Abstract
Background:
The blood urea nitrogen to serum albumin ratio (BAR) has emerged as a potential prognostic marker. This study investigated its association with clinical outcomes in patients with atrial fibrillation (AF).
Methods:
A retrospective cohort analysis was performed using data from the MIMIC-IV 2.2 database, including 4,977 patients diagnosed with AF. The primary outcome was 28-day mortality. Cox proportional hazards models were applied to evaluate the association between BAR and mortality, and restricted cubic spline (RCS) analysis was used to explore potential non-linear relationships.
Results:
Of the 4,977 patients analyzed, the 28-day mortality rate was 22.99%. Higher BAR levels were significantly associated with increased risk of mortality. Each one-unit increase in BAR was associated with a 2% higher risk of 28-day mortality (HR 1.02, 95% CI 1.01–1.03). Compared with the lowest quartile (Q1), patients in the highest quartile (Q4) had a significantly increased risk (HR 1.78, 95% CI 1.42–2.22). ROC analysis showed an area under the curve (AUC) of 0.65 for BAR in predicting 28-day mortality. Subgroup analyses confirmed the consistency and robustness of these findings across diverse clinical strata.
Conclusions:
BAR is an independent predictor of 28-day mortality in patients with AF. Higher BAR levels are strongly associated with worse outcomes, underscoring its potential utility as a risk stratification tool in this population.
1 Introduction
Atrial fibrillation (AF) is the most prevalent cardiac arrhythmia, characterized by rapid and disorganized electrical activity in the atria, resulting in an irregular heartbeat (1, 2). Despite significant progress in its diagnosis and treatment (3), the global prevalence and incidence of AF have markedly increased over the past three decades. In 2021 alone, the global incidence of AF reached 4.48 million (4). In Europe and the United States, approximately 25% of individuals over 55 years of age are projected to develop AF during their lifetime (5). AF poses a substantial public health burden due to its high rates of comorbidity, increased mortality risks, and escalating healthcare costs (6). Early identification of individuals at heightened risk for AF development is critical for effective clinical management (7, 8).
Blood-based biomarkers have shown promise in improving prognostic accuracy in AF (9). However, current tools such as the CHADS₂ and CHA₂DS₂-VASc scores are limited in individualized risk prediction. Traditional biomarkers—including troponin and natriuretic peptides—lack sufficient specificity for AF, as they are elevated in various cardiovascular conditions (10). Moreover, the cost and complexity of certain tests, such as echocardiography, can hinder widespread clinical adoption (11). Despite extensive efforts, a definitive, reliable prognostic biomarker for AF remains elusive (12).
Blood urea nitrogen (BUN) and serum albumin are well-established indicators of renal function, nutritional status, and systemic inflammation (13). Elevated BUN has been linked to poor outcomes in cardiovascular conditions (14), while hypoalbuminemia is a known predictor of mortality in critical illness, including AF (15).
The blood urea nitrogen to serum albumin ratio (BAR), reflecting both renal function and nutritional status, has emerged as a prognostic indicator in various conditions, including heart failure (16), acute myocardial infarction (17), chronic obstructive pulmonary disease (18), and sepsis (19). However, its role in predicting short-term outcomes in AF patients remains unclear. This study aims to explore the association between BAR and the prognosis of patients with AF.
2 Materials and methods
2.1 Database introduction
We conducted a retrospective cohort study using the MIMIC-IV v2.2 database, which contains de-identified electronic health records of ICU patients admitted to the Beth Israel Deaconess Medical Center from 2008–2019. The dataset includes demographics, vital signs, laboratory results, medications, and clinical outcomes for approximately 60,000 ICU admissions. The first author of this study, Yun Huang, successfully completed the required training and certification exam (certificate number: 62970244) and was granted access to the database.
2.2 Population selection criteria
Adult patients (≥18 years) with a diagnosis of AF at their first ICU admission were included. Exclusion criteria were ICU stay <24 h and missing BUN or albumin data. Of the 14,341 eligible AF patients, 4,977 met the inclusion criteria (Figure 1). Patients were divided into BAR quartiles: Q1: BAR < 5.37 (n = 1,244); Q2: 5.37 ≤ BAR < 8.40 (n = 1,243); Q3: 8.40 ≤ BAR < 14.58 (n = 1,244); Q4: BAR ≥ 14.58 (n = 1,246).
Figure 1
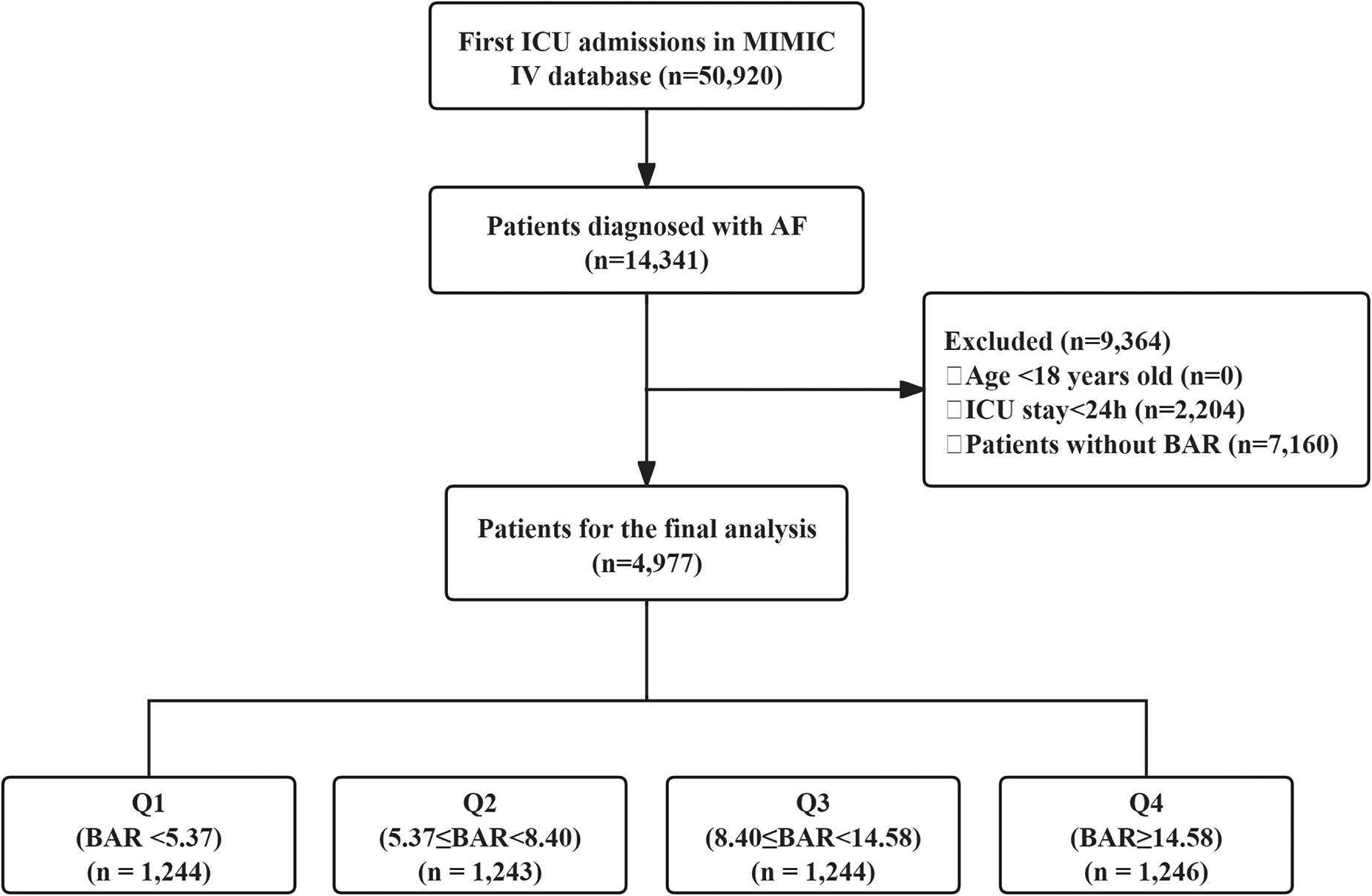
Flow chart of the study population. ICU, intensive care unit; MIMIC, medical information mart for intensive care IV; AF, atrial fibrillation; BAR, blood urea nitrogen to serum albumin ratio.
2.3 Data extraction and BAR calculation
Patient data were extracted from the MIMIC-IV database using Structured Query Language (SQL). Collected variables included demographics (age, gender, race) and vital signs at ICU admission, such as heart rate, mean blood pressure (MBP), respiratory rate, and oxygen saturation (SpO₂). Illness severity was assessed using the Sequential Organ Failure Assessment (SOFA) score, Glasgow Coma Scale (GCS), and Charlson Comorbidity Index (CCI). Comorbidities such as coronary artery disease (CAD), heart failure, hypertension, obesity, diabetes, acute kidney injury (AKI), and sepsis were documented. Laboratory data, including white blood cell count (WBC), red cell distribution width (RDW), red blood cell count (RBC), platelet count, BUN, creatinine, potassium, sodium, albumin, international normalized ratio (INR), and N-terminal pro-brain natriuretic peptide (NT-proBNP), were collected. Therapeutic interventions, including the use of aspirin, heparin, warfarin, non-vitamin K antagonist oral anticoagulants (NOACs), amiodarone, statins, vasoactive drugs, ablation, mechanical ventilation, and continuous renal replacement therapy (CRRT), were also recorded. The BAR was calculated by dividing BUN (mg/dl) by serum albumin concentration (g/dl).
2.4 Outcomes
The primary outcomes were 28-day all-cause mortality. Secondary outcomes included length of hospital and ICU stay, hospital and ICU mortality.
2.5 Statistical analysis
Baseline characteristics were analyzed across BAR quartiles using appropriate statistical methods. Continuous variables were reported as mean ± standard deviation (SD) or median with interquartile range (IQR), depending on data distribution, while categorical variables were presented as frequencies and percentages. Comparisons between groups were performed using one-way analysis of variance (ANOVA) or the Kruskal–Wallis test for continuous variables, and the chi-square test for categorical variables, as applicable.
To identify factors associated with 28-day outcomes, we employed least absolute shrinkage and selection operator (LASSO) regression. Cox proportional hazards models were subsequently used to examine the relationship between BAR and 28-day all-cause mortality. To address potential confounding, we developed three models to calculate HRs and 95% CI, and performed trend analyses across quartiles. Model 1 adjusted for age, gender, and race. Model 2 incorporated Model 1 variables plus heart rate, respiratory rate, SpO2, SOFA score, GCS, CCI, CAD, AKI, and sepsis. Model 3 further adjusted for WBC, RDW, creatinine, albumin, INR, NT-proBNP, aspirin, warfarin, NOACs, statins, vasoactive drugs, and CRRT. RCS analysis was conducted to explore non-linear relationships.
Kaplan–Meier survival curves were used to compare primary outcomes across BAR quartiles. Additionally, receiver operating characteristic (ROC) curves were generated. Stratified and interaction analyses were performed based on factors such as age, gender, race, SOFA score, CAD, hypertension, AKI, sepsis, and NT-proBNP levels.
All analyses were conducted using R software (version 4.4.1) and Free Statistics software (version 2.0). Statistical significance was set at P < 0.05.
3 Results
3.1 Patient characteristics
Baseline characteristics of the study population by BAR quartiles are summarized in Table 1. The mean age was 74.4 ± 12.2 years, and males comprised 57.46% of the cohort. Both age and gender distribution varied significantly across quartiles (P < 0.001). Patients in higher BAR quartiles exhibited lower MBP and SpO2 (both P < 0.001), higher heart and respiratory rates (both P < 0.001), and elevated SOFA and CCI scores. They were also more likely to receive treatments such as heparin, vasoactive drugs, ventilatory support, and CRRT (all P < 0.001). Comorbidities, including heart failure, diabetes, AKI, and sepsis, were more prevalent in higher quartiles (all P < 0.001). Significant differences in laboratory parameters were observed across quartiles, including WBC, RDW, RBC, BUN, creatinine, potassium, sodium, albumin, INR, and NT-proBNP levels (all P < 0.001). Patients in higher quartiles also had longer hospital and ICU stays (both P < 0.001). In Quartile 4, hospital mortality reached 30.98%, ICU mortality was 20.87%, and 28-day mortality was 35.71% (all P < 0.001). Hospital and ICU stays were longest in patients with invasive ventilation, with median durations of 12.0 days (IQR: 7.2–20.7) and 5.8 days (IQR: 3.1–10.7), respectively (Supplementary Table S1).
Table 1
| Variables | Total | Q1 | Q2 | Q3 | Q4 | P |
|---|---|---|---|---|---|---|
| (n = 4,977) | (BAR < 5.37) | (5.37 ≤ BAR < 8.40) | (8.40 ≤ BAR < 14.58) | (BAR ≥ 14.58) | ||
| (n = 1,244) | (n = 1,243) | (n = 1,244) | (n = 1,246) | |||
| Age (year) | 74.4 ± 12.2 | 71.7 ± 12.7 | 74.9 ± 12.0 | 75.7 ± 11.6 | 75.2 ± 11.9 | <0.001 |
| Gender, n (%) | <0.001 | |||||
| Female | 2,117 (42.54) | 602 (48.39) | 548 (44.09) | 525 (42.20) | 442 (35.47) | |
| Male | 2,860 (57.46) | 642 (51.61) | 695 (55.91) | 719 (57.80) | 804 (64.53) | |
| Race, n (%) | 0.051 | |||||
| Other | 1,439 (28.91) | 386 (31.03) | 324 (26.07) | 363 (29.18) | 366 (29.37) | |
| White | 3,538 (71.09) | 858 (68.97) | 919 (73.93) | 881 (70.82) | 880 (70.63) | |
| Vital signs | ||||||
| Heart rate (beats/min) | 88 (75, 105) | 86 (74, 103) | 87 (74, 103) | 90 (78, 108) | 89 (75, 106) | <0.001 |
| MBP (mmHg) | 81 (69, 93) | 85 (74, 97) | 83 (71, 95.8) | 78 (67, 90) | 76 (66, 88) | <0.001 |
| Respiratory rate (times/min) | 19 (16, 23) | 18 (15, 22) | 19 (16, 23) | 20 (16, 24) | 20 (17, 24) | <0.001 |
| SpO2 (%) | 96.4 ± 4.4 | 97.1 ± 3.6 | 96.6 ± 4.2 | 95.9 ± 4.9 | 95.9 ± 4.7 | <0.001 |
| Severity scores | ||||||
| SOFA | 5 (3, 8) | 3 (2, 5) | 5 (3, 7) | 5 (3, 8) | 7 (5, 10) | <0.001 |
| GCS | 15 (14, 15) | 15 (13, 15) | 15 (14, 15) | 15 (14, 15) | 15 (13, 15) | 0.227 |
| CCI | 6 (5, 8) | 5 (4, 7) | 6 (4, 8) | 7 (5, 8) | 8 (6, 9) | <0.001 |
| Comorbidity | ||||||
| CAD, n (%) | 1,138 (22.87) | 249 (20.02) | 288 (23.17) | 285 (22.91) | 316 (25.36) | 0.017 |
| Heart failure, n (%) | 2,518 (50.59) | 425 (34.16) | 607 (48.83) | 702 (56.43) | 784 (62.92) | <0.001 |
| Hypertension, n (%) | 2,051 (41.21) | 462 (37.14) | 525 (42.24) | 513 (41.24) | 551 (44.22) | 0.003 |
| Diabetes, n (%) | 1,694 (34.04) | 292 (23.47) | 370 (29.77) | 466 (37.46) | 566 (45.43) | <0.001 |
| AKI, n (%) | 4,197 (84.33) | 968 (77.81) | 1,064 (85.60) | 1,078 (86.66) | 1,087 (87.24) | <0.001 |
| Sepsis, n (%) | 3,247 (65.24) | 649 (52.17) | 761 (61.22) | 866 (69.61) | 971 (77.93) | <0.001 |
| Laboratory tests | ||||||
| WBC (K/μl) | 11.0 (7.7, 15.7) | 10.2 (7.5, 14.0) | 10.9 (7.8, 15.3) | 11.2 (7.8, 16.3) | 11.7 (8.0, 17.6) | <0.001 |
| RDW (%) | 14.9 (13.8, 16.7) | 14.1 (13.3, 15.3) | 14.6 (13.7, 16.1) | 15.2 (14.2, 16.9) | 16.0 (14.7, 17.9) | <0.001 |
| RBC (K/μl) | 3.5 (3.0, 4.1) | 3.8 (3.2, 4.3) | 3.6 (3.1, 4.2) | 3.5 (3.0, 3.9) | 3.2 (2.8, 3.8) | <0.001 |
| Platelet (K/μl) | 187 (133, 253) | 192 (139, 252) | 190 (138, 250) | 182 (132, 254) | 183 (126, 257) | 0.065 |
| BUN (mg/dl) | 26 (17, 42) | 13 (11, 16) | 21 (19, 24) | 32 (28, 38) | 61 (48, 79) | <0.001 |
| Creatinine (mg/dl) | 1.2 (0.8, 1.8) | 0.8 (0.6, 1.0) | 1.0 (0.8, 1.3) | 1.4 (1.1, 1.8) | 2.4 (1.7, 3.7) | <0.001 |
| Potassium (mmol/L) | 4.2 (3.8, 4.7) | 4.0 (3.6, 4.4) | 4.1 (3.8, 4.5) | 4.3 (3.9, 4.7) | 4.5 (4.0, 5.1) | <0.001 |
| Sodium (mmol/L) | 139 (135, 141) | 139 (136, 141) | 139 (136, 141) | 138 (135, 141) | 138 (134, 142) | <0.001 |
| Albumin (g/dl) | 3.1 (2.7, 3.5) | 3.4 (3.0, 3.8) | 3.2 (2.8, 3.6) | 3.0 (2.6, 3.4) | 2.8 (2.4, 3.2) | <0.001 |
| INR | 1.4 (1.2, 1.8) | 1.3 (1.1, 1.6) | 1.4 (1.2, 1.7) | 1.4 (1.2, 1.9) | 1.5 (1.3, 2.2) | <0.001 |
| NT-proBNP (pg/ml), n (%) | <0.001 | |||||
| <2,000 | 832 (16.72) | 232 (18.65) | 231 (18.58) | 203 (16.32) | 166 (13.32) | |
| >=2,000 | 1,411 (28.35) | 217 (17.44) | 299 (24.05) | 419 (33.68) | 476 (38.20) | |
| Missing | 2,734 (54.93) | 795 (63.91) | 713 (57.36) | 622 (50.00) | 604 (48.48) | |
| Treatment | ||||||
| Aspirin, n (%) | 2,070 (41.59) | 542 (43.57) | 581 (46.74) | 483 (38.83) | 464 (37.24) | <0.001 |
| Heparin, n (%) | 3,634 (73.02) | 856 (68.81) | 888 (71.44) | 914 (73.47) | 976 (78.33) | <0.001 |
| Warfarin, n (%) | 950 (19.09) | 215 (17.28) | 261 (21.00) | 265 (21.30) | 209 (16.77) | 0.003 |
| NOACs, n (%) | 301 (6.05) | 116 (9.32) | 72 (5.79) | 69 (5.55) | 44 (3.53) | <0.001 |
| Amiodarone, n (%) | 1,337 (26.86) | 298 (23.95) | 359 (28.88) | 348 (27.97) | 332 (26.65) | 0.033 |
| Statin, n (%) | 1,924 (38.66) | 516 (41.48) | 533 (42.88) | 474 (38.10) | 401 (32.18) | <0.001 |
| Vasoactive drug, n (%) | 2,467 (49.57) | 488 (39.23) | 624 (50.2) | 649 (52.17) | 706 (56.66) | <0.001 |
| Ablation, n (%) | 31 (0.62) | 14 (1.13) | 5 (0.40) | 8 (0.64) | 4 (0.32) | 0.048 |
| Ventilator | <0.001 | |||||
| No | 720 (14.47) | 226 (18.17) | 162 (13.03) | 171 (13.75) | 161 (12.92) | |
| Non-invasive | 2,044 (41.07) | 508 (40.84) | 485 (39.02) | 516 (41.48) | 535 (42.94) | |
| Invasive | 2,213 (44.46) | 510 (41.00) | 596 (47.95) | 557 (44.77) | 550 (44.14) | |
| CRRT, n (%) | 426 (8.56) | 34 (2.73) | 70 (5.63) | 104 (8.36) | 218 (17.5) | <0.001 |
| Clinical outcomes | ||||||
| Hospital stay (day) | 10.0 (6.1, 16.8) | 8.8 (5.8, 14.8) | 9.8 (6.1, 16.5) | 10.1 (6.2, 17.6) | 11.6 (6.7, 18.9) | <0.001 |
| ICU stay (day) | 3.4 (2.0, 6.7) | 2.9 (1.8, 5.5) | 3.4 (1.9, 6.3) | 3.5 (2.0, 6.9) | 3.9 (2.2, 7.7) | <0.001 |
| Hospital mortality, n (%) | 980 (19.69) | 129 (10.37) | 178 (14.32) | 287 (23.07) | 386 (30.98) | <0.001 |
| ICU mortality, n (%) | 648 (13.02) | 89 (7.15) | 113 (9.09) | 186 (14.95) | 260 (20.87) | <0.001 |
| 28-day mortality, n (%) | 1,144 (22.99) | 158 (12.7) | 210 (16.89) | 331 (26.61) | 445 (35.71) | <0.001 |
Baseline characteristics of the study participants.
MBP, mean blood pressure; SpO2, oxygen saturation; SOFA, sequential organ failure assessment; GCS, Glasgow coma scale; CCI, Charlson comorbidity index; CAD, coronary artery disease; AKI, acute kidney injury; WBC, white blood cell; RDW, red cell distribution width; RBC, red blood cell; BUN, blood urea nitrogen; INR, international normalized ratio; NT-proBNP, N-terminal pro-brain natriuretic peptide; NOACs, Non-vitamin K antagonist oral anticoagulants; CRRT, continuous renal replacement therapy; ICU, intensive care unit.
3.2 Baseline characteristics of the 28-day survivor and mortality groups
The baseline characteristics of the study population, stratified by 28-day survival and mortality groups, are shown in Table 2. Patients in the mortality group were older 76.7 ± 12.0 (P < 0.001), and exhibited higher heart rates, respiratory rates, SOFA scores, and BAR levels 11.61 (P < 0.001). AKI and sepsis were more prevalent in the mortality group (P < 0.001). Laboratory findings revealed higher BUN, creatinine, potassium, and RDW, alongside lower serum albumin. Treatment differences included greater use of vasoactive drugs and CRRT in the mortality group, while aspirin and statin use was lower (P < 0.001).
Table 2
| Variables | Total | 28-day survival | 28-day mortality | P |
|---|---|---|---|---|
| (n = 4,977) | (n = 3,833) | (n = 1,144) | ||
| Age (year) | 74.4 ± 12.2 | 73.7 ± 12.1 | 76.7 ± 12.0 | <0.001 |
| Gender, n (%) | 0.01 | |||
| Female | 2,117 (42.54) | 1,594 (41.59) | 523 (45.72) | |
| Male | 2,860 (57.46) | 2,239 (58.41) | 621 (54.28) | |
| Race, n (%) | <0.001 | |||
| Other | 1,439 (28.91) | 1,047 (27.32) | 392 (34.27) | |
| White | 3,538 (71.09) | 2,786 (72.68) | 752 (65.73) | |
| Vital signs | ||||
| Heart rate (beats/min) | 88 (75, 105) | 87 (74, 104) | 92 (77, 108) | <0.001 |
| MBP (mmHg) | 81 (69, 93) | 81 (70, 93) | 79 (67, 93) | 0.02 |
| Respiratory rate (times/min) | 19 (16, 23) | 19 (16, 23) | 20 (17, 24) | <0.001 |
| SpO2 (%) | 96.4 ± 4.4 | 96.6 ± 4.0 | 95.8 ± 5.4 | <0.001 |
| Severity scores | ||||
| SOFA | 5 (3, 8) | 5 (3, 7) | 7 (4, 10) | <0.001 |
| GCS | 15 (14, 15) | 15 (14, 15) | 15 (12, 15) | <0.001 |
| CCI | 6 (5, 8) | 6 (4, 8) | 7 (6, 9) | <0.001 |
| Comorbidity | ||||
| CAD, n (%) | 1,138 (22.87) | 850 (22.18) | 288 (25.17) | 0.034 |
| Heart failure, n (%) | 2,518 (50.59) | 1,916 (49.99) | 602 (52.62) | 0.12 |
| Hypertension, n (%) | 2,051 (41.21) | 1,612 (42.06) | 439 (38.37) | 0.03 |
| Diabetes, n (%) | 1,694 (34.04) | 1,293 (33.73) | 401 (35.05) | 0.41 |
| AKI, n (%) | 4,197 (84.33) | 3,148 (82.13) | 1,049 (91.70) | <0.001 |
| Sepsis, n (%) | 3,247 (65.24) | 2,342 (61.10) | 905 (79.11) | <0.001 |
| Laboratory tests | ||||
| WBC (K/μl) | 11.0 (7.7, 15.7) | 10.7 (7.6, 15.0) | 12.1 (8.3, 18.1) | <0.001 |
| RDW (%) | 14.9 (13.8, 16.7) | 14.7 (13.7, 16.3) | 15.7 (14.3, 17.7) | <0.001 |
| RBC (K/μl) | 3.5 (3.0, 4.1) | 3.5 (3.0, 4.1) | 3.4 (2.9, 4.0) | <0.001 |
| Platelet (K/μl) | 187 (133, 253) | 187 (135, 250) | 188 (125, 262) | 0.29 |
| BUN (mg/dl) | 26 (17, 42) | 24 (16, 39) | 34 (22, 53) | <0.001 |
| Creatinine (mg/dl) | 1.2 (0.8, 1.8) | 1.1 (0.8, 1.7) | 1.4 (0.9, 2.3) | <0.001 |
| Potassium (mmol/L) | 4.2 (3.8, 4.7) | 4.2 (3.8, 4.6) | 4.3 (3.8, 4.8) | <0.001 |
| Sodium (mmol/L) | 139 (135, 141) | 139 (136, 141) | 138 (135, 142) | 0.69 |
| Albumin (g/dl) | 3.1 (2.7, 3.5) | 3.2 (2.8, 3.6) | 3.0 (2.5, 3.4) | <0.001 |
| BAR | 8.40 (5.37, 14.58) | 7.60 (5.00, 12.94) | 11.61 (7.20, 19.68) | <0.001 |
| INR | 1.4 (1.2, 1.8) | 1.4 (1.2, 1.8) | 1.5 (1.2, 2.1) | <0.001 |
| NT-proBNP (pg/ml), n (%) | <0.001 | |||
| <2,000 | 832 (16.72) | 692 (18.05) | 140 (12.24) | |
| >=2,000 | 1,411 (28.35) | 1,096 (28.59) | 315 (27.53) | |
| missing | 2,734 (54.93) | 2,045 (53.35) | 689 (60.23) | |
| Treatment | ||||
| Aspirin, n (%) | 2,070 (41.59) | 1,659 (43.28) | 411 (35.93) | <0.001 |
| Heparin, n (%) | 3,634 (73.02) | 2,721 (70.99) | 913 (79.81) | <0.001 |
| Warfarin, n (%) | 950 (19.09) | 854 (22.28) | 96 (8.39) | <0.001 |
| NOACs | 301 (6.05) | 261 (6.81) | 40 (3.50) | <0.001 |
| Amiodarone, n (%) | 1,337 (26.86) | 1,012 (26.40) | 325 (28.41) | 0.18 |
| Statin, n (%) | 1,924 (38.66) | 1,569 (40.93) | 355 (31.03) | <0.001 |
| Vasoactive drug, n (%) | 2,467 (49.57) | 1,733 (45.21) | 734 (64.16) | <0.001 |
| Ablation, n (%) | 31 (0.62) | 28 (0.73) | 3 (0.26) | 0.08 |
| Ventilator | <0.001 | |||
| No | 720 (14.47) | 579 (15.11) | 141 (12.33) | |
| Non-invasive | 2,044 (41.07) | 1,675 (43.7) | 369 (32.26) | |
| Invasive | 2,213 (44.46) | 1,579 (41.19) | 634 (55.42) | |
| CRRT, n (%) | 426 (8.56) | 238 (6.21) | 188 (16.43) | <0.001 |
Baseline characteristics of the 28-day survivor and 28-day mortality group.
MBP, mean blood pressure; SpO2, oxygen saturation; SOFA, sequential organ failure assessment; GCS, glasgow coma scale; CCI, Charlson comorbidity index; CAD, coronary artery disease; AKI, acute kidney injury; WBC, white blood cell; RDW, red cell distribution width; RBC, red blood cell; BUN, blood urea nitrogen; BAR, blood urea nitrogen to albumin ratio; INR, international normalized ratio; NT-proBNP, N-terminal pro-brain natriuretic peptide; NOACs, Non-vitamin K antagonist oral anticoagulants; CRRT, continuous renal replacement therapy.
3.3 Association between BAR and 28-day all-cause mortality
LASSO regression identified 26 variables for adjustment (Supplementary Figure S1). Multivariable Cox models (Table 3) confirmed BAR as an independent predictor of 28-day mortality. Each unit increase in BAR was associated with a 2% higher mortality risk (HR: 1.02; 95% CI: 1.01–1.03). Compared with Q1, patients in Q4 had an HR of 1.78 (95% CI: 1.42–2.22; P for trend < 0.001).
Table 3
| Variables | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| 28-day mortality | ||||||
| BAR (continuous) | 1.03 (1.03, 1.04) | <0.001 | 1.01 (1.01, 1.02) | <0.001 | 1.02 (1.01, 1.03) | <0.001 |
| BAR (quartiles) | ||||||
| Q1 (BAR < 5.37) | 1 (ref) | 1 (ref) | 1 (ref) | |||
| Q2 (5.37 ≤ BAR < 8.40) | 1.33 (1.08, 1.64) | 0.007 | 1.00 (0.81, 1.23) | 0.996 | 1.01 (0.82, 1.25) | 0.914 |
| Q3 (8.40 ≤ BAR < 14.58) | 2.19 (1.81, 2.65) | <0.001 | 1.40 (1.16, 1.73 | 0.001 | 1.51 (1.23, 1.85) | <0.001 |
| Q4 (BAR ≥ 14.58) | 3.18 (2.65, 3.82) | <0.001 | 1.63 (1.34, 2.00) | <0.001 | 1.78 (1.42, 2.22) | <0.001 |
| P for trend | <0.001 | <0.001 | <0.001 | |||
Cox proportional hazard model assessing 28-day mortality in patients with AF.
Model 1: adjusted for age, gender and race.
Model 2: adjusted for Model 1 plus heart rate, MBP, respiratory rate, SpO2, SOFA, GCS, CCI, CAD, AKI, sepsis.
Model 3: adjusted for Model 2 plus WBC, RDW, creatinine, albumin, INR, NT-proBNP, aspirin, warfarin, NOACs, statin, vasoactive drug, CRRT.
MBP, mean blood pressure; SpO2, oxygen saturation; SOFA, sequential organ failure assessment; GCS, Glasgow coma scale; CCI, Charlson comorbidity index; CAD, coronary artery disease; AKI, acute kidney injury; WBC, white blood cell; RDW, red cell distribution width; INR, international normalized ratio; NT-proBNP, N-terminal pro-brain natriuretic peptide; NOACs, Non-vitamin K antagonist oral anticoagulants; CRRT, continuous renal replacement therapy.
3.4 Kaplan–Meier survival curve and RCS
Kaplan–Meier curves (Figure 2) demonstrated significantly lower survival in patients with higher BAR levels (log-rank P < 0.0001), with Q4 showing the steepest decline. RCS analysis revealed a significant non-linear association between BAR and 28-day mortality (Figure 3).
Figure 2
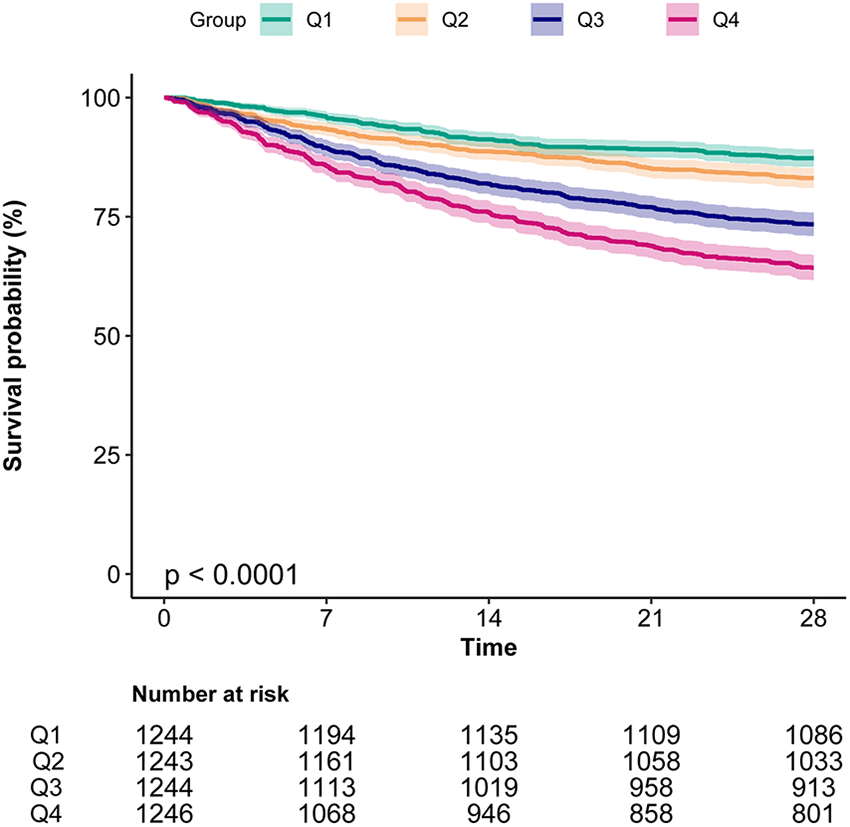
Kaplan–Meier curves indicates the association between the BAR and 28-day mortality of patients with AF. BAR, blood urea nitrogen to albumin ratio; AF, atrial fibrillation.
Figure 3
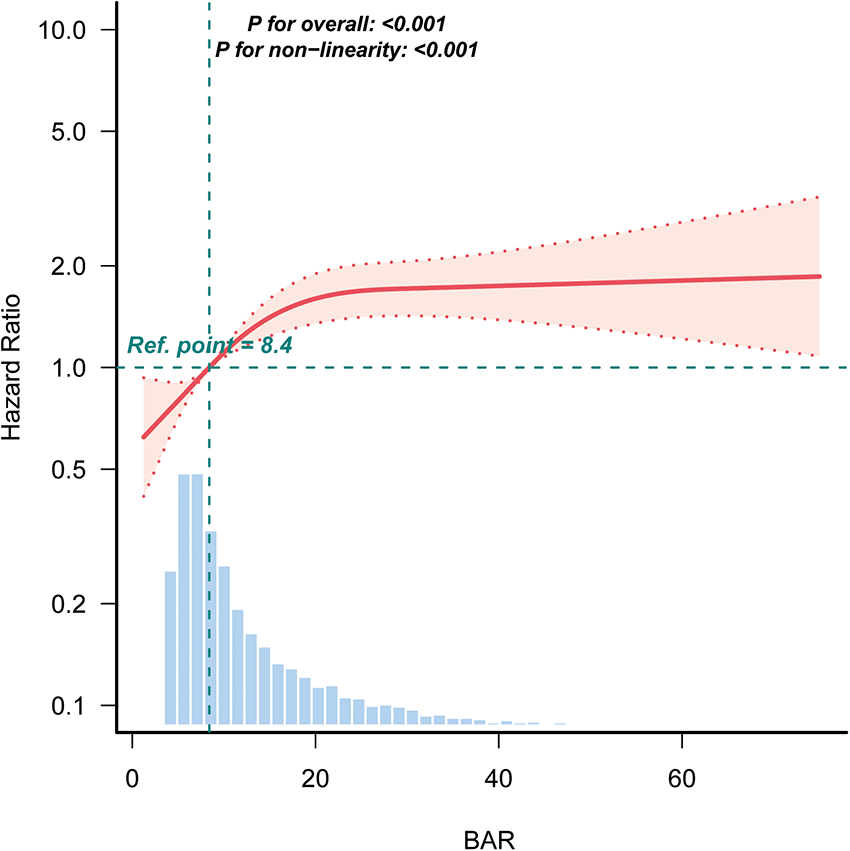
Restricted cubic spline analysis of the association between BAR and the risk of 28-day mortality in patients with AF. BAR, blood urea nitrogen to albumin ratio; AF, atrial fibrillation.
3.5 Prediction of 28-day all-cause mortality by BAR
The ROC curves compare the predictive performance of BAR, BUN, and albumin (Table 4 and Figure 4). BAR had the highest AUC for 28-day mortality (AUC = 0.65), indicating superior predictive ability.
Table 4
| Variables | Cut-off | Sensitivity | Specificity | PPV | NPV | AUC (95% CI) |
|---|---|---|---|---|---|---|
| BAR | 9.04 | 0.64 | 0.59 | 0.85 | 0.32 | 0.65 (0.63–0.67) |
| BUN | 29.5 | 0.58 | 0.62 | 0.83 | 0.32 | 0.63 (0.62–0.65) |
| Albumin | 2.75 | 0.41 | 0.75 | 0.67 | 0.19 | 0.59 (0.57–0.61) |
Performance of BAR in predicting 28-day mortality in AF patients.
BAR, blood urea nitrogen to albumin ratio; BUN, blood urea nitrogen; PPV, positive predictive value; NPV, negative predictive value; AUC, area under the curve.
Figure 4
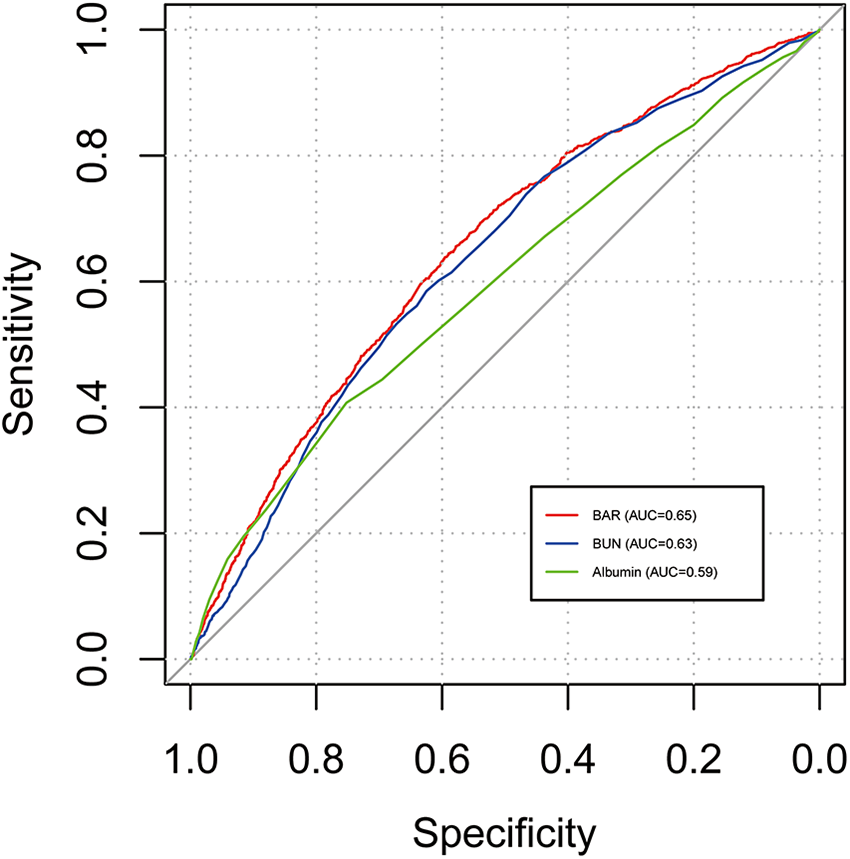
ROC curves of BAR for 28-day mortality of patients with AF. ROC, receiver operating characteristic; BAR, blood urea nitrogen to albumin ratio; AF, atrial fibrillation.
3.6 Subgroup analysis
Subgroup analysis indicates no significant interactions for age, gender, race, SOFA score, CAD, hypertension, AKI, sepsis, and NT-proBNP (all P for interaction > 0.05) (Figure 5).
Figure 5
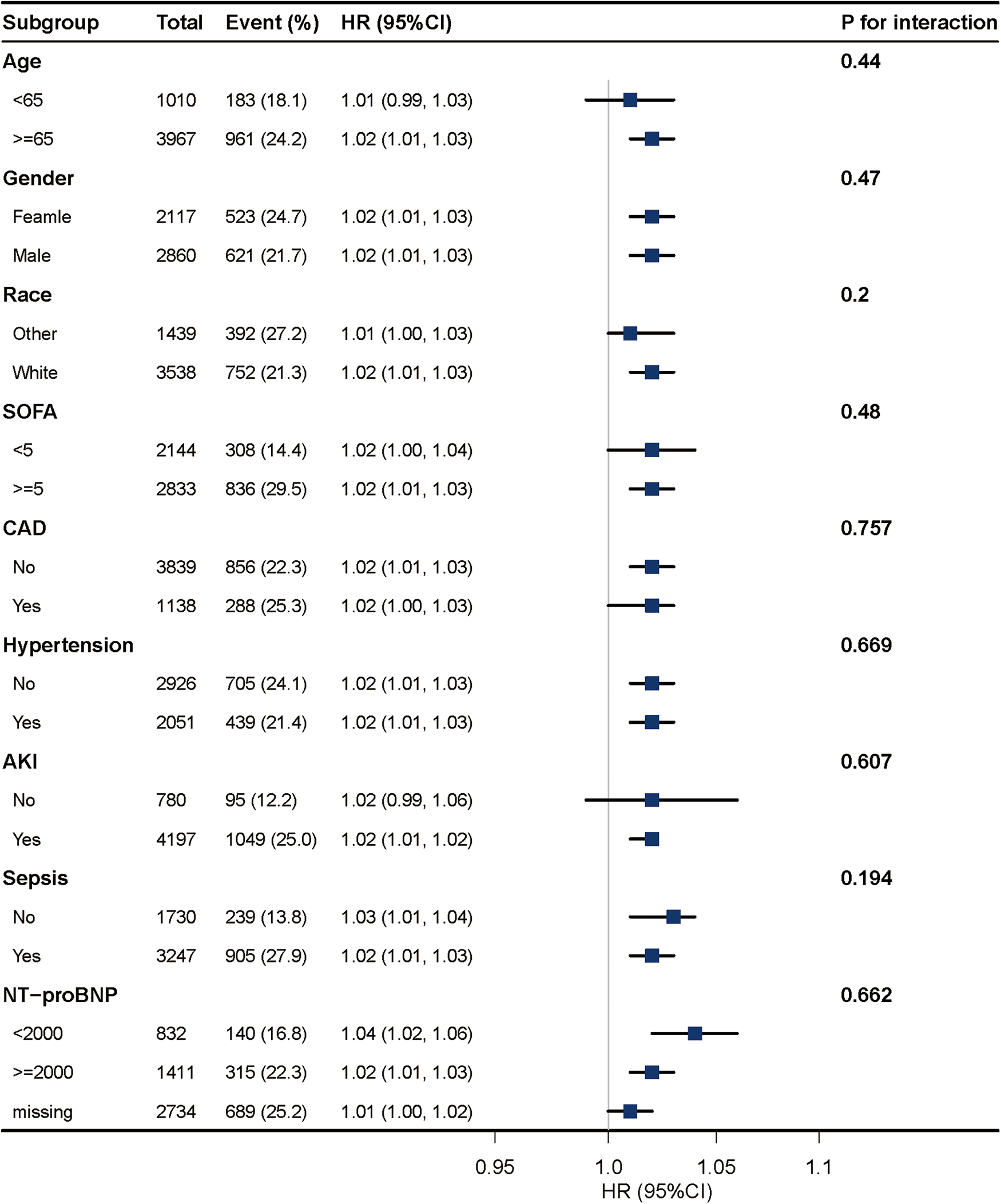
Association between BAR and 28-day mortality according to baseline characteristics. Each stratification was adjusted for all factors of Model 3 in Table 3 except for the stratification factor itself.
4 Discussion
This study demonstrates that the BAR is independently associated with 28-day mortality in critically ill patients with AF. The association remained significant after adjusting for demographic, clinical, and laboratory factors. Compared to BUN or albumin alone, BAR showed superior prognostic performance, supporting its utility as a simple and accessible biomarker for early risk stratification.
In critically ill patients with AF, BUN and albumin levels are important prognostic indicators. Elevated BUN typically indicates renal impairment or fluid retention (20, 21), both of which are closely associated with the onset and persistence of AF. Increased BUN levels lead to fluid accumulation, raising cardiac workload, promoting atrial dilation, and contributing to atrial fibrosis—all of which create a substrate for AF (22, 23). Moreover, elevated BUN is often associated with electrolyte imbalances, such as hyperkalemia, which can disrupt the heart's electrical function, increase the risk of arrhythmias, and prolong AF duration, thus worsening cardiac function and prognosis (20, 24). On the other hand, low albumin levels generally indicate chronic illness, malnutrition, or impaired liver function (25). As a plasma protein, albumin plays a critical role in maintaining colloid osmotic pressure and transporting drugs and nutrients. Low albumin levels suggest nutritional depletion or severe illness, leading to increased vascular permeability, edema, and fluid shifts. Additionally, hypoalbuminemia may indicate an underlying inflammatory state (26), which can accelerate cardiac and organ failure, reducing survival rates.
BAR serves as an effective marker of this complex interplay, providing a comprehensive measure of disease severity. Elevated BAR levels in AF patients often signal multiorgan dysfunction, including renal and hepatic impairment, which is common in critically ill patients (27, 28). Moreover, BAR has been associated with increased thromboembolic risk and atrial remodeling. High BUN levels reflect systemic stress and a prothrombotic state (13), while low albumin correlates with reduced anticoagulant efficacy and increased embolic risk (29). Unlike isolated biomarkers, BAR offers a holistic assessment of the metabolic, hemodynamic, and inflammatory shifts that characterize AF, making it a practical tool for risk stratification and treatment planning, especially in high-risk patients.
Although BAR was independently associated with 28-day mortality, its discriminatory power was moderate, with an AUC of 0.65. However, BAR showed better predictive performance compared with its individual components, with an AUC of 0.63 for BUN and 0.59 for albumin. This suggests that BAR may provide a more integrated assessment of renal function and nutritional status. These findings support its potential role as a composite prognostic biomarker. Nevertheless, due to its limited sensitivity and specificity, BAR may not be sufficient as a standalone risk stratification tool. Further research is needed to assess whether incorporating BAR into established risk prediction models, such as CHA2DS2-VASc or HAS-BLED, could improve prognostic accuracy and inform clinical decision-making in patients with atrial fibrillation.
Notably, our findings revealed relatively low rates of anticoagulant use, particularly in patients with high BAR levels (Q4 group), where warfarin was used in only 16.77% of cases and NOACs in just 3.53%. Current guidelines recommend oral anticoagulation as standard therapy for patients with atrial fibrillation, especially those with persistent or permanent AF at elevated thromboembolic risk. However, our dataset lacked detailed information on AF duration or classification, precluding analysis of whether the patients had newly diagnosed or chronic AF. The underuse of guideline-directed medical therapy (GDMT), including anticoagulants, may partially explain the higher mortality observed in the high-BAR group. Inadequate anticoagulation could contribute to increased thromboembolic complications or progression of atrial remodeling, compounding the risk associated with renal dysfunction and hypoalbuminemia. Future studies should incorporate detailed rhythm history and therapeutic adherence data to better delineate these associations.
In our study based on the MIMIC-IV database, the median ICU length of stay among intubated patients was 5.8 days (IQR: 3.1–10.7), which appears relatively short. Several factors may account for this. First, early ICU mortality was common in our cohort and could contribute to shorter ICU stays. Second, MIMIC includes a substantial proportion of patients with treatment-limiting orders such as Do-Not-Resuscitate (DNR) or Do-Not-Intubate (DNI), which may lead to earlier withdrawal of intensive care. Third, hospital-specific discharge practices and ICU resource limitations may have led to early transitions to step-down or general wards. Similar ICU durations have also been reported in previous studies using the MIMIC-III and IV datasets (30, 31). Importantly, the relatively short ICU stay may have implications for our results. It could lead to underestimation of disease progression or complications that typically manifest later during prolonged ICU admissions. Moreover, the early timing of data collection may favor early predictors of mortality, while longer-term prognostic factors may be less represented. Therefore, while our findings remain valid within the context of early ICU outcomes, caution is warranted when generalizing them to patients with prolonged ICU courses.
This study has several strengths, including the use of a large, well-curated critical care database and rigorous statistical adjustment. However, limitations should be acknowledged. First, the retrospective design inherently introduces potential selection bias and limits the ability to draw causal inferences. Second, this study was based on data from the MIMIC-IV database, which represents a single-center ICU cohort in the United States. As such, the generalizability of our findings to other healthcare settings, geographic regions, or patient populations may be limited. Third, BAR may be affected by unmeasured confounders such as hydration status, liver function, and chronic malnutrition, which were not fully accounted for due to data limitations. Fourth, echocardiographic data were not available in the MIMIC-IV database, which limited our ability to assess cardiac structure and function, including left ventricular ejection fraction and atrial size. Lastly, our study did not compare BAR with established AF-specific risk scores (e.g., CHA2DS2-VASc, ATRIA, or HAS-BLED), which limits our ability to evaluate the incremental prognostic value of BAR beyond current tools. In addition, while MIMIC-IV is a valuable resource, analyses based on such databases require careful attention to case definitions, data quality, and residual confounding. For example, evaluating the effect of anticoagulation is challenging, as treated patients may differ systematically in comorbidity burden or disease severity. Without careful statistical adjustment, treatment effects may be confounded. Moreover, data on anticoagulation intensity and adherence are limited, and MIMIC-IV does not capture post-discharge outcomes. This constrains assessment of long-term endpoints such as stroke, bleeding, or readmission. External validation in prospective cohorts is needed to confirm these findings.
In conclusion, BAR is an independent and easily accessible predictor of short-term mortality in critically ill patients with AF. Its integration into routine clinical assessment may enhance early risk stratification and inform personalized treatment strategies in this vulnerable population.
Statements
Data availability statement
Publicly available datasets were analyzed in this study. These data are available from the MIMIC-IV 2.2 database at https://physionet.org/content/mimiciv/2.2/.
Ethics statement
The studies involving humans were approved by the Institutional Review Board (IRB) of Beth Israel Deaconess Medical Center (Approval No: 2001P-001699/14). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
YH: Data curation, Writing – original draft. GW: Validation, Writing – original draft. XX: Validation, Writing – original draft. JM: Formal analysis, Software, Writing – original draft. CZ: Formal analysis, Software, Writing – original draft. YZ: Resources, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Correction Note
This article has been corrected with minor changes. These changes do not impact the scientific content of the article.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1533575/full#supplementary-material
References
1.
Brundel B Ai X Hills MT Kuipers MF Lip GYH de Groot NMS . Atrial fibrillation. Nat Rev Dis Primers (2022) 8(1):21. 10.1038/s41572-022-00347-9
2.
Baman JR Passman RS . Atrial fibrillation. Jama (2021) 325(21):2218. 10.1001/jama.2020.23700
3.
Joglar JA Chung MK Armbruster AL Benjamin EJ Chyou JY Cronin EM et al 2023 Acc/Aha/Accp/Hrs guideline for the diagnosis and management of atrial fibrillation: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. Circulation (2024) 149(1):e1–e156. 10.1161/cir.0000000000001193
4.
Cheng S He J Han Y Han S Li P Liao H et al Global burden of atrial fibrillation/atrial flutter and its attributable risk factors from 1990 to 2021. Europace (2024) 26(7)euae195.10.1093/europace/euae195
5.
Elliott AD Middeldorp ME Van Gelder IC Albert CM Sanders P . Epidemiology and modifiable risk factors for atrial fibrillation. Nat Rev Cardiol (2023) 20(6):404–17. 10.1038/s41569-022-00820-8
6.
Schnabel RB Yin X Gona P Larson MG Beiser AS McManus DD et al 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the framingham heart study: a cohort study. Lancet (2015) 386(9989):154–62. 10.1016/s0140-6736(14)61774-8
7.
Kääb S Holm H Kirchhof P . Genomic risk scores, biomolecules, and clinical conditions to predict atrial fibrillation: time to integrate what we can measure. Eur Heart J (2023) 44(3):232–4. 10.1093/eurheartj/ehac527
8.
Berg DD Ruff CT Morrow DA . Biomarkers for risk assessment in atrial fibrillation. Clin Chem (2021) 67(1):87–95. 10.1093/clinchem/hvaa298
9.
Noubiap JJ Sanders P Nattel S Lau DH . Biomarkers in atrial fibrillation: pathogenesis and clinical implications. Card Electrophysiol Clin (2021) 13(1):221–33. 10.1016/j.ccep.2020.10.006
10.
Hijazi Z Oldgren J Siegbahn A Granger CB Wallentin L . Biomarkers in atrial fibrillation: a clinical review. Eur Heart J (2013) 34(20):1475–80. 10.1093/eurheartj/eht024
11.
Smith JG Newton-Cheh C Almgren P Struck J Morgenthaler NG Bergmann A et al Assessment of conventional cardiovascular risk factors and multiple biomarkers for the prediction of incident heart failure and atrial fibrillation. J Am Coll Cardiol (2010) 56(21):1712–9. 10.1016/j.jacc.2010.05.049
12.
O'Neal WT Venkatesh S Broughton ST Griffin WF Soliman EZ . Biomarkers and the prediction of atrial fibrillation: state of the art. Vasc Health Risk Manag (2016) 12:297–303. 10.2147/vhrm.S75537
13.
Zhang L Xing M Yu Q Li Z Tong Y Li W . Blood urea nitrogen to serum albumin ratio: a novel mortality indicator in intensive care unit patients with coronary heart disease. Sci Rep (2024) 14(1):7466. 10.1038/s41598-024-58090-y
14.
Filippatos G Rossi J Lloyd-Jones DM Stough WG Ouyang J Shin DD et al Prognostic value of blood urea nitrogen in patients hospitalized with worsening heart failure: insights from the acute and chronic therapeutic impact of a vasopressin antagonist in chronic heart failure (Activ in Chf) study. J Card Fail (2007) 13(5):360–4. 10.1016/j.cardfail.2007.02.005
15.
Gremese E Bruno D Varriano V Perniola S Petricca L Ferraccioli G . Serum albumin levels: a biomarker to be repurposed in different disease settings in clinical practice. J Clin Med (2023) 12(18).601710.3390/jcm12186017
16.
Lin Z Zhao Y Xiao L Qi C Chen Q Li Y . Blood urea nitrogen to serum albumin ratio as a new prognostic indicator in critical patients with chronic heart failure. ESC Heart Fail (2022) 9(2):1360–9. 10.1002/ehf2.13825
17.
Zhao D Liu Y Chen S Xu Z Yang X Shen H et al Predictive value of blood urea nitrogen to albumin ratio in long-term mortality in intensive care unit patients with acute myocardial infarction: a propensity score matching analysis. Int J Gen Med (2022) 15:2247–59. 10.2147/ijgm.S349722
18.
Takano T Tsubouchi K Hamada N Ichiki K Torii R Takata S et al Update of prognosis and characteristics of chronic obstructive pulmonary disease in a real-world setting: a 5-year follow-up analysis of a multi-institutional registry. BMC Pulm Med (2024) 24(1):556. 10.1186/s12890-024-03347-5
19.
Wang Y Gao S Hong L Hou T Liu H Li M et al Prognostic impact of blood urea nitrogen to albumin ratio on patients with sepsis: a retrospective cohort study. Sci Rep (2023) 13(1):10013. 10.1038/s41598-023-37127-8
20.
Pickkers P Darmon M Hoste E Joannidis M Legrand M Ostermann M et al Acute kidney injury in the critically ill: an updated review on pathophysiology and management. Intensive Care Med (2021) 47(8):835–50. 10.1007/s00134-021-06454-7
21.
Seki M Nakayama M Sakoh T Yoshitomi R Fukui A Katafuchi E et al Blood urea nitrogen is independently associated with renal outcomes in Japanese patients with stage 3–5 chronic kidney disease: a prospective observational study. BMC Nephrol (2019) 20(1):115. 10.1186/s12882-019-1306-1
22.
Xintarakou A Tzeis S Psarras S Asvestas D Vardas P . Atrial fibrosis as a dominant factor for the development of atrial fibrillation: facts and gaps. Europace (2020) 22(3):342–51. 10.1093/europace/euaa009
23.
Wakili R Voigt N Kääb S Dobrev D Nattel S . Recent advances in the molecular pathophysiology of atrial fibrillation. J Clin Invest (2011) 121(8):2955–68. 10.1172/jci46315
24.
Bevc S Ekart R Hojs R . The assessment of acute kidney injury in critically ill patients. Eur J Intern Med (2017) 45:54–8. 10.1016/j.ejim.2017.09.041
25.
Bharadwaj S Ginoya S Tandon P Gohel TD Guirguis J Vallabh H et al Malnutrition: laboratory markers vs nutritional assessment. Gastroenterol Rep (Oxf) (2016) 4(4):272–80. 10.1093/gastro/gow013
26.
Don BR Kaysen G . Serum albumin: relationship to inflammation and nutrition. Semin Dial (2004) 17(6):432–7. 10.1111/j.0894-0959.2004.17603.x
27.
Magnocavallo M Bellasi A Mariani MV Fusaro M Ravera M Paoletti E et al Thromboembolic and bleeding risk in atrial fibrillation patients with chronic kidney disease: role of anticoagulation therapy. J Clin Med (2020) 10(1).8310.3390/jcm10010083
28.
Park S Lee S Kim Y Lee Y Kang MW Kim K et al Atrial fibrillation and kidney function: a bidirectional Mendelian randomization study. Eur Heart J (2021) 42(29):2816–23. 10.1093/eurheartj/ehab291
29.
Folsom AR Lutsey PL Heckbert SR Cushman M . Serum albumin and risk of venous thromboembolism. Thromb Haemost (2010) 104(1):100–4. 10.1160/th09-12-0856
30.
Johnson AEW Bulgarelli L Shen L Gayles A Shammout A Horng S et al Mimic-Iv, a freely accessible electronic health record dataset. Sci Data (2023) 10(1):1. 10.1038/s41597-022-01899-x
31.
Johnson AE Pollard TJ Shen L Lehman LW Feng M Ghassemi M et al Mimic-Iii, a freely accessible critical care database. Sci Data (2016) 3:160035. 10.1038/sdata.2016.35
Summary
Keywords
blood urea nitrogen to serum albumin ratio, atrial fibrillation, MIMIC-IV database, mortality, retrospective cohort study
Citation
Huang Y, Wang G, Xiang X, Mei J, Zhang C and Zhang Y (2025) Blood urea nitrogen to serum albumin ratio as a prognostic marker for 28-day mortality in atrial fibrillation: a retrospective cohort study. Front. Cardiovasc. Med. 12:1533575. doi: 10.3389/fcvm.2025.1533575
Received
24 November 2024
Accepted
25 April 2025
Published
09 May 2025
Corrected
28 May 2025
Volume
12 - 2025
Edited by
Rui Providencia, University College London, United Kingdom
Reviewed by
Vincenzo Santinelli, IRCCS San Donato Polyclinic, Italy
Tahir Yagdi, EGE University, Türkiye
Updates
Copyright
© 2025 Huang, Wang, Xiang, Mei, Zhang and Zhang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Yaxin Zhang yaxin1996@qq.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.