- 1Department of Medicine, Hamad Medical Corporation, Doha, Qatar
- 2Department of Medicine, Smt. NHL Municipal Medical College, Ahmedabad, Gujarat, India
- 3Department of Medicine, Shadan Institute of Medical Sciences, Hyderabad, India
- 4Department of Medicine, Government Kilpauk Medical College, Chennai, India
- 5Department of Medicine, Kasturba Medical College, Manipal, India
- 6Department of Medicine, Universitas Padjadjaran, Bandung, Indonesia
- 7Department of Medicine, University Notre-Dame of Haiti, P-au-P, Haiti/Medicine, Lakeside Medical Center, Belle-Glade, FL, United States
- 8Department of Medicine, Government Medical College, Patiala, India
- 9Department of Environmental Health, Johns Hopkins University, Baltimore, MD, United States
- 10Department of Medicine, King Edward Medical University, Lahore, Pakistan
- 11Department of Medicine, United Health Services, Johnson City, NY, United States
- 12National Heart and Lung Institute, Imperial College London, London, United Kingdom
Background: Acute heart failure (AHF) is a serious medical condition with considerable morbidity and mortality ranging from 20%–30% within the first month following hospital admission. We aimed to evaluate the efficacy and safety of sodium-glucose cotransporter-2 (SGLT2) inhibitors administered within the first five days of hospitalization for AHF.
Methods: We utilized various electronic resources such as MEDLINE, Embase, and the Cochrane Library to retrieve relevant randomized controlled trials (RCTs). The meta-analysis was performed using Revman, where the risk ratio (RR) and mean difference (MD) with a 95% confidence interval (CI) were used for dichotomous and continuous variablesrespectively.
Results: A total of seven trials were included in this review. SGLT2 inhibitors were associated with decreased all-cause mortality (RR = 0.61, 95% CI = 0.40, 0.95; P = 0.03), worsening of HF (RR = 0.59, 95%CI = 0.36, 0.97;P = 0.04), and GFR (MD: 1.05, 95% CI = 0.68, 1.43; P < 0.00001) compared with the control group. There were no significant differences between the two groups regarding readmission for HF, cardiovascular mortality, AKI, hypoglycemia, hypotension, and diuretic efficiency. SGLT2 inhibitors were associated with improved KCCQ-CSS scores (MD: −3.82, 95% CI = −7.51, −0.13; P = 0.04).
Conclusion: SGLT2 inhibitors demonstrate overall clinical benefits and a favorable safety profile in acute heart failure, although their impact on readmission rates is limited. Further research is needed to refine patient selection and optimize treatment strategies.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/view/CRD42024571563, PROSPERO (CRD42024571563).
Introduction
Heart failure (HF) is a prevalent and serious medical condition (1) and is characterized by significant morbidity and mortality, with a 5-year mortality rate ranging from 50%–75% (1). In addition to its detrimental impact on the quality of life, HF imposes a substantial economic burden, with costs in the United States estimated at $ 30.7 billion annually (2, 3). The prevalence of HF is increasing owing to enhanced life expectancy and improved therapeutic strategies. However, acute HF (AHF) remains a critical concern, with an annual mortality range of 20%–30% within the first month following hospitalization (4, 5). Furthermore, AHF significantly increases the risk of recurrent hospitalizations, with a 20% higher likelihood of re-hospitalization for decompensated HF (6).
Current treatment options for AHF, including loop diuretics, primarily focus on alleviating the congestion and symptoms. Although loop diuretics remain the cornerstone of AHF management, they have not demonstrated a meaningful reduction in mortality and are associated with adverse renal effects (7). The lack of effective treatments that improve long-term outcomes in AHF highlights the need for novel therapeutic strategies.
Sodium-glucose cotransporter-2 (SGLT2) inhibitors are a class of medications with unique antihyperglycemic effects. They act on the proximal convoluted tubules to inhibit glucose and sodium reabsorption, thereby promoting glucosuria and natriuresis in patients with type 2 diabetes (8). Beyond their role in diabetes management, SGLT2 inhibitors have shown outstanding cardiovascular benefits in clinical trials, significantly reducing the risk of cardiovascular morbidity and mortality (9–14). Notably, these medications have been found to lower the risk of hospitalization (worsening HF) and cardiovascular death, regardless of diabetes status and ejection fraction (EF), with benefits observed in both HF with reduced EF (HFrEF) and preserved EF (HFpEF) (15–18). This beneficial “class effect” has been consistently demonstrated across various SGLT2 inhibitors (9–14). The cardiac benefits of SGLT2 inhibitors are mediated primarily via natriuresis and glycosuria. They lead to several other downstream effects, such as improvement in blood pressure and reduction of oxidative stress and inflammation (19).
However, the role of SGLT2 inhibitors in AHF, particularly during the early phase of hospitalization, remains unclear. Previous studies have not adequately explored the initiation of these agents during the first days of AHF admission, and there has been considerable variability in the timing of SGLT2 inhibitor initiation. Moreover, recently published RCTs have highlighted the need for more updated reviews.
Our review aimed to fill this gap by systematically evaluating the efficacy and safety of SGLT2 inhibitors administered within the first five days of hospitalization for AHF. This meta-analysis provides crucial insights into the potential of SGLT2 inhibitors to improve outcomes in patients with AHF.
Materials and methods
This systematic review and meta-analysis was designed following the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions (20) and was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement (21). It was registered with PROSPERO with identifier number CRD42024571563. Ethical approval was not required for this study.
Eligibility criteria
The inclusion criteria were as follows: (1) study design: randomized controlled trials (RCTs) only; (2) patient population: patients presenting within 5 days of being admitted to the hospital with AHF; (3) intervention: SGLT2 inhibitors; (4) control: placebo or standard treatment; and (5) outcome: reporting at least one outcome of interest.
HF, whether as an acute decompensation of existing HF or as newly diagnosed (de novo) HF, and both HFrEF and HFpEF, were included regardless of diabetes status.
We excluded studies that: (1) recruited patients with AHF who were administered SGLT2 inhibitors after 5 days or discharged from the hospital; (2) were not RCTs i.e., observational studies (case series, cohorts, case-control studies) and reviews; and (3) were conducted in animal populations.
Information sources and search strategy
The following electronic databases and trial registers were searched for eligible studies from inception until May 15, 2024: Cochrane Central Register of Controlled Trials (CENTRAL, via The Cochrane Library), MEDLINE (via PubMed), Embase (via Ovid), and Clinical Trials.gov. We also utilized grey literature sources (ProQuest Dissertations and Theses Global, PQDT), reference lists of included studies, and related systematic reviews to retrieve all eligible studies. A combination of relevant keywords and MeSH terms such as “SGLT2”, “sodium-glucose cotransporter-2 inhibitors”, “SGLT2 inhibitors”, “de-novo heart failure”, “decompensated heart failure”, “acute heart failure” were used in multiple combinations in our search strategy. The literature search had no language restrictions.
Selection process
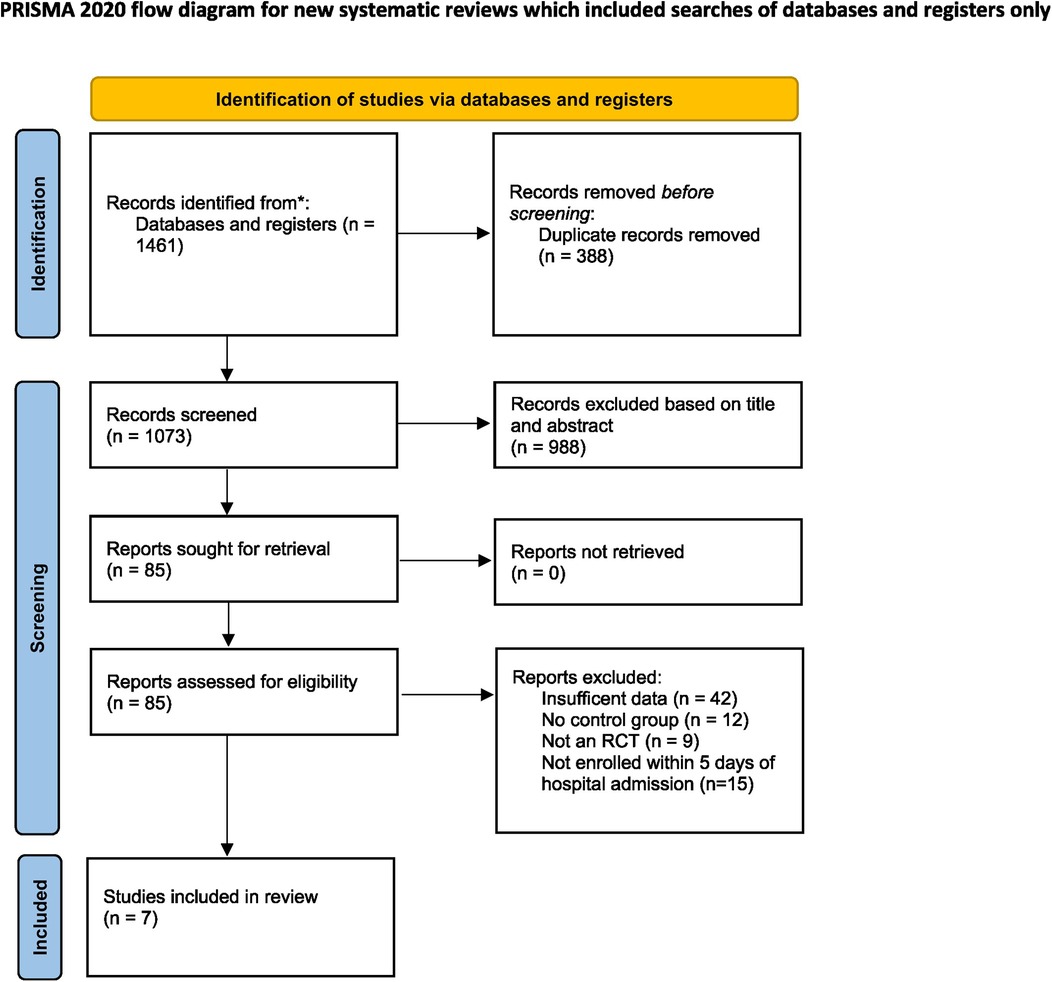
The articles retrieved through our literature search were uploaded to Rayyan, a software used for screening and retrieval of articles. Following deduplication, two reviewers independently screened the articles based on their titles and abstracts. The remaining articles were subjected to full-text screening based on the inclusion criteria. Any discordance between the two reviewers was resolved by a third reviewer. The selection process is illustrated using a PRISMA flowchart.
Data collection process and data items
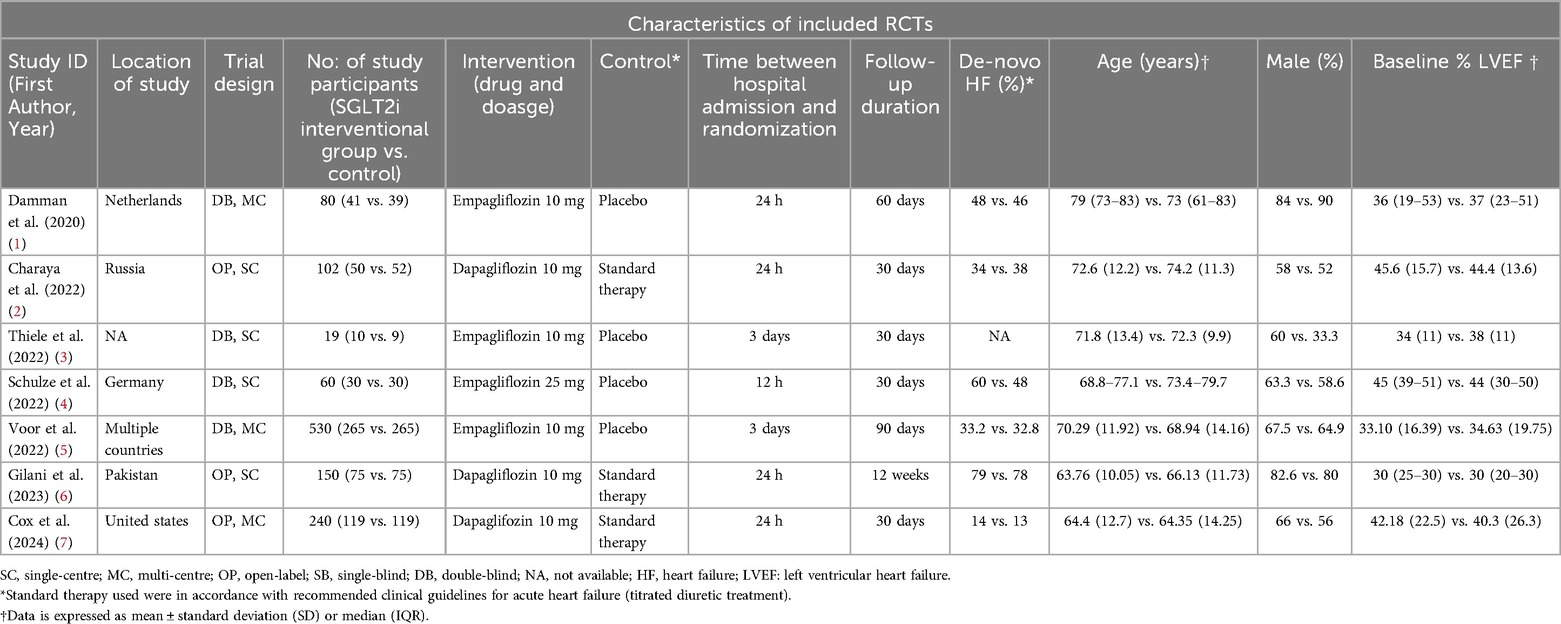
Two authors independently extracted data from the included studies using a structured Excel spreadsheet. The data items included study characteristics (trial design, author name, year of publication, trial name, sample size, and follow-up duration), patient characteristics (age, sex, details of comorbidities, baseline LVEF, and de novo HF), intervention and comparator details (dose, duration, and type of drug), and outcome measures. All-cause mortality and readmission for HF were the primary outcomes.
The secondary outcomes included cardiovascular mortality, worsening HF, Kansas City Cardiomyopathy Questionnaire Total Symptom Score (KCCQ-TSS) improvement, diuretic efficiency, glomerular filtration rate (GFR), acute kidney injury (AKI), urinary tract infection (UTI), hypoglycemia and hypotension. AKI was defined as an increase in blood creatinine level of 0.3 mg/dl (26.4 µmol/L) or more within 48 h. Diuretic efficiency was defined as weight change per 40 mg i/v furosemide.
Risk of bias assessment
The revised Cochrane Risk of Bias tool (RoB 2.0) was used to evaluate the risk of bias based on five potential items (2): (i) bias resulting from the process of randomization, (ii) bias secondary to deviation from the intended intervention, (iii) bias related to missed outcome data, (iv) bias in outcome measurement, and (v) bias due to the selective reporting of results. Two reviewers independently applied the RoB 2.0 tool and a third reviewer resolved any discrepancy between the two reviewers. The risk of bias was categorized as “low”, “high” or “some concern”.
Statistical analysis
Analyses were performed using Review Manager (RevMan version 5.4, Cochrane Collaboration, 2020). A random effects model was applied using the DerSimonian-Laird variance estimator. The risk ratio (RR) and mean difference (MD) with a 95% confidence interval (CI) were used for dichotomous and continuous data, respectively. Forest plots were used to present the results for each outcome.
We planned to construct a funnel plot to assess publication bias if the number of studies was greater than 10. The chi-square (χ2) test was used to detect heterogeneity and the I2 statistics was employed to assess its magnitude. I2 interpretation was performed according to the Cochrane Handbook for Systematic Reviews of Interventions, section 10.10. P < 0.10 was considered statistically significant for the χ2 test. A subgroup analysis of the primary outcomes was performed based on the type of SGLT2 inhibitor used in the trials. We also performed a subgroup analysis of all-cause mortality based on the follow-up duration of the RCTs.
Results
Study selection
A total of 1,461 studies were retrieved from various databases and registers. Following deduplication and screening, seven RCTs were included in this meta-analysis (22–28). The detailed selection process is depicted in the PRISMA flowchart (Figure 1).
Study characteristics
Of the seven included studies (1,181 patients), four evaluated empagliflozin, whereas dapagliflozin was an intervention in the remaining studies. Three studies had standard therapy as a comparator, while the remaining studies used placebo. The time taken between hospital admission and randomization was 24 h in three studies, 12 h in EMPAG-HF, 72 h in two studies, and less than 24 h in one study. Most studies had a follow-up duration of 30 days (57%), with the remaining studies having a follow-up period of more than 30 days. Detailed study and patient characteristics are presented in Table 1.
Risk of bias in included studies
Of the 7 studies, 3 studies were found to have some concerns of bias (Charaya et al., Thiele et al., Gilani et al.) as a result of bias due to deviations from the intended intervention, measurement of outcomes, during the randomization process, and in the selection of the reported results, while 4 studies were at low risk (Supplementary Figure 1). The key sources of bias included a lack of allocation concealment and the absence of a prespecified analysis plan, which may have led to selection bias and post-randomization imbalances. Furthermore, the failure to implement an intention-to-treat approach in these studies raises concerns about potential attrition bias, which could lead to an overestimation of treatment effects.
Results of the meta-analyses
Primary outcomes
All-cause mortality
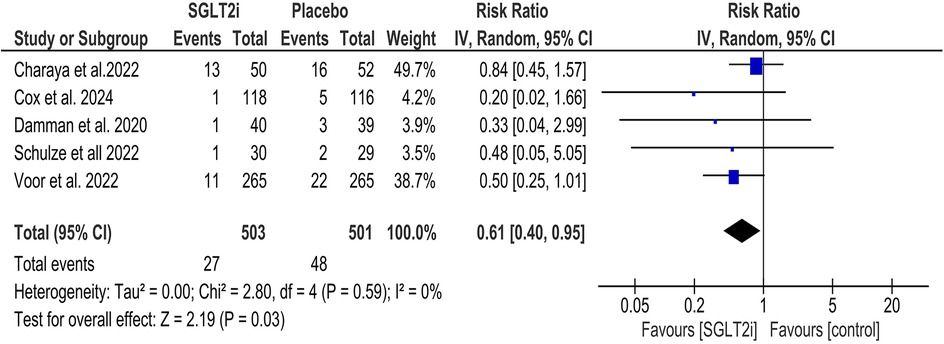
SGLT2 inhibitors significantly decreased all-cause mortality (RR = 0.61, 95% CI = 0.40, 0.95; Figure 2), and statistical heterogeneity was found to be minimal (I2 = 0%). Subgroup analysis was performed based on the type of SGLT2 inhibitor (empagliflozin and dapagliflozin), and it was found that there was no significant statistical difference between the two subgroups (P = 0.77; Supplementary Figure 2). Similarly, subgroup analysis of all-cause mortality based on the duration of follow-up yielded no significant difference between the subgroups (P = 0.60; Supplementary Figure 3).
Readmission for HF
SGLT2 inhibitors did not reduce the risk of readmission due to HF (RR = 0.87, 95% CI = 0.58, 1.31; Figure 3), and the statistical heterogeneity was minimal (I2 = 0%). Subgroup analysis was performed based on the type of SGLT2 inhibitor (empagliflozin and dapagliflozin), and no statistically significant difference was found between the two subgroups (P = 0.96; Supplementary Figure 4).
Secondary outcomes
Cardiovascular mortality
SGLT2 inhibitors were not superior to the placebo in reducing cardiovascular mortality in patients with AHF (RR = 0.67, 95% CI = 0.45, 1.00; Supplementary Figure 5) with minimal statistical heterogeneity (I2 = 0%).
AKI
There was no statistically significant difference found in the incidence of AKI between the two groups (RR = 1.46, 95% CI = 0.50, 4.24; Supplementary Figure 6). The statistical heterogeneity was found to be minimal (I2 = 0%).
Hypoglycemia
There was no statistically significant difference between the two groups in terms of hypoglycemic events (RR = 0.90, 95% CI = 0.42, 1.94; Supplementary Figure 7) with minimal statistical heterogeneity (I2 = 0%).
Worsening Hf
SGLT2 inhibitors have been found to significantly reduce the incidence of worsening HF compared to placebo (RR = 0.59, 95%CI = 0.36, 0.97; Supplementary Figure 8). The interstudy heterogeneity was minimal (I2 = 0%).
UTI
There was no statistically significant difference between the two subgroups regarding the incidence of UTIs (RR = 0.59, 95% CI = 0.31, 1.15; Supplementary Figure 9). The statistical heterogeneity was estimated to be minimal (I2 = 0%).
Hypotension
There was no statistically significant difference between the two subgroups in terms of the development of hypotensive episodes (RR = 0.63, 95% CI = 0.29, 1.97; Supplementary Figure 10), with minimal statistical heterogeneity (I2 = 0%).
Diuretic efficiency
No significant difference was found between the two groups in diuretic efficiency (MD −0.29, 95% CI = −0.81, 0.23; Supplementary Figure 11). The statistical heterogeneity was found to be high (I2 = 65%).
GFR
SGLT2 inhibitors were associated with significantly reduced GFR as compared to the control group (MD: 1.05, 95% CI = 0.68, 1.43; Supplementary Figure 12) with minimal statistical heterogeneity (I2 = 0%).
KCCQ-TSS score improvement
SGLT2 inhibitors were found to significantly improve the KCCQ-TSS score compared to the control group (MD: −3.82, 95% CI = −7.51, −0.13; Supplementary Figure 13). The heterogeneity was estimated to be minimal (I2 = 0%).
Discussion
In recent years, SGLT2 inhibitors have emerged as potential treatment options for patients with AHF, demonstrating notable efficacy in the management of this complex condition. Several systematic reviews and meta-analyses examining the efficacy of SGLT2 inhibitors in patients with AHF have provided significant insights into their clinical impact.
Our meta-analysis revealed that SGLT2 inhibitors provided a mortality benefit and reduced the rate of worsening HF. However, there was no significant reduction in cardiovascular mortality or HF readmission rates associated with their use. We also did not observe any significant risk of adverse events, aside from a reduction in GFR compared with the control group. Moreover, SGLT2 inhibitors significantly improved the KCCQ-TSS score in chronic HF populations, and recent data from the EMPULSE trial suggest similar improvements in quality of life among patients hospitalized with AHF. Our findings also indicate a substantial reduction in all-cause mortality by approximately 39%, highlighting the potential role of these agents as therapeutic options in the management of HF. This mortality benefit aligns with the existing literature on SGLT2 inhibitors, reinforcing their role as a treatment option for chronic HF management and in the acute setting.
The efficacy of SGLT2 inhibitors was initially evaluated in the EMPA-REG OUTCOME trial. In this trial, patients hospitalized for HF who received SGLT2 inhibitors experienced a significantly reduced risk of rehospitalization or death within the first three months following an initial HF event (HFE) (29). In another trial, the EMPULSE trial (26) evaluated patients over the first 90 days after hospital admission, a period often considered the vulnerable phase of HF. This trial included patients with no prior history of HF (acute de novo) who had not yet been treated for HF. This demonstrated that adding empagliflozin to standard therapy was well tolerated and produced clinical benefits similar to those seen in patients with chronic decompensated HF. Clinical benefits in the EMPULSE trial included a composite of death, number of HFEs [including hospitalizations for HF (HHFs), urgent HF visits, and unplanned outpatient visits], time to first HFE, and change from baseline in the KCCQ-CSS after 90 days of treatment. An extended pilot study of this trial, called EMPA-RESPONSE-AHF, also suggested a clinical benefit of empagliflozin in patients hospitalized for AHF (28). These results indicate that empagliflozin is highly regarded as an effective treatment for patients hospitalized with both de novo and decompensated AHF.
Several large-scale drug trials for hospitalized patients with AHF have failed to demonstrate substantial benefits, potentially due to the short duration of therapy (24–48 h) or the lack of continuation post-discharge (30–34). While the PIONEER-HF trial, with a design similar to EMPULSE, focused on NT-proBNP levels rather than clinical outcomes (35), subsequent trials such as EMPULSE and TRANSITION have provided evidence supporting the safety of initiating chronic HF therapies, such as SGLT2 inhibitors and sacubitril-valsartan, respectively, during the index hospitalization (pre-discharge) (36, 37). Nonetheless, questions remain regarding the mechanisms behind the lack significant reduction in re-hospitalization rates seen with SGLT2 inhibitors in meta-analyses for AHF, especially when compared to their pronounced impact on reducing re-hospitalizations in chronic HF populations. Our results also did not show a significant reduction in HF readmission with SGLT2 inhibitors. This raises critical questions regarding the long-term management of AHF. Although these agents reduce all-cause mortality and improve symptoms, their limited effect on preventing readmissions suggests that more research is needed to understand their role in preventing HF progression vs. stabilizing patients' post-acute events. A new trial is currently evaluating the effects of in-hospital dapagliflozin initiation in patients hospitalized with AHF post-stabilization between days 1 and 14 (38).
A review by Salah et al., which specifically addressed the initiation of SGLT2 inhibitors in patients hospitalized for AHF, demonstrated a significant reduction in the risk of rehospitalization for HF and improvement in patient-reported outcomes without an increase in adverse effects such as AKI, hypotension, or hypoglycemia. Similarly, a review by Kumar et al. (39) reported a significant reduction in cardiovascular death or HHF associated with SGLT2 inhibitor use alongside a decrease in HF symptoms and comparable rates of adverse events. Kumar et al. also reported a reduction in all-cause mortality associated with SGLT2 inhibitor use, whereas Salah et al. did not find a statistically significant effect. Moreover, review studies by Roy et al. (40) and Ahmad et al. (41) support the efficacy of SGLT2 inhibitors in reducing HHF and cardiovascular death across a broader patient population, not limited to AHF. Huang et al. (42) also demonstrated the efficacy of SGLT2 Inhibitors in patients with HFpEF. In support of this, another study showed that SGLT2 inhibitors are effective irrespective of EF status or the presence of diabetes mellitus (43). This suggests that the benefits of SGLT2 inhibitors are not confined to a specific type of HF. Therefore, SGLT2 inhibitors should be considered a valuable addition to the treatment regimen for patients with HF, including those with AHF. However, these meta-analyses included the SOLOIST-HF trial (44), which included patients who continued to use SGLT2 inhibitors even after discharge. In our meta-analysis, we analyzed trials that included patients treated within five days of AHF presentation to preserve homogeneity.
Our study performed a subgroup analysis of the specific SGLT2 inhibitors, empagliflozin and dapagliflozin. Interestingly, this analysis did not reveal any statistically significant differences in the efficacy between the two drugs. This finding suggests a class effect inherent to SGLT2 inhibitors, offering clinicians a level of reassurance regarding the interchangeable use of these agents in practice. The absence of a significant difference can guide treatment decisions, especially in resource-limited settings or when patient preferences must be considered. We also performed a subgroup analysis for all-cause mortality based on the duration of follow-up, but no significant differences were found between the subgroups. Another notable finding in our meta-analysis revealed that SGLT2 inhibitors reduced the worsening of HF compared to placebo, with a relative risk reduction of 41%. This large risk reduction highlights their potential role in symptom management, which is critical for improving the quality of life of patients with HF. There was also a statistically significant improvement in the KCCQ-TSS score. This presents a compelling argument for incorporating SGLT2 inhibitors into the standard care protocols for patients with AHF.
The use of SGLT2 inhibitors is believed to overcome the drawback of diuretic resistance often seen with diuretics alone in patients with AHF. This occurs by counteracting sodium absorption in PCT, thereby exerting a natriuretic effect (45, 46). However, our review did not find any significant improvements in diuretic efficacy, suggesting that other mechanisms may have been involved. Nevertheless, clinicians should be vigilant regarding individual patient responses and potential diuretic-related adverse effects. Inhibition of sodium and glucose reabsorption in the proximal tubule by SGLT2 inhibitors leads to an increased delivery of sodium to the macula densa. This triggers tubuloglomerular feedback, causing afferent arteriolar constriction, and thus, a reduction in eGFR. This decline is seen early within 2–4 weeks of initiation while showing some recovery by week 12 (47). However, this decrease in GFR appears to be transient, with no bearing on its protective effect on cardiovascular outcomes (48).
The safety profile of SGLT2 inhibitors appears robust, with no statistically significant differences noted between the SGLT2 inhibitor and control groups concerning AKI, hypoglycemia, UTIs, or hypotension. This suggests that when prescribed judiciously, SGLT2 inhibitors can be considered safe adjuncts in the treatment of AHF without imposing substantial risks commonly associated with HF pharmacotherapy.
Our study employed a comprehensive search strategy across multiple databases to capture all relevant RCTs. Additionally, we included only those studies that assessed the impact of SGLT2 inhibitors in patients within the first five days of AHF onset, enhancing the consistency of our findings.
However, several limitations of this meta-analysis should be acknowledged. The individual trials varied in key aspects, such as the type of control group, time from hospital admission to randomization, treatment duration, follow-up period, and HF severity, all of which contributed to heterogeneity. Importantly, not all the outcomes were evaluated in every study. For example, mortality was assessed in only five of the seven included trials, and safety outcomes such as adverse events were reported inconsistently across studies. This variability in reported outcomes limits the ability to draw definitive conclusions about certain endpoints and may introduce potential reporting bias. While statistical heterogeneity was minimal in most of our meta-analyses, these variations in study design and outcome definitions should be considered when interpreting our findings. Additionally, our study did not perform a subgroup analysis based on the type of AHF (recurrent vs. de novo) or EF (HFrEF vs. HFpEF), which may have provided further clinical insights. Certain safety outcomes were evaluated in only a few trials, limiting our ability to detect significant effects. Furthermore, some trials excluded patients with specific conditions, such as ESRD and ACS, making our findings less applicable to these populations. Finally, we relied on published summary data rather than individual patient-level data, which could have provided more precise patient-level insights, better identification of exposures and outcomes, and greater adjustment for confounders to reduce heterogeneity (49).
Given the limitations identified in this meta-analysis, future research should address the variability between trials. Standardizing aspects such as control group selection, treatment duration, follow-up periods, and the time from hospital admission to randomization will help reduce heterogeneity and improve the comparability of the results. Additionally, future studies should focus on different types of AHF (chronic decompensated vs. de novo) to better understand differential treatment effects. More robust investigations into the safety outcomes of SGLT2 inhibitors, particularly in underrepresented populations, such as those with ESRD or ACS, are needed.
In conclusion, the findings of this systematic review and meta-analysis underscore the overall clinical benefits and favorable profile of SGLT2 inhibitors in patients with AHF. However, their limited impact on readmission rates indicates the need for a nuanced approach to HF management that incorporates both pharmacological and non-pharmacological strategies. Further research is warranted to better characterize patient populations that derive the greatest benefit and to refine treatment strategies for optimal clinical outcomes.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
AR: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Validation, Writing – original draft, Writing – review & editing. TB: Data curation, Formal analysis, Investigation, Methodology, Validation, Writing – original draft. RF: Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft. AR: Data curation, Formal analysis, Investigation, Methodology, Resources, Visualization, Writing – original draft. JK: Data curation, Formal analysis, Investigation, Methodology, Validation, Writing – original draft. HM: Data curation, Formal analysis, Investigation, Methodology, Resources, Visualization, Writing – original draft. NG: Data curation, Formal analysis, Methodology, Software, Visualization, Writing – original draft. WL: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. FD: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. HL: Data curation, Formal analysis, Investigation, Resources, Writing – original draft. ME: Conceptualization, Data curation, Formal analysis, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing. WU: Conceptualization, Investigation, Project administration, Software, Validation, Visualization, Writing – review & editing. HA: Investigation, Methodology, Project administration, Software, Supervision, Writing – review & editing. RA: Funding acquisition, Project administration, Resources, Software, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
AR was employed by Hamad Medical Corporation.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1543153/full#supplementary-material
References
1. GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. (2018) 392:1798–858. doi: 10.1016/S0140-6736(18)32279-7
2. Savarese G, Becher PM, Lund LH, Seferovic P, Rosano GMC, Coats AJS. Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovasc Res. (2023) 118(17):3272–87. doi: 10.1093/cvr/cvac013
3. Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, et al. Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation. (2021) 143(8):e254–743. doi: 10.1161/CIR.0000000000000950
4. Crespo-Leiro MG, Anker SD, Maggioni AP, Coats AJ, Filippatos G, Ruschitzka F, et al. European Society of Cardiology Heart Failure Long-Term Registry (ESC-HF-LT): 1-year follow-up outcomes and differences across regions. Eur J Heart Fail. (2016) 18(6):613–25. doi: 10.1002/ejhf.566
5. Groenewegen A, Rutten FH, Mosterd A, Hoes AW. Epidemiology of heart failure. Eur J Heart Fail. (2020) 22(8):1342–56. doi: 10.1002/ejhf.1858
6. Arrigo M, Jessup M, Mullens W, Reza N, Shah AM, Sliwa K, et al. Acute heart failure. Nat Rev Dis Primer. (2020) 6(1):16. doi: 10.1038/s41572-020-0151-7
7. Funahashi Y, Chowdhury S, Eiwaz MB, Hutchens MP. Acute cardiorenal syndrome: models and heart-kidney connectors. Nephron. (2020) 144(12):629–33. doi: 10.1159/000509353
8. Boorsma EM, Beusekamp JC, Ter Maaten JM, Figarska SM, Danser AHJ, van Veldhuisen DJ, et al. Effects of empagliflozin on renal sodium and glucose handling in patients with acute heart failure. Eur J Heart Fail. (2021) 23(1):68–78. doi: 10.1002/ejhf.2066
9. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. (2015) 373(22):2117–28. doi: 10.1056/NEJMoa1504720
10. Neal B, Perkovic V, Matthews DR. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. (2017) 377(21):2099. doi: 10.1056/NEJMoa1611925
11. Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. (2019) 380(24):2295–306. doi: 10.1056/NEJMoa1811744
12. Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. (2019) 380(4):347–57. doi: 10.1056/NEJMoa1812389
13. Cannon CP, Pratley R, Dagogo-Jack S, Mancuso J, Huyck S, Masiukiewicz U, et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med. (2020) 383(15):1425–35. doi: 10.1056/NEJMoa2004967
14. McGuire DK, Shih WJ, Cosentino F, Charbonnel B, Cherney DZI, Dagogo-Jack S, et al. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: a meta-analysis. JAMA Cardiol. (2021) 6(2):148–58. doi: 10.1001/jamacardio.2020.4511
15. Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. (2021) 385(16):1451–61. doi: 10.1056/NEJMoa2107038
16. Solomon SD, McMurray JJV, Claggett B, de Boer RA, DeMets D, Hernandez AF, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. (2022) 387(12):1089–98. doi: 10.1056/NEJMoa2206286
17. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. (2020) 383(15):1413–24. doi: 10.1056/NEJMoa2022190
18. McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. (2019) 381(21):1995–2008. doi: 10.1056/NEJMoa1911303
19. Xiang B, Zhao X, Zhou X. Cardiovascular benefits of sodium-glucose cotransporter 2 inhibitors in diabetic and nondiabetic patients. Cardiovasc Diabetol. (2021) 20(1):78. doi: 10.1186/s12933-021-01266-x
20. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions. Wiley Online Books (2019). Available at: https://onlinelibrary.wiley.com/doi/book/10.1002/9781119536604 (Accessed December 6, 2023).
21. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J. (2021) 372:n71. doi: 10.1136/bmj.n71
22. Schulze PC, Bogoviku J, Westphal J, Aftanski P, Haertel F, Grund S, et al. Effects of early empagliflozin initiation on diuresis and kidney function in patients with acute decompensated heart failure (EMPAG-HF). Circulation. (2022) 146(4):289–98. doi: 10.1161/CIRCULATIONAHA.122.059038
23. Thiele K, Rau M, Hartmann N-UK, Möller M, Möllmann J, Jankowski J, et al. Empagliflozin reduces markers of acute kidney injury in patients with acute decompensated heart failure. ESC Heart Fail. (2022) 9:2233–8. doi: 10.1002/ehf2.13955
24. Fatima Gilani SF, Ali S, Farhat K, Noor M, Siddiqui MB, Waqar F. Early initiation of dapagliflozin and its effect on health related quality of life in acute heart failure: a randomised controlled trial. JPMA J Pak Med Assoc. (2024) 74(4):621–5. doi: 10.47391/JPMA.9813
25. Charaya K, Shchekochikhin D, Andreev D, Dyachuk I, Tarasenko S, Poltavskaya M, et al. Impact of dapagliflozin treatment on renal function and diuretics use in acute heart failure: a pilot study. Open Heart. (2022) 9(1):e001936. doi: 10.1136/openhrt-2021-001936
26. Voors AA, Angermann CE, Teerlink JR, Collins SP, Kosiborod M, Biegus J, et al. The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: a multinational randomized trial. Nat Med. (2022) 28(3):568–74. doi: 10.1038/s41591-021-01659-1
27. Cox ZL, Collins SP, Hernandez GA, McRae AT, Davidson BT, Adams K, et al. Efficacy and safety of dapagliflozin in patients with acute heart failure. J Am Coll Cardiol. (2024) 83(14):1295–306. doi: 10.1016/j.jacc.2024.02.009
28. Damman K, Beusekamp JC, Boorsma EM, Swart HP, Smilde TDJ, Elvan A, et al. Randomized, double-blind, placebo-controlled, multicentre pilot study on the effects of empagliflozin on clinical outcomes in patients with acute decompensated heart failure (EMPA-RESPONSE-AHF). Eur J Heart Fail. (2020) 22(4):713–22. doi: 10.1002/ejhf.1713
29. Savarese G, Sattar N, Januzzi J, Verma S, Lund LH, Fitchett D, et al. Empagliflozin is associated with a lower risk of post-acute heart failure rehospitalization and mortality: insights from the EMPA-REG OUTCOME trial. Circulation. (2019) 139(11):1458–60. doi: 10.1161/CIRCULATIONAHA.118.038339
30. Metra M, Teerlink JR, Cotter G, Davison BA, Felker GM, Filippatos G, et al. Effects of serelaxin in patients with acute heart failure. N Engl J Med. (2019) 381(8):716–26. doi: 10.1056/NEJMoa1801291
31. Massie BM, O’Connor CM, Metra M, Ponikowski P, Teerlink JR, Cotter G, et al. Rolofylline, an adenosine A1—receptor antagonist, in acute heart failure. N Engl J Med. (2010) 363(15):1419–28. doi: 10.1056/NEJMoa0912613
32. Konstam MA, Gheorghiade M, Burnett JC, Grinfeld L, Maggioni AP, Swedberg K, et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST outcome trial. Jama. (2007) 297(12):1319–31. doi: 10.1001/jama.297.12.1319
33. Packer M, O’Connor C, McMurray JJV, Wittes J, Abraham WT, Anker SD, et al. Effect of ularitide on cardiovascular mortality in acute heart failure. N Engl J Med. (2017) 376(20):1956–64. doi: 10.1056/NEJMoa1601895
34. Kozhuharov N, Goudev A, Flores D, Maeder MT, Walter J, Shrestha S, et al. Effect of a strategy of comprehensive vasodilation vs usual care on mortality and heart failure rehospitalization among patients with acute heart failure: the GALACTIC randomized clinical trial. JAMA. (2019) 322(23):2292. doi: 10.1001/jama.2019.18598
35. Velazquez EJ, Morrow DA, DeVore AD, Duffy CI, Ambrosy AP, McCague K, et al. Angiotensin–neprilysin inhibition in acute decompensated heart failure. N Engl J Med. (2019) 380(6):539–48. doi: 10.1056/NEJMoa1812851
36. Wachter R, Senni M, Belohlavek J, Straburzynska-Migaj E, Witte KK, Kobalava Z, et al. Initiation of sacubitril/valsartan in haemodynamically stabilised heart failure patients in hospital or early after discharge: primary results of the randomised TRANSITION study. Eur J Heart Fail. (2019) 21(8):998–1007. doi: 10.1002/ejhf.1498
37. Biegus J, Voors AA, Collins SP, Kosiborod MN, Teerlink JR, Angermann CE, et al. Impact of empagliflozin on decongestion in acute heart failure: the EMPULSE trial. Eur Heart J. (2023) 44(1):41–50. doi: 10.1093/eurheartj/ehac530
38. The TIMI Study Group. A Multicenter, Randomized, Double-Blind, Parallel Group, Placebo-Controlled Trial to Evaluate the Effect of In-Hospital Initiation of Dapagliflozin on Clinical Outcomes in Patients Who Have Been Stabilized During Hospitalization for Acute Heart Failure DAPAgliflozin and Effect on Cardiovascular Events in ACuTe Heart Failure-Thrombolysis in Myocardial Infarction 68 (DAPA ACT HF-TIMI 68). clinicaltrials.gov. Report No.: NCT04363697 (2025). Available at: https://clinicaltrials.gov/study/NCT04363697 (Accessed February 2, 2025).
39. Kumar K, Kheiri B, Simpson TF, Osman M, Rahmouni H. Sodium-Glucose cotransporter-2 inhibitors in heart failure: a meta-analysis of randomized clinical trials. Am J Med. (2020) 133(11):e625–30. doi: 10.1016/j.amjmed.2020.04.006
40. Roy R, Vinjamuri S, Baskara Salian R, Hafeez N, Meenashi Sundaram D, Patel T, et al. Sodium-glucose cotransporter-2 (SGLT-2) inhibitors in heart failure: an umbrella review. Cureus. (2023) 15(7):e42113. doi: 10.7759/cureus.42113
41. Ahmad Y, Madhavan MV, Stone GW, Francis DP, Makkar R, Bhatt DL, et al. Sodium-glucose cotransporter 2 inhibitors in patients with heart failure: a systematic review and meta-analysis of randomized trials. Eur Heart J Qual Care Clin Outcomes. (2022) 8(4):383–90. doi: 10.1093/ehjqcco/qcab072
42. Lou Y, Yang Q, Zhang W, Yu Y, Huang J. Efficacy of sodium-glucose cotransporter 2 inhibitors in heart failure with a preserved ejection fraction: a meta-analysis of randomized controlled trials. Rev Cardiovasc Med. (2022) 23(11):374. doi: 10.31083/j.rcm2311374
43. Nso N, Emmanuel K, Nassar M, Ayinde H. Heart failure prognosis in diabetic patients on SGLT2 (sodium-glucose cotransporter-2) inhibitors: a systematic review and meta-analysis. Circulation. (2021) 144(Suppl 1):12642. doi: 10.1161/circ.144.suppl_1.12642
44. Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. (2021) 384(2):117–28. doi: 10.1056/NEJMoa2030183
45. Stachteas P, Nasoufidou A, Patoulias D, Karakasis P, Karagiannidis E, Mourtzos MA, et al. The role of sodium-glucose co-transporter-2 inhibitors on diuretic resistance in heart failure. Int J Mol Sci. (2024) 25(6):3122. doi: 10.3390/ijms25063122
46. Hallow KM, Helmlinger G, Greasley PJ, McMurray JJV, Boulton DW. Why do SGLT2 inhibitors reduce heart failure hospitalization? A differential volume regulation hypothesis. Diabetes Obes Metab. (2018) 20(3):479–87. doi: 10.1111/dom.13126
47. Meraz-Muñoz AY, Weinstein J, Wald R. eGFR decline after SGLT2 inhibitor initiation: the tortoise and the hare reimagined. Kidney360. (2021) 2(6):1042–7. doi: 10.34067/KID.0001172021
48. Verbrugge FH, Dupont M, Steels P, Grieten L, Swennen Q, Tang WHW, et al. The kidney in congestive heart failure: ‘are natriuresis, sodium, and diuretics really the good, the bad and the ugly? Eur J Heart Fail. (2014) 16(2):133–42. doi: 10.1002/ejhf.35
Keywords: SGLT2 inhibitors, acute heart failure, de-novo heart failure, systematic review, meta-analysis
Citation: Rahil AI, Bhavsar T, Fatima R, Rajkumar A, Kumar J, Majidan HA, Gajjala N, Lefranc W, Deeksha F, Lingegowda H, Ehsan M, Ur Rehman W, Ahmad H and Ahmed R (2025) Efficacy and safety of SGLT2 inhibitors in acute heart failure: a systematic review and meta-analysis of randomized controlled trials. Front. Cardiovasc. Med. 12:1543153. doi: 10.3389/fcvm.2025.1543153
Received: 10 December 2024; Accepted: 18 April 2025;
Published: 1 May 2025.
Edited by:
Maria Concetta Pastore, University of Siena, ItalyReviewed by:
Nicolò Ghionzoli, University of Siena, ItalyAndrea Stefanini, University of Siena, Italy
Pongsathorn Gojaseni, Bhumibol Adulyadej Hospital, Thailand
Copyright: © 2025 Rahil, Bhavsar, Fatima, Rajkumar, Kumar, Majidan, Gajjala, Lefranc, Deeksha, Lingegowda, Ehsan, Ur Rehman, Ahmad and Ahmed. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Raheel Ahmed, ci5haG1lZDIxQGltcGVyaWFsLmFjLnVr
 Ali Ibrahim Rahil
Ali Ibrahim Rahil Tirth Bhavsar2
Tirth Bhavsar2 Harshitha Lingegowda
Harshitha Lingegowda Muhammad Ehsan
Muhammad Ehsan Wajeeh Ur Rehman
Wajeeh Ur Rehman Raheel Ahmed
Raheel Ahmed