Abstract
Background:
Atrial fibrillation (AF) is the most common sustained arrhythmia in adults and is associated with significant morbidity and mortality. Mitral stenosis (MS) is common in developing countries, affecting the younger population and posing a risk of atrial fibrillation.
Objectives:
This study aims to delineate the clinical characteristics and poor outcomes in patients who have recent non-valvular AF (NVAF) compared with AF related to MS (MS-AF). Furthermore, it seeks to assess the healthcare resource utilization associated with the management of AF patients in the two groups.
Patients and methods:
This is a prospective observational cohort conducted on 84 patients with recent AF. The patients were divided into two groups: the NVAF group (patients with no prosthetic valves or moderate/severe MS) and the MS-AF group (patients with AF in the presence of moderate/severe MS). The clinical characteristics, stroke risk, and anticoagulation regimens were assessed. AF-related outcomes (strokes, hospitalizations, major bleeding, and mortalities) were monitored and compared between the two groups.
Results:
The mean age of the studied AF patients was 49.80 ± 16.31 years, ranging from 25 to 89 years. Patients with MS-AF were significantly younger than patients with NVAF. Hypertension was the most prevalent risk factor associated with AF. Smoking, heart failure, and hypertension were more prevalent among patients with NVAF. The NVAF group received less anticoagulants than the MS-AF group. There were no statistically significant differences between the two groups regarding the overall incidence of death, stroke, myocardial infarction, TIA, or hospital admissions. In the overall studied group, all-cause mortality was higher among AF patients with a history of heart failure or stroke.
Conclusion:
Patients with NVAF had a significantly greater incidence of cardiovascular disease risk factors. However, AF related to mitral stenosis was associated with comparable worse outcomes.
Introduction
Atrial fibrillation (AF) is the most common sustained arrhythmia, and its incidence is increasing globally. It is associated with a substantial risk of stroke, heart failure, recurrent hospitalization, and death. It is also an important cause of healthcare burden and costs.
AF is broadly classified as either valvular or non-valvular, according to the recent ESC guidelines. The distinction between non-valvular atrial fibrillation (NVAF) and valvular atrial fibrillation—defined as AF in the presence of moderate-to-severe mitral stenosis (MS) or mechanical prosthetic heart valves—is clinically significant, as these groups differ not only in thromboembolic risk but also in recommended anticoagulation strategies (1). Globally, rheumatic heart disease remains the leading cause of mitral stenosis, with a particularly high prevalence in developing countries. Unlike degenerative mitral valve disease, rheumatic mitral stenosis tends to affect a younger demographic, often presenting in early adulthood. Patients with rheumatic mitral stenosis are also at a significantly increased risk of developing atrial fibrillation compared with individuals without structural mitral valve disease. Despite this elevated risk, they have been systematically excluded from most major clinical trials evaluating anticoagulation strategies in atrial fibrillation, leading to a gap in evidence-based management for this population.
The current study aims to evaluate the demographic data, worse outcomes, and healthcare resource utilization in the newly diagnosed AF related to mitral stenosis, compared with non-valvular AF.
Methods
Study population
This prospective observational cohort was conducted at a tertiary center (Assiut University Heart Hospital, Egypt) from July 2022 to July 2023. It enrolled patients presenting with recent-onset atrial fibrillation (3 months) in the presence of moderate-to-severe mitral stenosis (MS), along with a matched cohort of patients with recent NVAF patients.
All patients provided informed consent. The study was conducted in compliance with the principles of the Helsinki Declaration on human research and received approval from local institutional committees.
Inclusion criteria: all adult cases, male or female, aged 18 years or older at the time of enrollment, who presented with recent-onset AF.
Exclusion criteria: (1) AF occurring in specific clinical contexts, including secondary AF related to thyrotoxicosis, the postoperative period, or acute myocardial infarction, (2) patients with mechanical heart valves or valve disease (other than mitral valve) scheduled for surgical valve replacement, (3) coexisting medical conditions requiring long-term anticoagulation for reasons other than AF, or (4) current pregnancy or breastfeeding.
Definitions
AF was defined according to the 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation (1). NVAF was defined as AF in the absence of moderate/severe MS and prosthetic valves. MS severity was assessed according to the 2021 ESC/EACTS guidelines for the management of valvular heart disease (2). Moderate mitral stenosis is defined as MVA ≤2 cm2 and severe (or significant) MS MVA ≤1.5 cm2 as assessed by transthoracic echocardiography. Patients were classified into two groups: those with non-valvular atrial fibrillation (NVAF) and those with atrial fibrillation in the presence of moderate-to-severe mitral stenosis (MS-AF).
Study outcomes and follow-up
Demographic, clinical, laboratory, medication, and echocardiographic parameters were compared between the two groups. All enrolled patients were followed up at 6 months and 1 year. Adverse outcomes were recorded and compared between the two groups. They included all-cause mortality, ischemic cerebrovascular stroke, transient ischemic attack (TIA), myocardial infarction, major and minor bleeding events, and hospitalization. Major bleeding was defined as any bleeding event associated with one or more of the following: a drop in hemoglobin of ≥2 g/dl within 24 h, the need for ≥2 units of packed red blood cells, bleeding in critical sites (intracranial, intraspinal, intraocular, intra-articular, pericardial, retroperitoneal, intramuscular with compartment syndrome), or death. Minor bleeding was defined as any bleeding that did not meet the criteria for major bleeding. Hospitalization was defined as any initial hospital admission (even if <24 h) related to atrial fibrillation or associated adverse outcomes. Medication prescription and management decisions, including rate and rhythm control strategies, were made in accordance with standard clinical practice. The economic burden of AF management was estimated by calculating total healthcare resource utilization costs, derived by multiplying each unit of service by its corresponding estimated cost. Healthcare utilization was assessed based on the total number of hospital admissions, length of hospital stays, outpatient visits, and the number of ECG and echocardiographic examinations performed.
Certain considerations have been addressed regarding the handling of missing data and participant dropouts. Patients with missing critical outcome data during the 12-month follow-up were excluded from the respective analyses. The overall rate of missing data was low (<5%) and evenly distributed between the MS-AF and NVAF groups. Therefore, no imputation methods were applied. Patients lost to follow-up were not included in survival or event-based outcome analyses.
Statistical analysis
All statistical calculations were done by using Statistical Package for the Social Sciences (SPSS Inc., Chicago, IL, USA) version 22. Quantitative data were described using mean ± standard deviation (SD) and median with range, as appropriate. Qualitative data were summarized using frequencies (number of cases) and relative frequencies (percentages) when appropriate. Comparison of quantitative variables was done using Student’s t-test for normally distributed data and Mann–Whitney U test for non-normally distributed data. Friedman test was used for comparing continuous data over time. For comparing categorical data, Chi-square (χ2) test was performed. Fisher’s exact test was used when the expected frequency was <5. Kaplan–Meier's method with log-rank test was used for overall survival (OS) analysis. Hazard ratio (HR) with a 95% confidence interval (CI) and Cox regression analysis were calculated for the prediction of all causes of mortality risk factors among the studied participants. A p-value of <0.05 was considered statistically significant.
Results
The study included 84 patients with recent-onset atrial fibrillation (within the past 3 months). This included 42 patients with AF and moderate/severe mitral stenosis (MS-AF group) and a matched number of non-valvular AF patients (NVAF group). The mean age of all AF cases was 49.80 ± 16.31 (ranging from 25 to 89 years old). Hypertension was the most prevalent risk factor associated with AF (28 cases, 33.3%). Patients with MS-AF were significantly younger than patients with NVAF. History of smoking, heart failure, or hypertension was more prevalent among patients with NVAF (p = 0.046, 0.010, and <0.001, respectively). The two groups were comparable in terms of body mass index (BMI), gender, and the remaining risk factors associated with AF (p > 0.05 for all). Patients' characteristics in both groups are shown in Table 1.
Table 1
| Patients’ characteristics | Total (n = 84) | MS-AF (n = 42) | Non-valvular AF (n = 42) | p-value | |||
|---|---|---|---|---|---|---|---|
| Age (years) | 49.80 ± 16.31 | 41.98 ± 13.76 | 57.62 ± 14.97 | <0.001 | |||
| Age groups, n (%) | <0.001 | ||||||
| 18–40 | 36 | (42.9) | 27 | (64.3) | 9 | (21.4) | |
| 41–59 | 23 | (27.4) | 11 | (26.2) | 12 | (28.6) | |
| 60–74 | 16 | (19.0) | 3 | (7.1) | 13 | (31.0) | |
| ≥75 | 9 | (10.7) | 1 | (2.4) | 8 | (19.0) | |
| Sex, n (%) | 0.081 | ||||||
| Male | 42 | (50.0) | 17 | (40.5) | 25 | (59.5) | |
| BMI (kg/m2) | 21.52 ± 3.91 | 21.90 ± 3.60 | 21.14 ± 4.21 | 0.375 | |||
| Risk factors, n (%) | |||||||
| Family history AF | 3 | (3.6) | 1 | (2.4) | 2 | (4.8) | 1 |
| Smoking | 15 | (17.9) | 4 | (9.5) | 11 | (26.2) | 0.046 |
| Heart failure | 15 | (17.9) | 3 | (7.1) | 12 | (28.6) | 0.010 |
| Hypertension | 28 | (33.3) | 6 | (14.3) | 22 | (52.4) | <0.001 |
| Previous stroke | 7 | (8.3) | 4 | (9.5) | 3 | (7.1) | 1 |
| Diabetes mellitus | 21 | (25.0) | 11 | (26.2) | 10 | `(23.8) | 0.801 |
| CKD | 9 | (10.7) | 2 | (4.8) | 7 | (16.7) | 0.156 |
| CAD | 15 | (17.9) | 7 | (16.7) | 8 | (19.0) | 0.776 |
| COPD | 9 | (10.7) | 3 | (7.1) | 6 | (14.3) | 0.483 |
| PAD | 2 | (2.4) | 1 | (2.4) | 1 | (2.4) | 1 |
| Dyslipidemia | 8 | (9.5) | 2 | (4.8) | 6 | (14.3) | 0.265 |
| Thyroid disease | 1 | (1.2) | 0 | (0.0) | 1 | (2.4) | 1 |
Patients’ characteristics in the studied groups.
Bold values indicate significant p value.
AF, atrial fibrillation; BMI, body mass index; CKD, chronic kidney disease; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; MS-AF, AF related to mitral stenosis; PAD, peripheral arterial disease. Quantitative data are presented as mean ± SD, and qualitative data are presented as number (percentage). Significance defined by p < 0.05.
Patients with MS-AF had significantly larger left atrial volume than patients with NVAF (48.1 ± 4.9 cm3 vs. 41.3 ± 5.2 cm3, respectively; p < 0.001), while left ventricle ejection fraction (LVEF) (%) showed no significant difference between the two groups (p = 0.059). Systolic blood pressure was significantly higher in patients with NVAF. Stroke risk in patients with NVAF, as assessed by CHA2DS2-VASc score, showed that most of the patients (64.3%) were at high risk (Table 2).
Table 2
| Variables | MS-AF (n = 42) | Non-valvular AF (n = 42) | p-value | |
|---|---|---|---|---|
| Echocardiographic characteristics | ||||
| LVEF (%) | 58.57 ± 5.99 | 53.95 ± 14.44 | 0.059 | |
| LA volume (cm3) | 48.1 ± 4.9 | 41.3 ± 5.2 | <0.001 | |
| Blood pressure and HR, median (range) | ||||
| SBP (mmHg) | 110 (90–150) | 130 (90–180) | 0.010 | |
| DBP (mmHg) | 80 (60–90) | 80 (50–110) | 0.343 | |
| HR (beats/min) | 100 (80–150) | 100 (45–140) | 0.372 | |
| CHA2DS2-VASc score | ||||
| Low risk (0) | NA | 4 | (9.5%) | |
| Moderate risk (1) | NA | 11 | (26.2%) | |
| High risk (≥2) | NA | 27 | (64.3%) | |
Clinical and echocardiographic characteristics in studied groups.
Bold values indicate significant p value.
CHA2DS2-VASc = congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, stroke, vascular disease, age 65–74 years, sex category (female). HR, heart rate; LVEF, left ventricle ejection fraction; MS-AF, AF related to mitral stenosis; NA, not applicable. Quantitative data are presented as mean ± SD or median (range), and qualitative data are presented as number (percentage). Significance defined by p < 0.05.
Oral anticoagulants were used in all MS-AF patients and 27 (64.3%) NVAF patients (p < 0.001). Warfarin was used in all MS-AF patients and in 19 NVAF patients (70.4% of those who received anticoagulation). Novel oral anticoagulants (NOACs) were used in none of the MS-AF patients and in eight NVAF patients (29.6% of those who received anticoagulants). Patients with MS-AF were more likely to receive digoxin (p = 0.027) and diuretics (p = 0.001), while patients with NVAF were more likely to receive angiotensin-converting enzyme (ACE) inhibitors (p = 0.001). Medications received by the studied groups are shown in Table 3.
Table 3
| Medications | MS-AF AF (n = 42) | Non-valvular (n = 42) | p-value | ||
|---|---|---|---|---|---|
| Anticoagulant | 42 | (100.0) | 27 | (64.3) | <0.001 |
| Type of anticoagulant | <0.001 | ||||
| Warfarin | 42 | (100.0) | 19 | (70.4) | |
| NOACs | 0 | (0.0) | 8 | (29.6) | |
| Aspirin | 7 | (16.7) | 12 | (28.6) | 0.192 |
| Beta-blocker | 38 | (90.5) | 32 | (76.2) | 0.079 |
| CCB | 5 | (11.9) | 11 | (26.2) | 0.095 |
| Digoxin | 23 | (54.8) | 13 | (31.0) | 0.027 |
| ACE inhibitor | 2 | (4.8) | 18 | (42.9) | <0.001 |
| ARBs | 1 | (2.4) | 3 | (7.1) | 0.616 |
| ARNI | 0 | (0.0) | 2 | (4.8) | 0.494 |
| Diuretics | 34 | (81.0) | 20 | (47.6) | 0.001 |
| Other medication | 0.054 | ||||
| Penicillin | 4 | (9.5) | 0 | (0.0) | |
| Amiodaron | 1 | (2.4) | 0 | (0.0) | |
| Carbimazol | 0 | (0.0) | 1 | (2.4) | |
| Chemotherapy | 1 | (2.4) | 0 | (0.0) | |
Medication received by the studied groups.
Significance defined by p < 0.05. Bold values indicate significant p value.
ACE, angiotensin-converting enzyme; ARBs, angiotensin receptor blockers; ARNI, angiotensin receptor neprilysin inhibitor; CCB, calcium channel blocker; MS-AF, AF related to mitral stenosis; NOAC, novel oral anticoagulants.
In AF patients who received warfarin, there was no significant difference between the two groups regarding the therapeutic range (TTR) of anticoagulation (assessed by prothrombin time, prothrombin concentration, and INR) at baseline, 6 months, and 1 year. The percentage of days within the therapeutic range was 61% in MS-AF and 50% in NVAF patients, with no significant difference (p = 0.650) (Table 4).
Table 4
| INR level | MS-AF | Non-valvular AF | p-value | ||
|---|---|---|---|---|---|
| Baseline | 25 | (59.5%) | 10 | (52.6%) | 0.920 |
| Below therapeutic range | |||||
| Within therapeutic range | 12 | (29.3%) | 7 | (36.8%) | |
| Above therapeutic range | 5 | (12.2%) | 2 | (10.5%) | |
| After 6 months | 14 | (34.1%) | 8 | (42.1%) | 0.652 |
| Below therapeutic range | 19 | (46.3%) | 9 | (47.4%) | |
| Within therapeutic range | |||||
| Above therapeutic range | 8 | (19.5%) | 2 | (10.5%) | |
| After 12 months | 7 | (18.9%) | 3 | (21.4%) | 1 |
| Below therapeutic range | 25 | (67.6%) | 10 | (71.4%) | |
| Within therapeutic range | |||||
| Above therapeutic range | 5 | (13.5%) | 1 | (7.1%) | |
| Percent day within therapeutic range | 61% (20–100) | 50% (10–85) | 0.650 | ||
INR follow-up and time in therapeutic range (TTR) for patients who received warfarin.
The overall incidence of mortality, stroke, transient ischemic attacks, myocardial infarction, and hospital admissions was high among all AF patients, with a total of 11 deaths (eight cardiovascular, two non-cardiovascular, and one with unknown cause of death), seven strokes, and 47 hospitalizations during the follow-up period. There was no statistically significant difference between the two groups regarding deaths (11.9% vs. 14.3%, p = 0.746), stroke (7.1% vs. 9.5%, p = 1), transient ischemic attacks (0.0% vs. 7.1%, p = 0.241), myocardial infarction (4.8% vs. 2.4%, p = 1), or cardiac-related hospitalizations (61.9% vs. 50.0%, p = 0.272) for MS-AF and NVAF patients (Table 5). The incidence of major and minor bleeding was also comparable between the two groups, with no significant difference (p = 0.483).
Table 5
| One-year outcome | MS-AF (n = 42) | Non-valvular (n = 42) | p-value | ||
|---|---|---|---|---|---|
| All-cause mortality | 5 | (11.9) | 6 | (14.3) | 0.746 |
| Causes of death | 0.697 | ||||
| Cardiovascular death | 3 | (60.0) | 5 | (83.3) | |
| Non-cardiovascular death | 1 | (20.0) | 1 | (16.7) | |
| Unknown cause | 1 | (20.0) | 0 | (0.0) | |
| Stroke | 3 | (7.1) | 4 | (9.5) | 1 |
| Myocardial infarction | 2 | (4.8) | 1 | (2.4) | 1 |
| Transient ischemic attack | 0 | (0.0) | 3 | (7.1) | 0.241 |
| Major bleeding | 2 | (4.8) | 1 | (2.4) | 1 |
| Minor bleeding | 5 | (11.9) | 7 | (16.7) | 0.533 |
| Hospitalization | 26 | (61.9) | 21 | (50.0) | 0.272 |
Comparison of outcomes between the studied groups.
Qualitative data are presented as number (percentage). Significance defined by p < 0.05.
Among the whole studied AF patients, the mortality rate was 13.1% at a mean follow-up of 11 months. According to Kaplan–Meier analysis, the overall survival (OS) rate at 11 months was 86.9%. Among all clinical parameters, patient age and history of heart failure, previous stroke, and/or chronic kidney disease (CKD) were observed to significantly affect the overall survival of the studied participants. However, the overall survival was not affected by the type of AF (MS-AF vs. NVAF). Survival analysis of variable parameters is shown in Table 6 and Figure 1.
Table 6
| Demographic and clinical parameters |
OS (12 months) | |
|---|---|---|
| Estimate ± SE | p-value | |
| Age (years) | 0.003 | |
| <50 | 97.7 ± 2.2 | |
| ≥50 | 75.0 ± 6.8 | |
| Sex | 0.739 | |
| Male | 88.1 ± 5.0 | |
| Female | 85.6 ± 5.4 | |
| Smoking | 0.424 | |
| No | 85.4 ± 4.3 | |
| Yes | 93.3 ± 6.4 | |
| Heart failure | <0.001 | |
| No | 94.2 ± 2.8 | |
| Yes | 53.3 ± 12.9 | |
| Hypertension | 0.836 | |
| No | 87.4 ± 4.4 | |
| Yes | 85.7 ± 6.6 | |
| Previous stroke | <0.001 | |
| No | 90.9 ± 3.3 | |
| Yes | 42.9 ± 18.7 | |
| Diabetes mellitus | 0.413 | |
| No | 88.9 ± 4.0 | |
| Yes | 81.0 ± 8.6 | |
| CKD | 0.047 | |
| No | 80.3 ± 3.6 | |
| Yes | 66.7 ± 15.7 | |
| CAD | 0.376 | |
| No | 88.3 ± 3.9 | |
| Yes | 80.0 ± 10.3 | |
| COPD | 0.857 | |
| No | 86.6 ± 3.9 | |
| Yes | 88.9 ± 10.5 | |
| Dyslipidemia | 0.267 | |
| No | 88.1 ± 3.7 | |
| Yes | 75.0 ± 15.3 | |
| Type of AF | 0.759 | |
| MS-AF | 88.0 ± 5.0 | |
| NVAF | 85.7 ± 5.4 | |
Overall survival analysis according to demographic and clinical parameters.
Significance defined by p < 0.05. Bold values indicate significant p value.
AF, atrial fibrillation; BMI, body mass index; CKD, chronic kidney disease; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; MS-AF, AF related to mitral stenosis; NVAF, non-valvular AF.
Figure 1
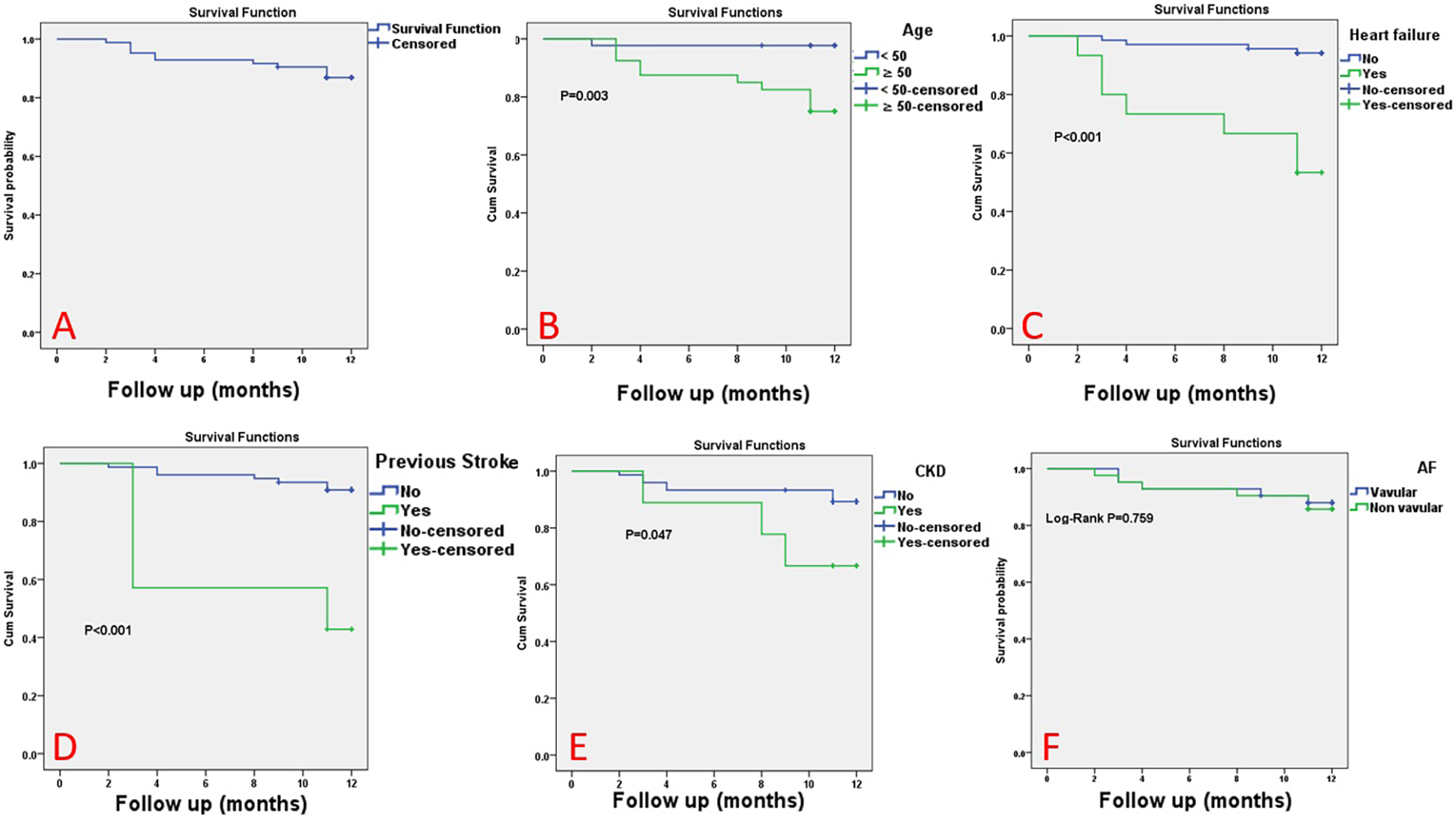
The Kaplan–Meier survival curve for the studied patients. The Kaplan–Meier survival curve for all studied atrial fibrillation (AF) patients (A), according to age (B), heart failure (C), previous stroke (D), chronic kidney disease (E), and type of AF (non-valvular AF vs. MS-related AF) (F).
According to multivariate analysis, AF patients with a history of heart failure and/or stroke had a greater likelihood of death. Among all AF patients, a history of heart failure was associated with a 10-fold increase in the risk of mortality (OR = 9.512, 95% CI: 12.169–41.706, p = 0.003), while a history of stroke was associated with a ninefold increase in the risk of mortality (OR = 8.773, 95% CI: 1.951–39.426, p = 0.005) (Table 7).
Table 7
| Variables | Multivariate analysis | ||
|---|---|---|---|
| HR | 95% CI | p-value | |
| Age (years) | |||
| <50 | Ref | ||
| ≥50 | 3.397 | 0.363–31.753 | 0.284 |
| Heart failure | |||
| No | Ref | ||
| Yes | 9.512 | 2.169–41.706 | 0.003 |
| History of stroke | |||
| No | Ref | ||
| Yes | 8.773 | 1.951–39.426 | 0.005 |
| CKD | |||
| No | Ref | ||
| Yes | 0.978 | 0.230–4.165 | 0.976 |
Cox regression analysis for prediction of death among the studied patients.
Bold values indicate significant p value.
CI, confidence interval; HR, hazard ratio. A p-value of ≤0.05 is significant.
Among all studied patients, 55 patients (65.5%) required inpatient readmission (46 cases for once, and 9 cases for two times). An echocardiography exam was performed once for 68 patients (81%) and twice for 16 patients (19%). The median hospital stay was two days (range, 0–12 days), the median number of outpatient visits was three (range, 0–8 times), and the median number of ECGs was four (range, 1–8 times). No significant differences were observed between the two groups regarding overall healthcare resource utilization (p > 0.05, for all), as shown in Table 8.
Table 8
| Outcome | Total (n = 84) | MS-AF (n = 42) | Non-valvular (n = 42) | p-value | |||
|---|---|---|---|---|---|---|---|
| Inpatient admission | 0.214 | ||||||
| 0 | 29 | (34.5) | 11 | (26.2) | 18 | (42.9) | |
| 1 | 46 | (54.8) | 27 | (64.3) | 19 | (45.2) | |
| 2 | 9 | (10.7) | 4 | (9.5) | 5 | (11.9) | |
| No. of echocardiography exams | 0.578 | ||||||
| 1 | 68 | (81.0) | 33 | (78.6) | 35 | (83.3) | |
| 2 | 16 | (19.0) | 9 | (21.4) | 7 | (16.7) | |
| Median hospital stays (days) | 2 (0–12) | 2 (0–9) | 2 (0–12) | 0.514 | |||
| Median outpatient visits | 3 (0–8) | 4 (0–7) | 3 (0–8) | 0.455 | |||
| ECG follow-up times | 4 (1–8) | 4 (2–8) | 5 (1–6) | 0.632 | |||
Healthcare resource utilization in both groups.
Quantitative data are presented as median (range).
Discussion
This single-center study, conducted in Egypt, aimed to compare the epidemiological characteristics and clinical outcomes of atrial fibrillation (AF) related to moderate-to-severe mitral stenosis (MS-AF) with those of non-valvular atrial fibrillation (NVAF). A total of 84 newly diagnosed AF patients were enrolled, comprising 42 consecutive patients with MS-AF and a matched cohort of 42 patients with NVAF. The mean age of the study population was 49.8 years, with the MS-AF group being significantly younger than the NVAF group. The overall male-to-female ratio was 1:1; however, male predominance was observed in the NVAF group, while females predominated in the MS-AF group. A history of smoking, heart failure, and hypertension was more frequently observed among patients with NVAF. In alignment with prior studies (3–6), hypertension emerged as the most prevalent risk factor for AF in the overall cohort.
Rheumatic fever remains the leading global cause of mitral stenosis, with a declining prevalence in developed countries; however, it continues to represent a significant health burden in many developing regions (7–9). Advancing age is a well-established risk factor for AF, which is frequently associated with a variety of underlying etiologies and comorbid conditions (7–9). In contrast, rheumatic mitral stenosis typically affects younger individuals and is strongly associated with a high incidence of AF, primarily due to chronic left atrial pressure and volume overload (10). In the present study, patients with MS-AF demonstrated significantly greater left atrial volume compared with those with NVAF.
Although AF is generally more common in men—with European data indicating incidence rates roughly 20% higher in men than in women (7–11)—our cohort showed a predominance of mitral stenosis–related AF in women. This sex difference likely reflects the higher prevalence of rheumatic mitral stenosis among females (12). Notably, many of these women are of childbearing age, placing them at increased risk of maternal cardiovascular complications and posing distinctive challenges for stroke prevention and anticoagulation management during pregnancy.
At 1-year follow-up, the overall incidence rates of all-cause mortality, stroke, and major bleeding were notably high, occurring in 13.1%, 8.3%, and 3.6% of patients, respectively, with no statistically significant differences observed between the MS-AF and NVAF groups. In patients with NVAF, the risk of stroke and thromboembolism is well recognized to increase with age and higher CHA₂DS₂-VASc scores (13); in our study, 64.3% of NVAF patients had scores ≥2, indicating elevated thromboembolic risk. Despite being significantly younger, patients with MS-AF demonstrated comparable rates of stroke and mortality to those with NVAF. This finding is supported by data from the Framingham Heart Study, which reported that the presence of mitral stenosis in patients with AF increases the risk of stroke by over 20-fold (14). Similarly, a study from sub-Saharan Africa reported no significant differences in stroke or mortality between patients with valvular AF (including mitral stenosis and prosthetic valves) and those with NVAF (15).
High early mortality following AF diagnosis has also been documented in several large-scale observational cohorts. In a community-based study, mortality was highest within the first 4 months of diagnosis (16), and another registry reported that 30-day mortality following newly diagnosed AF exceeded rates observed in subsequent months (17). A population-based cohort of older adults with newly diagnosed AF showed a 1-year mortality rate of 19.5% (18). Furthermore, findings from a Kenyan study showed 12-month mortality and stroke rates of 10% and 5% in patients with valvular AF and 15% and 5% in those with NVAF, respectively (15). The elevated stroke incidence reported in the current study aligns with previously published data from several sub-Saharan African settings (3, 19–21).
Overall survival in the study cohort was significantly influenced by patient age and a history of heart failure, prior stroke, and/or chronic kidney disease (CKD). On multivariate analysis, only a history of heart failure and/or stroke remained independently associated with increased mortality risk. Stroke and systemic embolism have been reported to account for approximately 10% of all deaths among patients with atrial fibrillation (22–24). In the ROCKET AF trial, the Kaplan–Meier estimated mortality rates were 4.2% at 1 year and 8.9% at 2 years, with deaths more frequently occurring among older patients, males, and those with a history of heart failure, vascular disease, or impaired renal function (25). In the same analysis, independent predictors of increased mortality included reduced creatinine clearance, chronic obstructive pulmonary disease, male sex, peripheral vascular disease, advanced age, diabetes, heart failure, elevated heart rate, prior stroke or transient ischemic attack, and geographic location (Latin America) (25).
Oral anticoagulation therapy has been consistently shown to reduce the risk of stroke and improve survival in patients with AF who are at elevated thromboembolic risk (26–28). In patients with rheumatic mitral valve stenosis and/or mechanical prosthetic heart valves, vitamin K antagonists (VKAs) remain the only anticoagulant class with established safety and efficacy. In contrast, for patients with non-valvular atrial fibrillation, current guidelines recommend non-vitamin K antagonist oral anticoagulants (NOACs) as the preferred option over VKAs due to their favorable risk–benefit profile (1).
All patients with mitral stenosis and atrial fibrillation were treated with warfarin, whereas 70.4% of non-valvular atrial fibrillation patients who were anticoagulated also received warfarin. Among MS-AF patients on warfarin, the proportion of individuals within the therapeutic INR range remained suboptimal: 29.3% at baseline, 46.3% at 6 months, and 67.6% at the 12-month follow-up. The overall percentage of time spent within the therapeutic range (TTR) during follow-up was 61%, which is below the recommended threshold.
Although warfarin remains a well-established and effective oral anticoagulant, its utility is limited by a narrow therapeutic window, requiring frequent INR monitoring and individualized dose adjustments. Clinical evidence consistently demonstrates that maintaining a TTR above 70% is critical for maximizing efficacy and minimizing adverse events. Suboptimal TTR has been associated with increased risks of both thromboembolic complications and bleeding. Even modest improvements in TTR can lead to meaningful reductions in adverse outcomes and improve overall clinical care. Maintaining a high TTR is crucial in patients receiving warfarin, as suboptimal TTR is strongly associated with increased risks of thromboembolic and hemorrhagic events (28). Given the high prevalence of mitral stenosis–associated atrial fibrillation in our region, optimizing anticoagulation control is not only clinically important but also a pressing public health priority, warranting structured interventions to improve INR monitoring, patient adherence, and long-term outcomes.
Although the overall use of anticoagulation in our cohort was comparable to rates reported in global AF registries and observational studies (29–31), our real-world data highlight a pattern of underutilization of oral anticoagulants and preferential use of warfarin over NOACs among patients with non-valvular AF. This discrepancy likely reflects multifactorial barriers to optimal anticoagulation, including financial constraints, limited access to NOACs, concerns about adherence, and physician-related hesitancy in adopting guideline-directed therapies.
Despite the relatively small sample size, the elevated stroke and mortality rates observed may reflect a higher baseline risk of AF-related adverse outcomes in our population, potentially influenced by delays in diagnosis, suboptimal anticoagulation control, or systemic healthcare limitations. Moreover, previous studies have identified racial and ethnic disparities in AF outcomes, suggesting that sociodemographic and regional factors may contribute to worse prognoses in certain populations (32).
AF imposes a substantial economic burden on healthcare systems, primarily driven by hospitalizations, stroke-related complications, and reduced productivity (33–35). Globally, AF-related hospital admissions have shown a marked upward trend over recent years (36). This burden is particularly pronounced in low- and middle-income countries, where limited healthcare infrastructure and financial constraints amplify the social and economic consequences, especially among younger patients affected by AF. In the current study, hospitalization rates were notably high, reaching 61.9% in the MS-AF group and 50% in the NVAF group. However, overall healthcare resource utilization—including hospital readmissions, length of stay, outpatient clinic visits, and the frequency of ECG and echocardiographic assessments—did not differ significantly between the two groups.
The higher prevalence of rheumatic MS may contribute to the increased prevalence of AF seen in developing countries such as Egypt. AF associated with rheumatic MS predominantly affects a younger demographic, yet demonstrates adverse outcomes that are comparable to, if not worse than, those seen in non-valvular AF. The high rates of stroke and mortality observed in this study underscore the serious public health implications of AF in our population. These findings emphasize the urgent need for comprehensive, community-based strategies aimed at early detection, primary prevention, and consistent implementation of evidence-based guidelines for AF management. Enhancing public health awareness, improving access to diagnostic tools, and optimizing long-term care pathways may collectively help mitigate the burden of AF in resource-limited settings.
Conclusion
Newly diagnosed atrial fibrillation is associated with high 1-year rates of stroke and mortality. Mitral stenosis–related AF, despite affecting a younger population with fewer cardiovascular risk factors and being managed with anticoagulation, demonstrates comparable adverse outcomes and healthcare resource utilization to non-valvular AF, highlighting the need for tailored management strategies in this subgroup.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Faculty of Medicine, Assiut University Ethical Committee with IRB number: 17101026. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
MAbo: Writing – original draft, Writing – review & editing. HH-A: Supervision, Validation, Writing – review & editing, Writing – original draft. MM: Data curation, Investigation, Writing – review & editing. AA: Formal analysis, Investigation, Validation, Writing – review & editing. MAbd: Formal analysis, Project administration, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AF, atrial fibrillation; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; MS-AF, AF related to mitral stenosis; NOACs, novel oral anticoagulants; NVAF, non-valvular atrial fibrillation; PAD, peripheral arterial disease; VKA, vitamin K antagonists.
References
1.
Hindricks G Potpara T Dagres N Arbelo E Bax JJ Blomström-Lundqvist C et al 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. (2021) 42(5):373–498. 10.1093/eurheartj/ehaa612
2.
Vahanian A Beyersdorf F Praz F Milojevic M Baldus S Bauersachs J et al 2021 ESC/EACTS guidelines for the management of valvular heart disease: developed by the task force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Rev Esp Cardiol (Engl Ed). (2022) 75(6):524. 10.1016/j.rec.2022.05.006
3.
Ntep-Gweth M Zimmermann M Meiltz A Kingue S Ndobo P Urban P et al Atrial fibrillation in Africa: clinical characteristics, prognosis, and adherence to guidelines in Cameroon. Europace. (2010) 12(4):482–7. 10.1093/europace/euq006
4.
Benjamin EJ Muntner P Alonso A Bittencourt MS Callaway CW Carson AP et al American Heart Association Council on epidemiology and prevention statistics committee and stroke statistics subcommittee. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation. (2019) 139(10):e56–528. 10.1161/CIR.0000000000000659
5.
Allan V Honarbakhsh S Casas JP Wallace J Hunter R Schilling R et al Are cardiovascular risk factors also associated with the incidence of atrial fibrillation? A systematic review and field synopsis of 23 factors in 32 population-based cohorts of 20 million participants. Thromb Haemost. (2017) 117(5):837–50. 10.1160/TH16-11-0825
6.
Kirchhof P Lip GY Van Gelder IC Bax J Hylek E Kaab S et al Comprehensive risk reduction in patients with atrial fibrillation: emerging diagnostic and therapeutic options–a report from the 3rd atrial fibrillation competence NETwork/European Heart Rhythm Association consensus conference. Europace. (2012) 14(1):8–27. 10.1093/europace/eur241
7.
Yadgir S Johnson CO Aboyans V Adebayo OM Adedoyin RA Afarideh M et al Global, regional, and national burden of calcific aortic valve and degenerative mitral valve diseases, 1990–2017. Circulation. (2020) 141(21):1670–80. 10.1161/CIRCULATIONAHA.119.043391
8.
Kingué S Ba SA Balde D Diarra MB Anzouan-Kacou JB Anisubia B et al The VALVAFRIC study: a registry of rheumatic heart disease in Western and Central Africa. Arch Cardiovasc Dis. (2016) 109(5):321–9. 10.1016/j.acvd.2015.12.004
9.
Iung B Vahanian A . Epidemiology of acquired valvular heart disease. Can J Cardiol. (2014) 30(9):962–70. 10.1016/j.cjca.2014.03.022
10.
Iung B Leenhardt A Extramiana F . Management of atrial fibrillation in patients with rheumatic mitral stenosis. Heart. (2018) 104(13):1062–8. 10.1136/heartjnl-2017-311425
11.
Chugh SS Havmoeller R Narayanan K Singh D Rienstra M Benjamin EJ et al Worldwide epidemiology of atrial fibrillation: a global burden of disease 2010 study. Circulation. (2014) 129(8):837–47. 10.1161/CIRCULATIONAHA.113.005119
12.
Zühlke L Engel ME Karthikeyan G Rangarajan S Mackie P Cupido B et al Characteristics, complications, and gaps in evidence-based interventions in rheumatic heart disease: the Global Rheumatic Heart Disease Registry (the REMEDY study). Eur Heart J. (2015) 36(18):1115–22a. 10.1093/eurheartj/ehu449
13.
Philippart R Brunet-Bernard A Clementy N Bourguignon T Mirza A Babuty D et al Prognostic value of CHA2DS2-VASc score in patients with “non-valvular atrial fibrillation” and valvular heart disease: the Loire Valley Atrial Fibrillation Project. Eur Heart J. (2015) 36(28):1822–30. 10.1093/eurheartj/ehv163
14.
Wolf PA Abbott RD Kannel WB . Atrial fibrillation as an independent risk factor for stroke: the Framingham study. Stroke. (1991) 22(8):983–8. 10.1161/01.STR.22.8.983
15.
Temu TM Lane KA Shen C Ng’ang’a L Akwanalo CO Chen PS et al Clinical characteristics and 12-month outcomes of patients with valvular and non-valvular atrial fibrillation in Kenya. PLoS One. (2017) 12(9):e0185204. 10.1371/journal.pone.0185204
16.
Miyasaka Y Barnes ME Bailey KR Cha SS Gersh BJ Seward JB et al Mortality trends in patients diagnosed with first atrial fibrillation: a 21-year community-based study. J Am Coll Cardiol. (2007) 49(9):986–92. 10.1016/j.jacc.2006.10.062
17.
Chamberlain AM Gersh BJ Alonso A Chen LY Berardi C Manemann SM et al Decade-long trends in atrial fibrillation incidence and survival: a community study. Am J Med. (2015) 128(3):260–7.e1. 10.1016/j.amjmed.2014.10.030
18.
Piccini JP Hammill BG Sinner MF Hernandez AF Walkey AJ Benjamin EJ et al Clinical course of atrial fibrillation in older adults: the importance of cardiovascular events beyond stroke. Eur Heart J. (2014) 35(4):250–6. 10.1093/eurheartj/eht483
19.
Zubaid M Rashed WA Alsheikh-Ali AA Almahmeed W Shehab A Sulaiman K et al Gulf survey of atrial fibrillation events (gulf SAFE): design and baseline characteristics of patients with atrial fibrillation in the Arab Middle East. Circ Cardiovasc Qual Outcomes. (2011) 4(4):477–82. 10.1161/CIRCOUTCOMES.110.959700
20.
Sliwa K Carrington MJ Klug E Opie L Lee G Ball J et al Predisposing factors and incidence of newly diagnosed atrial fibrillation in an urban African community: insights from the Heart of Soweto Study. Heart. (2010) 96(23):1878–82. 10.1136/hrt.2010.206938
21.
Shavadia J Yonga G Mwanzi S Jinah A Moriasi A Otieno H . Clinical characteristics and outcomes of atrial fibrillation and flutter at the Aga Khan University Hospital, Nairobi. Cardiovasc J Afr. (2013) 24(2):6–9. 10.5830/CVJA-2012-064
22.
Friberg L Hammar N Pettersson H Rosenqvist M . Increased mortality in paroxysmal atrial fibrillation: report from the Stockholm cohort-study of atrial fibrillation (SCAF). Eur Heart J. (2007) 28(19):2346–53. 10.1093/eurheartj/ehm308
23.
Steinberg JS Sadaniantz A Kron J Krahn A Denny DM Daubert J et al Analysis of cause-specific mortality in the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study. Circulation. (2004) 109(16):1973–80. 10.1161/01.CIR.0000118472.77237.FA
24.
Marijon E Le Heuzey JY Connolly S Yang S Pogue J Brueckmann M et al Causes of death and influencing factors in patients with atrial fibrillation: a competing-risk analysis from the randomized evaluation of long-term anticoagulant therapy study. Circulation. (2013) 128(20):2192–201. 10.1161/CIRCULATIONAHA.112.000491
25.
Pokorney SD Piccini JP Stevens SR Patel MR Pieper KS Halperin JL et al Cause of death and predictors of all-cause mortality in anticoagulated patients with nonvalvular atrial fibrillation: data from ROCKET AF. J Am Heart Assoc. (2016) 5(3):e002197. 10.1161/JAHA.115.002197
26.
Hart RG Pearce LA Aguilar MI . Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. (2007) 146(12):857–67. 10.7326/0003-4819-146-12-200706190-00007
27.
Ruff CT Giugliano RP Braunwald E Hoffman EB Deenadayalu N Ezekowitz MD et al Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. (2014) 383(9921):955–62. 10.1016/S0140-6736(13)62343-0
28.
Wan Y Heneghan C Perera R Roberts N Hollowell J Glasziou P et al Anticoagulation control and prediction of adverse events in patients with atrial fibrillation: a systematic review. Circ Cardiovasc Qual Outcomes. (2008) 1(2):84–91. 10.1161/CIRCOUTCOMES.108.796185
29.
Kirley K Qato DM Kornfield R Stafford RS Alexander GC . National trends in oral anticoagulant use in the United States, 2007 to 2011. Circ Cardiovasc Qual Outcomes. (2012) 5(5):615–21. 10.1161/CIRCOUTCOMES.112.967299
30.
Nieuwlaat R Capucci A Camm AJ Olsson SB Andresen D Davies DW et al Atrial fibrillation management: a prospective survey in ESC member countries: the Euro heart survey on atrial fibrillation. Eur Heart J. (2005) 26(22):2422–34. 10.1093/eurheartj/ehi505
31.
Wen-Hang QI ; Society of Cardiology, Chinese Medical Association. Retrospective investigation of hospitalised patients with atrial fibrillation in mainland China. Int J Cardiol. (2005) 105(3):283–7. 10.1016/j.ijcard.2004.12.042
32.
Magnani JW Norby FL Agarwal SK Soliman EZ Chen LY Loehr LR et al Racial differences in atrial fibrillation-related cardiovascular disease and mortality: the Atherosclerosis Risk in Communities (ARIC) study. JAMA Cardiol. (2016) 1(4):433–41. 10.1001/jamacardio.2016.1025
33.
Stewart S Murphy NF Walker A McGuire A McMurray JJ . Cost of an emerging epidemic: an economic analysis of atrial fibrillation in the UK. Heart. (2004) 90(3):286–92. 10.1136/hrt.2002.008748
34.
Blomstrom Lundqvist C Lip GY Kirchhof P . What are the costs of atrial fibrillation?Europace. (2011) 13(Suppl 2):ii9–12. 10.1093/europace/eur087
35.
Brüggenjürgen B Rossnagel K Roll S Andersson FL Selim D Müller-Nordhorn J et al The impact of atrial fibrillation on the cost of stroke: the Berlin acute stroke study. Value Health. (2007) 10(2):137–43. 10.1111/j.1524-4733.2006.00160.x
36.
Wong CX Brooks AG Leong DP Roberts-Thomson KC Sanders P. The increasing burden of atrial fibrillation compared with heart failure and myocardial infarction: a 15-year study of all hospitalizations in Australia. Arch Intern Med. (2012) 172(9):739–41. 10.1001/archinternmed.2012.878
Summary
Keywords
mitral stenosis, atrial fibrillation, non-valvular atrial fibrillation, NVAF, rheumatic heart disease
Citation
Aboelhassan M, Hasan-Ali H, Mohammed MT, Ashry A and Abdelmegid MA-KF (2025) Clinical outcomes in recently diagnosed atrial fibrillation related to mitral stenosis compared to non-valvular atrial fibrillation. Front. Cardiovasc. Med. 12:1555557. doi: 10.3389/fcvm.2025.1555557
Received
04 January 2025
Accepted
27 May 2025
Published
18 June 2025
Volume
12 - 2025
Edited by
Amer Harky, Liverpool Heart and Chest Hospital, United Kingdom
Reviewed by
Hassen Ibn Hadj Amor, University of Monastir, Tunisia
Sophia Khan, The University of Manchester, United Kingdom
Abdulla Tarmahomed, Birmingham Children's Hospital, United Kingdom
Updates
Copyright
© 2025 Aboelhassan, Hasan-Ali, Mohammed, Ashry and Abdelmegid.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Mohamed Aboelhassan mabolhassan10@gmail.com; mabolhassan@aun.edu.eg
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.