- Department of Anesthesiology, West China Hospital, Sichuan University, Chengdu, Sichuan, China
Background: Total aortic arch replacement surgery (TARS) for Acute type A aortic dissection is associated with high incidence of postoperative acute kidney injury (AKI), at least partly due to the lower body ischemia during circulatory arrest. This study aimed to evaluate whether retrograde inferior vena cava perfusion (RIVP) reduces the risk of AKI by providing oxygenated blood to the lower body.
Methods: This retrospective study utilized a medical recording system to screen patients who underwent TARS from January 1 to December 31, 2019. Patients were assigned to receive antegrade cerebral perfusion (ACP) only or ACP + RIVP during circulatory arrest. The primary outcome was postoperative AKI. Oxygen delivery, consumption, and extraction ratio during RIVP were also determined.
Results: Of all included 87 patients, postoperative AKI occurred in 35 (40%), of whom 23 (53.5%) were in the ACP, and 12 (27.3%) were in the ACP + RIVP (P = 0.013). In regression analysis, ACP + RIVP was associated with lower risk of AKI than ACP alone (adjusted OR 0.229; 95% CI 0.071–0.746). RIVP at a pressure of 22.5 ± 3.8 mmHg delivered 0.98 ± 0.34 ml/min/kg of oxygen to the lower body, and the partial oxygen pressure decreased from 359 ± 57 mmHg in RIVP blood to 64 ± 30 mmHg in returning blood. Oxygen extraction ratio was 44 ± 16%, which correlated negatively with peak postoperative creatinine levels (r = −0.58, P = 0.01) and creatinine increase (r = −0.61, P = 0.009). No correlations were found between oxygen delivery and postoperative creatinine or creatinine increase.
Conclusion: RIVP may reduce the risk of postoperative AKI in a manner that depends on the tissue oxygen extraction ratio.
Introduction
Acute type A aortic dissection (AAAD) is associated with mortality rates of 27%–45% (1–3). To reduce mortality, this condition is often immediately treated by replacing both the ascending aorta and the aortic arch with artificial vascular grafts under cardiopulmonary bypass (CPB) in a procedure called total aortic arch replacement surgery (TARS) (4). TARS requires a period of circulatory arrest during which arteries are reconstructed via anastomosis of the graft and the proximal descending aorta. This arrest period can cause ischemic injury in vital organs. The application of deep hypothermic circulatory and antegrade (ACP) or retrograde cerebral perfusion can mitigate cerebral ischemic injury (5–7). These techniques, however, still expose the lower body to substantial risk of ischemic injury. As a result, acute kidney injury (AKI) occurs in up to 54.5% of TARS patients (4, 8, 9).
Our group proposed a technique combining ACP with retrograde inferior vena cava perfusion (RIVP), which may provide oxygenated blood to the lower body during circulatory arrest in TARS (10, 11). Theoretically, RIVP may avoid ischemic injury of the low body during circulatory arrest, but whether it can reduce risk of AKI has not been explored in detail. Therefore, the present study utilized a medical recording system, tested whether RIVP reduces risk of AKI and its potential mechanism.
Materials and methods
Study subjects and ethics
Patients aged ≥18 years who were diagnosed with AAAD and underwent TARS with ACP combined with RIVP during circulatory arrest at West China Hospital between 1 January, 2019, and 31 December, 2019, were eligible for this study. Patients were excluded if they were pregnant or had renal dysfunction requiring dialysis before surgery. This single-center retrospective cohort study was approved by the Biomedical Ethics Committee of West China Hospital (2021–837). Because of the study's retrospective nature, the informed consent requirement was waived.
Patient and public involvement statement
Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
Data collection
Data on demographic and clinical characteristics, perioperative details, and blood analysis results were extracted from our hospital's electronic records system. Blood gas analysis values obtained from anesthesia and CPB record sheets. Information on patients undergoing ACP or ACP + RIVP was also available on the CPB record sheets. All preoperative examinations and test results closest to the operation day were included.
Anesthesia and surgical procedures
The procedures were performed according to our hospital's protocol. Briefly, patients received sevoflurane via inhalation along with intravenous infusions of propofol, sufentanil and cisatracurium. Cannulation for systemic perfusion was performed in the aortic arch, right axillary artery or femoral artery (Supplementary Table S1), according to the vascular dissection and surgeon's preferences. The superior and inferior venae cavae were cannulated for venous drainage. After cross-clamping the ascending aorta, cardiac arrest was achieved by intermittent antegrade infusion of cold blood cardioplegia and maintained using either antegrade or retrograde delivery methods.
Systemic perfusion was stopped and circulatory arrest was initiated when the nasopharyngeal temperature reached 24–26°C and the rectal temperature fell below 28°C. The aortic clamp was removed to open the aorta, and a four-branched artificial graft was employed for TARS. An elephant trunk was also inserted into the descending aorta, if necessary. After anastomosis of the graft with the descending aorta and left subclavian artery, systemic perfusion was performed via the fourth branch, and rewarming began.
The arch was reconstructed by anastomosis of the left common carotid artery and the innominate artery with the other two side branches of the hybrid prosthesis. Patients were progressively weaned off CPB.
In accordance with standard protocols at our hospital, red blood cells were transfused when hemoglobin concentration was <7 g/dl during CPB, <8 g/dl during surgery or <9.5 g/dl in the ICU.
ACP and RIVP treatment
For ACP, supra-aortic vessels were gently clamped during circulatory arrest, and ACP was achieved by axillary cannulation or direct innominate artery cannulation with a cannula (10–14 Fr) at 5–12 ml/kg/min under pump pressure of 50–80 mmHg. The systemic perfusion was initiated from the graft after anastomosis of the descending aorta and the graft (5, 12, 13).
The RIVP procedure involved securing the inferior vena cava using a perivascular band near the cannulation site, combined with distal occlusion of the venous drainage catheter. This configuration enabled retrograde perfusion through another pump delivering oxygenated blood into the venous system. Technical specifications for this protocol are detailed in our team's prior publication (10). The flow of RIVP was regulated to achieve a target venous pressure of 20–25 mmHg. This range of pressure was selected assuming that pressures below 20 mmHg may result in insufficient organ perfusion, while pressures exceeding 25 mmHg could lead to ascites (14). A pressure monitoring tube was connected to the pipeline distal to the roller pump, which was infused into the inferior vena cava, to measure RIVP pressure. RIVP was discontinued after anastomosis, and systemic perfusion was initiated from the graft as described for the ACP group.
Blood gases data and lactate
In the beginning of 2019, we detected the blood samples simultaneously from the arterial line and the descending aorta under the assistance of a surgeon in some patients with RIVP, then analyzed immediately on a blood gas analyzer (Cobas b 123; Roche, Basel, Switzerland), which allowed us to determine the oxygen delivery (DO2) and, oxygen consumption (VO2) of RIVP. The following data were then collected: oxygen partial pressure (PO2), oxygen saturation (SO2), and hemoglobin levels.
Oxygen content of the arterial line (CaO2) and of venous blood from the descending aorta (CvO2) was calculated using the equations (15):
where : arterial partial pressure of oxygen (mmHg); : venous partial pressure of oxygen (mmHg); : hemoglobin concentration (g/dl); : arterial and venous oxygen saturation (%)
DO2, VO2 and oxygen extraction ratio (ERO2) were calculated using the equations (15):
where is RIVP blood flow rate (L/min), and W is the patient's weight (kg).
Plasma lactate levels before CPB (baseline), before circulatory arrest, 5 min after lower body perfusion was restored, after rewarming to 32°C and at the end of CPB, were also collected. The increase in lactate was calculated as the lactate level at the end of CPB minus lactate level before CPB.
Outcomes
The primary outcome was the occurrence of postoperative AKI before hospital discharge. AKI, which was defined using the Acute Kidney Injury Network classification (16). AKI stage 1 was defined as an increase in serum creatinine ≥27 mol/L or an increase from baseline ≥150%. Stage 2 was defined as an increase in serum creatinine value ≥200% from baseline. Stage 3 was defined as an increase in serum creatinine ≥300% from baseline, an absolute serum creatinine level ≥354 mol/L, or requirement of dialysis.
Serum creatinine and urea nitrogen levels were collected before surgery (baseline), daily in the intensive care unit, and in the ward after surgery. Peak creatinine was defined as the postoperative maximum, and its increase was defined as the difference between the peak and the baseline.
Secondary outcomes were indices of oxygen metabolism during RIVP, including PO2 in the returned blood, DO2 of RIVP, as well as VO2 and ERO2 in the lower body.
Other outcomes were occurrence of the following composite adverse events after surgery: all-cause death, defined as death for any reason within 30 days after surgery or to hospital discharge, if the hospital time over 30 days; stroke, defined as an acute episode of focal or global neurologic dysfunction caused by brain injury as a result of hemorrhage or infarction in which the neurologic dysfunction lasts for >24 h (17); prolonged ventilation >24 h (17).
Statistical analysis
Continuous variables were reported as mean and standard deviation or median and 25th and 75th quartiles, and categorical variables as frequency and percentage. In the case of continuous variables, differences between groups were assessed for significance using the unpaired t test if the data showed a normal distribution, or the rank sum test if data were skewed. In the case of categorical variables, differences were assessed using the chi-squared test or Fisher's exact test when the theoretical frequency was less than 5. Pearson or Spearman correlation analysis was performed to examine the association between oxygen metabolism and plasma creatinine. Univariate and multivariate logistic regression was applied to calculate the odds ratios (ORs) and 95% confidence intervals (CIs) of ACP + RIVP relative to ACP patients. Data were analyzed using SPSS 22.0 (IBM Corp., Armonk, NY, USA) and GraphPad Prism version 9 (GraphPad Inc., San Diego, CA, USA). All P-values were two-sided, and P < 0.05 was considered significant.
Results
Patient characteristics
From January 1, 2019 to December 31, 2019, 88 patients with AAAD undergoing TARS were identified from the medical record system in the Division of Thoracic and Cardiovascular Surgery at West China Hospital. Of them, 1 patient was excluded because of preoperative dialysis. Of the remaining 87 patients, 43 patients with only ACP, and 44 patients with combination ACP and RIVP. Blood gas analyses of blood from inferior vena cava and the descending aorta were available in 19 of the 44 patients (43%), and they were included into calculating oxygen supply and consumption in the lower body during RIVP (Figure 1).
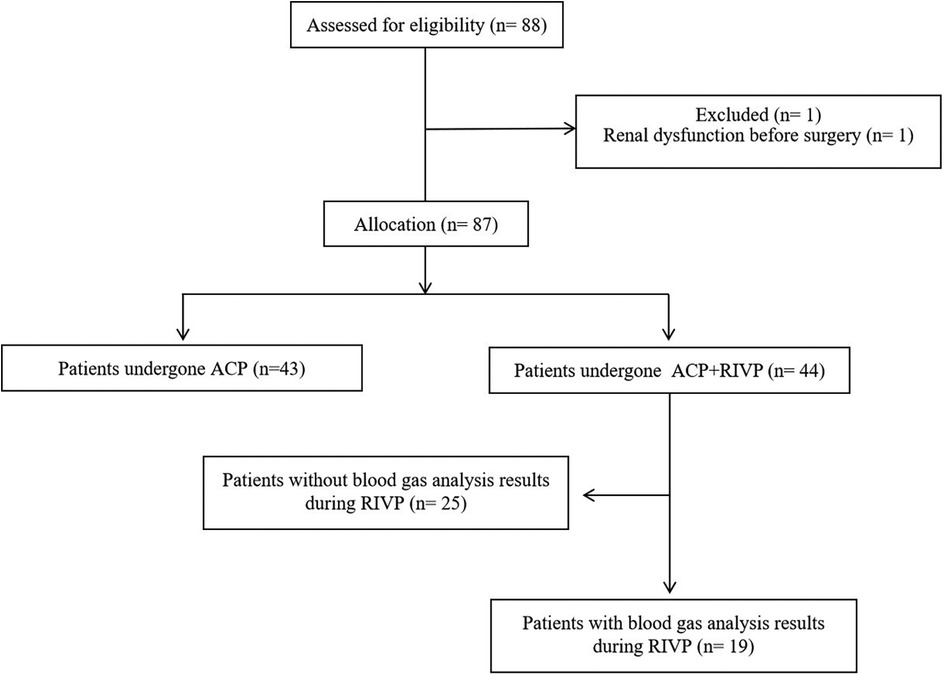
Figure 1. Flow diagram showing patient selection. ACP, antegrade cerebral perfusion; RIVP, retrograde inferior vena caval perfusion.
Of all 87 patients, the mean age was 45 years old, 75 (86%) were male, and 53 (61%) had hypertension. The demographic characteristics, including preoperative serum creatinine levels and renal artery involvement in dissection, were comparable between the two groups (Supplementary Table S2).
The surgical procedures, times of aortic cross-clamping and circulatory arrest were also comparable between the groups. However, CPB duration was shorter in the ACP + RIVP group (250 ± 53 vs. 276 ± 58 min, P = 0.031) (Supplementary Table S3).
During circulatory arrest, the lowest nasopharyngeal and rectal temperatures were higher in the ACP + RIVP group than in the ACP group (mean difference: 1.7°C, P < 0.001). ACP flow was 6.4 ± 2.1 ml/min/kg in the ACP group and 7.2 ± 2.2 ml/min/kg in the ACP + RIVP group at a pump pressure of 50–80 mmHg. In ACP + RIVP patients, RIVP blood flowed during circulatory arrest at 9.6 ± 3.2 ml/min/kg (range 2.54–16.34 ml/min/kg) at a pressure of 22.5 ± 3.8 mmHg (range 15–28 mmHg). Patients in the ACP group received more red blood cells than ACP + RIVP patients (P = 0.01, Supplementary Table S3).
DO2 did not differ between groups pre-arrest but was higher in the ACP + RIVP group post-arrest (6.2 ± 1.0 vs. 5.4 ± 1.3 ml/kg/min, P = 0.001) (Supplementary Table S3).
We obtained blood gas analyses results of the descending aorta during circulatory arrest from 19 of 44 (43%) RIVP patients for determination of oxygen metabolism. The demographic characteristics and perioperative data of these 19 patients were similar to those of the patients from whom without blood gas analyses results.
Acute kidney injury and other outcomes
Baseline renal function was comparable between groups (Supplementary Table S2). After surgery, AKI occurred in 35 out of 87 (40%) patients before hospital discharge, and it was classified as stage 1 injury in 19 patients, stage 2 in 11 patients, and stage 3 in 5 patients. The incidence of AKI was higher in the ACP group (23/43, 53.5%) than in ACP + RIVP patients (12/44, 27.3%; P = 0.014). In the logistic regression analysis, ACP + RIVP was associated with lower risk of AKI than ACP only (crude OR 0.326, 95% CI 0.130–0.785, P = 0.014). This risk reduction remained significant (adjusted OR 0.196, 95% CI 0.038–0.843, P = 0.036) after adjusting by sex, age, body mass index, hypertension, diabetes mellitus, New York Heart Association class, baseline creatinine level, renal arteries involved in dissection, bypass duration, red blood cell transfusion, lowest nasopharyngeal and rectal temperature (7, 18–20) (Table 1).
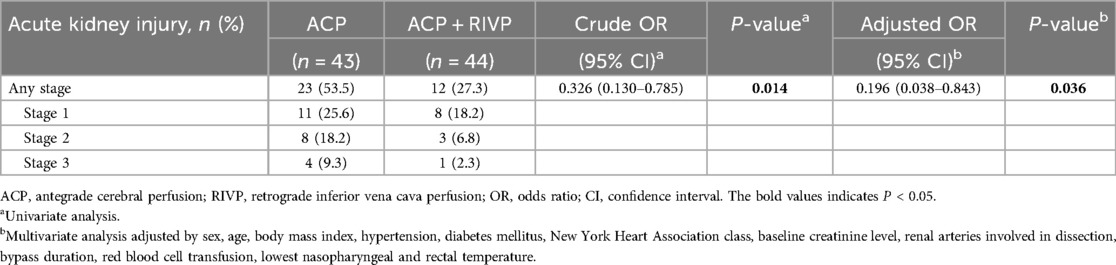
Table 1. Postoperative acute kidney injury in 87 patients stratified by perfusion strategy during circulatory arrest.
Patients receiving ACP + RIVP showed a significantly lower postoperative creatinine peak than patients receiving ACP only (139 ± 67 vs. 196 ± 118 μmol/L, P = 0.004), as well as smaller serum creatinine increase (58 ± 63 vs. 106 ± 103 μmol/L, P = 0.006; Figure 2A). ACP + RIVP patients also showed lower peak blood urea nitrogen (15 ± 9 vs. 21 ± 13 mmol/L, P = 0.017; Figure 2B).
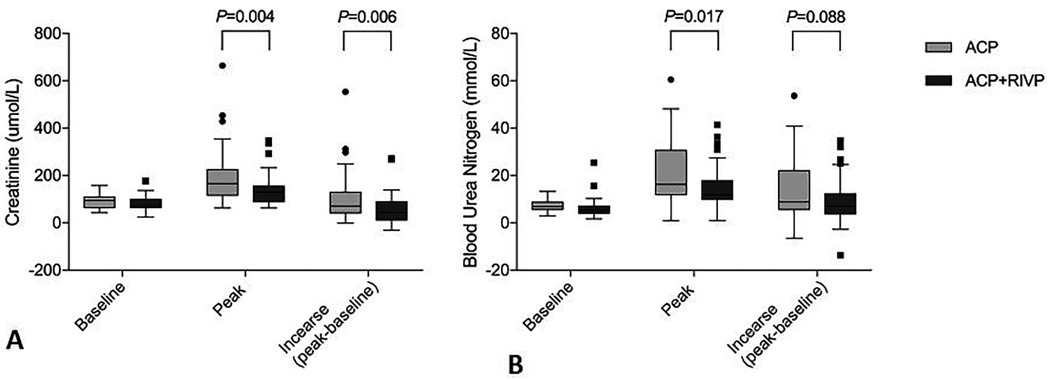
Figure 2. Serum creatinine (A) and blood urea nitrogen levels (B) in patients undergoing antegrade cerebral perfusion (ACP, n = 43) or ACP + retrograde inferior vena caval perfusion (ACP + RIVP, n = 44). Shown are the level at baseline, the peak level and the increase (peak—baseline). ACP, antegrade cerebral perfusion; RIVP, retrograde inferior vena caval perfusion.
Other outcomes occurred in 4 out of 87 (4.6%) of whom experienced all-cause death; 8 (9.2%), stroke; 51 (58.6%), prolonged ventilation. ACP + RIVP was associated with lower risk of prolonged ventilation than ACP only (crude OR 0.361, 95% CI 0.146–0.859, P = 0.023), but this risk reduction remained insignificant (adjusted OR 1.231, 95% CI 0.319–5.067, P = 0.765) after adjusting (Supplementary Table S4).
RIVP for metabolism during circulatory arrest and AKI
In 19 patients in whom blood gas analysis was performed, RIVP blood showed a PO2 of 359 ± 57 mmHg and an O2 content of 107 ± 14 ml/L; these values decreased to 64 ± 30 mmHg (range 30–166 mmHg) and 59 ± 18 ml/L (range 33–93 ml/L) in the blood returning from the descending aorta (Figure 3A). Based on SO2, PO2 and RIVP flow, we calculated DO2 to be 0.98 ± 0.34 ml/min/kg (range 0.26–1.75 ml/min/kg) and VO2 to be 0.43 ± 0.2 ml/min/kg (range 0.06–0.85 ml/min/kg). This corresponded to a mean ERO2 of 44 ± 16% (range 11.8–69.5%) (Figure 3B). The extraction ratio correlated strongly with residual oxygen in returned blood from the descending aorta (r = −0.87, P < 0.001) and with VO2 (r = 0.73, P < 0.001) but not with DO2 (r = −0.009, P = 0.97) (Figures 3C–E).
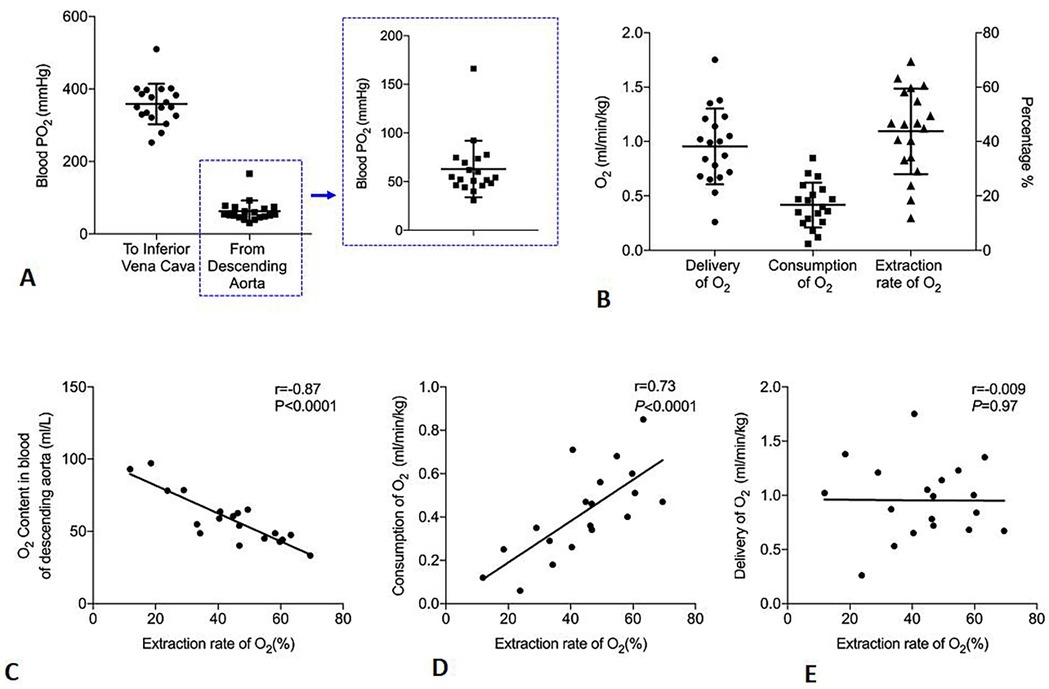
Figure 3. Oxygen (O2) metabolism in patients undergoing antegrade cerebral perfusion and retrograde inferior vena caval perfusion (ACP + RIVP) during circulatory arrest (n = 19). (A) Oxygen partial pressure (PO2) in perfused blood of inferior vena cava and returned blood from descending aorta. Data points for the descending aorta are shown in greater detail in the zoomed-in view on the right. (B) Oxygen delivery (DO2), oxygen consumption (VO2), and oxygen extraction ratio (ERO2). The y-axis on the right indicates the percentage of oxygen extraction ratio (ERO2). (C–E) Correlations of indices with oxygen extraction ratio (ERO2).
In the entire study cohort, lactate levels significantly increased after restoration of lower body perfusion, and hyperlacticemia persisted through the end of surgery. The ACP group had higher lactate levels than the ACP + RIVP group starting from 5 min after restoration of lower body perfusion until the end of CPB (Figure 4A).

Figure 4. Oxygen metabolism and renal function. (A) Lactate levels at different timepoints during surgery in patients who underwent antegrade cerebral perfusion only (ACP, n = 43) or ACP + retrograde inferior vena caval perfusion (ACP + RIVP, n = 44). (B–D) Correlations between lactate increase (peak—baseline) and various oxygen indices in 19 patients who underwent ACP + RIVP. (E,F) Negative correlation of oxygen extraction ratio (ERO2) with peak creatinine and creatinine increase in 19 patients who underwent ACP + RIVP. In these experiments, lactate peak and increase were defined with respect to the perioperative peak, while creatinine peak and increase were defined with respect to the postoperative peak. CPB, cardiopulmonary bypass; ACP, antegrade cerebral perfusion; RIVP, retrograde inferior vena caval perfusion; O2, oxygen.
In RIVP patients, lactate levels were elevated to 4.06 ± 1.68 mmol/L (range 1.6–8.3) at the end of CPB, corresponding to a lactate increase of 2.59 ± 1.61 mmol/L. This increase correlated negatively with ERO2 (r = −0.6, P = 0.006; Figure 4B), but not with DO2 (r = −0.07, P = 0.79) or VO2 (r = −0.4, P = 0.09) (Figures 4C-D).
Potential correlation between metabolic indicators and AKI was explored in blood gas analyses results obtained from the descending aorta during circulatory arrest in ACP + RIVP patients. ERO2 during RIVP negatively correlated with the creatinine peak (r = −0.58, P = 0.01; Figure 4E) and with the increase in creatinine (r = −0.61, P = 0.009; Figure 4F). In contrast, lactate increase during RIVP showed a weak correlation with creatinine peak (r = 0.46, P = 0.06) and creatinine increase (r = 0.43, P = 0.08).
Discussion
The main finding of this study was that RIVP during circulatory arrest in patients with TARS decreased the risk of AKI, and this decrease was dependent on tissue ERO2. Furthermore, we showed that RIVP under 22.5 ± 3.8 mmHg provided a blood flow of 9.6 ± 3.2 ml/kg/min with a rectal temperature of 27.7 ± 1.4°C. During RIVP under these conditions, PO2 in ascending aortic blood was higher than 30 mmHg, suggesting that RIVP can deliver sufficient oxygen to meet the metabolic requirements of vital organs in the lower body.
AAAD patients undergoing CPB for TARS present a high risk of postoperative AKI (4, 8), which likely stems from vascular dissection involving the renal arteries, as well as from ischemic injury during circulatory arrest to allow anastomosis of the graft and descending aorta. To reduce the ischemia of the lower body, various techniques have been applied, including left subclavian artery perfusion (21), balloon occlusion catheter perfusion in the descending thoracic aorta, femoral artery perfusion with antegrade aortic balloon occlusion (22), or intermittent lower body perfusion (23). These methods may be less appropriate for AAAD patients because of the risk of intimal injury and malperfusion (24). In the present study, ischemic injury was attenuated by RIVP, a novel technique without risk of intimal injury and malperfusion that provides blood flow from veins to vital organs in the lower body during circulatory arrest. Combining RIVP with ACP led to significantly lower risk of AKI in this study than ACP alone. Compared with our results published in 2021 (25), however, incidences of AKI in both groups were much reduced.
Similar to retrograde cerebral perfusion, RIVP may exert its protective effects by allowing better cooling and less embolization (26). Because RIVP also provides oxygenated blood to the lower body, we collected blood gas analyses results from RIVP blood and the returned blood from descending aorta in order to calculate DO2, VO2, and ERO2. O2 content in the blood that had returned from the descending aorta was much lower than that in RIVP blood. This difference was used to calculate the ERO2, which negatively correlated with peaks of lactate and creatinine as well as with increases in creatinine. These results suggest that the protective effect of RIVP on the kidney depends on the ERO2 by tissues.
Under normal conditions, VO2 remains fairly constant despite changes in DO2. If DO2 falls below a critical threshold, VO2 diminishes as well (15, 27, 28), and anaerobic metabolism occurs. In our study, DO2 correlated poorly with VO2, ERO2, and increases in lactate and creatinine. These observations suggest that, in our patients, lower body metabolism was independent of DO2, implying that RIVP delivered adequate amounts of oxygen to the kidney. Consistent with this idea, blood from the descending aorta was still able to release oxygen (PO2 > 30 mmHg).
In view that the ERO2 widely varied from 12%–69% in our patients, and that AKI was still higher in ACP + RIVP patients than in regular bypass patients, we hypothesize that risk of AKI depends on additional factors besides ischemic injury during circulatory arrest. For example, low ERO2 may be due to a decrease in oxygen uptake by tissue, rather than a decrease in DO2. Given that low temperature can lead to low ERO2 (29), it may be preferable to keep body temperature higher. Future studies should investigate whether RIVP can meet metabolic oxygen demand at higher body temperatures.
Anaerobic glycolysis still occurred during RIVP in our patients, as reflected in the significantly greater lactate levels in blood from the descending aorta than in RIVP blood flow. The fact that lactate levels correlated poorly with creatinine levels after surgery implies that anaerobic metabolism may occur not in kidney tissue but in other tissues such as the legs, which are not reached by RIVP blood flow because of venous valves (30).
This study presents several limitations. First, only 43% of the patients in the ACP + RIVP group were detected the blood gas with samples from the descending aorta for oxygen metabolism analysis, which may have introduced potential bias. Second, we were able to collect information from imaging studies on whether the dissection involved the renal arteries, but did not have information about whether this involvement resulted in malperfusion. Additionally, the volume of contrast agent administered during preoperative imaging studies could not be quantified. Third, the absence of the lowest hemoglobin may limit our ability to fully understand the differences in transfusion rates between the groups and their potential impact on patient outcomes. Fourth, in this study we did not evaluate other complications related to abdominal ischemia, such as paraplegia, gastrointestinal complications and acute liver injury. The reason was that the relatively low incidence of these complications would require a larger sample size to analyze differences between two groups. Finally, although the majority of perioperative variables were comparable between the two groups, minority variables such as coeliac trunk dissection rate, DO2 in the post-circulatory arrest period and other unmeasured confounding factors may still affect this retrospective study. Further prospective studies are needed to verify these aspects in the future.
Conclusion
Despite these limitations, the results of this retrospective study suggest that RIVP can significantly reduce risk of AKI by providing adequate oxygen supply to the lower body during circulatory arrest in patients undergoing TARS.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Biomedical Ethics Committee of West China Hospital (2021-837). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants' legal guardians/next of kin because this is a retrospective study.
Author contributions
XL: Data curation, Formal analysis, Investigation, Project administration, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. DL: Data curation, Formal analysis, Investigation, Project administration, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. JL: Data curation, Formal analysis, Investigation, Project administration, Software, Validation, Writing – original draft. ZT: Data curation, Formal analysis, Investigation, Software, Visualization, Writing – review & editing. JX: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Visualization, Writing – review & editing. LD: Conceptualization, Formal analysis, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the 1.3.5 Project for Disciplines of Excellence West China Hospital of Sichuan University (2017-120), and the Natural Science Foundation of Sichuan Province (2022NSFSC1411).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1514247/full#supplementary-material
References
1. Pacini D, Di Marco L, Fortuna D, Belotti LM, Gabbieri D, Zussa C, et al. Acute aortic dissection: epidemiology and outcomes. Int J Cardiol. (2013) 167(6):2806–12. doi: 10.1016/j.ijcard.2012.07.008
2. Olsson C, Thelin S, Ståhle E, Ekbom A, Granath F. Thoracic aortic aneurysm and dissection: increasing prevalence and improved outcomes reported in a nationwide population-based study of more than 14,000 cases from 1987 to 2002. Circulation. (2006) 114(24):2611–8. doi: 10.1161/CIRCULATIONAHA.106.630400
3. Zhang J, Jiang Y, Gao C, Feng J, Wang A. Risk factors for hospital death in patients with acute aortic dissection. Heart Lung Circ. (2015) 24(4):348–53. doi: 10.1016/j.hlc.2014.10.009
4. Takagi H, Umemoto T. A meta-analysis of total arch replacement with frozen elephant trunk in acute type A aortic dissection. Vasc Endovascular Surg. (2016) 50(1):33–46. doi: 10.1177/1538574415624767
5. Halkos ME, Kerendi F, Myung R, Kilgo P, Puskas JD, Chen EP. Selective antegrade cerebral perfusion via right axillary artery cannulation reduces morbidity and mortality after proximal aortic surgery. J Thorac Cardiovasc Surg. (2009) 138(5):1081–9. doi: 10.1016/j.jtcvs.2009.07.045
6. Girardi LN, Shavladze N, Sedrakyan A, Neragi-Miandoab S. Safety and efficacy of retrograde cerebral perfusion as an adjunct for cerebral protection during surgery on the aortic arch. J Thorac Cardiovasc Surg. (2014) 148(6):2927–33. doi: 10.1016/j.jtcvs.2014.07.024
7. Zierer A, El-Sayed Ahmad A, Papadopoulos N, Moritz A, Diegeler A, Urbanski PP. Selective antegrade cerebral perfusion and mild (28°C–30°C) systemic hypothermic circulatory arrest for aortic arch replacement: results from 1002 patients. J Thorac Cardiovasc Surg. (2012) 144(5):1042–9. doi: 10.1016/j.jtcvs.2012.07.063
8. Jakob H, Tsagakis K, Tossios P, Massoudy P, Thielmann M, Buck T, et al. Combining classic surgery with descending stent grafting for acute DeBakey type I dissection. Ann Thorac Surg. (2008) 86(1):95–101. doi: 10.1016/j.athoracsur.2008.03.037
9. Velayudhan BV, Idhrees AM, Mukesh K, Kannan RN. Mesenteric malperfusion in acute aortic dissection: challenges and frontiers. Semin Thorac Cardiovasc Surg. (2019) 31(4):668–73. doi: 10.1053/j.semtcvs.2019.03.012
10. Lin J, Xiong J, Luo M, Tan Z, Wu Z, Guo Y, et al. Combining cerebral perfusion with retrograde Inferior vena caval perfusion for aortic arch surgery. Ann Thorac Surg. (2019) 107(1):e67–67e69. doi: 10.1016/j.athoracsur.2018.08.013
11. Lin J, Tan Z, Yao H, Hu X, Zhang D, Zhao Y, et al. Retrograde inferior vena caval perfusion for total aortic arch replacement surgery (RIVP-TARS): study protocol for a multicenter, randomized controlled trial. Trials. (2019) 20(1):232. doi: 10.1186/s13063-019-3319-2
12. Kazui T, Washiyama N, Muhammad BA, Terada H, Yamashita K, Takinami M, et al. Total arch replacement using aortic arch branched grafts with the aid of antegrade selective cerebral perfusion. Ann Thorac Surg. (2000) 70(1):3–8. doi: 10.1016/s0003-4975(00)01535-6
13. Bachet J, Guilmet D, Goudot B, Dreyfus GD, Delentdecker P, Brodaty D, et al. Antegrade cerebral perfusion with cold blood: a 13-year experience. Ann Thorac Surg. (1999) 67(6):1874–8. doi: 10.1016/s0003-4975(99)00411-7
14. Rao PV, Stahl RF, Soller BR, Shortt KG, Hsi C, Cotter KJ, et al. Retrograde abdominal visceral perfusion: is it beneficial. Ann Thorac Surg. (1995) 60(6):1704–8. doi: 10.1016/0003-4975(95)00735-0
15. Vincent JL. Determination of oxygen delivery and consumption versus cardiac index and oxygen extraction ratio. Crit Care Clin. (1996) 12(4):995–1006. doi: 10.1016/s0749-0704(05)70288-8
16. Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. (2007) 11(2):R31. doi: 10.1186/cc5713
17. O'Brien SM, Feng L, He X, Xian Y, Jacobs JP, Badhwar V, et al. The society of thoracic surgeons 2018 adult cardiac surgery risk models: part 2-statistical methods and results. Ann Thorac Surg. (2018) 105(5):1419–28. doi: 10.1016/j.athoracsur.2018.03.003
18. Zhou H, Wang G, Yang L, Shi S, Li J, Wang M, et al. Acute kidney injury after total arch replacement combined with frozen elephant trunk implantation: incidence, risk factors, and outcome. J Cardiothorac Vasc Anesth. (2018) 32(5):2210–7. doi: 10.1053/j.jvca.2018.02.026
19. Kim WH, Park MH, Kim HJ, Lim HY, Shim HS, Sohn JT, et al. Potentially modifiable risk factors for acute kidney injury after surgery on the thoracic aorta: a propensity score matched case-control study. Medicine (Baltimore). (2015) 94(2):e273. doi: 10.1097/MD.0000000000000273
20. Arnaoutakis GJ, Vallabhajosyula P, Bavaria JE, Sultan I, Siki M, Naidu S, et al. The impact of deep versus moderate hypothermia on postoperative kidney function after elective aortic hemiarch repair. Ann Thorac Surg. (2016) 102(4):1313–21. doi: 10.1016/j.athoracsur.2016.04.007
21. Minatoya K, Ogino H, Matsuda H, Sasaki H, Tanaka H, Kobayashi J, et al. Evolving selective cerebral perfusion for aortic arch replacement: high flow rate with moderate hypothermic circulatory arrest. Ann Thorac Surg. (2008) 86(6):1827–31. doi: 10.1016/j.athoracsur.2008.07.024
22. Della Corte A, Scardone M, Romano G, Amarelli C, Biondi A, De Santo LS, et al. Aortic arch surgery: thoracoabdominal perfusion during antegrade cerebral perfusion may reduce postoperative morbidity. Ann Thorac Surg. (2006) 81(4):1358–64. doi: 10.1016/j.athoracsur.2005.11.062
23. Song SW, Yoo KJ, Shin YR, Lim SH, Cho BK. Effects of intermittent lower body perfusion on end-organ function during repair of acute DeBakey type I aortic dissection under moderate hypothermic circulatory arrest. Eur J Cardiothorac Surg. (2013) 44(6):1070–4. doi: 10.1093/ejcts/ezt145
24. Ueda Y, Miki S, Kusuhara K, Okita Y, Tahata T, Yamanaka K. Deep hypothermic systemic circulatory arrest and continuous retrograde cerebral perfusion for surgery of aortic arch aneurysm. Eur J Cardiothorac Surg. (1992) 6(1):36–41. doi: 10.1016/1010-7940(92)90096-g
25. Lin J, Qin Z, Liu X, Xiong J, Wu Z, Guo Y, et al. Retrograde inferior vena caval perfusion for total aortic arch replacement surgery: a randomized pilot study. BMC Cardiovasc Disord. (2021) 21(1):193. doi: 10.1186/s12872-021-02002-9
26. Pacini D, Leone A, Belotti LM, Fortuna D, Gabbieri D, Zussa C, et al. Acute type A aortic dissection: significance of multiorgan malperfusion. Eur J Cardiothorac Surg. (2013) 43(4):820–6. doi: 10.1093/ejcts/ezs500
27. Baigorri F, Russell JA. Oxygen delivery in critical illness. Crit Care Clin. (1996) 12(4):971–94. doi: 10.1016/s0749-0704(05)70287-6
28. Schertel ER, Brourman JD, Kling SM, Schmall LM, Tobias TA, Myerowitz PD. Vagal innervation influences the whole body oxygen consumption-delivery relationship in the dog. Shock. (1994) 2(2):127–32. doi: 10.1097/00024382-199408000-00008
29. Bentley TB, Meng H, Pittman RN. Temperature dependence of oxygen diffusion and consumption in mammalian striated muscle. Am J Physiol. (1993) 264(6 Pt 2):H1825–30. doi: 10.1152/ajpheart.1993.264.6.H1825
Keywords: acute type A aortic dissection, acute kidney injury, retrograde inferior vena cava perfusion, antegrade cerebral perfusion, ERO2
Citation: Liao X, Luo D, Lin J, Tan Z, Xiong J and Du L (2025) Retrograde inferior vena cava perfusion reduces the risk of acute kidney injury depending on the oxygen extraction ratio. A retrospective cohort study. Front. Cardiovasc. Med. 12:1514247. doi: 10.3389/fcvm.2025.1514247
Received: 25 October 2024; Accepted: 16 April 2025;
Published: 28 April 2025.
Edited by:
DeLisa Fairweather, Mayo Clinic Florida, United StatesReviewed by:
Stefano De Paulis, Agostino Gemelli University Polyclinic (IRCCS), ItalyLingjin Huang, Xiangya Hospital, China
Michael Jessen, University of Texas Southwestern Medical Center, United States
Copyright: © 2025 Liao, Luo, Lin, Tan, Xiong and Du. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiyue Xiong, eGlvbmdqaXl1ZTAyOEAxNjMuY29t; Lei Du, ZHVsZWlAc2N1LmVkdS5jbg==
†These authors have contributed equally to this work
 Xinyi Liao
Xinyi Liao Dan Luo†
Dan Luo† Jiyue Xiong
Jiyue Xiong Lei Du
Lei Du