Abstract
Purpose:
This meta-analysis aimed to compare the safety and efficacy of aspirin and indobufen in patients with coronary heart disease. The primary focus was on the incidence of cardiovascular events, bleeding events, and gastrointestinal reactions. Given the relatively limited research on indobufen, this study utilized aspirin as a control drug and employed meta-analysis to integrate existing clinical studies. The goal was to provide a reference for the clinical use of indobufen and to suggest directions for further largescale, multicenter prospective studies.
Methods:
This review adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. We conducted a comprehensive search of the PubMed, EMBASE, WOS, and Cochrane Library databases to identify all relevant literature on indobufen. A total of nine trials met the inclusion criteria, encompassing seven randomized controlled trails (RCTs) and two retrospective studies. Categorical variables were analyzed using odds ratio and random effects models.
Results:
The meta-analysis included nine trials, comprising seven RCTs and two retrospective studies. The pooled results indicated that indobufen significantly reduced the incidence of minor bleeding events, and gastrointestinal discomfort compared to aspirin. However, both drugs had similar effects on the incidence of recurrent angina pectoris, myocardial infarction and mortality due to coronary heart disease.
Conclusion:
Indobufen was associated with fewer gastrointestinal reactions and a low risk of bleeding, making it a viable option for patients with high-risk factors for bleeding and gastric ulcers. Despite this, indobufen's short history and limited evidence base compared to aspirin highlight the need for further research. Aspirin remains widely available, cost-effective, and the preferred drug for the primary and secondary prevention of cardiovascular and cerebrovascular diseases. Indobufen or other antiplatelet agents should only be considered when aspirin is not tolerated or contraindicated. Further clinical trials are necessary to determine whether indobufen can replace aspirin.
Systematic Review Registration:
https://www.crd.york.ac.uk/, identifier [CRD42024523477].
Background
The activation and aggregation of platelets are crucial processes in the pathogenesis of atherosclerosis thrombosis (1). Therefore, antiplatelet therapy is a cornerstone in the treatment of coronary heart disease. Indobufen and aspirin are both inhibitors of platelet cyclooxygenase (COX), reducing the production of thromboxane A2, a potent promoter of platelet aggregation. Despite their similar roles in inhibiting platelet aggregation and reducing thrombosis, they differ significantly in their mechanisms of action. Indobufen and aspirin are both inhibitors of platelet cyclooxygenase (COX), reducing the production of thromboxane A2, a potent promoter of platelet aggregation. Despite their similar roles in inhibiting platelet aggregation and reducing thrombosis, they differ significantly in their mechanisms of action.
Indobufen offers several advantages over aspirin due to its high selectivity for inhibiting platelet COX-1. Unlike aspirin, which has irreversible effects, the inhibitory effects of indobufen on platelet function are reversible and return to baseline within 24 h after discontinuation. Additionally, indobufen does not significantly impact prostacyclin, posing a lower risk of gastrointestinal injury, bleeding, and renal damage. It also does not affect uric acid metabolism. Indobufen's multi-target approach inhibits platelet aggregation induced by various factors, including ADP, AA, collagen, epinephrine, and platelet factor, enhancing its efficacy in overcoming drug resistance.
Consequently, guidelines and textbooks recommend indobufen as an alternative to aspirin for patients at a high risk of bleeding and those with gastric ulcers (2, 3). While aspirin's first-line status in preventing and treating cardiovascular and cerebrovascular diseases is well-supported by extensive evidence-based research, the evidence for indobufen remains comparatively limited.
This article systematically reviewed clinical trials and cohort studies on the efficacy and safety of indobufen in treating of coronary heart disease, usingaspirin as the control. By employing meta-analysis, we aimed to integrate existing clinical studies to provide a reference for the clinical use of indobufen and to underscore the need for further large-sample prospective research.
Methods
According to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement, this meta-analysis was performed in agreement (4). The protocol for this meta-analysis was registered on PROSPERO (Registration No: CRD42024523477).
Inclusion criteria
Study type: randomized controlled trial (RCT), cohort study or case-control study. Study population: patients with coronary heart disease. Intervention and control: Indobufen was used in the treatment group, and aspirin was used in the control group. Outcome index: adverse cardiovascular events (ACE) including recurrent angina, nonfatal myocardial infarction, and cardiac death, bleeding events [according Bleeding Academic Research Consortium (BARC) (5)] including minor bleeding (BARC grades 0–2), major bleeding (BARC grades 3–5) and any bleeding (BARC grade 0–5), and gastrointestinal adverse reactions.
Exclusion criteria
Letters, case reports, meetings, reviews, animal trials, or republished studies; indobufen was not used in the treatment group; Studies lacking a control group; Patients receiving oral or intravenous anticoagulant therapy for another condition, such as atrial fibrillation, pulmonary embolism, lower limb venous thrombosis, or an artificial heart valve.
Search strategy
One of the authors performed the search in PubMed, EMBASE, Web of Science, and the Cochrane Central Register of Controlled Trials from the inception dates to March 21, 2024, using the keywords “Indobufen” AND “Aspirin” AND “Coronary Diseases”. The detail of searching strategy for each database was shown in Supplementary Table S1.
Study selection
Two researchers (YR and XCZ) screened the retrieved literature strictly against inclusion and exclusion criteria. First, the documents that meet the inclusion criteria are read in full by reading the title and abstract, and the included papers are finally confirmed. If two researchers do not agree during the literature screening process, it will be left to the senior researcher (HL).
Data collection process
Data on relevant outcome measures that met the inclusion criteria were extracted from the literature, including author year, study design type, country, sample size, participants, TXA treatment, age, outcomes, etc.
Assessment of risk of bias and quality of evidence
Two researchers (QYY and YR) independently assessed the quality of all included trials based on Cochrane risk-of-bias criteria (6). The ROBINS-I tool evaluates bias risk for non-RCT across domains like confounding, selection, intervention classification, deviations, missing data, outcome measurement, and result reporting.
Data synthesis
The Meta-analysis was performed using Stata (version 17; StataCorp, 2021) software. The heterogeneity was assessed by using the Q test and I2 value calculation. If the heterogeneity was absent (P > 0.1 and I2 < 50%), the data was combined with a fixed effect model. The random effects model was used if the heterogeneity was present (P < 0.1 or I2 > 50%). The odds ratio (OR) and their associated 95% confidence interval (CI) were used to assess outcomes for dichotomous outcomes. Continuous outcomes were analyzed using mean, SD, and sample size to provide a mean difference (MD) between the indobufen and aspirin groups. A P value less than 0.05 suggested that the difference was statistically significant.
Sensitivity analyses
We performed a sensitivity analysis by excluding the largest trial, cluster randomized or quasi-randomized trials, and trials with a high risk of bias, using random effect models.
Results
After screening 202 studies, 43 duplicates were removed, and 143 irrelevant studies were excluded based on titles and abstracts. The full text of 16 articles were reviewed, resulting in the elimination of 12 articles. Among these, eight trials lacked results (7–13), two were conference abstract (14, 15), and two studies used dipyridamole in addition to aspirin in the control group (16, 17), preventing extraction of data specific to aspirin. Five studies were already included in previous meta-analysis. Ultimately, nine trials, comprising seven randomized controlled trials (RCTs) and two retrospective studies were included in this meta-analysis. The literature screening process is illustrated in Figure 1, and the essential characteristics of the included studies are detailed in Table 1.
Figure 1
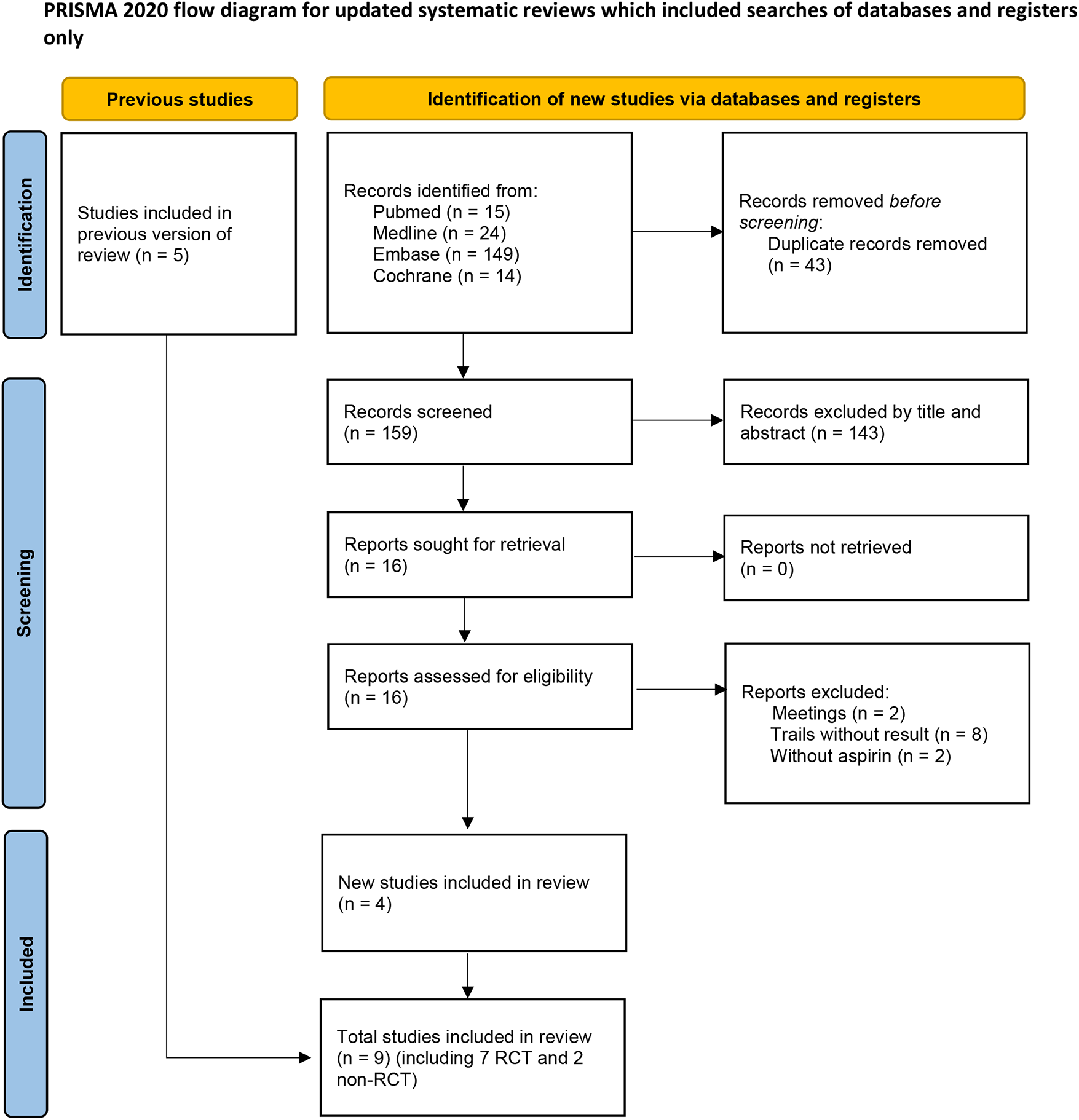
Flow diagram for search and selection of included studies.
Table 1
| Study | Country | Participants | Treatment | Design | Age | Sex (F/M) | Outcomes | No. of subject | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Aspirin | Indobufen | Aspirin | Indobufen | Aspirin | Indobufen | ||||||
| Bai et al. (18) | China | Patients after CABG | 100 mg aspirin qd + 75 mg clopidogrel qd. 100 mg indobufen qd + 75 mg clopidogrel qd. |
RCS | 59.7 ± 7.2 | 60.3 ± 6.6 | 18/57 | 14/62 | Adverse cardiovascular event, gastrointestinal bleeding | 76 | 76 |
| Wu et al. (19) | China | Coronary artery disease with symptoms of angina pectoris or documented ischemia | 100 mg aspirin qd + 75 mg clopidogrel qd. 100 mg indobufen bid + 75 mg clopidogrel qd. |
RCT | 61.2 ± 8.4 | 61.0 ± 8.3 | 846/1,447 | 737/1,521 | Cardiovascular death, nonfatal MI, ischemic stroke, ST, BARC type bleeding; | 2,293 | 2,258 |
| Shi et al. (14) | China | Stable coronary heart disease after PCI | aspirin 100 mg qd + clopidogrel 75 mg qd indobufen 100 mg bid + clopidogrel 75 mg qd indobufen 100 mg bid, aspirin 100 mg qd |
open label crossover study | 63.07 ± 6.46 | 2/54 | AA-PAR, ADP-PA, PRI-VASP, plasma/Urinary TXB2, Gastrointestinal adverse reactions, BARC type bleeding; | 56 | |||
| Yang et al. (15) | China | Patients with coronary atherosclerosis | asprini 100 mg qd; indobufen 100 mg bid |
RCT | 60.8 ± 10.7 | 60.7 ± 8.0 | 13/17 | 11/21 | PLAA, plasma TXB2, urinary 11-dh-TXB2, gastrointestinal adverse reactions, BARC type bleeding | 30 | 32 |
| Chen (20) | China | Patients with unstable angina pectoris | 100 mg aspirin qd + 75 mg clopidogrel qd. 100 mg indobufen bid + 75 mg clopidogrel qd. |
RCT | 69.46 ± 5.72 | 68.32 ± 4.72 | 35/40 | 39/36 | Clinical effect, bleeding events (oral bleeding) | 75 | 75 |
| Zhang (13) | China | ACS | 100 mg aspirin qd + 75 mg clopidogrel qd. 100 mg indobufen bid + 75 mg clopidogrel qd. |
RCT | 61.2 ± 7.4 | 62.2 ± 6.7 | 14/21 | 13/22 | Adverse cardiovascular events, bleeding events (defecate occult blood, ecchymosis) | 35 | 35 |
| Zhou (21) | China | NSTE-ACS | 100 mg aspirin qd + 75 mg clopidogrel qd. 100 mg indobufen bid + 75 mg clopidogrel qd. |
RCT | 68.5 | 67.3 | 14/16 | 13/17 | Adverse cardiovascular events, gastrointestinal adverse reactions, bleeding event (bleeding gums, nasal bleeding) | 30 | 30 |
| Wen (23) | China | PCI | 100 mg aspirin qd + 75 mg clopidogrel qd. 100 mg indobufen bid + 75 mg clopidogrel qd. |
RCT | 57 ± 9 | 58 ± 7 | 8/24 | 6/26 | Adverse cardiovascular event, gastrointestinal adverse reactions, bleeding event (bleeding gums,) | 32 | 32 |
| Lv et al. (22) | China | Unstable angina pectoris | 100 mg aspirin qd + 75 mg clopidogrel qd. 100 mg indobufen bid + 75 mg clopidogrel qd. |
RCT | 62.49 ± 4.35 | 61.61 ± 4.58 | 38/57 | 41/54 | Gastrointestinal adverse reactions, bleeding event (occult blood in stool) | 95 | 95 |
Characteristics of included studies.
CABG, Coronary artery bypass grafting; PCI, percutaneous transluminal coronary intervention; ACS, acute coronary syndrome; NSTE-ACS, non-ST segment elevation acute coronary syndromes; RCS, retrospective cohort study; RCT, randomized controlled trial; BARC, Bleeding Academic Research Consortium.
Risk of bias cochrane assessment tool version 2
Seven RCTs deemed to carry some risk due to lack of specification regarding the specific follow-up methods and blinding procedures (15, 19–24). Overall, there was some risk of bias across studies in seven trails (Figure 2).
Figure 2
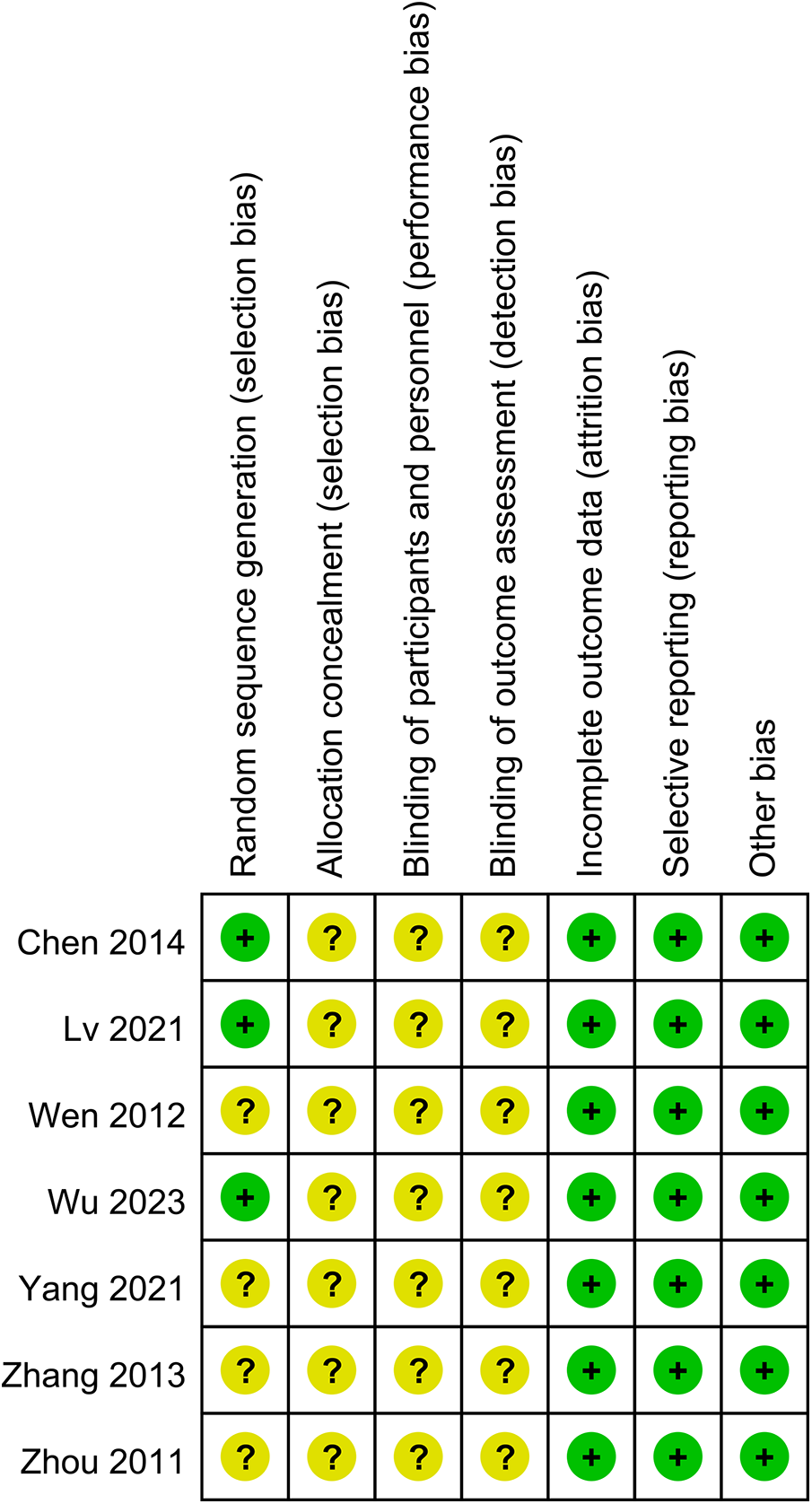
Risk of bias summary: review authors’ judgements about each risk of bias item for RCTs.
Risk of bias ROBINS—I tool
One study was assessed as having a low risk of bias (18) and one study was classified as having a serious risk of bias (14). These assessments primarily stemmed from issues related to selection bias, intervention bias, measurement bias, and reporting bias. The details of risk bias were shown in Table 2.
Table 2
| Study | Confounding bias | Selection bias | Classification bias | Intervention bias | Missing data bias | Measurement bias | Reporting bias | Overall risk of bias |
|---|---|---|---|---|---|---|---|---|
| Bai et al. (18) | Low | Low | Low | Low | Low | Low | Low | Low |
| Shi et al. (14) | Low | High | Low | Low | Low | Low | Low | High |
Risk of bias assessment with the ROBINS-I tool.
Adverse cardiovascular events
Five studies reported adverse cardiovascular events as a primary outcome (18, 19, 21, 23, 24). These events were categorized as recurrent angina, nonfatal myocardial infarction, and cardiac death.
Recurrence of angina pectoris
Three studies addressed the recurrence of angina pectoris (18, 21, 24). The pooled results showed no significant difference in the recurrence rate of angina pectoris between the indobufen and aspirin group (OR: 1.22, 95% CI: 0.51–2.93, I2 = 0%, P = 0.659; Figure 3).
Figure 3
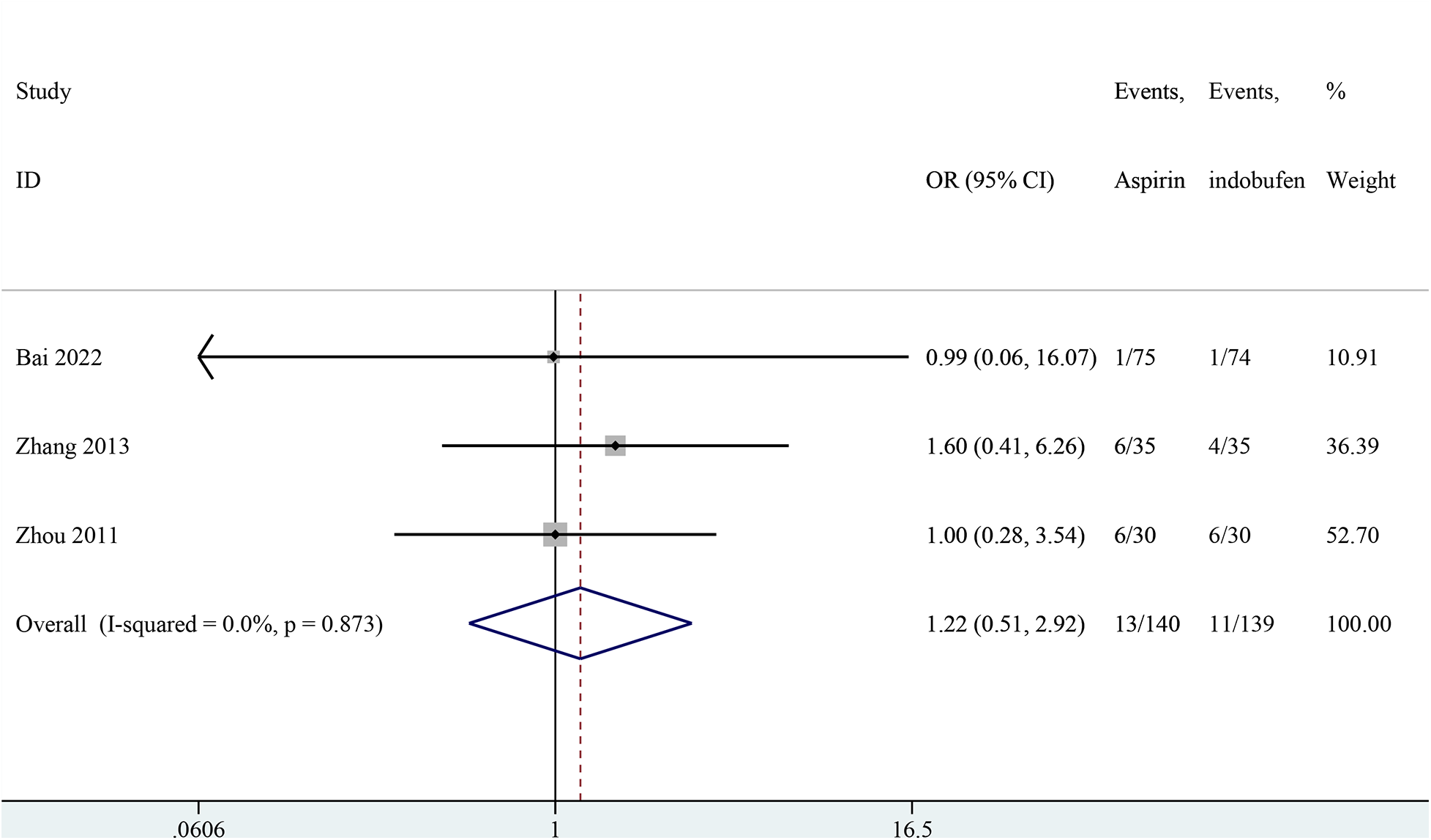
Forest plot of comparison: aspirin vs. indobufen; outcome: recurrence of angina pectoris.
Non-fatal myocardial infarction
Three studies reported non-fatal myocardial infarction (18, 19, 24). The pooled results indicated that no significant difference in the incidence of non-fatal myocardial infarction between the indobufen and aspirin group (OR: 1.36, 95% CI: 0.61–3.02, I2 = 0%, P = 0.451; Figure 4).
Figure 4
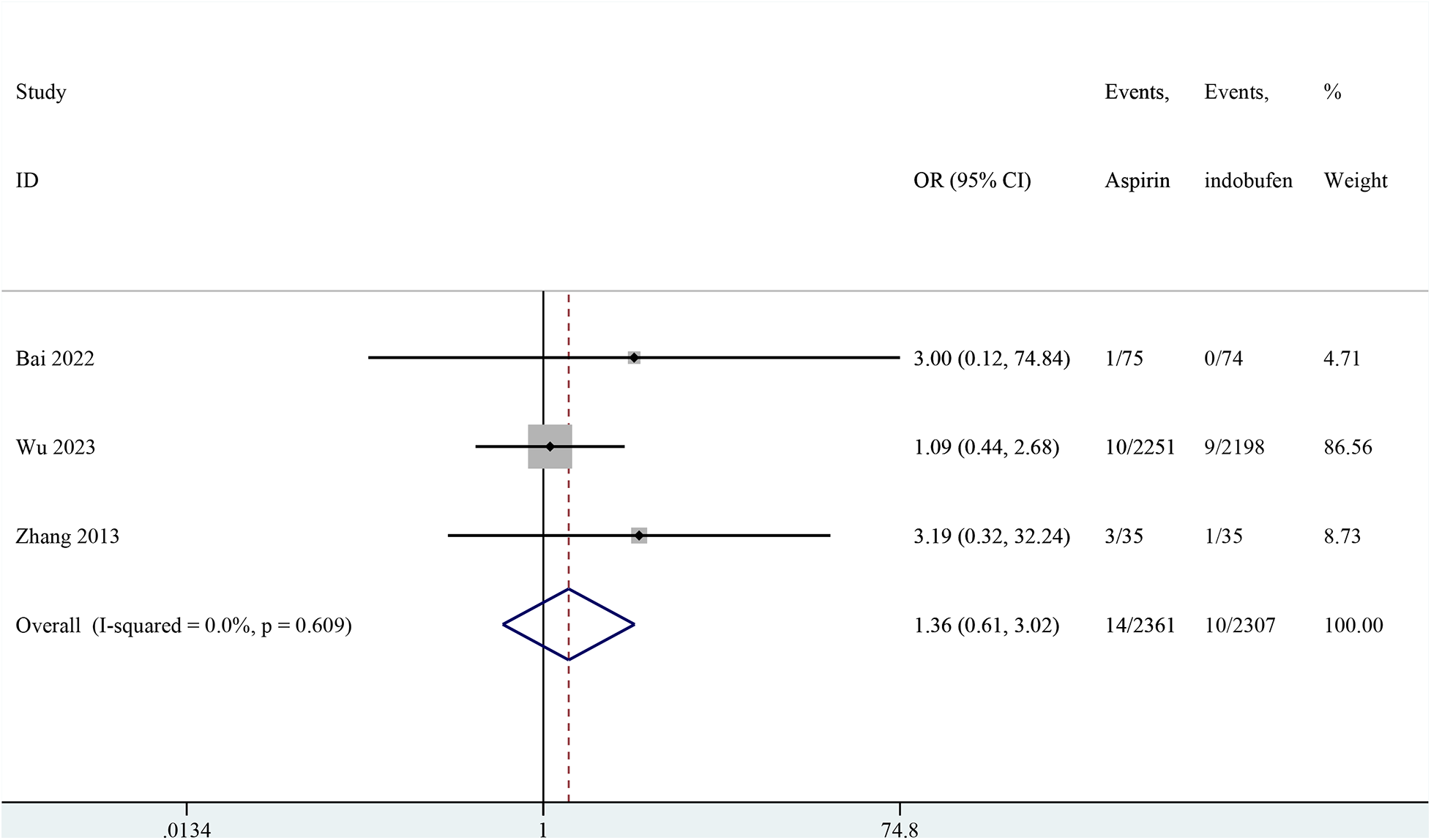
Forest plot of comparison: aspirin vs. indobufen; outcome: Non-fatal myocardial infarction.
Cardiovascular death
Four studies included cardiovascular death (18, 19, 23, 24). The combined results showed no significant difference in cardiovascular death between the indobufen and aspirin group (OR: 1.58, 95% CI: 0.52–4.86, I2 = 0%, P = 0.422; Figure 5).
Figure 5
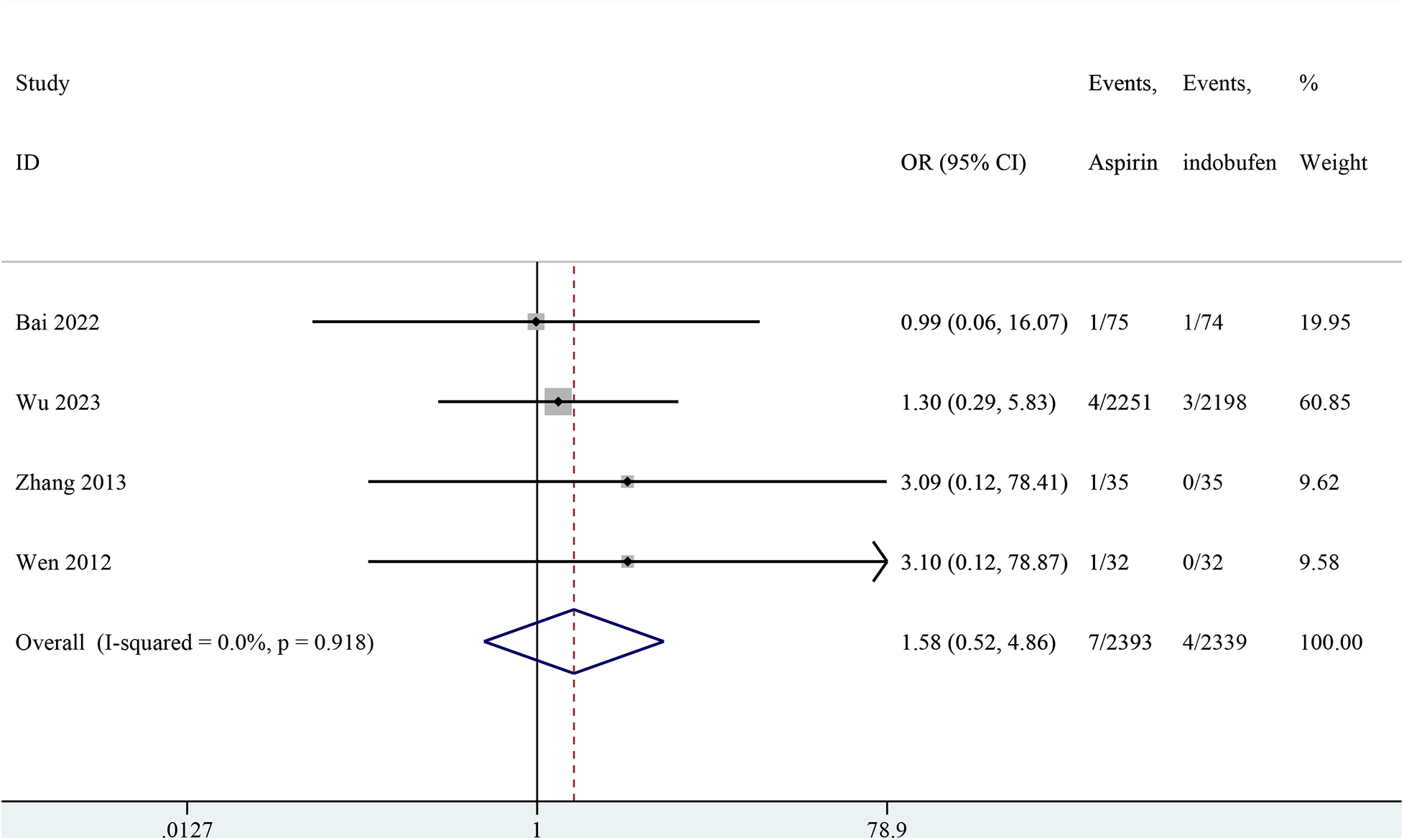
Forest plot of comparison: aspirin vs. indobufen; outcome: cardiovascular death.
Bleeding events
Nine studies reported on bleeding events (14, 15, 18–24). Bleeding events were classified according to academic research standards into minor bleeding (BARC grades 0–2), major bleeding (BARC grades 3–5) and any bleeding (BARC grade 0–5).
Minor bleeding
Nine studies reported minor bleeding events (14, 15, 18–24). Two studies were excluded from the pooled meta-analysis results because both groups in these studies reported zero events (14, 15). The pooled results showed a significant reduction in minor bleeding events with indobufen compared to aspirin (OR: 2.18, 95% CI: 1.54–3.10, I2 = 0%, P < 0.001; Figure 6).
Figure 6
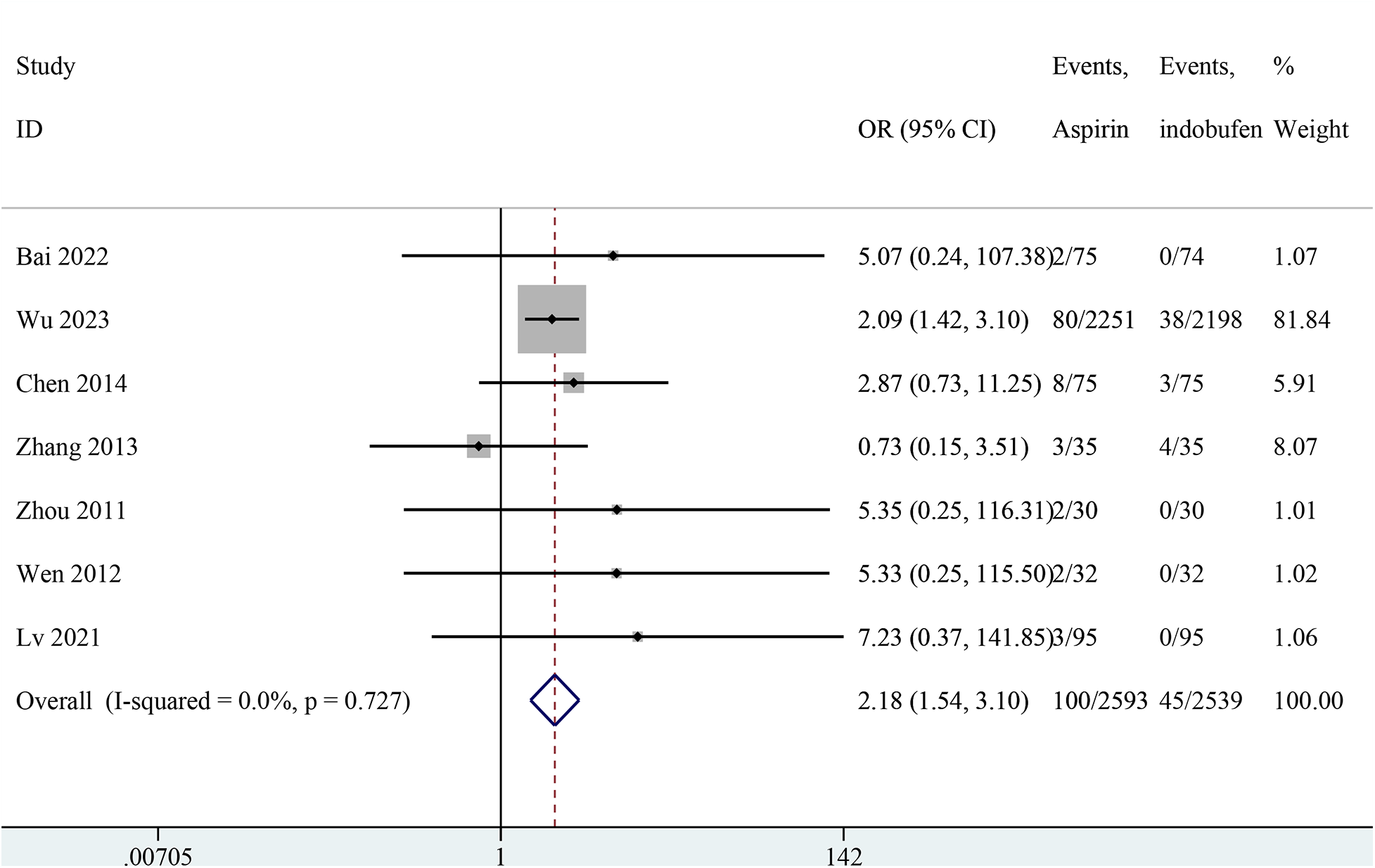
Forest plot of comparison: aspirin vs. indobufen; outcome: Minor bleeding.
Major bleeding
Two studies reported major bleeding events (15, 19). The pooled results showed no significant difference in major bleeding events between the indobufen and aspirin groups (OR: 0.91, 95% CI: 0.55–1.53, I2 = 0%, P = 0.732; Figure 7).
Figure 7
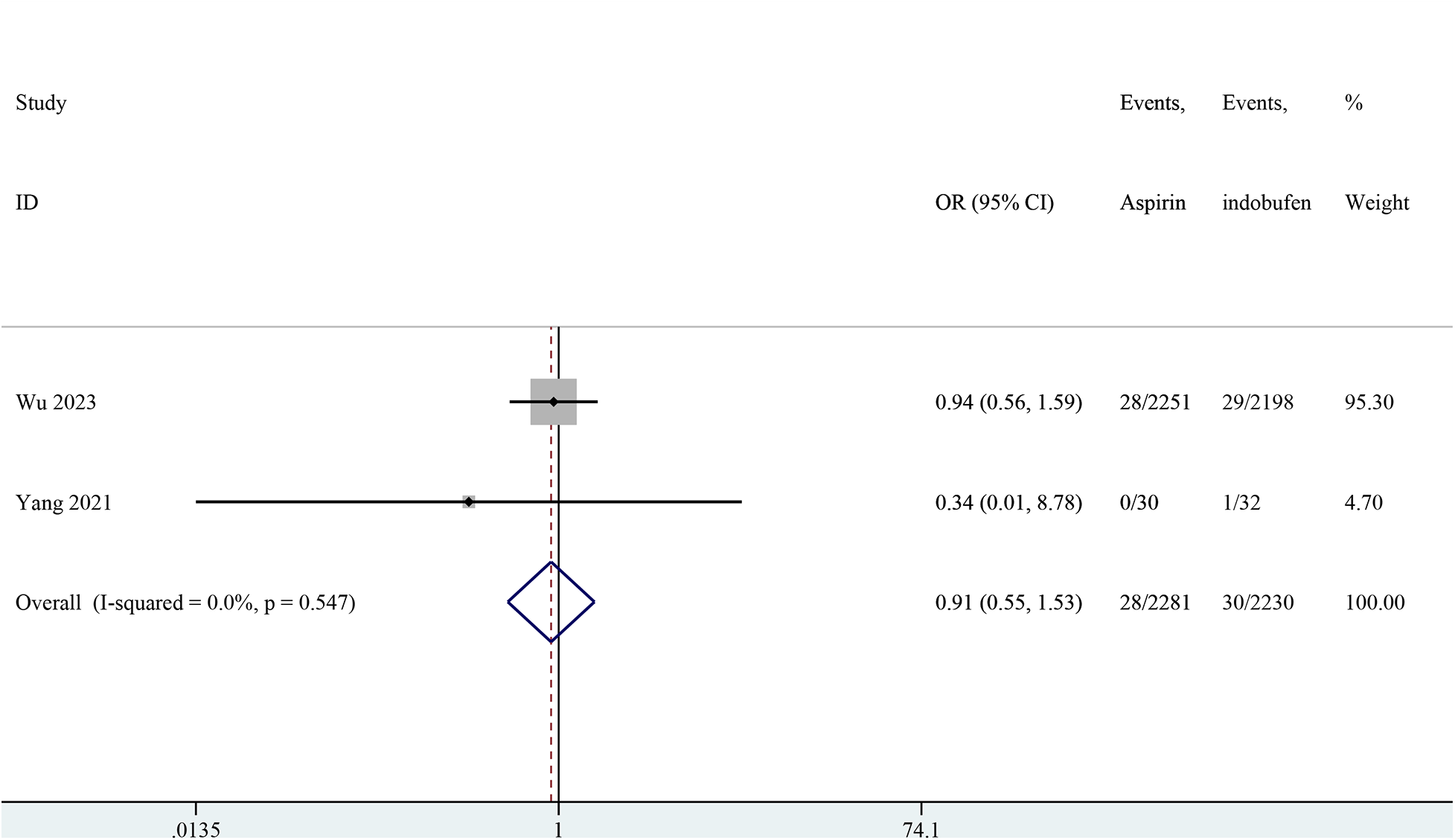
Forest plot of comparison: aspirin vs. indobufen; outcome: Major bleeding.
Any bleeding
Nine studies reported any bleeding events (14, 15, 18–24). One study was excluded from the pooled meta-analysis results because both groups in these studies reported zero events (14). The pooled results showed a significant reduction in any bleeding events with indobufen compared to aspirin (OR: 1.69, 95% CI: 1.27–2.25, I2 = 0%, P < 0.001; Figure 8).
Figure 8
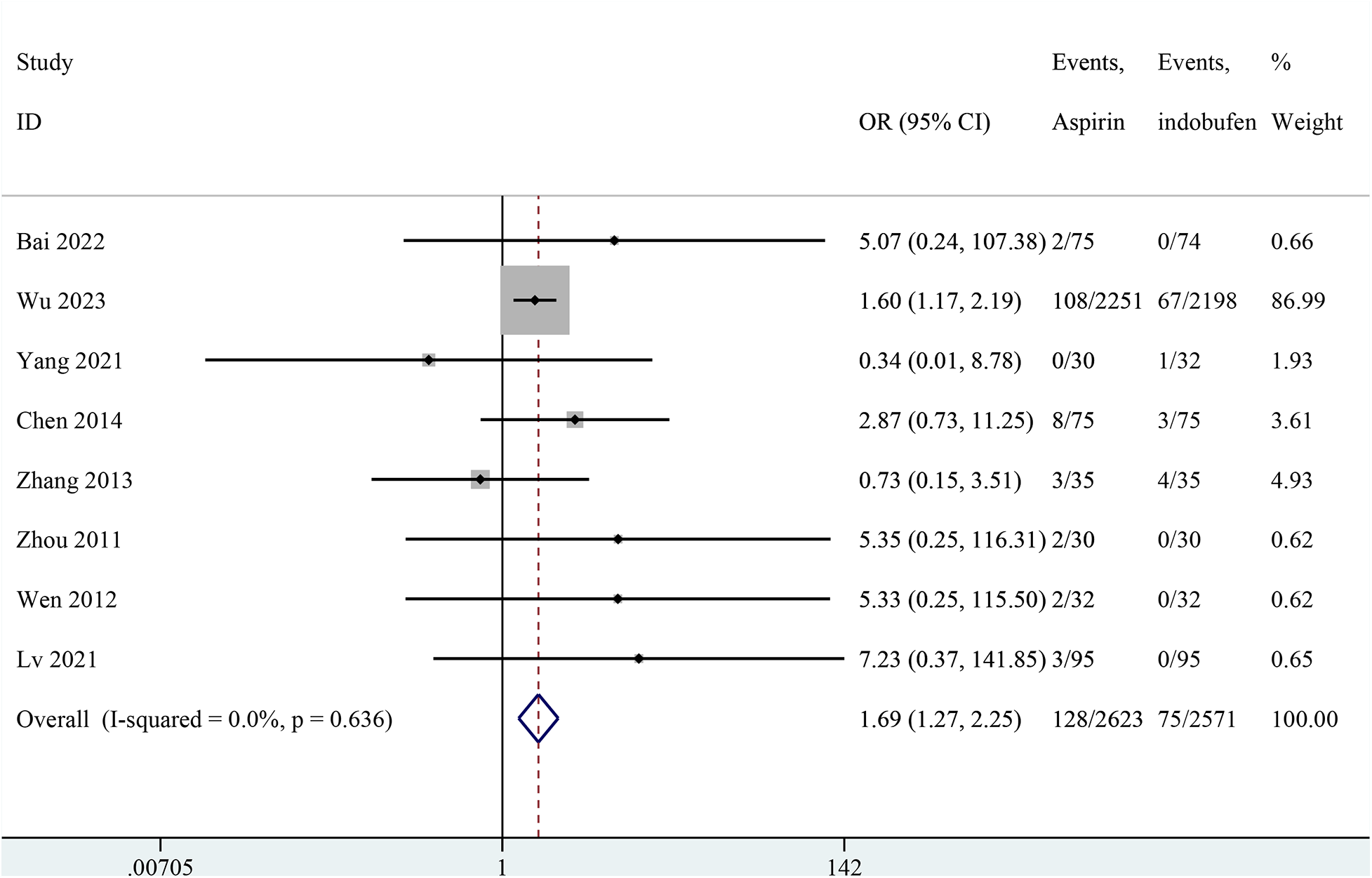
Forest plot of comparison: aspirin vs. indobufen; outcome: Any bleeding.
Gastrointestinal adverse
Six studies reported gastrointestinal adverse reactions (14, 15, 18, 21–23). The results showed that gastrointestinal reaction were significantly less frequent in the indobufen group compared to the aspirin group (OR: 2.77, 95% CI: 1.34–5.74, I2 = 0%, P = 0.006; Figure 9).
Figure 9
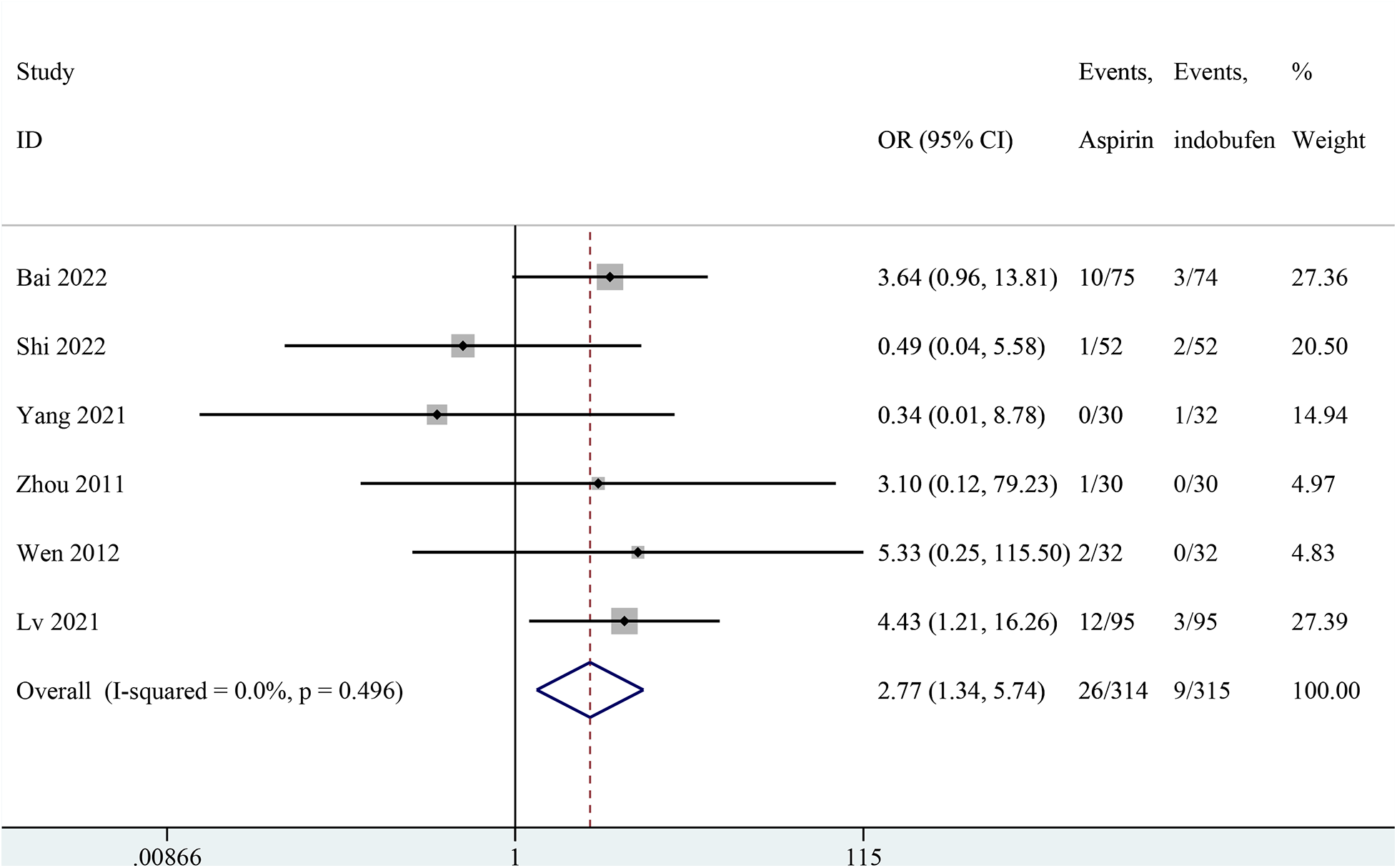
Forest plot of comparison: aspirin vs. indobufen; outcome: gastrointestinal adverse.
Sensitivity analysis
No individual study had a significant impact on the results. The results of sensitivity analysis were shown in Supplementary Figure S1–S7.
Publication bias
As fewer than ten trials were included, no publication bias assessment was performed by funnel plots.
Discussion
Data from this study reaffirm that indobufen significantly reduces the incidence of minor bleeding events (BARC grades 0–2) and gastrointestinal adverse reactions, highlighting it's superior safety profile compared to aspirin. In terms of effectiveness, indobufen was comparable to aspirin regarding in the incidence of recurrent angina pectoris, myocardial infarction, and death from coronary heart disease. This meta-analysis included seven RCTs, all involving patients from China, providing a reference for current clinical practice, particularly for long-term antiplatelet monotherapy in Asian patients with a history of digestive tract diseases. There was no heterogeneity among the studies (I2 = 0), suggests a reliable conclusion. Additionally, indobufen significantly reduced the risk of any bleeding events compared to aspirin, consistent with previous studies. Therefore, the use of indobufen can alleviate concerns about gastrointestinal adverse effects associated with aspirin use. However, while the meta-analysis showed no heterogeneity (I2 = 0), suggesting consistency in the findings, it is essential to acknowledge the broader context of heterogeneity in the literature. Differences in study designs, ranging from randomized controlled trials to retrospective cohort studies, can introduce variability in outcomes due to differing methodologies. The patient populations also varied widely, including those undergoing procedures like coronary artery bypass grafting and percutaneous coronary intervention, each with different baseline characteristics such as age and sex distribution, which could influence the results. Treatment regimens also differed, with some studies combining indobufen or aspirin with clopidogrel, potentially leading to synergistic effects not seen in monotherapy. Moreover, the outcomes assessed, such as adverse cardiovascular events and gastrointestinal bleeding, were defined and reported inconsistently across studies. Despite these variations, the consistent finding that indobufen reduces gastrointestinal bleeding and adverse reactions compared to aspirin provides robust evidence for its clinical use, particularly for patients at high risk of bleeding or with aspirin intolerance.
Our meta-analysis confirms that indobufen significantly reduces gastrointestinal bleeding events compared to aspirin, aligning with guidelines that recommend indobufen for its lower incidence of gastrointestinal reactions and bleeding (2). This makes indobufen a viable option for patients with aspirin intolerance or those at high risk for bleeding and gastric ulcers, showing promising prospects for clinical application. For instance, studies have demonstrated that indobufen's efficacy is comparable to that of aspirin combined with dipyridamole after coronary artery bypass grafting (CABG). Furthermore, in preventing coronary restenosis post-percutaneous transluminal coronary intervention (PCI), indobufen combined with clopidogrel has shown to be safer with fewer side effects than aspirin combined with clopidogrel (23). Additionally, indobufen has proven effective and safe in preventing thromboembolic events in patients with non-rheumatic atrial fibrillation (25).
A broader meta-analysis on indobufen's efficacy and safety, either alone or in combination with other conventional drugs for ischemic cardiovascular and cerebrovascular diseases, found significantly lower incidences of bleeding, gastrointestinal reactions, and overall adverse drug events in the indobufen group. Indobufen also showed lower rates of bleeding and gastrointestinal reactions than other antiplatelet drugs and was significantly superior to placebo or no treatment in preventing thromboembolic events (26). Xu et al. systematically reviewed the efficacy and safety of indobufen in preventing cardiovascular and cerebrovascular events, finding no significant differences between indobufen and aspirin in preventing recurrent angina, nonfatal myocardial infarction, and cardiac death. However, total bleeding events and gastrointestinal reactions were less frequent with indobufen (27). Tian et al. evaluated indobufen's efficacy, safety, and cost-effectiveness compared to anticoagulants in patients with nonvalvular atrial fibrillation, including 72,599 patients across five studies. They found that indobufen reduced net clinical benefit events and clinically relevant non-major bleeding events, with no major bleeding events reported during the observation period (28).
In conclusion, while aspirin has a broader range of indications, indobufen is better tolerated and safer for inhibiting platelet aggregation. Economically, however, indobufen is less favorable. The average daily cost of indobufen (27.8 yuan/day) is significantly higher than that of aspirin (0.48 yuan/day). Despite its advantage, indobufen is not yet positioned to replace aspirin as the frontline treatment due to the extensive evidence-based support for aspirin in preventing and treating cardiovascular and cerebrovascular diseases. Indobufen remains a recognized alternative for patients intolerant to aspirin, but more extensive evidence is needed to challenge aspirin's primary status.
Limitation
This study has several limitations. First, it included seven RCTs with high methodological quality evidence, one retrospective cohorts, and one open-label crossover study. RCTs are advantageous because they minimize bias and provide more robust causal inference by randomly assigning participants and controlling for various factors, making their conclusions more reliable. However, retrospective studies have inherent limitations due to the researchers' lack of control over data collection. The integrity and authenticity of records in these studies directly impact the reliability of the results, introducing significant bias. Open-label crossover studies are prone to ascertainment bias because researchers and participants are aware of the treatment assignment, which can influence the assessment of outcomes. For instance, researchers might subconsciously favor treatment plan A, leading to biased data collection for group A participants. researchers might subconsciously favor treatment plan A, leading to biased data collection for group A, participants.
Second, the study included patients with varying disease statuses, such as those with stable coronary artery disease, those who had undergone PCI, and those who had undergone CABG. Different studies evaluated treatment efficacy using diverse metrics, including the recanalization rate of the vascular bridge after aspirin and indobufen administration, coagulation function indicators such as FIB and D-dimer, plasma and urine Thromboxane B2 (TXB2) content, platelet aggregation rate (PAR), and platelet reactivity index (PRI-VASP, %). This variability limited the comparison of indicators in the final summary results, which foucused only on bleeding events, adverse gastrointestinal reactions, and cardiovascular events.
Thirdly, the dosing regimens in the included studies were inconsistent. Some studies compared aspirin or indobufen combined with clopidogrel, while others compared the efficacy and safety of aspirin and indobufen alone. The inability to account for the effect of concomitant clopidogrel use on gastrointestinal bleeding, gastrointestinal complaints, and cardiovascular event rates with introduced bias into the pooled analysis results.
Fourth, the literature search revealed that most studies comparing the safety and efficacy of indobufen and aspirin were conducted in China. This geographical limitation may introduce bias, as the results might not fully represent the global patient population. These limitations highlight the necessity for more diverse and comprehensive studies to provide clearer insights into the comparative safety and efficacy of indobufen and aspirin. While our findings are most applicable to Chinese or broader Asian populations, they offer valuable reference points for future research in different geographic and ethnic contexts. Future research should aim to include participants from various racial and ethnic backgrounds to validate these findings and ensure their global applicability. This approach will help to build a more comprehensive understanding of indobufen's benefits across diverse populations.
Conclusion
The antiplatelet effect of indobufen is similar to that of aspirin, but indobufen offers some distinct advantages, including high selectivity and reversible binding to its receptor. Indobufen is associated with fewer gastrointestinal reactions and a lower risk of bleeding, making it an attractive option for patients who are intolerant to aspirin or have a high risk of bleeding and gastric ulcers. However, indobufen has a shorter history of use, and the evidence supporting its efficacy and safety is not as robust as that for aspirin. This limited scope and short history undermine the strength of its conclusions. Aspirin is more accessible and cost-effective due to its lower price. From a pharmacoeconomic perspective, aspirin remains the first choice for the primary and secondary prevention of cardiovascular and cerebrovascular diseases. Switching to indobufen or another antiplatelet agent should only be considered when aspirin is not tolerated or is contraindicated. As more large-scale clinical trials are conducted, the potential for indobufen to replace aspirin remains uncertain. In summary, while indobufen is a valuable alternative for specific patient populations, aspirin continues to be the preferred agent for most patients due to its well-established efficacy, safety profile, and cost-effectiveness.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
XZ: Data curation, Investigation, Methodology, Software, Supervision, Writing – original draft. QY: Data curation, Formal Analysis, Project administration, Resources, Visualization, Writing – original draft. JJ: Methodology, Project administration, Validation, Writing – original draft. HL: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Writing – original draft, Writing – review & editing. YR: Conceptualization, Data curation, Formal Analysis, Methodology, Project administration, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This work was supported by the Enze Medical Center (Group) Scientific Research (No. 23EZA04) and Zhejiang Medicine and Health Scientific Research Project (No. 2024KY531). The funder had a specific role in the data collection, analysis, decision to publish, and preparation of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1412944/full#supplementary-material
References
1.
Davì G Patrono C . Platelet activation and atherothrombosis. N Engl J Med. (2007) 357(24):2482–94. 10.1056/NEJMra071014
2.
Yong Huo YW Gu Y Huang K Xu A Zheng Y Ge J . Expert consensus on the diagnosis and treatment of patients with intolerance and low response to commonly used oral antiplatelet drugs. Chin J Intervent Cardiol. (2021) 29(05):240–9.
3.
Committee of Thrombosis Prevention and Treatment SoCP, Chinese Medical Doctor Association, Coronary Heart Disease and Atherosclerosis CSoC, Chinese Medical Association, Cardiology EBoCJo. Chinese expert consensus on antiplatelet therapy in patients with acute coronary syndrome without revascularization. Chin J Cardiol. (2019) 47(6):430–42. 10.3760/cma.j.issn.0253-3758.2019.06.003
4.
Liberati A Altman DG Tetzlaff J Mulrow C Gøtzsche PC Ioannidis JP et al The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. (2009) 6(7):e1000100. 10.1371/journal.pmed.1000100.
5.
Dai C Liu M Yang Z Li Y Zhou Y Lu D et al Real-world performance of indobufen versus aspirin after percutaneous coronary intervention: insights from the ASPIRATION registry. BMC Med. (2024) 22(1):148. 10.1186/s12916-024-03374-3
6.
Cumpston M Li T Page MJ Chandler J Welch VA Higgins JP et al Updated guidance for trusted systematic reviews: a new edition of the cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. (2019) 10:Ed000142. 10.1002/14651858.Ed000142
7.
Chi CI . A randomized controlled trial of ind0bufen versus asPirin aftercoronary drug-eluting stentimplantaTION: the OPTION trial [Trial registry record] (2017). Available online at:https://trialsearchwhoint/Trial2aspx?TrialID=ChiCTR-IIR-17013505 (Accessed March 22, 2024).
8.
ChiCtr. A randomized controlled trial of indobufen versus aspirin plus dabigatran after stable coronary artery disease in atrial fibrillation[Trial registry record] (2020). Available online at:https://trialsearchwhoint/Trial2aspx?TrialID=ChiCTR2000029015 (Accessed March 22, 2024).
9.
ChiCtr. A randomized controlled study of indobufen and aspirin in the prevention of 1-year restenosis after off-pump coronary artery bypass[Trial registry record] (2021). Available online at:https://trialsearchwhoint/Trial2aspx?TrialID=ChiCTR2100051267 (Accessed March 22, 2024).
10.
Nct. Effect of NCX4016 on Walking Distance in Patients With Peripheral Arterial Occlusive Disease (PAOD)[Trial registry record] (2010). Available online at:https://clinicaltrialsgov/show/NCT01256775 (Accessed March 22, 2024).
11.
Nct. GaStroEsophageal effeCt of indobUfen Versus aspiRin in Patients Undergoing Dual antiplatElet Therapy[Trial registry record] (2019). Available online at:https://clinicaltrialsgov/show/NCT04129008 (Accessed March 22, 2024).
12.
Nct. Effect of Indobufen and Aspirin on Platelet Aggregation and Long Term Prognosis in Patients With Coronary Heart Disease[Trial registry record] (2020). Available online at:https://clinicaltrialsgov/show/NCT04308551 (Accessed March 22, 2024).
13.
Nct. A Comparative Study of Indobufen and Aspirin in Patients With Coronary Atherosclerosis[Trial registry record] (2021). Available online at:https://clinicaltrialsgov/show/NCT05105750 (Accessed March 22, 2024).
14.
Shi Q-P Luo X-Y Zhang B Wang X-G Zhao J Xie Q-F et al Effect of indobufen vs. aspirin on platelet accumulation in patients with stable coronary heart disease after percutaneous coronary intervention: an open-label crossover study [article]. Front Pharmacol. (2022) 13:1–10. 10.3389/fphar.2022.950719
15.
Yang M Ye Z Mei L Ullah I Tan C Wang G et al Pharmacodynamic effects of indobufen compared with aspirin in patients with coronary atherosclerosis [article; early access]. Eur J Clin Pharmacol. (2021) 77(12):1815–23. 10.1007/s00228-021-03177-y
16.
Rajah SM Nair U Rees M Saunders N Walker D Williams G et al Effects of antiplatelet therapy with indobufen or aspirin-dipyridamole on graft patency one year after coronary artery bypass grafting [article]. J Thorac Cardiovasc Surg. (1994) 107(4):1146–53. English. 10.1016/S0022-5223(94)70392-2
17.
Cataldo G Heiman F Lavezzari M Marubini E . Indobufen compared with aspirin and dipyridamole on graft patency after coronary artery bypass surgery: results of a combined analysis [article]. Coron Artery Dis. (1998) 9(4):217–22. English. 10.1097/00019501-199809040-00007
18.
Bai C Li JX Yu Y Liu R Gao MX Zhang F Li HY . A randomized controlled trial of indobufen versus aspirin in the prevention of bridging restenosis after coronary artery bypass grafting [article]. Chin J Cardiol. (2022) 50(5):466–70. Chinese. 10.3760/cma.j.cn112148-20210701-00560
19.
Wu H Xu L Zhao X Zhang H Cheng K Wang X et al Indobufen or aspirin on top of clopidogrel after coronary drug-eluting stent implantation (OPTION): a randomized, open-label, end point-blinded, noninferiority trial [article]. Circulation. (2023) 147(3):212–22. 10.1161/circulationaha.122.062762
20.
Chen L . Clinical analysis of treatment on the unstable angina pectoris with indobufen and clopidogrel. China Health Industry. (2014) 06(3):105–8. 10.16659/j.cnki.1672-5654.2014.18.056
21.
Zhou J Q H . Clinical observation of indobufen combined with clopidogrel in the treatment of non-ST-segment elevation acute coronary syndrome. Chin J Integr Med Cardio-/Cerebrovasc Dis. (2011) 9(2):3.
22.
Lv C Y C Wanlin X . Comparison in efficacy and safety of indobufen and aspirin in treatment of unstable angina pectoris. Chin J Evid Based Cardiovasc Med. (2021) 13(12):1093–495. 10.3969/j.issn.1674-4055.2021.12.21
23.
Wen J-LC-Y . To explore the prevention effect of indobufen on PCI patients with coronary artery restenosis. J Hunan Univer Tradit Chin Med. (2012) 32(10):29–32. 10.3969/j.issn.1674-070X.2012.10.013.029.03
24.
Zhang L . Clinical observation of indobufen combined with clopidogrel in the treatment of non-ST-segment elevation acute coronary syndrome. China Prac Med. (2013) 8(6):2. 10.14163/j.cnki.11-5547/r.2013.06.107
25.
Morocutti C Amabile G Fattapposta F Nicolosi A Matteoli S Trappolini M et al Indobufen versus warfarin in the secondary prevention of major vascular events in nonrheumatic atrial fibrillation. SIFA (studio italiano fibrillazione atriale) investigators. Stroke. (1997) 28(5):1015–21. 10.1161/01.str.28.5.1015
26.
Yang Xia LW Ken C Shujie D Suodi Z . Efficacy and safety of indobuprofen tablets for prevention and treatment of ischemic cardio-cerebrovascular disease: a meta-analysis. Chin J Clin Pharmacol. (2017) 33(04):359–62. 10.13699/j.cnki.1001-6821.2017.04.019
27.
Xu Rongbin YJ Shen H Feng S Jun M . Meta-analysis of the efficacy and safety of indobuprofen in the prevention of cardiovascular and cerebrovascular events. Chin J Evid-Based Cardiovasc Med. (2017) 9(05):532–8.
28.
Xia T Wanjie G Liying Z Luwen S Sheng H . Mesh meta and pharmacoeconomic evaluation of indobuprofen in atrial fibrillation. Chin J Hosp Pharm. (2019) 39(10):1071–5. 10.13286/j.cnki.chinhosppharmacyj.2019.10.17
Summary
Keywords
aspirin, indobufen, coronary heart disease, efficacy, meta-analysis
Citation
Zhang X, Yan Q, Jiang J, Luo H and Ren Y (2024) Safety and efficacy of aspirin and indobufen in the treatment of coronary heart disease: a systematic review and meta-analysis. Front. Cardiovasc. Med. 11:1412944. doi: 10.3389/fcvm.2024.1412944
Received
06 April 2024
Accepted
01 August 2024
Published
15 August 2024
Volume
11 - 2024
Edited by
Juncheng Wei, Temple University, United States
Reviewed by
Peerawat Jinatongthai, Ubon Ratchathani University, Thailand
Hector A. Cabrera-Fuentes, University of Giessen, Germany
Updates
Copyright
© 2024 Zhang, Yan, Jiang, Luo and Ren.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Hua Luo, 18732196660@163.com Yu Ren reny4147@enzemed.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.