- 1Department of Department of Cardiac Surgery, Affiliated Dong Yang Hospital of Wenzhou Medical University, Dong Yang, Zhejiang, China
- 2Department of Ultrasonography, Affiliated Dong Yang Hospital of Wenzhou Medical University, Dong Yang, Zhejiang, China
Deep sternal wound infection (DSWI) caused by Mycoplasma hominis after cardiac surgery is very rare and easily overlooked. This paper reports the case of a patient with DSWI after cardiac surgery. The patient had an unexplained fever postoperatively. On the seventh day after surgery, the incision opened, and there was pus leakage. On the 11th day after surgery, the pus culture indicated Mycoplasma hominis infection. The patient was cured after treatment with omadacycline and secondary surgery.
Introduction
Deep sternal wound infection (DSWI) is one of the most serious complications after cardiac surgery through the sternum, with an incidence rate worldwide ranging from 0.5% to 5.6% (1). Patient risk factors include female gender, age, diabetes, renal failure, smoking, obesity, breast size, steroid use, and chronic obstructive pulmonary disease (2, 3). Surgery-related risk factors include surgery duration, internal mammary artery use, re-thoracotomy, blood product use, intensive care unit stay, and duration of mechanical ventilation (4, 5). The most common pathogens are Methicillin-sensitive Staphylococcus aureus (45%), Methicillin-resistant S. aureus (16%), Gram-negative bacilli (17%), Coagulase-negative Staphylococci (13%), and Streptococci (5%) (6). Mycoplasma hominis is a rare pathogen for DSWI, with less than 20 cases of postcardiac surgery M. hominis mediastinitis reported globally in the past 50 years (6, 7). This paper reports a case of a patient who underwent valvular replacement and coronary artery bypass grafting and presented with fever and chest pain postoperatively. M. hominis was found in the incision pus culture. The patient showed improvement after standardized anti-infection treatment and pectoralis major muscle flap transfer and was discharged in good condition.
Case presentation
A 59-year-old man was admitted to the hospital for “chest tightness and shortness of breath after exertion for 1 month.” He had a history of “cerebral infarction” and underwent coronary stent implantation 1 year ago at a hospital in another underdeveloped province about 2,000 km far away. He came to our hospital for medical treatment through a health assistance project between the two provincial governments. He denied any other medical history. Echocardiography in our hospital revealed moderate stenosis with moderate insufficiency of the aortic valve. The ejection fraction (EF)% value was 68%. Coronary angiography indicated 85% stenosis in the middle of the right coronary artery and 80%–90% stenosis in the distal segment of the left circumflex branch. The patient's predicted mortality was 1.29% based on EuroSCORE II. The calculated additive EuroSCORE II value was 4.333.
The patient underwent mechanical aortic valve replacement combined with coronary artery bypass grafting on 26 September 2024. Two graft procedures were performed: a saphenous vein sequential graft, harvested from the right thigh, was anastomosed from the ascending aorta to the left circumflex coronary artery and subsequently to the posterior descending branch of the right coronary artery.
The total surgical time was 380 min, of which the cardiopulmonary bypass time was 127 min. The aortic cross-clamp time during surgery was 180 min.
After the patient was admitted to the surgical intensive care unit (SICU) after surgery, the SICU nurse reported that he had a swelling of the foreskin and several small ruptures in the local area (unfortunately, the photographs taken at that time are missing now). Local disinfection and foreskin reduction were immediately performed, and the swelling gradually reduced.
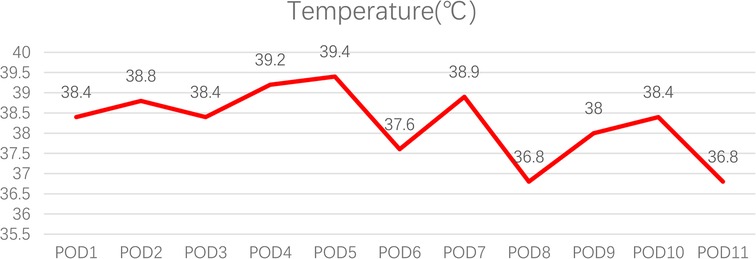
The patient had a fever on postoperation day (POD) 1, with the highest temperature reaching 39.4°C on POD 5. It was not until POD 11 that the body temperature returned to normal (Figure 1). The patient's white blood cell count and the percentage of neutrophils were slightly elevated. Procalcitonin levels decreased over time, which was considered to have no guiding significance for M. hominis infection. C-reactive protein (CRP) levels changed with time and treatment measures (Figure 2).
The urinary catheter was removed on POD 7, and the central venous catheter was removed on POD 8. Chest CT scans performed on POD 1 revealed mild consolidation in the bilateral lower lung lobes and minimal pleural effusion, with subsequent imaging on POD 6 demonstrating improvement in these findings; no mediastinal abnormalities were detected in either scan.
On POD 7 (3 October 2024), the patient's incision was dehisced, revealing a large amount of yellow-white pus exuding, with poor healing of subcutaneous tissue and sternum (Figure 3).
Before sternal dehiscence, localized erythema and tenderness around the incision were observed on POD 3. However, these signs were initially attributed to routine postoperative inflammation without special attention.
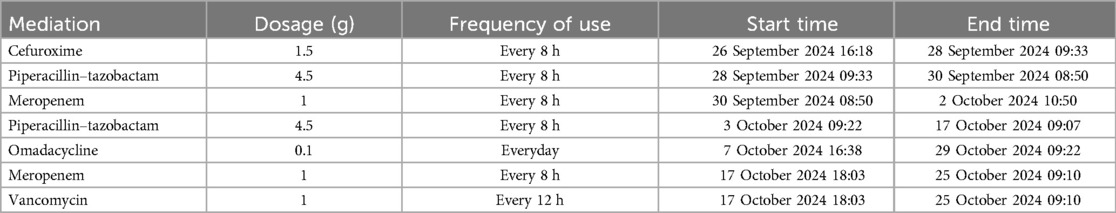
Multiple continuous samplings were taken from the incision for culture since sternal dehiscence. Finally, on POD 11, the first positive culture result came out, which indicated M. hominis (Figure 4). An antibiogram was not performed due to technical limitations in M. hominis susceptibility testing, which is rarely conducted in clinical practice, and the absence of institutional infrastructure for such specialized assays. After a multidisciplinary team discussion, Omadacycline was administered for anti-infection treatment. After using the medication, the incision condition gradually improved, and no positive results were cultured again. On POD 21, an open chest debridement and bilateral pectoralis major muscle flap plugging surgery was successfully performed. The patient was discharged 14 days after the secondary surgery.
The anti-infection treatment is detailed in Table 1.
At 1-, 3-, and 5-month follow-ups, the patient exhibited no fever recurrence, with complete sternal wound healing confirmed on physical examination. Cardiac function remained normal, as evidenced by transthoracic echocardiography and electrocardiography. In chest CT scans, no mediastinal abnormalities were observed. Laboratory assessments revealed normalized inflammatory markers and stable coagulation profiles (international normalized ratio: 1.8–2.5 under warfarin therapy). The patient resumed full-time work and daily activities without functional limitations.
The patient initially expressed frustration due to incision dehiscence and delayed diagnosis. Through detailed communication and empathic counseling, his understanding and adherence improved. At discharge, he reported high satisfaction with the care team's efforts and gratitude for the successful outcome.
Discussion
DSWI is a rare but potentially devastating complication of median sternotomy performed in cardiac surgery. Risk factors can be broadly divided into preoperative, intraoperative, and postoperative factors, including female sex, obesity, diabetes mellitus, smoking and chronic obstructive pulmonary disease (COPD), bilateral internal mammary artery harvesting, prolonged cardiopulmonary bypass time, and re-exploration for bleeding. In this case, the patient exhibited the following DSWI risks: smoking and single internal mammary artery harvesting. The risk factors had limited guiding significance for this patient.
M. hominis infection causing DSWI after cardiac surgery is very rare and difficult to diagnose. The main reason is that M. hominis has high requirements for culture, and ordinary culture methods make it difficult to obtain positive results (8). Currently, many studies have adopted molecular diagnosis and genomic sequencing methods to diagnose Mycoplasma infections (6, 7) and have achieved good results, but new technology means high costs and low popularity. So, conventional culture and testing remain the main methods for grassroots hospitals to find the causative bacteria of infection. First, qualified test specimens and multiple tests may increase the positive detection rate. Second, culture dishes with no bacterial growth within 48 h should not be directly reported as negative and discarded. Previous studies have proven that M. hominis requires at least 4 days or even longer under conventional culture to appear in colonies (9).
Many articles also mention an interesting phenomenon about M. hominis, which is that all patients with DSWI caused by postoperative M. hominis are male. M. hominis mainly colonizes in the human respiratory and urinary tract and, because of the anatomical differences between male and female urethras, the damage to the male urethra from catheterization is much greater than that to the female urethra. Therefore, many researchers believe that Mycoplasma infection is related to urethral injury. In this case, the patient did indeed have penile edema and local skin ulceration on the first and second days after surgery. In addition, the patient's graft vessel for the bypass was taken from the great saphenous vein of the right thigh, which is close to the perineal area. It is possible that strict aseptic operation was not maintained, leading to Mycoplasma infection. Therefore, perioperative perineal cleaning, preoperative catheterization operations, and strict aseptic operations during surgery are crucial for preventing infection.
In this case, the patient was treated with piperacillin–sulbactam and meropenem without therapeutic effect before the pathogen was identified. After the culture confirmed a Mycoplasma infection, omadacycline was used for anti-infection treatment. The decision to initiate Omadacycline was guided by a multidisciplinary team consultation involving infectious disease and emergency medicine specialists. Key factors included the following: confirmed M. hominis infection with suspected genitourinary origin, given the patient's postoperative penile edema and proximity of the saphenous vein graft harvest site to the perineal region; in vitro evidence demonstrating the potent activity of Omadacycline against Mycoplasma genitalium [minimal inhibit concentration (MIC) ≤0.5 mg/ml], even in isolates resistant to other tetracyclines (10); and pharmacokinetic advantages, including intravenous administration for rapid therapeutic effect, and broad-spectrum coverage against Gram-positive and Gram-negative pathogens, which mitigated the risks of polymicrobial infection.
Omadacycline is a tetracycline-class antibiotic that inhibits bacterial protein synthesis by binding to the 30S ribosomal subunit. It has antibacterial activity against aerobic Gram-positive bacteria such as Enterococcus faecalis, Enterococcus faecium, vancomycin-resistant enterococci, methicillin-resistant S. aureus, various streptococci, Corynebacterium jeikeium, and Enterobacteriaceae resistant to ceftazidime and carbapenems, as well as atypical pathogens (11). In previous case reports on anti-infection treatment with quinolones combined with doxycycline, with or without decortication surgery, 28.6% of patients died due to uncontrollable infection (6).
This case highlights the critical role of persistent culturing with prolonged incubation to isolate M. hominis, a fastidious pathogen rarely detected by conventional methods. To our knowledge, it is the first reported use of omadacycline for successfully treating DSWI caused by M. hominis after cardiac surgery, addressing a literature gap. Limitations include the single-center, single-case design and the inability to perform susceptibility testing due to institutional constraints, underscoring challenges in managing atypical infections in resource-limited settings.
Prospective multicenter studies with larger cohorts are warranted to validate the efficacy of omadacycline in DSWI caused by M. hominis, while advancing rapid molecular diagnostics and standardized susceptibility protocols could enhance the management of such rare, challenging infections.
Summary
M. hominis-induced deep sternal wound infection is a relatively rare complication after cardiac surgery, which is considered to be related to catheterization. M. hominis is difficult to culture and, therefore, molecular testing or genomic sequencing methods can be used to assist in diagnosis for units with the capability. For confirmed M. hominis infections in DSWI, omadacycline can be used for anti-infection treatment.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
Ethics statement
The studies involving humans were approved by the ethics committee of Dongyang People's Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
ZW: Conceptualization, Data curation, Formal analysis, Funding acquisition, Writing – original draft, Writing – review & editing. YS: Software, Supervision, Validation, Visualization, Writing – original draft. YC: Investigation, Methodology, Project administration, Resources, Writing – original draft. LC: Conceptualization, Data curation, Investigation, Methodology, Software, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Vos RJ, Van Putte BP, Kloppenburg GTL. Prevention of deep sternal wound infection in cardiac surgery: a literature review. J Hosp Infect. (2018) 100:411–20. doi: 10.1016/j.jhin.2018.05.026
2. Lepelletier D, Bourigault C, Roussel JC, Lasserre C, Leclère B, Corvec S, et al. Epidemiology and prevention of surgical site infections after cardiac surgery. Med Mal Infect. (2013) 43:403–9. doi: 10.1016/j.medmal.2013.07.003
3. Leung Wai Sang S, Chaturvedi R, Alam A, Samoukovic G, de Varennes B, Lachapelle K. Preoperative hospital length of stay as a modifiable risk factor for mediastinitis after cardiac surgery. J Cardiothorac Surg. (2013) 8:45. doi: 10.1186/1749-8090-8-45
4. Diez C, Koch D, Kuss O, Silber R-E, Friedrich I, Boergermann J. Risk factors for mediastinitis after cardiac surgery—a retrospective analysis of 1700 patients. J Cardiothorac Surg. (2007) 2:23. doi: 10.1186/1749-8090-2-23
5. Lu JCY, Grayson AD, Jha P, Srinivasan AK, Fabri BM. Risk factors for sternal wound infection and mid-term survival following coronary artery bypass surgery. Eur J Cardiothorac Surg. (2003) 23:943–9. doi: 10.1016/s1010-7940(03)00137-4
6. Trouillet J-L, Vuagnat A, Combes A, Bors V, Chastre J, Gandjbakhch I, et al. Acute poststernotomy mediastinitis managed with debridement and closed-drainage aspiration: factors associated with death in the intensive care unit. J Thorac Cardiovasc Surg. (2005) 129:518–24. doi: 10.1016/j.jtcvs.2004.07.027
7. Le Guern R, Loïez C, Loobuyck V, Rousse N, Courcol R, Wallet F. A new case of Mycoplasma hominis mediastinitis and sternal osteitis after cardiac surgery. Int J Infect Dis. (2015) 31:53–5. doi: 10.1016/j.ijid.2014.12.028
8. Houpikian P, Raoult D. Blood culture-negative endocarditis in a reference center: etiologic diagnosis of 348 cases. Medicine. (2005) 84:162–73. doi: 10.1097/01.md.0000165658.82869.17
9. Filthuth I, Emler S, Jacobs E, Auckenthaler R. Isolation of Mycoplasma hominis on CDC anaerobic blood agar. Eur J Clin Microbiol Infect Dis. (1996) 15:896–7. doi: 10.1007/BF01691229
10. Waites KB, Crabb DM, Atkinson TP, Geisler WM, Xiao L. Omadacycline is highly active in vitro against Mycoplasma genitalium. Microbiol Spectr. (2022) 10:e0365422. doi: 10.1128/spectrum.03654-22
Keywords: Mycoplasma hominis, deep mediastinitis, cardiac surgery, omadacycline, DSWI
Citation: Wang Z, Shi Y, Chen Y and Chen L (2025) Case Report: Deep sternal wound infection caused by Mycoplasma hominis after cardiac surgery. Front. Cardiovasc. Med. 12:1538389. doi: 10.3389/fcvm.2025.1538389
Received: 2 December 2024; Accepted: 22 April 2025;
Published: 12 May 2025.
Edited by:
Giuseppe Gatti, Azienda Sanitaria Universitaria Giuliano Isontina, ItalyReviewed by:
Maruti Haranal, U N Mehta Institute of Cardiology and Research, IndiaJosé María Arribas, Virgen de la Arrixaca University Hospital, Spain
Copyright: © 2025 Wang, Shi, Chen and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhaohui Wang, am9zZWtpbmc3NjI4NTkyQG91dGxvb2suY29t
 Zhaohui Wang
Zhaohui Wang Yong Shi
Yong Shi Yu Chen
Yu Chen Lijun Chen
Lijun Chen