- Research Area Biochemical Engineering, Institute of Chemical, Environmental and Bioscience Engineering, Technische Universität Wien, Vienna, Austria
Quality standard landscape in NK cell therapies
Natural killer (NK) cells are innate lymphocytes capable of directly targeting and killing tumor cells without prior sensitization (Vivier et al., 2024). Due to their inherent cytotoxicity, NK cells have emerged as a very promising modality for cancer immunotherapy, offering outstanding advantages over chimeric antigen receptor-T (CAR-T) cell therapies, such as reduced risks of the cytokine release syndrome and neurotoxicity (Vivier et al., 2024; Zhang et al., 2019; Lodoen and Lanier, 2006). CAR-engineered NK cells further enhance therapeutic potential by improving target specificity and cytotoxic efficacy, thereby complementing and extending the clinical success of current CAR-T cell therapies (Vivier et al., 2024; Zhong and Liu, 2024). However, the lack of formally defined critical quality attributes (CQAs) currently limits cross-trial and cross-study comparability, compromises safety evaluation, and impedes clinical efficacy optimization for NK cell-based therapies.
CQAs play a pivotal role in biopharmaceutical development as they describe the required product quality, safety and efficacy (FDA U.S. Department of Health and Human Services, 2012). According to the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) Guideline Q8 (R2), a CQA is a “physical, chemical, biological, or microbiological property or characteristic that should be within an appropriate limit, range, or distribution to ensure the desired product quality” (ICH, 2009). Despite their central importance, CQAs have not yet been formally defined for NK cell therapies yet. The current FDA guidance only roughly defines parameters for quality testing, recommending that CQAs should minimally consist of identity, quality, purity, and potency (FDA U.S. Department of Health and Human Services, 2024; FDA U.S. Department of Health and Human Services, 2023). Regulatory agencies currently refrain from defining generalized acceptance criteria or fixed limits, as these must be adapted to each manufacturing platform, therapeutic indication, and target patient group. While clearly defined CQAs with specified ranges would bring obvious benefits, overly rigid or premature standardization could stifle innovation, particularly for next-generation therapies tailored to specific indications or specific patient populations. In contrast, the current high variance in trial release criteria is complicating product comparison and poses challenges for a consistent regulatory evaluation. We are convinced that a harmonized effort for defining CQAs - anchored in a Quality by Design (QbD) framework and aligned with ICH and regulatory guidelines - is required and essential for ensuring the consistency, safety, and efficacy of NK cell therapies.
To potentially initiate this harmonization effort, we systematically analyzed the reported release criteria from all completed Phase 1 and 2 clinical trials of NK cell therapies targeting cancer, which were conducted in the US and Europe with reported release criteria (Shah et al., 2015; McKenna et al., 2012; Lapteva et al., 2014; Dolstra et al., 2017; Williams et al., 2017; Boyiadzis et al., 2017) (Table 1).
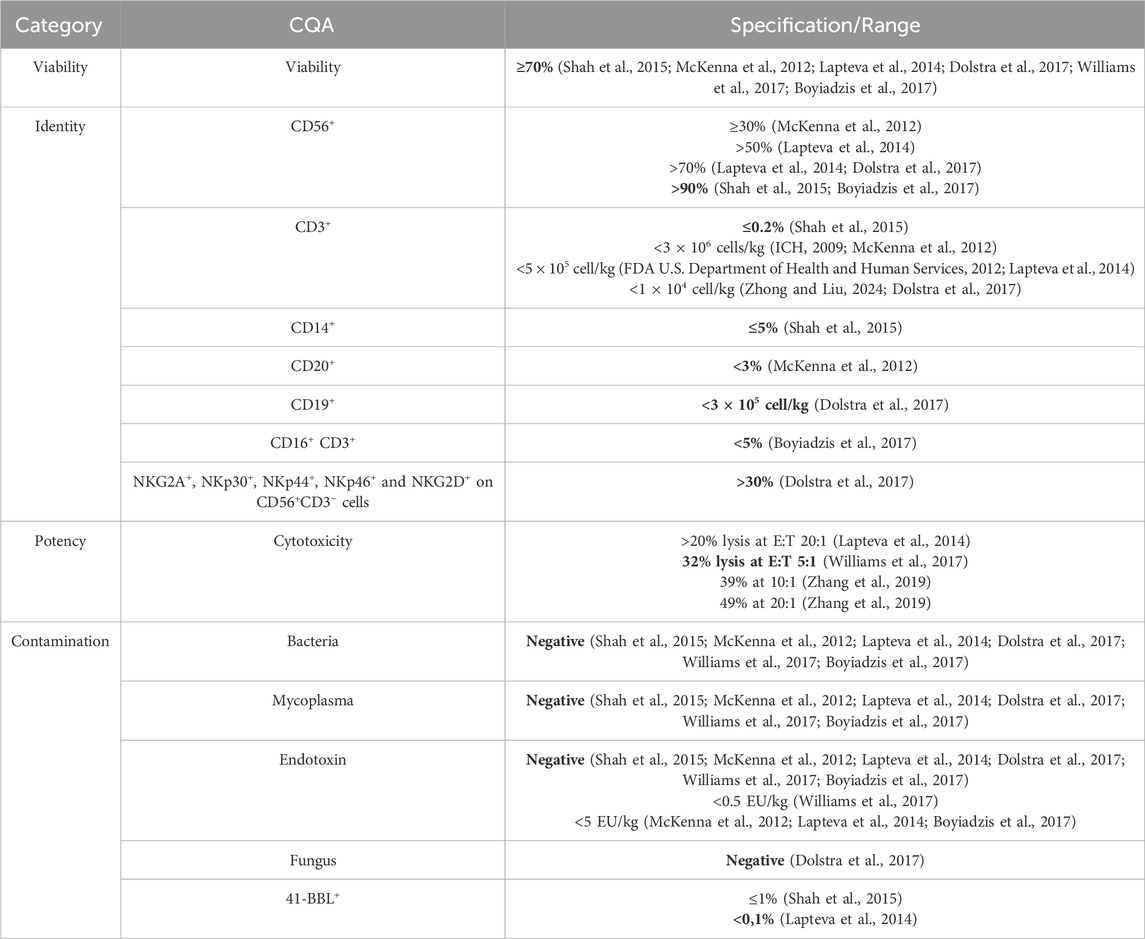
Table 1. Suggestions for CQAs for NK cell products, based on release criteria from phase 1 and 2 cancer immunotherapy trials using primary human NK and NK-92 cells in the EU and United States. Acceptance ranges were derived from reported thresholds, with the most stringent values highlighted in bold. 4-1BBL, commonly used as a stimulatory factor on feeder cells, is listed as a contaminant, as its unintended presence may indicate feeder cell carry-over.
In all the clinical trials the accepted viability range for NK cells was uniformly set at ≥70%, highlighting its importance as a benchmark linked to clinical performance. Purity was defined by the presence of other cell types in the final NK cell product. Therefore, cell types were identified by cell surface markers of NK cells (CD56+), T cells (CD3+), B cells (CD19+, CD20+), monocytes (CD14+), and NK-like T cells (CD16+CD3+). Activation markers (NKG2D, NKp30, NKp44, NKp46) and inhibitory receptors (NKG2A) were reported to a variable extent, and NK cell purity criteria of the final product ranged from ≥30% to ≥90% (Shah et al., 2015; McKenna et al., 2012; Lapteva et al., 2014; Dolstra et al., 2017; Boyiadzis et al., 2017). T and B cell contaminants were strictly limited between ≤0.2% and <3% (Shah et al., 2015; McKenna et al., 2012; Lapteva et al., 2014; Dolstra et al., 2017). While most studies reported acceptable purity ranges in relative units (i.e., percentage of impurities within the final product), others presented only absolute cell counts. To improve cross-study comparability and regulatory alignment, CQAs should consistently be reported in relative units. Contamination standards were uniform, requiring sterility and strict endotoxin limits (negative detection to <5 EU/kg) (Shah et al., 2015; McKenna et al., 2012; Lapteva et al., 2014; Dolstra et al., 2017; Williams et al., 2017; Boyiadzis et al., 2017) as well as the absence of feeder cell residuals, such as the stimulant 41-BBL (Shah et al., 2015; Lapteva et al., 2014). Potency assay norms also varied, with tumor cell lysis values reaching up to 49% at an effector-to-target ratio acronym: (E:T) of 20:1 (Zhang et al., 2019). However, cross-study and cross-trial comparability remain challenging, as no harmonized assay protocols, such as consistent E:T ratios or standardized target cell types, have been employed, underscoring the urgent need for assay harmonization and clear definition of CQAs.
We suggest the data summarized in Table 1 to be a potential foundation stone for the definition of CQAs with respective acceptance ranges. Defining CQAs early in development and validating appropriate assays will enhance product reliability and patient safety. Applying a QbD approach allows systematic identification and control of critical process parameters during manufacturing and their effects on CQAs. Integration of QbD principles with ICH Q8–Q11 and FDA/EMA frameworks can facilitate robust, reproducible production protocols and support smoother regulatory approvals (ICH, 2009). In our opinion, collaboration among key stakeholders, including academic researchers, biotechnology developers, manufacturers, sponsors and regulatory agencies, is essential to develop consensus guidelines and reference quality standards for NK cell products. Regulatory bodies should issue more detailed guidelines specific for NK cell products, including standardized thresholds for viability, potency, purity, and contaminant limits. While NK cells from different sources or isolation methods may display varying phenotypes, harmonized release criteria should ensure that every patient receives an NK cell product meeting certain safety, purity, and potency standards, irrespective of its origin. The proposed CQAs, derived from available clinical trial release criteria (Table 1), provide a foundational baseline for more refined, QbD-driven definition of CQA ranges tailored to specific disease indications. The analyzed clinical trial cohort was limited in size, which reflects the current scarcity of publicly available data on release criteria rather than a selective bias, underscoring the urgent need for greater transparency and standardized reporting in future NK cell trials. Harmonizing CQAs through this QbD-driven approach will be pivotal for NK cell therapy development - ensuring patient safety, optimizing clinical outcomes, and enabling more scalable, reproducible, and effective NK-based immunotherapies across diverse clinical settings worldwide.
Author contributions
VW: Conceptualization, Formal Analysis, Investigation, Methodology, Validation, Writing – original draft, Writing – review and editing. AS: Conceptualization, Data curation, Formal Analysis, Methodology, Writing – review and editing. SZ-B: Conceptualization, Data curation, Formal Analysis, Methodology, Writing – original draft. OS: Funding acquisition, Project administration, Resources, Supervision, Validation, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded in whole or in part by the Austrian Science Fund (FWF) (Grant- DOI: 10.55776/I5910) as well as by the FFG - Die Österreichische Forschungsförderungsgesellschaft (Project Title: Computational fluid dynamics aided natural killer cell expansion scale-up; Project No.: FFG 5126071). For open access purposes, the author has applied a CC by public copyright license to any author-accepted manuscript version arising from this submission. Open access funding provided by Technische Universität Wien.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Boyiadzis, M., Agha, M., Redner, R. L., Sehgal, A., Im, A., Hou, J. Z., et al. (2017). Phase 1 clinical trial of adoptive immunotherapy using “off-the-shelf” activated natural killer cells in patients with refractory and relapsed acute myeloid leukemia. Cytotherapy 19, 1225–1232. doi:10.1016/j.jcyt.2017.07.008
Dolstra, H., Roeven, M. W. H., Spanholtz, J., Hangalapura, B. N., Tordoir, M., Maas, F., et al. (2017). Successful transfer of umbilical cord blood CD34+ hematopoietic stem and progenitor-derived NK cells in older acute myeloid leukemia patients. Clin. Cancer Res. 23, 4107–4118. doi:10.1158/1078-0432.CCR-16-2981
FDA U.S. Department of Health and Human Services (2023). FDA U.S. department of Health and human services. Food Drug Adm. Available online at: https://www.fda.gov/media/170198/download.
FDA U.S. Department of Health and Human Services (2012). FDA U.S. Department of Health and human services. Food Drug Adm. Available online at: https://www.fda.gov/media/83904/download.
FDA U.S. Department of Health and Human Services (2024). FDA U.S. Department of Health and human services. Food Drug Adm. Available online at: https://www.fda.gov/vaccines-blood-biologics/guidance-complianceregulatory-information-biologics.
ICH (2009). ICH international conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. Available online at: https://database.ich.org/sites/default/files/Q8_R2_Guideline.pdf.
Lapteva, N., Szmania, S. M., van Rhee, F., and Rooney, C. M. (2014). Clinical grade purification and expansion of natural killer cells. Crit. Rev. Oncog. 19, 121–132. doi:10.1615/critrevoncog.2014010931
Lodoen, M. B., and Lanier, L. L. (2006). Natural killer cells as an initial defense against pathogens. Curr. Opin. Immunol. 18, 391–398. doi:10.1016/j.coi.2006.05.002
McKenna, D. H., Kadidlo, D. M., Cooley, S., and Miller, J. S. (2012). Clinical production and therapeutic applications of alloreactive natural killer cells. Methods Mol. Biol. 882, 491–507. doi:10.1007/978-1-61779-842-9_28
Shah, N. N., Baird, K., Delbrook, C. P., Fleisher, T. A., Kohler, M. E., Rampertaap, S., et al. (2015). Acute GVHD in patients receiving IL-15/4-1BBL activated NK cells following T-cell–depleted stem cell transplantation. Blood 125, 784–792. doi:10.1182/blood-2014-07-592881
Vivier, E., Rebuffet, L., Narni-Mancinelli, E., Cornen, S., Igarashi, R. Y., and Fantin, V. R. (2024). Natural killer cell therapies. Nature 626, 727–736. doi:10.1038/s41586-023-06945-1
Williams, B. A., Law, A. D., Routy, B., denHollander, N., Gupta, V., Wang, X. H., et al. (2017). A phase I trial of NK-92 cells for refractory hematological malignancies relapsing after autologous hematopoietic cell transplantation shows safety and evidence of efficacy. Oncotarget 8, 89256–89268. doi:10.18632/oncotarget.19204
Zhang, P., Yang, S., Zou, Y., Yan, X., Wu, H., Zhou, M., et al. (2019). NK cell predicts the severity of acute graft-versus-host disease in patients after allogeneic stem cell transplantation using antithymocyte globulin (ATG) in pretreatment scheme. BMC Immunol. 20, 46. doi:10.1186/s12865-019-0326-8
Keywords: NK cell, quality standand, ICH, CQA, clinical trial, cancer
Citation: von Werz V, Szarzynski A, Zigon-Branc S and Spadiut O (2025) Quality standards for NK cell immunotherapies. Front. Bioeng. Biotechnol. 13:1716975. doi: 10.3389/fbioe.2025.1716975
Received: 01 October 2025; Accepted: 29 October 2025;
Published: 11 November 2025.
Edited by:
Chunying Li, Georgia State University, United StatesReviewed by:
Diana Hernandez, Anthony Nolan, United KingdomCopyright © 2025 von Werz, Szarzynski, Zigon-Branc and Spadiut. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Oliver Spadiut, b2xpdmVyLnNwYWRpdXRAdHV3aWVuLmFjLmF0
 Valentin von Werz
Valentin von Werz Aleksander Szarzynski
Aleksander Szarzynski Sara Zigon-Branc
Sara Zigon-Branc Oliver Spadiut
Oliver Spadiut