Abstract
Background:
Literature on the association between high body mass index (BMI) and cardiac conduction defects (CCD) is scarce.
Methods:
The cross-sectional association between obesity and CCD was examined in 455,790 participants (56.1 years; 55.9% females) from the United Kingdom (UK) Biobank. CCD was defined by ICD codes as the presence of either atrioventricular block (AVB) or intraventricular block (IVB). Multivariable logistic regression models were used to assess the association between different levels of BMI and CCD.
Results:
About 2.7% (n = 12,169) of the participants exhibited CCD. Each 1-SD increase in BMI (4.68 kg/m2) was associated with increased odds of CCD (OR (95% CI): 1.03 (1.01, 1.06). In subgroup analysis, this association was stronger in older participants (>65 vs. <65 years), men than women, and participants with diabetes (interaction p-value < 0.05 for all). In a stratified analysis by CCD subtypes, each 1-SD of BMI was associated with increased odds of AVB, but not IVB [OR (95% CI): 1.04 (1.01, 1.07), 0.97 (0.89, 1.05), respectively]. Compared to normal BMI (25–29.9 Kg/m2), participants with marked obesity, defined as BMI >40 Kg/m2, had 20% increased odds of CCD (OR (95% CI): 1.20 (1.04, 1.39). No significant association was observed with BMI between 30 and 39.9 Kg/m2.
Conclusions:
Higher BMI levels are associated with an increased risk of CCD, which is probably triggered by AVB, and the association is stronger in men, the elderly, and those with diabetes; further research is needed to examine whether weight management in obesity will be accompanied by a reduction in the risk of CCD.
Introduction
Cardiac conduction defects (CCD) is a prevalent condition characterized by disruptions in the heart's normal electrical depolarization. It encompasses a spectrum of symptoms given its pathophysiological nature, affecting various levels of the cardiac conduction pathways. These symptoms range from benign electrocardiographic findings to potentially life-threatening heart rhythm disturbances or heart block (1, 2). CCD is an established predictor of heart failure and cardiovascular mortality (3). The usual course of treatment for symptomatic end-stage CCD is cardiac pacing. While pacemakers provide a solution for CCD, this treatment comes with its own set of challenges, such as financial burdens, infection risk, the need for serial generator changes, and potential adverse health effects associated with chronic pacing itself (4). In 2015 it was estimated that 12% of adults were obese worldwide, with an attributable 3 million deaths every year (5). More than two-thirds of the deaths associated with a high BMI are caused by CVD (5). Only few studies have examined the effects of lifestyle behaviors on bradyarrhythmia and CCD (6, 7). With the clear need for strategies to mitigate the burden of CCD, identifying modifiable risk factors is essential. Given the scant evidence, we aimed to assess association of high BMI with the risk of CCD and its subtypes in the general population using UK Biobank cohort.
Materials and methods
The UK Biobank is a prospective cohort study that enrolled over half a million participants aged 40–69 years between 2006 and 2010 from across the UK. Participants attended one of the 22 assessment centers located across England, Scotland, and Wales, where they completed touchscreen and nurse-led questionnaires, underwent physical measurements, and provided biological samples. Information on sociodemographic factors, habitual diet, lifestyle, medical history, and medication usage was collected through touchscreen questionnaires at recruitment (8, 9). The UK Biobank study received approval from the National Information Governance Board for Health and Social Care in England and Wales, the Community Health Index Advisory Group in Scotland, and the Northwest Multicenter Research Ethics Committee. All participants provided written informed consent, and the study was approved by the National Research Ethics Service. Those who were underweight (BMI < 18.5) or with missing variables needed for the analysis were excluded (<2% of the total cohort). Additionally, we excluded those with atrial fibrillation from our sample as shown in the flow chart in Figure 1 The final analysis included 455,790 participants.
Figure 1
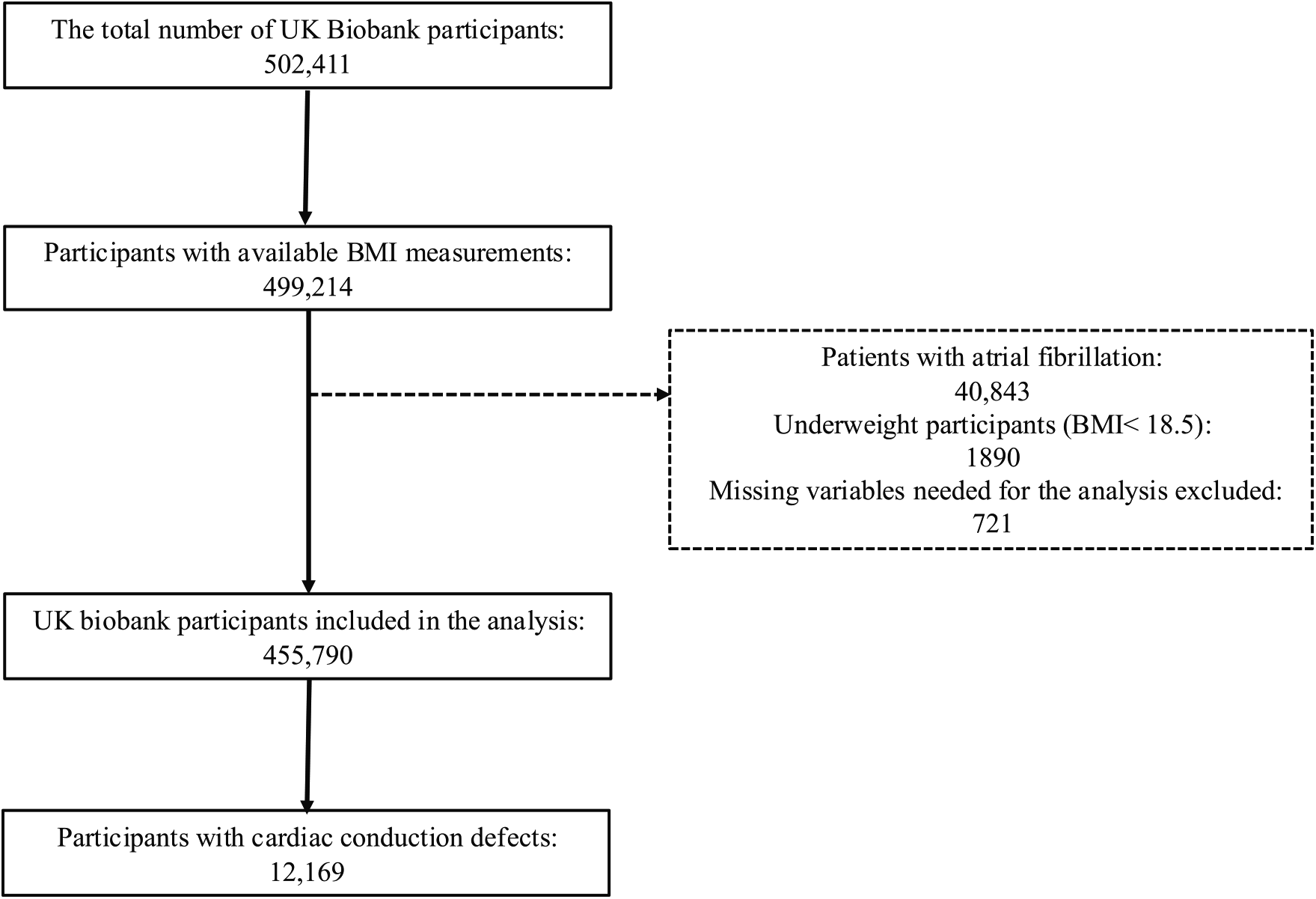
Flow chart for study population.
Body mass index (BMI), as a primary exposure, was measured as part of the baseline during the initial enrollment. BMI was calculated as weight in kilograms divided by height in meters squared. Body weight was measured to the nearest 0.1 kg using a Tanita BC418MA body composition analyzer (Tanita, Tokyo, Japan). Height was measured using a Seca 202 height measure with the head positioned in the Frankfort plane. Obesity is defined as a BMI equal to or exceeding 30 kg/m2. Furthermore, BMI is subcategorized into the following groups: normal weight: BMI 18.5–24.9 kg/m2, overweight: BMI 25.0–29.9 kg/m2, obese: BMI 30.0–39.9 kg/m2, severe obesity: BMI ≥ 40 kg/m2.
The primary outcome for this analysis is composite CCD, which is defined as first, second or third-degree atrioventricular block (AVB), complete or incomplete right bundle branch block (RBBB), complete or incomplete left bundle branch block (LBBB), left anterior fascicular block (LAFB), left posterior fascicular block (LPFB), bifascicular or trifasciuclar block or nonspecific intraventricular conduction delay. Cardiac conduction defects were subdivided into two groups: atrioventricular block (AVB) and intraventricular block (IVB). AVB included participants with first-, second-, or third-degree AV block. IVB defined as those with RBBB, LBBB, LPFB, LAFB, bifascicular block, trifascicular block or nonspecific intraventricular block. Similar classification has been utilized in prior literature (6, 7, 10, 11). Prevalent CCD was extracted from the first occurrence of health outcomes defined by the 10th three-character International Statistical Classification of Diseases (ICD-10).
Our analysis was adjusted for sociodemographic, behavioral, and lifestyle factors, common CVD risk factors and comorbidities. Information on participants' age, sex, ethnicity, educational levels, smoking status, alcohol intake frequency were self-reported based on validated questionnaires administered at baseline. Physical activity was determined using adapted questions from the validated short International Physical Activity Questionnaire. Social status was determined using the Townsend deprivation index with higher scores indicating higher levels of socioeconomic deprivation (12). Hypertension was defined as self-reported use of antihypertensive medication and/or systolic blood pressure ≥130 mmHg and/or diastolic blood pressure ≥80 mmHg, measured using the Omron HEM-7015IT digital blood pressure monitor by averaging two automated measures taken one minute apart. Prevalent comorbidities were determined based on self-reported physician diagnoses at baseline, along with relevant medication use or biomarker thresholds when applicable. Diabetes was defined by a self-reported diagnosis, use of glucose-lowering medications, or an HbA1c level of ≥6.5% at baseline. Coronary artery disease included a history of myocardial infarction, coronary angioplasty (with or without stenting), or coronary artery bypass grafting. Heart failure encompassed diagnoses of heart failure, cardiomyopathy, pulmonary edema, or hypertrophic cardiomyopathy. Valvular heart disease was defined by the presence of aortic or mitral stenosis or regurgitation, mitral valve prolapse, or any history of valve repair or replacement. Dyslipidemia was defined by self-reported use of lipid-lowering medications or elevated total cholesterol: >=6.2 mmol/L, high-density lipoprotein (HDL) cholesterol: <1.0 mmol/L, triglycerides: >=2.3 mmol/L, low-Density Lipoprotein (LDL) Cholesterol: >=4.1 mmol/L. Demographics and clinical characteristics of the participants were compared across BMI groups using student ANOVA for continuous variables and Chi-square for categorical variables. Given the large sample size, we reported effect sizes as eta-squared (η²) for continuous variables and Cramér's V for categorical variables. Odds ratios (OR) and 95% confidence intervals (CI) for the risk of CCD and its subtypes were estimated using multivariable logistic regression models across BMI groups, with the normal weight group as a reference. To examine the association between BMI and CCD, three multivariable-adjusted models were constructed. Model 1 adjusted for demographics (age, sex, and race) and model 2 adjusted for model 1 plus education level, social status, smoking status, alcohol consumption, diabetes, hypertension, dyslipidemia, and prior CVD including coronary heart disease, heart failure, atrial fibrillation and valvular heart disease. Final model 3 accounted for physical activity in addition to model 2. Similar models were utilized to examine the odds of CCD associated with 1-SD (4.76) increase in BMI. Subgroup analysis was conducted to examine the effect modification of the association between tertiles and I-SD of BMI by age (< 65 vs. >65 years), sex (men vs. women), race (white vs. non-white), smoking status, physical activity, and diabetes. Interaction with the main effect was tested in an adjusted model similar to model 3.
All statistical analyses were performed using JMP Pro 17. A P-value of less than 0.05 was coincided significant.
Results
Among 455,790 participants included, 2.7% (n = 12,169) had prevalent CCD. Prevalent conduction disease included 3,073 with first-degree AVB, 928 with 2nd degree AVB, 1,250 with 3rd degree AVB, 4,239 with RBBB, 3,795 with LBBB, 106 with either LAFB or LPFB, 456 with bifasicular block, 409 with trifasicular block, 101 with nonspecific IVB (3,011 participants had more than one type of CCD). Participants with more than one CCD were included in the respective CCD group if at least one type met the inclusion criteria for AVB, IVB, or CCD groups. The mean BMI of the total population was 27.3 ± 4.6, 43% were overweight and 24% were obese. Baseline characteristics of study population stratified by BMI groups are shown in (Table 1). The prevalence of CCD, AVB, and IVB increased with higher BMI levels, peaking in participants with severe obesity compared to the normal weight group (4.2% vs. 3.5% vs. 2.8% vs. 1.9%), respectively. Participants with highest levels of BMI tended to be female, white and had higher prevalence of diabetes, CVD and dyslipidemia.
Table 1
| BMI groups/number | BMI 18.5–24.9 | BMI 25.0–29.9 | BMI 30.0–39.9 | BMI > 40 | P-value | Effect size |
|---|---|---|---|---|---|---|
| N = 148,616 | N = 1,97,074 | N = 1,01,919 | N = 8,181 | |||
| Age, years | 55.3 | 56.5 | 56.4 | 54.5 | <0.001 | 0.006 |
| Sex, males N (%) | 49,783 (33.5%) | 101,326 (51.4%) | 47,775 (46.8%) | 2,327 (28.4%) | <0.001 | 0.164 |
| Race, Whites N (%) | 137,430 (92.5%) | 189,860 (96.3%) | 92,673 (90.9%) | 7,356 (89.9%) | <0.001 | 0.042 |
| Education level, years | 16.9 | 16.7 | 16.6 | 16.6 | <0.001 | <0.001 |
| Social Status, Townsend Deprivation Index | −1.5 | −1.4 | −0.9 | 0 | <0.001 | 0.010 |
| Physical activity, MET | ||||||
| Walking | 1,030 | 1,021 | 1,035 | 1,044 | 0.154 | 0.027 |
| Moderate | 935 | 920 | 932 | 940 | 0.323 | |
| Vigorous | 681 | 669 | 684 | 678 | 0.192 | |
| Smoking status N (%) | ||||||
| Never | 88,252 (59.4%) | 106,714 (54.1%) | 52,828 (51.8%) | 4,467 (54.6%) | <0.001 | 0.047 |
| Former | 43,048 (29.0%) | 69,203 (35.1%) | 38,492 (37.8%) | 2,897 (35.4%) | <0.001 | |
| Current | 16,755 (11.3%) | 20,170 (10.2%) | 9,965 (9.8%) | 763 (9.3%) | <0.001 | |
| Alcohol consumption N (%) | ||||||
| Never | 10,709 (7.2%) | 14,072 (7.1%) | 9,815 (9.6%) | 1,297 (15.9%) | <0.001 | 0.080 |
| Special occasions only | 14,818 (10.0%) | 20,268 (10.3%) | 15,229 (14.9%) | 2,110 (25.8%) | <0.001 | |
| 1 to 3 times per month | 15,479 (10.4%) | 20,943 (10.6%) | 13,601 (13.3%) | 1,423 (17.4%) | <0.001 | |
| Once or twice per week | 37,588 (25.3%) | 51,926 (26.3%) | 27,196 (26.7%) | 1,869 (22.8%) | <0.001 | |
| 3 to 4 times per week | 36,584 (24.6%) | 48,137 (24.4%) | 19,988 (19.6%) | 891 (10.9%) | <0.001 | |
| Daily or almost daily | 33,151 (22.3%) | 41,341 (21.0%) | 15,820 (15.5%) | 564 (6.9%) | <0.001 | |
| Prefer not to answer | 153 (0.1%) | 190 (0.1%) | 127 (0.1%) | 15 (0.2%) | <0.001 | |
| Diabetes, N (%) | 3,971 (2.7%) | 13,493 (6.8%) | 17,167 (16.8%) | 2,780 (34.0%) | <0.001 | 0.246 |
| Hypertension, N (%) | 25,843 (17.4%) | 57,451 (29.2%) | 44,097 (43.3%) | 4,666 (57.0%) | <0.001 | 0.239 |
| Coronary artery disease, N(%) | 7,846 (5.3%) | 17,945 (9.1%) | 12,381 (12.1%) | 1,045 (12.8%) | <0.001 | 0.111 |
| Heart failure, N (%) | 1,728 (1.2%) | 3,622 (1.8%) | 3,223 (3.2%) | 456 (5.6%) | <0.001 | 0.092 |
| Valvular disease, N (%) | 3,312 (2.2%) | 5,773 (2.9%) | 3,710 (3.6%) | 378 (4.6%) | <0.001 | 0.054 |
| Dyslipidemia, N (%) | 13,452 (9.1%) | 30,142 (15.3%) | 21,236 (20.8%) | 1,978 (24.2%) | <0.001 | 0.142 |
| Cardiac conduction defects (CCD), N (%) | 2,761 (1.9%) | 5,452 (2.8%) | 3,610 (3.5%) | 346 (4.2%) | <0.001 | 0.057 |
| Atrioventricular nodal block (AVB): | 1,113 (0.75%) | 2,352 (1.19%) | 1,626 (1.60%) | 160 (1.96%) | <0.001 | 0.028 |
| 1st degree heart block, N (%) | 572 (0.4%) | 1,384 (0.7%) | 1,017 (1.0%) | 100 (1.2%) | <0.001 | 0.035 |
| 2nd degree heart block, N (%) | 242 (0.2%) | 417 (0.2%) | 247 (0.2%) | 22 (0.3%) | <0.001 | 0.013 |
| 3rd degree heart block, N (%) | 299 (0.2%) | 551 (0.3%) | 362 (0.4%) | 38 (0.5%) | <0.001 | 0.018 |
| Intraventricular block (IVB): | 2,038 (1.37%) | 4,070 (2.07%) | 2,746 (2.69%) | 252 (3.08%) | <0.001 | 0.031 |
| RBBB, N (%) | 1,016 (0.7%) | 1,848 (0.9%) | 1,254 (1.2%) | 121 (1.5%) | <0.001 | 0.032 |
| LBBB, N (%) | 846 (0.6%) | 1,741 (0.9%) | 1,115 (1.1%) | 93 (1.1%) | <0.001 | 0.034 |
| LAFB or LPFB, N (%) | 22 (0.0%) | 49 (0.0%) | 34 (0.0%) | 1 (0.0%) | 0.001 | 0.005 |
| Bifascicular block, N (%) | 75 (0.1%) | 201 (0.1%) | 157 (0.2%) | 23 (0.3%) | <0.001 | 0.002 |
| Trifascicular block, N (%) | 59 (0.0%) | 182 (0.1%) | 156 (0.2%) | 12 (0.1%) | <0.001 | 0.011 |
| Nonspecific IVB, N (%) | 20 (0.0%) | 49 (0.0%) | 30 (0.0%) | 2 (0.0%) | <0.001 | 0.006 |
Baseline population characteristics.
RBBB, right bundle branch block; LBBB, left bundle branch block; LAFB, left anterior fascicular block; LPFB, left posterior fascicular block.
Effect sizes are reported as eta-squared (η²) for continuous variables and Cramér's V for categorical variables. Values ≥ 0.1 are considered clinically meaningful in this context.
In multivariable-adjusted logistic regression, increasing BMI levels were associated with higher odds of composite CCD. As shown in Table 2, in a model adjusted for demographics, prior CVD and its common risk factors, severe obesity was associated with 20% increased odds of CCD (OR (95% CI): 1.20 (1.04, 1.39). Similar patterns were observed for AVB and IVB, although the association with IVB did not reach statistical significance (OR (95% CI): 1.04 (1.01, 1.07), 0.97 (0.89, 1.05), respectively. In a continuous fashion, each 1-SD increase in BMI was associated with increased odds of CCD and AVB but not IVB, indicating a dose-response relationship (OR (95% CI): 1.03 (1.01, 1.06), 1.04 (1.01, 1.07), 0.97 (0.89, 1.05)), respectively. The association between BMI (modeled as 1-SD increase) and CCD was consistent across subgroups of the participants stratified by race, smoking status, and physical activity (Table 3). However, significant effect modification was observed, with the risk of CCD per 1-SD increase in BMI being more pronounced in older participants (>65 vs. <65), men compared to women, and participants with diabetes (interaction p-value = 0.035, <0.001, <0.001, respectively).
Table 2
| CCD subtype | Obesity class | Unadjusted Model | Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|---|---|---|
| Odds Ratio | p-value | Odds Ratio | p-value | Odds Ratio | p-value | Odds Ratio | p-value | ||
| (95% CI) | (95% CI) | (95% CI) | (95% CI) | ||||||
| Composite CCDa | Normal weight | Ref. | –. | Ref. | – | Ref. | – | Ref. | – |
| Overweight | 1.50 (1.43, 1.57) | <0.001 | 1.22 (1.16, 1.28) | <0.001 | 1.02 (0.97, 1.08) | 0.337 | 1.01 (0.95, 1.08) | 0.588 | |
| Obese | 1.93 (1.84, 2.03) | <0.001 | 1.65 (1.56, 1.74) | <0.001 | 1.05 (1.00, 1.12) | 0.093 | 1.03 (0.97, 1.11) | 0.288 | |
| Severe obesity | 2.33 (2.08, 2.61) | <0.001 | 2.65 (2.36, 2.98) | <0.001 | 1.23 (1.08, 1.40) | <0.001 | 1.20 (1.04, 1.39) | 0.013 | |
| Per 1-SD BMI increased | 1.25 (1.23, 1.27) | <0.001 | 1.26 (1.24, 1.29) | <0.001 | 1.04 (1.02, 1.06) | 0.026 | 1.03 (1.01, 1.06) | 0.004 | |
| AVBb | Normal weight | Ref. | –. | Ref. | – | Ref. | – | Ref. | – |
| Overweight | 1.44 (1.36, 1.52) | <0.001 | 1.31 (1.24, 1.39) | <0.001 | 1.07 (0.99, 1.14) | 064 | 1.01 (0.94, 1.08) | 0.810 | |
| Obese | 1.83 (1.73, 1.94) | <0.001 | 1.72 (1.62, 1.83) | <0.001 | 1.19 (1.10, 1.28) | <0.001 | 1.05 (0.98, 1.13) | 0.162 | |
| Severe obesity | 2.11 (1.84, 2.42) | <0.001 | 2.32 (2.02, 2.68) | <0.001 | 1.43 (1.21, 1.69) | <0.001 | 1.21 (1.02, 1.43) | 0.029 | |
| Per 1-SD BMI increased | 1.23 (1.21, 1.25) | <0.001 | 1.24 (1.21, 1.27) | <0.001 | 1.10 (1.08, 1.14) | <0.001 | 1.04 (1.01, 1.07) | 0.001 | |
| IVBc | Normal weight | Ref. | –. | Ref. | – | Ref. | – | Ref. | – |
| Overweight | 1.44 (1.36, 1.53) | <0.001 | 1.20 (1.14, 1.28) | <0.001 | 1.01 (0.95, 1.07) | 0.753 | 1.07 (0.88, 1.31) | 0.455 | |
| Obese | 1.86 (1.75, 1.98) | <0.001 | 1.63 (1.54, 1.74) | <0.001 | 1.02 (0.95, 1.09) | 0.620 | 0.81 (0.46, 1.43) | 0.477 | |
| Severe obesity | 2.06 (1.78, 2.38) | <0.001 | 2.3 (2.04, 2.75) | <0.001 | 1.02 (0.87, 1.19) | 0.799 | 1.09 (0.88, 1.34) | 0.821 | |
| Per 1-SD BMI increased | 1.23 (1.21, 1.25) | <0.001 | 1.24 (1.21, 1.27) | <0.001 | 1.01 (0.97, 1.03) | 0.900 | 0.97 (0.89, 1.05) | 0.492 | |
Association of BMI and cardiac conduction defects.
Normal weight: BMI 18.5–24.9, Overweight: BMI 25.0–29.9, Obese: BMI 30.0–39.9, Severe obesity: BMI > 40.
Model 1 adjusted for demographics (age, sex, race) Model 2 adjusted for Model 1 plus education level, social status, smoking status, alcohol consumption, diabetes, hypertension, dyslipidemia, and CVD (CAD, HF, AF, Valvular disease) Model 3 adjusted for model 2 plus physical activity.
Composite CCD: Cardiac conduction defects defined as AVB plus IVB.
AVB: Atrioventricular Block (1st,2nd, and 3rd degree AV block plus Mobitz II).
IVB: Intraventricular block (RBBB, LBBB, LPFB, LAFB and nonspecific intraventricular block).
1-SD BMI = 4.68.
Table 3
| Variables | Per 1-SD BMI increase | |
|---|---|---|
| Odds Ratio (95% CI) | Interaction p-value a | |
| Age | ||
| <65 | 1.01 (0.98, 1.03) | 0.041 |
| >65 | 1.25 (0.99, 1.73) | |
| Gender | ||
| Men | 1.09 (1.06, 1.12) | <0.001 |
| Women | 0.99 (0.95, 1.02) | |
| Race | ||
| Whites | 1.03 (1.00–1.06) | 0.216 |
| Non-whites | 1.08 (1.01–1.16) | |
| Diabetes | ||
| Yes | 1.08 (1.04, 1.12) | <0.001 |
| No | 0.99 (0.96, 1.02) | |
| Smoking status | ||
| Former | 1.00 (0.97, 1.03) | 0.557 |
| Current | 1.02 (0.96, 1.08) | |
| Physical Activity | ||
| Light | 1.01 (0.92, 1.11) | 0.476 |
| Moderate | 0.96 (0.87, 1.07) | 0.303 |
| Vigorous | 0.90 (0.74, 1.10) | 0.572 |
Association of BMI and cardiac conduction defects among sub-groups.
Model adjusted for demographics (age, sex, race), education level, social status, smoking status, alcohol consumption, diabetes, hypertension, dyslipidemia, CVD (coronary artery disease, heart failure, atrial fibrillation, valvular disease) and physical activity.
Discussion
In this cross-sectional analysis of the UK Biobank, a large-scale population survey, we demonstrated a dose-response relationship between higher BMI levels and an increased risk of CCD and its subtypes, particularly AVB. This association was particularly notable among older male participants with prevalent diabetes. These findings underscore the significance of obesity as a potential risk factor for CCD and further validate prior studies linking obesity to heart arrhythmias, including CCD. Integrating obesity into risk stratification and considering it as a potential target for prevention plans could help alleviate the burden of CCD.
Extensive research explored the relationship between obesity and tachyarrhythmia such as AF (13). Current guidelines recommend weight reduction measures for AF patients with obesity, as weight loss has been shown to mitigate the occurrence, progression, and recurrence of AFs (14–16). Despite these recommendations, there are no established clinical guidelines for the prevention of bradyarrhythmia and CCD.
After adjusting for confounders and prior CVD, our analysis consistently shows that obesity is associated with CCD, particularly AVB. Furthermore, despite showing higher odds for the IVB subtype, this association was not statistically significant. Varying effects of BMI across CCDs subtypes have been reported. For incidence, Liu et al. found an increased risk of CCD in participants with obesity, but no associations were observed with IVB except for LAFB and iRBBB in a Chinese cohort while Frimodt-Moller et al. reported higher incidence of infra-Hisian block in a US-based cohort with obesity (6, 7). In terms of electrocardiogram markers, obesity has been linked to prolonged PR interval and increased QRS duration (17, 18).
While the cross-sectional nature of our analysis precludes establishing causality, the association and variation across CCD subtypes can be explained by multifactorial mechanisms involving blood supply, cellular properties, and the anatomical location of the conduction system.
Potential pathophysiological explanations mainly derived by the linear association between epicardial adipose tissue (EAT) and obesity (19). Research has demonstrated the arrhythmogenicity and adverse cardiac effects associated with EAT (20). While literature mainly focused on tachyarrhythmias including AF and ventricular arrhythmias, similar concepts apply to slowing conduction pathways and bradyarrhythmia (21, 22). Peptides and adipokines diffuse freely between EAT and the subepicardial myocardium serving as an epitome for fibrosis (23). Fatty infiltrates in EAT often coexist with fibrosis, creating non-conducting barriers between myocyte strands and potentially altering gap junctions responsible for electrical impulse propagation (20, 24, 25). This fibro-fatty infiltration serves as a common substrate for both ventricular and atrial arrhythmias, with differences attributed to specific cellular properties and electrical impulse propagation, possibly explaining the heterogeneity observed between atrial and ventricular conduction defects (26).
The observed predominance of the association between obesity and AVB, rather than IVB, may be explained by several anatomical, metabolic, and electrophysiological differences between the AV node and the His–Purkinje system. Structurally, the AV node is a compact region with a single arterial supply in over 90% of individuals, making it particularly susceptible to ischemia in the context of obesity-related microvascular dysfunction (27, 28). Its limited vascular reserve and single-entry anatomy increase vulnerability to fibro-fatty infiltration and hypertrophy (28, 29). In contrast, the His bundle and bundle branches are typically supported by dual blood supply and insulated by fibrous tissue, which may delay ischemic or inflammatory remodeling (30). Moreover, the AV node's calcium-dependent and decremental conduction properties render it more sensitive to autonomic imbalance, inflammation, and metabolic stress (31), whereas the sodium-channel–dependent His–Purkinje fibers may require more advanced or prolonged remodeling before dysfunction becomes clinically evident (32, 33).
Epicardial adipose tissue (EAT), which increases with BMI and preferentially deposits around atrial and AV junctional structures, further contributes to this differential vulnerability (20, 27). EAT secretes pro-inflammatory and profibrotic cytokines that may disrupt ion channel expression, gap junction integrity, and conduction velocity in adjacent tissues. Histological evidence shows increased collagen deposition and reduced connexin-43 in areas of fatty infiltration, particularly around the AV node (27, 29). These combined anatomical and molecular factors may explain why AVB manifests earlier or more prominently with obesity, while IVB may require a longer duration or greater burden of pathologic adiposity to become clinically apparent (27–33). Further research is needed to confirm these mechanisms and assess whether interventions such as weight loss or metabolic therapies can differentially affect the progression of CCD subtypes.
With advancements in weight reduction medication in recent years, it is important to recognize the associated cardiac outcomes and their potential to influence on cardiac electrophysiology either through the pharmacological priorities of these agents or the achieved weight reduction (34, 35). For instance, recently approved medications such as Semaglutide and Tirzepatide have shown potential to improve cardiac outcomes and possibly influence its remodelling through metabolic modulation and weight reduction. In addition to the significant reductions in C-reactive protein and NT-proBNP reported in the STEP-HFpEF trial with semaglutide use in patients with obesity and HFpEF, a subsequent substudy demonstrated further favorable effects. Notably, reductions were observed in left atrial volume, EAT volume, and biomarkers of fibrosis such as galectin-3 and TGF-β (36, 37). These findings support the potential role of Semaglutide in modifying arrhythmogenic substrates associated with conduction disease.
Similarly, a substudy of the SUMMIT trial demonstrated that that Tirzepatide improved diastolic function and reduced epicardial fat and left atrial size- factors that are key in arrhythmogenic substrates, including conduction disturbances particularly in patients with obesity (38, 39). These findings indicate that newer pharmacologic treatments for obesity may alter the trajectory of cardiac conduction disease and warrant further investigation in this context.
Our analysis revealed that the effects of obesity and CCD were most pronounced in older male participants with prevalent diabetes, consistent with prior literature showing higher risk among older individuals and men across CCD subtypes (40). EAT, which is more abundant in men compared to women and increases linearly with BMI, secretes inflammatory and profibrotic cytokines that promote fibro-fatty infiltration of the AV node and surrounding myocardium (19).
Diabetes alone has been linked to increased risk of CCD through systemic inflammation, fibrosis, and autonomic dysfunction. When combined with obesity and aging, these effects are amplified by microvascular and endothelial dysfunction, further impairing blood flow to conduction tissue (41, 42). The combination of greater baseline EAT volume, accelerated fibro-fatty remodeling, and diabetes-associated microvascular disease may together create a synergistic substrate for conduction block in this high-risk subgroup.
Our results should be interpreted within certain limitations. Our study utilized ICD-9 codes to define clinical diagnoses, a method widely adopted in large-scale population-based research. However, ICD-9 codes may not fully capture the clinical complexity of certain conditions, potentially leading to diagnostic misclassification. As a result, there is a risk of imprecise identification of comorbidities and confounding variables, which could affect the accuracy of adjusted effect estimates. Despite adjusting for potential confounders and CVD risk factors, residual confounding by other comorbidities including obstructive sleep apnoea remains possible. Additionally, the UK Biobank study's sample may not fully represent the general UK population of the same age, as volunteers were older, more likely to be white, had higher socioeconomic status, and fewer cardiovascular disease risk factors. Furthermore, different classifications of BMI groups exist and are influenced by race and ethnicity, which could yield slightly different results. To counteract this, our analysis was adjusted for race and ethnicity. Additionally, some baseline variables were self-reported and captured through a nurse-led questionnaire, which may introduce recall bias and subjectivity despite the standardized data collection process. Despite these limitations, our analysis has several strengths, including a large sample size, numerous cases, and adjustments for common cardiovascular risk factors. Lifestyle habits and baseline covariates were prospectively ascertained using uniform methods according to predefined protocols.
Our findings show that higher BMI levels are linked to increased odds of CCD in the general population. Given the limited treatment options available for CCD, it is crucial to identify modifiable risk factors to mitigate its health and economic burden. Incorporating obesity into risk stratification and implementing preventive measures, particularly among older individuals with prevalent diabetes, could help alleviate the burden of CCD.
Statements
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://www.ukbiobank.ac.uk.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
MM: Methodology, Writing – original draft, Writing – review & editing. JK: Data curation, Formal analysis, Investigation, Software, Writing – original draft, Writing – review & editing. ES: Conceptualization, Investigation, Methodology, Resources, Validation, Writing – original draft, Writing – review & editing. PB: Conceptualization, Investigation, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
This article was presented as an abstract in HeartR ythm 2024 scientific sessions: https://doi.org/10.1016/j.hrthm.2024.03.679. There were no further contributions nor grant support to this project beyond those of the listed authors.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Bernstein AD Parsonnet V . Survey of cardiac pacing and implanted defibrillator practice patterns in the United States in 1997. Pacing Clin Electrophysiol. (2001) 24:842–55. 10.1046/j.1460-9592.2001.00842.x
2.
Da vies M Harris A . Pathological basis of primary heart block. Br Heart J. (1969) 31:219–26. 10.1136/hrt.31.2.219
3.
Bussink BE Holst AG Jespersen L Deckers JW Jensen GB Prescott E . Right bundle branch block: prevalence, risk factors, and outcome in the general population: results from the Copenhagen city heart study. Eur Heart J. (2013) 34:138–46. 10.1093/eurheartj/ehs291
4.
Clémenty N Carion PL Léotoing L Lamarsalle L Wilquin-Bequet F Brown B et al Infections and associated costs following cardiovascular implantable electronic device implantations: a nationwide cohort study. Europace. (2018) 20:1974–80. 10.1093/europace/eux387
5.
Ortega FB Lavie CJ Sui X . Health effects of overweight and obesity in 195 countries. N Engl J Med. (2017) 377:1495. 10.1056/NEJMc1710026
6.
Liu P Wang Y Zhang X Zhang Z Zhao N Ou W et al Obesity and cardiac conduction block disease in China. JAMA Netw Open. (2023) 6:e2342831. 10.1001/jamanetworkopen.2023.42831
7.
Frimodt-Møller EK Soliman EZ Kizer JR Vittinghoff E Psaty BM Biering-Sørensen T et al Lifestyle habits associated with cardiac conduction disease. Eur Heart J. (2023) 44:1058–66. 10.1093/eurheartj/ehac799
8.
Sudlow C Gallacher J Allen N Beral V Burton P Danesh J et al UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. (2015) 12:e1001779. 10.1371/journal.pmed.1001779
9.
Elliott P Peakman TC , UK Biobank. The UK biobank sample handling and storage protocol for the collection, processing and archiving of human blood and urine. Int J Epidemiol. (2008) 37:234–44. 10.1093/ije/dym276
10.
Frimodt-Møller EK Gottdiener JS Soliman EZ Kizer JR Vittinghoff E Psaty BM et al Inflammation and incident conduction disease. J Am Heart Assoc. (2023) 12:e027247. 10.1161/JAHA.123.027247
11.
Frimodt-Møller EK Vittinghoff E Kaur G Biering-Sørensen T Soliman EZ Marcus GM . Association between intensive vs standard blood pressure control and incident left ventricular conduction disease: a post hoc analysis of the SPRINT randomized clinical trial. JAMA Cardiol. (2023) 8:612–6. 10.1001/jamacardio.2023.0845
12.
Townsend P Phillimore P Beattie A . Health and Deprivation: Inequality and the North. London: Routledge (1988).
13.
Wong CX Sullivan T Sun MT Mahajan R Pathak RK Middeldorp M et al Obesity and the risk of incident, post-operative, and post-ablation atrial fibrillation: a meta-analysis of 626,603 individuals in 51 studies. JACC Clin Electrophysiol. (2015) 1:139–52. 10.1016/j.jacep.2015.04.004
14.
Writing Committee Members, JoglarJAChungMKArmbrusterALBenjaminEJChyouJYet al2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. J Am Coll Cardiol. (2024) 83:109–279. 10.1016/j.jacc.2023.08.017
15.
Lavie CJ Pandey A Lau DH Alpert MA Sanders P . Obesity and atrial fibrillation prevalence, pathogenesis, and prognosis: effects of weight loss and exercise. J Am Coll Cardiol. (2017) 70:2022–35. 10.1016/j.jacc.2017.09.002
16.
Abed HS Wittert GA Leong DP Shirazi MG Bahrami B Middeldorp ME et al Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation: a randomized clinical trial. JAMA. (2013) 310:2050–60. 10.1001/jama.2013.280521
17.
Magnani JW Lopez FL Soliman EZ Maclehose RF Crow RS Alonso A . P wave indices, obesity, and the metabolic syndrome: the atherosclerosis risk in communities study. Obesity (Silver Spring). (2012) 20:666–72. 10.1038/oby.2011.53
18.
Frank S Colliver JA Frank A . The electrocardiogram in obesity: statistical analysis of 1,029 patients. J Am Coll Cardiol. (1986) 7:295–9. 10.1016/S0735-1097(86)80494-6
19.
Rabkin SW . The relationship between epicardial fat and indices of obesity and the metabolic syndrome: a systematic review and meta-analysis. Metab Syndr Relat Disord. (2014) 12:31–42. 10.1089/met.2013.0107
20.
Chaumont C Suffee N Gandjbakhch E Balse E Anselme F Hatem SN . Epicardial origin of cardiac arrhythmias: clinical evidences and pathophysiology. Cardiovasc Res. (2022) 118:1693–702. 10.1093/cvr/cvab213
21.
Ernault AC Meijborg VMF Coronel R . Modulation of cardiac arrhythmogenesis by epicardial adipose tissue. J Am Coll Cardiol. (2021) 78:1730–45. 10.1016/j.jacc.2021.08.037
22.
Wong CX Abed HS Molaee P Nelson AJ Brooks AG Sharma G et al Pericardial fat is associated with atrial fibrillation severity and ablation outcome. J Am Coll Cardiol. (2011) 57:1745–51. 10.1016/j.jacc.2010.11.045
23.
Carpenter HM . Myocardial fat infiltration. Am Heart J. (1962) 63:491–6. 10.1016/0002-8703(62)90305-8
24.
Abed HS Samuel CS Lau DH Kelly DJ Royce SG Alasady M et al Obesity results in progressive atrial structural and electrical remodeling: implications for atrial fibrillation. Heart Rhythm. (2013) 10:90–100. 10.1016/j.hrthm.2012.08.043
25.
Azaouagh A Churzidse S Konorza T Erbel R . Arrhythmogenic right ventricular cardiomyopathy/dysplasia: a review and update. Clin Res Cardiol. (2011) 100:383–94. 10.1007/s00392-011-0295-2
26.
Wynn TA . Cellular and molecular mechanisms of fibrosis. J Pathol. (2008) 214:199–210. 10.1002/path.2277
27.
Park JS Ahn SG Hwang JW Lim HS Choi BJ Choi SY et al Impact of body mass index on the relationship of epicardial adipose tissue to metabolic syndrome and coronary artery disease in an Asian population. Cardiovasc Diabetol. (2010) 9:29. 10.1186/1475-2840-9-29
28.
Álvarez-García J Vives-Borrás M Gomis P Ordoñez-Llanos J Ferrero-Gregori A Serra-Peñaranda A et al Electrophysiological effects of selective atrial coronary artery occlusion in humans. Circulation. (2016) 133:2235–42. 10.1161/CIRCULATIONAHA.116.021700
29.
Kasper EK Hruban RH Baughman KL . Cardiomyopathy of obesity: a clinicopathologic evaluation of 43 obese patients with heart failure. Am J Cardiol. (1992) 70:921–4. 10.1016/0002-9149(92)90739-L
30.
Oh IY Cha MJ Lee TH Seo JW Oh S . Unsolved questions on the anatomy of the ventricular conduction system. Korean Circ J. (2018) 48:1081–96. 10.4070/kcj.2018.0335
31.
George SA Faye NR Murillo-Berlioz A Lee KB Trachiotis GD Efimov IR et al At the atrioventricular crossroads: dual pathway electrophysiology in the atrioventricular node and its underlying heterogeneities. Arrhythm Electrophysiol Rev. (2017) 6:179–85. 10.15420/aer.2017.30.1
32.
Grant AO . Molecular biology of sodium channels and their role in cardiac arrhythmias. Am J Med. (2001) 110:296–305. 10.1016/s0002-9343(00)00714-2
33.
Cardona A Arnold WD Kissel JT Raman SV Zareba KM . Myocardial fibrosis by late gadolinium enhancement cardiovascular magnetic resonance in myotonic muscular dystrophy type 1: highly prevalent but not associated with surface conduction abnormality. J Cardiovasc Magn Reson. (2019) 21:26. 10.1186/s12968-019-0535-6
34.
Guo Y Lin C Cai X Wu H Yan J Li Z et al The weight reduction mediated by anti-obesity medication and the cardiovascular outcome. iScience. (2024) 27:110867. 10.1016/j.isci.2024.110867
35.
Baser O Samayoa G Rodchenko K Isenman L Baser E Yapar N . The association between weight loss medications and cardiovascular complications. Obesity (Silver Spring). (2024) 32:1401–09. 10.1002/oby.24037
36.
Kosiborod MN Abildstrøm SZ Borlaug BA Butler J Rasmussen S Davies M et al Semaglutide in patients with heart failure with preserved ejection fraction and obesity. N Engl J Med. (2023) 389:1069–84. 10.1056/NEJMoa2306963
37.
Solomon SD Ostrominski JW Wang X Shah SJ Borlaug BA Butler J et al Effect of semaglutide on cardiac structure and function in patients with obesity-related heart failure. J Am Coll Cardiol. (2024) 84:1587–602. 10.1016/j.jacc.2024.08.021
38.
Packer M Zile MR Kramer CM Baum SJ Litwin SE Menon V et al Tirzepatide for heart failure with preserved ejection fraction and obesity. N Engl J Med. (2025) 392:427–37. 10.1056/NEJMoa2410027
39.
Kramer CM Borlaug BA Zile MR Ruff D DiMaria JM Menon V et al Tirzepatide reduces LV mass and paracardiac adipose tissue in obesity-related heart failure: SUMMIT CMR substudy. J Am Coll Cardiol. (2025) 85:699–706. 10.1016/j.jacc.2024.11.001
40.
Li YG Benditt DG Klingenheben T Hu K Feng D . Cardiac arrhythmias: update on mechanisms and clinical managements. Cardiol Res Pract. (2016) 2016:8023723. 10.1155/2016/8023723
41.
Kerola T Eranti A Aro AL Haukilahti MA Holkeri A Junttila MJ et al Risk factors associated with atrioventricular block. JAMA Netw Open. (2019) 2:e194176. 10.1001/jamanetworkopen.2019.4176
42.
Movahed MR . Diabetes as a risk factor for cardiac conduction defects: a review. Diabetes Obes Metab. (2007) 9:276–81. 10.1111/j.1463-1326.2006.00609
Summary
Keywords
BMI, obesity, cardiac conduction defects, heart block, UK biobank
Citation
Mostafa MA, Kingsley JA, Soliman EZ and Bhave PD (2025) Association between obesity and cardiac conduction defects. Front. Cardiovasc. Med. 12:1476935. doi: 10.3389/fcvm.2025.1476935
Received
06 August 2024
Accepted
27 June 2025
Published
09 July 2025
Volume
12 - 2025
Edited by
Hendrik Tevaearai Stahel, University Hospital of Bern, Switzerland
Reviewed by
Pietro Scicchitano, ASLBari—Azienda Sanitaria Localedella provincia di Bari (ASL BA), Italy
Hanqing Liu, Zhejiang University, China
Updates
Copyright
© 2025 Mostafa, Kingsley, Soliman and Bhave.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Prashant D. Bhave Prashant.Bhave@advocatehealth.org
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.