- Division of Cardiovascular Medicine, Department of Clinical Sciences, Danderyd Hospital, Karolinska Institutet, Stockholm, Sweden
Background: Methods to assess aortic stiffness are not validated during ongoing atrial fibrillation (AF) We aimed to determine whether aortic stiffness can be assessed reliably in patients during AF.
Methods and results: Carotid-to-femoral and aortic pulse wave velocity (cf/aoPWV), central blood pressure (BP), and augmentation index (AIx) were assessed by a two-site applanation method and a one-site cuff-based oscillometric method in 40 patients with persistent AF and repeated after cardioversion to SR. Mean age was 63 ± 8 years, 73% male, 50% hypertensive. For the two-site method, cfPWV values were slightly higher in AF than in SR (9.3 ± 1.8 vs. 8.5 ± 1.6 m/s, p < 0.001), whereas the one-site method provided similar values in AF and SR (10.1 ± 1.5 vs. 10.0 ± 1.8 m/s).The variability indices from the device was higher in AF for the two-site method (SD 2.5 ± 1.7 vs. 1.0 ± 0.5 m/s, p < 0.001) but similar in AF and SR with the one-site method (SD 0.7 ± 0.2 vs. 0.6 ± 0.2 m/s). Both methods yielded higher central BP (+4.8/+6.6 and +4.1/+5.7 mm Hg) and lower Aix (−6.8 and −9.1 mm Hg) in AF.
Conclusions: Aortic stiffness can be assessed during AF. Both methods yielded higher central BP and lower AIx in AF, but similar results for PWV in AF and SR, also when adjusted for BP changes. The two-site method showed high variability necessitating repeated measurements. The one-site method showed lower device-calculated variability and needed fewer repeated measurements.
1 Introduction
The most common underlying cause of atrial fibrillation (AF) is hypertension (1, 2), and these diseases combined entail a multifold risk of future cardiovascular events (2–4). The prevailing theory is that hypertension accounts for an increased left ventricular load, inducing stiffening and left ventricular hypertrophy, and thereby left atrial dilatation and fibrosis (2, 5). While studies show an association between left ventricular hypertrophy and AF (6), and pathological left atrial function in hypertensive subjects (7), the pathophysiology of the transition to AF is less well studied. Some studies suggest that an increased aortic stiffness may be a more important determinant than peripheral blood pressure (BP) in this process (8, 9). This is supported by studies showing an independent relation between aortic stiffness and future new onset AF (10–12).
Few studies have assessed the importance of aortic stiffness in patients with a diagnosis of AF. One important reason why, is that current methods to assess aortic stiffness have not been validated and are not recommended for patients with ongoing AF (5). Carotid-to-femoral pulse wave velocity (cfPWV) measured by applanation tonometry at two vascular sites is the most validated non-invasive measure of aortic stiffness in terms of prognostic importance (13). This method use ECG triggered time differences between the two vascular sites, and requires a stable heart rhythm for at least 10–20 s (14, 15). Thus, studies in patients with AF using this technique were performed after interventions to restore sinus rhythm (SR) (16, 17). Some studies have also used methodss based on multiple cuffs to estimate systemic arterial stiffness (18). They are, however, not validated for use during arrhythmia, and have demonstrated limited relationship to aortic stiffness (19).
Single cuff-based oscillometric devices use the arterial pressure curve during occlusion to identify the forward- and the reflected arterial pressure peaks, to estimate aortic PWV (aoPWV) in a beat to-beat manner. Such methods to assess aoPWV have been validated to non-invasive and invasive measurements of cfPWV (20, 21). The results using this method relate to the extent of atherosclerotic disease in healthy individuals (22), and preliminary findings show prediction of cardiovascular morbidity and mortality, and all-cause mortality (23, 24).
Non-invasive reliable methods to assess large artery vascular function during AF are needed to further understand the role of aortic stiffness in patients with AF. Beat-to-beat variations with BP measurements during AF show regression towards the mean (25), suggesting that a technique based on several repeated measurements of aortic stiffness indices could be an option during arrhythmia. This study aimed first to study whether aortic stiffness measurements can be performed during persistent AF, and second to assess the reliability and variability of measures of aortic stiffness during AF and after successful electric cardioversion to SR. Thus, intra-individual comparisons in AF and in SR of repeated measurements of cfPWV, central BP and augmentation index (AIx) were performed and indices of variability of measurements were evaluated with a two-site ECG-triggered applanation-based methodology. In addition, we performed repeated beat-to-beat assessments of aoPWV, central BP and AIx with a one-site single cuff-based oscillometric device.
2 Methods
2.1 Study population
In all, 45 patients with persistent AF scheduled for electric cardioversion at Danderyd University Hospital, Stockholm, Sweden, were invited to participate and accepted participation. Measurements were performed during persistent AF within 1 week before scheduled electric cardioversion, and repeated during SR, between 2 h and 1 week after electric cardioversion. We excluded 5 patients who did not obtain stable SR after the procedure. The study was approved of by the National Ethics Review Authority (# 2020-06355) and was performed in line with Helsinki Declarations of 1974 (as revised in 2013). All patients provided their informed consent.
2.2 Procedures
All investigations were performed in the supine position at rest in a quiet room at the Cardiovascular Research Laboratory, Danderyd University Hospital, Stockholm, Sweden. Patients were asked to continue with their prescribed medications but refrain from caffeine and nicotine. Heart rhythm was determined by a standard 12-lead ECG (Cardiolex Medical AB, Solna Sweden) before further examination. Brachial BP was measured with an oscillometric device (Arteriograph, TensioMed Kft, Budapest, Hungary) and an appropriate cuff size as a mean of three recordings. Mean arterial pressure (MAP) was obtained as calculated from the device. Weight and height were measured, and body mass calculated as weight/height2. Medical history was reported by the patients and verified against electronic health records.
2.2.1 Assessment of aortic stiffness
Assessments were performed with the two-site ECG-triggered device (SphygmoCor AtCor Pty, West Ryde, NSW, Australia) using applanation tonometry (Millar Instruments, Houston, TX, USA) to determine a pulse waveform from the right radial, carotid and femoral arteries, as described in detail elsewhere (14, 26). The integrated software determined central BP and calculated AIx as the ratio between augmentation pressure (augmentation from reflected wave to late systolic peak) and pulse pressure (PP). The cfPWV was determined by subsequent registrations from the carotid and femoral artery, using the ECG-triggered time difference as pulse wave travel time and the distance calculated by the subtracted distance method (27). We used 10 s recordings from each site, which is within the shorter recommended interval (15), since longer registrations yielded too few approved recordings due to the irregular rhythm. An operator index, given by the software as an indicator of the variability of recorded wave forms, (average pulse height variation, diastolic variation, shape deviation and pulse length variation) of >75% rendered an approved measurement for pulse wave analysis by the device. A standard deviation (SD) given by the software as an indicator of variability in measurements during the registration period of cfPWV <10% was needed for an approved recording. A “not measurable” recording indicated the inability of the device to find a sufficiently stable wave form for calculations.
We also used a one-site beat-to-beat single cuff-based oscillometric device (Arteriograph, TensioMed Kft, Budapest, Hungary). The device uses upper arm suprasystolic vascular occlusion and a piezoelectric sensor to register the forward (left ventricular ejection) and reflected wave form of the central pulse wave, as described in detail elsewhere (21, 26). The torniquet (applied on the right arm) automatically inflates and repeats the assessment of a series of pulse waves/heart beats 3 times. For each inflation, 3 consecutive pulse waves are averaged. From these assessments, the integrated software determined central BP and calculated AIx (as described above). The aoPWV was estimated, by using the return time from forward to reflected pulse wave and the distance from the suprasternal notch to the symphysis (direct measure). Standard deviation (SD) was given by the software, comparing the 3 repeated averaged values of aoPWV. A difference of <1 m/s was needed for an approved recording by the device. A “not measurable” recording (no calculation performed) indicated inability of the device to find a sufficiently stable wave form for calculations.
2.2.2 Number of measurements for each patient
While 2–3 approved measurements are generally recommended for the SphygmoCor and the Arteriograph, this study aimed to evaluate the reliability of measurements in conditions with assumed increased variability due to AF. Thus, we registered the number of all approved, not approved and not measurable recordings for each patient. We aimed for 5 approved measurements with each device on each visit; however, no more than 10 attempts were performed due to the potential discomfort for the patient.
2.3 Statistical analysis
Data are presented as mean values ± SD, or as fractions (%), where appropriate. Power calculations for paired comparisons (2 sided α 0.05, β 0.80) to demonstrate a difference of 1 m/s in PWV with a SD of 1.5 m/s required 21 patients. Intraindividual comparisons between measurements in AF and SR were performed by Student´s paired t-tests and intraclass correlation coefficients (ICC). To evaluate the potential influence of BP and heart rate (HR) on assessments of stiffness two models were performed. First, the association between stiffness indices (PWV and AIx) and central BP and HR in AF (visit 1) and in SR (visit 2) were investigated by linear regression using stiffness indices as dependent and central BP and HR as independent variables, adjusted for age, body mass index, and concomitant disease (hypertension, diabetes, ischemic heart disease). Second, the impact of changes in central BP (MAP) and HR between visit 1 and visit 2 were investigated, by calculating the difference of these parameters between the visits (value in AF, visit 1, minus the value in SR, visit 2). Linear regressions were then performed with stiffness measurements in AF as independent, difference for central MAP and HR as covariates, and stiffness measurements in SR as dependent variables. SPSS version 27 (IBM Corp. IBM SPSS Statistics for Windows. Armonk, NY, USA), was used. The significance level was set to a two-sided probability (p) < 0.05.
3 Results
3.1 General
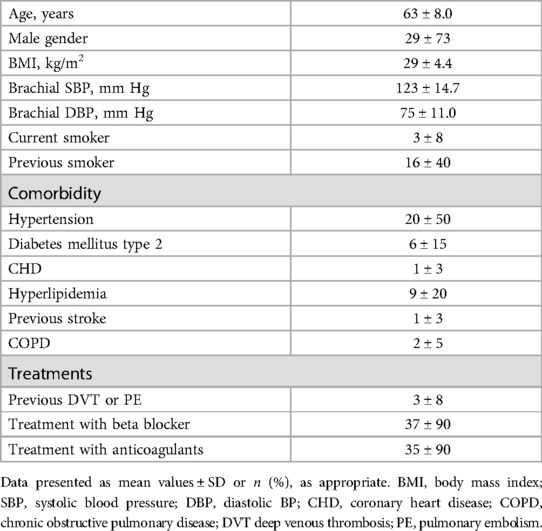
Baseline characteristics are presented in Table 1. Changes in medications between visits 1 and 2 occurred in 6 patients (beta blocker dose reduced in 4, digoxin dose reduced in 1, and angiotensinogen receptor blocker dose increased in 1). During AF, PWV could not be evaluated with the Sphygmocor in 2 patients, due to “not measurable” recordings indicated by the device (Table 2).
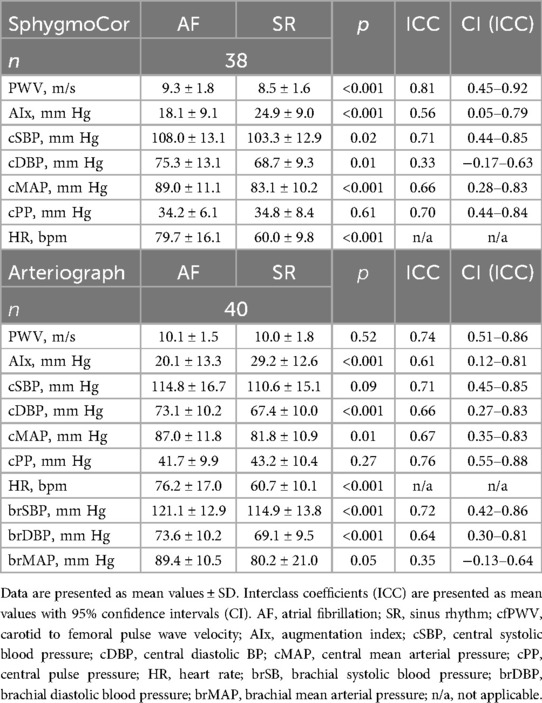
Table 2. Measurements in atrial fibrillation and sinus rhythm by the two-site methodology (SphygmoCor) and one site methodology (Arteriograph).
3.2 Assessments of central BP and AIx
Central BP measurements (Table 2) showed lower central systolic and diastolic BP values in SR than in AF for the two-site methodology (SphygmoCor; −4.8 ± 12.0/−6.6 ± 14.1 mm Hg) and for the one-site methodology (Arteriograph; −4.1 ± 15.0/−5.7 ± 9.5 mm Hg). As expected, this rendered a reduction in central MAP in SR, as compared to AF for both methods, while central pulse pressure (PP) remained unchanged, due to the equal reductions in both SBP and DBP (Table 2). Brachial BP values are also given in Table 2 for comparative reasons. AIx values were higher in SR, as compared to AF, for both the SphygmoCor (+6.8 ± 9.1 mm Hg) and the Arteriograph (+9.1 ± 12.4 mm Hg) (Table 2).
3.2.1 Associations of central MAP and HR to AIx in AF and in SR
Linear regressions to assess the independent (controlled for age, body mass index, and concomitant disease) relation of central MAP and HR to AIx in AF and in SR were performed. An independent relation between HR (but not central MAP) and AIx was seen both in AF and in SR with the SphygmoCor (in AF: β = −0.62, in SR: β = −0.57, both p < 0.001). With the Arteriograph there were independent relations of both central MAP and HR to AIx in both rhythms (MAP in AF: β = 0.32, p = 0.03, HR in AF β = −0.26, p = 0.04; MAP in SR β = 0.28, p = 0.04, HR in SR β = −0.43 p = 0.003).
3.2.2 Assessments of the impact of changes in central MAP and HR between visits for AIx
To control for the changes in central MAP and HR between visits 1 (AF) and 2 (SR), the difference in these values between visits was used as covariates in linear regressions using AIx in AF as independent, and AIx in SR as dependent variable. Relations between AIx in AF and in SR were strengthened by adjusting for the difference in central MAP and HR (SphygmoCor: β from 0.50 to 0.77; Arteriograph: β from 0.54 to 0.85). AIx in SR was also dependent on the difference in HR with the SphygmoCor (β = 0.61, p < 0.001), AIx in SR by the Arteriograph was dependent on the difference in both central MAP and HR (β = 0.41 and 0.51, both p < 0.001).
3.3 Assessments of PWV
Measurements of cfPWV by the two-site methodology (SphygmoCor) were 0.83 ± 1.1 m/s higher during AF, as compared to SR (Table 2, Figure 1). Assessments of aoPWV using one-site methodology (Arteriograph) were similar during AF and SR (0.15 ± 1.5 m/s difference) (Table 2, Figure 1). ICC results were similar with both methodologies (Table 2).
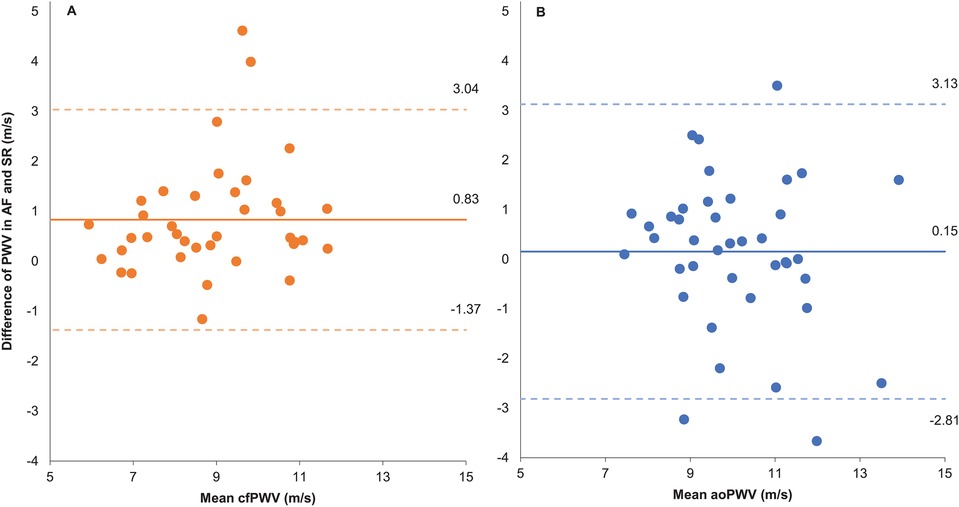
Figure 1. Bland-Altman plot. (A) Two-site methodology. (B) One-site methodology. Difference of pulse wave velocity (PWV) measured as AF – SR and mean PWV as the average PWV in AF and SR. AF, atrial fibrillation; SR, sinus rhythm; cfPWV, carotid to femoral PWV measured by two site method; aoPWV, aortic PWV estimated by one site method.
3.3.1 Associations of central MAP and HR to PWV in AF and in SR
Linear regressions to assess the independent (controlled for age, body mass index, and concomitant disease) relation of central MAP and HR to PWV in AF and in SR were performed. Central MAP and HR were related to cfPWV with the SphygmoCor in SR but not in AF (MAP in AF: β = 0.32, p = 0.09, HR in AF: β = 0.11, p = 0.50, MAP in SR: β = 0.48, p = 0.003; HR in SR: β = 0.30, p = 0.05). For assessed aoPWV with the Arteriograph these relations were slightly weaker to central MAP (MAP in AF: β = 0.25, p = 0.15; MAP in SR: β = 0.39, p = 0.01), and were not related to HR (HR in AF: β = 0.20, p = 0.20; HR in SR: β = 0.24, p = 0.10).
3.3.2 Assessments of the impact of changes in central MAP and HR between visits for PWV
To control for the changes in central MAP and HR between visits 1 (AF) and 2 (SR), the difference in these values between visits was used as covariates in linear regressions using PWV in AF as independent, and PWV in SR as dependent variable. There were no changes in the strength of the relation between neither cfPWV nor aoPWV in AF and in SR when adjusting for changes in central MAP and HR with either device. However, the difference in central MAP was related to cfPWV in SR with the SphygmoCor (β = 0.27, p = 0.01).
3.4 Variability of measurements by the device algorithms and by number of repeated measurements
With the SphygmoCor, intraindividual variability (expressed and device calculated as Operator Index and SD; see Methods) was greater in AF than in SR, and was reflected in the high numbers of performed and low numbers of accepted measurements in AF (Table 3, Figure 2). The number of “not measurable” readings was low and similar in AF and SR.
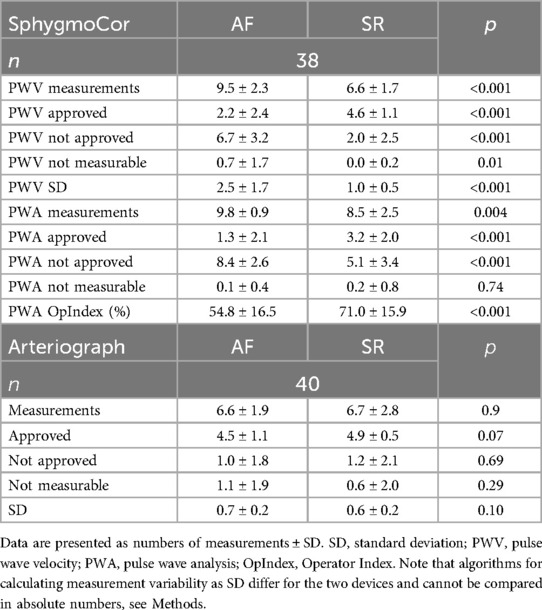
Table 3. Numbers of performed measurements (mean), and device calculated standard deviations and operator Index.
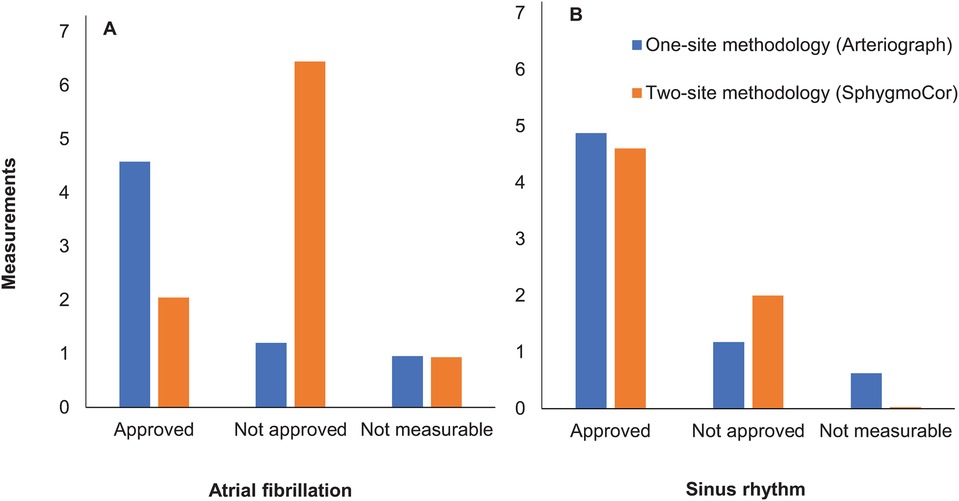
Figure 2. Number of performed measurements by the two methods in atrial fibrillation (A) vs. sinus rhythm (B). Mean values of numbers of approved, not approved and not measurable recordings for pulse wave velocity are presented.
The intraindividual variability with the Arteriograph (expressed and device calculated as SD; see Methods), was similar in AF and SR, with lower numbers of performed and higher numbers of accepted measurements (Table 3, Figure 2). The numbers of “not measurable” readings in AF and SR were low.
The number of measurements to achieve a reliable estimate of cfPWV and aoPWV appeared to be 6–7 with two-site methodology and 4 with the one-site methodology (Figure 3).
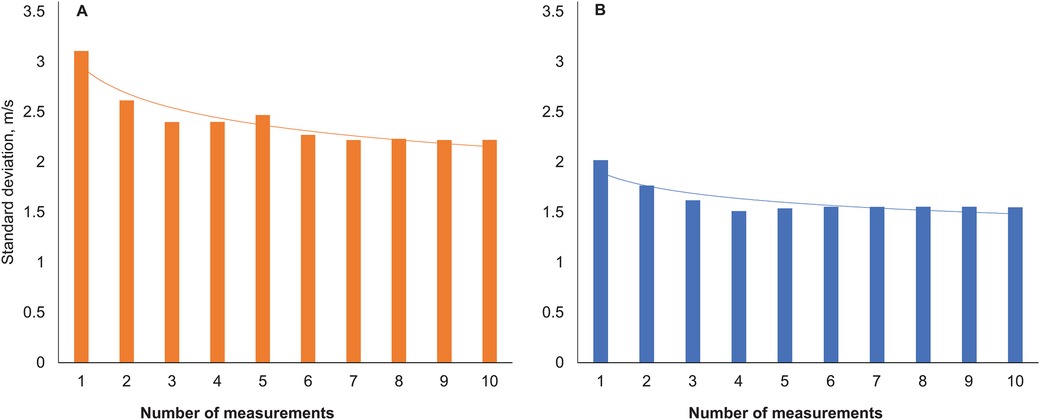
Figure 3. Standard deviations of pulse wave velocity with increasing numbers of measurements. Two-site method (A), one-site method (B).
4 Discussion
We studied whether aortic stiffness can be assessed reliably in patients during AF. A two-site ECG-triggered device using applanation tonometry (SphygmoCor) and a one-site methodology using oscillometric technique (Arteriograph) were employed in patients during AF and the results were compared intra-individually to recordings in SR after electric cardioversion. Our results show a decrease in central BP and HR, an increase in AIx, and similar values of PWV, suggesting that aortic stiffness can be non-invasively assessed during AF, provided a sufficient number of recordings are performed.
There are few studies on the reliability of measuring indices of aortic stiffness in AF. In the present study central systolic and diastolic BP decreased after electric cardioversion to SR. Results from intra-individual comparisons of brachial BP in AF and SR are not consistent but suggest a trend towards rhythm-dependent changes with lower systolic and higher diastolic BP in AF (28–31). If these changes are due to hemodynamic reasons or measurement errors remains unclear (32). However, our recent findings from intra-individual comparisons during ambulatory BP monitoring indicate that the beat-to-beat variations in BP in AF, as compared to SR, regress towards the mean with averaged values (25).
The calculation of AIx is dependent on central BP and, as expected, AIx increased after cardioversion from AF to SR in the present study. This is in agreement with an earlier report also using the SphygmoCor (33). Regression analyses showed a clear dependency on both BP and HR for AIx results, and relations between AIx in AF and SR were stronger in the adjusted models. Thus, an increase in AIx during SR, as compared to AF, should probably be interpreted as due to changes in BP and HR between visits. Of note, our findings on AIx with the two-site and the one-site methodology were consistent.
Caluwé et al. in a study like ours using the SphygmoCor device reported a BP dependent 1.1 m/s decrease in cfPWV after cardioversion from AF to SR (33). Those authors concluded that cfPWV measurements were reliable with the SphygmoCor, when adjusted for BP levels. Our study confirms and extends their findings by employing also a one-site single cuff-based oscillometric technique (Arteriograph) with beat-to-beat assessment of aortic stiffness measurements. Both methodologies provided similar results for PWV in AF and in SR with independent strength adjusted for the difference in BP and HR between AF and SR visits.
Our results with the two-site method on device-calculated intraindividual variability (both Operator Index for pulse waves and SD for measured PWV) (Figure 3) suggest that 6–7 repeated measurements in AF may be needed to reach stable cfPWV values. High indices of variability and low numbers of accepted measurements also in SR with this method was explained by many patients with frequent supraventricular extrasystoles during SR, which was almost as challenging for this procedure as persistent AF. Results with the one-site beat-to-beat method showed similar device-calculated intraindividual variability in AF and SR, an expected difference to the SphygmoCor device since the SphygmoCor algorithm is more sensitive to variability (see Methods). These results suggest that 4 repeated measurements in AF may be needed to assess indices of stiffness with the one-site beat-to-beat method.
There are some strengths with this study. We confirm that non-invasive assessment of aortic stiffness is feasible during AF and we provide new information on the use of a one-site beat-to-beat single cuff-based oscillometric method. Intraindividual comparisons in AF and SR with a short time frame between visits renders high statistical power and accounts for reducing potential confounding influence. There are also important limitations to consider. First, some individuals had greater visit to visit differences in the measured variables, which did not seem to be due to the magnitude of HR and BP changes. This study was not designed to determine precision on an individual level, which would have required a larger sample for further stratification on variables. However, the techniques investigated are primarily used in research, where comparisons on group level are more relevant. Second, uncertainties in BP measurement during AF affects the interpretation of our results. To our knowledge, no studies have yet reported invasive reference values for intraindividual comparisons of measures of BP in AF and SR. However, several non-invasive studies support the notion of regression towards the mean, making averaged BP values, especially MAP, and BP variability indices comparable in AF and SR (25, 34, 35). Third, the substantial difference in algorithms for calculating variability by the devices makes comparisons between methodologies difficult. Finally, there are no invasive studies of intraindividual comparison of PWV in AF and SR, and comparisons of invasive and non-invasive assessment of PWV during AF are missing, making assumptions for both comparisons in AF and SR and true variability of measurements in AF difficult. However, in the light of results indicating a regression towards the mean with BP in AF, it is plausible to believe that the same phenomenon might be true for aortic stiffness measures.
In conclusion, we show that it is feasible to measure aortic stiffness during AF with sufficient reliability to add useful information for cardiovascular risk assessment. The ECG-triggered two-site tonometry-based method (SphygmoCor) showed higher device calculated variability with lower numbers of approved readings necessitating several repeated measurements, than a one-site beat to beat oscillometric method (Arteriograph), using device calculated variability based on comparisons of several averaged values.
Data availability statement
The datasets presented in this article are not readily available as the dataset will only be shared for collaborative purposes to other academic researchers, and can for this purpose be used for further research questions/analyses, as long as these align with ethics approval for the study. Requests to access the datasets should be directed toa3Jpc3RpbmEubHVuZHdhbGxAa2kuc2U=.
Ethics statement
The studies involving humans were approved by National Ethics Review Authority. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
KL: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. MA: Data curation, Investigation, Methodology, Project administration, Software, Writing – original draft, Writing – review & editing. TK: Conceptualization, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. JS: Conceptualization, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The study was supported by the Heart Foundation of Danderyd University Hospital, Karolinska Institutet Research Foundations, the Swedish Medical Association, and Region Stockholm (NSV project).
Acknowledgments
The authors wish to thank Biomedical scientist Eva Andersson (in memoriam) and RN Liselotte Persson for expert technical assistance and coordination of the study.
Conflict of interest
KL has received advisory board and speaker honoraria from NovoNordic, Boeringer-Ingelheim and Bayer, all outside the submitted work. TK declares research grants to Karolinska Institutet from Medtronic and ReCor Medical, all outside the submitted work. JS has received speaker honoraria from AstraZeneca, Bayer, Boehringer-Ingelheim, Sanofi, and NovoNordic, and is a minor shareholder in Beat Vascular Health, and declares research grants to Karolinska Institutet from AstraZeneca and Medtronic, all outside the submitted work.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kornej J, Börschel CS, Benjamin EJ, Schnabel RB. Epidemiology of atrial fibrillation in the 21st century: novel methods and new insights. Circ Res. (2020) 127(1):4–20. doi: 10.1161/CIRCRESAHA.120.316340
2. Manolis AJ, Rosei EA, Coca A, Cifkova R, Erdine SE, Kjeldsen S, et al. Hypertension and atrial fibrillation: diagnostic approach, prevention and treatment. Position paper of the working group “hypertension arrhythmias and thrombosis’ of the European society of hypertension. J Hypertens. (2012) 30(2):239–52. doi: 10.1097/HJH.0b013e32834f03bf
3. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist C, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European association for cardio-thoracic surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European heart rhythm association (EHRA) of the ESC. Eur Heart J. (2021) 42(5):373–498. doi: 10.1093/eurheartj/ehaa612
4. Staerk L, Sherer JA, Ko D, Benjamin EJ, Helm RH. Atrial fibrillation: epidemiology, pathophysiology, and clinical outcomes. Circ Res. (2017) 120(9):1501–17. doi: 10.1161/CIRCRESAHA.117.309732
5. Vio R, Giordani AS, Stefil M, Madine J, Fairbairn T, Themistoclakis S, et al. Arterial stiffness and atrial fibrillation: shared mechanisms, clinical implications and therapeutic options. J Hypertens. (2022) 40(9):1639–46. doi: 10.1097/HJH.0000000000003223
6. Chatterjee S, Bavishi C, Sardar P, Agarwal V, Krishnamoorthy P, Grodzicki T, et al. Meta-analysis of left ventricular hypertrophy and sustained arrhythmias. Am J Cardiol. (2014) 114(7):1049–52. doi: 10.1016/j.amjcard.2014.07.015
7. Losi MA, Izzo R, De Marco M, Canciello G, Rapacciuolo A, Trimarco V, et al. Cardiovascular ultrasound exploration contributes to predict incident atrial fibrillation in arterial hypertension: the campania salute network. Int J Cardiol. (2015) 199:290–5. doi: 10.1016/j.ijcard.2015.07.019
8. Przewlocka-Kosmala M, Jasic-Szpak E, Rojek A, Kabaj M, Sharman JE, Kosmala W. Association of central blood pressure with left atrial structural and functional abnormalities in hypertensive patients: implications for atrial fibrillation prevention. Eur J Prev Cardiol. (2019) 26(10):1018–27. doi: 10.1177/2047487319839162
9. Roetker NS, Chen LY, Heckbert SR, Nazarian S, Soliman EZ, Bluemke DA, et al. Relation of systolic, diastolic, and pulse pressures and aortic distensibility with atrial fibrillation (from the multi-ethnic study of atherosclerosis). Am J Cardiol. (2014) 114(4):587–92. doi: 10.1016/j.amjcard.2014.05.041
10. Almuwaqqat Z, Claxton JS, Norby FL, Lutsey PL, Wei J, Soliman EZ, et al. Association of arterial stiffness with incident atrial fibrillation: a cohort study. BMC Cardiovasc Disord. (2021) 21(1):247. doi: 10.1186/s12872-021-02057-8
11. Chen LY, Leening MJ, Norby FL, Roetker NS, Hofman A, Franco OH, et al. Carotid intima-media thickness and arterial stiffness and the risk of atrial fibrillation: the atherosclerosis risk in communities (ARIC) study, multi-ethnic study of atherosclerosis (MESA), and the Rotterdam study. J Am Heart Assoc. (2016) 5(5):e002907. doi: 10.1161/JAHA.115.002907
12. Shaikh AY, Wang N, Yin X, Larson MG, Vasan RS, Hamburg NM, et al. Relations of arterial stiffness and brachial flow-mediated dilation with new-onset atrial fibrillation: the Framingham heart study. Hypertension. (2016) 68(3):590–6. doi: 10.1161/HYPERTENSIONAHA.116.07650
13. Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, et al. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. (2014) 63(7):636–46. doi: 10.1016/j.jacc.2013.09.063
14. Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. (2006) 27(21):2588–605. doi: 10.1093/eurheartj/ehl254
15. Spronck B, Terentes-Printzios D, Avolio AP, Boutouyrie P, Guala A, Jerončić A, et al. 2024 recommendations for validation of noninvasive arterial pulse wave velocity measurement devices. Hypertension. (2024) 81(1):183–92. doi: 10.1161/HYPERTENSIONAHA.123.21618
16. Pauklin P, Eha J, Tootsi K, Kolk R, Paju R, Kals M, et al. Atrial fibrillation is associated with increased central blood pressure and arterial stiffness. J Clin Hypertens (Greenwich). (2021) 23(8):1581–7. doi: 10.1111/jch.14323
17. Tsaknakis K, Xanthopoulos A, Papamichalis M, Chamaidi A, Tsaknakis T, Skoularigis J, et al. Relationship of CHA(2)DS(2)-VASc score to left atrial volume and arterial stiffness in patients with atrial fibrillation. Hellenic J Cardiol. (2020) 61(1):54–6. doi: 10.1016/j.hjc.2019.07.002
18. Nakamura K, Takagi T, Kogame N, Hashimoto H, Asami M, Toyoda Y, et al. The association of cardio-ankle vascular Index (CAVI) with biatrial remodeling in atrial fibrillation. J Atheroscler Thromb. (2021) 28(6):590–603. doi: 10.5551/jat.57737
19. Gavish B, Izzo JL Jr. Arterial stiffness: going a step beyond. Am J Hypertens. (2016) 29(11):1223–33. doi: 10.1093/ajh/hpw061
20. Baulmann J, Schillings U, Rickert S, Uen S, Düsing R, Illyes M, et al. A new oscillometric method for assessment of arterial stiffness: comparison with tonometric and piezo-electronic methods. J Hypertens. (2008) 26(3):523–8. doi: 10.1097/HJH.0b013e3282f314f7
21. Horvath IG, Nemeth A, Lenkey Z, Alessandri N, Tufano F, Kis P, et al. Invasive validation of a new oscillometric device (arteriograph) for measuring augmentation index, central blood pressure and aortic pulse wave velocity. J Hypertens. (2010) 28(10):2068–75. doi: 10.1097/HJH.0b013e32833c8a1a
22. Bocskei RM, Benczur B, Muller V, Bikov A, Szekely A, Kahan T, et al. Oscillometrically measured aortic pulse wave velocity reveals asymptomatic carotid atherosclerosis in a middle-aged, apparently healthy population. Biomed Res Int. (2020) 2020:8571062. doi: 10.1155/2020/8571062
23. Kahan T, Boutouyrie P, Cziráki A, Illyés M, Molnár F, Laurent S, et al. Aortic stiffness measured by a novel oscillometric method independently predicts cardiovascular morbidity and mortality: a study of 4,146 subjects. J Hypertens. (2013) 31 (e-Suppl A):e102.
24. Lenkey Z, Illyés M, Kahan T, Boutouyrie P, Laurent S, Molnár F, et al. Aortic pulse wave velocity measured by an oscillometric device independently predicts all-cause mortality in a cohort of 4,146 subjects. Eur Heart J. (2019) 40(Suppl 1):844. doi: 10.1093/eurheartj/ehz748.0301
25. Lundwall K, Kahan T, Omboni S. Blood pressure in atrial fibrillation and in sinus rhythm during ambulatory blood pressure monitoring: data from the TEMPLAR project. Hypertens Res. (2024) 47(2):435–44. doi: 10.1038/s41440-023-01473-x
26. Jekell A, Kahan T. The usefulness of a single arm cuff oscillometric method (arteriograph) to assess changes in central aortic blood pressure and arterial stiffness by antihypertensive treatment: results from the Doxazosin-Ramipril study. Blood Press. (2018) 27(2):88–98. doi: 10.1080/08037051.2017.1394791
27. Van Bortel LM, De Backer T, Segers P. Standardization of arterial stiffness measurements make them ready for use in clinical practice. Am J Hypertens. (2016) 29(11):1234–6. doi: 10.1093/ajh/hpw084
28. Maselli M, Giantin V, Franchin A, Attanasio F, Tramontano A, De Toni P, et al. Effect of restoring sinus rhythm in hypertensive patients with atrial fibrillation undergoing electrical cardioversion. Blood Press Monit. (2016) 21(6):335–9. doi: 10.1097/MBP.0000000000000211
29. Olbers J, Östergren J, Rosenqvist M, Skuladottir H, Klavebäck S, Ljungman P, et al. Changes in 24-h ambulatory blood pressure following restoration of sinus rhythm in patients with atrial fibrillation. J Hypertens. (2021) 39(2):243–9. doi: 10.1097/HJH.0000000000002623
30. Olsen R, Amlie A, Omvik P. Twenty-four-hour ambulatory blood pressure monitoring in atrial fibrillation. Blood Press Monit. (2002) 7(3):149–56. doi: 10.1097/00126097-200206000-00002
31. Sanders NA, Bertolone C, Jetter TL, Wasmund SL, Croci F, Solano A, et al. Restoring sinus rhythm results in blood pressure reduction in patients with persistent atrial fibrillation and a history of hypertension. J Cardiovasc Electrophysiol. (2012) 23(7):722–6. doi: 10.1111/j.1540-8167.2011.02280.x
32. Xie F, Xu J, Liu H, Li X, Wu Y, Su H. Different impact factors for accurate oscillometric blood pressure measurement between sinus rhythm and atrial fibrillation. J Hum Hypertens. (2021) 35(9):785–90. doi: 10.1038/s41371-021-00512-9
33. Caluwe R, De Vriese AS, Van Vlem B, Verbeke F. Measurement of pulse wave velocity, augmentation index, and central pulse pressure in atrial fibrillation: a proof of concept study. J Am Soc Hypertens. (2018) 12(8):627–32. doi: 10.1016/j.jash.2018.06.016
34. Mehlum MH, Liestøl K, Wyller TB, Hua TA, Rostrup M, Berge E. Blood pressure variability in hypertensive patients with atrial fibrillation in the VALUE trial. Blood Press. (2019) 28(2):77–83. doi: 10.1080/08037051.2018.1524707
Keywords: aortic stiffness, atrial fibrillation, pulse wave velocity, central blood pressure, augmentation index
Citation: Lundwall K, Al Nouh M, Kahan T and Spaak J (2024) Assessment of aortic stiffness during atrial fibrillation: solutions and considerations. Front. Cardiovasc. Med. 11:1449168. doi: 10.3389/fcvm.2024.1449168
Received: 14 June 2024; Accepted: 15 August 2024;
Published: 29 August 2024.
Edited by:
Tlili Barhoumi, King Abdullah International Medical Research Center (KAIMRC), Saudi ArabiaReviewed by:
Marek Rajzer, Jagiellonian University Medical College, PolandAudrey Adji, Victor Chang Cardiac Research Institute, Australia
Copyright: © 2024 Lundwall, Al Nouh, Kahan and Spaak. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kristina Lundwall, a3Jpc3RpbmEubHVuZHdhbGxAa2kuc2U=
†These authors have contributed equally to this work
 Kristina Lundwall
Kristina Lundwall Maria Al Nouh
Maria Al Nouh Thomas Kahan
Thomas Kahan Jonas Spaak
Jonas Spaak