- 1Center for Prevention and Treatment of Cardiovascular Diseases, The Second Affiliated Hospital of Nanchang University, Nanchang, Jiangxi, China
- 2Jiangxi Provincial Cardiovascular Disease Clinical Medical Research Center, Nanchang, Jiangxi, China
- 3Department of Cardiovascular Medicine, The Second Affiliated Hospital of Nanchang University, Nanchang, Jiangxi, China
Background: Previous studies have reported a linear association between brachial-ankle pulse wave velocity (baPWV) and stroke in hypertensive individuals, primarily in foreign countries, with few studies conducted in China. This study aimed to investigate the saturation effect of baPWV on the first stroke in adults with hypertension and propose a possible inflection point of baPWV at which the saturation effect occurs.
Methods: A total of 7,198 adults with hypertension and baseline baPWV were enrolled from the China Hypertension Registry Study. The outcome of this study was the first stroke. Cox proportional hazards regression, smoothing curve fitting (restricted cubic spline), Kaplan–Meier survival curve analysis, and subgroup analysis were used to investigate the association between baPWV and first stroke.
Results: A total of 281 patients experienced their first stroke during an average of four years of follow-up. There was a saturation effect of baPWV with an inflection value of 17.5 m/s on the first stroke. For baPWV < 17.5 m/s, each 1 m/s increment was associated with a 31% higher risk of first stroke (hazard ratio [HR]: 1.31, 95% confidence interval [CI]: 1.17, 1.47). For baPWV ≥ 17.5 m/s, there was no significant association between baPWV and first stroke (HR: 0.99, 95% CI: 0.96, 1.02) (for log-likelihood ratio test P < 0.001). Kaplan–Meier curves revealed a continual increase in the cumulative hazard for the first stroke from quartile 1–3 levels of baPWV (log-rank P < 0.001), whereas a non-significant difference in cumulative hazard between quartiles 3 and 4 was observed (log-rank P = 0.873).
Conclusion: BaPWV exhibited a saturation effect on the first stroke in hypertensive adults in China. Increased baPWV was positively associated with a higher risk of first stroke among hypertensive adults with a baPWV < 17.5 m/s.
1 Introduction
Stroke remains a leading cause of disability and mortality in China, imposing a substantial socioeconomic burden that has escalated steadily over the past three decades (1, 2). Although primary prevention and early intervention are recognized as optimal strategies for mitigating stroke-related morbidity and economic costs (3), the insufficient explanatory power of traditional risk factors in stroke pathogenesis highlights the need to investigate novel biomarkers of vascular dysfunction (4).
Emerging evidence positions arterial stiffness as a reliable feature of arterial structure and function (5), with proven prognostic value in hypertensive populations (6, 7). The European guidelines for the management of hypertension introduce the evaluation of arterial stiffness by pulse wave velocity (PWV) as a measure of cardiovascular target organ damage associated with hypertension. PWV is a relatively simple, noninvasive, and reproducible measurement for assessing arterial stiffness and is most widely used in clinical practice. Numerous studies have proven the accuracy of PWV as an independent predictor of cardiovascular events and mortality (8). Currently, the most commonly used indicators of PWV are carotid-femoral pulse wave velocity (cfPWV) and brachial ankle pulse wave velocity (baPWV). A study comparing aortic PWV and baPWV revealed that baPWV exhibited excellent validity and reproducibility and was an acceptable marker of vascular damage (9). Additionally, cfPWV is known as the gold standard for measuring arterial stiffness; however, its time-consuming nature and high technical requirements render it unsuitable for large-scale research. Therefore, baPWV is used as an alternative measurement of arterial stiffness (10). BaPWV exhibited stronger correlations with cardiovascular hemodynamics and coronary calcification than cfPWV (11, 12). Although international studies have reported a linear relationship between baPWV elevation and stroke incidence in hypertensive individuals (7, 13–15), critical knowledge gaps persist regarding population-specific risk patterns in China's unique healthcare context and potential threshold effects of baPWV values on disproportionate stroke risk.
To address these uncertainties, this prospective cohort study investigated the association between baPWV and first stroke in the Chinese hypertensive population, with particular emphasis on identifying potential nonlinear relationships.
2 Methods
2.1 Study participants
This study was a subset of the China Hypertension Registry Study (Registration website: http://www.chictr.org.cn/, Number: ChiCTR1800017274, Date: July 20, 2018), a real-world, prospective, and observational study conducted in Wuyuan, Jiangxi Province, China, from March 2018 to August 2018. The methodology for data acquisition and exclusion criteria has been previously described (16, 17). This study adhered to the Declaration of Helsinki and was approved by the Ethics Committee of the Second Affiliated Hospital of Nanchang University (Ethics No. 2018019) and the Institute of Biomedical Sciences, Anhui Medical University (Ethics No. CH1059). Written informed consent was obtained from all enrolled participants.
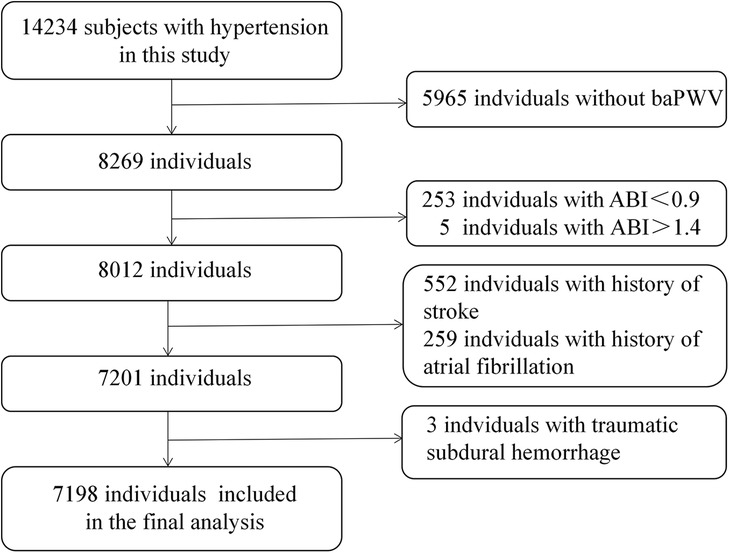
From the initial cohort of 14,234 hypertensive individuals recruited at baseline, we sequentially excluded individuals lacking baPWV values (n = 5,965) and those with ankle-brachial index (ABI) <0.90 (n = 253), ABI > 1.4 (n = 5), stroke (n = 552), atrial fibrillation (n = 259) and traumatic subdural hemorrhage (n = 3). Finally, this prospective study included 7,198 participants for analysis. The selection process for the analytic sample is presented in Figure 1.
2.2 Covariates
Based on the existing literature and clinical applications, covariates obtained from physical examinations, standard questionnaires, and laboratory measurements were selected as potential confounders. Physical examinations and standard questionnaires were administered by trained staff following a standard operating procedure. A fully adjusted model was developed using the following covariates. Categorical variables included gender, current smoking, current drinking, sleep duration, previous medical diagnoses (hypertension, dyslipidemia, diabetes, coronary heart disease, heart failure, atrial fibrillation, and stroke), and current medication based on drug packaging (antihypertensive, lipoprotein-lowering, glucose-lowering, and antiplatelet drugs). Continuous variables included age, body mass index (BMI), systolic blood pressure (SBP), diastolic blood pressure (DBP), homocysteine (Hcy), total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C). The BMI was calculated as weight (kg)/height (m2). Blood pressure was measured thrice using an electronic sphygmomanometer on the right arm positioned at the heart level after a 5-min rest, with a 30-s interval between measurements. The average of the three measurements was used.
2.3 BaPWV measurement
The automatic device of Omron-Colin BP-203RPE (Omron Health Care, Japan) was used to measure simultaneously baPWV and ankle-brachial index (ABI). After resting in the supine position for more than 5 min, four cuffs were wrapped around bilateral brachia and ankle arteries of participants, and connected to a plethysmographic sensor and oscillometric pressure sensor. Pulse volume waveform were recorded using semiconductor pressure sensors to assess the time interval between the initial increase in brachial and tibial waves.
The ABI value of each leg was calculated by the ankle SBP divided by the ipsilateral brachial SBP, and the lower value in two legs was used in the analysis. The baPWV was calculated by (La-Lb)/Tba. La is the path distance between the suprasternal notch and the ankle, Lb is the path distance between the suprasternal notch and the brachium, and Tba is the time interval between the brachial and ankle waveform. The larger values of baPWV on the left and right sides were used for the main analysis (18), and the average values on both sides were used for a sensitivity analysis.
2.4 Outcome assessment
Details on the definition and event adjudication were sourced from the literature (19). Stroke event identification followed a rigorous, multistep protocol. First, potential cases were preliminarily identified using retrospective patient interviews, medical record reviews (including hospitalizations and emergency department visits), and linkage with the national health insurance database. Subsequently, all suspected cases were systematically verified by retrieving and analyzing neuroimaging records (for instance, CT/MRI scans). Finally, confirmed cases were reviewed and adjudicated through independent discussion by an Endpoint Adjudication Committee comprising neurologists, neurosurgeons, and public health experts.
The primary outcome was the first occurrence of fatal or nonfatal symptomatic stroke (ischemic or hemorrhagic), excluding subarachnoid hemorrhage and silent strokes. Participants with preexisting stroke at baseline were excluded. Recurrent strokes were documented but not considered primary outcomes. The follow-up period was extended from the baseline survey completion date to August 15, 2022.
Patients with a history of stroke at baseline were excluded. The first attack of symptomatic stroke was considered the first stroke in the patient unless there was evidence against it. Any stroke after the first attack was considered a recurrent stroke, which was not the primary outcome of this study.
2.5 Statistical analysis
Continuous variables are presented as mean ± standard deviation (SD), and categorical variables are presented as frequencies and percentages (%). Differences in baseline characteristics according to baPWV categories were compared using a one-way analysis of variance for continuous variables and a chi-square test for categorical variables. Cox proportional hazard regression was used to evaluate the HRs and 95% CIs for the association between baPWV and first stroke, with adjustment for potential covariates in the three models. The proportional hazards assumption was evaluated using Schoenfeld residual tests, which revealed no violations (P > 0.05), indicating that the assumption of proportionality was satisfied. Potential covariates adjusted in the models were included due to their clinical importance, statistical significance in the univariable analysis, and the potential confounder effect estimates being individually changed by at least 10%. The cumulative hazards of the first stroke by baPWV categories were estimated using the Kaplan–Meier curve, and differences between groups were compared using the log-rank test. The generalized additive model and a restricted cubic spline were used to characterize the dose-response association of baPWV with the first stroke. Additionally, potential modifications to the association between baPWV and first stroke were evaluated using subgroup analyses.
A two-tailed P < 0.05 was considered statistically significant. The R statistical package (version 4.2.3; http://www.r-proje.ct.org) and Empower (R) software (version 4.1; http://www.empow.erstats.com) were used for statistical analyses.
3 Results
3.1 Baseline characteristics
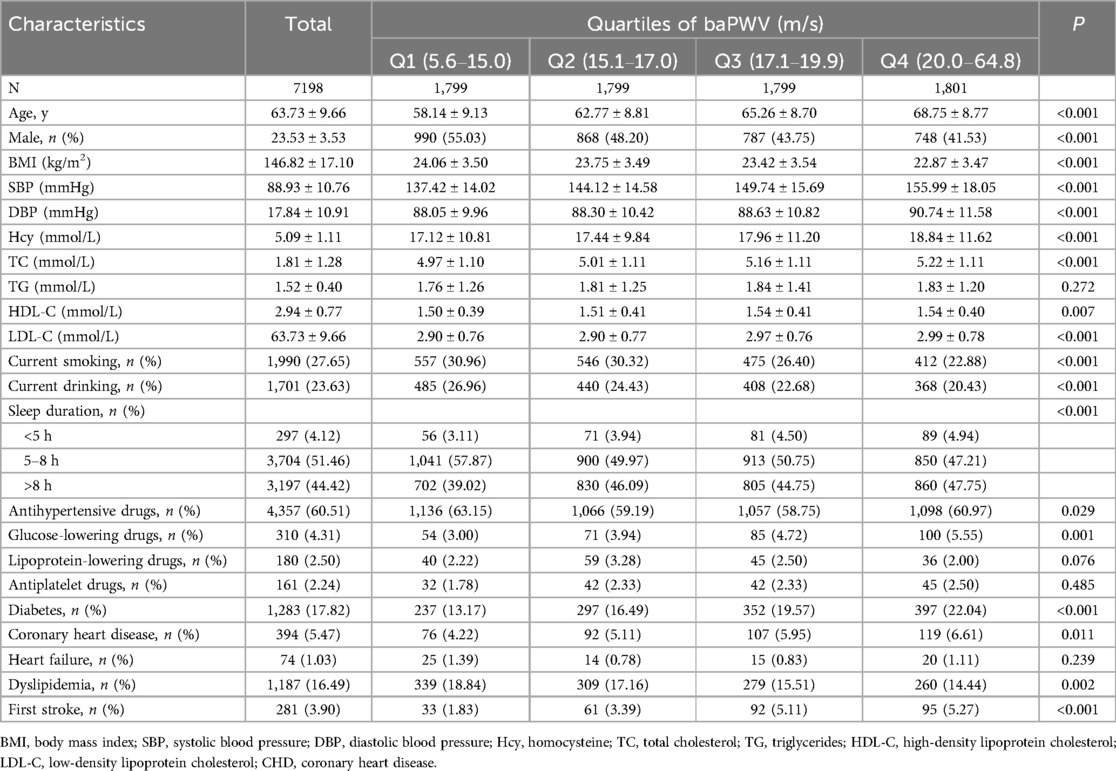
This study included 7,198 individuals with hypertension [mean age, 63.7 (9.7) years], and 47.1% of them were male. The mean ± SD value of baseline baPWV was 18.2 ± 5.4 m/s. The baseline characteristics of all participants stratified by the baPWV quartile are listed in Table 1. Participants with a higher baPWV were more likely to be female and have a higher age, SBP, DBP, Hcy, TC, HDL-C, and LDL-C levels, and a lower BMI. Additionally, they exhibited a longer sleep duration of <5 and >8 h, use of antihypertensive and glucose-lowering drugs, a history of diabetes and congenital heart defect (CHD), a lower rate of current smoking and current drinking, and a history of dyslipidemia (P < 0.05).
3.2 Association of baPWV with first stroke
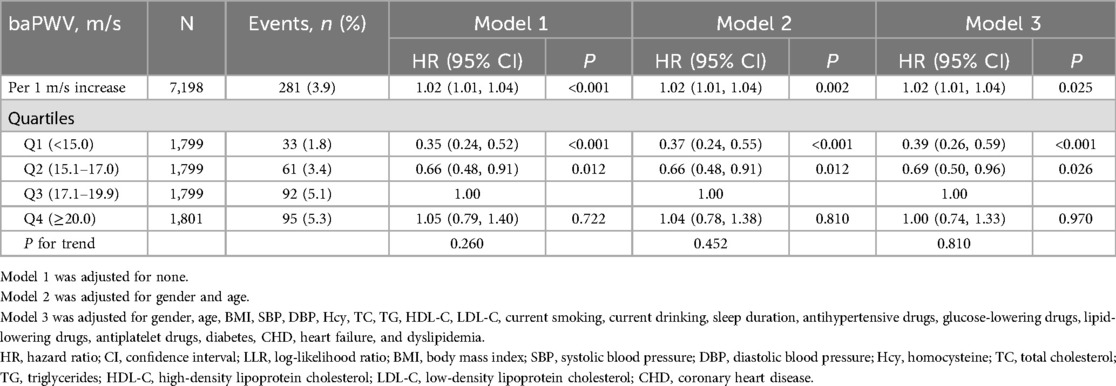
During an average follow-up period of four years, 281 cases were identified with the first stroke. The independent effects of baPWV on the first stroke are illustrated in Table 2. After adjusting for potential confounders in model 3, each 1 m/s increment of baPWV was associated with a 2% increase in the risk of the first stroke (HR: 1.02; 95% CI: 1.01, 1.04). When baPWV was measured in quartiles, the HRs (95% CIs) of the first stroke for participants in quartiles 1, 2, and 4 were 0.39 (0.26, 0.59), 0.69 (0.50, 0.96), and 1.00 (0.74, 1.33) respectively, compared with those in quartile 3 (P for trend = 0.810) (Table 2).
3.3 Saturation effect of baPWV on first stroke
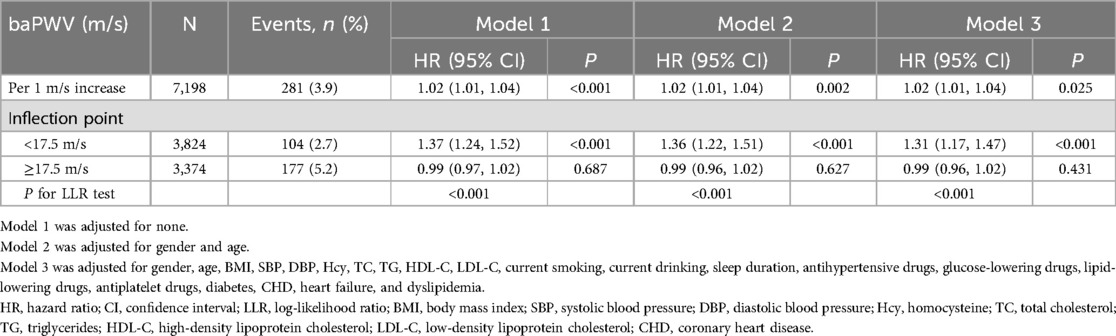
The smoothing curve fitting revealed a saturation effect of baPWV on the first stroke (Figure 2). The saturation effect analysis revealed that the inflection point of baPWV was 17.5 m/s. For baPWV < 17.5 m/s, the adjusted HR (95% CI) was 1.31 (1.17, 1.47), and the adjusted HR (95% CI) was 0.99 (0.96, 1.02) for baPWV ≥ 17.5 m/s (P for log-likelihood ratio test < 0.001) (Table 3). In addition, the Kaplan–Meier curves revealed a consistent increase in cumulative hazard for the first stroke from quartile 1 to quartile 3 of baPWV (log-rank P < 0.001), whereas there was a non-significant difference in cumulative hazard between quartiles 3 and 4 (log-rank P = 0.873) (Figure 3). To verify the robustness of our findings, we also conducted a sensitivity analysis using the average of both sides, and the conclusions remained consistent with the main analysis (Supplementary Table S1 and Table S2).
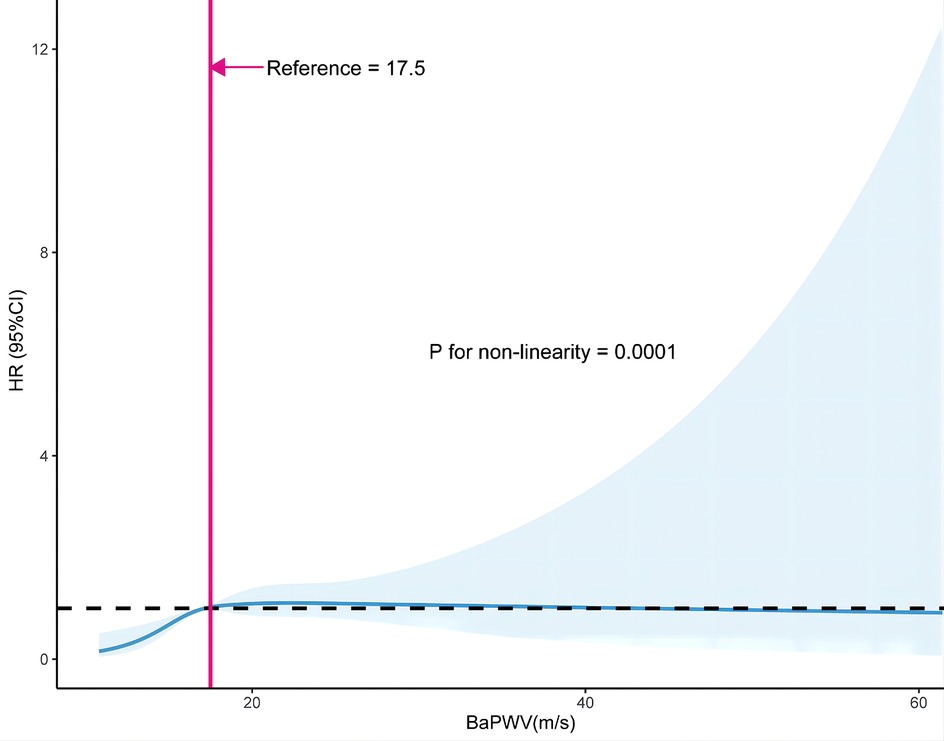
Figure 2. Restricted cubic spline curve of the association between baPWV and first stroke. The solid and dashed lines represent the estimated values and their corresponding 95% CI. Adjustment factors included gender, age, BMI, SBP, DBP, Hcy, TC, TG, HDL-C, LDL-C, current smoking, current drinking, sleep duration, antihypertensive drugs, glucose-lowering drugs, lipid-lowering drugs, antiplatelet drugs, diabetes, CHD, heart failure, and dyslipidemia. BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; Hcy, homocysteine; TC, total cholesterol; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; CHD, coronary heart disease.
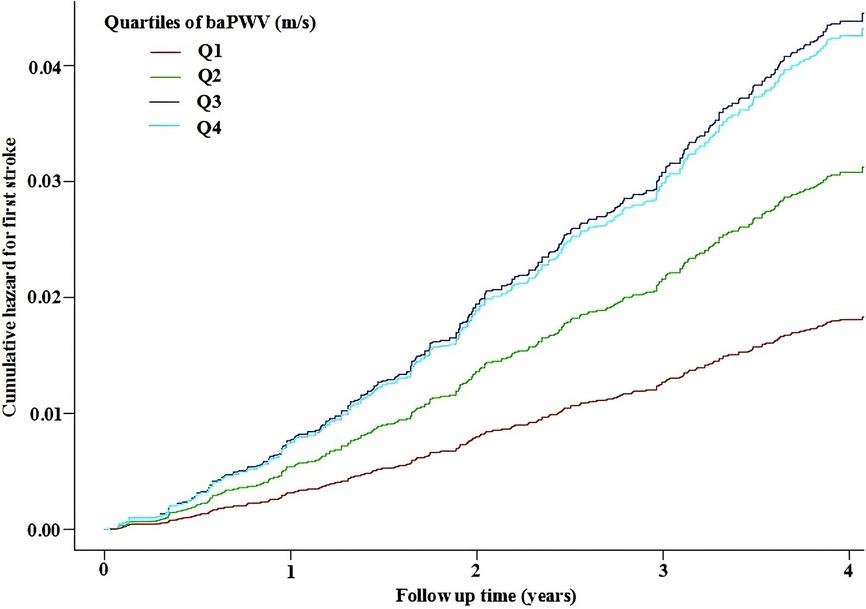
Figure 3. Kaplan–Meier curves for the cumulative hazard of the first stroke stratified by baPWV quartiles. Adjustment factors included gender, age, BMI, SBP, DBP, Hcy, TC, TG, HDL-C, LDL-C, current smoking, current drinking, sleep duration, antihypertensive drugs, glucose-lowering drugs, lipid-lowering drugs, antiplatelet drugs, diabetes, CHD, heart failure, and dyslipidemia. BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; Hcy, homocysteine; TC, total cholesterol; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; CHD, coronary heart disease.
3.4 Subgroup analyses
To determine the effect of covariates on the association between baPWV (per 1 m/s increment) and first stroke, stratified analyses were performed in two groups of participants separated by the inflection point of baPWV (17.5 m/s). The stratified analysis results revealed that the association of baPWV with the first stroke in various subgroups was consistent with the findings of the saturation effect analysis (Figure 4). In both the baPWV < 17.5 m/s and the baPWV ≥ 17.5 m/s groups, there were no significant interactions in the following subgroups: gender (male vs. Female), age (60 vs. ≥ 60 years), BMI (<24 vs. ≥ 24 kg/m2), current smokers (no vs. yes), hcy (15 vs. ≥ 15 mmol/L), current smoking (no vs. yes), current drinking (no vs. yes), diabetes (no vs. yes), CHD (no vs. yes), heart failure (no vs. yes), and dyslipidemia (no vs. yes) (P > 0.05 for interaction).
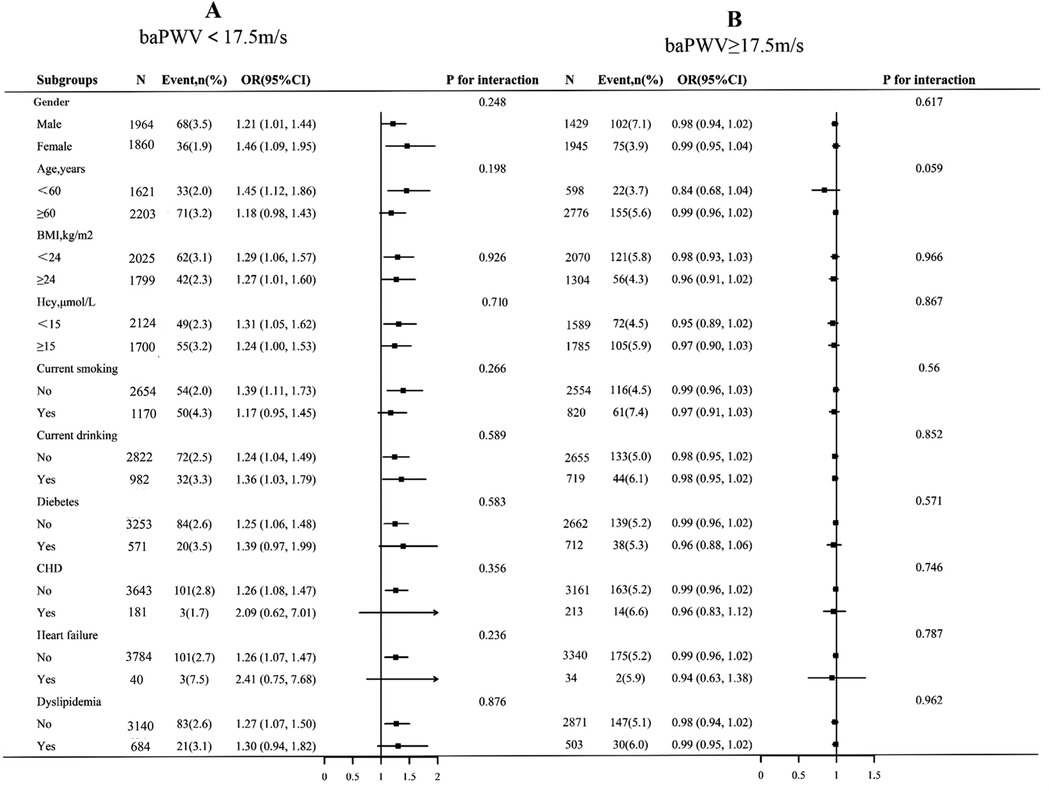
Figure 4. Subgroup analyses of the effect of baPWV on first stroke, grouped by baPWV < 17.5 m/s (A) and baPWV ≥ 17.5 m/s (B). Each subgroup analysis was adjusted, if not stratified, for gender, age, BMI, SBP, DBP, Hcy, TC, TG, HDL-C, LDL-C, current smoking, current drinking, sleep duration, antihypertensive drugs, glucose-lowering drugs, lipid-lowering drugs, antiplatelet drugs, diabetes, CHD, heart failure, and dyslipidemia. BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; Hcy, homocysteine; TC, total cholesterol; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; CHD, coronary heart disease.
4 Discussion
This cohort study demonstrated a saturation effect with the inflection point of 17.5 m/s of baPWV on the first stroke. Higher baPWV was positively associated with a higher risk of first stroke among hypertensive adults with baPWV < 17.5 m/s.
Several studies have reported associations between baPWV and cardiovascular and cerebrovascular diseases. A Meta-analysis of 12 cohort studies revealed that baPWV is an independent predictor of cardiovascular disease, and an increase of 1 m/s was associated with a 12% increase in the risk of cardiovascular events (20). Li C et al. followed up 19,217 Chinese patients with hypertension for three years and discovered that increased baPWV was significantly associated with new-onset stroke among patients with hypertension aged < 65 years (21). Hu L et al. followed up 9,787 patients with hypertension for 20.8 months in China and demonstrated that higher baPWV levels were associated with an increased risk of first stroke (22). Kim HL et al. investigated 2,561 Korean patients with hypertension with a follow-up period of 4.14 years and discovered that increased baPWV was associated with a higher risk of future cardiovascular events (5). Kawai et al. investigated 338 Japanese patients with essential hypertension with a mean follow-up of 6.3 years and discovered that patients with higher baPWV exhibited more cardiovascular events and stroke (7). Consistent with these findings, our results revealed that an increased baPWV (<17.5 m/s) was positively associated with a higher risk of first stroke among hypertensive adults. Compared to other studies, we discovered a threshold saturation effect between baPWV and stroke rather than a linear association. Consequently, we propose that baPWV within a certain range could be an effective index for predicting future cerebrovascular events in patients with hypertension.
Several mechanisms could explain the association between baPWV and the first stroke. First, increased arterial stiffness is related to decreased local cerebral blood flow and higher cerebrovascular reactivity (23). Changes in cerebrovascular hemodynamics and arteriolar damage can cause central nervous system damage, including stroke. As baPWV levels increase, endothelial function is impaired in patients with acute stroke (24). Second, increased arterial stiffness indicates a narrowing of the peripheral arteries, which is conducive to cardiovascular disease (25).
Identifying an effective threshold value for baPWV will aid in guiding treatment strategies for cardiovascular and cerebrovascular diseases. A Japanese study proposed a cutoff value of 18.3 m/s for baPWV to predict future cardiovascular events in hypertensive populations (26). Similarly, the Japanese Circulation Society proposed a baPWV of 18 m/s as the threshold for high-risk (27). A South Korean study supported a baPWV cutoff point of 16.3 m/s for predicting cardiovascular diseases in patients with hypertension (5). In Chinese studies, baPWV cutoff values of 21.43 (22) and 20 m/s (13) were proposed to predict future cerebrovascular events. We identified a threshold of 17.5 m/s for stroke risk prediction, consistent with the findings of a Japanese cohort study (7). Variations across studies may be attributed to differences in ethnicity, study endpoints, or follow-up durations. The observed threshold effect (17.5 m/s) can be mechanistically explained as follows: First, the pathophysiological threshold of arterial remodeling; when arterial stiffness reaches a critical level, the elastic reserve of the vascular wall may be completely depleted, stabilizing hemodynamic impacts of further stiffness progression, while other risk factors (for instance, blood pressure variability, and plaque vulnerability) may become more direct triggers of stroke (28, 29). Second, cerebrovascular compensatory mechanisms (30); under severe arterial stiffness, cerebral vessels may partially compensate for hypoperfusion through autoregulation (for instance, collateral circulation recruitment), leading to slower acceleration of stroke risk (30, 31). Additionally, the inclusion of patients with hypertension with baseline vascular damage exceeding that of the general population might obscure the additional risks associated with higher baPWV (32).
Our study has several advantages, including the large sample size, the study design, and advanced statistical techniques. Moreover, to our knowledge, this is the first report of the saturation effect of baPWV on the first stroke in adults with hypertension. However, this study has several limitations. First, although a few confounding covariates were adjusted, other potential confounding effects could not be completely excluded. Second, all enrolled patients were from China, limiting the generalizability of the findings to other populations. Third, the large number of random baPWV deletions resulted in a relatively small sample size. Fourth, accurate stroke subtyping (ischemic stroke and intracerebral hemorrhage) could not be performed in some cases due to incomplete neuroimaging data (for instance, missing or low-quality CT/MRI scans), which may limit subtype-specific interpretations.
5 Conclusions
BaPWV exhibited a saturation effect on the first stroke in hypertensive adults in China. Increased baPWV (<17.5 m/s) was positively associated with a higher risk of first stroke among hypertensive adults. Our results demonstrate that baPWV could be an effective and simple tool for assessing first stroke in China. Intervention strategies, including intensified blood pressure control, comprehensive vascular risk management, and dynamic monitoring of baPWV, should be prioritized for patients with hypertension with elevated baPWV. If risk levels persist or escalate, further evaluation of the target organ damage is warranted.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the Second Affiliated Hospital of Nanchang University (Ethics NO. 2018019) and the Institute of Biomedical Sciences, Anhui Medical University (Ethics NO. CH1059). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
WZ: Formal analysis, Funding acquisition, Investigation, Writing – original draft. LZ: Data curation, Investigation, Writing – review & editing. TW: Data curation, Investigation, Writing – review & editing. CY: Formal analysis, Funding acquisition, Investigation, Writing – review & editing. HB: Methodology, Supervision, Writing – review & editing. XC: Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (82460670), the Cultivation of backup projects for National Science and Technology Awards (20223AEI91007), Jiangxi Science and Technology Innovation Base Plan-Jiangxi Clinical Medical Research Center (20223BCG74012), Science and Technology Innovation Base Construction Project (20221ZDG02010), Jiangxi Provincial Natural Science Foundation (20224BAB206090, 20232BAB206140, 20232ACB216006), Jiangxi Provincial Health Commission Science and Technology Project (202130440,202210495, 202310528), Jiangxi Provincial Drug Administration Science and Technology Project (2022JS41, 2023JS26), Fund project of the Second Affiliated Hospital of Nanchang University (2021efyA01, 2023efyA05).
Acknowledgments
Thanks to all staffs and subjects who participated in the China Hypertension Registry Study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer FH declared a past co-authorship with the author to the handling editor.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1535366/full#supplementary-material
References
1. Wang W, Jiang B, Sun H, Ru X, Sun D, Wang L, et al. Prevalence, incidence, and mortality of stroke in China: results from a nationwide population-based survey of 480 687 adults. Circulation. (2017) 135(8):759–71. doi: 10.1161/CIRCULATIONAHA.116.025250
2. GBD 2016 Stroke Collaborators. Global, regional, and national burden of stroke, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. (2019) 18(5):439–58. doi: 10.1016/S1474-4422(19)30034-1
3. Meschia JF, Bushnell C, Boden-Albala B, Braun LT, Bravata DM, Chaturvedi S, et al. Guidelines for the primary prevention of stroke: a statement for healthcare professionals from the American Heart Association/American stroke association. Stroke. (2014) 45(12):3754–832. doi: 10.1161/STR.0000000000000046
4. Chen Y, Shen F, Liu J, Yang GY. Arterial stiffness and stroke: de-stiffening strategy, a therapeutic target for stroke. Stroke Vasc Neurol. (2017) 2(2):65–72. doi: 10.1136/svn-2016-000045
5. Kim HL, Lim WH, Seo JB, Kim SH, Zo ZH, Kim MA. Prediction of cardiovascular events using brachial-ankle pulse wave velocity in hypertensive patients. J Clin Hypertens (Greenwich). (2020) 22(9):1659–65. doi: 10.1111/jch.13992
6. Ohkuma T, Tomiyama H, Ninomiya T, Kario K, Hoshide S, Kita Y, et al. Proposed cutoff value of brachial-ankle pulse wave velocity for the management of hypertension. Circ J. (2017) 81(10):1540–2. doi: 10.1253/circj.CJ-17-0636
7. Kawai T, Ohishi M, Onishi M, Ito N, Takeya Y, Oguro R, et al. Prognostic impact of regional arterial stiffness in hypertensive patients. Heart Vessels. (2015) 30(3):338–46. doi: 10.1007/s00380-014-0485-8
8. Milan A, Tosello F, Fabbri A, Vairo A, Leone D, Chiarlo M, et al. Arterial stiffness: from physiology to clinical implications. High Blood Press Cardiovasc Prev. (2011) 18(1):1–12. doi: 10.2165/11588020-000000000-00000
9. Yamashina A, Tomiyama H, Takeda K, Tsuda H, Arai T, Hirose K, et al. Validity, reproducibility, and clinical significance of noninvasive brachial-ankle pulse wave velocity measurement. Hypertens Res. (2002) 25(3):359–64. doi: 10.1291/hypres.25.359
10. Tomiyama H, Matsumoto C, Shiina K, Yamashina A, Brachial-Ankle PWV. Current status and future directions as a useful marker in the management of cardiovascular disease and/or cardiovascular risk factors. J Atheroscler Thromb. (2016) 23(2):128–46. doi: 10.5551/jat.32979
11. Yu WC, Chuang SY, Lin YP, Chen CH. Brachial-ankle vs carotid-femoral pulse wave velocity as a determinant of cardiovascular structure and function. J Hum Hypertens. (2008) 22(1):24–31. doi: 10.1038/sj.jhh.1002259
12. Tanaka H, Munakata M, Kawano Y, Ohishi M, Shoji T, Sugawara J, et al. Comparison between carotid-femoral and brachial-ankle pulse wave velocity as measures of arterial stiffness. J Hypertens. (2009) 27(10):2022–7. doi: 10.1097/HJH.0b013e32832e94e7
13. Song Y, Xu B, Xu R, Tung R, Frank E, Tromble W, et al. Independent and joint effect of brachial-ankle pulse wave velocity and blood pressure control on incident stroke in hypertensive adults. Hypertension. (2016) 68(1):46–53. doi: 10.1161/HYPERTENSIONAHA.115.07023
14. Kim YB, Park K, Chung P, Kim JM, Moon HS, Youn YC. Brachial-ankle pulse wave velocity is associated with both acute and chronic cerebral small vessel disease. Atherosclerosis. (2016) 245:54–9. doi: 10.1016/j.atherosclerosis.2015.12.006
15. Munakata M, Konno S, Miura Y, Yoshinaga K. Prognostic significance of the brachial-ankle pulse wave velocity in patients with essential hypertension: final results of the J-TOPP study. Hypertens Res. (2012) 35(8):839–42. doi: 10.1038/hr.2012.53
16. Zhou W, Yan W, Wang T, Zhu LJ, Xu Y, Zhao J, et al. Independent and joint association of physical activity and sedentary behavior on all-cause mortality. Chin Med J (Engl). (2021) 134(23):2857–64. doi: 10.1097/CM9.0000000000001730
17. Li M, Zhan A, Huang X, Hu L, Zhou W, Wang T, et al. Positive association between triglyceride glucose index and arterial stiffness in hypertensive patients: the China H-type hypertension registry study. Cardiovasc Diabetol. (2020) 19(1):139. doi: 10.1186/s12933-020-01124-2
18. Song Y, Xu B, Xu R, Tung R, Frank E, Tromble W, et al. Independent and joint effect of brachial-ankle pulse wave velocity and blood pressure control on incident stroke in hypertensive adults. Hypertension. (2016) 68(1):46–53. doi: 10.1161/HYPERTENSIONAHA.115.07023
19. Huo Y, Li J, Qin X, Huang Y, Wang X, Gottesman RF, et al. Efficacy of folic acid therapy in primary prevention of stroke among adults with hypertension in China: the CSPPT randomized clinical trial. JAMA. (2015) 313(13):1325–35. doi: 10.1001/jama.2015.2274
20. Vlachopoulos C, Aznaouridis K, Terentes-Printzios D, Ioakeimidis N, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with brachial-ankle elasticity index: a systematic review and meta-analysis. Hypertension. (2012) 60(2):556–62. doi: 10.1161/HYPERTENSIONAHA.112.194779
21. Li C, Wang Z, Liu S, Guo S, Song Y, Liu L, et al. Association of brachial ankle pulse wave velocity with new onset stroke in hypertensive patients aged less than 65 with normal fasting glucose among Chinese community-based population. Front Endocrinol (Lausanne). (2022) 12:828286. doi: 10.3389/fendo.2021.828286
22. Hu L, Bi C, Liu L, Song Y, Zhang Y, Wang B, et al. Association between baseline brachial-ankle pulse wave velocity and short-term risk of first stroke among Chinese hypertensive adults. J Hum Hypertens. (2022) 36(12):1085–91. doi: 10.1038/s41371-021-00611-7
23. Jefferson AL, Cambronero FE, Liu D, Moore EE, Neal JE, Terry JG, et al. Higher aortic stiffness is related to lower cerebral blood flow and preserved cerebrovascular reactivity in older adults. Circulation. (2018) 138(18):1951–62. doi: 10.1161/CIRCULATIONAHA.118.032410
24. Tuttolomondo A, Casuccio A, Della Corte V, Maida C, Pecoraro R, Di Raimondo D, et al. Endothelial function and arterial stiffness indexes in subjects with acute ischemic stroke: relationship with TOAST subtype. Atherosclerosis. (2017) 256:94–9. doi: 10.1016/j.atherosclerosis.2016.10.044
25. Xu Y, Wu Y, Li J, Ma W, Guo X, Luo Y, et al. The predictive value of brachial-ankle pulse wave velocity in coronary atherosclerosis and peripheral artery diseases in urban Chinese patients. Hypertens Res. (2008) 31(6):1079–85. doi: 10.1291/hypres.31.1079
26. Cavalcante JL, Lima JA, Redheuil A, Al-Mallah MH. Aortic stiffness: current understanding and future directions. J Am Coll Cardiol. (2011) 57(14):1511–22. doi: 10.1016/j.jacc.2010.12.017
27. Kario K, Kai H, Rakugi H, Hoshide S, Node K, Maekawa Y, et al. Consensus statement on renal denervation by the Joint Committee of Japanese Society of Hypertension (JSH), Japanese Association of Cardiovascular Intervention and Therapeutics (CVIT), and the Japanese Circulation Society (JCS). Circ J. (2024) 88(10):1718–25. doi: 10.1253/circj.CJ-66-0225
28. Lacolley P, Regnault V, Segers P, Laurent S. Vascular smooth muscle cells and arterial stiffening: relevance in development, aging, and disease. Physiol Rev. (2017) 97(4):1555–617. doi: 10.1152/physrev.00003.2017
29. Thorin-Trescases N, Thorin E. Lifelong cyclic mechanical strain promotes large elastic artery stiffening: increased pulse pressure and old age-related organ failure. Can J Cardiol. (2016) 32(5):624–33. doi: 10.1016/j.cjca.2015.12.022
30. Faizy TD, Mlynash M, Kabiri R, Christensen S, Kuraitis GM, Mader MM, et al. The cerebral collateral cascade: comprehensive blood flow in ischemic stroke. Neurology. (2022) 98(23):e2296–306. doi: 10.1212/WNL.0000000000200340
31. Castro P, Azevedo E, Sorond F. Cerebral autoregulation in stroke. Curr Atheroscler Rep. (2018) 20(8):37. doi: 10.1007/s11883-018-0739-5
Keywords: brachial-ankle pulse wave velocity, stroke, hypertension, saturation effect, elderly
Citation: Zhou W, Zhu L, Wang T, Yu C, Bao H and Cheng X (2025) Saturation effect of brachial-ankle pulse wave velocity on first stroke in adults with hypertension: a prospective cohort study. Front. Cardiovasc. Med. 12:1535366. doi: 10.3389/fcvm.2025.1535366
Received: 19 February 2025; Accepted: 14 August 2025;
Published: 3 September 2025.
Edited by:
Daniel Bia, Universidad de la República, UruguayReviewed by:
Feng Hu, Fujian Medical University, ChinaYuichiro Toma, University of the Ryukyus, Japan
Copyright: © 2025 Zhou, Zhu, Wang, Yu, Bao and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chao Yu, eXVjaGFvOUBnbWFpbC5jb20=
 Wei Zhou
Wei Zhou Lingjuan Zhu
Lingjuan Zhu Tao Wang
Tao Wang Chao Yu
Chao Yu Huihui Bao
Huihui Bao Xiaoshu Cheng
Xiaoshu Cheng