Abstract
Introduction:
Dual antithrombotic therapy (DAT) combining oral anticoagulation (OAC), preferentially Non-vitamin K antagonist OAC (NOAC) and single antiplatelet therapy (SAPT) for a period of 6–12 months is recommended after percutaneous coronary intervention (PCI) in patients with an indication for OAC.
Objective:
To compare outcomes between vitamin K antagonist (VKA) and NOAC-treated patients in the nation-wide France PCI registry.
Methods:
All consecutive patients from the France PCI registry treated by PCI and discharged with OAC between 2014 and 2020 were included and followed one-year. Major bleeding was defined as Bleeding Academic Research Consortium (BARC) classification ≥3 and major adverse cardiac events (MACE) as the composite of all-cause mortality, myocardial infarction (MI), and ischemic stroke. A propensity-score analysis was used.
Results:
Of the 7,277 eligible participants, 2,432 (33.4%) were discharged on VKA and 4,845 (66.6%) on NOAC. After propensity-score adjustment, one-year major bleeding was less frequent in NOAC vs. VKA-treated participants [3.1% vs. 5.2%, −2.1% (−3.6% to −0.6%), p = 0.005 as well as the rate of MACE [9.2% vs. 11.9%, −2.7% (−5.0% to −0.4%), p = 0.02]. One-year mortality was also significantly decreased in NOAC vs. VKA-treated participants [7.4% vs. 9.9%, −2.6% (−4.7% to −0.5%), p = 0.02]. The area under ROC curves of the anticoagulant treatment propensity score was estimated at 0.93, suggesting potential indication bias
Conclusions:
NOAC seems to have a better efficacy and safety profile than VKA. However, potential indication bias were found.
Introduction
Patients with acute coronary syndrome (ACS) or who undergo percutaneous coronary intervention (PCI) require dual antiplatelet therapy (DAPT) with aspirin and a P2Y12 inhibitor to prevent myocardial infarction (MI) and stent thrombosis (1). The association of atrial fibrillation (AF) and coronary artery disease (CAD) is common and require the combination of oral anticoagulant (OAC) therapy to prevent ischaemic stroke and systemic embolism (2) with DAPT (3).
The AUGUSTUS trial (a two-by-two factorial, randomized, controlled clinical trial) demonstrated less bleeding in apixaban vs. VKA-treated AF patients with recent ACS or PCI [10.5% vs. 14.7%, hazard ratio, 0.69; 95% confidence interval (CI): 0.58–0.81; p < 0.001] on a background of concomitant P2Y12 inhibitor therapy for 6 months (4). There was also more bleeding events in aspirin vs. placebo-treated participants (16.1% vs. 9.0%; hazard ratio, 1.89; 95% CI: 1.59–2.24; p < 0.001) (4). Other trials comparing dual antithrombotic therapy (DAT), a combination of single antiplatelet therapy (SAPT) with a Non-vitamin K oral anticoagulant (NOAC), vs. triple antithrombotic therapy (TAT), a combination of VKA and DAPT in a similar setting, demonstrated a lower bleeding rate with DAT. However, none of these trials were designed to assess whether this was due to the use of NOAC or to aspirin cessation (5–7).
Our study aimed to evaluate the real-world setting in the nation-wide prospective FRANCE-PCI registry. Our objectives were to describe the use of VKA vs. NOAC over time and the one-year clinical outcome among participants who underwent PCI.
Methods
Study population
France PCI is a fully electronic, daily updated, high-quality, and low-cost national multicenter observational registry that includes consecutive patients undergoing coronary angiogram and/or PCI in 47 French centers (8, 9). France PCI is registered at clinicaltrials.org (NCT02778724) and all participants are informed of data collection and of the aims of the survey. Antithrombotic regimens were collected before PCI and after PCI at discharge. Participants who underwent PCI between January 2014 and December 2020 with an indication for long-term OAC at discharge were eligible for inclusion. Those without an indication for OAC or who had an ischemic or a bleeding event before discharge were excluded.
Data collection and outcomes
More than 150 epidemiological, clinical and procedural variables are collected as well as 1-year follow-up of all patients who have undergone PCI. These data are extracted from reporting software and monitored to ensure exhaustivity and optimal quality control. Data monitoring, reporting and extraction are supervised by the coordinating clinical research associate and a national medical coordinator.
Participants follow-up is the responsibility of the local on-site research technician at each participating centers. Major adverse events (MACE) defined as the composite of death, stent thrombosis (ARC-2 definition), myocardial infarction (ESC definition), unplanned coronary revascularization, major bleeding (BARC ≥ 3 definition) (10) and stroke were assessed at one-year. The primary safety endpoint was major bleeding (BARC ≥ 3). The primary efficacy endpoint was a composite of all-cause mortality, MI, and ischemic stroke. Individual components of the primary efficacy endpoints as well as the net clinical benefit were also evaluated.
Statistical analysis
A propensity score weighting, based on overlap weights and binary logistic regression explaining the exposure (NOAC vs. VKA or TAT vs. DAT) by adjustment variables was used (10). No survival model was required and endpoints were analyzed as binary variables given the exhaustive follow-up. Each treatment choice (anticoagulant and antiplatelet) was analyzed in a different propensity score analysis, but the other treatment was used as an adjustment variable. Other adjustment variables, listed in Table 1, were chosen a priori, as known prognostic factors or potential indication variables, and included in the propensity score. The year of admission (coded as a categorical variable from 2014 to 2020) was used as an adjustment variable in propensity scores analyses. Heteroscedasticity consistent of type 3 (HC3) sandwich estimator was used for propensity score analysis in a general linear model weighted by overlap weights of the propensity scored, explaining the binary endpoint by the binary exposure; multiple imputation with Rubin's rule for pooling variances was used to handle missing data.
Table 1
| Variables | Measurements |
|---|---|
| Indication for PCI | STEMI, NSTEMI, CCS |
| Emergency | Yes/no |
| Patient's age at admission | <65, 65–70, 70–75, 75–85, 80–85, ≥85 years |
| Sex | Male/female |
| Number of stents | 0, 1, 2, ≥3 |
| Syntax score | quintiles coded as nominal categorical variable |
| Body mass index at admission | (<18.5, 18.5–25, 25–30, ≥30 |
| History of myocardial infarction | Yes/no |
| History of ischemic stroke or transient ischemic attack | Yes/no |
| Hypertension | Yes/no |
| Renal failure | no failure, GFR > 50 ml/min, GFR 30–50, GFR < 30 without dialysis, dialysis |
| Diabetes | no, type I, type II |
| Tobacco use | never, former, current |
| LVEF before PCI | (<30, 30–40, 41–50, >50% |
| Anticoagulant use before admission | none, NOAC, VKA, Other |
| Antiplatelet use before admission | none, aspirin alone, other antiplatelet drug alone, aspirin + other antiplatelet drug) |
Adjustment variables included in the propensity score.
To account for the potential indication bias, an ecological sensitivity analysis was performed. All patients admitted on the same year were considered as equally exposed to the treatment (NOAC vs. VKA). This analysis was performed using a general linear model explaining the 1-year endpoint (binary variable) by the exposition rate to NOAC on the admission year and by the same adjustment variables as the propensity score analysis, except the admission year; the usual variance estimator was used. The strength of the indication bias was assessed by the area under the Receiver Operating Characteristic (ROC) curve of the propensity score for the exposure.
Results
Number of participants
Between 2014 and 2020, 56,334 participants of the France-PCI registry were enrolled of whom 7,277 were event-free at discharge and exposed either to VKA or NOACs (Figure 1). The proportion of participants treated by NOAC vs. VKA increased dramatically during the study period (Figure 2). In 2017, the proportion of participants discharged on NOAC became superior to VKA (57% vs. 41%). The number of included participants also increased exponentially from 327 in 2014 to 2,528 in 2020 (Supplementary Table S1).
Figure 1
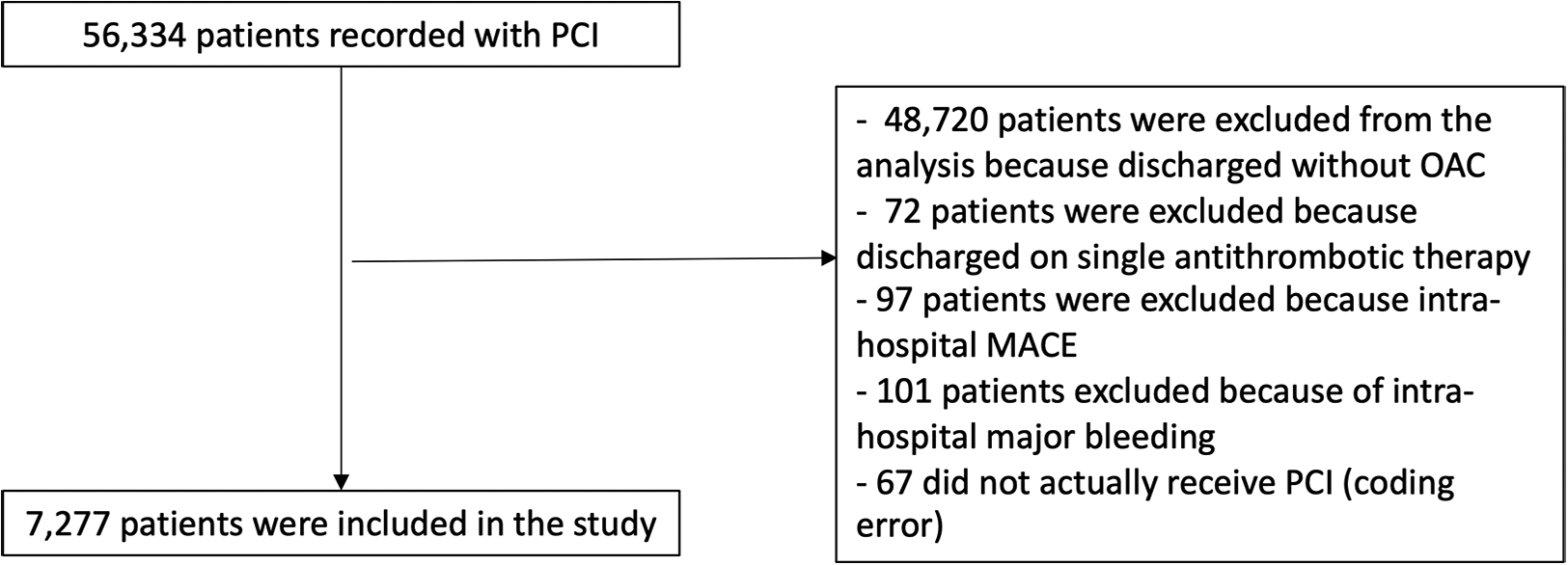
Study flowchart.
Figure 2
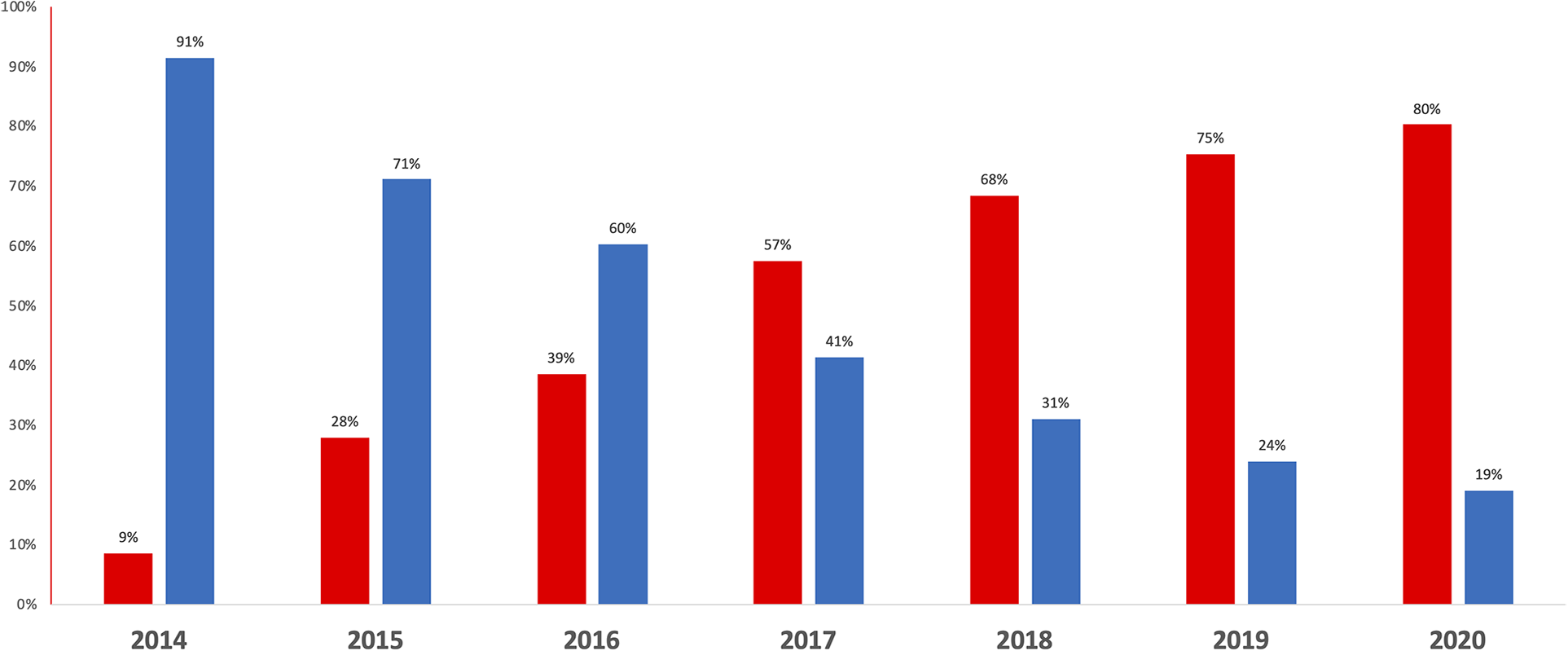
Proportion of patients prescribed long-term VKA (blue) and NOAC (red) at hospital discharge after PCI, between 2014 and 2020.
Baseline characteristics
Baseline characteristics of the studied population according to VKA (n = 2,432) vs. NOAC (n = 4,845) regimen are shown in Table 2. VKA-treated participants were more comorbid than NOAC-treated participants with slightly higher CHA2DS2-VASc score and a more frequent exposure to antiplatelet therapy before admission. The indications of PCI were similar between the two groups but the femoral approach was more frequent in VKA vs. NOAC participants and the syntax score and the number of treated vessels was also significantly higher. The proportion of patients receiving a DAT at discharge was significantly higher in the NOAC group. The proportion of patients treated by TAT vs. DAT at hospital discharge slightly decreased over the study period, from 286/327 (87.5%) in 2014 to 1,914/2,528 (75.7%) in 2020.
Table 2
| VKA N = 2,432 | NOAC N = 4,845 | p-value | |
|---|---|---|---|
| Demographics | |||
| Age, years, n (%) | 0.0007 | ||
| <65 | 392 (16.1%) | 704 (14.5%) | |
| 65–69 | 290 (11.9%) | 650 (13.4%) | |
| 70–74 | 345 (14.2%) | 844 (17.4%) | |
| 75–79 | 466 (19.2%) | 834 (17.2%) | |
| 80–84 | 533 (21.9%) | 986 (20.4%) | |
| ≥85 | 406 (16.7%) | 827 (17.1%) | |
| Female | 570 (23.4%) | 1,139 (23.5%) | 0.97 |
| BMI (kg/m2), n/N (%) | 0.38 | ||
| <18.5 | 27/2,404 (1.1%) | 48/4,788 (1%) | |
| 18.5–25 | 648/2,404 (27%) | 1,380/4,788 (28.8%) | |
| 25–30 | 1,006/2,404 (41.8%) | 1,976/4,788 (41.3%) | |
| ≥30 | 723/2,404 (30.1%) | 1,384/4,788 (28.9%) | |
| Medical history | |||
| Hypertension, n/N (%) | 1,683/2,424 (69.4%) | 3,285/4,834 (68%) | 0.21 |
| Dyslipidemia, n/N (%) | 1,287/2,376 (54.2%) | 2,398/4,745 (50.5%) | 0.004 |
| Diabetes, n/N (%) | <0.0001 | ||
| Type I diabetes | 239/2,424 (9.9%) | 334/4,831 (6.9%) | |
| Type II | 588/2,424 (24.3%) | 1,105/4,831 (22.9%) | |
| Smoking, n/N (%) | 0.34 | ||
| Former smoker | 675/2,423 (27.9%) | 1,422/4,828 (29.5%) | |
| Active smoker | 271/2,423 (11.2%) | 514/4,828 (10.6%) | |
| Myocardial infarction, n/N (%) | 423/2,421 (17.5%) | 677/4,828 (14%) | 0.0001 |
| CABG, n/N (%) | 285/2,429 (11.7%) | 362/4,843 (7.5%) | <0.0001 |
| PCI, n/N (%) | 731/2,427 (30.1%) | 1,424/4,839 (29.4%) | 0.56 |
| CHA2DS2-VASc, mean ± SD | 4.03 ± 1.33 | 3.91 ± 1.31 | 0.0003 |
| Stroke, n/N (%) | 161/2,430 (6.6%) | 315/4,838 (6.5%) | 0.89 |
| Peripheral artery disease, n/N (%) | 365/2,419 (15.1%) | 600/4,820 (12.4%) | 0.002 |
| Chronic kidney diease, n/N (%) | <0.0001 | ||
| GFR > 50 ml/min | 1,898/2,418 (78.5%) | 4,185/4,796 (87.3%) | |
| GFR 30–50 ml/min | 301/2,418 (12.4%) | 541/4,796 (11.3%) | |
| GFR < 30 ml/min | 120/2,418 (5%) | 63/4,796 (1.3%) | |
| Dialysis | 99/2,418 (4.1%) | 7/4,796 (0.1%) | |
| OAC regimen before admission, n/N (%) | <0.0001 | ||
| NOAC | 44/2,412 (1.8%) | 2,556/4,830 (52.9%) | |
| VKA | 1,336/2,412 (55.4%) | 218/4,830 (4.5%) | |
| Other | 3/2,412 (0.1%) | 7/4,830 (0.1%) | |
| None | 1,029/2,412 (42.7%) | 2,049/4,830 (42.4%) | |
| Antiplatelet therapy before admission, n/N %() | <0.0001 | ||
| Aspirin | 287/2,415 (11.9%) | 720/4,835 (14.9%) | |
| Other antiplatelet drug | 238/2,415 (9.9%) | 500/4,835 (10.3%) | |
| Aspirin + Other antiplatelet drug | 1,680/2,415 (69.6%) | 3,018/4,835 (62.4%) | |
| None | 210/2,415 (8.7%) | 597/4,835 (12.3%) | |
| Indication for PCI, n/N (%) | 0.38 | ||
| Non-STEMI | 826/2,431 (34%) | 1,622/4,845 (33.5%) | |
| STEMI | 200/2,431 (8.2%) | 446/4,845 (9.2%) | |
| Chronic coronary syndrome | 1,405/2,431 (57.8%) | 2,777/4,845 (57.3%) | |
| PCI performed in emergency, n/N (%) | 1,267/2,431 (52.1%) | 2,606/4,845 (53.8%) | 0.19 |
| LVEF before PCI, n/N (%) | <0.0001 | ||
| <30, n (%) | 231/2,063 (11.2%) | 355/3,996 (8.9%) | |
| 30–40, n (%) | 345/2,063 (16.7%) | 530/3,996 (13.3%) | |
| 41–50, n (%) | 367/2,063 (17.8%) | 665/3,996 (16.6%) | |
| >50, n (%) | 1,120/2,063 (54.3%) | 2,446/3,996 (61.2%) | |
| Unknown, n (%) | 369/2,432 (15.2%) | 849/4,845 (17.5%) | |
| Procedure | |||
| Angiographic results, n/N (%) | |||
| Monotroncular | 817/2,419 (33.8%) | 1,658/4,817 (34.4%) | 0.093 |
| Bitroncular | 821/2,419 (33.9%) | 1,664/4,817 (34.5%) | |
| Tritroncular | 734/2,419 (30.3%) | 1,365/4,817 (28.3%) | |
| Left main alone | 18/2,419 (0.7%) | 36/4,817 (0.7%) | |
| All lesions <50% | 29/2,419 (1.2%) | 94/4,817 (2%) | |
| Left main (alone or not) | 210/2,430 (8.6%) | 373/4,845 (7.7%) | 0.18 |
| Syntax score, n/N (%) | 0.001 | ||
| [0,5] | 495/2,249 (22%) | 1,085/4,306 (25.2%) | |
| [6,9] | 476/2,249 (21.2%) | 986/4,306 (22.9%) | |
| [10,17] | 664/2,249 (29.5%) | 1,192/4,306 (27.7%) | |
| [18,110] | 614/2,249 (27.3%) | 1,043/4,306 (24.2%) | |
| Unknown | 183/2,432 (7.5%) | 539/4,845 (11.1%) | |
| Approach, n/N (%) | <0.0001 | ||
| Femoral | 308/2,430 (12.7%) | 404/4,844 (8.3%) | |
| Radial | 2,097/2,430 (86.3%) | 4,412/4,844 (91.1%) | |
| Other | 25/2,430 (1%) | 28/4,844 (0.6%) | |
| Anticoagulation during PCI, n/N (%) | <0.0001 | ||
| UFH | 2,006/2,419 (82.9%) | 4,064/4,831 (84.1%) | |
| LMWH | 92/2,419 (3.8%) | 344/4,831 (7.1%) | |
| Other | 9/2,419 (0.4%) | 15/4,831 (0.3%) | |
| None | 312/2,419 (12.9%) | 408/4,831 (8.4%) | |
| Number of treated vessels, n (%) | 0.033 | ||
| 1 | 1,408 (57.9%) | 2,870 (59.2%) | |
| 2 | 649 (26.7%) | 1,337 (27.6%) | |
| ≥3 | 375 (15.4%) | 638 (13.2%) | |
| Number of stents, n (%) | 0.088 | ||
| 0 | 151 (6.2%) | 289 (6%) | |
| 1 | 1,305 (53.7%) | 2,664 (55%) | |
| 2 | 617 (25.4%) | 1,278 (26.4%) | |
| ≥3 | 359 (14.8%) | 614 (12.7%) | |
| Fluoroscopy time (min), mean ± SD | 12.92 ± 10.89 | 12.56 ± 9.93 | 0.16 |
| Discharge medication | |||
| VKA, n (%) | 2,432 (100%) | 0 | |
| NOAC, n (%) | 0 | 4,845 (100%) | |
| Apixaban | 2,726 (56.3%) | ||
| Rivaroxaban | 1,847 (38.1%) | ||
| Dabigatran | 272 (5.6%) | ||
| Antiplatelet therapy, n (%) | <0.0001 | ||
| Clopidogrel alone | 254 (10.4%) | 1,248 (25.8%) | |
| Aspirin alone | 73 (3%) | 77 (1.6%) | |
| Ticagrelor alone | 15 (0.6%) | 33 (0.7%) | |
| Clopidogrel + Aspirin | 2,005 (82.4%) | 3,405 (70.3%) | |
| Ticagrelor + Aspirin | 75 (3.1%) | 76 (1.6%) | |
| Prasugrel + Aspirin | 10 (0.4%) | 6 (0.1%) | |
| Antiplatelet treatment, n (%) | <0.0001 | ||
| DAT | 342 (14.1%) | 1,358 (28%) | |
| TAT ≤ 1 month | 237 (9.7%) | 377 (7.8%) | |
| TAT > 1 month | 1,612 (66.3%) | 2,777 (57.3%) | |
| TAT with unknown duration | 241 (9.9%) | 333 (6.9%) | |
Baseline characteristics of patients treated with VKA and NOAC.
BMI, body mass index; CABG, coronary artery bypass graft; PCI, percutaneous coronary intervention; GFR, glomerular filtration rate; NOAC, non-vitamin K antagonist oral anticoagulant; VKA, vitamin K antagonist; STEMI, ST elevation myocardial infarction; LVEF, left ventricular ejection fraction; UFH, unfractionated heparin; LMWH, low molecular weight heparin; DAT, dual antithrombotic therapy; TAT, triple antithrombotic therapy; SD, standard deviation.
Outcomes according to VKA vs. NOAC
The area under ROC curves of the anticoagulant treatment propensity score was estimated at 0.930 (or 0.896 if the admission year is removed from the propensity score), suggesting that the potential indication bias could be major. The fraction of missing information, according to multiple imputation was estimated at 0.38% for the primary outcome.
The 12-months incidence of major bleeding was significantly lower in the NOAC group, a difference that was sustained after propensity-score adjustment (Table 3 and Figure 3). The incidence of death, MI and ischemic stroke was significantly lower in the NOAC group, a difference driven by a significant reduction of death halved (−4.5% to −2.6%) by propensity score adjustments. The net clinical benefit was similar between the two groups.
Table 3
| Minimally adjusted analysis (propensity score)a | Fully adjusted analysis (propensity score)b | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| VKA (N = 2,432) | NOAC (N = 4,845) | Absolute risk reduction | 95% CI | p-value | VKA (N = 2,432) | NOAC (N = 4,845) | Absolute risk reduction | 95% CI | p-value | |
| Bleeding events | ||||||||||
| Major bleeding >BARC 3c | 5.6% | 3.3% | −2.3% | (−3.4% to −1.1%) | <0.0001 | 5.2% | 3.1% | −2.1% | (−3.6% to −0.6%) | 0.005 |
| Ischemic events | ||||||||||
| Death, MI, ischemic stroked | 13.4% | 8.5% | −4.9% | (−6.7% to −3.2%) | <0.0001 | 11.9% | 9.2% | −2.7% | (−5.0% to −0.4%) | 0.02 |
| Death | 11.5% | 7.0% | −4.5% | (−6.1% to −2.9%) | <0.0001 | 9.9% | 7.4% | −2.6% | (−4.7% to −0.5%) | 0.02 |
| MI | 1.8% | 1.2% | −0.6% | (−1.3%–0.1%) | 0.07 | 1.6% | 1.8% | 0.1% | (−0.9%–1.2%) | 0.78 |
| Ischemic stroke | 0.9% | 0.9% | 0.0% | (−0.5%–0.5%) | 0.91 | 0.9% | 1.1% | 0.1% | (−0.6%–0.9%) | 0.71 |
| Ischemic stroke, MI | 2.7% | 2.1% | −0.6% | (−1.5%–0.2%) | 0.16 | 2.5% | 2.8% | 0.3% | (−1.0%–1.5%) | 0.68 |
| Stent thrombosis | 0.3% | 0.4% | 0.1% | (−0.2%–0.4%) | 0.69 | 0.4% | 0.5% | 0.2% | (−0.3%–0.6%) | 0.53 |
| Death, MI, ischemic stroke, stent thrombosis, unplanned PCI | 16.6% | 11.8% | −4.8% | (−6.8% to −2.9%) | <0.0001 | 14.8% | 12.7% | −2.1% | (−4.7%–0.5%) | 0.11 |
| Bleeding and ischemic events | ||||||||||
| Major bleeding, death, MI, stroke | 16.9% | 10.9% | −6.0% | (−7.9% to −4.1%) | <0.0001 | 15.3% | 11.5% | −3.8% | (−6.3% to −1.2%) | 0.004 |
Minimally adjusted and fully adjusted 12-months clinical outcomes of patients prescribed long-term NOAC versus VKA at hospital discharge of a PCI, with propensity score weighting and multiple imputation.
MI, myocardial infarction; PCI, percutaneous coronary intervention.
Primary safety endpoint.
Primary efficacy endpoint.
Adjusted on year of admission.
Adjusted on year of admission, PCI indication, emergency/planned PCI, age, sex, number of stents, syntax score (quintiles), body mass index, history of myocardial infraction, history of stroke/transient ischemic attack, hypertension before admission, renal failure, diabetes, tobacco use, LVEF, anticoagulant treatment before admission, antiplatelet treatment before admission, antiplatelet treatment at discharge (TAT vs DAT).
Figure 3
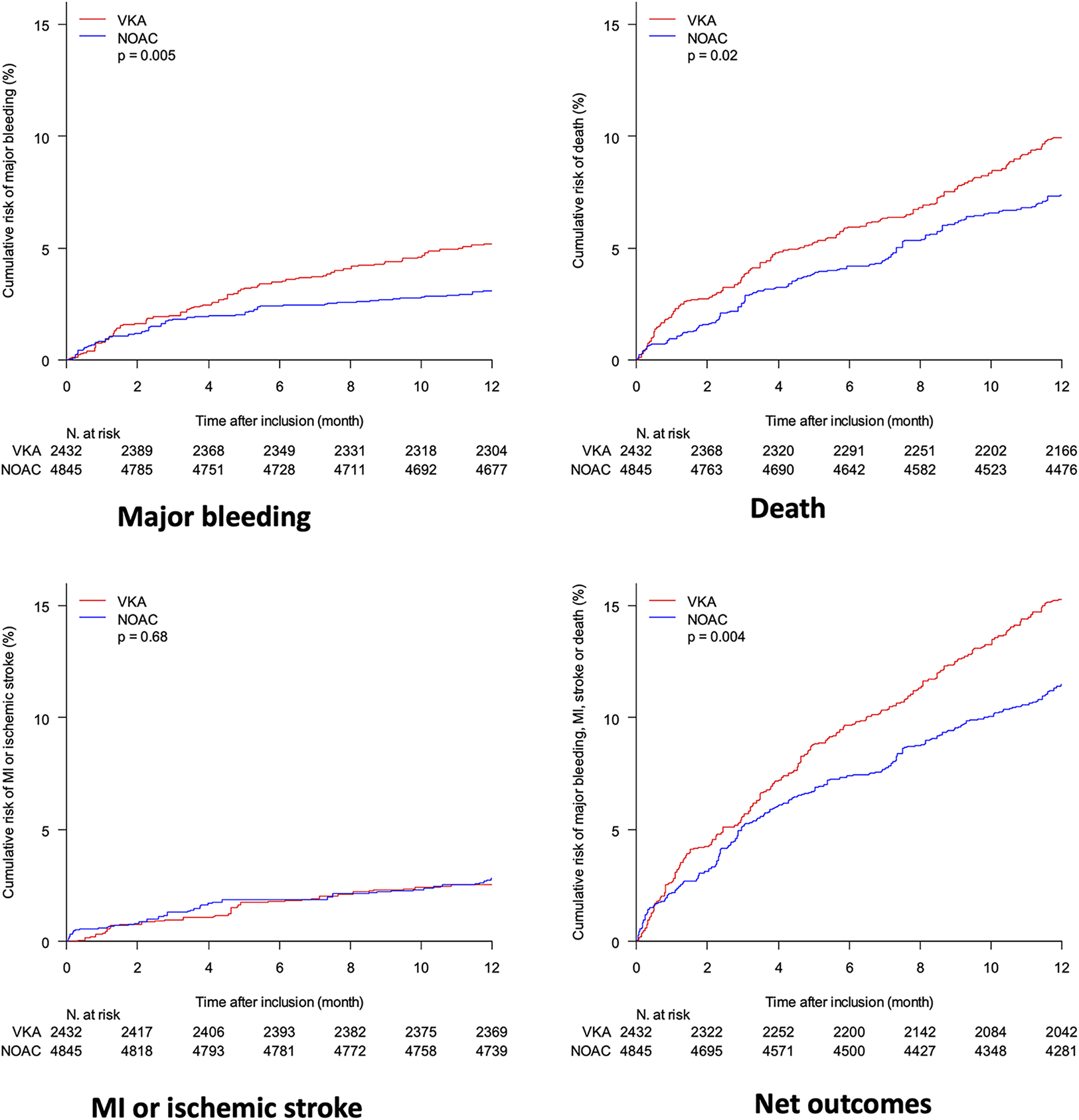
Comparison of propensity-score weighted cumulative risk of major bleeding, death, myocardial infraction (MI) and stroke and net outcomes (including death, MI, stroke and major bleeding) between patients on VKA (red) and NOAC (blue); adjustment variables are the same as in the primary analysis, including year of admission.
Outcomes did not change according to the year of inclusion (Supplementary Figure S1). The fully adjusted ecological sensitivity analysis did not find a significant effect of the NOAC prescription rate on the 12-months risk of major bleeding (+0.1%, 95% CI: −2.3% to +2.5%, p = 0.94), composite endpoint of death, MI and ischemic stroke (+1.7%, 95% CI: −2.0% to +5.3%, p = 0.37), or any other endpoint (Supplementary Table S2). However, confidence intervals were wider than in the primary analysis suggesting some loss of statistical power.
Outcomes according to DAT vs. TAT
Propensity score adjusted analyses of the effect of TAT vs. DAT are shown in Table 4. The area under ROC curve of the associated propensity score was 0.774. The composite endpoint of major bleeding, death, MI and stroke was lower in TAT vs. DAT (−3.0%, 95% CI: −4.9% to −1.1%, p = 0.002) in the minimally adjusted model, but this effect was attenuated and became non-significant after full adjustment (−2.0%, 95% CI: −4.1% to +0.1%, p = 0.07).
Table 4
| Minimally adjusted analysis (propensity score)a | Fully adjusted analysis (propensity score)b | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DAT (N = 1,700) | TAT (N = 5,577) | Absolute risk reduction | 95% CI | p-value | DAT (N = 1,700) | TAT (N = 5,577) | Absolute risk reduction | 95% CI | p-value | |
| Bleeding events | ||||||||||
| Major bleeding > BARC 3 | 4.6% | 3.8% | −0.8% | (−1.9%–0.3%) | 0.16 | 4.4% | 4.1% | −0.3% | (−1.6%–0.9%) | 0.58 |
| Ischemic events | ||||||||||
| Death, MI, ischemic stroke | 11.7% | 9.6% | −2.2% | (−3.9% to −0.4%) | 0.02 | 11.6% | 10.1% | −1.5% | (−3.4%–0.4%) | 0.12 |
| Death | 9.8% | 8.0% | −1.8% | (−3.4% to −0.1%) | 0.03 | 9.7% | 8.7% | −1.0% | (−2.8%–0.8%) | 0.26 |
| MI | 1.2% | 1.5% | 0.3% | (−0.3%–0.9%) | 0.34 | 1.3% | 1.4% | 0.2% | (−0.5%–0.8%) | 0.66 |
| Ischemic stroke | 1.1% | 0.8% | −0.3% | (−0.9%–0.2%) | 0.26 | 1.0% | 0.9% | −0.2% | (−0.8%–0.4%) | 0.58 |
| Ischemic stroke, MI | 2.4% | 2.3% | 0.0% | (−0.9%–0.8%) | 0.92 | 2.3% | 2.3% | 0.0% | (−0.9%–0.9%) | 0.95 |
| Stent thrombosis | 0.4% | 0.3% | −0.1% | (−0.5%–0.2%) | 0.41 | 0.4% | 0.3% | −0.1% | (−0.5%–0.2%) | 0.50 |
| Death, MI, ischemic stroke, stent thrombosis, unplanned PCI | 14.1% | 12.9% | −1.2% | (−3.1%–0.7%) | 0.23 | 13.9% | 13.3% | −0.5% | (−2.6%–1.5%) | 0.60 |
| Bleeding and ischemic events | ||||||||||
| Major bleeding, death, MI, stroke | 15.1% | 12.1% | −3.0% | (−4.9% to −1.1%) | 0.002 | 14.9% | 13.0% | −2.0% | (−4.1%–0.1%) | 0.07 |
Minimally adjusted and fully adjusted 12-months clinical outcomes of patients prescribed TAT vs. DAT at hospital discharge of a PCI, with propensity score weighting and multiple imputation.
Adjusted on year of admission.
Adjusted on year of admission, PCI indication, emergency/planned PCI, age, sex, number of stents, syntax score (quintiles), body mass index, history of myocardial infraction, history of stroke/transient ischemic attack, hypertension before admission, renal failure, diabetes, tobacco use, LVEF, anticoagulant treatment before admission, antiplatelet treatment before admission, anticoagulant treatment at discharge (NOAC vs VKA).
In linear models adjusted on the same variables as the primary analysis with exposure defined as the actual treatment that the patient was prescribed, there was no significant statistical interaction on any outcome although statistical precision of the interaction term was low (Supplementary Table S3).
Discussion
The main results of our study may be summarized as follow: (1) The proportion of patients treated with NOAC increased dramatically between 2014 and 2020; (2) Major bleeding and MACE were significantly lower in NOAC vs. VKA-treated participants. (3) Mortality was also lower, a difference not accounted by major bleeding reduction; (4) There was no statistical interaction with TAT and DAT regimen for all outcomes.
NOAC vs. VKA
From 2014 to 2021, the European guidelines concerning the optimal antithrombotic regimen in patients with long-term OAC treated with PCI have evolved from a TAT including an OAC and DAPT to a DAT including an OAC (preferentially a NOAC) and SAPT (preferentially clopidogrel) for a period of 6–12 months according to the clinical context and bleeding risk profile of the patient. These recommendations are based on the results of randomized multicenter studies (WOEST, PIONEER AF-PCI, RE-DUAL PCI, and ENTRUST-AF PCI trials) demonstrating lower bleeding outcomes with DAT as compared to TAT without increase in the risk of ischemic events (5–7, 11).
Our results are in accordance with the AUGUSTUS trial showing a significant reduction of major bleeding in patients treated with apixaban as compared to those on VKA [hazard ratio (HR) 0.69, 95% CI: 0.58–0.81, p < 0.001] (4). Unlike our study, the incidence of MACE was not significantly different between the apixaban and VKA groups (HR, 0.93, 95% CI: 0.75–1.16, p = NS) (4). Other randomized trials have compared a DAT including a single antiplatelet therapy and a NOAC vs. TAT including a vitamin K antagonist (VKA) and a dual antiplatelet therapy in patients with AF who were undergoing PCI. Those studies showed a lower incidence of bleeding with DAT but these three trials were not designed to assess whether the lower incidence of bleeding was due to the use of the standard or reduced doses of NOAC or to the removal of aspirin therapy (5–7).
A meta-analysis of benefits and risks associated with the use of NOAC vs. VKA in patients treated by PCI has been therefore reported (12). The primary outcome (composite of major bleeding according to ISTH definition and clinically relevant bleeding requiring medical intervention) occurred less frequently in patients receiving NOAC. Combination strategies with NOACs were associated with reduced risk of major bleeding events across different combination strategies as compared to VKA, with the most significant risk reduction when NOAC + SAPT was associated with a 37% relative risk reduction of major bleeding events as compared to TAT with VKA + DAPT (RR, 0.63; 95% CI: 0.50–0.80) (12). The reduction of major bleeding risks was considered as a class effect of NOACs. Again, unlike our study, combination strategies of NOAC vs. VKA resulted in a comparable risk of MACE. In our study, the incidence of MI and stroke was similar in patients receiving NOAC or VKA. The significant reduction of MACE was only driven by a significant reduction of the incidence of death. A post hoc analysis excluding all patients who had a major bleeding found an absolute difference of 1-year death at −3.79% (−5.36% to −2.22%, p < 0.0001) for NOAC vs. VKA without adjustment; after propensity-score adjustment it was −1.95% (−3.99%–0.09%, p = 0.06) for NOAC vs. VKA, confirming the hypothesis that most of the death reduction is unrelated to bleeding events. Moreover, the strong attenuation of this effect after statistical adjustments and disappearance in the ecological sensitivity analysis, suggest that this effect may be partly or fully explained by residual confounding.
Antiplatelet regimen
In our study, we did not find any significant effect of TAT vs. DAT. This result is not in accordance with the randomized studies and meta-analysis showing that DAT, particularly the association of a NOAC and a P2Y12 inhibitor, was associated with significantly less major or clinically relevant non-major bleeding compared to TAT (4–7, 11–13). There are several explanations for this result. First of all, only 23.4% of the studied population received a DAT between 2014 and 2020, leading to poor statistical precision. On the other hand, minimally adjusted analyses show that there was an indication bias with DAT given to patients with poor prognosis, so that the residual confounding bias may disadvantage DAT. Similarly, effects of TAT vs. DAT on the composite outcome of bleeding, death, MI or stroke, suggest that there were strong confounding factors, partly attenuated by adjustment.
Although for most of the duration of the study it was recommended to use a TAT by default for 1 month except in patients at high risk of bleeding, most patients receiving TAT had a prescription lasting more than 1 month. The latest guidelines recommend to use, as a default strategy, a DAT but were published in 2021.
Strengths and limitations
The main strength is the large sample size and the use of a multi-center monitored prospective registry with high quality follow-up data at 12-months and real-life patients with usual care practice. Our study has several limitations. First, this analysis was retrospectively conducted. Second, the indication bias remained uncontrolled as suggested by an attenuation of effects after adjustment, with a risk of residual confounding due to unobserved confounders and measurement errors on observed confounders. Third, the ecological analysis was not affected by indication bias but had lower statistical power and may have differential selection or measurement bias due to evolution of the quality of the registry over time. Fourth, the antithrombotic regimen strategy was not collected at the time of PCI but at discharge with potential unmeasured confounders which may have interacted with the decision. Fifth, NOAC dose regimen and VKA monitoring were not collected. Sixth, the indication of anticoagulant therapy was not available in our database but the vast majority of patients had atrial fibrillation. Finally, events were not adjudicated and whether events occurred on-treatment could not be assessed.
Conclusion
In the real-life setting, the use of NOAC vs. VKA after PCI was associated with less bleeding and less MACE at one-year follow-up. The benefit effect on MACE was mainly driven by a better survival. However, these effects should be interpreted with caution given that the indication of OAC remains a major bias with a risk of residual confounding due to unobserved confounders and measurement errors on observed confounders.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
ED: Conceptualization, Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. TV: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft. AG: Formal Analysis, Methodology, Writing – review & editing. TL: Data curation, Formal Analysis, Writing – review & editing. TB: Data curation, Formal Analysis, Writing – review & editing. RK: Supervision, Validation, Writing – review & editing. PM: Supervision, Validation, Writing – review & editing. HE: Supervision, Validation, Writing – review & editing. J-PC: Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing. GR: Conceptualization, Investigation, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
Publication costs were made possible thanks to the support of CRAN (Club Régional des Angioplasticiens de Normandie).
Conflict of interest
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer [PG] declared a shared parent affiliation with the author [J-PC] to the handling editor at the time of review.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1320001/full#supplementary-material
References
1.
Collet JP Thiele H Barbato E Barthélémy O Bauersachs J Bhatt DL et al 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. (2021) 42:1289–367. 10.1093/eurheartj/ehaa575
2.
Ruff CT Giugliano RP Braunwald E Hoffman EB Deenadayalu N Ezekowitz MD et al Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. (2014) 383:955–62. 10.1016/S0140-6736(13)62343-0
3.
Mekhael M Marrouche N Hajjar AHE Donnellan E . The relationship between atrial fibrillation and coronary artery disease: understanding common denominators. Trends Cardiovasc Med. (2022) S1050-1738(22)00123-2. 10.1016/j.tcm.2022.09.006. [Epub ahead of print].
4.
Lopes RD Heizer G Aronson R Vora AN Massaro T Mehran R et al AUGUSTUS investigators. antithrombotic therapy after acute coronary syndrome or PCI in atrial fibrillation. N Engl J Med. (2019) 380:1509–24. 10.1056/NEJMoa1817083
5.
Gibson CM Mehran R Bode C Halperin J Verheugt FW Wildgoose P et al Prevention of bleeding in patients with atrial fibrillation undergoing PCI. N Engl J Med. (2016) 375:2423–34. 10.1056/NEJMoa1611594
6.
Cannon CP Bhatt DL Oldgren J Lip GYH Ellis SG Kimura T et al RE-DUAL PCI steering committee and investigators. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med. (2017) 377:1513–24. 10.1056/NEJMoa1708454
7.
Vranckx P Valgimigli M Eckardt L Tijssen J Lewalter T Gargiulo G et al Edoxaban-based versus vitamin K antagonist-based antithrombotic regimen after successful coronary stenting in patients with atrial fibrillation (ENTRUST-AF PCI): a randomised, open-label, phase 3b trial. Lancet. (2019) 394:1335–43. 10.1016/S0140-6736(19)31872-0
8.
Rangé G Chassaing S Marcollet P Saint-Étienne C Dequenne P Goralski M et al The CRAC cohort model: a computerized low cost registry of interventional cardiology with daily update and long-term follow-up. Rev Epidemiol Sante Publique. (2018) 66:209–16. 10.1016/j.respe.2018.01.135
9.
Mezier A Motreff P Clerc JM Bar O Deballon R Demicheli T et al Is the duration of dual antiplatelet therapy (DAPT) excessive in post-angioplasty in chronic coronary syndrome? Data from the France-PCI registry (2014–2019). Front Cardiovasc Med. (2023) 10:1106503. 10.3389/fcvm.2023.1106503
10.
Li F Thomas LE Li F . Addressing extreme propensity scores via the overlap weights. Am J Epidemiol. (2019) 188:250–7. 10.1093/aje/kwy201
11.
Dewilde WJ Oirbans T Verheugt FW Kelder JC De Smet BJ Herrman JP et al Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet. (2013) 381:1107–15. 10.1016/S0140-6736(12)62177-1
12.
Eyileten C Postula M Jakubik D Toma A Mirowska-Guzel D Patti G et al Non-vitamin K oral anticoagulants (NOAC) versus vitamin K antagonists (VKA) for atrial fibrillation with elective or urgent percutaneous coronary intervention: a meta-analysis with a particular focus on combination type. J Clin Med. (2020) 9:1120. 10.3390/jcm9041120
13.
Gargiulo G Goette A Tijssen J Eckardt L Lewalter T Vranckx P et al Safety and efficacy outcomes of double vs. Triple antithrombotic therapy in patients with atrial fibrillation following percutaneous coronary intervention: a systematic review and meta-analysis of non-vitamin K antagonist oral anticoagulant-based randomized clinical trials. Eur Heart J. (2019) 40:3757–67. 10.1093/eurheartj/ehz732
Summary
Keywords
PCI, VKA, NOAC, bleeding, atrial fibrillation
Citation
Durand E, Verrez T, Gillibert A, Levesque T, Barbe T, Koning R, Motreff P, Eltchaninoff H, Collet J-P and Rangé G (2024) Safety and efficacy of NOAC vs. VKA in patients treated by PCI: a retrospective study of the FRANCE PCI registry. Front. Cardiovasc. Med. 10:1320001. doi: 10.3389/fcvm.2023.1320001
Received
11 October 2023
Accepted
19 December 2023
Published
16 January 2024
Volume
10 - 2023
Edited by
Andreas Schäfer, Hannover Medical School, Germany
Reviewed by
Paul Guedeney, Hôpitaux Universitaires Pitié Salpêtrière, France
Mirko Dragutin Colic, Institute for Cardiovascular Diseases Dedinje, Serbia
Naveen Anand Seecheran, The University of the West Indies St. Augustine, Trinidad and Tobago
Updates
Copyright
© 2024 Durand, Verrez, Gillibert, Levesque, Barbe, Koning, Motreff, Eltchaninoff, Collet and Rangé.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Eric Durand eric.durand@chu-rouen.fr
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.