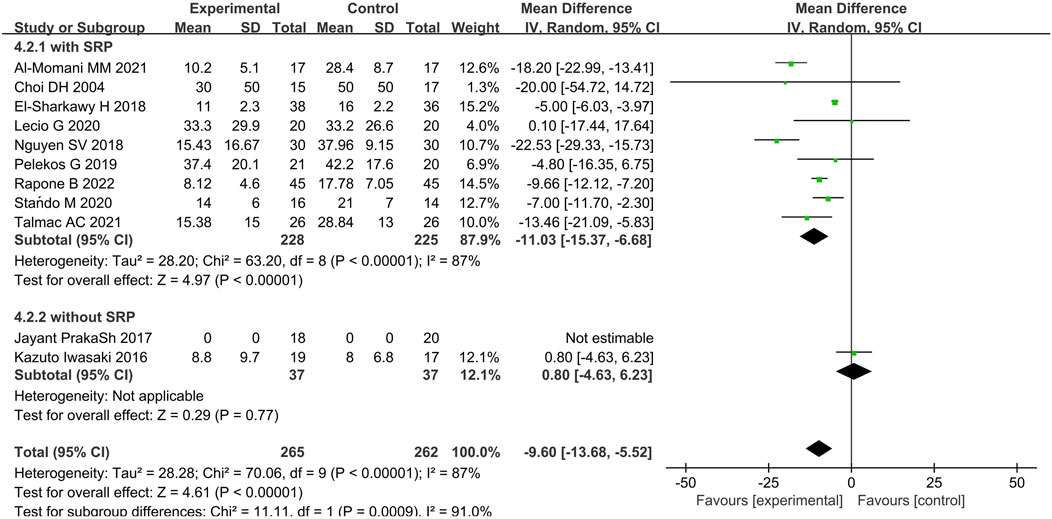
Figure 7. Forest plot of BOP (%) between the immunotherapy group and the non-immunotherapy group subgrouped according to the presence or absence of SRP (immunotherapy + SRP or immunotherapy versus no immunotherapy). CI = confidence interval.
3.5 Immunomodulator
Thirteen studies reported results regarding immunological parameters of the outcomes. Sometimes these parameters were reported as totals instead of concentrations, sometimes they were only provided in graphical form, which impeded direct comparison of these results. Additionally, time points of analysis were inconsistent among all the included studies, which further reduce the power of meta-analysis. In general, ten studies reporting results of immunological parameters were quantitatively analyzed (Figure 8).
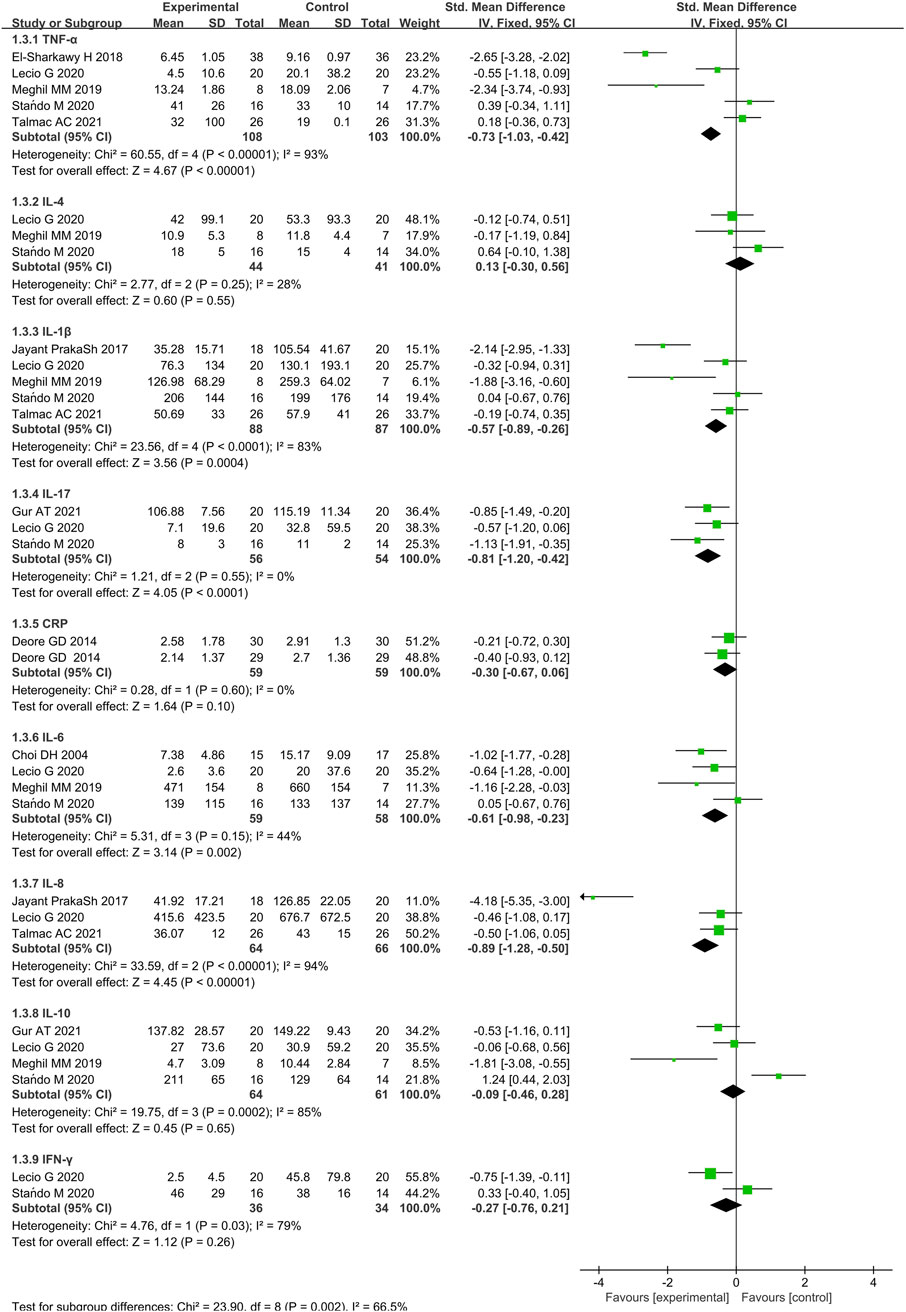
Figure 8. Forest plot of immunological parameters (pg/mL) at 3 months follow-up. CI = confidence interval.
3.5.1 Studies of IL-1β,IL-17,IL-6,IL-8,and TNF-α
TNF-α was investigated across 5 studies (El-Sharkawy et al., 2019; Talmac et al., 2022; Lecio et al., 2020; Stańdo et al., 2020; Meghil et al., 2019) (108 cases and 103 controls). Participants received immunotherapy had lower concentrations of TNF-α compared to the control (SMD = −0.73, 95% CI [-1.03, −0.42], p < 0.00001). The variability in differences regarding TNF-α levels was also significant (Q-value = 60.55; p < 0.00001; and I2 = 93%).
IL-1β measurements were extracted from 5 studies (Prakash et al., 2017; Talmac et al., 2022; Lecio et al., 2020; Stańdo et al., 2020; Meghil et al., 2019) (88 cases and 87 controls). IL-1β levels reduced significantly more when immunotherapies rather than placebo were combined with SRP (SMD = −0.57, 95% CI [-0.89, −0.26], p = 0.0004). The heterogeneity was large (I2 = 83%). Heterogeneity is higher in studies that measured this immune mediator because of different types of samples, sampling methods and relative assays (GCF or saliva).
IL-17 measurements were extracted from 3 studies (Lecio et al., 2020; Stańdo et al., 2020; Gur et al., 2022) (56 cases and 54 controls). IL-17 levels were significantly reduced in the SRP with immunotherapy participants at 3 months after treatment (p < 0.0001). The heterogeneity in IL-17 between studies was not significant (Q-value = 1.21; p = 0.55; and I2 = 0%).
IL-6 measurements were extracted from 4 studies (Choi et al., 2004; Lecio et al., 2020; Stańdo et al., 2020; Meghil et al., 2019) (59 cases and 58 controls). IL-6 levels were significantly lower in the test group compared to the control (p = 0.002). However, the heterogeneity of IL-6 among the studies was not significant as IL-17 (Q-value = 5.31; p = 0.15; and I2 = 44%).
IL-8 measurements were extracted from 3 studies (Prakash et al., 2017; Talmac et al., 2022; Lecio et al., 2020) (64 cases and 66 controls). The GCF concentrations of IL-8 were significantly lower in the test group compared to the control group (p < 0.00001). The variability in differences in IL-8 levels was significant (Q-value = 33.59; p < 0.00001; and I2 = 94%). The quantitative measures of included studies also emerged as a significant moderator as TNF-α.
3.5.2 Studies of IL-4, 10, CRP, and IFN-γ
The meta-analysis showed no significant difference in levels of IL-4 between test and control groups across 3 included studies (Lecio et al., 2020; Stańdo et al., 2020; Meghil et al., 2019). (SMD 1.80 [−0.89, 4.49], p = 0.55, 95% CI). The heterogeneity in levels of IL-4 between studies was not significant (Q-value = 2.77; p = 0.25; and I2 = 28%).
Results from 4 studies (Lecio et al., 2020; Stańdo et al., 2020; Meghil et al., 2019; Gur et al., 2022) (test/control = 64/61) measured IL-10 levels. No significant difference was noticed between the test and control groups for the expression of IL-10 (p = 0.65).
Identically, the levels of IFN-γ did not show statistical difference between the test and control groups across the 2 included studies (Lecio et al., 2020; Stańdo et al., 2020) (p = 0.26). The heterogeneity in levels of IFN-γ between studies was, however, significant (Q-value = 4.76; p = 0.03; and I2 = 79%).
Results from 2 studies (Deore et al., 2014a; Deore et al., 2014b) (59 cases and 59 controls) measured serum C-reactive protein (CRP) levels were also included in the meta-analysis. We used the fix-effects model, compared to controls, finding no significant difference in serum CRP levels in the test groups (SMD = −30, 95% CI [-0.67,0.06], p = 0.10). The heterogeneity in serum CRP levels between studies was also not significant (Q-value = 0.28; p = 0.60; and I2 = 0%).
3.5.3 Other mediators
Levels of IL-2, IL-5, IL-11, IL-12, IL-32, CXCL8, CCL-20, and transforming growth factor-β (TGF-β) were also measured in studies (Stańdo et al., 2020; Meghil et al., 2019; Gürkan et al., 2005; Gürkan et al., 2008). The meta-analysis could not be performed due to the limited number of studies.
4 Discussion
Periodontitis is the second cause of tooth loss worldwide (Ba et al., 2000). As an inflammatory disease, the main culprit identified is the bacterial biofilm growing on the tooth surfaces (Potempa et al., 2000). There are plenty of therapeutic approaches for periodontitis in clinic, among which SRP has been recognized as the gold standard for the treatment of periodontitis for decades (Aljateeli et al., 2014). This is because SRP focuses on the removal of pathogenic plaque and contributes to a chance to re-establish the metabolic balance between the environment (periodontal microbes) and host (local tissue). However, SRP is not always effective because the treated sites might be recolonized with the microbiota right away due to the breakdown of the previous healthy periodontal metabolic balance (Mombelli, 2000). This situation tends to occur at sites of deep periodontal pocket, which is one of the most challenging issues for clinicians (Graziani et al., 2019). Therefore, immunotherapies have been proposed in the treatment of periodontitis to re-establish the periodontal homestasis and modulate the repairing capability of host tissue (Yang et al., 2021). In this study, we included 22 clinical trials concerning immunomodulatory therapy for periodontal disease, including antibiotics, essential oils, laser and photodynamic therapy, probiotics, etc. Using intervention factors, the clinical manifestations of periodontal disease in clinical cases were discussed to infer the therapeutic effect. Some of these cases were also measured by local or systemic immune regulators, and changes in cytokines before and after the intervention were demonstrated (Choi et al., 2004; Deore et al., 2014a; Deore et al., 2014b; El-Sharkawy et al., 2019; Prakash et al., 2017; Talmac et al., 2022; Keceli et al., 2020; Lecio et al., 2020; Meghil et al., 2019; Al-Momani, 2021; Gur et al., 2022). We first analyzed the effect of immunomodulatory factors in the intervention treatment and further explored the immunomodulatory factors that may influence the treatment prognosis after periodontal intervention.
Many studies have found that periodontal disease can reverse the immune regulatory factors in the local and even systemic microenvironment after intervention treatment (Bokhari et al., 2012; Türer et al., 2017; D'Aiuto et al., 2018; Albuquerque-Souza et al., 2019). Li et al. reported biomimetic immunomodulation by crosstalk with nanoparticulate regulatory T cells in animal models of periodontitis, which inhibited the proliferation and activation of T cells, reduced secreting of TNF-α, IFN-γ, and IL-17a, suppressed excessive immune responses, alleviated inflammation and curbed alveolar bone resorption (Sun et al., 2023; Hienz et al., 2015). Preclinical studies also found resolvin E1 turned over bone loss, reversed inflammatory gene expression, and significantly reduced osteoclast density, inflammatory cell infiltration, and systemic CRP and IL-1β levels (Hasturk et al., 2007; Lee et al., 2016). The in vivo data from animal studies highlighted the potential mechanism for the improved efficacy of the immunomodulation in the treatment of periodontitis. Our study found that compared with the control group without intervention or with only periodontal scaling, the combination of SRP and immunomodulatory therapy showed a general improvement in PD, CAL, BOP, and more importantly, in the experimental group, pro-inflammatory factors (IL-1β, IL-6, IL-8, IL-17, and TNF-α) were notably reduced. The results of human trials are consistent with data from animal studies with immunotherapy.
To further clarify the role of immune intervention therapy in periodontitis, we conducted a further analysis on the effect of immunotherapy, and found that immunomodulatory treatment alone reduced PD compared with blank control group, but BOP did not respond obviously. However, conclusions regarding the comparison of ‘immunotherapy combined with SRP’ versus ‘immunotherapy alone’ should be interpreted with caution, as this subgroup analysis was based on a small sample size (only two included studies), necessitating careful extrapolation of these findings. However, the combination of immunomodulatory therapy and SRP can achieve good clinical outcomes, as indicated by PD, BOP, and CAL changes. Minagawa, et al. have proved that, resveratrol suppresses IL-1β, IL-8, and monocyte chemoattractant-1 (MCP-1) in human gingival epithelial cells, which partly explained the reaction of host cells towards immunomodulation treatment (Minagawa et al., 2015). Also, our findings further suggested that the progression of periodontitis may be due to the dysregulation of the periodontal local immune microenvironment.
During the progression of periodontitis, abundant inflammatory factors are stimulated in a spatiotemporal order. These factors trigger a series of pro-inflammatory or anti-inflammatory responses. The combat between protective and destructive responses decides the fate of compromised periodontal tissue. If the pro-inflammatory progress went out of control, the disease would eventually lead to alveolar bone resorption, gum atrophy, and tooth loosening (Cekici et al., 2000; Slots, 2000b; Fu et al., 2016). On the other hand, if these factors could reach a new balance, and contribute to the microenvironment of periodontal tissue, the regeneration progress might be activated (Gonzales et al., 2014; Pan et al., 2019). However, the interpretation of the concentration of a certain factor sometimes has two sides. For example, IL-6 may activate a classical pathway and a trans-signaling pathway, which may have predominantly anti- and proinflammatory activities respectively (Scheller et al., 2011). It has also been suggested that IL-17 contributes to disease progression in early-stage experimental periodontitis in mice, but has a protective role in the later stages (Wilharm et al., 2022). Our findings suggested that alterations in cytokine levels were associated with immunotherapy treatment outcomes in periodontitis. Further investigations are warranted to examine the potentiality of using inflammatory cytokines as real-time, sensitive, and reliable predictive markers for the comprehensive treatment system of periodontitis. It has to be pointed out that immunotherapy also has some drawbacks, especially when been systemically administrated which can cause adverse events (AEs). The unique mechanism of action of immunomodulatory therapy may elicit a toxicity spectrum different from that of traditional therapy, and the risk-benefit ratio needs to be evaluated separately.
A critical limitation of current immunomodulatory approaches is their tendency to target broad-spectrum inflammatory mediators, which may not resolve the complex periodontal inflammatory cascade effectively. Furthermore, the chronic nature of periodontitis requires prolonged systemic administration of some host-modulating therapies (HMTs), raising concerns about potential severe adverse events (AEs), such as increased risk for serious bacterial, fungal, and viral infections, or drug-induced Lupus, reported for anti-cytokine therapies. These safety concerns underscore the necessity to shift from broad-spectrum inhibition toward more precise, localized targets. The wide individual variation in patient susceptibility and response to treatment, influenced by genetics, systemic or environmental factors, further highlights the need for personalized treatment approaches and patient stratification in future studies.
This study is a pioneering and comprehensive meta-analysis (including 22 RCTs, all human trials) to assess the clinical efficacy of immunotherapy in the treatment of periodontitis. The results of the meta-analysis showed that adjunctive immunotherapies with SRP provided a note-worthy clinical benefit in clinical tests (PD, BOP, and CAL) when compared with the control. Meanwhile, immunomodulatory treatment significantly decreased levels of IL-1β,IL-17,IL-6,IL-8, and TNF-α.
This study has several limitations. Firstly, the sample size of the included studies was relatively limited. Large-scale, well-designed RCTs are also required to validate our conclusion and to identify which type of immunotherapy is more effective for different stages of periodontal inflammatory diseases. Follow-up periods across studies ranged from 4 weeks to 12 months, which may impair comparability of clinical outcomes. A major limitation is the high statistical heterogeneity observed across multiple analyses (e.g., PD: I2 = 64%, BOP: I2 = 87%). This high I2 value suggests substantial clinical and methodological diversity among the included trials. In addition, fixed-effects models were used to estimate the Ess of mediators, which may be inaccurate when heterogeneity is large. Further investigations are warranted to elucidate sources of heterogeneity of the results. Also, during the literature selection process, not all existing literature could be screened and some studies did not provide original data, also providing a potential bias source. Finally, restricting the inclusion criteria to English-language manuscripts may introduce language bias. The inclusion of studies assessing different biofluids (e.g., GCF, saliva) also introduces variability that should be acknowledged. Last but not least, the wide range of immunomodulatory therapies included may contribute to imprecision in the findings.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author contributions
YZ: Data curation, Investigation, Methodology, Visualization, Writing – original draft. XQ: Conceptualization, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review and editing. JY: Investigation, Resources, Supervision, Validation, Writing – review and editing. HR: Formal Analysis, Supervision, Validation, Writing – review and editing. BB: Conceptualization, Formal Analysis, Resources, Supervision, Writing – review and editing. ST: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Project administration, Resources, Software, Supervision, Validation, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The authors received financial supports from the Natural Science Foundation of China (81800981), Key Research and Development Program of Ningxia Hui Autonomous Region (2022BEG03160), Hubei Provincial Natural Science Foundation of China (2023AFB765,2024AFB599), Fund of Hubei Province Key Laboratory of Oral and Maxillofacial Development and Regeneration (2022kqhm003) and Huazhong University of Science and Technology Teaching Research Project (2025170).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fbioe.2025.1693365/full#supplementary-material
References
Al-Momani, M. M. (2021). Indocyanine-mediated antimicrobial photodynamic therapy promotes superior clinical effects in stage III and grade C chronic periodontitis among controlled and uncontrolled diabetes mellitus: a randomized controlled clinical trial. Photodiagnosis Photodyn. Ther. 35, 102379. doi:10.1016/j.pdpdt.2021.102379
AlAhmari, F., Ahmed, H. B., Al-Kheraif, A. A., Javed, F., and Akram, Z. (2019). Effectiveness of scaling and root planning with and without adjunct antimicrobial photodynamic therapy in the treatment of chronic periodontitis among cigarette-smokers and never-smokers: a randomized controlled clinical trial. Photodiagnosis Photodyn. Ther. 25, 247–252. doi:10.1016/j.pdpdt.2019.01.006
Albandar, J. M. (2000). Aggressive and acute periodontal diseases. Periodontology 65 (1), 7–12. doi:10.1111/prd.12013
Albuquerque-Souza, E., Balzarini, D., Ando-Suguimoto, E. S., Ishikawa, K. H., Simionato, M. R. L., Holzhausen, M., et al. (2019). Probiotics alter the immune response of gingival epithelial cells challenged by Porphyromonas gingivalis. J. Periodontal Res. 54 (2), 115–127. doi:10.1111/jre.12608
Aljateeli, M., Koticha, T., Bashutski, J., Sugai, J. V., Braun, T. M., Giannobile, W. V., et al. (2014). Surgical periodontal therapy with and without initial scaling and root planing in the management of chronic periodontitis: a randomized clinical trial. J. Clin. periodontology 41 (7), 693–700. doi:10.1111/jcpe.12259
Azuma, M. M., Cardoso, CDBM, Silva, C. C., Oliveira, P. H. C., Jacinto, R. D. C., Andrada, A. C., et al. (2022). The use of omega-3 fatty acids in the treatment of oral diseases. Oral Dis. 28 (2), 264–274. doi:10.1111/odi.13667
Baelum, V., and López, R. (2000). Periodontal disease epidemiology - learned and unlearned? Periodontology 62 (1), 37–58. doi:10.1111/j.1600-0757.2012.00449.x
Basic, A., and Dahlen, G. (2023). Microbial metabolites in the pathogenesis of periodontal diseases: a narrative review. Front. Oral Health 4, 1210200. doi:10.3389/froh.2023.1210200
Bevilacqua, L., Eriani, J., Serroni, I., Liani, G., Borelli, V., Castronovo, G., et al. (2012). Effectiveness of adjunctive subgingival administration of amino acids and sodium hyaluronate gel on clinical and immunological parameters in the treatment of chronic periodontitis. Ann. Stomatol. (Roma) 3 (2), 75–81.
Bokhari, S. A., Khan, A. A., Butt, A. K., Azhar, M., Hanif, M., Izhar, M., et al. (2012). Non-surgical periodontal therapy reduces coronary heart disease risk markers: a randomized controlled trial. J. Clin. Periodontol. 39 (11), 1065–1074. doi:10.1111/j.1600-051X.2012.01942.x
Booth, V., Ashley, F. P., and Lehner, T. (1996). Passive immunization with monoclonal antibodies against Porphyromonas gingivalis in patients with periodontitis. Infect. Immun. 64 (2), 422–427. doi:10.1128/iai.64.2.422-427.1996
Bostanci, V., Toker, H., Senel, S., Poyraz, O., Akpinar, A., Gorgun, E. P., et al. (2017). Evaluation of IL-1β, IL-1ra, and IL-10 levels and outcome of periodontal therapy in chronic periodontitis with familial mediterranean fever. Clin. Oral Investig. 21 (1), 469–475. doi:10.1007/s00784-016-1816-1
Capv, Da, Avt, E., de Carvalho, V. F., De Franco, R. M., Pannuti, C. M., Holzhausen, M., et al. (2017). Photodynamic therapy decrease immune-inflammatory mediators levels during periodontal maintenance. Lasers Med. Sci. 32 (1), 9–17. doi:10.1007/s10103-016-2076-7
Cekici, A., Kantarci, A., Hasturk, H., and Van Dyke, T. E. (2000). Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontology 64 (1), 57–80. doi:10.1111/prd.12002
Chee, B., Park, B., Fitzsimmons, T., Coates, A. M., and Bartold, P. M. (2016). Omega-3 fatty acids as an adjunct for periodontal Therapy—a review. Clin. Oral Investig. 20 (5), 879–894. doi:10.1007/s00784-016-1750-2
Chitsazi, M. T., Shirmohammadi, A., Pourabbas, R., Abolfazli, N., Farhoudi, I., Daghigh, A. B., et al. (2014). Clinical and microbiological effects of photodynamic therapy associated with non-surgical treatment in aggressive periodontitis. J. Dent. Res. Dent. Clin. Dent. Prospects 8 (3), 153–159. doi:10.5681/joddd.2014.028
Choi, D. H., Moon, I. S., Choi, B. K., Paik, J. W., Kim, Y. S., Choi, S. H., et al. (2004). Effects of sub-antimicrobial dose doxycycline therapy on crevicular fluid MMP-8, and gingival tissue MMP-9, TIMP-1 and IL-6 levels in chronic periodontitis. J. Periodontal Res. 39 (1), 20–26. doi:10.1111/j.1600-0765.2004.00696.x
Cobb, C. M. (2002). Clinical significance of non-surgical periodontal therapy: an evidence-based perspective of scaling and root planing. J. Clin. Periodontol. 29 (Suppl. 2), 22–32. doi:10.1034/j.1600-051x.29.s2.4.x
D'Aiuto, F., Gkranias, N., Bhowruth, D., Khan, T., Orlandi, M., Suvan, J., et al. (2018). Systemic effects of periodontitis treatment in patients with type 2 diabetes: a 12 month, single-centre, investigator-masked, randomised trial. Lancet Diabetes Endocrinol. 6 (12), 954–965. doi:10.1016/s2213-8587(18)30038-x
Demkovych, A., Kalashnikov, D., Hasiuk, P., Zubchenko, S., and Vorobets, A. (2023). The influence of microbiota on the development and course of inflammatory diseases of periodontal tissues. Front. Oral Health 4, 1237448. doi:10.3389/froh.2023.1237448
Deore, G. D., Gurav, A. N., Patil, R., Shete, A. R., Naiktari, R. S., and Inamdar, S. P. (2014a). Herbal anti-inflammatory immunomodulators as host modulators in chronic periodontitis patients: a randomised, double-blind, placebo-controlled, clinical trial. J. Periodontal Implant Sci. 44 (2), 71–78. doi:10.5051/jpis.2014.44.2.71
Deore, G. D., Gurav, A. N., Patil, R., Shete, A. R., Naiktari, R. S., and Inamdar, S. P. (2014b). Omega 3 fatty acids as a host modulator in chronic periodontitis patients: a randomised, double-blind, palcebo-controlled, clinical trial. J. Periodontal Implant Sci. 44 (1), 25–32. doi:10.5051/jpis.2014.44.1.25
Eick, S., Renatus, A., Heinicke, M., Pfister, W., Stratul, S. I., and Jentsch, H. (2013). Hyaluronic acid as an adjunct after scaling and root planing: a prospective randomized clinical trial. J. Periodontol. 84 (7), 941–949. doi:10.1902/jop.2012.120269
Eke, P. I., Wei, L., Borgnakke, W. S., Thornton-Evans, G., Zhang, X., Lu, H., et al. (2000). Periodontitis prevalence in adults ≥ 65 years of age, in the USA. Periodontology 72 (1), 76–95. doi:10.1111/prd.12145
El-Sharkawy, H. M., Anees, M. M., and Van Dyke, T. E. (2016). Propolis improves periodontal status and glycemic control in patients with type 2 diabetes mellitus and chronic periodontitis: a randomized clinical trial. J. Periodontol. 87 (12), 1418–1426. doi:10.1902/jop.2016.150694
El-Sharkawy, H., Elmeadawy, S., Elshinnawi, U., and Anees, M. (2019). Is dietary melatonin supplementation a viable adjunctive therapy for chronic periodontitis?—A randomized controlled clinical trial. J. Periodontal Res. 54 (2), 190–197. doi:10.1111/jre.12619
Fu, Y. W., Li, X. X., Xu, H. Z., Gong, Y. Q., and Yang, Y. (2016). Effects of periodontal therapy on serum lipid profile and proinflammatory cytokines in patients with hyperlipidemia: a randomized controlled trial. Clin. Oral Investig. 20 (6), 1263–1269. doi:10.1007/s00784-015-1621-2
Gonzales, J. R., Groeger, S., Johansson, A., and Meyle, J. (2014). T helper cells from aggressive periodontitis patients produce higher levels of interleukin-1 beta and interleukin-6 in interaction with Porphyromonas gingivalis. Clin. Oral Investig. 18 (7), 1835–1843. doi:10.1007/s00784-013-1162-5
Graziani, F., Karapetsa, D., Alonso, B., and Herrera, D. (2000). Nonsurgical and surgical treatment of periodontitis: how many options for one disease? Periodontology 75 (1), 152–188. doi:10.1111/prd.12201
Graziani, F., Gennai, S., Petrini, M., Bettini, L., and Tonetti, M. (2019). Enamel matrix derivative stabilizes blood clot and improves clinical healing in deep pockets after flapless periodontal therapy: a randomized clinical trial. J. Clin. Periodontol. 46 (2), 231–240. doi:10.1111/jcpe.13074
Grover, V., Jain, A., Kapoor, A., Malhotra, R., and Chahal, G. S. (2016). The gender Bender effect in periodontal immune response. Endocr. metabolic and immune Disord. drug targets 16 (1), 12–20. doi:10.2174/1871530316666160107111301
Gur, A. T., Guncu, G. N., Akman, A. C., Pinar, A., Karabulut, E., and Nohutcu, R. M. (2022). Evaluation of GCF IL-17, IL-10, TWEAK, and sclerostin levels after scaling and root planing and adjunctive use of diode laser application in patients with periodontitis. J. Periodontol. 93 (8), 1161–1172. doi:10.1002/jper.21-0494
Gürkan, A., Cinarcik, S., and Hüseyinov, A. (2005). Adjunctive subantimicrobial dose doxycycline: effect on clinical parameters and gingival crevicular fluid transforming growth factor-beta levels in severe, generalized chronic periodontitis. J. Clin. Periodontol. 32 (3), 244–253. doi:10.1111/j.1600-051X.2005.00663.x
Gürkan, A., Emingil, G., Çınarcık, S., and Berdeli, A. (2008). Post-treatment effects of subantimicrobial dose doxycycline on clinical parameters and gingival crevicular fluid transforming growth factor-β1 in severe, generalized chronic periodontitis. Int. J. Dent. Hyg. 6 (2), 84–92. doi:10.1111/j.1601-5037.2007.00268.x
Hajishengallis, G., Chavakis, T., and Lambris, J. D. (2000). Current understanding of periodontal disease pathogenesis and targets for host-modulation therapy. Periodontol 84 (1), 14–34. doi:10.1111/prd.12331
Hasturk, H., Kantarci, A., Goguet-Surmenian, E., Blackwood, A., Andry, C., Serhan, C. N., et al. (2007). Resolvin E1 regulates inflammation at the cellular and tissue level and restores tissue homeostasis in vivo. J. Immunol. 179 (10), 7021–7029. doi:10.4049/jimmunol.179.10.7021
Hienz, S. A., Paliwal, S., and Ivanovski, S. (2015). Mechanisms of bone resorption in periodontitis. J. Immunol. Res. 2015, 1–10. doi:10.1155/2015/615486
Higgins, J. P. T., Thompson, S. G., Deeks, J. J., and Altman, D. G. (2003). Measuring inconsistency in meta-analyses. Bmj 327 (7414), 557–560. doi:10.1136/bmj.327.7414.557
Higgins, J. P. T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M. J., et al. (2019). Cochrane handbook for systematic reviews of interventions. Newark: John Wiley and Sons Incorporated.
Iwasaki, K., Maeda, K., Hidaka, K., Nemoto, K., Hirose, Y., and Deguchi, S. (2016). Daily intake of heat-killed Lactobacillus plantarum L-137 decreases the probing depth in patients undergoing supportive periodontal therapy. Oral Health Prev. Dent. 14 (3), 207–214. doi:10.3290/j.ohpd.a36099
Jepsen, S., Suvan, J., and Deschner, J. (2000). The association of periodontal diseases with metabolic syndrome and obesity. Periodontol 83 (1), 125–153. doi:10.1111/prd.12326
Kassebaum, N. J., Bernabe, E., Dahiya, M., Bhandari, B., Murray, C. J. L., and Marcenes, W. (2014). Global burden of severe periodontitis in 1990-2010: a systematic review and metaregression. J. Dent. Res. 93 (11), 1045–1053. doi:10.1177/0022034514552491
Keceli, H. G., Ercan, N., Karsiyaka, H. M., Kisa, U., Mesut, B., and Olgun, E. (2020). The effect of the systemic folic acid intake as an adjunct to scaling and root planing on clinical parameters and homocysteine and C-reactive protein levels in gingival crevicular fluid of periodontitis patients: a randomized placebo-controlled clinical trial. J. Clin. Periodontol. 47 (5), 602–613. doi:10.1111/jcpe.13276
Lecio, G., Ribeiro, F. V., Pimentel, S. P., Reis, A. A., Da, SRVC, Nociti-Jr, F., et al. (2020). Novel 20% doxycycline-loaded PLGA nanospheres as adjunctive therapy in chronic periodontitis in type-2 diabetics: randomized clinical, immune and microbiological trial. Clin. Oral Investig. 24 (3), 1269–1279. doi:10.1007/s00784-019-03005-9
Lee, C. T., Teles, R., Kantarci, A., Chen, T., McCafferty, J., Starr, J. R., et al. (2016). Resolvin E1 reverses experimental periodontitis and dysbiosis. J. Immunol. 197 (7), 2796–2806. doi:10.4049/jimmunol.1600859
Manresa, C., Sanz-Miralles, E. C., Twigg, J., and Bravo, M. (2018). Supportive periodontal therapy (SPT) for maintaining the dentition in adults treated for periodontitis. Cochrane Database Syst. Rev. 1 (1), CD009376. doi:10.1002/14651858.cd009376.pub2
Martin-Cabezas, R., Davideau, J. L., Tenenbaum, H., and Huck, O. (2016). Clinical efficacy of probiotics as an adjunctive therapy to non-surgical periodontal treatment of chronic periodontitis: a systematic review and meta-analysis. J. Clin. Periodontol. 43 (6), 520–530. doi:10.1111/jcpe.12545
Meghil, M. M., Hutchens, L., Raed, A., Multani, N. A., Rajendran, M., Zhu, H., et al. (2019). The influence of vitamin D supplementation on local and systemic inflammatory markers in periodontitis patients: a pilot study. Oral Dis. 25 (5), 1403–1413. doi:10.1111/odi.13097
Minagawa, T., Okui, T., Takahashi, N., Nakajima, T., Tabeta, K., Murakami, S., et al. (2015). Resveratrol suppresses the inflammatory responses of human gingival epithelial cells in a SIRT1 independent manner. J. Periodontal Res. 50 (5), 586–593. doi:10.1111/jre.12238
Minić, I., Pejčić, A., and Bradić-Vasić, M. (2022). Effect of the local probiotics in the therapy of periodontitis A randomized prospective study. Int. J. Dent. Hyg. 20 (2), 401–407. doi:10.1111/idh.12509
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 6 (7), e1000097. doi:10.1371/journal.pmed.1000097
Mombelli, A. (2000). Microbial colonization of the periodontal pocket and its significance for periodontal therapy. Periodontology 76 (1), 85–96. doi:10.1111/prd.12147
Mysak, J., Podzimek, S., Sommerova, P., Lyuya-Mi, Y., Bartova, J., Janatova, T., et al. (2014). Porphyromonas gingivalis: major periodontopathic pathogen overview. J. Immunol. Res. 2014, 1–8. doi:10.1155/2014/476068
Nędzi-Góra, M., WrÓblewska, M., and Górska, R. (2020). The effect of Lactobacillus salivarius SGL03 on clinical and microbiological parameters in periodontal patients. Pol. J. Microbiol. 69 (4), 441–451. doi:10.33073/pjm-2020-047
Nguyen, S. V., Nguyen, M. T. H., Tran, B. C., Ho, M. T. Q., Umeda, K., and Rahman, S. (2018). Evaluation of lozenges containing egg yolk antibody against Porphyromonas gingivalis gingipains as an adjunct to conventional non-surgical therapy in periodontitis patients: a randomized controlled clinical trial. J. Periodontol. 89 (11), 1334–1339. doi:10.1002/jper.18-0037
Niazi, F. H., Noushad, M., Tanvir, S. B., Ali, S., Al-Khalifa, K. S., Qamar, Z., et al. (2020). Antimicrobial efficacy of indocyanine green-mediated photodynamic therapy compared with Salvadora persica gel application in the treatment of moderate and deep pockets in periodontitis. Photodiagnosis Photodyn. Ther. 29, 101665. doi:10.1016/j.pdpdt.2020.101665
Nile, C. J., Apatzidou, D. A., Awang, R. A., Riggio, M. P., Kinane, D. F., and Lappin, D. F. (2016). The effect of periodontal scaling and root polishing on serum IL-17E concentrations and the IL-17A:IL-17E ratio. Clin. Oral Investig. 20 (9), 2529–2537. doi:10.1007/s00784-016-1749-8
Page, M. J., Moher, D., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ (Online) 372, n160. doi:10.1136/bmj.n160
Pan, W., Wang, Q., and Chen, Q. (2019). The cytokine network involved in the host immune response to periodontitis. Int. J. Oral Sci. 11 (3), 30. doi:10.1038/s41368-019-0064-z
Pelekos, G., Ho, S. N., Acharya, A., Leung, W. K., and McGrath, C. (2019). A double-blind, paralleled-arm, placebo-controlled and randomized clinical trial of the effectiveness of probiotics as an adjunct in periodontal care. J. Clin. Periodontol. 46 (12), 1217–1227. doi:10.1111/jcpe.13191
Penala, S., Kalakonda, B., Pathakota, K. R., Jayakumar, A., Koppolu, P., Lakshmi, B. V., et al. (2016). Efficacy of local use of probiotics as an adjunct to scaling and root planing in chronic periodontitis and halitosis: a randomized controlled trial. J. Res. Pharm. Pract. 5 (2), 86–93. doi:10.4103/2279-042x.179568
Potempa, J., Banbula, A., and Travis, J. (2000). Role of bacterial proteinases in matrix destruction and modulation of host responses. Periodontol 24, 153–192. doi:10.1034/j.1600-0757.2000.2240108.x
Pradeep, A. R., Garg, V., Raju, A., and Singh, P. (2016). Adjunctive local delivery of Aloe vera gel in patients with type 2 diabetes and chronic periodontitis: a randomized, controlled clinical trial. J. Periodontol. 87 (3), 268–274. doi:10.1902/jop.2015.150161
Prakash, J., Bhatnagar, V., Nath, SSYC, Pulikkotil, S., and Prajapati, V. K. (2017). Effect of Punica granatum extract gel on gingival crevicular fluid levels of Interleukin- 1β, Interleukin-8 and CCL28 levels: randomised controlled clinical trial. J. Clin. and Diagnostic Res. (11), 12–17.
Qamar, Z., Almohana, S. A., Khalid, A. A., Khalid, A. A., Almohana, A. A., and Zeeshan, T. (2021). Clinical evaluation of the effects of topical indocyanine-Green mediated photosensitiser vs Aloe vera gel as adjunct therapy to scaling and root planing in chronic periodontitis patients. Oral Health Prev. Dent. 19 (1), 489–494. doi:10.3290/j.ohpd.b2082037
Qin, X., Hoda, M. N., Susin, C., Wheeler, J. N., Marshall, B., Perry, L., et al. (2017a). Increased innate lymphoid cells in periodontal tissue of the murine model of periodontitis: the role of AMP-activated protein kinase and relevance for the human condition. Front. Immunol. 8, 922. doi:10.3389/fimmu.2017.00922
Qin, X., Liu, J. Y., Wang, T., Pashley, D. H., Al-Hashim, A. H., Abdelsayed, R., et al. (2017b). Role of indoleamine 2,3-dioxygenase in an inflammatory model of Murine gingiva. J. Periodontal Res. 52 (1), 107–113. doi:10.1111/jre.12374
Ramanauskaite, A., Fretwurst, T., and Schwarz, F. (2021). Efficacy of alternative or adjunctive measures to conventional non-surgical and surgical treatment of peri-implant mucositis and peri-implantitis: a systematic review and meta-analysis. Int. J. Implant Dent. 7 (1), 112. doi:10.1186/s40729-021-00388-x
Ramos, U. D., Ayub, L. G., Reino, D. M., Grisi, M. F., Taba, M., Souza, S. L., et al. (2016). Antimicrobial photodynamic therapy as an alternative to systemic antibiotics: results from a double-blind, randomized, placebo-controlled, clinical study on type 2 diabetics. J. Clin. Periodontol. 43 (2), 147–155. doi:10.1111/jcpe.12498
Rapone, B., Ferrara, E., Santacroce, L., Topi, S., Gnoni, A., Dipalma, G., et al. (2022). The gaseous ozone therapy as a promising antiseptic adjuvant of periodontal treatment: a randomized controlled clinical trial. Int. J. Environ. Res. Public Health 19 (2), 985. doi:10.3390/ijerph19020985
Rashidi, M. F., Haerian-Ardakani, A., Nabi-Maybodi, M., and Nasrabadi, N. (2016). Effect of 1% phenytoin muco-adhesive paste on improvement of periodontal status in patients with chronic periodontitis: a randomized blinded controlled clinical study. J. Dent. (Shiraz) 17 (3 Suppl. l), 256–261.
Sanjay, L. S., Rukhshana, N. S., Vibhuti, M., Amit, V., Prajapati, N., Shah, D., et al. (2025). Clinical study comparing herbal plant extract as a supportive treatment for chronic periodontitis. J. Pharm. and bioallied Sci. 17 (Suppl. 1), S757–s759. doi:10.4103/jpbs.jpbs_361_25
Scheller, J., Chalaris, A., Schmidt-Arras, D., and Rose-John, S. (2011). The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta 1813 (5), 878–888. doi:10.1016/j.bbamcr.2011.01.034
Slots, J. (2000a). Low-cost periodontal therapy. Periodontology 60 (1), 110–137. doi:10.1111/j.1600-0757.2011.00429.x
Slots, J. (2000b). Periodontitis: facts, fallacies and the future. Periodontol 75 (1), 7–23. doi:10.1111/prd.12221
Stańdo, M., Piatek, P., Namiecinska, M., Lewkowicz, P., and Lewkowicz, N. (2020). Omega-3 polyunsaturated fatty acids EPA and DHA as an adjunct to non-surgical treatment of periodontitis: a randomized clinical trial. Nutrients 12 (9), 2614. doi:10.3390/nu12092614
Sun, L., Wang, L., Moore, B. B., Zhang, S., Xiao, P., Decker, A. M., et al. (2023). IL-17: balancing protective immunity and pathogenesis. J. Immunol. Res. 2023, 1–9. doi:10.1155/2023/3360310
Talmac, A. C., Yayli, N. Z. A., Calisir, M., and Ertugrul, A. S. (2022). Comparing the efficiency of Er,Cr:YSGG laser and diode laser for the treatment of generalized aggressive periodontitis. Ir. J. Med. Sci. 191 (3), 1331–1339. doi:10.1007/s11845-021-02705-0
Trindade, D., Carvalho, R., Machado, V., Chambrone, L., Mendes, J. J., and Botelho, J. (2023). Prevalence of periodontitis in dentate people between 2011 and 2020: a systematic review and meta-analysis of epidemiological studies. J. Clin. periodontology 50 (5), 604–626. doi:10.1111/jcpe.13769
Türer, Ç. C., Durmuş, D., Balli, U., and Güven, B. (2017). Effect of non-surgical periodontal treatment on gingival crevicular fluid and serum endocan, vascular endothelial growth Factor-A, and tumor necrosis factor-alpha levels. J. Periodontol. 88 (5), 493–501. doi:10.1902/jop.2016.160279
Wilharm, A., Binz, C., Sandrock, I., Rampoldi, F., Lienenklaus, S., Blank, E., et al. (2022). Interleukin-17 is disease promoting in early stages and protective in late stages of experimental periodontitis. PLoS One 17 (3), e0265486. doi:10.1371/journal.pone.0265486
Yang, B., Pang, X., Li, Z., Chen, Z., and Wang, Y. (2021). Immunomodulation in the treatment of periodontitis: progress and perspectives. Front. Immunol. 12, 781378. doi:10.3389/fimmu.2021.781378
Yokoyama, K., Sugano, N., Shimada, T., Shofiqur, R. A., Ibrahim, E.-S. M., Isoda, R., et al. (2007). Effects of egg yolk antibody against Porphyromonas gingivalis gingipains in periodontitis patients. J. Oral Sci. 49 (3), 201–206. doi:10.2334/josnusd.49.201
Zhang, H., Zhang, Y., Chen, X., Li, J., Zhang, Z., and Yu, H. (2021). Effects of statins on cytokines levels in gingival crevicular fluid and saliva and on clinical periodontal parameters of middle-aged and elderly patients with type 2 diabetes mellitus. PLoS One 16 (1), e0244806. doi:10.1371/journal.pone.0244806
Keywords: periodontitis, clinical trials, immunomodulation, immunotherapy, systematic review
Citation: Zhang Y, Qin X, Yang J, Rogers HM, Baban B and Tian S (2025) Clinical effect of immunomodulatory therapy in periodontitis: a systematic review and meta-analysis. Front. Bioeng. Biotechnol. 13:1693365. doi: 10.3389/fbioe.2025.1693365
Received: 27 August 2025; Accepted: 27 October 2025;
Published: 20 November 2025.
Edited by:
Qianju Wu, Xiamen Stomatological Hospital, ChinaReviewed by:
Longwei Hu, Shanghai Jiao Tong University, ChinaYi Sun, University Hospitals Leuven, Belgium
Copyright © 2025 Zhang, Qin, Yang, Rogers, Baban and Tian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Siwei Tian, dGlhbnN3QDEzOS5jb20=
†These authors have contributed equally to this work
 Yubing Zhang
Yubing Zhang Xu Qin1,3,4†
Xu Qin1,3,4† Babak Baban
Babak Baban