- 1Vascular and Endovascular Surgery Division, Department of General Surgery and Surgical Specialties, Policlinico Umberto I, “Sapienza” University of Rome, Rome, Italy
- 2Department of General Surgery, Policlinico Umberto I, “Sapienza” University of Rome, Rome, Italy
Background: The Perclose ProStyle™/ProGlide™ Suture-Mediated Closure and Repair (SMCR) System is designed to close the common femoral artery (CFA) access during percutaneous endovascular procedures. Instructions for use (IFU) recommend the use of at least two devices, per single access, and the pre-close technique for arterial sheath sizes greater than 8 F. Besides, recent clinical studies suggest that a single ProStyle™/ProGlide™ pre-implantation can safely close percutaneous access for larger diameters.
Aim: The purpose of this study was to analyse the efficacy of a single pre-implanted ProStyle™/ProGlide™ in closing the Common Femoral Artery (CFA) access site in patients undergoing aortic endovascular procedures using sheaths with diameter 12–16 French (F).
Methods: We performed a prospective study including 72 consecutive patients who underwent aortic endovascular surgery from December 2022 to June 2024 in our University Hospital. In this group, only a single pre-implanted ProStyle™/ProGlide™ was used to close the access site in the CFA after using sheaths with diameters 12–16 F. The primary endpoint was technical success, defined as the absence of intraoperative open surgical conversion. The secondary endpoint was clinical success, defined as the absence of bleeding, pseudoaneurysms, and arteriovenous fistulas in the peri and postoperative period. We compared the results of this group with a cohort of patients in whom two ProStyle™/ProGlide™ were used to close the access site in the CFA.
Result: Technical success was achieved in all cases (100%). Clinical success was achieved in 98% of cases. Only two minor bleedings occurred: one resulted in a small pseudoaneurysm, completely thrombosed 48 h after the procedure. One patient suffered from CFA dissection, requiring an open surgical endarterectomy. There were no statistically significant differences of clinical and technical success rates between the two groups.
Conclusions: This study demonstrates that a Single ProStyle™/ProGlide™ preimplantation can be safe and effective in the closure of vascular accesses up to 16 Fr, with a low complication rate.
1 Introduction
Endovascular procedures have become the preferred treatment for patients with complex aortic diseases (1–4). Percutaneous access for EVAR (PEVAR) (5) is an accepted alternative to traditional EVAR with surgical cut-down (SEVAR), reducing procedure's invasiveness (5–8) and surgical site complications (6). Technical success rate of PEVAR has improved over the years, from 62%–100% (9–16). Most studies have utilised the Prostar™ (Abbott Perclose, Redwood City, Calif) closure device; the newest version of this device, the ProStyle™/ProGlide™ device, presents higher possibilities for successful closure (12, 17, 18).
Improvements in technical details (18–20) such as percutaneous devices capable of closing ever larger accesses and endovascular devices have led to wider use of total percutaneous techniques of endovascular procedures (21), even in challenging anatomies (20), with positive midterm outcomes (7). Several techniques are available for vascular haemostasis, including suture-based, collagen-based, clip-based devices or their combination (19, 22). Femoral calcification seems to be the only predictor of percutaneous access failure (23).
Percutaneous approach is usually performed under local anaesthesia, contributing to reduced operative time, hospital stay, and lower perioperative complications related to general anaesthesia and surgical cutdown (24–30).
The aim of this study was to analyse the efficacy of using a single ProStyle™/ProGlide™ in closing access sites with sheath diameters greater than 12 French (F) (4. 7 mm) up to 16 F (5. 3 mm), in patients undergoing endovascular aortic procedures.
2 Materials and methods
Two groups of patients were compared: patients requiring a single ProStyle™/ProGlide™ per percutaneous femoral artery site (OneStyle group), and patients who underwent standard protocol ProStyle™/ProGlide™ with two preimplanted devices per femoral access site (Control Group). Data were collected prospectively for the first group, and they were compared with data of the second group, which were collected retrospectively.
The first group included patients who underwent elective percutaneous endovascular aortic procedures from December 2022 to June 2024 at a tertiary University hospital, while the control group consisted of patients who underwent the same endovascular procedures from 2018–2023. Patients were matched 1–1 according to age, sex, clinical conditions, and type of endovascular surgery.
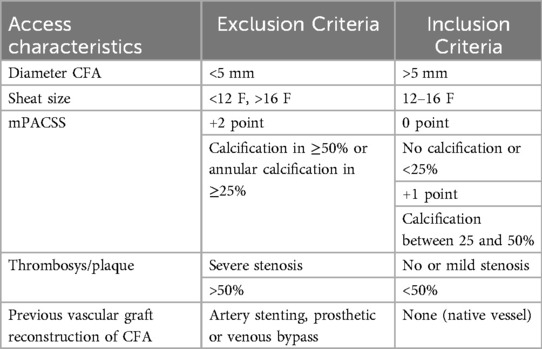
Exclusion criteria included femoral diameter of less than 5 mm; different access vessels than the CFA; access size sheath <12 F and >16 F; the Modified Peripheral Arterial Calcium Scoring System (mPACSS) (31) score of 2; arterial stenosis of more than 50%, previous vascular grafts reconstruction with bypass surgery or stenting of the femoral artery (Table 1).
We analysed technical and clinical success using a single ProStyle™/ProGlide™ to close the access site in the Common Femoral Artery (CFA) with sheaths diameter from 12 Fr up to 16 F.
All patients were followed postoperatively with a Duplex Ultrasound (DUS) examination and computed tomography angiography (CTA) performed at 1 month.
The primary endpoint of this study was technical success, defined as the correct device implantation and the absence of intraoperative open surgical conversion. Secondary endpoint was the clinical success, defined as the absence of bleeding, pseudoaneurysms, and arteriovenous fistulas in the postoperative period.
Outcomes of interest were classified according to Bleeding Academic Research Consortium (BARC) criteria (32) that were subdivided into life-threatening or disabling bleeding (bleeding in a critical organ, hypovolemic shock, or hypotension requiring vasopressors), major bleeding (overt bleeding requiring transfusion of 2 or 3 units of whole blood or packed red blood cells or requiring surgery), and minor bleeding (access site hematoma) (32).
2.1 The ProStyle™/ProGlide™ device
The Perclose ProStyle™/ProGlide™ Suture-Mediated Closure and Repair (SMCR) System (Abbott Vascular, Abbott Park, IL, USA) is one of the most recent VCDs able to deliver a single monofilament polypropylene suture and positioning the pre-tied suture knot to the top of the access site. It is indicated for the closure of access sites in the common femoral artery (CFA) using 5 F to 21 F sheaths.
The product's instructions for use (IFU) suggest at least two devices and the pre-close technique for arterial sheath sizes greater than 8 F. No contraindications are known for the use of this device (33). However, some recent clinical studies suggest that a single pre-implanted ProStyle™/ProGlide™ could safely close percutaneous access even in larger sizes access (34, 35).
2.2 Access site/anatomical characteristics
Aorto-iliac pathology and femoral accesses anatomical and morphological characteristics were assessed preoperatively by CTA and DUS exams. We considered the mPACSS classification to quantify the vessel calcification (31) (Table 2). In case of plaque with lumen reduction more than 30% and less than 50%, accesses were defined as characterized by moderate atherosclerotic disease.
All vessel measurements were performed using a dedicated software and center lumen line reconstruction from OsiriX MD software—version 12 (PIXMEO, Bernex, Switzerland).
2.3 Technique
The standard preimplantation closure technique involved the deployment of pre-implanted ProStyle™/ProGlide™ at the beginning of the procedure. Vascular access was performed under ultrasound guidance, and the CFA was punctured at the centre of its upper wall, in a non-calcified/atheromatous area. The ProStyle™/ProGlide™ device is then mounted on the wire, positioning and deploying at 12 o'clock for a single preimplantation procedure or at 10 and 2 o'clock for a double preimplantation procedure (Control group). Then it is subsequently removed, allowing the ProStyle™/ProGlide™ suture to be deployed. The endovascular procedure is then performed; after the endograft deployment, the sheath is removed, and the ProStyle™/ProGlide™ is tightened over the access wire. The wire is gently tugged to ensure the ProStyle™/ProGlide™ suture has a secure grip around the wire and no bleeding is observed. Two minutes of manual compression is performed for additional safety in all cases.
An ultrasound control is performed at the end of the procedure and a compression bandage is applied for the next 24 h. Patients were re-evaluated with a duplex ultrasound at one-month follow-up.
The procedures were performed by vascular surgeons with extensive experience in the use of ProStyle™/ProGlide™ devices, and operators were the same for both groups.
2.4 Statistical analysis
Continuous variables are collected as median and interquartile range (IQR) or mean and standard deviation (SD); categorical data are presented as numbers and percentages. Continuous data were compared with the Mann–Whitney U test or t-test, whereas categorical data were compared using Fisher's exact or the Chi-square test. A multivariable logistic regression was performed to identify independent predictors (1) sex, (2) sheath diameters, and (3) time of manual compression in comparing bilateral use of a single ProStyle™/ProGlide™ with non-single ProStyle™/ProGlide™ use. Analysis was performed according to major/minor complications criteria, and patients were categorized according to their initial group allocation. All variables with p-values < 0.1 is included in the univariate analysis. For all analyses, a two-sided p-value < 0.05 was considered significant.
As the entire cohort was highly unbalanced regarding sex distribution, with the aim of obtaining a more homogeneous population for properly comparing, a 1:1 automatic propensity matching was performed using a logistic regression model adjusted for demographic data and comorbidities. The model included all available risk factors listed in the electronic data set.
All analyses were performed using SPSS Statistics version 28.0 [International Business Machines Corporation (IBM Corp.), Armonk, NY, USA].
3 Results
3.1 Patient population
3.1.1 Prospective study: single-style group (use of only one ProStyle™/ProGlide™ system)
We prospectively enrolled 72 consecutive patients, for a total of 100 vascular accesses, from December 2022 to June 2024, undergoing transfemoral endovascular aorto-iliac procedures with access site closure using a single ProStyle™/ProGlide™ system. EVAR and iliac branch devices used are listed in Table 3.
Baseline characteristics, comorbidities, operative reports, complications, and discharge summaries were collected by a thorough review of the individual patient medical charts and grouped by access.
3.1.2 Control group (use of two ProStyle™/ProGlide™ system)
The retrospective analysis collected data from 84 consecutive patients from August 2018 to September 2023 who underwent transfemoral endovascular aorto-iliac procedures with access site closure using two ProStyle™/ProGlide™ systems for a total of 100 accesses.
3.2 Data analysis
A total of 156 patients were included in the study. Of those, 72 patients were treated with endovascular aorto-iliac procedure using one single ProStyle™/ProGlide™ system for primary access site closure and 84 patients with two devices (Control group), for a total of 200 endovascular accesses. In 39 patients, a single closure device was used bilaterally.
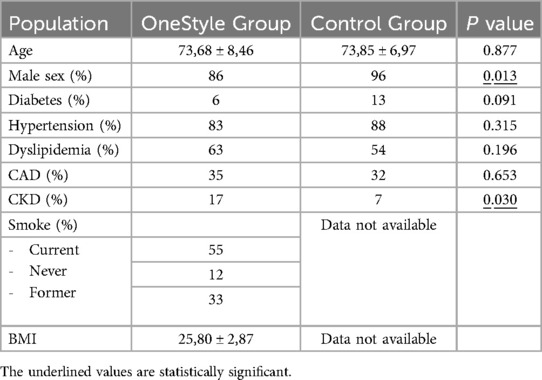
Single ProStyle™/ProGlide™ and double ProStyle™/ProGlide™ groups were similar for all demographic characteristics except sex, sheath diameters, and chronic kidney disease (CKD) in remote medical history (Tables 4–6). A propensity score matching was used to analyse all these variables and no significant differences were observed between the groups for sex and CKD.
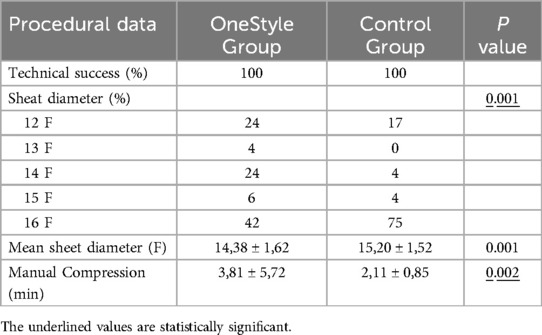
The sheath diameter value was the only factor that was statistically significative at multivariate regression analysis after adjustment for all confounding variables (p = 0.001). After adjusting for the initial imbalance in the sheath diameters parameter using propensity score matching, the logistic regression analysis shows that the two groups can be considered clinically comparable, and the sheath diameters value did not have a significant impact on the analyzed outcomes as bleeding and pseudoaneurysm.
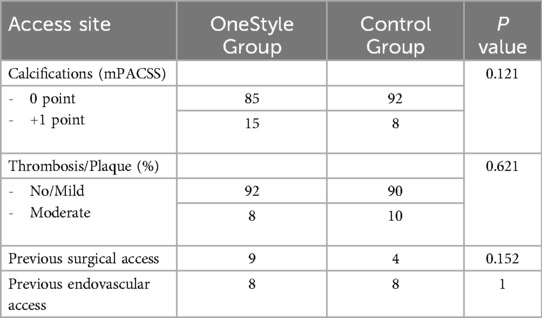
No significant differences were found between the two groups regarding the characteristics of the accesses (Tables 5, 6).
Technical success was achieved in all cases with no need for intraoperative open surgical conversion. No further ProStyle™/ProGlide™ or alternative closure systems were required.
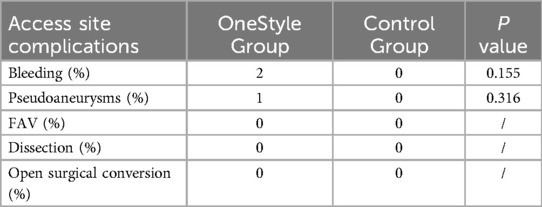
Clinical success was achieved in 98% of the accesses when only one ProStyle™/ProGlide™ was used: two cases were followed by minor bleeding (2%), according to the BARC classification, requiring 40-minute manual compression to achieve complete haemostasis. One of the two cases hesitated in a small pseudoaneurysm, which was completely thrombosed at the 48 h DUS control and remained unchanged in size at the 1-month CTA. It completely resolved 6 months after the procedure. No further cases of bleeding, pseudoaneurysms, or arteriovenous fistulas were found on DUS examination in the immediate post-operative and 24 h after the surgical procedure.
Clinical success was achieved in 100% of the cases in the Control Group.
No statistically significant differences were found between the two groups regarding both bleeding (2 vs. 0, p = 0,155) and pseudoaneurysm (1 vs. 0, p = 0,316) (Tables 7 and 8).
In the prospective group (OneStyle), twenty-two accesses required more than 2 min of manual compression (22%), with an average time of 3, 81 min (Range: 2–40 min) to achieve complete hemostasis. In the two cases of minor bleedings, the suture placed by the closure system probably did not completely close the breach on the artery, so manual compression was necessary to achieve complete haemostasis In the Control group, manual compression exceeding 2 min was needed in only two cases (2/100, 2%), with an average time of manual compression of 2, 11 min (Range: 2–10 min (p = 0,002).
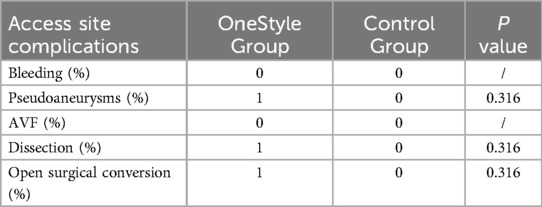
One patient who had closure of the CFA access with only one ProStyle™/ProGlide™ suffered from CFA dissection, which required open endarterectomy (Figures 1, 2).
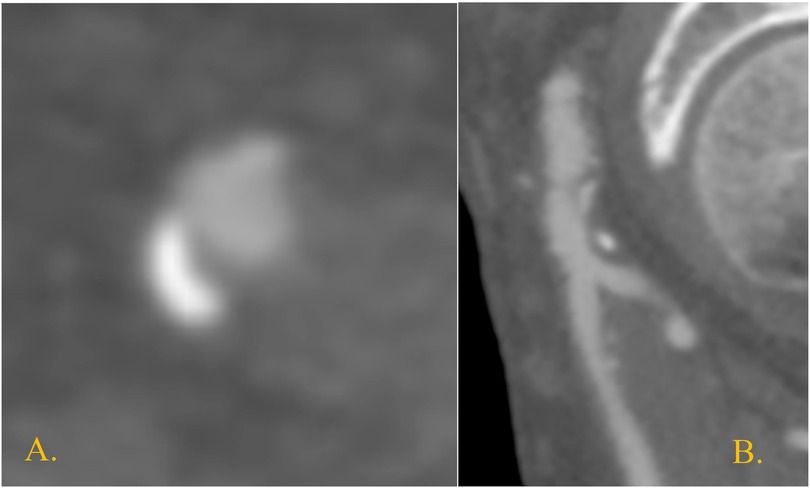
Figure 1. Right CFA at preoperative CTA in the only case of postoperative focal dissection. It shows a posterior atheroma plaque at the level of the endovascular access (A) transversal section; (B) sagittal section.
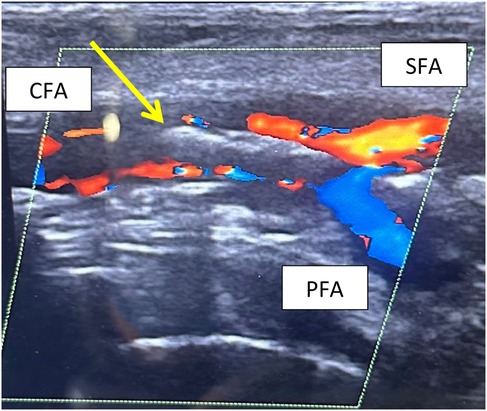
Figure 2. Postoperative CFA dissection at DUS examination. The arrow evidences the double binary sign due to the lifting of the atherosclerotic plaque.
4 Discussion
In our clinical practice, we have successfully utilized the ProStyle™/ProGlide™ system and the pre-close technique for achieving hemostasis at the CFA access during endovascular aortic procedures. Initially, we followed the manufacturer's instructions, deploying one suture knot at a time, and found that a single device was sufficient to ensure complete hemostasis in most cases. This led us to adopt the strategy of using a single ProStyle™/ProGlide™ device for all patients with access sites requiring sheaths smaller than 14 F, since 2019. This approach has proven successful, becoming our standard protocol. Based on this experience, in 2022, we started this prospective study analysing the efficacy of using a single ProStyle™/ProGlide™ in case of sheath up to 16 F. We observed only one case of a pseudoaneurysm, which fully thrombosed within 48 h and reabsorbed spontaneously within six months, and a single case of CFA dissection over a two-year period. However, the dissection was likely caused by the device's suture deployment mechanism, rather than by the use of a single device. In fact, in this patient, the preoperative ultrasound examination revealed moderate atherosclerotic plaque in the femoral artery, which likely led to the suture engaging the plaque instead of the arterial wall, causing a focal dissection. This was managed by femoral endarterectomy due to the patient's claudication symptoms. Such complications, though rare, have been reported in the literature, with a rate of about 1.62% according to Saadi et al. in 2017 (36).
Supporting our results, several studies in the literature have demonstrated the safety and effectiveness of using a single ProStyle™/ProGlide™ device for vascular access closure. In 2020 Krista Dunn et al. (35) reported a retrospective study of 116 patients undergoing EVAR procedure with bilateral percutaneous femoral access. They experienced 6.11% access-site complications: 3.8% required surgical conversions and 2.3% required additional ProGlide™ insertion to achieve adequate hemostasis, although this was not an issue in our study. Even if the median sheath outer diameter was similar to ours (maximum of 18F), they reported a notable number of open surgical conversions, compared to our center results.
Similarly, Taku Ichihashi et al. (34) (2016) studied the use of a single ProGlide™ device in 50 patients undergoing PEVAR, reporting positive outcomes with no surgical corrections needed, though one case required additional manual compression. The sheath diameters in their study were larger than those in the previous study till 22 F sheath, a quite significant data for our discussion.
By the way, larger studies come from interventional cardiology (37), such as those by Kodama et al. (38) (2016), Bazarbashi Najdat et al. (39) (2019) and Joerg Reifart et al. (40), comparing the single-device technique with double-device systems in transcatheter aortic valve implantation (TAVI) procedures. While the single-device approach was generally safe and effective, these studies indicated that some patients required additional closure devices, especially when there were larger sheath sizes or complications related to hemostasis. In contrast, our study showed no such need for additional devices, which suggests a higher success rate in achieving hemostasis with the single-device approach.
In addition, a technique proposed by Gurbhej Singh et al. (41) (2020) involved downsizing the sheath after device deployment to 8 F, before tightening the ProGlide™ suture. This approach increases the procedure time and material use, which did not align with our goal of optimizing efficiency.
Moreover, we documented a single case of isolated CFA dissection, due to the suture deployment mechanism of the ProStyle™/ProGlide™ system rather than the use of a single device. The navigation of the device deployment system, inside the arterial lumen, may cause the plaque to lift and focal dissection when in contact with any atheroma plaques of the common femoral artery. This occurrence is evidenced in Figure 2, where is showed the common femoral focal dissection in post-operative period. This kind of adverse event is reported in literature as a possible complication using this device, as described by Saadi et al. in 2017 whit a rate of 1.62% (36). In our study, only one case was registered out of a total of 200 accesses (0.5%). In our opinion, the role of ultrasound-guided puncture is essential in ensuring safe access to the CFA, minimizing potential complications. The main advantage of our approach is its cost-effectiveness. Using a single device reduces the overall cost compared to the double-device or SEVAR techniques while maintaining the same level of safety and effectiveness. This reduction in device-related costs is significant in the broader context of healthcare economics, potentially improving the affordability of endovascular procedures both locally and globally.
Our monocentric study confirms the possibility to use a single ProStyle™/ProGlide™ closure system for achieving complete hemostasis, with no significant differences in outcomes compared to the double-device approach. Nevertheless, a minor statistical difference in the time for manual compression (only 1. 7 min) was observed, but this did not affect the overall success rate. Our results also suggest a better rate of surgical conversion compared to other studies, with similar positive outcomes for both complex aortic procedures and interventional cardiology treatments.
4.1 Limitations
This study is based on a single center experience, with a limited number of patients. Moreover, there is a difference between the two groups in terms of sheeth size although it was not statistically significant in defining outcomes.
However, the sample size reached with the prospective and retrospective cohort of patients enhanced no statistical differences in the outcomes measured. Given the low event rates (e.g., 2% bleeding), the manuscript must acknowledge the limitations in detecting true differences, leading to low statistical power.
However, given the very low complication rate observed, direct comparison with other studies in the literature is challenging.
In order to confirm our findings, the need for further research in randomised or multicentre studies is necessary.
5 Conclusions
In conclusion, our study supports the use of a single ProStyle™/ProGlide™ device for hemostasis in endovascular aortic procedures, offering a reliable, cost-effective alternative to more complex strategies, with similar complication-free outcomes and potential for reducing overall healthcare costs.
Total percutaneous endovascular procedures have become an integral part of the clinical practice in an increasing number of centers. These procedures reduce hospitalization time, intensive care unit stay, in hospital readmission and help lower the overall costs of endovascular treatments, which remain.
The results of our study, demonstrate the safety and efficacy of using a Single ProStyle™/ProGlide™ vascular pre-close technique for the closure of vascular accesses up to 16 F, with very low rate of bleedings and pseudoaneurysms, and no cases of open surgical conversion. Employing a single closure system further reduces the cost of endovascular procedures by utilizing fewer materials and expediting the process, without compromising the security of the procedure.
Moreover, the use of the ONESTYLE technique requires no learning curve for professionals already familiar with the ProStyle™/ProGlide™ device, making it a seamless addition to existing practices.
However, our data are limited by the monocentric nature of the study, and the difference in term of sheath sizes between two groups can be considered as a procedural bias in our study. Further research in randomized studies or multicentred studies would be beneficial to fully validate the effectiveness of this technique.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
RC: Conceptualization, Data curation, Formal analysis, Writing – original draft. AD: Investigation, Methodology, Validation, Writing – review & editing. MA: Formal analysis, Supervision, Validation, Writing – review & editing, Data curation, Investigation, Writing – original draft. FM: Resources, Validation, Visualization, Writing – review & editing. CD: Data curation, Writing – review & editing. AM: Investigation, Supervision, Writing – review & editing. AD: Writing – review & editing, Investigation. AS: Supervision, Writing – original draft, Writing – review & editing. GG: Writing – review & editing, Methodology, Resources. LM: Project administration, Validation, Writing – review & editing, Resources. WM: Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chaikof EL, Dalman RL, Eskandari MK, Jackson BM, Lee WA, Mansour MA, et al. The society for vascular surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. (2018) 67(1):2–77.e2. doi: 10.1016/j.jvs.2017.10.044
2. Wanhainen A, Verzini F, Van Herzeele I, Allaire E, Bown M, Cohnert T, et al. Editor’s choice—European society for vascular surgery (ESVS) 2019 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur J Vasc Endovasc Surg. (2019) 57(1):8–93. doi: 10.1016/j.ejvs.2018.09.020
3. Veith FJ, Marin ML, Cynamon J, Schonholz C, Parodi J. 1992: Parodi, Montefiore, and the first abdominal aortic aneurysm stent graft in the United States. Ann Vasc Surg. (2005) 19(5):749–51. doi: 10.1007/s10016-005-6858-9
4. Sirignano P, Piffaretti G, Ceruti S, Orso M, Picozzi M, Ricci G, et al. Insight from an Italian Delphi consensus on EVAR feasibility outside the instruction for use: the SAFE EVAR study. J Cardiovas Surg. (2024) 65(3):273–9. doi: 10.23736/S0021-9509.23.12906-5
5. Nelson PR, Kracjer Z, Kansal N, Rao V, Bianchi C, Hashemi H, et al. A multicenter, randomized, controlled trial of totally percutaneous access versus open femoral exposure for endovascular aortic aneurysm repair (the PEVAR trial). J Vasc Surg. (2014) 59(5):1181–93. doi: 10.1016/j.jvs.2013.10.101
6. Buck DB, Karthaus EG, Soden PA, Ultee KHJ, Van Herwaarden JA, Moll FL, et al. Percutaneous versus femoral cutdown access for endovascular aneurysm repair. J Vasc Surg. (2015) 62(1):16–21. doi: 10.1016/j.jvs.2015.01.058
7. Lee WA, Brown MP, Nelson PR, Huber TS, Seeger JM. Midterm outcomes of femoral arteries after percutaneous endovascular aortic repair using the Preclose technique. J Vasc Surg. (2008) 47(5):919–23. doi: 10.1016/j.jvs.2007.12.029
8. Torsello GB, Kasprzak B, Klenk E, Tessarek J, Osada N, Torsello GF. Endovascular suture versus cutdown for endovascular aneurysm repair: a prospective randomized pilot study. J Vasc Surg. (2003) 38(1):78–82. doi: 10.1016/S0741-5214(02)75454-2
9. Traul DK, Clair DG, Gray B, O’Hara PJ, Ouriel K. Percutaneous endovascular repair of infrarenal abdominal aortic aneurysms: a feasibility study. J Vasc Surg. (2000) 32(4):770–6. doi: 10.1067/mva.2000.107987
10. Howell M, Villareal R, Krajcer Z. Percutaneous access and closure of femoral artery access sites associated with endoluminal repair of abdominal aortic aneurysms. J Endovasc Ther. (2001) 8(1):68–74. doi: 10.1177/152660280100800112
11. Teh LG, Sieunarine K, Van Schie G, Goodman MA, Lawrence-Brown M, Prendergast FJ, et al. Use of the percutaneous vascular surgery device for closure of femoral access sites during endovascular aneurysm repair: lessons from our experience. Eur J Vasc Endovasc Surg. (2001) 22(5):418–23. doi: 10.1053/ejvs.2001.1495
12. Patel PJ, Kelly Q, Hieb RA, Lee CJ. Current status of percutaneous endografting. Semin Intervent Radiol. (2015) 32(3):278–88. doi: 10.1055/s-0035-1556826
13. Starnes BW, Andersen CA, Ronsivalle JA, Stockmaster NR, Mullenix PS, Statler JD. Totally percutaneous aortic aneurysm repair: experience and prudence. J Vasc Surg. (2006) 43(2):270–6. doi: 10.1016/j.jvs.2005.11.004
14. Watelet J, Gallot JC, Thomas P, Douvrin F, Plissonnier D. Percutaneous repair of aortic aneurysms: a prospective study of suture-mediated closure devices. Eur J Vasc Endovasc Surg. (2006) 32(3):261–5. doi: 10.1016/j.ejvs.2006.01.022
15. Rachel ES, Bergamini TM, Kinney EV, Jung MT, Kaebnick HW, Mitchell RA. Percutaneous endovascular abdominal aortic aneurysm repair. Ann Vasc Surg. (2002) 16(1):43–9. doi: 10.1007/s10016-001-0127-3
16. Miceli F, Dajci A, Di Girolamo A, Nardis P, Ascione M, Cangiano R, et al. Early and mid-term outcomes of isolated type 2 endoleak refractory to an embolization procedure. J Clin Med. (2025) 14(2):502. doi: 10.3390/jcm14020502
17. Jaffan AAA, Prince EA, Hampson CO, Murphy TP. The preclose technique in percutaneous endovascular aortic repair: a systematic literature review and meta-analysis. Cardiovasc Intervent Radiol. (2013) 36(3):567–77. doi: 10.1007/s00270-013-0593-3
18. Lee WA, Brown MP, Nelson PR, Huber TS. Total percutaneous access for endovascular aortic aneurysm repair (“Preclose” technique). J Vasc Surg. (2007) 45(6):1095–101. doi: 10.1016/j.jvs.2007.01.050
19. Noori VJ, Eldrup-Jørgensen J. A systematic review of vascular closure devices for femoral artery puncture sites. J Vasc Surg. (2018) 68(3):887–99. doi: 10.1016/j.jvs.2018.05.019
20. Sirignano P, Mansour W, Capoccia L, Cuozzo S, Camparini S, de Donato G, et al. Endovascular aortic repair in patients with challenging anatomies: the EXTREME study. EuroIntervention. (2021) 16(18):E1544–50. doi: 10.4244/EIJ-D-19-00547
21. Melani C, Bastianon M, Mozzetta G, Di Gregorio S, Di Bartolo M, Capone A, et al. Multicenter real-life study on access-related outcomes after EVAR: percutaneous is the way. Ital J Vasc Endovasc Surg. (2023) 29(4). doi: 10.23736/S1824-4777.22.01559-5
22. Najem M, Martin G, Patrone L, Malina M, Theivacumar NS. Pledget reinforcement and tractional compression as adjunctive techniques for suture-mediated closure (SMC) in percutaneous endovascular aneurysm repair (pEVAR): a retrospective observational cohort study. Ann Vasc Surg. (2021) 73:369–74. doi: 10.1016/j.avsg.2020.11.021
23. Pratesi G, Barbante M, Pulli R, Fargion A, Dorigo W, Bisceglie R, et al. Italian Percutaneous EVAR (IPER) registry: outcomes of 2381 percutaneous femoral access sites' Closure for aortic stent-graft. J Cardiovasc Surg (Torino). (2015) 56(6):889–98.26372021
24. Hajibandeh S, Hajibandeh S, Adasonla K, Antoniou SA, Barrie J, Madan M, et al. Loco-regional versus general anaesthesia for elective endovascular aneurysm repair—results of a cohort study and a meta-analysis. Vasa. (2018) 47(3):209–17. doi: 10.1024/0301-1526/a000688
25. Kerré S, Kustermans L, Vandendriessche T, Bosmans J, Haine SE, Miljoen H, et al. Cost-effectiveness of contemporary vascular closure devices for the prevention of vascular complications after percutaneous coronary interventions in an all-comers PCI population. EuroIntervention. (2014) 10(2):191–7. doi: 10.4244/EIJV10I2A32
26. Franz R, Hartman J, Wright M. Comparison of anesthesia technique on outcomes of endovascular repair of abdominal aortic aneurysms: a five-year review of monitored anesthesia care with local anesthesia vs. general or regional anesthesia. J Cardiovasc Surg (Torino). (2011) 52(4):567–77. Available online at: https://pubmed.ncbi.nlm.nih.gov/22034673/
27. Resnic FS, Arora N, Matheny M, Reynolds MR. A cost-minimization analysis of the angio-seal vascular closure device following percutaneous coronary intervention. Am J Cardiol. (2007) 99(6):766–70. doi: 10.1016/j.amjcard.2006.10.032
28. Rickli H, Unterweger M, Sütsch G, Brunner-La Rocca HP, Sagmeister M, Ammann P, et al. Comparison of costs and safety of a suture-mediated closure device with conventional manual compression after coronary artery interventions. Catheter Cardiovasc Interv. (2002) 57(3):297–302. doi: 10.1002/ccd.10294
29. Roche-Nagle G, Hazel M, Rajan DK. Financial impact of PEVAR compared with standard endovascular repair in Canadian hospitals. Can Assoc Radiol J. (2018) 69(2):215–9. doi: 10.1016/j.carj.2017.08.003
30. Troisi N, Bertagna G, Berchiolli R, Abdelrahman A, Abd Elhamid MS, Abdelhamid M, et al. RIvaroxaban and vascular surgery (rIVaS): insights from a multicenter, worldwide web-based survey. Int Angiol. (2024) 43(2):306–8. doi: 10.23736/S0392-9590.24.05146-0
31. Nugteren MJ, Ünlü Ç, Samim M, Scheffer HJ, de Borst GJ, Hazenberg CEVB. Inter- and intra-observer agreement of the peripheral arterial calcium scoring system in patients undergoing (infra)Popliteal endovascular interventions. Cardiovasc Intervent Radiol. (2024) 47(11):1441–9. doi: 10.1007/s00270-024-03839-1
32. Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, et al. Standardized bleeding definitions for cardiovascular clinical trials (BARC). Circulation. (2011) 123(23):2736–47. doi: 10.1161/CIRCULATIONAHA.110.009449
33. Abbott Cardiovascular. Perclose™ ProStyle™ SMCR System Instrucition for Use (2025). Available online at: https://www.cardiovascular.abbott/int/en/hcp/products/peripheral-intervention/vessel-closure/perclose-prostyle-suture-mediated-closure-system/overview.html (Accessed January 04, 2025).
34. Ichihashi T, Ito T, Kinoshita Y, Suzuki T, Ohte N. Safety and utility of total percutaneous endovascular aortic repair with a single Perclose ProGlide closure device. J Vasc Surg. (2016) 63(3):585–8. doi: 10.1016/j.jvs.2015.08.111
35. Dunn K, Jessula S, Herman CR, Smith M, Lee MS, Casey P. Safety and effectiveness of single proglide vascular access in patients undergoing endovascular aneurysm repair. J Vasc Surg. (2019) 70(4):e110–1. doi: 10.1016/j.jvs.2019.07.047
36. Saadi EK, Saadi M, Saadi R, Tagliari AP, Mastella B. Totally percutaneous access using perclose proglide for endovascular treatment of aortic diseases. Braz J Cardiovasc Surg. (2017) 32(1):43–8. doi: 10.21470/1678-9741-2016-0065
37. Barbash IM, Barbanti M, Webb J, Molina-Martin De Nicolas J, Abramowitz Y, Latib A, et al. Comparison of vascular closure devices for access site closure after transfemoral aortic valve implantation. Eur Heart J. (2015) 36(47):3370–9. doi: 10.1093/eurheartj/ehv417
38. Kodama A, Yamamoto M, Shimura T, Kagase A, Koyama Y, Tada N, et al. Comparative data of single versus double proglide vascular preclose technique after percutaneous transfemoral transcatheter aortic valve implantation from the optimized catheter valvular intervention (OCEAN-TAVI) Japanese multicenter registry. Catheter Cardiovasc Interv. (2017) 90(3):E55–62. doi: 10.1002/ccd.26686
39. Bazarbashi N, Ahuja K, Gad MM, Sammour YM, Kaur M, Karrthik A, et al. The utilization of single versus double Perclose devices for transfemoral aortic valve replacement access site closure: insights from Cleveland clinic aortic valve center. Catheter Cardiovasc Interv. (2020) 96(2):442–7. doi: 10.1002/ccd.28585
40. Reifart J, Liebetrau C, Weferling M, Dörr O, Renker M, Bhumimuang K, et al. Single versus double use of a suture-based closure device for transfemoral aortic valve implantation. Int J Cardiol. (2021) 331:183–8. doi: 10.1016/j.ijcard.2021.01.043
Keywords: vascular access, vascular access closure devices, aortic endovascular procedure, pre-close technique, vascular-diagnosis
Citation: Cangiano R, Di Girolamo A, Ascione M, Miceli F, D’Amico C, Molinari A, Dajci A, Sterpetti A, Gagliardo G, di Marzo L and Mansour W (2025) Safety and effectiveness of single ProStyle™/ProGlide™ for aortic endovascular procedures: single-style study. Front. Cardiovasc. Med. 12:1559131. doi: 10.3389/fcvm.2025.1559131
Received: 11 January 2025; Accepted: 15 June 2025;
Published: 3 July 2025.
Edited by:
Shahzad Raja, Harefield Hospital, United KingdomReviewed by:
Francesco Cabrucci, Lankenau Institute for Medical Research, United StatesFrancis Smit, University of the Free State, South Africa
Andreas Zuckermann, Meduniwien
Copyright: © 2025 Cangiano, Di Girolamo, Ascione, Miceli, D’Amico, Molinari, Dajci, Sterpetti, Gagliardo, di Marzo and Mansour. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marta Ascione, bWFydGEuYXNjaW9uZUB1bmlyb21hMS5pdA==
 Rocco Cangiano
Rocco Cangiano Alessia Di Girolamo
Alessia Di Girolamo Marta Ascione
Marta Ascione Francesca Miceli1
Francesca Miceli1 Antonio Sterpetti
Antonio Sterpetti Luca di Marzo
Luca di Marzo Wassim Mansour
Wassim Mansour