- 1Department of Medicine, Penn State Health Milton S. Hershey Medical Center, Hershey, PA, United States
- 2Division of Infectious Diseases, Department of Medicine, Penn State Health Milton S. Hershey Medical Center, Hershey, PA, United States
- 3Department of Public Health Sciences, Penn State Health Milton S. Hershey Medical Center, Hershey, PA, United States
- 4Division of Cardiology, Department of Medicine, Penn State Health Milton S. Hershey Medical Center, Hershey, PA, United States
Background: Cardiovascular disease is the leading cause of mortality and morbidity worldwide, and polypills have established efficacy in preventing poor outcomes. However, evidence on the optimal polypill combination is lacking. The objective of the study is to estimate the optimal polypill combination that maximizes cardiovascular outcomes.
Methods: MEDLINE/PubMed, Scopus, and Cochrane Database of Systematic Reviews databases were searched from January 1, 1960, to March 30, 2025. Studies that provided data on the association between polypill and cardiovascular outcomes were included. We estimated the effect of various polypill combinations by random-effects meta-analyses using the generic inverse variance method. Subgroup and meta-regression analyses were conducted to explore potential effect modification in the association between polypill combinations and cardiovascular outcomes.
Results: Thirty studies comprising 35,833 individuals met the inclusion criteria from 6 continents. The estimated pooled effects of polypill use on major adverse cardiovascular events (MACE), cardiovascular death, and all-cause mortality were RR 0.78 (95% CI, 0.63–0.97), 0.75 (95% CI, 0.63–0.89), 0.88 (95% CI, 0.79–0.98), respectively. The pooled relative risk of MACE outcome was 21% lower in combination of 4 or more pills [0.79 (95% CI, 0.55–1.15), n = 6 studies] vs. to 22% and 3 or less combination of medication classes (RR: 0.78 95% CI: 0.70–0.86), n = 4 studies). Polypill combinations containing moderate or high-intensity statins were associated with lower risk of MACE outcomes RR 0.79 95% CI: 0.70–0.97), n = 2 studies compared to combinations with low-intensity statins RR 0.78 95% CI: 0.59–1.03, n = 8). All polypills for MACE outcomes contained RAAS inhibitors. Calcium channel blockers, RAAS inhibitors and diuretics-containing polypills were associated with the highest reduction in blood pressure. Certainty of evidence for MACE ranged from low to high, with most trials rated as moderate to high.
Conclusions: In this meta-analysis, polypills with 3 cardiovascular classes that contain a high-intensity statins, aspirin and RAAS inhibitors appeared to have greater reduction in MACE outcomes. The presence of a diuretic and a calcium channel blocker in the polypill was associated with greater reductions in systolic and diastolic blood pressure.
1 Introduction
Cardiovascular disease (CVD) is the leading cause of death globally, with ischemic heart disease and stroke comprising more than 80% of this group. Over the last two decades, the rate of cardiovascular disease-associated death, adjusted for age, has steadily declined, but this rate of decline has recently plateaued in high-income countries (1, 2). High low-density lipoprotein (LDL) cholesterol and high blood pressure are two significant risk factors for cardiovascular disease-associated mortality and morbidity (3, 4). Randomized trials showed that drugs to lower three risk factors—LDL cholesterol (5), blood pressure (6–8), and platelet function (with aspirin)—reduce the incidence of ischemic heart disease (IHD) events and stroke (9).
About 1 in 4 people do not adhere well to prescribed drug therapy (10). Poor adherence is considered a significant factor in treatment failure and is one of the leading challenges to healthcare professionals (11). In general, poor adherence to medications has also been linked to worse clinical outcomes (12). Non-adherence to cardiovascular medications has been associated with an increased risk of morbidity and mortality (13, 14). Further, in chronic coronary artery disease setting, non-adherence to cardioprotective medications (statins and/or angiotensin-converting enzyme inhibitors) was associated with a 10% to 40% relative increase in the risk of cardiovascular hospitalizations and a 50%–80% relative increase in the risk of mortality (15).
Polypills have been shown to improve medication adherence for CVD (16, 17). The reason behind the increased adherence to using a polypill for the prevention of CVD is that it simplifies medication intake (18). In several studies, fixed-dose polypills have been shown to be superior to usual care in reducing high blood pressure and high LDL cholesterol (19–21).
Findings from previous meta-analyses on the efficacy of the polypill have been inconsistent. Meta-analyses by Kandil, Virk, and Joseph confirm the efficacy of polypill interventions in primary prevention of cardiovascular disease (CVD) (21–23). They collectively show that polypills reduce the risk of major adverse cardiovascular events (MACE), including CVD mortality, myocardial infarction, stroke, and cardiovascular death, while also lowering systolic blood pressure (SBP) and low-density lipoprotein cholesterol (LDL-C). On the other hand, a 2022 meta-analysis did not demonstrate the benefit of polypill in reducing all-cause and CVD mortality. Additionally, the components of a polypill can vary significantly, and in some cases, some drugs, such as aspirin, are not included despite their proven efficacy in the primary and secondary prevention of cardiovascular disease and perhaps this can explain the inconsistencies in these meta-analyses (24–26).
To address the inconsistency in the aforementioned meta-analyses, we explored the effects of various medication combinations—comprising statins, multiple blood pressure–lowering drugs, and aspirin—on CVD outcomes. Our approach is distinctive in that it goes beyond the general focus on polypills in previous studies, aiming to assess a broader array of medication subsets to identify the most effective polypill composition for heart disease prevention. By methodically evaluating the additive potential of different drug combinations, our study aims to fill a literature gap, offering a novel contribution to cardiovascular disease prevention. We compare the impacts of various polypill formulations on mortality and disease outcomes from a primary and secondary prevention standpoint to provide insights on the most beneficial combinations with additive effect, guiding future research and informing clinical practice with effective preventive strategies.
2 Methods
2.1 Data sources and searches
We searched the MEDLINE/PubMed, Scopus, and Cochrane Database of Systematic Reviews databases for studies published since inception through March 30, 2025, using a combination of Medical Subject Headings (MeSH) and keywords in the title and abstract related to polypill use in cardiovascular disease prevention. We used the terms Polypill combined with cardiovascular disease (e.g., myocardial infarction, stroke, blood pressure, cholesterol types) combined with adherence to search peer-reviewed publications. This study was reported according to the Meta-analysis of Observational Studies in Epidemiology (MOOSE) reporting guidelines and the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guidelines (27, 28).
We also searched the reference lists of retrieved articles to identify additional relevant studies. We included studies that evaluated: (1) the effect of polypill use on medication adherence; (2) the effect of polypill use on primary cardiovascular disease prevention; and (3) the effect of polypill use on secondary cardiovascular disease outcomes. Studies not conducted in humans, case reports, letters to the editor, case series, case-control studies, practice guidelines, meta-analyses, literature reviews, and commentaries were excluded. We did not impose any restrictions based on the language of the articles or country of study. Some studies used the same population in several articles. We excluded articles with overlapping study populations.
2.2 Study selection
Studies were selected according to Participant (P), Intervention (I), Comparator [C], Outcome (O), and Study type (S) [PICOS] criteria (29):
Population: Adults aged ≥18 years with or at risk for cardiovascular disease, eligible for either primary or secondary prevention.
Intervention: Polypills were defined as fixed-dose combination therapy including at least two cardiovascular medications (e.g., statins, antihypertensives, aspirin).
Comparator: Standard of care, which included monotherapy (e.g., individual components of the polypill administered separately) or placebo.
Outcomes:
Primary outcomes:
1. Major Adverse Cardiovascular Events (MACE): As defined by each study, typically including composite outcomes such as cardiovascular death, myocardial infarction, stroke, or hospitalization for heart failure.
2. Cardiovascular mortality
3. All-cause mortality
Secondary outcomes:
1. Change in systolic and diastolic blood pressure (mmHg)
2. Change in low-density lipoprotein cholesterol (LDL-C, mg/dl)
3. Medication adherence, defined as the proportion of days covered (PDC), self-reported compliance, or pill counts as reported by individual studies.
Study Design: Randomized controlled trials and observational cohort studies.
2.3 Data extraction
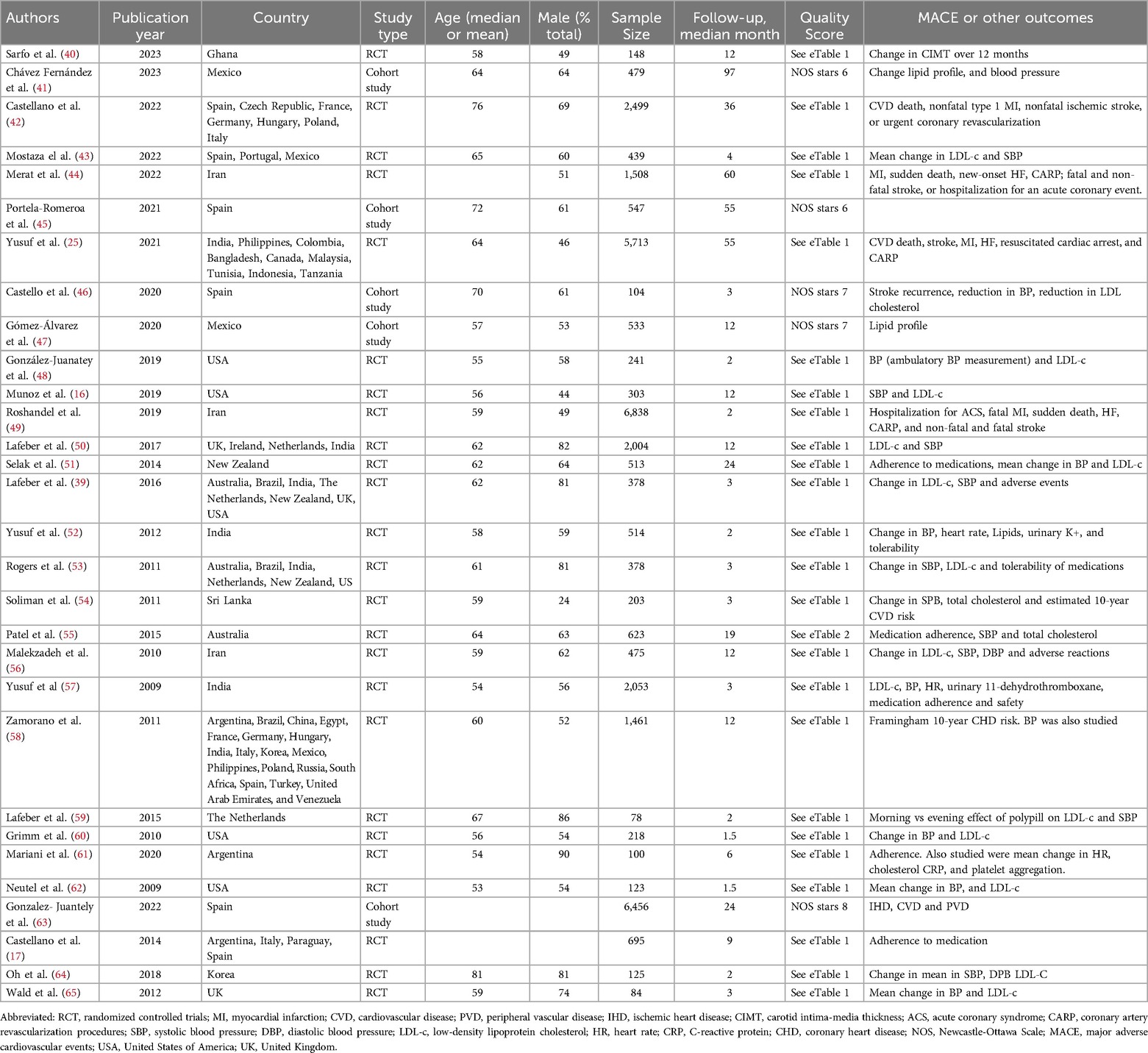
A standardized data extraction form was developed and three investigators (S.M., H.H., and H.Y) independently screened the titles and abstracts of articles, obtained the full-text articles, and performed data extraction on those meeting the inclusion criteria. Three investigators (S.M., H.H., and P.S) jointly reviewed a random subset of articles to ensure selection accuracy. Disagreements about the included articles were resolved by the senior investigator (P.S.). A detailed account of the inclusion/exclusion process is shown in Figure 1. The following information was extracted: year of study publication, country and time frame, follow-up time, study-level descriptive statistics [mean (SD)/ median (IQR) age in years, proportion (%) female, male and obese], the content of medications in each polypill, and adherence information. For primary outcomes of major adverse cardiovascular events (MACE, cardiovascular mortality and all-cause mortality, we extracted risk ratios (RR) and 95% confidence intervals or the raw values values in each group. For secondary outcomes, we extracted change in systolic and diastolic blood pressure (mmHg), change in low-density lipoprotein cholesterol (LDL-C, mg/dl) from baseline and the proportion of patients (in percentage) who were adherent to polypills.
2.4 Risk of bias and evidence grading
Three authors (S.M. HH and PS) independently assessed the quality of the articles included in our analysis. The risk of bias of the included RCTs was evaluated with the Cochrane Collaboration's Risk of Bias 2 tool, which evaluates five key domains: the randomization process, deviations from intended interventions, missing outcome data, measurement of outcomes, and selection of reported results (30). For nonrandomized observational studies, methodological quality was assessed with the Newcastle–Ottawa Scale (NOS) (31). As described by the NOS criteria, we assigned a maximum of 4 stars for selection, 2 stars for comparability, and 3 stars for exposure and outcome assessment. Studies with fewer than 5 stars were considered low quality; 5–7 stars, moderate quality; and more than 7 stars, high quality. Studies were included regardless of the risk of bias and quality scores, but a sub-group analysis was conducted by study type (RCT vs. non-RCT) in accordance with the framework for combining RCTs and non-RCTs (32).
To evaluate the certainty of evidence for the major adverse cardiovascular events outcome we applied the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) framework (33). RCTs were assessed across five domains: risk of bias, inconsistency, indirectness, imprecision, and publication bias. Certainty was rated as high, moderate, low, or very low.
2.5 Data synthesis and statistical analysis
We adopted a narrative approach describing the number of studies, study settings, and types of polypills. Descriptive statistics are reported as population proportions and medians (interquartile range). We applied random-effects models to estimate the association between polypills and primary outcomes of interest, including MACE, cardiovascular mortality, and all-cause mortality (34). We reported relative risks (RRs) and associated 95% confidence intervals. A random-effects meta-analysis was performed using the generic inverse variance method. We estimated all parameters by maximizing the pseudolikelihood. Individual and pooled estimates are displayed using forest plots. Between-study variation was assessed using I2, which describes the percentage of total variation across studies that is due to heterogeneity rather than chance, expressed as a percentage [low (25%), moderate (50%), and high (75%)] (35).
We conducted random-effects subgroup analysis and metaregression analysis to investigate the sources of heterogeneity. We examined the associations of each explanatory variable included in the metaregression associated with cardiovascular outcomes. These variables included study-level median or mean age, the proportion of males, and the year of study. Differences in risk estimates were estimated using various combinations of polypills. We also examined the effects of various polypill combinations on blood pressure and LDL cholesterol reduction.
To evaluate possible publication bias, we visually inspected the funnel plot for asymmetry by plotting the study effect size against S.E.s of the effect size (36). We performed the Egger linear regression test and the Begg rank correlation test (37). The Duval and Tweedie trim and fill procedure was used to adjust for the publication bias (38). An influence and outlier study sensitivity analysis were undertaken to estimate the association of each study with the overall pooled estimate. The metagen and forest function from the R package meta were used for the analysis. All statistical analyses were performed with R software, version 4.3.2 (R Foundation). The significance level was set at P < .05, and all P values were 2-tailed.
3 Results
3.1 Overview of the results
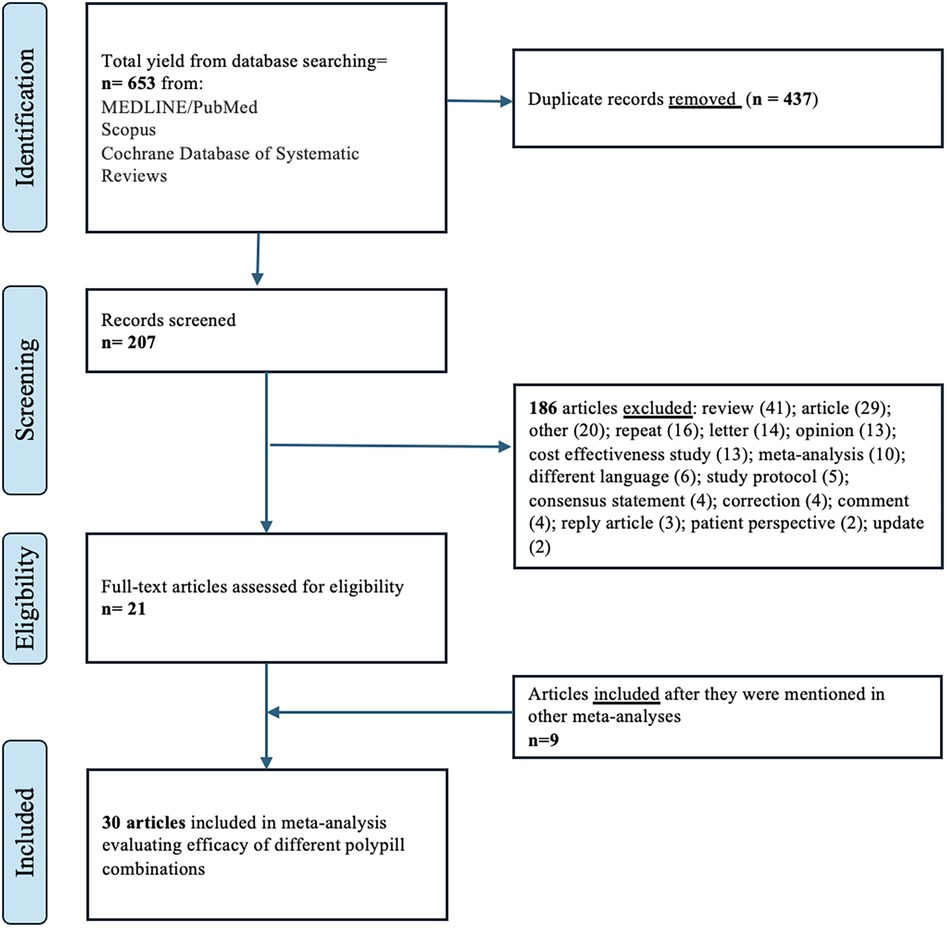
The initial literature search yielded 653 articles Figure 1; of these, we excluded 437 duplicates. After a review of titles and abstracts, we excluded 186 articles if they (1) were cost-effective studies; (2) were case series, case-control, reviews, letters, and opinions; (3) did not have a control group. We included 30 articles in this meta-analysis; 24 studies were randomized controlled trials and 6 were cohort studies. 22 studies focused on primary prevention, while 8 focused on secondary prevention. A study by Yusuf et al. was used in calculating the effect estimates in polypills with and without aspirin (25). Similarly, the study by Lafeber et al. evaluated the effect of polypills in the morning and the evening (39). Although both the PolyIran and PolyIran-Liver trials recruited participants from the Golestan Cohort Study, the trials employed distinct randomization schemes and eligibility criteria. It is therefore unlikely that the same randomized participants were included in both trials. Therefore, we included both studies in our meta-analysis.
The final studies were from 6 continents and are categorized by World Health Organization regions as follows: Africa (1 study, 3%), Asia (7 studies, 23%), Europe (7 studies, 23%), multiple continents (7 studies, 23%), North America (6 studies, 20%), Oceania (2 studies, 7%), and South America (1 study, 3%). The present analysis included a total sample of 35,833 individuals. The number of individuals included in individual studies ranged widely (78 to maximum 6,838), with a median of 477 patients (interquartile range, 207–1,270). Details of each study included in the meta-analysis are provided in Table 1. The median age of participants was 61 years (IQR 58–64) and median proportional of male sex was 61 (IQR 52–73).
3.2 The association of polypill use and major adverse cardiovascular events
First, we estimated the effect of polypill on major adverse cardiovascular events. Compared to individual agents, polypills were associated with a 22% lower risk of major adverse cardiovascular events: RR: 0.78 (95% CI, 0.63–0.97, I2 = 96%) (Figure 2A). When stratified by primary vs. secondary prevention, there was no significant difference in effect estimates: RR: 0.75 (95% CI, 0.55–1.02; I² = 95%) for primary prevention and RR: 0.88 (95% CI, 0.71–1.08; I² = 86%) for secondary prevention (p for subgroup difference = 0.40) (Figure 2B). All studies except one were RCTs and sensitivity analysis of comparing RCT vs. non-RCT did not cause meaningful differences in the effect estimates RR: 0.78 (95% CI, 0.61–1.00, I2 = 96%) vs. RR: 0.80(95% CI, 0.70–0.92) (Supplementary Figure S1). In all included studies that estimated MACE outcomes, the polypills evaluated contained either an angiotensin receptor blocker (ARB) or an angiotensin-converting enzyme (ACE) inhibitor.
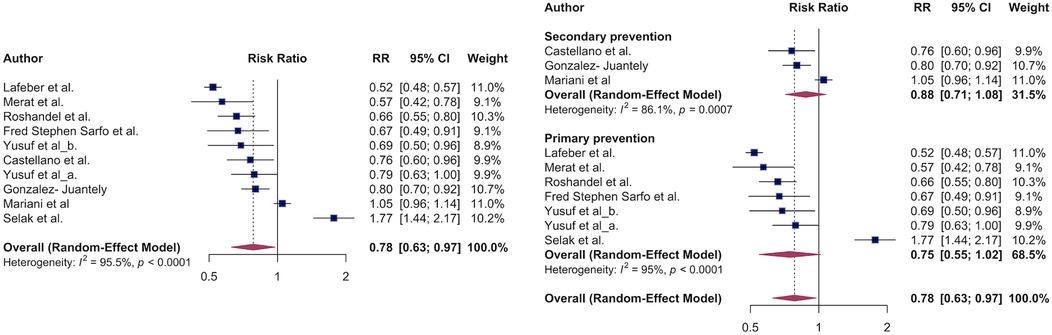
Figure 2. The association of major adverse cardiovascular events and polypill. Overall survival benefit of polypill on MACE outcomes (A). Polypill effect on MACE stratified by primary vs. secondary CVD prevention.
Due to significant heterogeneity in the overall effect estimate, various subgroup analyses were conducted as summarized below:
3.2.1 Aspirin
Next, we evaluated efficacy of aspirin-containing polypills on cardiovascular disease (CVD) primary outcomes by conducting subgroup analysis by comparing MACE outcomes between aspirin-containing polypill vs. polypills without aspirin. Most of studies analyzed, except one, included aspirin in the polypill. There was no between-group difference in the effect estimates: (RR 0.78, 95% CI: 0.61–1.00, I2 = 96%, for aspirin containing polypill) vs. RR of 0.79 (95% CI: 0.63–1.00, I2 = NA) for polypills without aspirin (Figure 3A).
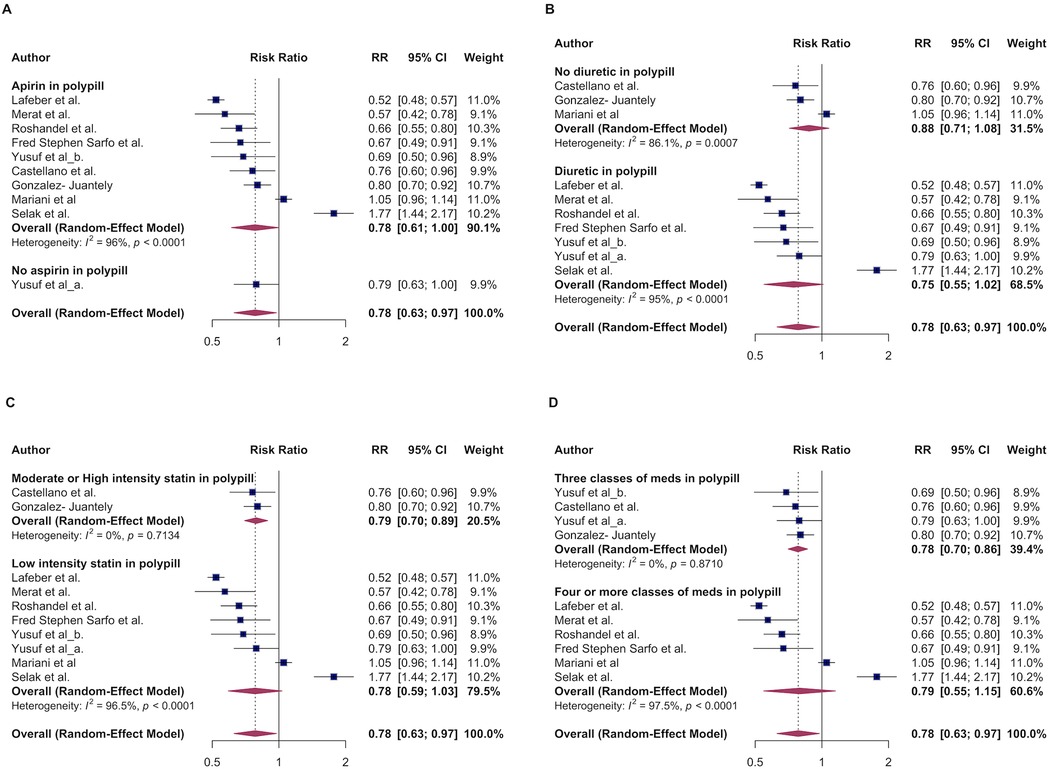
Figure 3. The association of major adverse cardiovascular events and polypill stratified by: aspirin-containing vs non-aspirin containing polypill (A); Diuretic vs lack of diuretic in a polypill (B). High/moderate intensity vs. low intensity statin in a polypill (C), three or fewer vs. more than three classes of medications in a polypill (D).
3.2.2 Diuretics
Next, we explored whether adding a diuretic to the poly-pill is associated with better MACE outcomes compared to the non-diuretic containing poly-pill. The pooled RR for the diuretic group was 0.75 (95% CI: 0.55–1.02, I² = 95%), compared to poly-pill without diuretic, RR of 0.88 (95% CI: 0.71–1.08 I² = 86) (Figure 3B) indicating a lack of between-group difference in MACE outcomes.
3.2.3 Number of pills per fixed dose combination
We conducted various permutations of the number of components in the fixed-dose polypill to identify the optimal combination that confers maximum benefit. Of note majority of polypills combinations included 3 and more pills. We therefore compared MACE outcomes by having 4 or more vs. 3 or less medications in a fixed-dose combination. Studies with four or more pills in combination, the pooled RR estimate was 0.79, 95% CI (0.55–1.15, I² = 98%) suggesting a potential benefit but not statistically significant (Figure 3C). For the studies with 3 pills in combination, the pooled risk ratio was 078 (95% CI: 0.70- 0.86, I² = 0%), indicating significant reduction in risk. The low heterogeneity (I² = 0%) suggests more consistent results among these studies. In summary, the analysis of permutation of the pills number suggests that the most benefits are observed with when a fixed-dose strategy has on average three pills.
3.2.4 Statins: high/moderate intensity vs. low intensity
Furthermore, polypills were analyzed based on their statin component. The fixed-dose polypills containing high- or moderate-intensity statins were compared to those with low-intensity statins, with the results highlighting differential impacts on primary MACE outcomes. Polypills with low-intensity statins demonstrated a 22% reduction in MACE outcomes (RR 0.78; 95% CI: 0.59–1.03) (Figure 3D), suggesting a lack of association. The high heterogeneity (I² = 97%) in this group indicates significant variability among the included studies. In contrast, polypills containing high- or moderate-intensity statins demonstrated a significant reduction in cardiovascular events, with an RR 0.79 (95% CI: 0.70–0.86), and low heterogeneity (I² = 0%), suggesting more consistent findings and statistical significance.
3.2.5 Geographic location of studies
The analysis of polypill effects on MACE outcomes across different continents reveals varied efficacy. The one study from Africa demonstrated a significant reduction in risk (RR 0.67, 95% CI: 0.49–0.91). In Europe, the pooled effect estimate indicated a significant reduction in MACE outcomes (RR 0.79, 95% CI: 0.70–0.89) with low heterogeneity (I² = 0%), indicating consistent findings. Studies from Asia presented the most significant benefit with a risk ratio of 0.63 (95% CI: 0.54–0.74) (Supplementary Material 2) and low heterogeneity. Conversely, studies spanning multiple continents demonstrated a significant reduction (RR 0.72, 95% CI: 0.54–0.94) but with high heterogeneity (I² = 94%), suggesting large variations in the outcomes. The one study from Oceania indicated an increased risk of MACE (RR 1.77, 95% CI: 1.44–2.17), while the study from South America showed no significant effect (RR 1.05, 95% CI: 0.96–1.14). Overall, the polypill demonstrated a significant benefit in reducing MACE outcomes, with the greatest reduction observed in studies conducted in Asia.
3.2.6 Meta-regression
To explore further potential sources of heterogeneity in our meta-analysis, we conducted meta-regression analyses using study-level covariates, including year of publication, mean or median age of participants, and the proportion of male participants. None of these covariates significantly influenced the pooled effect size. Specifically, meta-regression yielded a relative risk of 0.95 (95% CI, 0.88–1.02) for year of publication, 0.99 (95% CI, 0.95–1.04) for age, and 1.00 (95% CI, 0.99–1.02) for the proportion of men enrolled.
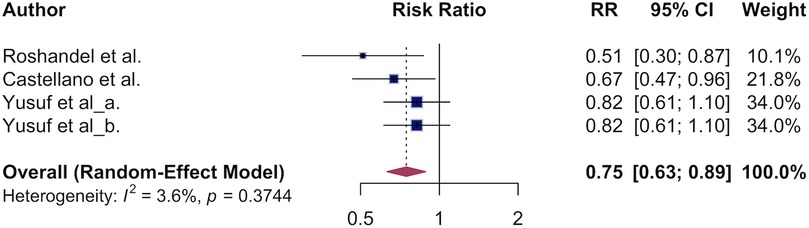
3.3 The association of polypill and primary CVD mortality
The estimated pooled overall survival benefit of the polypill for cardiovascular death was 0.75 (95% CI, 0.63–0.89, Figure 4) translating to a 25% lower CVD mortality in the polypill vs. usual care. Low heterogeneity was observed (I² = 0%), indicating consistent findings across studies. Due to low variation across studies and the limited number of studies pooled for CVD mortality, we were unable to conduct a meaningful subgroup analysis.
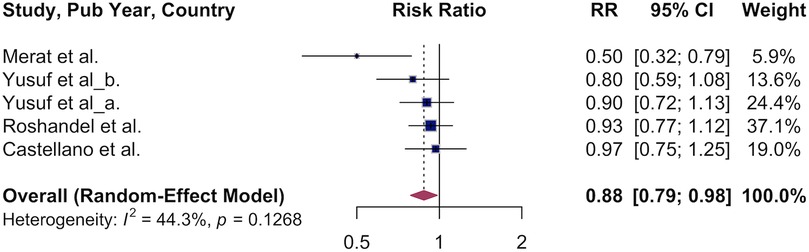
3.4 Association of polypill and all-cause mortality
Next, we pooled the results of studies that examined the effect of polypill use vs. usual care on all-cause mortality. We observed a 12% lower overall survival in the polypill group: RR 0.88 (95% CI, 0.79–0.98, Figure 5). Variation between studies was moderate (I² = 44%). Due to the small number of studies, subgroup analyses were not conducted.
3.5 Polypills use and mean change in blood pressure
Across polypill trials with available data, treatment with a cardiovascular polypill was associated with overall reductions in blood pressure and LDL cholesterol. The mean reduction in systolic blood pressure (SBP) was −7.38 mmHg in the polypill group compared to −4.65 mmHg with standard care, corresponding to a net benefit of SBP reduction of 2.72 mmHg. For diastolic blood pressure (DBP), the polypill achieved a mean reduction of −3.79 mmHg, vs. −1.97 mmHg with control, corresponding to a net benefit of 1.82 mmHg. However, the strongest systolic reduction was observed in the polypill combination that contained a calcium channel blocker with a median reduction of 9.0 mm Hg systolic blood pressure (Figure 6A) and 5.0 mm Hg diastolic blood pressure (Figure 6B). Polypills containing a diuretic and a RAAS inhibitor were also associated with a substantial reduction in diastolic blood pressure (mean of 4 mm Hg).
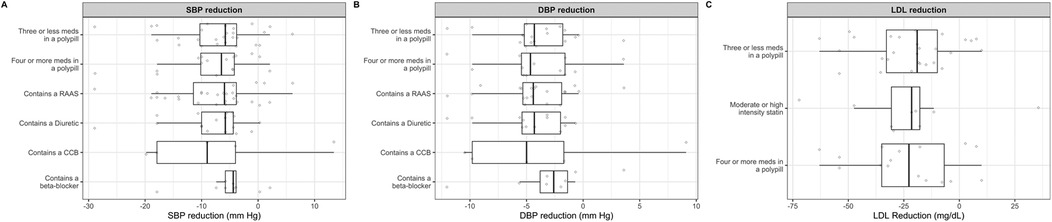
Figure 6. Effect of various combinations of polypills on mean change of systolic blood pressure (A), diastolic blood pressure (B) and low-density lipoprotein cholesterol (D).
3.6 Association between polypills and LDL reduction
Regarding lipid lowering, the mean LDL-C reduction was −23.07 mg/dl in the polypill group compared to −11.0 mg/dl in the control group, resulting in a greater LDL-C lowering of approximately 12.0 mg/dl with polypill therapy. Across the different polypill combinations, the effect on low density lipoprotein cholesterol reduction varied. Polypills containing an average of three or fewer medications, four or more medications, and moderate- or high-intensity statins were associated with median LDL cholesterol reductions of 19, 22 and 22 mg/dl, respectively (Figure 6C).
3.7 Correlation between polypills and adherence
The mean adherence to polypill vs. usual care was 85% and 79% respectively. The correlation analysis between adherence to polypills and health outcomes evaluated three key metrics: MACE outcomes, CVD deaths and all-cause mortality. No significant correlation was observed between polypill use and CVD deaths, MACE, or all-cause mortality (Supplementary Figure S3).
3.8 Publication bias and certainty of evidence
Due to an adequate number of studies, publication bias was assessed only for MACE outcomes. Visual inspection of a funnel plot of the included studies did not indicate asymmetry indicative of lack of publication bias (Supplementary Figure S4). Egger's test for publication bias (p = 0.85) and Begg's test were non-significant (p = 0.39). To identify outlier studies, we further performed influence sensitivity analyses by excluding and replacing one study at a time (Leave-One-Out method) from the meta-analysis and calculated the RR for the remaining studies. No substantial change in any of the pooled RRs was observed when individual studies were removed in turn, indicating that no single study had a considerable influence on the overall pooled estimate (Supplementary Figure S5). The overall certainty of evidence for MACE ranged from low to high across studies, with the majority rated as moderate to high; full GRADE assessments are provided in Supplementary Table S2.
4 Discussion
In this meta-analysis of 30 studies including over 30,000 participants, we demonstrated that polypills are effective in reducing the risk of major adverse cardiovascular events MACE, cardiovascular mortality, and all-cause mortality by 22%, 25%, and 12%, respectively. The optimal pills that showed the greatest effect in reducing MACE outcomes contained 3 cardiovascular classes of medications that contain a high-intensity statins, aspirin and RAAS inhibitors. The presence of a diuretic, calcium channel blocker, and RAAS inhibitor in the polypill was associated with greater reductions in both systolic and diastolic blood pressure.
Previous meta-analyses included fewer studies and smaller sample sizes (66, 67). Bahiru et al. analyzed data from 9,059 patients and focused on the primary prevention of cardiovascular events, showing that polypills improved blood pressure and cholesterol control but did not significantly impact all-cause mortality or atherosclerotic cardiovascular disease (ASCVD) events. However, we included 30 studies with a combined sample size of over 30,000 patients, providing a more robust and comprehensive analysis. This larger dataset allows for more reliable and generalizable conclusions. Additionally, we evaluated both primary and secondary prevention, demonstrating that polypills effectively reduce cardiovascular outcomes in both contexts. Prior analyses did not break down the effects of individual components within the polypills including recent meta-analysis (68). Conversely, we examined in detail different polypill combinations, evaluating the additive effects of various medications. This approach revealed that polypills containing a high-intensity statins, RAAS inhibitors and potentially aspirin provided the most significant reductions in MACE outcomes.
By examining the additive effects of different polypill components, we identified the most effective combinations and the optimal number of medications in the polypill needed to reduce cardiovascular events. Polypills with multiple components can simultaneously target various cardiovascular risk factors. For instance, a polypill might include a statin for cholesterol management, an antihypertensive for blood pressure control, and an antiplatelet agent like aspirin for reducing clot formation. The combined effects of different classes of medications can provide additive benefits. Statins and antihypertensives together may produce greater reductions in MACE than either medication alone (69).
While improved adherence is a well-recognized benefit of polypill use, our analysis did not find a significant correlation between adherence and cardiovascular outcomes. This indicates that the benefits of polypills may extend beyond merely improving adherence. The additional benefits could be due to the pharmacodynamic interactions between the drugs, leading to more effective control of risk factors such as blood pressure and cholesterol levels.
The findings of our meta-analysis have significant implications for public health and clinical practice. Polypills should be considered for broader use in primary and secondary cardiovascular disease prevention. Pharmaceutical companies should be encouraged to develop and manufacture polypill combinations that include aspirin, diuretics, and statins, as these have shown the most promise in reducing cardiovascular events. From our analysis, 3 classes of cardiovascular medications in a pill were also associated with increased efficacy in reduced MACE outcomes. Our meta-analysis provides an opportunity for further research and optimization of polypill strategies, informing future approaches to preventative cardiovascular therapy.
5 Strengths and limitations
One of the strengths of our study is the significantly large dataset, including more than 30, 000 patients overall. The larger sample size and rigorous methodology ensure more reliable and generalizable results. Second, our detailed and in-depth component analysis provides new insights into the most effective polypill combinations, and what would be the optimal way to design one to target cardiovascular primary outcomes as well as blood pressure and LDL cholesterol. Third, we included primary and secondary prevention in our meta-analysis which makes data more applicable to primary and secondary prevention. Finally, to further broaden on generalizability of our findings, our meta-analysis included patients from around the world, representing diverse countries and racial backgrounds, and included both sexes, which makes it more holistic and applicable to various populations regardless of sex and race/ethnicity. However, the findings from the present meta-analysis should be interpreted considering some limitations. The included studies varied significantly in their design, populations, and methodologies. This heterogeneity can introduce variability in the results and make it challenging to draw unified conclusions. Additionally, adherence was self-reported, and no specific objective measures were used to accurately assess adherence to usual care vs. polypill therapy, which may have influenced the reported adherence rates. Also, we conducted subgroup analysis of primary from secondary prevention in MACE outcomes, we did not have sufficient papers in each group to perform a detailed stratified analysis by type of prevention—it has been known that there are some differences when it comes to secondary compared to primary and importance of cardiovascular preventative medications such as aspirin that is indicated more in secondary vs. primary. So, it would be appropriate for a future project to conduct a detailed analysis between primary and secondary prevention to observe any major differences.
6 Conclusions
In this meta-analysis, polypills with 3 cardiovascular classes that contain a high-intensity statins, aspirin and RAAS inhibitors appeared to have greater reduction in MACE outcomes. The presence of a diuretic, a RAAS inhibitor and a calcium channel blocker in the polypill was associated with greater reductions in systolic and diastolic blood pressure.
Data availability statement
The datasets presented in this study can be found in online repositories. The data can be found here: https://github.com/ssentongojeddy/Polypill-CVDMeta-analyis.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
HY: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. SM: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. BF: Writing – original draft, Writing – review & editing. HH: Data curation, Investigation, Writing – review & editing. PS: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. MF: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1558579/full#supplementary-material
References
1. Harikrishnan S, Jeemon P, Mini G, Thankappan K, Sylaja P. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the global burden of disease study 2017. Lancet. (2018) 392(10159):1736–88. doi: 10.1016/S0140-6736(18)32203-7
2. Oren E. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the global burden of disease study 2016. Lancet. (2017) 390(10100):1151. doi: 10.1016/S0140-6736(17)32152-9
3. Murray CJ, Aravkin AY, Zheng P, Abbafati C, Abbas KM, Abbasi-Kangevari M, et al. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet. (2020) 396(10258):1223–49. doi: 10.1016/S0140-6736(20)30752-2
4. Yusuf S, Joseph P, Rangarajan S, Islam S, Mente A, Hystad P, et al. Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high-income, middle-income, and low-income countries (PURE): a prospective cohort study. Lancet. (2020) 395(10226):795–808. doi: 10.1016/S0140-6736(19)32008-2
5. Law MR, Wald NJ, Rudnicka A. Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta-analysis. Br Med J. (2003) 326(7404):1423. doi: 10.1136/bmj.326.7404.1423
6. Law M, Wald N, Morris J. Lowering blood pressure to prevent myocardial infarction and stroke: a new preventive strategy. Health Technol Assessment. (2003) 7(31):1–94. doi: 10.3310/hta7310
7. MacMahon S, Peto R, Collins R, Godwin J, Cutler J, Sorlie P, et al. Blood pressure, stroke, and coronary heart disease: part 1, prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet. (1990) 335(8692):765–74. doi: 10.1016/0140-6736(90)90878-9
8. Collins R, Peto R, MacMahon S, Godwin J, Qizilbash N, Hebert P, et al. Blood pressure, stroke, and coronary heart disease: part 2, short-term reductions in blood pressure: overview of randomised drug trials in their epidemiological context. Lancet. (1990) 335(8693):827–38. doi: 10.1016/0140-6736(90)90944-Z
9. Collaboration AT. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. Br Med J. (2002) 324(7329):71–86. doi: 10.1136/bmj.324.7329.71
10. DiMatteo MR. Variations in patients’ adherence to medical recommendations: a quantitative review of 50 years of research. Med Care. (2004) 42(3):200–9. doi: 10.1097/01.mlr.0000114908.90348.f9
11. Miller NH, Hill M, Kottke T, Ockene IS. The multilevel compliance challenge: recommendations for a call to action: a statement for healthcare professionals. Circulation. (1997) 95(4):1085–90. doi: 10.1161/01.CIR.95.4.1085
12. Simpson SH, Eurich DT, Majumdar SR, Padwal RS, Tsuyuki RT, Varney J, et al. A meta-analysis of the association between adherence to drug therapy and mortality. Br Med J. (2006) 333(7557):15. doi: 10.1136/bmj.38875.675486.55
13. Spertus JA, Kettelkamp R, Vance C, Decker C, Jones PG, Rumsfeld JS, et al. Prevalence, predictors, and outcomes of premature discontinuation of thienopyridine therapy after drug-eluting stent placement: results from the PREMIER registry. Circulation. (2006) 113(24):2803–9. doi: 10.1161/CIRCULATIONAHA.106.618066
14. Jn R. Relationship between adherence to evidence-based pharmacotherapy and long-term mortality after acute myocardial infarction. JAMA. (2007) 297:177–86. doi: 10.1001/jama.297.2.177
15. Ho PM, Magid DJ, Shetterly SM, Olson KL, Maddox TM, Peterson PN, et al. Medication nonadherence is associated with a broad range of adverse outcomes in patients with coronary artery disease. Am Heart J. (2008) 155(4):772–9. doi: 10.1016/j.ahj.2007.12.011
16. Muñoz D, Uzoije P, Reynolds C, Miller R, Walkley D, Pappalardo S, et al. Polypill for cardiovascular disease prevention in an underserved population. N Engl J Med. (2019) 381(12):1114–23. doi: 10.1056/NEJMoa1815359
17. Castellano JM, Sanz G, Peñalvo JL, Bansilal S, Fernández-Ortiz A, Alvarez L, et al. A polypill strategy to improve adherence: results from the FOCUS project. J Am Coll Cardiol. (2014) 64(20):2071–82. doi: 10.1016/j.jacc.2014.08.021
18. Abushouk AI, Sayed A, Munir M, Ghanem E, Abdelfattah O, Michos ED, et al. Fixed-dose combination (polypill) for cardiovascular disease prevention: a meta-analysis. Am J Prev Med. (2022) 63(3):440–9. doi: 10.1016/j.amepre.2022.03.027
19. Webster R, Salam A, De Silva HA, Selak V, Stepien S, Rajapakse S, et al. Fixed low-dose triple combination antihypertensive medication vs usual care for blood pressure control in patients with mild to moderate hypertension in Sri Lanka: a randomized clinical trial. Jama. (2018) 320(6):566–79. doi: 10.1001/jama.2018.10359
20. Thom S, Poulter N, Field J, Patel A, Prabhakaran D, Stanton A, et al. Effects of a fixed-dose combination strategy on adherence and risk factors in patients with or at high risk of CVD: the UMPIRE randomized clinical trial. Jama. (2013) 310(9):918–29. doi: 10.1001/jama.2013.277064
21. Simon ST, Kini V, Levy AE, Ho PM. Medication adherence in cardiovascular medicine. Br Med J. (2021) 374:n1493. doi: 10.1136/bmj.n1493
22. Kandil OA, Motawea KR, Aboelenein MM, Shah J. Polypills for primary prevention of cardiovascular disease: a systematic review and meta-analysis. Front Cardiovasc Med. (2022) 9:880054. doi: 10.3389/fcvm.2022.880054
23. Virk GS, Sharma A, Khan MR, Shah K, Mengar J, Chaudhari SS, et al. The effectiveness of polypill for the prevention of cardiovascular disease: a meta-analysis of randomized controlled trials. Cureus. (2023) 15(10):e47032. doi: 10.7759/cureus.47032
24. Zheng SL, Roddick AJ. Association of aspirin use for primary prevention with cardiovascular events and bleeding events: a systematic review and meta-analysis. Jama. (2019) 321(3):277–87. doi: 10.1001/jama.2018.20578
25. Yusuf S, Joseph P, Dans A, Gao P, Teo K, Xavier D, et al. Polypill with or without aspirin in persons without cardiovascular disease. N Engl J Med. (2021) 384(3):216–28. doi: 10.1056/NEJMoa2028220
26. Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, Peto R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. (2009) 373(9678):1849–60. doi: 10.1016/S0140-6736(09)60503-1
27. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Jama. (2000) 283(15):2008–12. doi: 10.1001/jama.283.15.2008
28. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J. (2021) 372:n71. doi: 10.1136/bmj.n71
29. Methley AM, Campbell S, Chew-Graham C, McNally R, Cheraghi-Sohi S. PICO, PICOS and SPIDER: a comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv Res. (2014) 14(1):1–10. doi: 10.1186/s12913-014-0579-0
30. Higgins JP, Altman DG. Assessing risk of bias in included studies. In: Higgins JPT, Sally Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions: Cochrane Book Series. Chichester: John Wiley & Sons (2008). p. 187–241.
31. Wells GA, Shea B, O’Connell D, Robertson J, Peterson J, Welch V, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-analyses. Ottawa, ON: Ottawa Hospital Research Institute (2000).
32. Sarri G, Patorno E, Yuan H, Guo JJ, Bennett D, Wen X, et al. Framework for the synthesis of non-randomised studies and randomised controlled trials: a guidance on conducting a systematic review and meta-analysis for healthcare decision making. BMJ Evid Based Med. (2022) 27(2):109–19. doi: 10.1136/bmjebm-2020-111493
33. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. Br Med J. (2008) 336(7650):924–6. doi: 10.1136/bmj.39489.470347.AD
34. Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. (2010) 1(2):97–111. doi: 10.1002/jrsm.12
35. Veroniki AA, Jackson D, Viechtbauer W, Bender R, Bowden J, Knapp G, et al. Methods to estimate the between-study variance and its uncertainty in meta-analysis. Res Synth Methods. (2016) 7(1):55–79. doi: 10.1002/jrsm.1164
36. Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. (2001) 54(10):1046–55. doi: 10.1016/S0895-4356(01)00377-8
37. Sterne JAC, Egger M. Regression methods to detect publication and other bias in meta-analysis. In: Rothstein HR, Sutton AJ, Borenstein M, editors. Publication Bias in Meta-Analysis: Prevention, Assessment and Adjustments. Chichester: John Wiley & Sons (2005). p. 99–110.
38. Duval S, Tweedie R. A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. J Am Stat Assoc. (2000) 95(449):89–98. doi: 10.1080/01621459.2000.10473905
39. Lafeber M, Webster R, Visseren FL, Bots ML, Grobbee DE, Spiering W, et al. Estimated cardiovascular relative risk reduction from fixed-dose combination pill (polypill) treatment in a wide range of patients with a moderate risk of cardiovascular disease. Eur J Prev Cardiol. (2016) 23(12):1289–97. doi: 10.1177/2047487315624523
40. Sarfo FS, Voeks J, Adamu S, Agyei BA, Agbenorku M, Adu-Darko N, et al. A cardiovascular polypill for secondary stroke prevention in a tertiary centre in Ghana (SMAART): a phase 2 randomised clinical trial. Lancet Glob Health. (2023) 11(10):e1619–28. doi: 10.1016/S2214-109X(23)00347-9
41. Chávez Fernández JA, Ramírez Mendoza M, Kassck Ipinaa H, Sánchez Ángeles LA, González Chávez A, Escobedo G, et al. The cardiovascular polypill as baseline treatment improves lipid profile and blood pressure regardless of body mass index in patients with cardiovascular disease. The bacus study. PLoS One. (2023) 18(8):e0290544. doi: 10.1371/journal.pone.0290544
42. Castellano JM, Pocock SJ, Bhatt DL, Quesada AJ, Owen R, Fernandez-Ortiz A, et al. Polypill strategy in secondary cardiovascular prevention. N Engl J Med. (2022) 387(11):967–77. doi: 10.1056/NEJMoa2208275
43. Mostaza JM, Suárez-Fernández C, Cosín-Sales J, Gómez-Huelgas R, Brotons C, Araujo FP, et al. Safety and efficacy of a cardiovascular polypill in people at high and very high risk without a previous cardiovascular event: the international VULCANO randomised clinical trial. BMC Cardiovasc Disord. (2022) 22(1):560. doi: 10.1186/s12872-022-03013-w
44. Merat S, Jafari E, Radmard AR, Khoshnia M, Sharafkhah M, Nateghi Baygi A, et al. Polypill for prevention of cardiovascular diseases with focus on non-alcoholic steatohepatitis: the PolyIran-liver trial. Eur Heart J. (2022) 43(21):2023–33. doi: 10.1093/eurheartj/ehab919
45. Portela-Romero M, Cinza-Sanjurjo S, Conde-Sabarís P, Rodríguez-Mañero M, Mazón-Ramos P, Rey-Aldana D, et al. Real-life effect on the control of risk factors associated with initiation of the cardiovascular polypill created from equipotent drugs. Rev Clín Esp. (2022) 222(3):131–7. doi: 10.1016/j.rceng.2021.05.008
46. Ros-Castelló V, Natera-Villalba E, Gómez-López A, Sánchez-Sánchez A, Chico-García JL, García-Madrona S, et al. Use of the cardiovascular polypill in secondary prevention of cerebrovascular disease: a real-life tertiary hospital cohort study of 104 patients. Cerebrovasc Dis Extra. (2020) 10(3):166–73. doi: 10.1159/000511064
47. Gómez-Álvarez E, Verdejo J, Ocampo S, Ponte-Negretti CI, Ruíz E, Ríos MM. The CNIC-polypill improves atherogenic dyslipidemia markers in patients at high risk or with cardiovascular disease: results from a real-world setting in Mexico. IJC Heart Vasc. (2020) 29:100545. doi: 10.1016/j.ijcha.2020.100545
48. Gonzalez-Juanatey JR, Tamargo J, Torres F, Weisser B, Oudovenko N. Pharmacodynamic study of the cardiovascular polypill. Is there any interaction among the monocomponents? Rev Esp Cardiol. (2021) 74(1):51–8. doi: 10.1016/j.rec.2019.11.008
49. Roshandel G, Khoshnia M, Poustchi H, Hemming K, Kamangar F, Gharavi A, et al. Effectiveness of polypill for primary and secondary prevention of cardiovascular diseases (PolyIran): a pragmatic, cluster-randomised trial. Lancet. (2019) 394(10199):672–83. doi: 10.1016/S0140-6736(19)31791-X
50. Lafeber M, Spiering W, Visseren FL, Grobbee DE, Bots ML, Stanton A, et al. Impact of switching from different treatment regimens to a fixed-dose combination pill (polypill) in patients with cardiovascular disease or similarly high risk: the influence of baseline medication on LDL-cholesterol, blood pressure and calculated risk reduction when switching to a cardiovascular polypill. Eur J Prev Cardiol. (2017) 24(9):951–61. doi: 10.1177/2047487317695616
51. Selak V, Elley CR, Bullen C, Crengle S, Wadham A, Rafter N, et al. Effect of fixed dose combination treatment on adherence and risk factor control among patients at high risk of cardiovascular disease: randomised controlled trial in primary care. Br Med J. (2014) 348:g3318. doi: 10.1136/bmj.g3318
52. Yusuf S, Pais P, Sigamani A, Xavier D, Afzal R, Gao P, et al. Comparison of risk factor reduction and tolerability of a full-dose polypill (with potassium) versus low-dose polypill (polycap) in individuals at high risk of cardiovascular diseases: the second Indian polycap study (TIPS-2) investigators. Circ Cardiovasc Qual Outcomes. (2012) 5(4):463–71. doi: 10.1161/CIRCOUTCOMES.111.963637
53. Group PC. An international randomised placebo-controlled trial of a four-component combination pill (“polypill”) in people with raised cardiovascular risk. PLoS One. (2011) 6(5):e19857. doi: 10.1371/journal.pone.0019857
54. Soliman EZ, Mendis S, Dissanayake WP, Somasundaram NP, Gunaratne PS, Jayasingne IK, et al. A polypill for primary prevention of cardiovascular disease: a feasibility study of the world health organization. Trials. (2011) 12:1–6. doi: 10.1186/1745-6215-12-3
55. Patel A, Cass A, Peiris D, Usherwood T, Brown A, Jan S, et al. A pragmatic randomized trial of a polypill-based strategy to improve use of indicated preventive treatments in people at high cardiovascular disease risk. Eur J Prev Cardiol. (2015) 22(7):920–30. doi: 10.1177/2047487314530382
56. Malekzadeh F, Marshall T, Pourshams A, Gharravi M, Aslani A, Nateghi A, et al. A pilot double-blind randomised placebo-controlled trial of the effects of fixed-dose combination therapy (‘polypill’) on cardiovascular risk factors. Int J Clin Pract. (2010) 64(9):1220–7. doi: 10.1111/j.1742-1241.2010.02412.x
57. Study TIP. Effects of a polypill (polycap) on risk factors in middle-aged individuals without cardiovascular disease (TIPS): a phase II, double-blind, randomised trial. Lancet. (2009) 373(9672):1341–51. doi: 10.1016/S0140-6736(09)60611-5
58. Zamorano J, Erdine S, Pavia A, Kim JH, Al-Khadra A, Westergaard M, et al. Proactive multiple cardiovascular risk factor management compared with usual care in patients with hypertension and additional risk factors: the CRUCIAL trial. Curr Med Res Opin. (2011) 27(4):821–33. doi: 10.1185/03007995.2011.555754
59. Lafeber M, Grobbee DE, Schrover IM, Thom S, Webster R, Rodgers A, et al. Comparison of a morning polypill, evening polypill and individual pills on LDL-cholesterol, ambulatory blood pressure and adherence in high-risk patients; a randomized crossover trial. Int J Cardiol. (2015) 181:193–9. doi: 10.1016/j.ijcard.2014.11.176
60. Grimm R, Malik M, Yunis C, Sutradhar S, Kursun A. Simultaneous treatment to attain blood pressure and lipid goals and reduced CV risk burden using amlodipine/atorvastatin single-pill therapy in treated hypertensive participants in a randomized controlled trial. Vasc Health Risk Manag. (2010) 6:261–71. doi: 10.2147/VHRM.S7710
61. Mariani J, Rosende A, De Abreu M, Gonzalez Villa Monte G, D’Imperio H, Antonietti L, et al. Multicap to improve adherence after acute coronary syndromes: results of a randomized controlled clinical trial. Ther Adv Cardiovasc Dis. (2020) 14:1753944720912071. doi: 10.1177/1753944720912071
62. Neutel JM, Bestermann WH, Dyess EM, Graff A, Kursun A, Sutradhar S, et al. The use of a single-pill calcium channel blocker/statin combination in the management of hypertension and dyslipidemia: a randomized, placebo-controlled, multicenter study. J Clin Hypertens. (2009) 11(1):22–30. doi: 10.1111/j.1751-7176.2008.00058.x
63. González-Juanatey JR, Cordero A, Castellano JM, Masana L, Dalmau R, Ruiz E, et al. The CNIC-polypill reduces recurrent major cardiovascular events in real-life secondary prevention patients in Spain: the NEPTUNO study. Int J Cardiol. (2022) 361:116–23. doi: 10.1016/j.ijcard.2022.05.015
64. Oh GC, Han JK, Han KH, Hyon MS, Doh JH, Kim MH, et al. Efficacy and safety of fixed-dose combination therapy with telmisartan and rosuvastatin in Korean patients with hypertension and dyslipidemia: TELSTA-YU (TELmisartan-rosuvaSTAtin from YUhan), a multicenter, randomized, 4-arm, double-blind, placebo-controlled, phase III study. Clin Ther. (2018) 40(5):676–691.e1. doi: 10.1016/j.clinthera.2018.03.010
65. Wald DS, Morris JK, Wald NJ. Randomized polypill crossover trial in people aged 50 and over. PLoS One. (2012) 7(7):e41297. doi: 10.1371/journal.pone.0041297
66. Elley CR, Gupta AK, Webster R, Selak V, Jun M, Patel A, et al. The efficacy and tolerability of ‘polypills’: meta-analysis of randomised controlled trials. PLoS One. (2012) 7(12):e52145. doi: 10.1371/journal.pone.0052145
67. Bahiru E, De Cates AN, Farr MR, Jarvis MC, Palla M, Rees K, et al. Fixed-dose combination therapy for the prevention of atherosclerotic cardiovascular diseases. Cochrane Database Syst Rev. (2017) (3):CD009868. doi: 10.1002/14651858.CD009868.pub3
68. Mohamed MM, Osman M, Kheiri B, Saleem M, Lacasse A, Alkhouli M. Polypill for cardiovascular disease prevention: systematic review and meta-analysis of randomized controlled trials. Int J Cardiol. (2022) 360:91–8. doi: 10.1016/j.ijcard.2022.04.085
Keywords: polypill, cardiovascular disease, major adverse cardiac events, mortality, meta-analyses
Citation: Yazgi H, Mattikalli S, Fang B, Heselton H, Ssentongo P and Farbaniec M (2025) Efficacy of different polypill combinations for primary and secondary cardiovascular disease prevention: a systematic review and meta-analysis. Front. Cardiovasc. Med. 12:1558579. doi: 10.3389/fcvm.2025.1558579
Received: 10 January 2025; Accepted: 9 May 2025;
Published: 9 June 2025.
Edited by:
DeLisa Fairweather, Mayo Clinic Florida, United StatesReviewed by:
Yongliang Jia, Zhengzhou University, ChinaElena Cuadrado-Payán, Hospital Clinic of Barcelona, Spain
Copyright: © 2025 Yazgi, Mattikalli, Fang, Heselton, Ssentongo and Farbaniec. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Paddy Ssentongo, cHNzZW50b25nb0BwZW5uc3RhdGVoZWFsdGgucHN1LmVkdQ==
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share senior authorship
 Habib Yazgi1,†
Habib Yazgi1,† Shivani Mattikalli
Shivani Mattikalli Paddy Ssentongo
Paddy Ssentongo