Abstract
Background:
Treatment and control of hypertension are important for the prevention of cardiovascular diseases. The autonomic nervous system plays a major role in the development and progression of hypertension and has become a new research hotspot in cardiovascular disease. Exercise as a non-pharmacologic intervention has likewise received much attention in the field of cardiovascular disease.
Objective:
To determine the effects of exercise on the sympathetic and parasympathetic nervous systems of hypertensive patients. The effects of aerobic, resistance, and combined aerobic and resistance exercise on autonomic function in hypertensive patients will be compared and analyzed to explore more appropriate exercise modalities for hypertensive patients.
Methods:
Databases such as Web of Science, PubMed, Embase, Cochrane Library, and CNKI were searched to collect randomized controlled trials (RCTs) evaluating exercise (aerobic, resistance, and aerobic combined with resistance exercise) as an intervention for the autonomic nervous system in hypertension. The Cochrane evaluation tool and Jadad scale were used to evaluate the methodological quality of the included literature. RevMan software was used for statistical and sensitivity analyses, and Stata software was used for net analysis and assessment of publication bias.
Results:
This study included 20 studies with 794 hypertensive patients. Exercise improved the joint effect sizes of the basic phenotype in hypertensive patients [SMD = 0.89, 95% CI (0.69, 1.10)] as well as blood pressure variability in hypertensive patients [WMD = 0.89, 95% CI (0.51, 1.27)]. The effect of exercise on hypertensive patients was more centered on the sympathetic nervous system [SMD = 0.29, 95% CI (0.17, 0.40)] and was not significant on the parasympathetic nervous system in hypertensive patients [SMD = −0.08, 95% CI (−0.31, 0.14)]. In addition, the efficacy of aerobic combined resistance exercise on the regulation of blood pressure and the autonomic nervous system in hypertensive patients was the most significant (p < 0.05).
Conclusion:
The regulation of exercise in hypertensive patients is dominated by the sympathetic nervous system. The efficacy of aerobic combined resistance exercise on the autonomic nervous system of hypertensive patients is particularly prominent and plays an important role in improving the blood pressure level of patients, among other things.
Systematic Review Registration:
https://www.crd.york.ac.uk/PROSPERO/, identifier CRD42025634362.
1 Introduction
Hypertension is a chronic multifactorial disease characterized by a persistent increase in blood pressure (1). As the leading modifiable risk factor for cardiovascular disease morbidity and mortality, with an impact on tens of millions of people worldwide (2). Therefore, active treatment and control of hypertension is important for the prevention of cardiovascular disease (3).
Autonomic nervous system (ANS) dysfunction is a key factor in the development of hypertension (4). The sympathetic and parasympathetic branches of the ANS work in tandem, and the delicate balance between them is critical for cardiovascular stability, effectively regulating key physiological processes such as heart rate and blood pressure (5). In addition, exercise is recognized as an effective means of lowering blood pressure in hypertensive patients and avoiding elevated blood pressure in pre-hypertension (6). For example, in recent years meta-analyses have found that exercise has a significant improvement in the regulation of the autonomic nervous system in hypertensive patients (7, 8). However, despite the numerous studies focusing on exercise to improve autonomic nervous system regulation in hypertensive patients, most of them are limited to overall improvements in indices, and there are no clear conclusions as to whether these improvements predominantly affect the sympathetic nervous system (SNS) or the parasympathetic nervous system (PNS).
Exercise is a key nonpharmacologic intervention for hypertension treatment in all hypertension guidelines (2). Exercise can be broadly categorized into two types, one being aerobic and the other being resistance. Most physical activities are some combination of two types (9). Exercise can be effective in improving ANS balance (10). For example, during exercise, cardiac tone in the vagus nerve gradually subsides, and sympathetic cardiac and vasodilatory tone gradually increases (11). Studies have demonstrated the effects of aerobic, resistance, and aerobic combined resistance exercise on autonomic function improvement (12, 13). For example, differences in the effect of exercise on improving autonomic regulation between hypertensive and normotensive patients were explored in a meta-analysis by Ayesha Miraj Abidi et al. (8). The effect of acute resistance exercise on autonomic activity in hypertensive patients was explored in a meta-analysis by Paulo Farinatti et al. (14). However, there are no systematic evaluations or meta-analyses to assess the differences in the effects of the three exercise modalities, aerobic, resistance, and aerobic combined with resistance, on the improvement of autonomic function in hypertensive patients (15).
Although, a large number of randomized controlled trials have explored the effects of exercise (including aerobic, resistance and combined exercise) on the autonomic nervous system in hypertensive patients, there is heterogeneity in the findings. Existing studies are generally at risk of bias in the original study. Thus, this study aimed to explore the focus of exercise in improving the function of the autonomic nervous system (sympathetic and parasympathetic nervous systems) in hypertensive patients through systematic evaluation and meta-analysis, comparatively analyze the differences in efficacy between different forms of exercise, resolve the differences and controversies in existing studies, provide evidence-based medical evidence for complementary clinical treatments, and promote the development of non-pharmacological treatments of hypertension, thereby improving patients’ quality of life and Health.
2 Materials and methods
This study used a systematic review and meta-analysis to comprehensively evaluate the improvement effect of exercise in hypertensive patients, focusing primarily on the autonomic nervous system. The Preferred Reporting Items for Systematic Reviews and Meta-Analysis of Individual Participant Data (PRISMA) checklist was followed to ensure rigor and transparency of the study (16). In addition, to improve the verifiability of the study, the study was registered on the Prospero International Prospective Systematic Review Registry Platform under the registration number CRD42025634362.
2.1 Inclusion criteria
This study was a systematic evaluation using PICOS principles to assess the efficacy of exercise (aerobic, resistance and aerobic combined resistance) interventions in hypertensive patients. To ensure the inclusiveness of the study, no restriction was imposed on the type of study during the literature search. Moreover, during the literature selection process, we selected only randomized controlled trials and included only those that met the indicators for judging autonomic nervous system outcomes.
Population: All patients who met the diagnostic criteria for hypertension, regardless of symptom presentation. (The diagnosis of hypertension is based on the criteria of the World Health Organization (WHO) and the International Society of Hypertension (ISH). Grade 1 hypertension: systolic blood pressure between 140 and 159 mmHg, diastolic blood pressure between 90 and 99 mmHg; Grade 2 hypertension: systolic blood pressure between 160 and 179 mmHg, diastolic blood pressure between 100 and 109 mmHg; grade 3 hypertension: systolic blood pressure ≥180 mmHg, diastolic blood pressure ≥110 mmHg). Grade 3 hypertension: systolic blood pressure ≥180 mmHg, diastolic blood pressure ≥110 mmHg).
Intervention: Aerobic exercise, resistance exercise, and combined aerobic resistance exercise.
Comparator: Hypertensive patients without exercise intervention or standard treatment.
Outcome: Resting Indicator: Systolic blood pressure (SBP), diastolic blood pressure (DBP), mean blood pressure (MBP), heart rate (HR). Dynamic indicators: heart rate variability [high frequency power (HF); low frequency power (LF), high frequency power to low frequency power ratio (LF/HF), root mean square of successive RR interval differences (RMSSD), the number of successive RR interval differences greater than 50 ms accounted for the total RR Percentage of the number of phases (pNN50), total power (TP), standard deviation of all normal sinus RR intervals (SDNN), and blood pressure variability [24-h systolic blood pressure coefficient of variation (SBP-24 h CV), daytime systolic blood pressure coefficient of variation (SBP-Daytime CV), nighttime systolic blood pressure coefficient of variation (SBP-Nighttime CV), 24-h diastolic blood pressure coefficient of variation (DBP-24 h CV), daytime diastolic blood pressure coefficient of variation (DBP-Daytime CV), and nighttime diastolic blood pressure coefficient of variation (DBP-Nighttime CV)].
Study design: Randomized controlled trial (RCT).
2.2 Exclusion criteria
(1) Non-randomized studies and non-controlled studies were excluded. (2) Case studies or case-specific studies were excluded. (3) Studies that combined dietary modification and other lifestyle changes were excluded. (4) Studies with incomplete or unextractable data of outcome indicators were excluded. (5) Review articles were excluded. (6) Animal experiments were excluded. (7) Duplicate publications were excluded. (8) articles in non-Chinese, non-English, and other languages were excluded. (9) Studies with subjects without hypertension were excluded.
2.3 Literature search strategy
To ensure the comprehensiveness of the information sources, this study searched Web of Science, PubMed, Embase, Cochrane Library, China Knowledge Network (CNKI), and Wanfang (WANFANG DATA) databases and also reviewed published literature reviews and meta-analyses to find more relevant references and identify and include more potentially relevant studies. Search terms were combined with MESH terms and free terms and set according to the PICOS principle. (Table 1) The search deadline was December 31, 2024, and it aimed to collect randomized controlled trials on the effects of exercise on the autonomic nervous system in hypertensive patients. The search languages were English and Chinese.
Table 1
| Retrieval formula | Subject headings | Free word | Chinese search items |
|---|---|---|---|
| #1 | Hypertension | Hypertension OR Blood Pressure, High OR Blood Pressures, High OR High Blood Pressure OR High Blood Pressures | 高血压 |
| #2 | Exercise | Exercise OR Exercises OR Exercise, Physical OR Exercises, Physical OR Physical Exercise OR Physical Exercises OR Physical Activity OR Activities, Physical OR Activity, Physical OR Physical Activities OR Exercise, Aerobic OR Aerobic Exercise OR Aerobic Exercises OR Exercises, Aerobic OR Exercise, Isometric OR Exercises, Isometric OR Isometric Exercises OR Isometric Exercise OR Acute Exercise OR Acute Exercises OR Exercise, Acute OR Exercises, Acute OR Exercise Training OR Exercise Trainings OR Training, Exercise OR Trainings, Exercise | 运动 |
| #3 | Aerobic exercise | Aerobic exercise OR Exercises OR Exercise, Physical OR Exercises, Physical OR Physical Exercise OR Physical Exercises OR Physical Activity OR Activities, Physical OR Activity, Physical OR Physical Activities OR Exercise, Aerobic OR Aerobic Exercise OR Aerobic Exercises OR Exercises, Aerobic OR Exercise, Isometric OR Exercises, Isometric OR Isometric Exercises OR Isometric Exercise OR Acute Exercise OR Acute Exercises OR Exercise, Acute OR Exercises, Acute OR Exercise Training OR Exercise Trainings OR Training, Exercise OR Trainings, Exercise | 有氧运动 |
| #4 | Resistance resistance exercise | Resistance resistance exercise OR Training, Resistance OR Strength Training OR Training, Strength OR Weight-Lifting Strengthening Program OR Strengthening Programs, Weight-Lifting OR Strengthening Program, Weight-Lifting OR Weight Lifting Strengthening Program OR Weight-Lifting Strengthening Programs OR Weight-Lifting Exercise Program OR Exercise Programs, Weight-Lifting OR Exercise Program, Weight-Lifting OR Weight Lifting Exercise Program OR Weight-Lifting Exercise Programs OR Weight-Bearing Strengthening Program OR Strengthening Programs, Weight-Bearing OR Strengthening Program, Weight-Bearing OR Weight Bearing Strengthening Program OR Weight-Bearing Strengthening Programs OR Weight-Bearing Exercise Program OR Exercise Programs, Weight-Bearing OR Exercise Program, Weight-Bearing OR Weight Bearing Exercise Program OR Weight-Bearing Exercise Programs | 抗阻运动 |
| #5 | Autonomic nerves system | autonomic nerves system OR Autonomic Pathway OR Pathway, Autonomic OR Pathways, Autonomic OR Autonomic Nerves OR Autonomic Nerve OR Nerve, Autonomic OR Nerves, Autonomic | 自主神经系统 |
| #6 | Sympathetic nervous system | sympathetic nervous system OR Nervous Systems, Sympathetic OR Nervous System, Sympathetic OR Sympathetic Nervous Systems OR Systems, Sympathetic Nervous OR System, Sympathetic Nervous | 交感神经系统 |
| #7 | Parasympathetic Nervous System | Parasympathetic Nervous System OR Nervous System, Parasympathetic OR Nervous Systems, Parasympathetic OR Parasympathetic Nervous Systems OR System, Parasympathetic Nervous OR Systems, Parasympathetic Nervous | 副交感神经系统 |
Literature search strategy and search terms.
2.4 Study selection
Jingfeng Wang and Xia Fan conducted an initial screening of all retrieved literature and excluded literature unrelated to the study topic based on the title and abstract. After the initial screening, a detailed reading of the literature was conducted to assess whether it met the inclusion criteria. The rescreening process involved a more rigorous assessment, including full-text reading, assessment of the study design, sample characteristics, and specifics and quality of the intervention. In the event of discrepancies in the screening or eligibility assessment process, Xiaolin Li made the final decision to ensure the fairness and accuracy of the selection process.
2.5 Data extraction
For literature included after eligibility assessment, data extraction was performed, including first author, year of publication, type of study population, sample size, age, interventions, substance use, and outcome indicators. For literature with incomplete data descriptions or lacking key information, authors were contacted via email to obtain missing data to ensure data completeness and reliability.
In the data extraction stage, to ensure accuracy, Jingfeng Wang and Fan Xia extracted the mean and standard deviation (SD) of all outcome indicators from the included literature, respectively. If the means and SD could not be obtained directly, they were derived from the extracted variables using the appropriate calculation method. The calculation methods were as follows: (1) Interquartile range (IQR), , (n ≤ 25) or (n > 25); (2) Estimated from the range, , or ; (3) Estimated from t-test or F-test results, that is, ; (4) From the results of variance analysis, that is, ; (5) From the regression analysis results, ; (6) Estimated from the Kaplan–Meier survival curve, the area under the survival curve (AUC), survival function [S(t)], , SD: needed to combine the variance formula of survival time; (7). If the data were presented in bar charts, WebPlotDigitizer was used to convert the graphical data into quantitative data, and Prism was used to calculate the mean and standard deviation of the data (17). The extracted data results were cross-checked. If any inconsistency was found during the data verification process, Xiaolin Li intervened and made the final decision.
2.6 Literature quality evaluation
To ensure the quality of the included studies, we used the Cochrane Risk of Bias Assessment tool and the Jadad scale (18). Cochrane's Risk of Bias Assessment tool addressed aspects such as random allocation, allocation concealment, implementation of blinding, data integrity, selective reporting, and other potential sources of bias. Through a quality assessment chart, the degree of compliance with each criterion was indicated using three color labels (low risk, high risk, and unclear) and was described and analyzed in the study results. The Jadad scale is designed for RCTs to quantify the quality of the literature, standardize study evaluation, guide study involvement, and assist in grading evidence by scoring four items: randomization, blinding, withdrawal and exit, and allocation concealment.
2.7 Statistical analysis
Data extraction, organization, and graphing of the included studies were performed using WebPlotDigitizer 4.8, Endnote X9, Excel, and GraphPad Prism 8.0.2, and meta-analysis was performed using Review Manager 5.4 and Stata18 software. Weighted mean difference (WMD) was used when the units of the outcome indicator were the same, and standardized mean difference (SMD) was used when the units of the outcome indicator were different or when the means differed significantly, with a significance level of p = 0.05, both expressed as 95% CI. Heterogeneity was assessed based on the Q-test combined with the p-value of I². According to the Cochrane Handbook, a fixed-effects model was selected when the heterogeneity test result I² ≤ 50%; a random-effects model was selected when the heterogeneity test result I² > 50%; and a random-effects model was selected when both I² ≤ 50% and I² > 50% in the subgroup analysis (19, 20).
2.8 Other analyses
In this study, a subgroup analysis was performed to assess the same types of evaluation metrics as subgroup analysis criteria, specifically divided into basic metrics, heart rate variability, and blood pressure variability. By comparing these corresponding metrics, this allowed for gaining a deeper understanding of where the effects of exercise on the autonomic nervous system were more centered.
Through sensitivity analyses, we further explored sources of outcome heterogeneity. In this process, each outcome indicator was included in the analysis and was excluded on a case-by-case basis to assess its impact on the joint effect size. If the excluded effect sizes were not significantly different from the overall effect sizes and the point estimates were within the 95% CI, this indicated good stability of the findings.
In this study, Egger's test was performed using Stata software to assess overall publication bias. Egger's test assesses bias by analyzing the regression relationship between effect sizes and their standard errors and identifies small sample effects. Small sample studies are prone to random error, which often leads to extreme effect size estimates. Identifying this pattern through the Egger's test allows for the exploration of potential assessment bias in the included literature, thereby increasing the transparency and reliability of this meta-analysis.
3 Results
3.1 Results of literature analysis
3.1.1 Results of the literature search
An initial search for articles related to the effects of exercise on the autonomic nervous system (sympathetic and parasympathetic nervous systems) in hypertensive patients identified a total of 2,176 publications. After exclusion of duplicate articles, 1,684 literatures remained. Based on the inclusion and exclusion criteria, 1,571 articles were further excluded after reading the titles and abstracts, leaving 113 articles for full-text reading. Finally, a total of 20 articles were included in this study (Figure 1).
Figure 1
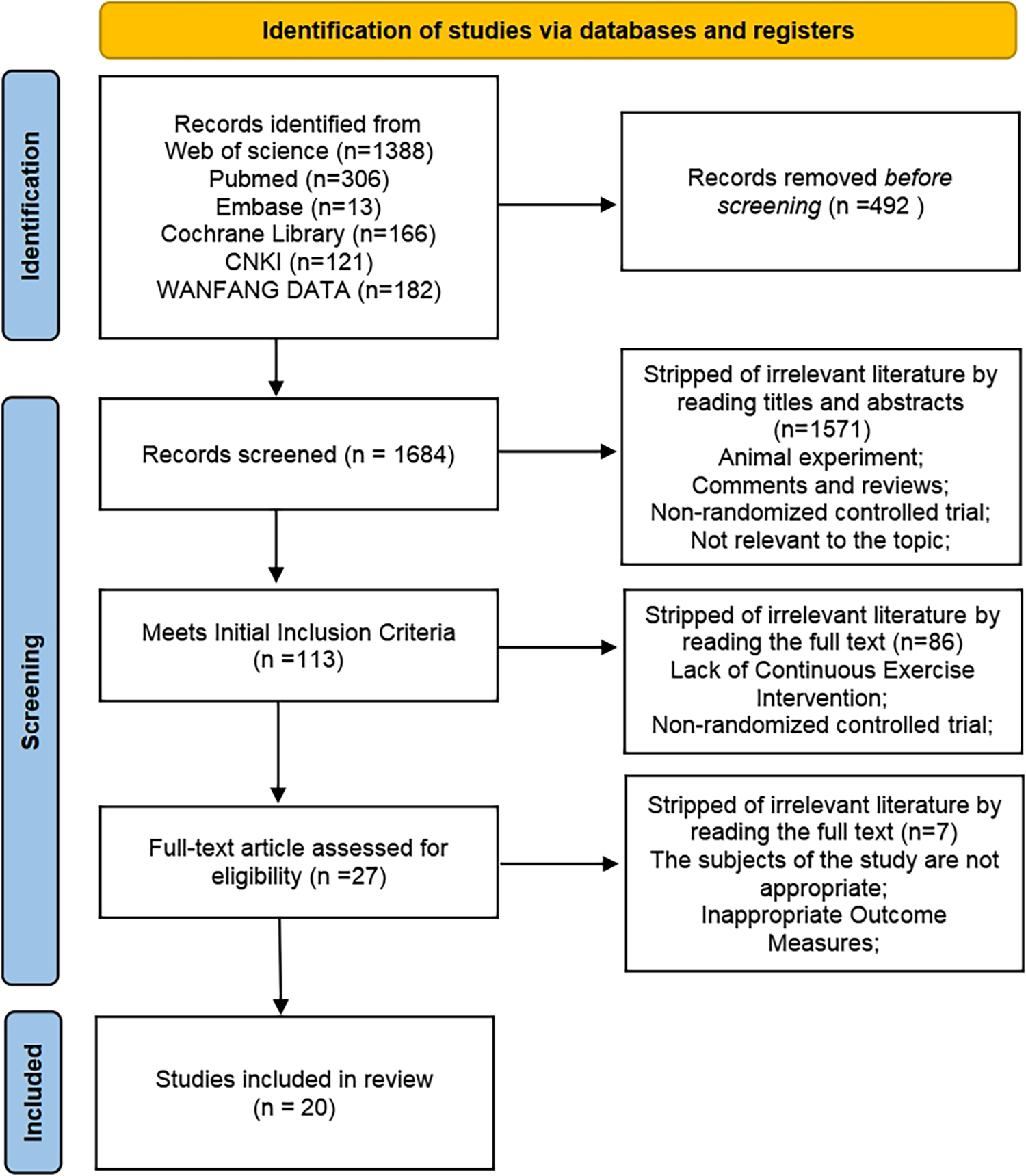
Literature search and screening process.
3.1.2 Characteristics of the included literature
Twenty papers were included in this study for meta-analysis, including 794 participants. There were a total of three exercise modalities studied, including 16 aerobic exercise interventions, four resistance exercise interventions, and four aerobic combined resistance exercise interventions. All controls included in the study did not receive any form of exercise intervention but received usual care, health education, or maintenance of daily activities. A wide range of health-related measurements were included in the literature, such as blood pressure, heart rate, and BMI (Table 2).
Table 2
| Researchers; year | Gender | Age mean(SD) | Height (cm) | Body weight(kg) | BMI(kg/㎡) | SBP (mmHg) | DBP (mmHg) | HR (bmp) | Exercise intensity and duration | Mode of exercise | Sample size |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fu et al. (2024) (58) | Male | 57.59 (14.2) | 165.91 (5.87) | 73.92 (13.86) | 26.83 (4.77) | 130.2 (10) | 82.2 (9.3) | 75.5 (7.9) | Tai Chi/aerobics: 60 min/time, 40%–60% heart rate reserve, 3 times/week, lasting 12 weeks | AE | 43 |
| Female | 37 | ||||||||||
| Rodrigues et al. (2023) (59) | Male | 59 (4) | 1.62 (0.06) | 79.5 (13.1) | 29.8 (4.1) | 120 (12) | 78 (9) | 73 (11) | Interval Aerobic exercise: 8 sets of 2-min brisk walking up and down 20 cm steps with 2 min between sets. Continuous aerobic exercise: 30 min of intensity walking at 50% heart rate reserve | AE | 12 |
| Female | |||||||||||
| Domingues et al. (2022) (22) | Male | 48.4 (8.5) | 1.70 (0.1) | 86.1 (18) | 29.6 (5.2) | 127.5 (13.6) | 77.2 (11.1) | 73 (9.9) | 12 min/session, 3 sessions, 2 min interval between sessions | AE | 22 |
| Female | 22 | ||||||||||
| Roque Marçal (2022) (2) | Male | 68 (7) | N | N | 31 (5) | 12,618) | 74 (9) | 75 (9) | High-intensity interval exercise: 21 min of high-intensity aerobic exercise (jogging/running at RPE levels 15–17), alternating with 2 min of active recovery (walking at RPE levels 9–11). Moderate-intensity interval exercise: 26 min of moderate-intensity aerobic exercise (walking at RPE levels 11–13), alternating 2 min of active recovery (walking at RPE levels 9–11). This study adopted a crossover design, meaning that each participant had to undergo all the interventions. As a result, the sample size for each group was the same | AE | 9 |
| Female | 67 (7) | N | N | 32 (4) | 12,618) | 74 (9) | 76 (9) | 11 | |||
| Sardeli et al. (2022) (60) | Male | 65.3 (2.57) | N | 78.55 (7.15) | 29.66 (2.19) | 132.5 (11.21) | 82.75 (5.46) | N | Twice a week, on Mondays and Thursdays, strength training (15 min duration) was followed by the aerobic training (50 min duration) and once per week, on Fridays, aerobic training was performed alone (50 min duration), with the protocol consisting of continuous walking and/or running on a treadmill. Strength training consisted of one set of 15 repetitions for each of seven strength exercises for the major muscle groups (leg extension and flexion, leg press, heel lift, bench press, pulley, and abdominal) | CARE | 46 |
| Female | |||||||||||
| Fraccari-Pires et al. (2022) (61) | Male | 57 (6.68) | N | N | 31.5 (3.52) | 140.5 (6.7) | 81.5 (4.27) | 64.5 (5.44) | Aerobic (ARE) session was performed in an electronic treadmill for 45 min at 50%–60% of maximal HR (HRmax) obtained from the ergometric stress test. Resistance(RES) consisted of 6 exercises with 4 sets of 12 submaximal repetitions at moderate intensity (3–5 on the adapted Borg scale). Exercises were performed in the following order: (1) chair squat, (2) vertical bench press, (3) seated knee raise, (4) seated row, (5) dorsiflexion and plantar flexion, and (6) shoulder abduction. A 1 min rest interval was adopted between sets and exercises. All exercises were performed in the full range of motion, and muscle contractions—concentric and eccentric—were performed at moderate velocity (2 s for each). Combined (COM) consisted of AER exercise performed at 50%–60% HRmax for 25 min plus RES based on 6 exercises with 2 sets of 12 submaximal repetitions at moderate intensity according to modified Borg scale. All exercise sessions lasted up to 60 min and were supervised by an exercise physiologist | AE, RE, CARE | 9 |
| Female | 11 | ||||||||||
| Caminiti et al. (2021) (50) | Male | 68.3 (5.05) | N | 87.2 (4.26) | 29.75 (1.86) | 121.9 (17.28) | 80.95 (7.59) | N | Each exercise session included 10 min of warm-up, 10 min of cool-down, and 60 min of aerobic exercise on a treadmill. 40 min of aerobic training on treadmill; 20 min of resistance training consisting in the following exercises: leg press and extension, shoulder press, chest press, low row, and vertical traction. Patients performed 10 repetitions per set, two sets for each exercise at 60% of 1 RM, with 2 min rest between sets | AE, CARE | 55 |
| Female | |||||||||||
| Casonatto et al. (2020) (62) | Male | 57.15 (10.3) | 1.63 (0.06) | 78.45 (9.73) | 29.35 (3.29) | 138.5 (9.22) | 84 (5.07) | N | 40 min run/walk, 60%–70% of heart rate reserve | AE | 20 |
| Female | |||||||||||
| Oliveira-Dantas et al. (2020) (63) | Male | 64.7 (4.7) | N | N | 28.4 (1.79) | N | N | N | The resistance training intervention period lasted 10 weeks. The supervised group sessions were held twice a week for the first 5 weeks, and after this initial period, the frequency increased to 3 times per week, totaling 25 exercise sessions. The short-term resistance training program consisted of 9 exercises, which were conducted in the following order: seated leg press; seated rowing machine; trunk flexion; knee flexion machine, bench press, trunk extension machine, push press, standing plantar flexion, and front pull-down. | RE | 25 |
| Female | |||||||||||
| Brito et al. (2019) (64) | Male | 50 (4.66) | 1.71 (0.05) | 88.45 (7.86) | 30.15 (1.88) | 133.5 (4.5) | 90.5 (3.62) | N | Four weeks before training, the exercise time was increased from 30 min to 45 min. Starting from the fifth week, the intensity was increased every 2 weeks, from the heart rate corresponding to the anaerobic threshold to 10% below the respiratory compensation point | AE | 50 |
| Female | |||||||||||
| Masroor et al. (2018) (38) | Male | 40.61 (2.47) | 154.9 (3.12) | 70.7 (5.94) | 30.5 (2.54) | 145.5 (5.56) | 84.8 (3.08) | 77.9 (5.51) | Combined aerobic and resistance training trained on an inclined (5.0%) treadmill 3 days/week for 4 weeks. In addition, resistance training was provided twice a week, resulting in a total of 5 training days/week. Each session commenced with a 5-min warm-up performed at 40% of HRmax on treadmill, followed by either an aerobic or resistance exercise bout. Aerobic training was performed at 50%–80% of HRmax (intensity was progressed gradually from 50% to 80% HRmax across 4 weeks) for 20 min, followed by 5 min of cool down at 40% of HRmax. Resistance training constituted 3 sets of 10 repetitions of 5 exercises: bicep curls, triceps extensions, abdominal crunches, leg curls, and knee extensions, at an intensity of 50%–80% of 1RM (intensity was progressed gradually from 50% to 80% of 1 RM over 4 weeks).30, 31 HR, SBP, and DBP were closely monitored during and after exercise. Exercise training was performed 5 times/week for 4 weeks | CARE | 28 |
| Female | |||||||||||
| Vale et al. (2018) (65) | Male | 57.73 (6.11) | 1.56 (0.08) | 65.77 (10.37) | 26.90 (3.74) | 130.5 (8.45) | 78.06 (3.65) | 67.52 (3.39) | After determination of the loads of 6RM and 15RM, the participants performed three different experimental protocols: a control session, a RT session with 6RM, and a RT session with 15RM. The rest intervals between sets and exercises lasted for 2 min | RE | 15 |
| Female | |||||||||||
| Pushpanathan et al. (2015) (66) | Male | 43.36 (4.45) | 1.64 (0.05) | 73.47 (7.39) | 27.06 (2.38) | 125.6 (5.57) | 81.82 (4.16) | 72.63 (6.29) | Yoga: 45 min per session, 3 times a week, for a total of 12 weeks | AE | 44 |
| Female | 11 | ||||||||||
| Sun and Bai (2015) (67) | Male | ≥60 | N | 76.87 (11.64) | 27.35 (4.76) | 147.96 (10.62) | 97.61 (5.84) | 77.9 (5.1) | 3 times per week. The exercise intensity in the first week is 30% of VO2 max. From then on, the intensity will be increased by 10% of VO2 max each week. When the intensity reaches 60% of VO2 max, it will remain constant until the end of the experiment. The exercise lasts for a total of 3 months. | AE | 44 |
| Female | 34 | ||||||||||
| Zhang (2015) (68) | Male | 48.3 (7) | 1.66 (0.04) | 71.4 (7.6) | 26 (3) | 143 (16) | 95 (10) | 77 (6) | Jogging, 40 min per session, 3 times a week, for a total of 24 weeks. During the first 12 weeks, the exercise intensity was 60%–70% of VO2 max, and in the last 12 weeks, it increased to 70%–75% of VO2 max | AE | 12 |
| Female | 18 | ||||||||||
| Pagonas et al. (2014) (21) | Male | 67.7 (43–77) | N | N | 29.5 (4.5) | 133.1 (12.1) | 73.8 (6.4) | N | The initial duration of the training was 30 min. in the first week, the training consisted of five 3-min loads; between loads, the patient walked at half speed for 3 min. in the second week, the duration of the exercise was gradually increased to four 5-min loads per day; in the third week, to three 8-min loads per day; in the fourth week, to three 10-min loads per day; and in the fifth week, to two 15-min loads per day. The sixth week and subsequent weeks, the exercise volume was gradually increased to 30, 32, and 36 min per day and was performed without interruption. Weeks, the amount of exercise was gradually increased to 30, 32, and 36 min per day and was performed without interruption. Training was performed three times a week for 8–12 weeks. | AE | 66 |
| Female | |||||||||||
| Huang (2014) (69) | Male | 54.4 (8) | 1.65 (0.03) | 77.4 (12.1) | 28.6 (4.6) | 145 (8) | 96 (4) | 77 (4) | Intensity of 60% VO2 max for the first 6 weeks, increasing to 70% VO2 max for 40 min/session for the second 6 weeks | AE, RE | 19 |
| Female | 14 | ||||||||||
| Knoepfli-Lenzin C (2010) | Male | 38 (5) | N | N | 27 (3) | 137.6 (3.848) | 90.31 (3.389) | 74 (8) | Soccer group: 12 weeks of alternating high and low loads for aerobic training; running group: 12 weeks of continuous running exercise at 80% of maximum heart rate | AE | 47 |
| Female | |||||||||||
| Wang et al. (2007) (70) | Male | 63.6 (2.5) | 155.6 (6.3) | 65.7 (7.7 | N | 140∼160 | 90∼95 | N | Walking: 3 times per week, 1 h each time, exercise intensity 46%–60% heart rate reserve for 12 weeks. | AE | 50 |
| Female | |||||||||||
| Laterza et al. (2007) (32) | Male | 44 (1) | 1.70 (0.02) | 77 (3) | 26 (1) | 145 (4) | 94 (2) | 76 (3.32) | 60 min of exercise three times a week for 4 months. Exercise intensity was determined by the HR level, which corresponded to the anaerobic threshold to 70% VO2 max | AE | 13 |
| Female | 7 |
Baseline data before exercise intervention in hypertensive patients.
N: No information.
3.1.3 Quality assessment of the included literature
In the area of random sequence generation, 20 studies were considered low risk. In the area of allocation concealment, 16 studies also showed low risk. However, four studies did not provide sufficient information on concealment of allocations. In the areas of blinding of participants and personnel and blinding of outcome assessment, 13 and 10 studies implemented rigorous blinding measures and were considered low risk, but 2 studies had significant blinding problems in outcome assessment. In the incomplete outcome data domain, four studies lacked a description of exit. Sensitivity analyses were conducted on these studies to fully understand and explain the impact of missing data on the findings. In the selective reporting domain, none of the 20 studies mentioned this risk. In assessing other potential biases, a systematic evaluation was conducted, and it was concluded that the included studies did not exhibit significant other biases (Figure 2).
Figure 2
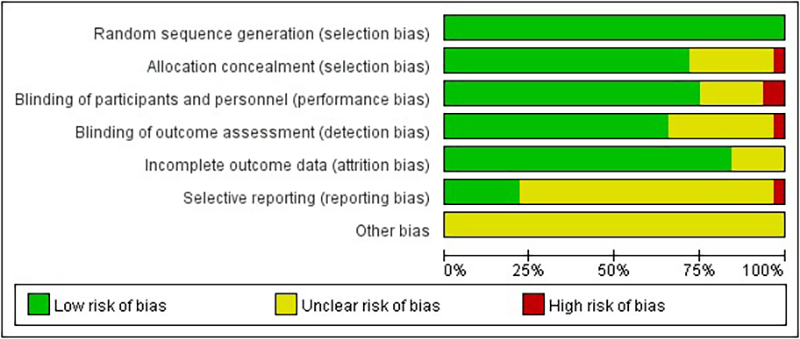
Quality evaluation distribution chart.
A modified version of the Jadad score was assessed for the 20 randomized controlled trials included. All studies used randomized allocation, of which 15 used blinding and the rest were not fully described or were not blinded, reducing the score. 16 trials reported complete withdrawal and dropout, and 4 trials were unclear about complete reporting of withdrawal and dropout. 6 study allocation concealment was not described (Table 3).
Table 3
| Item | Randomization | Blinding | Withdrawal | Allocation concealment | Totals |
|---|---|---|---|---|---|
| Fu et al. (2024) (58) | 2 | 2 | 2 | 1 | 7 |
| Rodrigues et al. (2023) (59) | 2 | 1 | 2 | 1 | 6 |
| Domingues et al. (2022) (22) | 2 | 2 | 2 | 0 | 6 |
| Roque Marçal et al. (2022) (2) | 2 | 1 | 2 | 1 | 6 |
| Sardeli et al. (2022) (60) | 2 | 2 | 2 | 1 | 7 |
| Fraccari-Pires (2022) (61) | 2 | 2 | 1 | 1 | 6 |
| Caminiti et al. (2021) (50) | 2 | 2 | 2 | 0 | 6 |
| Casonatto et al. (2020) (62) | 2 | 2 | 2 | 1 | 7 |
| Oliveira-Dantas et al. (2020) (63) | 2 | 1 | 2 | 1 | 6 |
| Brito et al. (2019) (64) | 2 | 2 | 2 | 1 | 7 |
| Masroor et al. (2018) (38) | 2 | 2 | 2 | 0 | 6 |
| Vale et al. (2018) (65) | 2 | 2 | 1 | 1 | 6 |
| Pushpanathan et al. (2015) (66) | 2 | 2 | 2 | 1 | 7 |
| Sun and Bai (2015) (67) | 2 | 2 | 1 | 1 | 6 |
| Zhang (2015) (68) | 2 | 0 | 2 | 1 | 5 |
| Pagonas et al. (2014) (21) | 2 | 2 | 2 | 0 | 6 |
| Huang (2014) (69) | 2 | 2 | 2 | 0 | 6 |
| Knoepfli-Lenzin (2010) (71) | 2 | 2 | 2 | 0 | 6 |
| Wang et al. (2007) (70) | 2 | 0 | 2 | 1 | 5 |
| Laterza et al. (2007) (32) | 2 | 2 | 1 | 1 | 6 |
Jadad quality evaluation of randomized controlled trials.
3.1.4 Publication bias analysis
For the basic indicators, there was no publication bias in the SBP, DBP, and HR groups (p > 0.05), while there was a publication bias in the MBP group (p < 0.05). For the evaluation of the sympathetic nervous system as well as the autonomic nervous system balance, there was no publication bias in the LF (ms²) group, LF (nu), and LF/HF group (p > 0.05). For the indexes evaluating the parasympathetic nervous system, there was no publication bias in the HF (ms²), HF (nu), RMSSD, and pNN50% groups (p > 0.05). For blood pressure variability indices, publication bias was observed in the SBP-24 h CV group and the DBP-24 h CV group (p < 0.05) (Table 4).
Table 4
| Item | Relevant indicators | Egger's test | p value |
|---|---|---|---|
| Basic data | SBP | 0.454 | p > 0.05 |
| DBP | 0.311 | p > 0.05 | |
| MBP | 0.017 | p < 0.05 | |
| HR | 0.290 | p > 0.05 | |
| Blood pressure variability | SBP-24 h CV | 0.021 | p < 0.05 |
| DBP-24-h CV | 0.011 | p < 0.05 | |
| Sympathetic nervous system and autonomic nervous system balance | LF (ms²) | 0.261 | p > 0.05 |
| LF (nu) | 0.174 | p > 0.05 | |
| LF/HF | 0.079 | p > 0.05 | |
| Parasympathetic nervous system response | HF (ms²) | 0.305 | p > 0.05 |
| HF (nu) | 0.658 | p > 0.05 | |
| RMMSD | 0.072 | p > 0.05 | |
| pNN50 | 0.149 | p > 0.05 | |
| Total effect of autonomic nervous system | TP | 0.219 | p > 0.05 |
| SDNN | 0.301 | p > 0.05 |
Egger test for literature publication bias results.
The data of blood pressure variability indicators (SBP-daytime CV, SBP-nighttime CV, DBP-daytime CV, DBP-nighttime CV) in the included literature were limited, so the publication bias analysis was not conducted.
3.1.5 Sensitivity analysis
Excluding any single study, there was no statistically significant difference between the joint effect size and the overall effect size for the remaining studies. The point estimate after each exclusion consistently fell within the 95% CI of the overall effect size.
3.1.6 Heterogeneity analysis
In the subgroup analysis of basic indicators of hypertension patients, the heterogeneity index (I²) of SBP was 78%, showing high heterogeneity. The I² of DBP was 79%, showing high heterogeneity. The I² of MBP was 82%, indicating high heterogeneity. The I² of HR was 76%, showing high heterogeneity. The combined effect I² was 77%, with high heterogeneity (Figure 3). In the subgroup analysis of blood pressure variability indicators in hypertensive patients, the I² of SBP-24 h CV was 78%, showing high heterogeneity. The I² of SBP-Daytime CV was 0%, showing low heterogeneity. The I² of SBP-Nighttime CV was 76%, showing high heterogeneity. The I² of DBP-24 h CV was 66%, showing moderate heterogeneity. The I² of DBP-Daytime CV was 0%, indicating low heterogeneity. The I² of DBP-Nighttime CV was 0%, with low heterogeneity. The combined effect I² was 63%, with moderate heterogeneity (Figure 4). In the subgroup analysis of parasympathetic nervous system indicators in hypertensive patients, the I² of HF (ms²) was 88%, showing high heterogeneity. The I² of HF (nu) was 54%, showing moderate heterogeneity. The I² of RMSSD was 70%, showing moderate heterogeneity. The I² of pNN50 was 54%, indicating moderate heterogeneity. The combined effect I² was 74%, with moderate heterogeneity (Figure 5). In the subgroup analysis of sympathetic nervous system and autonomic nervous system balance indicators in hypertensive patients, the I² of LF(ms²) was 0%, showing low heterogeneity. The I² of LF (nu) was 0%, indicating low heterogeneity. The I² of LF/HF was 78%, showing moderate heterogeneity. The combined effect I² was 49%, with low heterogeneity (Figure 6). In the subgroup analysis of the total frequency domain and time domain indicators of heart rate variability in hypertensive patients, the I² of TP was 83%, showing moderate heterogeneity. The I² of SDNN was 72%, indicating moderate heterogeneity. The combined effect I² was 79%, with high heterogeneity (Figure 7).
Figure 3
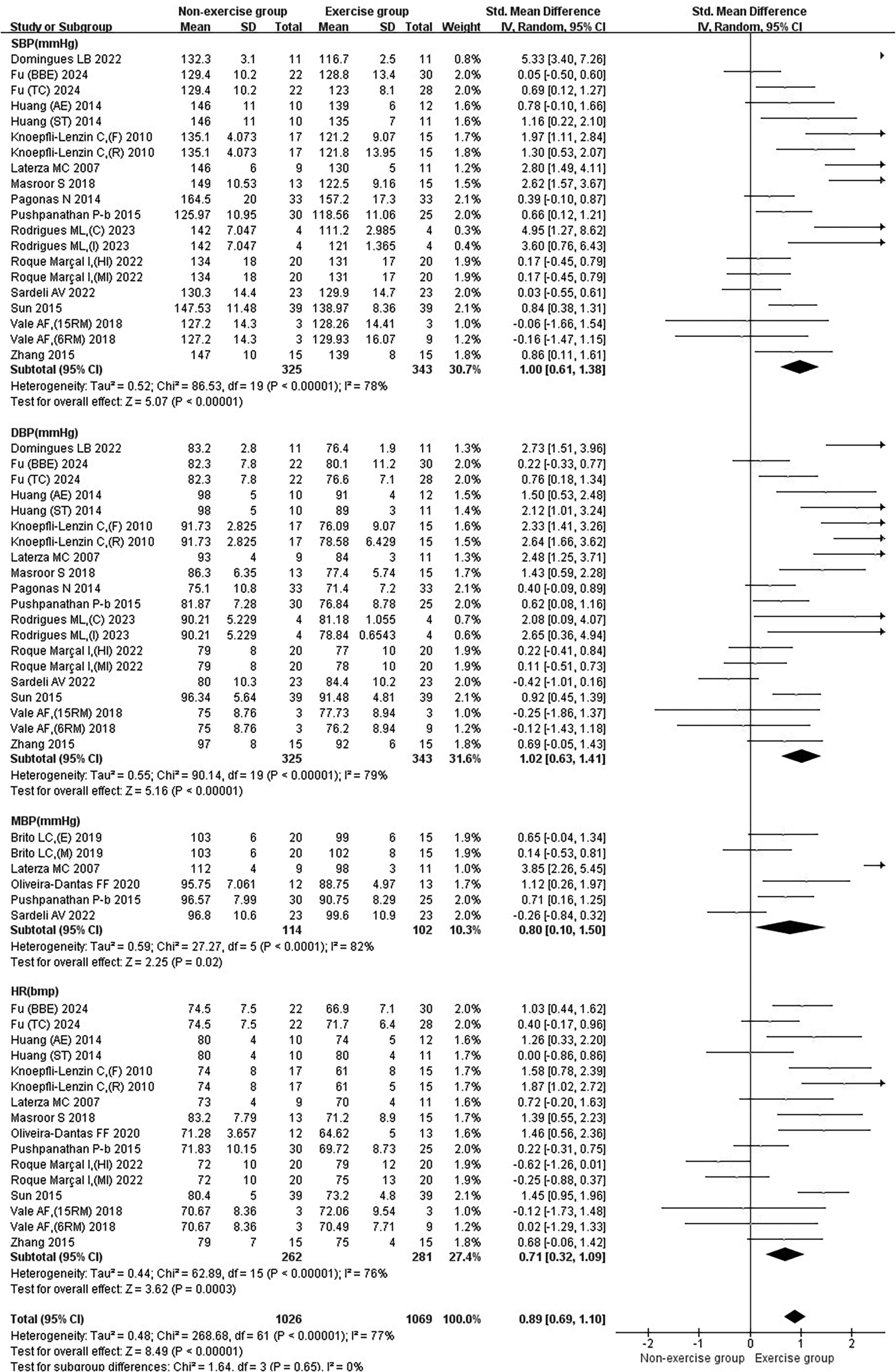
Subgroup analysis of basic indicators in patients with hypertension treated with exercise.
Figure 4
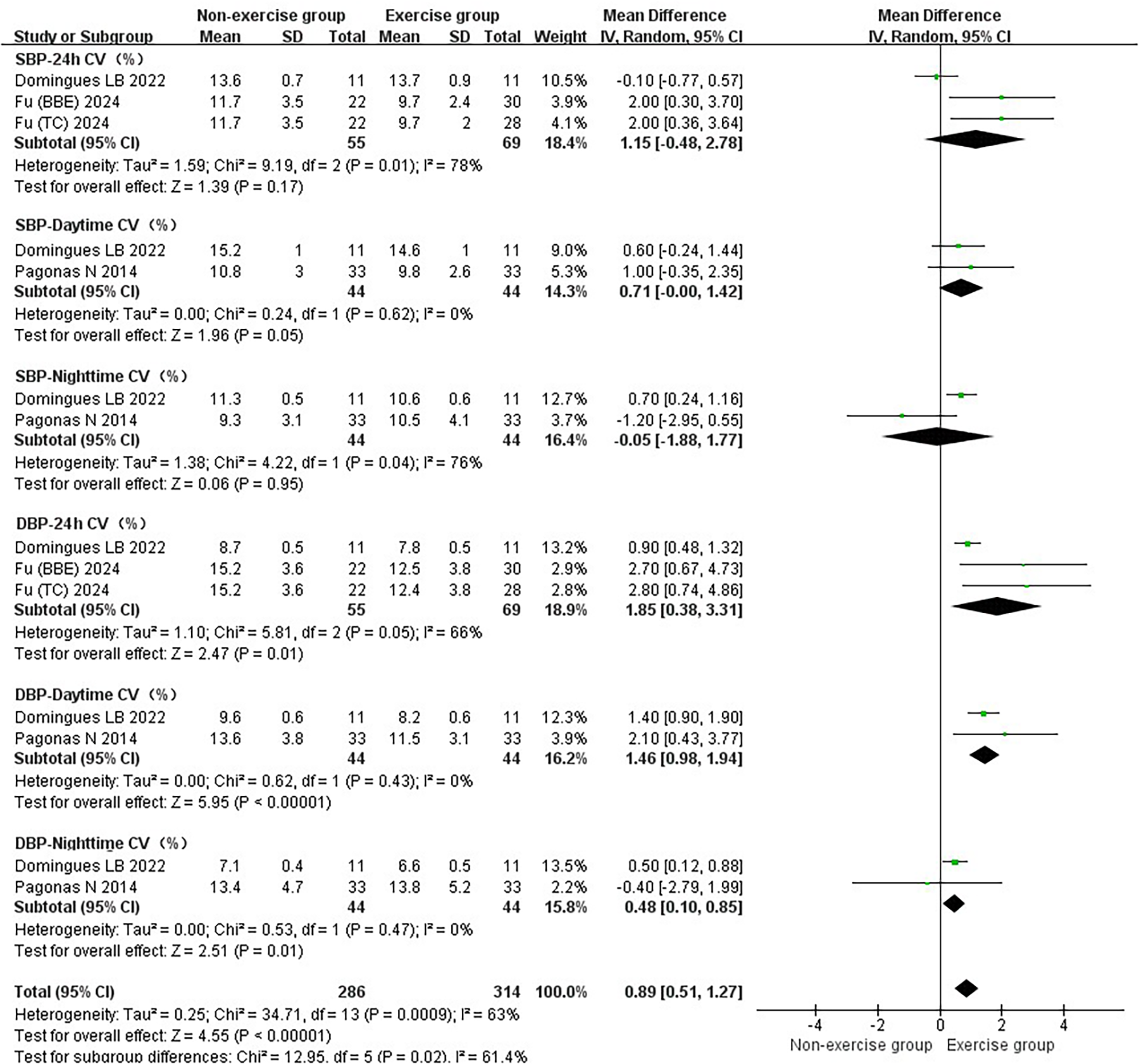
Subgroup analysis of exercise intervention on blood pressure variability in patients with hypertension.
Figure 5
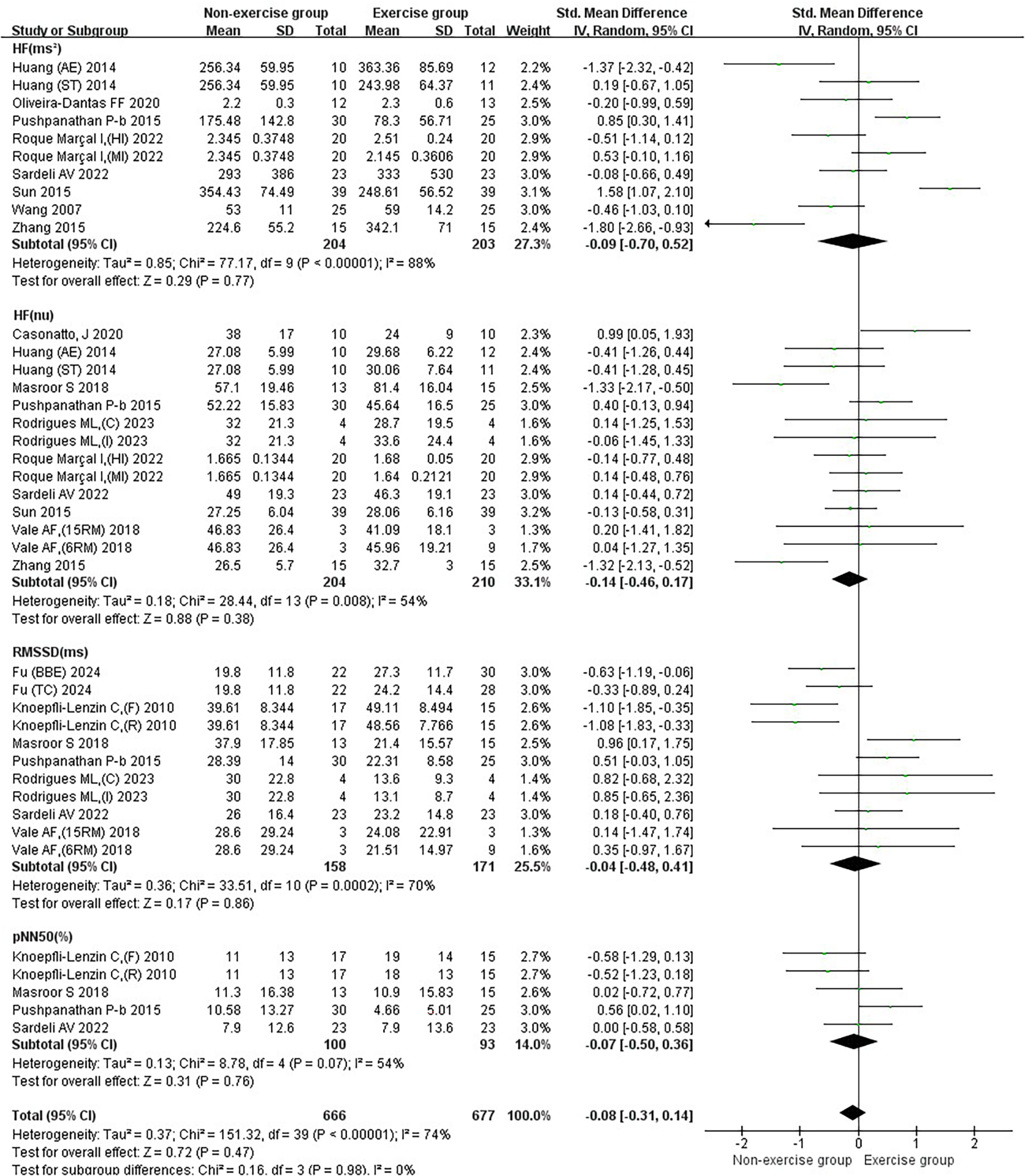
Subgroup analysis of exercise intervention on parasympathetic nervous system in patients with hypertension.
Figure 6
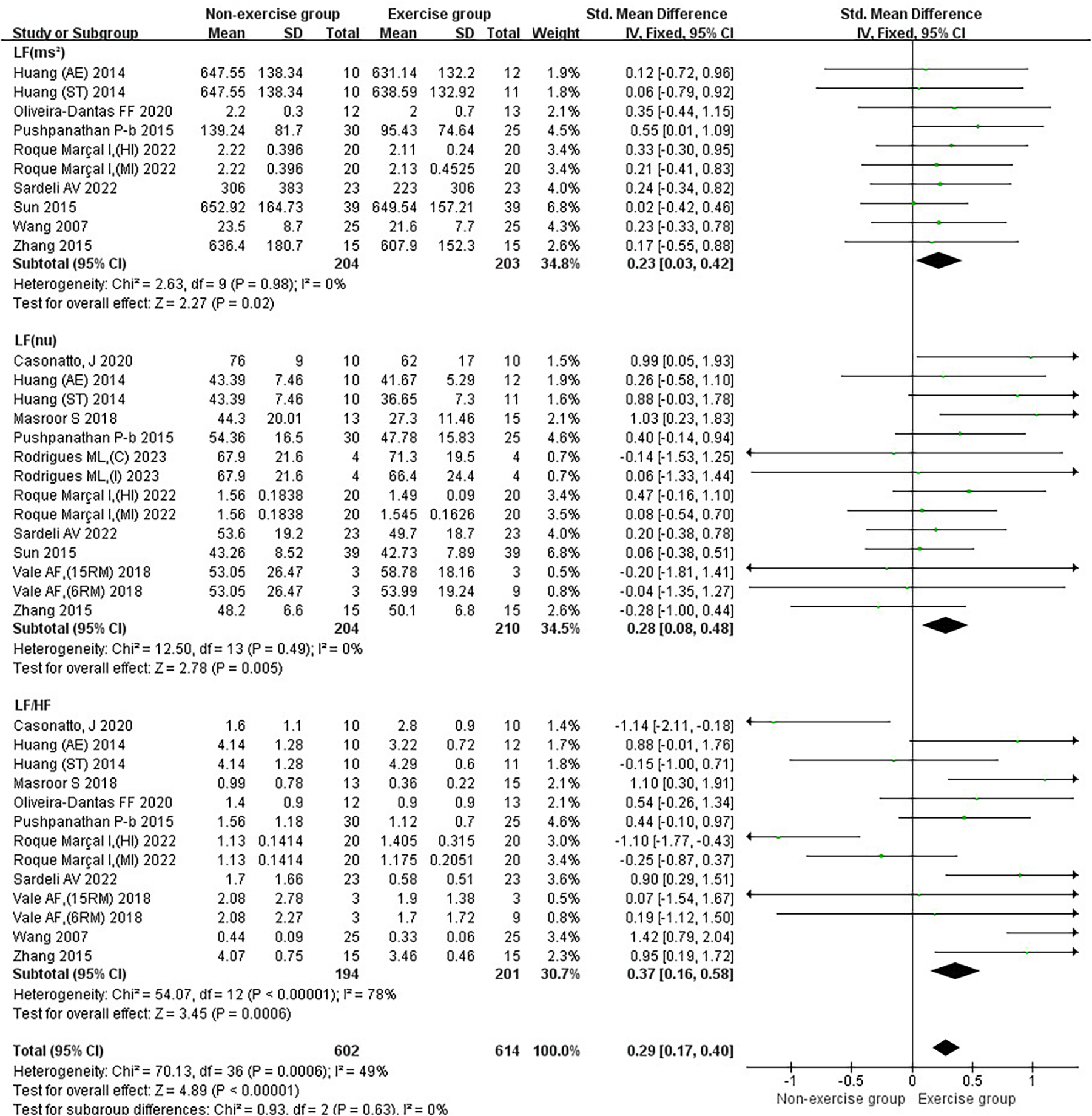
Subgroup analysis of exercise intervention on sympathetic nervous system and autonomic nervous system balance in patients with hypertension.
Figure 7
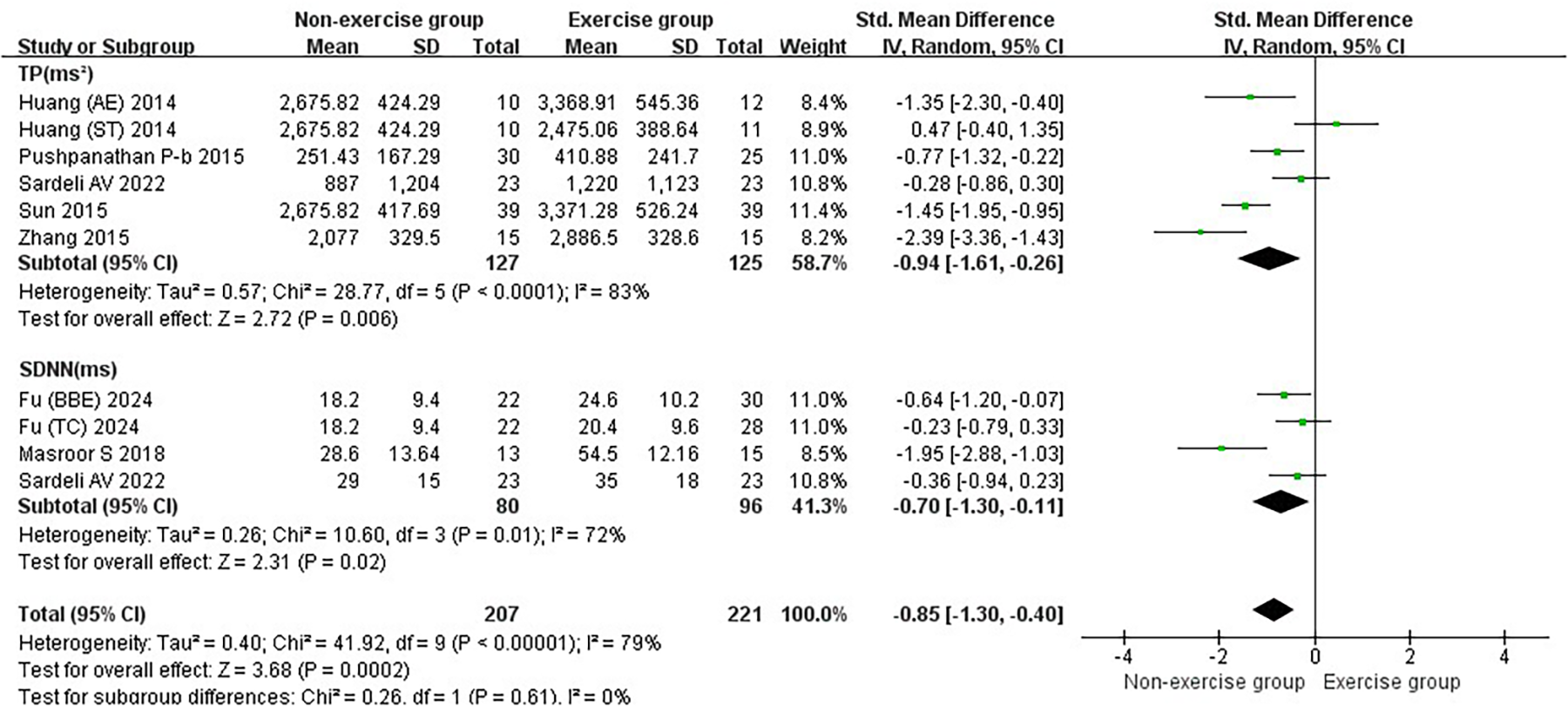
Subgroup analysis of the total effect of exercise intervention on heart rate variability in the frequency domain and time domain in hypertensive patients.
3.2 Meta-analysis results
3.2.1 Results of the effect of exercise on basic indicators in hypertensive patients
Exercise significantly reduced the joint effect sizes of blood pressure as well as heart rate in hypertensive patients [SMD = 0.89, 95% CI (0.69, 1.10)]. Exercise significantly reduced SBP [SMD = 1.00, 95% CI (0.61, 1.38)], DBP [SMD = 1.02, 95% CI (0.63, 1.41)], MBP [SMD = 0.80, 95% CI (0.10, 1.50)], and HR [SMD = 0.71, 95% CI (0.32, 1.09)] (Figure 3).
Aerobic and combined aerobic resistance exercise significantly reduced SBP in hypertensive patients (p < 0.05). Aerobic combined resistance exercise had a significantly higher effect on SBP than resistance exercise (p < 0.05) (Figure 8A). Aerobic combined resistance exercise significantly reduced DBP in hypertensive patients (p < 0.05). Aerobic combined resistance exercise had a significantly higher effect on DBP than resistance exercise (p < 0.05) (Figure 8B). Aerobic combined resistance exercise significantly reduced MBP in hypertensive patients (p < 0.05). Aerobic combined resistance exercise had a significantly higher effect on MBP than resistance exercise (p < 0.05) (Figure 8C). Resistance and aerobic combined resistance exercise significantly reduced HR in hypertensive patients (p < 0.05) (Figure 9J).
Figure 8
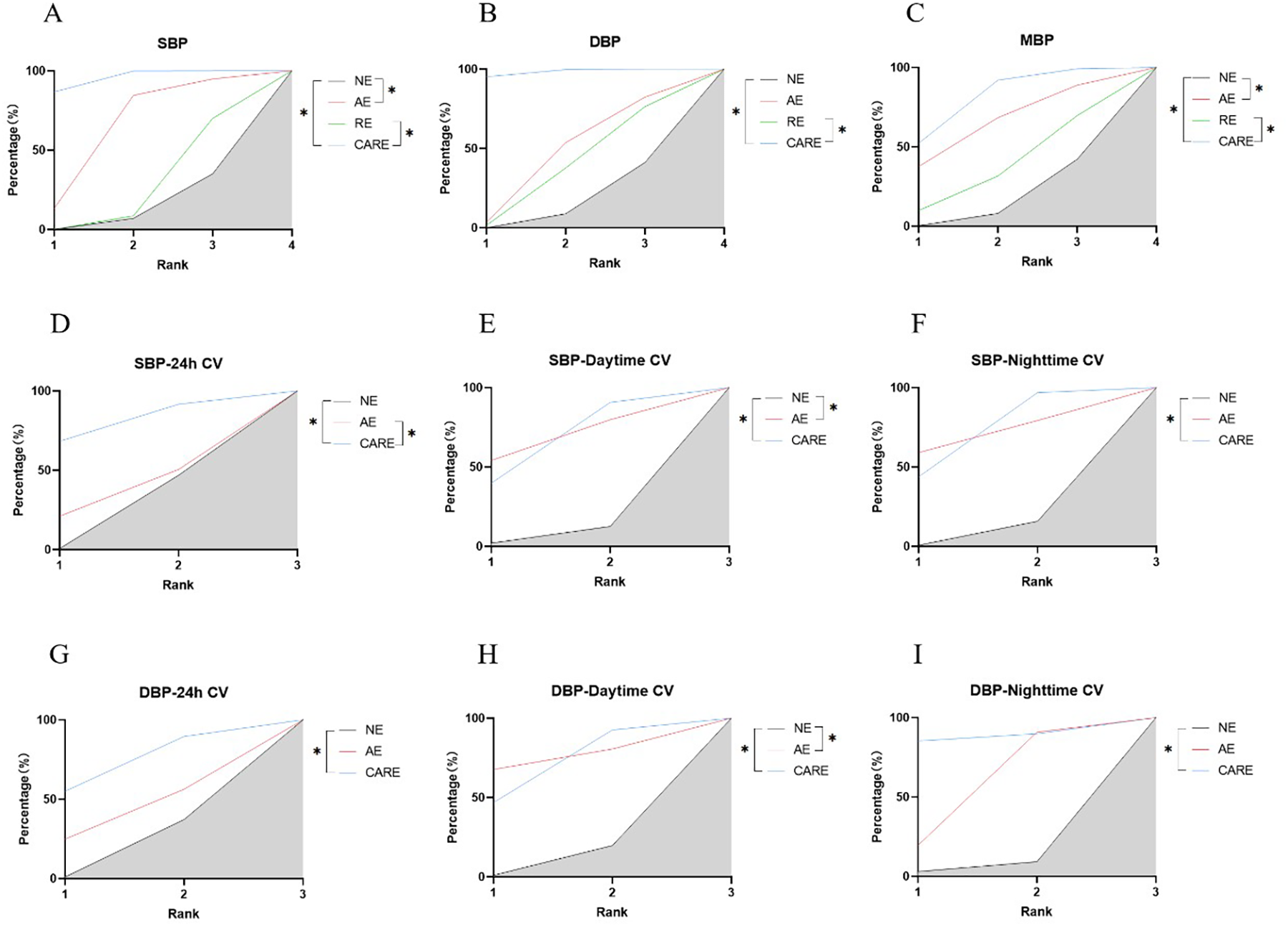
SUCRA plots of the effects of aerobic exercise, resistance exercise, and combined aerobic resistance exercise on the blood pressure and blood pressure variability of patients with hypertension. (A) Systolic blood pressure (SBP); (B) diastolic blood pressure (DBP); (C) mean blood pressure (MBP); (D) 24-hour systolic blood pressure coefficient of variation (SBP-24h CV); (E) daytime systolic blood pressure coefficient of variation (SBP-Daytime CV); (F) nighttime systolic blood pressure coefficient of variation (SBP-Nighttime CV); (G) 24-hour diastolic blood pressure coefficient of variation (DBP-24h CV); (H) daytime diastolic blood pressure coefficient of variation (DBP-Daytime CV); (I) nighttime diastolic blood pressure coefficient of variation (DBP-Nighttime CV); *p < 0.05. Note: SUCRA, Surface under the cumulative ranking curve; The literature on resistance training lacks data on blood pressure variability indicators; Therefore, only a network analysis was conducted on the effects of aerobic exercise and combined aerobic resistance exercise on this indicator; NE, no exercise; AE, aerobic exercise; RE, resistance exercise; CARE, Combined aerobic resistance exercise.
Figure 9
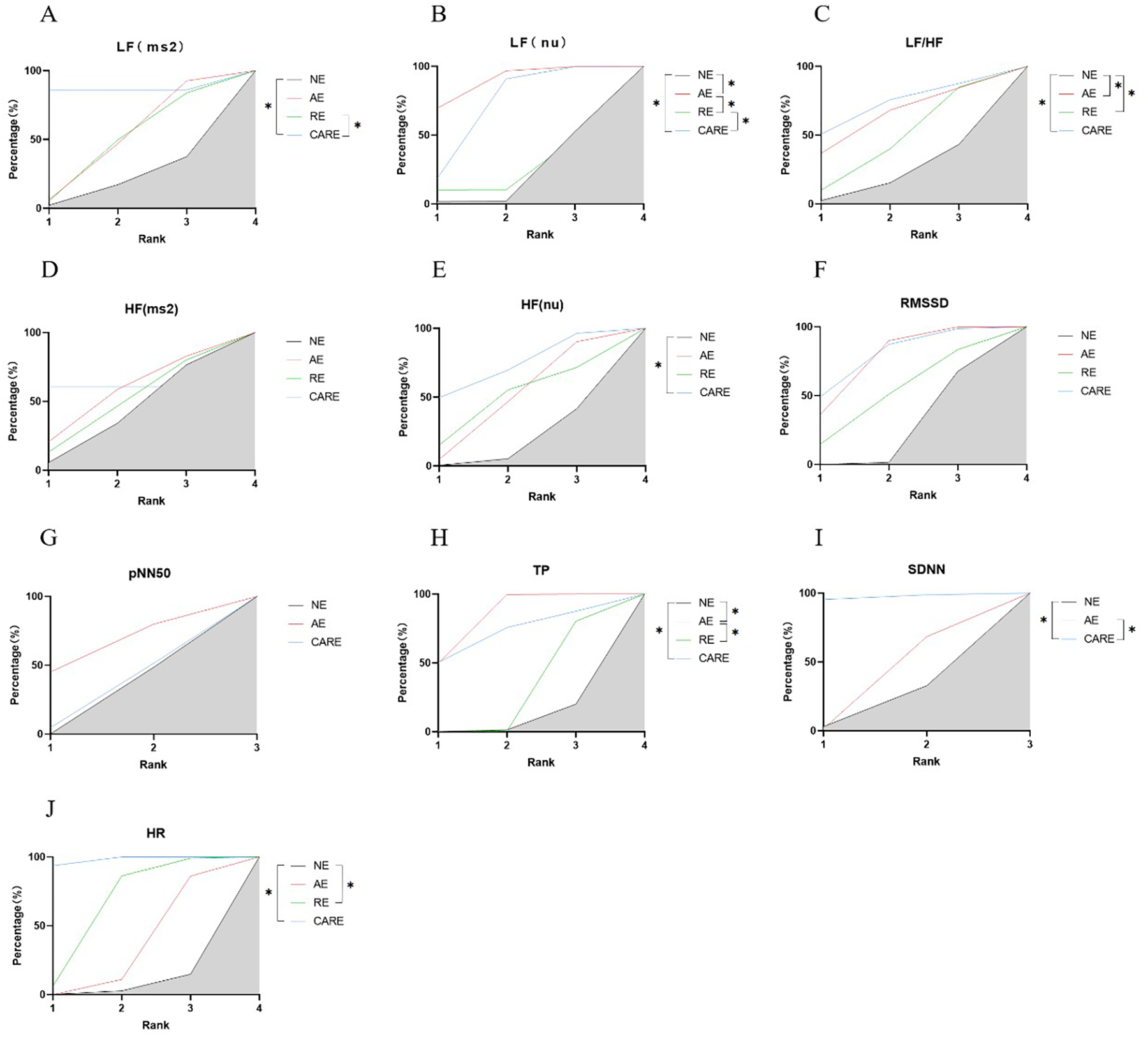
SUCRA plots of the effects of aerobic exercise, resistance exercise, and combined aerobic and resistance exercise on the heart rate variability in patients with hypertension. (A) low frequency power (LF); (B) Normalized low-frequency power; (C) low frequency power to high frequency power ratio (LF/HF); (D) high frequency power (HF); (E) Normalized high-frequency power; (F) root mean square of successive RR interval differences (RMSSD); (G) the number of successive RR interval differences greater than 50ms accounted for the total RR Percentage of the number of phases (pNN50); (H) total power (TP); (I) standard deviation of all normal sinus RR intervals (SDNN); (J) heart rate (HR); *p < 0.05. Note: The literature on resistance training lacks data on pNN50 and SDNN indicators; Therefore, only network analyses were conducted on the effects of aerobic exercise and combined aerobic resistance exercise on these indicators.
3.2.2 Results of the effect of exercise on blood pressure variability in hypertensive patients
Exercise significantly reduced the joint effect size of hypertension being a patient's blood pressure variability [WMD = 0.89, 95% CI (0.51, 1.27)]. The exercise did not show significant reduction in SBP-24 h CV [WMD = 1.15, 95% CI (−0.48, 2.78)], SBP-Daytime CV [WMD = 0.71, 95% CI (−0.00, 1.42)], and SBP-Nighttime CV [WMD = −0.05, 95% CI (−1.88, 1.77)]. The exercise significantly reduced the DBP-24 h CV [WMD = 1.85, 95% CI (0.38, 3.31)], DBP-Daytime CV [WMD = 1.46, 95% CI (0.98, 1.94)] and DBP-Nighttime CV [WMD = 0.48, 95% CI (0.10, 0.85)] (Figure 4).
Aerobic combined resistance exercise significantly reduced SBP-24 h CV in hypertensive patients (p < 0.05). Aerobic combined resistance exercise had a significantly higher effect on SBP-24 h CV than aerobic exercise (p < 0.05) (Figure 8D). Aerobic and aerobic combined resistance exercise significantly reduced SBP-Daytime CV in hypertensive patients (p < 0.05) (Figure 8E). Aerobic combined resistance exercise significantly reduced SBP-Nighttime CV in hypertensive patients (p < 0.05) (Figure 8F). Aerobic combined resistance exercise significantly reduced DBP-24 h CV in hypertensive patients (p < 0.05) (Figure 8G). Aerobic and combined aerobic resistance exercise significantly reduced DBP-Daytime CV in hypertensive patients (p < 0.05) (Figure 8H). Aerobic and combined aerobic resistance exercise significantly reduced DBP-Nighttime CV in hypertensive patients (p < 0.05) (Figure 8I).
3.2.3 Results of the effect of exercise on the parasympathetic nervous system in hypertensive patients
The effect of exercise on the joint effect size of the parasympathetic nervous system in hypertensive patients was not significant [SMD = −0.08, 95% CI (−0.31, 0.14)]. Exercise did not significantly increase HF (ms²) [SMD = −0.09, 95%CI (−0.70, 0.52)], HF(nu) [SMD = −0.14, 95%CI (−0.46, 0.17)], RMSSD [SMD = −0.04, 95%CI (−0.48, 0.41)], and pNN50 [SMD = −0.07, 95%CI (−0.50, 0.36)] in patients with hypertension (Figure 5).
Aerobic combined resistance exercise significantly increased HF (nu) in hypertensive patients (p < 0.05) (Figure 9E).
3.2.4 Results of the effect of exercise on the balance of the sympathetic nervous system as well as the autonomic nervous system in patients with hypertension
Exercise significantly reduced the joint effect sizes of sympathetic as well as autonomic nervous system homeostasis in hypertensive patients [SMD = 0.29, 95% CI (0.17, 0.40)]. Exercise had a significant reduction effect on LF (ms²) [SMD = 0.23, 95%CI (0.03, 0.42)], LF (nu) [SMD = 0.28, 95%CI (0.08, 0.48)], and LF/HF [SMD = 0.37, 95%CI (0.16, 0.58)] in hypertensive patients. Combined with the results of the effects of exercise on the parasympathetic nervous system in hypertensive patients, it suggested that exercise improves the balance of the autonomic nervous system in hypertensive patients mainly focusing on the sympathetic nervous system (Figure 6).
Aerobic combined resistance exercise significantly reduced LF (ms²) in hypertensive patients (p < 0.05). Aerobic combined resistance exercise had a significantly higher effect on LF (ms²) than resistance exercise (p < 0.05) (Figure 9A). Aerobic and aerobic combined resistance exercise significantly reduced LF (nu) in hypertensive patients (p < 0.05). Aerobic combined resistance exercise had a significantly higher effect on LF (nu) than resistance exercise (p < 0.05) (Figure 9B). Aerobic, resistance and aerobic combined resistance exercise significantly reduced LF/HF in hypertensive patients (p < 0.05) (Figure 9C).
3.2.5 Results of the effect of exercise on the combined frequency-domain and time-domain effects of heart rate variability in hypertensive patients
Exercise significantly increased the joint effect size of the combined frequency-domain and time-domain effects of heart rate variability in hypertensive patients [SMD = −0.85, 95% CI (−1.30, −0.40)]. Exercise had a significant increase on TP [SMD = −0.94, 95% CI (−1.61, −0.26)] and SDNN [SMD = −0.70, 95% CI (−1.30, −0.11)] in hypertensive patients (Figure 7).
Aerobic and aerobic combined resistance exercise significantly increased TP in hypertensive patients (p < 0.05). The effect of aerobic exercise on TP was significantly higher than resistance exercise (p < 0.05) (Figure 9H). Aerobic combined resistance exercise significantly increased SDNN in hypertensive patients (p < 0.05). Aerobic combined resistance exercise had a significantly higher effect on TP than aerobic exercise (p < 0.05) (Figure 9I).
4 Discussion
This study analyzed the effect of exercise on ANS in hypertensive patients with the intention of determining the efficacy of exercise on SNS as well as PNS specifically in hypertensive patients. And the effects of specific forms of exercise including aerobic, resistance and aerobic combined resistance exercise on ANS in hypertensive patients were comparatively analyzed to explore more suitable forms of exercise for hypertensive patients to practice. This study found that either aerobic, resistance, or aerobic combined with resistance exercise can have beneficial effects (decrease in systolic and diastolic blood pressure, improvement in heart rate) in hypertensive patients.
4.1 Effect of exercise on blood pressure variability in hypertensive patients
Blood pressure variability (BPV) refers to fluctuations in blood pressure over time, and there is growing evidence that BPV is an independent predictor of cardiovascular risk (21). Any means of inducing a reduction in blood pressure variability may be a complementary therapeutic target for blood pressure control in hypertensive patients (22). Physical activity as a nonpharmacologic strategy for the prevention and treatment of hypertension (23). In turn, traditional exercises such as aerobic and resistance training are the focus of these studies (24). A recent meta-analysis suggests that aerobic exercise is a cost-effective way to improve BPV and demonstrates that aerobic exercise reduces BPV, in adult hypertensive patients (25). In this study, this phenomenon was also observed, especially in terms of the improvement of diastolic blood pressure. In addition, BPV has an important association with the autonomic cardiovascular system, and higher BP fluctuations may be associated with damage to the ANS, leading to hypertension (26). For example, in a meta-analysis exploring the effects of resistance training on autonomic control of the heart in healthy and diseased individuals, it was found that resistance training had little to no effect on autonomic control of the heart in healthy individuals, but it improved autonomic control of the heart in patients with (27). Although the mechanisms associated with the modulation of BPV after exercise are not fully understood, the reduction in blood pressure variability appears to be achieved by decreasing sympathetic activity and systemic vascular resistance (28).
4.2 Effects of exercise on the sympathetic and parasympathetic nervous system in hypertensive patients
The cardiovascular response to exercise is largely determined by neurocirculatory control mechanisms, which help regulate vascular resistance and, together with local vasodilatory mechanisms, promote blood flow to active muscles and organs (29). In a meta-analysis focused on exploring the effects of exercise training on muscle sympathetic nerve activity (MSNA), researchers found that individuals with cardiovascular disease had more significant changes in MSNA compared to individuals without cardiovascular disease. This analysis revealed an association between exercise and response, as evidenced by the fact that individuals whose sympathetic nerve activity was at a higher level prior to the exercise intervention showed a more significant decrease after receiving the exercise intervention. This finding may be valuable in improving cardiovascular health (30). Exercise has been shown to reduce elevated SNS activity in heart failure, hypertension, and the aging heart and vascular system (31). For example, 4 months of aerobic training reduced MSNA and enhanced arterial pressure reflex regulation in patients with hypertension (32). In contrast, activation of cardiac sympathetic modulation was also found in a meta-analysis of healthy individuals exposed to acute resistance exercise (33). In addition, it has been shown that the sympathetic drive reduction after exercise is more pronounced in patients with essential hypertension than in normotensive populations, which may underlie the antihypertensive effects of exercise (11). During recovery from exercise, vasodilation and reduced sympathetic nerve activity contribute significantly to post-exercise hypotension (34). These studies provide some evidence that exercise-induced blood pressure reductions in hypertensive patients may be dominated by sympathetic mechanisms.
In addition, the second arm of the ANS is the PNS, which regulates a wide range of functions from blood pressure and heart rate to respiration and immune responses (35). Reactivation of the PNS can be measured by heart rate recovery (reduction in heart rate after exercise). In the elderly, physical activity also has important antiarrhythmic effects and improves the influence of vagal components on hemodynamic parameters (36). Long-term aerobic exercise significantly reduces sympathetic excitability and improves sympathetic and vagal balance, leading to quiet heart rate and blood pressure reduction (37). Furthermore, in a recent meta-analysis the benefits of aerobic combined with resistance exercise in improving the vagus nerve in middle-aged hypertensive women were found, yet two other systematic studies negated the role of resistance exercise in optimizing vagal regulation (7, 15, 38, 39). Suggesting the controversy of exercise for parasympathetic nervous system improvement. In addition, it has been shown that when arterial pressure falls, the SNS is immediately activated, leading to increased cardiac output and peripheral vasoconstriction (40). In contrast to increased sympathetic activity, parasympathetic activity is decreased in hypertensive patients, indicating an imbalance between sympathetic and parasympathetic activity (41). However, the specific effect of exercise on SNS as well as PNS in hypertensive patients remains unclear.
Heart rate variability (HRV) not only expresses ANS status, but also assesses PNS and SNS activity (42, 43). A growing number of studies have linked autonomic nervous system imbalances (assessed by HRV) to several pathophysiologic conditions, particularly in cardiovascular disease (44). For example, 12 weeks of aerobic combined resistance training significantly increased HF (nu) and decreased the LF/HF ratio in diabetics (38). In another study, 4 weeks of aerobic exercise training (20 min at 80% of maximum heart rate intensity) improved SDNN and total power and reduced LF, LF/HF, and systolic and diastolic blood pressure in patients with prehypertension (15). Similarly, 4 weeks of resistance exercise effectively lowered systolic blood pressure and slightly improved HRV frequency domain index in hypertensive patients, thereby modulating cardiac autonomic balance (7, 45). In the present study, we also used HRV (time and frequency domains) to analyze the effect of exercise on SNS as well as PNS specifically in hypertensive patients. In subgroup analyses targeting the frequency domain of HRV, statistically significant results were found for LF (ms²), LF/HF, and TP, suggesting improved ANS function. However, further analysis of normalized LF (nu) and HF (nu) revealed that HF, which responds to fluctuations in PNS, did not change significantly in response to the intervention of movement, whereas LF was mainly driven by SNS, and this decrease suggests a negative transfer of SNS activity (46). However, there is still some controversy regarding the interpretation of LF, and it has been suggested that LF (which is co-regulated by the SNS and PNS) may reflect sympathetic-parasympathetic balance rather than merely sympathetic activity (47). Thus, LF cannot be used alone as the sole indicator of sympathetic activity, but needs to be combined with other parameters. The RMSSD and pNN50, which are closely related to the PNS, were also found not to be significantly altered by the intervention of exercise in a subgroup analysis targeting the HRV time domain (13), and SDNN showed a significant increase, which likewise proves the major modulating role of the SNS in the improvement of the symptoms of patients with hypertension by exercise.
4.3 Effects of aerobic, resistance, and combined exercise on the autonomic nervous system in hypertensive patients
The form of exercise has a great influence on the improvement of the functioning of the organism. For example, exercise training, especially aerobic exercise, is a well-recognized tool for lowering blood pressure in hypertensive subjects and for avoiding elevated blood pressure in prehypertension (6). Resistance exercise has also been proposed for the control of hypertension, but it has been reported to have a smaller antihypertensive effect compared to aerobic exercise (48). However, one study found that a combined exercise approach was more acceptable to hypertensive patients and had higher long-term adherence in an unsupervised home training program (49). Over time, this may translate into a more sustained blood pressure response. In addition, the combination of aerobic and resistance exercise can elicit additional positive adaptations in the cardiovascular system, which, in addition to the effects on blood pressure, may help reduce overall cardiovascular risk in hypertensive subjects (50). Similarly, the recommendations of the American College of Sports Medicine (ACSM) specify aerobic and resistance exercise in programs for people with hypertension, and combined exercise of the two showed more significant improvements in blood pressure than exercise alone (51). Although the role of aerobic and resistance training in enhancing cardiovascular fitness is well documented in the scientific literature, the efficacy of its impact on ANS control remains unclear (38). Based on the existing literature, we conducted a retrospective analysis of the effects of aerobic exercise, resistance exercise and combined exercise on blood pressure regulation and ANS in hypertensive patients. The results showed that aerobic exercise combined with resistance exercise has the best effect on ANS (especially SNS), which suggests that in the future, the combination of the two should be emphasized in the treatment of hypertension, and the time and intensity of the combination should be constantly improved and updated.
4.4 Limitations
Only published literature was included in this study, excluding unpublished literature, which may have affected the comprehensiveness of this study. The included literature did not discuss the form of medication (type of medication, duration of medication use), which may have introduced bias due to differences in medication use. In addition, in the literature examining resistance exercise on the autonomic nervous system in hypertensive patients, there are fewer outcome indicators involving blood pressure variability, which may have influenced the judgment of resistance exercise in this study. Although the included studies demonstrated rigor in randomization, uncertainty remains regarding blinding and transparency of reporting. Therefore, the credibility of their conclusions needs to be assessed with caution (52). Egger's test was used to assess publication bias, and the results of MBP as well as blood pressure variability suggested the presence of potential publication bias, which may be influenced by the small sample effect. The limited number of studies may lead to insufficient statistical power, which may affect the interpretation and reliability of the results (53). Heterogeneity (I² ≥ 50%) was present among the included studies. It may mainly stem from the fact that: the age range of hypertensive patients in each study ranged from 38 ± 5 to 68 ± 7 years, with different autonomic nervous system functions and responses to exercise in patients of different ages; and the duration of hypertension affects the degree of autonomic nervous system damage and adaptability to exercise interventions (54). In addition, because the included literature did not differentiate between genders, hypertensive patients of different genders may have differentiated autonomic nervous system modulation patterns and exercise adaptations due to differences in hormone levels (55). In addition, the details of the exercise protocols varied, such as the intensity of exercise from 50% to 75% of maximal heart rate for aerobic exercise and the percentage of individual maximal repetitions for resistance exercise, and these differences may have increased the uncertainty of the study results and reduced the stability of the combined effect sizes (56). To further identify potential sources of heterogeneity, this study conducted a sensitivity analysis. This analysis considered all outcome indicators and systematically excluded each study to see its effect on the overall joint effect size. The results suggest that the impact of individual studies on the overall analyzed results is limited. However, despite the results of this meta-analysis, limitations still exist. Furthermore, as the gold standard for measuring sympathetic nerve activity, muscle sympathetic nerve activity (MSNA) was not included in the analysis due to the low availability of relevant literature and the insufficient compatibility with the non-invasive indicators of this study (57). This may have limited the depth of assessment of the autonomic nervous system response. Future studies need to be more rigorous in the design, implementation, and analysis phases to obtain more accurate and generalizable conclusions to provide a more reliable basis for exercise interventions in hypertensive patients.
5 Conclusion
Exercise can regulate hypertensive patients through the autonomic nervous system, and can significantly improve the clinical symptoms of hypertensive patients. Moreover, the autonomic nervous system regulation of hypertensive patients by exercise mainly focuses on sympathetic nerves. Aerobic, resistance, and a combination of both exercise modalities all have positive effects on hypertensive patients. Among them, the efficacy of combined exercise in affecting the autonomic nervous system (especially the sympathetic nervous system) of hypertensive patients is more obvious, and it is more effective in improving the blood pressure and heart rate level of the patients. These results provide valuable guidance for clinical complementary therapies, suggesting that future investigations into the mechanisms of exercise interventions in hypertensive patients could focus on the sympathetic nervous system, and that the protocol for exercise to improve hypertension could focus on aerobic combination training, with continual refinement and updating of the duration as well as the intensity of this form of exercise.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
J-FW: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. S-JM: Supervision, Writing – review & editing. FX: Supervision, Writing – review & editing. X-LL: Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work is supported by the Natural Science Foundation of Heilongjiang Province (LH2024G003).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Hicklin HE Gilbert ON Ye F Brooks JE Upadhya B Hypertension as a road to treatment of heart failure with preserved ejection fraction. Curr Hypertens Rep. (2020) 22(10):82. 10.1007/s11906-020-01093-7
2.
Roque Marçal I Teixeira Do Amaral V Fernandes B Martins de Abreu R Alvarez C Veiga Guimarães G et al Acute high-intensity interval exercise versus moderate-intensity continuous exercise in heated water-based on hemodynamic, cardiac autonomic, and vascular responses in older individuals with hypertension. Clin Exp Hypertens. (2022) 44(5):427–35. 10.1080/10641963.2022.2065288
3.
Prasertsri P Phoemsapthawee J Kuamsub S Poolpol K Boonla O . Effects of long-term regular continuous and intermittent walking on oxidative stress, metabolic profile, heart rate variability, and blood pressure in older adults with hypertension. J Environ Public Health. (2022) 2022:5942947. 10.1155/2022/5942947
4.
He B Ji D Zhang B . Hypertension and its correlation with autonomic nervous system dysfunction, heart rate variability and chronic inflammation. Blood Press. (2024) 33(1):2405156. 10.1080/08037051.2024.2405156
5.
Collard D Westerhof BE Karemaker JM Stok WJ Postema PG Krediet CTP et al Automated analysis of finger blood pressure recordings provides insight in determinants of baroreflex sensitivity and heart rate variability-the HELIUS study. Med Biol Eng Comput. (2023) 61(5):1183–91. 10.1007/s11517-023-02768-4
6.
Fomenky YN . Impact of regular physical activity on high blood pressure management. (2022).
7.
Deng Y Zeng X Tang C Hou X Zhang Y Shi L . The effect of exercise training on heart rate variability in patients with hypertension: a systematic review and meta-analysis. J Sports Sci. (2024) 42(13):1272–87. 10.1080/02640414.2024.2388984
8.
Abidi A Mujaddadi A Raza S Moiz J . Effect of physical exercise on cardiac autonomic modulation in hypertensive individuals: a systematic review and meta-analysis. Curr Hypertens Rev. (2023) 19:149–72. 10.2174/1573402119666230803090330
9.
Mitchell J . Abnormal cardiovascular response to exercise in hypertension: contribution of neural factors. Am J Physiol Regul Integr Comp Physiol. (2017) 312:R851–63. 10.1152/ajpregu.00042.2017
10.
Xiao X Deng X Zhang G Liu M Fu D Yang P et al Monitoring of the regulatory ability and regulatory state of the autonomic nervous system and its application to the management of hypertensive patients: a study protocol for randomised controlled trials. BMJ Open. (2023) 13(6):e063434. 10.1136/bmjopen-2022-063434
11.
O'Sullivan S Bell C . The effects of exercise and training on human cardiovascular reflex control. J Auton Nerv Syst. (2000) 81:16–24. 10.1016/S0165-1838(00)00148-X
12.
Besnier F Labrunée M Pathak A Pavy-Le Traon A Galès C Sénard JM et al Exercise training-induced modification in autonomic nervous system: an update for cardiac patients. Ann Phys Rehabil Med. (2017) 60(7):27–35. 10.1016/j.rehab.2016.07.002
13.
Ricca-Mallada R Migliaro ER Silvera G Chiappella L Frattini R Ferrando-Castagnetto F . Functional outcome in chronic heart failure after exercise training: possible predictive value of heart rate variability. Ann Phys Rehabil Med. (2017) 60(2):87–94. 10.1016/j.rehab.2016.12.003
14.
Kazeminia M Daneshkhah A Jalali R Vaisi-Raygani A Salari N Mohammadi M . The effect of exercise on the older adult’s blood pressure suffering hypertension: systematic review and meta-analysis on clinical trial studies. Int J Hypertens. (2020) 2020:2786120. 10.1155/2020/2786120
15.
Saeidi M Ravanbod R . Effects of resistance added on aerobic training on autonomic function in cardiac patients. Anatol J Cardiol. (2022) 26:80–9. 10.5152/AnatolJCardiol.2021.215
16.
Rethlefsen M Page M . PRISMA 2020 and PRISMA-S: common questions on tracking records and the flow diagram. J Med Libr Assoc. (2022) 110:253–7. 10.5195/jmla.2022.1449
17.
Drevon D Fursa S Malcolm A . Intercoder reliability and validity of WebPlotDigitizer in extracting graphed data. Behav Modif. (2017) 41:323–39. 10.1177/0145445516673998
18.
Jørgensen L Paludan-Müller AS Laursen DR Savović J Boutron I Sterne JA et al Evaluation of the Cochrane tool for assessing risk of bias in randomized clinical trials: overview of published comments and analysis of user practice in Cochrane and non-Cochrane reviews. Syst Rev. (2016) 5:80. 10.1186/s13643-016-0259-8
19.
Migliavaca CB Stein C Colpani V Barker TH Ziegelmann PK Munn Z et al Meta-analysis of prevalence: I2 statistic and how to deal with heterogeneity. Res Synth Methods. (2022) 13(3):363–7. 10.1002/jrsm.1547
20.
Nino A Brignardello-Petersen R . How to read a network meta-analysis. Eur Urol Focus. (2023) 9:701–4. 10.1016/j.euf.2023.10.018
21.
Pagonas N Dimeo F Bauer F Seibert F Kiziler F Zidek W et al The impact of aerobic exercise on blood pressure variability. J Hum Hypertens. (2014) 28(6):367–71. 10.1038/jhh.2013.121
22.
Domingues L Carpes L Fuchs S Ferrari R . Effects of a single beach tennis session on short-term blood pressure variability in individuals with hypertension: a randomized crossover trial. Blood Press Monit. (2022) 27:185–91. 10.1097/MBP.0000000000000586
23.
Pescatello LS MacDonald HV Ash GI Lamberti LM Farquhar WB Arena R et al Assessing the existing professional exercise recommendations for hypertension: a review and recommendations for future research priorities. Mayo Clin Proc. (2015) 90(6):801–12. 10.1016/j.mayocp.2015.04.008
24.
Ferrari R Cadore E Périco B Kothe G . Acute effects of body-weight resistance exercises on blood pressure and glycemia in middle-aged adults with hypertension. Clin Exp Hypertens. (2021) 43:63–8. 10.1080/10641963.2020.1806293
25.
Lin M Lin Y Li Y Lin X . Effect of exercise training on blood pressure variability in adults: a systematic review and meta-analysis. PLoS One. (2023) 18(10):e0292020. 10.1371/journal.pone.0292020
26.
Maseli A Aeschbacher S Schoen T Fischer A Jung M Risch M et al Healthy lifestyle and blood pressure variability in young adults. Am J Hypertens. (2017) 30(7):690–9. 10.1093/ajh/hpx034
27.
Bhati P Moiz J Menon G Hussain M . Does resistance training modulate cardiac autonomic control? A systematic review and meta-analysis. Clin Auton Res. (2019) 29:75–103. 10.1007/s10286-018-0558-3
28.
Del Pinto R Pietropaoli D Dobre M Ferri C . Prognostic importance of long-term SBP variability in high-risk hypertension. J Hypertens. (2020) 38:2237–44. 10.1097/HJH.0000000000002552
29.
Wan H Bunsawat K Amann M . Autonomic cardiovascular control during exercise. Am J Physiol Heart Circ Physiol. (2023) 325:H675–86. 10.1152/ajpheart.00303.2023
30.
Meyer SE Kimber M Maier LE Matenchuk B Moldenhauer R de Waal S et al The impact of exercise training on muscle sympathetic nerve activity: a systematic review and meta-analysis. J Appl Physiol (1985). (2024) 137(2):429–44. 10.1152/japplphysiol.00060.2024
31.
Zanesco A Antunes E . Effects of exercise training on the cardiovascular system: pharmacological approaches. Pharmacol Ther. (2007) 114:307–17. 10.1016/j.pharmthera.2007.03.010
32.
Laterza MC de Matos LD Trombetta IC Braga AM Roveda F Alves MJ et al Exercise training restores baroreflex sensitivity in never-treated hypertensive patients. Hypertension. (2007) 49(6):1298–306. 10.1161/HYPERTENSIONAHA.106.085548
33.
Marasingha-Arachchige S Rubio-Arias J Alcaraz P Chung L . Factors that affect heart rate variability following acute resistance exercise: a systematic review and meta-analysis. J Sport Health Sci. (2022) 11:376–92. 10.1016/j.jshs.2020.11.008
34.
Ferrari R Umpierre D Vogel G Vieira PJC Santos LP de Mello RB et al Effects of concurrent and aerobic exercises on postexercise hypotension in elderly hypertensive men. Exp Gerontol. (2017) 98:1–7. 10.1016/j.exger.2017.08.012
35.
Shanks J Ramchandra R . Angiotensin II and the cardiac parasympathetic nervous system in hypertension. Int J Mol Sci. (2021) 22(22):12305. 10.3390/ijms222212305
36.
Lee K Picard G Beske S Hwang G Taylor J . Effects of fitness and age on the response to vagotonic atropine. Auton Neurosci Basic Clin. (2008) 139:60–7. 10.1016/j.autneu.2008.01.007
37.
Carter J Ray C . Sympathetic neural adaptations to exercise training in humans. Auton Neurosci Basic Clin. (2015) 188:36–43. 10.1016/j.autneu.2014.10.020
38.
Masroor S Bhati P Verma S Khan M Hussain ME . Heart rate variability following combined aerobic and resistance training in sedentary hypertensive women: a randomised control trial. Indian Heart J. (2018) 70(Suppl 3):S28–35. 10.1016/j.ihj.2018.03.005
39.
Picard M Tauveron I Magdasy S Benichou T Bagheri R Ugbolue UC et al Effect of exercise training on heart rate variability in type 2 diabetes mellitus patients: a systematic review and meta-analysis. PLoS One. (2021) 16(5):e0251863. 10.1371/journal.pone.0251863
40.
Galougahi K Ashley E Ali Z . Redox regulation of vascular remodeling. Cell Mol Life Sci. (2016) 73:349–63. 10.1007/s00018-015-2068-y
41.
Valensi P . Autonomic nervous system activity changes in patients with hypertension and overweight: role and therapeutic implications. Cardiovasc Diabetol. (2021) 20(1):170. 10.1186/s12933-021-01356-w
42.
Ernst G . Heart-rate variability-more than heart beats?Front Public Health. (2017) 5:240. 10.3389/fpubh.2017.00240
43.
Tiwari R Kumar R Malik S Raj T Kumar P . Analysis of heart rate variability and implication of different factors on heart rate variability. Curr Cardiol Rev. (2021) 17(5):e160721189770. 10.2174/1573403X16999201231203854
44.
Xhyheri B Manfrini O Mazzolini M Pizzi C Bugiardini R . Heart rate variability today. Prog Cardiovasc Dis. (2012) 55:321–31. 10.1016/j.pcad.2012.09.001
45.
Trevizani GA Seixas MB Benchimol-Barbosa PR Vianna JM da Silva LP Nadal J . Effect of resistance training on blood pressure and autonomic responses in treated hypertensives. J Strength Cond Res. (2018) 32(5):1462–70. 10.1519/JSC.0000000000001995
46.
Billman G . Heart rate variability—a historical perspective. Front Physiol. (2011) 2:86. 10.3389/fphys.2011.00086
47.
Pearson M Smart N . Exercise therapy and autonomic function in heart failure patients: a systematic review and meta-analysis. Heart Fail Rev. (2018) 23:91–108. 10.1007/s10741-017-9662-z
48.
Cornelissen W Fagard R . Effect of resistance training on resting blood pressure: a meta-analysis of randomized controlled trials. J Hypertens. (2005) 23:251–9. 10.1097/00004872-200502000-00003
49.
Timmons JF Minnock D Hone M Cogan KE Murphy JC Egan B . Comparison of time-matched aerobic, resistance, or concurrent exercise training in older adults. Scand J Med Sci Sports. (2018) 28(11):2272–83. 10.1111/sms.13254
50.
Caminiti G Iellamo F Mancuso A Cerrito A Montano M Manzi V et al Effects of 12 weeks of aerobic versus combined aerobic plus resistance exercise training on short-term blood pressure variability in patients with hypertension. J Appl Physiol. (2021) 130(4):1085–92. 10.1152/japplphysiol.00910.2020
51.
Son W Sung K Bharath L Choi K Park S . Combined exercise training reduces blood pressure, arterial stiffness, and insulin resistance in obese prehypertensive adolescent girls. Clin Exp Hypertens. (2017) 39:546–52. 10.1080/10641963.2017.1288742
52.
Voracek M Kossmeier M Tran U . Which data to meta-analyze, and how? A specification-curve and multiverse-analysis approach to meta-analysis. Z Psychol. (2019) 227:64–82. 10.1027/2151-2604/a000357
53.
Rodgers M Pustejovsky J . Evaluating meta-analytic methods to detect selective reporting in the presence of dependent effect sizes. Psychol Methods. (2021) 26:141–60. 10.1037/met0000300
54.
Taylor KA Wiles JD Coleman DA Leeson P Sharma R O'Driscoll JM . Neurohumoral and ambulatory haemodynamic adaptations following isometric exercise training in unmedicated hypertensive patients. J Hypertens. (2019) 37(4):827–36. 10.1097/HJH.0000000000001922
55.
Almeida-Santos MA Barreto-Filho JA Oliveira JL Reis FP da Cunha Oliveira CC Sousa AC . Aging, heart rate variability and patterns of autonomic regulation of the heart. Arch Gerontol Geriatr. (2016) 63:1–8. 10.1016/j.archger.2015.11.011
56.
Boutcher Y Boutcher S . Exercise intensity and hypertension: what’s new?J Hum Hypertens. (2017) 31:157–64. 10.1038/jhh.2016.62
57.
Urbancsek R Forgács IN Papp TB Boczán J Barta J Édes I et al Az izom szimpatikus idegaktivitás vizsgálatának elméleti alapjai és története [Theory and history of the study of muscle sympathetic nerve activity]. Orv Hetil. (2020) 161(29):1190–9. Hungarian. 10.1556/650.2020.31780
58.
Fu JH Chen ZH Liu LL Fang H Ma YX Cui MZ et al The influence of tai chi and fitness exercises on autonomic nervous system in patients with essential hypertension. Chin J Hypertens. (2024) 32:763–71. 10.16439/j.issn.1673-7245.2024.08.010
59.
Rodrigues ML Carrijo VHV Amaral AL Cunha ACR Tavares JB Costa JG et al Acute effect of interval step exercise versus continuous walk exercise on cardiovascular parameters in hypertensive postmenopausal women: a clinical, controlled, and randomized study. J Bodyw Mov Ther. (2023) 35:124–9. 10.1016/j.jbmt.2023.04.058
60.
Sardeli AV Gáspari AF Dos Santos WM de Araujo AA de Angelis K Mariano LO et al Comprehensive time-course effects of combined training on hypertensive older adults: a randomized control trial. Int J Environ Res Public Health. (2022) 19(17):11042. 10.3390/ijerph191711042
61.
Fraccari-Pires N Coelho-Júnior HJ Gambassi BB Cabral de Faria AP Ritter AMV Gasparetti CS et al Cardiovascular autonomic responses to aerobic, resistance and combined exercises in resistance hypertensive patients. BioMed Res Int. (2022) 2022:8202610. 10.1155/2022/8202610
62.
Casonatto Oliveira LS Grandolf K . Post-aerobic-exercise autonomic responses in hypertensives—a randomized controlled trial. Arterial Hypertens. (2020) 24:74–82. 10.5603/AH.a2020.0009
63.
Oliveira-Dantas FF Brasileiro-Santos MDS Thomas SG Silva AS Silva DC Browne RAV et al Short-term resistance training improves cardiac autonomic modulation and blood pressure in hypertensive older women: a randomized controlled trial. J Strength Cond Res. (2020) 34(1):37–45. 10.1519/JSC.0000000000003182
64.
Brito LC Peçanha T Fecchio RY Rezende RA Sousa P DA Silva-Júnior N et al Morning versus evening aerobic training effects on blood pressure in treated hypertension. Med Sci Sports Exerc. (2019) 51(4):653–62. 10.1249/MSS.0000000000001852
65.
Vale AF Carneiro JA Jardim PCV Jardim TV Steele J Fisher JP et al Acute effects of different resistance training loads on cardiac autonomic modulation in hypertensive postmenopausal women. J Transl Med. (2018) 16(1):240. 10.1186/s12967-018-1615-3
66.
Pushpanathan P Madanmohan T Swaminathan RP Senthil KS Ananda BB Chandrasekhar M . Randomized controlled trial of 12-week yoga therapy as lifestyle intervention in patients of essential hypertension and cardiac autonomic function tests. Natl J Physiol Pharm Pharmacol. (2015) 6:19–26. 10.5455/njppp.2015.5.2408201572
67.
Sun XL Bai QJ . The impact of aerobic exercise on cardiovascular autonomic nervous function in elderly patients with refractory hypertension. Chin J Gerontol. (2015) 35:1005–9202. 10.14163/j.cnki.11-5547/r.2019.10.070
68.
Zhang XS . The regulation of long-term aerobic exercise on cardiovascular autonomic nervous function in patients with essential hypertension. J Henan Univ (Nat Sci). (2015) 45:73–7. 10.15991/j.cnki.411100.2015.01.013
69.
Huang W . The impact of different exercise modes on cardiovascular autonomic nervous function in patients with refractory hypertension. Chin J Sports Med. (2014) 33:431–9. 10.16038/j.1000-6710.2014.05.001
70.
Wang ST Zeng YG Zhao J . The impact of brisk walking exercise on autonomic nervous function in patients with essential hypertension. Modern Prev Med. (2007) 34:2848–51. 10.3969/j.issn.1003-8507.2007.15.020
71.
Knoepfli-Lenzin C Sennhauser C Toigo M Boutellier U Bangsbo J Krustrup P et al Effects of a 12-week intervention period with football and running for habitually active men with mild hypertension. Scand J Med Sci Sports. (2010) 20(Suppl 1):72–9. 10.1111/j.1600-0838.2009.01089.x
Summary
Keywords
aerobic exercise, resistance exercise, hypertension, autonomic nervous system, meta-analysis
Citation
Wang J-f, Mao S-j, Xia F and Li X-l (2025) Effects of aerobic and resistance exercise on patients with hypertension: a systematic review and meta-analysis focusing on the sympathetic nervous system. Front. Cardiovasc. Med. 12:1569638. doi: 10.3389/fcvm.2025.1569638
Received
01 February 2025
Accepted
21 July 2025
Published
18 August 2025
Volume
12 - 2025
Edited by
Marco Alfonso Perrone, University of Rome Tor Vergata, Italy
Reviewed by
Haijun Duan, Shaanxi Normal University, China
Nirupama Ramadas, University of North Carolina at Chapel Hill, United States
Daniel Craighead, University of Minnesota Twin Cities, United States
Updates
Copyright
© 2025 Wang, Mao, Xia and Li.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Xiao-lin Li 1318706074@qq.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.