- 1Department of Cardiovascular Intervention, Nguyen Tri Phuong Hospital, Ho Chi Minh City, Vietnam
- 2Faculty of Medicine, Nguyen Tat Thanh University, Ho Chi Minh City, Vietnam
Background: Coronavirus disease 2019 (COVID-19) has disrupted the management of acute coronary syndrome (ACS), with emerging evidence suggesting increased complications and mortality among patients undergoing percutaneous coronary intervention (PCI). However, data from low- and middle-income settings such as Vietnam remain limited. This study aimed to evaluate the clinical characteristics and outcomes of ACS patients with COVID-19 undergoing PCI at a tertiary hospital in Vietnam.
Methods: This retrospective cohort study was conducted at a tertiary hospital in Ho Chi Minh City from 2019 to 2022. Adult patients diagnosed with ACS who underwent PCI were included and stratified by COVID-19 status confirmed via RT-PCR. All patients received standard guideline-directed therapy, including dual antiplatelet and anticoagulant regimens, and were followed for 1 year to assess clinical outcomes.
Result: A total of 118 patients were included, comprising 26 COVID-19-positive and 92 COVID-19-negative individuals. Baseline characteristics and cardiovascular risk factors were generally comparable between the two groups. While procedural success rates were similar, COVID-19-positive patients demonstrated higher thrombus burden and significantly increased rates of ICU admission, prolonged hospitalization, and MACCE at all timepoints. COVID-19 severity, cardiogenic shock, and multivessel disease emerged as independent predictors of adverse outcomes.
Conclusion: In this Vietnamese cohort, COVID-19 infection was associated with worse clinical outcomes following PCI for ACS. These findings highlight the need for early risk stratification and resource planning during pandemic conditions. However, the small sample size, single-center design, and observational nature of the study limit its generalizability, and causal inferences should be drawn with caution.
1 Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), emerged as a global health crisis in late 2019 and was declared a pandemic by the World Health Organization (WHO) in March 2020 (1). Since then, it has placed unprecedented strain on healthcare systems worldwide, reshaping the management of both acute and chronic conditions. In particular, care for patients with acute coronary syndrome (ACS) has been disrupted, affecting every stage of diagnosis and treatment, compromising outcomes and straining established protocols (2).
Percutaneous coronary intervention (PCI) remains the gold standard for treating ACS, particularly in ST-segment elevation myocardial infarction (STEMI). However, emerging evidence indicates that ACS patients with concurrent COVID-19 infection experience worse in-hospital outcomes, including elevated thrombus burden, higher rates of cardiogenic shock, prolonged hospitalization and increased mortality (3, 4). These adverse outcomes have been attributed to delays in care, the diversion of critical care resources, and the systemic inflammatory and prothrombotic effects of COVID-19 (5, 6).
While studies from high-income countries have begun to characterize the interplay between COVID-19 and ACS outcomes, data from low- and middle-income countries, including Vietnam, remain limited. Vietnam's healthcare system, with its centralized tertiary hospitals and constrained critical care capacity during pandemic surges, may present unique challenges in maintaining standard ACS care (7). Moreover, the distinct characteristics of the Vietnamese population such as younger average age and differing cardiovascular risk profiles could modulate disease presentation and outcomes (8).
Understanding how COVID-19 infection and the evolving pandemic landscape influenced ACS management and outcomes is essential for improving future preparedness, particularly in resource-limited settings. Specifically, the effects of pandemic waves, COVID-19 severity, and vaccination coverage on procedural characteristics and major adverse cardiac and cerebrovascular events (MACCE) remain underexplored in Vietnam.
This study aims to evaluate the clinical features, procedural outcomes, and MACCE rates among ACS patients undergoing PCI during the COVID-19 pandemic in a Vietnamese tertiary center. By identifying predictors of poor outcomes, we aim to provide insight relevant to both local clinical decision-making and national care strategies in resource-limited settings.
2 Material and method
2.1 Design of the study
This retrospective descriptive study was carried out at a tertiary care hospital in Ho Chi Minh City, Vietnam, during the COVID-19 pandemic, spanning January 2020 to December 2022. Ethical approval for the study was obtained from the hospital's Ethical Review Board.
2.2 Study population
The study population consisted of adult patients diagnosed with ACS who underwent PCI, with or without a positive COVID-19 result confirmed by polymerase chain reaction (PCR). All participants underwent thorough evaluations, including medical history documentation, clinical examinations, laboratory tests, cardiac biomarker analyses, and echocardiography upon admission. The diagnosis of ACS was established according to the Fourth Universal Definition of Myocardial Infarction (9).
2.3 Catheterization procedure
Percutaneous coronary interventions were performed in accordance with the European Society of Cardiology (ESC) guidelines (10). Before the procedure, patients received loading doses of aspirin (342 mg) and an adenosine diphosphate (ADP) receptor inhibitor (either clopidogrel 600 mg or ticagrelor 180 mg). Intravenous unfractionated heparin (UFH) was administered during the procedure. Coronary angiography was conducted through the radial or femoral artery to identify the culprit lesion, which was subsequently crossed with an angioplasty guidewire. Once the lesion was successfully crossed, predilation was performed at the operator's discretion using a semi-compliant balloon. Drug-eluting stents (DES) were deployed, with postdilation carried out as needed to ensure optimal stent apposition. Stent size and length were selected based on angiographic assessment. Following PCI, patients were monitored in the Cardiovascular Intervention Department and discharged upon maintaining hemodynamic stability for at least 24 h. Postprocedure anticoagulation therapy, including UFH or lowmolecular-weight heparin (LMWH), was provided at the operator's discretion.
2.4 Follow-up
After discharge, patients were prescribed dual antiplatelet therapy, consisting of daily aspirin (81 mg) combined with clopidogrel (75 mg/day) or ticagrelor (90 mg twice daily), for a minimum of 12 months, provided no contraindications were present. Additional medications, including statins, beta-blockers, angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), and spironolactone were prescribed following ESC guidelines (10). Follow-up evaluations were scheduled at 7 days and 30 days post-discharge, followed by monthly outpatient visits.
2.5 Outcome measures
The primary endpoint of the study was the occurrence of major adverse cardiac and cerebral events (MACCE), including cardiovascular mortality, non-fatal myocardial infarction, non-fatal cerebrovascular events, and ACS requiring hospitalization. Secondary outcomes included post-procedure complications such as contrast-induced nephropathy (CIN), bleeding, hematoma, intensive care unit (ICU) admission, hospital stay duration, and procedural duration.
2.6 Statistical analysis
All statistical analyses were performed using IBM SPSS Statistics version 26.0 (IBM Corp., Armonk, NY, USA). The normality of continuous variables was assessed using the Shapiro–Wilk test. Variables following a normal distribution were expressed as mean ± standard deviation (SD) and compared using the independent-samples t-test. Non-normally distributed variables were presented as median with interquartile range (IQR) and compared using the Mann–Whitney U test. Categorical variables were summarized as frequencies and percentages and compared using the Chi-square test or Fisher's exact test, as appropriate. For comparisons involving more than two groups, the one-way ANOVA, Kruskal–Wallis test, or Fisher–Freeman–Halton exact test was used based on the data distribution. When multiple comparisons were conducted, Bonferroni correction was applied to adjust for type I error inflation.
Multivariate logistic regression analyses were performed to identify independent predictors of MACCE during hospitalization, at 30 days, and at 1 year. Variables with a p-value <0.1 in univariate analysis or those considered clinically relevant were included using a backward stepwise elimination method. To minimize overfitting, an events-per-variable ratio >10 was ensured. Multicollinearity was assessed using variance inflation factor (VIF), and model assumptions were verified before inclusion. Model calibration was assessed using the Hosmer–Lemeshow goodness-of-fit test, and explanatory power was measured using Nagelkerke's pseudo-R2.
3 Result
3.1 Patient characteristics
In Vietnam, the COVID-19 pandemic unfolded in four distinct waves, each characterized by unique viral variants and evolving challenges. The first wave, spanning January to April 2020, was associated with the Wuhan variant. The second wave, from July to December 2020, was driven by the D614G variant. The third wave, occurring between January and March 2021, corresponded to the emergence of the Alpha variant. The fourth and most prolonged wave, extending from April 2021 to early 2022, was marked by the predominance of the Delta and Omicron variants (11).
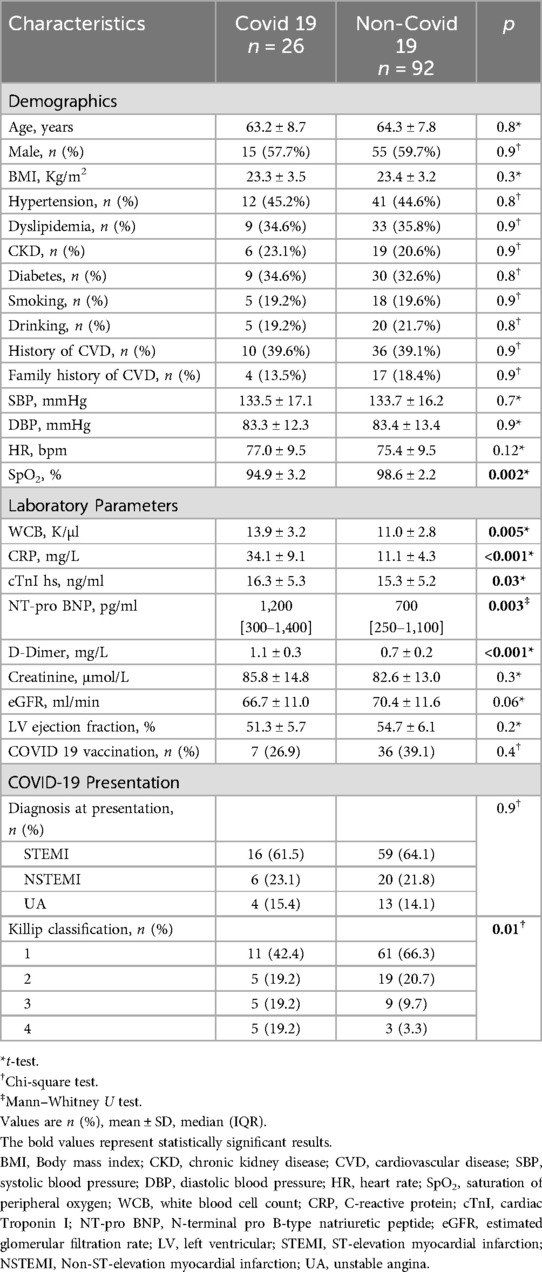
This study included 118 ACS patients who underwent PCI, comprising 26 COVID-19-positive and 92 non-COVID patients. Baseline characteristics such as age, sex, body mass index, and cardiovascular risk factors were comparable between groups. Vital signs were generally similar, except for lower oxygen saturation in the COVID-19 group (94.9 ± 3.2% vs. 98.6 ± 2.2%; p = 0.002). COVID-19-positive patients also exhibited significantly higher inflammatory and thrombotic markers, including CRP, white blood cell count, D-dimer, and Troponin I (p < 0.01), as well as elevated NT-proBNP. Although left ventricular ejection fraction (LVEF) and estimated glomerular filtration rate (eGFR) trended lower in COVID-19 patients, these differences were not statistically significant. While ACS type distribution was similar between groups, a greater proportion of COVID-19 patients presented with advanced Killip class (≥III: 38.4% vs. 13.0%; p = 0.01), indicating more severe heart failure at presentation (Table 1).
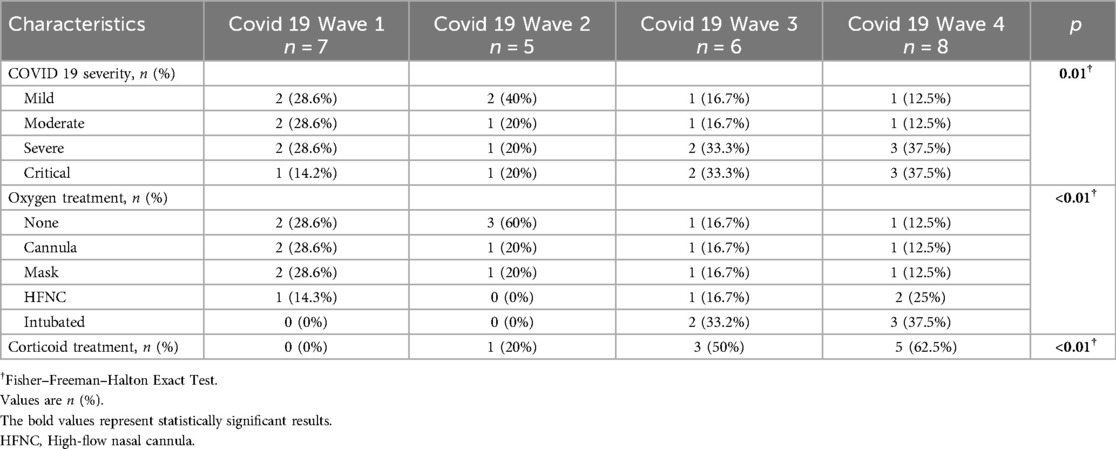
Analysis of clinical trends across successive pandemic waves revealed a progressive escalation in disease severity. Patients in Waves 1 and 2 demonstrated relatively stable inflammatory profiles, with white blood cell (WBC) counts comparable to those observed in non-COVID-19 individuals. In contrast, Waves 3 and 4 exhibited significantly elevated WBC counts (Wave 3: 14.1 ± 3.9; Wave 4: 15.4 ± 2.5 K/µl; p < 0.01) and modestly increased D-dimer levels (Wave 4: 1.2 ± 0.4 mg/L vs. Wave 1: 1.0 ± 0.4 mg/L; p = 0.7). These changes were paralleled by a rise in the proportion of patients requiring oxygen therapy or admission to the intensive care unit (ICU). The prevalence of Killip class III–IV heart failure increased markedly, affecting over 50% of patients in Wave 4 compared to only 14.3% in Wave 1. Although comorbidities and demographic variables remained consistent, the cumulative clinical burden—including cardiogenic shock and reduced LVEF—was more pronounced in the later waves. These findings reflect an evolving clinical phenotype, with later waves associated with more severe inflammation, thrombotic activity, and hemodynamic compromise (Table 2, Supplementary Table S1).
3.2 Coronary lesions and intervention details
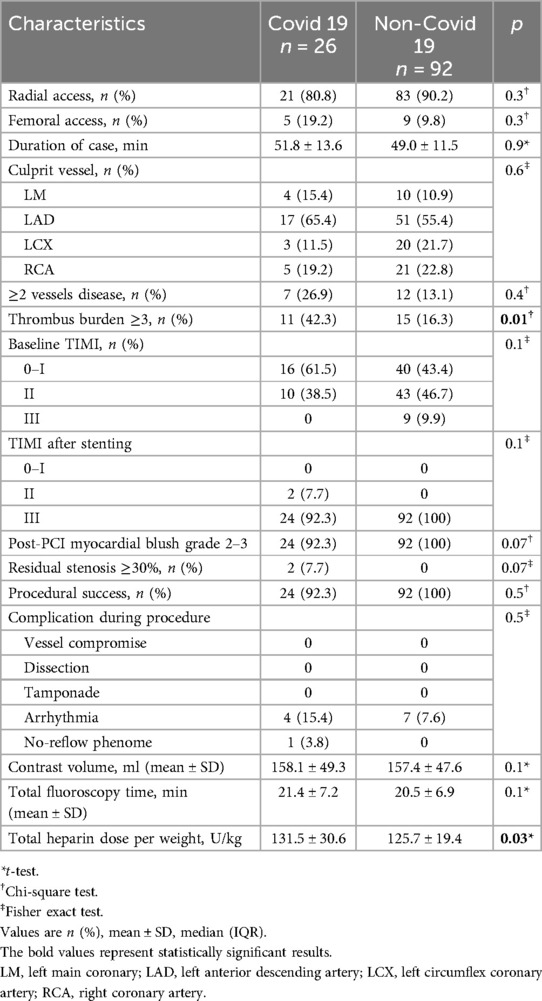
Procedural features were largely comparable between COVID-19 and non-COVID-19 patients. Radial access was predominant in both groups (80.8% vs. 90.2%; p = 0.3), with no significant differences in PCI duration, contrast volume, or fluoroscopy time. The LAD was the most common culprit vessel in both group (p = 0.6). Multivessel disease was more frequent in the COVID-19 group (26.9% vs. 13.1%; p = 0.4), while thrombus burden was higher (grade ≥3: 42.3% vs. 16.3%; p = 0.01). Poor baseline TIMI flow (0–I) was more common in COVID-19 patients (61.5% vs. 43.4%; p = 0.1), although final TIMI 3 flow was achieved in most cases (92.3% vs. 100%; p = 0.1). Myocardial blush grade ≥2 and complete lesion resolution were slightly lower in the COVID-19 group (both 92.3% vs. 100%; p = 0.07). Procedural success remained high (92.3% vs. 100%; p = 0.5), with no significant differences in arrhythmias or no-reflow events (p = 0.5). The only significant difference was a higher heparin dose in COVID-19 patients (131.5 ± 30.6 vs. 125.7 ± 19.4 U/kg; p = 0.03) (Table 3).
Across the four COVID-19 waves (Supplementary Table S2), procedural patterns suggested increasing complexity. Radial access continued to be the predominant approach (71.4–87.5%; p = 0.5), while the LAD was the leading culprit vessel across all waves. PCI duration rose from 45.6 ± 10.3 min in Wave 1 to 56.3 ± 18.8 min in Wave 4 (p = 0.4), paralleled by increased thrombus burden (grade ≥3: 28.6%–50%) and more frequent baseline TIMI ≤ I (62.5% in Wave 4). Myocardial blush grade 2–3 declined slightly in Waves 1 and 4 (85.7% and 87.5%). Heparin dose increased across waves, peaking in Wave 4 (143.6 ± 28.2 U/kg; p = 0.03). Despite the more complex presentations, procedural success remained ≥85% across all waves. Arrhythmias and no-reflow phenomena were infrequent overall but appeared more often in later waves, reflecting evolving thrombo-inflammatory burden.
3.3 Clinical outcomes
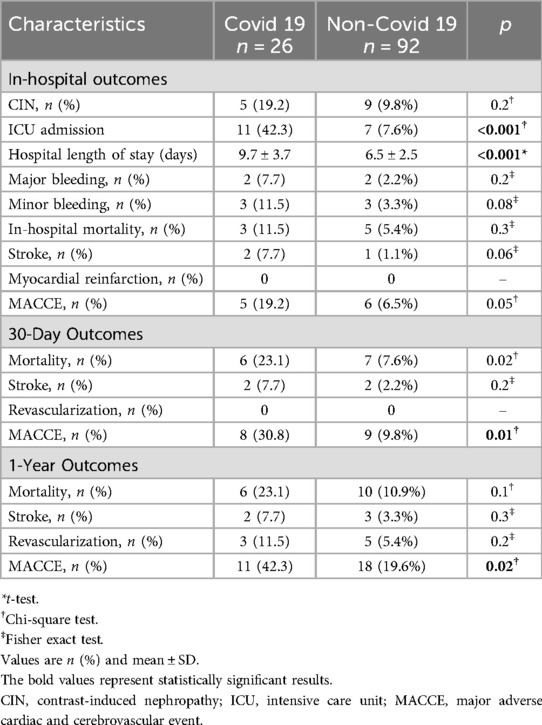
COVID-19 patients experienced significantly worse in-hospital outcomes, including higher ICU admission (42.3% vs. 7.6%) and longer hospital stays (9.7 ± 3.7 vs. 6.5 ± 2.5 days; both p < 0.001). Although not statistically significant, rates of contrast-induced nephropathy (19.2% vs. 9.8%), major bleeding (7.7% vs. 2.2%), stroke (7.7% vs. 1.1%; p = 0.06), and in-hospital mortality (11.5% vs. 5.4%) were numerically higher, contributing to increased MACCE (19.2% vs. 6.5%; p = 0.05). At 30 days, COVID-19 patients had higher mortality (23.1% vs. 7.6%; p = 0.02) and MACCE (30.8% vs. 9.8%; p = 0.01), with these differences persisting at 1 year (MACCE: 42.3% vs. 19.6%; p = 0.02). Mortality at 1 year was higher but not significant (23.1% vs. 10.9%; p = 0.1). Revascularization and stroke remained more frequent but without statistical significance (Table 4).
Outcomes among COVID-19 patients worsened across pandemic waves. ICU admissions rose from 14.3% in Wave 1 to 62.5% in Wave 4 (p < 0.01), with hospital stays increasing accordingly (7.3 ± 2.1 to 12.4 ± 4.2 days; p < 0.01). MACCE incidence increased in-hospital (0%–37.5%), at 30 days (14.3%–50%), and at 1 year (14.3%–62.5%; all p < 0.01), reflecting greater disease severity in later waves. Mortality followed a similar trend but was not statistically significant (Supplementary Table S3).
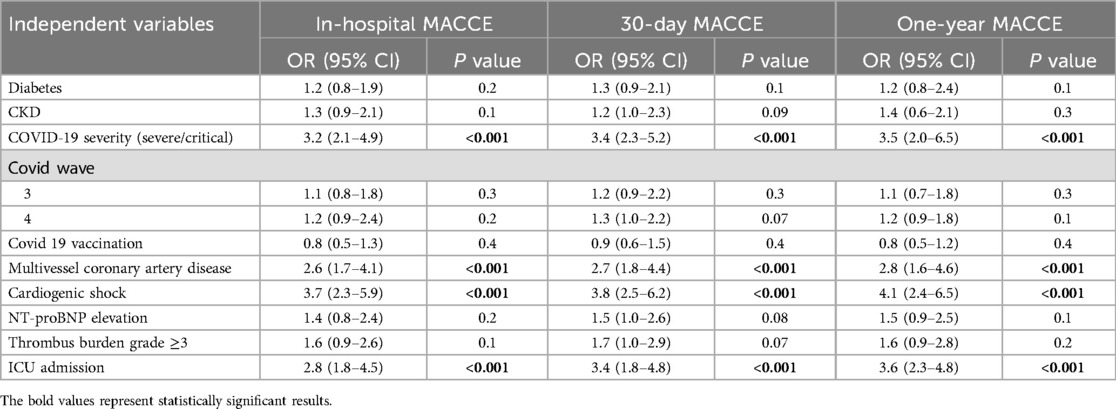
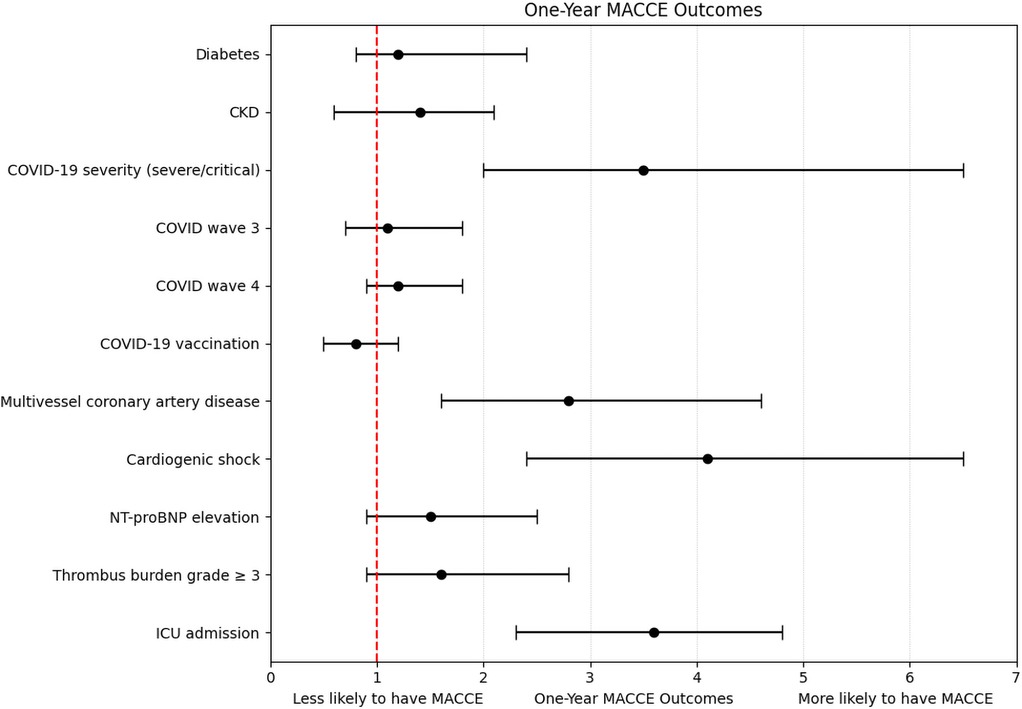
A total of 22 in-hospital MACCE events, 26 MACCE events at 30 days, and 30 events at 1-year follow-up were included in the respective logistic regression models. Severe COVID-19, cardiogenic shock, ICU admission, and multivessel disease consistently emerged as independent predictors across all timepoints (ORs: 2.6–4.1; all p < 0.001). Other variables, including diabetes, CKD, elevated biomarkers, vaccination status, and pandemic wave, were not significant (Table 5, Figure 1). The models demonstrated good calibration (Hosmer–Lemeshow p > 0.05) and modest explanatory power (pseudo-R2: 0.18–0.22) (Supplementary Table S4, Figure S1).
4 Discussion
COVID-19 presents considerable challenges in managing patients with ACS due to its hyperinflammatory and pro-thrombotic state, which exacerbates pre-existing cardiovascular conditions (12). The virus induces endothelial dysfunction, platelet activation, and cytokine storm, ultimately contributing to plaque destabilization, thrombus formation, and increased cardiovascular risks (13, 14). These systemic effects further aggravate underlying coronary artery disease, complicating PCI procedures and increase the risk of severe complications such as arrhythmias, cardiogenic shock, no-reflow phenomena, and coronary artery re-occlusion (15).
In this study, we assessed PCI outcomes of ACS patients with and without COVID-19 infection during four distinct pandemic waves in Vietnam. Although baseline characteristics were similar, COVID-positive patients exhibited more severe clinical and laboratory profiles, including lower oxygen saturation, higher inflammatory and thrombotic markers, and an elevated incidence of cardiogenic shock. Coronary angiography revealed a markedly higher thrombus burden among COVID-positive patients These findings support existing evidence of COVID-19-induced vascular inflammation and thrombosis that contribute to adverse outcomes (14, 16). Despite these challenges, primary PCI achieved comparable reperfusion rate in both groups.
However, even with procedural success, COVID-positive patients still experienced worse clinical outcomes, including increased ICU admissions, longer hospital stays, and higher rates of in-hospital mortality and stroke. This trend was especially pronounced during Wave 4, dominated by the Delta variant, which was associated with the most severe clinical presentations, highest thrombus burden, and elevated 1-year MACCE rate (62.5%, p = 0.02). Delta variant is known for its increased transmissibility and its ability to trigger a more intense inflammatory and pro-thrombotic response compared to earlier variants (17, 18). Multivessel disease and cardiogenic shock were also more common in COVID-19 patients, which may have further contributed to poor prognosis despite timely PCI.
Many large-scale studies from high-income countries have documented poorer in-hospital outcomes in ACS patients with concurrent COVID-19. For instance, a study by Markson et al. (19) found significantly higher incidences of hospital mortality, cardiac arrest, cardiogenic shock, and respiratory failure in ACS patients with COVID-19 compared to non-COVID counterparts (19). Similarly, Krishnaraj S. Rathod's study demonstrated that STEMI patients with COVID-19 were associated with higher rates of cardiac arrest, larger thrombus burdens, more extensive infarctions, and worse clinical outcomes (19). A systematic review by Nicholas W. S. Chew emphasized substantial delays in door-to-balloon time during the pandemic and identified higher in-hospital mortality rates (OR = 1.27; 95% CI: 1.09–1.49). Subgroup analysis revealed that low- and middle-income countries experienced a significantly higher mortality rate during the pandemic, while high-income nations showed a similar trend but did not reach statistical significance (18).
In the multivariate analysis, severe or critical COVID-19 infection, multivessel coronary artery disease, cardiogenic shock, and ICU admission emerged as independent predictors of MACCE across all timepoints. The strong relationship between cardiogenic shock and adverse outcomes is consistent with existing evidence, likely attributable to systemic hypoperfusion, myocardial injury, and inflammatory dysregulation (20). Similarly, ICU admission may serve as a marker of overall disease severity and is frequently accompanied by complications such as ventilator-associated pneumonia or acute kidney injury. Notably, conventional predictors such as NT-proBNP and diabetes did not show statistical significance. This may reflect the overwhelming impact of acute inflammatory and thrombotic processes and COVID-specific complications, which could overshadow the contribution of chronic comorbidities, particularly in smaller cohorts.
These findings add to the limited body of evidence from Southeast Asia, highlighting the distinct healthcare approaches and challenges encountered in Vietnam during the COVID-19 pandemic. While the country implemented a strong public health response during the early waves of the pandemic, prolonged lockdowns, delayed referral systems, and healthcare worker shortages disrupted the continuity of care, particularly for chronic cardiovascular diseases (21, 22). The situation was particularly critical during the Delta wave, when healthcare systems were overwhelmed, resulting in delayed diagnoses, limited ICU availability, and restricted access to advanced interventional tools (7). These systemic pressures likely contributed to poorer clinical outcomes, independent of patient-level factors. Disruptions in care delivery may also explain the delayed presentation of ACS cases observed in the later pandemic waves.
Our study offers an insight into the interaction between COVID-19 and ACS in a resource-limited setting; however, the findings should be interpreted with caution due to inherent limitations, including its retrospective, single-center design and relatively small sample size. Despite these constraints, the results provide a representative overview of the challenges encountered in ACS management during the pandemic and illustrate key procedural adaptations implemented at a tertiary care center in Vietnam. These observations emphasize the need for flexible and resilient healthcare strategies to sustain essential cardiac services in the face of future public health crises.
4.1 Study limitations
This study has several limitations should be considered. First, its retrospective, single-center design limits generalizability. Only ACS patients undergoing PCI were included, potentially introducing selection bias by excluding those managed conservatively or unable to access care, particularly during resource-constrained periods of the pandemic. Strict lockdown policies and overwhelmed healthcare services also contributed to a small sample size, reducing statistical power and increasing the risk of type II error. Second, although multivariate logistic regression was conducted, the limited number of events per variable raises concerns about model robustness. Outcomes were not formally adjudicated, introducing potential misclassification. Additionally, in-hospital variables such as door-to-balloon time, pharmacologic treatments, and ventilatory support were not captured, limiting the analysis of treatment-related influences on outcomes. Third, vaccination status and SARS-CoV-2 variants were not analysed due to incomplete documentation, which may have affected disease severity and clinical outcomes across waves. Intravascular imaging techniques were also not utilized due to resource constraints during the pandemic and their high cost, limiting procedural assessment. Lastly, the findings reflect a tertiary hospital in Ho Chi Minh City and may not represent the broader Vietnamese healthcare context, particularly rural or provincial settings with fewer resources. Regional disparities in PCI access, care quality, and patient follow-up were not examined but may significantly impact outcomes.
Future multicenter studies or a national ACS-PCI registry are warranted to better capture variations in patient characteristics, healthcare access, and outcomes across Vietnam. These efforts would provide more representative data to inform equitable policy and strengthen preparedness for future public health crises.
5 Conclusion
This study provides real-world insight into the impact of COVID-19 on PCI outcomes in a Vietnamese tertiary care setting, particularly during the fourth wave dominated by the Delta variant. An increase in thrombotic burden, procedural complexity, and adverse outcomes was observed among COVID-19 patients, especially in later waves. These findings underscore the need for timely risk stratification, optimized procedural planning, and sustained vaccination efforts in managing ACS during public health emergencies. However, due to the study's retrospective design, single-center scope, and limited sample size, the results should be interpreted as exploratory rather than definitive. Larger, multicenter studies are needed to validate these observations and inform broader clinical and policy strategies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethical Committee at Nguyen Tri Phương Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
DL: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing. NN: Formal analysis, Investigation, Resources, Visualization, Writing – original draft, Writing – review & editing. QV: Conceptualization, Data curation, Formal analysis, Investigation, Project administration, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors would like to acknowledge Dr. Bui The Hoa, Head of the Cardiovascular Intervention Department at Nguyen Tri Phuong Hospital, for his contribution to this research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1563415/full#supplementary-material
Abbreviations
ACEIs, angiotensin-converting enzyme inhibitor; ACS, acute coronary syndrome; ADP, adenosine diphosphate; ARBs, angiotensin receptor blockers; CKD, chronic kidney disease; CIN, contrast-induced nephropathy; COVID-19, coronavirus disease 2019; cTnI, Cardiac Troponin I; CRP, C-reactive protein; ESC, european society of cardiology; ICU, intensive care unit; LAD, left anterior descending artery; LCx, circumflex artery; LM, Left main; LMWH, low-molecular-weight heparin; PCI, percutaneous coronary intervention; MACCE, major adverse cardiac and cerebrovascular events; NSTEMI, non-ST-segment elevation myocardial infarction; NT-proBNP, N-terminal pro B-type natriuretic peptide; RCA, right coronary artery; STEMI, ST-segment elevation myocardial infarction; TIMI, thrombolysis in myocardial infarction; UA, unstable angina; UFH, unfractionated heparin; WHO, world health organization.
References
1. Wu YC, Chen CS, Chan YJ. The outbreak of COVID-19: an overview. J Chin Med Assoc. (2020) 83:217–20. doi: 10.1097/JCMA.0000000000000270
2. Guddeti RR, Yildiz M, Nayak KR, Alraies MC, Davidson L, Henry TD, et al. Impact of COVID-19 on acute myocardial infarction care. Heart Fail Clin. (2023) 19:221–9. doi: 10.1016/j.hfc.2022.08.004
3. Ghasemzadeh N, Kim N, Amlani S, Madan M, Shavadia JS, Chong AY, et al. A review of ST-elevation myocardial infarction in patients with COVID-19. Heart Fail Clin. (2023) 19:197–204. doi: 10.1016/j.hfc.2022.08.007
4. El-Qushayri AE, Dahy A, Benmelouka AY, Kamel AMA. The effect of COVID-19 on the in-hospital outcomes of percutaneous coronary intervention in patients with acute coronary syndrome: a large scale meta-analysis. Am J Med Open. (2023) 9:100032. doi: 10.1016/j.ajmo.2023.100032
5. Clifford CR, Le May M, Chow A, Boudreau R, Fu AYN, Barry Q, et al. Delays in ST-elevation myocardial infarction care during the COVID-19 lockdown: an observational study. CJC Open. (2020) 3:565–73. doi: 10.1016/j.cjco.2020.12.009
6. Gong X, Zhou L, Dong T, Ding X, Zhao H, Chen H, et al. Impact of COVID-19 pandemic on STEMI undergoing primary PCI treatment in Beijing, China. Am J Emerg Med. (2022) 53:68–72. doi: 10.1016/j.ajem.2021.11.034
7. Minh LHN, Khoi Quan N, Le TN, Khanh PNQ, Huy NT. COVID-19 Timeline of Vietnam: important milestones through four waves of the pandemic and lesson learned. Front Public Health. (2021) 9:709067. doi: 10.3389/fpubh.2021.709067
8. Nguyen RT, Meyer O, Chu J, Le V, Ho TV, Le A, et al. Social determinants of health, cardiovascular risk factors, and atherosclerotic cardiovascular disease in individuals of Vietnamese origin. Am J Cardiol. (2023) 189:11–21. doi: 10.1016/j.amjcard.2022.11.028
9. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol. (2018) 72:2231–64. doi: 10.1016/j.jacc.2018.08.1038
10. Byrne RA, Rossello X, Coughlan JJ, Barbato E, Berry C, Chieffo A, et al. 2023 ESC guidelines for the management of acute coronary syndromes. Eur Heart J. (2023) 44:3720–826. doi: 10.1093/eurheartj/ehad191
11. Nguyen TS. An overview of strategies and response to the COVID-19 pandemic in Vietnam. J Epidemiol. (2024) 34:605–8. doi: 10.2188/jea.JE20240181
12. Abdel Moneim A, Radwan MA, Yousef AI. COVID-19 and cardiovascular disease: manifestations, pathophysiology, vaccination, and long-term implication. Curr Med Res Opin. (2022) 38:1071–9. doi: 10.1080/03007995.2022.2078081
13. Aljadah M, Khan N, Beyer AM, Chen Y, Blanker A, Widlansky ME. Clinical implications of COVID-19-related endothelial dysfunction. JACC Adv. (2024) 3:101070. doi: 10.1016/j.jacadv.2024.101070
14. Dhakal BP, Sweitzer NK, Indik JH, Acharya D, William P. SARS-CoV-2 infection and cardiovascular disease: cOVID-19 heart. Heart Lung Circ. (2020) 29:973–87. doi: 10.1016/j.hlc.2020.05.101
15. Hannan EL, Zhong Y, Cozzens K, Osinaga A, Efferen L, Jacobs AK, et al. Impact of COVID-19 on percutaneous coronary intervention utilization and mortality in New York. Catheter Cardiovasc Interv. (2023) 101:980–94. doi: 10.1002/ccd.30648
16. Semeraro N, Colucci M. The prothrombotic state associated with SARS-CoV-2 infection: pathophysiological aspects. Mediterr J Hematol Infect Dis. (2021) 13:e2021045. doi: 10.4084/MJHID.2021.045
17. Samieefar N, Rashedi R, Akhlaghdoust M, Mashhadi M, Darzi P, Rezaei N. Delta variant: the new challenge of COVID-19 pandemic, an overview of epidemiological, clinical, and immune characteristics. Acta Biomed. (2022) 93:e2022179. doi: 10.23750/abm.v93i1.12210
18. Hu Z, Huang X, Zhang J, Fu S, Ding D, Tao Z. Differences in clinical characteristics between delta variant and wild-type SARS-CoV-2 infected patients. Front Med (Lausanne). (2021) 8:792135. doi: 10.3389/fmed.2021.792135
19. Markson FE, Akuna E, Lim CY, Khemani L, Amanullah A. The impact of COVID-19 on hospitalization outcomes of patients with acute myocardial infarction in the USA. Am Heart J Plus. (2023) 32:100305. doi: 10.1016/j.ahjo.2023.100305
20. Wójcik M, Karpiak J, Zaręba L, Przybylski A. High in-hospital mortality and prevalence of cardiogenic shock in patients with ST-segment elevation myocardial infarction and concomitant COVID-19. Postepy Kardiol Interwencyjnej. (2023) 19:22–30. doi: 10.5114/aic.2023.124212
21. Hoang SV, Nguyen KM, Le HTA, Tung AT, Huynh PVT, Le TV, et al. The effects of the COVID-19 lockdown on patients with chronic cardiovascular disease in Vietnam. J Infect Dev Ctries. (2022) 16:268–75. doi: 10.3855/jidc.15002
Keywords: COVID-19, STEMI, percutaneous coronary intervention, acute coronary syndrome, outcomes
Citation: Le DCP, Nguyen NTM and Vo QD (2025) Outcomes of percutaneous coronary intervention in COVID-19-positive acute coronary syndrome patients: a retrospective study in Vietnam. Front. Cardiovasc. Med. 12:1563415. doi: 10.3389/fcvm.2025.1563415
Received: 20 January 2025; Accepted: 24 June 2025;
Published: 10 July 2025.
Edited by:
Nhat Tu Le, Houston Methodist Research Institute, United StatesReviewed by:
Małgorzata Ostrowska, Nicolaus Copernicus University in Toruń, PolandPriyanka Banerjee, Houston Methodist Research Institute, United States
Copyright: © 2025 Le, Nguyen and Vo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Quan Duy Vo, ZHIuZHV5cXVhbkBnbWFpbC5jb20=
 Duy Cao Phuong Le1,2
Duy Cao Phuong Le1,2 Nguyet Thi Minh Nguyen
Nguyet Thi Minh Nguyen Quan Duy Vo
Quan Duy Vo