Abstract
Background:
Gout is caused by hyperuricemia and is associated with cardiovascular diseases. Treatment for hyperuricemia primarily involves urate-lowering medications. Some trials showed higher cardiovascular mortality rates with febuxostat compared to allopurinol in gout patients. However, data on the cardiovascular safety of benzbromarone compared to allopurinol is limited, and there is no data comparing benzbromarone to febuxostat. This study aims to assess the cardiovascular safety of benzbromarone, febuxostat, and allopurinol in gout patients.
Methods:
A comprehensive search was conducted across PubMed and EMBASE from their inception to August 2024. Inclusion criteria were randomized controlled trials (RCTs) and cohort studies including adult patients with the diagnosis of gout, with urate-lowering medications. The outcome was the incidence of major adverse cardiovascular events. This systematic review and network meta-analysis were recorded in INPLASY with the ID INPLASY202460049.
Results:
A total of 176 studies were identified through the database search. There were 119 articles identified in EMBASE and 57 articles identified in PubMed. Following screening and review, 17 qualified studies (5 RCTs) were included in the network meta-analysis. The relative cardiovascular event risk (risk ratio, RR) for benzbromarone compared to febuxostat is 0.82 (95% CI 0.61–1.09), and for benzbromarone compared to allopurinol, the RR is 0.87 (95% CI 0.75–1.01). The RR for febuxostat compared to allopurinol is 1.08 (95% CI 0.97–1.20).
Conclusion:
Our network meta-analysis suggests a subtle trend indicating a lower risk of cardiovascular events for benzbromarone compared to both febuxostat and allopurinol in gout patients, although not statistically significant.
Systematic Review Registration:
https://inplasy.com/inplasy-2024-6-0049/, identifier INPLASY202460049.
1 Introduction
Gout is a metabolic disorder with hyperuricemia and urate crystal deposition followed by chronic inflammation and recurrent flares of acute arthritis. It is associated with increased risks of developing cardiovascular disease. Patients with gout have greater risks of mortality and many comorbidities, including chronic kidney disease, cardiovascular diseases, hypertension, and type 2 diabetes mellitus in comparison to those without gout (1–3). Urate-lowering medications play the pivotal role in the management of gout. Among the medications, xanthine oxidase inhibitors, febuxostat and allopurinol, are prescribed worldwide, whereas uricosuric agent, benzbromarone, is more often administered in some East Asia countries (4).
A number of studies have focused on the cardiovascular effects of xanthine oxidase inhibitors such as allopurinol and febuxostat due to their capability to suppress xanthine oxidase activity and reduce oxidative stress (5–7). Allopurinol had shown effects of scavenging oxygen radicals, improving insulin sensitivity and anti-inflammatory actions (6, 7). Xanthine oxidase inhibitors have long been the pharmacologic candidate to modulate cardiovascular risk, based on their theoretically blockade of oxidative stress.
Benzbromarone is an uricosuric drug that enhances urate excretion by inhibiting urate transporter 1 (URAT1), the primary apical urate transporter in the kidney's proximal tubule. This inhibition decreases urate reabsorption and increases its excretion in the urine. Benzbromarone was never approved in the United States, and it was withdrawn from the European market in 2003 due to hepatotoxicity concerns. However, the drug remains widely used in the Asia-Pacific region (8).
The Cardiovascular Safety of Febuxostat and Allopurinol in Patients with Gout and Cardiovascular Morbidities (CARES) trial was a multicenter, double-blind, noninferiority trial involving patients with gout and cardiovascular disease. The result revealed noninferiority of developing adverse cardiovascular events between the febuxostat and the allopurinol group. But it demonstrated higher rates of all-cause and cardiovascular death in the febuxostat group (9). Thereafter, the U.S. Food and Drug Administration (FDA) issued warnings, indicating that febuxostat might increase cardiovascular mortality and all-cause mortality compared with allopurinol. The issues of cardiovascular safety about using urate-lowering medications have drawn great attention, and several studies have been conducted to assess this issue. The Febuxostat vs. Allopurinol Streamlined Trial (FAST) was another multicenter, prospective, open-label, non-inferiority trial to evaluate the safety issues of treatment among patients with gout. The result, on the contrary, showed that the febuxostat group was noninferior to the allopurinol group with respect to both the rate of composite adverse cardiovascular events and all-cause mortality (10). The conclusion that usage of febuxostat would increase the risk of cardiovascular death remains in doubt.
On the other hand, a potentially deadly side effect of using allopurinol has been well established in the past decades. This is a severe cutaneous adverse reaction induced by allopurinol, more frequently seen in patients with variant of HLA-B*58:01 allele. Unfortunately, the prevalence of HLA-B*58:01 is around 12%–20% in some Asian populations (11, 12). The risk of developing this serious adverse event seems high in these populations, making allopurinol undesirable as the first-line treatment for urate-lowering therapy and gout prevention. Comparing to allopurinol, febuxostat and benzbromarone are commonly prescribed in Taiwan (13). With regards to the cardiovascular safety of benzbromarone, there have been only two studies published. One study found benzbromarone was associated with a decreased cardiovascular risk compared to allopurinol; however, another study showed no significant difference in cardiovascular risk between allopurinol and benzbromarone (14, 15). There was no clinical trials directly comparing the cardiovascular safety outcomes between febuxostat and benzbromarone. Therefore, we conducted this network meta-analysis to evaluate the cardiovascular safety of benzbromarone, febuxostat, and allopurinol in gout patients.
2 Methods
The manuscript was prepared according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement. The protocol of this study was registered and approved by INPLASY under the registration number of INPLASY202460049.
2.1 Eligibility criteria
This meta-analysis was based on the following PICO (population, intervention, comparison, outcome) to frame our study. The population included adult patients (≥18 years) with the diagnosis of gout. The intervention group was treated with benzbromarone, while the comparison group was treated with febuxostat or allopurinol. The outcome was the incidence of major adverse cardiovascular events, defined as a composite of cardiovascular death, non-fatal myocardial infarction, non-fatal stroke, and unstable angina with urgent coronary revascularization.
2.2 Information source and search strategy
PubMed and EMBASE were the main sources of electronic bibliographic database for literature search and were investigated from inception to August 2024. We used a combination of various terms for searching, including benzbromarone, febuxostat, allopurinol, cardiovascular, and gout as keywords. We only adopted articles written in English with full text available. Randomized clinical trials (RCTs) and comparative observational studies were both included. The types of studies such as animal studies, case series, case reports, reviews, abstracts, editorials, comments, and letters to the editor were not included in our meta-analysis.
2.3 Data collection
The data were extracted by an investigator according to the pre-defined PICO. The accuracy of extracted data was confirmed by another investigator. If the article did not provide adequate statistical parameters for analysis, such as hazard ratio or odds ratio, the article would not be included for network meta-analysis. Any discrepancies in the extracted data were identified and discussed in order to reach a consensus. The effect measures of interest included cardiovascular death, non-fatal myocardial infarction, non-fatal stroke, and unstable angina with urgent coronary revascularization. The data collected included the number of participants, country, and follow-up periods.
2.4 Risk of bias assessment
We applied Cochrane risk of bias tool to appraise the methodological quality. The domains included sequence generation, allocation sequence concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases. Assessment discrepancies were resolved by discussion until reaching consensus.
2.5 Statistical analysis
The network meta-analysis involved direct comparisons conducted using Review Manager (RevMan) Version 5.4 software (The Cochrane Collaboration, 2020) with a random-effects model, while indirect comparisons were performed using RStudio 2024.04.02 Build 764 and R 4.4.1 (2024-06-14 ucrt). The forest plots were generated for assessing the effects of individual study and pooled results. A funnel plot was created using Review Manager to illustrate the distribution of individual study effect and to evaluate the possibility of publication bias. A subgroup analysis of RCTs vs. observational trials was performed to evaluate the potential heterogeneity. A sensitivity analysis was performed to address the potential issue of overlapping populations. The hazard ratio with 95% confidence interval was used for the outcome of time-to-event data, and the odds ratio was used as a substitute if the hazard ratio was unavailable. All effect measures were converted to risk ratio for pooling results. P < 0.05 was considered as statistical significance. Cochran's Q test, with significance at P < 0.1, and I2 statistic, with 50% as substantial heterogeneity, were used to test the heterogeneity among the included studies.
3 Results
A total of 176 studies were identified through the database search. There were 119 articles identified in EMBASE and 57 articles identified in PubMed. Following screening and review, 17 qualified studies were included in the network meta-analysis. The excluded studies were primarily conference abstracts, letters, review papers, protocols, meta-analyses, and post-hoc analyses of randomized controlled trials. Among the studies included, 12 cohort studies and 5 RCTs were adopted for further analysis. A flow diagram depicting the process of evidence search and selection is shown in Figure 1. Among one of the 12 cohort studies, the high cardiovascular risk population and low cardiovascular risk population were analyzed separately. The network graph of the studies according to febuxostat, benzbromarone, and allopurinol is shown in Figure 2.
Figure 1
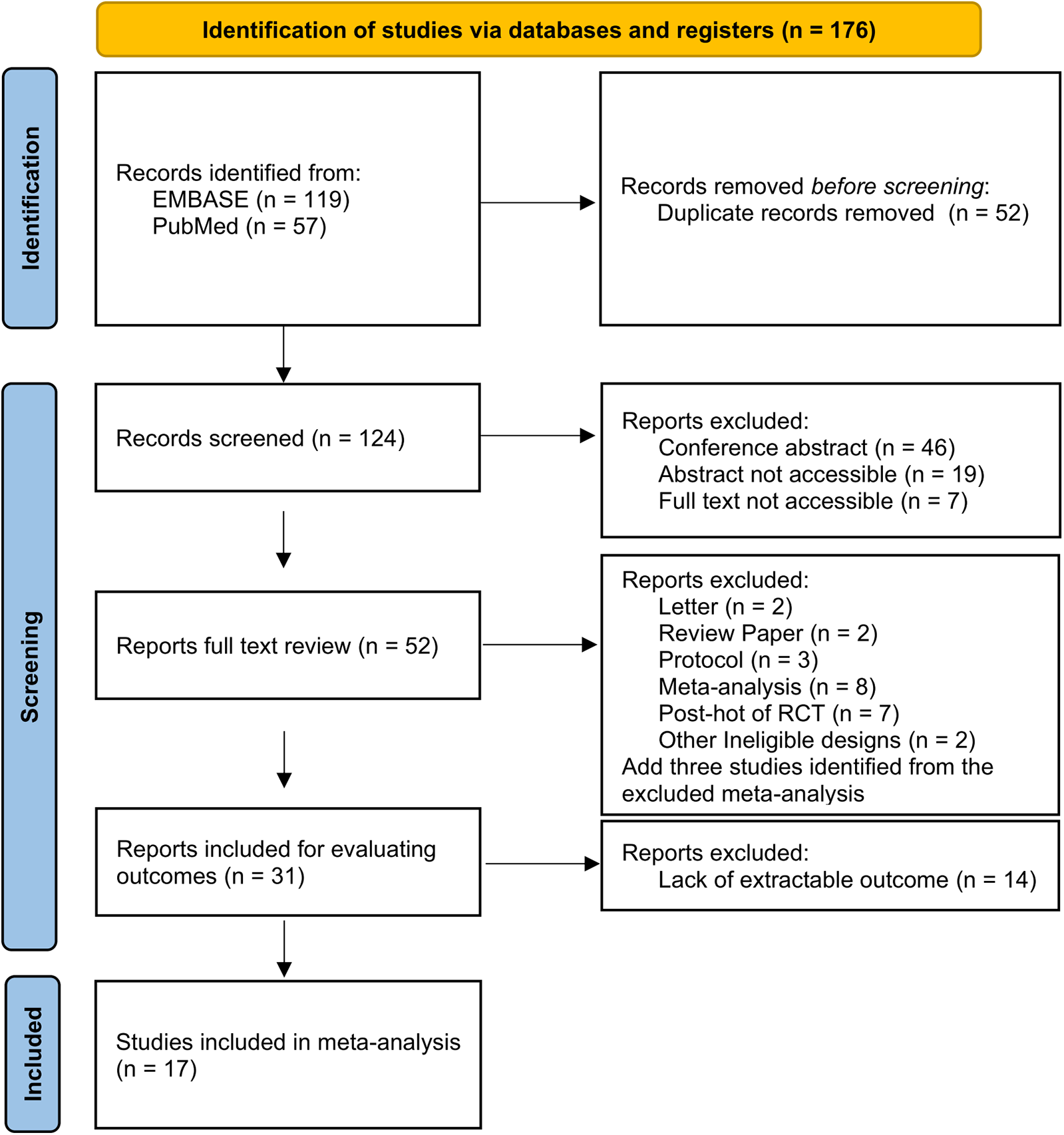
Flow diagram of the evidence search and selection.
Figure 2
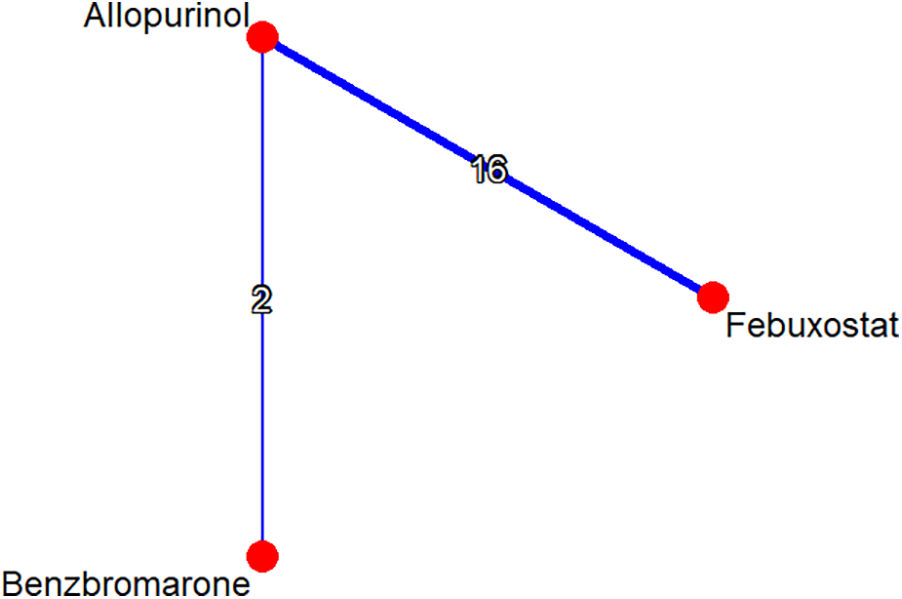
Network graph of studies according to febuxostat, benzbromarone, and allopurinol. The graph shows lines between treatments that have direct comparisons; thicker lines indicate that more comparison studies have been conducted.
The sample size of the studies ranged from 535 to 321,860 patients. The studies were conducted across different continents, including North America, Europe, and Asia. In total, there were 977,420 patients included in this analysis. Among them, there were 363,955 patients in the febuxostat group, 28,607 patients in the benzbromarone group, and 584,858 patients in the allopurinol group. All the five RCTs were febuxostat against allopurinol. Two observational studies were benzbromarone vs. allopurinol, whereas the rest ten were febuxostat vs. allopurinol. The characteristics of the included RCT studies are summarized in Table 1, and the characteristics of the included observational studies are summarized in Table 2.
Table 1
| Author country | Study design | Participants included | Intervention | Follow-up periods | Outcomes |
|---|---|---|---|---|---|
| O’Dell et al. (2022) USA | RCT | 472 vs. 468 | Febuxostat vs. allopurinol | 72 weeks | OR (95% CI) |
| (21) | 0.98 (0.37–2.68) | ||||
| Mackenzie et al. (2020) Europe | RCT | 3,063 vs. 3,065 | Febuxostat vs. allopurinol | A median of 6 years | HR (95% CI) |
| (10) | 0.85 (0.70–1.03) | ||||
| White et al. (2018) USA | RCT | 3,098 vs. 3,092 | Febuxostat vs. allopurinol | A median of 32 months | HR (95% CI) |
| (9) | 1.03 (0.87–1.23) | ||||
| Becher et al. (2010) USA | RCT | 1,513 vs. 756 | Febuxostat vs. allopurinol | 6 months | OR (95% CI) |
| (22) | 0.50 (0.07–3.73) | ||||
| Schumacher et al. (2008) USA | RCT | 267 vs. 268 | Febuxostat vs. allopurinol | 28 weeks | OR (95% CI) |
| (23) | 5.10 (0.56–241.85) |
Summary of baseline characteristics for the included RCTs.
Table 2
| Author country | Sample size & study design | Participants included | Intervention | Follow-up periods | Outcomes |
|---|---|---|---|---|---|
| Jeong et al. (2023) Korea | Retrospective cohort | 27,761 vs. 27,761 | Febuxostat vs. allopurinol | A median of 467 days | HR (95% CI) |
| (24) | (1:1 match) | 1.17 (1.10–1.24) | |||
| Shin et al. (2022) Korea | Retrospective cohort | 160,930 vs. 160,930 | Febuxostat vs. allopurinol | A mean of 250 days | HR (95% CI) |
| (25) | (1:1 match) | 1.03 (0.95–1.12) | |||
| Weisshaar et al. (2022) Austria | Retrospective cohort | 7,767 vs. 20,301 | Febuxostat vs. allopurinol | A median of 640 days | HR (95% CI) |
| (26) | 1.72 (1.59–1.89) | ||||
| Cheng et al. (2022) Taiwan | Retrospective cohort | Low risk | Febuxostat vs. allopurinol | A median of 640 days vs. 907 days | HR (95% CI) |
| (27) | 61,424 vs. 61,424 | 1.15 (1.07–1.24); HR (95% CI) | |||
| High risk | |||||
| 12,795 vs. 12,795 | 1.26 (1.15–1.37) | ||||
| (1:1 match) | |||||
| Eun et al. (2022) Korea | Retrospective cohort | 7,868 vs. 23,603 | Benzbromarone vs. allopurinol | 14,862 PYs vs. 6,705 PYs | HR (95% CI) |
| (14) | (1:3 match) | 0.96 (0.77–1.20) | |||
| Kang et al. (2021) Korea | Retrospective cohort | 20,739 vs. 103,695 | Benzbromarone vs. allopurinol | A mean of 1.16 years | HR (95% CI) |
| (15) | (1:5 match) | 0.82 (0.71–0.95) | |||
| Ju et al. (2020) | Retrospective cohort | 276 vs. 828 | Febuxostat vs. allopurinol | A median of 1.97 years | HR (95% CI) |
| Hong Kong | (1:3 match) | 0.67 (0.42–1.09) | |||
| (28) | |||||
| Kang et al. (2019) Korea | Retrospective cohort | 9,910 vs. 39,640 | Febuxostat vs. allopurinol | A median of 32 months | HR (95% CI) |
| (29) | (1:4 match) | 0.92 (0.76–1.11) | |||
| Chen et al. (2019) Taiwan | Retrospective cohort | 5,262 vs. 5,257 | Febuxostat vs. allopurinol | A mean of 2 years | HR (95% CI) |
| (30) | (1:1 match) | 1.23 (0.98–1.56) | |||
| Su et al. (2019) Taiwan | Retrospective cohort | 44,111 vs. 44,111 | Febuxostat vs. allopurinol | A mean of 200 days vs. 160 days | HR (95% CI) |
| (31) | (1:1 match) | 1.07 (0.97–1.18) | |||
| Zhang et al. (2018) USA | Retrospective cohort | 24,936 vs. 74,808 | Febuxostat vs. allopurinol | A mean of 1.1 years | HR (95% CI) |
| (32) | (1:3 match) | 1.01 (0.94–1.08) | |||
| Foody et al. (2017) USA | Retrospective cohort | 370 vs. 2,056 | Febuxostat vs. allopurinol | A median of 9 months | HR (95% CI) |
| (33) | 0.52 (0.30–0.91) |
Summary of baseline characteristics for the included observational studies.
The forest plots illustrating cardiovascular safety of febuxostat, allopurinol, and benzbromarone is provided as Figures 3,4. The relative cardiovascular event risk (risk ratio, RR) for febuxostat compared to allopurinol is 1.08 (95% CI 0.97–1.20), and for benzbromarone compared to allopurinol the RR is 0.87 (95% CI 0.75–1.01). The league table with network meta-analysis estimates for cardiovascular outcomes is provided as Table 3. The analysis demonstrated that the relative cardiovascular event risk (RR) for benzbromarone compared to febuxostat is 0.82 (95% CI 0.61–1.09). The forest plot for cardiovascular safety of febuxostat, benzbromarone, and allopurinol using febuxostat as the reference is provided in Supplementary Figure S1.
Figure 3
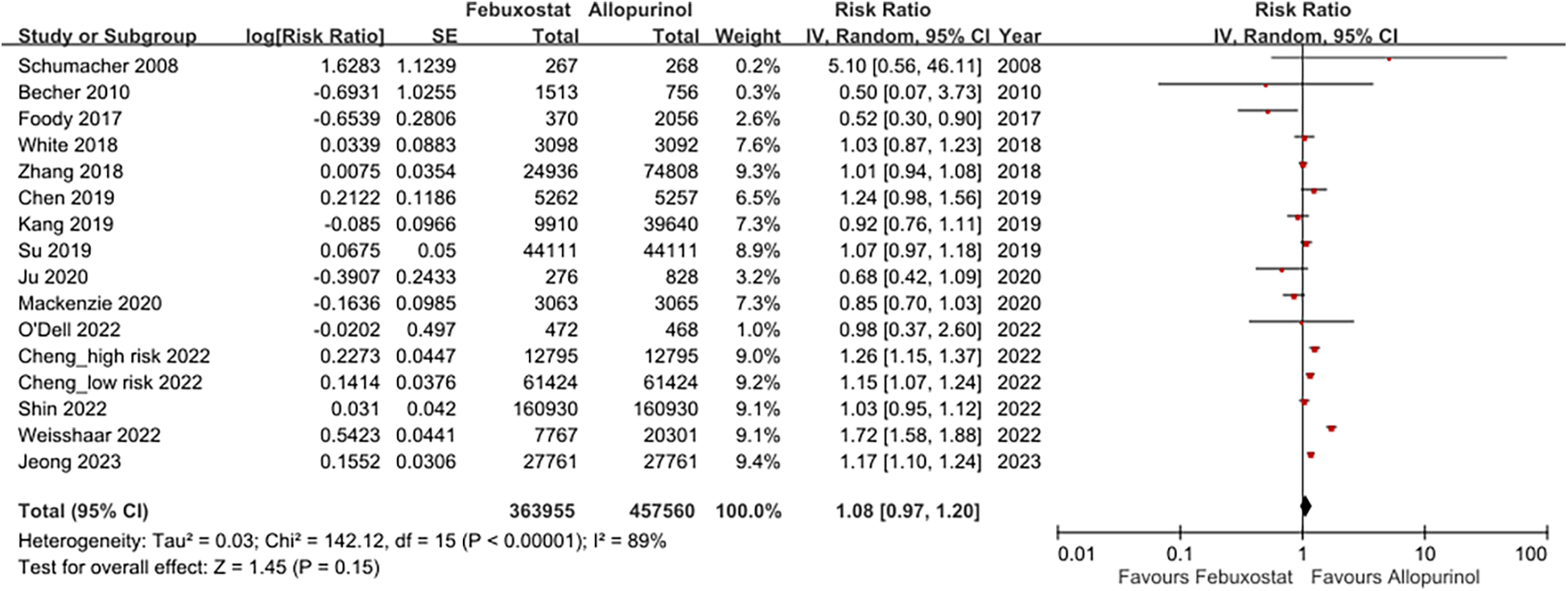
This forest plot provides a summary of the risk ratio for cardiovascular events of febuxostat and allopurinol across the included studies, highlighting both the individual and pooled effects.
Figure 4
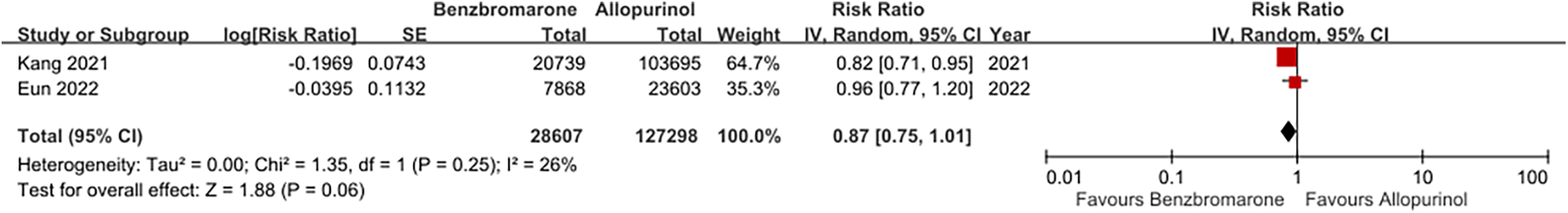
This forest plot provides a summary of the risk ratio for cardiovascular events of benzbromarone and allopurinol across the included studies, highlighting both the individual and pooled effects.
Table 3
| Benzbromarone | ||
| 0.88 (0.67; 1.15) | Allopurinol | |
| 0.82 (0.61; 1.09) | 0.93 (0.84; 1.03) | Febuxostat |
The league table compares relative cardiovascular event risk for each drug pair, including direct and indirect comparisons.
Values are risk ratios (95% credible interval).
The funnel plot was used to assess publication bias in this network meta-analysis. The Egger test was performed, and the P values was 0.32. The plot shows that the effect estimates are distributed symmetrically, suggesting there is no strong evidence of publication bias. The funnel plot for assessing the studies included in this meta-analysis is provided in Figure 5.
Figure 5
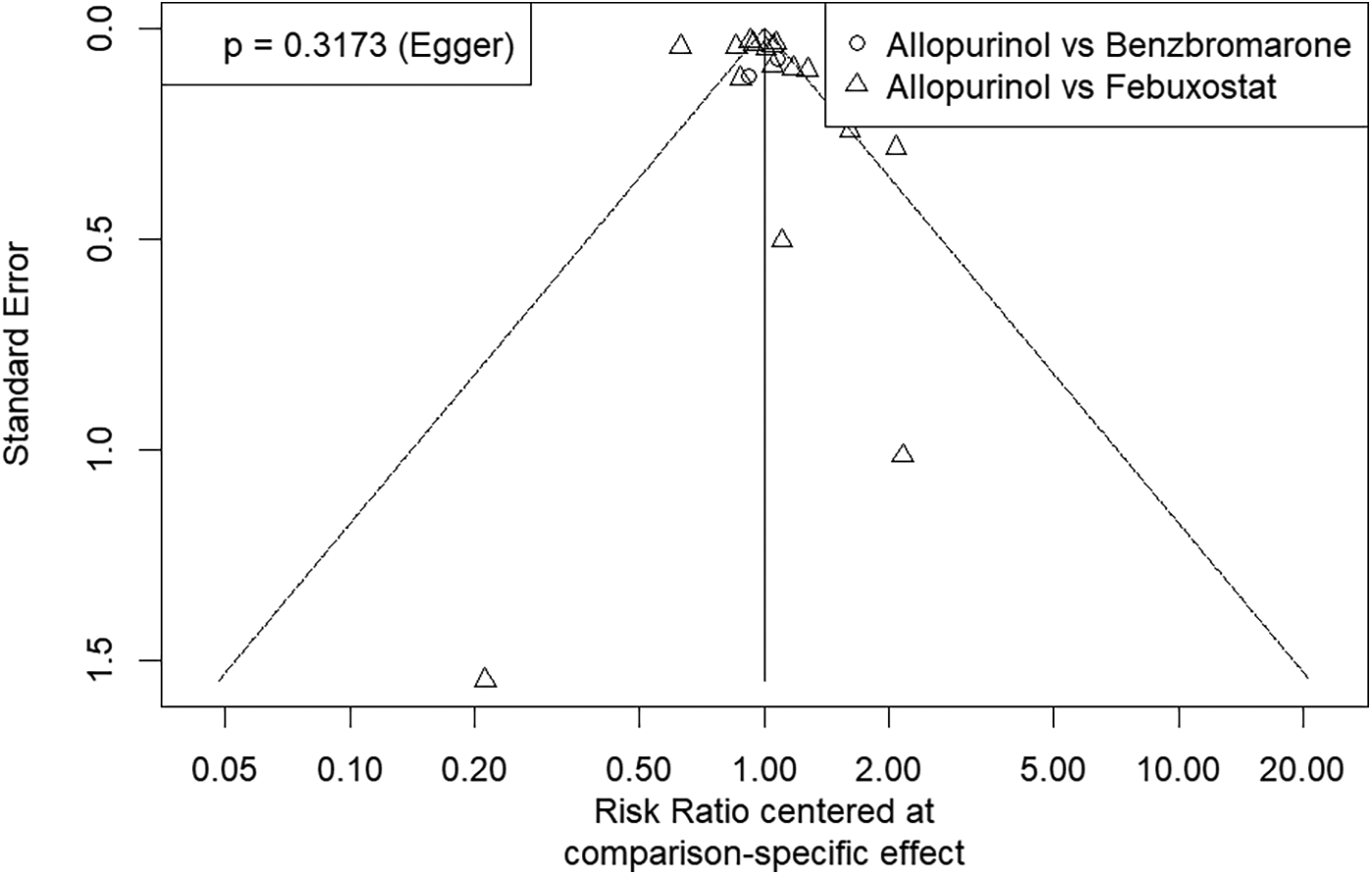
Funnel plot for assessing publication bias of studies included in this network meta-analysis. The symmetrical distribution of study effects depicted in this plot suggests a low likelihood of publication bias.
In studies comparing the cardiovascular risks of febuxostat and allopurinol, there was high heterogeneity among these studies with I2 = 89%. We separated RCTs and observational studies for further subgroup analysis. In the RCTs, the RR of using febuxostat compared to allopurinol was 0.95 with 95% CI 0.80–1.12, and the heterogeneity of these RCTs was low as I2 = 18%. Among observational studies, the RR of using febuxostat compared to allopurinol was 1.11 (95% CI 0.99–1.24), and the heterogeneity among these observational studies with I2 = 92%. The forest plot illustrating this subgroup analysis is provided in Supplementary Figure S2.
Possible overlapping of populations in two cohort studies comparing benzbromarone and febuxostat was noted (14, 15). A sensitivity analysis was performed to address these concerns. After excluding one of the two studies, the analysis showed that excluding Eun et al. (2022) resulted in an RR of 0.76 (95% CI 0.52–1.11) for the difference in the risk of cardiovascular events between benzbromarone and febuxostat users, while excluding Kang et al. (2021) resulted in an RR of 0.89 (95% CI 0.59–1.35). The league table with network meta-analysis estimates for cardiovascular outcomes excluding one of the two studies is provided in Supplementary Table S1 and Table S2.
We evaluated the risk of bias in the studies using the Cochrane bias tool. Overall, we found a moderate to high risk of bias due to issues such as selection of participants and blinding of participants and outcome assessors. In order to minimize the impact of confounders, many observational studies used propensity score analysis. The bias risk assessments for each included study is summarized in Supplementary Figure S3. A graphical representation of each bias item presented as percentages is provided in Supplementary Figure S4.
4 Discussion
Treatment for hyperuricemia primarily involves low-purine diets and urate-lowering medications. The main types of urate-lowering medications are xanthine oxidase inhibitors (e.g., allopurinol and febuxostat) and uricosurics (e.g., benzbromarone). Taiwan is one of the few countries marketing benzbromarone, a drug not commonly available in the United States or European Union. Therefore, publications about benzbromarone especially in comparison to febuxostat are limited. Since benzbromarone is not a xanthine oxidase inhibitor, including benzbromarone in the studies on cardiovascular risk of urate-lowering medications may further clarify the cardiovascular risks associated with these treatments.
Gout and hyperuricemia are both cardiovascular risk factors. Urate-lowering medications, theoretically, can reduce the cardiovascular risk by lowering uric acid level and reduce gout activity. Furthermore, recent studies suggested that circulating xanthine oxidoreductase, independent of its enzymatic product of uric acid, is a novel biomarker associated with the progression of cardiovascular diseases (16–19).
Both xanthine oxidase inhibitors and uricosurics are effective for reducing levels of uric acid (8, 20). Different urate-lowering medications, such as xanthine oxidase inhibitors and uricosurics, might impact cardiovascular outcomes differently due to their varying mechanisms of action. If the cardiovascular benefits are primarily linked to reduced serum uric acid levels, then both uricosurics and xanthine oxidase inhibitors should theoretically improve cardiovascular outcomes. Conversely, if the cardiovascular advantages are resulted from antioxidant effects through xanthine oxidase inhibition, then uricosurics might not be as protective. Our study showed a lower risk of cardiovascular events for benzbromarone compared to both febuxostat and allopurinol in gout patients, although not yet reaching statistical significance. There should be more studies to evaluate the hypothesis that the antioxidant effects provided by xanthine oxidase inhibition are central to cardiovascular benefits.
The major limitation of our network meta-analysis was high heterogeneity. A subgroup analysis of RCTs vs. observational trials was performed to evaluate the potential source of heterogeneity. Selection bias, performance bias, detection bias, and attrition bias may also exist in the included cohort studies. Many cohort studies used health insurance databases, which may partially explain the presence of these biases.
5 Conclusion
Our network meta-analysis suggests a subtle trend indicating a lower risk of cardiovascular events for benzbromarone compared to both febuxostat and allopurinol in gout patients. Further direct comparative trials between benzbromarone and febuxostat/allopurinol are necessary to confirm and validate these findings.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
T-SL: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Visualization, Writing – original draft. Y-LL: Data curation, Investigation, Writing – original draft. C-HH: Data curation, Investigation, Writing – original draft. S-CH: Supervision, Validation, Writing – review & editing. W-YS: Methodology, Software, Validation, Writing – review & editing. W-SY: Conceptualization, Supervision, Writing – review & editing. C-LC: Conceptualization, Methodology, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1541307/full#supplementary-material
References
1.
Hansildaar R Vedder D Baniaamam M Tausche AK Gerritsen M Nurmohamed MT . Cardiovascular risk in inflammatory arthritis: rheumatoid arthritis and gout. Lancet Rheumatol. (2021) 3(1):E58–70. 10.1016/S2665-9913(20)30221-6
2.
Zhu YY Pandya BJ Choi HK . Comorbidities of gout and hyperuricemia in the US general population: NHANES 2007–2008. Am J Med. (2012) 125(7):679–787.e1. 10.1016/j.amjmed.2011.09.033
3.
Richette P Clerson P Périssin L Flipo RM Bardin T . Revisiting comorbidities in gout: a cluster analysis. Ann Rheum Dis. (2015) 74(1):142–7. 10.1136/annrheumdis-2013-203779
4.
Hsu Y Lee W-T Hsu C-h Lin H-Y . A comparison between the guidelines for the gout and hyperuricemia from different associations. J Intern Med Taiwan. (2022) 33(5):365–77. 10.6314/jimt.202210_33(5).05
5.
Kang EH Kim SC . Cardiovascular safety of urate lowering therapies. Curr Rheumatol Rep. (2019) 21(9):48. 10.1007/s11926-019-0843-8
6.
Das DK Engelman RM Clement R Otani H Prasad MR Rao PS . Role of Xanthine-oxidase inhibitor as free-radical scavenger—a novel mechanism of action of allopurinol and oxypurinol in myocardial salvage. Biochem Bioph Res Co. (1987) 148(1):314–9. 10.1016/0006-291x(87)91112-0
7.
Takir M Kostek O Ozkok A Elcioglu OC Bakan A Erek A et al Lowering uric acid with allopurinol improves insulin resistance and systemic inflammation in asymptomatic hyperuricemia. J Invest Med. (2015) 63(8):924–9. 10.1097/Jim.0000000000000242
8.
Azevedo VF Kos IA Vargas-Santos AB Pinheiro GDC Paiva ED . Benzbromarone in the treatment of gout. Adv Rheumatol. (2019) 59(1):37. 10.1186/s42358-019-0080-x
9.
White WB Saag KG Becker MA Borer JS Gorelick PB Whelton A et al Cardiovascular safety of febuxostat or allopurinol in patients with gout. New Engl J Med. (2018) 378(13):1200–10. 10.1056/NEJMoa1710895
10.
Mackenzie IS Ford I Nuki G Hallas J Hawkey CJ Webster J et al Long-term cardiovascular safety of febuxostat compared with allopurinol in patients with gout (FAST): a multicentre, prospective, randomised, open-label, non-inferiority trial. Lancet. (2020) 396(10264):1745–57. 10.1016/S0140-6736(20)32234-0
11.
Ko TM Tsai CY Chen SY Chen KS Yu KH Chu CS et al Use of HLA-B*58:01 genotyping to prevent allopurinol induced severe cutaneous adverse reactions in Taiwan: national prospective cohort study. BMJ. (2015) 351:h4848. 10.1136/bmj.h4848
12.
Hershfield MS Callaghan JT Tassaneeyakul W Mushiroda T Thorn CF Klein TE et al Clinical pharmacogenetics implementation consortium guidelines for human leukocyte antigen-B genotype and allopurinol dosing. Clin Pharmacol Ther. (2013) 93(2):153–8. 10.1038/clpt.2012.209
13.
Tsai PH Kuo CF Liu JR Li PR See LC . Effect of febuxostat on adverse events and mortality in gout in Taiwan: an interrupted time series analysis. Int J Rheum Dis. (2023) 26(3):471–9. 10.1111/1756-185X.14558
14.
Eun Y Han H Kim K Kang S Lee S Kim H et al Cardiovascular risk associated with allopurinol or benzbromarone treatment in patients with gout. Ther Adv Musculoskel. (2022) 14:1759720X221116409. 10.1177/1759720X221116409
15.
Kang EH Park EH Shin A Song JS Kim SC . Cardiovascular risk associated with allopurinol vs. benzbromarone in patients with gout. Eur Heart J. (2021) 42(44):4578–88. 10.1093/eurheartj/ehab619
16.
Polito L Bortolotti M Battelli MG Bolognesi A . Xanthine oxidoreductase: a leading actor in cardiovascular disease drama. Redox Biol. (2021) 48:102195. 10.1016/j.redox.2021.102195
17.
Furuhashi M . New insights into purine metabolism in metabolic diseases: role of xanthine oxidoreductase activity. Am J Physiol-Endoc M. (2020) 319(5):E827–34. 10.1152/ajpendo.00378.2020
18.
Fujishima Y Kita S Nishizawa H Maeda N Shimomura I . Cardiovascular significance of adipose-derived adiponectin and liver-derived xanthine oxidoreductase in metabolic syndrome. Endocr J. (2023) 70(7):663–75. 10.1507/endocrj.EJ23-0160
19.
Kotozaki Y Satoh M Nasu T Tanno K Tanaka F Sasaki M . Human plasma Xanthine oxidoreductase activity in cardiovascular disease: evidence from a population-based study. Biomedicines. (2023) 11(3):754. 10.3390/biomedicines11030754
20.
Liu D Zhou B Li Z Zhang Z Dai X Ji Z et al Effectiveness of benzbromarone versus febuxostat in gouty patients: a retrospective study. Clin Rheumatol. (2022) 41(7):2121–8. 10.1007/s10067-022-06110-5
21.
O'Dell JR Brophy MT Pillinger MH Neogi T Palevsky PM Wu H et al Comparative effectiveness of allopurinol and febuxostat in gout management. NEJM Evid. (2022) 1(3). 10.1056/evidoa2100028
22.
Becker MA Schumacher HR Espinoza LR Wells AF MacDonald P Lloyd E et al The urate-lowering efficacy and safety of febuxostat in the treatment of the hyperuricemia of gout: the CONFIRMS trial. Arthritis Res Ther. (2010) 12(2):R63. 10.1186/ar2978
23.
Schumacher HR Jr Becker MA Wortmann RL Macdonald PA Hunt B Streit J et al Effects of febuxostat versus allopurinol and placebo in reducing serum urate in subjects with hyperuricemia and gout: a 28-week, phase III, randomized, double-blind, parallel-group trial. Arthritis Rheum. (2008) 59(11):1540–8. 10.1002/art.24209
24.
Jeong H Choi E Suh A Yoo M Kim B . Risk of cardiovascular disease associated with febuxostat versus allopurinol use in patients with gout: a retrospective cohort study in Korea. Rheumatol Int. (2023) 43(2):265–81. 10.1007/s00296-022-05222-0
25.
Shin A Choi SR Han MJ Ha YJ Lee YJ Lee EB et al Cardiovascular safety associated with febuxostat versus allopurinol among patients with gout: update with accumulated use of febuxostat. Semin Arthritis Rheu. (2022):56:152080. ARTN 152080. 10.1016/j.semarthrit.2022.152080
26.
Weisshaar S Litschauer B Reichardt B Gruber F Leitner S Sibinovic S et al Cardiovascular risk and mortality in patients with hyperuricemia treated with febuxostat or allopurinol: a retrospective nation-wide cohort study in Austria 2014–2017. Rheumatol Int. (2022) 42(9):1597–603. 10.1007/s00296-022-05139-8
27.
Cheng CL Yen CT Su CC Lee CH Huang CH Yang YHK . Sex difference in heart failure risk associated with febuxostat and allopurinol in gout patients. Front Cardiovasc Med. (2022) 9:891606. 10.3389/fcvm.2022.891606
28.
Ju CS Lai RWC Li KHC Hung JKF Lai JCL Ho J et al Comparative cardiovascular risk in users versus non-users of xanthine oxidase inhibitors and febuxostat versus allopurinol users. Rheumatology. (2020) 59(9):2340–9. 10.1093/rheumatology/kez576
29.
Kang EH Choi HK Shin A Lee YJ Lee EB Song YW et al Comparative cardiovascular risk of allopurinol and febuxostat in patients with gout: a nation-wide cohort study. Rheumatology. (2019) 58(12):2122–9. 10.1093/rheumatology/kez189
30.
Chen CH Chen CB Chang CJ Lin Y Wang CW Chi CC et al Hypersensitivity and cardiovascular risks related to allopurinol and febuxostat therapy in Asians: a population-based cohort study and meta-analysis. Clin Pharmacol Ther. (2019) 106(2):391–401. 10.1002/cpt.1377
31.
Su CY Shen LJ Hsieh SC Lin LY Lin FJ . Comparing cardiovascular safety of febuxostat and allopurinol in the real world: a population-based cohort study. Mayo Clin Proc. (2019) 94(7):1147–57. 10.1016/j.mayocp.2019.03.001
32.
Zhang M Solomon DH Desai RJ Kang EH Liu J Neogi T et al Assessment of cardiovascular risk in older patients with gout initiating febuxostat versus allopurinol: population-based cohort study. Circulation. (2018) 138(11):1116–26. 10.1161/Circulationaha.118.033992
33.
Foody J Turpin RS Tidwell BA Lawrence D Schulman KL . Major cardiovascular events in patients with gout and associated cardiovascular disease or heart failure and chronic kidney disease initiating a Xanthine oxidase inhibitor. Am Health Drug Benef. (2017) 10(8):393–400.
Summary
Keywords
febuxostat, allopurinol, benzbromarone, cardiovascular, gout
Citation
Lin T-S, Lin Y-L, Hsu C-H, Hsieh S-C, Shau W-Y, Yang W-S and Chen C-L (2025) A systematic review and network meta-analysis of cardiovascular safety of benzbromarone compared to febuxostat and allopurinol in patients with gout. Front. Cardiovasc. Med. 12:1541307. doi: 10.3389/fcvm.2025.1541307
Received
07 March 2025
Accepted
27 June 2025
Published
10 July 2025
Volume
12 - 2025
Edited by
Juncheng Wei, Temple University, United States
Reviewed by
Julianna Desmarais, Oregon Health and Science University, United States
Liping Jiang, University of Illinois Chicago, United States
Updates
Copyright
© 2025 Lin, Lin, Hsu, Hsieh, Shau, Yang and Chen.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Chi-Ling Chen chlnchen@ntu.edu.tw
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.