Abstract
Objective:
Kawasaki disease is an acute immune vasculitis that often has a poor prognosis when complicated by coronary artery lesions. Our study aims to construct a risk model for Kawasaki disease complicated by coronary artery lesions and validate it in different clinical characteristic subgroups, optimizing personalized and precise management of Kawasaki disease to improve patient outcomes.
Methods:
First, we compared each factor between the groups with and without coronary artery damage. We then used LASSO analysis to further filter for factors that were more significant in predicting outcomes. The selected factors were used to construct the risk model. The model was evaluated using ROC curves, calibration curves, and DCA, and was internally validated using 5-fold cross-validation. Finally, we also conducted subgroup analyses based on factors such as age stages and sex.
Results:
Through univariate analysis, LASSO analysis, and correlation analysis, we identified WBC, PLT, CRP, ALB, Na, Time to IVIG treatment, and symptoms of limb as the key factors for constructing the risk model. The model achieved an area under the curve of 0.815(95%CI: 0.779–0.851). Additionally, calibration curves, DCA, and 10-fold cross-validation demonstrated that the model has good predictive performance. The predictive efficacy of the model was also satisfactory across various subgroups.
Conclusions:
Our study has constructed a risk model for Kawasaki disease complicated by coronary artery lesions in the Chinese population that demonstrates good predictive performance, and it has been validated successfully across multiple subgroups.
1 Introduction
Kawasaki disease (KD) is an acute immune-mediated vasculitis that primarily affects children under the age of five. KD is most prevalent in East Asian countries such as China and Japan. Approximately 25% of KD patients experience the most severe complication, coronary artery lesions (CAL) (1). CAL refers to the inflammation and fibrosis of the coronary artery intima caused by Kawasaki disease, leading to arterial stenosis, thrombosis, and subsequent myocardial ischemia (2). CAL is the leading cause of mortality in KD cases. Currently, the diagnosis of KD relies mainly on clinical symptoms such as the duration of fever, mucosal changes, and limb edam, which can lead to delayed or missed diagnoses (3, 4). This is particularly concerning in atypical KD cases, where delays in acute-phase treatment may increase the risk of CAL. Early prediction of whether children with Kawasaki disease will develop coronary artery lesions is crucial, as it can facilitate timely interventions and treatment to mitigate or prevent further coronary artery damage.
However, the pathogenesis of KD remained incompletely understood, making the prediction of its complication, CAL, a challenging area. Studies demonstrated that younger children were more prone to developing CAL (5, 6). Infants and toddlers, in particular, faced a higher risk due to their smaller coronary arteries, which were more susceptible to inflammation. Additionally, research identified certain clinical features and laboratory indicators in the early stages of the disease as associated with the risk of coronary artery lesions in KD patients. These included prolonged high fever, elevated white blood cell counts, increased platelet counts, and significantly elevated CRP levels (7–10).
As a systemic inflammatory condition, KD presents significant clinical variability and prognostic heterogeneity due to diverse patient characteristics. Factors such as gender, age, ethnicity, and disease duration can substantially influence disease progression and treatment response (11). Studies utilizing PCA analysis have identified multiple subgroups within KD, revealing significant differences in CAL outcomes among these subgroups. Therefore, our study aimed to develop a risk model for CAL in KD and validate it across different clinical characteristic subgroups. The reproducibility and universality of these subgroups are still needed. This approach aided not only in identifying clinical features and predictive efficacy for specific patient populations but also provided important insights for the development of personalized treatment strategies. Gaining a deeper understanding of the differences among these subgroups may have optimized the management of Kawasaki disease and improved patient prognosis.
2 Materials and methods
2.1 Participants
Our study retrospectively collected clinical data from 1,795 cases of Kawasaki disease diagnosed at Kunming Children's Hospital between December 2014 and December 2023. These cases represent an expansion based on previous research. All cases met the criteria for Kawasaki disease established by the American Heart Association (AHA). The study was conducted in accordance with the principles of the Declaration of Helsinki and received approval from the Ethics Committee of Kunming Children's Hospital (Ethics Approval No.: 2023-05-016-K01).
2.2 Diagnostic and inclusion/exclusion criteria
Diagnostic Criteria: All cases in our study conformed to the AHA standards for Kawasaki disease, which include complete Kawasaki disease (CKD) and incomplete Kawasaki disease (IKD). Coronary artery lesions (CAL) were defined as a maximum Z-score of the coronary arteries greater than 2.5 within 60 days of onset (12). IVIG non-response was defined as persistent fever above 38°C after 36 h post-standard initial treatment, or recurrent fever within two weeks after initial treatment accompanied by at least one major clinical manifestation of Kawasaki disease, after excluding other potential causes of fever.
Inclusion Criteria: (1) Hospitalized children diagnosed with Kawasaki disease according to the “Expert Consensus on the Diagnosis and Acute Treatment of Kawasaki Disease”; (2) Complete clinical data available for KD patients. Exclusion Criteria: (1) Patients with underlying conditions such as cardiovascular diseases, liver diseases, kidney diseases, hematologic disorders, immune system diseases, and endocrine or metabolic genetic disorders; (2) Children with a history of previous Kawasaki disease; (3) KD patients who received IVIG, aspirin, or glucocorticoids prior to hospitalization; (4) KD patients with incomplete clinical data; (5) CAL occurred before IVIG treatment.
2.3 Inclusion factors
The data for our study originated from the results of examinations conducted upon initial hospitalization before any treatment was implemented. The included variables comprised three demographic characteristics (age at onset, gender, and ethnicity), ten laboratory results (hemoglobin (HB), white blood cell count (WBC), platelet (PLT), percentage of neutrophils (N), percentage of lymphocytes (L), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), alanine aminotransferase (ALT), total bilirubin (TBIL), gamma-glutamyl transferase (GGT), Albumin (ALB), Globulin (GLB), Bile Acid, Potassium (K), Sodium (Na), Chloride (Cl), Magnesium (Mg), Phosphorus (P), Calcium (Ca), Prothrombin Time (PT), Activated Partial Thromboplastin Time (APTT), Fibrinogen (Fbg), Thrombin Time (TT), D-dimer (D.dimer), Immunoglobulin A (IgA), Immunoglobulin M (IgM), and Immunoglobulin G (IgG)), and five physical examination findings (oral mucosal involvement, conjunctival injection, cervical lymphadenopathy, symptoms of limb, and rash). Additionally, data on the number of days with fever (Fever days), days of intravenous immunoglobulin (IVIG) use (Time to IVIG treatment), and echocardiography were included.
2.4 Model construction
Initially, all cases were divided into two groups: CAL and nCAL, based on the occurrence of CAL. Univariate statistical analyses were performed on clinical data between the two groups, and variables with P < 0.05 were included in subsequent analyses. Next, we used the “glmnet” R package to perform Least Absolute Shrinkage and Selection Operator (LASSO) to further filter the factors that are more important for predicting the outcome. LASSO is a regularization method for linear regression. It achieves variable selection and model simplification by introducing an L1 regularization term into the loss function. The main advantages of LASSO include its ability to shrink the coefficients of unimportant variables to zero, thereby retaining only the key variables, reducing model complexity and preventing overfitting. During the selection process, 5-fold cross-validation was used to select the optimal lambda value, namely lambda.min or lambda.1se. A larger lambda indicates a stronger regularization effect of the model, resulting in fewer selected independent variables. Variables were selected based on the optimal and maximum lambda values. Additionally, we performed correlation analysis on the selected factors using the “corrplot” R package to avoid multicollinearity among highly correlated factors. Subsequently, we utilized the “rmda” R package to conduct multifactorial logistic regression analysis, retaining the optimal model factors as our final key factors. A nomogram of the optimal model was created using the “rms” R package.
2.5 Model evaluation and validation
We initially evaluated the model using the “pROC” R package to plot the receiver operating characteristic (ROC) curve, with the area under the curve (AUC) representing the effectiveness of the model. Calibration of the model was assessed using calibration curves generated by the “ResourceSelection” R package, and decision curve analysis (DCA) was performed using the “decision_curve” function to further evaluate the model's accuracy. Finally, we conducted 5-fold cross-validation using the createFolds function.
2.6 Subgroup analysis
To evaluate the predictive performance of the model across different subgroups of Kawasaki disease, we categorized the 1,795 KD patients into multiple subgroups. These included sex (male and female), age stages (Infancy, Toddlerhood, Preschool age, Reach 7 years), ethnicity (Ethnic minorities, Han ethnicity), diagnostic criteria based on typical features (IKD and CKD), duration of fever (less than 5 days and 5 days or more), and illness duration in relation to the common increase in platelet count (illness duration less than 1 week and reaching 1 week). We calculated the AUC for each subgroup to assess the model's predictive value.
2.7 Statistical analysis
All statistical analyses were performed using R version 4.4.1. For normally distributed continuous data, means were expressed as mean ± sd and independent samples t-tests were used for comparisons between groups. Non-normally distributed data were described as median (P25, P75), and comparisons were conducted using non-parametric rank-sum tests. Categorical data were expressed as rates, with comparisons between groups performed using chi-square tests or Fisher's exact probability method. A p < 0.05 was considered statistically significant.
3 Results
3.1 Clinical characteristics description and comparison of two groups
Our study included a total of 2,686 KD patients. Among them, 12 cases met exclusion criterion (1); 27 cases met exclusion criterion (2); 589 cases met exclusion criterion (3); 433 cases met exclusion criterion (4); and 116 cases met exclusion criterion (5). In the end, a total of 1,795 Kawasaki disease patients who met the diagnostic and inclusion/exclusion criteria with complete clinical data were included. Among the included patients, 1,130 (62.95%) were male and 665 (37.05%) were female. The majority of patients were of Han ethnicity, with 1,524 cases (84.90%), while 271 cases (15.10%) were from ethnic minorities. The average age of the patients was 2.62 years. Based on whether the Z-score was less than 2.5, patients were divided into the CAL group (195 cases, 10.86%) and the nCAL group (1,600 cases, 89.14%).
A comparison of clinical data between the CAL and nCAL groups revealed significant statistical differences in various parameters, including WBC, PLT, ESR, CRP, ALB, Na, fever days, Time to IVIG treatment, HB, PLR, ALT, GGT, bile acid, Ca, IgA, IgM, and symptoms of limb (Table 1). Notably, the type of KD was not included in subsequent analyses as a clinical characteristic.
Table 1
| Factor | Total (n = 1,795) | CAL (n = 195) | nCAL (n = 1,600) | p |
|---|---|---|---|---|
| Age | 2.62 ± 2.01 | 2.88 ± 2.39 | 2.59 ± 1.95 | 0.11 |
| WBC | 14.19 ± 5.37 | 18.39 ± 9.03 | 13.68 ± 4.47 | <0.001 |
| PLT | 359.24 ± 127.04 | 474.49 ± 180.88 | 345.2 ± 111 | <0.001 |
| N | 61.49 ± 17.38 | 63.19 ± 16.95 | 61.28 ± 17.43 | 0.14 |
| L | 28.81 ± 14.82 | 27.32 ± 14.66 | 28.99 ± 14.84 | 0.136 |
| ESR | 60.92 ± 28 | 66.1 ± 28.82 | 60.29 ± 27.84 | 0.008 |
| CRP | 77.6 ± 50.42 | 107.08 ± 57.98 | 74.01 ± 48.22 | <0.001 |
| ALB | 34.89 ± 3.74 | 32.64 ± 4.88 | 35.16 ± 3.47 | <0.001 |
| GLB | 26.25 ± 5.99 | 26.02 ± 5.27 | 26.28 ± 6.08 | 0.52 |
| Na | 135.38 ± 3.23 | 132.74 ± 4.22 | 135.7 ± 2.93 | <0.001 |
| Cl | 99.5 ± 4 | 99.54 ± 3.94 | 99.49 ± 4.01 | 0.857 |
| Mg | 0.89 ± 0.15 | 0.88 ± 0.13 | 0.89 ± 0.15 | 0.623 |
| PT | 12.8 ± 3.19 | 12.7 ± 3.3 | 12.81 ± 3.18 | 0.651 |
| APTT | 32.64 ± 7.19 | 32.58 ± 7.25 | 32.65 ± 7.19 | 0.901 |
| Fbg | 5.28 ± 1.42 | 5.3 ± 1.31 | 5.27 ± 1.44 | 0.759 |
| TT | 16.64 ± 2.75 | 16.35 ± 2.41 | 16.68 ± 2.79 | 0.081 |
| D-dimer | 0.51 ± 6.25 | 0.36 ± 1.23 | 0.52 ± 6.61 | 0.381 |
| Fever days | 5.65 ± 2.13 | 6.6 ± 2.71 | 5.53 ± 2.01 | <0.001 |
| Time to IVIG treatment | 6.17 ± 1.85 | 7.08 ± 2.5 | 6.06 ± 1.72 | <0.001 |
| HB | 113 (105–121) | 110 (101–118.5) | 113 (106–121) | <0.001 |
| NLR | 2.38 (1.35–4.16) | 2.8 (1.46–4.6) | 2.34 (1.34–4.11) | 0.104 |
| PLR | 101.83 (69.65–147.19) | 118.11 (82.65–170.86) | 99.9 (68.46–144.58) | <0.001 |
| ALT | 28.7 (15–67.45) | 43 (20.5–92.5) | 27 (14–64) | <0.001 |
| GGT | 37 (16–94) | 57 (26–129) | 34 (15–90) | <0.001 |
| TBIL | 9.2 (6.5–17.4) | 9.2 (6.2–30.35) | 9.2 (6.5–16.5) | 0.526 |
| Bile Acid | 7.3 (4.8–11.4) | 8.2 (5.3–12.8) | 7.1 (4.8–11.3) | 0.037 |
| K | 4.3 (3.8–4.8) | 4.2 (3.8–4.8) | 4.3 (3.8–4.8) | 0.386 |
| P | 1.39 (1.17–1.58) | 1.38 (1.1–1.55) | 1.39 (1.17–1.58) | 0.121 |
| Ca | 2.23 (2.13–2.33) | 2.21 (2.1–2.3) | 2.23 (2.13–2.33) | 0.007 |
| IgA | 0.7 (0.4–0.96) | 0.77 (0.46–1.05) | 0.69 (0.4–0.95) | 0.048 |
| IgM | 1.2 (0.84–1.42) | 1.2 (0.98–1.45) | 1.18 (0.82–1.42) | 0.043 |
| IgG | 6.9 (4.9–9.18) | 7.3 (5.36–9.18) | 6.81 (4.88–9.18) | 0.061 |
| Sex | 0.224 | |||
| Female | 665 | 64 (32.82) | 601 (37.56) | |
| Male | 1,130 | 131 (67.18) | 999 (62.44) | |
| Ethic | 0.39 | |||
| No | 1,524 | 161 (82.56) | 1,363 (85.19) | |
| Yes | 271 | 34 (17.44) | 237 (14.81) | |
| Oral mucosal involvement | 0.999 | |||
| No | 326 | 35 (17.95) | 291 (18.19) | |
| Yes | 1,469 | 160 (82.05) | 1,309 (81.81) | |
| Conjunctival injection | 0.463 | |||
| No | 247 | 23 (11.79) | 224 (14) | |
| Yes | 1,548 | 172 (88.21) | 1,376 (86) | |
| Rash | 0.491 | |||
| No | 501 | 59 (30.26) | 442 (27.62) | |
| Yes | 1,294 | 136 (69.74) | 1,158 (72.38) | |
| Cervical lymphadenopathy | 0.107 | |||
| No | 846 | 103 (52.82) | 743 (46.44) | |
| Yes | 949 | 92 (47.18) | 857 (53.56) | |
| Symptoms of limb | <0.001 | |||
| No | 936 | 73(37.44) | 863(53.94) | |
| Yes | 859 | 122(62.56) | 737(46.06) |
Comparison of clinical data between the CAL and nCAL groups.
3.2 Screening for key factors
To ensure the operational simplicity of the risk model, the aforementioned 13 factors were included in a LASSO analysis to identify those with greater predictive value. In the LASSO analysis, we selected the optimal λ value of 0.0244 through 10-fold cross-validation. A larger λ value indicates a higher degree of regularization for the model, leading to a reduced number of selected independent variables. Ultimately, we filtered out WBC, PLT, CRP, ALB, Na, Time to IVIG treatment, and symptoms of limb (Figures 1A,B). Therefore, these 7 factors were included in the subsequent model construction.
Figure 1
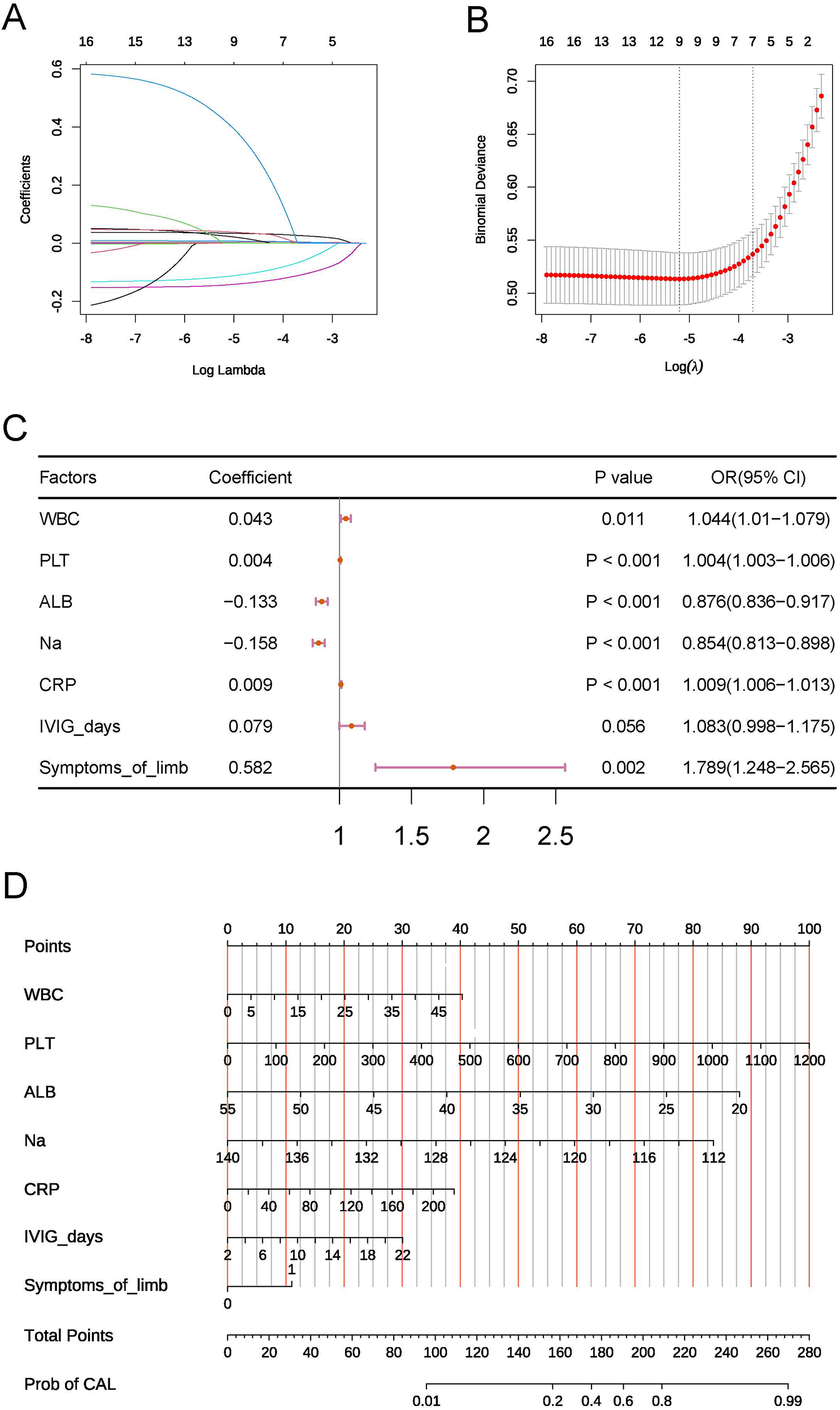
(A,B) Key variables selected by LASSO. (C) Forest plot of the multivariable logistic regression model. (D) Nomogram of the risk model.
To address potential multicollinearity, correlation analysis was performed on the seven key factors. Pearson correlation coefficients among all factors were below 0.5 (maximum absolute value = 0.34), indicating a low risk of multicollinearity bias.
3.3 Construction and evaluation of the risk model
The seven key factors were incorporated into a multifactorial logistic regression analysis model, and higher WBC, higher PLT, higher CRP, lower ALB, lower serum Na ion levels, longer Time to IVIG treatment, and the presence of symptoms of limb were identified as independent risk factors for KD complicated by CAL (Figure 1C). We also constructed a nomogram to visualize the risk model (Figure 1D).
To assess the predictive efficacy of the model, we first plotted the ROC curves for the seven independent risk factors, with PLT showing the highest AUC value of 0.732 (Figure 2A). We then plotted the ROC curve for the multifactorial logistic regression model, which yielded an AUC value of 0.819 (95% CI: 0.783–0.854), an accuracy of 0.911, a sensitivity of 0.282, and a specificity of 0.988 (Figure 2B). The calibration curve demonstrated satisfactory consistency for the CAL risk prediction model (Figure 2C), and the DCA further confirmed the model's accuracy (Figure 2D). To validate the predictive performance of the model, we conducted 5-fold cross-validation, which indicated that the constructed model has good predictive efficacy (Figure 2E).
Figure 2
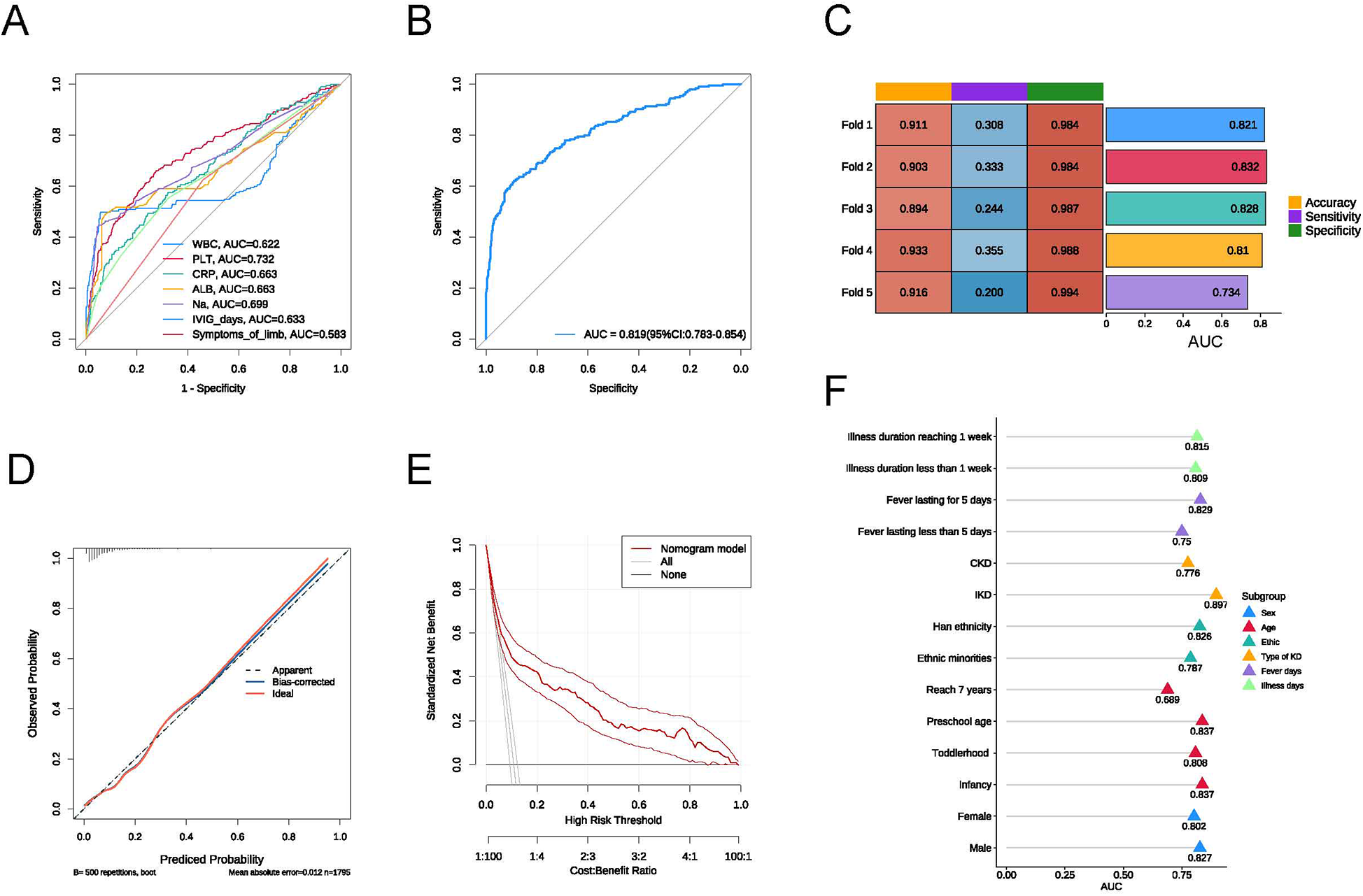
(A) ROC curve of the risk model; (B) ROC curves for individual factors: WBC, PLT, CRP, ALB, Na, time to IVIG treatment, and symptoms of limb; (C) results of the 5-fold cross-validation; (D) calibration curve of the risk model; (E) DCA curve of the risk model; (F) AUC values for each subgroup.
3.4 Subgroup analysis
To assess the predictive performance of our model under different conditions, we conducted subgroup analyses based on sex, age, ethnicity, type of KD, fever days, and illness duration. In each subgroup, the model exhibited good predictive value (Figure 2E). The AUC for illness duration reaching 1 week (0.815) was higher than the AUC for illness duration less than 1 week (0.809); the AUC for KD with fever lasting for 5 days (0.829) was higher than that for fever lasting less than 5 days (0.750); the AUC for IKD (0.897) was significantly higher than the AUC for CKD (0.776); the AUC for Han ethnicity (0.826) was higher than the AUC for ethnic minorities (0.787); and the AUC for males (0.827) was higher than the AUC for females (0.802). Among the various pediatric stages, the AUC for infancy was 0.837, for toddlerhood was 0.808, for preschool age was 0.837, and the lowest AUC for children reaching 7 years was 0.689.
4 Discussion
KD is an acute immune vasculitis, and its mechanism remains unclear. As the incidence of CAL increased, early prediction and precise treatment of KD with varying characteristics became increasingly important. Our study conducted a retrospective analysis of the demographic, clinical, and laboratory features of 1,795 KD patients. We constructed a multifactor logistic regression model to predict CAL after variable selection using the LASSO and performed 5-fold cross-validation. We also conducted subgroup validation analyses based on different clinical characteristics. Compared to previous studies, our research utilized a larger KD cohort for model construction while addressing the heterogeneity between KD clinical features and outcomes through subgroup validation.
CAL is a serious complication of KD and a leading cause of acquired heart disease in children. The early pathology of CAL involves acute, self-limiting necrotizing arteritis, typically manifesting within two weeks of KD onset. This condition manifests as neutrophilic inflammation of the vascular endothelium, leading to damage of the intima and other vascular layers (13). Various cytokines and PLT adhere at the injury site, forming saccular aneurysms, which increase the risk of aneurysm rupture or thrombosis. Identifying relevant predictive factors in KD with CAL is crucial. Our study identified WBC, PLT, CRP, ALB, Na, Time to IVIG treatment, and symptoms of limb as significant predictors of CAL. Elevated WBC levels reflected an inflammatory state in the body, indicating exacerbated vascular inflammation and increased CAL risk. Early in KD, PLT counts usually remained within normal ranges or were slightly elevated. However, as the disease progressed, particularly during the second to third week, PLT counts often rose significantly. Previous studies also indicated that elevated PLTs could serve as an independent risk factor for CAL in KD. This may result from vascular inflammation and injury in KD patients, which leads to PLT aggregation at the sites of inflammation and injury (14, 15). When coronary arteries become inflamed, PLTs aggregate extensively, releasing inflammatory mediators such as PLT factors, inflammatory cytokines, and adhesion molecules (16). The massive activation of PLTs may also lead to functional abnormalities, making them more likely to adhere to damaged vascular endothelial cells, thus perpetuating and exacerbating vascular inflammatory responses, further increasing CAL incidence. Notably, PLTs and leukocytes interact, further promoting inflammatory responses and cellular infiltration, exacerbating the degree of vascular inflammation. CRP, the most common inflammatory marker, rises rapidly following inflammation or infection due to the release of cytokines and interleukins that stimulate the liver to synthesize and release CRP (5, 17). Higher CRP levels in KD often indicate more severe vascular inflammation, which increases the likelihood of coronary artery damage. Previous studies found that CAL patients had significantly higher CRP levels than those without CAL (17, 18). IVIG, the first-line treatment for KD, significantly reduced the incidence of CAL; however, a small number of patients still experienced CAL (19–21). Recent studies have also found that hyponatremia is a risk factor for the occurrence of CAL in KD (22). During the acute phase of KD, high levels of cytokines such as IL-6 and TNF-α can directly stimulate the hypothalamus to release antidiuretic hormone, leading to increased renal water reabsorption. This “dilutional hyponatremia” reflects the intensity of the systemic inflammatory response, and severe inflammation is a core trigger for CAL. Additionally, inflammatory factors (such as IL-1β) can directly inhibit the sodium pump function of endothelial cells, resulting in intracellular sodium retention and cellular edema, which disrupts the integrity of the endothelial barrier. This allows lipoproteins and inflammatory cells to more easily infiltrate the vascular wall, promoting the development of coronary artery lesions. First, low albumin weakens antioxidant capacity and increases the release of pro-inflammatory factors (IL-6, TNF-α), which exacerbates endothelial damage and oxidative stress, leading to damage of the coronary artery wall (23). In addition, albumin deficiency promotes a hypercoagulable state (elevated fibrinogen) and microthrombus formation, while impaired NO metabolism triggers vasospasm, collectively aggravating the risk of coronary ischemia and aneurysm. Current research indicates that the timing of IVIG administration correlates closely with the occurrence and duration of CAL. Delayed or insufficient IVIG treatment may prevent timely control of inflammation, thereby increasing CAL risk. Symptoms of limb constitute a novel predictor for CAL in KD patients. Although the precise mechanisms underlying limb involvement in KD remain incompletely understood, we hypothesize that this association likely stems from multiple factors. First, limb changes, such as edema and erythema of the hands and feet, represent a readily apparent localized manifestation of systemic inflammation in KD. The presence of limb symptoms may indicate a more intense systemic inflammatory response, which, in turn, can lead to more severe coronary artery endothelial damage, a critical step in CAL progression. Second, immune complexes formed during KD can deposit in small vessels, including those in the extremities, leading to local vasculitis and tissue injury. These pathological changes may mirror similar processes occurring in coronary microvasculature, thereby promoting CAL development. Furthermore, the release of pro-inflammatory cytokines and vasoactive substances in KD can impair vascular function, resulting in vasoregulatory abnormalities in both the limbs and coronary arteries (10).
In the subgroup analysis, we found that our model had better predictive value for the subgroup characteristics. For the IKD subgroup, IKD is often misdiagnosed or treatment is delayed due to subtle clinical signs, which can easily lead to the occurrence of CAL. Our risk model can help in the early prediction of CAL risk in children suspected of having IKD. It can heighten our vigilance for high-risk populations with CAL, guiding pediatricians to initiate treatment promptly and closely monitor coronary changes in KD patients (24–26). In all age stages of children, the predictive performance of our model was suboptimal for patients over seven years old. This may be due to the relatively mature immune system of children over seven, with more developed immune regulatory mechanisms (such as Treg cell function) that can effectively suppress excessive inflammation (like IL-1β and TNF-α storms) and reduce persistent endothelial damage. Additionally, with increasing age, the proportion of smooth muscle layer and collagen/elastin fibers in the coronary artery wall increases, enhancing vascular mechanical strength and the ability to resist inflammatory damage. In contrast, the vascular walls of infants and toddlers are thinner and have fewer elastic fibers, making them more susceptible to dilation or aneurysm due to inflammation. Moreover, for younger children, symptoms are often not promptly communicated to parents, leading to delays in diagnosis and treatment. This prolonged inflammatory response increases the risk of vascular damage. Additionally, younger patients have underdeveloped vasculature, which weakens their immune resistance and repair capacity, making them more vulnerable to severe vascular damage (27, 28). Therefore, our risk model is particularly effective in identifying high-risk CAL in younger children, especially those under one year of age. It can aid in the early prevention of CAL occurrences and improve long-term outcomes.
In prior studies in China, WBC, PLT, NT-proBNP, DD, ALB, and T cell subsets effectively predicted KD complications like CAL with delayed IVIG treatment (16, 23, 29). Higher WBC and PLT, lower ALB matched our findings, but IVIG was given later in our study, possibly due to delayed recognition of KD in lower-tier hospitals. Our study analyzed Na and other electrolytes (K, Cl, Mg, P, Ca), finding hyponatremia a key predictor for CAL. We identified both hyponatremia and limb symptoms as predictive factors, linking systemic metabolic disturbances to localized vascular inflammation, addressing a gap in CAL prediction related to electrolyte imbalances and clinical symptoms. Though IgA and IgM were not included in the risk model, they were higher in KD patients with CAL, suggesting stronger immune inflammation may lead to CAL. In summary, our study combines indicators of inflammation (CRP and WBC), immune metabolism (ALB and PLT), and treatment (Time to IVIG treatment), laying groundwork for a standardized clinical predictive system.
This retrospective, single-center study is subject to limitations, including potential recall bias. The lack of external validation across diverse regions, ethnicities, and time points also restricts the generalizability of our findings and may impact the predictive performance of the model in broader clinical settings. To address these limitations and enhance the reliability and applicability of future models, we recommend prioritizing large-scale, multi-center, prospective studies incorporating diverse populations.
5 Conclusions
In conclusion, our study developed a risk model with good predictive efficacy for CAL in KD patients in China. It also provided robust subgroup validation across age, sex, ethnicity, KD type, fever duration, and disease course. This model serves as a theoretical basis for individualized precision treatment of KD, aiming to improve outcomes and protect the cardiovascular health of KD patients.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of Kunming Children's Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants' legal guardians/next of kin because our study is a retrospective collection of case data from the case management system, and therefore, we obtained a waiver of informed consent from the ethics committee.
Author contributions
CG: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. ZS: Conceptualization, Data curation, Writing – original draft. QL: Data curation, Methodology, Writing – original draft. HL: Data curation, Writing – original draft. ZW: Conceptualization, Data curation, Methodology, Writing – original draft. HG: Methodology, Software, Writing – review & editing. YL: Investigation, Software, Writing – review & editing. XL: Conceptualization, Data curation, Funding acquisition, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. LD: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Construction and Application Demonstration of a Smart Medical Platform-Based System for Intelligent Diagnosis and Treatment of Pediatric Diseases (202102AA100021), Kunming Health Science and Technology Talent Training Project for the backup talent cultivation plan in the field of medical technology (2023-SW(Backup)-50), and The Yunnan Provincial Department of Education Scientific Research Fund Project (2023J0678).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
McCrindle BW Rowley AH Newburger JW Burns JC Bolger AF Gewitz M et al Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. (2017) 135(17):e927–99. 10.1161/CIR.0000000000000484
2.
Chen Y Yang M Zhang M Wang H Zheng Y Sun R et al Single-Cell transcriptome reveals potential mechanisms for coronary artery lesions in Kawasaki disease. Arterioscler Thromb Vasc Biol. (2024) 44:866–82. 10.1161/ATVBAHA.123.320188
3.
Morana E Guida F Andreozzi L Frazzoni L Baselli LA Lami F et al Coronary arteries lesions in Kawasaki disease: risk factors in an Italian cohort. Biomedicines. (2024) 12:2010. 10.3390/biomedicines12092010
4.
Tanaka A Inoue M Hoshina T Koga H . Correlation of coronary artery abnormalities with fever pattern in patients with Kawasaki disease. J Pediatr. (2021) 236:95–100. 10.1016/j.jpeds.2021.05.020
5.
An HS Kim GB Song MK Lee SY Kwon HW Lee JW et al The occurrence of coronary artery lesions in Kawasaki disease based on C-reactive protein levels: a retrospective cohort study. Pediatr Rheumatol. (2021) 19:78. 10.1186/s12969-021-00566-6
6.
Gong C Liu K Li B Li Y Gao H Wang Z et al Analysis and validation of clinical subgroups of Kawasaki disease in children in China: a retrospective study. BMJ Paediatr Open. (2024) 8:e002650. 10.1136/bmjpo-2024-002650
7.
La Vecchia A Stracquadaino R Mauri L Baselli LA Abdallah R Cucchetti M et al Risk factors and scores for prediction of coronary artery aneurysms in Kawasaki disease: a European monocentric study. BMC Pediatr. (2024) 24:139. 10.1186/s12887-024-04623-3
8.
Liu M-Y Liu H-M Wu C-H Chang C-H Huang G-J Chen C-A et al Risk factors and implications of progressive coronary dilatation in children with Kawasaki disease. BMC Pediatr. (2017) 17:139. 10.1186/s12887-017-0895-8
9.
McCrindle BW Cifra B . The role of echocardiography in Kawasaki disease. Int J Rheum Dis. (2018) 21:50–5. 10.1111/1756-185X.13216
10.
Liu HH Chen WX Niu MM Jiang Q Qiu Z Fan GZ et al A new scoring system for coronary artery abnormalities in Kawasaki disease. Pediatr Res. (2022) 92:275–83. 10.1038/s41390-021-01752-8
11.
Wang H Shimizu C Bainto E Hamilton S Jackson HR Estrada-Rivadeneyra D et al Subgroups of children with Kawasaki disease: a data-driven cluster analysis. Lancet Child Adolesc Health. (2023) 7:697–707. 10.1016/S2352-4642(23)00166-9
12.
Damluji AA van Diepen S Katz JN Menon V Tamis-Holland JE Bakitas M et al Mechanical complications of acute myocardial infarction: a scientific statement from the American Heart Association. Circulation. (2021) 144:e16–35. 10.1161/CIR.0000000000000985
13.
Li S-M Liu W-T Yang F Yi Q-J Zhang S Jia H-L . Phosphorylated proteomics analysis of human coronary artery endothelial cells stimulated by Kawasaki disease patients serum. BMC Cardiovasc Disord. (2019) 19:21. 10.1186/s12872-018-0982-2
14.
Ae R Abrams JY Maddox RA Schonberger LB Nakamura Y Shindo A et al Platelet count variation and risk for coronary artery abnormalities in Kawasaki disease. Pediatr Infect Dis J. (2020) 39:197–203. 10.1097/INF.0000000000002563
15.
Wu R Jiang W Sun Y Wu L Di Y Wang J et al Indicators of oxidative stress in the prediction of coronary artery lesions in patients with Kawasaki disease. JCR J Clin Rheumatol. (2023) 29:126–31. 10.1097/RHU.0000000000001925
16.
Zhang C Chen L Chen S Bian Y Shen J Zhang P et al Predictive role of IL-2R and IL-10 in the anti-inflammatory response and antiplatelet therapy of Kawasaki disease: a retrospective study. Mediators Inflamm. (2022) 2022:1–13. 10.1155/2022/4917550
17.
Shuai S Zhang H Zhang R Tang M Luo E Yang Y et al Prediction of coronary artery lesions based on C-reactive protein levels in children with Kawasaki disease: a retrospective cohort study. J Pediatr (Rio J). (2023) 99:406–12. 10.1016/j.jped.2023.02.005
18.
Kim GB . Reality of Kawasaki disease epidemiology. Korean J Pediatr. (2019) 62:292–6. 10.3345/kjp.2019.00157
19.
Zeidman LA Fahey CD Grinblatt DL Harsanyi K . Immunoglobulin for Concurrent Guillain-Barré and immune thrombocytopenic Purpura. Pediatr Neurol. (2006) 34:60–2. 10.1016/j.pediatrneurol.2005.06.012
20.
Kitoh T Ohara T Muto T Okumura A Baba R Koizumi Y et al Increased pentraxin 3 levels correlate with IVIG responsiveness and coronary artery aneurysm formation in Kawasaki disease. Front Immunol. (2021) 12:624802. 10.3389/fimmu.2021.624802
21.
Balch A Wilkes J Thorell E Pavia A Sherwin CMT Enioutina EY . Changing trends in IVIG use in pediatric patients: a retrospective review of practices in a network of major USA pediatric hospitals. Int Immunopharmacol. (2019) 76:105868. 10.1016/j.intimp.2019.105868
22.
Masuda H Ae R Koshimizu T Matsumura M Kosami K Hayashida K et al Serum sodium level associated with coronary artery lesions in patients with Kawasaki disease. Clin Rheumatol. (2022) 41:137–45. 10.1007/s10067-021-05881-7
23.
Wang Y Xing J Gao H Zhao H Li M Liu Z . Predictive value of serum N-terminal pro-brain natriuretic peptide, D-dimer, albumin combined with T-cell subsets in detecting coronary artery damage in children with Kawasaki disease. Br J Hosp Med. (2025) 86:1–14. 10.12968/hmed.2024.0630
24.
Xu Y Yuan Y Mou L Hui L Zhang X Yao X et al scRNA + TCR-seq reveals the pivotal role of dual receptor T lymphocytes in the pathogenesis of Kawasaki disease and during IVIG treatment. Front Immunol. (2024) 15:1457687. 10.3389/fimmu.2024.1457687
25.
Zhang M Wang C Li Q Wang H Li X . Risk factors and an early predictive model for kawasaki disease shock syndrome in Chinese children. Ital J Pediatr. (2024) 50:22. 10.1186/s13052-024-01597-x
26.
Dai L Zhang L He J Huang R Tang W Guo H et al Diagnostic value of syndecan-1 for coronary artery lesions and correlation analysis of laboratory indicators in Kawasaki disease patients. Ital J Pediatr. (2024) 50:209. 10.1186/s13052-024-01772-0
27.
Blaney MM Williams RV Areinamo IA Sauer M Tani LY Ou Z et al The impact of the American Heart Association guidelines on patients treated for incomplete Kawasaki disease. Cardiol Young. (2022) 32:1066–70. 10.1017/S1047951121003632
28.
Shi H Qiu H Jin Z Li C Yang X Huang C et al Coronary artery lesion risk and mediating mechanism in children with complete and incomplete Kawasaki disease. J Investig Med. (2019) 67:950–6. 10.1136/jim-2018-000898
29.
Zhang X He Y Shao Y Hang B Xu Z Chu M . Factors affecting the duration of coronary artery lesions in patients with the Kawasaki disease: a retrospective cohort study. Pediatr Rheumatol Online J. (2021) 19:96. 10.1186/s12969-021-00589-z
Summary
Keywords
Kawasaki disease, nomogram, coronary artery lesions, cardiovascular diseases, children's diseases
Citation
Gong C, Su Z, Li Q, Li H, Wang Z, Gao H, Li Y, Liu X and Deng L (2025) A risk stratification model for coronary artery lesions in Kawasaki disease: focus on subgroup-specific utility. Front. Cardiovasc. Med. 12:1543767. doi: 10.3389/fcvm.2025.1543767
Received
17 December 2024
Accepted
16 April 2025
Published
29 April 2025
Volume
12 - 2025
Edited by
Hiroyuki Wakiguchi, Oita University, Japan
Reviewed by
Yoji Uejima, Saitama Children’s Medical Center, Japan
Kentaro Ueno, Kagoshima University Hospital, Japan
Updates
Copyright
© 2025 Gong, Su, Li, Li, Wang, Gao, Li, Liu and Deng.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Xiaomei Liu 1043443230@qq.com Lili Deng 676253354@qq.com
†These authors share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.