- 1Division of Cardiovascular Medicine, Department of Medicine, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA, United States
- 2Department of Trial Design and Development, Baim Institute for Clinical Research, Boston, MA, United States
- 3Cardiovascular, Metabolism, Retina and Pulmonary Hypertension, Johnson & Johnson Pharmaceutical Research and Development, Raritan, NJ, United States
Background: Stent thrombosis (ST) is an uncommon but serious complication of stent implantation. This study aimed to explore factors associated with early, late, and very late ST to help guide risk assessment and clinical decision-making on ST.
Methods: The analysis included patients who received stent placement for the index acute coronary syndrome (ACS). Cumulative incidence of ST was assessed at 30 days (early ST), 31–360 days (late ST), 361–720 days (very late ST), and up to 720 days. Cox proportional hazards models were used to assess associations between ST and various factors, including patient characteristics [i.e., age, sex, ACS presentation, history of hypertension, smoking, diabetes, prior myocardial infarction (MI), heart failure, prior ischemic stroke, and cancer], laboratory tests [i.e., positive cardiac biomarker, hemoglobin, platelet count, white blood cell (WBC) count], and treatment [i.e., drug-eluting stent (DES) vs. bare-metal stent (BMS) and anticoagulant with rivaroxaban vs. placebo].
Results: Among the 8,741 stented patients, 155 ST events (2.25%) occurred by Day 720. The cumulative incidences of early, late, and very late ST were 0.80%, 0.81%, and 0.77%, respectively. After multivariable adjustment, age ≥ 75 [hazard ratio (HR) = 2.13 (95% confidence interval, CI: 1.26–3.60)], a history of prior MI [HR = 1.81 (95% CI: 1.22–2.68)], low hemoglobin level [HR = 2.34 (95% CI: 1.59–3.44)], and high WBC count [HR = 1.58 (95% CI: 1.02–2.46)] were associated with a greater risk of overall ST, whereas DES [HR = 0.56 (95% CI: 0.38–0.83)] and rivaroxaban therapy [HR = 0.63 (95% CI: 0.44–0.88)] were associated with a lower risk of overall ST up to 720 days. Low hemoglobin level and high WBC count were associated with early ST (low hemoglobin: HR = 2.35 [95% CI: 1.34–4.12]; high WBC count: HR = 2.11 [95% CI: 1.17–3.81]). Low hemoglobin level and prior MI were associated with a greater risk of late ST (low hemoglobin: HR = 2.32 [95% CI: 1.26–4.27]; prior MI: HR = 2.98 [95% CI: 1.67–5.31]), whereas DES was associated with a lower risk of late ST [HR = 0.33 (95% CI: 0.16–0.67)]. Age ≥75 years was associated with very late ST.
Conclusion: The study identified positive and negative associations with early, late, and very late ST. These variables may be useful in constructing risk assessment models for ST.
Clinical Trial Registration: http://www.clinicaltrials.gov, identifier NCT00809965.
Introduction
Stent thrombosis (ST) is a rare but potentially catastrophic complication of stent implantation that carries a high risk of mortality (1). It typically presents as myocardial infarction (MI), usually with ST segment elevation MI (STEMI), or death (2). Dual antiplatelet therapy (DAPT) has been demonstrated to prevent ST in patients with acute coronary syndrome (ACS) undergoing percutaneous coronary intervention (PCI) (3). The current guidelines from the American College of Cardiology (ACC)/American Heart Association (AHA) include a Class I recommendation to continue DAPT for at least 6 months after revascularization for stable ischemic heart disease and at least 12 months after an ACS (4). Prolonged DAPT beyond 6–12 months was placed as a Class IIb recommendation and may be considered based on the assessment of ischemic-bleeding tradeoff (4).
There is a pressing need to understand better the factors associated with increased risk of ST. Several clinical factors (e.g., ACS presentation, diabetes mellitus, current cigarette smoking, and reduced left ventricular ejection fraction) and procedural characteristics (e.g., stent undersizing, stent underdeployment, small stent diameter, greater stent length, and bifurcation stents) have been identified as predictors of ST (5–7). Yet, little is known about how laboratory tests may assist in stratifying ACS patients according to their ST risk. Few studies have assessed the prognostic significance of hemoglobin values and platelet counts in predicting ST (8, 9). However, it remains uncertain whether elevations in cardiac biomarkers or inflammatory markers during the index ACS event are linked to subsequent ST risk. As such, this study aimed to validate the association of hemoglobin and platelet count with the risk of ST in an international, multicenter Phase 3 trial with a relatively large sample size. Furthermore, the study sought to extend previous findings by investigating the predictive value of other available laboratory measurements, including cardiac biomarkers and white blood cell (WBC) counts (as a marker for inflammation), in relation to ST. In addition, due to the low event rate of ST, risk factors of ST based on the timing [i.e., early ST (within 30 days of implantation), late ST (between 1 month and 1 year from stent implantation), or very late ST (more than 1 year after stent implantation)] have not been well characterized in prior studies. As the underlying mechanisms of ST may differ across various periods (10), this substudy explored factors associated with early, late, and very late ST, including patient characteristics, laboratory tests, and treatments in the ATLAS ACS 2-TIMI 51 trial (A Randomized, Double-Blind, Placebo-Controlled, Event-Driven Multicenter Study to Evaluate the Efficacy and Safety of Rivaroxaban in Subjects With a Recent Acute Coronary Syndrome).
Methods
The rationale and design of the ATLAS ACS 2-TIMI 51 trial (National Clinical Trial Identifier Number: NCT00809965) have been described previously (11, 12). In brief, the ATLAS ACS 2-TIMI 51 trial enrolled patients (≥18 years of age) who presented with symptoms suggestive of ACS. Enrollment occurred within 7 days of hospital admission, and patients were randomly assigned in a 1:1:1 fashion to twice-daily administration of either 2.5 or 5.0 mg of rivaroxaban or a placebo. Patients received the first dose of the study drug as soon as possible after randomization, but no sooner than 4 h after the final dose of intravenous unfractionated heparin, 2 h after the final dose of bivalirudin, and 12 h after the final dose of other intravenous or subcutaneous anticoagulants (e.g., enoxaparin or fondaparinux). All patients received standard medical therapy, including low-dose aspirin and a thienopyridine (either clopidogrel or ticlopidine) at a dose recommended according to the national or local guidelines. The patients were seen at 4 weeks, 12 weeks, and after that every 12 weeks, with a maximum follow-up of 31 months. The trial was conducted from November 26, 2008, to September 19, 2011. The primary efficacy endpoint of the ATLAS ACS 2 TIMI 51 trial was a composite of death from cardiovascular causes, MI, and stroke. ST was a predefined, independently adjudicated endpoint classified as definite, probable, or possible based on the Academic Research Consortium (ARC) definitions (13). The endpoint of the present analysis was ST (including definite, probable, and possible ST).
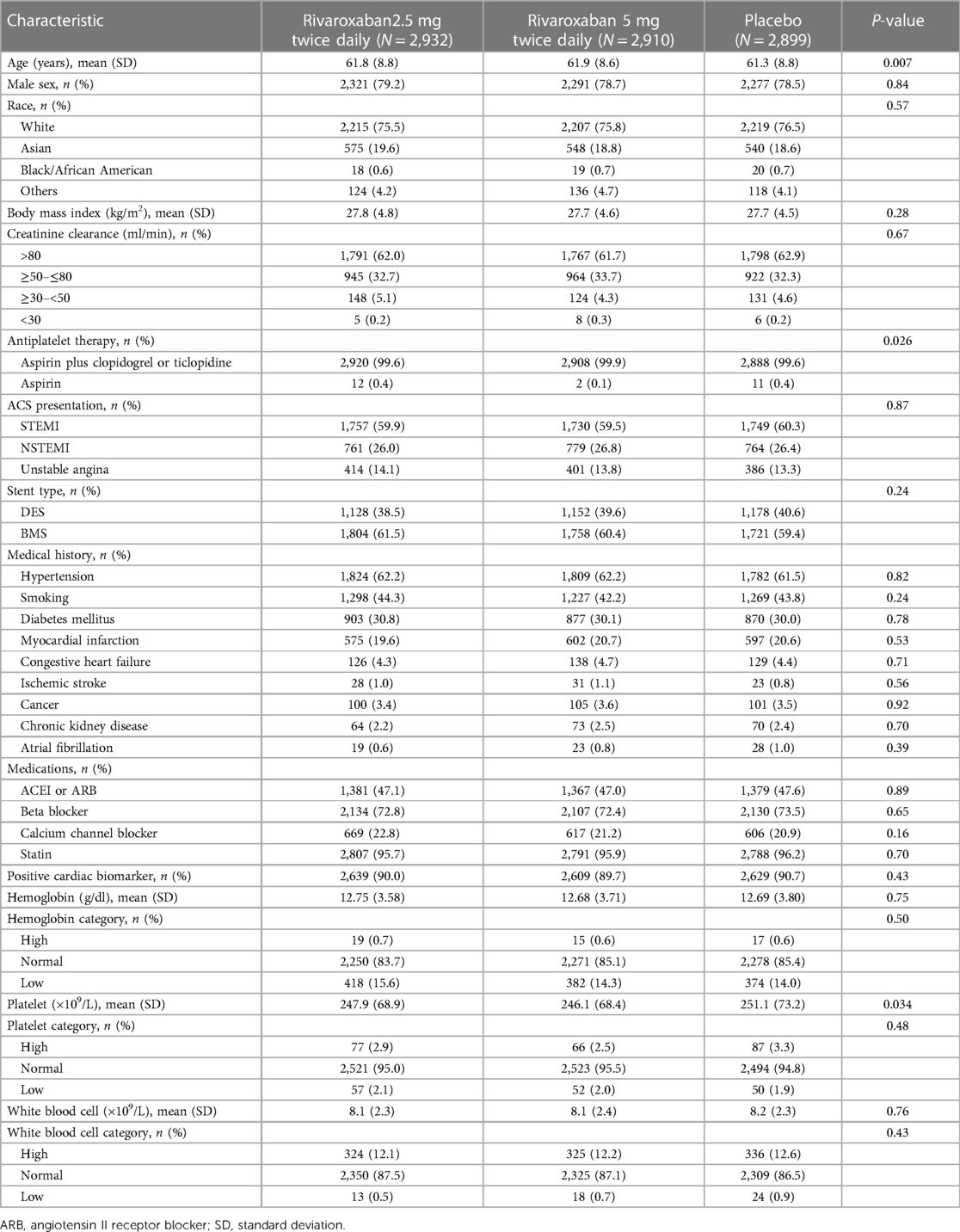
The present analysis was restricted to patients who received stent placement during the primary PCI for the index ACS. Patients with a history of coronary stent implantation were excluded from the analysis, because the specific date of their prior stent placement was not recorded, and it was not possible to accurately assess the time elapsed from stent placement to the occurrence of ST. The analysis was performed using a modified intention-to-treat (mITT) approach, described previously (12). A positive cardiac biomarker was defined as either a troponin level above the 99th percentile or a creatine kinase myocardial band above the upper limit of the normal range (according to the local laboratories), or both. The normal hemoglobin ranges were 12.5–17.0 g/dl for men and 11.5–15.8 g/dl for women. The normal ranges of platelet count and WBC count were 130–394 × 109/L and 3.8–10.7 × 109/L, respectively.
Statistical analysis
The analyses in this substudy were post hoc and exploratory. Baseline characteristics of study participants stratified by treatment allocation (rivaroxaban 2.5 mg twice daily vs. rivaroxaban 5 mg twice daily vs. placebo) were compared using the chi-squared test for categorical variables and the one-way ANOVA or Kruskal–Wallis test for continuous variables, as appropriate.
The cumulative incidence of ST was assessed by Kaplan–Meier estimates at 30 days (early ST), 31–360 days (late ST), 361–720 days (very late ST), and up to 720 days, with Day 1 defined as the day of stent implantation. In addition, univariable and multivariable associations with ST were explored by fitting a Cox proportional hazards model. Candidate variables included patient characteristics (i.e., age, sex, ACS presentation, history of hypertension, smoking, diabetes mellitus, prior MI, congestive heart failure, prior ischemic stroke, and cancer), baseline laboratory tests (i.e., positive cardiac biomarker, hemoglobin, platelet count, WBC count), and treatment (i.e., the type of stent and anticoagulant with rivaroxaban vs. placebo). In addition, landmark analyses with the landmark time of 30, 31–360, and 361–720 days were performed to assess the association with early, late, and very late ST, respectively.
A nominal threshold of P < 0.05 was used to determine the significance of the association. All analyses were performed with SAS software version 9.4 (SAS Institute, Inc., NC, United States).
Results
Patient characteristics
Of the 15,342 patients included in the mITT analysis, 8,741 (57.0%) patients received coronary stent placement during PCI for the index ACS. Among those, 2,932 (33.5%) patients were assigned to receive rivaroxaban 2.5 mg twice daily, 2,910 (33.3%) were assigned to receive rivaroxaban 5 mg twice daily, and 2,899 (33.2%) were assigned to receive a placebo. As provided in Table 1, patient characteristics were generally balanced across patients with different treatment allocations, except that the mean age in the rivaroxaban arms was higher, the proportion of patients receiving DAPT differed, and the mean platelet level in the placebo arm was higher. Most patients received DAPT with low-dose aspirin plus a thienopyridine (99.7%) and statin (95.9%). Approximately 59.9%, 26.4%, and 13.7% of the patients presented with STEMI, non-ST segment elevation myocardial infarction (NSTEMI), and unstable angina, respectively. A total of 3,458 (39.6%) received drug-eluting stent (DES) implantation, and 5,283 (60.4%) received bare-metal stent (BMS) implantation. The rates of thrombolysis in myocardial infarction (TIMI) major bleeding not related to coronary artery bypass graft surgery (CABG) were 1.73%, 1.71%, and 0.52% in patients who received rivaroxaban 2.5 mg twice daily, rivaroxaban 5 mg twice daily, and placebo, respectively. The rates of TIMI minor bleeding were 0.80%, 1.22%, and 0.49%, respectively. The rates of bleeding event requiring medical attention were 11.14%, 14.10%, and 6.54%, respectively.
A total of 155 ST events occurred by Day 720, including 72 early ST events (within 30 days), 62 late ST events (from 31 to 360 days), and 21 very late ST events (from 361 to 720 days). Of the 155 ST events, there were 75 categorized as definite ST, 41 as probable ST, and 38 as possible ST (with one ST event remaining uncategorized). A total of 129 patients had only one ST event (64, 48, and 17 were early, late, and very late ST events, respectively), and 13 patients had two ST events (8, 14, and 4 were early, late, and very late ST events, respectively). The cumulative incidence of overall ST at 720 days was 2.25% [95% confidence interval (CI): 1.83%–2.68%] (Figure 1A). The cumulative incidence of early, late, and very late ST was 0.80% (95% CI: 0.61%–0.98%), 0.81% (95% CI: 0.59%–1.02%), and 0.77% (95% CI: 0.41%–1.12%), respectively (Figure 1B).
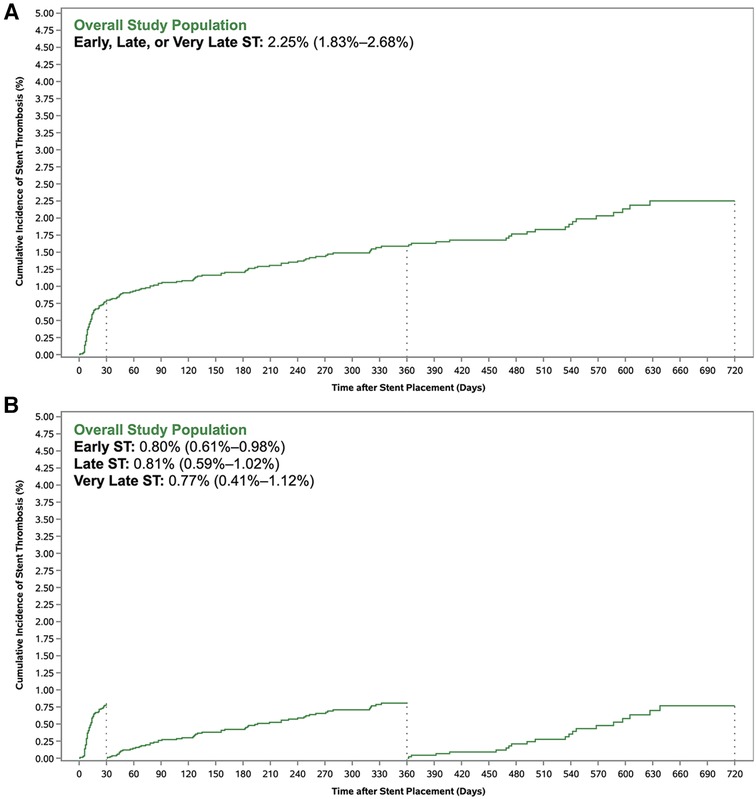
Figure 1. Cumulative incidence of stent thrombosis with (A) standard Kaplan–Meier method and (B) landmark analysis.
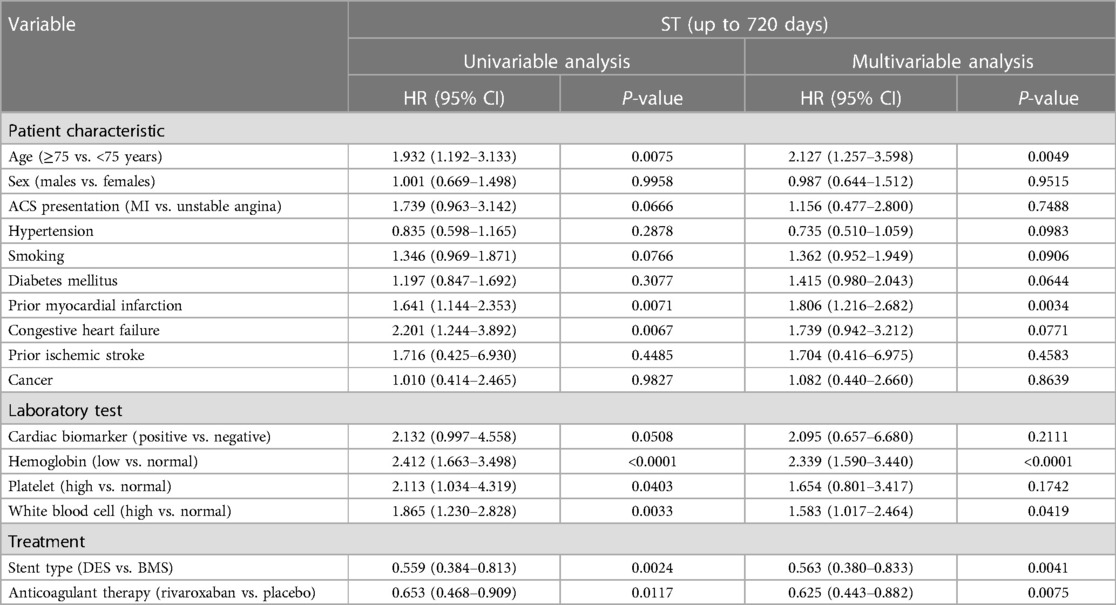
Factors associated with stent thrombosis up to 720 days
In univariable analysis, the factors associated with ST up to 720 days included age ≥ 75 years, a history of prior MI, congestive heart failure, low hemoglobin level, high platelet count, high WBC count, DES, and rivaroxaban therapy (Table 2). After multivariable adjustment, age ≥ 75 years [hazard ratio (HR) = 2.127 (95% CI: 1.257–3.598)], a history of prior MI [HR = 1.806 (95% CI: 1.216–2.682)], low hemoglobin level [HR = 2.339 (95% CI: 1.590–3.440)], and high WBC count [HR = 1.583 (95% CI: 1.017–2.464)] remained associated with a greater risk of ST up to 720 days, whereas DES [HR = 0.563 (95% CI: 0.380–0.833)] and rivaroxaban therapy [HR = 0.625 (95% CI: 0.443–0.882)] were associated with a lower risk of ST up to 720 days. Hemoglobin or WBC category did not significantly modify the effect of rivaroxaban on the risk of ST (interaction P = 0.95 and P = 0.98, respectively).
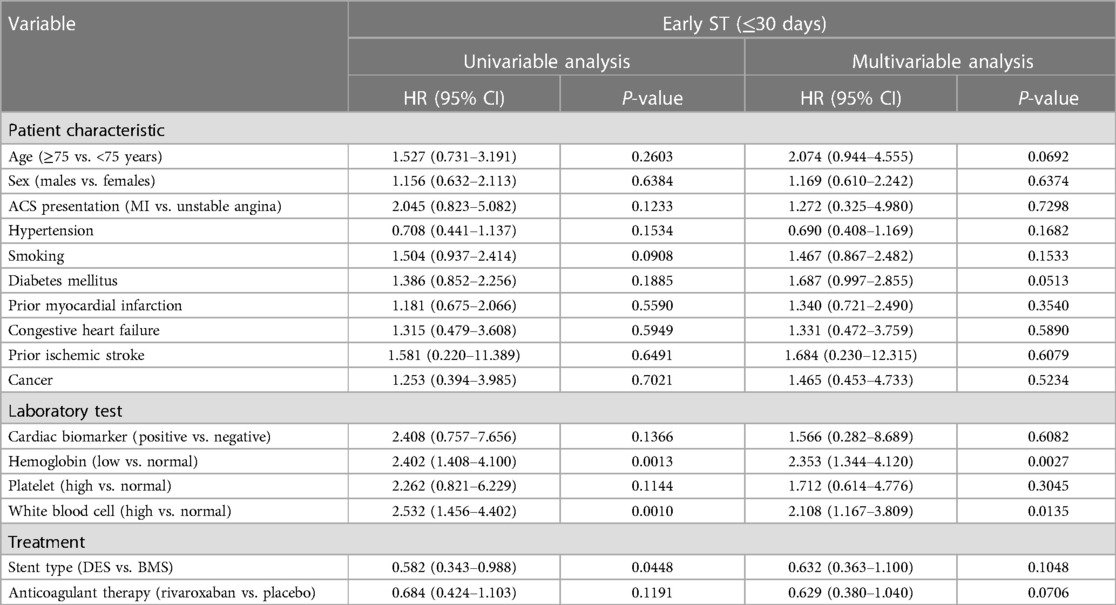
Factors associated with early stent thrombosis
In univariable analysis, the factors associated with early ST (within 30 days) included low hemoglobin level, high WBC count, and DES (Table 3). After multivariable adjustment, only low hemoglobin level [HR = 2.353 (95% CI: 1.344–4.120)] and high WBC count [HR = 2.108 (95% CI: 1.167–3.809)] remained associated with early ST.
Factors associated with late stent thrombosis
In univariable analysis, the factors associated with late ST (31–360 days) included age ≥75 years, a history of prior MI, congestive heart failure, low hemoglobin level, and DES (Table 4). After multivariable adjustment, prior MI [HR = 2.978 (95% CI: 1.671–5.309)] and low hemoglobin level [HR = 2.315 (95% CI: 1.256–4.265)] remained associated with late ST, whereas DES [HR = 0.334 (95% CI: 0.165–0.673)] was protective against late ST.
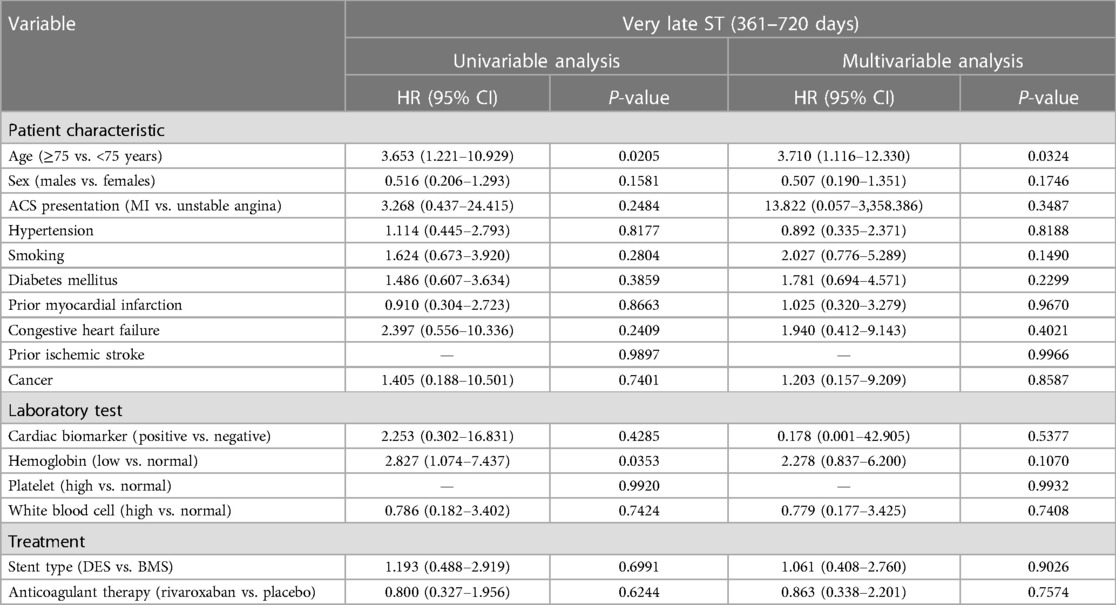
Factors associated with very late stent thrombosis
In univariable analysis, the factors associated with very late ST (361–720 days) included age ≥75 years and low hemoglobin level (Table 5). After multivariable adjustment, only age ≥75 years [HR = 3.710 (95% CI: 1.116–12.330)] remained associated with very late ST.
Summary of factors associated with stent thrombosis
The results of independent factors with positive (including age ≥75 years, a history of prior MI, low hemoglobin level, and high WBC count) and negative (including DES and rivaroxaban) association with ST up to 720 days (Panel A), early ST (Panel B), late ST (Panel C), and very late ST (Panel D) were summarized in Figure 2.
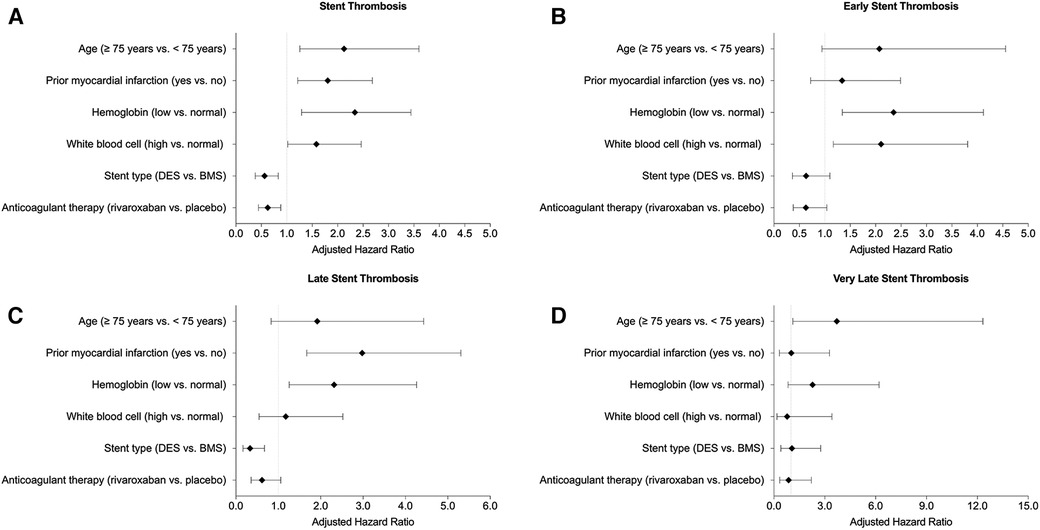
Figure 2. Summary of association with (A) all stent thrombosis, (B) early stent thrombosis, (C) late stent thrombosis, and (D) very late stent thrombosis.
With respect to factors with a positive association, high WBC count was predictive of ST up to 720 days and early ST. Low hemoglobin level was predictive of ST up to 720 days, early ST, and late ST. Prior MI was predictive of ST up to 720 days and late ST. Age ≥75 years was predictive of ST up to 720 days and very late ST. With respect to factors with a negative association, DES was protective against ST up to 720 days and late ST. Rivaroxaban therapy was protective against ST for up to 720 days.
Landmark analyses of the six factors were provided in Supplementary Figures S1–S6. Compared with patients aged <75 years, those aged ≥75 years had a significantly greater risk of late and very late ST (Supplementary Figure S1). The association between prior MI and ST was primarily driven by the substantial risk difference from 31 to 360 days (i.e., late ST) (Supplementary Figure S2). Compared with patients with normal hemoglobin levels, anemic patients had a significantly greater ST risk within the first year (i.e., early and late ST) (Supplementary Figure S3). Patients with leukocytosis had a significantly greater risk for ST only within the first 30 days following stent implantation (i.e., early ST) (Supplementary Figure S4). Compared with BMS, DES was associated with a significantly reduced ST risk within the first year, encompassing early and late ST events. During the second year, there was no significant difference in ST risk between DES and BMS (Supplementary Figure S5). While patients who received rivaroxaban had a reduced ST risk at 2 years, its effect on early, late, or very late ST was not statistically significant (Supplementary Figure S6).
Discussion
In the ATLAS ACS 2-TIMI 51 trial, the incidence of early, late, and very late ST was 0.80%, 0.81%, and 0.77% among patients with a recent ACS. These findings were consistent with prior studies, which reported an incidence of <1% for early ST, 0.5%–1.0% for late ST, and 0.2%–0.4% for very late ST (5). While confirming the established factors associated with ST (such as advanced age, prior MI, and DES), the study extends the current knowledge by demonstrating the usefulness of commonly available blood tests, including WBC and hemoglobin, in predicting early and late ST. In a patient-level pooled analysis of ACS patients undergoing PCI with stent implantation from the HORIZON-AMI and ACUITY trials, Dangas et al. developed a 1-year ST risk score that included type of ACS, smoking status, insulin-treated diabetes mellitus, history of PCI, baseline platelet count, absence of early heparin use, aneurysm or ulceration, baseline and final TIMI flow grade, and the number of vessels treated (9). While we did not find an association between ST and the type of ACS, smoking status, or platelet count, we observed that high WBC counts and low hemoglobin levels were associated with a greater ST risk. As part of the standard measures of complete blood count by automated instruments in the hematology laboratory, WBC and hemoglobin are of low cost and can be readily incorporated into ST risk assessment.
The data suggested that a high WBC count was independently associated with an increased risk of ST by 30 days. Previous analysis of the ATLAS ACS 2-TIMI 51 trial indicates an association between ischemic events and high WBC count (14–16). The plausible mechanisms for such association included alteration of platelet function, tissue factor formation, endothelial injury, and extrinsic pathway activation (14). Specifically, these mechanisms can be classified into two major categories: (1) mechanisms that directly involve WBC in the development of ischemic events: (i) direct effect of WBC surface antigen (CD11b/CD18) on activation of the coagulation cascade and the subsequent plaque formation (17), (ii) plugging capillary lumen caused by high viscosity, and (iii) platelet-WBC aggregation caused by WBC surface antigens (18); (2) mechanisms that indirectly involve WBCs as an index of inflammation and coagulation: (i) decreased epicardial and myocardial perfusion (19) and (ii) endothelial injury due to release of oxygen-free radicals and other factors that may promote complement activation, cause reperfusion injury, and provoke an inflammatory response, which may persist after the resolution of the acute event and last up to 6 months (20, 21).
In agreement with previous studies, the present study indicated that low hemoglobin levels were associated with an increased risk of ST (8, 22, 23). While the exact mechanisms remain unclear, anemic patients may be more likely to experience thrombotic events (24, 25). The presence of anemia might reflect an underlying condition that predisposes patients to thrombosis, such as iron deficiency, chronic inflammation, malignancy, and chronic kidney disease (26–28). For instance, studies have suggested that lower hemoglobin and serum iron levels are associated with elevated levels of coagulation factor VIII and accelerated platelet aggregation, which can contribute to ST (29, 30). Therefore, low hemoglobin levels could be considered an integrative marker of multiple pathologic processes in ACS patients. Another possible explanation is that anemia may be associated with endothelial dysfunction, blood stasis, or a hypercoagulable state, leading to a greater risk of thrombus formation (31–33).
Previous data have confirmed the superiority of second-generation DES over BMS in terms of the risk of ST at 1 year (34, 35). A pooled analysis of data from recent trials with 26,616 patients comparing the outcomes after implantation of new-generation DES vs. BMS over an average follow-up of 3.2 years demonstrated lower definite ST with DES [HR = 0.63 (95% CI: 0.50–0.80), P < 0.001] (35). This is similar to our findings that showed a lower risk of ST with DES over a follow-up period of 2 years [HR = 0.563 (95% CI) 0.380–0.833], P = 0.004). However, Brodie et al. (36) demonstrated DES as the only protective factor of ST (HR = 3.73, 95% CI: 1.81–7.88). It is worth mentioning that Brodie et al. compared the first-generation DES with BMS. The first-generation DES had been associated with higher rates of late and very late ST, with a reported very late ST rate of up to 2% with first-generation stents as compared with 0.2%–0.4% per year with newer DES (5). The higher thrombosis risk resulted in the discontinuation of first-generation DES and the development of the new generation of DES (37). The mechanisms of newer generation DES and the reduction of ST are not well understood. Some investigators argued that this reduction occurs due to a more extended period of DAPT with the application of DES. However, the LEADERS FREE trial demonstrated that ST might occur irrespective of DAPT duration. In the LEADERS FREE trial, patients at high risk of bleeding were randomized to receive either DES or BMS, and 1-month DAPT was applied irrespective of stent type. The trial found a significantly lower rate of ST with DES compared to BMS at 30 days and over a period of 390 days (38). The protection against late ST by DES may be due to the effect of delayed endothelialization of the coronary lumen by DES-released antiproliferative drugs (10). There is no clear consensus on whether DES is more effective than BMS beyond 1 year (35). In the present analysis, DES usage was associated with a lower risk of overall ST up to 720 days, primarily attributed to a reduction in late ST events. Nonetheless, we did not observe a significant difference in the risk of very late ST between DES and BMS usage [HR = 1.061 95% CI: (0.408–2.760), P = 0.9026]. The lack of a discernible advantage with DES could potentially be influenced by the prolonged duration of DAPT, typically spanning a minimum of 6–12 months, in patients who underwent DES implantation. Future studies are warranted to focus on reducing thrombotic events beyond 1 year.
In this analysis, patients 75 years or older were at a higher risk of developing very late ST. There have been mixed results with respect to whether younger or older age was associated with an increased risk of ST. In a study conducted among 124 patients with ST, a 1-year decrease in age was associated with an 8% increase in very late ST (39). Two previous reports also suggested younger age as a predictor of ST (40, 41). However, these studies were performed when first-generation DES was used, which may have affected the results. It is, therefore, not known whether the association of younger age with increased risk of ST applies to the newer generation of DES. Of note, it has been demonstrated that premature discontinuation of P2Y12 inhibitors was associated with older age, which may help explain the higher ST risk (42). In addition, consistent with previous reports, a history of previous MI was another predictor of late ST in this analysis (36). Older patients are more likely to have a complicated cardiac history, including previous MI, thus contributing to an increased ST risk.
Limitations
The current findings should be interpreted in the context of several limitations. First, only 155 adjudicated ST events occurred through the end of follow-up by 720 days, and the landmark analysis may be underpowered. Second, the maximum follow-up was 31 months, which may be inadequate for analyzing very late ST. Third, although the majority (99.7%) of patients received DAPT therapy at baseline, information about DAPT interruption was not available for analysis, which might have affected the precision of the estimates. It is important to emphasize that, although clopidogrel remains commonly used as part of the DAPT regimen, contemporary clinical practice guidelines now recommend more potent P2Y12 inhibitors, such as prasugrel and ticagrelor. This recommendation stems from head-to-head comparisons in recent clinical trials, demonstrating the superiority of these next-generation inhibitors over clopidogrel in preventing ischemic events. Furthermore, recent evidence from the M.O.Ca. registry suggests a potential protective role of cangrelor in reducing in-hospital definite ST among ACS patients when administered in addition to the current standard-of-care P2Y12 inhibitors (43). Aside from antiplatelet therapy, the range in collection times for the various laboratory investigations was not controlled for in the study. Fourth, it is important to note that several established lesion-, device-, and procedure-related risk factors, such as angiographic characteristics, the type of DES employed, stent dimensions (diameter and length), the utilization of intracoronary imaging, and the practice of bifurcation stenting, were not included in the study due to unavailability. The potential complex interactions among these factors could significantly influence the timeframes of ST. It is essential to consider these limitations when interpreting the results of the current study. Fifth, in the ATLAS ACS 2-TIMI 51 trial, participants were enrolled within 7 days (with a mean ± SD of 1.5 ± 0.9 days) after being admitted to the hospital because of an acute coronary syndrome, once their condition had been stabilized and initial management strategies (e.g., revascularization) were completed. In this study, Day 1 was defined as the day of stent implantation, marking the initiation of stent exposure. This approach was chosen to accurately capture the timing when patients became at risk for ST, as opposed to using the day of randomization or treatment initiation in the main analysis. Nevertheless, the incidence of early ST might have been underestimated in this study, as acute ST events (i.e., ST events that occurred within 24 h of stent placement) prior to study enrollment were not accounted for. Sixth, to ensure a sufficient event frequency at each landmark time for exploring potential predictors in the multivariable analysis, we included definite, probable, and possible ST as the endpoint. Under the ARC classification scheme, definite ST exhibits a high level of specificity, but is prone to potentially underestimating the actual occurrence. Conversely, possible ST offers enhanced sensitivity, but lacks diagnostic certainty (44). It is important to note that incorporating possible ST into the analysis could have introduced some uncertainties, and the results from the exploratory analysis should be validated in future studies. Seventh, according to the practice guidelines, DES is preferred over BMS in patients undergoing PCI (45). While the study findings were consistent with the guideline recommendations, it should be highlighted that the role of BMS is limited in current practice. Eighth, it is important to consider the risk of overfitting in the multivariable analysis when examining the type of ST by timeframe. Last, this is a post hoc analysis of a subset of stented ACS patients who agreed to participate in a clinical trial with specific enrollment criteria. The findings from the present study do not suggest causality and should be considered exploratory and hypothesis-generating.
Conclusion
The study identified factors associated with early ST (high WBC count and low hemoglobin), late ST (low hemoglobin, prior MI, and DES), very late ST (advanced age), and overall ST (advanced age, prior MI, low hemoglobin, high WBC, DES, and rivaroxaban). These variables may be useful in constructing risk assessment models for ST.
Data availability statement
The datasets presented in this article are not readily available because they contain restricted data. Requests to access the datasets should be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by local ethics committee and institutional review board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
GC: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Visualization, Writing – original draft. FA: Writing – review & editing. JL: Writing – review & editing. SM: Writing – review & editing. CF: Writing – review & editing, Project administration. SK: Writing – review & editing. WO: Writing – review & editing. EB: Writing – review & editing. AP: Writing – review & editing. CMG: Funding acquisition, Resources, Supervision, Validation, Writing – review & editing.
Funding
The authors declare financial support was received for the research, authorship, and/or publication of this article.
The ATLAS ACS 2-TIMI 51 trial was supported by research grants from Johnson & Johnson Pharmaceutical Research & Development and Bayer Healthcare AG.
Conflict of interest
GC receives research grant support paid to the Beth Israel Deaconess Medical Center and Harvard Medical School from Bayer, Janssen Scientific Affairs, and CSL Behring. EB is employed by and has an equity interest in Johnson & Johnson. AP is employed by and has an equity interest in Johnson & Johnson. CMG receives consultant fees from Portola Pharmaceuticals and reports grants from Angel Medical Corporation and CSL Behring; grants and other support from Bayer Corporation; grants and personal fees from Janssen and Johnson & Johnson; and personal fees from The Medicines Company, Boston Clinical Research Institute, Cardiovascular Research Foundation, Eli Lilly, Gilead Sciences Inc., Novo Nordisk, Pfizer, Web MD, UpToDate in Cardiovascular Medicine, Amarin Pharma, Amgen, Arena Pharmaceuticals, Bayer Corporation, Boehringer Ingelheim, Chiesi, Merck & Co, PharmaMar, Sanofi, Somahlution, St Francis Hospital, and Verreseon Corporation.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1269011/full#supplementary-material
References
1. Byrne RA, Joner M, Kastrati A. Stent thrombosis and restenosis: what have we learned and where are we going? The Andreas Grüntzig Lecture ESC 2014. Eur Heart J. (2015) 36(47):3320–31. doi: 10.1093/eurheartj/ehv511
2. Biondi-Zoccai GG, Agostoni P, Sangiorgi GM, Airoldi F, Cosgrave J, Chieffo A, et al. Incidence, predictors, and outcomes of coronary dissections left untreated after drug-eluting stent implantation. Eur Heart J. (2006) 27(5):540–6. doi: 10.1093/eurheartj/ehi618
3. Kikkert WJ, Damman P. Optimal duration of dual antiplatelet therapy for coronary artery disease. Netherlands Heart J. (2018) 26(6):321–33. doi: 10.1007/s12471-018-1113-5
4. Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention, 2011 ACCF/AHA guideline for coronary artery bypass graft surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease, 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction, 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes, and 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. Circulation. (2016) 134(10):e123–55. doi: 10.1161/CIR.0000000000000404
5. Gori T, Polimeni A, Indolfi C, Räber L, Adriaenssens T, Münzel T. Predictors of stent thrombosis and their implications for clinical practice. Nat Rev Cardiol. (2019) 16(4):243–56. doi: 10.1038/s41569-018-0118-5
6. Yeh RW, Secemsky EA, Kereiakes DJ, Normand SL, Gershlick AH, Cohen DJ, et al. Development and validation of a prediction rule for benefit and harm of dual antiplatelet therapy beyond 1 year after percutaneous coronary intervention. JAMA. (2016) 315(16):1735–49. doi: 10.1001/jama.2016.3775
7. Kuramitsu S, Ohya M, Shinozaki T, Otake H, Horie K, Kawamoto H, et al. Risk factors and long-term clinical outcomes of second-generation drug-eluting stent thrombosis. Circ Cardiovasc Interv. (2019) 12(6):e007822. doi: 10.1161/CIRCINTERVENTIONS.119.007822
8. Pilgrim T, Vetterli F, Kalesan B, Stefanini GG, Räber L, Stortecky S, et al. The impact of anemia on long-term clinical outcome in patients undergoing revascularization with the unrestricted use of drug-eluting stents. Circ Cardiovasc Interventions. (2012) 5(2):202–10. doi: 10.1161/CIRCINTERVENTIONS.111.965749
9. Dangas GD, Claessen BE, Mehran R, Xu K, Fahy M, Parise H, et al. Development and validation of a stent thrombosis risk score in patients with acute coronary syndromes. JACC Cardiovasc Interv. (2012) 5(11):1097–105. doi: 10.1016/j.jcin.2012.07.012
10. Torrado J, Buckley L, Durán A, Trujillo P, Toldo S, Raleigh JV, et al. Restenosis, stent thrombosis, and bleeding complications: navigating between Scylla and Charybdis. J Am Coll Cardiol. (2018) 71(15):1676–95. doi: 10.1016/j.jacc.2018.02.023
11. Gibson CM, Mega JL, Burton P, Goto S, Verheugt F, Bode C, et al. Rationale and design of the anti-Xa therapy to lower cardiovascular events in addition to standard therapy in subjects with acute coronary syndrome-thrombolysis in myocardial infarction 51 (ATLAS-ACS 2 TIMI 51) trial: a randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of rivaroxaban in subjects with acute coronary syndrome. Am Heart J. (2011) 161(5):815–821.e6. doi: 10.1016/j.ahj.2011.01.026
12. Mega JL, Braunwald E, Wiviott SD, Bassand JP, Bhatt DL, Bode C, et al. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med. (2012) 366(1):9–19. doi: 10.1056/NEJMoa1112277
13. Garcia-Garcia HM, McFadden EP, Farb A, Mehran R, Stone GW, Spertus J, et al. Standardized end point definitions for coronary intervention trials: the Academic Research Consortium-2 consensus document. Circulation. (2018) 137(24):2635–50. doi: 10.1161/CIRCULATIONAHA.117.029289
14. Alkhalfan F, Nafee T, Yee MK, Chi G, Kalayci A, Plotnikov A, et al. Relation of white blood cell count to bleeding and ischemic events in patients with acute coronary syndrome (from the ATLAS ACS 2-TIMI 51 trial). Am J Cardiol. (2020) 125(5):661–9. doi: 10.1016/j.amjcard.2019.12.007
15. Bhatt DL, Chew DP, Lincoff AM, Simoons ML, Harrington RA, Ommen SR, et al. Effect of revascularization on mortality associated with an elevated white blood cell count in acute coronary syndromes. Am J Cardiol. (2003) 92(2):136–40. doi: 10.1016/S0002-9149(03)00527-7
16. Sabatine MS, Morrow DA, Cannon CP, Murphy SA, Demopoulos LA, DiBattiste PM, et al. Relationship between baseline white blood cell count and degree of coronary artery disease and mortality in patients with acute coronary syndromes: a TACTICS-TIMI 18 substudy. J Am Coll Cardiol. (2002) 40(10):1761–8. doi: 10.1016/S0735-1097(02)02484-1
17. Hirahashi J, Hishikawa K, Kaname S, Tsuboi N, Wang Y, Simon DI, et al. Mac-1 (CD11b/CD18) is a critical molecular link between inflammation and thrombosis following glomerular injury1. Circulation. (2009) 120(13):1255. doi: 10.1161/CIRCULATIONAHA.109.873695
18. Ernst E, Hammerschmidt DE, Bagge U, Matrai A, Dormandy JA. Leukocytes and the risk of ischemic diseases. JAMA. (1987) 257(17):2318–24. doi: 10.1001/jama.1987.03390170074031
19. Barron HV, Cannon CP, Murphy SA, Braunwald E, Gibson CM. Association between white blood cell count, epicardial blood flow, myocardial perfusion, and clinical outcomes in the setting of acute myocardial infarction: a thrombolysis in myocardial infarction 10 substudy. Circulation. (2000) 102(19):2329–34. doi: 10.1161/01.CIR.102.19.2329
20. van der Wal AC, Becker A, Van der Loos C, Das P. Site of intimal rupture or erosion of thrombosed coronary atherosclerotic plaques is characterized by an inflammatory process irrespective of the dominant plaque morphology. Circulation. (1994) 89(1):36–44. doi: 10.1161/01.CIR.89.1.36
21. Mulvihill NT, Foley JB. Inflammation in acute coronary syndromes. Heart. (2002) 87(3):201–4. doi: 10.1136/heart.87.3.201
22. Tunçez A, Çetin MS, Çetin EHÖ, Yılmaz S, Korkmaz A, Uçar FM. Association between RDW and stent thrombosis in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Medicine (Baltimore). (2017) 96(5):e5986. doi: 10.1097/MD.0000000000005986
23. Yazji K, Abdul F, Elangovan S, Ul Haq MZ, Ossei-Gerning N, Morris K, et al. Baseline anemia in patients undergoing percutaneous coronary intervention after an acute coronary syndrome—a paradox of high bleeding risk, high ischemic risk, and complex coronary disease. J Interv Cardiol. (2017) 30(5):491–9. doi: 10.1111/joic.12406
24. Stolz E, Valdueza JM, Grebe M, Schlachetzki F, Schmitt E, Madlener K, et al. Anemia as a risk factor for cerebral venous thrombosis? An old hypothesis revisited. Results of a prospective study. J Neurol. (2007) 254(6):729–34. doi: 10.1007/s00415-006-0411-9
25. Chi G, Gibson CM, Hernandez AF, Hull RD, Kazmi SHA, Younes A, et al. Association of anemia with venous thromboembolism in acutely ill hospitalized patients: an APEX trial substudy. Am J Med. (2018) 131(8):972.e1–7. doi: 10.1016/j.amjmed.2018.03.031
26. Palta S, Saroa R, Palta A. Overview of the coagulation system. Indian J Anaesth. (2014) 58(5):515–23. doi: 10.4103/0019-5049.144643
27. Huang MJ, Wei RB, Wang Y, Su TY, Di P, Li QP, et al. Blood coagulation system in patients with chronic kidney disease: a prospective observational study. BMJ Open. (2017) 7(5):e014294. doi: 10.1136/bmjopen-2016-014294
28. Zöller B, Li X, Sundquist J, Sundquist K. Autoimmune diseases and venous thromboembolism: a review of the literature. Am J Cardiovasc Dis. (2012) 2(3):171–83. PMID: 22937487
29. Livesey JA, Manning RA, Meek JH, Jackson JE, Kulinskaya E, Laffan MA, et al. Low serum iron levels are associated with elevated plasma levels of coagulation factor VIII and pulmonary emboli/deep venous thromboses in replicate cohorts of patients with hereditary haemorrhagic telangiectasia. Thorax. (2012) 67(4):328–33. doi: 10.1136/thoraxjnl-2011-201076
30. Scharbert G, Wetzel L, Berlinger L, Kozek-Langenecker S. Effect of anemia on coagulation and platelet function: a whole blood in vitro study. Critical Care. (2011) 15(Suppl 1):P445. doi: 10.1186/cc9865
31. Solovey A, Lin Y, Browne P, Choong S, Wayner E, Hebbel RP. Circulating activated endothelial cells in sickle cell anemia. N Engl J Med. (1997) 337(22):1584–90. doi: 10.1056/NEJM199711273372203
32. Hebbel RP. Ischemia-reperfusion injury in sickle cell anemia: relationship to acute chest syndrome, endothelial dysfunction, arterial vasculopathy, and inflammatory pain. Hematol Oncol Clin North Am. (2014) 28(2):181–98. doi: 10.1016/j.hoc.2013.11.005
33. Yildiz A, Oflaz H, Pusuroglu H, Mercanoglu F, Genchallac H, Akkaya V, et al. Left ventricular hypertrophy and endothelial dysfunction in chronic hemodialysis patients. Am J Kidney Dis. (2003) 41(3):616–23. doi: 10.1053/ajkd.2003.50123
34. Bønaa KH, Mannsverk J, Wiseth R, Aaberge L, Myreng Y, Nygård O, et al. Drug-eluting or bare-metal stents for coronary artery disease. N Engl J Med. (2016) 375(13):1242–52. doi: 10.1056/NEJMoa1607991
35. Piccolo R, Bonaa KH, Efthimiou O, Varenne O, Baldo A, Urban P, et al. Drug-eluting or bare-metal stents for percutaneous coronary intervention: a systematic review and individual patient data meta-analysis of randomised clinical trials. Lancet. (2019) 393(10190):2503–10. doi: 10.1016/S0140-6736(19)30474-X
36. Brodie B, Pokharel Y, Garg A, Kissling G, Hansen C, Milks S, et al. Predictors of early, late, and very late stent thrombosis after primary percutaneous coronary intervention with bare-metal and drug-eluting stents for ST-segment elevation myocardial infarction. JACC Cardiovasc Interv. (2012) 5(10):1043–51. doi: 10.1016/j.jcin.2012.06.013
37. Valgimigli M, Tebaldi M, Borghesi M, Vranckx P, Campo G, Tumscitz C, et al. Two-year outcomes after first-or second-generation drug-eluting or bare-metal stent implantation in all-comer patients undergoing percutaneous coronary intervention: a pre-specified analysis from the PRODIGY study (PROlonging dual antiplatelet treatment after grading stent-induced intimal hyperplasia studY). JACC Cardiovasc Interv. (2014) 7(1):20–8. doi: 10.1016/j.jcin.2013.09.008
38. Urban P, Meredith IT, Abizaid A, Pocock SJ, Carrié D, Naber C, et al. Polymer-free drug-coated coronary stents in patients at high bleeding risk. N Engl J Med. (2015) 373(21):2038–47. doi: 10.1056/NEJMoa1503943
39. Park KW, Hwang S-J, Kwon D-A, Oh B-H, Park Y-B, Chae I-H, et al. Characteristics and predictors of drug-eluting stent thrombosis. Circ J. (2011) 75(7):1626–32. doi: 10.1253/circj.CJ-10-1160
40. Waksman R, Kirtane AJ, Torguson R, Cohen DJ, Ryan T, Räber L, et al. Correlates and outcomes of late and very late drug-eluting stent thrombosis: results from DESERT (international drug-eluting stent event registry of thrombosis). JACC Cardiovasc Interv. (2014) 7(10):1093–102. doi: 10.1016/j.jcin.2014.04.017
41. Van Werkum JW, Heestermans AA, Zomer AC, Kelder JC, Suttorp M-J, Rensing BJ, et al. Predictors of coronary stent thrombosis: the Dutch stent thrombosis registry. J Am Coll Cardiol. (2009) 53(16):1399–409. doi: 10.1016/j.jacc.2008.12.055
42. Windecker S, Meier B. Late coronary stent thrombosis. Circulation. (2007) 116(17):1952–65. doi: 10.1161/CIRCULATIONAHA.106.683995
43. Pepe M, Carulli E, Larosa C, Napoli G, Nestola PL, Carella MC, et al. Comparative effectiveness of cangrelor in patients with acute coronary syndrome undergoing percutaneous coronary intervention: an observational investigation from the M.O.Ca. registry. Sci Rep. (2023) 13(1):10685. doi: 10.1038/s41598-023-37084-2
44. Holmes DR Jr, Kereiakes DJ, Garg S, Serruys PW, Dehmer GJ, Ellis SG, et al. Stent thrombosis. J Am Coll Cardiol. (2010) 56(17):1357–65. doi: 10.1016/j.jacc.2010.07.016
45. Lawton JS, Tamis-Holland JE, Bangalore S, Bates ER, Beckie TM, Bischoff JM, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. (2022) 145(3):e4–17. doi: 10.1161/CIR.0000000000001039
Keywords: stent thrombosis (ST), acute coronary syndrome (ACS), percutaneous coronary intervention (PCI), myocardial infarction (MI), rivaroxaban, factor Xa inhibitor, anticoagulant, landmark analysis
Citation: Chi G, AlKhalfan F, Lee JJ, Montazerin SM, Fitzgerald C, Korjian S, Omar W, Barnathan E, Plotnikov A and Gibson CM (2024) Factors associated with early, late, and very late stent thrombosis among patients with acute coronary syndrome undergoing coronary stent placement: analysis from the ATLAS ACS 2-TIMI 51 trial. Front. Cardiovasc. Med. 10:1269011. doi: 10.3389/fcvm.2023.1269011
Received: 28 July 2023; Accepted: 19 December 2023;
Published: 8 January 2024.
Edited by:
Hugo Ten Cate, Maastricht University Medical Centre, NetherlandsReviewed by:
Oskar Angerås, Sahlgrenska University Hospital, SwedenMartino Pepe, University of Bari Aldo Moro, Italy
© 2024 Chi, Alkhalfan, Lee, Montazerin, Fitzgerald, Korjian, Omar, Barnathan, Plotnikov and Gibson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: C. Michael Gibson bWdpYnNvbkBwZXJmdXNlLm9yZw==
Abbreviations ACC, American College of Cardiology; ACEI, angiotensin-converting enzyme inhibitors; ACS, acute coronary syndrome; AHA, American Heart Association; ARB, angiotensin II receptor blockers; ARC, Academic Research Consortium; BMS, bare-metal stent; CI, confidence interval; DAPT, dual antiplatelet therapy; DES, drug-eluting stent; HR, hazard ratio; MI, myocardial infarction; mITT, modified intention-to-treat; NSTEMI, non-ST segment elevation myocardial infarction; PCI, percutaneous coronary intervention; ST, stent thrombosis; STEMI, ST segment elevation myocardial infarction; TIMI, thrombolysis in myocardial infarction; WBC, white blood cell
 Gerald Chi
Gerald Chi Fahad AlKhalfan
Fahad AlKhalfan Jane J. Lee2
Jane J. Lee2 Clara Fitzgerald
Clara Fitzgerald Serge Korjian
Serge Korjian Elliot Barnathan
Elliot Barnathan Alexei Plotnikov
Alexei Plotnikov