Abstract
Background:
The objective of the study was to analyze and compare the effectiveness and safety of rivaroxaban in patients with atrial fibrillation (AF) and heart failure (HF).
Methods:
The clinical profile and outcomes of the FARAONIC study were indirectly compared with those of the ROCKET-AF trial and other national and international observational registries.
Results:
In FARAONIC, the median age was 73.7 years, 34.1% were women, and the median CHA2DS2-VASc was 4.1. In the rivaroxaban arm of ROCKET-AF in patients with HF, these statistics were 72 years, 39.1%, and 5.1, respectively. In the national/international registries of patients with HF receiving rivaroxaban, these statistics were 74.0–75.3 years, 40.8%–41.4%, and 3.2–4.5, respectively. In the GLORIA-AF (dabigatran) and ETNA-AF (edoxaban) trials, these numbers were 69.9–75.3 years, 39.3%–41.6%, and 3.8–4.4, respectively. Among the HF populations, annualized rates of stroke or systemic embolism were 0.75% in FARAONIC (vs. 1.90% in ROCKET-AF, 0.92%–1.2% in national/international registries with rivaroxaban, 0.82% in GLORIA-AF, and 0.88% in ETNA-AF). Rates of major bleeding in FARAONIC were 1.55% (vs. 1.4%–3.86% in the national/international registries with rivaroxaban, 1.20% in GLORIA-AF, and 1.65% in ETNA-AF).
Conclusion:
In clinical practice, AF patients with HF, anticoagulated with rivaroxaban are old, have many comorbidities and have a high thromboembolic risk. Despite this, rates of adverse events are low.
1 Introduction
Heart failure (HF) and atrial fibrillation (AF) are two common cardiovascular conditions that frequently coexist (1–3). The prevalence of both conditions is increasing globally (4). AF can precipitate HF but it can also be a consequence of HF (1–3, 5). Patients with AF have a nearly fivefold increased risk of HF (6). In HF trials, the prevalence of AF ranges from 10% to 50% (7). Conversely, in clinical trials with direct oral anticoagulants (DOACs), among patients with AF, approximately 27%–65% of patients had HF concomitantly at baseline (8–11).
The concomitance of HF and AF markedly worsens the prognosis (12), and the risk of developing thromboembolic complications compared with the risk of each condition separately (1–3). Importantly, the stroke risk in patients with AF and HF is increased across the entire spectrum of left ventricular ejection fraction (3, 13). In this context, guidelines recommend chronic oral anticoagulation in these patients to reduce the risk of thromboembolic complications (14, 15).
The ROCKET-AF trial showed that compared with warfarin, rivaroxaban was as effective for the prevention of stroke or systemic embolism, had a similar risk of major bleeding, and had a higher risk for major gastrointestinal bleeding, but had a significantly lower risk of intracranial and fatal hemorrhages (16). A specific substudy of the ROCKET-AF trial showed that the benefit of rivaroxaban was independent of HF status at baseline (8). Nevertheless, it is important to ascertain whether these results can be extended to real-life populations (17). The FARAONIC study was a prospective, multicenter, cohort study of patients with AF and HF, chronically treated with rivaroxaban, and aimed to determine the risk factors associated with worsening HF. This study showed that after 24 months of follow-up, approximately 25% of the patients developed HF worsening, and nearly 3% of the patients had a thromboembolic event and major bleeding, with a very low risk of intracranial bleeding and no cases of fatal hemorrhage (Figure 1) (18).
Figure 1
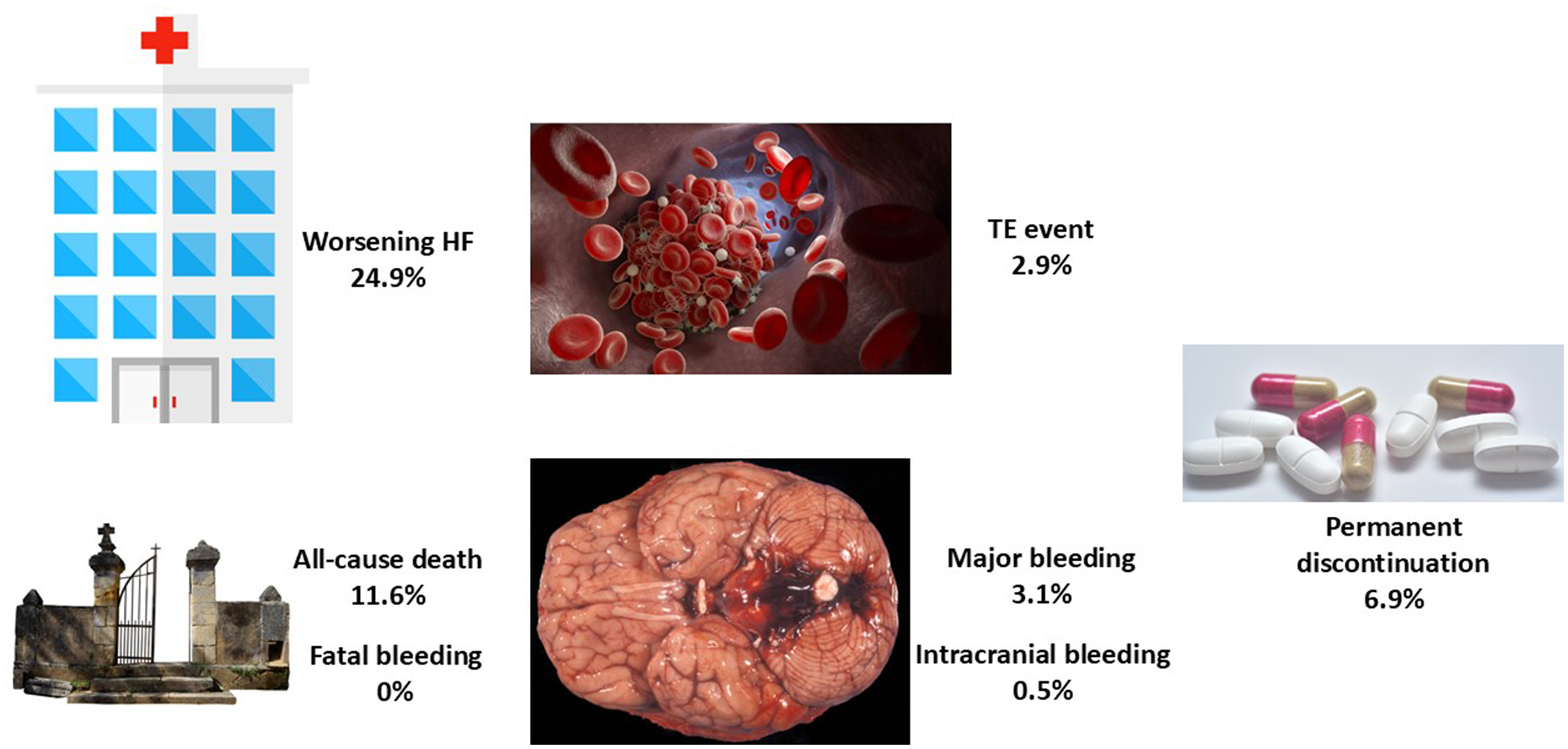
Main events in the FARAONIC registry after 2 years of follow-up. HF, heart failure; TE, thromboembolic event. Figure created with data from Manito et al. (18).
However, in the last few years, new clinical trials and a number of national and international studies on the use of rivaroxaban in clinical practice, according to HF status have been published (19–21). As a result, it is essential to ascertain whether the FARAONIC study results are comparable not only to the ROCKET-AF trial but also to other registries of rivaroxaban-treated patients. The aim of this article was to evaluate, through indirect comparisons between different national or international real-life studies, the clinical profile and outcomes of patients with AF and HF anticoagulated with rivaroxaban. In addition, data from the FARAONIC study were compared with two other observational and prospective studies of patients treated with other DOACs (22, 23).
2 Methods
FARAONIC was a Spanish multicenter, prospective, observational cohort study that included patients with non-valvular AF and chronic HF (regardless of ejection fraction) who received treatment with rivaroxaban for ≥4 months before being enrolled. A total of 672 patients from 71 Spanish centers were recruited, of whom 552 (82.1%) were included in the per-protocol analysis. In total, 51.3% of the patients had HF with preserved ejection fraction, 31.3% HF with reduced ejection fraction, and 17.4% HF with mildly reduced ejection fraction. Patients were followed up over a 2-year period (18).
First, to initially place the efficacy and safety of rivaroxaban in context, the results of phase III clinical trials with DOACs compared with warfarin according to baseline HF status were analyzed. ROCKET-AF was a double-blind clinical trial in which 14,264 patients with AF and a high stroke risk were randomized to rivaroxaban or warfarin. A total of 9,033 (63.7%) patients had HF. HF was defined as a history of HF or a left ventricular ejection fraction <40% (8). RE-LY was a clinical trial that compared two fixed and blinded doses of dabigatran (110 and 150 mg twice daily) with open-label dose-adjusted warfarin in 18,113 AF patients at increased risk for stroke, of whom 4,904 (27.1%) had HF at baseline. HF was defined as the presence of New York Heart Association (NYHA) class II–IV HF symptoms (fatigue and dyspnea) in the 6 months before being enrolled, in patients with a history of previous admission for congestive HF (9). ARISTOTLE was a double-blind randomized trial comparing apixaban with warfarin in patients with AF at risk of stroke. Information on investigator-reported HF and ventricular function was obtained from the trial case report forms and only patients with a report of both HF status and left ventricular function were included in this analysis. A total of 18,201 patients were initially randomized, of which 14,671 (81%) had information on both HF status and left ventricular systolic function. Of these, 18.6% had HF with reduced ejection fraction and 21.9% HF with preserved ejection fraction (10). ENGAGE AF-TIMI 48 was a randomized, double-blind clinical trial comparing edoxaban with warfarin. HF was defined as the presence or previous history of HF stage C or D according to the American College of Cardiology/American Heart Association (ACC/AHA) definition. Patients were classified as HF and NYHA classes I–II, HF and NYHA classes III–IV, and no HF. Patients in ACC/AHA stage B (asymptomatic left ventricular dysfunction) were considered as not having HF. Of the 14,071 patients randomized to warfarin or high-dose edoxaban, 5,926 (42%) had no history of HF at baseline, 6,344 (45%) mild HF, and 1,801 (13%) severe HF (11).
Second, the clinical profile and outcomes of the FARAONIC study were compared with those of two clinical trials (ROCKET-AF and AFIRE) and two national registries (EMIR and US database). In the AFIRE trial, Japanese patients with AF and stable coronary artery disease were randomized to receive rivaroxaban monotherapy or combination therapy with rivaroxaban and an antiplatelet agent. In this study, 2,215 patients were included; 36% (n = 788) had a history of HF (19). EMIR was a non-interventional and observational study that included adults with AF who had been administered rivaroxaban according to clinical practice for ≥6 months before being enrolled. Patients were recruited from 79 Spanish centers and followed up for 2.5 years. The information source was in all cases the medical record and the patient during the routine visits. A total of 1,433 patients were included in the final analysis, of whom 326 (22.7%) had HF at baseline (20). The US database was a retrospective claims database analysis of US Truven MarketScan data that combines two separate databases (a commercial and a Medicare supplemental database) from 1 November 2011 to 31 December 2016. For this study, patients with oral anticoagulant-naïve AF, HF, and ≥12 months of insurance coverage were identified. A total of 3,418 patients who received rivaroxaban were analyzed. Patients were followed up until an event, rivaroxaban discontinuation/switch, insurance disenrollment, or end of follow-up (21).
Finally, to put into context the results of rivaroxaban compared with those of other DOACs, two prospective registries were analyzed (GLORIA-AF and ETNA-AF). GLORIA-AF was a large, international, observational registry program that included patients with newly diagnosed AF at risk of stroke and CHA2DS2-VASc ≥1 in 44 countries from five geographical regions. Among the 4,873 dabigatran-treated patients, 1,169 (24.0%) had HF and 2-year outcomes were reported. HF was defined as NYHA classes II–IV or ejection fraction ≤40% (22). The Global ETNA-AF program included data from multiple prospective, observational, non-interventional regional studies of patients with AF receiving edoxaban for stroke prevention. Data from 27,333 patients, of whom 5,258 had HF history with 2-year annualized rates, were analyzed (23).
Whereas ROCKET-AF, RE-LY, ARISTOTLE, ENGAGE AF-TIMI 48, and AFIRE were randomized clinical trials, FARAONIC, EMIR, GLORIA-AF, and ETNA-AF were observational and prospective studies and the US database was an observational and retrospective study. Biodemographic data, comorbidities, NYHA functional class, HF treatments, and thromboembolic (CHADS2, CHA2DS2-VASc) and bleeding (HAS-BLED) risk were recorded if available. Adverse events, including stroke or systemic embolism, all-cause death, major bleeding, intracranial hemorrhage, and major adverse cardiovascular events (MACEs) were recorded from all studies if available. In addition, the proportion of patients that developed HF worsening (hospitalization or visit to the emergency department) and the proportion of permanent discontinuation of rivaroxaban during the follow-up in the FARAONIC study were also determined.
2.1 Statistical analysis
A descriptive analysis was performed and data were compared numerically (indirect comparisons). Quantitative variables were reported with mean or median, as available, and qualitative variables as relative frequencies (percentages). Events were recorded from the original publication of the clinical trials and registries, including stroke or systemic embolism, all-cause death, major bleeding, intracranial bleeding, and MACEs, when available. The annual incidence of events was calculated in the FARAONIC study and annual rates expressed as events per 100 patient-years were recorded for the rest of studies from the original publications. The data were analyzed using the statistical package SPSS (v18.0 or superior).
3 Results
In the FARAONIC study, at baseline, the mean age was 73.7 ± 10.9 years, 65.9% were men, and 33.9% were considered frail. With regard to AF, 53.9% of patients had permanent AF, CHA2DS2-VASc was 4.1 ± 1.5, and HAS-BLED was 1.6 ± 0.9. Comorbidities were common, as 77.5% had arterial hypertension, 39.1% had previous coronary artery disease, 37.3% had diabetes, and 32.4% had chronic kidney disease. Furthermore, 51.3% of the patients had HF with preserved ejection fraction. Regarding HF treatments at baseline, 90.6% received diuretics, 85.5% a renin-angiotensin system inhibitor (36.7% received an angiotensin-converting enzyme inhibitor, 23.8% an angiotensin II receptor blocker, and 25.0% sacubitril/valsartan), 79.7% a beta blocker, 51.4% an aldosterone antagonist, 23.0% digoxin, and 3.1% ivabradine. After 24 months of follow-up, 11.6% of the patients had died, 2.9% had a thromboembolic event, 3.1% had a major bleeding event, 0.5% had an intracranial bleeding event, and no patient had a fatal hemorrhage (18).
3.1 ROCKET-AF, RE-LY, ARISTOTLE, and ENGAGE AF-TIMI 48 trials, according to baseline HF status
In ROCKET-AF, the patients with HF were younger and had higher levels of hypertension, diabetes, history of myocardial infarction, and thromboembolic risk, but less prior cerebrovascular disease compared to those patients without HF (8). In RE-LY, the patients with HF were younger and had less prior history of cerebrovascular disease and hypertension, but higher levels of diabetes, history of coronary artery disease, and a higher thromboembolic risk (9). In ARISTOTLE, the HF population was younger and had less history of cerebrovascular disease, but higher levels of ischemic heart disease and thromboembolic risk (10). In ENGAGE AF-TIMI 48, the patients with HF were younger and less likely to have prior cerebrovascular disease and diabetes. However, the patients with HF were more likely to have hypertension and had a higher thromboembolic risk (11) (Table 1).
Table 1
| Clinical characteristics | ROCKET-AF (rivaroxaban arm) | RE-LY (dabigatran 110 arm) | RE-LY (dabigatran 150 arm) | ARISTOTLE (overall) | ENGAGE AF-TIMI 48 (overall) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HF (n = 4,530; 64.0%) | No HF (n = 2,551; 36.0%) | HF (n = 1,641; 27.3%) | No HF (n = 4,374; 72.7%) | HF (n = 1,640; 27.0%) | No HF (n = 4,436; 73.0%) | HFrEF (n = 2,736; 18.6%) | HFpEF (n = 3,207; 21.9%) | No HF (n = 8,728; 59.5%) | HF (n = 8,145; 58%) | No HF (n = 5,926; 42%) | |
| Age, years | 72 | 74 | 68.5 | 72.5 | 68 | 72.8 | 68 | 69 | 71 | 70 | 75 |
| Female, % | 39.1 | 40.3 | 30.4 | 37.7 | 35.3 | 37.4 | 21 | 42 | 35 | 37.4 | 38.1 |
| Stroke or TIAa, % | 42.9 | 70.0 | 16.5a | 23.7a | 18.7a | 23.7a | 16 | 17 | 20 | 21.2 | 37.9 |
| CHADS2 | 3.7 | 3.2 | 2.6 | 2.0 | 2.7 | 2.0 | 2.2 | 2.7 | 1.9 | 3.0 | 2.6 |
| CHA2DS2-VASc | 5.1 | 4.5 | NR | NR | NR | NR | NR | NR | NR | 4.5 | 4.1 |
| LVEF <40%b, % | 33.3 | 0 | 44.0 | 11.2 | 44.0 | 11.1 | 86 | 0 | 0 | 49.0b | 10.1b |
| NYHA class, % | NR | NR | NR | NR | NR | NYHA I–II: 77.9 NYHA III–IV: 22.1 |
NR | ||||
| I | 13.7 | 27 | 16 | 73 | |||||||
| II | 56.4 | 50 | 62 | 24 | |||||||
| III | 28.3 | 22 | 21 | 2 | |||||||
| IV | 1.7 | 1 | <1 | <1 | |||||||
| Hypertension, % | 92.8 | 85.7 | 74.9 | 80.2 | 75.0 | 80.4 | 75 | 89 | 90 | 94.0 | 93.1 |
| Diabetes, % | 42.3 | 36.7 | 25.6 | 22.6 | 27.9 | 21.3 | 27 | 25 | 25 | 30.4 | 43.9 |
| Coronary artery disease, % | NR | NR | 31.8 | 26.0 | 31.4 | 26.9 | 43 | 48 | 29 | NR | NR |
| Myocardial infarction, % | 20.8 | 9.1 | NR | NR | NR | NR | 28 | 18 | 11 | 14.3 | 7.7 |
| Previous VKA use, % | 58.7 | 68.8 | NR | NR | NR | NR | 61 | 51 | 63 | 43.5 | 38.0 |
| Beta blockers, % | 68.7 | 56.8 | 68.1 | 61.1 | 70.4 | 61.6 | 75 | 69 | 62 | 71.1 | 59.4 |
| Digitalis, % | 44.7 | 27.4 | NR | NR | NR | NR | 47 | 39 | 24 | NR | NR |
| ACEi, % | 61.6 | 41.5 | 57.2 | 40.2 | 58.7 | 40.4 | 81 | 77 | 66 | 71.0 | 58.7 |
| ARB | NR | NR | 21.8 | 24.9 | 21.5 | 25.2 | |||||
| Diuretics, % | 71.4 | 39.4 | 72.0 | 42.8 | 72.5 | 43.5 | 73 | 70 | 46 | 72.1 | 43.7 |
Clinical characteristics of the patients included in the ROCKET-AF, RE-LY, ARISTOTLE, and ENGAGE AF-TIMI 48 trials, according to baseline HF status.
NR, not reported; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; HF, heart failure; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; TIA, transient ischemic attack; VKA, vitamin K antagonists.
Stroke or TIA or systemic embolism.
<50%.
The median follow-up in the four clinical trials ranged from 1.5 to 2.8 years. Among DOAC arms of the clinical trials, the annualized event rates for stroke or systemic embolism ranged from 0.99% to 1.90% in the HF population (vs. from 1.0% to 2.1% in the non-HF population). Annualized event rates for major bleeding were 1.95%–3.26% and 2.17%–3.39%, for HF and non-HF patients, respectively. Annualized event rates for intracranial bleeding were 0.15%–0.40% and 0.23%–0.64%, for HF and non-HF patients, respectively. Annualized event rates for all-cause death were 4.36%–6.99% and 2.17%–3.20%, for HF and non-HF patients, respectively (Table 2).
Table 2
| Adverse events | ROCKET-AF (rivaroxaban arm) | RE-LY (dabigatran 110 arm) | RE-LY (dabigatran 150 arm) | ARISTOTLE (apixaban) | ENGAGE AF-TIMI 48 (edoxaban) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HF (n = 4,530; 64.0%) | No HF (n = 2,551; 36.0%) | HF (n = 1,641; 27.3%) | No HF (n = 4,374; 72.7%) | HF (n = 1,640; 27.0%) | No HF (n = 4,436; 73.0%) | HFrEF | HFpEF | No HF | HF (n = 8,145; 58%) | No HF (n = 5,926; 42%) | |
| Median follow-up, years | 1.9 | 2.0 | 2.0 | 1.5 | 2.8 | ||||||
| Stroke or SE | 1.90 | 2.10 | 1.90 | 1.41 | 1.44 | 1.00 | 0.99 | 1.51 | 1.16 | NYHA I–II: 1.52 NYHA III–IV: 1.83 |
1.54 |
| All-cause death | 5.05 | 3.20 | NR | NR | NR | NR | 6.99 | 4.05 | 2.17 | NYHA I–II: 4.36 NYHA III–IV: 6.50 |
2.89 |
| Major bleeding | 14.22b | 16.12b | 3.26 | 2.73 | 3.10 | 3.39 | 2.77 | 1.95 | 2.17 | NYHA I–II: 2.61 NYHA III–IV: 2.49 |
2.98 |
| Intracranial hemorrhage | 0.40 | 0.64 | 0.22 | 0.23 | 0.26 | 0.34 | 0.18 | 0.15 | 0.38 | NYHA I–II: 0.35 NYHA III–IV: 0.32 |
0.46 |
Adverse eventsa in the ROCKET-AF, RE-LY, ARISTOTLE, and ENGAGE AF-TIMI 48 trials, according to baseline HF status.
3.2 ROCKET-AF trial and FARAONIC, AFIRE, EMIR, and US database registries, according to baseline HF status
The clinical characteristics of the patients with AF treated with rivaroxaban in the ROCKET-AF trial and different registries, according to baseline HF status, are presented in Table 3. In the patients with HF, the median age of the patients included in the registries (73.7–75.3 years) was higher than that of the ROCKET-AF trial (72 years). The HF population included in the registries had fewer comorbidities than the patients included in the ROCKET-AF trial and lower thromboembolic risk (CHA2DS2-VASc 3.9–4.5 vs. 5.1, respectively). There were some differences in the proportion of patients treated with HF drugs depending on the registries. With regard to clinical events in the HF population, annualized rates/incidence of stroke or systemic embolism were 0.75%–0.98% in the registries (vs. 1.90% in the ROCKET-AF trial). These rates for major bleeding, intracranial bleeding, and all-cause death were 1.4%–3.86% vs. 14.22% (including major or clinically relevant non-major bleeding), 0.25%–0.27% vs. 0.40%, and 3.14%–5.8% vs. 5.05%, respectively (Table 4) (8, 18–21).
Table 3
| Clinical characteristics | ROCKET-AF (rivaroxaban arm) | FARAONIC (rivaroxaban) | AFIRE (monotherapy arm) | EMIR (rivaroxaban) | US database (rivaroxaban) | |||
|---|---|---|---|---|---|---|---|---|
| HF (n = 4,530; 64.0%) | No HF (n = 2,551; 36.0%) | HF (n = 552) | HF (n = 389; 35.5%) | No HF (718; 64.5%) | HF (n = 326; 22.7%) | No HF (n = 1,107; 77.3%) | HF (n = 3,418; 100%) | |
| Age, years | 72 | 74 | 73.7 | 75.3 | 73.8 | 75.3 | 73.8 | 74 |
| Female, % | 39.1 | 40.3 | 34.1 | NR | NR | 40.8 | 53.2 | 41.4 |
| Stroke or TIAa, % | 42.9 | 70.0 | 12.5 | NR | NR | 12.9 | 12.4 | 7.7a |
| CHADS2 | 3.7 | 3.2 | NR | NR | NR | NR | NR | NR |
| CHA2DS2-VASc | 5.1 | 4.5 | 4.1 | NR | NR | 4.5 | 3.2 | 3.9 |
| LVEF <40%, % | 33.3 | 0 | 31.3 | NR | NR | 28.8 | 0 | NR |
| NYHA class, % | NR | NR | NR | NR | NR | NR | ||
| I | 13.7 | 17.4 | ||||||
| II | 56.4 | 58.7 | ||||||
| III | 28.3 | 23.2 | ||||||
| IV | 1.7 | 0.7 | ||||||
| Hypertension, % | 92.8 | 85.7 | 77.5 | NR | NR | 80.1 | 79.1 | 82.9 |
| Diabetes, % | 42.3 | 36.7 | 37.3 | NR | NR | 36.5 | 24.3 | 35.2 |
| CAD | NR | NR | 39.1 | 100 | 100 | 28.2 | 12.9 | NR |
| Myocardial infarction, % | 20.8 | 9.1 | NR | — | 14.4 | NR | 13.2 | |
| Previous VKA use, % | 58.7 | 68.8 | 44.9 | NR | NR | NR | NR | NR |
| β-Blocker, % | 68.7 | 56.8 | 79.7 | NR | NR | NR | NR | 64.5 |
| Digitalis, % | 44.7 | 27.4 | 23.0 | NR | NR | NR | NR | 11.1 |
| ACEi, % | 61.6 | 41.5 | 85.5 | NR | NR | NR | NR | 61.6 |
| ARB | NR | NR | ||||||
| Diuretics, % | 71.4 | 39.4 | 90.6 | NR | NR | NR | NR | 72.8 |
Clinical characteristics of the patients with AF treated with rivaroxaban in the ROCKET-AF trial and the FARAONIC, AFIRE, EMIR, and US database registries, according to baseline HF status.
NR, not reported; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; HF, heart failure; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; TIA, transient ischemic attack; VKA, vitamin K antagonists; CAD, coronary artery disease.
Ischemic stroke.
Table 4
| Adverse events | ROCKET-AF (rivaroxaban arm) | FARAONIC (rivaroxaban) | AFIRE (monotherapy arm) | EMIR (rivaroxaban) | US database (rivaroxaban) | |||
|---|---|---|---|---|---|---|---|---|
| HF (n = 4,530; 64.0%) | No HF (n = 2,551; 36.0%) | HF (n = 552) | HF (n = 389; 35.5%) | No HF (718; 64.5%) | HF (n = 326; 22.7%) | No HF (n = 1,107; 77.3%) | HF (n = 3,418; 100%) | |
| Median follow-up, years | 1.9 | 2.0 | 2.0 | 2.5 | 1.4 | |||
| Stroke or SEb,d | 1.90 | 2.10 | 0.75 | 0.92b | 0.98b | 1.2d | 0.6d | 0.98 |
| All-cause death | 5.05 | 3.20 | 5.8 | 3.14 | 1.17 | 5.5 | 2.0 | NR |
| Major bleeding | 14.22c | 16.12c | 1.55 | 1.60 | 1.62 | 1.4 | 0.9 | 3.86 |
| Intracranial hemorrhage | 0.40 | 0.64 | 0.25 | NR | NR | NR | NR | 0.27 |
| MACE | NR | NR | NR | 3.89 | 2.25 | 3.0 | 0.5 | NR |
Adverse eventsa of the patients with AF treated with rivaroxaban in the ROCKET-AF trial and the FARAONIC, AFIRE, EMIR, and US database registries, according to baseline HF status.
NR, not reported; MACE, major adverse cardiac event.
Annual incidence of events was calculated in the FARAONIC study and annual rates expressed as events per 100 patient-years were recorded for the rest of the studies.
Ischemic stroke.
Major or clinically relevant non-major bleeding.
Stroke + SE + TIA; SE: systemic embolism.
3.3 FARAONIC, GLORIA-AF, and ETNA-AF registries
Three registries with different DOACs (FARAONIC, rivaroxaban; GLORIA-AF, dabigatran; and ETNA-AF, edoxaban) were analyzed to determine the clinical profile and outcomes in the HF population (Tables 5, 6). The patients with HF included in these registries were old (age 69.9–75.3 years), 10.1%–13.0% had prior cerebrovascular disease, 29.8%–39.1% had coronary artery disease, and 23.9%–37.3% had diabetes and a high thromboembolic risk (CHA2DS2-VASc 3.8–4.4). In all registries, a high proportion of patients were receiving HF drugs. The follow-up or outcomes were similar in the three registries (2 years). In the HF population in GLORIA-AF and ETNA-AF, annualized rates for stroke or systemic embolism, major bleeding, intracranial bleeding, and all-cause death were 0.75%–0.88%, 1.20%–1.65%, 0.25%–0.36%, and 4.76%–6.08%, respectively. With regard to the dose of DOACs used in each registry in the HF population, in FARAONIC, 69% of patients were receiving rivaroxaban 20 mg (31% rivaroxaban 15 mg); in GLORIA-AF, 50.1% were receiving dabigatran 150 mg, 47.3% dabigatran 110 mg, and 2.0% dabigatran 75 mg; and in ETNA, AF 36.9% were receiving edoxaban 60 mg and 63.1% edoxaban 30 mg (18, 22, 23).
Table 5
| Clinical characteristics | FARAONIC (rivaroxaban) | GLORIA-AF (dabigatran) | ETNA-AF (edoxaban) | ||
|---|---|---|---|---|---|
| HF (n = 552) | HF (n = 1,169; 24.2%) | No HF (n = 3,658;75.8%) | HF (n = 5,258; 19.2%) | No HF (n = 22,075;80.8%) | |
| Age, years | 73.7 | 69.9 | 70.3 | 75.3 | 73.3 |
| Female, % | 34.1 | 39.3 | 46.0 | 41.6 | 41.9 |
| Stroke or TIAa, % | 12.5 | 10.1 | 17.5 | 13.0a | 11.5a |
| CHADS2 | NR | NR | NR | NR | NR |
| CHA2DS2-VASc | 4.1 | 3.8 | 3.0 | 4.4 | 3.0 |
| LVEF <40%, % | 31.3 | 38.2 | 0 | NR | NR |
| NYHA class, % | NR | NR | NR | ||
| I | 17.4 | 9.6 | |||
| II | 58.7 | 50.6 | |||
| III | 23.2 | 24.7 | |||
| IV | 0.7 | 4.6+ | |||
| Hypertension, % | 77.5 | 76.8 | 77.7 | 76.7 | 73.5 |
| Diabetes, % | 37.3 | 23.9 | 22.4 | 29.6 | 21.7 |
| CAD | 39.1 | 29.8 | 15.5 | NR | NR |
| Myocardial infarction, % | NR | 15.2 | 6.6 | 9.1 | 2.5 |
| Previous VKA use, % | 44.9 | NR | NR | NR | NR |
| β-blocker, % | 79.7 | 70.9 | 60.2 | NR | NR |
| Digitalis, % | 23.0 | 19.9 | 7.2 | NR | NR |
| ACEi, % | 85.5 | 45.9 | 29.3 | NR | NR |
| ARB | 25.5 | 28.9 | |||
| Diuretics, % | 90.6 | 66.6 | 31.3 | NR | NR |
Clinical characteristics of the patients with AF included in the FARAONIC (rivaroxaban), GLORIA-AF (dabigatran), and ETNA-AF (edoxaban) registries, according to baseline HF status.
NR, not reported; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; HF, heart failure; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; TIA, transient ischemic attack; VKA, vitamin K antagonists; CAD, coronary artery disease.
Ischemic stroke.
+For 386 patients (10.5%), NYHA class was unknown.
Table 6
| Adverse events | FARAONIC (rivaroxaban) | GLORIA-AF (dabigatran) | ETNA-AF (edoxaban) | ||
|---|---|---|---|---|---|
| HF (n = 552) | HF (n = 1,169; 24.2%) | No HF (n = 3,658; 75.8%) | HF (n = 5,258; 19.2%) | No HF (n = 22,075; 80.8%) | |
| Median follow-up, years | 2.0 | 2.0 | 2.0 | ||
| Stroke or SE | 0.75 | 0.82b | 0.60b | 0.88c | 0.71c |
| All-cause death | 5.8 | 4.76 | 1.80 | 6.08 | 2.52 |
| Major bleeding | 1.55 | 1.20 | 0.92 | 1.65 | 0.88 |
| Intracranial hemorrhage | 0.25 | NR | NR | 0.36 | 0.27 |
| MACE | NR | NR | NR | NR | NR |
Adverse eventsa of the patients with AF included in the FARAONIC (rivaroxaban), GLORIA-AF (dabigatran), and ETNA-AF (edoxaban) registries, according to baseline HF status.
SE, systemic embolism; NR, not reported; MACE, major adverse cardiac event.
Annual incidence of events was calculated in the FARAONIC study and annual rates expressed as events per 100 patient-years were recorded for the rest of the studies.
Stroke.
Ischemic stroke.
4 Discussion
The FARAONIC study showed in a diverse sample of real-life patients with AF and HF anticoagulated with rivaroxaban that these patients were old, had many comorbidities, and had a high thromboembolic risk. Despite this, the rates of thromboembolism, death, and major bleeding remained low. These numbers were lower than those reported in ROCKET-AF and other clinical trials with DOACs but in line with other national or international registries.
Although the most important complication in patients with AF is the development of ischemic stroke that is associated with great morbidity and mortality (24), anticoagulated patients with AF have a substantial residual risk of other outcomes, such as mortality or MACEs. This is even more important in patients with concomitant HF (1–3). In this context, the optimal treatment strategies for patients with HF and AF remain unclear. Traditionally, in recent decades, vitamin K antagonists have been used to reduce the risk of stroke in AF patients with HF. However, they have many limitations that increase in HF, in which suboptimal levels of warfarin anticoagulation control are more common, leading to more complications (25). However, a meta-analysis of four clinical trials that compared DOACs and warfarin showed that overall, DOACs were more effective, with a lower risk of death and bleeding (26). For these reasons, it is crucial to ascertain whether the benefits of DOACs over warfarin remain in the HF population. In our study, we analyzed the clinical profile and clinical outcomes of four phase III clinical trials with DOACs. Despite the worse clinical profile and higher thromboembolic risk of patients with HF, clinical trials showed that the relatively better efficacy and safety of DOACs over warfarin persisted in the HF population (8–11, 27), suggesting that the use of DOACs should be preferred over vitamin K antagonists in this population (1–3, 14, 15). In fact, a recent meta-analysis showed that in patients with HF and AF, compared with warfarin, DOACs significantly reduced the risk of stroke or systemic embolism by 17%, all-cause mortality by 15%, major bleeding by 11%, and intracranial hemorrhage by 46%. These beneficial effects extended to the overall spectrum of patients with HF (28). In summary, oral anticoagulation should be recommended for all patients with AF and HF, independent of HF type, making DOAC the first choice in this population (29).
Compared with the rivaroxaban arm of the ROCKET-AF trial, patients included in the FARAONIC study had a better clinical profile, with a lower proportion of comorbidities and thromboembolic risk (8, 18). This is related to the ROCKET-AF inclusion criteria that represented the more advanced clinical scenario of patients with AF included in all phase III clinical trials with DOACs (8–11). By contrast, the FARAONIC study included patients with AF and HF who were representative of clinical practice, as no strict selection criteria were defined. In fact, the clinical profile of the FARAONIC study was similar to that of other national registries including patients with HF and AF, anticoagulated with rivaroxaban (18, 20, 21). In the registries of patients who received rivaroxaban, annual rates of stroke or systemic embolism were low (0.75%–0.98% vs. 1.90% in the ROCKET-AF trial), suggesting that rivaroxaban was effective in the treatment of this very high-risk population in clinical practice, with a low risk of bleeding, particularly intracranial hemorrhage (18, 20, 21). In addition, no cases of fatal bleeding were observed in FARAONIC (18).
Furthermore, the EMIR study showed that HF was independently associated with the development of MACEs, but not with thromboembolic or bleeding events (20). This means that despite proper anticoagulation, patients with AF and HF still have a residual risk of developing cardiovascular complications. The optimal management of patients with AF and HF should not be limited to anticoagulation but include a comprehensive therapeutic approach (14, 15). In this context, choosing the most appropriate oral anticoagulant is mandatory. Thus, experimental data in a mouse model have shown that rivaroxaban may suppress the progression of ischemic cardiomyopathy and reduce cardiac dysfunction of ischemic origin and clinical studies have suggested that rivaroxaban could reduce the risk of myocardial infarction and cardiovascular death in the AF population (30–32).
In addition, we compared the clinical profile and rates of adverse events of the FARAONIC study with some international clinical registries with other DOACs, i.e., GLORIA-AF (dabigatran) and ETNA-AF (edoxaban) (18, 22, 23). Although only indirect comparisons can be performed, it seems that despite a similar clinical profile, the incidence of stroke or systemic embolism was lower in the FARAONIC study, with a similar risk of bleeding. This could be related to the higher use of reduced DOAC doses reported in the GLORIA-AF and ETNA-AF registries compared with the FARAONIC study, which may lead to reduced effectiveness in clinical practice (33). This could be explained by the fact that the GLORIA-AF and ETNA-AF registries started earlier, and physicians were not as confident with the use of DOACs as in the FARAONIC study. In addition, in the FARAONIC study, persistence with rivaroxaban was very high (permanent discontinuation of 6.9%) after 24 months of follow-up (18). This is important because this indicates not only the good tolerability of rivaroxaban but also because medication persistence is crucial to assure good efficacy in patients with chronic conditions. In addition, the once-daily dose of rivaroxaban could also enhance medication adherence, particularly in this polymedicated population (34).
HF treatments were underused in the FARAONIC study and in the other registries. However, it should be noted that 51.3% of patients included in the FARAONIC study had HF with preserved ejection fraction and at the moment of recruitment (between March 2018 and July 2019), no specific drugs had been approved for this indication (18). Regardless, this type of study clearly indicates that more efforts should be made to improve the management of our patients, not only from an HF perspective but with a holistic approach, treating all the comorbidities (14, 15).
This study has some limitations. Due to the design of this study, only indirect comparisons between the studies were made and these were descriptive and non-adjusted. As a result, no definite conclusions can be obtained from these comparisons and no more than hypotheses can be suggested. In this context, these data should be confirmed in further studies, with longer follow-ups. However, observational studies are the best design to reflect clinical practice. In addition, the high number of patients included in the national and international registries may reduce these potential biases, providing relevant information about a population that has not been well characterized.
| Center | Principal researcher |
|---|---|
| Hospital del Mar | Nuria Farré López |
| Hospital Universitario Lucus Augusti | Margarita Regueiro Abel |
| Hospital Basurto | Ainara Lozano Bahamonde |
| Consulta Privada Dr. Torres | Francisco Torres Calvo |
| Complejo Hospitalario de Santiago | Rosa María Agra Bermejo |
| Clínica Cardiología Vera | Eduardo Sebastián López Sánchez |
| Consulta Cardiológica Ricardo Fajardo Molina | Ricardo Fajardo Molina |
| CHOU Ourense | Gloria López Barros |
| Hospital de Galdakao/Usansolo | Mª Angeles Eneriz |
| Hospital Universitario Ramón y Cajal | Susana del Prado |
| Complejo Hospitalario de Navarra | Ana Carmen Abecia Ozcariz |
| Consorci Sanitario de Terrassa | Joan Martinez Tur |
| Complejo Hospitalario de Ferrol (H. Arquitecto Marcide) | Manuel López Pérez |
| Hospital Regional de Málaga Carlos Haya | José María Pérez Ruiz |
| Hospital Virgen de la Victoria | Jose Manuel Garcia Pinilla |
| Hospital Universitari de Girona Doctor Josep Trueta | Julia Roure Fernandez |
| Hospital Rey Juan Carlos I de Móstoles | Elena Mejia Martinez |
| Hospital Rio Hortega de Valladolid | Mª del Mar de la Torre Carpente |
| Consulta Dr. Enrique Galve Basilio | Enrique Galve Basilio |
| Hospital Doce de Octubre | Daniel Ferreiro |
| Cardioempordà | Sara Darnés Soler |
| Hospital Clínico Universitario de Valladolid | Pedro Ángel de Santos Castro |
| Hospital Virgen de las Nieves | Silvia López-Fernández |
| Hospital Puerto Real | Fco. Javier Camacho Jurado |
| Hospital Universitario San Cecilio | Jesús Gabriel Sanchez Ramos |
| Hospital La Paz | Isabel Antorrena |
| Hospital Universitario Donostia | Irene Rilo Miranda |
| Hospital Puerta del Mar | Daniel Bartolome Mateos |
| Hospital San Carlos | Francisco Manuel Brun Romero |
| Hospital Clínico Universitario de Salamanca | Elisabete Alzola Martinez |
| Complejo Asist. Univ. León | José Ignacio Iglesias Garriz |
| Hospital Costa de la Luz | María Rosario Perez Tristancho |
| Hospital de Burgos | Esther Sánchez Corral |
| Hospital Rio Carrión (Complejo Aistencial Universitario) | Jose Ignacio Cuende Melero |
| Hospital Comarcal Monforte de Lemos | Ricardo Izquierdo |
| Clínica Clivina | María Rosa Fernández Olmo |
| Complejo Asistencial de Soria (Hospital Santa Barbara) | Margarita Carrera Izquierdo |
| Fundación Hayge | Pere Álvarez García |
| Hospital Poniente | Juan A. Montes Romero |
| Hospital Universitario La Zarzuela (Sanitas) | Santiago de Dios |
| Hospital Virgen Macarena | Alejandro Recio Mayoral |
| Complejo Hospitalario de Pontevedra (Hospital de Montecelo) | Juan Carlos Rodríguez García |
| Hospital de Sierrallana | Pilar Ortiz Oficialdegui |
| Hospital Clínic i Provincial | Ana García Alvarez |
| Hospital Clínico Universitario Lozano Blesa | Juan Ignacio Perez Calvo |
| Hospital Miguel Servet | Ana Portoles Ocampo |
| Hospital Royo Vilanova | David Bierge Valero |
| Hospital Sanchinarro | Francisco Javier Parra |
| Hospital Monteprincipe | Francisco J. Rodriguez Rodrigo |
| Hospital Sant Pau | Sonia Mirabet Perez |
| Hospital Arrixaca | Domingo Pascual Figal |
| Hospital Morales Meseguer | Diego Miguel Giménez Cervantes |
| Hospital Moises Broggi | Roman Freixa Pamias |
| Hospital de Cruces | Ángel Sebastián Leza |
| Hospital de Bellvitge | Josep Comin Colet |
| Hospital Infanta Leonor de Madrid | David Vaqueriza Cubillo |
| Hospital Nuestra Señora de Sonsoles | Rosa Ana Lopez Jiménez |
| Hospital del Sagrat Cor | Martin Luis Descalzo |
| Hospital Sant Joan de Déu de Martorell | María Ysabel Saldarriaga Infante |
| Complejo Hospitalario Ruber Juan Bravo | María Carmen Gómez Rubín |
| Hospital Universitari Germans Trias i Pujol | Javier Santesmases Ejarque |
| Hospital de la Princesa | Berta Moyano |
| Hospital Universitari Vall d'Hebron | Teresa Soriano Sanchez |
| Hospital General San Jorge | Maria Teresa Villarroel Salcedo |
| Hospital Infanta Sofía | Diego Iglesias Del Valle |
| Hospital Virgen de la Luz | José Antonio Nieto Rodriguez |
| Centro Médico Lamar | Monzer Khanji Khatib |
| Clínica Nuestra Señora del Rosario | Maria Carmen Alonso Gutierrez |
| Hospital San Rafael | Gonzalo Peña Pérez |
| Hospital Povisa | Fernando Soto Loureiro |
5 Conclusion
In conclusion, in clinical practice, patients with AF and HF, anticoagulated with rivaroxaban, are old, have many comorbidities, and have a high thromboembolic risk. Despite this, the rates of adverse outcomes, including stroke, all-cause death, and bleeding, are low.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Research Ethical Committee of Parc de Salut Mar. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
JC: Conceptualization, Investigation, Methodology, Validation, Writing – review & editing. NM: Conceptualization, Investigation, Methodology, Validation, Writing – review & editing. AR: Conceptualization, Investigation, Methodology, Validation, Writing – review & editing. IL: Conceptualization, Investigation, Methodology, Validation, Writing – review & editing. MC: Conceptualization, Investigation, Methodology, Validation, Writing – review & editing. EB: Conceptualization, Investigation, Methodology, Validation, Writing – review & editing. MB: Conceptualization, Investigation, Methodology, Validation, Writing – review & editing. NF: Conceptualization, Investigation, Methodology, Validation, Writing – review & editing. JGP: Conceptualization, Investigation, Methodology, Validation, Writing – review & editing. JJ-C: Conceptualization, Investigation, Methodology, Validation, Writing – review & editing. CR: Conceptualization, Investigation, Methodology, Validation, Writing – review & editing. JGD: Conceptualization, Investigation, Methodology, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Funding was recieved for the research of this study from Bayer Hispania SL.
Acknowledgments
Writing and editorial assistance was provided by Content Ed Net. The authors thank the centers and researchers who participated in the study:
Conflict of interest
The authors received honoraria from Bayer Hispania SL for their participation as researchers in the FARAONIC study. As sponsor of the FARAONIC study, the funder was involved in the study design, collection, analysis, interpretation of data, the writing of this article and the decision to submit it for publication. Nuria Farré has received honoraria from Novartis and Bristol outside the submitted work. CR is an employee of Bayer Hispania SL.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Carlisle MA Fudim M DeVore AD Piccini JP . Heart failure and atrial fibrillation, like fire and fury. JACC Heart Fail. (2019) 7(6):447–56. 10.1016/j.jchf.2019.03.005
2.
Gopinathannair R Chen LY Chung MK Cornwell WK Furie KL Lakkireddy DR et al Managing atrial fibrillation in patients with heart failure and reduced ejection fraction: a scientific statement from the American Heart Association. Circ Arrhythm Electrophysiol. (2021) 14(6):HAE0000000000000078. 10.1161/HAE.0000000000000078
3.
Reddy YNV Borlaug BA Gersh BJ . Management of atrial fibrillation across the spectrum of heart failure with preserved and reduced ejection fraction. Circulation. (2022) 146(4):339–57. 10.1161/CIRCULATIONAHA.122.057444
4.
Anter E Jessup M Callans DJ . Atrial fibrillation and heart failure: treatment considerations for a dual epidemic. Circulation. (2009) 119(18):2516–25. 10.1161/CIRCULATIONAHA.108.821306
5.
Batul SA Gopinathannair R . Atrial fibrillation in heart failure: a therapeutic challenge of our times. Korean Circ J. (2017) 47(5):644–62. 10.4070/kcj.2017.0040
6.
Ruddox V Sandven I Munkhaugen J Skattebu J Edvardsen T Otterstad JE . Atrial fibrillation and the risk for myocardial infarction, all-cause mortality and heart failure: a systematic review and meta-analysis. Eur J Prev Cardiol. (2017) 24(14):1555–66. 10.1177/2047487317715769
7.
Ling LH Kistler PM Kalman JM Schilling RJ Hunter RJ . Comorbidity of atrial fibrillation and heart failure. Nat Rev Cardiol. (2016) 13(3):131–47. 10.1038/nrcardio.2015.191
8.
van Diepen S Hellkamp AS Patel MR Becker RC Breithardt G Hacke W et al Efficacy and safety of rivaroxaban in patients with heart failure and nonvalvular atrial fibrillation: insights from ROCKET AF. Circ Heart Fail. (2013) 6(4):740–7. 10.1161/CIRCHEARTFAILURE.113.000212
9.
Ferreira J Ezekowitz MD Connolly SJ Brueckmann M Fraessdorf M Reilly PA et al Dabigatran compared with warfarin in patients with atrial fibrillation and symptomatic heart failure: a subgroup analysis of the RE-LY trial. Eur J Heart Fail. (2013) 15(9):1053–61. 10.1093/eurjhf/hft111
10.
McMurray JJ Ezekowitz JA Lewis BS Gersh BJ van Diepen S Amerena J et al Left ventricular systolic dysfunction, heart failure, and the risk of stroke and systemic embolism in patients with atrial fibrillation: insights from the ARISTOTLE trial. Circ Heart Fail. (2013) 6(3):451–60. 10.1161/CIRCHEARTFAILURE.112.000143
11.
Magnani G Giugliano RP Ruff CT Murphy SA Nordio F Metra M et al Efficacy and safety of edoxaban compared with warfarin in patients with atrial fibrillation and heart failure: insights from ENGAGE AF-TIMI 48. Eur J Heart Fail. (2016) 18(9):1153–61. 10.1002/ejhf.595
12.
Wang TJ Larson MG Levy D Vasan RS Leip EP Wolf PA et al Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham heart study. Circulation. (2003) 107(23):2920–5. 10.1161/01.CIR.0000072767.89944.6E
13.
Banerjee A Taillandier S Olesen JB Lane DA Lallemand B Lip GY et al Ejection fraction and outcomes in patients with atrial fibrillation and heart failure: the Loire Valley Atrial Fibrillation Project. Eur J Heart Fail. (2012) 14(3):295–301. 10.1093/eurjhf/hfs005
14.
McDonagh TA Metra M Adamo M Gardner RS Baumbach A Böhm M et al 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. (2021) 42(36):3599–726. 10.1093/eurheartj/ehab368
15.
Hindricks G Potpara T Dagres N Arbelo E Bax JJ Blomström-Lundqvist C et al 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. (2021) 42(5):373–498. 10.1093/eurheartj/ehaa612
16.
Patel MR Mahaffey KW Garg J Pan G Singer DE Hacke W et al Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. (2011) 365(10):883–91. 10.1056/NEJMoa1009638
17.
Barrios V Escobar C . From clinical trials to clinical practice. Experience with rivaroxaban in the anticoagulant treatment of patients with non-valvular atrial fibrillation. Semergen. (2017) 43(3):222–9. 10.1016/j.semerg.2016.01.016
18.
Manito N Cepeda-Rodrigo JM Farré N Castillo Orive M Galve E Jiménez-Candil J et al Factors associated with disease progression in patients with atrial fibrillation and heart failure anticoagulated with rivaroxaban. Clin Cardiol. (2023) 47:e24189. Online ahead of print. 10.1002/clc.24189
19.
Yazaki Y Nakamura M Iijima R Yasuda S Kaikita K Akao M et al Clinical outcomes of rivaroxaban monotherapy in heart failure patients with atrial fibrillation and stable coronary disease: insights from the AFIRE trial. Circulation. (2021) 144(17):1449–51. 10.1161/CIRCULATIONAHA.121.055374
20.
Anguita Sánchez M Marín F Masjuan J Cosín-Sales J Vázquez Rodríguez JM Barrios V et al Impact of heart failure on the clinical profile and outcomes in patients with atrial fibrillation treated with rivaroxaban. Data from the EMIR study. Cardiol J. (2022) 29(6):936–47. 10.5603/CJ.a2022.0091
21.
Martinez BK Bunz TJ Eriksson D Meinecke AK Sood NA Coleman CI . Effectiveness and safety of rivaroxaban vs. warfarin in patients with non-valvular atrial fibrillation and heart failure. ESC Heart Fail. (2019) 6(1):10–5. 10.1002/ehf2.12365
22.
Dubner SJ Teutsch C Huisman MV Diener HC Halperin J Rothman KJ et al Characteristics and 2-year outcomes of dabigatran treatment in patients with heart failure and atrial fibrillation: GLORIA-AF. ESC Heart Fail. (2020) 7(5):2679–89. 10.1002/ehf2.12857
23.
Siller-Matula J Unverdorben M Wang CC Koretsune Y Pecen L Borrow A et al The real-world effectiveness and safety of edoxaban treatment in 27,333 global ETNA-AF programme patients with and without a history of heart failure. Eur Heart J. (2022) 43(Suppl. 2):ehac544.2700. 10.1093/eurheartj/ehac544.2700
24.
Masjuán J Álvarez-Sabín J Blanco M de Felipe A Gil-Núñez A Gállego-Culleré J et al Current management of antithrombotic treatment in patients with non valvular atrial fibrillation and prior history of stroke or transient ischemic attack. Rev Neurol. (2014) 59(1):25–36. 10.33588/rn.5901.2014037
25.
Lee TC Qian M Lip GYH Di Tullio MR Graham S Mann DL et al Heart failure severity and quality of warfarin anticoagulation control (from the WARCEF trial). Am J Cardiol. (2018) 122(5):821–7. 10.1016/j.amjcard.2018.05.024
26.
Ruff CT Giugliano RP Braunwald E Hoffman EB Deenadayalu N Ezekowitz MD et al Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. (2014) 383(9921):955–62. 10.1016/S0140-6736(13)62343-0
27.
Savarese G Giugliano RP Rosano GM McMurray J Magnani G Filippatos G et al Efficacy and safety of novel oral anticoagulants in patients with atrial fibrillation and heart failure: a meta-analysis. JACC Heart Fail. (2016) 4(11):870–80. 10.1016/j.jchf.2016.07.012
28.
Wulamiding K Xu Z Chen Y He J Wu Z . Non-vitamin K antagonist oral anticoagulants versus warfarin in atrial fibrillation patients with heart failure and preserved, mildly reduced, and reduced ejection fraction: a systemic review and meta-analysis. Front Cardiovasc Med. (2022) 9:949726. 10.3389/fcvm.2022.949726
29.
Brown LAE Boos CJ . Atrial fibrillation and heart failure: factors influencing the choice of oral anticoagulant. Int J Cardiol. (2017) 227:863–8. 10.1016/j.ijcard.2016.09.086
30.
Liu J Nishida M Inui H Chang J Zhu Y Kanno K et al Rivaroxaban suppresses the progression of ischemic cardiomyopathy in a murine model of diet-induced myocardial infarction. J Atheroscler Thromb. (2019) 26(10):915–30. 10.5551/jat.48405
31.
Bode MF Auriemma AC Grover SP Hisada Y Rennie A Bode WD et al The factor Xa inhibitor rivaroxaban reduces cardiac dysfunction in a mouse model of myocardial infarction. Thromb Res. (2018) 167:128–34. 10.1016/j.thromres.2018.05.015
32.
Loffredo L Perri L Violi F . Myocardial infarction and atrial fibrillation: different impact of anti-IIa vs anti-Xa new oral anticoagulants: a meta-analysis of the interventional trials. Int J Cardiol. (2015) 178:8–9. 10.1016/j.ijcard.2014.10.124
33.
Escobar C Martí-Almor J Pérez Cabeza A Martínez-Zapata MJ . Direct oral anticoagulants versus vitamin K antagonists in real-life patients with atrial fibrillation. A systematic review and meta-analysis. Rev Esp Cardiol. (2019) 72(4):305–16. 10.1016/j.rec.2018.03.009
34.
Ozaki AF Choi AS Le QT Ko DT Han JK Park SS et al Real-world adherence and persistence to direct oral anticoagulants in patients with atrial fibrillation: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. (2020) 13(3):e005969. 10.1161/CIRCOUTCOMES.119.005969
Summary
Keywords
atrial fibrillation, anticoagulation, heart failure, rivaroxaban, clinical practice
Citation
Cepeda JM, Manito N, Recio Mayoral A, Lekuona I, Castillo Orive M, Blanco Labrador E, Blasco MT, Farré N, García Pinilla JM, Jiménez-Candil J, Rafols C and Gomez Doblas JJ (2025) Effectiveness and safety of rivaroxaban in patients with atrial fibrillation and heart failure in clinical practice: an indirect comparison of national and international registries. Front. Cardiovasc. Med. 12:1451499. doi: 10.3389/fcvm.2025.1451499
Received
19 June 2024
Accepted
18 March 2025
Published
27 May 2025
Volume
12 - 2025
Edited by
Michael Henein, Umeå University, Sweden
Reviewed by
Iris Parrini, Hospital Mauritian Turin, Italy
Eric Rytkin, Northwestern University, United States
Updates
Copyright
© 2025 Cepeda, Manito, Recio Mayoral, Lekuona, Castillo Orive, Blanco Labrador, Blasco, Farré, García Pinilla, Jiménez-Candil, Rafols and Gomez Doblas.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Jose Maria Cepeda jmcepedarodrigo@gmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.