- 1Division of Pediatric Cardiology and Congenital Heart Disease, Department of Cardiology and Vascular Medicine, National Cardiovascular Center of Harapan Kita, Universitas Indonesia, Jakarta, Indonesia
- 2Department of Cardiology and Vascular Medicine, Sultan Sulaiman Government Hospital, Serdang Bedagai, Indonesia
- 3Department of Cardiology and Vascular Medicine, National Cardiovascular Center of Harapan Kita, Universitas Indonesia, Jakarta, Indonesia
Objectives: The purpose of this study was to assess the clinical outcome after right ventricular outflow tract (RVOT) stenting in late presenter patient with unrepaired Fallot physiology.
Background: In younger patients, RVOT stenting is an alternative to mBTT shunt; however, there have been few reports of this palliative technique in late presenter population, including adults.
Methods: This was a single-center, retrospective study of nonrandomized, palliated Fallot patients. Clinical outcomes such as left ventricular ejection fraction and saturation were measured in 32 individuals following RVOT stenting in adults (n = 10) and children (n = 22). The Statistical Package for Social Science (SPSS) 26.0 software was used to analyze the statistical data.
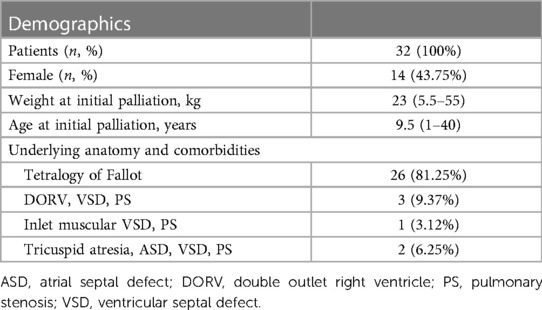
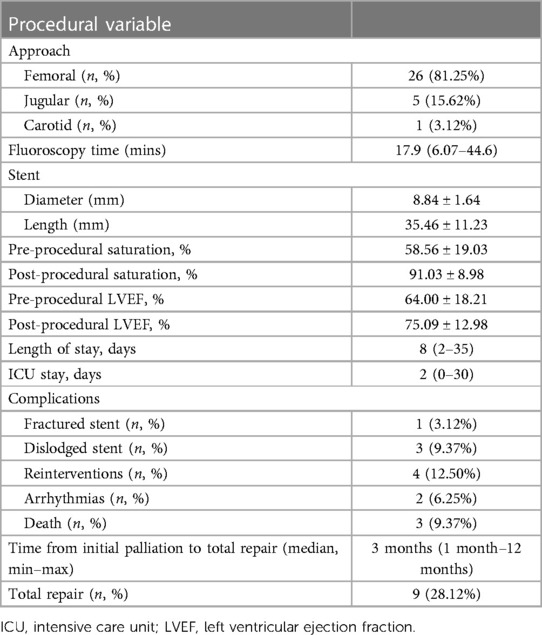
Results: During the procedure, the average stent diameter and length were 8.84 ± 1.64 mm and 35.46 ± 11.23 mm, respectively. Adult patients received slightly longer stents than pediatric patients (43.60 ± 11.64 mm vs. 31.77 ± 9.07 mm). Overall, patients' saturation increased from 58.56 ± 19.03% to 91.03 ± 8.98% (p < 0.001), as did their left ventricular ejection fraction (LVEF) from 64.00 ± 18.21% to 75.09 ± 12.98% (p = 0.001). Three patients improved their LVEF from 31 to 55%, 31 to 67%, and 26 to 50%. The median length of stay was 8 (2–35) days, with an ICU stay of 2 (0–30) days. The median time from RVOT stent palliation to total repair was 3 months (range: 1 month–12 months).
Conclusions: RVOT stenting is a safe and effective method for increasing saturation and ejection fraction not only in newborn infants but also in late presenters, including adults with unrepaired Fallot physiology.
1 Introduction
The initial palliative strategy of patients with ventricular shunts and narrow right ventricular outflow tract (RVOT) with reduced pulmonary blood flow remains challenging. During the last decades, creation of modified Blalock–Thomas–Taussig (mBTT) shunt has been well established, however complication and mortality has yet to be very high (1, 2). To countermeasure this issue, right ventricular outflow tract (RVOT) stenting has been developed as initial palliation of symptomatic patients. However, the efficacy of RVOT stenting is only highlighted in current literature for newborns and young children (3, 4).
Late presentations in developing countries is common, making the problems more complicated. Previously, uncorrected Fallot physiology in late presenter patients was exclusively treated conservatively with medication, especially with significant desaturation and diminished ventricular function. Tetralogy of Fallot patients frequently showed late in low-income countries, accounting for 85.1% of cases (5, 6).
Nonetheless, there have been few reports of the benefit of RVOT stenting in patients with unrepaired Fallot physiology, particularly in late presenter patients (7, 8). Therefore, this institutional experience aims to investigate the outcome of RVOT stenting in late presenter patients with Fallot physiology.
2 Methods
2.1 Study design and setting
Between December 2019 and April 2023, thirty-two patients with unrepaired ventricular shunts and severely narrow right ventricular outflow tract physiology resulting in profound desaturation were considered for RVOT stenting in a retrospective, single-center study. The local hospital ethics review board approved RVOT stenting as an alternative to mBTT shunt. The primary endpoints were saturation and ejection fraction before and after palliation in late presenter tetralogy of Fallot patients (beyond one year old). Subjects were chosen after a multidisciplinary team meeting in a weekly surgical conference. The decision not to operate is justified by evidence-based medicine and in the patient's best interest considering very high mortality in this patient's group. The main indications for RVOT stenting in late presenters and adults in our center are (1) Profound desaturation (typically below 40%–50%), (2) Low ejection fraction (LVEF <40%), (3) Patient who had a high risk for performing mBTT shunt, and (4) Patient who had a high risk for total repair despite adequate pulmonary artery size (4, 5).
Each procedure were performed while the patients were sedated and mechanically ventilated. To enable for biplane angiography, patients were positioned on the table with their arms lifted. To prevent hypothermia, all patients were given a warming device. A sterile dressing was applied to the patient's chest and upper abdomen to enable for intraprocedural echocardiographic assessment with a standard ultrasonography probe in a sterile sleeve. The preferred method was right femoral or right internal jugular venous access, while in certain circumstances a carotid approach may be employed (9).
The first right ventricular angiography was conducted with a diagnostic catheter positioned near the apex of the right ventricle with a 30″ RAO, 30″ cranial tilt, and a straight lateral projection. Heparin 50 IU per kgBW was administered to the patient. The diagnostic catheter was removed from the right ventricle and inserted in the superior vena cava following the angiography. The right ventricular outflow tract, pulmonary valve annulus, and branch pulmonary arteries were measured and compared to earlier or simultaneous ultrasonography data (9).
2.2 Stent choice
The size and type of stent were chosen based on Kirklin's full size and the anticipated length of palliation. Our center had stent lengths of 28 mm, 39 mm, and 56 mm available. The type and diameter of the stent were chosen in general based on the patient's body weight, the length of the RVOT infundibulum, and the need for palliative surgery. We employed peripheral vascular stents in adult and adolescents patients, and coronary stents in young children above the age of one year. The stent's diameter is typically 1–2 mm bigger than the measured infundibulum during diastole, and the stent's length should include the distal muscular section of the RVOT, the pulmonary valve, and/or the main branch of the pulmonary artery before the bifurcation (9).
Following stent selection, the suitable delivery sheath or guide catheter was chosen. A 4–5 F Right Judkins catheter was pushed past the tip of the delivery sheath after the delivery sheath was inserted in the superior vena cava. On the hub of the Judkins catheter, a spinning haemostatic valve was attached to the pressure line and side arm contrast syringe. A 0.014″ coronary wire was threaded through the haemostatic valve near the catheter's tip. After withdrawing the entire system, the right ventricle was reintroduced under pressure monitoring and the right ventricular outflow tract was intubated using the catheter. Instead of the wire, side arm test injections were utilized to check the location of the catheter. The catheter was then moved to the distal branch pulmonary artery while continuous pressure was recorded and an angiography was performed. The coronary wire was inserted into the distal branch pulmonary artery, and the delivery sheath or guide catheter was advanced into the distal branch pulmonary artery over the diagnostic catheter. The diagnostic catheter was then withdrawn from the coronary wire, and the sheath was aspirated and flushed (9).
The wire was wrapped around the pre-mounted stent, which was then advanced to the desired spot within the RVOT. The stent was slightly exposed, and side arm test injections were repeated. When the position was deemed satisfactory, the stent was totally exposed. The delivery balloon was manually inflated with one hand while the stent system was managed with the other. Following stent implantation, the balloon was gradually deflated as the delivery sheath was advanced over the balloon to re-sheath it. This enabled for a secure placement of the delivery system's tip into the stent (9). Predilatation with a coronary or vascular balloon should be considered if necessary, especially in cases of multilayer stenosis. Following the procedure, furosemide IV 1 mg/kgBW IV and milrinone drip 0.375 mg/kgBW IV were administered. A repeat angiography and a brief ultrasound assessment were performed. The balloon was subsequently advanced over the pulmonary valve in all cases for valvuloplasty. Oxygen saturations were regularly measured.
Another cardiac ultrasonography assessment and right ventricular angiography were performed. An additional blood gas study was performed. Finally, the coronary wire was removed under fluoroscopic control through the delivery sheath, the delivery sheath was removed, and manual hemostasis was applied. Hemoglobin should be 14g% after the procedure.
2.3 Statistical presentations
Exploratory analysis with graphical and tabular displays evaluated evidence in favor of trends and associations. Normally distributed data was presented as mean ± standard deviation. Data that is skewed is presented as the median, minimum, and maximum. Where appropriate, categorical data is expressed as counts and percentages. Boxplot graphs depicted the difference in saturation and ejection percent between the pre- and post-palliation procedures. The Shapiro–Wilk test was used to determine the normality of numerical data because the subjects were less than 50. The paired t-test was used to compare the change in both saturation and LVEF before and after the procedure. The Statistical Package for Social Science (SPSS) 26.0 software was used for all data analyses (10). p < 0.05 values were considered significant.
3 Results
The RVOT stenting was performed on 32 individuals, with the patient flow diagram depicted in Figure 1. Table 1 shows the baseline parameters of this investigation. In two cases, mBTT shunt was performed initially; however, due to blocked mBTT shunt, the patients were scheduled for rescue (secondary) RVOT stenting (rescue RVOT stent was performed to improve the patient clinical condition). The patient was followed for a median of 13.5 (1–31) months. There were 14 female patients (43.75%). The median age at stent implantation was 9.5 (1–40) years. The median weight ranged from 23 (5.5–55) kilos. During presentation, ten adult patients (31.25%) had unpalliated Fallot physiology. Nine were diagnosed with tetralogy of Fallot.
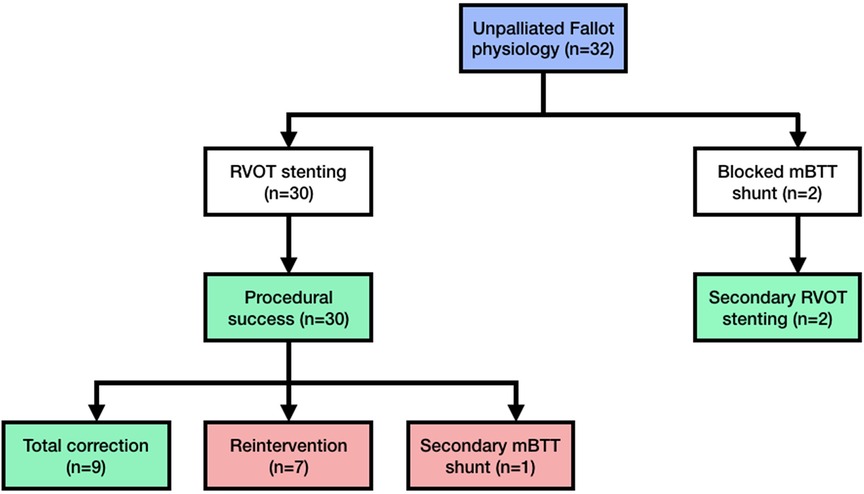
Figure 1. Patient flow diagram showing the cohort of unrepaired late presenter fallot physiology. Every patient who received RVOT stenting was included in this diagram. This study did not record any intraprocedural patients deaths. mBTT modified-Blalock–Thomas–Taussig; RVOT right ventricular outflow tract.
3.1 Procedural details
The procedural details was summarized in Table 2. The approach for this procedure was 26 femoral, 5 jugular and 1 carotid. All adults patient was performed using the femoral approach. Median fluoroscopy time was 17.9 (6.07–44.6) minutes. During the learning curve stage, the procedure time was greatly reduced. During the procedure, the average stent diameter and length were 8.84 1.64 mm and 35.46 ± 11.23 mm, respectively. Adult patients received slightly longer stents than pediatric patients (43.60 ± 11.64 mm vs. 31.77 ± 9.07 mm). Overall, patients' saturation went from 58.56 ± 19.03% to 91.03 ± 8.98% (p < 0.001). Adults' saturation went from 65.20 ± 15.36 to 92.00 ± 5.29 (p = 0.005), while pediatrics' saturation went from 55.54 ± 20.08 to 90.59 ± 10.32 (p < 0.001). The overall left ventricular ejection fraction (LVEF) increased from 64.00 ± 18.21% to 75.09 ± 12.98% (p = 0.001) with adults' LVEF went from 60.33 ± 19.37 to 70.66 ± 12.13 (p = 0.097) and pediatrics' LVEF saturation went from 65.50 ± 17.96 to 76.90 ± 13.15 (p = 0.002). Figures 2, 3 illustrate boxplots of the saturation and ejection fractions comparing adult and pediatric populations. Three patients improved their LVEF from 31 to 55%, 31 to 67%, and 26 to 50%. The median length of stay was 8 (2–35) days, with an ICU stay of 2 (0–30) days.
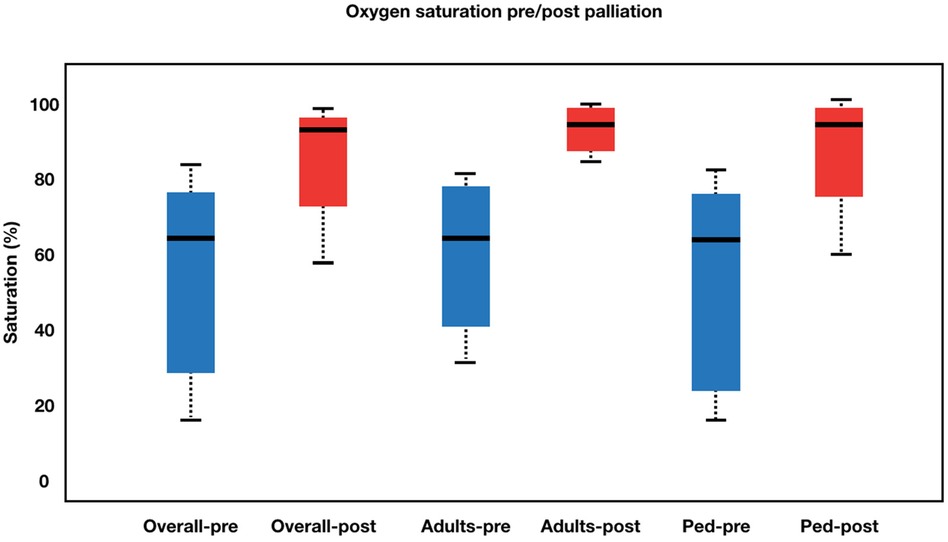
Figure 2. Boxplot diagram illustrating oxygen saturation pre- and post-palliation. Overall, patients’ saturation went from 58.56 ± 19.03% to 91.03 ± 8.98% (p < 0.001). Adults’ saturation went from 65.20 ± 15.36 to 92.00 ± 5.29 (p = 0.005), while pediatrics’ saturation went from 55.54 ± 20.08 to 90.59 ± 10.32 (p < 0.001). Pre-palliation is shown in blue. Post-palliation is shown in red. Box is first to third quartile. Median is thick line in box. The whiskers extend to the data point that is no more than 1.5 times the interquatile range from the box.
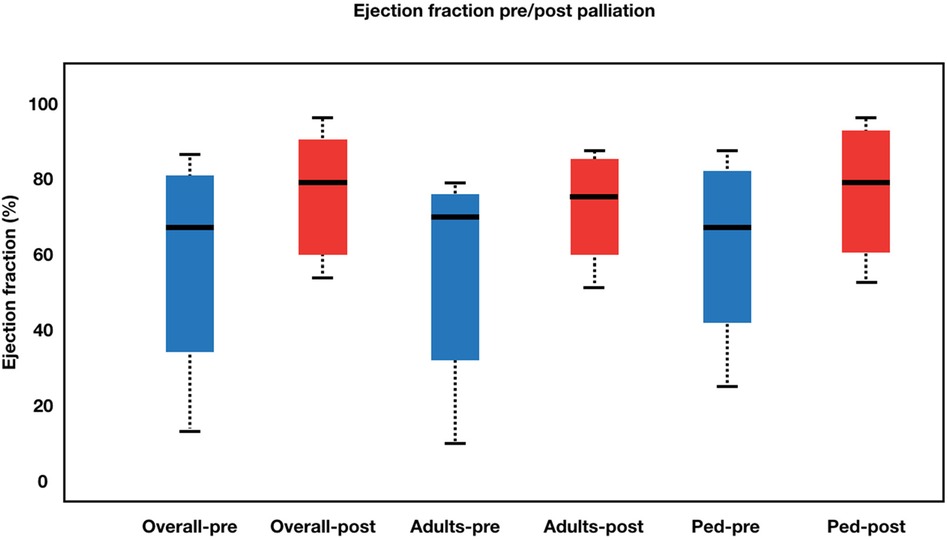
Figure 3. Boxplot diagram illustrating ejection fraction pre- and post-palliation. The overall left ventricular ejection fraction (LVEF) increased from 64.00 ± 18.21% to 75.09 ± 12.98% (p = 0.001) with adults’ LVEF went from 60.33 ± 19.37 to 70.66 ± 12.13 (p = 0.097) and pediatrics’ LVEF saturation went from 65.50 ± 17.96 to 76.90 ± 13.15 (p = 0.002).Pre-palliation is shown in blue. Post-palliation is shown in red. Box is first to third quartile. Median is thick line in box. The whiskers extend to the data point that is no more than 1.5 times the interquatile range from the box.
3.2 Complications
One fractured stent was discovered in an 18-year-old woman who had reintervention using the stent-in-stent approach (see Figure 4). The patient clinical condition improved and was able to undergo total repair one week after the second RVOT stent. Three patients (9.37%) experienced a dislodged stent (two in the right atrium and one in the descending aorta), requiring a rescue RVOT stent. In three cases of dislodged stent, total repair was performed not directly after the complication, but several months after the initial palliation with the median time from RVOT stent palliation to total repair 3 months (range: 1 month–12 months). Referral to surgical ward was only to remove the dislodged stent. Arrhythmias were found in two young patients, one with supraventricular tachycardia and the other with severe bradycardia.
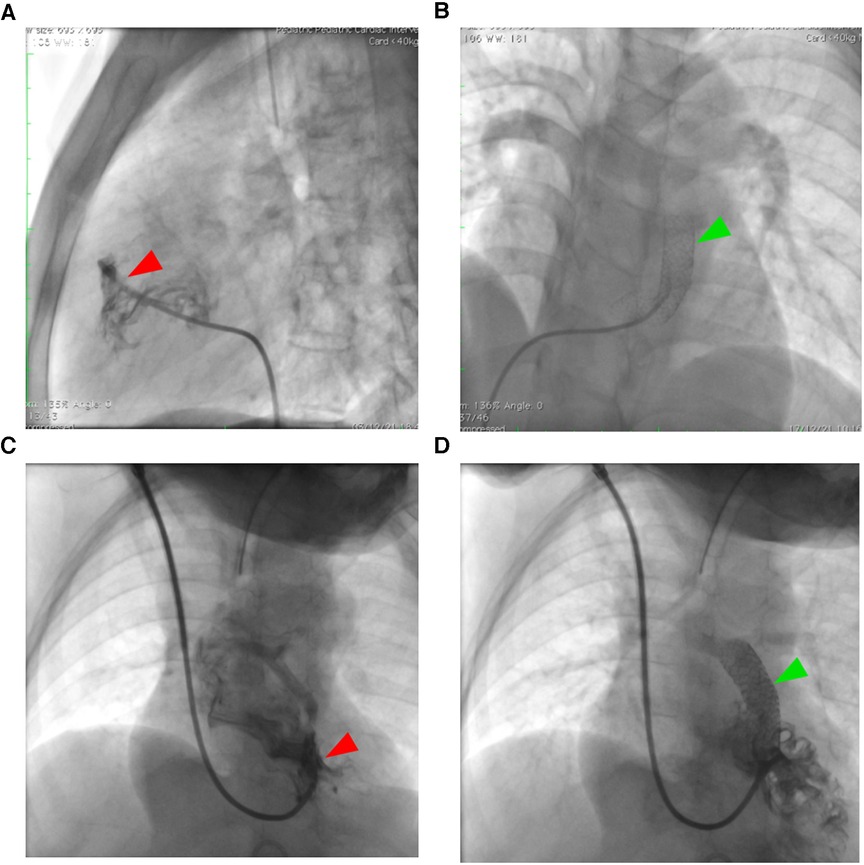
Figure 4. Right ventricular outflow tract stenting in unpalliated Fallot physiology. Adult patients (A,B) with tetralogy of Fallot face particular difficulties with RVOT stenting in comparison to pediatric population (C,D) because of their greater muscular infundibular pulmonary stenosis (red arrowhead), which increases the risk of stent fracture as seen in figure (B) The green arrowhead points to the stent.
In both the adult and pediatric populations, there was no intraprocedural death. There was no post-procedural death in the adult Fallot physiology populations. However, three pediatric patients died from sepsis during in-hospital treatment. Total repair was performed on nine patients, four adults and five children.
4 Discussions
4.1 Right ventricular outflow tract stenting in Fallot physiology patients
Total repair was the definitive treatment for TOF patients, which was usually done before the age of one year (11). However, not all TOF patients may benefit from total repair because the remainder required palliative care for issues such as cyanotic spells, severe and broad RVOT blockage, stenotic or hypoplastic branch pulmonary artery, or ventricular dysfunction. Similarly, patients with ventricular shunts and pulmonary stenosis physiology or a blocked mBTT shunt may benefit from RVOT stenting prior to definitive treatment, as in our instance (12–14). Quandt et al. discovered that 26.6% of TOF patients required palliative care before undergoing total repair. The mBTT shunt was one of the most commonly performed palliative procedures, with a death rate ranging from 2.3 to 18.4% (4). The mBTT shunt produced various unintended consequences, including pulmonary artery deformation and stenosis, as well as the existence of cicatrix formation, which could complicate the total repair surgery (1). RVOT stents were known to have a better prognosis than mBTT shunts (2). Luxford et al. shown in their study that high-risk infants undergoing RVOT stent had lower morbidity and mortality than those undergoing mBTT shunt, and achieved an effective bridge to definitive surgery (15). However, we did not directly compare the efficacy of the procedures between mBTT shunt and RVOT stent in this study because of the different nature of the patient undergoing those two procedures in our center (This pilot study primarily included late presenter and adult patients, whereas mBBT shunts were typically performed in neonates in our center).
TOF patients commonly presented late in nations with lower incomes, accounting for 85.1% of cases. Due to limited resources, TOF is frequently identified late in adulthood. Despite advancements in diagnostic technologies and a better understanding of the ailment compared to earlier centuries, certain delayed presentations are still being documented (5, 6). Unfortunately, no additional late presenter Fallot type lesion has been studied. In our particular instance, ten patients with Fallot physiology presented as adults. Because Fallot physiology in adults is uncommon, they were initially treated with different diagnoses. Furthermore, there are no acknowledged recommendations or consensus on the age of late-presenter TOF patients or how to manage them (16–19). The majority of late-presenter TOF patients who lived into adulthood had chronic polycythemia with hyperviscosity and consumptive coagulopathy with varying manifestations. Though uncorrected TOF survival is uncommon, it has been observed that approximately 10% of affected individuals live to adulthood, with only 5% reaching the age of 40 (5, 6). Chronic hypoxia and fibrosis are thought to be the causes of late-presenter TOF patients' left or right ventricular dysfunction. Pre-operative elevated hematocrit (>45%), age greater than 4 years, and RVEDP ratio greater than 12 mmHg were identified as risk factors for cardiomyocyte degeneration and interstitial fibrosis in RVOT, which could result in ventricular dysfunction (20, 21). According to Chowdury et al., 29.3% of TOF patients aged 4–15 years and 81.8% of those aged >15 years had right ventricular dysfunction and low cardiac output (20).
4.2 RVOT stenting as a potential primary palliative treatment for unrepaired adults Fallot physiology
Previously, most RVOT stenting research focused on the effects of saturation and pulmonary artery growth in babies (22–26). However, in this investigation, we discovered an intriguing occurrence in which saturation and ejection fraction improved dramatically, particularly in adults with unrepaired Fallot physiology. The decision not to operate is justified by evidence-based medicine and in the patient's best interest considering very high mortality in this patient's group. The stage approach with initial palliation should be the preferential approach. Palliative non-surgical treatments may thus be considered in individuals with unrepaired Fallot physiology, cyanosis, and ventricular dysfunction, with the goal of improving ventricular dysfunction before to total repair. Li et al. demonstrated that pre-operative ventricular function predicts improved post-operative ventricular function (27). The palliative technique also served to prepare the left ventricle for enhanced blood flow when total repair was performed.
The placement of an RVOT stent is intended to enhance pulmonary blood flow, which will increase systemic saturation and possibly repair ventricular function in late-presenter TOF patients. Prolonged hypoxia in people with unrepaired Fallot physiology and borderline ventricular function resulted in hybernating myocardium. As a result, when the hybernating myocardial regained blood supply, the EF improved considerably. In contrast to pediatric populations, children had high EF despite being severely cyanotic due to their still well-maintained reserve (8).
Arrhythmia, cerebrovascular accident, tamponade, stent thrombosis, malposition and embolisation, and vascular access problems such as hemorrhage and thrombosis have previously been documented following RVOT stent insertion. Post-procedural problems were confined to a transitory conduction disruption, clinically inconsequential RVOT perforation, and proximal stent embolisation (22–26). We also discovered various issues in adult populations, such as fractured or dislodged stents, which we believe are caused by hypertrophied infundibular stenosis. However, reintervention resolves the majority of these issues (8). Interestingly, all of our adult patients improved clinically and were able to undergo total repair with no intra- or post-procedural death reported.
4.3 Long-term outcome: stent fracture and restenosis
Regarding RVOT stenting in late presenter tetralogy of Fallot, there were not many long-term data available, particularly for adult populations. Main causes of reduced patency with RVOT stenting include intimal hyperplasia, early thrombosis, and stent fractures. In the RVOT, fractures have been identified as a major risk factor for late stent failure. The stent was exposed to distortive stresses during the heart contractions due to the presence of very tight infundibular stenosis and hyperdynamic motion, which are common in patients who present with TOF late (7, 8). Roughly 1%–8% of cases are reported to have a stent fracture; higher prevalence is noted in structures with excessive motion and hinge movement (28). The stent's torsion and muscle compression in the RVOT context may be comparable to those in the femoropopliteal artery. Untreated stent fractures may increase the risk of in-stent restenosis and stent thrombosis (7, 8).
Total repair should ideally be carried out three to six months following initial palliation. Because of this reason, the stent was not intended to be durable for long-term use. Thus, by (1) developing stents to better respond to the dynamic mechanics of the RVOT region and (2) determining the ideal implantation site to decrease mechanical stresses, the rate of stent fracture and its related problems could be reduced.
5 Limitations
The limitations of this study stem from its retrospective approach and limited cohort size. The cohort is a high-risk, diverse collection of patients with distinct anatomical substrates. Because our institution's practice has evolved over time, a consistent technique was not used on all patients.
6 Conclusions
RVOT stenting is a safe and effective method for increasing saturation and left ventricular ejection fraction in late presenter patients with unrepaired Fallot physiology. In a few cases, repeat RVOT stenting is required prior to definitive correction of very tight pulmonary stenosis, this method allows for somatic growth and pulmonary artery rehabilitation before to definitive total repair.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Institutional Review Board of National Cardiovascular Center Harapan Kita with No. LB.02.01/VII/009/KEP009/2023. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
RP: Conceptualization, Writing – original draft, Writing – review & editing. YK: Data curation, Investigation, Writing – review & editing. SS: Formal Analysis, Methodology, Writing – review & editing. AS: Writing – review & editing. DS: Data curation, Writing – review & editing. BM: Writing – review & editing. IP: Writing – review & editing. OLe: Writing – review & editing. OLi: Writing – review & editing.
Funding
The author(s) declare have no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We would like to thank those who have supported us in the making of this study. We are especially grateful to the Department of Cardiology and Vascular Medicine, Faculty of Medicine Universitas Indonesia, for their guidance and assistance in teaching the authors about research methodology and for proof-reading this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Dirks V, Pretre R, Knirsch W, Buechel ERV, Seifert B, Schweiger M, et al. Modified Blalock–Taussig shunt: a not-so-simple palliative procedure. Eur J Cardio-Thoracic Surg. (2013) 44(6):1096–102. doi: 10.1093/ejcts/ezt172
2. Ghaderian M, Ahmadi A, Sabri MR, Behdad S, Dehghan B, Mahdavi C, et al. Clinical outcome of right ventricular outflow tract stenting versus Blalock–Taussig shunt in tetralogy of Fallot: a systematic review and meta-analysis. Curr Probl Cardiol. (2021) 46(3):1–14. doi: 10.1016/j.cpcardiol.2020.100643
3. Tanidir I, Bulut M, Kamali H, Ozturk E, Yucel I, Guzeltas A. Right ventricular outflow tract stenting during neonatal and infancy periods: a multi-center, retrospective study. Turk Gogus Kalp Dama. (2020) 28(3):442–9. doi: 10.5606/tgkdc.dergisi.2020.18970
4. Quandt D, Ramchandani B, Stickley J, Mehta C, Bhole V, Barron D. Stenting of the right ventricular outflow tract promotes better pulmonary arterial growth compared with modified Blalock–Taussig shunt palliation in tetralogy of Fallot-type lesions. JACC Cardiovasc Interv. (2017) 10:1774–84. doi: 10.1016/j.jcin.2017.06.023
5. McCartney S, Machovec K, Jooste E. Uncorrected tetralogy of Fallot, biventricular dysfunction, and a large pericardial effusion. J Cardiothorac Vasc Anesth. (2015) 29(5):1391–5. doi: 10.1053/j.jvca.2015.06.033
6. Murni IK, Wirawan MT, Patmasari L, Sativa ER, Arafuri N, Nugroho S, et al. Delayed diagnosis in children with congenital heart disease: a mixed-method study. BMC Pediatr. (2021) 21(1):1–7. doi: 10.1186/s12887-021-02667-3
7. Prakoso E, Kurniawati Y, Siagian SN, Sakti DDA, Mendel B, Lilyasari O. Stenting of right ventricular outflow tract as a bridge to definite treatment in 40-year old tetralogy of Fallot patient: a case report. J Am Coll Cardiol. (2022) 79(9_Supplement):893. doi: 10.1016/S0735-1097(22)01884-8
8. Prakoso R, Agita Sembiring A, Dwisepto Aulia Sakti D, Mendel B, Lilyasari O. Double stenting in 19-year-old patient with tetralogy of Fallot with prior fractured stent. J Am Coll Cardiol Case Rep. (2022) 4(20):1375–8. doi: 10.1016/j.jaccas.2022.07.043
9. Quandt D, Penford G, Ramchandani B, Bhole V, Mehta C, Stumper O. Stenting of the right ventricular outflow tract as primary palliation for Fallot-type lesions. J Congenit Cardiol. (2017) 1(1):1–3. doi: 10.1186/s40949-017-0005-7
11. Shirazi M, Ghavanini A, Sajjadi S. Early postoperative results after total correction of tetralogy of Fallot in older patients: is primary repair always justified? Pediatr Cardiol. (2001) 22(3):238–41. doi: 10.1007/s002460010211
12. Barron D, Jegatheeswaran A. How and when should tetralogy of Fallot be palliated prior to complete repair? Pediatr Card Surg Annu. (2021) 21:77–84. doi: 10.1053/j.pcsu.2021.02.002
13. Rashid U, Qureshi A, Hyder S. Pattern of congenital heart disease in a developing country tertiary care center: factors associated with delayed diagnosis. Ann Pediatr Cardiol. (2016) 9:210–5. doi: 10.4103/0974-2069.189125
14. Heinisch PP, Guarino L, Hutter D, Bartkevics M, Erdoes G, Eberle B, et al. Late correction of tetralogy of Fallot in children. Swiss Med Wkly. (2019) 149:w20096. doi: 10.4414/smw.2019.20096
15. Luxford JC, Adams PE, Roberts PA, Mervis J. Right ventricular outflow tract stenting is a safe and effective bridge to definitive repair in symptomatic infants with tetralogy of Fallot. Heart Lung Circ. (2023) 32(5):638–44. doi: 10.1016/j.hlc.2023.02.010
16. Baumgartner H, Backer JD, Babu-Narayan SV, Budts W, Chessa M, Diller G, et al. 2020 ESC guidelines for the management of adult congenital heart disease. Eur Heart J. (2021) 42:563–645. doi: 10.1093/eurheartj/ehaa554
17. Stout KK, Daniels CJ, Aboulhosn JA, Bozkurt B, Broberg CS, Colman JM. 2018 AHA/ACC guideline for the management of adults with congenital heart disease: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Circulation. (2019) 139:e698–800. doi: 10.1161/CIR.0000000000000603
18. Ven J, Bosch E, Bogers A, Helbing W. Current outcomes and treatment of tetralogy of Fallot. F1000Res. (2019) 8:2–7. F1000 Faculty Rev-1530. doi: 10.12688/f1000research.17174.1
19. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart failure society of Amer. Circulation. (2017) 136:e137–61. doi: 10.1161/CIR.0000000000000509
20. Chowdhury UK, Sathia S, Ray R, Singh R, Pradeep KK, Venugopal P. Histopathology of the right ventricular outflow tract and its relationship to clinical outcomes and arrhythmias in patients with tetralogy of Fallot. J Thorac Cardiovasc Surg. (2006) 132(2):270–4. doi: 10.1016/j.jtcvs.2006.04.001
21. Hafez MO, Morsy SM, Mahfoz RA, Ali AR. Myocardial injury in children with unoperated congenital heart diseases. Cardiol Res Pract. (2015) 2015:104818. doi: 10.1155/2015/104818
22. Lingaswamy D, Koepcke L, Krishna MR, Kottayil BP, Sunil GS, Moynihan K, et al. Catheter-based palliation for infants with tetralogy of Fallot. Cardiol Young. (2020) 30(10):1469–72. doi: 10.1017/S1047951120002334
23. Castleberry C, Gudausky T, Berger S, Tweddel J, Pelech A. Stenting of the right ventricular outflow tract in the high-risk infant with cyanotic teratology of Fallot. Pediatr Cardiol. (2014) 35:423–30. doi: 10.1007/s00246-013-0796-z
24. Abumehdi M, Sasikumar D, Chaudhari M, Bhole V, Botha P, Mehta C, et al. Stenting of the right ventricular outflow tract as an initial intervention in tetralogy of Fallot with pulmonary stenosis and major aortopulmonary collateral arteries. Cardiol Young. (2021) 31:452–9. doi: 10.1017/S1047951120004278
25. Pizzuto A, Cuman M, Assanta N, Franchi E, Marrone C, Pak V, et al. Right ventricular outflow tract stenting as palliation of critical tetralogy of fallot: techniques and results. Heart. (2021) 2:278–87. doi: 10.3390/hearts2020022
26. Kwaśniak E, Haponiuk I, Chojnicki M, Jaworski R, Szofer-Sendrowska A, Steffens M. Right ventricular outflow tract stenting in double outlet right ventricle with critical pulmonary stenosis and hypoplastic pulmonary arteries. Postep Kardiol Interwencyjnej. (2016) 12(3):267–70. doi: 10.5114/aic.2016.61651
27. Li Y, Wang X, Lv Q, Wang J, Yang Y, He L, et al. Impact of surgical correction of tetralogy of Fallot on short-term right and left ventricular function as determined by 2-dimensional speckle tracking echocardiography. Medicine (Baltimore). (2016) 95(31):e4426. doi: 10.1097/MD.0000000000004426
Keywords: adults, ejection fraction, late presenter, tetralogy of Fallot, palliative, RVOT stent
Citation: Prakoso R, Kurniawati Y, Siagian SN, Sembiring AA, Sakti DDA, Mendel B, Pratiwi I, Lelya O and Lilyasari O (2024) Right ventricular outflow tract stenting for late presenter unrepaired Fallot physiology: a single-center experience. Front. Cardiovasc. Med. 11:1340570. doi: 10.3389/fcvm.2024.1340570
Received: 18 November 2023; Accepted: 19 January 2024;
Published: 1 February 2024.
Edited by:
Francesco Sturla, IRCCS San Donato Polyclinic, ItalyReviewed by:
Ender ÖDEMİŞ, Koç University Hospital, TürkiyeBranko Mimic University of Leicester, United Kingdom
Claudia Cattapan, University of Padua, Italy
© 2024 Prakoso, Kurniawati, Siagian, Sembiring, Sakti, Mendel, Pratiwi, Lelya and Lilyasari. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Radityo Prakoso a2FyYWphbmg3MEBnbWFpbC5jb20=
 Radityo Prakoso
Radityo Prakoso Yovi Kurniawati
Yovi Kurniawati Sisca Natalia Siagian
Sisca Natalia Siagian Aditya Agita Sembiring
Aditya Agita Sembiring Damba Dwisepto Aulia Sakti1
Damba Dwisepto Aulia Sakti1 Brian Mendel
Brian Mendel Indah Pratiwi
Indah Pratiwi Oktavia Lilyasari
Oktavia Lilyasari