Abstract
Background:
Proper prognostic biomarker is of great importance for clinical decision-making in patients with acute myocardial infarction (AMI) undergoing percutaneous coronary intervention (PCI). Although recently emerges plenty of novel inflammatory biomarkers, the canonical inflammatory mediator C-reactive protein still plays an important role in prognosing adverse post-infarction complications.
Methods:
PubMed, Embase, and Medline were systematically searched from the establishment of databases up to December 2021, conforming with standards set forth by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.
Results:
A total of 23 studies were eventually eligible for this meta-analysis, including 18,715 individuals. Our findings showed that elevated C-reactive protein (CRP) had a statistically significant superiority in predicting all-cause mortality (OR: 3.22, 95% CI: [2.71, 3.84], p < 0.00001), cardiovascular death (OR: 3.26, 95% CI: [2.30, 4.61], p < 0.00001), major adverse cardiovascular events (MACEs) (OR: 2.85, 95% CI [2.08, 3.90], p < 0.00001), heart failure (OR: 2.29, 95% CI: [1.48, 3.54], p = 0.0002), recurrent myocardial infarction (OR: 1.76, 95% CI: [1.28, 2.43], p < 0.001), and restenosis (OR: 1.71, 95% CI: [1.18, 2.47], p = 0.004). Subgroup analysis implies that CRP had better performance in predicting plenty of hospitalization and short-term (<12 months) adverse prognosis than long-term prognosis and Asian patients with elevated CRP were under more risk in adverse prognosis after PCI than Europeans.
Conclusion:
Our meta-analysis suggests that CRP is a prospective predictor of the prognosis in patients with AMI undergoing PCI, especially in hospitalization and short-term and in the Asian group.
Introduction
Despite advances in the therapies for coronary artery disease, such as percutaneous coronary intervention (PCI) which has saved a lot of lives since its application (1), myocardial infarction is still the main cause of morbidity and mortality among all cardiovascular diseases worldwide (2, 3). For patients with acute myocardial infarction (AMI) undergoing PCI, inflammatory response plays a pivotal role in the whole pathophysiological process. Since inflammation can promote endothelial cell injury (4), vascular remodeling (5), and plaque destabilization (6), it is now regarded as another risk factor for AMI besides traditionally acknowledged risk factors like hypertension and dyslipidemia (7). In addition, the pro-inflammatory response occurs in the early stage of AMI and may exacerbate after PCI, thus contributing to the death of myocardial cells (8).
During AMI, the release of the intracellular content and destruction of the extracellular matrix (9) leads to the generation of a cascade of inflammatory infiltration and mediators (10). Biomarkers from inflammatory infiltration are based on the accumulation of plasma inflammatory cells (11) [e.g., neutrophil-to-lymphocyte ratio (NLR) (12), systemic immune-inflammation index (SII) (13), and novel subclasses of Tregs (14)], which are easily influenced by heterogeneity under ethnicity and individuals. With respect to inflammatory mediators, plasma molecules consisting of common inflammatory pathways are routinely measured in clinical practice and have been studied as prognostic biomarkers, such as IL-1b (15) or cytokine concentrations and proinflammatory to anti-inflammatory cytokine ratios (16). However, some limitations like a small sample size are unneglectable in all these studies, requiring for in-depth investigation. It is noteworthy that C-reactive protein (CRP), among plenty of canonical inflammatory mediators, has exhibited excellent capacity in predicting post-STEMI adverse prognoses in comprehensive comparison studies (16–19). It might result from that CRP, as a canonical downstream of multiple inflammatory pathways (8, 17, 18, 20), actively participating in the inflammatory process (21), stimulating the secretion of various pro-inflammatory cytokines, promoting the release of reactive oxygen, and inducing the switch of quiescent macrophage to pro-inflammatory M1 subtype (22). Even the relationship between the prognosis of AMI and other novel biomarkers has been extensively explored, such as plasma long pentraxin-3 (23) and YKL-40 (24), but none have been proven as practically useful as hsCRP.
Hence, CRP is a typically and frequently used biomarker of inflammation, which may give rise to an increased cardiovascular risk (6). The relationship between the increase of plasma CRP concentration and the prognosis of patients with AMI, such as mortality (25, 26), cardiovascular mortality (27, 28), the rate of major adverse cardiovascular events (MACEs) (29, 30), heart failure (5), recurrent myocardial infarction (31, 32), and restenosis (33, 34), has been thoroughly studied in the past decades. A single study alone can only partially depict the whole profile of the association between CRP and poor prognosis in AMI after CRP. Besides, although Mincu et al. (35) had made a persuasive meta-analysis in 2016 including seven studies and 6,993 patients, the detailed subgroup analyses remain to be in-depth investigated. Therefore, we conducted this meta-analysis in an attempt to elucidate the relationship between the elevation of CRP and the prognosis of patients with AMI undergoing PCI and figure out its special traits in clinical implication.
Methods
A meta-analysis conformed with standards set forth by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (36).
Search strategy
PubMed, Embase, and Medline were systematically searched from the establishment of databases up to December 2021. The search strategy was edited following the principle of each database and included keywords related to CRP, AMI, and PCI. Previous meta-analyses and other reviews related to the topic were reviewed to identify studies not included in this search strategy. We also scanned the bibliographies of the included articles and relevant reviews for further reference.
Inclusion and exclusion criteria
After removing duplicates, titles and abstracts were screened to identify potentially relevant studies. All potentially relevant studies proceeded to full-text review by either reviewer independently and studies that met all the following criteria were included as follows: (1) the study was designed as randomized studies, prospective, or retrospective observational design studies; (2) the study population was patients with AMI (including STEMI and NSTEMI) treated with PCI; (3) the target variant was CRP and concrete information of CRP including cut-off, patient number in different groups, measuring methodology and time was available; and (4) the study reported follow-up duration in detail and outcomes of patients.
Exclusion criteria as follows: (1) articles were reviews, animal studies, laboratory studies, conference documents, and letters; (2) the population of studies included patients who suffered from other coronary diseases like stable or unstable angina; (3) patients were treated with other therapies like conservative medication, thrombolysis, or coronary artery bypass graft; (4) patients were not divided into groups by CRP or cut-off was unavailable; (5) incidence of outcomes in each group could not be directly attained or indirectly calculated; and (6) studies were without access to full text for quality assessment or data extraction.
Any discrepancy was settled by getting through full texture to reach a consensus.
Data extraction
Two of the authors independently performed data extraction, using a standard data extraction form that contained publication details (name of the first author, year of publication, and region), characteristics of the studied population (sample size, age, and gender distribution), and traits of CRP (high-sensitivity, cut-off, and time of blood taking), the follow-up, and outcomes.
Outcomes
The end points were as follows: all-cause mortality, cardiovascular death, MACE, heart failure, recurrent myocardial infarction, restenosis, and revascularization. All-cause mortality was defined as the death without specific causes. MACE was a composite definition usually consisting of death, cardiovascular death, recurrent MI, revascularization, stroke, and heart failure. In some studies, more adverse events were composited into MACEs like cardiopulmonary resuscitation and malignant arrhythmia; on the contrary, some selected only few events as MACE. Restenosis is defined as a narrowing of vessel diameter greater than 50% to that of the reference vessel (37), consisting of stent restenosis and in-stent restenosis (38). The former is defined as the presence of an acute coronary syndrome with angiographic or autopsy evidence of thrombus or occlusion. In this meta-analysis, we did not conduct the subgroup analysis between different types of restenosis due to the limitation of research quantity.
Data analysis
All analyses were conducted using the Review Manager 5.4. The results of our meta-analysis were present as adjusted ORs with 95% confidence intervals (CI). The heterogeneity was presented with estimation using the I2 statistic. When I2 statistic is less than 50%, the measured data were pooled in the study and analyzed using a fixed-effects meta-analysis model with inverse variance weighting. On the contrary, a random-effects model is selected. All p-values were two-tailed with the statistical significance set at 0.05.
Results
Study selection
The study selection process is shown in Figure 1. After selection and evaluation, 23 studies were eventually eligible for this meta-analysis (5, 25–34, 39–50) (Table 1). Overall, there were 18,715 patients involved in our analysis, 12,109 in the elevated CRP group and 6,606 in the normal CRP group. The follow-up period varied from in-hospital to 48.3 months. CRP in 17 (5, 27, 28, 30–34, 39, 41, 43–47, 49, 50) studies were measured with high-sensitivity methodology and in one (29) not mentioned. The cut-off values in different experiments were not exactly the same, while 2 mg/L (5, 28, 30, 32, 43), 3 mg/L (25, 27, 33, 40, 41, 45, 49), and 5 mg/L (31, 39, 42) were the most common cut-off values in included experiments. Instead of presupposed cut-off, some studies divided patients by points of bisection (34, 46–48, 50), trisection (28, 30), quadrisection (41, 44) according to CRP value. The diversion point ranged from 2 to 9 mg/L (46) and was selected as the cut-off. The majority of population was originated from Europe (5, 29, 32, 41, 48) and Asian (25–28, 30, 31, 33, 34, 39, 40, 42–47, 49, 50).
FIGURE 1
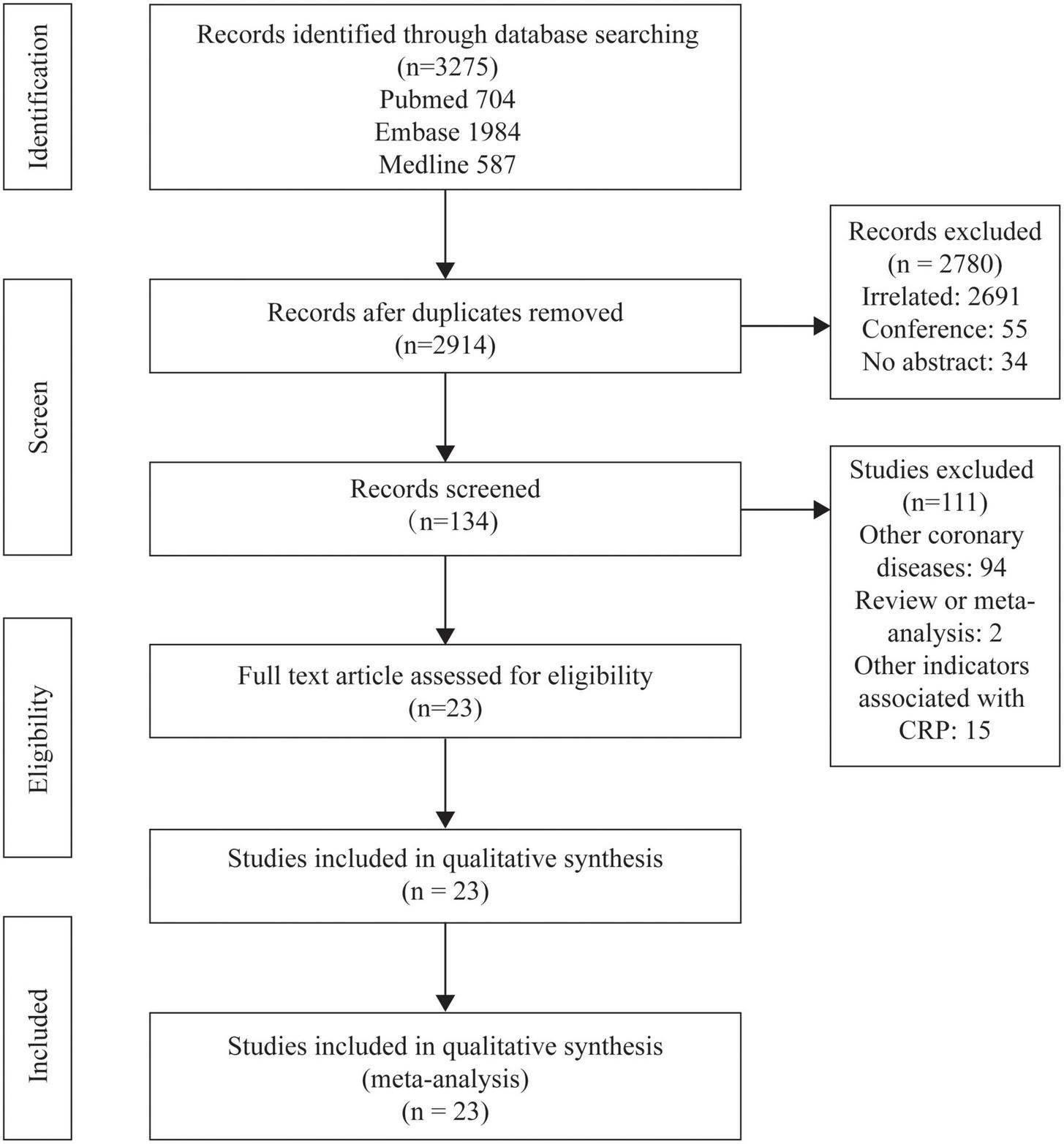
Flowcharts showing relevant studies.
TABLE 1
| First author |
Year | Region | Number of patients included (age, %males) | Types of MI | Follw-up* | hs-CRP | Cut-off (mg/L) | Measuring time | Endpoints | Results |
| Bicciré, F. G. | 2021 | Europe | 220 (68 ± 12.1, 58%) | STEMI | In-hospital | N/A | 17 | Peak value | MACEs | Low SA is not infrequent in patients admitted for STEMI treatment and is associated with worse outcomes independently from troponin and CRP levels. |
| Wang, Y. | 2021 | Asian | 2318 (58.8 ± 11.9, 79.8%) | STEMI | 30 months | Yes | 2.0 | After PCI | MACEs | Systemic inflammation (hsCRP≥2mg/L) can modulate Lp(a)-associated MACE risk in STEMI-PCI patients. |
| Świątkiewicz, I. | 2021 | Europe | 204 (56.17, 76.5%) | STEMI | 6 months | Yes | 2.0 | 1 month after discharge | HF | Persistent elevation in CRP concentration post-STEMI can serve as a risk marker and aid in identifying patients at increased risk of HF and HF-related mortality in multi-year period. |
| Jiang, H. | 2021 | Asian | 203 (60.35 ± 10.24, 66.0%) | STEMI | 12 months | Yes | 2.0 | Within 72 h inhospitalization | All-cause death, cardiovascular death, HF, reMI | The presence of hs-CRP was not only significantly associated with platelet inhibition function, but was also a prognostic marker in STEMI. |
| Kang, D. O. | 2019 | Asian | 4410 (62.7 ± 12.4, 77.3%) | STEMI and NSTEMI | 36 months | Yes | 3.0 | On admission | All-cause death, cardiovascular death, MACEs, reMI | The prognostic impact of elevated hs-CRP at baseline was most evident during the first 6 months after AMI. |
| Her, A. Y. | 2017 | Asian | 146 (57.1 ± 12.4, 81.4%) | STEMI | 24 months | Yes | 2.7 | On admission | MACEs | Elevated hs-CRP and NLR levels were significantly associated with MACE in STEMI patients successfully treated with DES and had incremental predictive values over conventional risk factors. |
| Shin, H. C. | 2017 | Asian | 381 (61.64 ± 11.0, 76.1%) | STEMI and NSTEMI | 24 months | No | 7.6 | Before PCI | All-cause death, cardiovascular death, MACEs, reMI, restonisis | Elevated levels of both NLR and CRP are associated with increased risk of long-term mortality in AMI patients with PCI. |
| Wang, C. H. | 2015 | Asian | 241 (63.70 ± 11.96, 77.6%) | STEMI | 48.3 months | Yes | 3.0 | After PCI | All-cause death, cardiovascular death, MACEs, reMI, restonisis | Renal dysfunction and elevated hsCRP predict a high long-term incidence of MACE in patients with acute STEMI with primary PCI, with the combination being of prognostic significance for long-term mortality and MI in these patients. |
| Shacham, Y. | 2015 | Asian | 562(62 ± 13, 80%) | STEMI | 1 month | Yes | 9.0 | Before PCI | All-cause death and HF | Admission serum hs-CRP level (>9 mg/l) is an independent predictor for AKI following primary PCI in STEMI patients. |
| Jian-Wei, Z. | 2014 | Asian | 1452(64.50 ± 13.29, 81.68%) | STEMI | In-hospital | Yes | 3.0 | Before PCI | All-cause death and MACEs | Higher baseline hsCRP level (>6.50 mg/L) was an independent and significant predictor of CIN after p-PCI. A high level of hsCRP was strongly associated with inhospital mortality and composite MACE. |
| He, Y. T. | 2013 | Asian | 220(62.38 ± 12.28, 82.73%) | STEMI | In-hospital | Yes | 6.26 | On admission | All-cause death | hs-CRP is positively correlated with CIN incidence. |
| Kim, K. H. | 2013 | Asian | 5123(62.94 ± 13.38, 74.12%) | STEMI | 24 months | Yes | 3.0 | On admission | MACEs | For STEMI patients with a long ischemic time (≥6 hours), an elevated level of hs-CRP is a poor prognostic factor of long-term cardiovascular outcomes. |
| Ahmed, K. | 2012 | Asian | 5647(60.4 ± 12.2, 75.2%) | STEMI and NSTEMI | 12 months | Yes | 2.0 | On admission | All-cause death and MACEs | Higher baseline hs-CRP level (≥4.08 mg/dL) in overweight/obese AMI patients showed significant association with 12-month all-cause mortality independent of other prognostic markers. |
| Schoos, M. M. | 2011 | Europe | 258(60.83, 75.97%) | STEMI | 36 months | Yes | 2.0 | Before pPCI | All-cause death, cardiovascular death, reMI and restenosis | BMS implantation should be preferred when hs-CRP is <2 mg/L and DES when hs-CRP is >2 mg/L to decrease long-term adverse outcomes including stent thrombosis in patients with STEMI treated with pPCI. |
| Damman, P. | 2011 | Europe | 1034(62 ± 13, 73%) | STEMI | 30 months | No | 7.0 | Before pPCI | All-cause death | The sole use or addition of a multimarker to a model including established risk factors improves the prediction of mortality in STEMI patients undergoing PPCI. |
| L. I. Gui-Hua | 2009 | Asian | 84(58 ± 11, 65.48%) | STEMI | 3–12 months | No | 5.0 | Within 6h onset | Cardiovascular death, MACEs and HF | The CRP levels within 6h after attack of AMI can be taken as one of the indexes to predict the prognosis of PCI. |
| Ortolani, P. | 2008 | Europe | 758(68.00, 70.45%) | STEMI | in-hospital | Yes | 3.1 | On admission | All-cause death, MACEs, reMI and restenosis | hs-CRP levels at admission independently predict in-hospital and long-term clinical outcome, potentially negatively influencing survival. |
| Jeong, Y. H. | 2008 | Asian | 207(57.3 ± 12.0, 81.6%) | STEMI | 12 months | Yes | 5.0 | On admission | All-cause death, MACEs, HF and reMI | cTnI and hs-CRP levels on admission give no addictive information on classic TIMI risk score for predicting long-term cardiovascular outcomes in STEMI patients treated with primary DES implantation and intensive medical therapy. |
| Yip, H. K. | 2005 | Asian | 146(60.0 ± 10.8, 86.3%) | STEMI | 1 months | Yes | 2.37 | Before PCI | All-cause death, MACEs and restenosis | Prospective evaluation ofthe hsCRP in STEMI of onset < 6 h allows accurate risk stratification of individuals at risk of 30-day MACE after primary PCI. |
| Liu, Jun | 2004 | Asian | 76(N/A) | STEMI | 6 months | No | 3.0 | Within 6 h onset | All-cause death, MACEs and reMI | CRP levels within six hours after the onset of AMI might predict early and late outcome after primary PCI. |
| Magadle, R. | 2004 | Asian | 230(63,58 ± 8.80, 75.22%) | STEMI | 12 months | Yes | 5.0 | Before PCI | All-cause death, MACEs, reMI and restenosis | Preprocedural serum CRP level might be considered a powerful predictor of early but not late complications in patients undergoing PTCA/stent procedures. |
| Hong, Y. J. | 2003 | Asian | 208(59.43 ± 9.96, 79.81%) | STEMI | 12 months | No | 1.0 | On admission | All-cause death, MACEs, reMI and restenosis | An leevated CRP is an independent prognostic marker in patients with acute myocaridal infarction after primary or rescue PCI. |
| Tomoda, H. | 2000 | Asian | 234(62.42 ± 10, 77.35%) | STEMI | 6 months | No | 3.0 | Within 6 h onset | All-cause death, MACEs, reMI and restenosis | CRP levels within 6 h after the onset of AMI reflect the vulnerability of culprit coronary lesions and predict adverse coronary events after primary PTCA/stenting. |
Characteristics of studies included in the meta-analysis.
*The longest follow time of studies.
C-reactive protein and outcomes
Elevated CRP was associated with increased all-cause mortality (OR: 3.22, 95% CI [2.71, 3.84], p < 0.00001, I2 = 46%) (25–28, 31–34, 39–41, 43, 44, 46–49) and cardiovascular mortality (OR: 3.26, 95% CI [2.30, 4.61], p < 0.00001, I2 = 34%) (27, 28, 32–34, 42). In addition to mortality, elevated CRP were also related to higher incidence of various malignant cardiovascular events, such as MACEs (OR: 2.85, 95% CI [2.08, 3.90], p < 0.00001, I2 = 81%) (25–27, 29–31, 33, 34, 39–43, 45, 47, 49, 50), heart failure (OR: 2.29, 95% CI [1.48, 3.54], p = 0.0002, I2 = 0%) (5, 28, 31, 42, 46), recurrent myocardial infarction (OR: 1.76, 95% CI [1.28, 2.43], p < 0.001, I2 = 33%) (25–28, 31–34, 39–41), and restenosis (OR: 1.71, 95% CI [1.18, 2.47], p = 0.004, I2 = 0%) (25, 26, 32–34, 39, 41, 42, 47). All these results have shown the potential of CRP as the prognostic biomarker for multiple outcomes in patients with AMI after PCI (Figure 2). With respect to the situation that different variants like duration of follow-up, traits of CRP, and racial distributions may lead to different outcomes, we processed the data in the trials and conducted subgroup analyses to shrink heterogeneity, while sensitivity analysis verified the stability of results (Supplementary Table 1).
FIGURE 2
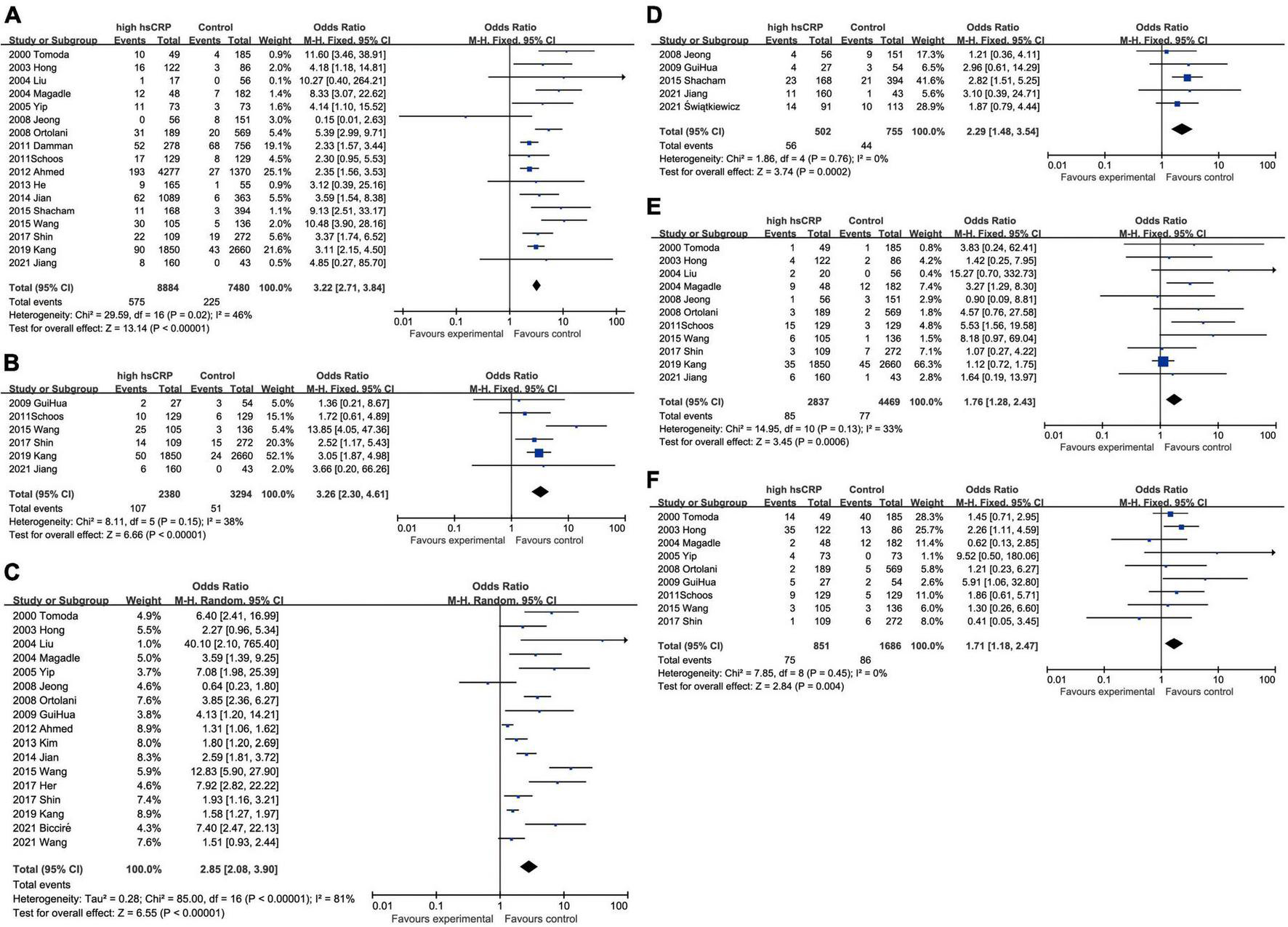
Forest plots of studies assessing the association between elevated CRP and different outcomes among patients with AMI with PCI: (A) CRP and all-cause mortality; (B) CRP and cardiovascular mortality; (C) CRP and MACEs; (D) CRP and heart failure; (E) CRP and recurrent myocardial infarction; and (F) CRP and restenosis.
Subgroup analysis
C-reactive protein and all-cause mortality
All-cause mortality was one of the most concerning outcomes of AMI. Increased CRP was invariably a powerful predictor to all-cause mortality in hospital (OR: 4.52, 95% CI [2.81, 7.27], p < 0.00001, I2 = 39%), <12 months (OR: 4.51, 95% CI [2.73, 7.45], p < 0.00001, I2 = 54%) and ≥12 months (OR: 3.18, 95% CI [2.19, 4.63], p < 0.00001, I2 = 52%) (Figure 3A). When we took the marginal value of CRP into subgroup analysis, 3 mg/L (OR: 5.10, 95% CI [3.21, 8.12], p < 0.00001, I2 = 48%) was the most frequently chosen cut-off value and had been shown a more powerful capacity than 2 mg/L (OR: 2.37, 95% CI [1.64, 3.42], p < 0.00001, I2 = 0) and 5 mg/L (OR: 1.34, 95% CI [0.02, 113.29], p = 0.90, I2 = 88%) to predict the mortality. Five milligrams per liter exhibited no statistical significance. Although 9 mg/L had shown to be the best predictor (OR: 9.13, 95% CI [2.51, 33.17], p = 0.008), only one study was included. Besides, elevated hsCRP (OR: 3.31, 95% CI [2.68, 4.09], p < 0.00001, I2 = 49%) had slight advantage than CRP (OR: 3.00, 95% CI [2.20, 4.08], p < 0.00001, I2 = 46%). In addition, the upregulated CRP was shown to be less associated with mortality in European (OR: 3.09, 95% CI [1.73, 5.51], p < 0.00001, I2 = 43%) than in Asian (OR: 4.18, 95% CI [2.95, 5.93], p < 0.00001, I2 = 65%) (Figure 3).
FIGURE 3
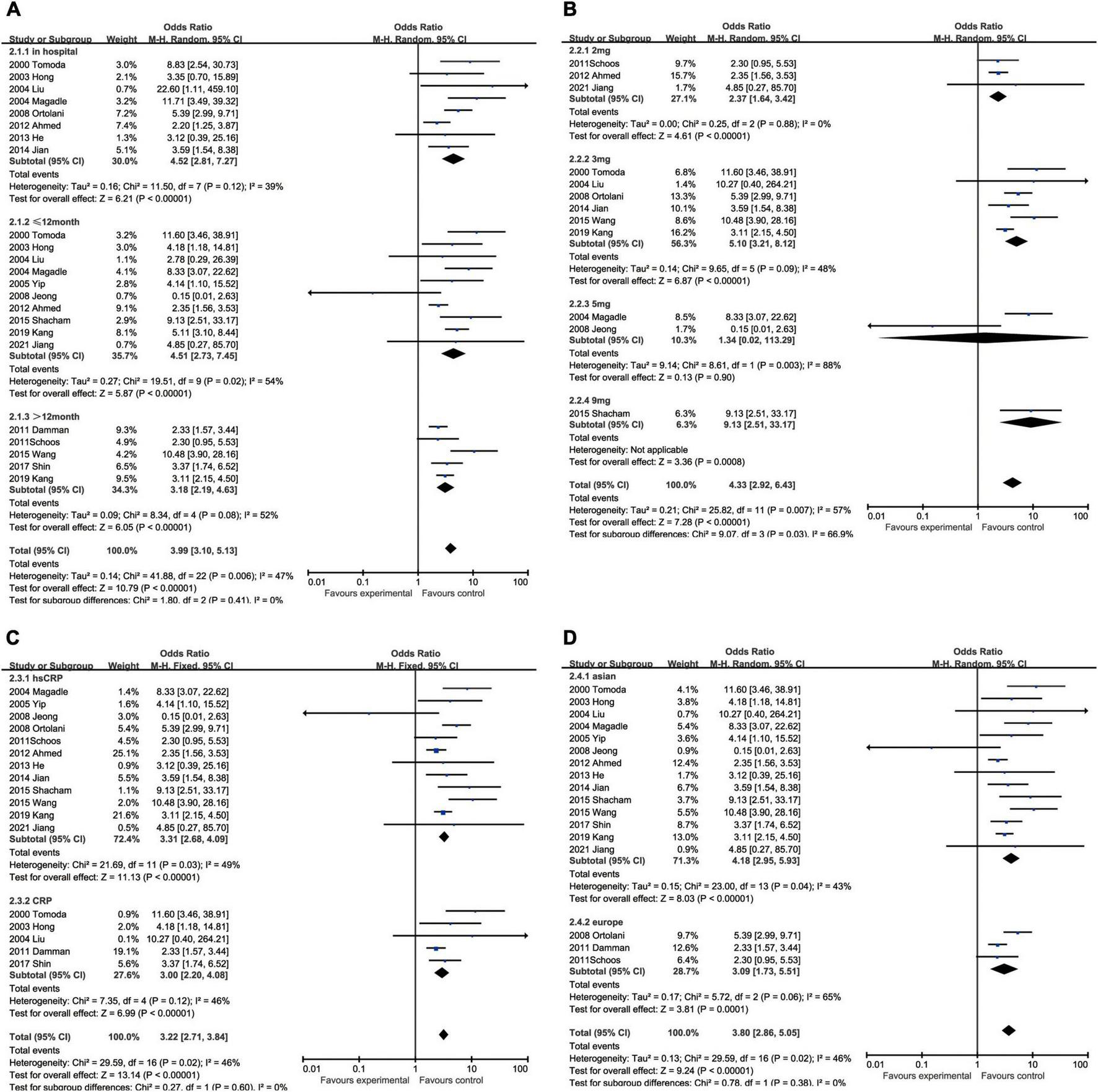
Forrest plots of subgroup analysis assessing all-cause mortality associated with increased CRP: (A) subgroups based on follow-up; (B) subgroups based on cut-off value of hsCRP; (C) subgroups based on CRP and hsCRP; and (D) subgroups based on ethnicities.
C-reactive protein and cardiovascular mortality
The augment of CRP amplified the possibility of cardiovascular death within 12 months after hospitalization (OR: 4.50, 95% CI [2.48, 8.15], p < 0.00001, I2 = 0) and the influence still existed when the follow-up was prolonged to more than 12 months (OR: 3.33, 95% CI [1.74, 6.37], p < 0.00001, I2 = 59%). With respect to the critical value of CRP, 3 mg/L (OR: 5.84, 95% CI [1.32, 25.80], p = 0.02, I2 = 81%) was usually the most recommended, while the others like 2 mg/L (OR: 1.88, 95% CI [0.70, 5.01], p = 0.21, I2 = 0) and 5 mg/L (OR: 1.36, 95% CI [0.21, 8.67], p = 0.74) had no statistical significance. The measuring methodologies did not exert obvious impact on the incidence of cardiovascular death, although hsCRP (OR: 3.78, 95% CI [1.63, 8.77], p = 0.002, I2 = 57%) was presumed to perform better than CRP (OR: 2.31, 95% CI [1.14, 4.68], p = 0.02, I2 = 0). The ethnicity had also an effect on cardiovascular death, while Asian (OR 3.53, 95% CI [2.44, 5.10], p < 0.00001, I2 = 42%) were at higher risk than European (OR: 1.72 [0.61, 4.89], p = 0.20) and the latter had no statistical significance (Figure 4).
FIGURE 4
Forrest plots of subgroup analysis assessing cardiovascular mortality associated with increased CRP: (A) subgroups based on follow-up; (B) subgroups based on cut-off value of hsCRP; (C) subgroups based on CRP and hsCRP; and (D) subgroups based on ethnicities.
C-reactive protein and major adverse cardiovascular events
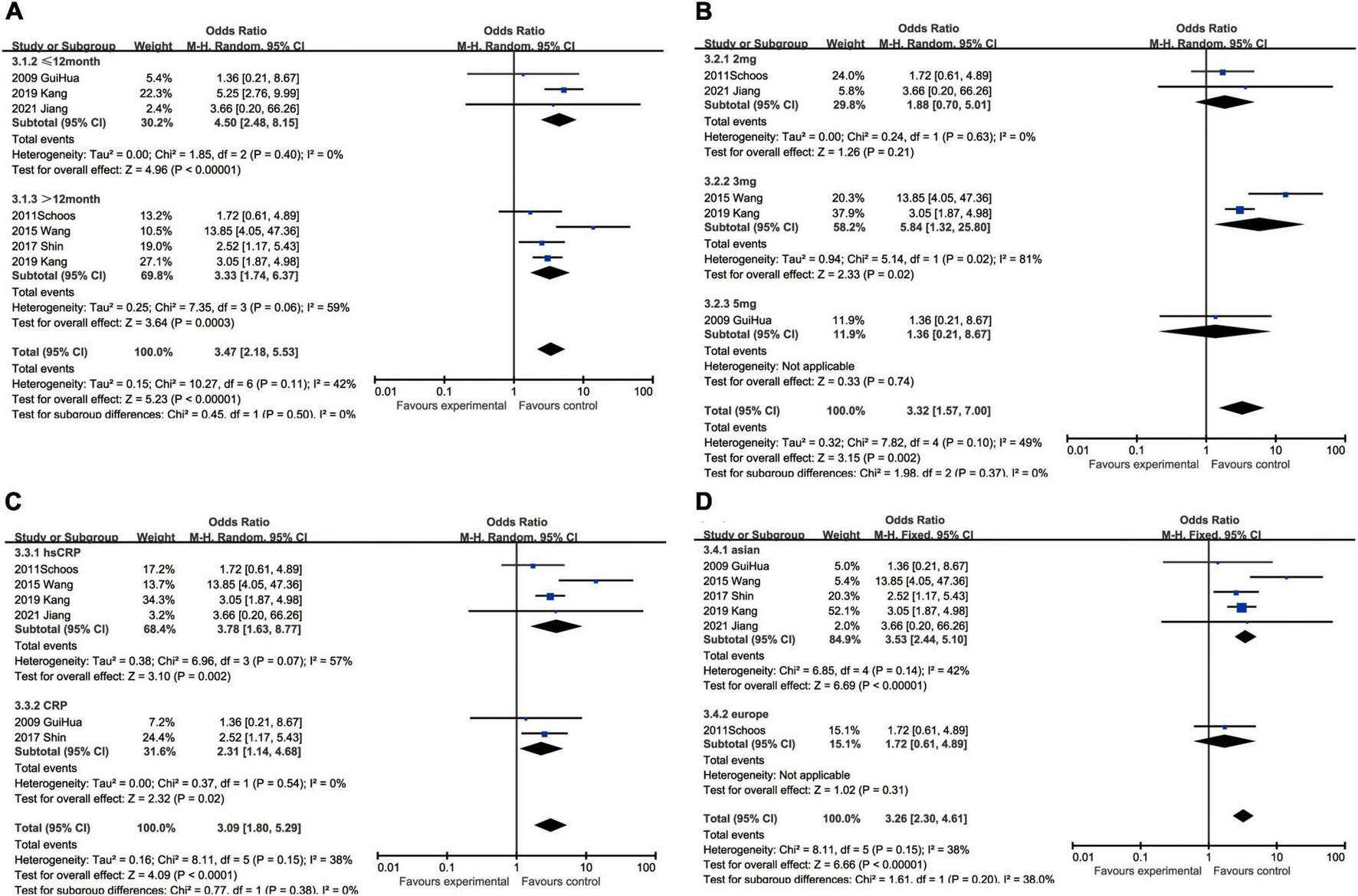
In regard to MACEs, elevated CRP might result in increased hospitalized malignant events (OR: 3.66, 95% CI [2.54, 5.28], p < 0.00001, I2 = 37%) with medium heterogeneity. We also analyzed the association between ascending CRP and incidence of MACEs within 12 months (OR: 1.76, 95% CI [1.24, 2.51], p = 0.002, I2 = 69%) or over 12 months (OR: 2.68, 95% CI [1.62, 4.46], p = 0.0001, I2 = 84%), but greater heterogeneity was accompanied with the elongation of follow-up. Three milligrams per liter (OR: 3.51, 95% CI [2.10, 5.87], p < 0.00001, I2 = 86%) was a superior threshold in dividing patients with AMI with higher or lower risk of MACEs, while mediocre performances exhibited in other cut-off such as 2 mg/L (OR: 1.34, 95% CI [1.11, 1.63], p = 0.003, I2 = 0) and 5 mg/L (OR: 2.09, 95% CI [0.63, 6.89], p = 0.23, I2 = 73%) which had no statistical significance. As for CRP measuring methodology, CRP (OR: 3.37, 95% CI [1.75, 6.46], p = 0.0003, I2 = 53%) was more efficient to predict MACEs than hsCRP (OR: 2.55, 95% CI [1.78, 3.65], p < 0.00001, I2 = 85%). Considering ethnics, the subgroup analysis implied that Caucasian patients (OR: 4.43, 95% CI [2.59, 7.57], p < 0.00001, I2 = 15%) were more susceptible to MACEs than Asian (OR: 2.62, 95% CI [1.90, 3.61], p < 0.00001, I2 = 80%) (Figure 5).
FIGURE 5
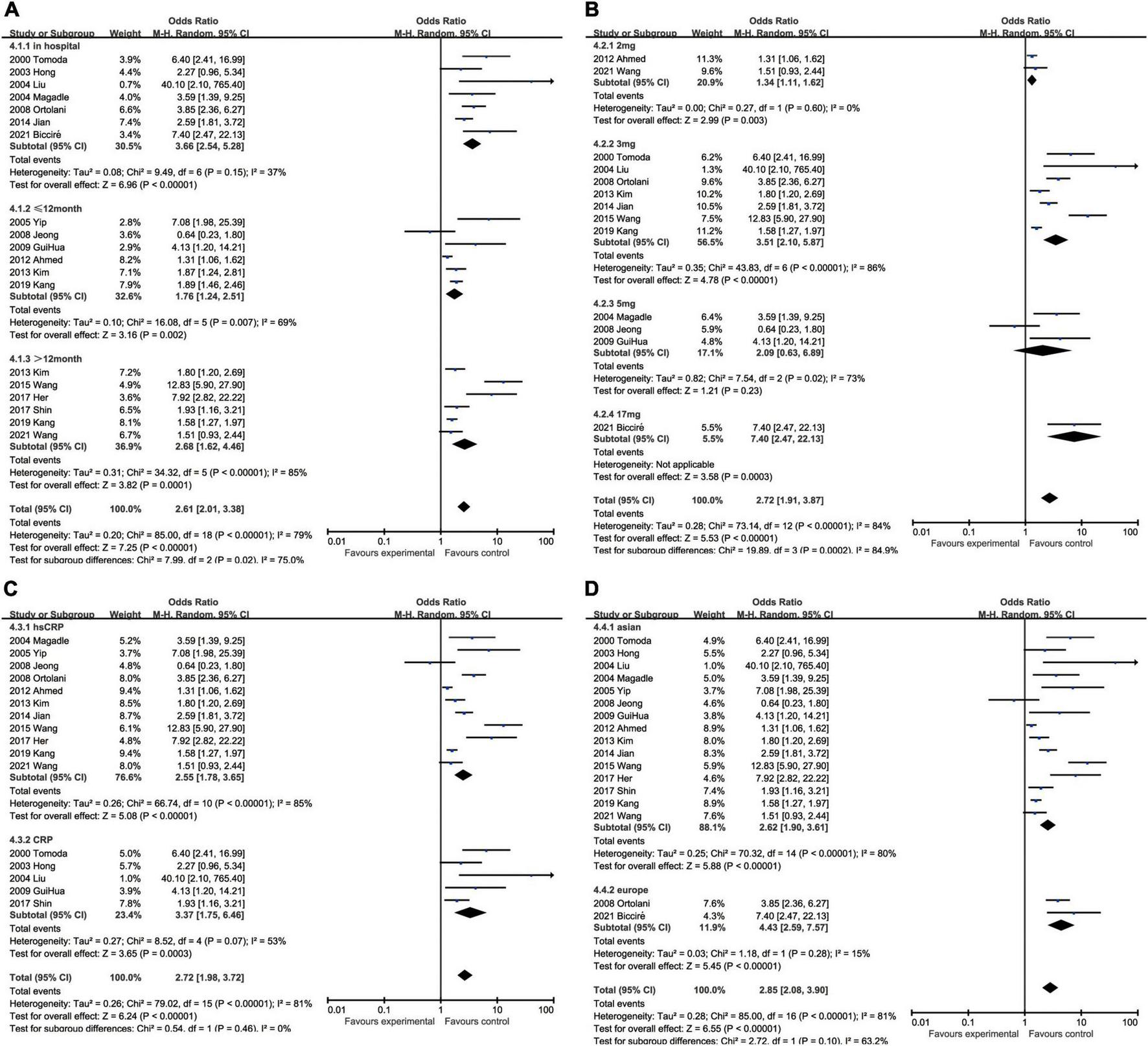
Forrest plots of subgroup analysis assessing MACEs associated with increased CRP: (A) subgroups based on follow-up; (B) subgroups based on cut-off value of hsCRP; (C) subgroups based on CRP and hsCRP; and (D) subgroups based on ethnicities.
C-reactive protein and heart failure
Although heart failure was reported in only five studies containing 1,257 patients, the marginal values of hsCRP were variably selected. When we put different cut-off values into different subgroups for analysis, no statistical significance could be found in some subgroups, namely 2 mg/L group (OR: 2.07, 95% CI [0.94, 4.58], p = 0.07, I2 = 0) and 5 mg/L group (OR: 1.69, 95% CI [0.66, 4.33], p = 0.27, I2 = 0). As the critical value was upregulated to 9 mg/L (OR: 2.82, 95% CI [1.51, 5.25], p = 0.001), statistical significance was eventually exhibited. In addition, hsCRP (OR: 2.24, 95% CI [1.43, 3.53], p = 0.0005, I2 = 0) could be a predictor of incidence of heart failure, while CRP (OR: 2.96, 95% CI [0.61, 14.29], p = 0.18) had no statistical significance. Asian patients (OR: 2.46, 95% CI [1.49, 4.06], p = 0.0004, I2 = 0) were under statistically significant risk to heart failure, comparing with European (OR: 1.87, 95% CI [0.79, 4.44], p = 0.15) whose subgroup analysis result had no statistical significance (Figure 6).
FIGURE 6
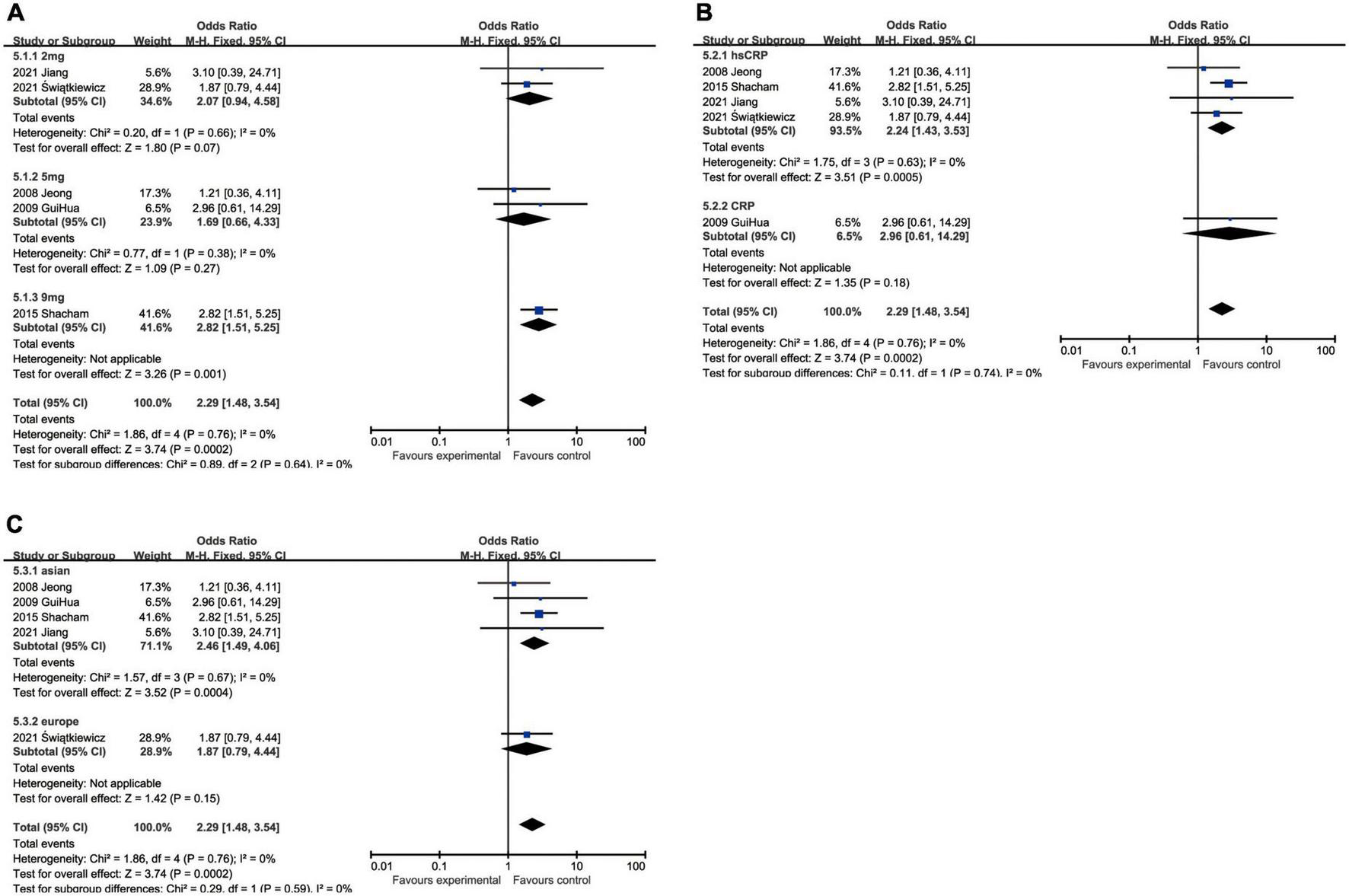
Forrest plots of subgroup analysis assessing heart failure associated with increased CRP: (A) subgroups based on cut-off value of hsCRP; (B) subgroups based on CRP and hsCRP; and (C) subgroups based on ethnicities.
C-reactive protein and recurrent myocardial infarction
With regard to recurrent myocardial infarction, subgroup analysis indicated that enhanced CRP could foresee soaring incidence of recurrent myocardial infarction in hospitalization (OR: 5.32, 95% CI [1.10, 25.60], p = 0.04, I2 = 0), within 12 months (OR: 1.90, 95% CI [1.24, 2.92], p = 0.003, I2 = 0), while CRP showed no statistical significance over 12 months (OR: 2.17, 95% CI [0.81, 5.80], p = 0.12, I2 = 64%). Three milligrams per liter (OR: 6.69, 95% CI [2.16, 20.75], p = 0.0010, I2 = 0) had a satisfactory performance as a cut-off value of CRP, whereas 2 mg/L (OR: 4.11, 95% CI [1.38, 12.24], p = 0.01, I2 = 0) and 5 mg/L (OR: 2.60, 95% CI [1.12, 6.06], p = 0.03, I2 = 6%) were also equal to predicting recurrent myocardial infarction. Elevated hsCRP (OR: 2.49, 95% CI [1.24, 5.01], p = 0.01, I2 = 51%) was considered to be a potential risk factor, especially comparing to CRP (OR: 1.75, 95% CI [0.67, 4.53], p = 0.25, I2 = 0) measured by normal sensitivity methodology which had no statistical significance. When we focused on the impact of ethnics on heterogeneity, European (OR: 5.27, 95% CI [1.86, 14.96], p = 0.002, I2 = 0) were more susceptible to recurrent myocardial infarction than Asian (OR: 1.51, 95% CI [1.07, 2.14], p = 0.02, I2 = 19%) (Figure 7).
FIGURE 7
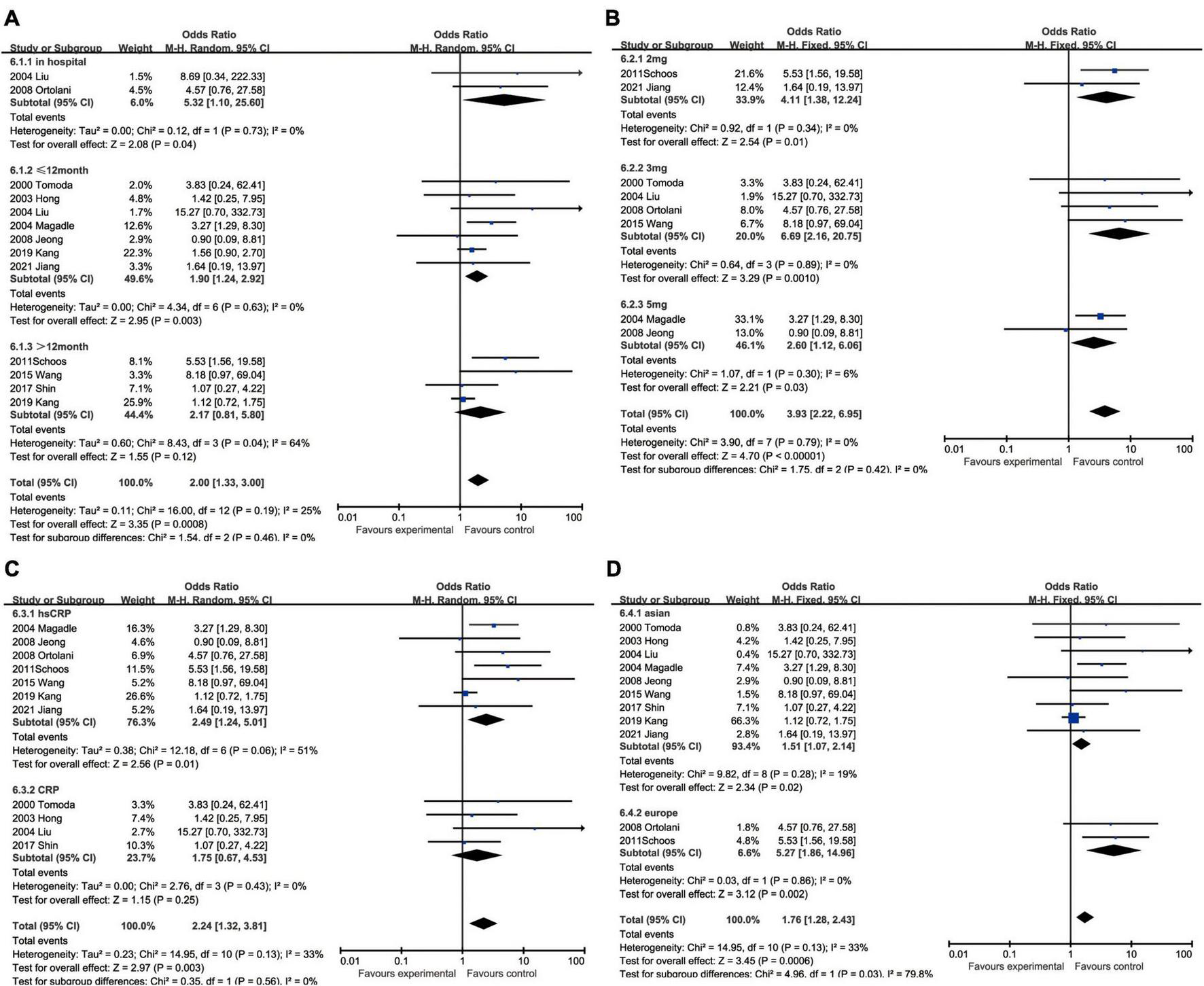
Forrest plots of subgroup analysis assessing recurrent myocardial infarction associated with increased CRP: (A) subgroups based on cut-off value of hsCRP; (B) subgroups based on CRP and hsCRP; (C) subgroups based on ethnicities; and (D) subgroups based on ethnicities.
C-reactive protein and restenosis
The subgroup analysis demonstrated that the predicting capacity of increased CRP had a relatively narrow time window within 12 months (OR: 1.91, 95% CI [1.24, 2.94], p = 0.003, I2 = 30%), whereas neither in hospitalization (OR: 1.21, 95% CI [0.23, 6.27], p = 0.82) nor over 12 months (OR: 1.26, 95% CI [0.57, 2.81], p = 0.57, I2 = 0) had statistical significance. None of the cut-off performed statistically significant, namely 2 mg/L group (OR: 1.86, 95% CI [0.61, 5.71], p = 0.28), 3 mg/L (OR: 1.39, 95% CI [0.76, 2.55], p = 0.28, I2 = 0), 5 mg/L (OR: 1.59, 95% CI [0.60, 4.22], p = 0.35, I2 = 73%) and 7.6 mg/L (OR: 0.41, 95% CI [0.05, 3.45], p = 0.54). The subgroup analysis could help us make the decision between CRP (OR: 1.82, 95% CI [1.16, 2.86], p = 0.009, I2 = 32%) and hsCRP (OR: 1.50, 95% CI [0.79, 2.84], p = 0.22, I2 = 0) as a prognostic factor for restenosis for reason of that the latter had no statistical significance. Ethnically, CRP could predict restenosis in Asians ((OR = 1.72, 95% CI [1.15, 2.57], p = 0.008, I2 = 22%), but not in Europeans (OR: 1.63, 95% CI [0.66, 4.07], p = 0.29, I2 = 0) (Figure 8).
FIGURE 8
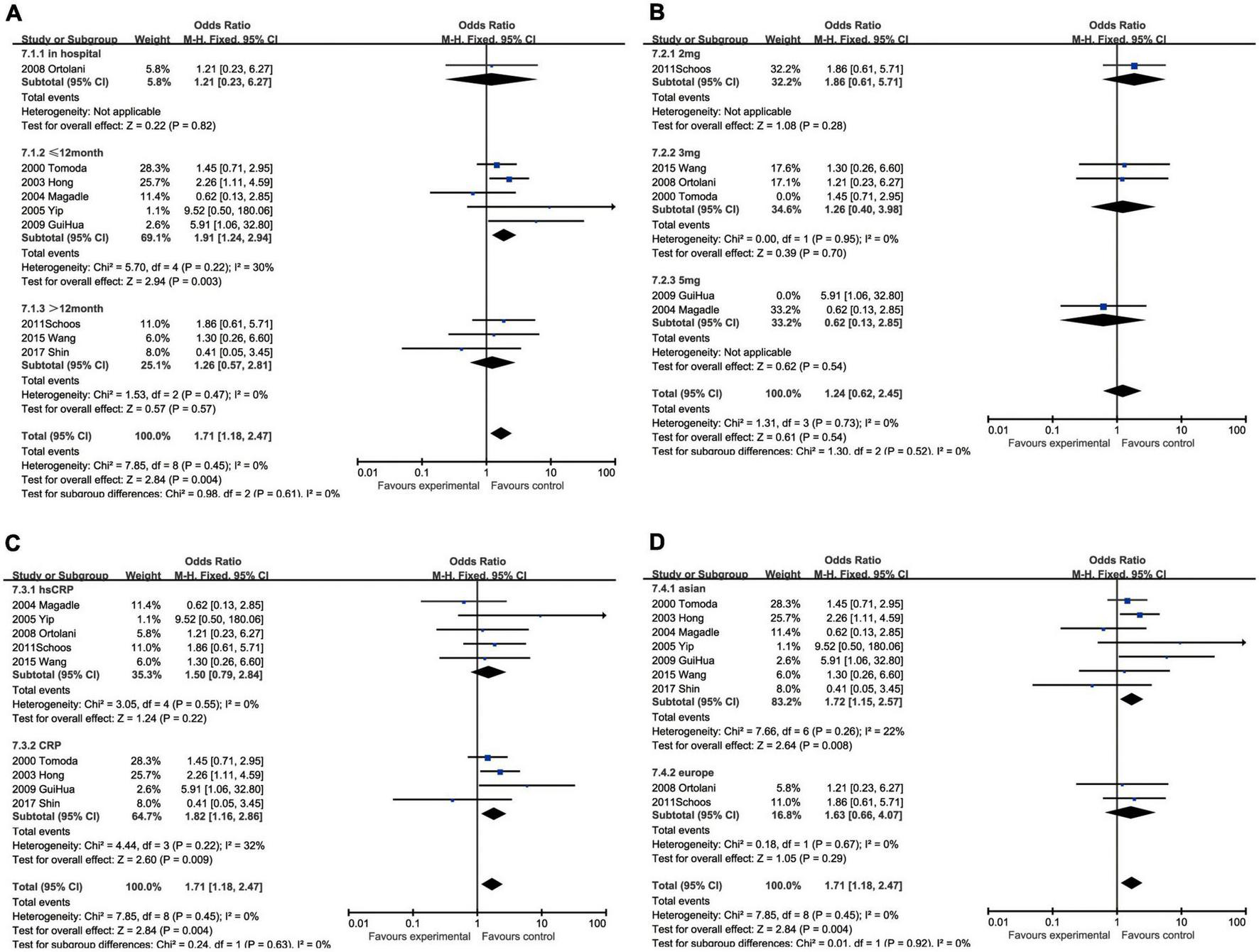
Forrest plots of subgroup analysis assessing restenosis associated with increased CRP: (A) subgroups based on cut-off value of hsCRP; (B) subgroups based on CRP and hsCRP; (C) subgroups based on ethnicities; and (D) subgroups based on ethnicities.
Discussion
Briefly summarizing the results of our meta-analysis, the elevation of CRP is significantly associated with the boost in multiple adverse prognoses for the patients with AMI undergoing PCI, especially in all-cause mortality and cardiovascular death. Furthermore, it is noteworthy that increased CRP has more prognostic value in hospitalization and short-term outcomes and the Asian group.
Recent years have emerged plenty of studies investigating diverse post-infarction prognostic inflammatory biomarkers originating from either inflammation infiltration or plasma inflammatory factors. Biomarkers based on inflammatory infiltration have been verified from simple white blood cell counts (51) to counts (8) or ratios (52, 53) of a different specific subclass of innate immune cells. NLR was most reported to be related to the hospital and long-term prognosis of patients with STEMI after PCI (12). Scoring systems have also been developed from white blood cell counts, such as SII (54) foreseeing no-reflow phenomenon in patients with STEMI after PCI (13). Nevertheless, these biomarkers cannot get rid of the disadvantages of WBC counts that are easily influenced by race and sex and it is difficult to establish a reference range for the whole population (52) and are in need of further verification. Though single-cell proteomic and transcriptomic analyses uncovered distinct features of novel subclasses in atherosclerotic lesions (55), it is promising but remains to be explored in clinical transformation looking forward to high-quality randomized controlled trials.
Comparing to inflammatory infiltration, conventional plasma inflammatory mediators are routinely measured in clinical practice and have been verified as prospective prognostic biomarkers. For example, IL-1b was associated with 90-day all-cause mortality, cardiovascular mortality, and MACEs but defected in predicting nonfatal cardiovascular events (15), while Kilic et al. (16) suggested cytokine concentrations and proinflammatory to anti-inflammatory cytokine ratios as markers of a high risk of new coronary events. Moreover, comprehensive studies or reviews (16, 19, 56–58) were conducted comparing the prognostic performance of multiple conventional inflammatory factors like IL-6, IL-10, TNF-α, and CRP. Among these mediators, CRP has exhibited a predominant power. Lippi et al. (56) compared prognostic significance of 12 cytokines or growth factors in patients with myocardial ischemia and among these biomarkers, CRP displayed the most notable risk estimates identifying potential post-infarction heart failure victims. Ritschel et al. (57) found that neither IL-6 nor sgp130 levels were related to future events by cox regression models, but patients with elevated CRP levels had a higher risk of death. Furthermore, compared with part of inflammatory factors, CRP has its own advantages such as no diurnal variation, insensitivity to external factors like diet and stable measured value (17). In the last decades, elevated CRP has been studied and verified in plenty of cohorts and is widely acknowledged as a cardiovascular risk factor. In the previous meta-analysis by Mincu et al. (35), high preprocedural CRP levels prognosed a significant increase of the incidence of in-hospital and follow-up adverse end points. However, recent years novel cohorts have been reported and associated CRP with more post-infarction events, such as heart failure (5).
In the current study analysis, the subgroup provided us new prospective on the clinical application of the relationship between elevated CRP and poor AMI prognosis. First, though CRP displayed a favorable predicting capacity of hospitalization, short-term (<12 months) or long-term (>12 months) prognosis, it differed between end points. Comparing with all outcomes, CRP can better foresee hospitalization incidence of MACEs and recurrent myocardial infarction and short-term restenosis while both all-term all-cause mortality and cardiovascular mortality. Świątkiewicz et al. (5) had reported that persistent elevation in CRP was associated with increased risk of hospitalization for HF and HF-related mortality in long-term (>6 years) follow-up, which further completed the function of CRP as a predictor. Second, as the most adopted thresholds, 2 and 3 mg/L can both serve as an optimal cut-off value for post-infarction adverse prognosis. In general, standard clinical assays for CRP with the normal sensitivity range from 3 to 8 mg/L (59), which limits its effective application to vascular risk prediction. To solve the limitation of CRP, high-sensitive CRP with excellent fidelity and reproducibility was developed and its comparability was proven (60). In the current study, hsCRP has better performance in predicting all-cause mortality, cardiovascular mortality, and recurrent myocardial infarction. However, CRP had its superiority in MACEs, heart failure, and restenosis, which implies unknown underlying impact factors in need of further investigation. Third, elevated CRP is a more important sign of adverse prognosis in Asian patients than in European. With respect to ethnic groups, we found that CRP had better performance in predicting the all-cause mortality, cardiovascular mortality, the incidence of heart failure and restenosis in Asians, and the incidence of MACEs and recurrent myocardial infarction in Europeans, while it was not statistically significant to predict the cardiovascular mortality, the incidence of heart failure and restenosis in Europeans. The diversity might originate from the differences in genetic backgrounds and environments, different including criteria, and selection biases (61). A meta-analysis of 7,703 subjects has shown CRP gene polymorphisms associated with decreased risk of MI among Asian populations, while no statistical significance was found among Caucasian populations (62).
There are also limitations in our study. First, as is known, there are two types of AMI (i.e., STEMI and NSTEMI). The majority of our including studies focused on the relationship between CRP and STEMI. Although three studies (27, 34, 43) incorporate patients with NSTEMI, the detailed information could not be extracted from the source articles. In consideration of different inflammation conditions (58) and outcome profiles (63) under STEMI or NSTEMI, these diversities may lead our results to be less appropriate for NSTEMI. Second, in-depth studies have been conducted in an attempt to improve the sensitivity and specificity of CRP, attempting to adjust or combine hsCRP with its change velocity (64, 65), cTnT (66), albumin (67–69), and fibrinogen (70). These studies extended the practicability of CRP but are not included in our study due to lack of enough high-quality cohorts. Finally, there is publication bias existing in all-cause mortality and MACEs. As these studies were carefully selected following PRISMA and the results remain stable after sensitivity analysis, we preferred not to delete studies.
In brief, the current meta-analysis suggests that CRP is a prospective predictor of the diverse adverse prognoses in patients with AMI undergoing PCI. CRP had better performance in predicting plenty of hospitalization and short-term (<12 months) adverse prognoses than long-term prognoses and it is noteworthy that elevated CRP in Asian patients had more predictive value than in European.
Statements
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
HZ conceptually designed the work. LD and YJ critically reviewed the manuscript. MD provided constructive recommendations in data processing and article polishing. SL and HJ conducted the main work and prepared the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.1013501/full#supplementary-material
References
1.
Zhao L Li Y Xu T Luan Y Lv Q Wang Y et al Impact of increased inflammation biomarkers on periprocedural myocardial infarction in patients undergoing elective percutaneous coronary intervention: a cohort study. J Thorac Dis. (2020) 12:5398–410. 10.21037/jtd-20-1605
2.
Thygesen K Alpert JS White HD . Universal definition of myocardial infarction.J Am Coll Cardiol. (2007) 50:2173–95.
3.
Sahoo S Losordo DW . Exosomes and cardiac repair after myocardial infarction.Circ Res. (2014) 114:333–44.
4.
Takahashi N Dohi T Endo H Funamizu T Wada H Doi S et al Residual inflammation indicated by high-sensitivity C-reactive protein predicts worse long-term clinical outcomes in Japanese patients after percutaneous coronary intervention. J Clin Med. (2020) 9:1033. 10.3390/jcm9041033
5.
Świątkiewicz I Magielski P Kubica J . C-reactive protein as a risk marker for post-infarct heart failure over a multi-year period.Int J Mol Sci. (2021) 22:3169. 10.3390/ijms22063169
6.
Rossi VA Denegri A Candreva A Klingenberg R Obeid S Räber L et al Prognostic value of inflammatory biomarkers and GRACE score for cardiac death and acute kidney injury after acute coronary syndromes. Eur Heart J Acute Cardiovasc Care. (2021) 10:445–52. 10.1093/ehjacc/zuab003
7.
Liu Y Jia SD Yao Y Tang XF Xu N Jiang L et al Impact of high-sensitivity C-reactive protein on coronary artery disease severity and outcomes in patients undergoing percutaneous coronary intervention. J Cardiol. (2020) 75:60–5.
8.
Ong SB Hernández-Reséndiz S Crespo-Avilan GE Mukhametshina RT Kwek XY Cabrera-Fuentes HA et al Inflammation following acute myocardial infarction: multiple players, dynamic roles, and novel therapeutic opportunities. Pharmacol Ther. (2018) 186:73–87. 10.1016/j.pharmthera.2018.01.001
9.
Kologrivova I Shtatolkina M Suslova T Ryabov V . Cells of the immune system in cardiac remodeling: main players in resolution of inflammation and repair after myocardial infarction.Front Immunol. (2021) 12:664457. 10.3389/fimmu.2021.664457
10.
Prabhu SD Frangogiannis NG . The biological basis for cardiac repair after myocardial infarction: from inflammation to fibrosis.Circ Res. (2016) 119:91–112.
11.
Eltzschig HK Eckle T . Ischemia and reperfusion–from mechanism to translation.Nat Med. (2011) 17:1391–401. 10.1038/nm.2507
12.
Zhang S Diao J Qi C Jin J Li L Gao X et al Predictive value of neutrophil to lymphocyte ratio in patients with acute ST segment elevation myocardial infarction after percutaneous coronary intervention: a meta-analysis. BMC Cardiovasc Disord. (2018) 18:75. 10.1186/s12872-018-0812-6
13.
Esenboğa K Kurtul A Yamantürk YY Tan TS Tutar DE . Systemic immune-inflammation index predicts no-reflow phenomenon after primary percutaneous coronary intervention.Acta Cardiol. (2021) 77:59–65. 10.1080/00015385.2021.1884786
14.
Xia N Lu Y Gu M Li N Liu M Jiao J et al A unique population of regulatory T cells in heart potentiates cardiac protection from myocardial infarction. Circulation. (2020) 142:1956–73. 10.1161/CIRCULATIONAHA.120.046789
15.
Silvain J Kerneis M Zeitouni M Lattuca B Galier S Brugier D et al Interleukin-1β and risk of premature death in patients with myocardial infarction. J Am Coll Cardiol. (2020) 76:1763–73.
16.
Kilic T Ural D Ural E Yumuk Z Agacdiken A Sahin T et al Relation between proinflammatory to anti-inflammatory cytokine ratios and long-term prognosis in patients with non-ST elevation acute coronary syndrome. Heart. (2006) 92:1041–6. 10.1136/hrt.2005.080382
17.
Libby P . Inflammation in atherosclerosis-no longer a theory.Clin Chem. (2021) 67:131–42. 10.1093/clinchem/hvaa275
18.
Libby P Crea F . Clinical implications of inflammation for cardiovascular primary prevention.Eur Heart J. (2010) 31:777–83. 10.1093/eurheartj/ehq022
19.
van Diepen S Newby LK Lopes RD Stebbins A Hasselblad V James S et al Prognostic relevance of baseline pro- and anti-inflammatory markers in STEMI: an APEX AMI substudy. Int J Cardiol. (2013) 168:2127–33. 10.1016/j.ijcard.2013.01.004
20.
Ridker PM . From C-reactive protein to interleukin-6 to interleukin-1: moving upstream to identify novel targets for atheroprotection.Circ Res. (2016) 118:145–56. 10.1161/CIRCRESAHA.115.306656
21.
Sproston NR Ashworth JJ . Role of C-reactive protein at sites of inflammation and infection.Front Immunol. (2018) 9:754. 10.3389/fimmu.2018.00754
22.
Del Giudice M Gangestad SW . Rethinking IL-6 and CRP: why they are more than inflammatory biomarkers, and why it matters.Brain Behav Immun. (2018) 70:61–75. 10.1016/j.bbi.2018.02.013
23.
Dharma S Sari NY Santoso A Sukmawan R Rao SV . Association of plasma pentraxin 3 concentration with angiographic and clinical outcomes in patients with acute ST-segment elevation myocardial infarction treated by primary angioplasty.Catheter Cardiovasc Interv. (2020) 96:1233–9. 10.1002/ccd.28626
24.
Yang L Dong H Lu H Liao Y Zhang H Xu L et al Serum YKL-40 predicts long-term outcome in patients undergoing primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. Medicine. (2019) 98:e14920. 10.1097/MD.0000000000014920
25.
Tomoda H Aoki N . Prognostic value of C-reactive protein levels within six hours after the onset of acute myocardial infarction.Am Heart J. (2000) 140:324–8. 10.1067/mhj.2000.108244
26.
Hong YJ Jeong MH Park OY Kim W Kim JH Ahn YK et al The role of C-reactive protein on the long-term clinical outcome after primary or rescue percutaneous coronary intervention. Korean J Intern Med. (2003) 18:29–34.
27.
Kang DO Park Y Seo JH Jeong MH Chae SC Ahn TH et al Time-dependent prognostic effect of high sensitivity C-reactive protein with statin therapy in acute myocardial infarction. J Cardiol. (2019) 74:74–83. 10.1016/j.jjcc.2018.12.022
28.
Jiang H Wang H Liang B Sun L Bai L . Prognostic implication of systemic inflammatory state on antiplatelet effect in patients after percutaneous coronary intervention for ST-elevation myocardial infarction: a retrospective cohort study.Medicine. (2021) 100:e27214. 10.1097/MD.0000000000027214
29.
Bicciré FG Pastori D Tanzilli A Pignatelli P Viceconte N Barillà F et al Low serum albumin levels and in-hospital outcomes in patients with ST segment elevation myocardial infarction. Nutr Metab Cardiovasc Dis. (2021) 31:2904–11.
30.
Wang Y Zhao X Zhou P Liu C Chen R Sheng Z et al Impact of postprocedural high-sensitivity c-reactive protein on lipoprotein(a)-associated cardiovascular risk with ST-segment elevation myocardial infarction with percutaneous coronary intervention. Am J Cardiol. (2021) 150:8–14. 10.1016/j.amjcard.2021.03.038
31.
Jeong YH Lee SW Lee CW Hong MK Kim JJ Park SW et al Biomarkers on admission for the prediction of cardiovascular events after primary stenting in patients with ST-elevation myocardial infarction. Clin Cardiol. (2008) 31:572–9.
32.
Schoos MM Kelbæk H Kofoed KF Køber L Kløvgaard L Helqvist S et al Usefulness of preprocedure high-sensitivity C-reactive protein to predict death, recurrent myocardial infarction, and stent thrombosis according to stent type in patients with ST-segment elevation myocardial infarction randomized to bare metal or drug-eluting stenting during primary percutaneous coronary intervention. Am J Cardiol. (2011) 107:1597–603.
33.
Wang CH Zhang SY Fang Q Shen ZJ Fan ZJ Jin XF et al Renal dysfunction and hsCRP predict long-term outcomes of percutaneous coronary intervention in acute myocardial infarction. Am J Med Sci. (2015) 349:413–20.
34.
Shin HC Jang JS Jin HY Seo JS Yang TH Kim DK et al Combined use of neutrophil to lymphocyte ratio and c-reactive protein level to predict clinical outcomes in acute myocardial infarction patients undergoing percutaneous coronary intervention. Korean Circ J. (2017) 47:383–91. 10.4070/kcj.2016.0327
35.
Mincu RI Jánosi RA Vinereanu D Rassaf T Totzeck M . Preprocedural C-reactive protein predicts outcomes after primary percutaneous coronary intervention in patients with ST-elevation myocardial infarction a systematic meta-analysis.Sci Rep. (2017) 7:41530. 10.1038/srep41530
36.
Moher D Liberati A Tetzlaff J Altman DG Prisma Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. 10.1371/journal.pmed.1000097
37.
Marx SO Totary-Jain H Marks AR . Vascular smooth muscle cell proliferation in restenosis.Circ Cardiovasc Interv. (2011) 4:104–11.
38.
Byrne RA Joner M Kastrati A . Stent thrombosis and restenosis: what have we learned and where are we going? The andreas gruntzig lecture ESC 2014.Eur Heart J. (2015) 36:3320–31. 10.1093/eurheartj/ehv511
39.
Magadle R Hertz I Merlon H Weiner P Mohammedi I Robert D . The relation between preprocedural C-reactive protein levels and early and late complications in patients with acute myocardial infarction undergoing interventional coronary angioplasty.Clin Cardiol. (2004) 27:163–8. 10.1002/clc.4960270314
40.
Liu J Fu X Ma N Wu W Li S Li L. Prognostic value of C-reactive protein levels within six hours after the onset of acute anterior myocardial infarction. J Clin Cardiol. (2004) 20:158–61.
41.
Ortolani P Marzocchi A Marrozzini C Palmerini T Saia F Taglieri N et al Predictive value of high sensitivity C-reactive protein in patients with ST-elevation myocardial infarction treated with percutaneous coronary intervention. Eur Heart J. (2008) 29:1241–9. 10.1093/eurheartj/ehm338
42.
Gui-Hua LI Yu-Ying MA . Influence of C reactive protein level in early acute myocardial infarction on prognosis after perceous coronary interventionutan.Chin J Arterioscler. (2009) 17:406–8.
43.
Ahmed K Jeong MH Chakraborty R Cho KH Sim DS Hong YJ et al Prognostic impact of baseline high-sensitivity C-reactive protein in patients with acute myocardial infarction undergoing percutaneous coronary intervention based on body mass index. Korean Circ J. (2012) 42:164–72. 10.4070/kcj.2012.42.3.164
44.
He YT Tan N Liu YH Chen SQ Liu Y Huang SJ et al [Association between high-sensitivity C-reactive protein and contrast-induced nephropathy after primary percutaneous coronary intervention]. Zhonghua Xin Xue Guan Bing Za Zhi. (2013) 41:394–8.
45.
Kim KH Kim W Kang WY Hwang SH Cho SC Kim W et al The impact of ischemic time on the predictive value of high-sensitivity C-reactive protein in ST-segment elevation myocardial infarction patients treated by primary percutaneous coronary intervention. Korean Circ J. (2013) 43:664–73. 10.4070/kcj.2013.43.10.664
46.
Shacham Y Leshem-Rubinow E Steinvil A Keren G Roth A Arbel Y . High sensitive C-reactive protein and the risk of acute kidney injury among ST elevation myocardial infarction patients undergoing primary percutaneous intervention.Clin Exp Nephrol. (2015) 19:838–43. 10.1007/s10157-014-1071-1
47.
Yip HK Hang CL Fang CY Hsieh YK Yang CH Hung WC et al Level of high-sensitivity C-reactive protein is predictive of 30-day outcomes in patients with acute myocardial infarction undergoing primary coronary intervention. Chest. (2005) 127:803–8. 10.1378/chest.127.3.803
48.
Damman P Beijk MA Kuijt WJ Verouden NJ van Geloven N Henriques JP et al Multiple biomarkers at admission significantly improve the prediction of mortality in patients undergoing primary percutaneous coronary intervention for acute ST-segment elevation myocardial infarction. J Am Coll Cardiol. (2011) 57:29–36. 10.1016/j.jacc.2010.06.053
49.
Jian-Wei Z Yu-Jie Z Shu-Jun C Qing Y Shi-Wei Y Bin N . Impact of preprocedural high-sensitivity C-reactive protein on contrast-induced nephropathy in patients undergoing primary percutaneous coronary intervention.Angiology. (2014) 65:402–7. 10.1177/0003319713482177
50.
Her AY Cho KI Singh GB An DS Jeong YH Koo BK et al Plaque characteristics and inflammatory markers for the prediction of major cardiovascular events in patients with ST-segment elevation myocardial infarction. Int J Cardiovasc Imaging. (2017) 33:1445–54. 10.1007/s10554-017-1135-x
51.
Chung S Song YB Hahn JY Chang SA Lee SC Choe YH et al Impact of white blood cell count on myocardial salvage, infarct size, and clinical outcomes in patients undergoing primary percutaneous coronary intervention for ST-segment elevation myocardial infarction: a magnetic resonance imaging study. Int J Cardiovasc Imaging. (2014) 30:129–36. 10.1007/s10554-013-0303-x
52.
Bain BJ . Ethnic and sex differences in the total and differential white cell count and platelet count.J Clin Pathol. (1996) 49:664–6.
53.
Akpek M Sahin O Elcik D Kaya MG. The association of neutrophil/lymphocyte ratio with coronary flow and in-hospital mace in patients with STEMI undergoing primary PCI. Eur Heart J. (2012) 33:788.
54.
Hu B Yang XR Xu Y Sun YF Sun C Guo W et al Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. (2014) 20:6212–22.
55.
Fernandez DM Rahman AH Fernandez NF Chudnovskiy A Amir ED Amadori L et al Single-cell immune landscape of human atherosclerotic plaques. Nat Med. (2019) 25:1576–88.
56.
Lippi G Cervellin G . Risk assessment of post-infarction heart failure. Systematic review on the role of emerging biomarkers.Crit Rev Clin Lab Sci. (2014) 51:13–29. 10.3109/10408363.2013.863267
57.
Ritschel VN Seljeflot I Arnesen H Halvorsen S Eritsland J Fagerland MW et al Circulating levels of IL-6 receptor and gp130 and long-term clinical outcomes in ST-elevation myocardial infarction. J Am Heart Assoc. (2016) 5:e003014. 10.1161/JAHA.115.003014
58.
Hjort M Eggers KM Lindhagen L Baron T Erlinge D Jernberg T et al Differences in biomarker concentrations and predictions of long-term outcome in patients with ST-elevation and non-ST-elevation myocardial infarction. Clin Biochem. (2021) 98:17–23. 10.1016/j.clinbiochem.2021.09.001
59.
Ridker PM . High-sensitivity C-reactive protein: potential adjunct for global risk assessment in the primary prevention of cardiovascular disease.Circulation. (2001) 103:1813–8.
60.
Rifai N Tracy RP Ridker PM . Clinical efficacy of an automated high-sensitivity C-reactive protein assay.Clin Chem. (1999) 45:2136–41.
61.
Schoos MM Munthe-Fog L Skjoedt MO Ripa RS Lønborg J Kastrup J et al Association between lectin complement pathway initiators, C-reactive protein and left ventricular remodeling in myocardial infarction-a magnetic resonance study. Mol Immunol. (2013) 54:408–14. 10.1016/j.molimm.2013.01.008
62.
Zhu Y Liu T He H Sun Y Zhuo F . C-reactive protein gene polymorphisms and myocardial infarction risk: a meta-analysis and meta-regression.Genet Test Mol Biomarkers. (2013) 17:873–80. 10.1089/gtmb.2013.0340
63.
Cox DA Stone GW Grines CL Stuckey T Zimetbaum PJ Tcheng JE et al Comparative early and late outcomes after primary percutaneous coronary intervention in ST-segment elevation and non-ST-segment elevation acute myocardial infarction (from the CADILLAC trial). Am J Cardiol. (2006) 98:331–7. 10.1016/j.amjcard.2006.01.102
64.
Zahler D Rozenfeld KL Stein M Milwidsky A Berliner S Banai S et al C-reactive protein velocity and the risk of acute kidney injury among ST elevation myocardial infarction patients undergoing primary percutaneous intervention. J Nephrol. (2019) 32:437–43. 10.1007/s40620-019-00594-2
65.
Yun KH Jeong MH Oh SK Rhee SJ Park EM Lee EM et al Response of high-sensitivity C-reactive protein to percutaneous coronary intervention in patients with acute coronary syndrome. Heart Vessels. (2009) 24:175–80.
66.
Holzknecht M Tiller C Reindl M Lechner I Troger F Hosp M et al C-reactive protein velocity predicts microvascular pathology after acute ST-elevation myocardial infarction. Int J Cardiol. (2021) 338:30–6.
67.
Ren H Zhao L Liu Y Tan Z Luo G Deng X . The High-sensitivity C-reactive protein to prealbumin ratio predicts adverse cardiovascular events after ST-elevation myocardial infarction.Heart Surg Forum. (2021) 24:E153–7. 10.1532/hsf.3307
68.
Acet H Güzel T Aslan B Isik MA Ertas F Catalkaya S . Predictive value of C-reactive protein to albumin ratio in ST-segment elevation myocardial infarction patients treated with primary percutaneous coronary intervention.Angiology. (2021) 72:244–51. 10.1177/0003319720963697
69.
Liu ZY Tang JN Cheng MD Jiang LZ Guo QQ Zhang JC et al C-reactive protein-to-serum albumin ratio as a novel predictor of long-term outcomes in coronary artery disease patients who have undergone percutaneous coronary intervention: analysis of a real-world retrospective cohort study. Coron Artery Dis. (2021) 32:191–6. 10.1097/MCA.0000000000001021
70.
Shacham Y Leshem-Rubinow E Ben Assa E Rogowski O Topilsky Y Roth A et al Comparison of C-reactive protein and fibrinogen levels in patients having anterior wall ST-segment elevation myocardial infarction with versus without left ventricular thrombus (from a primary percutaneous coronary intervention cohort). Am J Cardiol. (2013) 112:57–60. 10.1016/j.amjcard.2013.02.052
Summary
Keywords
acute myocardial infarction, percutaneous coronary intervention (PCI), C-reactive protein (CRP), adverse prognosis, inflammatory/anti-inflammatory factors, coronary arterial disease
Citation
Liu S, Jiang H, Dhuromsingh M, Dai L, Jiang Y and Zeng H (2022) Evaluation of C-reactive protein as predictor of adverse prognosis in acute myocardial infarction after percutaneous coronary intervention: A systematic review and meta-analysis from 18,715 individuals. Front. Cardiovasc. Med. 9:1013501. doi: 10.3389/fcvm.2022.1013501
Received
07 August 2022
Accepted
24 October 2022
Published
16 November 2022
Volume
9 - 2022
Edited by
Tommaso Gori, Johannes Gutenberg University Mainz, Germany
Reviewed by
Feng-Hua Ding, Shanghai Jiao Tong University School of Medicine, China; Ilan Merdler, Tel Aviv Sourasky Medical Center, Israel
Updates
Copyright
© 2022 Liu, Jiang, Dhuromsingh, Dai, Jiang and Zeng.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hesong Zeng, zenghs@tjh.tjmu.edu.cn
†These authors have contributed equally to this work and share first authorship
This article was submitted to Coronary Artery Disease, a section of the journal Frontiers in Cardiovascular Medicine
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.