Abstract
Background:
Chronic kidney disease (CKD) is a significant global health challenge, particularly in low- and middle-income countries where hypertension is a prominent risk factor. This study aimed to assess the prevalence of CKD and its associated risk factors among hypertensive adults at the Burao General Hospital in Somaliland, Somalia.
Methods:
A cross-sectional study was conducted from January to July 2024, enrolling 262 hypertensive adults using consecutive sampling. Data were collected using structured questionnaires addressing sociodemographic factors, clinical characteristics, and lifestyle choices. Proteinuria levels and estimated glomerular filtration rates were assessed to confirm CKD diagnosis.
Results:
The prevalence of CKD among hypertensive patients was 52.67% (95% CI: 46.6%–58.7%). Significant associations were observed between CKD and factors such as age, proteinuria, diabetes, blood pressure control, and body mass index (BMI). Specifically, proteinuria was strongly linked to CKD (AOR: 10.72, 95% CI: 5.74–20.04). Individuals aged 34–41 years (AOR: 3.39, 95% CI: 0.99–11.54) and those classified as overweight (AOR: 3.37, 95% CI: 1.65–6.88) were at greater risk for CKD.
Conclusion:
The findings highlight a critical association between hypertension and CKD, emphasizing the need for targeted interventions to effectively manage hypertension and address modifiable risk factors. Understanding these relationships is vital for developing healthcare policies aimed at reducing CKD prevalence among adults in Burao City, Somaliland.
1 Background
Chronic kidney disease (CKD) is a significant global health issue, particularly in low- and middle-income countries, where access to healthcare can be limited (1, 2). One of the leading risk factors for CKD is hypertension, which greatly increases the risk of both kidney and cardiovascular diseases (3, 4).
The correlation between hypertension and CKD has been well established in the literature (5–7). Epidemiological studies have consistently shown that hypertension is both a cause and consequence of CKD, creating a vicious cycle of kidney damage and worsening of blood pressure control (3, 8). A large meta-analysis of cohort studies found that individuals with hypertension had a 1.81 times higher risk of developing CKD than those without hypertension (95% CI: 1.47–2.23) (9–11). Additionally, clinical trials have demonstrated that intensive blood pressure control can slow CKD progression (12). The SPRINT trial showed that targeting a systolic blood pressure of <120 mmHg, compared to <140 mmHg, reduced the risk of CKD progression by 32% in non-diabetic adults (13, 14).
The prevalence of hypertension is notably high in Burao and Hargeisa City, Somaliland; however, its impact on kidney health has not been thoroughly examined (15). While there is evidence of a clear link between hypertension and CKD globally, few studies have focused specifically on adults in this region, highlighting a critical gap in our understanding (16, 17).
This study investigated the effect of hypertension on CKD among adults visiting Burao General Hospital, specifically examining associated factors such as age, diabetes, protein levels in urine, and body mass index. We hypothesized that uncontrolled hypertension is linked to a higher risk of CKD, particularly in patients with additional health. Understanding these relationships is essential for developing effective strategies to reduce the impact of CKD on individuals with high blood pressure in Burao.
The findings of this study are expected to provide valuable insights and inform healthcare policies aimed at improving kidney health in Somaliland. Therefore, this study aimed to explore the prevalence of hypertension among adults with CKD and to assess the impact of various associated factors in Burao General Hospital, Burao City, Somaliland, and Somalia.
2 Materials and Methods
2.1 Study area
This cross-sectional study was conducted at the Burao General Hospital, located in Burao City, Somaliland, Somalia. The hospital is the primary healthcare provider in the Togdheer region, serving a population of over 400,000 (18) Burao General Hospital offers comprehensive medical services, including diagnostic and treatment facilities for hypertension and chronic kidney disease (CKD), making it an ideal setting for this study (19, 20). Given the hospital's strategic location and role as a referral center, it manages a significant number of patients with hypertensive and kidney-related conditions (20). This study focused on adults visiting the hospital, representing a mix of urban and rural populations, and aimed to explore the prevalence and impact of hypertension on CKD.
2.2 Study design and period
The study was conducted from January to July 2024 using a hospital-based cross-sectional study design.
2.3 Study population and sample size determination
The source population for this study consisted of adult patients with chronic kidney disease who had hypertension in Burao City, Somaliland. The study population included all individuals diagnosed with chronic kidney disease presenting in Burao City. The overall sample size determined for this study was 262 participants based on the prevalence of chronic kidney disease among hypertensive patients in Ethiopia.
2.4 Eligibility criteria
2.4.1 Inclusion criteria
Adult hypertensive patients aged 18 years or older attending Burao General Hospital between January and July 2024, irrespective of eGFR values were includes.
2.4.2 Exclusion criteria
In contrast, pregnant patients with hypertension during the study period and individuals who developed the outcome of interest prior to or at the start of the follow-up period were excluded. Additionally, patients with an eGFR < 60 ml/min/1.73 m2 were also not considered for inclusion in the study.
2.5 Sampling techniques
A consecutive sampling technique was implemented, systematically incorporating each patient into the study to ensure comprehensive representation.
2.6 Data collection instrument and techniques
This study used a structured questionnaire for face-to-face interviews and patient chart review. The questionnaire was initially developed in English, translated into Somali, and back-translated into English to ensure accuracy. It covered sociodemographic information, clinical characteristics, and lifestyle-related factors associated with the prevalence of chronic kidney disease among patients at Burao General Hospital, Somaliland. Data collection was carried out by four students under the supervision of one supervisor, and interviews were conducted directly with the respondents. Data quality control was ensured through a 5% pre-test, leading to minor modifications based on expert suggestions. The supervisor checked the data daily for completeness and the principal investigator reviewed the data before and after administration. Backup data were stored on an external hard drive and each questionnaire was coded to prevent duplication and errors.
2.7 Measurement tools
Data on body mass index (BMI) and blood pressure control status were collected and analyzed as categorical variables. BMI was classified as normal vs. overweight/obese, while blood pressure control was classified as controlled (<140/90 mmHg) vs. uncontrolled (≥140/90 mmHg), as per the patient records. Therefore, mean values and standard deviations were not calculated for these variables. For the urine dipstick test, the patient was asked to provide a random midstream urine sample in a 50 ml container before admission for laboratory analysis. The samples were tested for protein and creatinine levels using a Uriplus 900 urinalysis strip. The manufacturer's grades for proteinuria are as follows:
- •
0: Absent
- •
Traces: 15–30 mg/dl
- •
1+: 30–100 mg/dl
- •
2+: 100–300 mg/dl
- •
3+: 300–1,000 mg/dl
- •
4+: Greater than 1,000 mg/dl
Estimated glomerular filtration rate (eGFR) was used to evaluate kidney function by estimating the volume of blood filtered by the kidneys over time. This can be calculated using formulae such as the Cockcroft-Gault equation (adjusted for body surface area [BSA]):
BSA can be calculated using the Mosteller formula, as follows:
here, weight was measured in kilograms and height in centimeters.
2.8 Data processing and analysis
The data were coded, entered, and cleaned using EpiData Manager version 4.2, then exported to STATA version 17 for further analysis. Chronic kidney disease was confirmed through a review of medical charts at hospitals. Baseline demographic, clinical, laboratory, and social factors were considered independent variables in the analysis. A binary logistic regression model was used to assess the relationship between the outcomes and these independent variables. Variables with a P-value of less than 0.25 in the bivariate analysis were included in the multivariable binary logistic regression. Odds ratios with 95% confidence intervals were calculated, and a P-value below 0.05 was considered indicative of a significant association. Multicollinearity was assessed using the Variance Inflation Factor (VIF). A threshold of 2.5 was used to indicate potential multicollinearity. All variables included in the multivariate logistic regression model had VIF values well below this cutoff.
2.9 Variables
2.9.1 Dependent variable
Chronic kidney disease.
2.9.2 Independent variables
Sociodemographic factors included age, sex, education level, occupation, income, and marital status. Clinical characteristics: blood pressure status, baseline diastolic and systolic blood pressure, hypertension stage, type of hypertension, number of medications, duration of medication, types of medications, presence of diabetes mellitus, dyslipidemia, and obesity. Lifestyle factors: Smoking and alcohol consumption.
2.10 Operational definitions
2.10.1 Alcohol exposure
Participants were considered alcohol consumers if their medical records indicated any intake of alcoholic beverages.
2.10.2 Chronic kidney disease (CKD)
Defined according to KDIGO guidelines as either an estimated glomerular filtration rate (eGFR) < 60 ml/min/1.73 m2, or evidence of kidney damage (proteinuria ≥1+ on urine dipstick) persisting for at least 3 months, documented in medical records (21, 22).
2.10.3 Diabetes mellitus
Diabetes mellitus is defined as a disorder in which the body does not produce sufficient insulin or fails to respond to insulin effectively, leading to consistently elevated blood sugar (glucose) levels.
2.10.4 Hypertension
Hypertension was identified in participants with a documented diagnosis of elevated blood pressure (BP ≥ 140/90 mmHg) or in those on antihypertensive medications. The target BP control was defined as BP < 140/90 mmHg for non-diabetic patients and BP < 130/80 mmHg for diabetic patients (23).
3 Result
3.1 Socio demographic characteristics
The demographic data indicated that the majority of participants were aged 34–41 years (28.24%), followed by those aged 27–33 (14.50%) and 42–48 (19.85%). The sample consisted of 47.33% males and 52.67% females. In terms of marital status, most participants were married (79.01%), with smaller percentages identifying single (8.78%), divorced (9.92%), and widowed (2.29%) (Table 1).
Table 1
| Variables | Category | Frequency | Percentage |
|---|---|---|---|
| Age | 20–26 | 19 | 7.25% |
| 27–33 | 38 | 14.50% | |
| 34–41 | 74 | 28.24% | |
| 42–48 | 52 | 19.85% | |
| 49–55 | 40 | 15.27% | |
| <56 | 39 | 14.89% | |
| Sex | Male | 124 | 47.33% |
| Female | 138 | 52.67% | |
| Marital statues | Single | 23 | 8.78% |
| Married | 207 | 79.01% | |
| Divorced | 26 | 9.92% | |
| Widow | 6 | 2.29% |
Sociodemographic characteristics of the participants (N = 262).
3.2 Clinical characteristics and lifestyle factor
The findings of the study revealed that a significant majority of the participants (72.14%) had systolic hypertension, primarily classified as primary hypertension (64.89%). Most individuals (61.45%) were prescribed one antihypertensive medication. Proteinuria was detected in 42.75% of the participants, whereas diabetes mellitus was present in 39.08%. Dyslipidemia affects 28.24% of the population. The majority of the participants (76.34%) had a normal BMI, while 23.66% were classified as overweight or obese. Regarding blood pressure control, 84.56% of the participants had controlled hypertension and 15.44% had uncontrolled hypertension. As BMI and blood pressure were recorded in categorical formats, the mean and standard deviation values were not calculated for these parameters. Additionally, 45.5% of the participants reported being smokers, indicating a significant prevalence of these clinical factors (Table 2).
Table 2
| Variables | Category | Frequency | Percentage |
|---|---|---|---|
| Types of hypertensions | Systolic HTN | 189 | 72.14% |
| Mixed | 73 | 27.86% | |
| Classification of hypertension | Primary | 170 | 64.89% |
| Secondary | 92 | 35.11% | |
| Number of drugs | One | 161 | 61.45% |
| Two | 72 | 27.48% | |
| Three and above | 29 | 11.07% | |
| Proteinuria | Yes | 112 | 42.75% |
| No | 150 | 57.25% | |
| Diabetic mellitus | Yes | 102 | 39.08% |
| No | 159 | 60.92% | |
| Dyslipidemia | Yes | 74 | 28.24% |
| No | 188 | 71.76% | |
| Blood pressure status | Controlled | 219 | 84.56% |
| Uncontrolled | 40 | 15.44% | |
| Body mass index | Normal | 200 | 76.34% |
| Overweight/obese | 62 | 23.66% | |
| Smoking | Yes | 120 | 45.5% |
| No | 144 | 54.5% |
Clinical characteristics and lifestyle factor.
3.3 The prevalence of chronic kidney diseases
The overall prevalence of CKD among hypertensive patients was found to be 52.67% (95% CI: 46.6%–58.7%), which included those with reduced kidney function and/or evidence of kidney damage (proteinuria).
3.4 Factors associated with chronic kidney diseases
This study examined the factors associated with chronic kidney disease (CKD) among adult hypertensive patients at the Burao General Hospital in 2024. A total of 124 patients diagnosed with CKD were compared with 153 hypertensive patients without CKD. Bivariate analysis revealed several notable associations; however, the focus was on the significant factors determined through multivariable logistic regression analysis (AOR). The age group of 34–41 years showed a significant association with CKD, with an adjusted odds ratio of 3.39 (95% CI: 0.99–11.54; P = 0.05), indicating that individuals in this age range are more likely to develop CKD compared to the reference age group (20–26 years). However, this association should be interpreted with caution given the borderline statistical significance (P = 0.05) and wide confidence interval (0.99–11.54).
The presence of proteinuria was strongly associated with CKD, yielding an AOR of 10.72 (95% CI: 5.74–20.04; P = 0.00), underscoring the critical role of proteinuria as a risk factor for CKD in hypertensive patients. Additionally, diabetic patients had a significant association with CKD, with a COR of 9.74 (95% CI: 5.49–17.29; P = 0.00), highlighting the exacerbating effect of diabetes on renal health in hypertensive individuals. Uncontrolled blood pressure was associated with a decreased likelihood of CKD (AOR: 0.22; 95% CI: 0.09–0.53; P = 0.00), suggesting that effective management of hypertension may mitigate the risk of developing CKD.
Finally, overweight individuals were significantly more likely to have CKD, with an AOR of 3.37 (95% CI: 1.65–6.88; P = 0.00), indicating that maintaining a healthy weight is crucial in preventing CKD among hypertensive patients. These findings emphasize the prevalence and impact of hypertension on CKD, particularly the importance of age, proteinuria, diabetes, blood pressure control, and BMI as critical factors in the management and prevention of chronic kidney disease among adults in Burao (Table 3). Before conducting the multivariate analysis, we assessed the multicollinearity among the independent variables. All VIF values ranged from 1.10 to 1.30 (mean VIF = 1.16), indicating no evidence of problematic multicollinearity (Figure 1).
Table 3
| Variable name | Responses | Chronic kidney disease | COR (95% CI) | P-value | AOR (95% CI) | P-value | |
|---|---|---|---|---|---|---|---|
| Yes | No | ||||||
| Age | 20–26 | 13 | 6 | Ref | Ref | ||
| 27–33 | 19 | 19 | 2.16 (0.68, 6.89) | 0.19 | 2.20 (0.66, 7.26) | 0.19 | |
| 34–41 | 36 | 38 | 2.28 (0.78, 6.66) | 0.12 | 2.20 (0.67, 7.22) | 0.19 | |
| 42–48 | 19 | 33 | 3.76 (1.22, 11.53) | 0.02* | 3.39 (0.99, 11.54) | 0.05* | |
| 49–55 | 17 | 23 | 1.72 (0.92, 9.28) | 0.06 | 2.50 (0.69, 9.06) | 0.16 | |
| >56 | 20 | 19 | 2.05 (0.64, 6.52) | 0.22 | |||
| Sex | Female | 61 | 77 | 0.76 (0.47, 1.24) | 0.28 | ||
| Male | 63 | 61 | Ref | ||||
| Marital statues | Single | 13 | 10 | Ref | |||
| Married | 96 | 111 | 1.50 (0.63, 3.58) | 0.35 | |||
| Divorced | 14 | 12 | 1.11 (0.36, 3.44) | 0.85 | |||
| Widow | 1 | 5 | 6.49 (0.65, 64.82) | 0.11 | |||
| Types of hypertensions | Systolic | 92 | 97 | Ref | |||
| Mixed | 32 | 41 | 1.21 (0.70, 2.09) | 0.48 | |||
| Classification of hypertension | Primary | 86 | 84 | Ref | |||
| Secondary | 38 | 54 | 1.45 (0.87, 2.42) | 0.15 | |||
| Number of drugs | One | 76 | 85 | Ref | |||
| Two | 30 | 42 | 1.25 (0.71, 2.19) | 0.43 | |||
| Three and above | 18 | 11 | 0.54 (0.24, 1.22) | 0.14 | |||
| Proteinuria | Yes | 86 | 26 | Ref | Ref | ||
| No | 38 | 112 | 9.74 (5.49, 17.28) | <0.001* | 10.72 (5.74, 20.04) | <0.001* | |
| Diabetes mellitus | Yes | 56 | 46 | Ref | |||
| No | 67 | 92 | 9.74 (5.49, 17.29) | <0.001* | |||
| Dyslipidemia | Yes | 40 | 67 | Ref | |||
| No | 84 | 92 | 1.45 (0.84, 2.499) | 0.17 | |||
| Blood pressure | Controlled | 95 | 124 | Ref | Ref | ||
| Uncontrolled | 28 | 12 | 0.32 (0.15, 0.67) | <0.001* | 0.22 (0.09, 0.53) | <0.001* | |
| Body mass index | Normal | 103 | 97 | Ref | Ref | ||
| Overweight | 21 | 41 | 2.07 (1.14, 3.75) | 0.17 | 3.37 (1.65, 6.88) | <0.001* | |
Factors associated with chronic kidney diseases among adult hypertensive patient in Burao general hospital in Burao, Somaliland, 2024.
NB: *indicates there is statistically significant association, P ≤ 0.05.
Figure 1
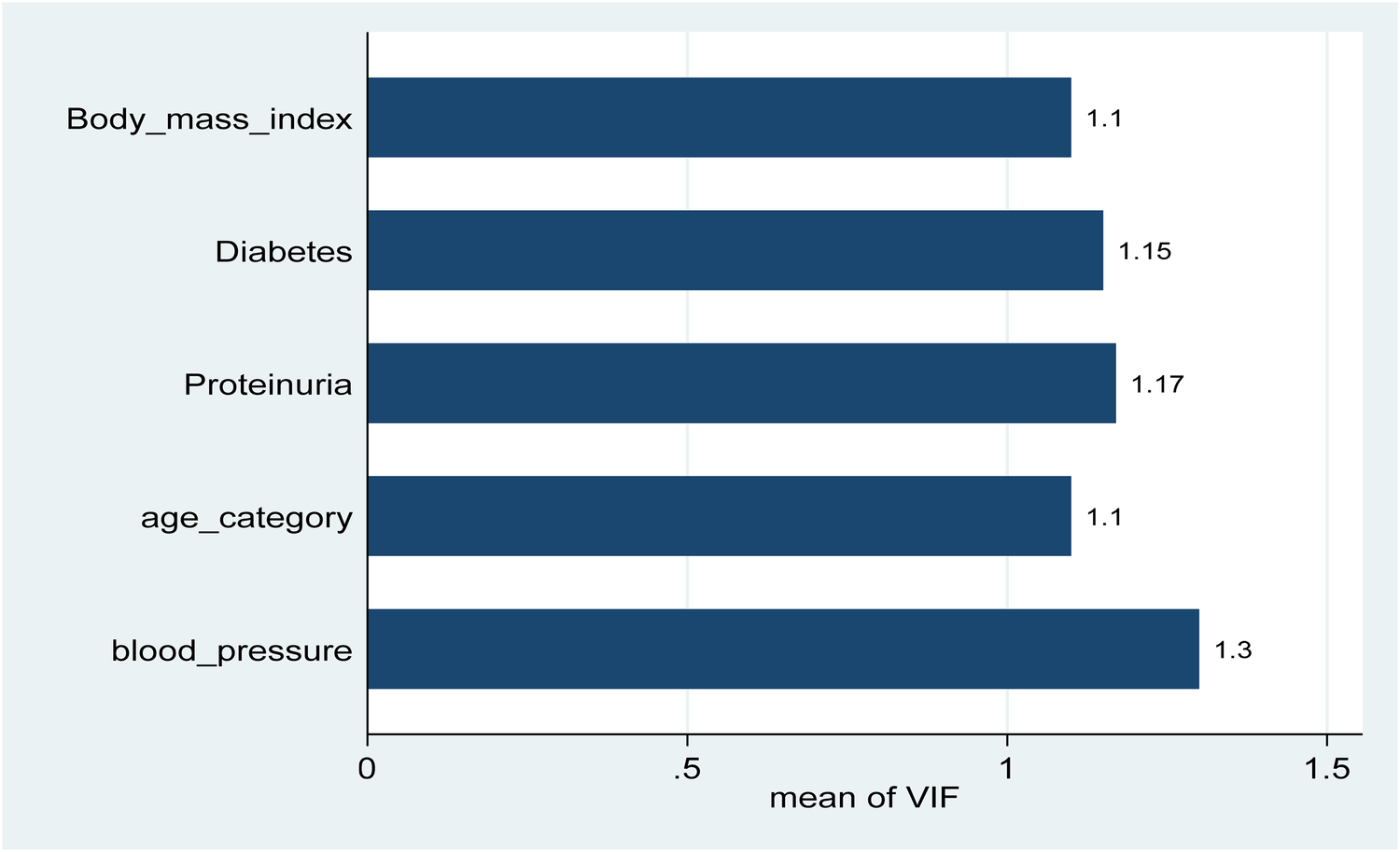
Variance inflation factors (VIF) for independent variables were included in the multivariable logistic regression model. All variables had VIF values ranged from 1.10 to 1.30 (mean VIF = 1.16), all well below the common threshold of 2.5, indicating no evidence of problematic multicollinearity.
4 Discussion
This study examined the prevalence and impact of hypertension on chronic kidney disease (CKD) among adults in Burao General Hospital, Somaliland, revealing that 52.67% (95% CI: 46.6%–58.7%) of hypertensive patients also had CKD. Significant factors associated with CKD include age, proteinuria, diabetes, blood pressure, and body mass index (BMI). In patients aged 34–41 showing a higher likelihood of developing CKD. Notably, the observed association for the 34–41 age group, while statistically significant at P = 0.05, had a wide confidence interval crossing 1 (0.99–11.54), suggesting considerable uncertainty around this estimate. This finding emphasizes the need for cautious interpretation and further studies with larger sample sizes to confirm this finding.
Proteinuria emerged as a highly significant risk factor (AOR = 10.72; 95% CI: 5.74–20.04; P < 0.001), underscoring its central role as both a marker and contributor to renal damage. Diabetes mellitus was also strongly associated with CKD (AOR = 9.74), consistent with extensive literature linking chronic hyperglycemia to progressive renal injury (24–26). Overweight individuals had markedly higher odds of CKD (AOR = 3.37; 95% CI: 1.65–6.88; P < 0.001) (27, 28), reflecting the contribution of elevated BMI to kidney disease risk. These findings underscore the importance of identifying and managing these associated factors, which may help reduce the burden of CKD, although causality cannot be inferred owing to the cross-sectional nature of the study.
The prevalence of CKD observed in this study aligns with the findings from other regions, although the figures vary significantly. Studies in Ethiopia reported a prevalence of 39% among hypertensive patients, whereas research in Tanzania reported a lower prevalence of 12.4% (23, 29). Conversely, a higher prevalence of 57.1% has been reported in Nigeria among similar populations (30), indicating substantial regional differences that may reflect variations in healthcare access, screening practices, patient demographics, and genetic factors.
Blood pressure control was statistically significant in relation to CKD outcomes. Patients with uncontrolled hypertension were significantly less likely to be free from CKD, with an adjusted odds ratio of 0.22 (95% CI: 0.09–0.53; P < 0.001), indicating that uncontrolled blood pressure markedly increased the risk of developing CKD. This finding reinforces the critical importance of achieving and maintaining optimal blood pressure targets to mitigate kidney damage in hypertensive individuals, consistent with previous evidence that prolonged elevated blood pressure accelerates nephron loss and glomerular injury (1, 31, 32). Hypertension contributes to CKD progression by inducing increased intraglomerular pressure and promoting glomerulosclerosis, leading to long-term impairment of the filtration capacity. Diabetes mellitus accelerates kidney damage through chronic hyperglycemia, causing thickening of the glomerular basement membrane and mesangial expansion (33, 34). Obesity further exacerbates this risk by inducing hyperfiltration, activating pro-inflammatory pathways, and contributing to insulin resistance (35, 36).
Socioeconomic factors likely play a substantial role in shaping these outcomes. Many participants came from low-income settings with limited access to routine healthcare services, potentially delaying the diagnosis and management of hypertension and diabetes. Lower health literacy, economic constraints affecting diet and medication adherence, and lifestyle limitations may have compounded these risks.
These findings reinforce the existing evidence that emphasizes the critical importance of integrating chronic disease management, routine screening for kidney dysfunction, and broader public health interventions. Targeted strategies addressing both medical and socioeconomic factors are essential to effectively mitigate the burden of CKD in low-resource settings.
5 Conclusion
This study investigated the prevalence and impact of hypertension on chronic kidney disease (CKD) among adults attending Burao General Hospital in Somaliland. The findings revealed a significant prevalence of CKD (52.67%) among patients with hypertension, underscoring the critical relationship between uncontrolled hypertension and CKD development. The key risk factors identified included age, diabetes, proteinuria, and body mass index. These results highlight the urgent need for strengthened hypertension management and monitoring of associated factors to potentially reduce the burden of CKD in this population; however, causality cannot be established owing to the study design.
6 Recommendations
Several recommendations should be implemented to effectively address the prevalence of hypertension and its impact on chronic kidney disease (CKD) in Burao City. First, the Burao General Hospital should develop and implement comprehensive hypertension management programs aimed at the early detection and effective management of hypertension. These programs will help prevent CKD progression in at-risk populations. In addition, increasing public awareness through community health education campaigns is essential. Such initiatives can inform the local community about the risks associated with hypertension and CKD, highlighting the importance of regular checkups and lifestyle modifications.
Establishing routine screening protocols for CKD in hypertensive patients is another critical step. This would include regular urine tests for proteinuria and the assessment of kidney function, which can facilitate early intervention and management. Integrating lifestyle modification strategies into patient management plans is vital. Encouraging healthy eating, regular physical activity, and smoking cessation can significantly improve the health outcomes of individuals with hypertension.
Finally, collaborating with local health authorities will be crucial in improving access to healthcare services and resources for managing hypertension and CKD. By working together, healthcare providers can enhance service delivery, ensuring that patients receive the necessary support and resources to effectively manage their conditions. These recommendations, supported by existing literature, aim to mitigate the impact of hypertension on kidney health in the community.
7 Strengths and limitations
7.1 Strengths
This study had several notable strengths. It employs structured questionnaires and clinical data, which provide a robust dataset for analyzing factors associated with chronic kidney disease (CKD). Focusing specifically on adults in Burao City addresses a critical research gap, offering tailored insights into the local context. Additionally, the use of multivariate logistic regression allows for the identification of significant predictors of CKD, enhancing our understanding of the relationship between hypertension and kidney health.
7.2 Limitations
However, this study had several limitations. Its cross-sectional design restricts its ability to establish temporal or causal relationships between hypertension, associated factors, and CKD. Therefore, all observed relationships should be interpreted strictly as an association. Additionally, BMI and blood pressure data were only available in categorical form, which limited our ability to report the mean values or analyze these variables as continuous outcomes. Reliance on self-reported data may introduce a recall bias, potentially affecting data accuracy. The findings also have limited generalizability because of the specific population studied in Burao City. Finally, the exclusion of non-respondents could result in selection bias, further impacting the representativeness of the sample.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Review Committee of the University of Burao, School of Postgraduate Studies and Research under reference number UoB/SPGSR/32/2024. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
DY: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. YA: Data curation, Methodology, Project administration, Supervision, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We extend our sincere gratitude to all those who contributed to the successful completion of this study on the prevalence and impact of hypertension on chronic kidney disease among adults in Burao, Somaliland. First, we would like to thank the management and staff of Burao General Hospital for their support and cooperation, especially the healthcare providers, who assisted in facilitating access to patient records and provided invaluable insights into hypertension and kidney disease management. We are deeply grateful to all patients who participated in this study for their willingness to share personal health information, without which this research would not have been possible. Their contributions are vital for advancing our understanding of the chronic diseases in this region. Additionally, we acknowledge the contributions of the data collection team and supervisors to their dedication and thoroughness during the study. Finally, we extend our thanks to our colleagues and peers for their constructive feedback and support, which significantly enriched the quality and outcomes of this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AOR, adjusted odds ratio; BSA, body surface area; BMI, body mass index; CKD, chronic kidney disease; CI, confidence interval; eGFR, estimated glomerular filtration rate; HTN, hypertension; WHO, World Health Organization.
References
1.
Lv J-C Zhang L-X . Prevalence and disease burden of chronic kidney disease. Ren Fibros Mech Ther. (2019) 1165:3–15. 10.1007/978-981-13-8871-2_1
2.
Burnier M Damianaki A . Hypertension as cardiovascular risk factor in chronic kidney disease. Circ Res. (2023) 132(8):1050–63. 10.1161/circresaha.122.321762
3.
Botdorf J Chaudhary K Whaley-Connell A . Hypertension in cardiovascular and kidney disease. Cardiorenal Med. (2011) 1(3):183–92. 10.1159/000329927
4.
Nerbass FB Calice-Silva V Pecoits-Filho R . Sodium intake and blood pressure in patients with chronic kidney disease: a salty relationship. Blood Purif. (2018) 45(1-3):166–72. 10.1159/000485154
5.
Santos PCJL Krieger JE Pereira AC . Renin–angiotensin system, hypertension, and chronic kidney disease: pharmacogenetic implications. J Pharmacol Sci. (2012) 120(2):77–88. 10.1254/jphs.12R03CR
6.
Qiu J Zhao L Cheng Y Chen Q Xu Y Lu Y et al Exploring the gut mycobiome: differential composition and clinical associations in hypertension, chronic kidney disease, and their comorbidity. Front Immunol. (2023) 14:1317809. 10.3389/fimmu.2023.1317809
7.
Ameer OZ . Hypertension in chronic kidney disease: what lies behind the scene. Front Pharmacol. (2022) 13:949260. 10.3389/fphar.2022.949260
8.
Liu ZZ Bullen A Li Y Singh P . Renal oxygenation in the pathophysiology of chronic kidney disease. Front Physiol. (2017) 8:385. 10.3389/fphys.2017.00385
9.
Weldegiorgis M Woodward M . The impact of hypertension on chronic kidney disease and end-stage renal disease is greater in men than women: a systematic review and meta-analysis. BMC Nephrol. (2020) 21:1–9. 10.1186/s12882-020-02151-7
10.
Chen W Feng J Ji P Liu Y Wan H Zhang J . Association of hyperhomocysteinemia and chronic kidney disease in the general population: a systematic review and meta-analysis. BMC Nephrol. (2023) 24(1):247. 10.1186/s12882-023-03295-y
11.
Deschamps-Lenhardt S Martin-Cabezas R Hannedouche T Huck O . Association between periodontitis and chronic kidney disease: systematic review and meta-analysis. Oral Dis. (2019) 25(2):385–402. 10.1111/odi.12834
12.
Tsai W-C Wu H-Y Peng Y-S Yang J-Y Chen H-Y Chiu Y-L et al Association of intensive blood pressure control and kidney disease progression in nondiabetic patients with chronic kidney disease: a systematic review and meta-analysis. JAMA Intern Med. (2017) 177(6):792–9. 10.1001/jamainternmed.2017.0197
13.
Chang AR Loser M Malhotra R Appel LJ . Blood pressure goals in patients with CKD: a review of evidence and guidelines. Clin J Am Soc Nephrol. (2019) 14(1):161–9. 10.2215/CJN.07440618
14.
Jovanovich A Ginsberg C You Z Katz R Ambrosius WT Berlowitz D et al FGF23, frailty, and falls in SPRINT. J Am Geriatr Soc. (2021) 69(2):467–73. 10.1111/jgs.16895
15.
Nooh F Ali MI Chernet A Probst-Hensch N Utzinger J . Prevalence and risk factors of hypertension in Hargeisa, Somaliland: a hospital-based cross-sectional study. Diseases. (2023) 11(2):62. 10.3390/diseases11020062
16.
Lee H Kwon SH Jeon JS Noh H Han DC Kim H . Association between blood pressure and the risk of chronic kidney disease in treatment-naïve hypertensive patients. Kidney Res Clin Pract. (2022) 41(1):31. 10.23876/j.krcp.21.099
17.
Ranzani OT Kalra A Di Girolamo C Curto A Valerio F Halonen JI et al Urban-rural differences in hypertension prevalence in low-income and middle-income countries, 1990–2020: a systematic review and meta-analysis. PLoS Med. (2022) 19(8):e1004079. 10.1371/journal.pmed.1004079
18.
Development. SMoHa. Health sector strategic 2017–2021.
19.
World Health Organization. Health service delivery in Somalia(2021).
20.
Burao general hospital annual Report. Statistics on hypertension and CKD (2023).
21.
Patel SS Raman VK Zhang S Deedwania P Zeng-Treitler Q Wu WC et al Identification and outcomes of KDIGO-defined chronic kidney disease in 1.4 million US veterans with heart failure. Eur J Heart Fail. (2024) 26(5):1251–60. 10.1002/ejhf.3210
22.
Levey AS . Defining AKD: the spectrum of AKI, AKD, and CKD. Nephron. (2022) 146(3):302–5. 10.1159/000516647
23.
Bahrey D Gebremedhn G Mariye T Girmay A Aberhe W Hika A et al Prevalence and associated factors of chronic kidney disease among adult hypertensive patients in Tigray teaching hospitals: a cross-sectional study. BMC Res Notes. (2019) 12:1–5. 10.1186/s13104-019-4610-8
24.
Provenzano M Coppolino G Faga T Garofalo C Serra R Andreucci M . Epidemiology of cardiovascular risk in chronic kidney disease patients: the real silent killer. Rev Cardiovasc Med. (2019) 20(4):209–20. 10.31083/j.rcm.2019.04.548
25.
Sandsmark DK Messé SR Zhang X Roy J Nessel L Hamm LL et al Proteinuria, but not eGFR, predicts stroke risk in chronic kidney disease. Stroke. (2015) 46(8):2075–80. 10.1161/STROKEAHA.115.009861
26.
Lepenies J Eardley KS Kienitz T Hewison M Ihl T Stewart PM et al Renal TLR4 mRNA expression correlates with inflammatory marker MCP-1 and profibrotic molecule TGF-β1 in patients with chronic kidney disease. Nephron Clin Pract. (2011) 119(2):c97–104. 10.1159/000324765
27.
Kanbay M Copur S Siriopol D Yildiz AB Berkkan M Tuttle KR et al The risk for chronic kidney disease in metabolically healthy obese patients: a systematic review and meta-analysis. Eur J Clin Investig. (2023) 53(1):e13878. 10.1111/eci.13878
28.
Tanaka M Mori K Takahashi S Higashiura Y Ohnishi H Hanawa N et al Metabolic dysfunction–associated fatty liver disease predicts new onset of chronic kidney disease better than fatty liver or nonalcoholic fatty liver disease. Nephrol Dial Transplant. (2023) 38(3):700–11. 10.1093/ndt/gfac188
29.
Ploth DW Mbwambo JK Fonner VA Horowitz B Zager P Schrader R et al Prevalence of CKD, diabetes, and hypertension in rural Tanzania. Kidney Int Rep. (2018) 3(4):905–15. 10.1016/j.ekir.2018.04.006
30.
Akpor OA Adeoye AO Ibitoba FA Akpor OB . Prevalence of chronic kidney disease among diabetes and hypertensive patients in a teaching hospital in Ekiti State, Southwest Nigeria. Open Public Health J. (2022) 15(1):1–3. 10.2174/18749445-v15-e221220-2022-99
31.
Hamrahian SM Falkner B . Hypertension in chronic kidney disease. Adv Exp Med Biol. (2017) 956:307–25. 10.1007/5584_2016_84
32.
Gluba-Brzozka A Franczyk B Rysz J . Cholesterol disturbances and the role of proper nutrition in CKD patients. Nutrients. (2019) 11(11):2820. 10.3390/nu11112820
33.
Li X-Q Tian W Liu X-X Zhang K Huo J-C Liu W-J et al Corosolic acid inhibits the proliferation of glomerular mesangial cells and protects against diabetic renal damage. Sci Rep. (2016) 6(1):26854. 10.1038/srep26854
34.
Robles-Osorio ML Sabath E . Tight junction disruption and the pathogenesis of the chronic complications of diabetes mellitus: a narrative review. World J Diabetes. (2023) 14(7):1013. 10.4239/wjd.v14.i7.1013
35.
Lakkis JI Weir MR . Obesity and kidney disease. Prog Cardiovasc Dis. (2018) 61(2):157–67. 10.1016/j.pcad.2018.07.005
36.
Silva G Bentes ACSN Daher EDF Matos S . Obesity and kidney disease. Braz J Nephrol. (2017) 39:65–9. 10.5935/0101-2800.20170011
Summary
Keywords
chronic kidney disease, hypertension, Burao, Somaliland, proteinuria
Citation
Yosef DK and Ali YA (2025) Chronic kidney disease prevalence and associated risk factors in hypertensive adults at Burao general hospital, Burao City, Somaliland: a cross-sectional study. Front. Cardiovasc. Med. 12:1503233. doi: 10.3389/fcvm.2025.1503233
Received
14 October 2024
Accepted
18 July 2025
Published
07 August 2025
Volume
12 - 2025
Edited by
Ravi Nistala, University of Missouri, United States
Reviewed by
Rahul Kakalij, University of Nebraska Medical Center, United States
Yaman Walid Kassab, National University of Science and Technology (Muscat), Oman
Updates
Copyright
© 2025 Yosef and Ali.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Dek Kahin Yosef dekkahin888@gmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.