- 1Department of Cardiology Center, The First Hospital of Jilin University, Changchun, China
- 2Department of Endocrinology and Metabolism, The First Hospital of Jilin University, Changchun, China
Cardiovascular diseases (CVD) remain the primary cause of morbidity and mortality in developed countries, highlighting the urgent need to identify biomarkers associated with CVD and its risk factors. Vascular adhesion protein-1 (VAP-1), a 170 kDa surface molecule expressed predominantly by endothelial cells, smooth muscle cells, and adipocytes, has garnered significant attention in this field. Beyond its role in inducing inflammatory mediators, VAP-1 is closely linked to coronary artery disease, heart failure, diabetes, obesity, and other CVDs, along with their associated risk factors. Notably, elevated plasma VAP-1 activity has been observed in patients with CVD and diabetes. The toxic metabolites produced by its enzymatic activity contribute to vascular endothelial injury and oxidative stress, thereby accelerating atherosclerosis and diabetes-related cardiovascular complications. Consequently, understanding the pathophysiological roles of VAP-1 in CVD has become a major research focus. This review examines the effects of VAP-1 on CVD pathogenesis and explores the therapeutic potential of VAP-1 inhibitors in managing these conditions.
1 Introduction
Vascular adhesion protein-1 (VAP-1), also known as semicarbazide-sensitive amine oxidase (SSAO) or primary amine oxidase, belongs to the family of copper-containing amine oxidases. This protein was first identified in 1992 as a novel adhesion molecule capable of mediating endothelial cell-lymphocyte interactions, encoded by the amine oxidase copper-containing 3 (AOC3 gene, official gene nomenclature) (1). Notably, the SSAO family also encompasses AOC1 (encoding diamine oxidase) and AOC2 (encoding retina-specific amine oxidase), whereas VAP-1/SSAO specifically refers to the AOC3 gene product (2). SSAO is ubiquitously expressed in mammalian tissues and plasma, with interspecies variations in isoform composition, structure, and ligand specificity (3–6). Ruminants show the highest plasma SSAO activity, while human tissues display greater activity than rodent and porcine systems (7). The mature, functional form of VAP-1 is a heavily sialylated 170-kDa dimer that adopts a heart-shaped configuration, with each monomer containing three key domains (D2, D3, and D4) characteristic of dimeric proteins (8). Immunoblotting under non-reducing conditions primarily detects the 170-kDa dimer, which likely consists of two 90-kDa glycoprotein subunits, as reduction or boiling disrupts its integrity. In contrast, metabolic labeling studies also reveal a 90-kDa species, which may represent a monomeric or proteolytic fragment observed primarily in organ cultures (9, 10). Each subunit comprises an extracellular region containing the catalytic site, a transmembrane domain, and a short intracellular N-terminal tail that lacks known binding motifs, suggesting it may not play a role in signal transduction (8, 11). VAP-1 at the plasma membrane typically forms homodimers consisting of two ∼90 kD glycoproteins, with the extracellular portion of each monomer consisting of three structural domains (D2- D4) (9, 12). The D2 and D3 structural domains adopt the same α-β fold, characterized by alternating α-helices and β-strands. The large D4 domains in each subunit are catalytic domains containing residues involved in the modification of topaquinone and its localization, catalytic bases, and copper-coordinated histidine, which form the surface of the dimer and each of which also contains a catalytic site buried on the surface of the deep cleavage site (12). The catalytic activity of VAP-1/SSAO depends on key structural components, including a quinone cofactor, copper ions, and six glycosylation sites. Glycosylation, particularly near the catalytic entrance, influences substrate specificity (13). The molecule's sensitivity to oxidative agents is attributed to its incorporation of 2 mol copper ions, 1 mol carbonyl cofactors, and a 6-hydroxydopamine quinone residue per mol of protein (14).
Functionally, VAP-1 exhibits dual biological roles as both an amine oxidase and an adhesion molecule (15–17). Its oxidase activity catalyzes the oxidative deamination of short-chain primary amines, generating aldehydes, hydrogen peroxide (H2O2), and ammonia (16). In contrast, its adhesion molecule activity facilitates the selective binding of lymphocytes to vascular endothelium in a sialic acid-dependent manner (17). These dual roles are intricately linked to cardiovascular disease (CVD) pathogenesis.VAP-1 exists in one main form, a membrane-bound form, mainly on the surface of vascular endothelium, smooth muscle cells, and adipocytes (18–20). Additionally, studies have reported the presence of VAP-1 in myofibroblasts, identifying it as a novel biomarker (21). From a functional point of view, VAP-1 expressed in smooth muscle cells has SSAO activity and catalyzes exogenous and endogenous deamination of primary amines but does not have the ability to bind to lymphocytes (19). In contrast, VAP-1 localized on vascular endothelium possesses both adhesive functions and enzymatic activity (22, 23). In addition to the membrane-bound form of VAP-1, another form is soluble vascular adhesion protein-1 (sVAP-1), which is present in the blood circulation (24, 25). Membrane-bound VAP-1 is cleaved by matrix metalloproteinases (MMPs) to generate sVAP-1 (25–27). Both membrane-bound and soluble forms of VAP-1 exhibit similar enzymatic properties, sharing at least 80% sequence homology (24). However, it is believed that the membrane-bound form of VAP-1 exhibits higher activity than its soluble counterpart. The membrane-bound VAP-1 can rapidly relocate to the cell surface under acute inflammatory conditions to exert its functions, whereas sVAP-1 primarily accumulates at elevated concentrations in various chronic diseases with a relatively slower response (28, 29). Moreover, the deamination of endogenous substrates by membrane-bound VAP-1 can directly damage endothelial cells, enhance oxidative glycation, and increase oxidative stress mediators (16). In contrast, the effects of sVAP-1 are further constrained by its concentration in the bloodstream.
Elevated sVAP-1 levels have been documented in various CVDs and diabetes, including coronary artery disease, aortic stenosis, hypertension, heart failure, and stroke (30, 31). Furthermore, sVAP-1 is significantly elevated in patients with nonalcoholic fatty liver disease (NAFLD), suggesting its potential role as a pathogenic link between NAFLD and CVD (32). The widespread expression of VAP-1 within the vascular system underscores its pivotal role in inflammatory processes and its contribution to CVD pathophysiology. In the past decade, clinical studies have established VAP-1 as a novel biomarker for CVD and a promising therapeutic target. Understanding its specific mechanisms in disease progression may enable the development of innovative strategies for CVD management. This review focuses on the intricate relationship between VAP-1 and CVD, as well as the therapeutic potential of targeting VAP-1.
2 Pathophysiological mechanisms of VAP-1 in cardiovascular diseases
CVD represent a significant global health challenge, with atherosclerotic cardiovascular disease (ASCVD) being the primary contributor to mortality worldwide. The incidence and fatality rates of ASCVD, which encompasses coronary heart disease (CHD), ischemic stroke (IS), and peripheral vascular disease, continue to rise (33–35). Atherosclerosis, the pathological basis of ASCVD, leads to acute and chronic ischemic events due to progressive arterial lumen narrowing, plaque instability, intraplaque hemorrhage, rupture, and thrombus formation (36, 37). The growing burden of CVD, exacerbated by socioeconomic development and an aging population, has emerged as a major public health issue, with younger individuals increasingly affected (33–35). T cells, B cells, neutrophils, macrophages, and NK cells all play respective roles in CVD (particularly T cells and macrophages), and VAP-1 exerts a recruitment effect on all of them (22, 38–44). VAP-1, a multifunctional adhesion molecule, plays a crucial role in the pathogenesis of atherosclerosis. It exerts its effects through inflammation regulation, vascular endothelial damage induction, glucose and lipid metabolism modulation, and plaque stability alteration. Strong associations between VAP-1 and both the development and prognosis of ASCVD have been established in numerous studies.
The pathophysiological mechanisms of VAP-1 involvement in cardiovascular disease are depicted in Figure 1.
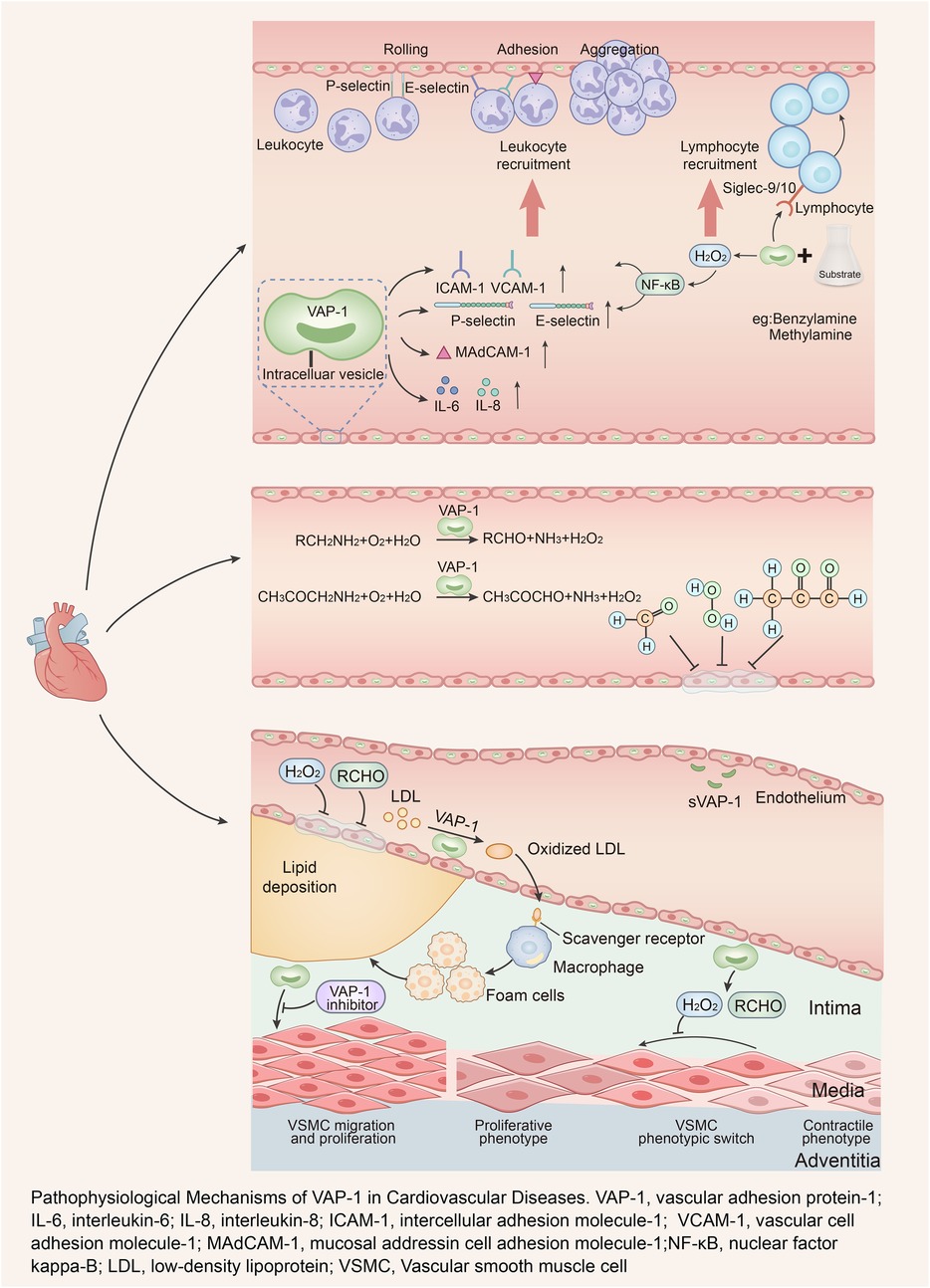
Figure 1. Pathophysiological mechanisms of VAP-1 in cardiovascular diseases. VAP-1, vascular adhesion protein-1; IL-6, interleukin-6; IL-8, interleukin-8; ICAM-1, intercellular adhesion molecule-1; VCAM-1, vascular cell adhesion molecule-1; MAdCAM-1, mucosal addressin cell adhesion molecule-1; NF-κB, nuclear factor kappa-B; LDL, low-density lipoprotein; VSMC, vascular smooth muscle cell.
2.1 VAP-1 in inflammatory responses
Atherosclerosis is fundamentally a chronic inflammatory disease, characterized by highly specific cellular and molecular reactions throughout its progression—from plaque initiation to instability (45, 46). VAP-1 significantly contributes to this process by mediating leukocyte adhesion, migration, and chemotaxis, thereby amplifying vascular inflammation. As an adhesion molecule, VAP-1 interacts with Siglec-9 and Siglec-10 receptors on lymphocytes to facilitate their binding to endothelial cells (2, 42, 47). Its SSAO activity further enhances the expression of adhesion molecules, including E-selectin, P-selectin, interleukin-6 (IL-6), interleukin-8 (IL-8), intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1), which collectively strengthen leukocyte-endothelial interactions (48–52). Moreover, VAP-1 promotes leukocyte migration to inflammatory sites by inducing the secretion of mucosal addressin cell adhesion molecule-1 (MAdCAM-1) and establishing hydrogen peroxide gradients in the extracellular matrix (53, 54). Leukocyte surface glycoproteins, belonging to the sialic acid adhesion molecule family, serve as ligands for VAP-1, forming transient covalent bonds with its amine groups to mediate leukocyte extravasation (42, 55). Inhibition of VAP-1 has been shown to reduce granulocyte adhesion, increase rolling velocity and jump frequency, and decrease CXCL1 and MAdCAM-1 levels, thereby mitigating leukocyte recruitment and migration (22, 51, 56, 57). The above mechanisms provide a theoretical basis for VAP-1-mediated leukocyte infiltration into plaques. Jaakkola et al. reported that VAP-1 expression was significantly elevated in coronary vessels surrounding myocardial infarction regions, with increased leukocyte extravasation observed (58). Additionally, blocking endothelial VAP-1 reduced granulocyte adhesion by 60% in ischemia-reperfusion models. These findings underscore the pivotal role of VAP-1 in leukocyte trafficking during atherosclerosis.
2.2 VAP-1-induced vascular endothelial injury
Endothelial dysfunction, a critical initiating factor in atherosclerosis, is driven by inflammation, immune responses, and oxidative stress (59, 60). VAP-1 contributes to endothelial injury through its enzymatic activity, which catalyzes the oxidative deamination of physiological substrates such as methylamine and aminoacetone. This process generates reactive byproducts, including formaldehyde, methylglyoxal (MGO), H2O2, and ammonia (61). Tobacco smoking and adrenal hormone hypersecretion constitute established cardiovascular risk factors, both of which promote increased systemic methylamine concentrations (62, 63). Elevated concentrations or enzymatic activity of VAP-1/SSAO have been observed in patients with tobacco use, atherosclerosis, hypertension, heart failure, aortic stenosis, coronary artery disease, and diabetes mellitus, potentially leading to enhanced conversion of methylamine and aminoacetone (64–71). Formaldehyde, a highly reactive and toxic byproduct, disrupts endothelial integrity and is implicated in atherosclerosis pathogenesis (61, 72). It reacts with amines or amides to form irreversible protein-DNA crosslinks (DPCs), impairing DNA structure and function (72). Inadequate repair of these crosslinks during DNA replication can result in aberrant gene expression, contributing to endothelial dysfunction and vascular stiffening (73, 74). In mice susceptible to AS, increased methylamine deamination was found to result in a significant increase in formaldehyde production, which further supports that VAP-1-mediated production of this toxin may contribute to the development of AS (75). Similarly, VAP-1-mediated MGO production is highly cytotoxic (76). Hyperglycemia, oxidative stress, inflammation, and exogenous sources of MGO all lead to elevated MGO levels and reduced glyoxalase 1 (Glo1) activity, and an MGO-Glo1 imbalance will lead to vascular dysfunction (77). Downregulation of Glo1 allows for the perpetuation of MGO accumulation, which has been linked to age-related diseases such as diabetes mellitus, obesity, central nervous system disorders, and cardiovascular diseases that are closely associated with endothelial dysfunction (77–79). MGO reacts mainly with arginine residues on proteins, of which methylglyoxal hydroimidazolone (MG-H1) is the most common MGO-derived AGE (advanced glycosylation end product) modification found in vivo, which leads to structural alterations, inactivation, and degradation of the target protein (80). Thus, elevated MGO levels impair endothelial function in various ways and in different regions of the organism. Another product generated by the catalytic reaction of VAP-1, H2O2, can not only be converted to oxygen free radicals, resulting in elevated levels of oxidative stress, which in turn damages endothelial cells and contributes to the pathogenesis of a variety of cardiovascular diseases (81). In addition, H2O2 can co-modify various proteins with aldehydes and glucose to produce advanced glycated end products (AGEs) (82, 83), which are important factors leading to atherosclerosis (84). In transgenic mice with endothelial-specific overexpression of human VAP-1/SSAO, increased formation of AGEs in glomeruli and upregulation of AGE receptor expression were observed (26). Clinical studies have further confirmed that serum VAP-1 levels show significant positive correlations with systemic oxidative stress (ROS) and AGE levels in humans (85). Collectively, these mechanisms highlight the detrimental impact of VAP-1's enzymatic byproducts on vascular endothelial health, reinforcing its critical role in atherosclerosis development.
2.3 VAP-1 in the regulation of glucose and lipid metabolism
Disturbances in glucose and lipid metabolism are well-established major risk factors for atherosclerosis (86). The role of VAP-1 in regulating glucose uptake has been a major focus of translational medical research. Research has shown that VAP-1 substrates, such as benzylamine, combined with low-dose vanadate, improve glucose tolerance by enhancing glucose transporter-4 (GLUT-4) expression in adipocytes and reducing hyperglycemia (87). In type 2 diabetes models like Goto-Kakizaki rats, both acute and chronic administration of benzylamine (with vanadate) stimulated glucose utilization in adipocytes and muscle cells, upregulated GLUT-4 expression, and alleviated insulin resistance in muscle tissue (88). In vitro experiments using liver slice models developed by Karim et al. (89) demonstrated an oxidase activity-dependent increase in both glucose uptake and GLUT-4 expression. However, transgenic mice overexpressing VAP-1 initially showed improved glucose tolerance, which was later offset by vascular complications typical of diabetes, such as glomerulosclerosis, atherosclerosis, and hypertension (26). Clinical studies have corroborated a positive correlation between fasting sVAP-1 levels and fasting blood glucose. Elevated sVAP-1 levels have also been observed during oral glucose tolerance tests, with levels peaking two hours after glucose loading, further correlating with fasting sVAP-1 levels (90). In cholesterol-rich diet-fed KKAy diabetic mice, SSAO inhibitors reduced body weight and atherosclerotic lesions (91, 92). Interestingly, VAP-1 knockout mice exhibited mild obesity and reduced leukocyte infiltration in adipose tissue but maintained normal blood glucose and glucose tolerance (93). These findings imply a role for VAP-1 in glucose regulation, though its precise mechanisms remain uncertain. One study proposed that VAP-1 exerts insulin-like effects by inducing Caveolin-1 mRNA expression and catalyzing endogenous amines to produce hydrogen peroxide. This activity was absent in adipocytes from AOC3 knockout mice (94–96). While elevated sVAP-1 levels under hyperglycemic conditions might confer hypoglycemic effects, conflicting data exist. For instance, studies using the VAP-1 inhibitor PXS-4728A reported reduced blood glucose levels in apolipoprotein E-deficient mice after 7 and 15 weeks of treatment (70). Therefore, the precise mechanisms underlying VAP-1-mediated regulation of blood glucose levels remain to be fully elucidated.
VAP-1 also plays a role in lipid metabolism. Hydrogen peroxide, a key product of its catalytic reactions, generates ROS (97, 98). These ROS oxidatively modify low-density lipoprotein (LDL), resulting in dysfunctional LDL that bypasses normal metabolic pathways and is absorbed by scavenger receptors, leading to foam cell formation and lipid accumulation within vascular walls (99–103). Metabolites of VAP-1 lead to endothelial cell damage, lipid deposition in the vessel wall, and ultimately, the formation of atherosclerotic lesions. A large clinical study found that MACROD2 gene expression could promote VAP-1 production in adipose tissue, and its level was positively correlated with body mass index (104), and higher levels of VAP-1 were also observed in obese mice, suggesting that obesity and related genetic factors may stimulate the production of VAP-1 in adipose tissue (94). VAP-1 inhibitors have demonstrated effects such as suppressed adipogenesis, reduced body weight, and lower cholesterol levels in mice. Conversely, AOC3 knockout mice showed increased weight, fat mass, and total cholesterol levels (96, 105–108). In addition, VAP-1 has been found to be negatively correlated with HDL cholesterol levels (104, 109). Notably, Weston et al. (32) demonstrated that genetic ablation of AOC3 attenuated the severity of hepatic steatosis in mice, whereas expression of enzymatically inactive VAP-1 failed to produce this protective effect. These results suggest that VAP-1 plays a multifaceted role in lipid metabolism, though further exploration is necessary. In summary, VAP-1 significantly influences glucose and lipid metabolism, both of which are integral to atherosclerosis development. However, its precise regulatory mechanisms remain to be fully elucidated.
2.4 VAP-1 in atherosclerosis
Vascular smooth muscle cells (VSMCs) play a critical role in maintaining atherosclerotic plaque stability. Reductions in VSMC numbers or phenotypic changes directly contribute to atherosclerosis progression (110–112). Current evidence suggests that inhibition of VAP-1 reduces monocyte adhesion and transendothelial migration, suppresses macrophage recruitment and activation, and decreases smooth muscle cell (SMC) migration and proliferation, thereby significantly attenuating the formation or progression of atherosclerotic lesions (70, 108). Studies in VAP-1 knockout mice have demonstrated increased VSMC content within plaques and a phenotypic shift from contractile to synthetic states, resulting in higher collagen deposition. This structural remodeling produces thicker fibrous caps, improving plaque stability (31, 113–115). Similarly, semicarbazide treatment in LDL receptor knockout (LDLr−/−) mice for 6–9 weeks yielded comparable improvements in plaque composition (113). Further research using PXS-4728A in cholesterol-fed rabbit models of atherosclerosis demonstrated that 12-week treatment reduced VSMC migration and proliferation (108). Discrepancies in study outcomes may stem from differences in animal models, inhibitor selection, or treatment durations. Overall, these findings underscore the therapeutic potential of targeting VAP-1 to improve atherosclerotic plaque stability and mitigate associated cardiovascular risks.
3 VAP-1 and coronary heart disease (CHD)
A large prospective cohort study demonstrated that elevated sVAP-1 levels are strongly associated with an increased risk of major adverse cardiovascular events (MACE), including CHD, unstable angina, acute myocardial infarction, coronary revascularization, and stroke (109). Serum VAP-1 independently predicts 10-year all-cause mortality, cardiovascular mortality, and cancer mortality in subjects with type 2 diabetes (116). Additionally, elevated sVAP-1 levels were linked to increased mortality risk. These findings suggest that plasma VAP-1 serves as a reliable biomarker for CHD presence and severity. Furthermore, inhibition of VAP-1 activity, such as through PXS-4728A, has been proposed as a potential therapeutic strategy for atherosclerosis management (31, 70). Clinical studies have consistently shown higher plasma VAP-1 levels in CHD patients compared to healthy controls, with a positive correlation between sVAP-1 concentrations and CHD severity (70). Elevated sVAP-1 levels were also associated with both the number and extent of coronary artery stenoses. Even after adjusting for confounding factors, sVAP-1 levels remained an independent predictor of CHD severity (OR = 2.09, 95% CI: 1.29–3.38; P = 0.003) (70). This highlights the potential of VAP-1 as a diagnostic and prognostic tool for CHD. Preclinical studies further validate the therapeutic potential of VAP-1 inhibition. In apolipoprotein E-deficient mice, VAP-1/SSAO inhibitors significantly reduced atherosclerotic plaque area in both preventive and therapeutic regimens. For instance, 15 weeks of PXS-4728A treatment alongside a high-fat diet reduced plaque formation to a degree comparable to atorvastatin (2.5 mg·kg−1·d−1 for 15 weeks) (70). These findings suggest that VAP-1 inhibitors may represent a novel class of therapeutics for CHD. The association between VAP-1 and calcific aortic stenosis (CAS) also underscores its clinical relevance. In patients undergoing aortic valve replacement, elevated VAP-1 mRNA expression was observed in calcified regions of the aortic valve (117, 118). Plasma VAP-1 levels were found to increase proportionally with CAS severity (69, 118), indicating its potential role in the progression of valvular diseases. Beyond CHD, VAP-1 has been implicated in hyperglycemia-induced atherosclerosis. Studies in low-risk populations have identified plasma VAP-1 concentrations as predictors of carotid intima-media thickness (IMT) and plaque formation (109, 119). Karadi et al. demonstrated that serum SSAO activity correlates with carotid artery stenosis severity and plaque scores in diabetic patients (120). In non-diabetic subjects, glucose loading during oral glucose tolerance tests increased sVAP-1 levels, which were independently associated with serum AGEs and carotid IMT (85). Aalto et al. conducted a longitudinal study involving 2,138 healthy individuals and reported a positive correlation between sVAP-1 activity, IMT, and carotid plaque formation. This suggests a potential role for sVAP-1 in subclinical atherosclerosis development (121). The therapeutic implications of VAP-1 inhibition extend beyond reducing atherosclerotic lesion size and inflammation to stabilizing plaques and preventing adverse clinical events. For example, in a rat model of myocardial ischemia-reperfusion injury, elevated myocardial SSAO activity was associated with increased leukocyte infiltration, endothelial P-selectin expression, and oxidative stress markers. Treatment with SSAO inhibitors—semicarbazide (a non-specific agent concurrently inhibiting both SSAO and Lysyl Oxidase), hydralazine (an irreversible SSAO inhibitor with additional inhibitory effects on aldehyde oxidase and NADPH oxidase), and LJP 1207 (a selective SSAO inhibitor)—significantly ameliorated the aforementioned pathological alterations and reduced infarct size (122). These findings emphasize the critical role of VAP-1/SSAO in CHD development and post-myocardial infarction tissue damage. While immune and inflammatory mechanisms are well-established contributors to CHD progression, most anti-inflammatory therapies have failed to demonstrate significant benefits in clinical trials. This highlights the need for precise inflammatory targets. Current evidence positions VAP-1 as a promising therapeutic target for CHD, offering new avenues for disease management and prevention.
4 VAP-1 and essential hypertension
Essential hypertension, a common cardiovascular disease among middle-aged and elderly individuals, is influenced by both environmental and genetic factors. It is a significant risk factor for severe clinical events such as coronary heart disease, heart failure, stroke, and end-stage renal disease. The disease is characterized by a high prevalence, insidious onset, low awareness rate, high disability rate, and the need for lifelong medication (123). Although the exact pathogenesis of essential hypertension remains unclear, most studies suggest it involves a chronic low-grade inflammatory process (124, 125). Inflammatory responses disrupt vascular microenvironment homeostasis by driving monocyte and macrophage infiltration during vascular injury. This triggers the secretion of adhesion molecules and other vascular active substances, promoting endothelial cell proliferation, migration, and differentiation. Such pathological vascular remodeling processes are integral to the development, progression, and complications of hypertension (126–128). Previous studies have suggested that inflammatory response increases the prevalence of hypertension and is closely related to the diagnosis, treatment, and prognosis of patients with hypertension, making it an important area of research in the pathogenesis of hypertension (129). Meanwhile, recent studies have shown that the increase in the levels of inflammatory factors precedes the increase in clinical blood pressure levels, which may have potentially important clinical applications in the prediction and diagnosis of hypertension (130). Maciorkowska et al. reported elevated circulating renin and VAP-1 levels in hypertensive patients with poorly controlled blood pressure (67). As a marker of systemic inflammation, VAP-1 is implicated in various inflammatory pathways (119, 131). Inhibition of VAP-1 has been shown to reduce levels of inflammatory mediators such as ICAM-1, MCP-1, and TNF-α, thereby alleviating inflammation (132). These findings suggest that VAP-1 may contribute to the development of hypertension via inflammatory mechanisms, potentially influencing its prevalence, diagnosis, and treatment. Additionally, excessive SSAO activity associated with VAP-1 may affect vascular elasticity by increasing elastin cross-linking and altering the structure of newly synthesized elastin. However, studies involving SSAO-deficient arteries have shown conflicting results. For instance, SSAO−/−mice exhibited increased arterial diameter with mechanical properties comparable to those of normal arteries, challenging the hypothesis that SSAO significantly alters elastic fiber organization and vascular responsiveness (27, 133). Overall, there is a strong correlation between VAP-1 and essential hypertension, likely mediated by inflammatory processes. Pharmacological targeting of VAP-1 may offer a novel approach to reducing inflammation and slowing hypertension progression. However, further research is needed to fully elucidate the role of VAP-1 in hypertension pathogenesis and to develop targeted therapeutic strategies.
5 VAP-1 and obesity
Obesity, a major risk factor for cardiovascular diseases, is closely linked to metabolic disorders such as diabetes and dyslipidemia (134–136). VAP-1 has garnered significant attention in obesity research due to its insulin-like effects and high expression on adipocyte membranes. In adipocytes, VAP-1 colocalizes with GLUT-4 within endosomal compartments, facilitating glucose uptake by stimulating GLUT-4 translocation to the plasma membrane (88, 137). VAP-1 enzymatic byproducts, including hydrogen peroxide, enhance glucose uptake and inhibit adipogenesis when combined with SSAO substrates and vanadate (138). These effects mimic insulin action. Jargaud et al. demonstrated that AOC3 knockout (AOC3KO) and VAP-1 mutant (AOC3KI) mice, which lack functional SSAO activity, exhibited greater fat deposition than controls, despite normal food intake (96). Thus, adipocyte-expressed VAP-1 deserves more attention in improving blood glucose and controlling obesity. While AOC3KO mice showed mild obesity (96), other studies reported a negative correlation between circulating sVAP-1 levels and obesity (90). Conversely, serum VAP-1 activity has been positively associated with body mass index (BMI) (139, 140). Increased SSAO activity in adipose tissue has been linked to low-grade inflammation observed in obesity and diabetic obesity (137). However, to date, there is no conclusive evidence of a link between VAP-1 and obesity. Current evidence indicates that VAP-1 expression is significantly upregulated in differentiated adipocytes compared to preadipocytes, paralleling an increase in enzymatic activity (20). In certain obese individuals, membrane-bound VAP-1 may remain localized within adipose tissue rather than being cleaved into circulation, potentially contributing to adipocyte proliferation and glucose transport while reducing circulating sVAP-1 levels (93, 121). However, the mechanisms governing sVAP-1 shedding and its regulation remain unclear and may depend on the severity of obesity and its associated comorbidities. Further research is required to elucidate the complex role of VAP-1 in obesity pathophysiology, particularly its contributions to adipose tissue inflammation and metabolic dysfunction.
6 VAP-1 and diabetes
Elevated plasma SSAO levels and activity are strongly associated with the onset and progression of diabetes. SSAO catalyzes the oxidative deamination of endogenous substrates such as methylamine and aminoacetone, producing toxic metabolites that directly damage vascular endothelial cells, promote glycation, and enhance oxidative stress. These processes exacerbate diabetes and contribute to its vascular complications. The primary mechanisms by which SSAO/VAP-1 influences diabetes pathogenesis involve oxidative stress and the formation of AGEs. Increased SSAO activity is closely linked to late-stage complications of diabetes, including atherosclerosis, retinopathy, and nephropathy. The link between sVAP-1 and diabetes was initially identified in individuals with type 1 diabetes (141, 142). sVAP-1 levels and SSAO activity were higher in individuals with type 1 diabetes, and plasma sVAP-1 was positively correlated with blood glucose (141, 142). Subsequently, plasma sVAP-1 was also found to be elevated in studies of type 2 diabetic patients compared to normal subjects (143). In addition, it has been shown that in patients with gestational diabetes, sVAP-1 levels are higher than in normoglycemic pregnant women (144). In the Taiwan Lifestyle Cohort Study, prediabetic individuals were found to have higher serum sVAP-1 levels compared to normoglycemic controls, further implicating sVAP-1 as a potential biomarker for early metabolic dysregulation (90). VAP-1 is also linked to diabetes complications such as retinopathy and nephropathy. Plasma VAP-1 levels correlate positively with vascular endothelial growth factor (VEGF) levels, which are elevated in diabetic retinopathy (132). Several studies suggest that VAP-1 is associated with the pathogenesis of diabetic retinopathy (30, 145, 146). Animal studies testing VAP-1 inhibitors have demonstrated therapeutic potential. For instance, RTU-1096 prevented retinal thickening in mice following laser photocoagulation, while the oral VAP-1 inhibitor 1H-imidazol-2-amine significantly reduced ocular permeability in diabetic rats (51, 147). In addition, several other studies have shown that VAP-1 is associated with the development of diabetic nephropathy (148–150). The VAP-1 inhibitor ASP8232 significantly reduced albuminuria in a phase 2 trial involving diabetic nephropathy patients (151). In patients with type 2 diabetes, we found that serum VAP-1 levels predicted the incidence of end-stage renal disease (ESRD). After adjusting for other risk factors, each standard deviation increase in serum VAP-1 was associated with a hazard ratio (HR) of 1.55 for ESRD risk (148). Beyond vascular complications, Valente et al. reported that VAP-1 levels were significantly elevated in hippocampal vessels of diabetic patients with Alzheimer's disease compared to those with Alzheimer's alone. This was accompanied by increased markers of oxidative stress, AGEs, and inflammation (152). Additionally, acute fluctuations in plasma VAP-1 levels have been observed in non-diabetic individuals during oral glucose tolerance tests, where SSAO/VAP-1 levels rose significantly 30 min post-glucose loading and remained elevated for 2 h. These changes correlated with systemic oxidative stress, AGEs, and carotid IMT, suggesting that VAP-1 could serve as a biomarker for hyperglycemia-induced atherosclerosis (85). These findings highlight the critical role of VAP-1 in diabetes onset and progression through its contributions to inflammation, oxidative stress, and AGE production. Targeting VAP-1 with inhibitors represents a promising therapeutic approach for diabetes and its complications.
7 VAP-1 and heart failure (HF)
HF, the terminal stage of many cardiovascular diseases, remains a leading cause of mortality worldwide. Epidemiological studies report high global prevalence and fatality rates for HF (153). In patients with chronic HF, Boomsma et al. found elevated plasma SSAO levels, with further increases observed in those with diabetes or more severe disease. These findings suggest that plasma SSAO may serve as a useful biomarker for assessing HF severity and prognosis (64). In a subsequent 3.4-year follow-up study of 372 patients, baseline plasma SSAO levels were significantly higher in those who died during the study period compared to survivors. This supports the role of SSAO as a prognostic marker for mortality in chronic HF (68). Similarly, Marinho et al. evaluated SSAO and monoamine oxidase (MAO) activity in patients with hypertensive heart disease and left ventricular systolic dysfunction (across NYHA HF classes II–IV). Both SSAO and MAO activity were significantly higher in patients than in controls, with the highest SSAO levels observed in NYHA class IV patients. These findings suggest that amine oxidases contribute to endothelial damage in HF pathogenesis (154). In addition, results from a prospective multicenter cohort study of patients undergoing hemodialysis showed that higher VAP-1 plasma levels were strongly associated with an increased likelihood of myocardial infarction, heart failure, cerebral infarction, cerebral hemorrhage, and other cardiovascular events (155). The role of VAP-1 in endothelial injury mechanisms during HF progression is particularly notable. Endothelial activation, especially in HF with preserved ejection fraction (HFpEF), plays a critical role in clinical HF. Therapies targeting endothelial activation may help prevent the progression of cardiovascular risk factors into overt HF (156). Given its involvement in leukocyte adhesion and inflammation, VAP-1 inhibition holds promise for improving HF prognosis. Despite strong evidence supporting its diagnostic and prognostic value, the specific mechanisms through which VAP-1 contributes to HF remain underexplored. Future research should investigate the therapeutic potential of VAP-1 inhibitors to better manage HF and enhance patient outcomes.
8 Potential of VAP-1 inhibitors as emerging therapeutics
Elevated VAP-1 activity is closely associated with the onset and progression of various diseases. By inhibiting VAP-1 activity, the oxidative deamination process can be suppressed, reducing the production of toxic metabolites and thereby minimizing endothelial damage, oxidative stress, and AGE formation. Research has shown that VAP-1 inhibition effectively decreases leukocyte extravasation and tissue inflammation (2, 56, 157, 158). VAP-1 inhibitors and anti-VAP-1 antibodies have demonstrated promising therapeutic potential in various animal models and inflammatory diseases. VAP-1's involvement in the pathogenesis of numerous conditions, such as rheumatoid arthritis, chronic liver inflammation and fibrosis, neuroinflammatory diseases, Parkinson's disease, Alzheimer's disease, and cancer, highlights its importance as a therapeutic target in the pharmaceutical industry (131, 159–164). Among small-molecule VAP-1 inhibitors, ASP8232 has shown favorable safety and tolerability profiles in phase II trials for diabetic nephropathy and diabetic macular edema (151, 165). However, its efficacy for macular edema was limited, leading to the trial's termination (165). Conversely, ASP8232 demonstrated greater promise in the ALBUM study by reducing albuminuria in diabetic nephropathy patients (151). Other VAP-1 inhibitors, such as MDL-72974A and aminoguanidine, have been shown to prevent obesity and atherosclerosis in KKAy mice (91, 92). PXS-4728A is a novel, orally available small-molecule inhibitor of VAP-1/SSAO that demonstrates irreversible and highly selective characteristics (5). It effectively suppresses neutrophil migration during acute pulmonary inflammation, lung infection, and airway hyperresponsiveness, thereby inhibiting airway inflammation and fibrosis while improving pulmonary function (57, 166). In addition to its potential therapeutic effects on atherosclerosis as described in Section 2.4, PXS-4728A has also been shown to significantly ameliorate renal fibrosis, with particularly prominent effects in diabetic nephropathy (70, 108, 167, 168). In myocardial ischemia-reperfusion injury models, SSAO inhibitors (e.g., SCZ, HYD, LJP 1207) administered shortly before reperfusion significantly reduced SSAO activity, disrupted leukocyte-endothelial adhesion, decreased leukocyte infiltration, and mitigated myocardial injury (122). However, certain VAP-1 inhibitors exert additional cardiovascular effects. For instance, hydralazine has been shown to reduce blood pressure at higher doses (169). Despite these advances, clinical trials evaluating VAP-1-targeted therapies for hypertension, coronary heart disease, heart failure, or other cardiovascular conditions are lacking. Further research is needed to explore these therapeutic possibilities. Ongoing efforts to develop high-efficiency, selective, and low-toxicity VAP-1 inhibitors are crucial for translating these findings into clinical practice. Future studies should focus on investigating novel VAP-1 inhibitors for the treatment and prevention of cardiovascular diseases.
9 Conclusion and future perspectives
Plasma VAP-1 levels and activity are markedly elevated in conditions such as inflammation, diabetes, atherosclerosis, and congestive heart failure. VAP-1 contributes to atherosclerosis progression through its enzymatic activity, facilitating leukocyte transendothelial migration and producing toxic metabolites that damage vascular endothelial cells. These functions position VAP-1 as a valuable biomarker for cardiovascular disease diagnosis and prognosis, aiding in the prediction of hypertension and coronary heart disease risk, as well as the assessment of major adverse event rates in CHD and HF patients. As a glycoprotein that mediates leukocyte migration and is readily detectable in circulation, VAP-1 holds significant potential as a future biomarker for cardiovascular diseases. Extensive research has explored its tissue distribution, substrates, and inhibitors, identifying it as a viable therapeutic target across multiple pathological contexts. However, the precise mechanisms underlying VAP-1's pathophysiological roles, along with the pharmacological effects of its inhibitors, remain insufficiently understood. Addressing these knowledge gaps will be critical for advancing VAP-1-targeted therapies. The development of novel, high-efficiency, and specific VAP-1 inhibitors will be instrumental in improving cardiovascular disease outcomes. Continued research efforts are necessary to fully elucidate the pathological significance of VAP-1 and to harness its therapeutic potential in future clinical applications.
Author contributions
CC: Conceptualization, Writing – original draft. WZho: Methodology, Writing – original draft. HZ: Writing – original draft. WZha: Writing – original draft. YW: Visualization, Writing – original draft. QD: Writing – original draft. BS: Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Salmi M, Jalkanen S. A 90-kilodalton endothelial cell molecule mediating lymphocyte binding in humans. Science. (1992) 257(5075):1407–9. doi: 10.1126/science.1529341
2. Salmi M, Jalkanen S. Vascular adhesion protein-1: a cell surface amine oxidase in translation. Antioxid Redox Signal. (2019) 30(3):314–32. doi: 10.1089/ars.2017.7418
3. Elliott J, Callingham BA, Sharman DF. Amine oxidase enzymes of sheep blood vessels and blood plasma: a comparison of their properties. Comp Biochem Physiol C Comp Pharmacol Toxicol. (1992) 102(1):83–9. doi: 10.1016/0742-8413(92)90048-c
4. Bligt-Linden E, Arunachalam R, Parkash V, Salminen TA. Structural comparison of the active site channels in rodent and primate vascular adhesion protein-1. J Neural Transm (Vienna). (2013) 120(6):947–50. doi: 10.1007/s00702-013-0974-4
5. Kubota R, Reid MJ, Lieu KL, Orme M, Diamond C, Tulberg N, et al. Comparison of inhibitor and substrate selectivity between rodent and human vascular adhesion protein-1. Mediators Inflamm. (2020) 2020:3270513. doi: 10.1155/2020/3270513
6. Holt A, Smith DJ, Cendron L, Zanotti G, Rigo A, Di Paolo ML. Multiple binding sites for substrates and modulators of semicarbazide-sensitive amine oxidases: kinetic consequences. Mol Pharmacol. (2008) 73(2):525–38. doi: 10.1124/mol.107.040964
7. Boomsma F, van Dijk J, Bhaggoe UM, Bouhuizen AM, van den Meiracker AH. Variation in semicarbazide-sensitive amine oxidase activity in plasma and tissues of mammals. Comp Biochem Physiol C Toxicol Pharmacol. (2000) 126(1):69–78. doi: 10.1016/s0742-8413(00)00101-8
8. Airenne TT, Nymalm Y, Kidron H, Smith DJ, Pihlavisto M, Salmi M, et al. Crystal structure of the human vascular adhesion protein-1: unique structural features with functional implications. Protein Sci. (2005) 14(8):1964–74. doi: 10.1110/ps.051438105
9. Salmi M, Jalkanen S. Human vascular adhesion protein 1 (vap-1) is a unique sialoglycoprotein that mediates carbohydrate-dependent binding of lymphocytes to endothelial cells. J Exp Med. (1996) 183(2):569–79. doi: 10.1084/jem.183.2.569
10. Smith DJ, Salmi M, Bono P, Hellman J, Leu T, Jalkanen S. Cloning of vascular adhesion protein 1 reveals a novel multifunctional adhesion molecule. J Exp Med. (1998) 188(1):17–27. doi: 10.1084/jem.188.1.17
11. Ernberg K, McGrath AP, Peat TS, Adams TE, Xiao X, Pham T, et al. A new crystal form of human vascular adhesion protein 1. Acta Crystallogr Sect F Struct Biol Cryst Commun. (2010) 66(Pt 12):1572–8. doi: 10.1107/s1744309110041515
12. Salmi M, Jalkanen S. Homing-associated molecules Cd73 and vap-1 as targets to prevent harmful inflammations and cancer spread. FEBS Lett. (2011) 585(11):1543–50. doi: 10.1016/j.febslet.2011.04.033
13. Jakobsson E, Nilsson J, Ogg D, Kleywegt GJ. Structure of human semicarbazide-sensitive amine oxidase/vascular adhesion protein-1. Acta Crystallogr D Biol Crystallogr. (2005) 61(Pt 11):1550–62. doi: 10.1107/s0907444905028805
14. Lizcano JM, Tipton KF, Unzeta M. Time-dependent activation of the semicarbazide-sensitive amine oxidase (ssao) from ox lung microsomes. Biochem J. (2000) 351(Pt 3):789–94. doi: 10.1042/bj3510789
15. Pannecoeck R, Serruys D, Benmeridja L, Delanghe JR, van Geel N, Speeckaert R, et al. Vascular adhesion protein-1: role in human pathology and application as a biomarker. Crit Rev Clin Lab Sci. (2015) 52(6):284–300. doi: 10.3109/10408363.2015.1050714
16. Stolen CM, Yegutkin GG, Kurkijärvi R, Bono P, Alitalo K, Jalkanen S. Origins of serum semicarbazide-sensitive amine oxidase. Circ Res. (2004) 95(1):50–7. doi: 10.1161/01.Res.0000134630.68877.2f
17. Salmi M, Jalkanen S. Vap-1: an adhesin and an enzyme. Trends Immunol. (2001) 22(4):211–6. doi: 10.1016/s1471-4906(01)01870-1
18. Salmi M, Kalimo K, Jalkanen S. Induction and function of vascular adhesion protein-1 at sites of inflammation. J Exp Med. (1993) 178(6):2255–60. doi: 10.1084/jem.178.6.2255
19. Jaakkola K, Kaunismäki K, Tohka S, Yegutkin G, Vänttinen E, Havia T, et al. Human vascular adhesion protein-1 in smooth muscle cells. Am J Pathol. (1999) 155(6):1953–65. doi: 10.1016/s0002-9440(10)65514-9
20. Moldes M, Fève B, Pairault J. Molecular cloning of a major mrna species in murine 3t3 adipocyte lineage. Differentiation-dependent expression, regulation, and identification as semicarbazide-sensitive amine oxidase. J Biol Chem. (1999) 274(14):9515–23. doi: 10.1074/jbc.274.14.9515
21. Hsia LT, Ashley N, Ouaret D, Wang LM, Wilding J, Bodmer WF. Myofibroblasts are distinguished from activated skin fibroblasts by the expression of Aoc3 and other associated markers. Proc Natl Acad Sci U S A. (2016) 113(15):E2162–71. doi: 10.1073/pnas.1603534113
22. Koskinen K, Vainio PJ, Smith DJ, Pihlavisto M, Ylä-Herttuala S, Jalkanen S, et al. Granulocyte transmigration through the endothelium is regulated by the oxidase activity of vascular adhesion protein-1 (vap-1). Blood. (2004) 103(9):3388–95. doi: 10.1182/blood-2003-09-3275
23. Sole M, Unzeta M. Vascular cell lines expressing ssao/vap-1: a new experimental tool to study its involvement in vascular diseases. Biol Cell. (2011) 103(11):543–57. doi: 10.1042/BC20110049
24. Mátyus P, Dajka-Halász B, Földi A, Haider N, Barlocco D, Magyar K. Semicarbazide-sensitive amine oxidase: current status and perspectives. Curr Med Chem. (2004) 11(10):1285–98. doi: 10.2174/0929867043365305
25. Abella A, García-Vicente S, Viguerie N, Ros-Baró A, Camps M, Palacín M, et al. Adipocytes release a soluble form of vap-1/ssao by a metalloprotease-dependent process and in a regulated manner. Diabetologia. (2004) 47(3):429–38. doi: 10.1007/s00125-004-1346-2
26. Stolen CM, Madanat R, Marti L, Kari S, Yegutkin GG, Sariola H, et al. Semicarbazide sensitive amine oxidase overexpression has dual consequences: insulin mimicry and diabetes-like complications. Faseb j. (2004) 18(6):702–4. doi: 10.1096/fj.03-0562fje
27. Göktürk C, Nilsson J, Nordquist J, Kristensson M, Svensson K, Söderberg C, et al. Overexpression of semicarbazide-sensitive amine oxidase in smooth muscle cells leads to an abnormal structure of the aortic elastic laminas. Am J Pathol. (2003) 163(5):1921–8. doi: 10.1016/s0002-9440(10)63550-x
28. Petzinna SM, Bauer CJ, Schafer VS. Vascular-adhesion protein 1 in giant cell arteritis and polymyalgia rheumatica. Front Med (Lausanne) (2024) 11:1448157. doi: 10.3389/fmed.2024.1448157
29. Unzeta M, Hernandez-Guillamon M, Sun P, Sole M. Ssao/vap-1 in cerebrovascular disorders: a potential therapeutic target for stroke and Alzheimer’s disease. Int J Mol Sci. (2021) 22(7):3365. doi: 10.3390/ijms22073365
30. Murata M, Noda K, Fukuhara J, Kanda A, Kase S, Saito W, et al. Soluble vascular adhesion protein-1 accumulates in proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. (2012) 53(7):4055–62. doi: 10.1167/iovs.12-9857
31. Li H, Du S, Niu P, Gu X, Wang J, Zhao Y. Vascular adhesion protein-1 (vap-1)/semicarbazide-sensitive amine oxidase (ssao): a potential therapeutic target for atherosclerotic cardiovascular diseases. Front Pharmacol. (2021) 12:679707. doi: 10.3389/fphar.2021.679707
32. Weston CJ, Shepherd EL, Claridge LC, Rantakari P, Curbishley SM, Tomlinson JW, et al. Vascular adhesion protein-1 promotes liver inflammation and drives hepatic fibrosis. J Clin Invest. (2015) 125(2):501–20. doi: 10.1172/JCI73722
33. Timmis A, Aboyans V, Vardas P, Townsend N, Torbica A, Kavousi M, et al. European Society of Cardiology: the 2023 atlas of cardiovascular disease statistics. Eur Heart J. (2024) 45(38):4019–62. doi: 10.1093/eurheartj/ehae466
34. Martin SS, Aday AW, Almarzooq ZI, Anderson CAM, Arora P, Avery CL, et al. 2024 Heart disease and stroke statistics: a report of US and global data from the American Heart Association. Circulation (2024) 149(8):e347–913. doi: 10.1161/cir.0000000000001209
35. Aggarwal R, Yeh RW, Joynt Maddox KE, Wadhera RK. Cardiovascular risk factor prevalence, treatment, and control in US adults aged 20 to 44 years, 2009 to March 2020. JAMA. (2023) 329(11):899–909. doi: 10.1001/jama.2023.2307
36. Libby P, Buring JE, Badimon L, Hansson GK, Deanfield J, Bittencourt MS, et al. Atherosclerosis. Nat Rev Dis Primers. (2019) 5(1):56. doi: 10.1038/s41572-019-0106-z
37. Jebari-Benslaiman S, Galicia-García U, Larrea-Sebal A, Olaetxea JR, Alloza I, Vandenbroeck K, et al. Pathophysiology of atherosclerosis. Int J Mol Sci. (2022) 23(6):3346. doi: 10.3390/ijms23063346
38. Xia D, Zheng Q, Liu Y, Wang L, Wei D. Targeting immune cell metabolism: a promising therapeutic approach for cardiovascular disease. Immunology. (2025) 175(2):134–50. doi: 10.1111/imm.13913
39. Martin P, Sanchez-Madrid F. T cells in cardiac health and disease. J Clin Invest. (2025) 135(2):e185218. doi: 10.1172/JCI185218
40. Chen J, Zhu Z, Wang Y, Yu J, Zhang X, Xu Y. Cardiac resident macrophages in cardiovascular disease: from physiology to pathology. Heart. (2025) 111(9):391–400. doi: 10.1136/heartjnl-2024-324333
41. Aalto K, Autio A, Kiss EA, Elima K, Nymalm Y, Veres TZ, et al. Siglec-9 is a novel leukocyte ligand for vascular adhesion protein-1 and can be used in pet imaging of inflammation and cancer. Blood. (2011) 118(13):3725–33. doi: 10.1182/blood-2010-09-311076
42. Kivi E, Elima K, Aalto K, Nymalm Y, Auvinen K, Koivunen E, et al. Human siglec-10 can bind to vascular adhesion protein-1 and serves as its substrate. Blood. (2009) 114(26):5385–92. doi: 10.1182/blood-2009-04-219253
43. Silvola JM, Virtanen H, Siitonen R, Hellberg S, Liljenback H, Metsala O, et al. Leukocyte trafficking-associated vascular adhesion protein 1 is expressed and functionally active in atherosclerotic plaques. Sci Rep. (2016) 6:35089. doi: 10.1038/srep35089
44. Irjala H, Salmi M, Alanen K, Grenman R, Jalkanen S. Vascular adhesion protein 1 mediates binding of immunotherapeutic effector cells to tumor endothelium. J Immunol. (2001) 166(11):6937–43. doi: 10.4049/jimmunol.166.11.6937
45. McLaren JE, Michael DR, Ashlin TG, Ramji DP. Cytokines, macrophage lipid metabolism and foam cells: implications for cardiovascular disease therapy. Prog Lipid Res. (2011) 50(4):331–47. doi: 10.1016/j.plipres.2011.04.002
46. Libby P. Inflammation and the pathogenesis of atherosclerosis. Vascul Pharmacol. (2024) 154:107255. doi: 10.1016/j.vph.2023.107255
47. Viitanen R, Moisio O, Lankinen P, Li XG, Koivumäki M, Suilamo S, et al. First-in-humans study of (68)Ga-dota-siglec-9, a pet ligand targeting vascular adhesion protein 1. J Nucl Med. (2021) 62(4):577–83. doi: 10.2967/jnumed.120.250696
48. Jalkanen S, Karikoski M, Mercier N, Koskinen K, Henttinen T, Elima K, et al. The oxidase activity of vascular adhesion protein-1 (vap-1) induces endothelial E- and P-selectins and leukocyte binding. Blood. (2007) 110(6):1864–70. doi: 10.1182/blood-2007-01-069674
49. Solé M, Esteban-Lopez M, Taltavull B, Fábregas C, Fadó R, Casals N, et al. Blood-brain barrier dysfunction underlying Alzheimer’s disease is induced by an ssao/vap-1-dependent cerebrovascular activation with enhanced aβ deposition. Biochim Biophys Acta Mol Basis Dis. (2019) 1865(9):2189–202. doi: 10.1016/j.bbadis.2019.04.016
50. Xu Q, Chen X, Yu T, Tang Q, Zhou Z, Wang H, et al. Downregulation of vap-1 in oscc suppresses tumor growth and metastasis via nf-Κb/il-8 signaling and reduces neutrophil infiltration. J Oral Pathol Med. (2022) 51(4):332–41. doi: 10.1111/jop.13285
51. Matsuda T, Noda K, Murata M, Kawasaki A, Kanda A, Mashima Y, et al. Vascular adhesion protein-1 blockade suppresses ocular inflammation after retinal laser photocoagulation in mice. Invest Ophthalmol Vis Sci. (2017) 58(7):3254–61. doi: 10.1167/iovs.17-21555
52. Sun P, Hernandez-Guillamón M, Campos-Martorell M, Simats A, Montaner J, Unzeta M, et al. Simvastatin blocks soluble ssao/vap-1 release in experimental models of cerebral ischemia: possible benefits for stroke-induced inflammation control. Biochim Biophys Acta Mol Basis Dis (2018) 1864(2):542–53. doi: 10.1016/j.bbadis.2017.11.014
53. Tanaka S, Tanaka T, Kawakami T, Takano H, Sugahara M, Saito H, et al. Vascular adhesion protein-1 enhances neutrophil infiltration by generation of hydrogen peroxide in renal ischemia/reperfusion injury. Kidney Int. (2017) 92(1):154–64. doi: 10.1016/j.kint.2017.01.014
54. Liaskou E, Karikoski M, Reynolds GM, Lalor PF, Weston CJ, Pullen N, et al. Regulation of mucosal addressin cell adhesion molecule 1 expression in human and mice by vascular adhesion protein 1 amine oxidase activity. Hepatology. (2011) 53(2):661–72. doi: 10.1002/hep.24085
55. Salmi M, Yegutkin GG, Lehvonen R, Koskinen K, Salminen T, Jalkanen S. A cell surface amine oxidase directly controls lymphocyte migration. Immunity. (2001) 14(3):265–76. doi: 10.1016/s1074-7613(01)00108-x
56. Tohka S, Laukkanen M, Jalkanen S, Salmi M. Vascular adhesion protein 1 (vap-1) functions as a molecular brake during granulocyte rolling and mediates recruitment in vivo. Faseb j. (2001) 15(2):373–82. doi: 10.1096/fj.00-0240com
57. Schilter HC, Collison A, Russo RC, Foot JS, Yow TT, Vieira AT, et al. Effects of an anti-inflammatory vap-1/ssao inhibitor, pxs-4728a, on pulmonary neutrophil migration. Respir Res. (2015) 16(1):42. doi: 10.1186/s12931-015-0200-z
58. Jaakkola K, Jalkanen S, Kaunismäki K, Vänttinen E, Saukko P, Alanen K, et al. Vascular adhesion protein-1, intercellular adhesion molecule-1 and P-selectin mediate leukocyte binding to ischemic heart in humans. J Am Coll Cardiol. (2000) 36(1):122–9. doi: 10.1016/s0735-1097(00)00706-3
59. Gimbrone MA J, García-Cardeña G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res. (2016) 118(4):620–36. doi: 10.1161/circresaha.115.306301
60. Zuchi C, Tritto I, Carluccio E, Mattei C, Cattadori G, Ambrosio G. Role of endothelial dysfunction in heart failure. Heart Fail Rev. (2020) 25(1):21–30. doi: 10.1007/s10741-019-09881-3
61. Manasieva V, Thakur S, Lione LA, Baydoun AR, Skamarauskas J. The impact of semicarbazide sensitive amine oxidase activity on rat aortic vascular smooth muscle cells. Int J Mol Sci. (2023) 24(5):4946. doi: 10.3390/ijms24054946
62. Yu PH, Lai CT, Zuo DM. Formation of formaldehyde from adrenaline in vivo; a potential risk factor for stress-related angiopathy. Neurochem Res. (1997) 22(5):615–20. doi: 10.1023/a:1022478221421
63. Yu PH. Increase of formation of methylamine and formaldehyde in vivo after administration of nicotine and the potential cytotoxicity. Neurochem Res. (1998) 23(9):1205–10. doi: 10.1023/a:1020786219966
64. Boomsma F, van Veldhuisen DJ, de Kam PJ, Man in't Veld AJ, Mosterd A, Lie KI, et al. Plasma semicarbazide-sensitive amine oxidase is elevated in patients with congestive heart failure. Cardiovasc Res. (1997) 33(2):387–91. doi: 10.1016/s0008-6363(96)00209-x
65. Wang YC, Li HY, Wei JN, Lin MS, Shih SR, Hua CH, et al. Serum vascular adhesion protein-1 level is higher in smokers than non-smokers. Ann Hum Biol. (2013) 40(5):413–8. doi: 10.3109/03014460.2013.788679
66. Chen DW, Zhao RM, Jin Y, Zhang J, Han C, Jiang SQ, et al. Plasma soluble vascular adhesion protein-1 concentration correlates with arterial stiffness: a cross-sectional study. Arch Gerontol Geriatr. (2015) 61(1):67–71. doi: 10.1016/j.archger.2015.04.007
67. Maciorkowska D, Zbroch E, Malyszko J. Circulating renalase, catecholamines, and vascular adhesion protein 1 in hypertensive patients. J Am Soc Hypertens. (2015) 9(11):855–64. doi: 10.1016/j.jash.2015.08.002
68. Boomsma F, de Kam PJ, Tjeerdsma G, van den Meiracker AH, van Veldhuisen DJ. Plasma semicarbazide-sensitive amine oxidase (ssao) is an independent prognostic marker for mortality in chronic heart failure. Eur Heart J. (2000) 21(22):1859–63. doi: 10.1053/euhj.2000.2176
69. Mercier N, Pawelzik SC, Pirault J, Carracedo M, Persson O, Wollensack B, et al. Semicarbazide-Sensitive amine oxidase increases in calcific aortic valve stenosis and contributes to valvular interstitial cell calcification. Oxid Med Cell Longev. (2020) 2020:5197376. doi: 10.1155/2020/5197376
70. Wang SH, Yu TY, Tsai FC, Weston CJ, Lin MS, Hung CS, et al. Inhibition of semicarbazide-sensitive amine oxidase reduces atherosclerosis in apolipoprotein E-deficient mice. Transl Res. (2018) 197:12–31. doi: 10.1016/j.trsl.2018.03.001
71. El-Maghrabey MH, Kishikawa N, Ohyama K, Imazato T, Ueki Y, Kuroda N. Determination of human serum semicarbazide-sensitive amine oxidase activity via flow injection analysis with fluorescence detection after online derivatization of the enzymatically produced benzaldehyde with 1,2-diaminoanthraquinone. Anal Chim Acta. (2015) 881:139–47. doi: 10.1016/j.aca.2015.04.006
72. Zhang Y, Yang Y, He X, Yang P, Zong T, Sun P, et al. The cellular function and molecular mechanism of formaldehyde in cardiovascular disease and heart development. J Cell Mol Med. (2021) 25(12):5358–71. doi: 10.1111/jcmm.16602
73. Lin Z, Luo W, Li H, Zhang Y. The effect of endogenous formaldehyde on the rat aorta endothelial cells. Toxicol Lett. (2005) 159(2):134–43. doi: 10.1016/j.toxlet.2005.05.003
74. Gubisne-Haberle D, Hill W, Kazachkov M, Richardson JS, Yu PH. Protein cross-linkage induced by formaldehyde derived from semicarbazide-sensitive amine oxidase-mediated deamination of methylamine. J Pharmacol Exp Ther. (2004) 310(3):1125–32. doi: 10.1124/jpet.104.068601
75. Yu PH, Deng YL. Endogenous formaldehyde as a potential factor of vulnerability of atherosclerosis: involvement of semicarbazide-sensitive amine oxidase-mediated methylamine turnover. Atherosclerosis. (1998) 140(2):357–63. doi: 10.1016/s0021-9150(98)00142-7
76. Nigro C, Leone A, Longo M, Prevenzano I, Fleming TH, Nicolò A, et al. Methylglyoxal accumulation de-regulates Hoxa5 expression, thereby impairing angiogenesis in glyoxalase 1 knock-down mouse aortic endothelial cells. Biochim Biophys Acta Mol Basis Dis. (2019) 1865(1):73–85. doi: 10.1016/j.bbadis.2018.10.014
77. Nigro C, Leone A, Raciti GA, Longo M, Mirra P, Formisano P, et al. Methylglyoxal-glyoxalase 1 balance: the root of vascular damage. Int J Mol Sci. (2017) 18(1):188. doi: 10.3390/ijms18010188
78. Maessen DE, Stehouwer CD, Schalkwijk CG. The role of methylglyoxal and the glyoxalase system in diabetes and other age-related diseases. Clin Sci (Lond). (2015) 128(12):839–61. doi: 10.1042/cs20140683
79. Schalkwijk CG, Stehouwer CDA. Methylglyoxal, a highly reactive dicarbonyl compound, in diabetes, its vascular complications, and other age-related diseases. Physiol Rev. (2020) 100(1):407–61. doi: 10.1152/physrev.00001.2019
80. Schalkwijk CG. Vascular age-ing by methylglyoxal: the past, the present and the future. Diabetologia. (2015) 58(8):1715–9. doi: 10.1007/s00125-015-3597-5
81. Byon CH, Heath JM, Chen Y. Redox signaling in cardiovascular pathophysiology: a focus on hydrogen peroxide and vascular smooth muscle cells. Redox Biol. (2016) 9:244–53. doi: 10.1016/j.redox.2016.08.015
82. Cepas V, Collino M, Mayo JC, Sainz RM. Redox signaling and advanced glycation endproducts (ages) in diet-related diseases. Antioxidants (Basel). (2020) 9(2):142. doi: 10.3390/antiox9020142
83. Jing C, Zhang G, Liu Z, Xu Q, Li C, Cheng G, et al. Peroxidasin promotes diabetic vascular endothelial dysfunction induced by advanced glycation end products via Nox2/hocl/akt/enos pathway. Redox Biol. (2021) 45:102031. doi: 10.1016/j.redox.2021.102031
84. Schalkwijk CG, Micali LR, Wouters K. Advanced glycation endproducts in diabetes-related macrovascular complications: focus on methylglyoxal. Trends Endocrinol Metab. (2023) 34(1):49–60. doi: 10.1016/j.tem.2022.11.004
85. Li HY, Lin MS, Wei JN, Hung CS, Chiang FT, Lin CH, et al. Change of serum vascular adhesion protein-1 after glucose loading correlates to carotid intima-medial thickness in non-diabetic subjects. Clin Chim Acta. (2009) 403(1–2):97–101. doi: 10.1016/j.cca.2009.01.027
86. Poznyak A, Grechko AV, Poggio P, Myasoedova VA, Alfieri V, Orekhov AN. The diabetes mellitus-atherosclerosis connection: the role of lipid and glucose metabolism and chronic inflammation. Int J Mol Sci. (2020) 21(5):1835. doi: 10.3390/ijms21051835
87. Marti L, Abella A, Carpéné C, Palacín M, Testar X, Zorzano A. Combined treatment with benzylamine and low dosages of vanadate enhances glucose tolerance and reduces hyperglycemia in streptozotocin-induced diabetic rats. Diabetes. (2001) 50(9):2061–8. doi: 10.2337/diabetes.50.9.2061
88. Abella A, Marti L, Camps M, Claret M, Fernández-Alvarez J, Gomis R, et al. Semicarbazide-sensitive amine oxidase/vascular adhesion protein-1 activity exerts an antidiabetic action in goto-kakizaki rats. Diabetes. (2003) 52(4):1004–13. doi: 10.2337/diabetes.52.4.1004
89. Karim S, Liaskou E, Fear J, Garg A, Reynolds G, Claridge L, et al. Dysregulated hepatic expression of glucose transporters in chronic disease: contribution of semicarbazide-sensitive amine oxidase to hepatic glucose uptake. Am J Physiol Gastrointest Liver Physiol. (2014) 307(12):G1180–90. doi: 10.1152/ajpgi.00377.2013
90. Kuo CH, Wei JN, Yang CY, Ou HY, Wu HT, Fan KC, et al. Serum vascular adhesion protein-1 is up-regulated in hyperglycemia and is associated with incident diabetes negatively. Int J Obes (Lond). (2019) 43(3):512–22. doi: 10.1038/s41366-018-0172-4
91. Yu PH, Wang M, Deng YL, Fan H, Shira-Bock L. Involvement of semicarbazide-sensitive amine oxidase-mediated deamination in atherogenesis in kkay diabetic mice fed with high cholesterol diet. Diabetologia. (2002) 45(9):1255–62. doi: 10.1007/s00125-002-0903-9
92. Yu PH, Wang M, Fan H, Deng Y, Gubisne-Haberle D. Involvement of ssao-mediated deamination in adipose glucose transport and weight gain in obese diabetic kkay mice. Am J Physiol Endocrinol Metab. (2004) 286(4):E634–41. doi: 10.1152/ajpendo.00272.2003
93. Bour S, Caspar-Bauguil S, Iffiú-Soltész Z, Nibbelink M, Cousin B, Miiluniemi M, et al. Semicarbazide-Sensitive amine oxidase/vascular adhesion protein-1 deficiency reduces leukocyte infiltration into adipose tissue and favors fat deposition. Am J Pathol. (2009) 174(3):1075–83. doi: 10.2353/ajpath.2009.080612
94. Papukashvili D, Rcheulishvili N, Deng Y. Attenuation of weight gain and prevention of associated pathologies by inhibiting ssao. Nutrients. (2020) 12(1):184. doi: 10.3390/nu12010184
95. Shepherd EL, Karim S, Newsome PN, Lalor PF. Inhibition of vascular adhesion protein-1 modifies hepatic steatosis in vitro and in vivo. World J Hepatol. (2020) 12(11):931–48. doi: 10.4254/wjh.v12.i11.931
96. Jargaud V, Bour S, Tercé F, Collet X, Valet P, Bouloumié A, et al. Obesity of mice lacking vap-1/ssao by Aoc3 gene deletion is reproduced in mice expressing a mutated vascular adhesion protein-1 (vap-1) devoid of amine oxidase activity. J Physiol Biochem. (2021) 77(1):141–54. doi: 10.1007/s13105-020-00756-y
97. Mailloux RJ. An update on methods and approaches for interrogating mitochondrial reactive oxygen Species production. Redox Biol. (2021) 45:102044. doi: 10.1016/j.redox.2021.102044
98. Jeong SJ, Park JG, Oh GT. Peroxiredoxins as potential targets for cardiovascular disease. Antioxidants (Basel). (2021) 10(8):1244. doi: 10.3390/antiox10081244
99. Tsimikas S, Witztum JL. Measuring circulating oxidized low-density lipoprotein to evaluate coronary risk. Circulation. (2001) 103(15):1930–2. doi: 10.1161/01.cir.103.15.1930
100. Ehara S, Ueda M, Naruko T, Haze K, Itoh A, Otsuka M, et al. Elevated levels of oxidized low density lipoprotein show a positive relationship with the severity of acute coronary syndromes. Circulation. (2001) 103(15):1955–60. doi: 10.1161/01.cir.103.15.1955
101. Zhang Q, Ai Y, Dong H, Wang J, Xu L. Circulating oxidized low-density lipoprotein is a strong risk factor for the early stage of coronary heart disease. IUBMB Life. (2019) 71(2):277–82. doi: 10.1002/iub.1966
102. Yang H, Mohamed AS, Zhou SH. Oxidized low density lipoprotein, stem cells, and atherosclerosis. Lipids Health Dis. (2012) 11:85. doi: 10.1186/1476-511x-11-85
103. Lubrano V, Balzan S. Lox-1 and ros, inseparable factors in the process of endothelial damage. Free Radic Res. (2014) 48(8):841–8. doi: 10.3109/10715762.2014.929122
104. Chang YC, Hee SW, Lee WJ, Li HY, Chang TJ, Lin MW, et al. Genome-wide scan for circulating vascular adhesion protein-1 levels: macrod2 as a potential transcriptional regulator of adipogenesis. J Diabetes Investig. (2018) 9(5):1067–74. doi: 10.1111/jdi.12805
105. Carpéné C, Iffiú-Soltesz Z, Bour S, Prévot D, Valet P. Reduction of fat deposition by combined inhibition of monoamine oxidases and semicarbazide-sensitive amine oxidases in obese zucker rats. Pharmacol Res. (2007) 56(6):522–30. doi: 10.1016/j.phrs.2007.09.016
106. Carpéné C, Abello V, Iffiú-Soltész Z, Mercier N, Fève B, Valet P. Limitation of adipose tissue enlargement in rats chronically treated with semicarbazide-sensitive amine oxidase and monoamine oxidase inhibitors. Pharmacol Res. (2008) 57(6):426–34. doi: 10.1016/j.phrs.2008.04.005
107. Bour S, Daviaud D, Gres S, Lefort C, Prévot D, Zorzano A, et al. Adipogenesis-related increase of semicarbazide-sensitive amine oxidase and monoamine oxidase in human adipocytes. Biochimie. (2007) 89(8):916–25. doi: 10.1016/j.biochi.2007.02.013
108. Wang SH, Yu TY, Hung CS, Yang CY, Lin MS, Su CY, et al. Inhibition of semicarbazide-sensitive amine oxidase reduces atherosclerosis in cholesterol-fed New Zealand white rabbits. Sci Rep. (2018) 8(1):9249. doi: 10.1038/s41598-018-27551-6
109. Aalto K, Havulinna AS, Jalkanen S, Salomaa V, Salmi M. Soluble vascular adhesion protein-1 predicts incident major adverse cardiovascular events and improves reclassification in a Finnish prospective cohort study. Circ Cardiovasc Genet. (2014) 7(4):529–35. doi: 10.1161/circgenetics.113.000543
110. Chen Z, Ouyang C, Zhang H, Gu Y, Deng Y, Du C, et al. Vascular smooth muscle cell-derived hydrogen sulfide promotes atherosclerotic plaque stability via tfeb (transcription factor eb)-mediated autophagy. Autophagy. (2022) 18(10):2270–87. doi: 10.1080/15548627.2022.2026097
111. Grootaert MOJ, Bennett MR. Vascular smooth muscle cells in atherosclerosis: time for a Re-assessment. Cardiovasc Res. (2021) 117(11):2326–39. doi: 10.1093/cvr/cvab046
112. Bennett MR, Sinha S, Owens GK. Vascular smooth muscle cells in atherosclerosis. Circ Res. (2016) 118(4):692–702. doi: 10.1161/circresaha.115.306361
113. Zhang M, Liu L, Zhi F, Niu P, Yang M, Zhu X, et al. Inactivation of semicarbazide-sensitive amine oxidase induces the phenotypic switch of smooth muscle cells and aggravates the development of atherosclerotic lesions. Atherosclerosis. (2016) 249:76–82. doi: 10.1016/j.atherosclerosis.2016.03.039
114. Peng Y, Wang J, Zhang M, Niu P, Yang M, Yang Y, et al. Inactivation of semicarbazide-sensitive amine oxidase stabilizes the established atherosclerotic lesions via inducing the phenotypic switch of smooth muscle cells. PLoS One. (2016) 11(4):e0152758. doi: 10.1371/journal.pone.0152758
115. Filip A, Taleb S, Bascetin R, Jahangiri M, Bardin M, Lerognon C, et al. Increased atherosclerotic plaque in Aoc3 knock-out in apoe(-/-) mice and characterization of Aoc3 in atherosclerotic human coronary arteries. Front Cardiovasc Med. (2022) 9:848680. doi: 10.3389/fcvm.2022.848680
116. Li HY, Jiang YD, Chang TJ, Wei JN, Lin MS, Lin CH, et al. Serum vascular adhesion protein-1 predicts 10-year cardiovascular and cancer mortality in individuals with type 2 diabetes. Diabetes. (2011) 60(3):993–9. doi: 10.2337/db10-0607
117. Anger T, Carson W, Weyand M, Daniel WG, Hoeher M, Garlichs CD. Atherosclerotic inflammation triggers osteogenic bone transformation in calcified and stenotic human aortic valves: still a matter of debate. Exp Mol Pathol. (2009) 86(1):10–7. doi: 10.1016/j.yexmp.2008.11.001
118. Anger T, Pohle FK, Kandler L, Barthel T, Ensminger SM, Fischlein T, et al. Vap-1, Eotaxin3 and mig as potential atherosclerotic triggers of severe calcified and stenotic human aortic valves: effects of statins. Exp Mol Pathol. (2007) 83(3):435–42. doi: 10.1016/j.yexmp.2007.02.008
119. Danielli M, Thomas RC, Quinn LM, Tan BK. Vascular adhesion protein-1 (vap-1) in vascular inflammatory diseases. Vasa. (2022) 51(6):341–50. doi: 10.1024/0301-1526/a001031
120. Karádi I, Mészáros Z, Csányi A, Szombathy T, Hosszúfalusi N, Romics L, et al. Serum semicarbazide-sensitive amine oxidase (ssao) activity is an independent marker of carotid atherosclerosis. Clin Chim Acta. (2002) 323(1–2):139–46. doi: 10.1016/s0009-8981(02)00189-4
121. Aalto K, Maksimow M, Juonala M, Viikari J, Jula A, Kähönen M, et al. Soluble vascular adhesion protein-1 correlates with cardiovascular risk factors and early atherosclerotic manifestations. Arterioscler Thromb Vasc Biol. (2012) 32(2):523–32. doi: 10.1161/atvbaha.111.238030
122. Yang W, Li H, Luo H, Luo W. Inhibition of semicarbazide-sensitive amine oxidase attenuates myocardial ischemia-reperfusion injury in an in vivo rat model. Life Sci. (2011) 88(7-8):302–6. doi: 10.1016/j.lfs.2010.12.003
123. Messerli FH, Williams B, Ritz E. Essential hypertension. Lancet. (2007) 370(9587):591–603. doi: 10.1016/s0140-6736(07)61299-9
124. McMaster WG, Kirabo A, Madhur MS, Harrison DG. Inflammation, immunity, and hypertensive end-organ damage. Circ Res. (2015) 116(6):1022–33. doi: 10.1161/circresaha.116.303697
125. Zhang Z, Zhao L, Zhou X, Meng X, Zhou X. Role of inflammation, immunity, and oxidative stress in hypertension: new insights and potential therapeutic targets. Front Immunol. (2022) 13:1098725. doi: 10.3389/fimmu.2022.1098725
126. Guzik TJ, Touyz RM. Oxidative stress, inflammation, and vascular aging in hypertension. Hypertension. (2017) 70(4):660–7. doi: 10.1161/hypertensionaha.117.07802
127. Caillon A, Schiffrin EL. Role of inflammation and immunity in hypertension: recent epidemiological, laboratory, and clinical evidence. Curr Hypertens Rep. (2016) 18(3):21. doi: 10.1007/s11906-016-0628-7
128. Solak Y, Afsar B, Vaziri ND, Aslan G, Yalcin CE, Covic A, et al. Hypertension as an autoimmune and inflammatory disease. Hypertens Res. (2016) 39(8):567–73. doi: 10.1038/hr.2016.35
129. Mikolajczyk TP, Szczepaniak P, Vidler F, Maffia P, Graham GJ, Guzik TJ. Role of inflammatory chemokines in hypertension. Pharmacol Ther. (2021) 223:107799. doi: 10.1016/j.pharmthera.2020.107799
130. Plante TB, Juraschek SP, Howard G, Howard VJ, Tracy RP, Olson NC, et al. Cytokines, C-reactive protein, and risk of incident hypertension in the regards study. Hypertension. (2024) 81(6):1244–53. doi: 10.1161/hypertensionaha.123.22714
131. Becchi S, Buson A, Balleine BW. Inhibition of vascular adhesion protein 1 protects dopamine neurons from the effects of acute inflammation and restores habit learning in the Striatum. J Neuroinflammation. (2021) 18(1):233. doi: 10.1186/s12974-021-02288-8
132. Yoshikawa N, Noda K, Shinoda H, Uchida A, Ozawa Y, Tsubota K, et al. Serum vascular adhesion protein-1 correlates with vascular endothelial growth factor in patients with type ii diabetes. J Diabetes Complications. (2013) 27(2):162–6. doi: 10.1016/j.jdiacomp.2012.09.001
133. Mercier N, Osborne-Pellegrin M, El Hadri K, Kakou A, Labat C, Loufrani L, et al. Carotid arterial stiffness, elastic fibre network and vasoreactivity in semicarbazide-sensitive amine-oxidase null mouse. Cardiovasc Res. (2006) 72(2):349–57. doi: 10.1016/j.cardiores.2006.08.008
134. Powell-Wiley TM, Poirier P, Burke LE, Després JP, Gordon-Larsen P, Lavie CJ, et al. Obesity and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. (2021) 143(21):e984–e1010. doi: 10.1161/cir.0000000000000973
135. Piché ME, Tchernof A, Després JP. Obesity phenotypes, diabetes, and cardiovascular diseases. Circ Res. (2020) 126(11):1477–500. doi: 10.1161/circresaha.120.316101
136. Cole JB, Florez JC. Genetics of diabetes Mellitus and diabetes complications. Nat Rev Nephrol. (2020) 16(7):377–90. doi: 10.1038/s41581-020-0278-5
137. Enrique-Tarancón G, Marti L, Morin N, Lizcano JM, Unzeta M, Sevilla L, et al. Role of semicarbazide-sensitive amine oxidase on glucose transport and Glut4 recruitment to the cell surface in adipose cells. J Biol Chem. (1998) 273(14):8025–32. doi: 10.1074/jbc.273.14.8025
138. Marti L, Morin N, Enrique-Tarancon G, Prevot D, Lafontan M, Testar X, et al. Tyramine and vanadate synergistically stimulate glucose transport in rat adipocytes by amine oxidase-dependent generation of hydrogen peroxide. J Pharmacol Exp Ther. (1998) 285(1):342–9. doi: 10.1016/S0022-3565(24)37382-3
139. Mészáros Z, Szombathy T, Raimondi L, Karádi I, Romics L, Magyar K. Elevated serum semicarbazide-sensitive amine oxidase activity in non-insulin-dependent diabetes Mellitus: correlation with body mass Index and Serum triglyceride. Metab Clin Exp. (1999) 48(1):113–7. doi: 10.1016/s0026-0495(99)90019-7
140. Weiss HG, Klocker J, Labeck B, Nehoda H, Aigner F, Klingler A, et al. Plasma amine oxidase: a postulated cardiovascular risk factor in nondiabetic obese patients. Metab Clin Exp. (2003) 52(6):688–92. doi: 10.1016/s0026-0495(03)00028-3
141. Boomsma F, Derkx FH, van den Meiracker AH, Man in ‘t Veld AJ, Schalekamp MA. Plasma semicarbazide-sensitive amine oxidase activity is elevated in diabetes mellitus and correlates with glycosylated haemoglobin. Clin Sci (Lond). (1995) 88(6):675–9. doi: 10.1042/cs0880675
142. Salmi M, Stolen C, Jousilahti P, Yegutkin GG, Tapanainen P, Janatuinen T, et al. Insulin-regulated increase of soluble vascular adhesion protein-1 in diabetes. Am J Pathol. (2002) 161(6):2255–62. doi: 10.1016/s0002-9440(10)64501-4
143. Li HY, Wei JN, Lin MS, Smith DJ, Vainio J, Lin CH, et al. Serum vascular adhesion protein-1 is increased in acute and chronic hyperglycemia. Clin Chim Acta. (2009) 404(2):149–53. doi: 10.1016/j.cca.2009.03.041
144. Dincgez Cakmak B, Dundar B, Ketenci Gencer F, Yildiz DE, Bayram F, Ozgen G, et al. Assessment of relationship between serum vascular adhesion protein-1 (vap-1) and gestational diabetes mellitus. Biomarkers. (2019) 24(8):750–6. doi: 10.1080/1354750x.2019.1684562
145. Noda K, Miyahara S, Nakazawa T, Almulki L, Nakao S, Hisatomi T, et al. Inhibition of vascular adhesion protein-1 suppresses endotoxin-induced uveitis. Faseb j. (2008) 22(4):1094–103. doi: 10.1096/fj.07-9377com
146. Murata M, Noda K, Kawasaki A, Yoshida S, Dong Y, Saito M, et al. Soluble vascular adhesion protein-1 mediates spermine oxidation as semicarbazide-sensitive amine oxidase: possible role in proliferative diabetic retinopathy. Curr Eye Res. (2017) 42(12):1674–83. doi: 10.1080/02713683.2017.1359847
147. Inoue T, Morita M, Tojo T, Nagashima A, Moritomo A, Miyake H. Novel 1h-imidazol-2-amine derivatives as potent and orally active vascular adhesion protein-1 (vap-1) inhibitors for diabetic macular edema treatment. Bioorg Med Chem. (2013) 21(13):3873–81. doi: 10.1016/j.bmc.2013.04.011
148. Li HY, Lin HA, Nien FJ, Wu VC, Jiang YD, Chang TJ, et al. Serum vascular adhesion protein-1 predicts end-stage renal disease in patients with type 2 diabetes. PLoS One. (2016) 11(2):e0147981. doi: 10.1371/journal.pone.0147981
149. Tuttle KR, Agarwal R, Alpers CE, Bakris GL, Brosius FC, Kolkhof P, et al. Molecular mechanisms and therapeutic targets for diabetic kidney disease. Kidney Int. (2022) 102(2):248–60. doi: 10.1016/j.kint.2022.05.012
150. Yen IW, Li HY. The role of vascular adhesion protein-1 in diabetes and diabetic complications. J Diabetes Investig. (2024) 15(8):982–9. doi: 10.1111/jdi.14209
151. de Zeeuw D, Renfurm RW, Bakris G, Rossing P, Perkovic V, Hou FF, et al. Efficacy of a novel inhibitor of vascular adhesion protein-1 in reducing albuminuria in patients with diabetic kidney disease (album): a randomised, placebo-controlled, phase 2 trial. Lancet Diabetes Endocrinol. (2018) 6(12):925–33. doi: 10.1016/s2213-8587(18)30289-4
152. Valente T, Gella A, Solé M, Durany N, Unzeta M. Immunohistochemical study of semicarbazide-sensitive amine oxidase/vascular adhesion protein-1 in the hippocampal vasculature: pathological synergy of Alzheimer’s disease and diabetes mellitus. J Neurosci Res. (2012) 90(10):1989–96. doi: 10.1002/jnr.23092
153. Savarese G, Becher PM, Lund LH, Seferovic P, Rosano GMC, Coats AJS. Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovasc Res. (2023) 118(17):3272–87. doi: 10.1093/cvr/cvac013
154. Marinho C, Arduíno D, Falcão LM, Bicho M. Alterations in plasma semicarbazide-sensitive amine oxidase activity in hypertensive heart disease with left ventricular systolic dysfunction. Rev Port Cardiol. (2010) 29(1):37–47.20391898
155. Kim DK, Lee YH, Kim JS, Kim YG, Lee SY, Ahn SY, et al. Circulating vascular adhesion protein-1 level predicts the risk of cardiovascular events and mortality in hemodialysis patients. Front Cardiovasc Med. (2021) 8:701079. doi: 10.3389/fcvm.2021.701079
156. Patel RB, Colangelo LA, Bielinski SJ, Larson NB, Ding J, Allen NB, et al. Circulating vascular cell adhesion molecule-1 and incident heart failure: the multi-ethnic study of atherosclerosis (mesa). J Am Heart Assoc. (2020) 9(22):e019390. doi: 10.1161/jaha.120.019390
157. Wang EY, Gao H, Salter-Cid L, Zhang J, Huang L, Podar EM, et al. Design, synthesis, and biological evaluation of semicarbazide-sensitive amine oxidase (ssao) inhibitors with anti-inflammatory activity. J Med Chem. (2006) 49(7):2166–73. doi: 10.1021/jm050538l
158. Xu H, Testai FD, Valyi-Nagy T, Pavuluri MN, Zhai F, Nanegrungsunk D, et al. Vap-1 blockade prevents subarachnoid hemorrhage-associated cerebrovascular dilating dysfunction via repression of a neutrophil recruitment-related mechanism. Brain Res. (2015) 1603:141–9. doi: 10.1016/j.brainres.2015.01.047
159. Vakal S, Jalkanen S, Dahlström KM, Salminen TA. Human copper-containing amine oxidases in drug design and development. Molecules. (2020) 25(6):1293. doi: 10.3390/molecules25061293
160. Becchi S, Buson A, Foot J, Jarolimek W, Balleine BW. Inhibition of semicarbazide-sensitive amine oxidase/vascular adhesion protein-1 reduces lipopolysaccharide-induced neuroinflammation. Br J Pharmacol. (2017) 174(14):2302–17. doi: 10.1111/bph.13832
161. Milczek EM, Bonivento D, Binda C, Mattevi A, McDonald IA, Edmondson DE. Structural and mechanistic studies of mofegiline inhibition of recombinant human monoamine oxidase B. J Med Chem. (2008) 51(24):8019–26. doi: 10.1021/jm8011867
162. Kinoshita T, Sayem MA, Yaguchi T, Kharma B, Morii K, Kato D, et al. Inhibition of vascular adhesion protein-1 enhances the anti-tumor effects of immune checkpoint inhibitors. Cancer Sci. (2021) 112(4):1390–401. doi: 10.1111/cas.14812
163. Marttila-Ichihara F, Auvinen K, Elima K, Jalkanen S, Salmi M. Vascular adhesion protein-1 enhances tumor growth by supporting recruitment of gr-1 + Cd11b+ myeloid cells into tumors. Cancer Res. (2009) 69(19):7875–83. doi: 10.1158/0008-5472.Can-09-1205
164. Marttila-Ichihara F, Castermans K, Auvinen K, Oude Egbrink MG, Jalkanen S, Griffioen AW, et al. Small-molecule inhibitors of vascular adhesion protein-1 reduce the accumulation of myeloid cells into tumors and attenuate tumor growth in mice. J Immunol. (2010) 184(6):3164–73. doi: 10.4049/jimmunol.0901794
165. Nguyen QD, Sepah YJ, Berger B, Brown D, Do DV, Garcia-Hernandez A, et al. Primary outcomes of the Vidi study: phase 2, double-masked, randomized, active-controlled study of Asp8232 for diabetic macular edema. Int J Retina Vitreous. (2019) 5:28. doi: 10.1186/s40942-019-0178-7
166. Jarnicki AG, Schilter H, Liu G, Wheeldon K, Essilfie AT, Foot JS, et al. The inhibitor of semicarbazide-sensitive amine oxidase, pxs-4728a, ameliorates key features of chronic obstructive pulmonary disease in a mouse model. Br J Pharmacol. (2016) 173(22):3161–75. doi: 10.1111/bph.13573
167. Katagiri D, Hamasaki Y, Doi K, Negishi K, Sugaya T, Nangaku M, et al. Interstitial renal fibrosis due to multiple cisplatin treatments is ameliorated by semicarbazide-sensitive amine oxidase inhibition. Kidney Int. (2016) 89(2):374–85. doi: 10.1038/ki.2015.327
168. Wong MY, Saad S, Wong MG, Stangenberg S, Jarolimek W, Schilter H, et al. Semicarbazide-Sensitive amine oxidase inhibition ameliorates albuminuria and glomerulosclerosis but does not improve tubulointerstitial fibrosis in diabetic nephropathy. PLoS One. (2020) 15(6):e0234617. doi: 10.1371/journal.pone.0234617
Keywords: VAP-1, SSAO, cardiovascular diseases, biomarker, atherosclerotic
Citation: Chen C, Zhong W, Zheng H, Zhao W, Wang Y, Dong Q and Shen B (2025) The role of VAP-1 in cardiovascular disease: a review. Front. Cardiovasc. Med. 12:1549157. doi: 10.3389/fcvm.2025.1549157
Received: 20 December 2024; Accepted: 6 May 2025;
Published: 19 May 2025.
Edited by:
Yue Liu, Xiyuan Hospital, ChinaReviewed by:
Chris John Weston, University of Birmingham, United KingdomHung-Yuan Li, National Taiwan University Hospital, Taiwan
Copyright: © 2025 Chen, Zhong, Zheng, Zhao, Wang, Dong and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Botao Shen, c2hlbmJ0QGpsdS5lZHUuY24=
 Chengqian Chen
Chengqian Chen Wentao Zhong
Wentao Zhong Hao Zheng
Hao Zheng Wei Zhao1
Wei Zhao1 Yushi Wang
Yushi Wang Botao Shen
Botao Shen