Abstract
Introduction:
Free wall rupture is a rare complication of myocardial infarction, occurring as early as a few hours after myocardial infarction. It usually happens after transmural myocardial infarction and has a very high mortality rate. The diagnosis of free wall rupture requires echocardiographic evidence of pericardial leakage or pericardial tamponade. The patient's cause of death is often attributed to other causes such as cardiac arrhythmia unless determined by open heart surgery or autopsy. Diagnostic tools for post-infarction cardiac free wall rupture are limited to date.
Case presentation:
We present a case of myocardial infarction in a patient who presented with atypical chest pain. Subsequent coronary angiography revealed small vessel disease, however, the patient's severe clinical presentation was not consistent with small vessel disease. The initial clinical presentation did not rule out aortic dissection, prompting further investigation through aortic computed tomography. This imaging technique revealed a rupture of the free wall of the heart accompanied by a large amount of bloody pericardial effusion. Unfortunately, attempts to puncture and drain the effusion were unsuccessful and the patient eventually succumbed.
Conclusions:
When patients present with more severe clinical manifestations that are not consistent with myocardial infarction, it is important to be alert to the possibility of cardiac rupture in addition to identifying the possibility of aortic dissection. Aortic CTA may be able to confirm the diagnosis of cardiac rupture.
1 Introduction
Cardiac rupture is one of the complications of myocardial infarction and is often characterized by acute episodes of shortness of breath, chest pain, shock, sweating, unexplained vomiting, cold clammy skin, and fainting (1). Risk factors for cardiac rupture after myocardial infarction include older adults, women, hypertension (2–6), single-vessel disease, and first-time infarction (7, 8). Pericardial effusion is the most common manifestation of cardiac rupture during acute myocardial infarction. It has extremely limited diagnostic tools and can often only be clarified by open-heart surgery or occasionally by echocardiography or angiography showing blood leakage into the pericardial cavity (9). A ruptured heart can lead to rapid death, making prompt diagnosis and treatment crucial1. We will present a patient with myocardial infarction who had a rupture of the free wall of the heart detected not by cardiac ultrasound or coronary angiography but by aortic computed tomography angiography (CTA).
2 Case presentation
We report a 47-year-old middle-aged male admitted to the emergency department with severe posterior back and lumbar pain. The patient's general state was poor, characterized by agitation, dyspnea, inability to lie down, cyanosis, and clammy cold skin. Electrocardiogram showed ST-segment elevation in leads II, III and AVF (Figure 1). Based on the patient's symptoms and history of hypertension it was considered that aortic dissection was not excluded and that the elevation of the ST segment in the inferior wall lead could be due to a cumulative right coronary artery opening. It was then decided to further identify the patient by drawing blood for laboratory tests.
Figure 1
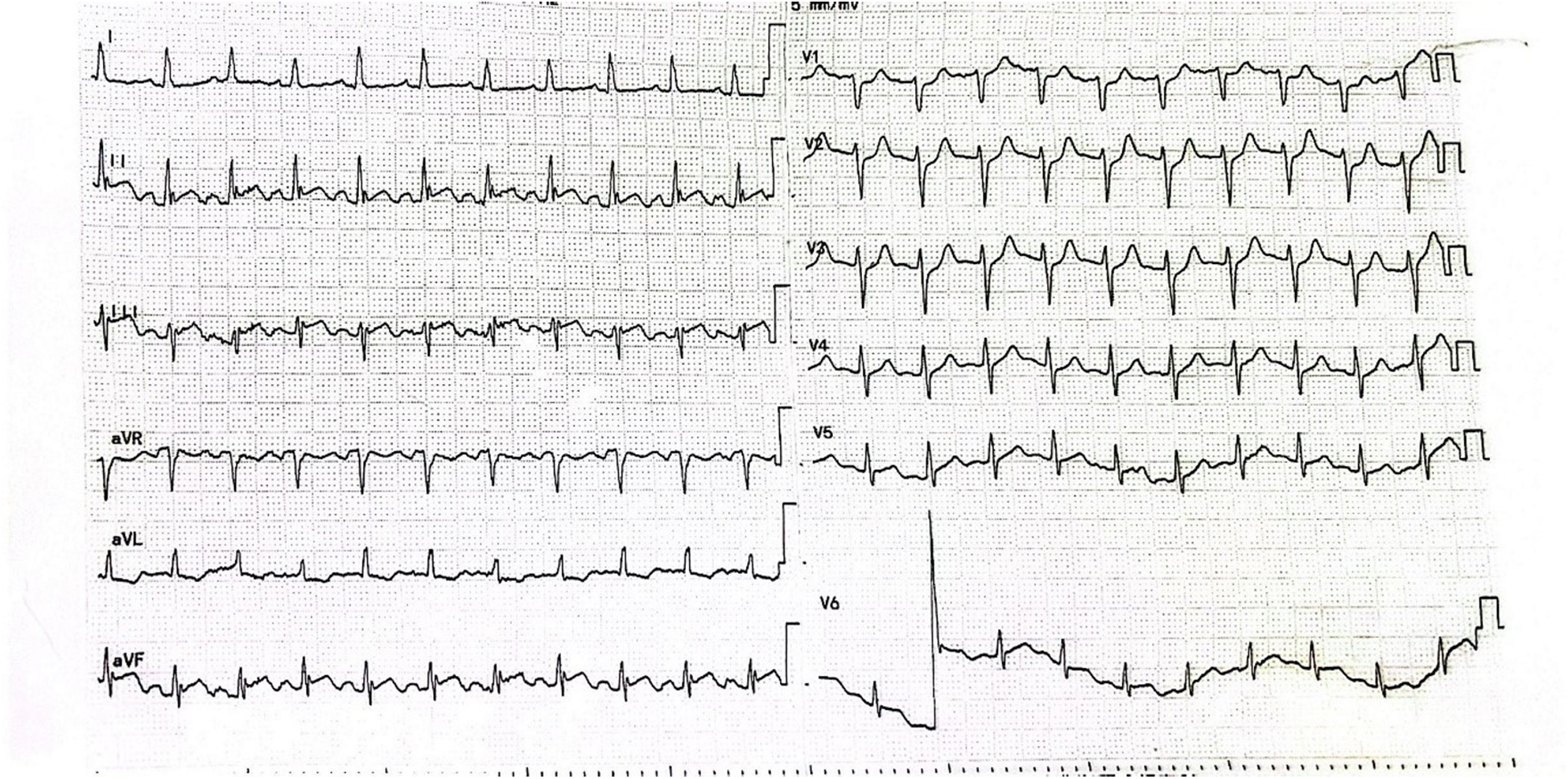
Patient's electrocardiogram on admission.
After waiting for 10 min, the laboratory results showed TroponinI 0.87 ng/ml (0.01–0.023), D-dimer: 978 ng/ml (80–500), N-Terminal Pro-Brain Natriuretic Peptide 599 ng/L (300–500), Lactate 25 mmol/L (0.5–1.6), Glucose 30 mmol/L (3.9–5.8), Potassium 2.7 mmol/L (3.4–4.5). Considering that the patient's troponin was markedly elevated and his D-dimer was not, the decision was made to proceed with coronary arteriography. The coronary angiography of the patient showed normal left coronary artery opening and main body; more than 70% systolic stenosis in the middle muscular bridge of the left anterior descending branch, about 50% stenosis in the middle of the diagonal branch, TIMI flow grade 3; normal opening of the left coronary artery circumflex, no obvious stenosis in the main body, distal occlusion, TIMI flow grade 0; normal opening of the right coronary artery, 30%–40% stenosis in the middle, TIMI flow grade 3 (Figure 2A). During the operation, the patient's blood pressure decreased, and we gave fluid replacement and continuous noninvasive ventilator-assisted breathing. The vessels in the distal segment of the occluded circumflex were small (Figure 2A), which was inconsistent with the clinical manifestations of critical illness. Considering the possibility of aortic dissection, an urgent aortic CTA was performed.
Figure 2
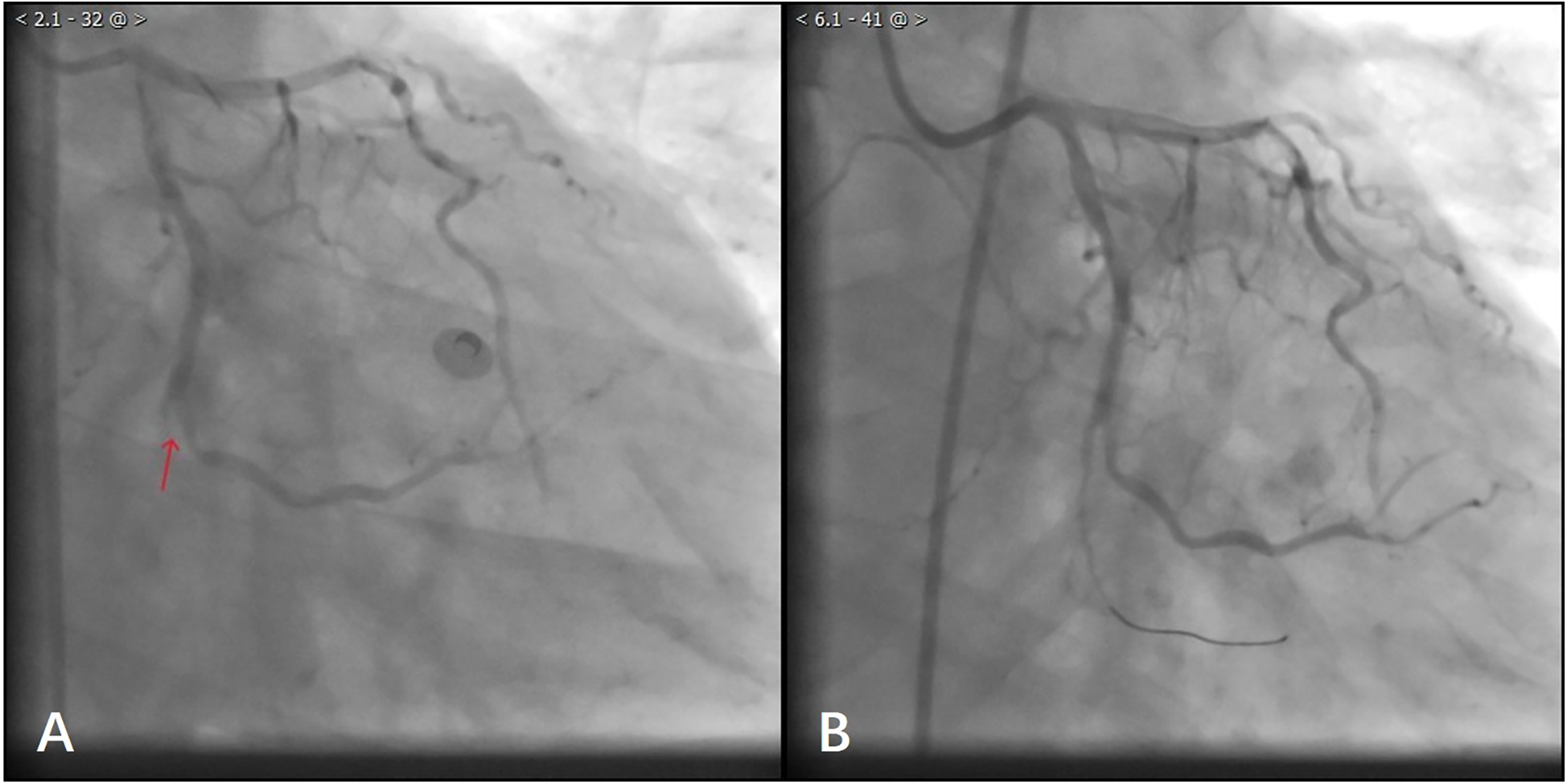
(A) Patient with angiographic occlusion of vessels distal to the gyratory branch. (B) Blood flow through the distal vessels of the gyratory branch after balloon dilatation.
During this period, although the patient underwent echocardiography, the bedside ultrasound did not find pericardial tamponade, but only moderate amount of pericardial effusion due to the patient's obesity, restlessness and the limitations of the ultrasound operator's level. Subsequent aortic CTA revealed a significant volume of pericardial effusion without evidence of aortic dissection. The clinical manifestations were initially attributed to the combined effects of pericardial effusion and coronary pathology. To further clarify the cause of death, a senior radiologist meticulously reviewed the imaging studies and identified contrast medium extravasation from the myocardium into the pericardial cavity (Figure 3). After aortic dissection was ruled out, the patient was returned to the cath lab for further treatment of distal circumflex branch occlusion and pericardiocentesis. Procedure: The guide wire was sent through the catheter and passed through the distal end of the circumflex branch occlusion segment successfully. Blood flow passed through the circumflex branch occlusion segment after balloon expansion. The TIMI grade was 3 (Figure 2B). Unfortunately, pericardiocentesis under DSA guidance was not successful.
Figure 3
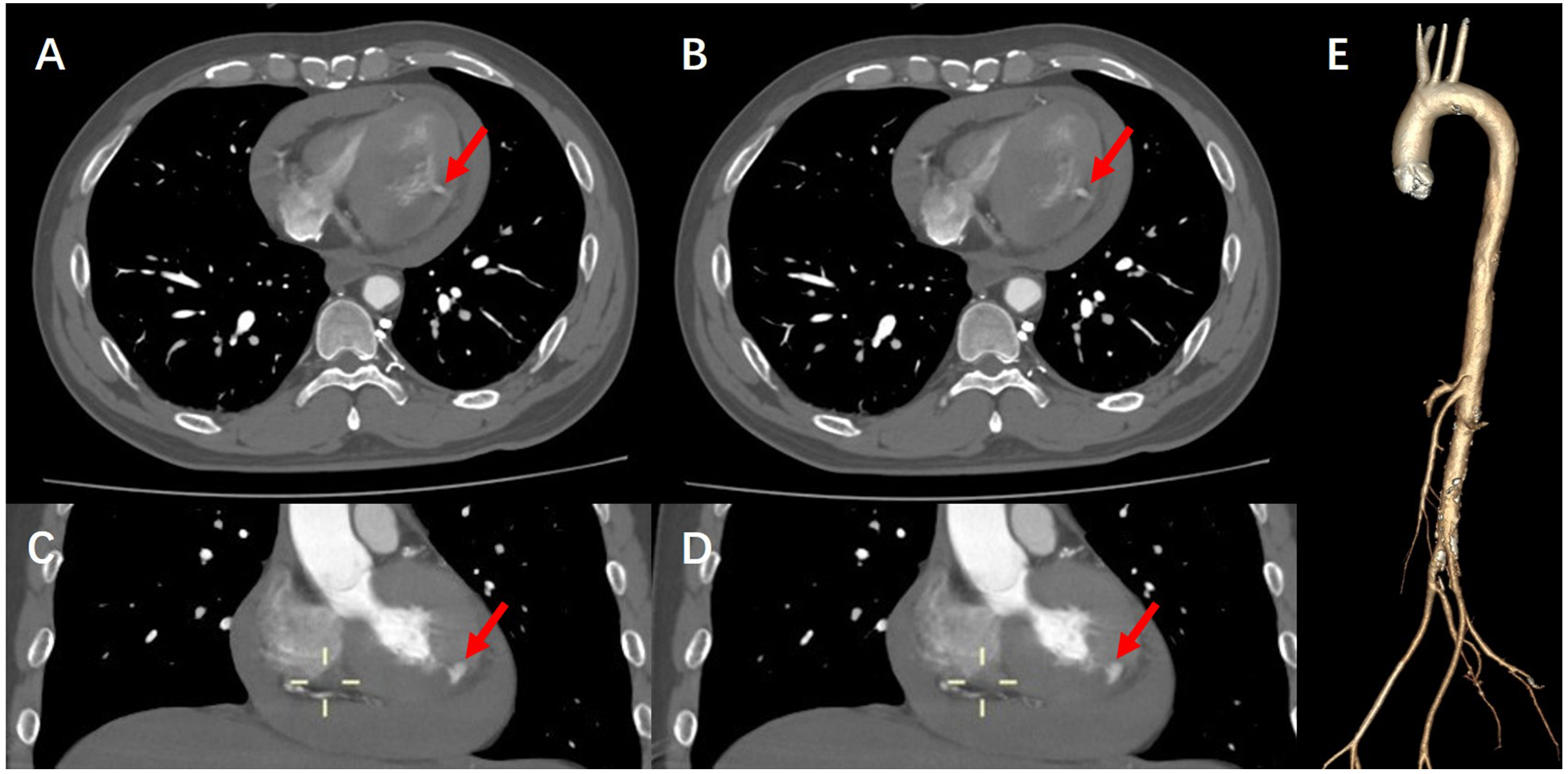
(A,B) CTA of the aorta demonstrate contrast extravasation into the myocardium, along with right-sided heart failure and pericardial effusion. (C,D) Coronal views of aortic CTA further reveal contrast leakage into the myocardial tissue. (E) Three-dimensional reconstruction of the aorta confirms the absence of aortic dissection, intramural hematoma, or other vascular anomalies.
The patient's symptoms of generalized cyanosis and dyspnea have been increasing. The patient was then transferred to the cardiac care unit (CCU) for ultrasound-guided pericardiocentesis. After transfer to the intensive care unit, the patient's ultrasound-guided pericardiocentesis remained unsuccessful due to a large amount of gas interference in the chest cavity. Given the possibility of pneumothorax, the patient underwent a computed tomography (CT) scan of the lungs (Figure 4). Pulmonary CT indicated pneumothorax, increased pericardial effusion, and more collapsed right ventricle. Upon transfer back to the intensive care unit, the patient suddenly experienced respiratory arrest, coma, slowed heart rate, and undetectable blood pressure. Emergency rescue of the patient, unfortunately, the patient did not rescue successfully, and eventually died.
Figure 4
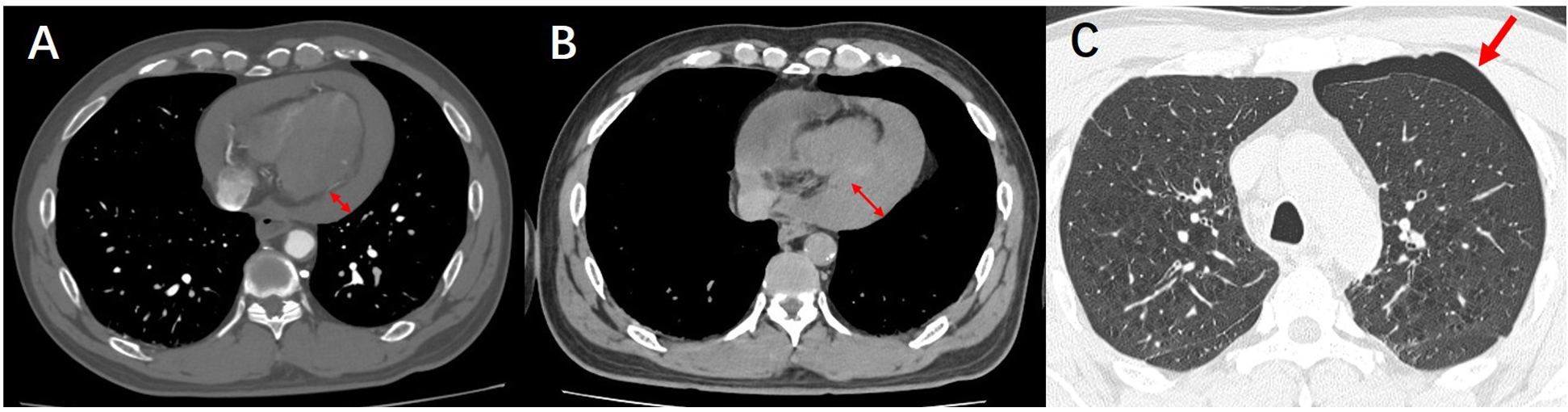
(A) CTA demonstrates pericardial effusion. (B) Follow-up lung CT reveals an increased volume of pericardial effusion. (C) Follow-up lung CT shows newly developed pneumothorax.
3 Discussion
Cardiac rupture is one of the most serious complications of acute myocardial infarction. Although its incidence is only 2%–4%, the mortality rate is as high as 90%. This higher mortality rate is closely related to pericardial tamponade (9). Cardiac rupture is not a sudden full-layer rupture of the myocardium. Instead, a rupture occurs in the endocardium first, and blood flows from the rupture into the myocardium to form a myocardial entrapment hematoma, which gradually penetrates to the epicardium, and ultimately ruptures the whole layer to occur pericardial pressure occlusion.
Once a heart rupture has occurred, timely and accurate diagnosis is important (1). Patients presenting with acute catastrophic rupture usually die immediately and are often diagnosed by autopsy (10). In some patients, the clinical manifestation of the subacute process is accompanied by persistent or recurrent severe chest pain after acute myocardial infarction, with entrapment hematoma in the myocardium, and a drop in blood pressure and deterioration of the condition. The blood pressure continues to remain at a low level for several hours to more than ten hours, with a gradual accumulation of seeping blood in the pericardial cavity, followed by the phenomenon of pericardial tamponade.
Early diagnosis and timely emergency surgical treatment of such patients can be expected to be successful. Given its convenience and rapid availability, echocardiography remains recognized as the first-line diagnostic modality for cardiac rupture (11, 12). Although echocardiography demonstrates moderate sensitivity (70%) and high specificity (90%) in detecting cardiac rupture, its diagnostic accuracy heavily depends on operator expertise and patient cooperation (13), which can be particularly challenging in critically ill patients. Therefore, when echocardiography fails to provide a definitive diagnosis, supplementary imaging modalities should be considered. In our case, no extravasation of contrast media was found in the patient during coronary angiography, but the clinical manifestations were inconsistent with coronary vascular disease. However, in order to rule out aortic dissection, we performed an aortic CTA examination and observed contrast agent shuttling through the myocardium and reaching the pericardium, definitively diagnosing the patient's heart rupture diagnosis.
Unfortunately, at the initial diagnosis, we did not alert the patient to the possibility of heart rupture and did not perform pericardial puncture in time. When DSA and cardiac ultrasound were not successfully drained, we considered surgery for the patient, but unfortunately the available surgeon did not arrive at the hospital and the patient died.
This case suggests that when coronary angiography and echocardiography fail to detect heart rupture, especially when there are high risk factors for heart rupture, we may be able to detect heart rupture in patients with myocardial infarction through aortic CTA. At the same time, we should be alert to the possibility of heart rupture when the angiography results are small coronary artery lesions, but the patient has critical clinical manifestations.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of China-Japan Union Hospital of Jilin University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JL: Writing – original draft. WC: Writing – review & editing. ZL: Writing – review & editing. NL: Writing – review & editing. CM: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by The Effects of High-Intensity Interval Training on Cardiac Function in Rats with Hypertrophic Cardiomyopathy (2021YX0341). The funding body had no role in the design or writing of the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Pallangyo A Pyuza JJ Nkya G Amsi P Andongolile A Makata AM et al Ventricular silent rupture leading to sudden death: navigating diagnostic challenges in a resource-constraint setting. Clin Case Rep. (2024) 12(1):e8439. 10.1002/ccr3.8439
2.
Christensen DJ Ford M Reading J Castle CH . Effect of hypertension on myocardial rupture after acute myocardial infarction. Chest. (1977) 72(5):618–22. 10.1378/chest.72.5.618
3.
Pollak H Nobis H Mlczoch J . Frequency of left ventricular free wall rupture complicating acute myocardial infarction since the advent of thrombolysis. Am J Cardiol. (1994) 74(2):184–6. 10.1016/0002-9149(94)90098-1
4.
Shapira I Isakov A Burke M Almog C . Cardiac rupture in patients with acute myocardial infarction. Chest. (1987) 92(2):219–23. 10.1378/chest.92.2.219
5.
Menon V Webb JG Hillis LD Sleeper LA Abboud R Dzavik V et al Outcome and profile of ventricular septal rupture with cardiogenic shock after myocardial infarction: a report from the SHOCK trial registry. SHould we emergently revascularize occluded coronaries in cardiogenic shocK? J Am Coll Cardiol. (2000) 36(3 Suppl A):1110–6. 10.1016/S0735-1097(00)00878-0
6.
Batts KP Ackermann DM Edwards WD . Postinfarction rupture of the left ventricular free wall: clinicopathologic correlates in 100 consecutive autopsy cases. Hum Pathol. (1990) 21(5):530–5. 10.1016/0046-8177(90)90010-3
7.
Mann JM Roberts WC . Rupture of the left ventricular free wall during acute myocardial infarction: analysis of 138 necropsy patients and comparison with 50 necropsy patients with acute myocardial infarction without rupture. Am J Cardiol. (1988) 62(13):847–59. 10.1016/0002-9149(88)90881-8
8.
Saffitz JE Fredrickson RC Roberts WC . Relation of size of transmural acute myocardial infarct to mode of death, interval between infarction and death and frequency of coronary arterial thrombus. Am J Cardiol. (1986) 57(15):1249–54. 10.1016/0002-9149(86)90198-0
9.
Figueras J Cortadellas J Soler-Soler J . Left ventricular free wall rupture: clinical presentation and management. Heart. (2000) 83(5):499–504. 10.1136/heart.83.5.499
10.
Montegut FJ Jr . Left ventricular rupture secondary to myocardial infarction. Report of survival with surgical repair. Ann Thorac Surg. (1972) 14(1):75–8. 10.1016/S0003-4975(10)65202-2
11.
Liu S Glavinovic T Tam JW . Early diagnosis and management of myocardial rupture. Can J Cardiol. (2015) 31(1):88–90. 10.1016/j.cjca.2014.09.026
12.
Wang X Nie F Ye N Liu X Yang S Guo F et al Successful occlusion of ventricular septal rupture in myocardial infarction under the guidance of echocardiography. J Cardiothorac Surg. (2019) 14(1):133. 10.1186/s13019-019-0954-3
13.
Krishnan U McCann GP Hickey M Schmitt M . Role of contrast-enhanced magnetic resonance imaging in detecting early adverse remodeling and subacute ventricular wall rupture complicating myocardial infarction. Heart Vessels. (2008) 23(6):430–2. 10.1007/s00380-008-1063-8
Summary
Keywords
cardiac rupture, free wall rupture, aortic computed tomography angiography, diagnosis, myocardial infarction
Citation
Liu J, Chen W, Li Z, Liu N and Mao C (2025) Case Report: Rupture of the cardiac free wall after myocardial infarction confirmed by aortic computed tomography angiography. Front. Cardiovasc. Med. 12:1502336. doi: 10.3389/fcvm.2025.1502336
Received
26 September 2024
Accepted
29 May 2025
Published
19 June 2025
Volume
12 - 2025
Edited by
Junjie Xiao, Shanghai University, China
Reviewed by
Kenichi Hongo, Jikei University School of Medicine, Japan
Monika Bhandari, King George's Medical University, India
Updates
Copyright
© 2025 Liu, Chen, Li, Liu and Mao.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Cuiying Mao maocy@jlu.edu.cn
†These authors share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.