- Department of Vascular and Interventional Radiology, Nanjing First Hospital, Nanjing Medical University, Nanjing, China
Objective: To compare the efficacy and safety outcomes between percutaneous mechanical thrombectomy (PMT) and catheter-directed thrombolysis (CDT) as the first endovascular revascularization (EVR) strategy for arterial acute mesenteric ischemia (AMI) and identify risk factors for 30-day mortality.
Methods: This was a single-center retrospective study. Between May 2014 and March 2024, consecutive patients with arterial AMI who received EVR using PMT or CDT as the first strategy were included. The baseline characteristics, imaging information, procedure-related information, complications, and clinical outcomes of patients were analyzed and compared. Binary logistic regression analysis was used to identify potential risk factors for 30-day mortality with an odds ratio (OR) and 95% confidence interval (CI).
Results: Forty-seven patients (PMT, n = 29; CDT, n = 18) were included. The mean age was 74.3 ± 7.6 years, and 66.0% were female. Successful revascularization was achieved in 89.4% of patients, and the 30-day mortality rate was 31.9%. There was no significant difference in successful revascularization, complications, and clinical outcomes between PMT and CDT as the first strategy. High plasma lactate (adjusted OR 1.73 per 1.0 mmol/L increase, 95% CI: 1.13–2.66; p = 0.012) and D-dimer (adjusted OR 1.73 per 1.0 mg/L increase; 95% CI: 1.20–2.50; p = .003) were associated with a high 30-day mortality rate.
Conclusions: PMT and CDT were associated with high revascularization rates and few complications. High plasma lactate and D-dimer may be associated with high 30-day mortality.
Introduction
Acute mesenteric ischemia (AMI) is considered an uncommon source of abdominal pain, and the incidence is approximately 6/100,000 person-years (1). Despite the development of treatment in the last decades, AMI remains a lethal scenario with short-term mortality of over 50% (1–3), and arterial AMI is the most lethal scenario among all subtypes (2).
Compared with Open surgical revascularization (OSR), endovascular revascularization (EVR) is characterized as minimally invasive and may be more appropriate for elderly and frail patients (4). Moreover, EVR has shown superiorities in improved clinical outcomes and cost-effectiveness (5–7). As such, EVR techniques have been increasingly advocated as the first strategy in recent years (8, 9). Various EVR techniques, including percutaneous mechanical thrombectomy (PMT), catheter-directed thrombolysis (CDT), percutaneous transluminal angioplasty (PTA), and stent placement, have been introduced in the treatment of arterial AMI depending on the cause of occlusion (10), but the optimal EVR technique for arterial AMI remains undetermined (11).
The present study aimed to investigate the differences regarding the efficacy and safety between PMT and CDT as the first EVR technique for the treatment of arterial occlusive AMI. In addition, the potential risk factors for 30-day mortality were evaluated.
Methods
Study design
This was a single-center retrospective observational study. Between May 2014 and March 2024, consecutive patients with computed tomography angiography (CTA) confirmed arterial occlusive AMI who received EVR using either PMT or CDT as the first EVR technique were included. Patients who underwent PMT as the first technique and required adjunctive CDT therapy were included in the PMT group. The patients who received direct stent placement for revascularization were excluded. In the study center, EVR was performed as the first-line revascularization treatment for arterial AMI patients. Two researchers independently searched the electronic medical recording system to identify potential candidates using the International Classification of Diseases 10th version (ICD-10) code K55.0.
Patient demographics, duration of symptom onset to diagnosis, etiology of arterial AMI, manifestations, comorbidities, and pre-procedure laboratory tests were collected and compared. The pre-procedure imaging information was reviewed. Procedure-related information, initial and final revascularization efficacy, complications, and clinical outcomes were noted and compared. The study was conducted in accordance with the Declaration of Helsinki. For patients who underwent stent thrombectomy, explicit written consent for the off-label use of the stent retriever was obtained from patients or their relatives before the procedures. The Institutional Review Board of the study hospital approved this study protocol, and informed consent was waived due to the retrospective study design.
Revascularization procedure and post-procedure management
In the present study, both PMT (including manual aspiration and stent thrombectomy) and CDT were performed as the first EVR techniques. The decision of the different first EVR techniques was predominantly made by the operators based on the severity of symptoms, anatomic locations of occlusion, and experiences. For instance, patients with milder symptoms and distal occlusion with a small SMA diameter may receive CDT first. Patients with severe symptoms and target vessel diameter suitable for PMT devices may receive PMT first.
Previous studies have described the detailed process for manual aspiration and stent thrombectomy (12, 13). In summary, for patients who received manual aspiration, a 6-Fr guiding sheath (Super Arrow-Flex; Teleflex, USA) was initially placed in the superior mesenteric artery (SMA), and then a 6-Fr aspiration catheter (Mach 1 Peripheral Guide Catheter; Boston Scientific, USA) was advanced into the thrombus gently, and aspiration was performed using a 60 ml syringe. For patients who underwent stent thrombectomy, a 6-Fr guiding sheath (Super Arrow-Flex) was initially placed in the SMA. A 2.8-Fr microcatheter (Progreat; Terumo, Japan) and a 6-Fr guiding catheter (Mach 1 Peripheral Guide Catheter) were introduced coaxially. After successfully crossing the occlusion, a Solitaire AB stent (Medtronic, USA) was deployed across the thrombus. After sufficient stent expansion, the stent was retrieved through the guiding catheter, and manual aspiration using the guiding catheter was synchronously performed. The Rotarex (Straub Medical, Switzerland) thrombectomy was performed as the previously described approach (14).
CDT was performed as the first EVR technique or as an adjunctive procedure in patients with residual thrombus after the initial PMT procedure. CDT was also performed for the residual branch vessel involvement that notably compromised distal infusion. A 4-Fr infusion catheter (UniFuse; AngioDynamics, USA; or Fountain; Merit Medical Systems, USA) or an end-hole catheter was placed in the target vessel. Recombinant tissue plasminogen activator (Actilyse; Boehringer Ingelheim, Germany) was continuously infused at 10.0–20.0 mg/24 h (15). Follow-up angiography was performed every 12–24 h.
No additional endovascular treatment was performed for distal embolization as this occlusion rarely deteriorated perfusion of the intestine owing to the abundant mesenteric collateral network (16).
PTA and stent placement were performed to correct coexisting atherosclerotic stenosis >50%. A self-expandable stent (EverFlex; Medtronic, USA; or Smart Control; Cordis, USA) was used in the present study.
After the revascularization procedure, patients were monitored for any complications, potential symptom aggravation, or signs of bowel necrosis. Patients received bowel rest, parenteral nutritional support, pain management, antithrombotic therapy, and intravenous broad-spectrum antibiotics (17). If patients exhibited pre-procedure and post-procedure signs of bowel necrosis, laparotomy with resection of the necrotic bowel was performed as needed. The decision to perform a second-look laparotomy is made based on the surgeon's interpretation of the initial laparotomy.
For embolic AMI, anticoagulation therapy with low-molecular weight heparin, rivaroxaban, or warfarin was prescribed based on per patient's condition. Lifelong anticoagulation was recommended if there were no contraindications. For thrombotic AMI, antiplatelet therapy with aspirin and/or clopidogrel was prescribed (4).
Definitions
Embolic occlusion was defined as a thrombus surrounded by contrast material in a noncalcified SMA in conjunction with the acute onset of symptoms (18). Thrombotic occlusion was defined as a thrombotic occlusion in a calcified or stenosis SMA (18). Complete revascularization was defined as complete SMA stem recanalization without residual thrombus on angiography. Partial revascularization was defined as the partial SMA stem recanalization with residual thrombus but not significantly compromising distal vessel opacification on angiography. Successful revascularization was defined as both complete and partial revascularization. Failed revascularization was defined as the inability to remove the thrombus after treatment. Short bowel syndrome was defined as the need for parenteral nutrition support (19). Proximal SMA was defined as the segment between the ostium and the origin of the inferior pancreaticoduodenal artery. Middle SMA was defined as the segment between the pancreaticoduodenal and the ileocolic artery. Distal SMA was defined as the vascular bed downstream from the ileocolic artery (20). The signs suggested bowel necrosis include persistent peritonitis, pneumatosis intestinalis, portomesenteric venous gas, and free peritoneal gas (21).
Statistical analysis
Data were processed and analyzed using the SPSS statistical package (26.0; IBM SPSS Statistics, USA). The distribution of continuous data was tested using the Shapiro–Wilk test. Normally distributed data were presented as mean ± standard deviation, and asymmetrically distributed data were presented as the median and interquartile range (IQR). The Student t-test or Manne–Whitney U test was performed to compare the difference between continuous data, and the chi-square test or Fisher's exact test was used for categorical data where appropriate. The potential risk factors for 30-day mortality were initially identified using univariable analysis and subsequently estimated using the logistic regression models with an odds ratio (OR) and 95% confidence interval (CI). A two-tailed p-value <.050 was considered statistically significant.
Results
Patients
During the study period, 47 patients with arterial occlusive AMI were included. Embolic and thrombotic occlusion accounted for 80.9% and 19.1% of the patients. The mean age was 74.3 ± 7.6 years, and 66.0% were female. Figure 1 presents the detailed patient inclusion flowchart and the first EVR technique choice.
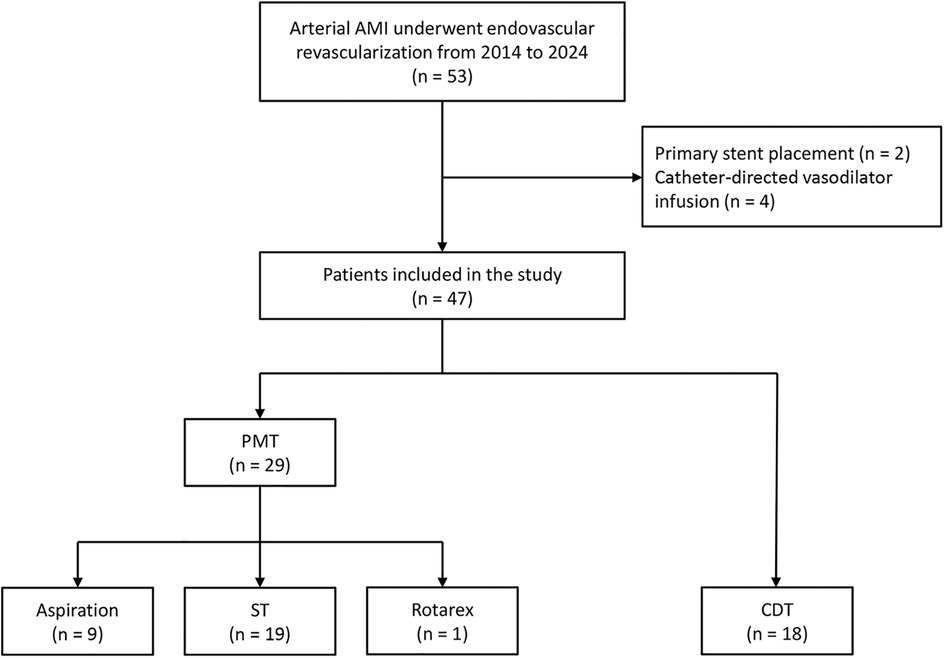
Figure 1. The flowchart of patients with acute mesenteric ischemia inclusion and first endovascular revascularization choice.
The median duration from symptom onset to admission was 15.0 h (IQR: 8.0–24.0 h). Thrombotic and embolic occlusion etiology accounted for 19.1% and 80.9% of patients, respectively. All patients had acute abdominal pain, and other common manifestations included diarrhea (42.6%) and vomiting (29.8%). The median symptom duration in the PMT group was significantly lower than in the CDT group (10.0 h vs. 24.0 h, p = .003). The main comorbidities were hypertension (64.8%) and atrial fibrillation (59.6%). Pre-procedure laboratory tests found notable elevated white blood cell count and D-dimer value. SMA occlusion since proximal, middle, and distal SMA was noted in 10 (21.3%), 29 (61.7%), and 8 (17.0%) patients, respectively. There was no significant difference regarding occlusion location between PMT and CDT groups (Table 1). SMA branch involvement, synchronous embolization, and evidence of bowel necrosis were noted in 12.8%, 6.4%, and 10.6% of patients, respectively.
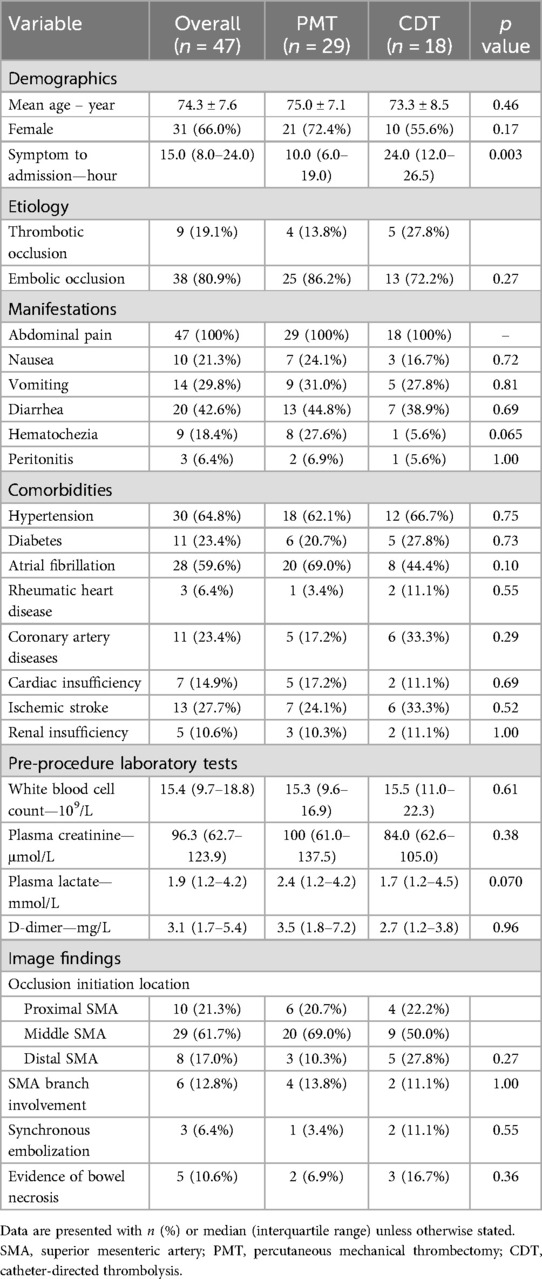
Table 1. Comparison of baseline characteristics and image findings of 47 patients with arterial acute mesenteric ischemia underwent PMT and CDT as primary revascularization.
Clinical outcomes and comparison between PMT and CDT as the first technique
Adjunctive PTA and stent placement were required in 10.6% and 10.6% of patients, respectively (Table 2). Finally, successful revascularization was achieved in 89.3% of patients. Five patients encountered unsuccessful revascularization due to the following reasons: 3 patients had multiple revascularization attempts but still failed, and the patients were unable to tolerate the subsequent attempts; 2 patients encountered severe vessel dissection during revascularization. All patients who experienced unsuccessful EVR refused open surgical revascularization, and they all died at 30-day follow-up. The overall procedure-related complications, including dissection, new distal embolization, and bleeding events, were encountered in 4.3%, 8.5%, and 4.3% of patients, respectively. The two bleeding events were minor, with hematoma around the sheath, and the bleeding events were successfully managed with manual compression and thrombolysis cessation. The overall bowel resection and second-look laparotomy was required in 12.8% and 14.9% of the patients, respectively. The median length of hospital stay was 7.0 days (IQR: 2.0–11.0 days). The overall in-hospital mortality and 30-day mortality were 10.6% (5/47) and 31.9% (15/47), respectively. The reasons for death were multiple organ dysfunction syndrome (80%) and septic shock (20%). Short bowel syndrome was noted in 16.7% (1/6) of surviving patients who received bowel resection at 30-day follow-up. No recurrent thrombosis or readmission was noted at the 30-day follow-up.

Table 2. Outcomes of 47 patients underwent PMT and CDT as the primary revascularization for arterial AMI.
For patients who underwent PMT as the first EVR technique, initial completed, partial, and unsuccessful revascularization was achieved in 55.2% (16/29), 24.1% (7/29), and 20.7% (6/29) of patients, respectively, and adjunctive local thrombolysis was performed in 24.1% (7/29) of patients. After all adjunctive procedures, completed and partial revascularization was achieved in 69.0% (20/29) and 20.7% (6/29) of patients, respectively. Figure 2 shows a successful PMT procedure without adjunctive procedures. For patients who underwent CDT as the first EVR technique, initial completed, partial, and unsuccessful revascularization was achieved in 50% (9/18), 33.3% (6/18), and 16.7% (3/18) of patients, respectively. After adjunctive procedures, completed and partial revascularization was achieved in 55.6% (10/18) and 33.3% (6/18) of patients, respectively. Figure 3 shows a successful CDT procedure.
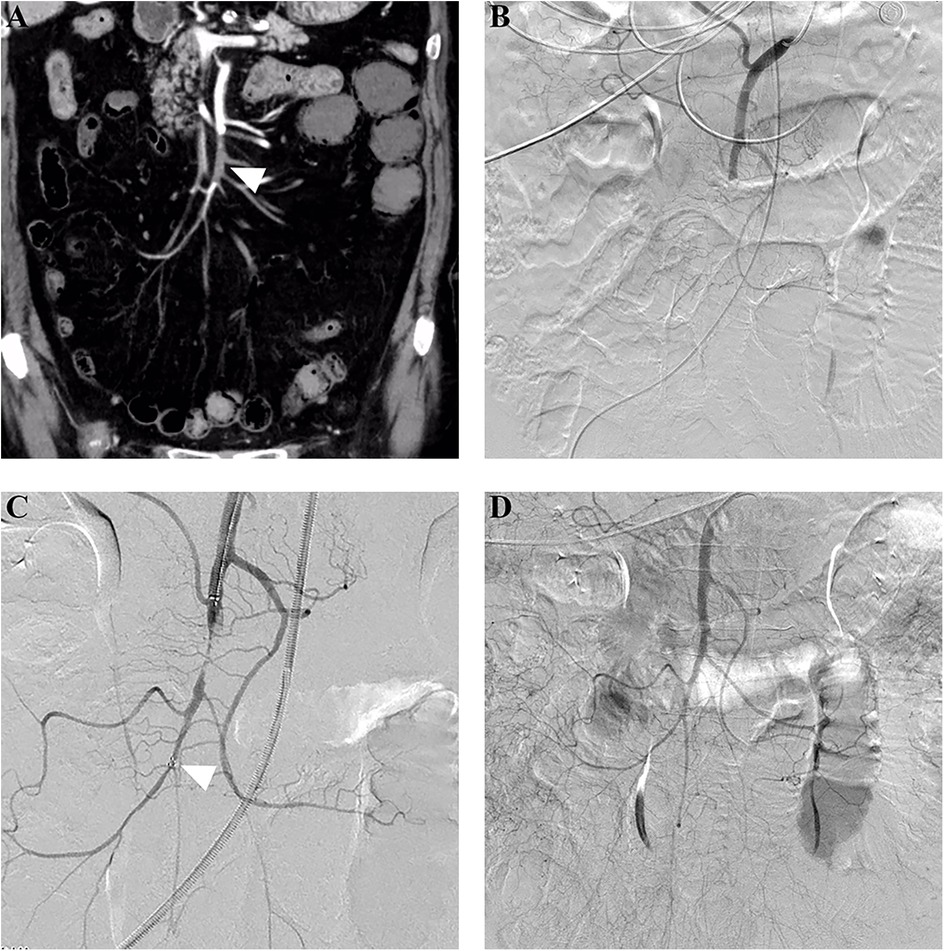
Figure 2. Successful stent thrombectomy in a patient with acute embolic SMA occlusion. (A) An 80-year-old woman presented with acute abdominal pain for 24 h, and subsequent computed tomography angiography revealed embolic occlusion (arrowhead) in the middle SMA stem. (B) Pre-procedure angiography further confirmed the occlusion without distal SMA stem and branch visualization. (C) Stent thrombectomy using a 6 mm × 30 mm Solitaire AB stent was performed. The arrowhead indicated the distal mark of the stent. (D) Post-procedure angiography showed complete SMA revascularization after one thrombectomy attempt. SMA,superior mesenteric artery.
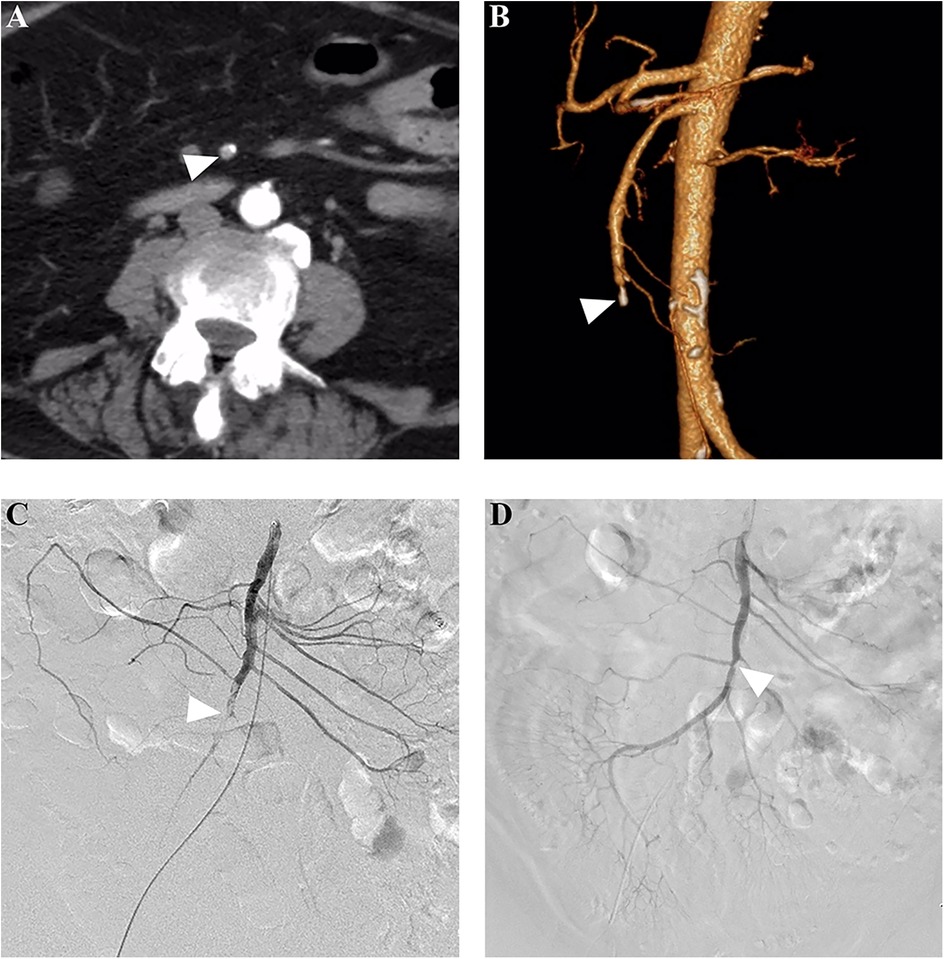
Figure 3. Successful catheter-directed thrombolysis in a patient with acute thrombotic SMA occlusion. (A) A 72-year-old woman presented with acute abdominal pain for 24 h, and subsequent computed tomography angiography revealed a filling defect (arrowhead) in the SMA stem. (B) The three-dimensional reconstruction showed distal SMA stem occlusion and calcification (arrowhead) in the proximal segment of the occluded artery. (C) Pre-procedure angiography showed a filling defect (arrowhead), and an end-hole catheter was placed for local thrombolysis using rt-PA at a rate of 10 mg/24 h. (D) After a thrombolysis duration of 2.0 days, complete revascularization was achieved, and the arrowhead showed a slight stenosis caused by the pre-existing calcification. SMA, superior mesenteric artery.
There was no significant difference regarding PTA/stent placement, revascularization rate, procedure-related complications, bowel resection, second-look laparotomy, short-term mortality, or short bowel syndrome between PMT and CDT as the first EVR technique (Table 2).
Risk factors for 30-day mortality
No significant difference regarding the quality of revascularization between survivors and nonsurvivors was noted (Table 3). The univariable analysis identified significant differences in plasma lactate (1.5 mmol/L vs. 3.4 mmol/L, p = .038) and D-dimer (2.8 mg/L vs. 6.4 mg/L, p = .002) levels between survivor and nonsurvivor at 30 days (Table 3). Univariable logistic regression analysis found that plasma lactate and D-dimer levels were associated with an increased 30-day mortality (Table 4). Moreover, the significant association of plasma lactate (adjusted OR: 1.73; 95% CI: 1.13–2.66; p = .012) and D-dimer (adjusted OR: 1.73; 95% CI: 1.20–2.50; p = .003) remained in the multivariable logistic regression adjusted for age, female, and symptom duration (Table 4).
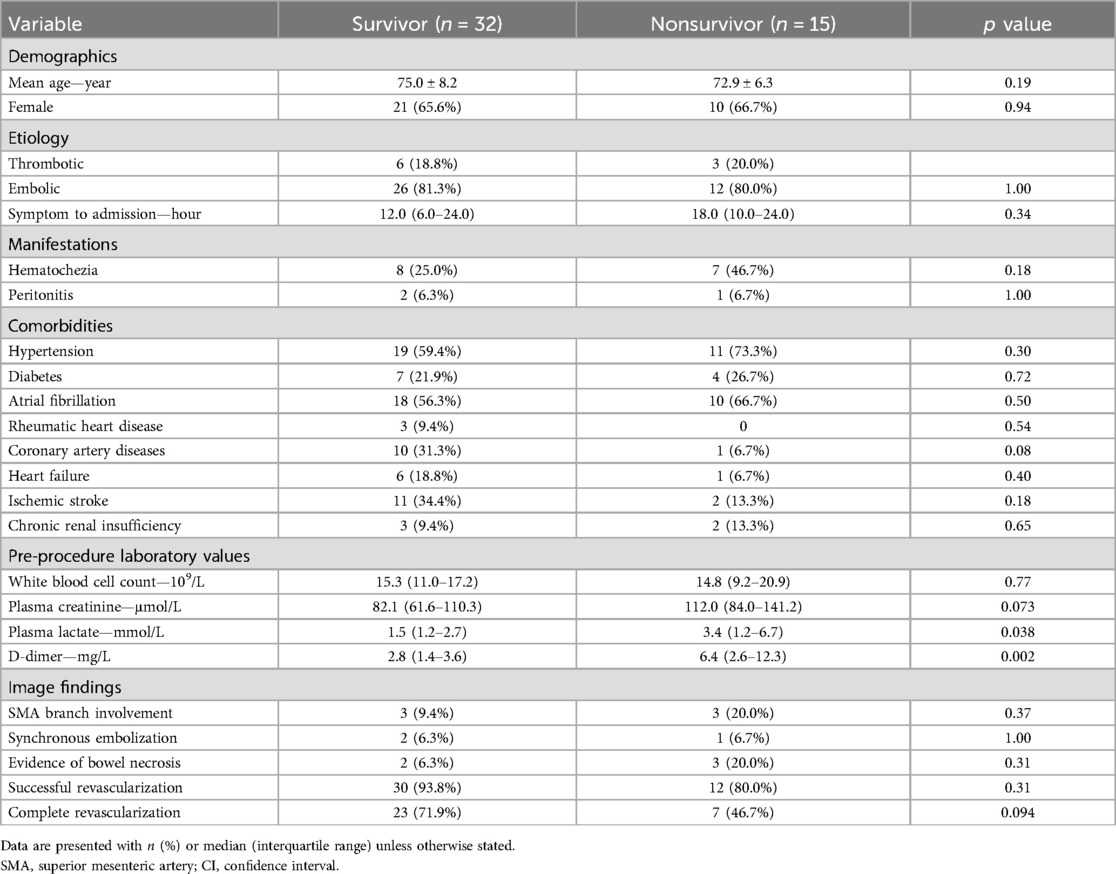
Table 3. Comparison of the potential risk factors for 30-day mortality between survivor and nonsurvivor.
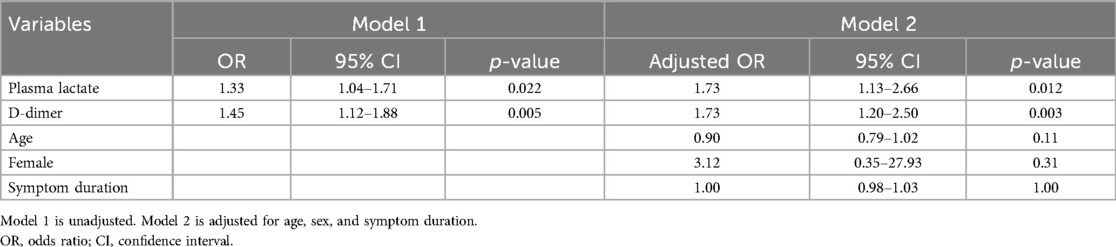
Table 4. Logistic regression models for the association between potential risk factors and 30-day mortality.
Discussion
The present study found that both PMT and CDT for the treatment of arterial AMI were associated with high successful revascularization and low complication rates. There was no significant difference in clinical outcomes between PMT and CDT. Elevated plasma lactate and D-dimer values were associated with increased 30-day mortality risk.
Arterial AMI remains a huge therapeutic challenge regardless of the increasingly advocated EVR first strategy (9, 22). In the recently published prospective multicenter AMESI study, the 30-day mortality for arterial AMI remained nearly 50% (2). Despite recent studies showing no significant difference regarding short-term mortality between EVR and OSR (5, 23), EVR first strategy is still preferred in patients with frail status or high morbidity burden (17).
Limited comparable evidence regarding endovascular treatment was available (11). CDT is a simple EVR technique, and it is associated with a satisfactory success rate and few complications (24, 25). In the present study, initial successful thrombolysis was achieved in 83.3% of patients, which is consistent with the previous publication (24). CDT is largely limited by a prolonged revascularization time, increased bleeding risk (24), and possible worsening during non-effective thrombolysis. As such, guidelines did not recommend CDT for patients with peritonitis and high bleeding risk (17).
Compared with CDT, PMT can promptly restore the compromised blood flow, thus potentially improving clinical outcomes. Manual aspiration using a large bore catheter is a simple and feasible PMT modality. Although the reported success rate was satisfactory (73.3%–100%) (26, 27), blood loss during aspiration may be a considerable issue (27). Moreover, the absence of revascularization after several passes should be considered a failure, and adjunctive thrombolysis or other PMT techniques may be needed. Stent thrombectomy is an important treatment modality for acute ischemic stroke with large vessel occlusion (28). This technique has been introduced to treat arterial AMI and achieved a satisfactory success rate (12). Previous studies suggested that Rotarex thrombectomy is associated with high technical success (14) and a satisfactory recanalization rate (14, 29). However, procedure-related complications, including vessel perforations and catheter tip fracture, have been reported (14, 29). Moreover, the rationale for Rotarex devices is thrombus fragmentation in combination with suction, which may be associated with the risk of distal embolization (29). The overall final successful revascularization rate for PMT in the present study was 89.7%, which is comparable to a previous study (29). The high successful revascularization rate in the present study may be associated with sufficient adjunctive therapies to address residual thrombus or pre-existing stenosis.
Few studies compared the outcomes between different EVR techniques. In a retrospective study including 31 patients, mechanical aspiration using the Indigo system (Penumbra, Inc., USA) failed to provide better results than manual aspiration (30). The present study compared PMT vs. CDT as the first EVR technique for arterial AMI, and no significant differences in clinical outcomes were observed. Unfortunately, the present study was unable to perform device-specific subgroup analyses due to the retrospective design and small sample size. Of note, the median symptom duration in the PMT group was significantly lower than in the CDT group, which may suggest that patients in the CDT group have less severe ischemia and are more able to tolerate thrombolysis.
Unfortunately, the present study failed to identify the association between successful revascularization and mortality, which may be attributed to the insufficient power of the study. But even in a larger study, the association between quality of revascularization and outcomes has not been shown (22). Various predictive factors for poor prognosis of AMI have been reported, such as advanced age (23, 31, 32), higher comorbidity burden (32), high C-reactive protein (22), and decreased bowel enhancement (22). The present study identified elevated plasma lactate and D-dimer values were associated with increased 30-day mortality. Elevated lactate levels are often associated with various severe pathological conditions such as sepsis and multiple organ dysfunction syndrome, which may lead to high mortality rates (33). The elevated D-dimer may be associated with a prolonged ischemia time (34) and more extensive thrombus, which may also lead to a poor prognosis. Patients with elevated lactate and elevated D-dimer levels may have advanced ischemia, and OSR should be considered in these patients to promptly restore blood flow and directly evaluate the bowel gangrene.
Some important limitations to the present study should be noticed. First, this was a single-center retrospective study with a limited sample size in a relatively long study period. Selection bias and other confounding factors inherent to the study design may have biased the results. For instance, patients had severe symptoms may have received PMT, and patients with milder symptoms may have received CDT. Additionally, a small cohort inherently restricts statistical power, particularly in detecting subtle differences between PMT and CDT. Second, three different techniques were classified as PMT, which might have influenced the results since the efficacy may vary among the different techniques. Third, the adjunctive use of thrombolysis and stent placement could bias an evaluation of the effect of PMT or CDT as standalone.
In conclusion, PMT and CDT were associated with a high successful revascularization rate and a low complication rate in the treatment of arterial occlusive AMI. No significant clinical differences between PMT and CDT were noted. Elevated plasma lactate and D-dimer values may be associated with increased 30-day mortality.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Institutional Review Board of Nanjing First Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants' legal guardians/next of kin because of the retrospective study design.
Author contributions
YS: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. YZ: Data curation, Formal analysis, Investigation, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. HH: Data curation, Formal analysis, Software, Visualization, Writing – original draft, Writing – review & editing. LC: Data curation, Formal analysis, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing. HS: Data curation, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing. JG: Conceptualization, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Tamme K, Reintam Blaser A, Laisaar KT, Mandul M, Kals J, Forbes A, et al. Incidence and outcomes of acute mesenteric ischaemia: a systematic review and meta-analysis. BMJ Open. (2022) 12(10):e062846. doi: 10.1136/bmjopen-2022-062846
2. Reintam Blaser A, Mandul M, Bjorck M, Acosta S, Bala M, Bodnar Z, et al. Incidence, diagnosis, management and outcome of acute mesenteric ischaemia: a prospective, multicentre observational study (AMESI study). Crit Care. (2024) 28(1):32. doi: 10.1186/s13054-024-04807-4
3. Clair DG, Beach JM. Mesenteric ischemia. N Engl J Med. (2016) 374(10):959–68. doi: 10.1056/NEJMra1503884
4. Aboyans V, Ricco JB, Bartelink MEL, Bjorck M, Brodmann M, Cohnert T, et al. Editor’s choice—2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European society for vascular surgery (ESVS). Eur J Vasc Endovasc Surg. (2018) 55(3):305–68. doi: 10.1016/j.ejvs.2017.07.018
5. Shi Y, Zhao B, Zhou Y, Chen L, Su H, Gu J. Endovascular revascularization vs open surgical revascularization as the first strategy for arterial acute mesenteric ischemia: a systematic review and meta-analysis. J Vasc Surg. (2024) 80(6):1883–93.e2. doi: 10.1016/j.jvs.2024.07.084
6. Salsano G, Salsano A, Sportelli E, Petrocelli F, Dahmane M, Spinella G, et al. What is the best revascularization strategy for acute occlusive arterial mesenteric ischemia: systematic review and meta-analysis. Cardiovasc Intervent Radiol. (2018) 41(1):27–36. doi: 10.1007/s00270-017-1749-3
7. Erben Y, Protack CD, Jean RA, Sumpio BJ, Miller SM, Liu S, et al. Endovascular interventions decrease length of hospitalization and are cost-effective in acute mesenteric ischemia. J Vasc Surg. (2018) 68(2):459–69. doi: 10.1016/j.jvs.2017.11.078
8. Zettervall SL, Lo RC, Soden PA, Deery SE, Ultee KH, Pinto DS, et al. Trends in treatment and mortality for mesenteric ischemia in the United States from 2000 to 2012. Ann Vasc Surg. (2017) 42:111–9. doi: 10.1016/j.avsg.2017.01.007
9. Eslami MH, Rybin D, Doros G, McPhee JT, Farber A. Mortality of acute mesenteric ischemia remains unchanged despite significant increase in utilization of endovascular techniques. Vascular. (2016) 24(1):44–52. doi: 10.1177/1708538115577730
10. Gries JJ, Virk HUH, Chen B, Sakamoto T, Alam M, Krittanawong C. Advancements in revascularization strategies for acute mesenteric ischemia: a comprehensive review. J Clin Med. (2024) 13(2):570. doi: 10.3390/jcm13020570
11. Ierardi AM, Tsetis D, Sbaraini S, Angileri SA, Galanakis N, Petrillo M, et al. The role of endovascular therapy in acute mesenteric ischemia. Ann Gastroenterol. (2017) 30(5):526–33. doi: 10.20524/aog.2017.0164
12. Shi Y, Gu J, Chen L, Shi W, Ahmed MJ, Huang H, et al. Mechanical thrombectomy using the solitaire AB device for acute embolic mesenteric ischemia. J Vasc Interv Radiol. (2019) 30(1):43–8. doi: 10.1016/j.jvir.2018.08.005
13. Shi Y, Su H, Chen L, Huang H, Lu Z, Gu J. Endovascular revascularization as primary treatment for acute embolic mesenteric ischemia: stent thrombectomy plus aspiration versus aspiration alone. J Vasc Interv Radiol. (2022) 33(3):295–303. doi: 10.1016/j.jvir.2021.12.008
14. Freitas B, Bausback Y, Schuster J, Ulrich M, Braunlich S, Schmidt A, et al. Thrombectomy devices in the treatment of acute mesenteric ischemia: initial single-center experience. Ann Vasc Surg. (2018) 51:124–31. doi: 10.1016/j.avsg.2017.11.041
15. Acosta S, Bjorck M. Modern treatment of acute mesenteric ischaemia. Br J Surg. (2014) 101(1):e100–8. doi: 10.1002/bjs.9330
16. Karkkainen JM, Acosta S. Acute mesenteric ischemia (part II)—vascular and endovascular surgical approaches. Best Pract Res Clin Gastroenterol. (2017) 31(1):27–38. doi: 10.1016/j.bpg.2016.11.003
17. Bjorck M, Koelemay M, Acosta S, Bastos Goncalves F, Kolbel T, Kolkman JJ, et al. Editor’s choice—management of the diseases of mesenteric arteries and veins: clinical practice guidelines of the European society of vascular surgery (ESVS). Eur J Vasc Endovasc Surg. (2017) 53(4):460–510. doi: 10.1016/j.ejvs.2017.01.010
18. Karkkainen JM, Lehtimaki TT, Saari P, Hartikainen J, Rantanen T, Paajanen H, et al. Endovascular therapy as a primary revascularization modality in acute mesenteric ischemia. Cardiovasc Intervent Radiol. (2015) 38(5):1119–29. doi: 10.1007/s00270-015-1064-9
19. Block TA, Acosta S, Bjorck M. Endovascular and open surgery for acute occlusion of the superior mesenteric artery. J Vasc Surg. (2010) 52(4):959–66. doi: 10.1016/j.jvs.2010.05.084
20. Tual A, Garzelli L, Nuzzo A, Corcos O, Castier Y, Ben Abdallah I, et al. Strengthening the description of superior mesenteric artery occlusions in acute mesenteric ischaemia: proposition for an anatomical classification. Eur J Vasc Endovasc Surg. (2023) 65(6):802–8. doi: 10.1016/j.ejvs.2023.01.041
21. Kanasaki S, Furukawa A, Fumoto K, Hamanaka Y, Ota S, Hirose T, et al. Acute mesenteric ischemia: multidetector CT findings and endovascular management. Radiographics. (2018) 38(3):945–61. doi: 10.1148/rg.2018170163
22. Garzelli L, Dufay R, Tual A, Corcos O, Cazals-Hatem D, Vilgrain V, et al. Predictors of survival without intestinal resection after first-line endovascular revascularization in patients with acute arterial mesenteric ischemia. Radiology. (2024) 311(3):e230830. doi: 10.1148/radiol.230830
23. Goto D, Yanishi K, Ozawa T, Yoshimura J, Kawamata H, Fujioka A, et al. Comparison of endovascular therapy and open surgical revascularization in patients with acute superior mesenteric artery occlusion: a large-scale analysis based on the JROAD-DPC database. J Am Heart Assoc. (2024) 13(12):e035017. doi: 10.1161/JAHA.124.035017
24. Bjornsson S, Bjorck M, Block T, Resch T, Acosta S. Thrombolysis for acute occlusion of the superior mesenteric artery. J Vasc Surg. (2011) 54(6):1734–42. doi: 10.1016/j.jvs.2011.07.054
25. Yanar F, Agcaoglu O, Sarici IS, Sivrikoz E, Ucar A, Yanar H, et al. Local thrombolytic therapy in acute mesenteric ischemia. World J Emerg Surg. (2013) 8(1):8. doi: 10.1186/1749-7922-8-8
26. Raupach J, Lojik M, Chovanec V, Renc O, Strycek M, Dvorak P, et al. Endovascular management of acute embolic occlusion of the superior mesenteric artery: a 12-year single-centre experience. Cardiovasc Intervent Radiol. (2016) 39(2):195–203. doi: 10.1007/s00270-015-1156-6
27. Jia Z, Jiang G, Tian F, Zhao J, Li S, Wang K, et al. Early endovascular treatment of superior mesenteric occlusion secondary to thromboemboli. Eur J Vasc Endovasc Surg. (2014) 47(2):196–203. doi: 10.1016/j.ejvs.2013.09.025
28. Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, et al. Thrombectomy within 8 h after symptom onset in ischemic stroke. N Engl J Med. (2015) 372(24):2296–306. doi: 10.1056/NEJMoa1503780
29. Thurner A, Peter D, Dalla Torre G, Flemming S, Kickuth R. Safety, efficacy and outcome of rotational thrombectomy assisted endovascular revascularisation of the superior mesenteric artery in acute thromboembolic mesenteric ischaemia. Rofo. (2024) 196(10):1055–62. doi: 10.1055/a-2234-0333
30. Garzelli L, Ben Abdallah I, Nuzzo A, Corcos O, Castier Y, Ronot M. Endovascular thrombectomy for acute arterial mesenteric ischaemia: no benefit of mechanical over manual thrombus aspiration. Eur J Vasc Endovasc Surg. (2022) 64(1):128–9. doi: 10.1016/j.ejvs.2022.05.020
31. Newton WB 3rd, Sagransky MJ, Andrews JS, Hansen KJ, Corriere MA, Goodney PP, et al. Outcomes of revascularized acute mesenteric ischemia in the American college of surgeons national surgical quality improvement program database. Am Surg. (2011) 77(7):832–8. doi: 10.1177/000313481107700715
32. Bette S, Habeeballah O, Luitjens JH, Kroencke T, Scheurig-Muenkler C, Decker JA. Treatment of acute mesenteric ischemia between 2010 and 2020—a German nation-wide study. BMC Gastroenterol. (2023) 23(1):300. doi: 10.1186/s12876-023-02926-w
33. Leone M, Bechis C, Baumstarck K, Ouattara A, Collange O, Augustin P, et al. Outcome of acute mesenteric ischemia in the intensive care unit: a retrospective, multicenter study of 780 cases. Intensive Care Med. (2015) 41(4):667–76. doi: 10.1007/s00134-015-3690-8
34. Cakir M, Yildirim D, Sarac F, Donmez T, Mirapoglu S, Hut A, et al. In the experimental model of acute mesenteric ischemia, the correlation of blood diagnostic parameters with the duration of ischemia and their effects on choice of treatment. J Invest Surg. (2019) 32(6):507–14. doi: 10.1080/08941939.2018.1437486
Keywords: acute mesenteric ischemia, endovascular revascularization, catheter-directed thrombolysis, risk factors, 30-day mortality, percutaneous mechanical thrombectomy
Citation: Shi Y, Zhou Y, Huang H, Chen L, Su H and Gu J (2025) Percutaneous mechanical thrombectomy versus catheter-directed thrombolysis for the treatment of arterial acute mesenteric ischemia and risk factors for 30-day mortality. Front. Cardiovasc. Med. 12:1553170. doi: 10.3389/fcvm.2025.1553170
Received: 30 December 2024; Accepted: 2 May 2025;
Published: 16 May 2025.
Edited by:
Dragos Cretoiu, Carol Davila University of Medicine and Pharmacy, RomaniaReviewed by:
Wenhan Wu, Shanghai Jiao Tong University, ChinaQiyang Xu, Ningbo Second Hospital, China
Minh Bao Luan Tran, University of Medicine and Pharmacy at Ho Chi Minh City, Vietnam
Copyright: © 2025 Shi, Zhou, Huang, Chen, Su and Gu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianping Gu, Z3VqaWFucGluZ19uakAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Yadong Shi†
Yadong Shi† Haobo Su
Haobo Su Jianping Gu
Jianping Gu