- Department of Primary and Long-Term Care, University of Groningen, University Medical Centre Groningen, Groningen, Netherlands
Introduction
Myocardial infarction (MI) is a critical endpoint in cardiovascular clinical outcomes trials, representing a diverse entity with distinct subtypes. The classification introduced in 2007 includes five MI subtypes: type 1 (spontaneous atherosclerotic), type 2 (oxygen supply-demand mismatch), type 3 (cardiac death without biomarker elevation), type 4 (percutaneous intervention-related), and type 5 (surgery-related) (1). Type 2 MI is caused by an imbalance between myocardial oxygen supply and demand in the absence of acute atherothrombotic plaque disruption, but it often occurs in the presence of underlying atherosclerotic coronary artery disease. This cause distinguishes it from type 1 MI, which is typically caused by coronary artery disease and acute thrombosis. Type 2 MI carries a higher mortality risk than type 1 MI (2).
The prevalence and incidence of type 2 MI are increasing. Studies have reported its prevalence among emergency department patients with suspected MI to range from 26% to 58% (3). The reported incidence of type 2 MI varies between 7% and 35%, depending on heterogeneity in populations and diagnostic criteria (4). With an aging population and rising comorbidities, its incidence is expected to grow exponentially (5–7).
The growing incidence of type 2 MI underscores the need for effective preventive therapies. Several recent clinical outcomes trials testing cholesterol- and glucose-lowering drugs have reported the effects on type 2 MI (8–11). Type 2 MIs were found to substantially contribute to the primary endpoints in these trials. These results necessitate a careful evaluation of the suitability of type 2 MI as a primary efficacy endpoint in clinical trials, particularly for medications aimed at reducing atherosclerosis-related events. In this paper, we examine the incidence of type 2 MI in these trials, evaluate proposed pathophysiological mechanisms, and explore the implications of including type 2 MI in primary endpoints.
Risk factors for type 2 MI
Type 2 MI shares many cardiovascular risk factors with type 1 MI (12). Atherosclerosis has been reported to be present in about 30%–50% of type 2 MI cases (13, 14) and affects the prognosis negatively (15). Also, many patients with type 2 MI may have hyperlipidemia (12) or hypertension (16). Tachyarrhythmia may also precipitate type 2 MI through increased myocardial oxygen demand (16). Nonetheless, type 2 MI also has non-cardiovascular risk factors including operative stress, sepsis, anaemia, and respiratory failure (12). Viral and bacterial infections are also associated with an increased risk of type 2 MI (17, 18). Moreover, it is more common in females, older adults, and those with multiple comorbidities (18). Given its complex risk profile, a plausible mechanism linking lipid- and glucose-lowering therapies to type 2 MI is essential for including it as an efficacy endpoint (19, 20). Such a mechanism would strengthen the validity of the reported effects on composite endpoints.
PCSK9-inhibitors and type 2 MI
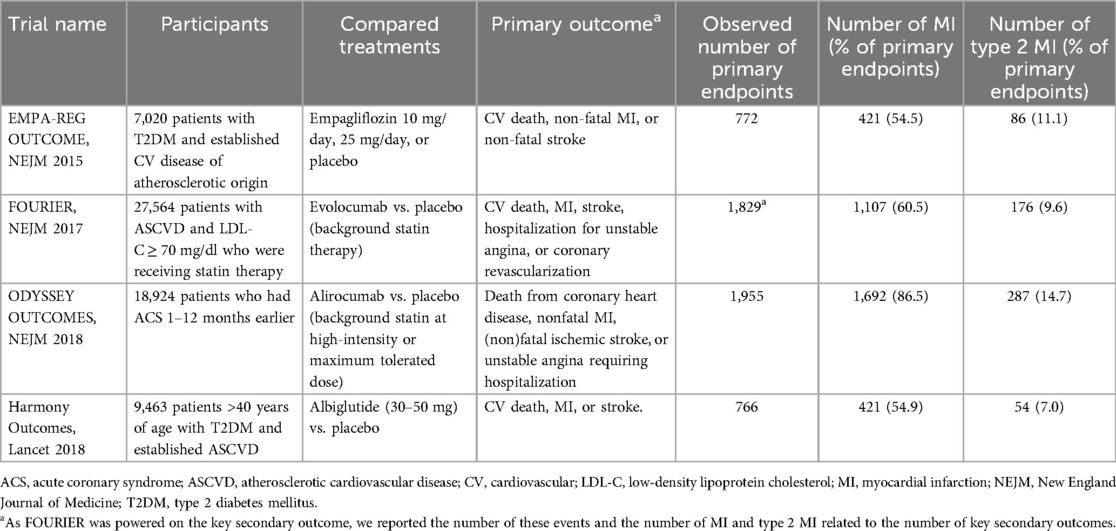
Recent trials involving PCSK9 inhibitors have included type 2 MI in their primary endpoints (Table 1). The ODYSSEY OUTCOMES trial compared alirocumab to placebo in post-acute coronary syndrome patients on high-intensity or maximum-tolerated statin therapy. The primary endpoint included coronary heart disease death, nonfatal MI, fatal and nonfatal ischemic stroke, and unstable angina requiring hospitalization (8). MI itself was a composite outcome that included the various types of MI. In ODYSSEY OUTCOMES, 287 of 1,692 MIs (17.0%) were type 2, among 1,955 (14.7%) primary endpoints (8, 21). Alirocumab reduced the risk of type 2 MI (HR 0.77; 95% CI 0.61–0.97) (21).
The FOURIER trial evaluated evolocumab vs. placebo in patients with prior MI or stroke on statin therapy (9). The primary composite endpoint included cardiovascular death, MI, stroke, hospitalization for unstable angina, and coronary revascularization. The trial was powered on the secondary endpoint of cardiovascular death, MI, or stroke. Again, MI was a composite outcome that included type 2 MI. FOURIER reported 176 type 2 MI out of 1,107 MIs (15.9%) among 1,829 secondary endpoints (9.6%) (9, 22),. Evolocumab showed a non-significant increase in type 2 MI risk (HR 1.09; 95% CI 0.82–1.44) (22). Thus, the effects of alirocumab and evolocumab on type 2 MI were inconsistent.
LDL-C lowering and type 2 MI
The ODYSSEY OUTCOMES investigators attributed alirocumab's reduced type 2 MI risk to improved myocardial oxygen supply by preventing plaque progression or promoting regression (21). However, FOURIER's evolocumab group achieved a lower mean LDL-C (30 mg/dl or 0.8 mmol/L) than ODYSSEY OUTCOMES' alirocumab group (58 mg/dl or 1.5 mmol/L) (8, 9). Hence, a greater plaque regression would be expected with evolocumab, but this did not correlate with a reduced type 2 MI risk. Also, a multivariable Cox regression analysis did not show an association between baseline LDL-C and the risk of type 2 MI in ODYSSEY OUTCOMES (21). Notably, to our knowledge, this is the only study to date specifically investigating the association between baseline LDL-C levels and incident type 2 MI.
The ODYSSEY OUTCOMES investigators suggested that the observed differences in effects between the trials could stem from differences in patient populations, event numbers, follow-up duration, definitions, and adjudication processes (21). However, in our view FOURIER and ODYSSEY OUTCOMES shared many similarities in these respects: both trials investigated LDL-C lowering drugs in populations with a high cardiovascular risk, employed the Third Universal Definition of Myocardial Infarction for classifying MI events, and utilized local blinded clinical events committees for the adjudication of events. Also, the proportion of type 2 MI among total MIs was very close: 15.9% in FOURIER and 17.0% in ODYSSEY OUTCOMES.
The IMPROVE-IT trial that evaluated ezetimibe against placebo could have provided valuable insights about the effects of LDL-C lowering and the risk of type 2 MI as well (23). As the third major clinical outcomes trial on intensive lipid-lowering therapy, it randomized patients at the time when the classification of MI subtypes, including type 2 MI, was already introduced (24). Unfortunately, no distinction between subtypes of MI was made in this trial. So, the question remains what explains the discrepancy in the effect of evolocumab and alirocumab on risk of type 2 MI.
Another potential explanation for the discrepancy in the risk of type 2 MI between the PCSK9 inhibitors may lie in the observed differences in the risk of severe infection. A systematic review and meta-analysis examined the association between PCSK9 inhibitor use and infection risk (25). In FOURIER, the evolocumab group showed an increased risk of both severe viral (HR 1.26; 95% CI 0.81–1.96) and bacterial infections (HR 1.06; 95% CI 0.83–1.35) compared to placebo but power was insufficient to be certain. ODYSSEY OUTCOMES similarly showed an increased but not statistically significant risk of severe viral infections (HR 1.10; 95% CI 0.72–1.61) for alirocumab, but a lower risk of severe bacterial infections (HR 0.81; 95% CI 0.62–1.08) (25).
Glucose-lowering and type 2 MI
Two clinical outcomes trials evaluating glucose-lowering drugs have also reported type 2 MI results. EMPA-REG OUTCOME evaluated the cardiovascular outcomes of empagliflozin 10 or 25 mg added to the participants' existing treatment regimen, compared to placebo. The study included patients with type 2 diabetes at high cardiovascular risk. The primary endpoint was a composite of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke (11). In the end, 86 of 421 MIs (20.4%) were classified as type 2 MIs, among 772 (11.1%) primary endpoints (11, 26). Empagliflozin showed an adjusted rate ratio of 0.67 (95% CI, 0.41–1.10) for type 2 MI compared to placebo (26).
The HARMONY OUTCOMES trial assessed the cardiovascular efficacy of albiglutide in patients with type 2 diabetes at high cardiovascular risk. Participants were randomized to receive albiglutide (30–50 mg) or placebo, against a background of cardiovascular medication. The primary endpoint was a composite of cardiovascular death, MI, or stroke. In total, 54 of 421 MIs (12.8%) were type 2 MIs, among 766 (7.0%) primary endpoints (10, 27). Albiglutide exhibited a hazard ratio of 0.65 (95% CI, 0.46–0.92) for type 2 MI (27).
Both empagliflozin and albiglutide demonstrated a reduction in the risk of type 2 MI. The beneficial effect of empagliflozin, an SGLT2-inhibitor, was attributed to an improved cardiac oxygen supply-demand balance through multiple mechanisms, including increased hemoglobin levels, shifted cardiac metabolism, reduced plasma volume, and decreased myocardial oxygen demand (11). For albiglutide, an GLP-1 agonist, no explanatory mechanism was provided by the investigators. Nevertheless, other researchers have proposed that the cardiovascular benefits of GLP-1 agonists may arise from multiple mechanisms. These include direct cardioprotection, vasodilation, natriuresis, and anti-inflammatory effects (28), which could play a role in reducing the risk of type 2 MI. Further evidence is needed to substantiate the observed beneficial effects on type 2 MI. Several other cardiovascular outcome trials investigating glucose-lowering drugs also included type 2 MI as part of the primary outcomes, but the results regarding type 2 MI have not been published yet (29–32).
Conclusion
As the prevalence and incidence of type 2 MI is growing, there is a pressing need for more evidence about the impact of cardiovascular drug therapy in the management and prevention of type 2 MI in the absence of atherosclerosis (33). Including type 2 MI in the primary endpoints of cardiovascular outcome trials requires careful consideration to ensure the validity and interpretability of study results. The appropriateness of this approach may vary depending on the intervention being studied and the available evidence supporting its effects on type 2 MI risk. A crucial first step is establishing a plausible pathophysiological mechanism between the intervention and the risk of type 2 MI (19, 20). Future clinical trials should explicitly report the impact of the investigated therapy on the risk of type 2 MI. Additionally, clinical trials specifically designed for populations at high risk of type 2 MI are needed to provide deeper insights into the effects of medications aimed at preventing atherosclerosis. For the time being, researchers should exercise caution when considering type 2 MI as an efficacy endpoint in trials about preventive cardiovascular drugs. It may be more appropriate to focus on well-established atherosclerosis-related endpoints for assessing efficacy of drugs aimed at reducing atherosclerosis, while monitoring type 2 MI as a safety outcome.
Author contributions
FB: Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing. HL: Conceptualization, Investigation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal definition of myocardial infarction (2018). Eur Heart J. (2019) 40(3):237–69. doi: 10.1093/eurheartj/ehy462
2. White K, Kinarivala M, Scott I. Diagnostic features, management and prognosis of type 2 myocardial infarction compared to type 1 myocardial infarction: a systematic review and meta-analysis. BMJ Open. (2022) 12:e055755. doi: 10.1136/bmjopen-2021-055755
3. DeFilippis AP, Chapman AR, Mills NL, De Lemos JA, Arbab-Zadeh A, Newby LK, et al. Assessment and treatment of patients with type 2 myocardial infarction and acute nonischemic myocardial injury. Circulation. (2019) 140:1661–78. doi: 10.1161/CIRCULATIONAHA.119.040631
4. Rafiudeen R, Barlis P, White HD, Van Gaal W. Type 2 MI and myocardial injury in the era of high-sensitivity troponin. Eur Cardiol Rev. (2022) 17:e03. doi: 10.15420/ecr.2021.42
5. Merlo AC, Bona RD, Ameri P, Porto I. Type 2 myocardial infarction: a diagnostic and therapeutic challenge in contemporary cardiology. Intern Emerg Med. (2022) 17(2):317–24. doi: 10.1007/s11739-021-02920-8
6. Raphael CE, Roger VL, Sandoval Y, Singh M, Bell M, Lerman A, et al. Incidence, trends, and outcomes of type 2 myocardial infarction in a community cohort. Circulation. (2020) 141(6):454–63. doi: 10.1161/CIRCULATIONAHA.119.043100
7. Global Cardiovascular Risk Consortium, Magnussen C, Ojeda FM, Leong DP, Alegre-Diaz J, Amouyel P, et al. Global effect of modifiable risk factors on cardiovascular disease and mortality. N Engl J Med. (2023) 389(14):1273–85. doi: 10.1056/NEJMoa2206916
8. Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. (2018) 379(22):2097–107. doi: 10.1056/NEJMoa1801174
9. Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. (2017) 376(18):1713–22. doi: 10.1056/NEJMoa1615664
10. Hernandez AF, Green JB, Janmohamed S, D’Agostino RB, Granger CB, Jones NP, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (harmony outcomes): a double-blind, randomised placebo-controlled trial. Lancet. (2018) 392(10157):1519–29. doi: 10.1016/S0140-6736(18)32261-X
11. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. (2015) 373(22):2117–28. doi: 10.1056/NEJMoa1504720
12. Gupta S, Vaidya SR, Arora S, Bahekar A, Devarapally SR. Type 2 versus type 1 myocardial infarction: a comparison of clinical characteristics and outcomes with a meta-analysis of observational studies. Cardiovasc Diagn Ther. (2017) 7(4):348–58. doi: 10.21037/cdt.2017.03.21
13. Sarkisian L, Saaby L, Poulsen TS, Gerke O, Jangaard N, Hosbond S, et al. Clinical characteristics and outcomes of patients with myocardial infarction, myocardial injury, and nonelevated troponins. Am J Med. (2016) 129(4):446.e5–e21. doi: 10.1016/j.amjmed.2015.11.006
14. Bularga A, Hung J, Daghem M, Stewart S, Taggart C, Wereski R, et al. Coronary artery and cardiac disease in patients with type 2 myocardial infarction: a prospective cohort study. Circulation. (2022) 145(16):1188–200. doi: 10.1161/CIRCULATIONAHA.121.058542
15. Thygesen K, Jaffe AS. Should myocardial infarction type 2 be regarded as two separate entities? Eur Heart J. (2019) 40(33):2810–2. doi: 10.1093/eurheartj/ehz451
16. Coscia T, Nestelberger T, Boeddinghaus J, Lopez-Ayala P, Koechlin L, Miró Ò, et al. Characteristics and outcomes of type 2 myocardial infarction. JAMA Cardiol. (2022) 7(4):427. doi: 10.1001/jamacardio.2022.0043
17. Caldeira D, Nogueira-Garcia B. Myocardial infarction and viral triggers: what do we know by now? Eur Heart J Suppl. (2023) 25:A12–6. doi: 10.1093/eurheartjsupp/suac122
18. Putot A, Buet-Derrida S, Avondo A, Ray P, Maza M, Zeller M, et al. Infection and type 2 myocardial infarction: a large observational study from emergency department. Arch Cardiovasc Dis Suppl. (2019) 11(1):146. doi: 10.1016/j.acvdsp.2018.10.325
19. Juszczak E, Altman DG, Hopewell S, Schulz K. Reporting of multi-arm parallel-group randomized trials: extension of the CONSORT 2010 statement. J Am Med Assoc. (2019) 321(16):1610–20. doi: 10.1001/jama.2019.3087
20. USFDA. ICH harmonised tripartite guideline. Statistical principles for clinical trials. International conference on harmonisation E9 expert working group. Stat Med. (1999) 18(15):1905–42.10532877
21. White HD, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, et al. Effects of alirocumab on types of myocardial infarction: insights from the ODYSSEY OUTCOMES trial. Eur Heart J. (2019) 40(33):2801–9. doi: 10.1093/eurheartj/ehz299
22. Wiviott SD, Giugliano RP, Morrow DA, De Ferrari GM, Lewis BS, Huber K, et al. Effect of evolocumab on type and size of subsequent myocardial infarction: a prespecified analysis of the FOURIER randomized clinical trial. JAMA Cardiol. (2020) 5(7):787–93. doi: 10.1001/jamacardio.2020.0764
23. Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. (2015) 372(25):2387–97. doi: 10.1056/NEJMoa1410489
24. Thygesen K, Alpert JS, White HD. Universal definition of myocardial infarction. Circulation. (2007) 116:2634–53. doi: 10.1161/CIRCULATIONAHA.107.187397
25. Zhou Z, Zhang W, Burgner D, Tonkin A, Zhu C, Sun C, et al. The association between PCSK9 inhibitor use and sepsis: a systematic review and meta-analysis of 20 double-blind, randomized, placebo-controlled trials. Am J Med. (2023) 136(6):558–567.e20. doi: 10.1016/j.amjmed.2023.02.025
26. Fitchett D, Zinman B, Inzucchi SE, Wanner C, Anker SD, Pocock S, et al. Effect of empagliflozin on total myocardial infarction events by type and additional coronary outcomes: insights from the randomized EMPA-REG OUTCOME trial. Cardiovasc Diabetol. (2024) 23:248. doi: 10.1186/s12933-024-02328-6
27. Krychtiuk K, Jones S, Del Prato S, Green J, Chiswell KE, Murphy S, et al. Effects of albiglutide on types and numbers of myocardial infarction in patientswith type 2 diabetes and cardiovascular disease in the harmony outcomes trial. J Am Coll Cardiol. (2023) 81:1132. doi: 10.1016/S0735-1097(23)01576-0
28. Ferhatbegović L, Mršić D, Macić-Džanković A. The benefits of GLP1 receptors in cardiovascular diseases. Front Clin Diabetes Healthc. (2023) 4:1293926. doi: 10.3389/fcdhc.2023.1293926
29. Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. (2019) 380(4):347–57. doi: 10.1056/NEJMoa1812389
30. Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. (2017) 377(7):644–57. doi: 10.1056/NEJMoa1611925
31. Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. (2016) 375(19):1834–44. doi: 10.1056/NEJMoa1607141
32. Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. (2019) 394(10193):121–30. doi: 10.1016/S0140-6736(19)31149-3
Keywords: type 2 MI, PCSK 9 inhibitors, GLP 1 analog, SLGT inhibitors, efficacy outcomes, safety outcomes
Citation: van Bruggen FH and Luijendijk HJ (2025) Type 2 MI a legitimate efficacy endpoint in cardiovascular trials? A critical appraisal. Front. Cardiovasc. Med. 12:1564432. doi: 10.3389/fcvm.2025.1564432
Received: 21 January 2025; Accepted: 9 June 2025;
Published: 19 June 2025.
Edited by:
Mario Daidone, University of Palermo, ItalyReviewed by:
Istvan Szokodi, University of Pécs, HungaryCopyright: © 2025 van Bruggen and Luijendijk. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: F. H. van Bruggen, Zi5oLnZhbi5icnVnZ2VuQHVtY2cubmw=
 F. H. van Bruggen
F. H. van Bruggen H. J. Luijendijk
H. J. Luijendijk