- 1Duke Clinical Research Institute, Duke University Medical Center, Durham, NC, United States
- 2Department of Cardiology, Keio University School of Medicine, Tokyo, Japan
- 3Department of Cardiology, Saiseikai Central Hospital, Tokyo, Japan
Patients with cancer face a high short-term risk of arterial thromboembolism. One of the most fatal manifestations of arterial thromboembolism is myocardial infarction (MI), and patients with cancer face a 3-fold greater risk of MI than patients without cancer. The individual risk for arterial thrombotic events in patients with cancer is determined by the complex interaction of baseline cardiovascular risk factors, cancer type and stage, chemotherapeutic regimen, and other general contributing factors for thrombosis. Managing MI in patients with cancer is a clinical challenge, particularly due to cancer's unique pathophysiology, which makes it difficult to balance thrombotic and bleeding risks in this specific patient population. When patients with cancer present with MI, a limited proportion are treated with guideline-recommended therapy, such as antiplatelet therapy or invasive revascularization. Despite the limited evidence, existing reports consistently suggest similar clinical benefits of guideline-recommended therapy when administered to patients with cancer presenting with MI. In this review, we briefly summarize the available evidence, clinical challenges, and future perspectives on simultaneous management of MI and cancer, with a focus on invasive strategy.
Introduction
Advances in cancer treatments have significantly contributed to a decline in cancer-specific mortality rates; as a result, cardiovascular disease has become the leading cause of death among cancer patients (1). The incidence of myocardial infarction (MI), in particular, is higher in patients with active cancer compared to those without cancer (2). The increased risk of thrombotic events in cancer patients is partially attributable to the pro-coagulant state in this population, and partially due to the adverse effects of some chemotherapeutic agents (2). Data from a nationwide Swedish report indicated that the overall incidence of coronary heart disease was 152 per 100,000 person year in patients with cancer compared with 143 per 100,000 person year in patients without cancer, with the risk of heart disease being more prominent during the first 6 months post-cancer diagnosis than in the periods following this 6-month time window (3). Navi et al. reported similar findings from the Surveillance, Epidemiology, and End Results (SEER)–Medicare database; patients with cancer have a 3-fold greater risk of MI when compared with patients without cancer (6-month cumulative incidence of 2.0 vs. 0.7%, respectively; hazard ratio, HR, 2.9 [95% confidence interval, CI, 2.8–3.1]) (4). Although there is increasing recognition that cancer and heart disease coexist, there are limited data on how to optimally manage these high-risk patients with both comorbidities. In this mini review, we provide an overview of the pathophysiologic mechanism, presentation, and management of MI in patients with cancer, with a particular focus on invasive strategy.
Possible Mechanisms Causing Increased MI Risk in Patients With Cancer
The individual risk for arterial thrombotic events in cancer patients is determined by the complex interaction of multiple elements including baseline cardiovascular risk factors, cancer type and stage, chemotherapeutic regimen, and other general contributing factors for thrombosis (2). Conventional cardiovascular risk factors such as age, hypertension, dyslipidemia, smoking, and diabetes are often present in cancer patients. Lupus anticoagulant and hyperhomocysteinemia are other known contributing risk factors of arterial thrombosis (5). Additionally, the risk of arterial thrombosis substantially varies among different types of cancer. Navi et al. have shown that patients with lung, gastric, or pancreatic cancers had the highest rates of arterial thrombotic events, including MI and stroke (4). Among hematologic malignancies, patients with multiple myeloma have an increased risk of arterial thrombosis (6).
Aside from clinical factors, biological factors produced by tumor cells also contribute to activate hemostatic system in cancer patients. Cancer cells can activate the hemostatic system through the expression of pro-coagulant factors including tissue factor and cancer pro-coagulant, release of inflammatory cytokines (i.e., TNF-α, IL-1β) and microparticles, and adhesion to host vascular cells (Figure 1) (7–9).
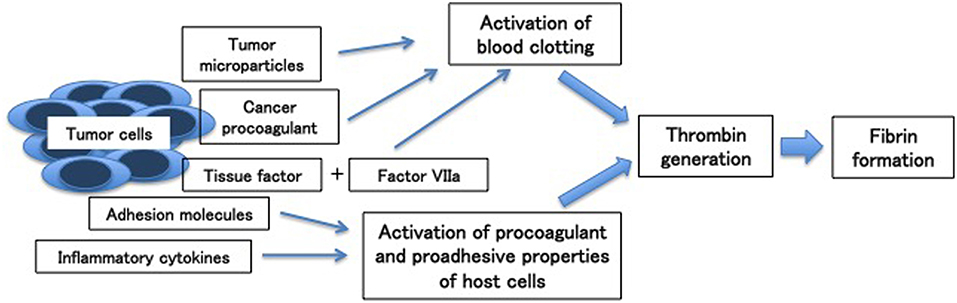
Figure 1. Mechanisms of cancer-associated thrombosis [Adapted from (7)].
Tissue factor, constitutively expressed on the malignant cell surface of a variety of cancers, plays an important role in thrombin by forming a complex with factor VIIa, triggering blood coagulation by activating factor IX and factor X. Unlike tissue factor, cancer procoagulant, another procoagulant factor, directly activates factor X independently of factor VII.
Microparticles, small plasma membrane vesicles, are also released by tumor cells and associated with hypercoagulable state of cancer. Their mechanism is related to intravascular thrombin generation by exposure of phosphatidylserine and procoagulant proteins such as tissue factor. In addition, tumor cells release inflammatory cytokines, which can stimulate the prothrombotic features of vascular cells.
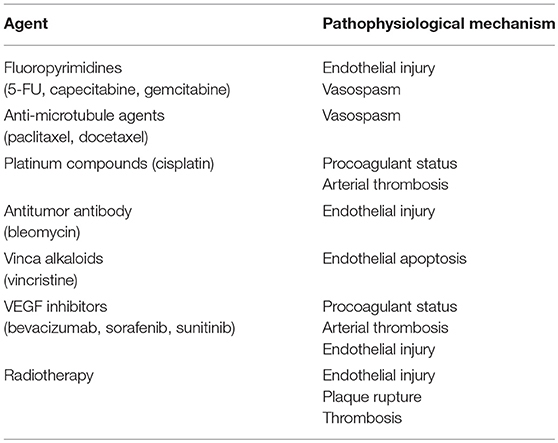
Several chemotherapy agents are known to increase the risk of myocardial ischemia through different mechanisms, including direct vasospastic effect, endothelial injury, acute arterial thrombosis, and the long-term effect on lipid metabolism, resulting in plaque destabilization. (Table 1) (5, 10, 11) For example, some cancer therapies cause endothelial damage resulting in arterial thrombosis. There are two main groups of chemotherapeutics that may have a detrimental effect on the function of endothelial cells: VEGF inhibitors and Bcr-Abl tyrosine kinase inhibitors. VEGF is an essential factor for endothelial cells to grow and survive. Inhibiting the VEGF pathway causes devastating injury to endothelial cells, resulting in progression of atherosclerosis, provocation of ischemia, and arterial thrombosis (2). The Bcr-Abl signaling pathway synergistically works with the VEGF pathway and plays a vital role in endothelial cell survival; therefore, chemotherapeutics for hematologic malignancies (e.g., chronic myeloid leukemia) that inhibit Bcr-Abl inhibitors result in an increased risk of arterial thrombosis (2).
Capecitabine- and 5-fluorouracil (5-FU)-associated cardiotoxicity is often overlooked, but both drugs are known to increase the risk of MI, especially when administered with a continuous 24-h 5-FU + leucovorin infusion for 5 days (12). Reports have also indicated that cisplatin is linked to an increased risk of MI. Moore et al. reported that 20 out of 932 patients who were treated with cisplatin-based chemotherapy experienced arterial thrombosis, including 2 MIs (13). Some molecular target drugs have also been implicated in the occurrence of MI. In patients with metastatic colorectal, breast, or non-small cell lung carcinoma, the combination therapy of bevacizumab, humanized monoclonal antibody against VEGF, and chemotherapy has been associated with an increased risk of arterial thrombosis compared with chemotherapy alone (5.5 vs. 3.1 events per 100 person-years) (14). A meta-analysis demonstrated that arterial thromboembolic events occurred more frequently in patients treated with sunitinib and sorafenib (which are VEGF receptor tyrosine kinase inhibitors) than in control patients, with an overall incidence of 1.7% for sorafenib and 1.4% for sunitinib (15). Radiotherapy, particularly applied to the supradiaphragmatic area, is also known to be associated with an increased risk of ischemic heart disease, mostly due to ostial lesions (16).
Clinical Manifestation
Cancer patients may experience different presentations of an acute MI. A University of Texas MD Anderson Cancer Center report highlighted how symptoms can be atypical in cancer patients, with dyspnea (rather than chest pain) being the most common presentation (17). Furthermore, of 456 cancer patients diagnosed with MI, 85% of them had non-ST-segment elevation MI (NSTEMI) and 15% had ST-segment elevation MI (STEMI) (17). As a result of these findings, patients with cancer who present with atypical symptoms (such as dyspnea) should be screened for acute MI. The initial cardiac workup for acute MI, which is based on repeated electrocardiograms and cardiac biomarkers, should be the same for patients with and without cancer. Munoz et al. found that >10% of cancer patients with acute coronary syndrome (ACS) presentation were ultimately diagnosed with Takotsubo cardiomyopathy, which is a non-ischemic transient cardiac syndrome that does not require antithrombotic therapy (18). Distinguishing between ACS and other cardiac syndromes like Takotsubo cardiomyopathy is important, since identifying the appropriate cardiac diagnosis may allow unnecessary antithrombotic medications (such as unfractionated heparin or P2Y12 inhibitors) to be discontinued in order to avoid unnecessary bleeding.
How to Manage MI in Cancer Patients
Prevention
Ischemic workup should be done in high-risk patients to detect pre-existing coronary artery disease, which is known to be a risk factor for chemotherapy-induced MI, before administering cancer drugs known to cause cardiac ischemia (Table 1) (19).
Medical Therapy
There are limited data that sufficiently address the management of cardiac ischemic disease in patients with cancer. Especially in an acute phase, there is scant data regarding optimal management for cancer patients presenting with MI. Further studies are warranted to clarify optimal antithrombotic regimen, such as unfractionated heparin vs. low molecular heparin vs. bivalirudin. Aspirin and beta-blockers are the primary drugs used to treat patients with MI in acute and chronic settings, but it is unknown if the safety and efficacy of aspirin and beta-blockers is equally preserved in cancer patients. Yusuf et al. retrospectively analyzed 456 cancer patients with MI, including 70 who presented with STEMI (17); of these patients, only 211 (46.3%) received aspirin. One-year survival was higher in patients treated with aspirin (34%) than in those without (18%). After adjustment for demographic baseline differences, aspirin use was significantly associated with improved survival at 1-year. Similarly, less than half (48.5%) of patients were treated with a beta-blocker, and 1-year survival was higher in those who received a beta-blocker (36.0%) compared with those who did not (16.0%). The survival benefit persisted even after multiple adjustments. Yusuf et al. also evaluated the efficacy of statin and angiotensin-converting enzyme inhibitors, but they were not associated with improved survival at 1 year. Despite a relatively small sample size and unmeasured confounders, Yusuf et al.'s study provides persuasive evidence in support of using aspirin and a beta-blocker when cancer patients suffer from MI. The National Registry of Acute Myocardial Infarction in Switzerland (AMIS Plus) reported consistently reduced standard of care treatment for MI in patients with cancer vs. those without (20); consequently, further efforts are required to facilitate rigorous implementation of these cardioprotective medications to improve patient outcomes.
Invasive Management
Cancer patients with MI are less likely to be treated with catheter-based revascularization (i.e., PCI), even for STEMI. The MD Anderson Cancer Center found that among 456 cancer patients with MI, only 11 (2.8%) underwent PCI. Of those presenting with STEMI, only 5.7% underwent PCI (17). Pothineni et al. analyzed the United States National Inpatient Sample and found that the utilization of PCI in STEMI patients with cancer varied according to the type of cancer, ranging from 17.3% in colon cancer to 30.8% in breast cancer (21). In the National Registry of Acute Myocardial Infarction in Switzerland (AMIS Plus), which is a large Swiss registry of patients with ACS, patients with a history of cancer were less likely to undergo PCI than those without (67.8 vs. 73.4%; adjusted odds ratio [OR] 0.76, 95% CI 0.67–0.88) (20). Notably, patients treated with PCI were less likely to die than those who did not receive revascularization (17, 21). There are a couple potential reasons for why a minority of patients with cancer received revascularization therapy: (1) a poor prognosis due to the cancer itself; and/or (2) concern for bleeding complications with prolonged dual antiplatelet therapy (DAPT) resulting from stent implantation. Due to advances in stent technology, the recommended duration of DAPT after stent implantation has been getting shorter, which may allow the PCI indication for cancer patients with MI to be expanded (22).
To date, there are several moderate-scale observational studies comparing clinical outcomes between patients with and without a history of cancer undergoing PCI (23–26). The CREDO-Kyoto Registry Cohort-2 (N = 12,180; history of cancer, 9.1%; ACS, 27.2%) found that the cumulative 5-year incidences of cardiovascular death were significantly higher in a group of patients with cancer vs. one without (12.4 vs. 7.5%, p < 0.001) (23). Even after adjustment, the excess risk of cardiovascular death in the cancer group relative to the non-cancer group remained significant. Findings were similar for other cardiovascular-related outcomes—the adjusted risks for all-cause death, non-cardiac death, heart failure readmission, and major bleeding were higher in cancer patients than in non-cancer patients, while the risks for MI and stroke were not different between groups. Subgroup analysis in ACS patients (N = 3,309, 27.2%) demonstrated consistent findings with those in the main analysis.
The Bleeding Complications in a Multicenter Registry of Patients Discharged after an Acute Coronary Syndrome (BleeMACS) project (N = 15,401; history of cancer, 6.4%), which was an international multicenter observational registry with 15 participating hospitals. BleeMACS compared the clinical outcomes of patients with and without cancer, who were also diagnosed with ACS and treated with PCI (25). After 1 year of follow-up, adverse cardiovascular events (i.e., a composite of all-cause death, MI, and bleeding events) and bleeding were significantly higher in cancer patients than non-cancer patients (adverse cardiovascular events: 15.2 vs. 5.3%; bleeding: 6.5 vs. 3.0%). After adjustment, the increased risks of adverse cardiovascular and bleeding events in cancer patients remained significant. An analysis from the Duke database (N = 15,008; history of cancer, 3.3%; ACS, 72.0%) found that after a 14-year follow-up, cardiovascular mortality was not different between groups (31.4 vs. 27.7%, p = 0.31), but the rate of all-cause death was significantly higher in patients with a history of cancer than in those without (79.7 vs. 49.3%, p < 0.01) (24). These varying results are likely due to differences in the definitions of cancer and outcomes, as well as differences in study population and follow-up duration.
When PCI is considered as a treatment option for cancer patients presenting with MI, higher risk of stent thrombosis should be taken into account. Several registries have demonstrated an underlying hypercoagulable state that predisposes cancer patients to a higher risk of stent thrombosis. The Dutch Stent Thrombosis Registry found that active cancer was associated with stent thrombosis (27). Among 437 patients diagnosed with definite stent thrombosis, 46 patients (10.5%) had active cancer. Similarly, the Coronary Revascularization Demonstrating Outcome Study in Kyoto (CREDO-Kyoto) Percutaneous Coronary Intervention (PCI)/Coronary Artery Bypass Grafting (CABG) Registry Cohort-2 reported that patients with active cancer who were undergoing PCI trended toward higher adjusted risk for definite or probable stent thrombosis as compared with patients without cancer, although this finding did not reach statistical significance (23). A retrospective chart review of patients treated with a bare metal stent at a single center in Germany also reported a higher rate of in-stent thrombosis in patients with cancer compared with those without cancer (5.6 vs. 0.8%) (28).
Intervention: Choice of Devices
Stent implantation undoubtedly remains the gold standard of PCI. Bare metal stents (BMS) were initially favored for patients with a high bleeding risk who were not able to tolerate prolonged DAPT; however, due to the development of drug-eluting stents (DES), prolonged duration of DAPT after DES placement is no longer necessary. The 2017 European Society of Cardiology Focused Update on Dual Antiplatelet Therapy in Coronary Artery Disease recommends 6 months of DAPT after stent implantation post-ACS event in patients with a high bleeding risk, regardless of stent type (22). In the 2016 American College of Cardiology (ACC)/American Heart Association (AHA) Focused Update on Duration of Dual Antiplatelet Therapy in Patients with Coronary Artery Disease, at least 1 year of DAPT is recommended for ACS patients treated with PCI, regardless of stent type (Class I), and P2Y12 therapy discontinuation after 6 months is considered a reasonable option for patients with high bleeding risk (Class IIb) (29). The other main concern surrounding DES deployment was a higher risk of stent thrombosis, but this concern has already been resolved by the development of second-generation DES (30). Given these improvements in DES, there may be no reason for physicians to recommend BMS over DES, even for those patients whose cardiac treatment is complicated by cancer.
Drug-coated balloon (DCB) is also an emerging technology in the field of PCI. Recently, the Basel Kosten Effektivitäts Trial–Drug-Coated Balloons vs. Drug-Eluting Stents in Small Vessel Interventions (BASKET-SMALL2) trial demonstrated the non-inferiority of DCB compared to DES regarding major adverse cardiovascular events (composite of cardiac death, non-fatal MI, and target-vessel revascularization) up to 12 months in PCIs for de novo lesions (<3 mm in diameter) in coronary vessels (31). This emerging technology could provide a novel management approach to MI, particularly for cancer patients in whom prolonged antiplatelet therapy often poses a major clinical dilemma. Nevertheless, current manufacturers still recommend 3 months of DAPT following DCB treatment; therefore, the clinical benefit of DCB over metallic stent when treating cancer patients presenting with MI is negated.
Intervention: Access Site
Another important key to reducing bleeding complications is appropriate access site selection. Given the high bleeding risk in patients with cancer, appropriate access site selection is particularly critical. In terms of reducing bleeding complications, radial access is generally considered favorable to femoral. There is no favored access site recommendation in the current 2013 American College of Cardiology Foundation (ACCF)/AHA Guideline for the Management of ST-Elevation Myocardial Infarction (32, 33), but in the 2017 ESC Guidelines for the Management of Acute Myocardial Infarction in Patients Presenting with ST-Segment Elevation, radial access is recommended for PCI in patients with ACS, including Class I STEMI (34, 35). Femoral access needs to be considered for PCI in hemodialysis patients or in patients in whom radial access is difficult to obtain. Every effort should be taken to avoid access site complications, such as the use of smaller sheath sizes, a lower dose of intra-arterial or intravenous unfractionated heparin, or a femoral angiogram after PCI (36). Due to the high-risk profile of bleeding complications, the Society for Cardiovascular Angiography and Interventions (SCAI) Expert Consensus Statement recommends a transradial approach for cancer patients who are excellent candidates for both radial and femoral access (36).
Patient With Thrombocytopenia
Cancer patients frequently develop thrombocytopenia after chemotherapy, with an incidence ranging from 10 to 25% (37). The standard approaches to treating an MI, such as antiplatelet, anticoagulant, and thrombolytic therapies exacerbate bleeding risk and, consequently, are typically withheld from patients with thrombocytopenia. Nonetheless, accumulating evidence may support the implementation of these standard approaches—even for this specific population. In hopes of addressing the efficacy and safety of antiplatelet therapy in MI patients with thrombocytopenia, investigators from the MD Anderson Cancer Center reported a case series (38) that demonstrated how, in patients with thrombocytopenia, the risk of bleeding varies and may depend on the underlying cause, instead of absolute antiplatelet counts. The investigators also showed that 7-day survival was higher in patients who received aspirin vs. those who did not (90 vs. 6%), although treatment selection bias should be taken into account (39). Iliescu et al. reviewed 30 cases with chronic thrombocytopenia (defined as absolute platelet count <100,000/mm3) who underwent coronary stenting and found that all procedures were completed without major bleeding complications and platelet transfusion (40). The SCAI Expert Consensus Statement recommends not to transfuse platelets prophylactically in cancer patients undergoing cardiac catheterization with thrombocytopenia, unless platelet counts are <20,000/ml and the oncology/hematology team recommends transfusion (36). SCAI also encourages reduced platelet count thresholds for cardiovascular therapies, recommending aspirin initiation in patients with platelet counts >10,000/ml and DAPT initiation (with aspirin and clopidogrel) if platelet counts are >30,000/ml. Due to a lack of evidence, prasugrel, ticagrelor, and glycoprotein IIb/IIIa inhibitors should not be used in patients with platelet counts <50,000/ml (36).
Conclusion
In cancer patients, endothelial dysfunction that is caused by the cancer cell(s) itself, as well as chemotherapeutics, promotes platelet aggregation, which results in an increased risk of arterial thrombosis (including MI). Optimal management of MI among patients with cancer remains a clinical challenge. Available evidence is only derived from small- or moderate-sized observational data; there are currently no randomized clinical trials investigating the optimal management of cancer patients at high risk for thrombosis. The studies that do exist contain inconsistent definitions of cancer, making it difficult to compare them to each other, and challenging to combine datasets. Looking to the future, prospective studies that involve cardiologists and oncologists who agree to universal definitions of MI and cancer will enhance our understanding of the best way to treat this high-risk population. Device improvement may enable the wide application of invasive management to this specific patient population, but further evidence is needed to optimally treat patients with cancer and MI.
Author Contributions
TI and CM contributed the study concept and design. TI contributed the manuscript drafting. AE and CM contributed the critical revision of the manuscript for important intellectual content, as well as the study supervision.
Conflict of Interest Statement
TI discloses the following relationships—Research Grant: JSPS Overseas Research fellowship and Boston Scientific. CM's disclosure can be viewed in the Supplementary Data Sheet 1.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We acknowledge Erin Campbell, MS (Duke Clinical Research Institute) for her editorial contributions.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2019.00057/full#supplementary-material
Data Sheet 1. CM's disclosure.
References
1. Patnaik JL, Byers T, DiGuiseppi C, Dabelea D, Denberg TD. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: a retrospective cohort study. Breast Cancer Res. (2011) 13:R64. doi: 10.1186/bcr2901
2. Tuzovic M, Herrmann J, Iliescu C, Marmagkiolis K, Ziaeian B, Yang EH. Arterial thrombosis in patients with cancer. Current treatment options in cardiovascular medicine. (2018) 20:40. doi: 10.1007/s11936-018-0635-x
3. Zoller B, Ji J, Sundquist J, Sundquist K. Risk of coronary heart disease in patients with cancer: a nationwide follow-up study from Sweden. Eur J Cancer. (2012) 48:121–8. doi: 10.1016/j.ejca.2011.09.015
4. Navi BB, Reiner AS, Kamel H, Iadecola C, Okin PM, Elkind MSV, et al. Risk of arterial thromboembolism in patients with cancer. J Am Coll Cardiol. (2017) 70:926–38. doi: 10.1016/j.jacc.2017.06.047
5. Zamorano JL, Lancellotti P, Rodriguez Munoz D, Aboyans V, Asteggiano R, Galderisi M, et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur J Heart Fail. (2017) 19:9–42. doi: 10.1002/ejhf.654
6. Kristinsson SY, Pfeiffer RM, Bjorkholm M, Goldin LR, Schulman S, Blimark C, et al. Arterial and venous thrombosis in monoclonal gammopathy of undetermined significance and multiple myeloma: a population-based study. Blood. (2010) 115:4991–8. doi: 10.1182/blood-2009-11-252072
7. Falanga A, Russo L, Verzeroli C. Mechanisms of thrombosis in cancer. Thromb Res. (2013) 131(Suppl. 1):S59–62. doi: 10.1016/S0049-3848(13)70024-0
8. Falanga A, Marchetti M, Russo L. The mechanisms of cancer-associated thrombosis. Thromb Res. (2015) 135(Suppl. 1):S8–11. doi: 10.1016/S0049-3848(15)50432-5
9. Falanga A, Russo L, Milesi V, Vignoli A. Mechanisms and risk factors of thrombosis in cancer. Critic Rev Oncol Hematol. (2017) 118:79–83. doi: 10.1016/j.critrevonc.2017.08.003
10. Herrmann J, Yang EH, Iliescu CA, Cilingiroglu M, Charitakis K, Hakeem A, et al. Vascular toxicities of cancer therapies: the old and the new–an evolving avenue. Circulation. (2016) 133:1272–89. doi: 10.1161/CIRCULATIONAHA.115.018347
11. Al-Hawwas M, Tsitlakidou D, Gupta N, Iliescu C, Cilingiroglu M, Marmagkiolis K. Acute coronary syndrome management in cancer patients. Curr Oncol Rep. (2018) 20:78. doi: 10.1007/s11912-018-0724-8
12. Kosmas C, Kallistratos MS, Kopterides P, Syrios J, Skopelitis H, Mylonakis N, et al. Cardiotoxicity of fluoropyrimidines in different schedules of administration: a prospective study. J Cancer Res Clin Oncol. (2008) 134:75–82. doi: 10.1007/s00432-007-0250-9
13. Moore RA, Adel N, Riedel E, Bhutani M, Feldman DR, Tabbara NE, et al. High incidence of thromboembolic events in patients treated with cisplatin-based chemotherapy: a large retrospective analysis. J Clin Oncol. (2011) 29:3466–73. doi: 10.1200/JCO.2011.35.5669
14. Scappaticci FA, Skillings JR, Holden SN, Gerber HP, Miller K, Kabbinavar F, et al. Arterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumab. J Natl Cancer Instit. (2007) 99:1232–9. doi: 10.1093/jnci/djm086
15. Choueiri TK, Schutz FA, Je Y, Rosenberg JE, Bellmunt J. Risk of arterial thromboembolic events with sunitinib and sorafenib: a systematic review and meta-analysis of clinical trials. J Clin Oncol. (2010) 28:2280–5. doi: 10.1200/JCO.2009.27.2757
16. Orzan F, Brusca A, Conte MR, Presbitero P, Figliomeni MC. Severe coronary artery disease after radiation therapy of the chest and mediastinum: clinical presentation and treatment. Br Heart J. (1993) 69:496–500. doi: 10.1136/hrt.69.6.496
17. Yusuf SW, Daraban N, Abbasi N, Lei X, Durand JB, Daher IN. Treatment and outcomes of acute coronary syndrome in the cancer population. Clin Cardiol. (2012) 35:443–50. doi: 10.1002/clc.22007
18. Munoz E, Iliescu G, Vejpongsa P, Charitakis K, Karimzad K, Lopez-Mattei J, et al. Takotsubo stress cardiomyopathy: “good news” in cancer patients? J Am Coll Cardiol. (2016) 68:1143–4. doi: 10.1016/j.jacc.2016.06.027
19. Chang HM, Moudgil R, Scarabelli T, Okwuosa TM, Yeh ETH. Cardiovascular complications of cancer therapy: best practices in diagnosis, prevention, and management: part 1. J Am Coll Cardiol. (2017) 70:2536–51. doi: 10.1016/j.jacc.2017.09.1096
20. Rohrmann S, Witassek F, Erne P, Rickli H, Radovanovic D. Treatment of patients with myocardial infarction depends on history of cancer. Eur Heart J Acute Cardiovasc Care. (2018) 7:639–45. doi: 10.1177/2048872617729636
21. Pothineni NV, Shah NN, Rochlani Y, Saad M, Kovelamudi S, Marmagkiolis K, et al. Temporal trends and outcomes of acute myocardial infarction in patients with cancer. Ann Transl Med. (2017) 5:482. doi: 10.21037/atm.2017.11.29
22. Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F, Jeppsson A, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. (2018) 39:213–60. doi: 10.1093/eurheartj/ehx419
23. Nakatsuma K, Shiomi H, Morimoto T, Watanabe H, Nakagawa Y, Furukawa Y, et al. Influence of A history of cancer on long-term cardiovascular outcomes after coronary stent implantation (an observation from coronary revascularization demonstrating outcome study-kyoto registry cohort-2). Eur Heart J Qual Care Clin Outcomes. (2018) 4:200–7. doi: 10.1093/ehjqcco/qcy014
24. Hess CN, Roe MT, Clare RM, Chiswell K, Kelly J, Tcheng JE, et al. Relationship between cancer and cardiovascular outcomes following percutaneous coronary intervention. J Am Heart Assoc. (2015) 4:e001779. doi: 10.1161/JAHA.115.001779
25. Iannaccone M, D'Ascenzo F, Vadala P, Wilton SB3, Noussan P1, Colombo F, et al. Prevalence and outcome of patients with cancer and acute coronary syndrome undergoing percutaneous coronary intervention: a BleeMACS substudy. Eur Heart J Acute Cardiovasc Care. (2017) 7:631–8. doi: 10.1177/2048872617706501
26. Tabata N, Sueta D, Yamamoto E, Takashio S, Arima Y, Araki S, et al. Impact of current and past cancer history on the risk of cardiovascular events following percutaneous coronary intervention: a Kumamoto University Malignancy and Atherosclerosis (KUMA) study. Eur Heart J Qual Care Clin Outcomes. (2017) 4:290–300. doi: 10.1093/ehjqcco/qcx047
27. van Werkum JW, Heestermans AA, Zomer AC, Kelder JC, Suttorp MJ, Rensing BJ, et al. Predictors of coronary stent thrombosis: the Dutch Stent Thrombosis Registry. J Am Coll Cardiol. (2009) 53:1399–409. doi: 10.1016/j.jacc.2008.12.055
28. Gross CM, Posch MG, Geier C, Olthoff H, Krämer J, Dechend R, et al. Subacute coronary stent thrombosis in cancer patients. J Am Coll Cardiol. (2008) 51:1232–3. doi: 10.1016/j.jacc.2007.11.061
29. Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, et al. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: an update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention, 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease, 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction, 2014 AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes, and 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery. Circulation. (2016) 134:e123–55. doi: 10.1161/CIR.0000000000000404
30. Philip F, Stewart S, Southard JA. Very late stent thrombosis with second generation drug eluting stents compared to bare metal stents: network meta-analysis of randomized primary percutaneous coronary intervention trials. Catheter Cardiovasc Intervent. (2016) 88:38–48. doi: 10.1002/ccd.26458
31. Jeger RV, Farah A, Ohlow MA, Mangner N, Möbius-Winkler S, Leibundgut G, et al. Drug-coated balloons for small coronary artery disease (BASKET-SMALL 2): an open-label randomised non-inferiority trial. Lancet. (2018) 392:849–56. doi: 10.1016/S0140-6736(18)31719-7
32. O'Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, de Lemos JA, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. (2013) 61:485–510. doi: 10.1016/j.jacc.2012.11.019
33. Amsterdam EA, Wenger NK, Brindis RG, Casey DE Jr, Ganiats TG, Holmes DR Jr, et al. 2014 AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. (2014) 64:e139–228. doi: 10.1016/j.jacc.2014.09.016
34. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. (2018) 39:119–77. doi: 10.1093/eurheartj/ehx393
35. Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J. (2016) 37:267–315. doi: 10.1093/eurheartj/ehv320
36. Iliescu CA, Grines CL, Herrmann J, Yang EH, Cilingiroglu M, Charitakis K, et al. SCAI Expert consensus statement: evaluation, management, and special considerations of cardio-oncology patients in the cardiac catheterization laboratory (endorsed by the cardiological society of india, and sociedad Latino Americana de Cardiologia intervencionista). Catheter Cardiovasc Intervent. (2016) 87:E202–23. doi: 10.1002/ccd.26375
37. Elting LS, Rubenstein EB, Martin CG, Kurtin D, Rodriguez S, Laiho E, et al. Incidence, cost, and outcomes of bleeding and chemotherapy dose modification among solid tumor patients with chemotherapy-induced thrombocytopenia. J Clin Oncol. (2001) 19:1137–46. doi: 10.1200/JCO.2001.19.4.1137
38. Yusuf SW, Iliescu C, Bathina JD, Daher IN, Durand JB. Antiplatelet therapy and percutaneous coronary intervention in patients with acute coronary syndrome and thrombocytopenia. Texas Heart Instit J. (2010) 37:336–40.
39. Sarkiss MG, Yusuf SW, Warneke CL, Botz G, Lakkis N, Hirch-Ginsburg C, et al. Impact of aspirin therapy in cancer patients with thrombocytopenia and acute coronary syndromes. Cancer. (2007) 109:621–7. doi: 10.1002/cncr.22434
Keywords: myocardial infarction, cancer, arterial thrombosis, chemotherapy, invasive strategy
Citation: Inohara T, Endo A and Melloni C (2019) Unmet Needs in Managing Myocardial Infarction in Patients With Malignancy. Front. Cardiovasc. Med. 6:57. doi: 10.3389/fcvm.2019.00057
Received: 30 November 2018; Accepted: 23 April 2019;
Published: 17 May 2019.
Edited by:
Anna Falanga, Ospedale Papa Giovanni XXIII, ItalyReviewed by:
Plinio Cirillo, University of Naples Federico II, ItalyValerio De Stefano, Università Cattolica del Sacro Cuore, Italy
Copyright © 2019 Inohara, Endo and Melloni. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chiara Melloni, Y2hpYXJhLm1lbGxvbmlAZHVrZS5lZHU=
 Taku Inohara1,2
Taku Inohara1,2 Chiara Melloni
Chiara Melloni